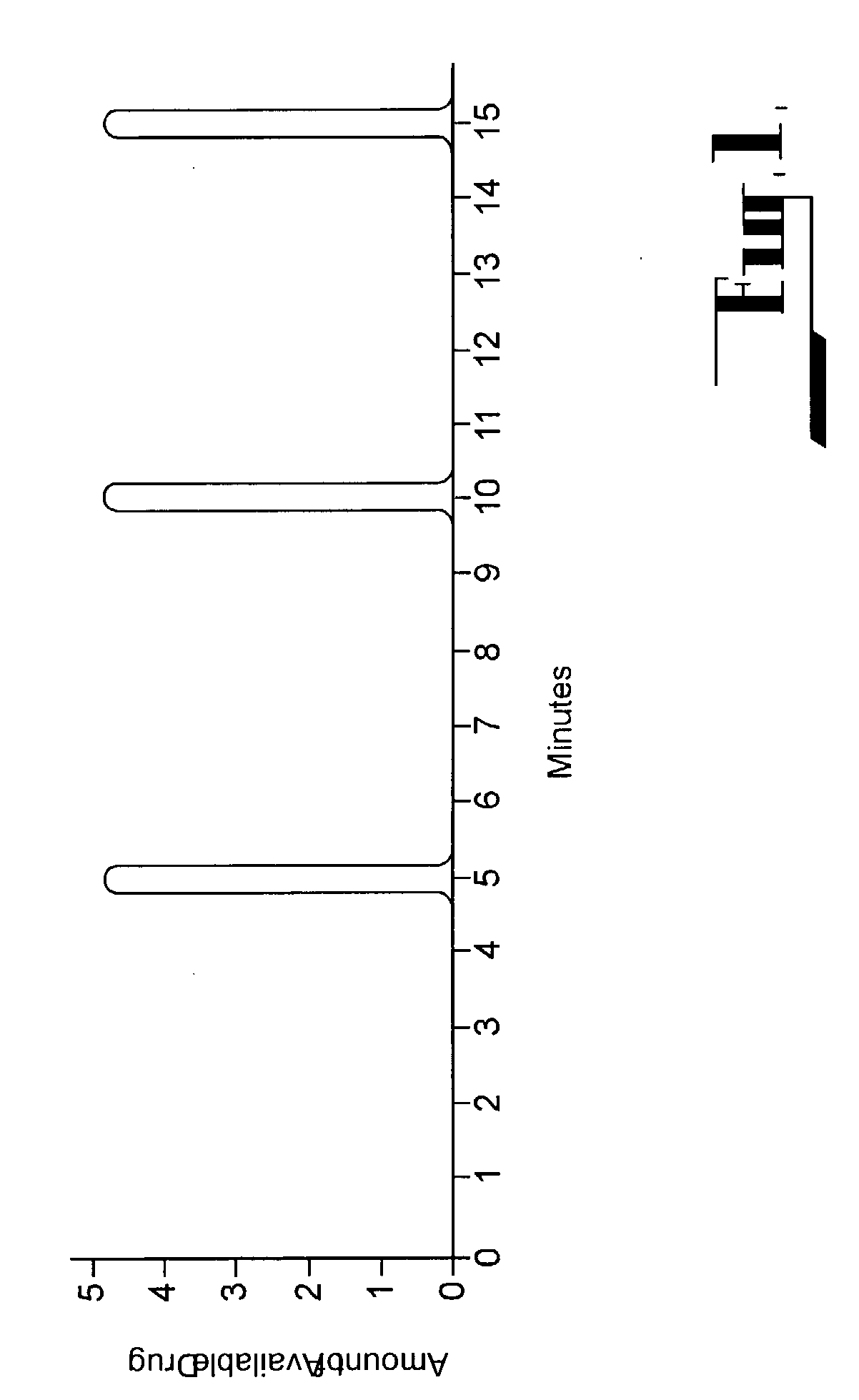
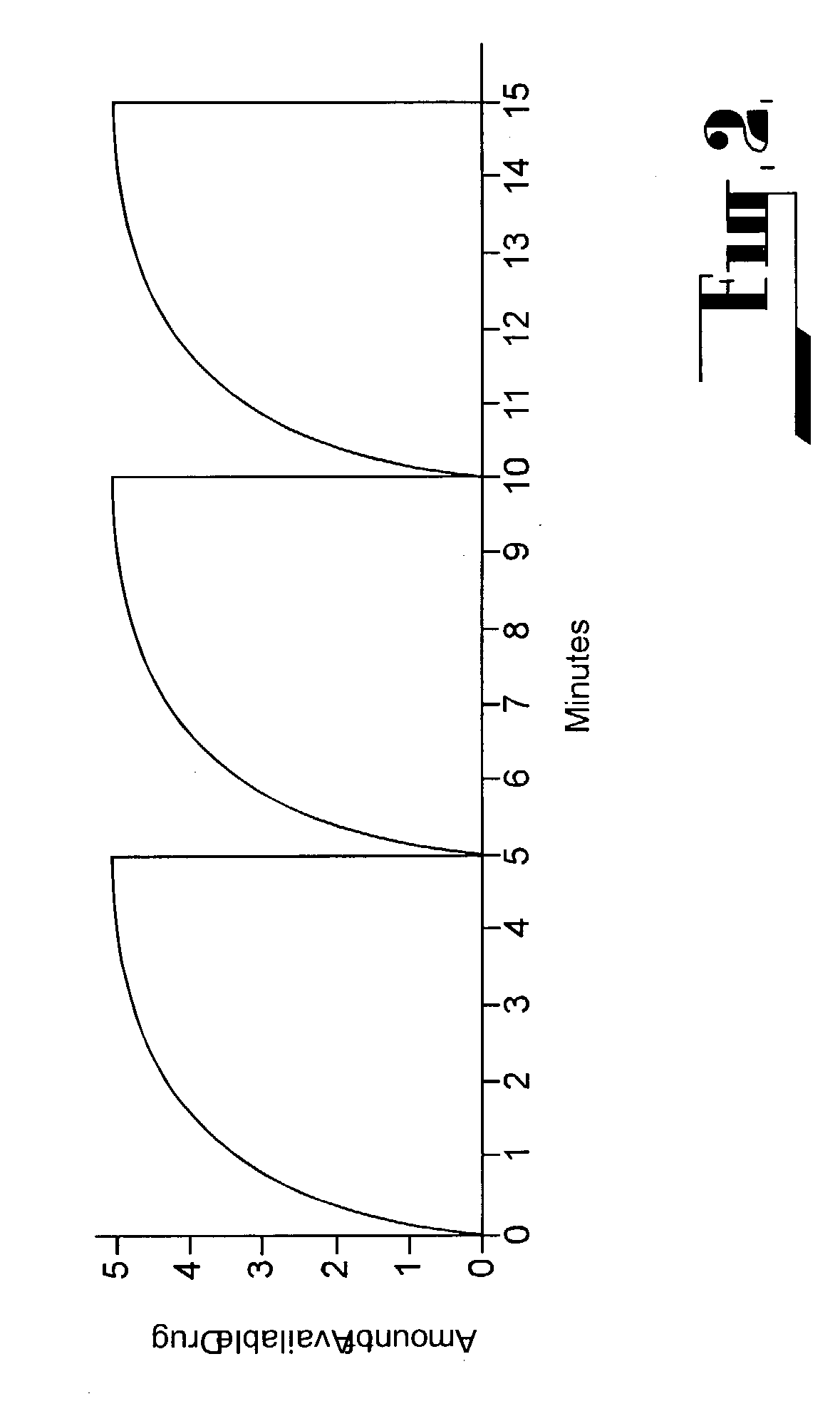
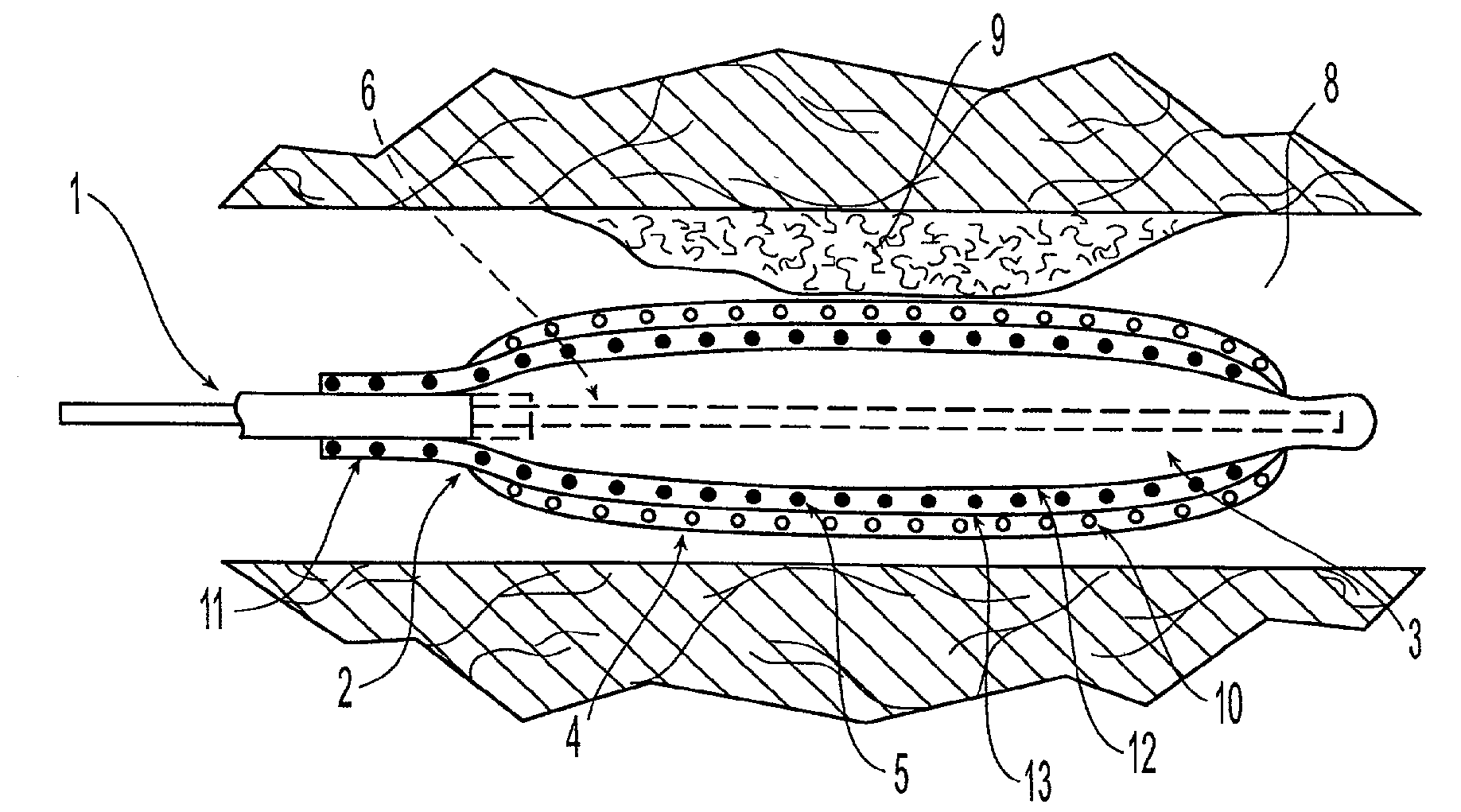
