VARIABLE REGIONS FOR NKp46 BINDING PROTEINS
A variable region and variable technology, applied in the field of variable regions of NKp46 binding proteins, can solve problems such as NK cell-specific bispecific antibodies that have not yet been developed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0222] Generation of anti-huNKp46 antibodies
[0223] Part A: Generation of anti-huNKp46 antibodies
[0224] Balb / c mice were immunized with recombinant human NKp46 extracellular domain recombinant Fc protein. Mice received a primary immunization (intraperitoneal injection) with an emulsion of 50 μg NKp46 protein and complete Freund's adjuvant, and a second immunization (intraperitoneal injection) with an emulsion of 50 μg NKp46 protein and incomplete Freund's adjuvant , and finally a boost (iv) with 10 μg of NKp46 protein. Immune splenocytes were fused with X63.Ag8.653 immortalized B cells 3 days after boost and cultured in the presence of irradiated splenocytes.
[0225] Primary screening: Supernatants (SN) of growing clones were tested in primary screening by flow cytometry using cell lines expressing human NKp46 constructs on the cell surface. Briefly, for FACS screening, the presence of reactive antibody in the supernatant was revealed by using PE-labeled goat anti-mou...
example 2
[0302] Identification of bispecific antibody formats that bind FcRn but not FcγR for targeting effector cell receptors
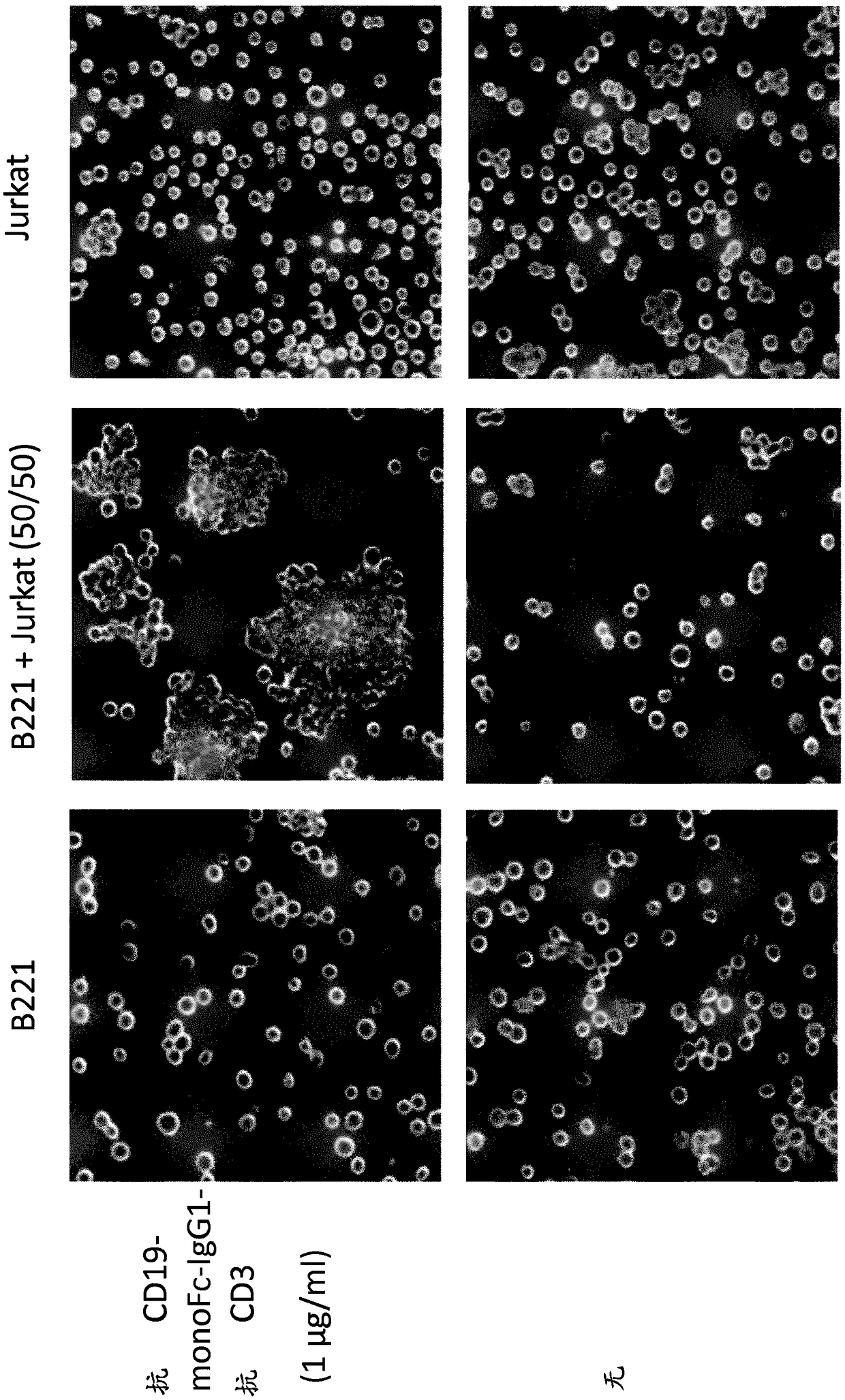
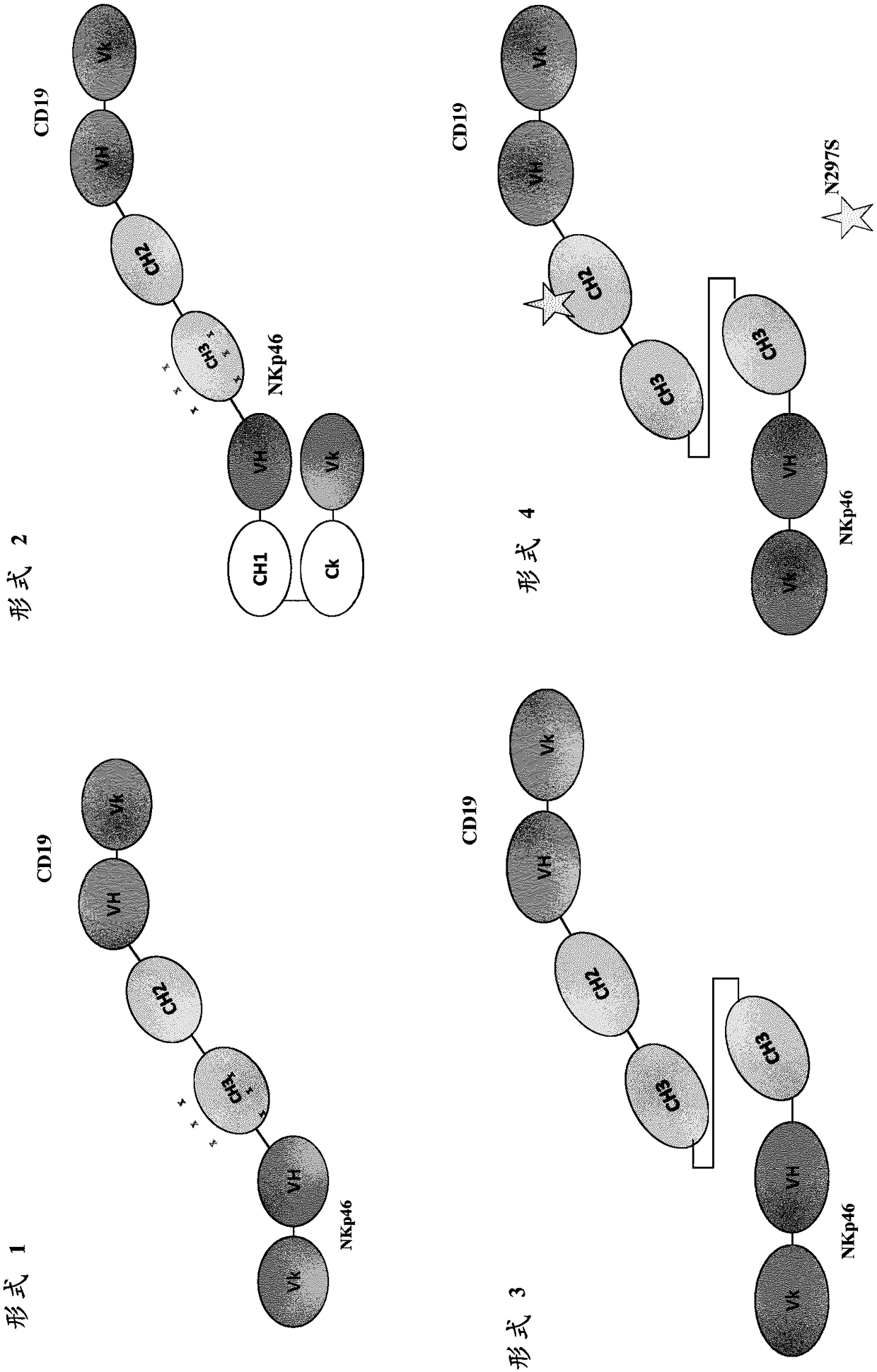
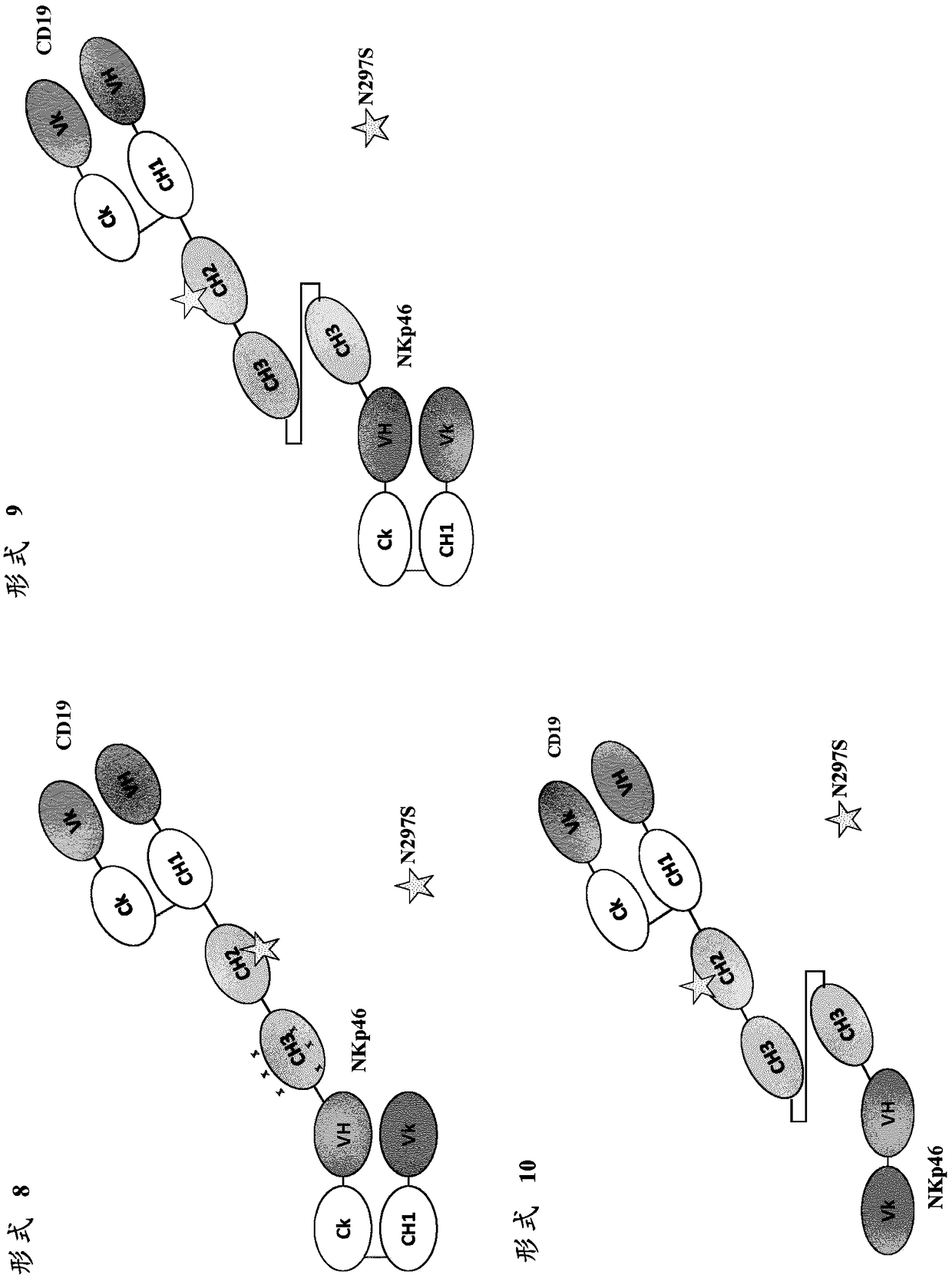
[0303] The purpose of this experiment was to develop a new bispecific protein format that places an Fc domain on a polypeptide together with an anti-NKp46 binding domain and an anti-target antigen binding domain. The bispecific protein binds monovalently to NKp46 through its anti-NKp46 binding domain. The monomeric Fc domain retains at least partial binding to the human neonatal Fc receptor (FcRn), but does not substantially bind to human CD16 and / or other human Fcγ receptors. Thus, bispecific proteins do not induce Fc[gamma]-mediated (eg CD16-mediated) target cell lysis.
Embodiment 2-1
[0304] Example 2-1 Construction and binding analysis of anti-CD19-IgG1-Fcmono-anti-CD3
[0305] Since anti-NKp46 bispecific antibodies that could indicate whether this protein is functional have not been produced, CD3 was used instead of NKp46 as a model antigen to study the function of new monovalent bispecific proteins before targeting NK cells by NKp46.
[0306] A bispecific Fc based on a scFv specific for the tumor antigen CD19 (anti-CD19 scFv) and an scFv specific for the activating receptor CD3 on T cells (anti-CD3 scFv) was used to assess the binding and binding of novel monomeric bispecific polypeptides to FcRn and CD19 binding function. The domain arrangement of the final polypeptide is referred to as the "F1" format (the asterisk in the CH2 domain indicates the optional N297S mutation, not included in the polypeptides tested here).
[0307] Fc-containing bispecific monomeric polypeptides were constructed based on a scFv specific for the tumor antigen CD19 (anti-CD19...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com