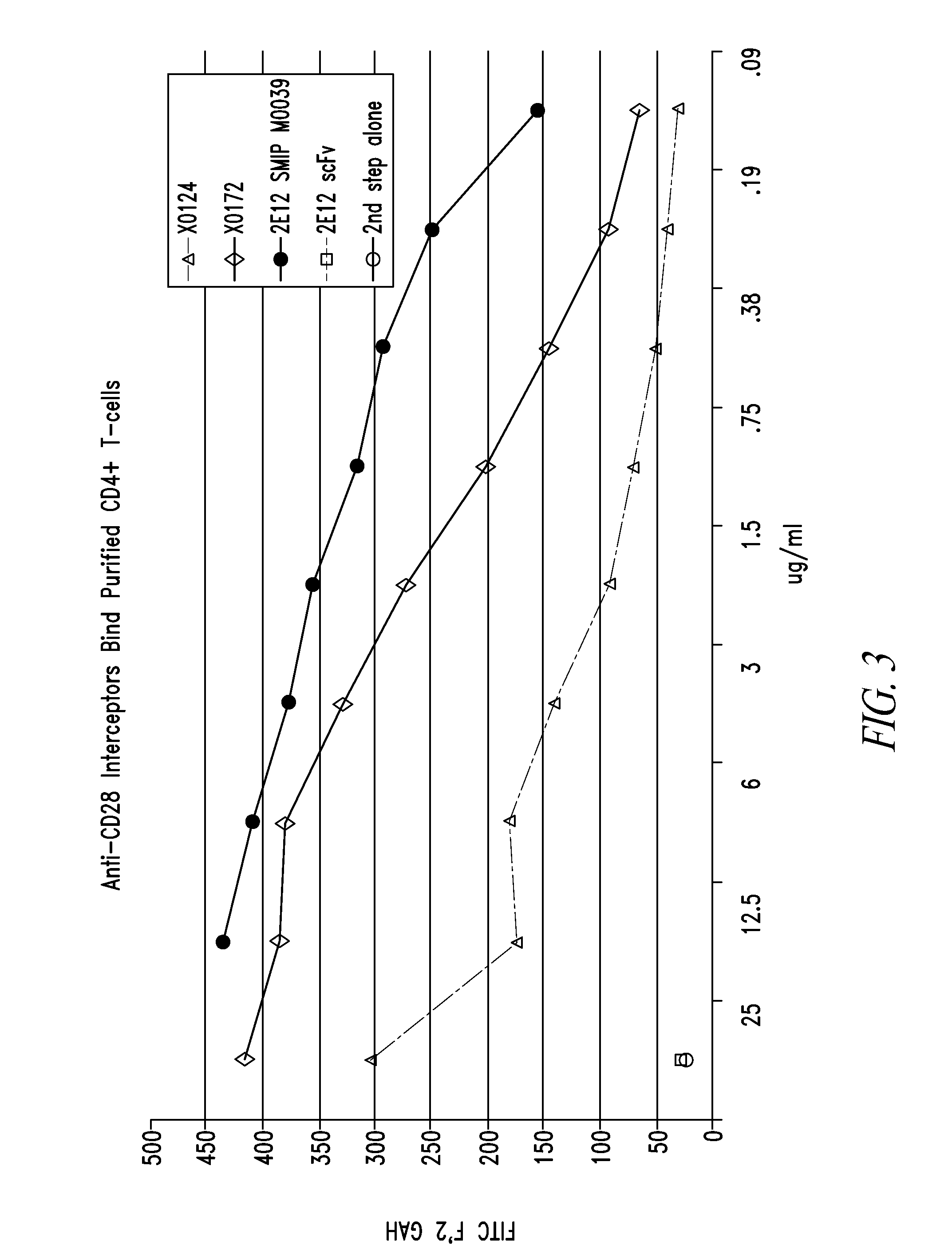
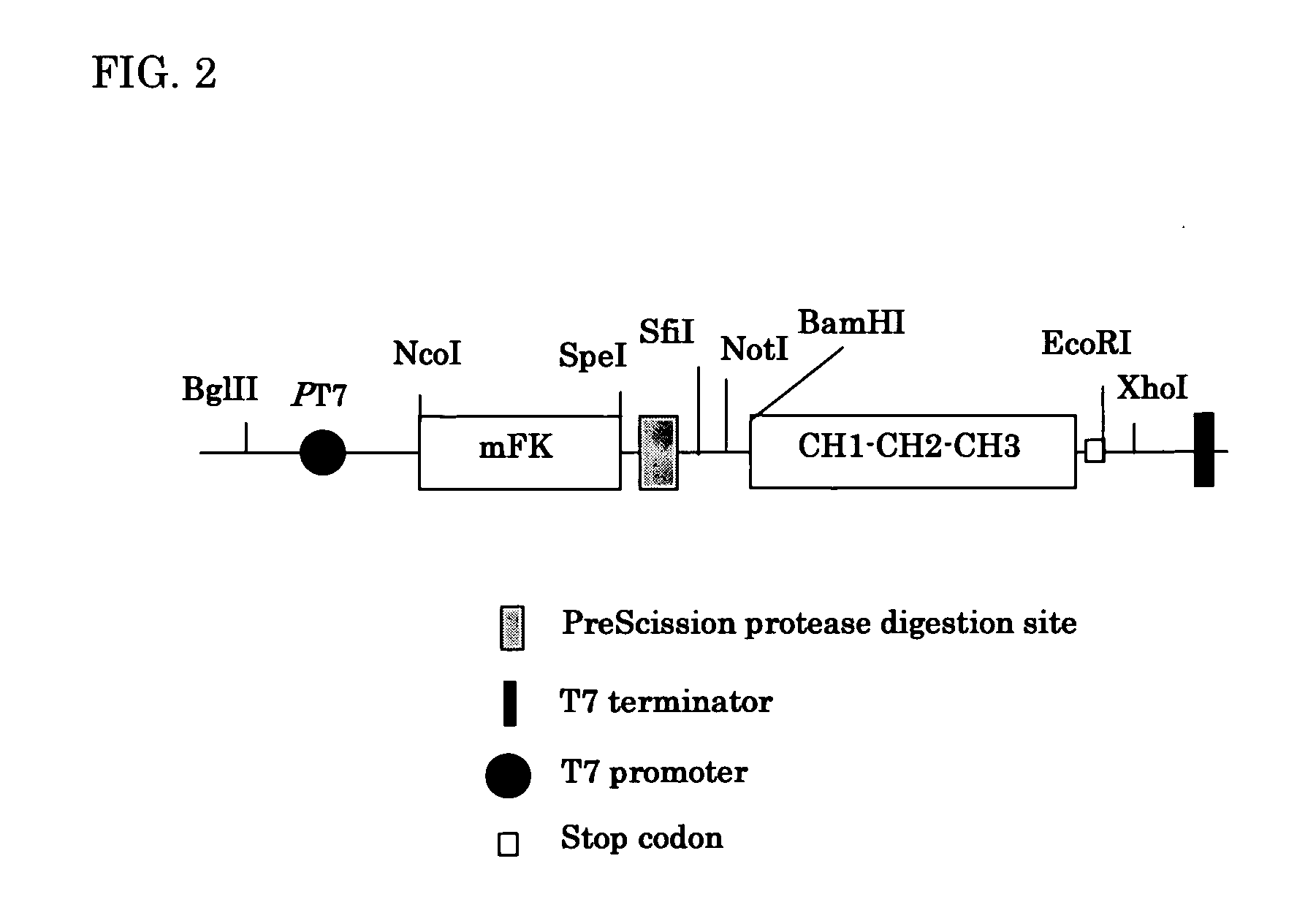
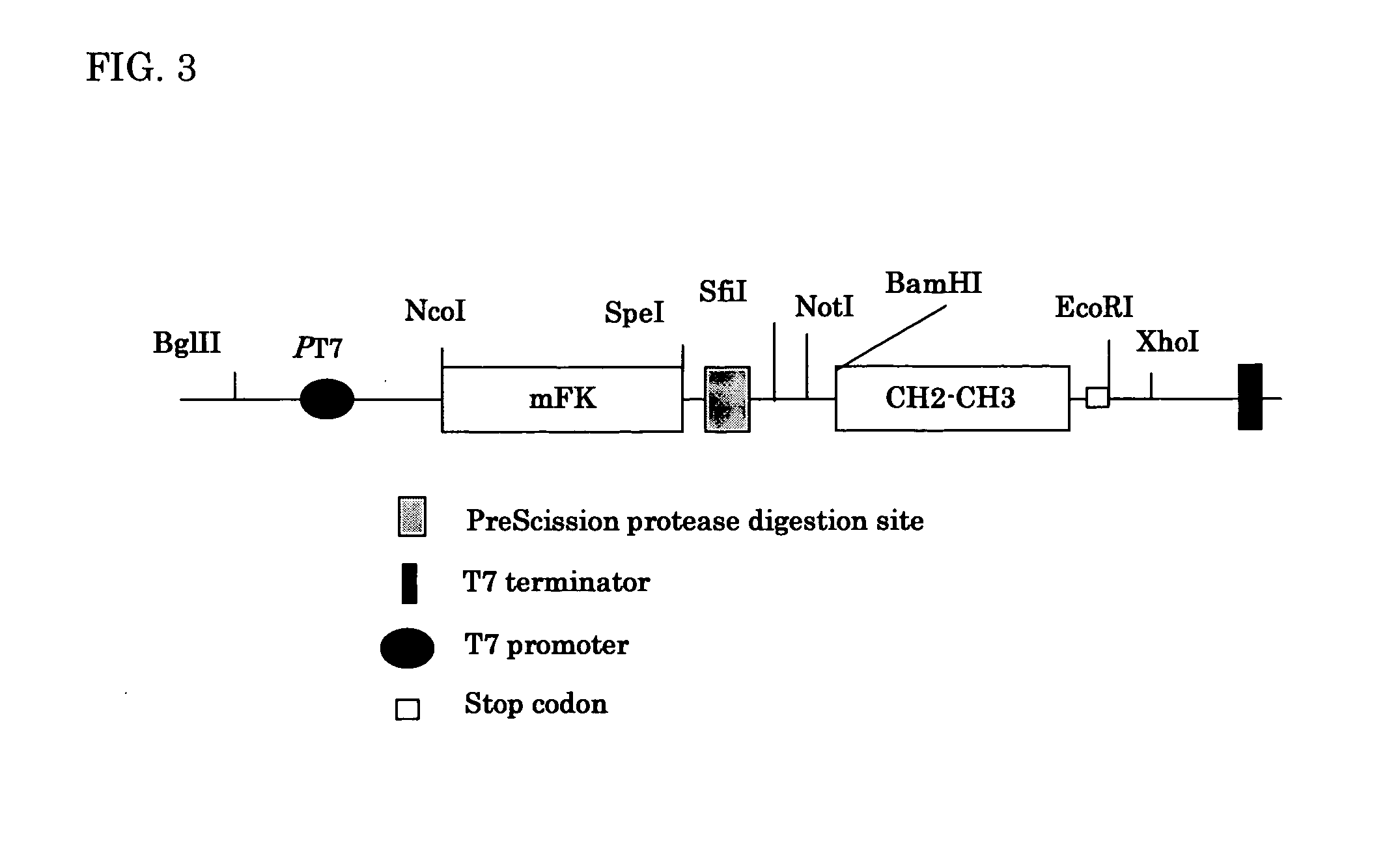
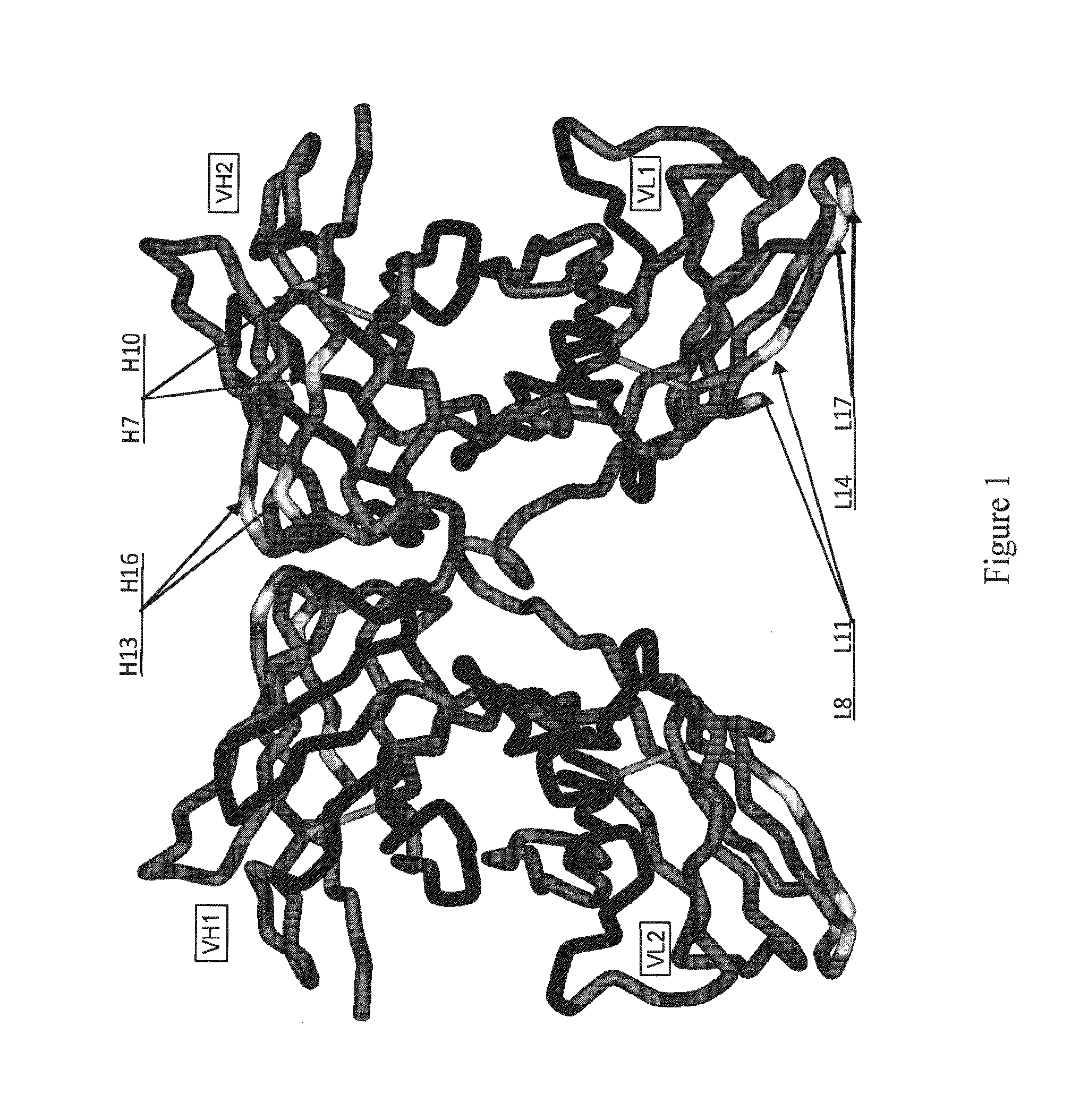
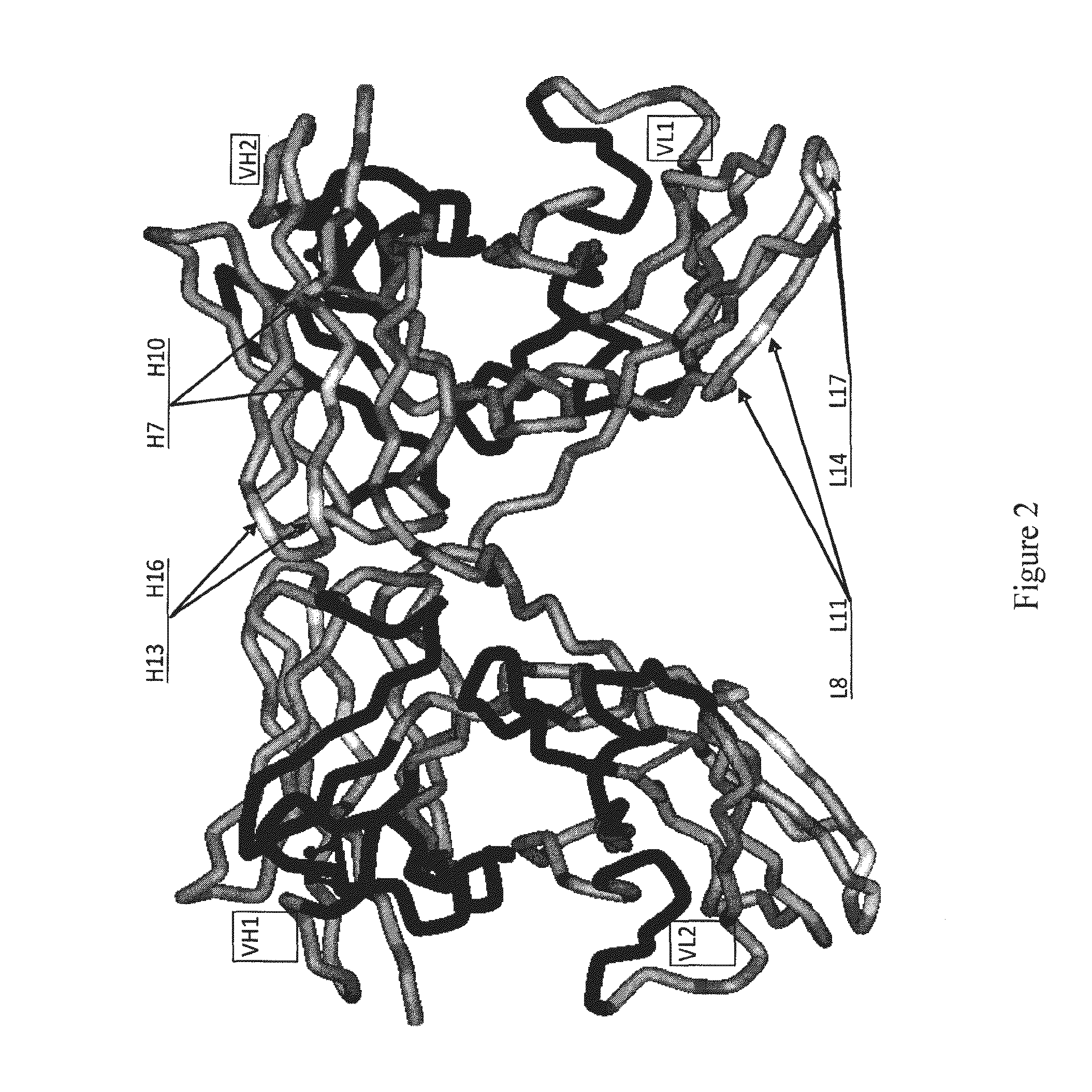
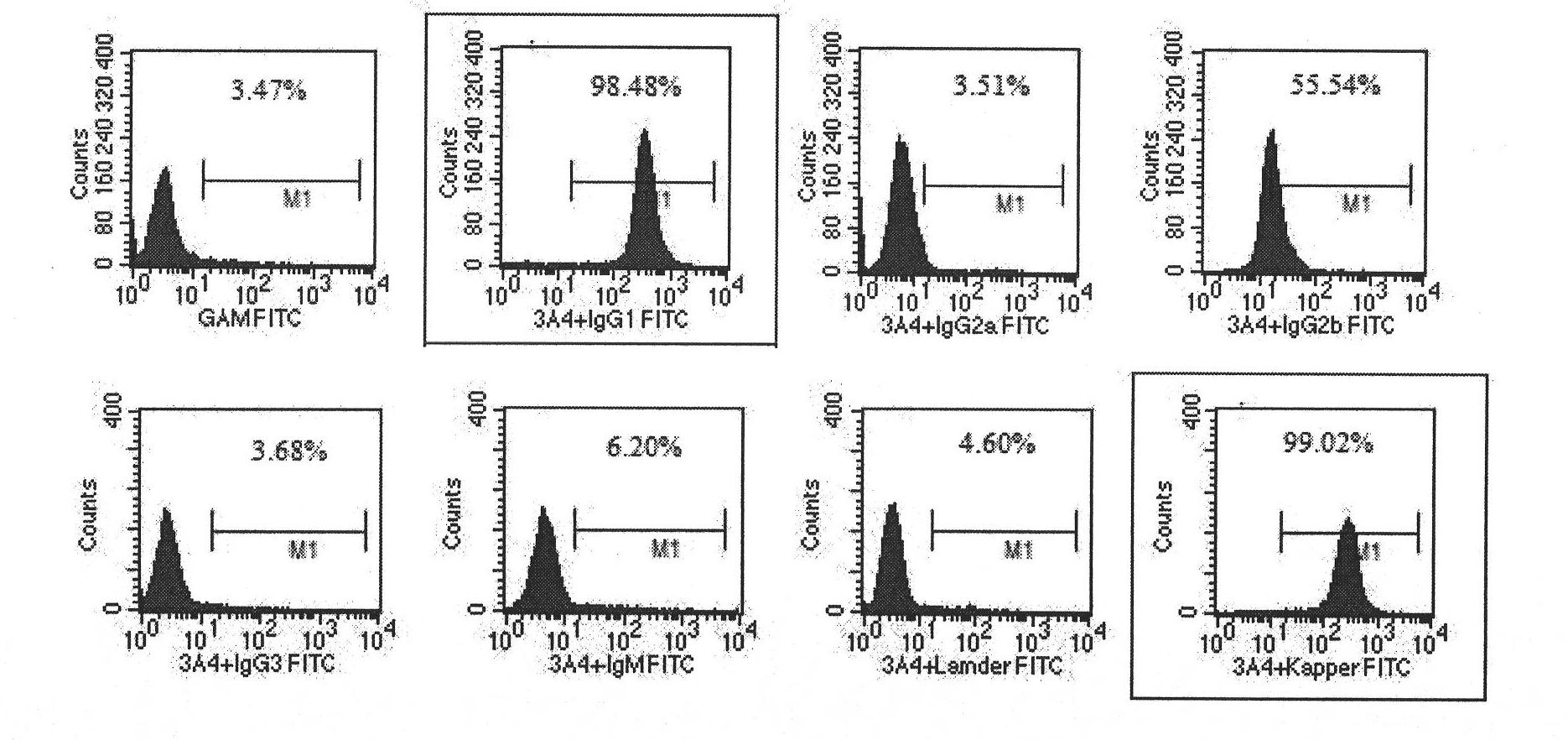
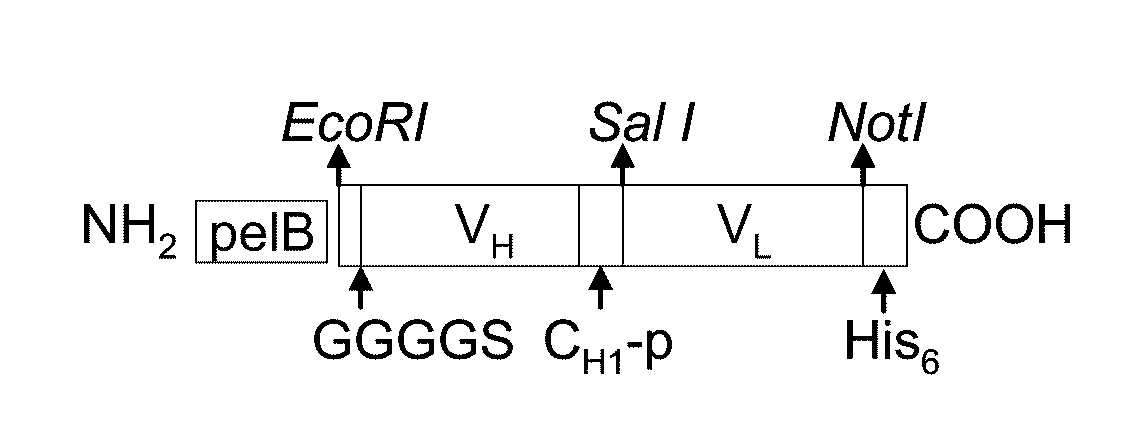
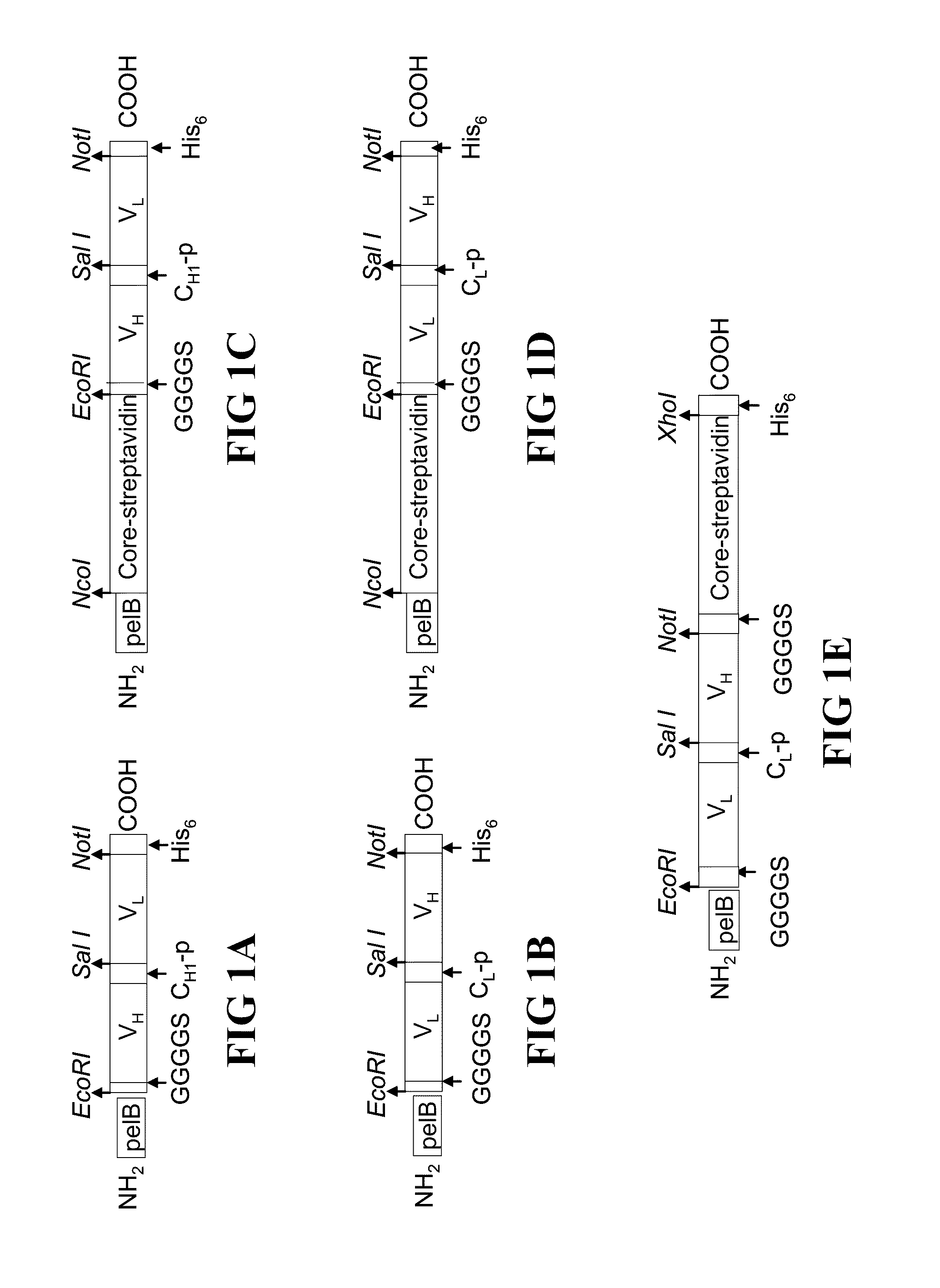
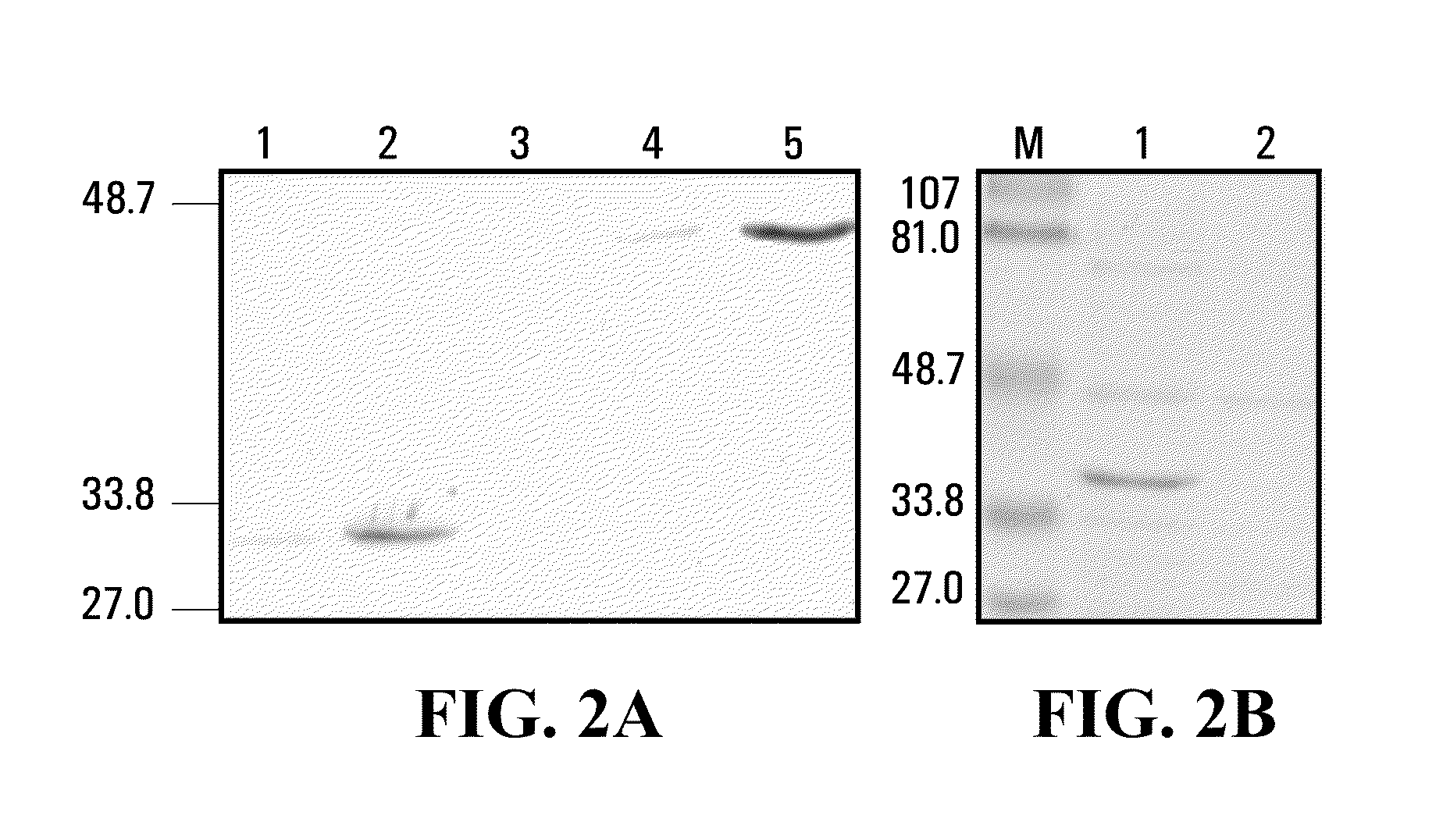
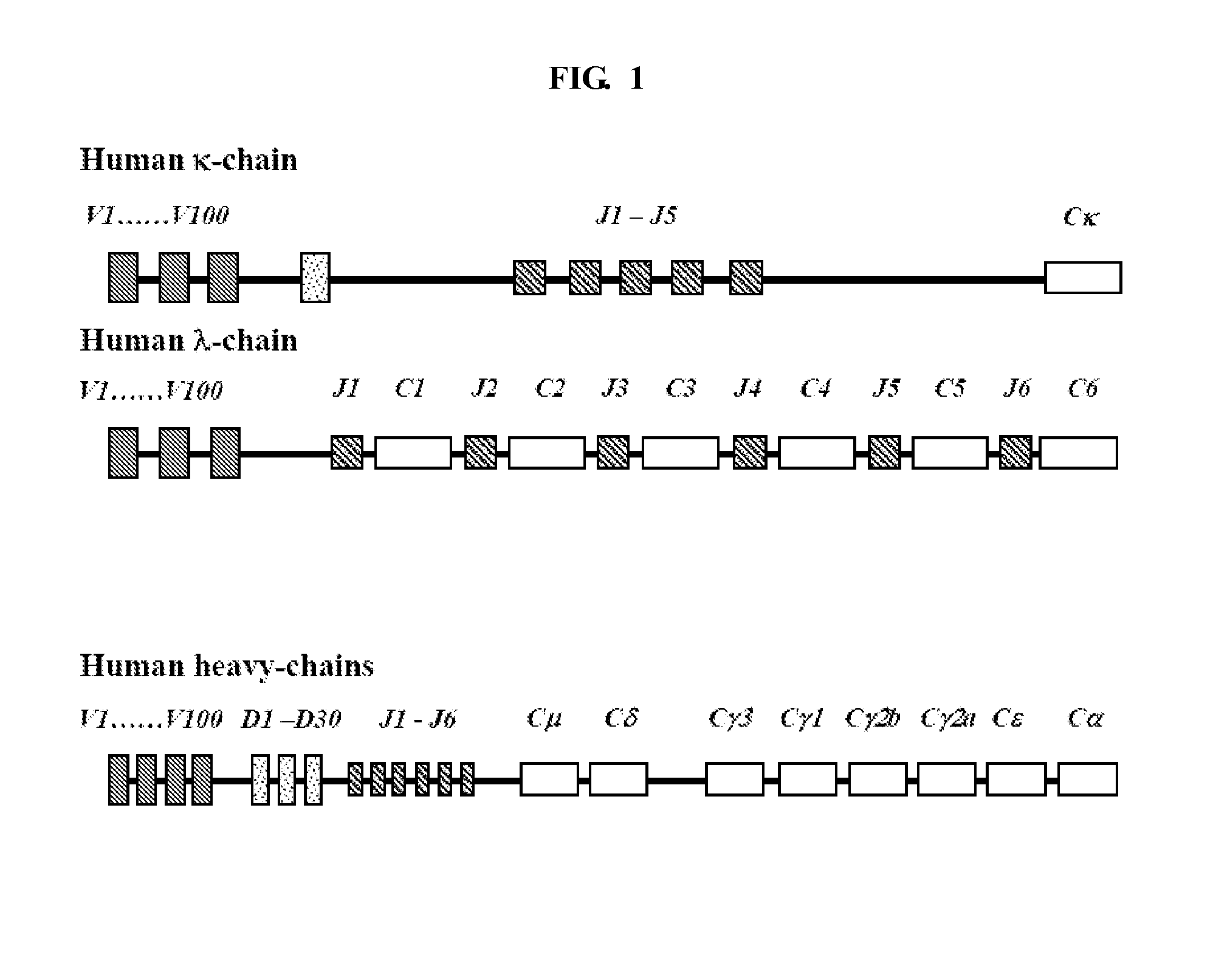