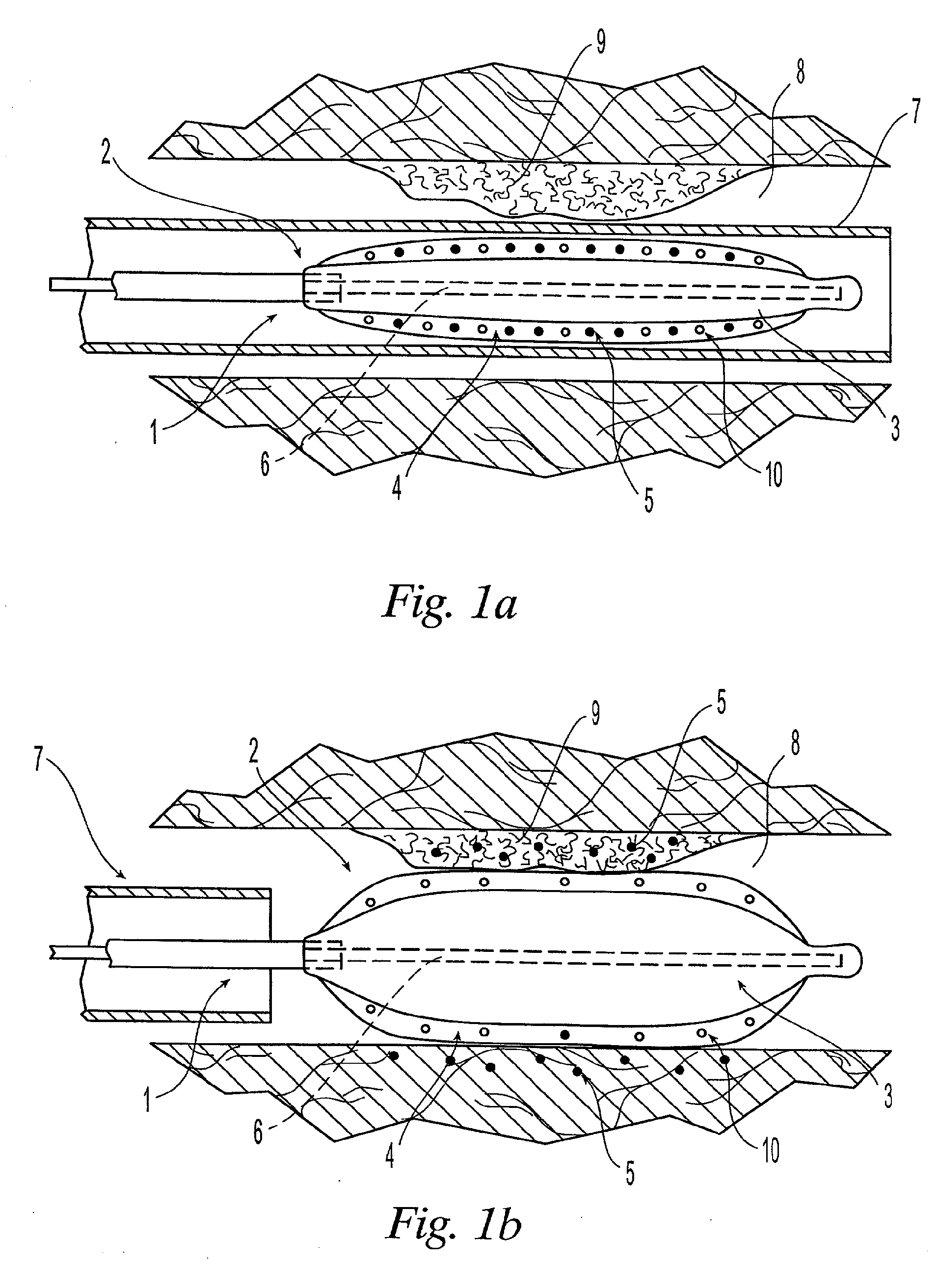
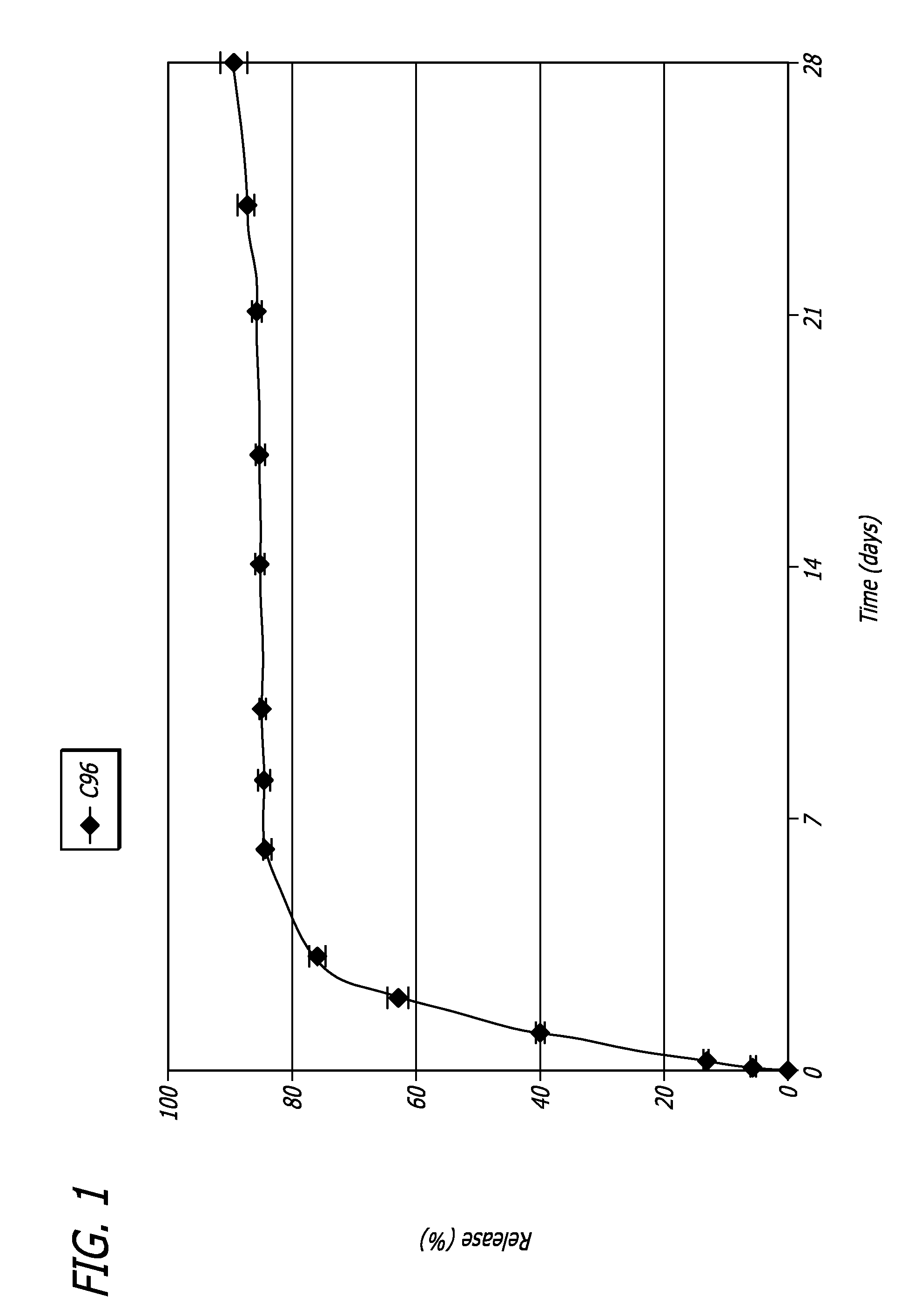
