Anti-hu-OX40 antigen nano antibody and application thereof
A technology of hu-ox40 and nano-antibody, which is applied in the direction of antibody, application, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc. It can solve the problems of enhancing activation and proliferation, complex anti-tumor immune response, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Preparation of membrane-expressing human OX40 stably transfected cell line
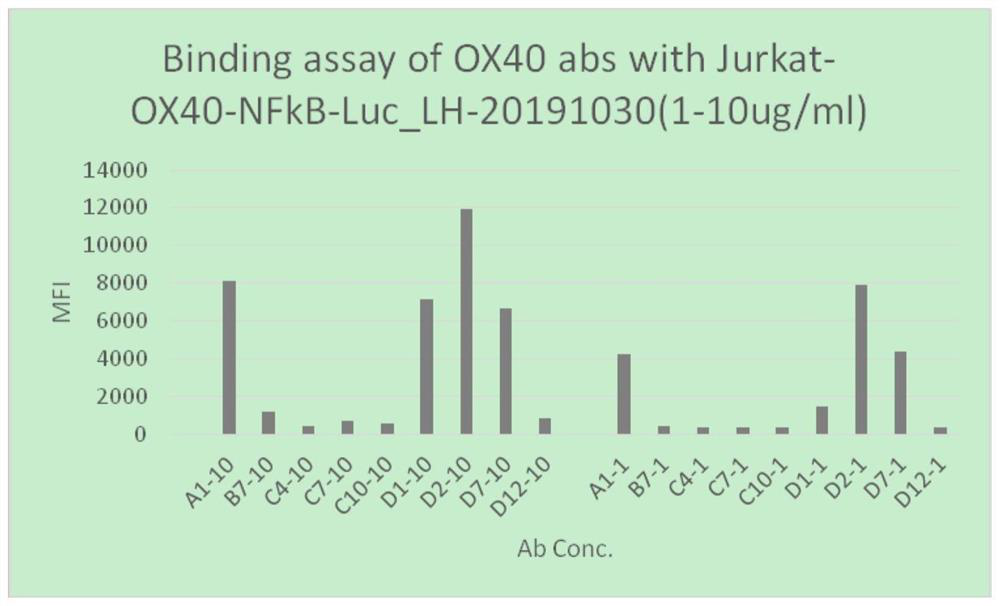
[0041] Whole-gene synthesis of the full-length human OX40 gene, including signal peptide, extracellular segment, transmembrane region and intracellular segment, with appropriate restriction sites at both ends. Using the method of enzyme digestion, the full-length gene of OX40 was constructed into the eukaryotic expression vector GC-ID (modified from pMH3 plasmid, Hangzhou Anpu), and the GC-ID-OX40 plasmid was obtained and then transfected into CHO-DG44 cells to construct Membrane expression of human OX40 stably transfected cell line CHO-DG44-OX40 was used to detect the binding activity of OX40 mAb by flow cytometry; Jurkat-OX40-NFκB-Luc cells were prepared in the same process for the detection of OX40 antibody binding to OX40 at the cellular level Or OX40 antibody NFκB cell pathway activation detection.
