Vaccine based on cyclodextrin grafted chitosan, preparation method and application
A technology of cyclodextrin and chitosan, applied in the field of biomedical engineering, can solve problems such as hindering biomedical application and poor solubility, and achieve the effects of good in vivo delivery and antigen storage, good solubility, and improved immune stimulation ability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
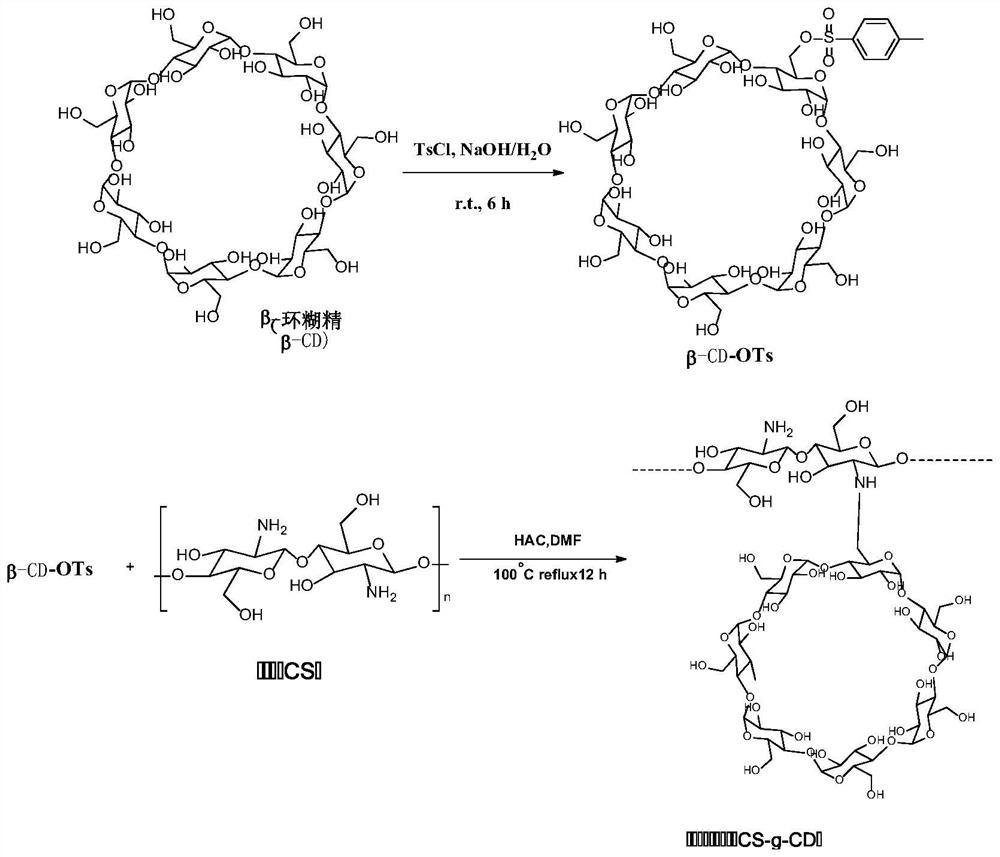
[0039] Embodiment 1: synthetic cyclodextrin grafted chitosan (CS-g-CD)
[0040] Synthetic route, see figure 1. β-Cyclodextrin (50.0 g) was dissolved in 200 mL of double-distilled water to obtain a suspension; p-toluenesulfonyl chloride (15.0 g) was then slowly added, and the mixture was stirred at room temperature. After 20% w / v NaOH solution (50 mL) was added to the suspension for 6 hours, the residue was removed by filtration. The pH of the filtrate was adjusted to 7 with dilute hydrochloric acid, and the solution was left at 4°C overnight to obtain a precipitate. The precipitate was filtered and recrystallized to obtain the precipitate, which was lyophilized to obtain β-CD-OTs.