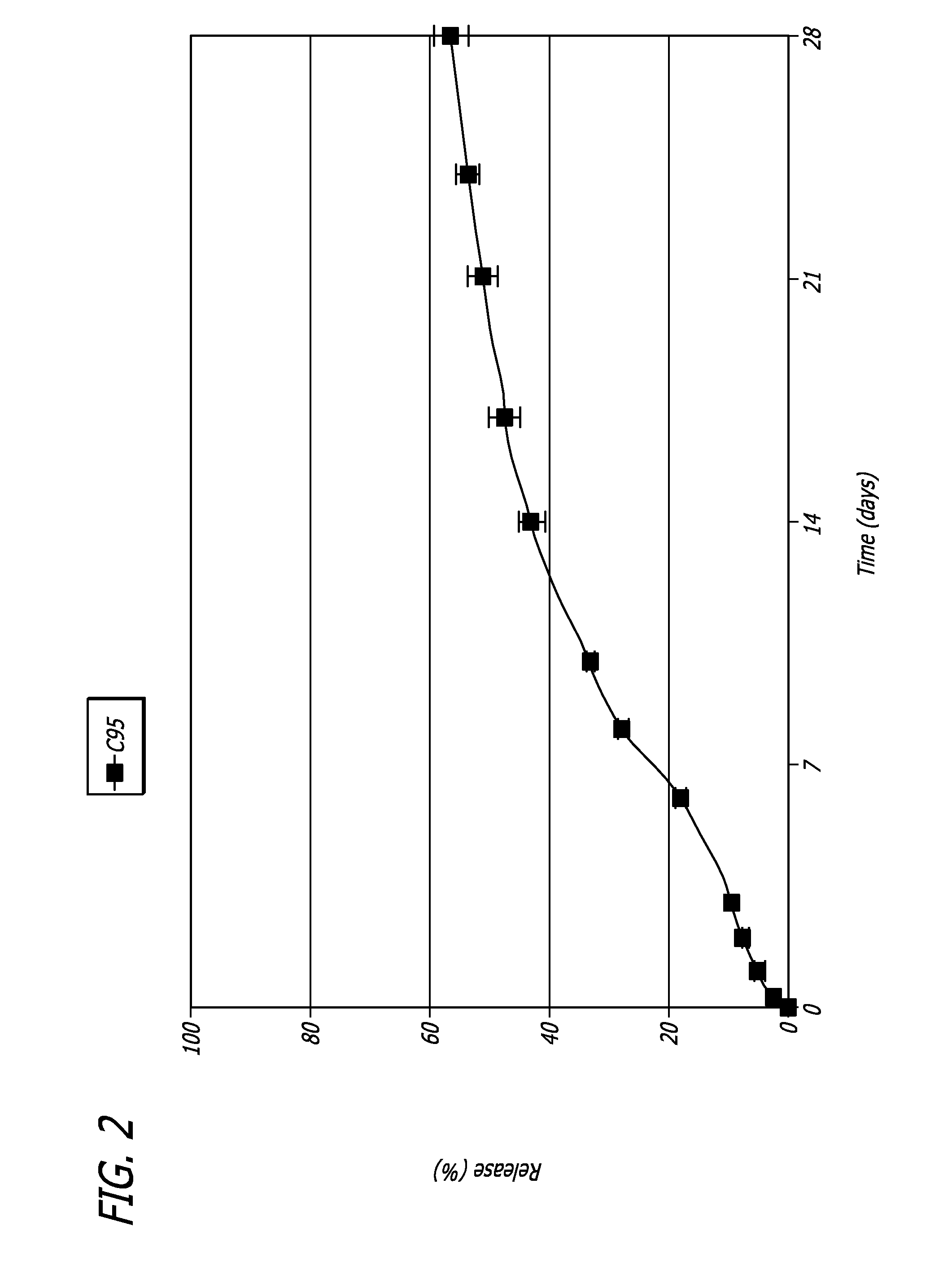
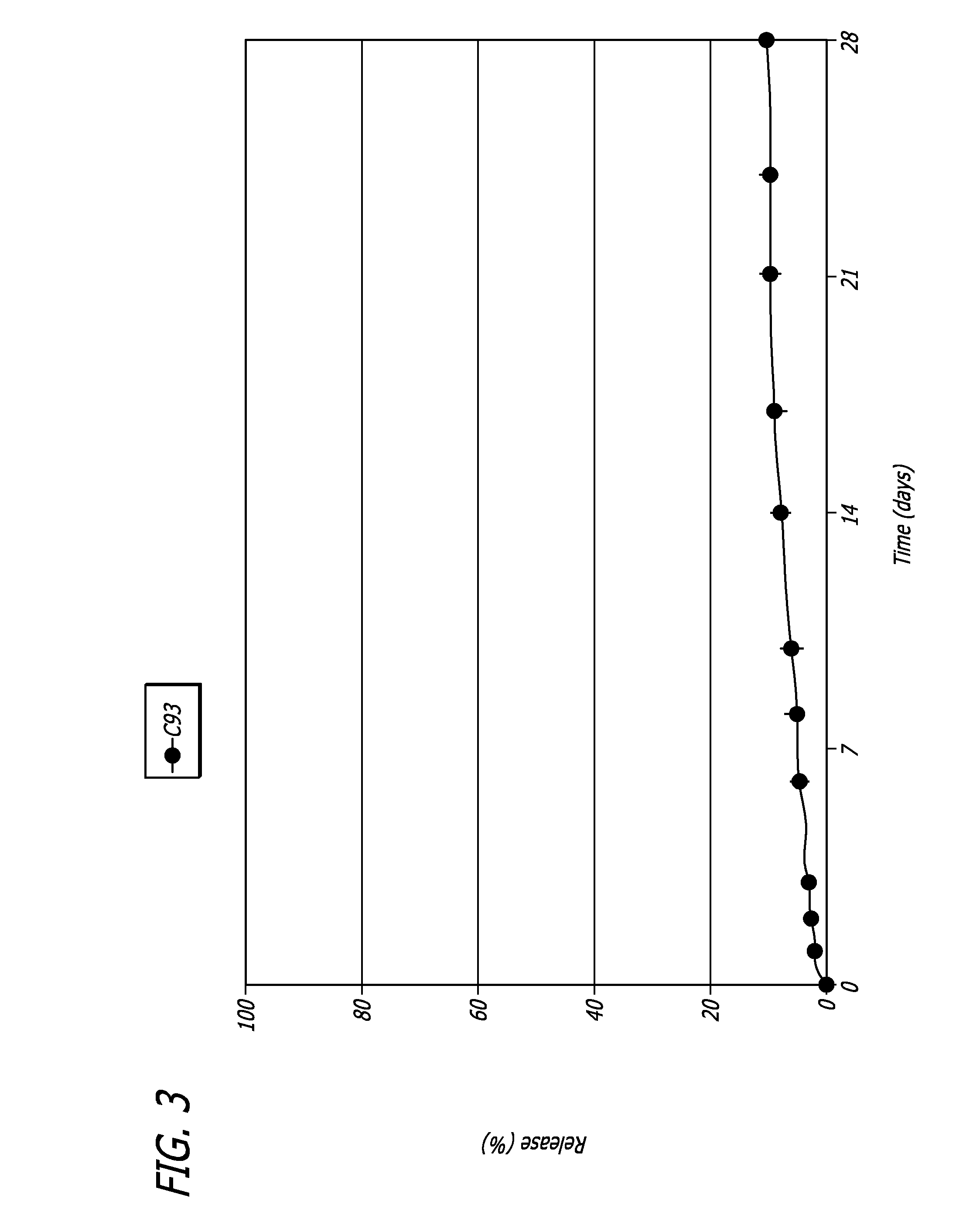
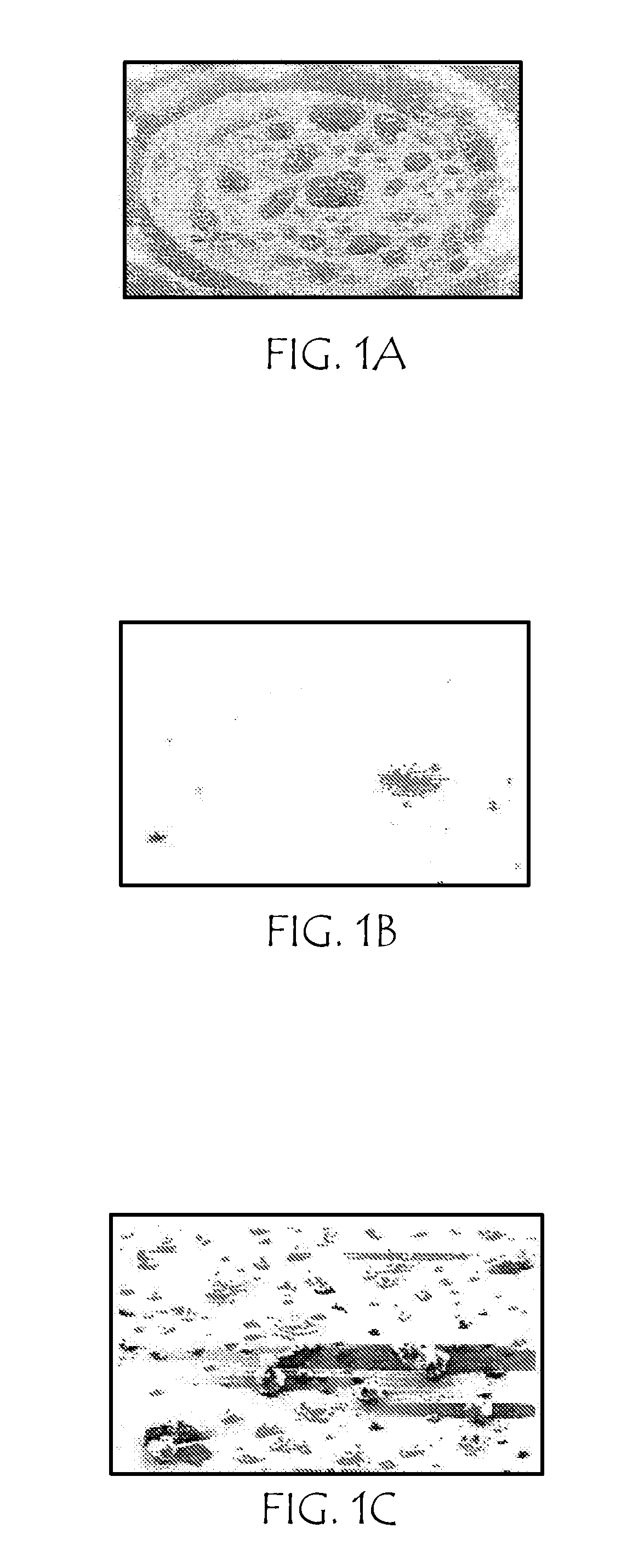