Embodiment 2
[0042] Example 2: Preparation of rhOX40-Avi-His-bio antigenic protein
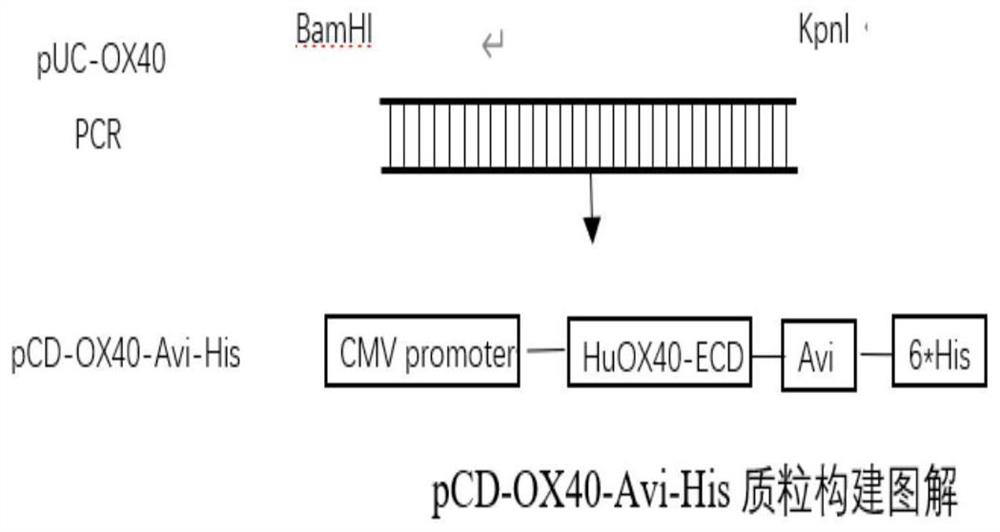
[0043] For the synthetic OX40 full-length gene, appropriate primers were designed to construct the immunoglobulin variable region (IgV, 29aa-214aa) domain of the extracellular segment into the vector pCD-Avi-His ( It was transformed from pCDNA3.1+, Invitrogen Company) to obtain the eukaryotic expression plasmid pCD-OX40-Avi-His ( figure 1 ). figure 1 Schematic diagram for the construction of the pCD-OX40-Avi-His plasmid, which can be transfected into eukaryotic cells to obtain the expressed rhOX40-Avi-His protein.
[0044] Biotin was covalently linked to the rhOX40-Avi-His protein containing the Avi-tagged peptide sequence using BirA biotinylase to complete biotinylase labeling. Both BiaA enzyme and Biotin solution were from GeneCopoeia, and the Hitrap Desalting column was from GE. , to obtain rhOX40-Avi-His-bio protein with higher concentration and better labeling efficiency, which is used for antibody ...
Embodiment 3
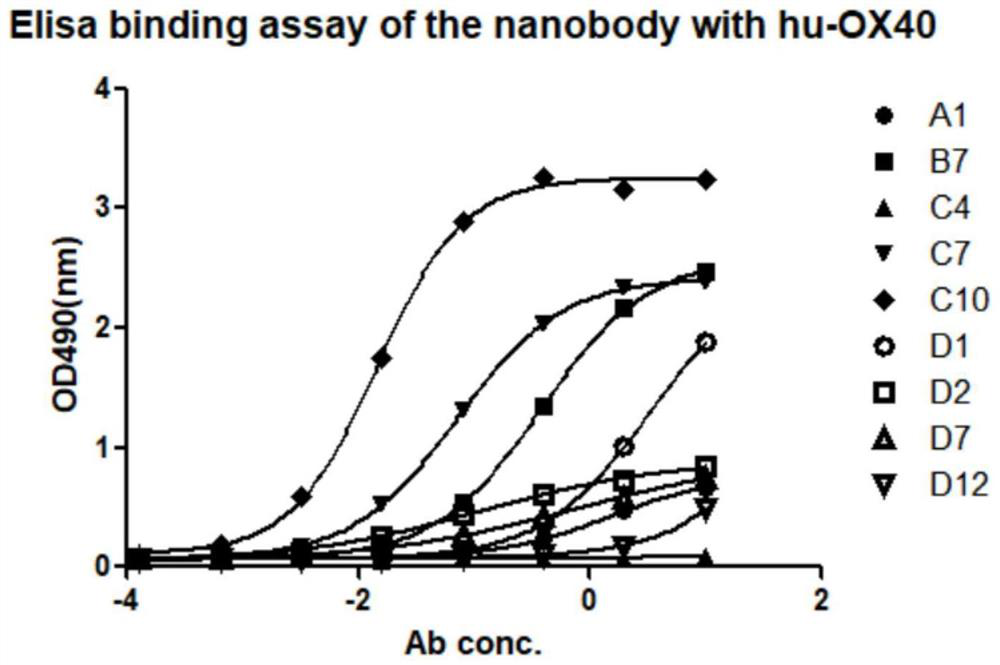
[0045] Example 3: OX40 mAb screening
[0046] Using the prepared rhOX40-Avi-His-bio antigenic protein, 3 rounds of liquid phase screening were performed on 3 fully synthetic human nanobody libraries built by our company, and picked from the bacterial culture plates in the second and third rounds A large number of monoclonal, soluble nanobodies were prepared.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com