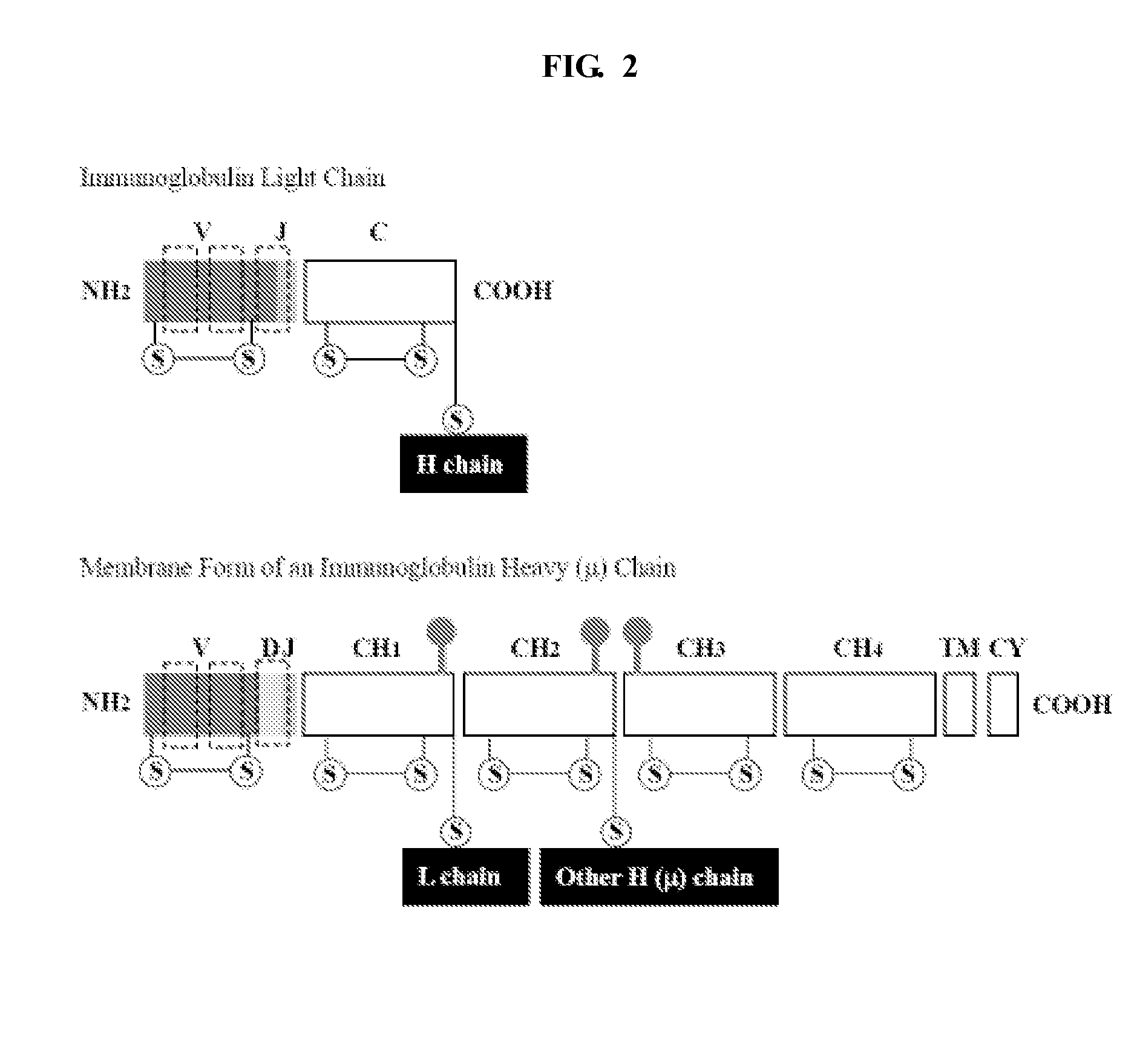
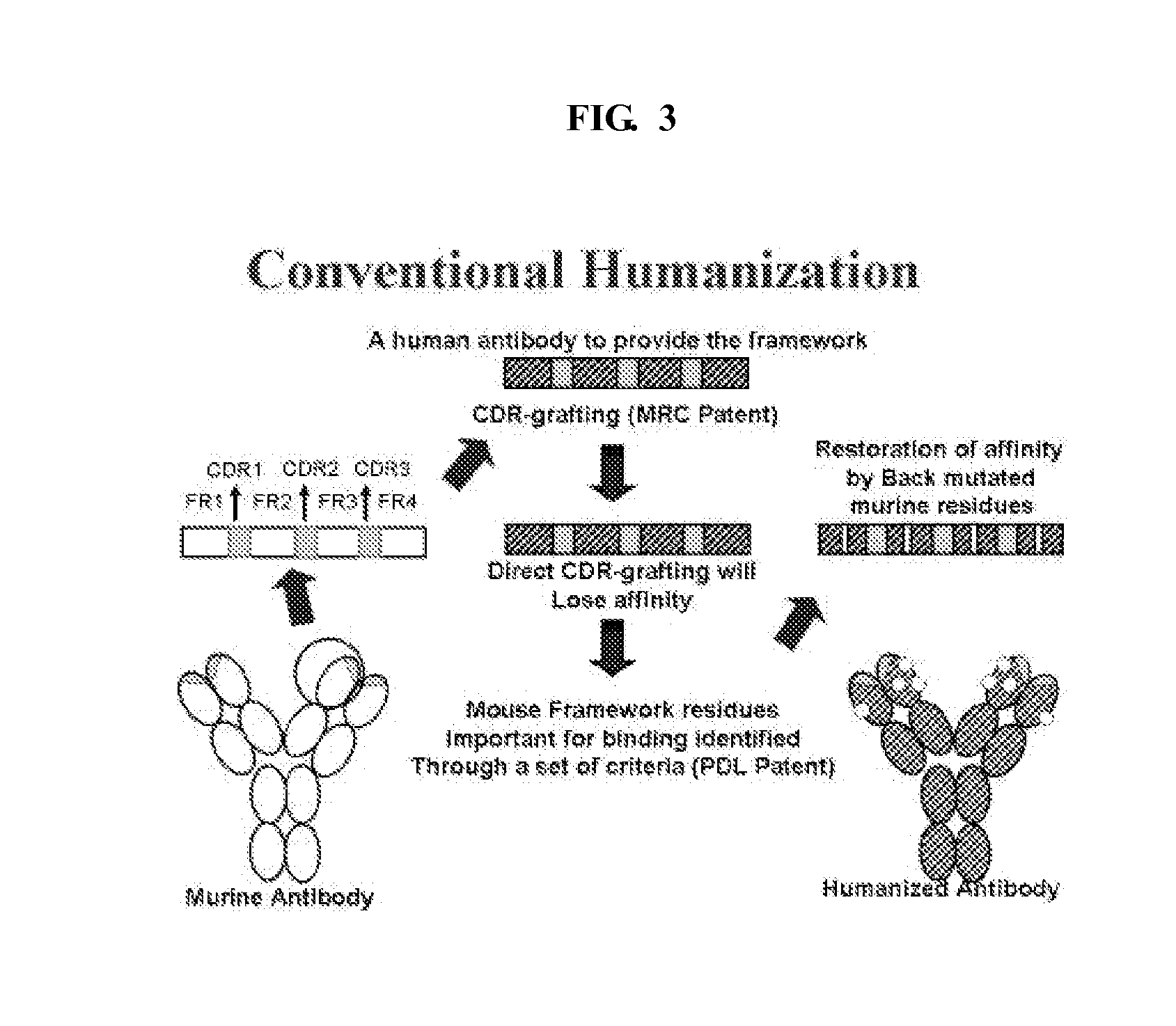
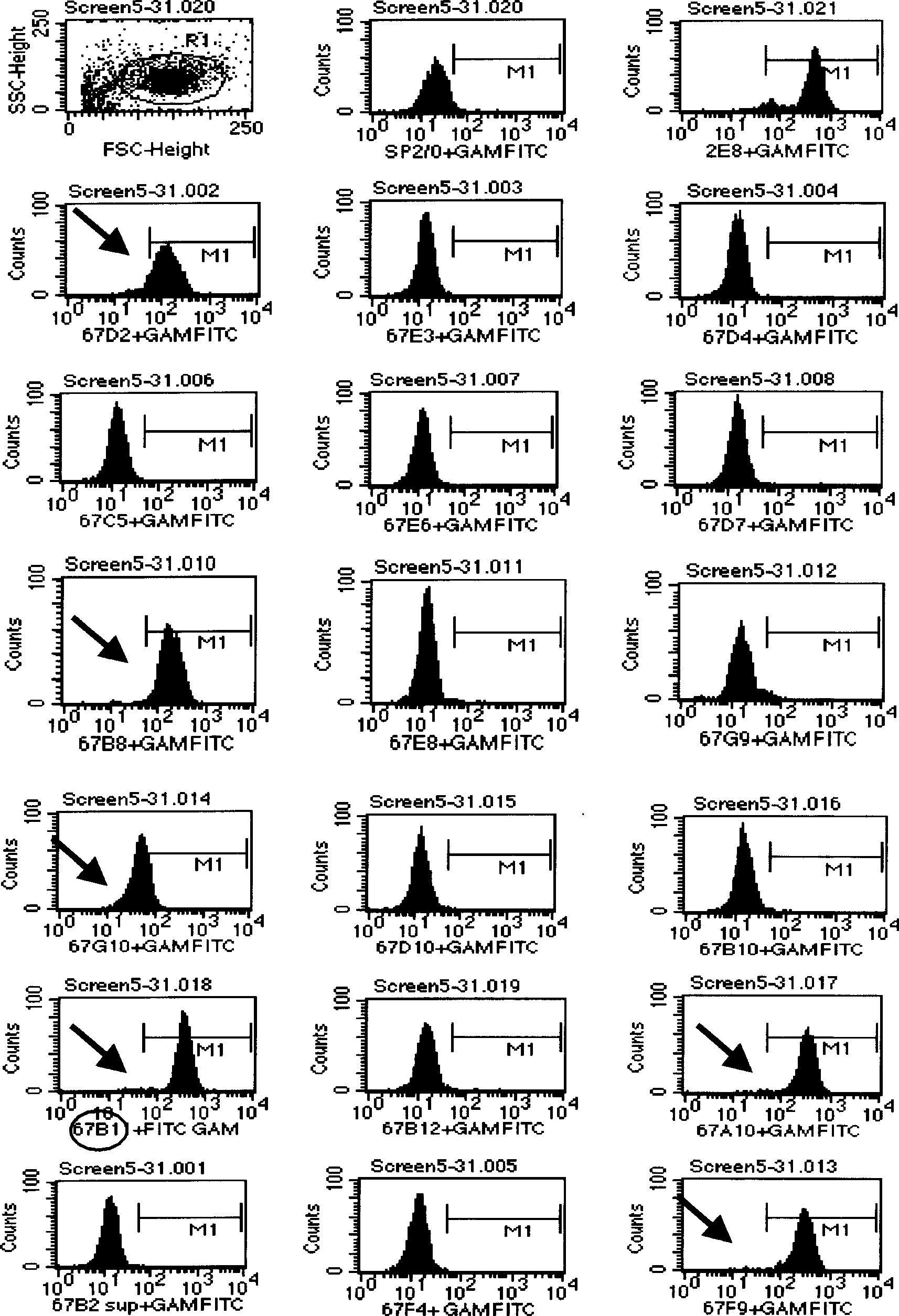
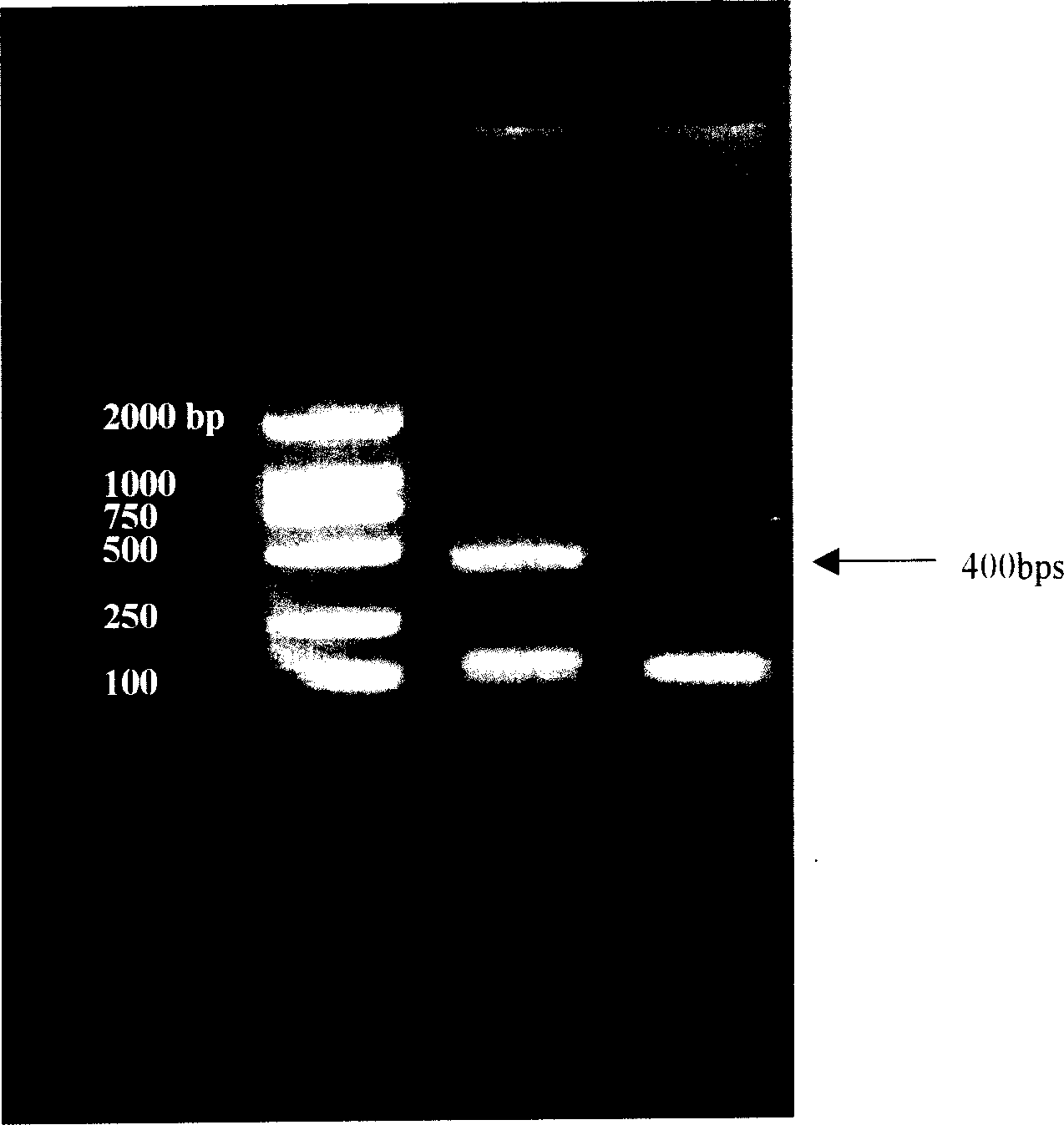
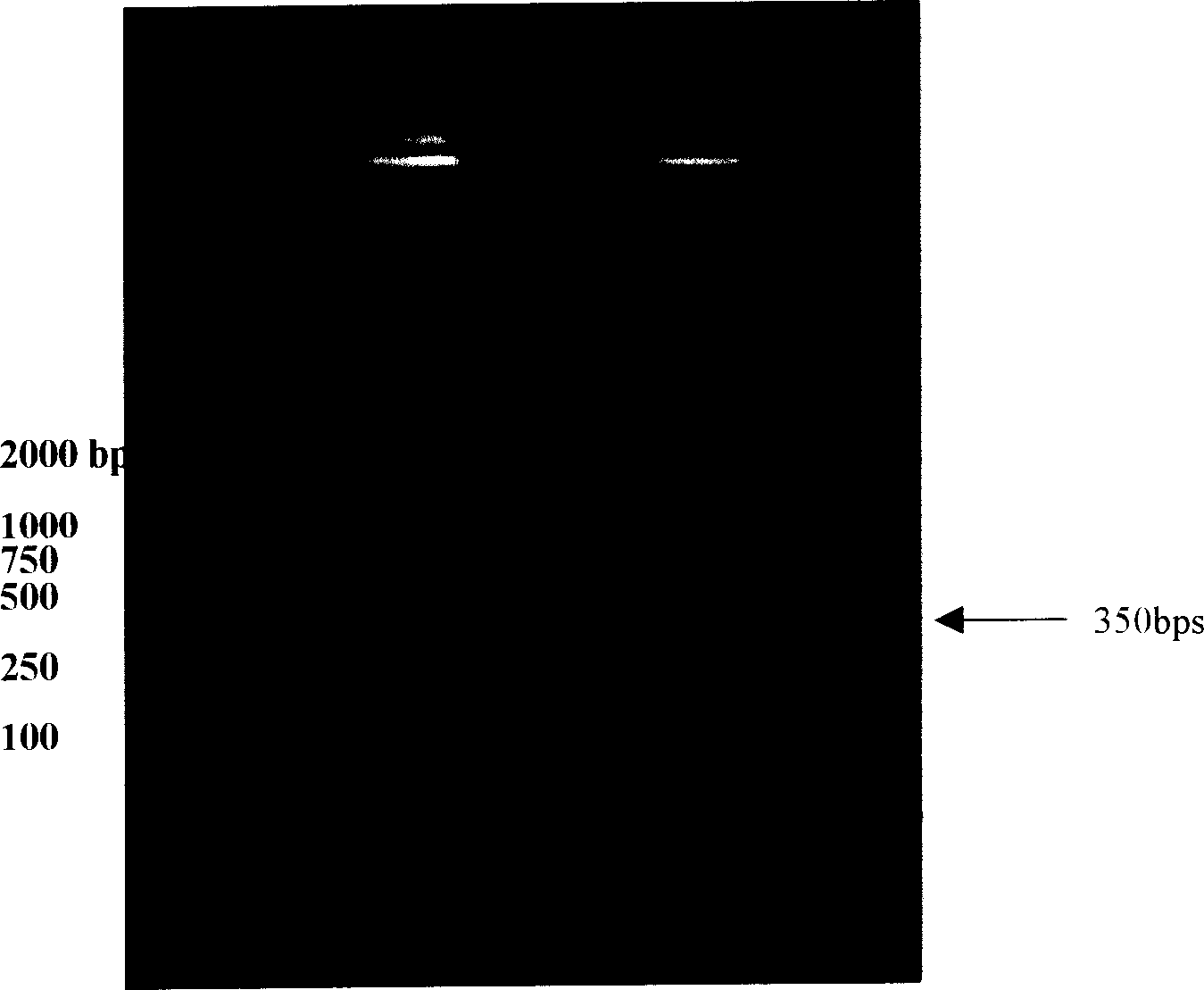
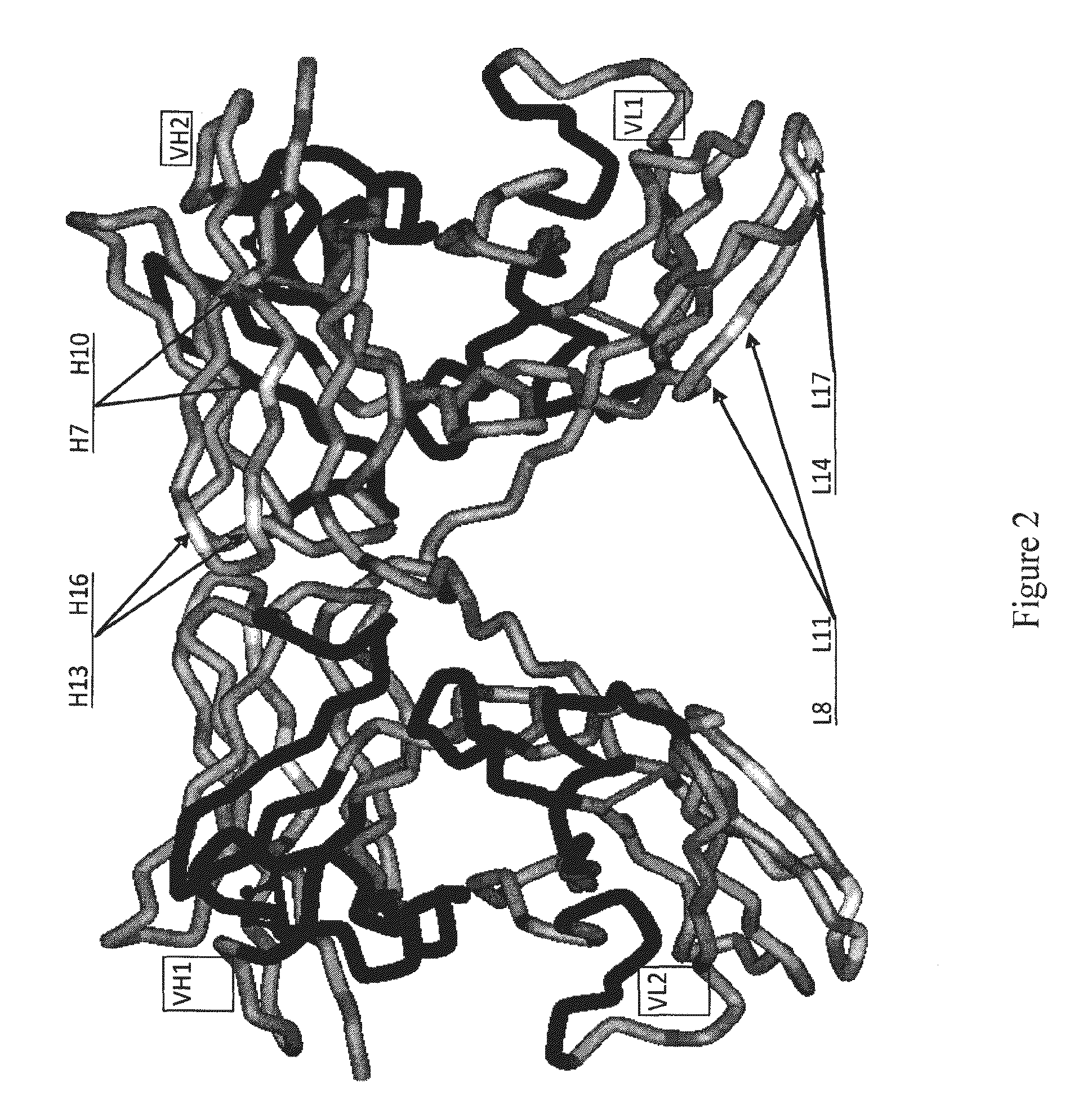
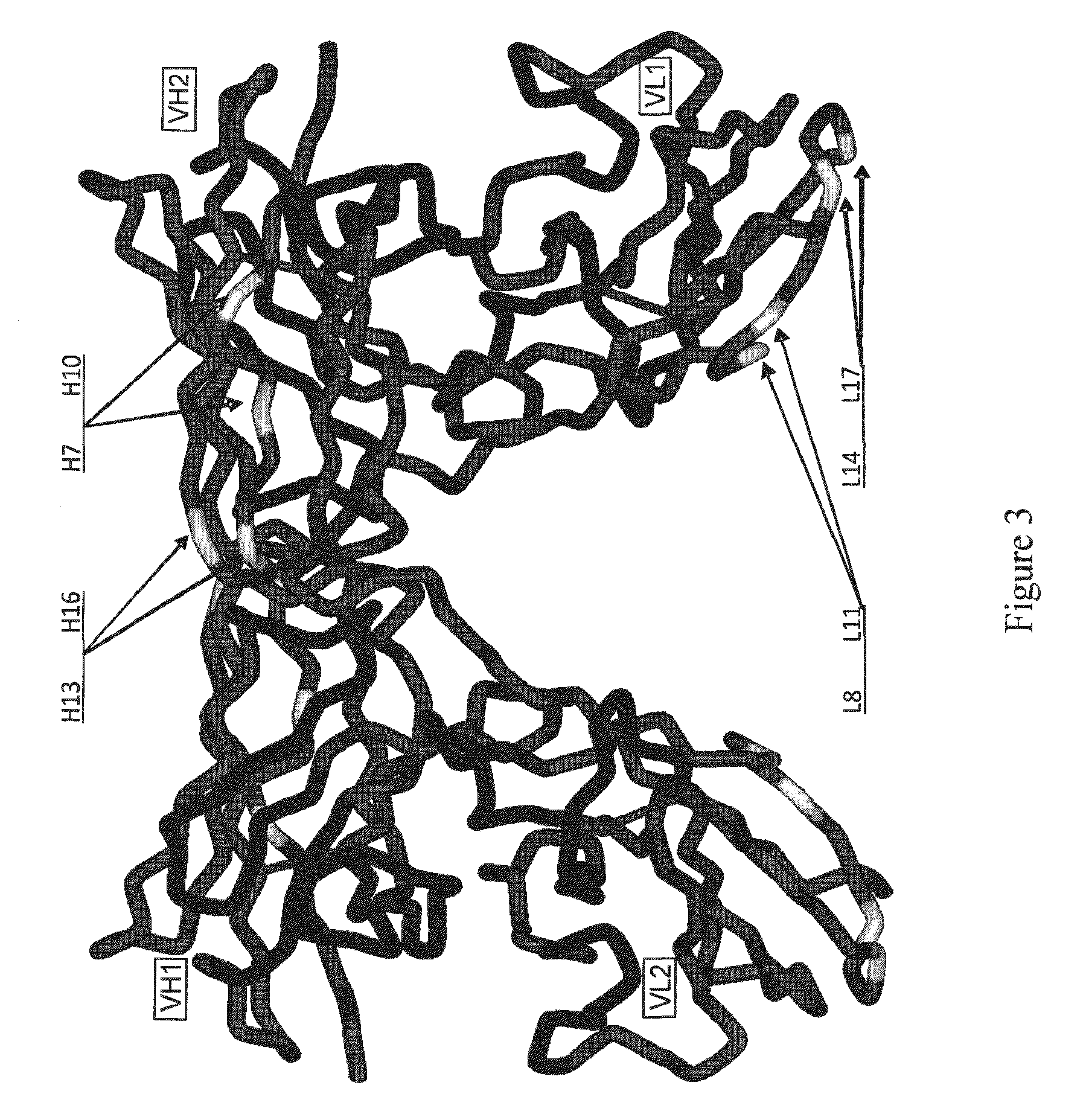
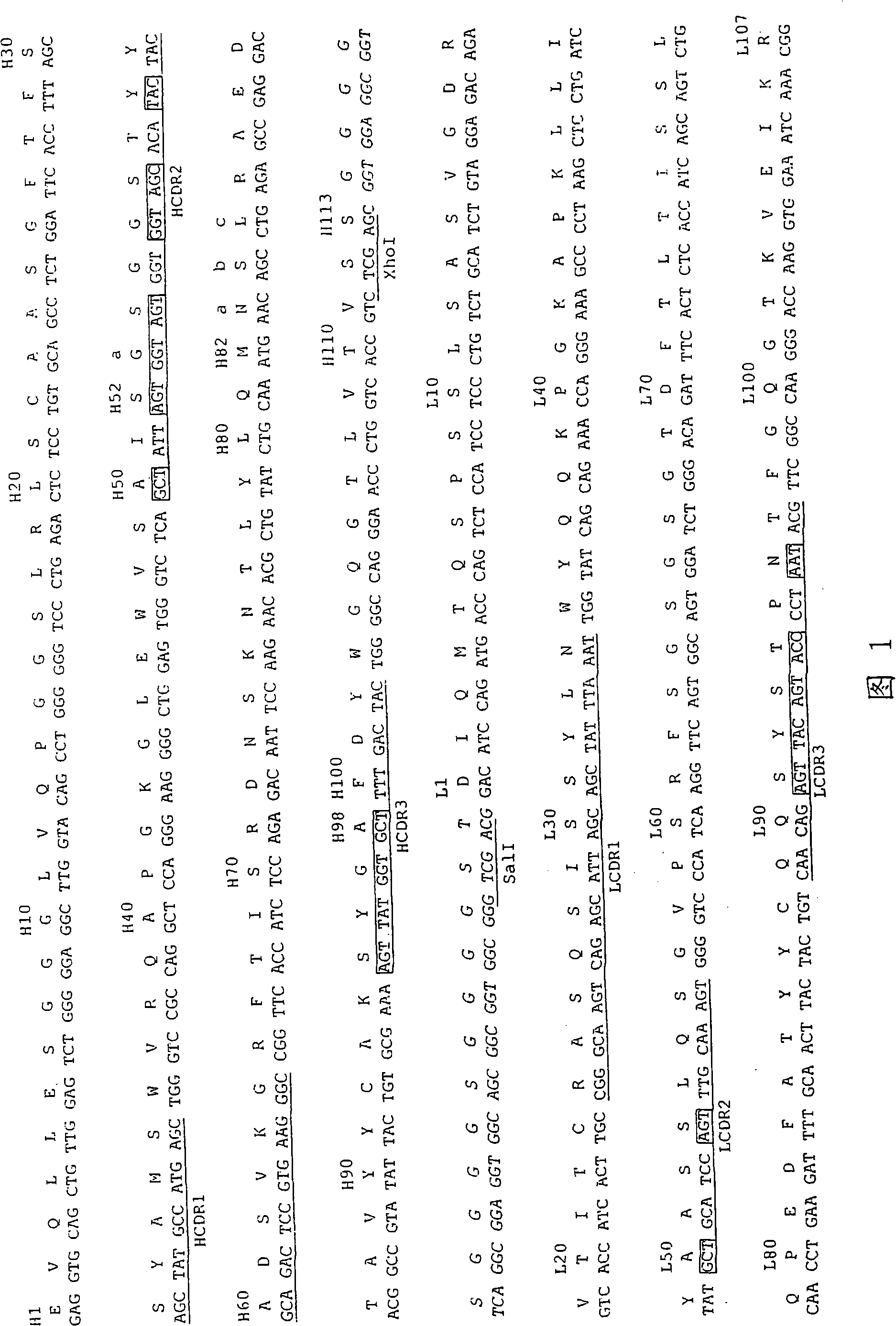
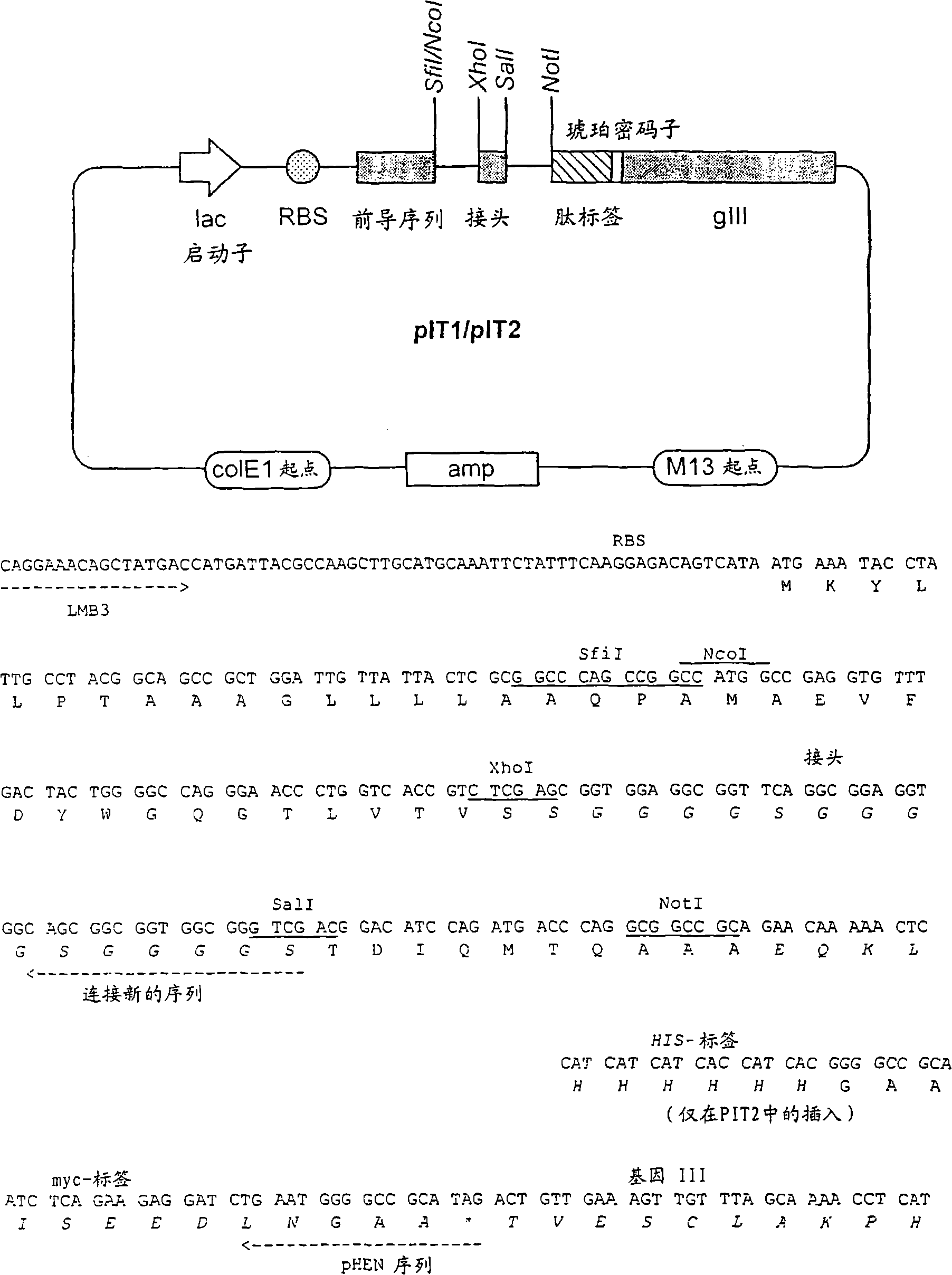
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33 results about "Immunoglobulin Variable Region" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Description of Immunoglobulin Variable Region. Immunoglobulin Variable Region: That region of the immunoglobulin molecule that varies in its amino acid sequence and composition, and comprises the binding site for a specific antigen. It is located at the N-terminus of the Fab fragment of the immunoglobulin.