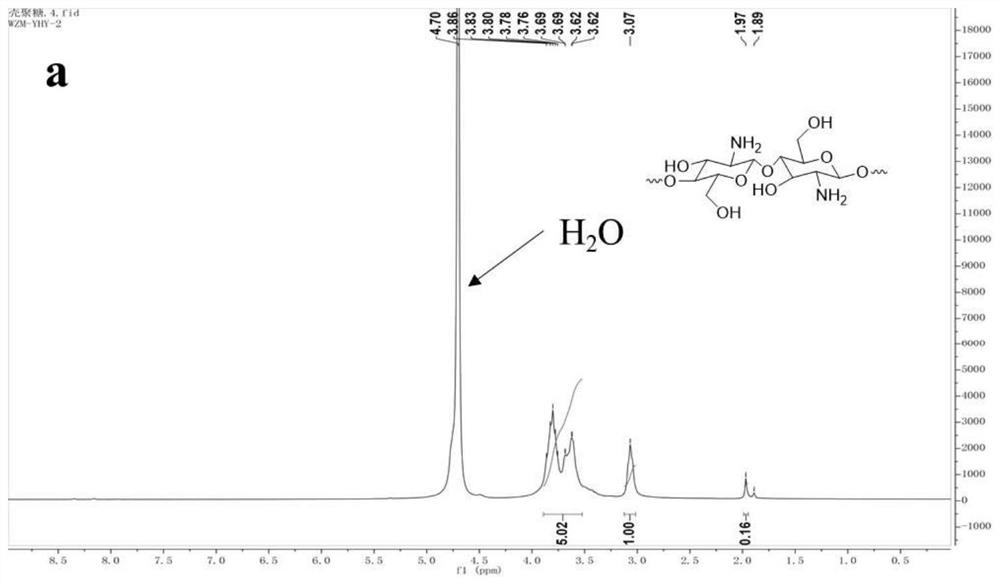
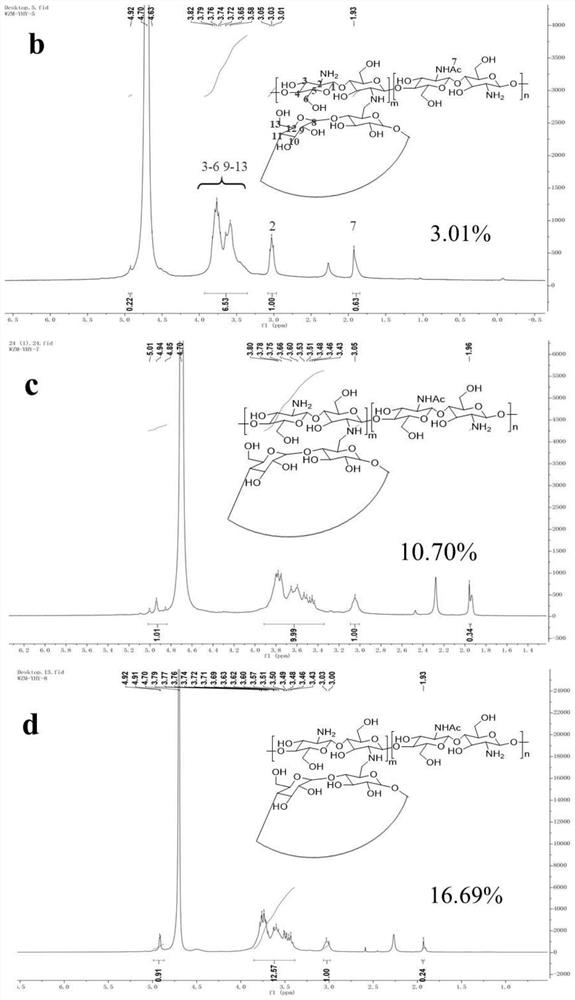
[0041] Chitosan CS (1.00 g) was dissolved in 1% (v / v) acetic acid (80 mL). The N,N-dimethylformamide (DMF, 40mL) solution of β-CD-OTs (1.00-5.00g) was added to the chitosan solution, and cyclodextrins with different grafting degrees were obtained according to different ratios grafted chitosan....
Embodiment 2
[0045] Embodiment 2: the synthesis of MUC1 polypeptide compound
[0046] The synthesis of MUC1 polypeptide compounds is carried out through the solid-phase synthesis strategy proposed by R. Bruce Merrifield. The process is to sequentially link amino acids from the C-terminal to the N-terminal on the solid-phase resin carrier, and finally cut the polypeptide from the solid-phase carrier.
[0047] MUC1, adamantane-modified MUC1 (ada-ACP-MUC1), and adamantane-modified MUC1(Tn) (ada-ACP-MUC1(Tn)) polypeptide compounds (polypeptide structure such as Figure 4 shown). Wherein, ACP is a 6-aminocaproic acid linking arm, and Tn is a glycosylation modification. The specific method of synthesis is as follows:
[0048] 1. Resin swelling, weigh a certain amount of Fmoc-Ala-OH Wang resin (0.05mmol) and place it in a 10mL polypeptide solid-phase synthesis tube, add 6mL N,N-dimethylformamide (DMF) solution, and swell for 3h. Drain the solvent and wash 3 times with DMF and DCM respectively; ...
Embodiment 3
[0055] Example 3 Cyclodextrin-grafted chitosan and MUC1 polypeptide compound (i.e. MUC1 antigen) assembled into nanoparticles
[0056] This embodiment has constructed the vaccine preparation of MUC1 and cyclodextrin grafted chitosan, such as Figure 4 shown. The cyclodextrin-grafted chitosan used in this example is the product 3 with a higher grafting degree, and the grafting degree is 16.69%.
[0057] CS-g-CD and several different MUC1 antigens were further assembled into nanoparticles, that is, cyclodextrin-grafted chitosan (product 3, CS-g-CD) was formulated into a 1 mg / mL solution with acetic acid , then adjust its pH to 4.5 with 0.1M NaOH solution, and stir for 1 h; then, add the above-mentioned polypeptide compounds MUC1, ada-ACP-MUC1, ada-ACP-MUC1 (Tn) respectively, stir at room temperature, and add sodium tripolyphosphate solution ( 1 mg / mL, 0.29 mL), stirred at room temperature for 1 h, and dialyzed for 3 days with a 7000 Da dialysis bag to obtain nanoparticles. de...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| degree of grafting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com