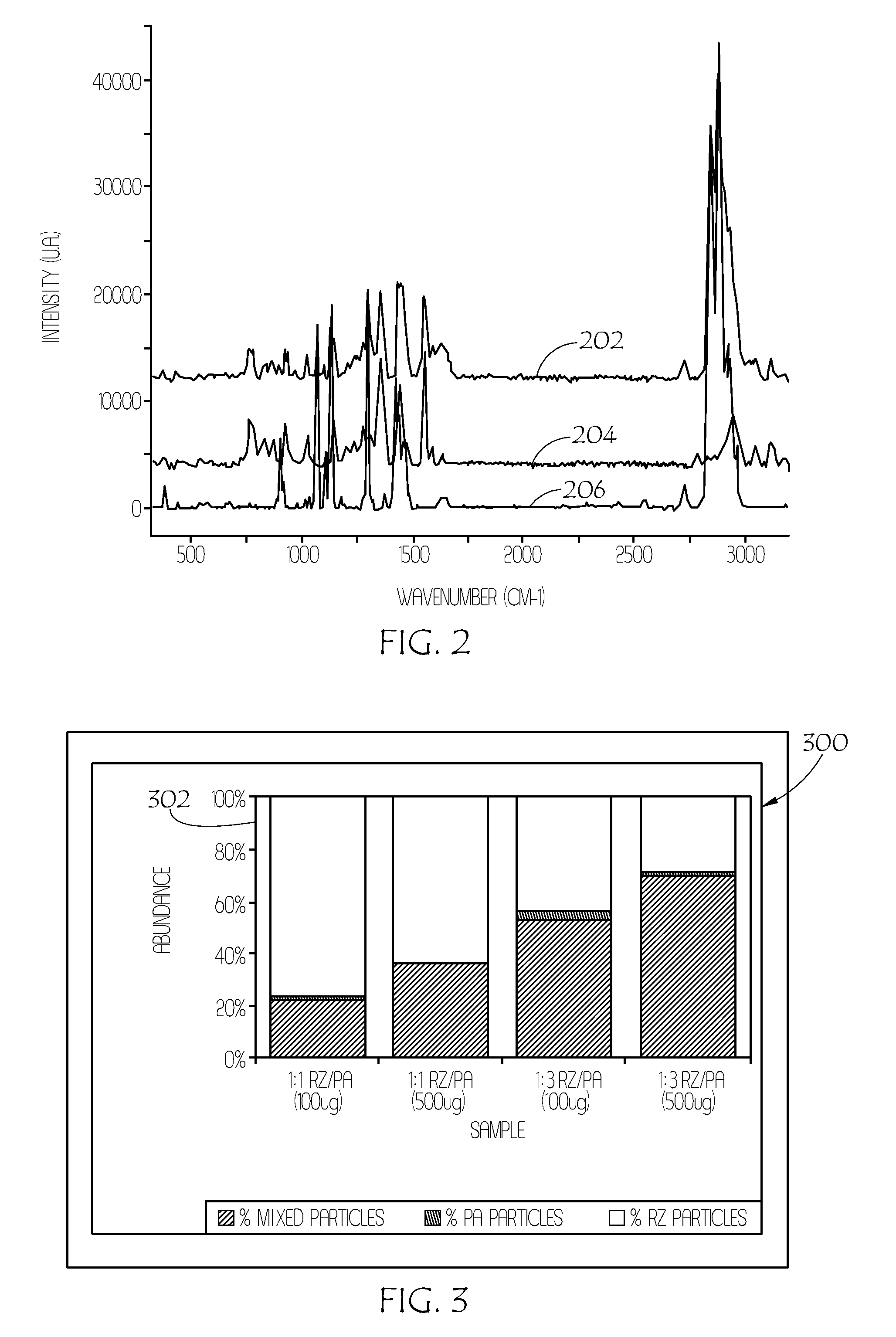
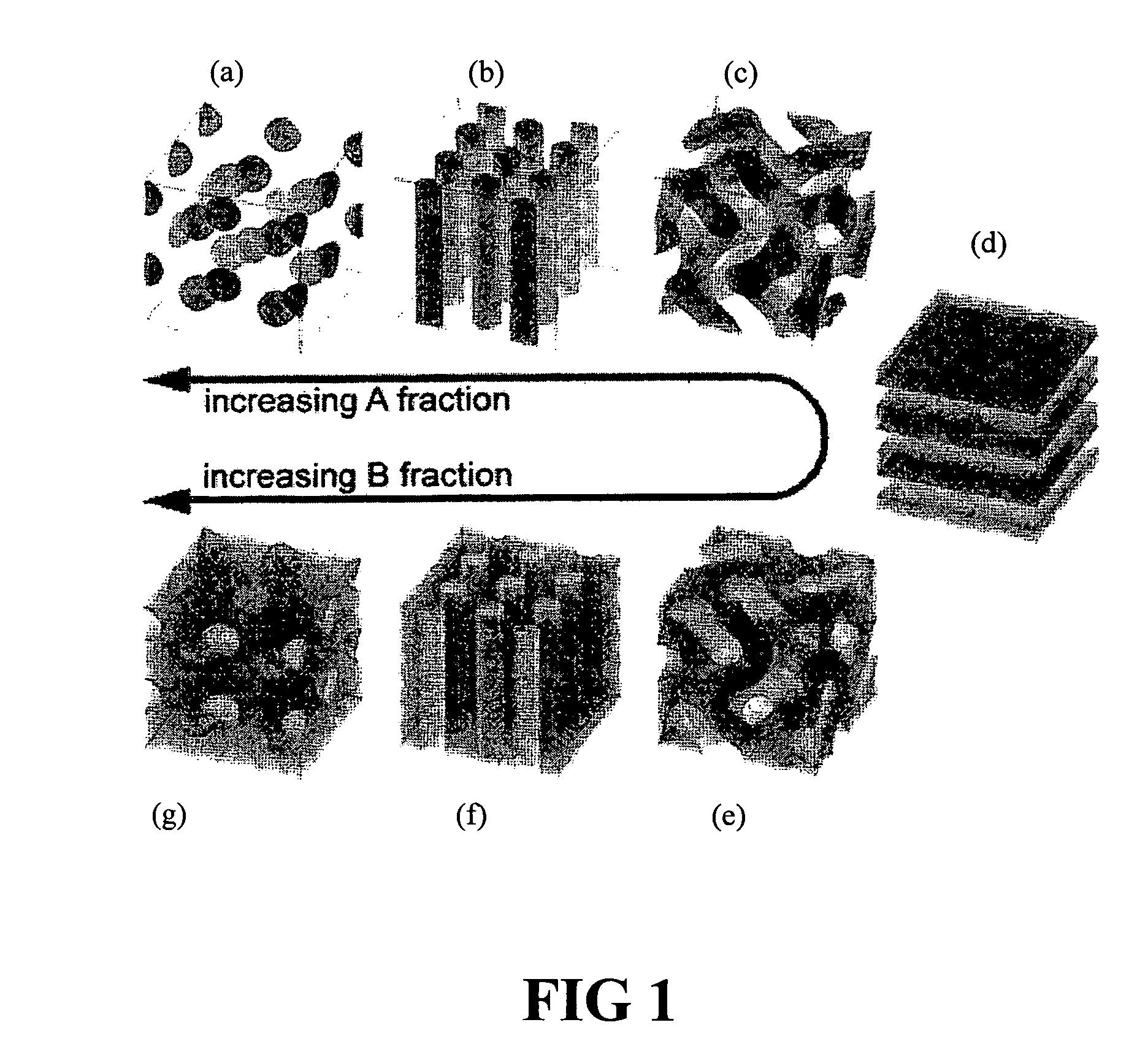