Increasing antibody affinity by altering glycosylation of immunoglobulin variable region
InactiveUS6933368B2Enhanced antigen binding propertyAnimal cellsImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody AffinitiesImmunoglobulin Variable Region
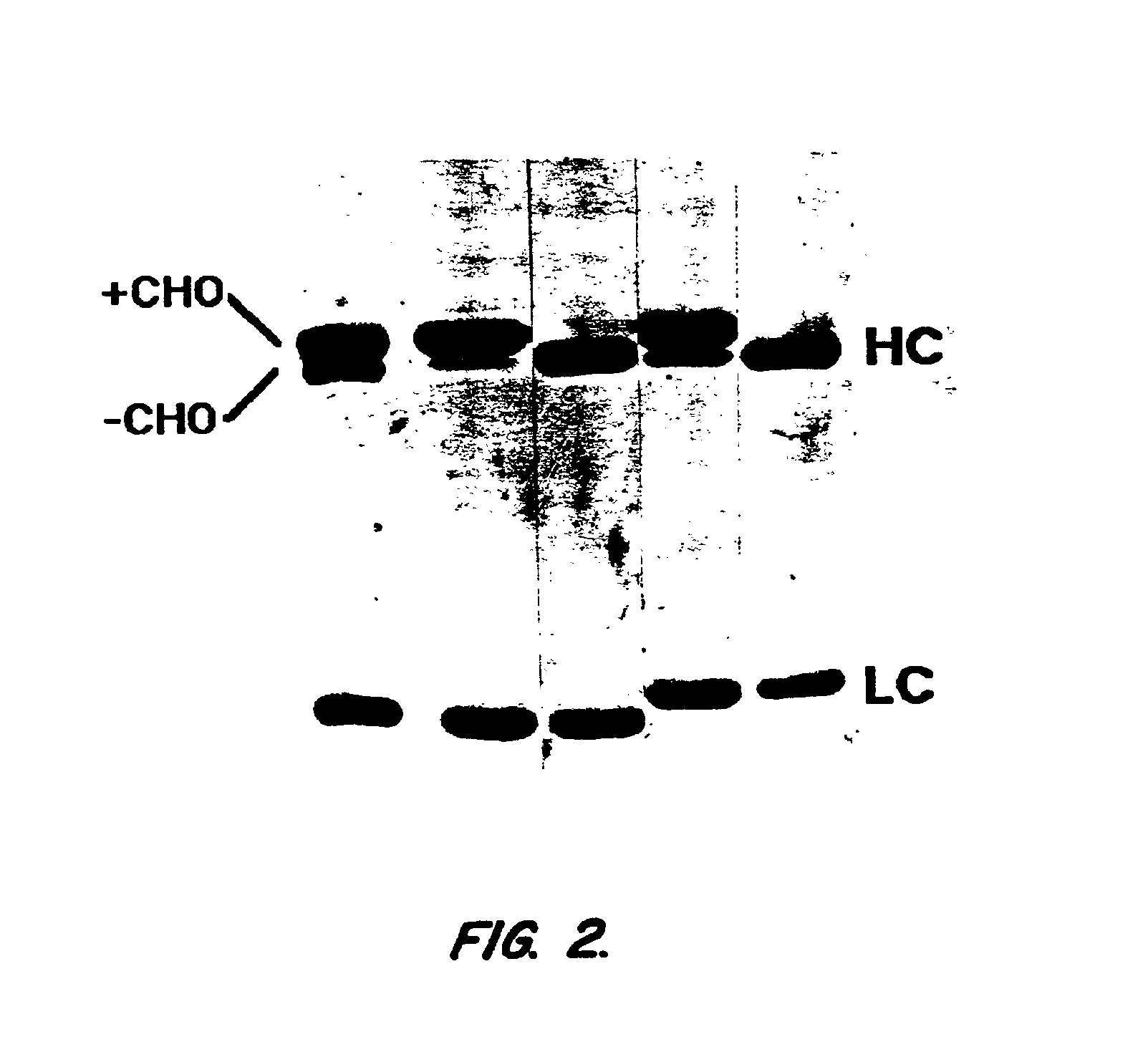
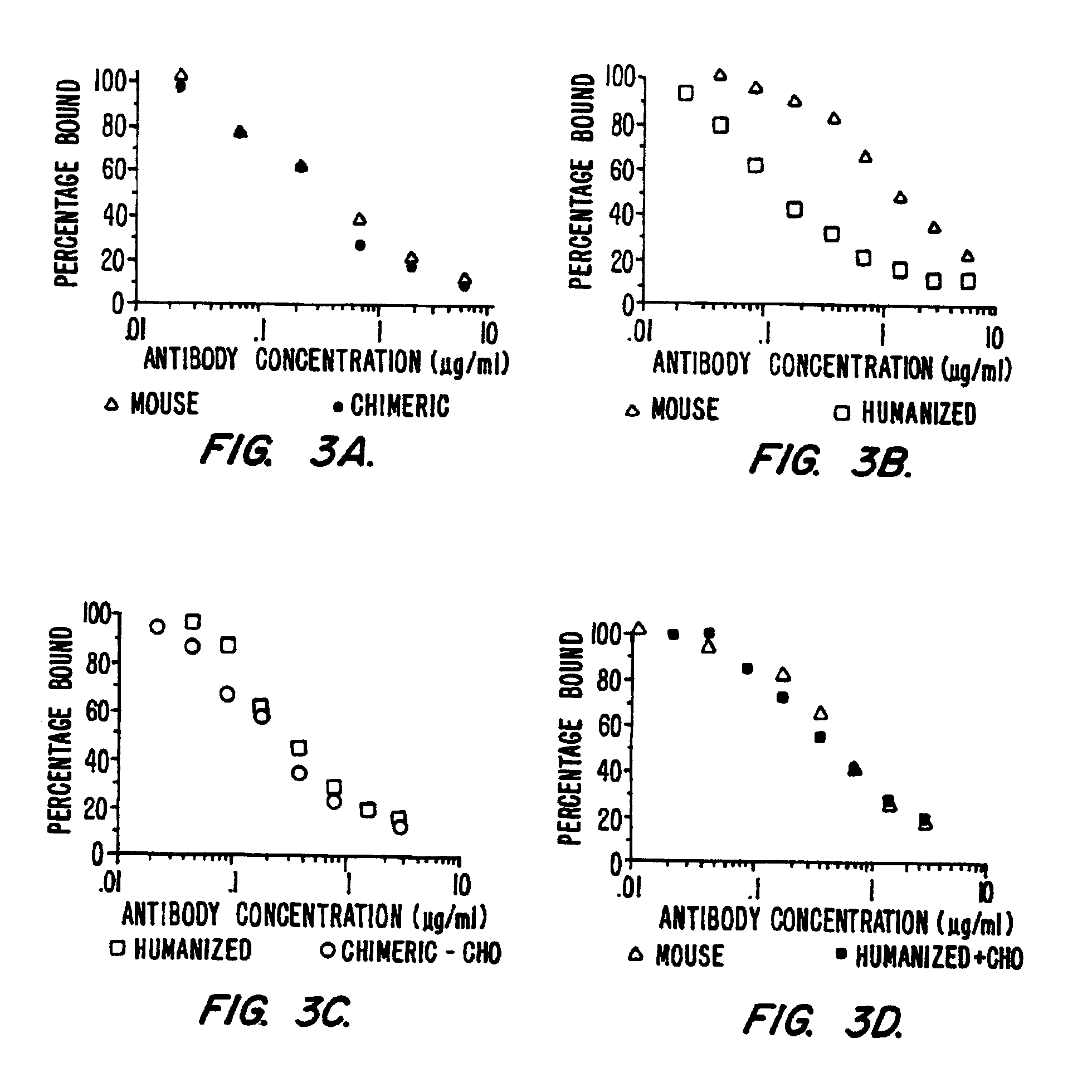
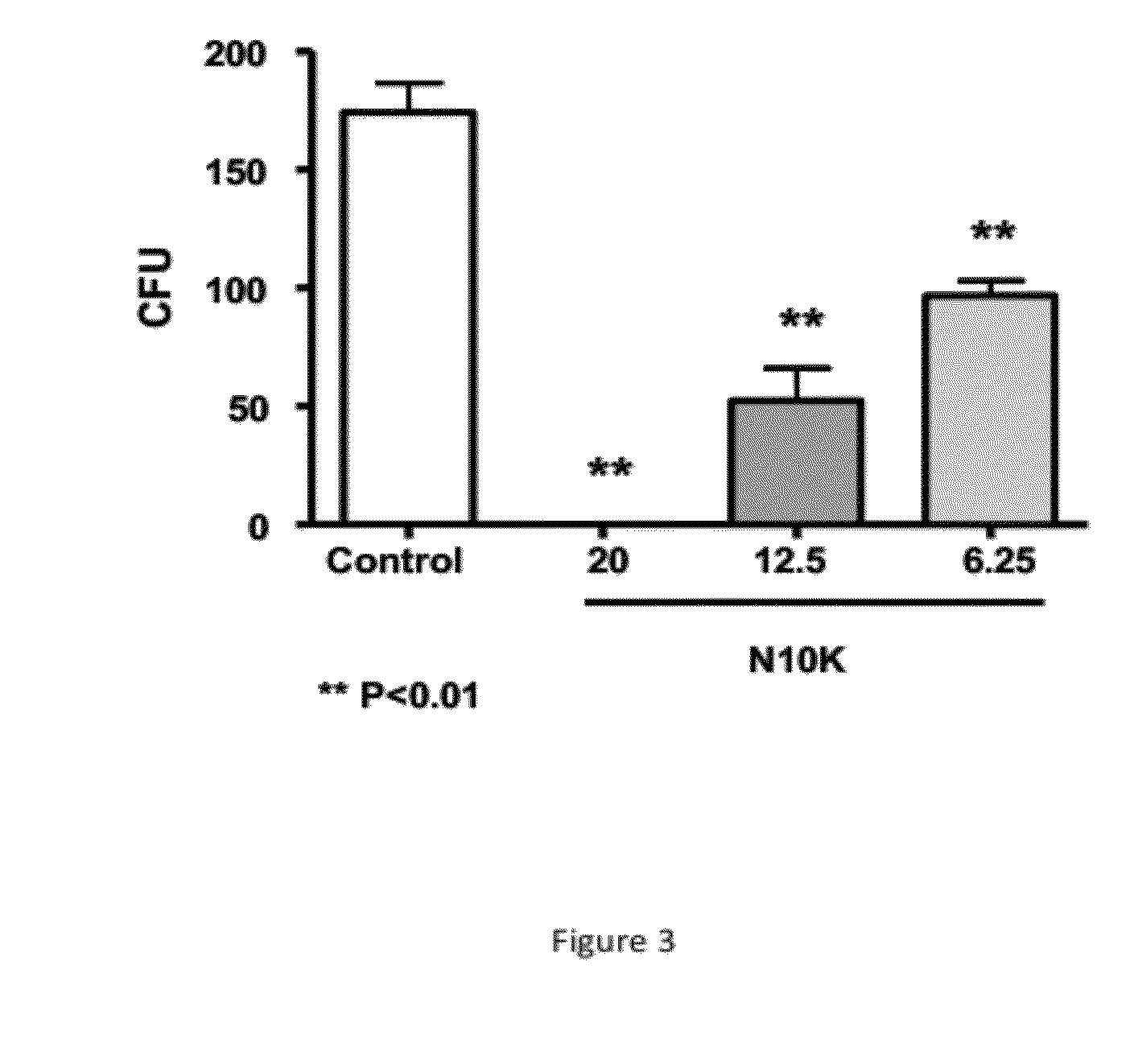
The present invention provides methods for producing mutationally-altered immunoglobulins and compositions containing such mutationally-altered immunoglobulins, wherein the mutationally-altered immunoglobulins have at least one mutation that alters the pattern of glycosylation in a variable region and thereby modifies the affinity of the immunoglobulin for a preselected antigen. The methods and compositions of the invention provide immunoglobulins that possess increased affinity for antigen. Such glycosylation-altered immunoglobulins are suitable for diagnostic and therapeutic applications.
Owner:FACET BIOTECH CORP
Heterodimer Binding Proteins and Uses Thereof
InactiveUS20130129723A1Animal cellsFused cellsImmunoglobulin Joining RegionImmunoglobulin light chain
The present disclosure provides polypeptide heterodimers formed between two different single chain fusion polypeptides via natural heterodimerization of an immunoglobulin CH1 region and an immunoglobulin light chain constant region (CL). The polypeptide heterodimer comprises two or more binding domains that specifically bind one or more targets (e.g., a receptor). In addition, both chains of the heterodimer further comprise an Fc region portion. The present disclosure also provides nucleic acids, vectors, host cells and methods for making polypeptide heterodimers as well as methods for using such polypeptide heterodimers, such as in directing T cell activation, inhibiting solid malignancy growth, and treating autoimmune or inflammatory conditions.
Owner:EMERGENT PRODUCTS DEVELOPMENT SEATTLE LLC
Immuno-conjugates and methods for producing them
ActiveUS20120171115A1Reducing and preventing residuePermit productionFungiBacteriaImmunoglobulin Heavy Chain Variable RegionDisulphide bonds
The present invention provides an isolated protein comprising an immunoglobulin variable region comprising at least two cysteine residues positioned within framework region (FR) 2 and / or at least two cysteine residues positioned within framework region (FR3), wherein if at least two of the cysteine residues in FR2 and / or FR3 are not conjugated to a compound then an intra-framework disulphide bond is capable of forming between the cysteine residues. Preferably the protein comprises an immunoglobulin heavy chain variable region (VH) and an immunoglobulin light chain variable region (VL), wherein at least one of the variable regions comprises the two cysteine residues. The present invention also provides conjugates of the protein and another compound.
Owner:AVIPEP
Polyclonal antibody composition for treating allergy
InactiveUS20050180967A1Good curative effectEfficient removalImmunoglobulins against animals/humansImmunoglobulins against plantsMicrosphereBULK ACTIVE INGREDIENT
A pharmaceutical composition for treating allergy is described. The composition comprises as an active ingredient a recombinant polyclonal antibody or a mixture of different monoclonal antibodies capable of reacting with or binding to an allergen together with one or more pharmaceutically acceptable excipients. The composition may be used topically as a solution, dispersion, powder, or in the form of microspheres. The polyclonal antibody is preferably a recombinant polyclonal antibody produced by phage display technology. The pairing of specific immunoglobulin variable region light chain and heavy chain maintained from the original polyclonal immune response or selected by panning using the allergen in question is preferably maintained by bulk transfer of the pairs into an expression vector.
