Nucleoside analogue as well as preparation method and application thereof
A nucleoside analog and reaction technology, which can be used in the preparation of sugar derivatives, chemical instruments and methods, drug combinations, etc., and can solve the problems of poor oral bioavailability and low intestinal permeability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The present invention also provides a method for preparing nucleoside analogs described in the above technical scheme, comprising the following steps:
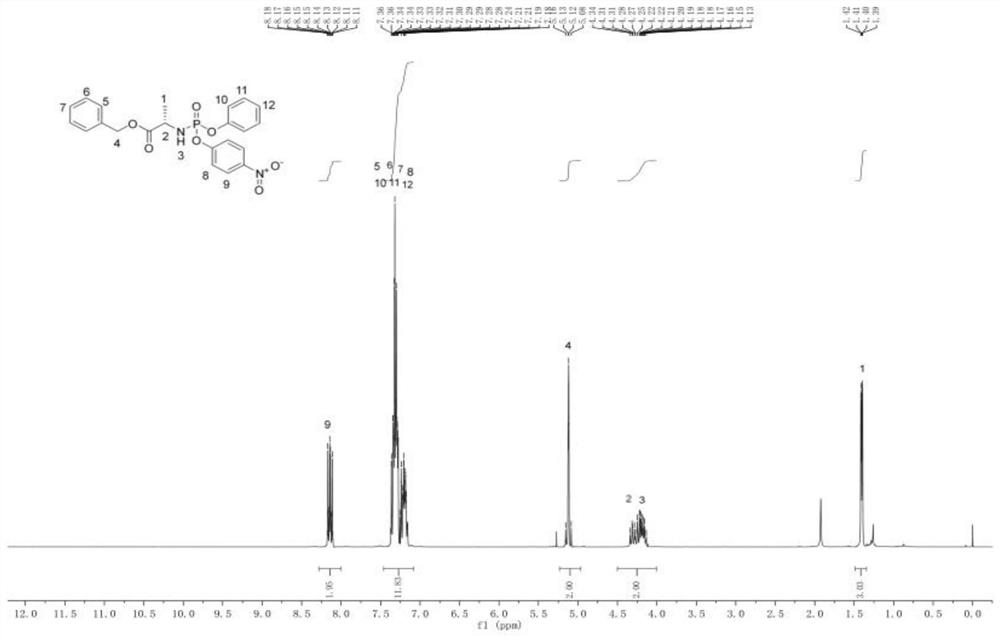
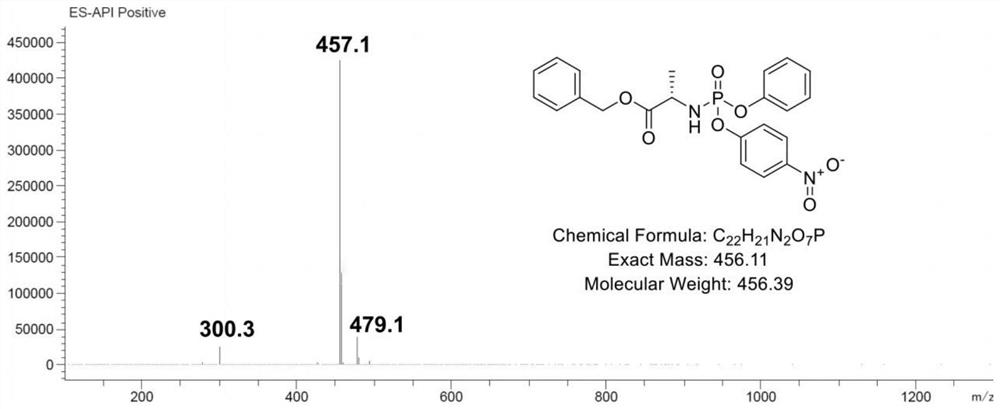
[0033] The L-alanine benzyl ester hydrochloride (formula a) after dissolving is mixed with triethylamine, carries out neutralization reaction, obtains free L-alanine benzyl ester, and free L-alanine benzyl ester Mix with phenyl phosphate dichloride (b) to carry out the first substitution reaction to obtain an intermediate product A, and mix the intermediate product A with p-nitrophenol (c) and triethylamine to perform a second substitution reaction to obtain the formula Compound shown in 1,
[0034]
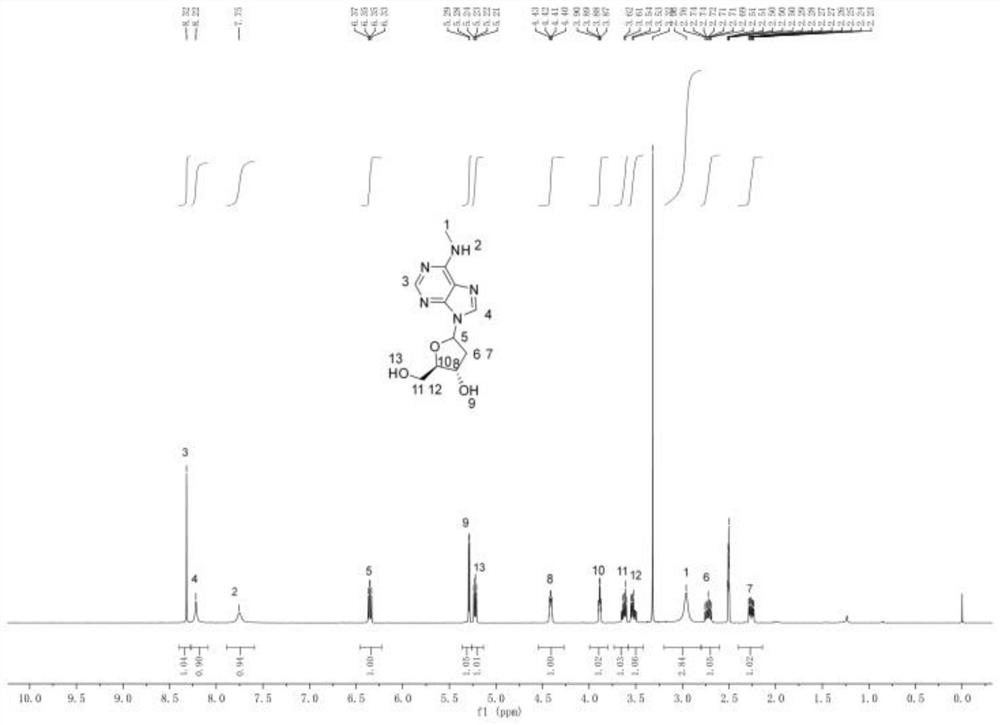
[0035] Dissolving N6-methyl deoxyadenosine (formula 2) in a mixed solution of anhydrous tetrahydrofuran and N-methylpyrrolidone, adding tert-butylmagnesium chloride for activation reaction, to obtain activated N6-methyl deoxyadenosine, and The activated N6-methyl deoxyadenosine is mixed with the compound shown in formu...
Embodiment 1
[0055] Carry out the synthesis of nucleoside analogs of the present invention according to the following reaction formula:
[0056]
[0057] 1) The first step is to synthesize compound 1 (i.e. the compound shown in formula 1):
[0058]
