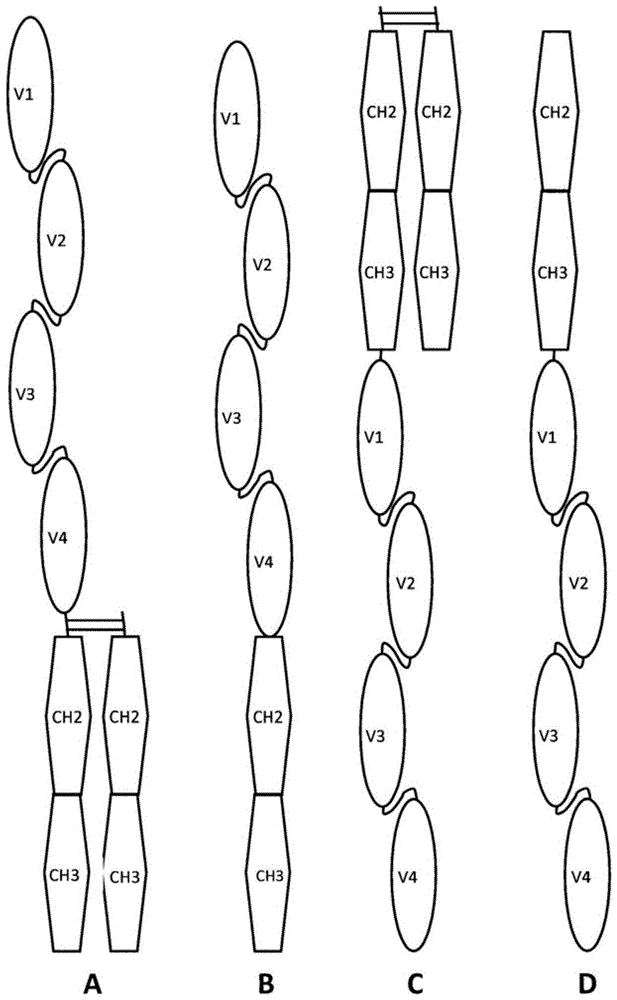
Bispecific bivalent scfv-Fc molecules
A technology of identity and linker, applied in the field of protein engineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0129] Example 1: Construction of anti-HER2CD3ε and anti-FOLR1 / CD3ε Bi-Fc molecules and single-chain bispecific molecules
[0130] Bi-Fc molecules were generated using the method essentially described above. et al. (2000), Blood95(6):2098-2103. In more detail, the construct encoding heterodimer anti-HER2 / CD3εBi-Fc was prepared as follows. DNA fragments encoding the VH region (SEQ ID NO: 5) and the VL region (SEQ ID NO: 6) of the anti-HER2 IgG antibody and the VH region (SEQ ID NO: 7) and the VL region (SEQ ID NO: 8) of the anti-human CD3ε IgG antibody were used in the forward direction and reverse primers were amplified by PCR and spliced together by a flexible linker. The resulting DNA fragment encoding a linear fusion DNA encoding two scFvs joined by a linker is referred to herein as a single-chain anti-HER2 / CD3ε (SEQ ID NO: 9). This construct was subcloned into a mammalian expression vector for antibody production.
[0131] A heterodimeric anti-HER2 / CD3ε Bi-Fc (SEQ I...
Embodiment 2
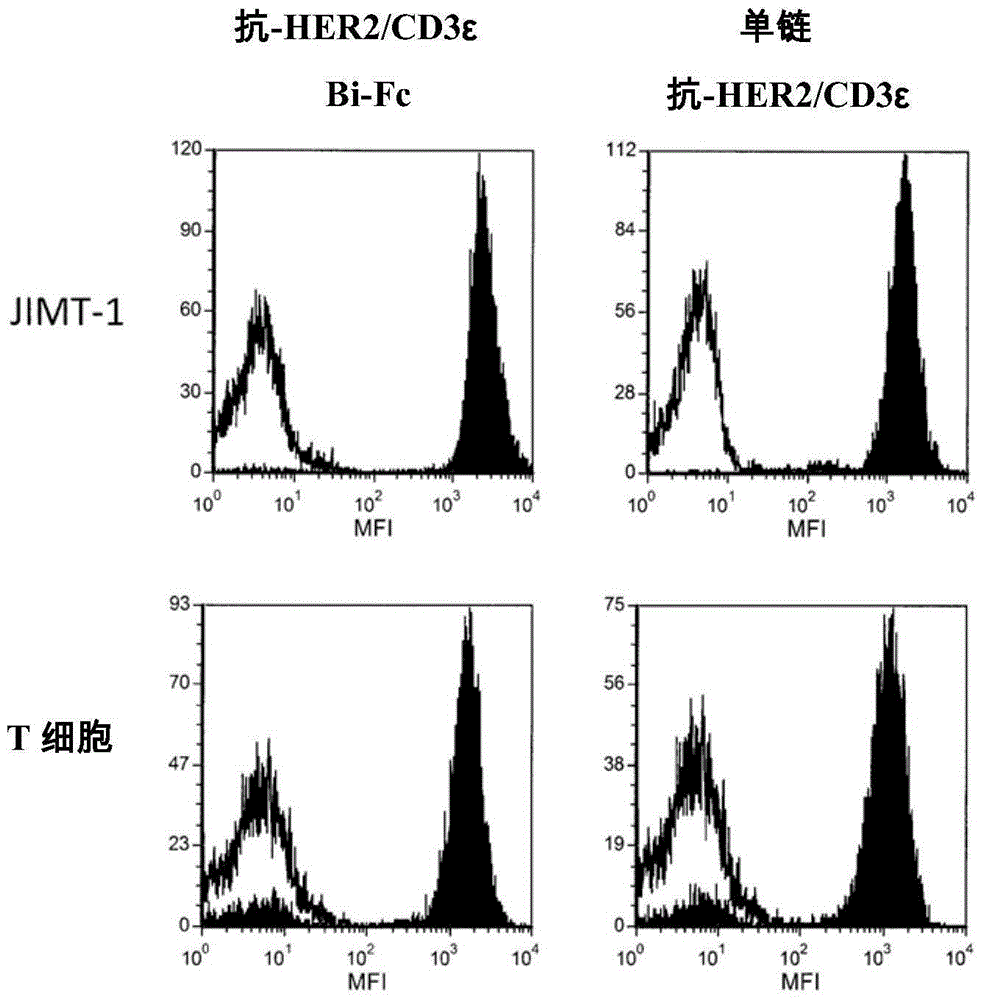
[0133] Example 2: Determination of Binding of BiTE:Fc Molecules to Target Cells and Immune Effector Cells
[0134] Binding of heterodimeric anti-HER2 / CD3ε Bi-Fc and single chain anti-HER2 / CD3ε to CD3 expressing T cells and HER2 expressing JIMT-1 cells was assessed as follows. Human pan-T cells (purified using Human PanT Cell Isolation Kit II, MiltenyiBiotec, Auburn, CA) or purified JIMT-1 cells in the absence or presence