Owner:SYMPHOGEN AS
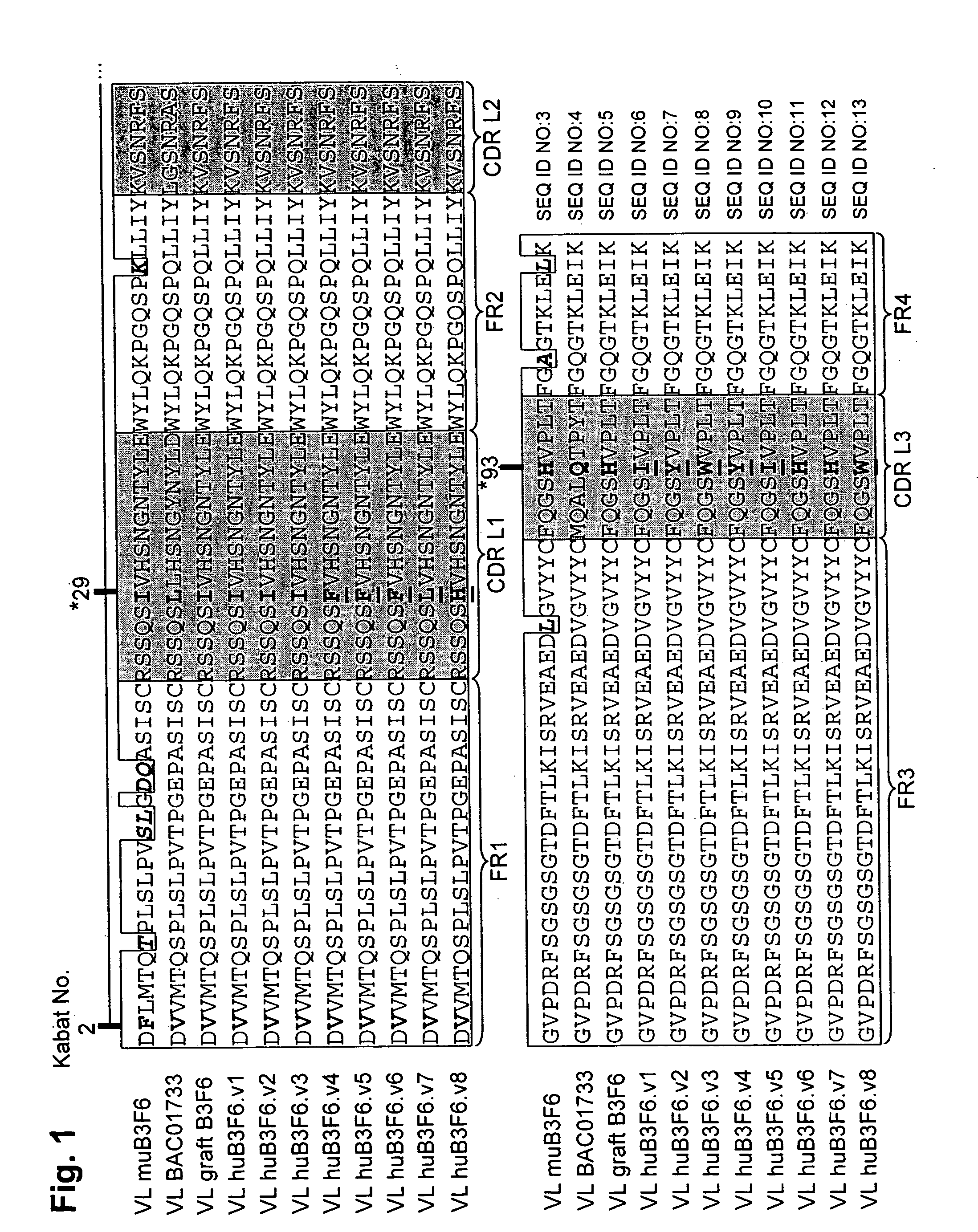
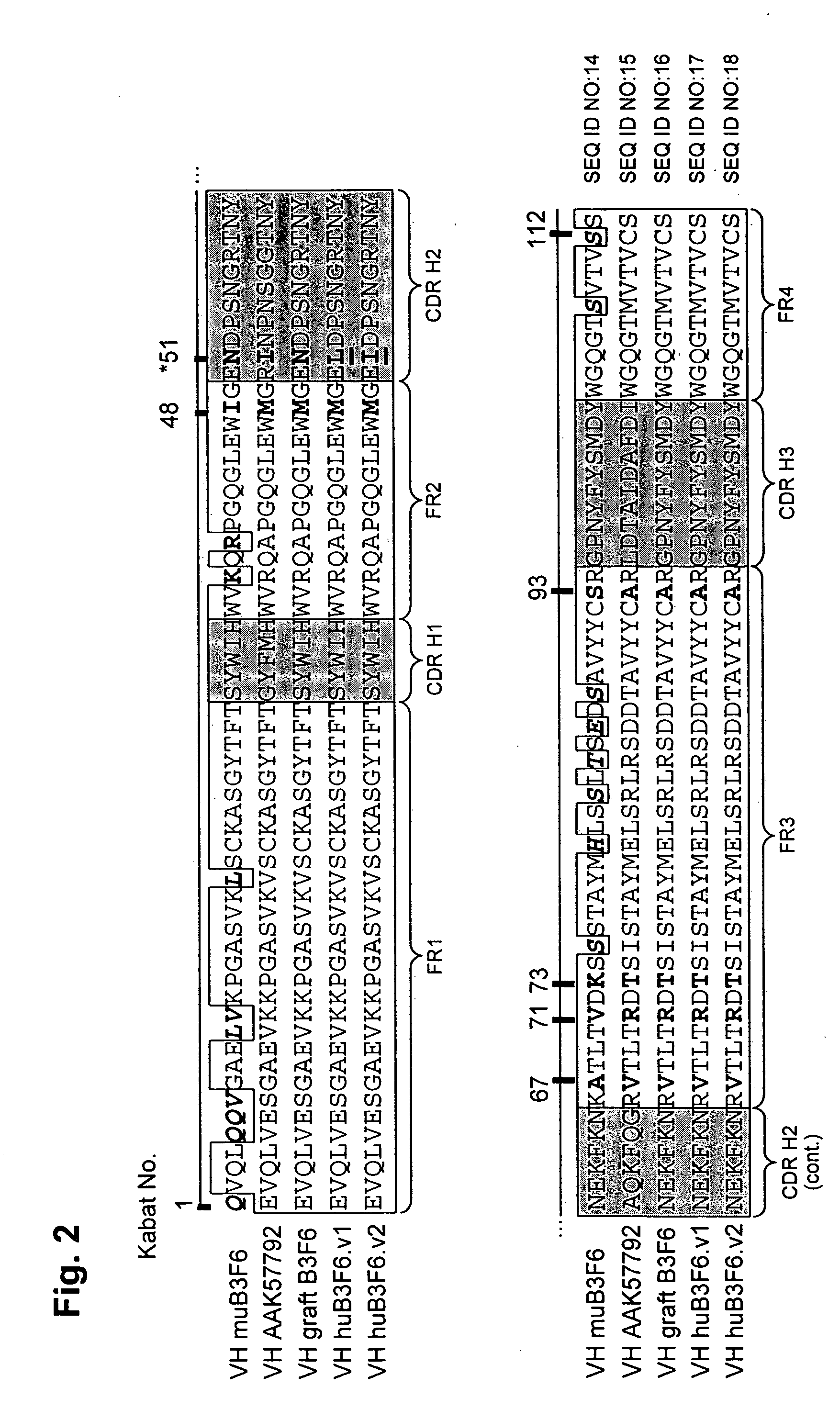
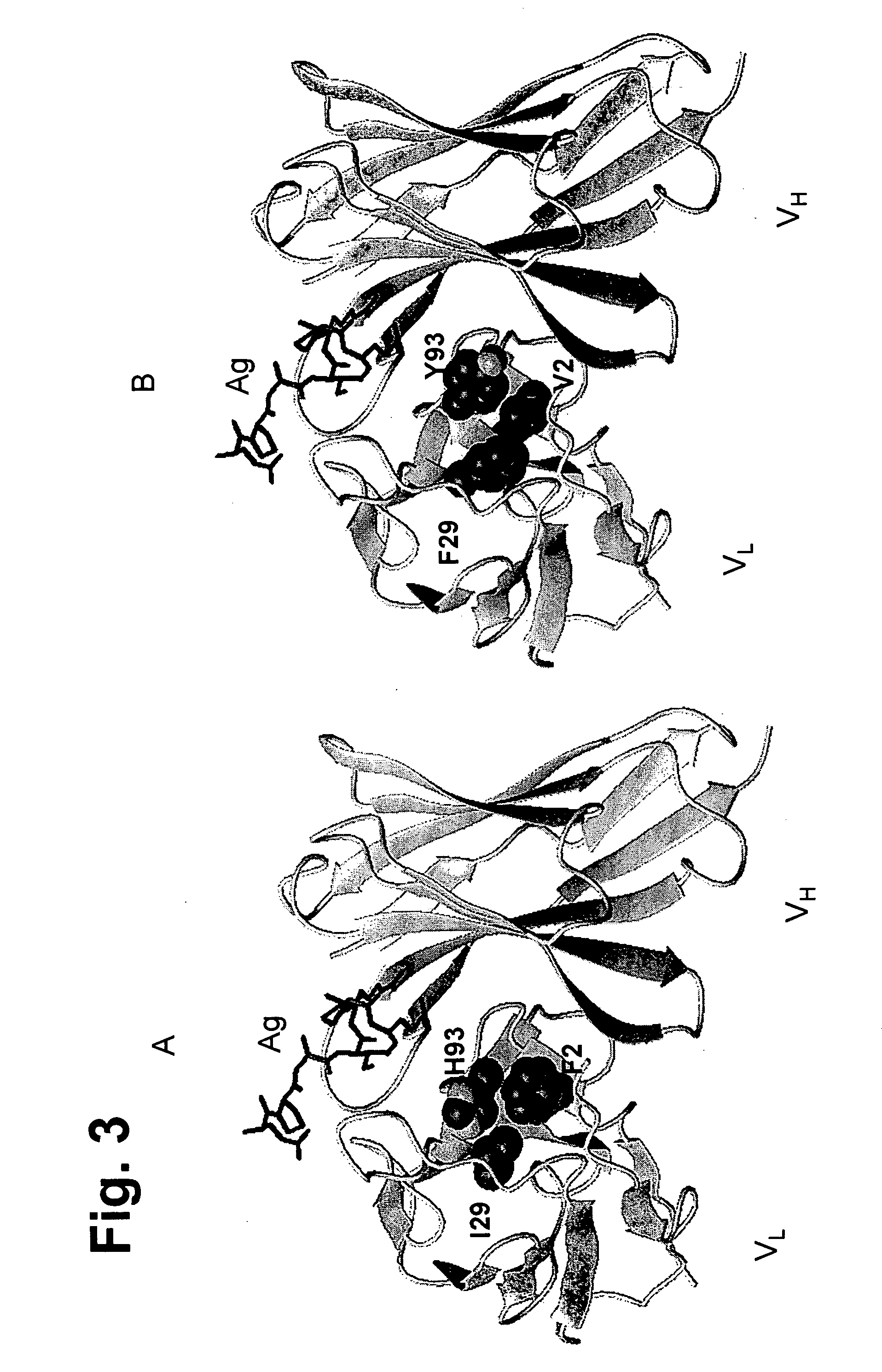
Methods of humanizing immunoglobulin variable regions through rational modification of complementarity determining residues
ActiveUS20060258852A1Low immunogenicityMinimizing immune responseAntipyreticAnalgesicsVariable domainHumanized antibody
The present invention is based, at least in part, on the discovery that strategic modifications of non-human donor antibody CDR residue(s) can be used to humanize antibodies. Such modifications modulate the 3D structural fit between donor antibody CDRs and human acceptor antibody framework regions that comprise the variable domains of a CDR-grafted antibody. Whereas prior art methods of humanization have relied on making framework substitutions (in which selected human framework residues are backmutated to the corresponding amino acid residue present in the non-human donor antibody), the instant invention is based, at least in part, on a method of humanizing antibodies in which selected CDR residues, and optionally adjacent FR residues, are changed in order to accommodate differences in FR amino acid sequences between donor and acceptor antibodies.
Owner:BIOGEN MA INC
Methods for producing recombinant polyclonal immunoglobulins
InactiveUS20050260710A1Improve stabilityEasy to getBacteriaAntibody mimetics/scaffoldsRecombinant DNAMinimum risk
The purpose of the present invention is to provide methods for producing recombinant polyclonal immunoglobulins using recombinant DNA techniques, which provides constant supply of immunoglobulin preparations with minimum risk of infection. A method of the present invention comprises the following steps: (1) isolating a plurality of types of genes from cDNAs derived from tissues or cells expressing immunoglobulins, said genes encoding a plurality of types of polypeptides respectively, and said polypeptides containing a plurality of types of immunoglobulin variable regions respectively, and preparing mixture of the genes; (2) preparing a plurality of types of recombinant vectors into which a plurality of types of genes are introduced respectively by contacting said mixture of the genes with vectors, and preparing mixture of the recombinant vectors; (3) preparing a plurality of types of transformants into which a plurality of types of recombinant vectors are introduced respectively by contacting said mixture of the recombinant vectors with host cells, and preparing mixture of the transformants; and (4) culturing said mixture of the transformants, and obtaining mixture of polypeptides from the transformants culture, wherein said polypeptides contain a plurality of types of immunoglobulin variable regions respectively.
Owner:SUZUKI CO LTD Y +2
Immuno-conjugates and methods for producing them
ActiveUS20120164068A1Reducing and preventing residuePermit productionSenses disorderFungiCompound aImmunoglobulin Heavy Chain Variable Region
The present invention provides an isolated protein comprising an immunoglobulin variable region comprising at least two cysteine residues positioned within framework region 1 such that if at least two of the cysteine residues are not conjugated to another compound a disulphide bond forms between the cysteine residues. Preferably the protein comprises an immunoglobulin heavy chain variable region and an immunoglobulin light chain variable region, wherein at least one of the variable regions comprises the two cysteine residues. The present invention also provides a protein that binds to TAG72. The present invention also provides conjugates of the protein and another compound.
Owner:AVIPEP
Methods of humanizing immunoglobulin variable regions through rational modification of complementarity determining residues
ActiveUS7678371B2Low immunogenicityMinimizing immune responseAntipyreticAnalgesicsVariable domainHumanized antibody
Owner:BIOGEN MA INC
Dual specific single domain antibodies specific for a ligand and for the receptor of the ligand
InactiveCN1745102AImmunoglobulins against cytokines/lymphokines/interferonsImmunoglobulins against cell receptors/antigens/surface-determinantsVariable domainSingle-domain antibody
The invention provides a dual-specific ligand, comprising an immunoglobulin variable region having a first binding specificity for a target ligand and a complementary or immunoglobulin variable region having a second binding specificity for a receptor of the target ligand. Noncomplementary immunoglobulin variable regions.
Owner:DORMANTIS LTD
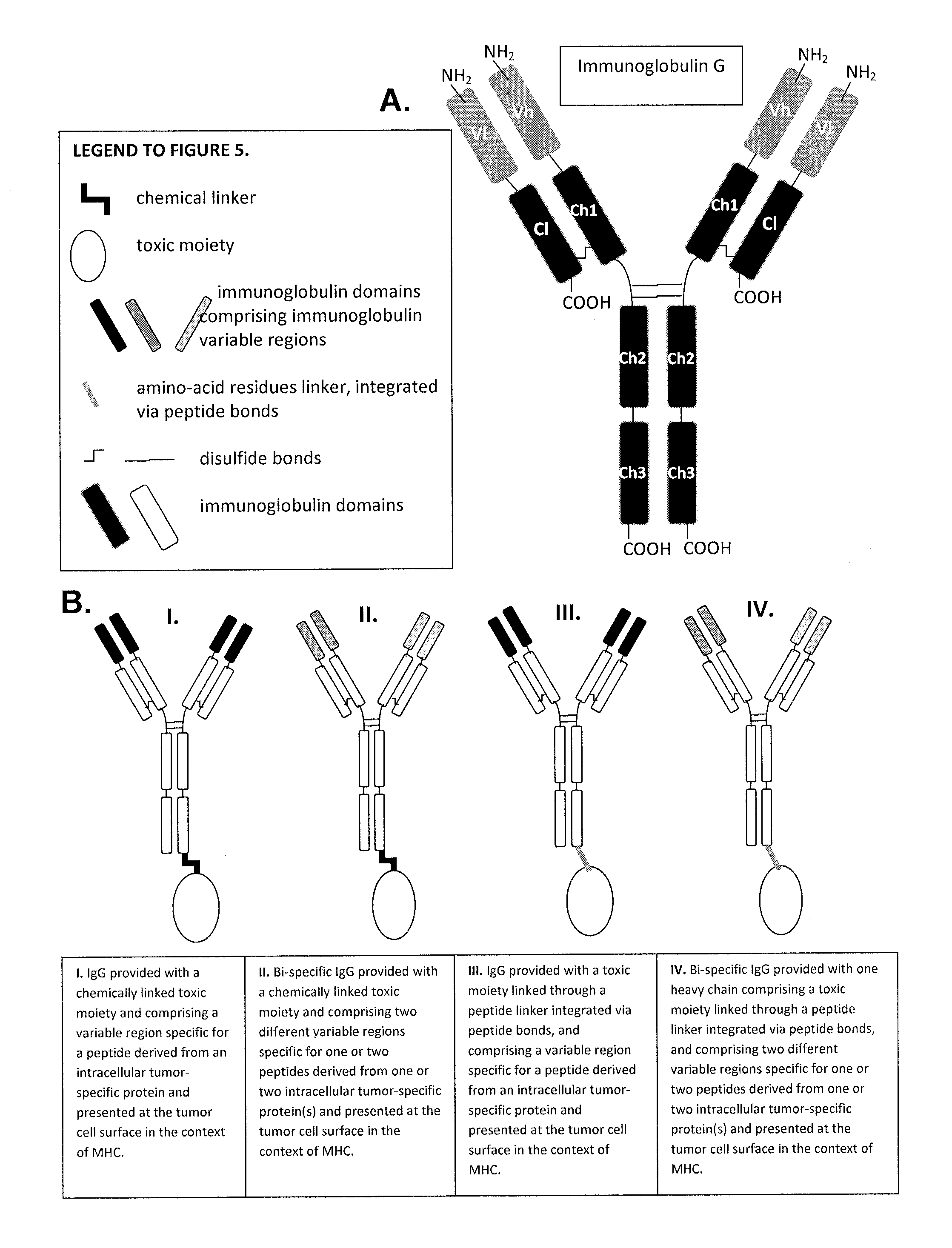
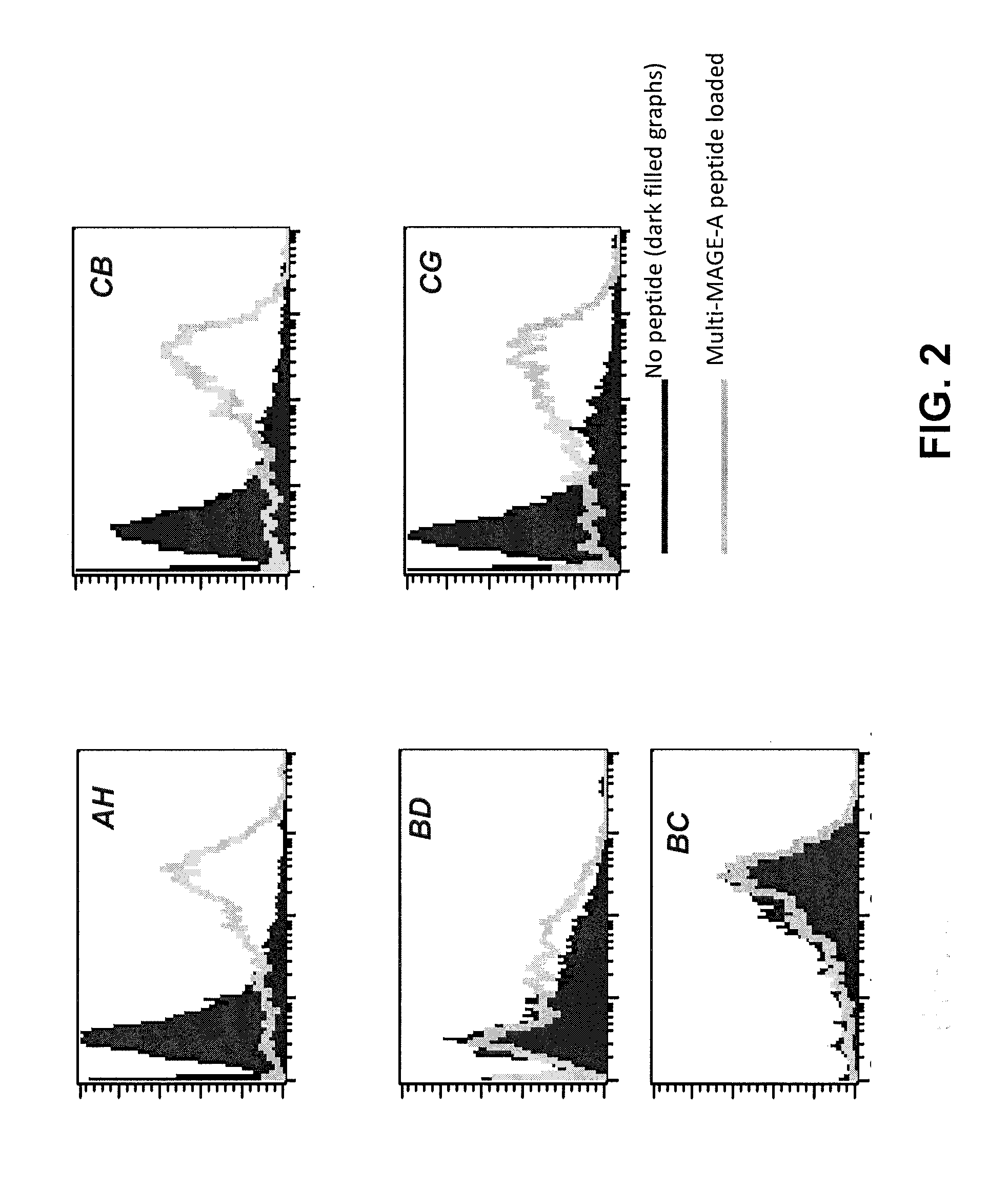
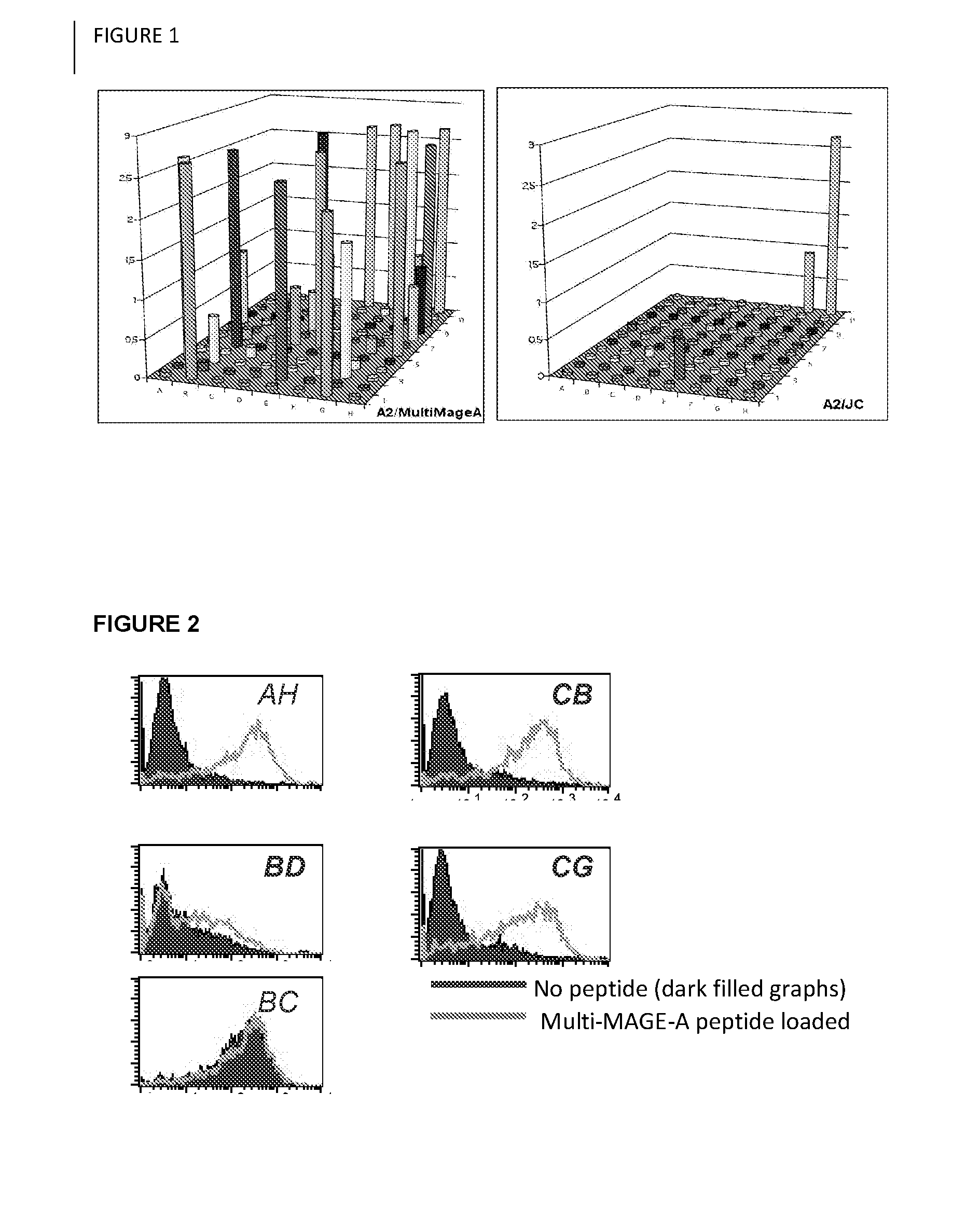
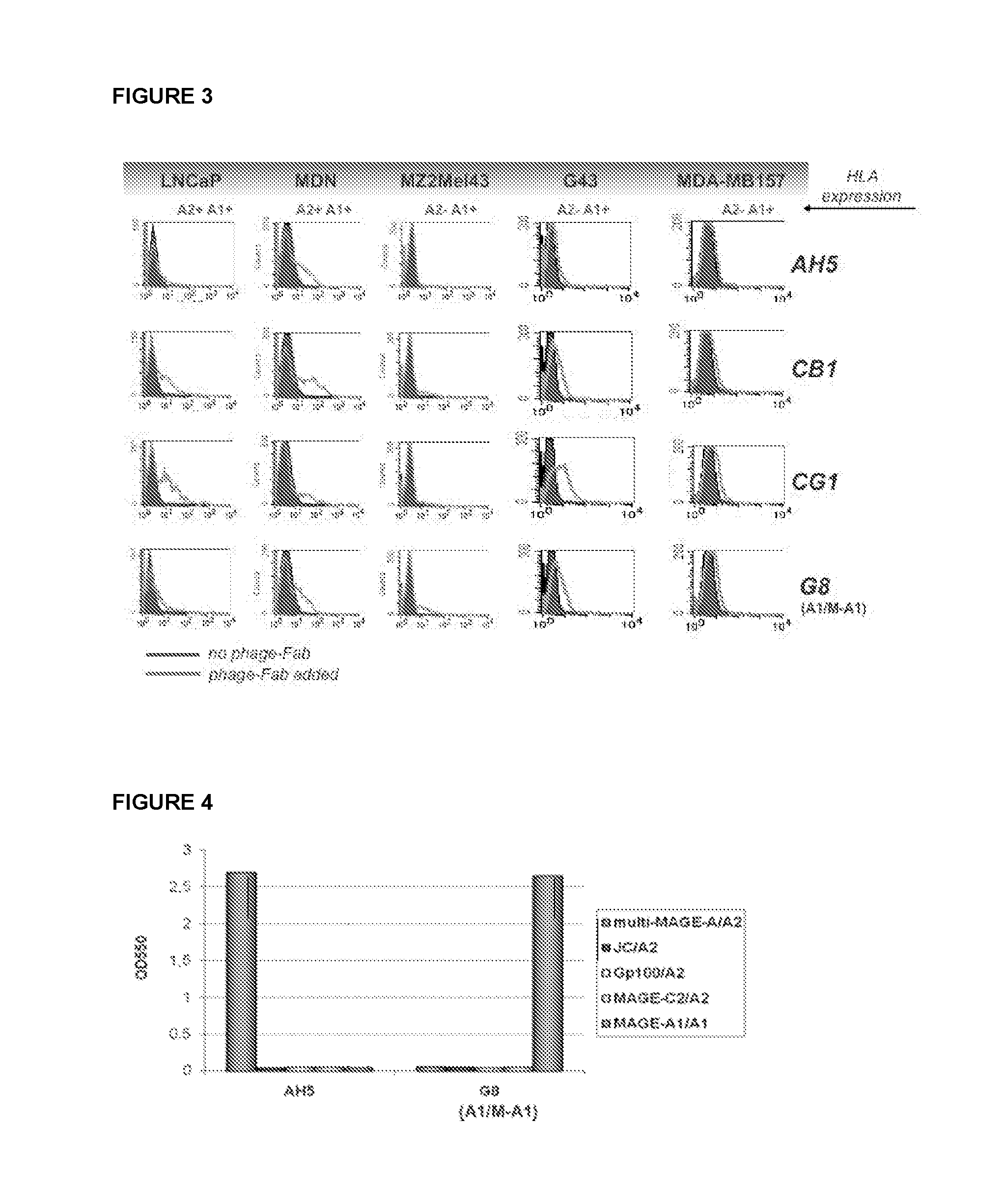
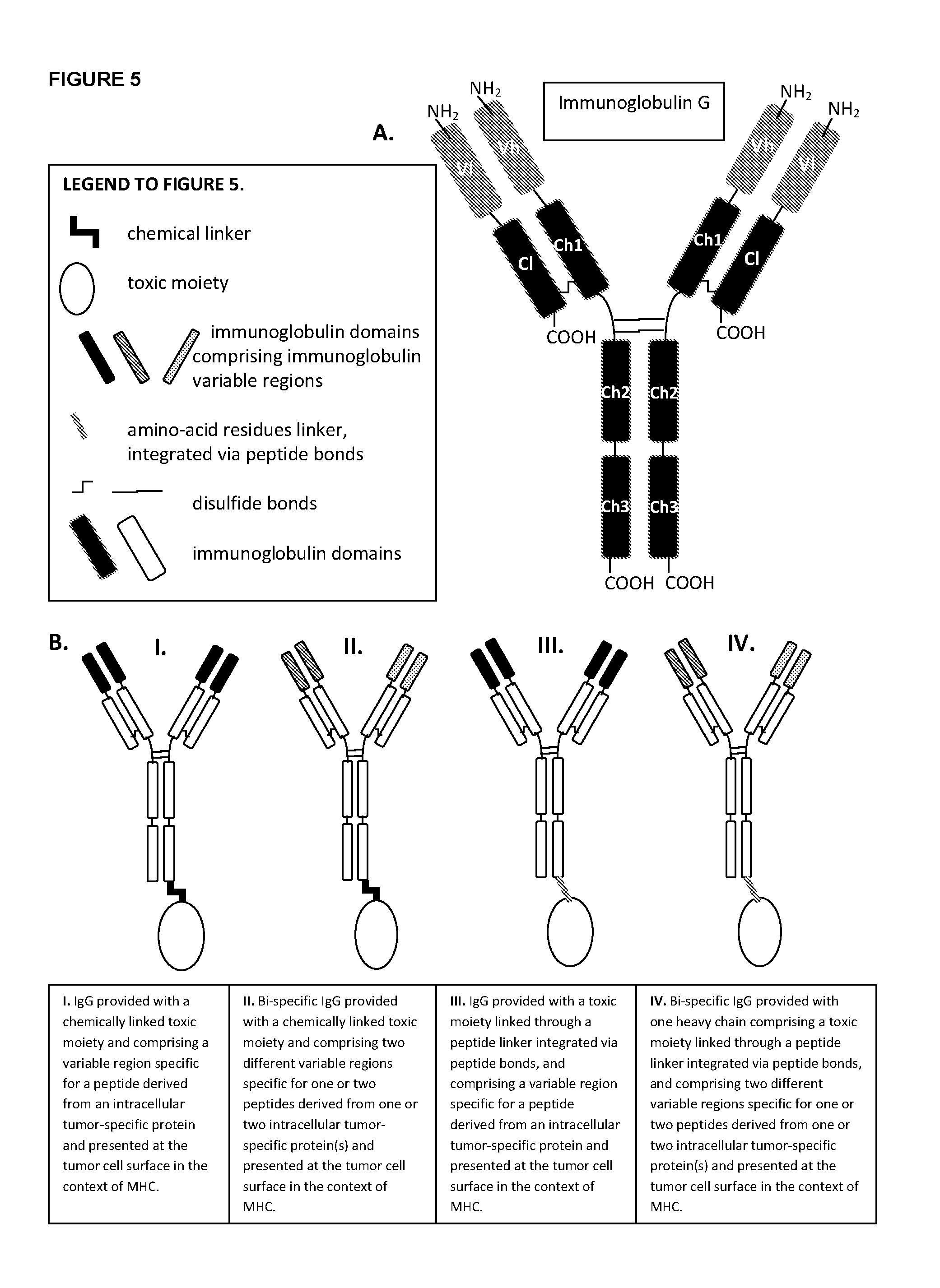
Aberrant cell-restricted immunoglobulins provided with a toxic moiety
InactiveUS20130183307A1Good flexibilityResistant to proteaseImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDiseaseAutoimmune disease
Described are immunoglobulins provided with a toxic moiety, comprising at least an immunoglobulin variable region that specifically binds to an MHC-peptide complex preferentially associated with aberrant cells. These immunoglobulins provided with a toxic moiety are preferably used in selectively modulating biological processes. The provided immunoglobulins provided with a toxic moiety are of particular use in pharmaceutical compositions for the treatment of diseases related to cellular aberrancies, such as cancers and autoimmune diseases.
Owner:APO T
Aberrant cell-restricted immunoglobulins provided with a toxic moiety
ActiveUS20150056198A1Improve treatment outcomesModulate effectImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDiseaseAutoimmune disease
Described are immunoglobulins provided with a toxic moiety, comprising at least an immunoglobulin variable region that specifically binds to an MHC-peptide complex preferentially associated with aberrant cells. These immunoglobulins provided with a toxic moiety are preferably used in selectively modulating biological processes. The provided immunoglobulins provided with a toxic moiety are of particular use in pharmaceutical compositions for the treatment of diseases related to cellular aberrancies, such as cancers and autoimmune diseases.