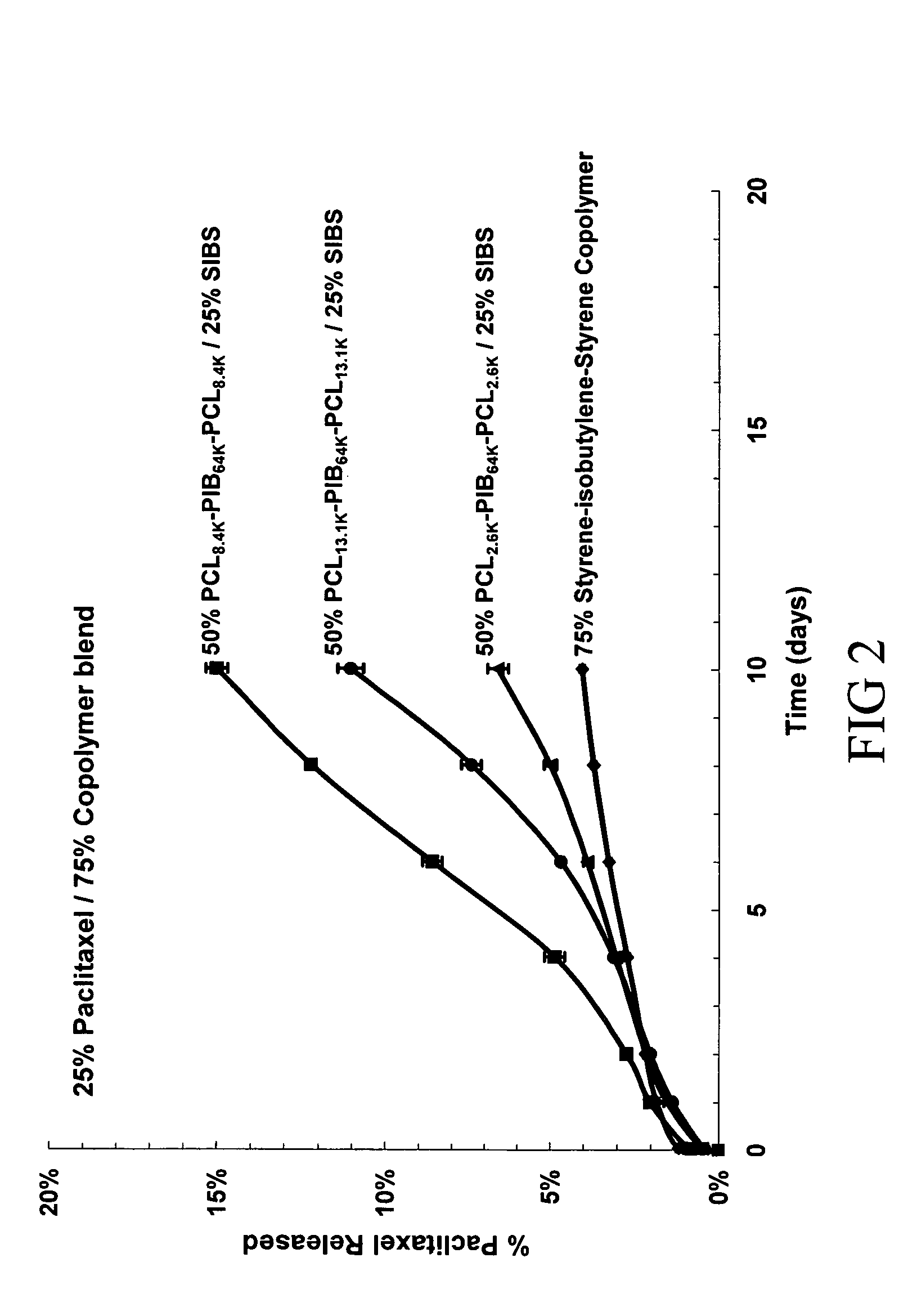
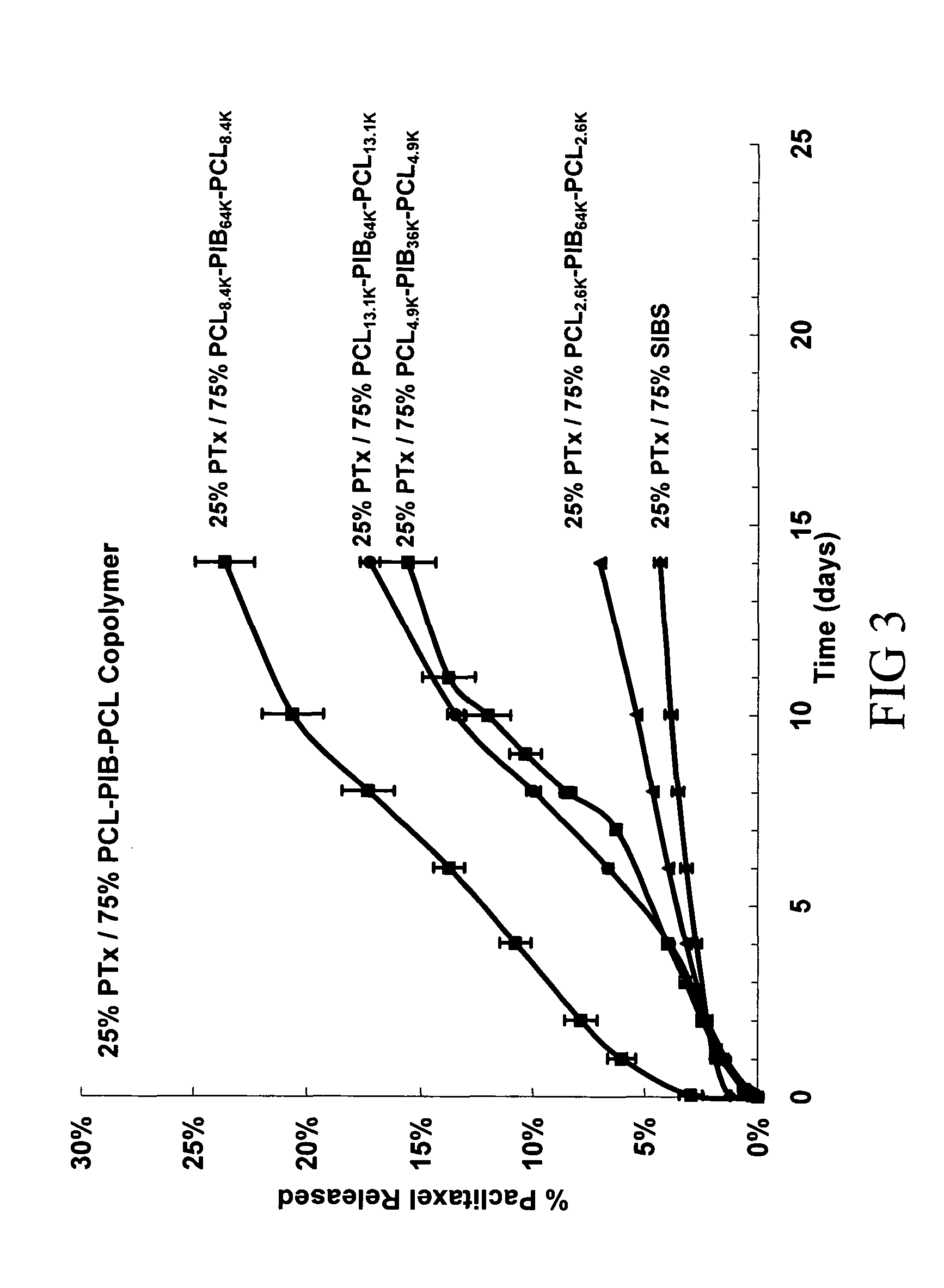
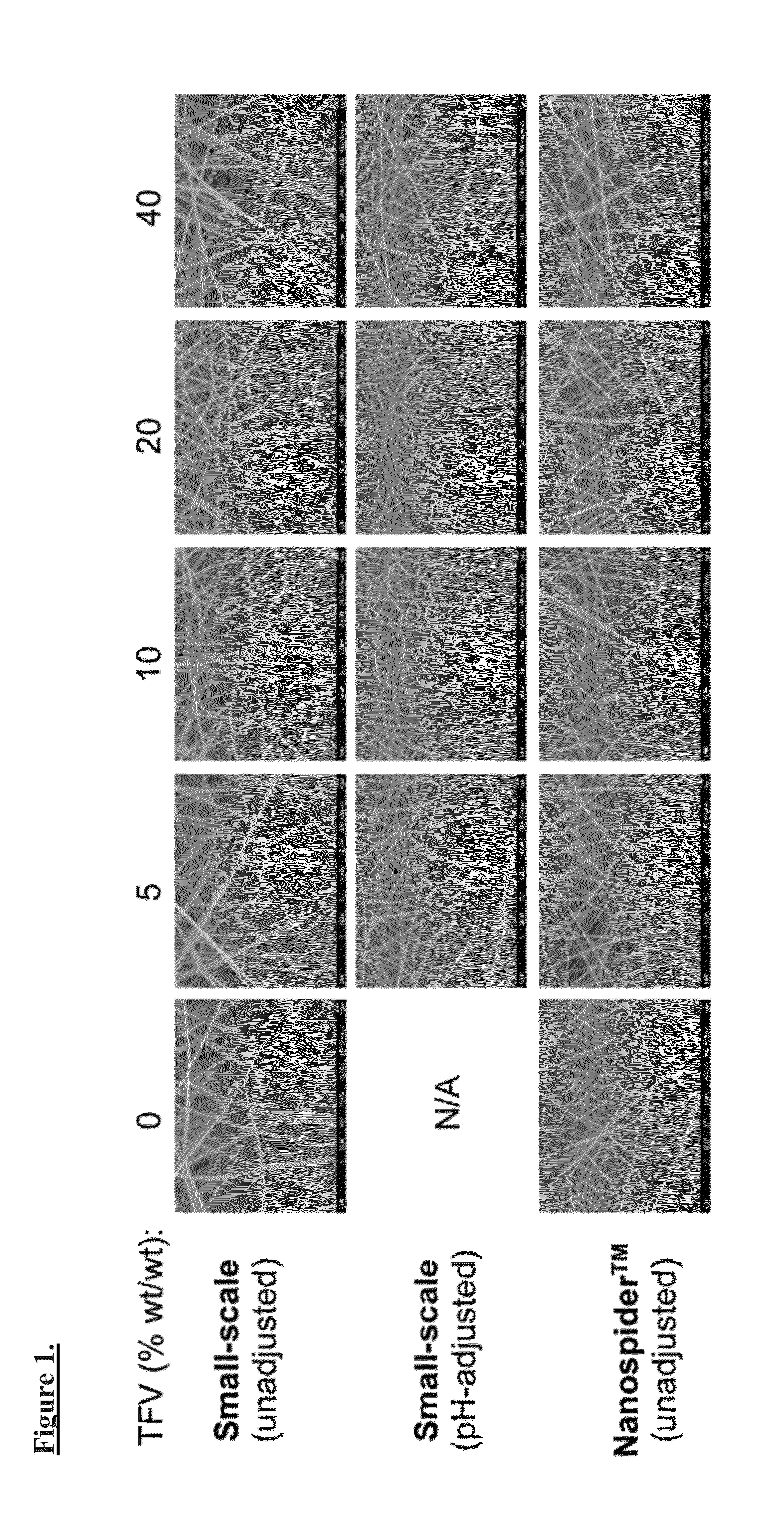
