Kit of high-efficiency novel DC autovaccine, method for preparing high-efficiency novel DC autovaccine and application of DC autovaccine
A kit and a new type of technology are applied in the field of preparing high-efficiency new autologous DC vaccines, which can solve the problem of difficulty in exerting anti-tumor effects, and achieve the enhancement of pDC immune stimulation function, specific immune killing and anti-tumor, and DC migration ability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Culture of DC by GM-CSF+IL-4+FLT3-L+ATRA cytokine combination
[0040] Prepare a novel autologous DC vaccine with high efficiency as follows, including the following steps:
[0041] 1) Preparation of mononuclear cells: extract 50-100ml of peripheral blood from tumor patients under sterile conditions or about 80-100ml of peripheral blood mononuclear cells from patients with apheresis, transfer them into a 50ml centrifuge tube, add an equal volume of normal saline to dilute, Then transfer it to the top of the lymphocyte separation medium according to the volume of 2:1 or 1:1, 2000 rpm, slowly rise and fall (rise 1 fall 0), centrifuge for 20-30 minutes, absorb the buffy coat layer, which is a single nucleus Cells were washed twice with normal saline or Hanks solution, centrifuged at 1000 rpm for 10 minutes, the supernatant was discarded, and the precipitate was PBMCs;
[0042] 2) Preparation of DC precursor cells: PBMCs in a culture flask, 37°C, 5% CO 2 Incubate for 1-2 ...
Embodiment 2
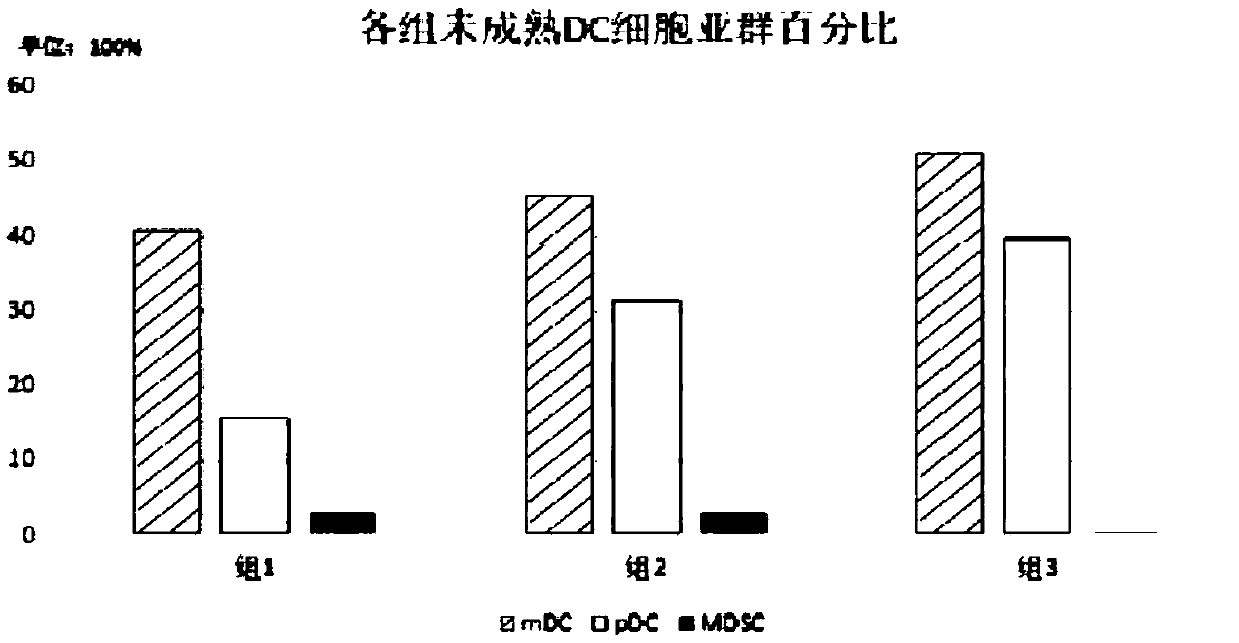
[0051] The semi-adherent PBMCs in step 2) of Example 1 were cultured with 500-1000U / mlGM-CSF+500-1000U / mlIL-4+50-200ng / mlFLT3-L+1-5uMATRA+1-5% autologous plasma Immature DCs were obtained after 4-5 days in the culture medium, and mature DCs were obtained as follows:
[0052] 1) Loading the obtained immature cells with 100ug / ml antigen for 24h;
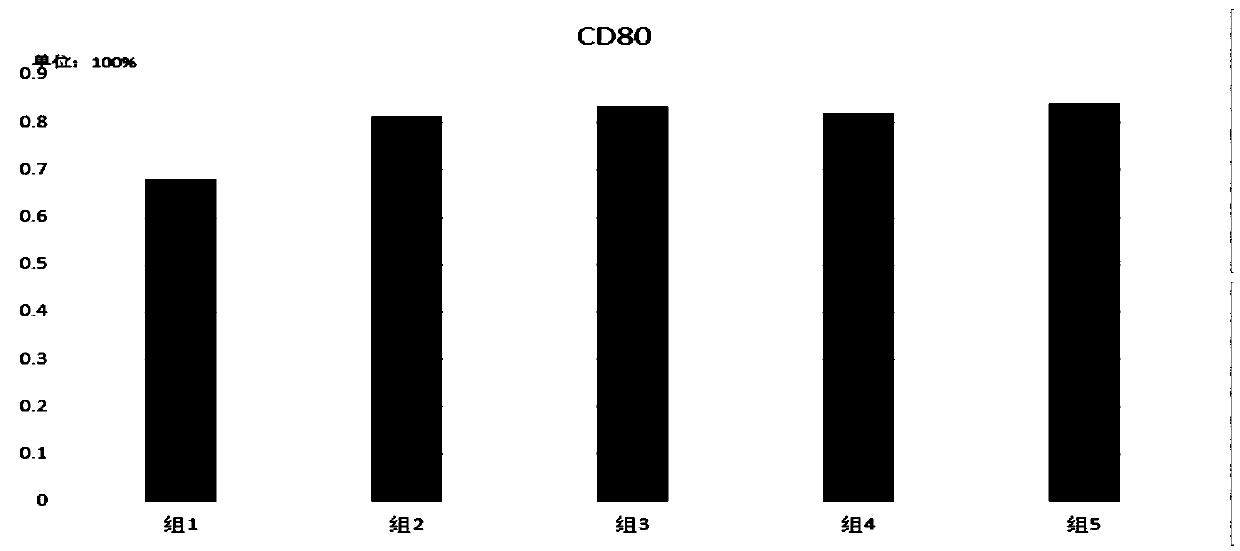
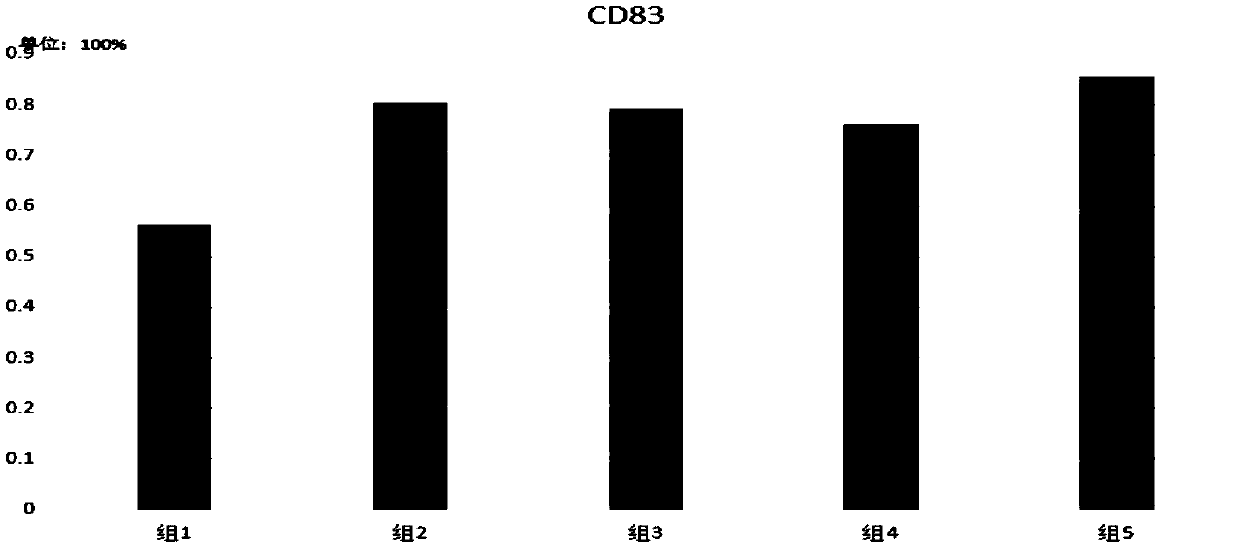
[0053] 2) Divide the cells in the above step 1) into 2 groups:
[0054] Group 1: stimulated with 500-2000U / ml TNF-α for 24h;
[0055] Group 2: Stimulated with TLRs agonists mixed with 0.1-5KE / mlOK432+1-5uMCPGODN2336 for 24h;
[0056] Group 3: stimulated with TLRs agonists mixed with 0.1-5KE / mlOK432+1-5uMCPGODN2006 for 24h;
[0057] Group 4: Stimulated with TLRs agonists mixed with 0.1-5KE / mlOK432+1-5uMCPGODN2395 for 24h;
[0058] Group 5: (DC vaccine of the present invention) stimulated with TLRs agonists mixed with 0.1-5KE / mlOK432+1-5uMCPGODN21798 for 24h;
[0059] The DC maturation markers of each group in step 2) were detected ...
Embodiment 3
[0067] The DC vaccine obtained by the OK432+CPGODN21798 combined stimulation method with the strongest ability to secrete IL-12p70 and the lowest expression of PD-L1 was selected and compared with the DC vaccine obtained by the TNF-α method to detect the migration ability of mature DC and the ability to induce T cell proliferation and activation.
[0068] The semi-adherent PBMCs in the step 2) of embodiment one are divided into 2 groups:
[0069] Group 1 (traditional DC vaccine): After cultured with GM-CSF+IL-4 for 4-5 days, the antigen was loaded for 24 hours, and TNF-α stimulated maturation for 24 hours;
[0070]Group 2 (DC vaccine of the present invention, the same DC vaccine as Group 5 in Example 2): after culturing for 4-5 days with GM-CSF+IL-4+FLT3-L+ATRA, 100ug / ml antigen loading for 24h, and then Stimulate maturation with OK432+CPGODN21798;
[0071] The prepared DC vaccine was mixed with T cells and cultured for 24 hours.
[0072] WesternBlot detects the expression l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com