Owner:APO T
Immuno-conjugates and methods for producing them
ActiveUS9315581B2Avoid bindingReducing and preventing residueUltrasonic/sonic/infrasonic diagnosticsBacteriaImmunoglobulin Heavy Chain Variable RegionHeavy chain
The present invention provides an isolated protein comprising an immunoglobulin variable region comprising at least two cysteine residues positioned within framework region (FR) 2 and / or at least two cysteine residues positioned within framework region (FR3), wherein if at least two of the cysteine residues in FR2 and / or FR3 are not conjugated to a compound then an intra-framework disulphide bond is capable of forming between the cysteine residues. Preferably the protein comprises an immunoglobulin heavy chain variable region (VH) and an immunoglobulin light chain variable region (VL), wherein at least one of the variable regions comprises the two cysteine residues. The present invention also provides conjugates of the protein and another compound.
Owner:AVIPEP
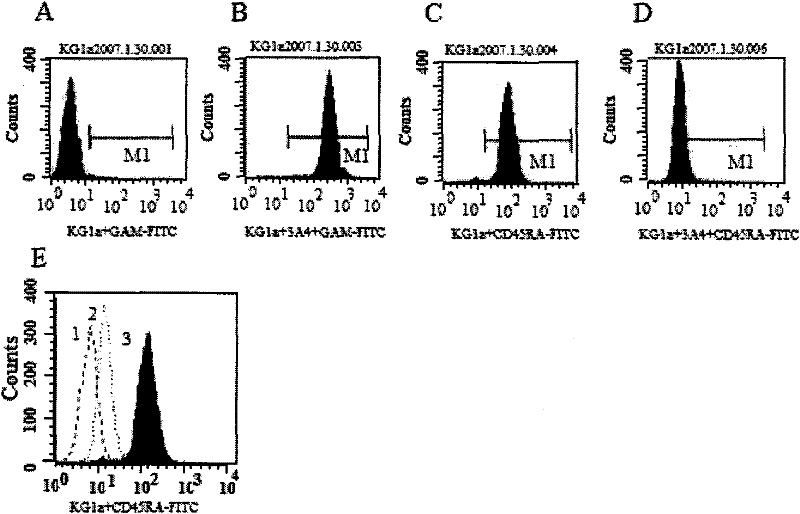
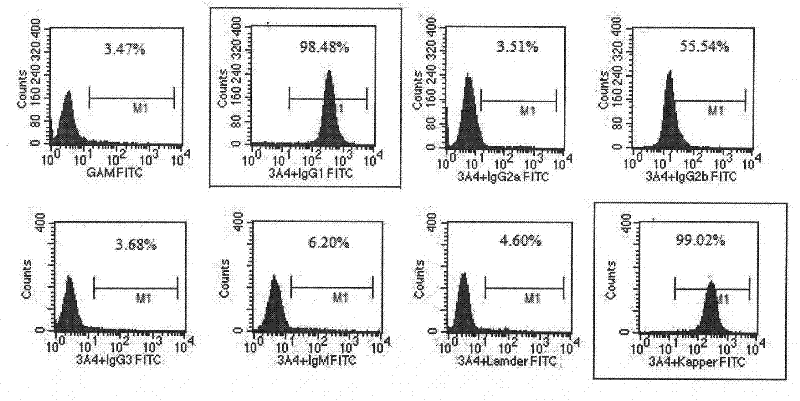
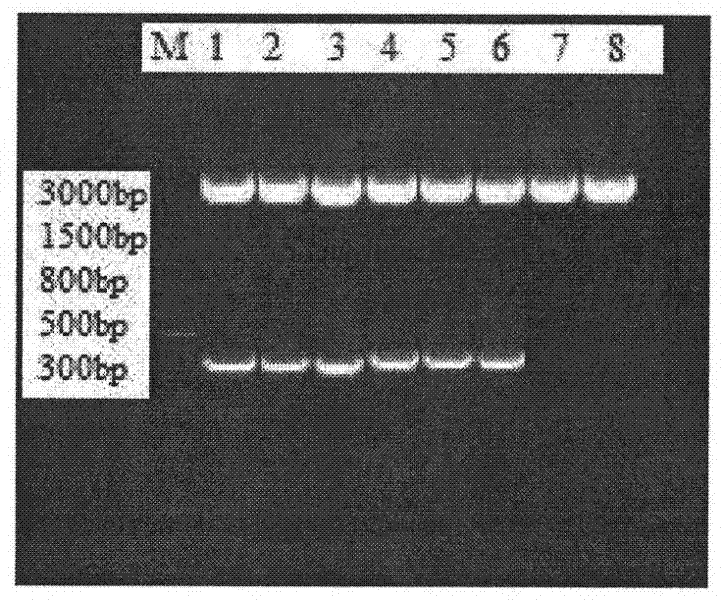
Anti-human CD45RA rat immune globulin variable region gene and application
ActiveCN101831434AImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsNucleotideLeukemia
The invention provides an anti-human CD45RA rat immune globulin variable region gene, which comprises nucleotide sequences of SEQIDNO.1 and SEQIDNO.2 and encoded nucleotide sequences of SEQIDNO.3 and SEQIDNO.4. Research proves that anti-human CD45RA rat immune globulin variable region gene can effectively distinguish leukemia patients, leukemia cell strain CD34+CD38-CD123+leukemia stem cells and CD34+CD38+CD123+ offspring leukemia cells, has the application prospects of leukemia targeted therapy, and can be used in preparing targeted therapy blood system malignant tumor medicines.
Owner:杭州永申生物科技有限公司
Bifunctional fusion molecules for the delivery of antigens to professional antigen-presenting cells
InactiveUS20100129383A1Faster clearance rateLow immunogenicityImmunoglobulinsBlood/immune system cellsAntigenImmunoglobulin Variable Region
The invention provides a bifunctional fusion molecule comprising: a first functional domain comprising a first immunoglobulin variable region, a second immunoglobulin variable region and a linker for connecting the first and second variable regions; a second functional domain comprising a moiety for binding to an antigenic agent; wherein the first and second functional domains are linked; and wherein the first functional domain specifically binds to a surface molecule of a professional antigen-presenting cell, and uses thereof.
Owner:THE GOVERNORS OF THE UNIV OF ALBERTA
Construction and use of a functionally human antibody library with maximized repertoire diversity
InactiveUS20100137156A1Increase diversityPeptide librariesLibrary screeningHeavy chainNatural immune system
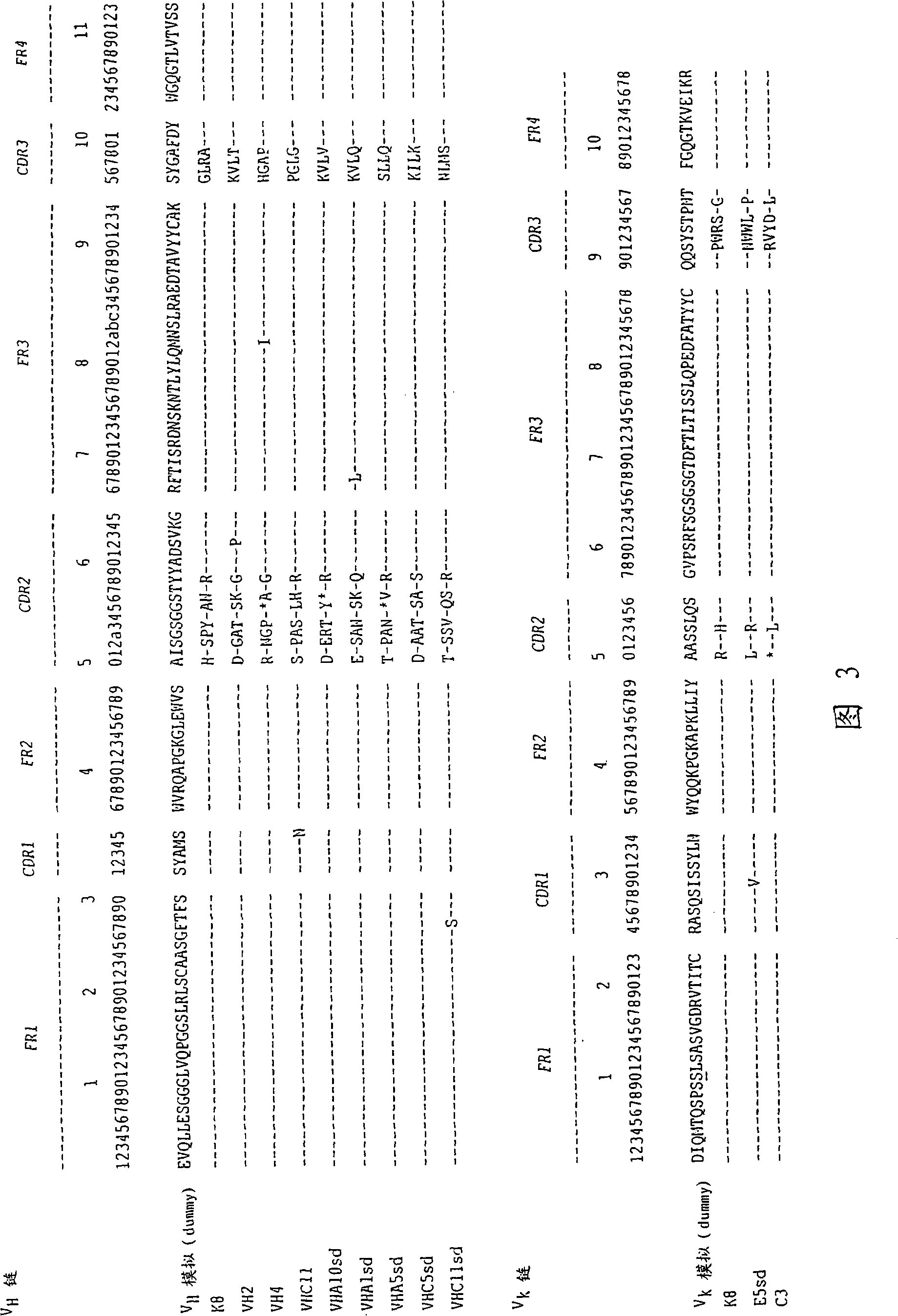
Immunoglobulin libraries are provided that contain randomly assembled FR1, CDR1, FR2, CDR2, FR3, CDR3 and FR4 sequences of heavy or light chain immunoglobulin variable regions. The libraries exhibit a degree of repertoire diversity not found in natural immune systems and can be used to express novel immunoglobulins. The libraries can be used for screening antibodies with the target specificity of interest. The resultant antibodies can be fully human and non-immunogenic.
Owner:SINOMAB BIOSCI
Anti human CD19 mouse immune globulin variable zone gene and uses
ActiveCN1884535AGenetic material ingredientsImmunoglobulins against animals/humansCell membraneFhit gene
This invention provides variable region gene of anti-human CD19 Mouse IgE ELISA, which has the nucleotide sequence of SEQ ID NO. 1 and SEQ ID NO. 2 as well as the amino acid sequence of SEQ ID NO. 3 and SEQ ID NO. 4. The mono-clone anuibody of this invention has the characteristic that identify B lymphocyte tumour cell membrane CD19 antigen but not identify other tissue and cell composition. The in vitro indicates that this antibody can selectively kill the B-ALL cell, so this kind of antibody has broad potential application prospect in curing B lymphocytes malignant tumor, including leukaemia, lymphoma. It can be applied to the medicine that curing B lymphocytes malignant tumor.
Owner:杭州永申生物科技有限公司
Phage antibody library and application thereof in avian influenza immunodetection
InactiveCN103806110AImprove efficiencyImprove throughputPeptide librariesFermentationHigh affinity antibodySpecific antibody
The invention relates to an antibody library. An immune globulin light chain variable region VL gene sequence is inserted between Xba and Sac restriction enzyme cutting sites of a Pcomb3 vector. An immune globulin heavy chain variable region VH gene sequence is inserted between Spe and Xho endo restriction enzyme cutting sites. The antibody library is characterized in that two gene fragments comprise the possible combination of antibody variable region light chain and heavy chain, and the antibody library can realize affinity enrichment with an avian influenza virus antigen, and can be applied to avian influenza immunodetection. The antibody library has the advantages that a high affinity antibody can be acquired without animal immunity; a preparation period is short; the antibody library is a phage antibody library which is constructed by taking mice natural immune globulin variable region light chain and heavy chain genes as sources, theoretically contains all possible antibodies in a mice, and is a specificity antibody library which can directly acquire different avian influenza viruses through screening; and the screening has the advantages of high efficiency, high flux, large selection base and high specificity, thus the phage antibody library has a wide application prospect in the aspect of the disease detection of avian influenza and other animals.