[0059] Under argon protection, 21.568g of L-alanine benzyl ester hydrochloride (0.1mol, 1eq) was dissolved in 200ml of dichloromethane, 21.25g of triethylamine (0.21mol, 2.1eq) was added, and the temperature was lowered to -78 °C, 23.2 g of phenyl phosphate dichloride (0.11 mol, 1.1 eq) was added dropwise, and after the addition was completed, it was kept at -78 °C for 30 min. Raised to room temperature for 3h. Then it was lowered to 0° C., and 13.91 g of p-nitrophenol (0.1 mol, 1 eq) and 10.12 g of triethylamine (0.1 mol, 1 eq) were added. After the addition, keep warm for 30 minutes, then rise to room temperature and react for 5 hours, and spot the plate (petroleum ether: ethyl acetate). After the reaction was completed, the solutio...
Embodiment 2
[0069] Examples of enhanced bioavailability
[0070] Add 0.1mM N6-methyldeoxyadenosine (dm6A) and the nucleoside analog QKY-613 of the present invention to Hela cells respectively, add DMSO to the control group (Ctrl), add PBS buffer to wash three times after 12 hours, and wash three times every 1 *10^7 cells were added with 100 microliters of cell metabolite extraction reagent (volume ratio of methanol, acetonitrile and water: 40:40:20), after repeated freezing and thawing with liquid nitrogen and 37°C, at 4°C, 1,3000rpm / min centrifuged for 15min, and the supernatant was taken to obtain cell metabolites. Quantitative detection of N6-Methyl-dATP in the extracted metabolites by liquid chromatography tandem mass spectrometry. The result shows that the drug QKY-613 cell availability of the new synthesis of the present invention is obviously higher than dm6A, as table 1 and Figure 5 shown in Table 1 and Figure 5 is the free intracellular N6-methyldeoxyadenosine triphosphate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com