of 10 μg / mL heterodimer anti-HER2 / CD3εBi- In the case of Fc or single chain anti-HER2 / CD3ε, they were incubated at 4°C for 16 hours. Cellular binding of the heterodimeric anti-HER2 / CD3ε Bi-Fc was detected using an allophycocyanin (APC)-labeled anti-human Fc secondary antibody. using mouse anti- The antibody was then detected with an APC-labeled mouse Ig-specific antibody containing Labeled single chain anti-HER2 / CD3ε.
[0135] shown in figure 2 In the fluorescence-activated cell sorting (FACS) histogram of , unfilled curves represent data from cells in...
Embodiment 3
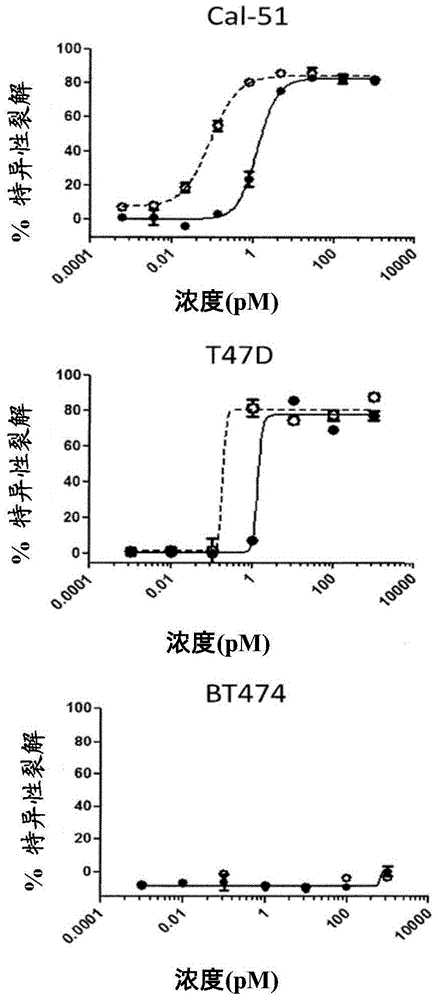
[0136] Example 3: Lysis of Tumor Cell Lines in the Presence of Bi-Fc and T Cells
[0137] Detection of the aforementioned heterodimeric anti-HER2 / CD3ε and anti-FOLR1 / CD3ε Bi-Fc and single-chain anti-HER2 / CD3ε and anti-FOLR1 / CD3ε molecules to determine their function in tumor cells expressing HER2 or FOLR1 as Activity of target cells in a T-cell-dependent cell lysis (TDCC) assay. Briefly, panT cells were isolated from healthy human donors using the Human PanT Cell Isolation Kit II (MiltenyiBiotec, Auburn, CA). The T cells were cultured with CFSE-labeled tumor target cells at a ratio of 10:1, with or without the presence of image 3 with Figure 4 Heterodimer anti-HER2 / CD3ε or anti-FOLR1 / CD3ε Bi-Fc or single chain anti-HER2 / CD3ε or anti-FOLR1 / CD3ε described in Example 1 at varying concentrations indicated in . As controls, some samples contained T cells and tumor target cells, but no Bi-Fc or single chain molecules.
[0138] Target cells for anti-FOLR1 / CD3ε heterodimeric Bi-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com