Owner:BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU INSPECTION & QUARANTINE TECH CENT +2
Staphylococcus aureus-specific egg yolk immunoglobulin variable region single-chain antibody and application
ActiveCN104961826AStrong specificityImprove bindingAntibacterial agentsBacteriaStaphylococcus cohniiSingle-Chain Antibodies
The invention discloses a staphylococcus aureus-specific egg yolk immunoglobulin (IgY) variable region single-chain antibody. The specific egg yolk immunoglobulin variable region antibody has a nucleotide sequence shown by SEQ ID No:2 and an amino acid sequence shown by SEQ ID No:1. The single-chain antibody has relatively strong binding capacity with staphylococcus aureus, namely that the screened antibody has an obvious inhibition effect on the growth of staphylococcus aureus, and the inhibition effect and inhibition behavior are similar to those of penicillin and streptomycin, so that the single-chain antibody has a perfect application prospect in preparation of staphylococcus aureus antibody drugs or detection reagents. Moreover, the single-chain antibody has high specificity to staphylococcus aureus, and thus the laboratory early diagnosis and clinical treatment level of the staphylococcus aureus can be improved.
Owner:DALIAN UNIV OF TECH
Mutant immunoglobulin kappa chain variable region-binding peptide
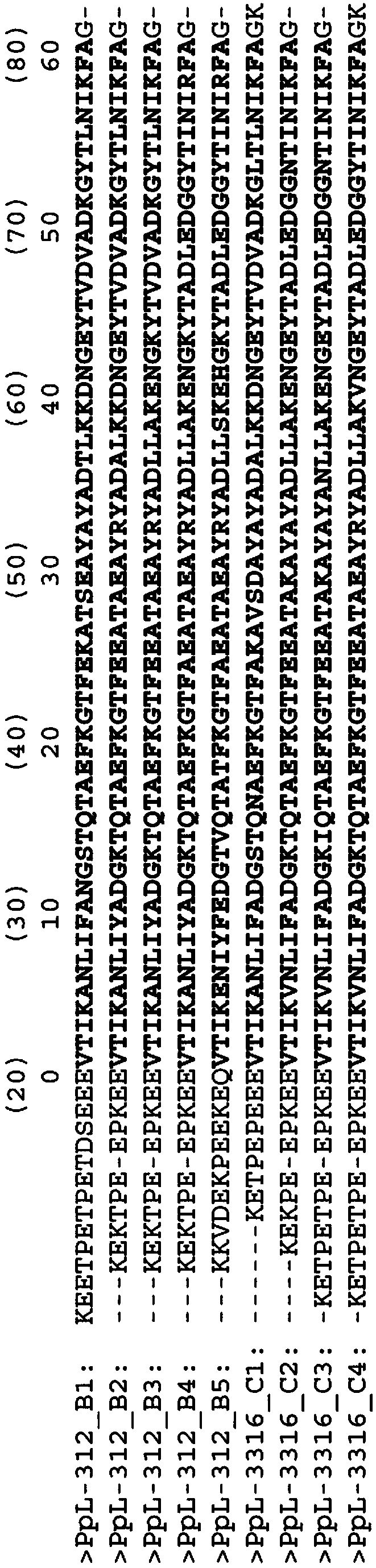
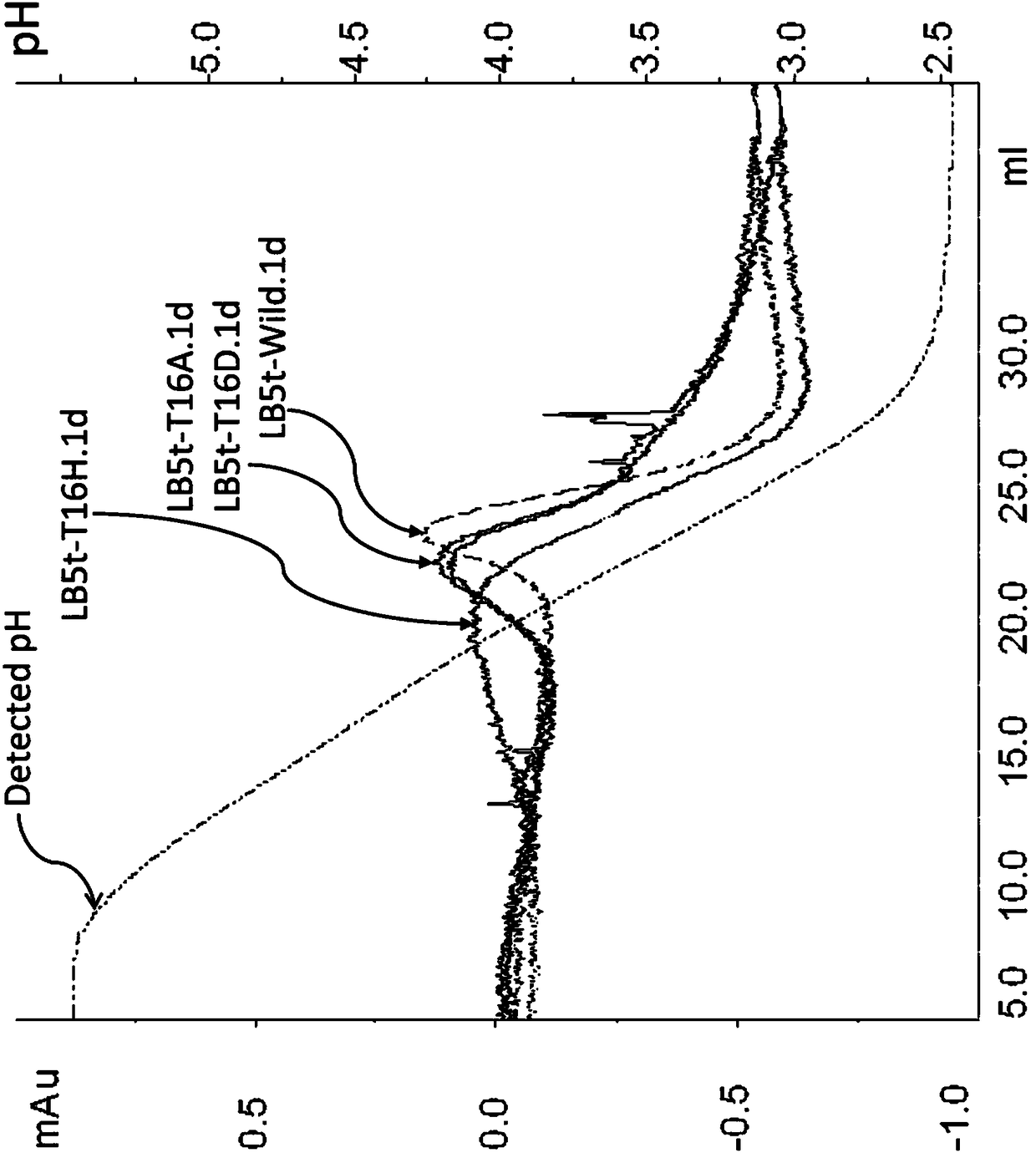
InactiveCN108064286AStrong selective adsorption capacityReduce capacityFungiBacteriaImmunoglobulin kappa-ChainsPeptostreptococcus magnus
The purpose of the present invention is to provide: a mutant immunoglobulin kappa chain variable region-binding peptide characterized by the acid dissociation pH of an immunoglobulin kappa chain variable region-containing protein being shifted to the neutral side compared to prior to the introduction of a mutation; an affinity separation matrix comprising the peptide as a ligand; and a method forproducing a kappa chain variable region-containing protein using the affinity separation matrix. Another purpose of the present invention is to provide DNA that encodes the peptide, a vector containing the DNA, and a transformant that is transformed by the vector. The problem addressed by the present invention is solved by using a peptide into which a mutation has been introduced at an appropriatesite as an affinity ligand for a kappa chain variable region binding domain of protein L derived from a Peptostreptococcus magnus strain.
Owner:KANEKA CORP
Aberrant cell-restricted immunoglobulins provided with a toxic moiety
InactiveUS20150202318A1Improved specificity and selectivityLower Level RequirementsImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsOrganismal ProcessAutoimmune disease
Described are immunoglobulins provided with a toxic moiety, comprising at least an immunoglobulin variable region that specifically binds to an MHC-peptide complex preferentially associated with aberrant cells. These immunoglobulins provided with a toxic moiety are preferably used in selectively modulating biological processes. The provided immunoglobulins provided with a toxic moiety are of particular use in pharmaceutical compositions for the treatment of diseases related to cellular aberrancies, such as cancers and autoimmune diseases.
Owner:APO T
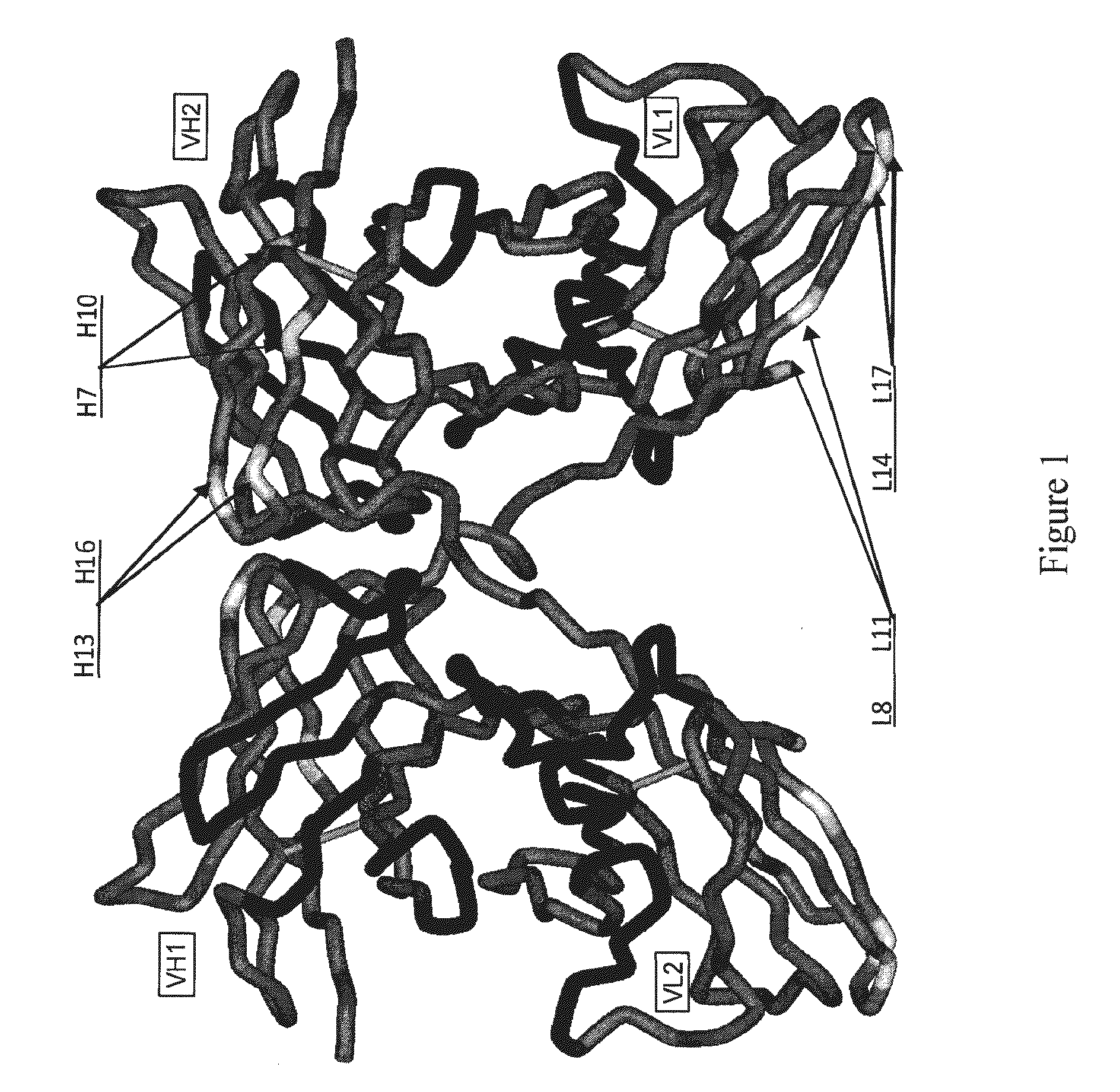
Recombinant antibody vector
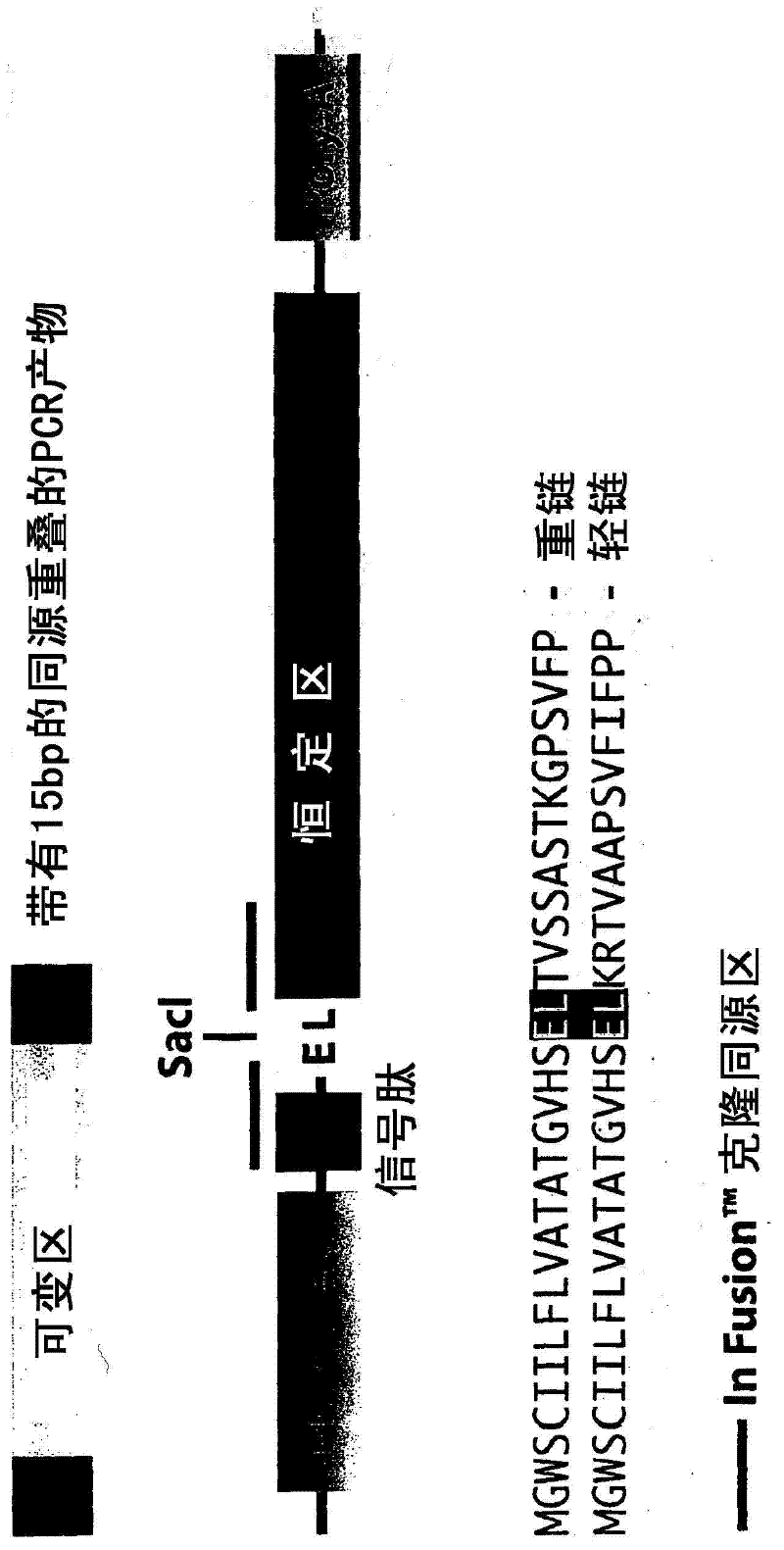
A recombinant antibody vector for producing a single chain recombinant antibody comprises: (a) a contiguous nucleotide sequence: (i) that comprises a restriction endonuclease site that encodes an amino acid sequence of an immunoglobulin variable region; and (ii) that encodes an immunoglobulin constant region amino acid sequence in the same reading frame as (i), wherein another nucleotide sequence encoding (iii) an immunoglobulin variable region amino acid sequence, is insertable into the restriction endonuclease site in the same reading frame as (ii); and (b) one or more regulatory nucleotide sequences operably linked or connected to said nucleotide sequence, wherein the amino acid sequence in (i) comprises amino acids conserved in different immunoglobulin variable regions. The restriction endonuclease site may be a SacI site which encodes the conserved amino acids glutamate and leucine. In frame insertion of the nucleotide sequence of (iii) is facilitated by ligase independent cloning.
Owner:ACYTE BIOTEC
Bispecific bivalent scfv-Fc molecules
InactiveCN105229031AAntibacterial agentsAntibody mimetics/scaffoldsPharmaceutical formulationImmunoglobulin Variable Region
Described herein is a bispecific molecule containing an Fc polypeptide chain and immunoglobulin variable regions. Also provided are pharmaceutical formulations comprising such molecules, nucleic acids encoding such molecules, host cells containing such nucleic acids, methods of making such molecules, and methods of using such molecules.