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
499 results about "Controlled drugs" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
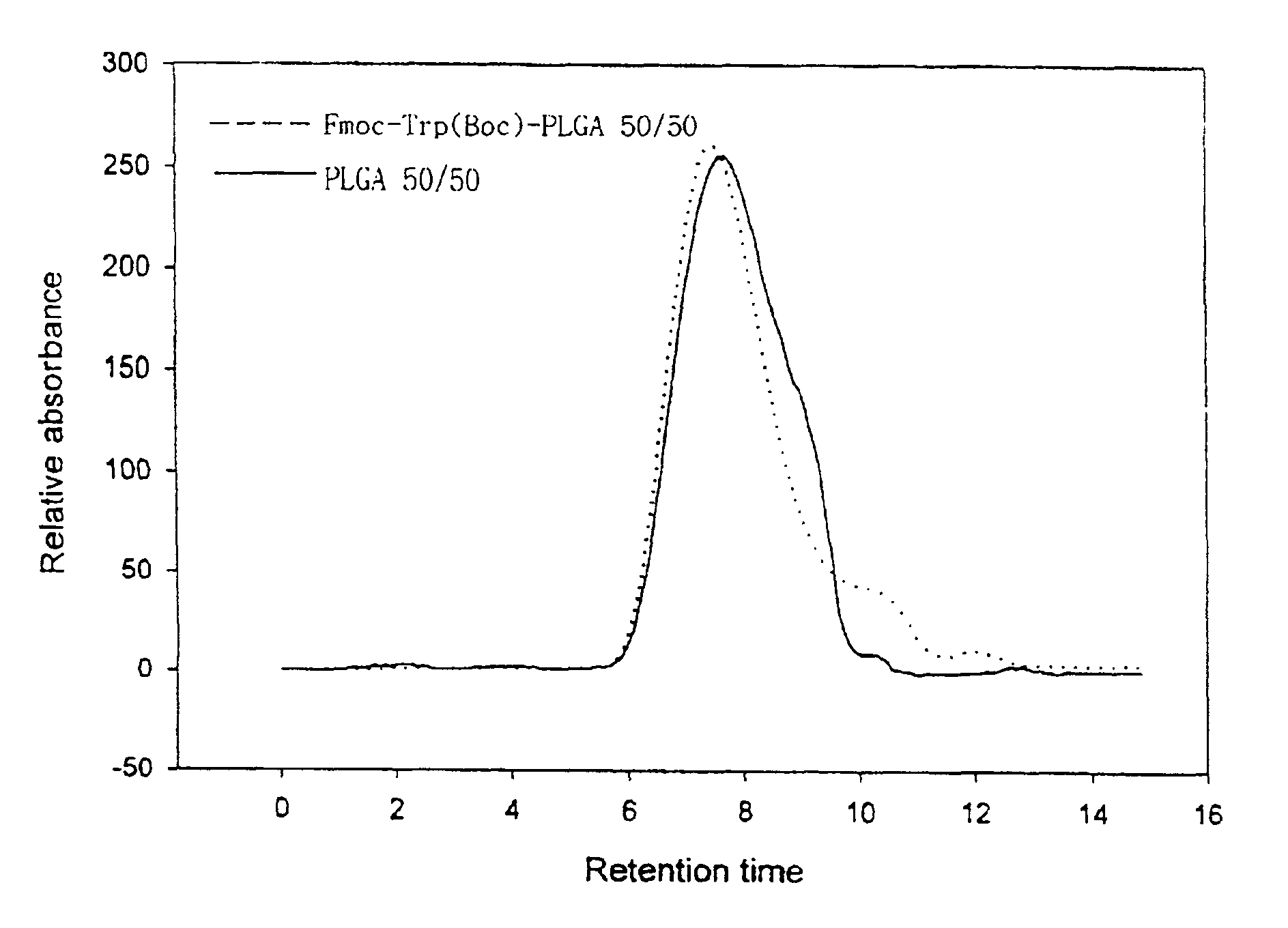