Owner:AMGEN INC
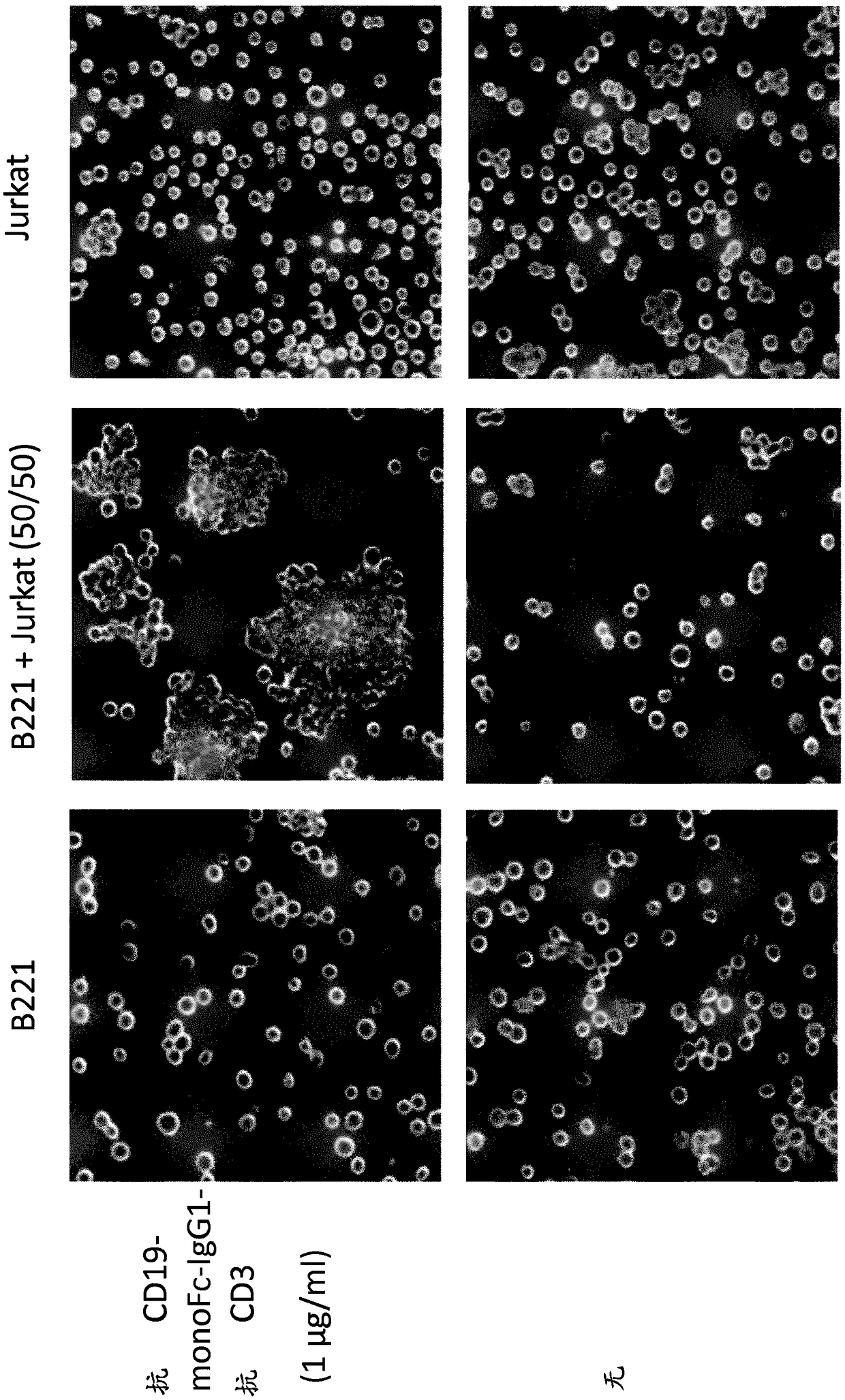
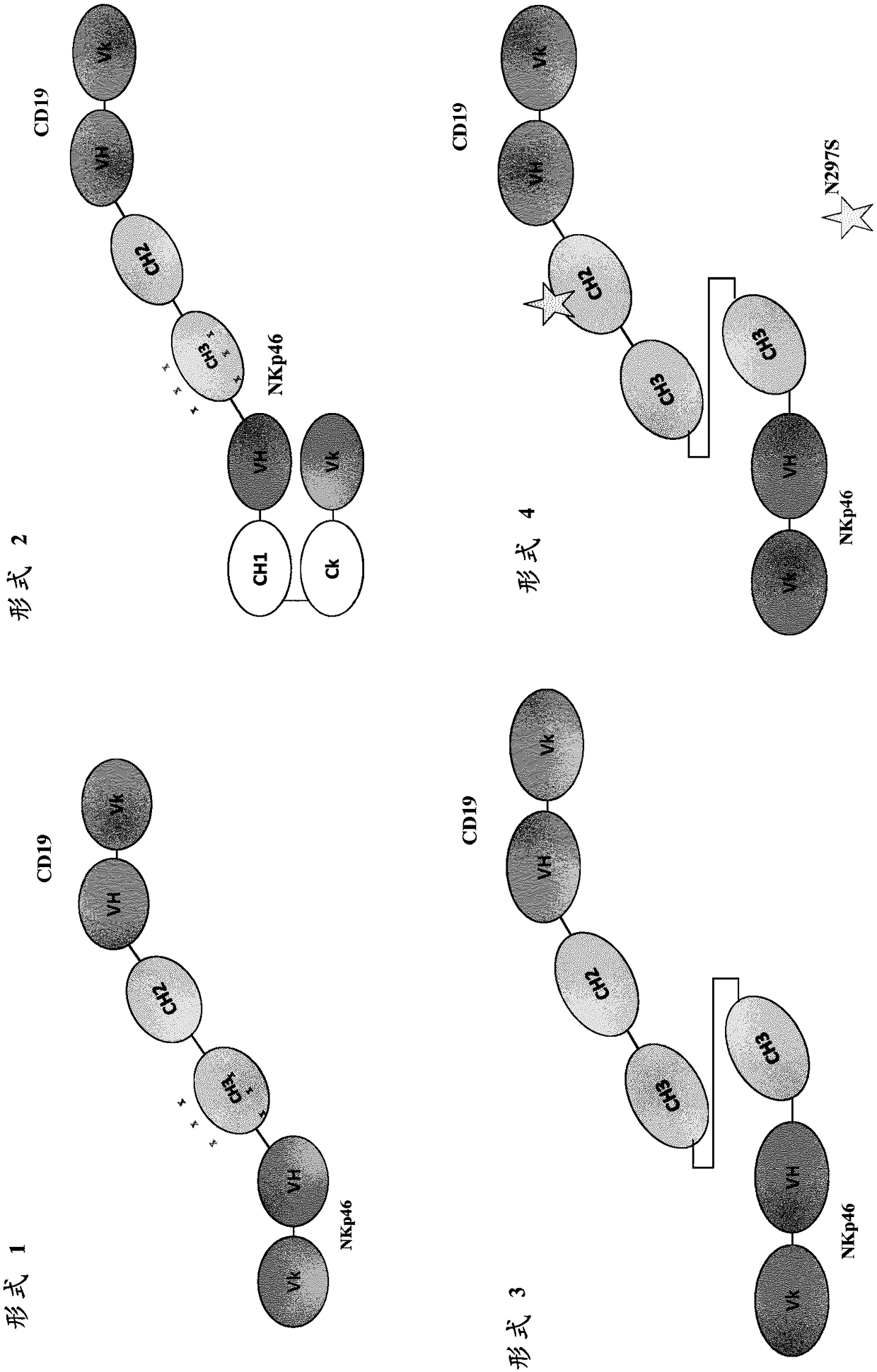
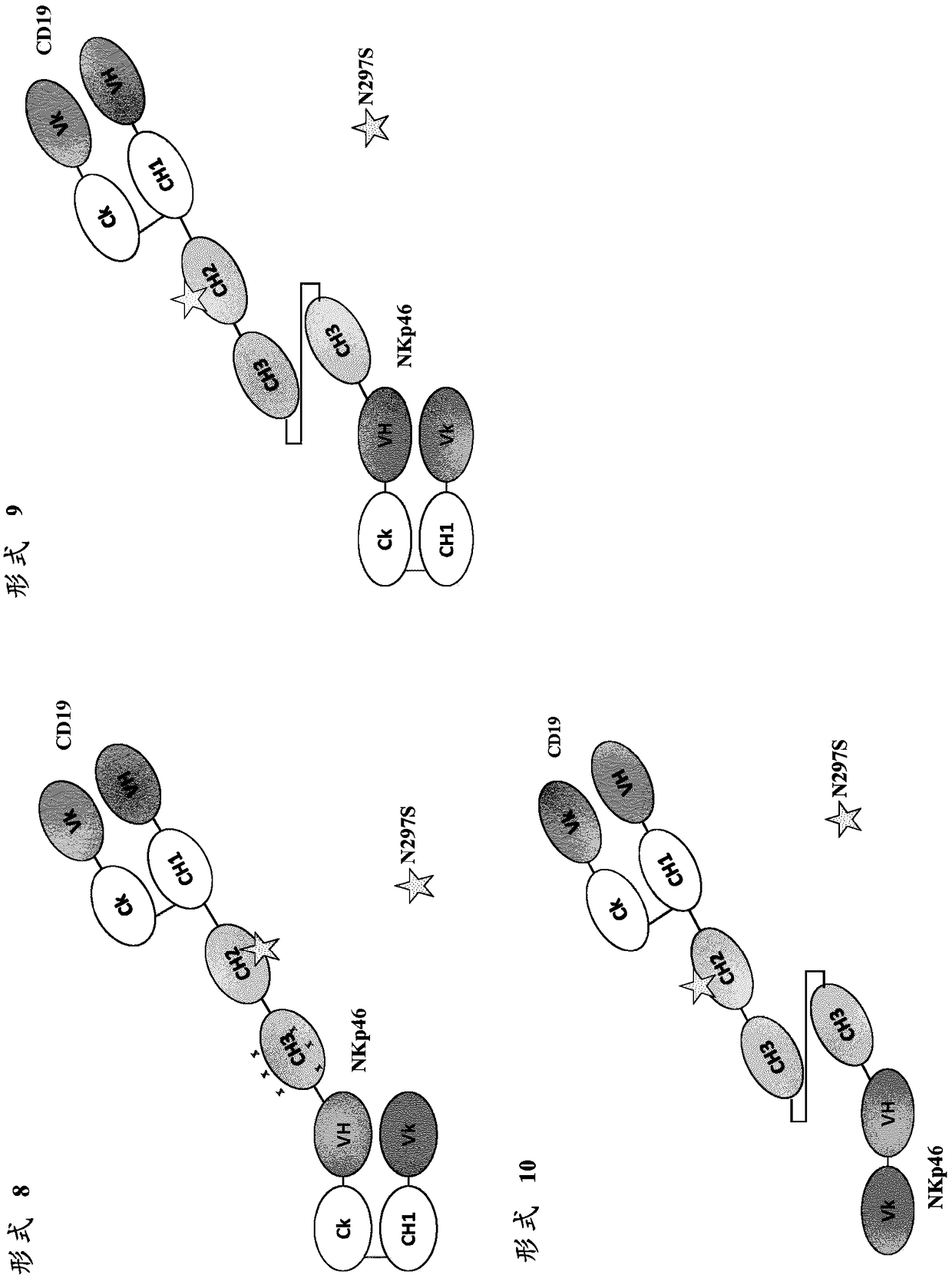
VARIABLE REGIONS FOR NKp46 BINDING PROTEINS
NKp46-binding immunoglobulin variable regions, and proteins such as antibodies and multispecific proteins that comprise the variable regions are provided. The proteins can bind and specifically redirect NK cells to lyse a target cell of interest. The proteins have utility in the treatment of disease, notably cancer or infectious disease.
Owner:INNATE PHARMA SA
Immuno-conjugates and methods for producing them
ActiveUS9290577B2Avoid bindingReducing and preventing residueSenses disorderNervous disorderImmunoglobulin Heavy Chain Variable RegionCompound a
Owner:AVIPEP
Immunoglobulin single variant antigen binding domain and specific construct thereof
The invention provides a dual-specific ligand comprising a first immunoglobulin variable domain having a first binding specificity and a complementary or non-complementary immunoglobulin variable domain having a second binding specificity.
Owner:DORMANTIS LTD
Antimicrobial, antiviral, anticancer and immunomodulatory peptides and uses therefore
InactiveUS20120121574A1Antibacterial agentsAntimycoticsComplementarity determining regionImmunomodulatory peptide
Polypeptides derived from constant domains of antibody light (L) and / or heavy (H) chains as well as from complementary determining regions (CDRs) of immunoglobulin variable regions are disclosed possessing broad spectrum biological activities including, among others, antifungal, antibacterial, antiviral, anticancer and / or immunomodulatory activity in vitro, ex vivo and / or in vivo.
Owner:POLONELLI LUCIANO
A kind of staphylococcus aureus-specific chicken egg yolk immunoglobulin variable region single-chain antibody and its application
ActiveCN104961826BStrong specificityImprove bindingAntibacterial agentsBacteriaSingle-Chain AntibodiesStaphylococcus cohnii
Owner:DALIAN UNIV OF TECH
BCR transgenic mice with common leader sequence
PendingCN114867345AImmunoglobulins against animals/humansAntibody medical ingredientsAntigenPolynucleotide
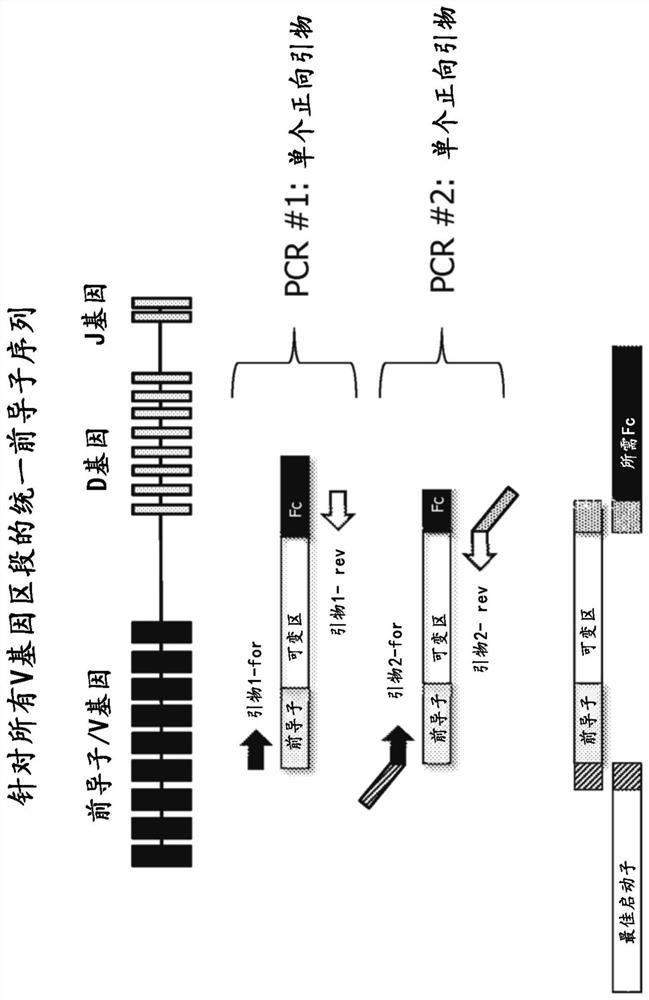
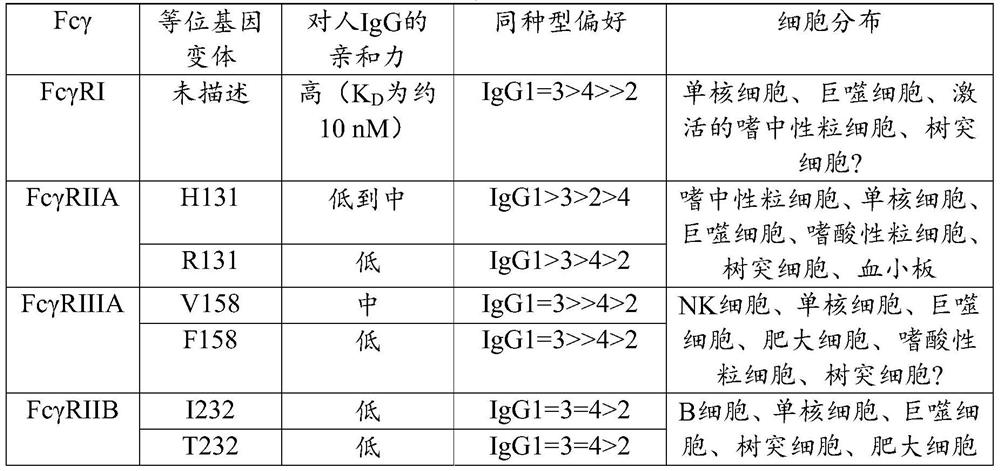
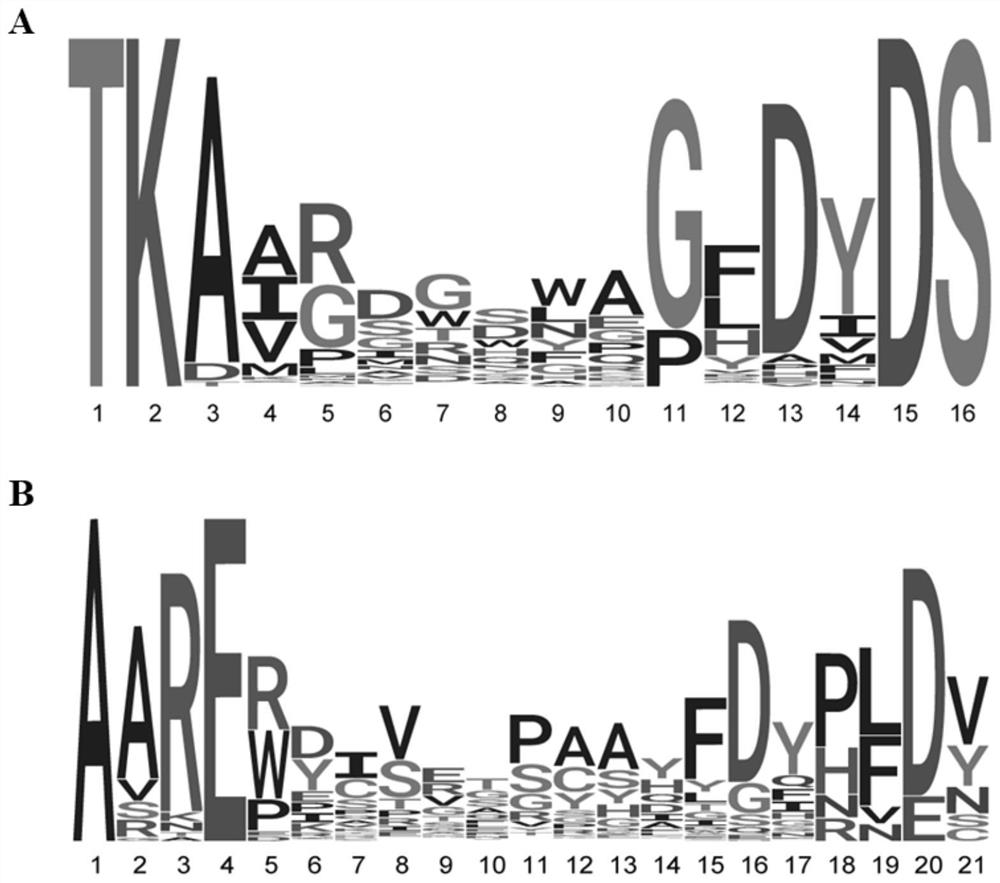
The invention provides transgenic animals comprising some or all components of human heavy and / or light chain immunoglobulin variable region loci, methods of making such animals, methods of making human antibodies using such animals, and methods of treatment using human antibodies prepared in such animals, wherein the animal comprises in its genome a plurality of human heavy chain V gene segments and / or a plurality of human light chain V gene segments, or both, all of the plurality of human heavy chain V gene segments immediately following a same first leader peptide coding sequence and all of the plurality of human light chain V gene segments immediately following a same second leader peptide coding sequence. The invention also provides a polynucleotide construct comprising two or more human heavy chain or light chain precursor / V gene segments comprising the same leader peptide coding sequence. Such animals, constructs, and methods can be used to efficiently produce an optimal diverse population of antibodies against an antigen of interest, such as a therapeutic antigen of interest.
Owner:BRISTOL MYERS SQUIBB CO
Primer group for amplifying B lymphocyte immune repertoire and application of primer group
PendingCN112301099AImprove featuresHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationForward primerMedicine
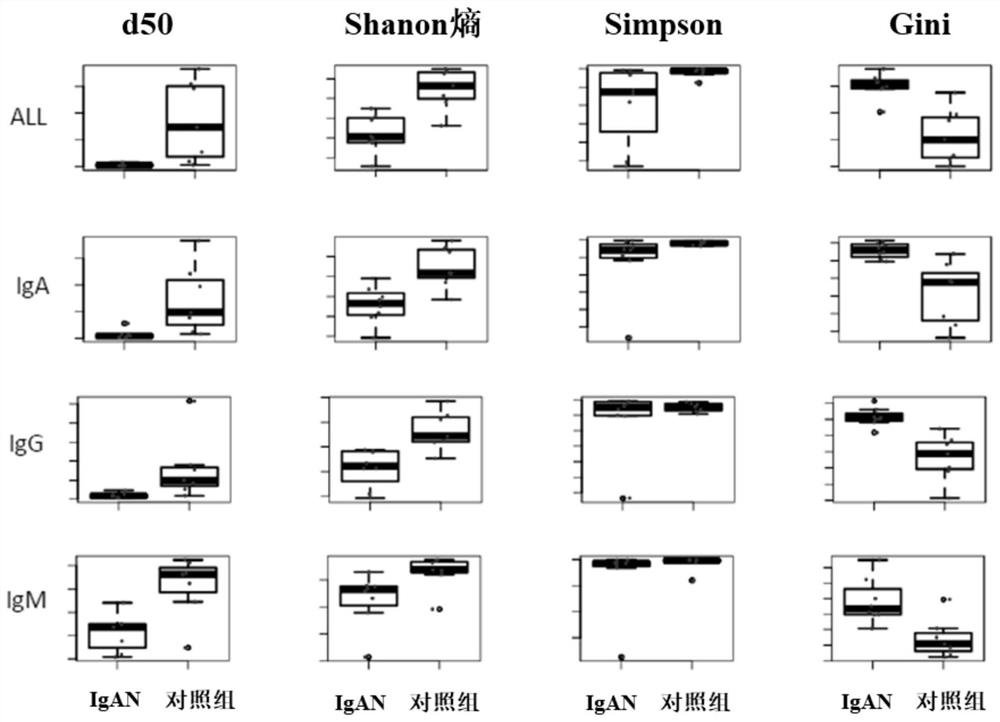
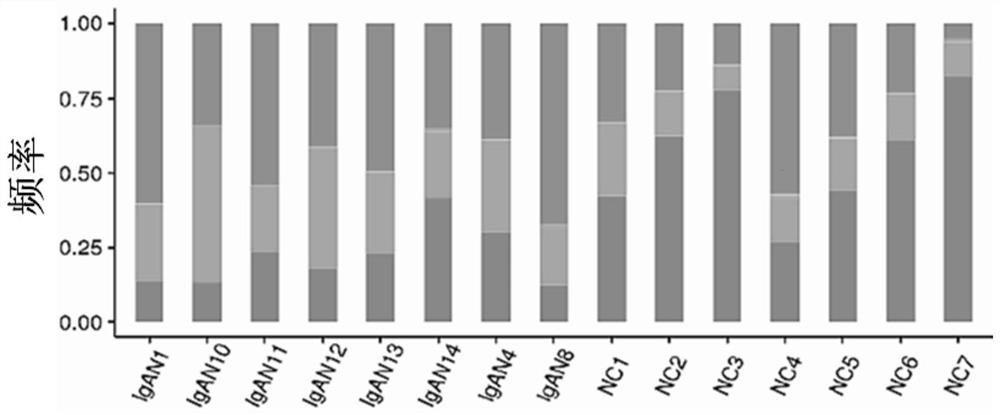
The invention provides a primer group for amplifying a B lymphocyte immune repertoire and an application of the primer group. The primer group comprises a group of forward primers and a group of reverse primers. The forward primers comprise a linker sequence 1, a bar code sequence 1 and a specific sequence aiming at an immunoglobulin variable region complementation determining region 3, which areconnected in sequence from a 5' end to a 3' end, wherein the specific sequence aiming at the immunoglobulin variable region complementary determining region 3 is shown as any one of SEQ ID NO. 1 to SEQ ID NO. 12; and the reverse primers sequentially comprise a linker sequence 2, a bar code sequence 2 and a specific sequence aiming at an immunoglobulin gene constant region from the 5' end to the 3'end. The primer group is used for amplifying B lymphocytes of an IgAN patient and a normal control group, library information is collected, and the length and diversity changes of CDR3 of the IgAN patient are analyzed through sequencing.
Owner:SOUTH UNIVERSITY OF SCIENCE AND TECHNOLOGY OF CHINA
Anti-human CD45RA rat immune globulin variable region gene and application
ActiveCN101831434BImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsFhit geneLeukemia
The invention provides an anti-human CD45RA rat immune globulin variable region gene, which comprises nucleotide sequences of SEQIDNO.1 and SEQIDNO.2 and encoded nucleotide sequences of SEQIDNO.3 and SEQIDNO.4. Research proves that anti-human CD45RA rat immune globulin variable region gene can effectively distinguish leukemia patients, leukemia cell strain CD34+CD38-CD123 stem cells and CD34+CD38+CD123 offspring leukemia cells, has the application prospects of leukemia targeted therapy, and can be used in preparing targeted therapy blood system malignant tumor medicines.
Owner:杭州永申生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com