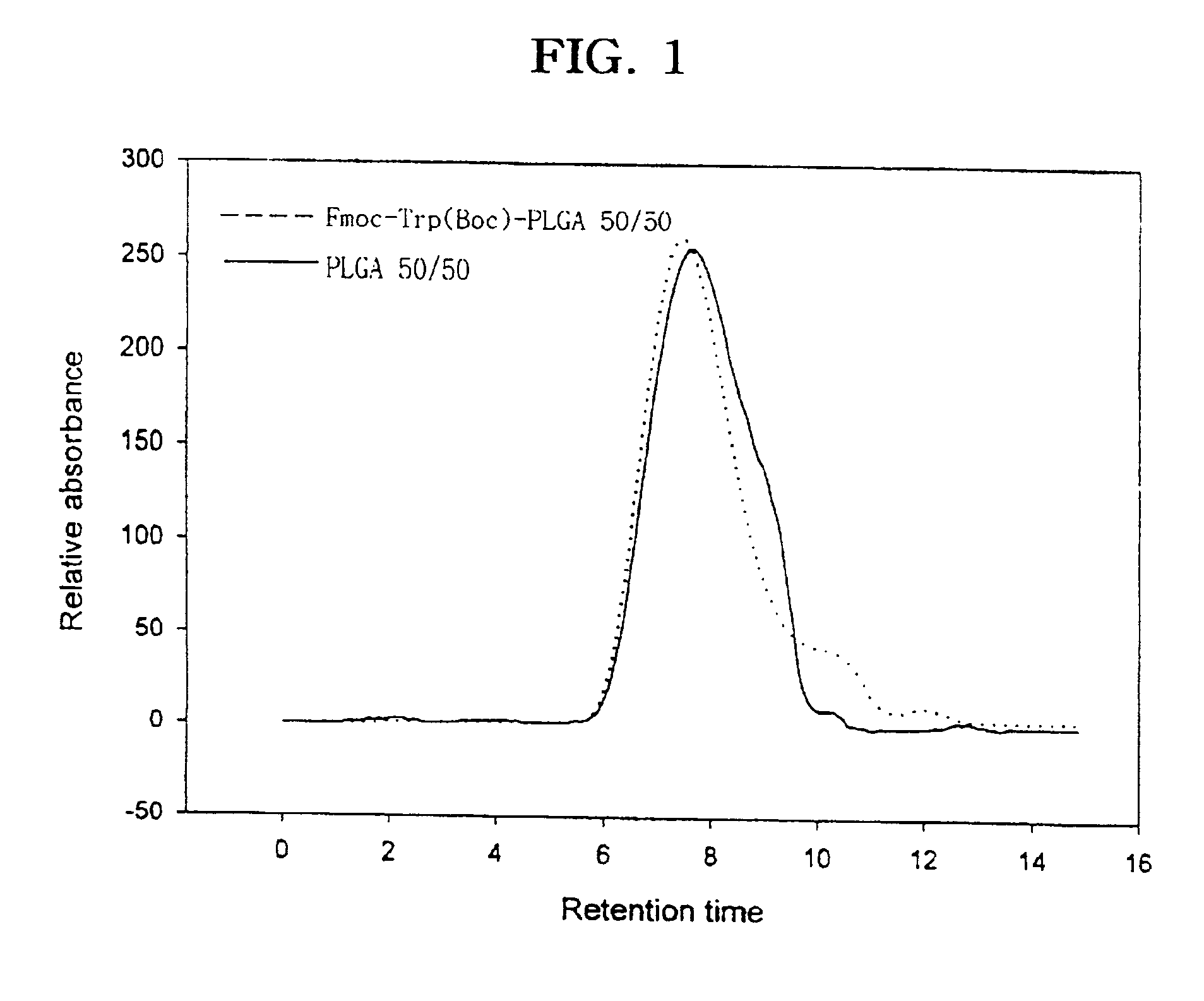
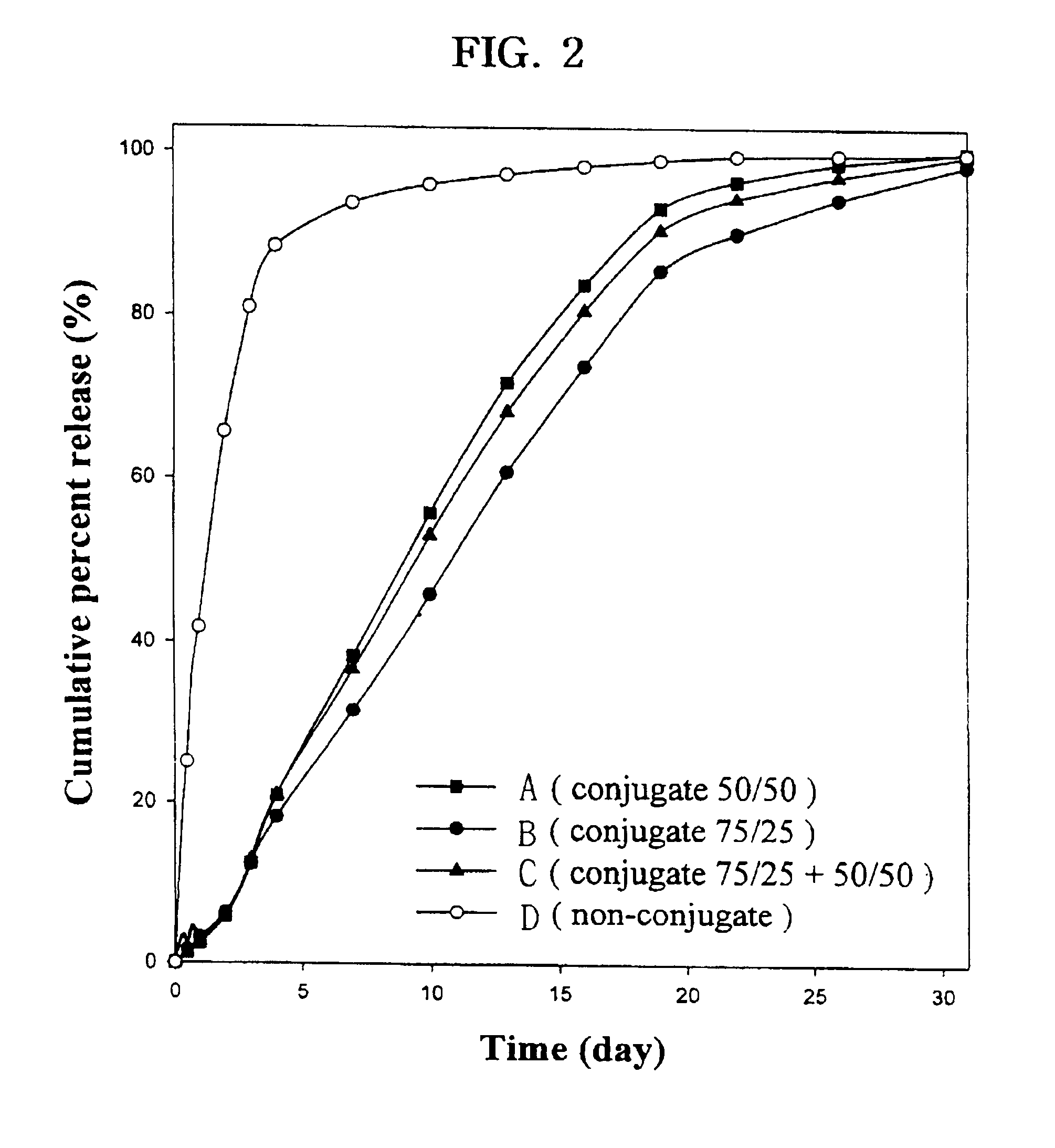
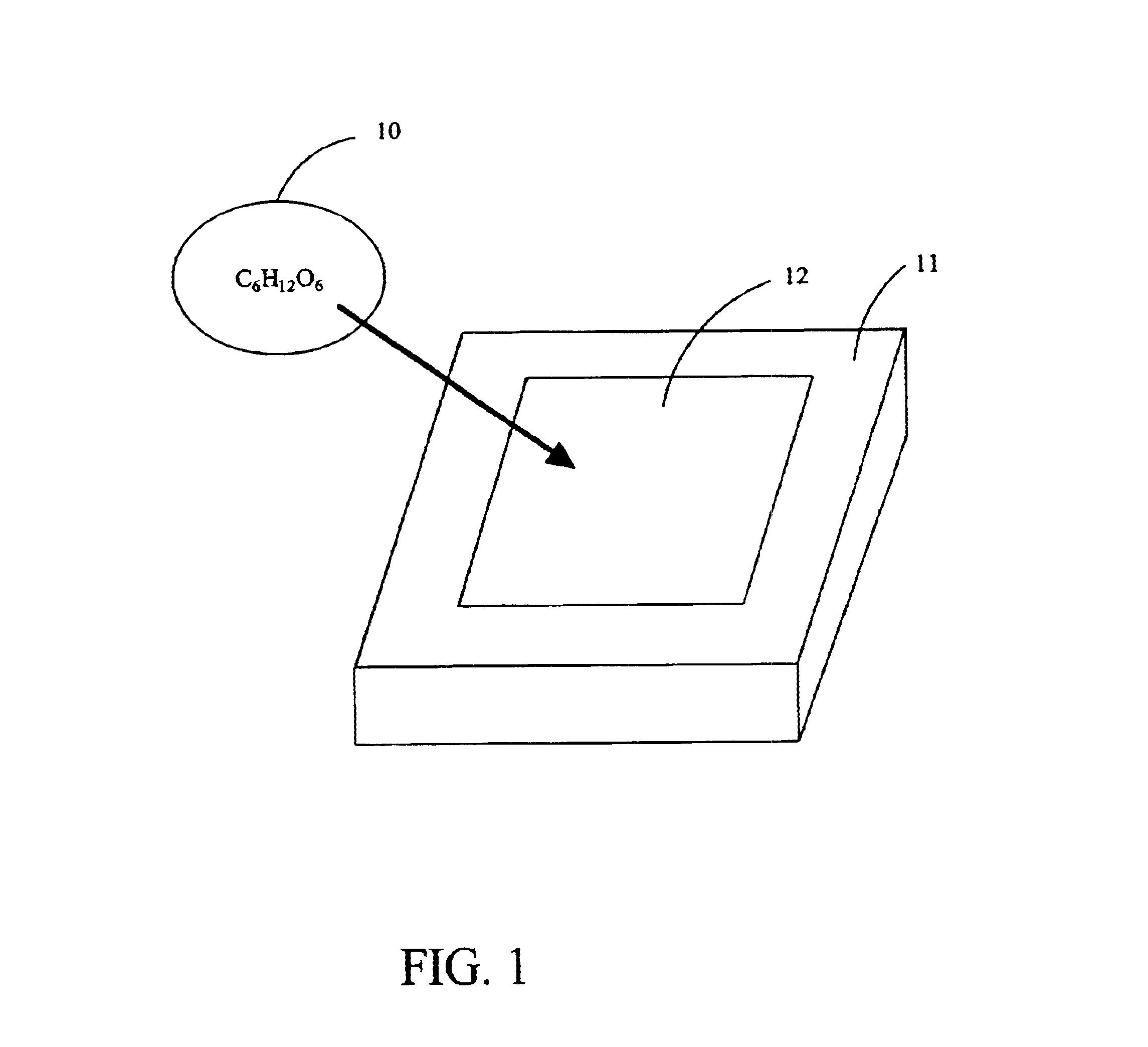
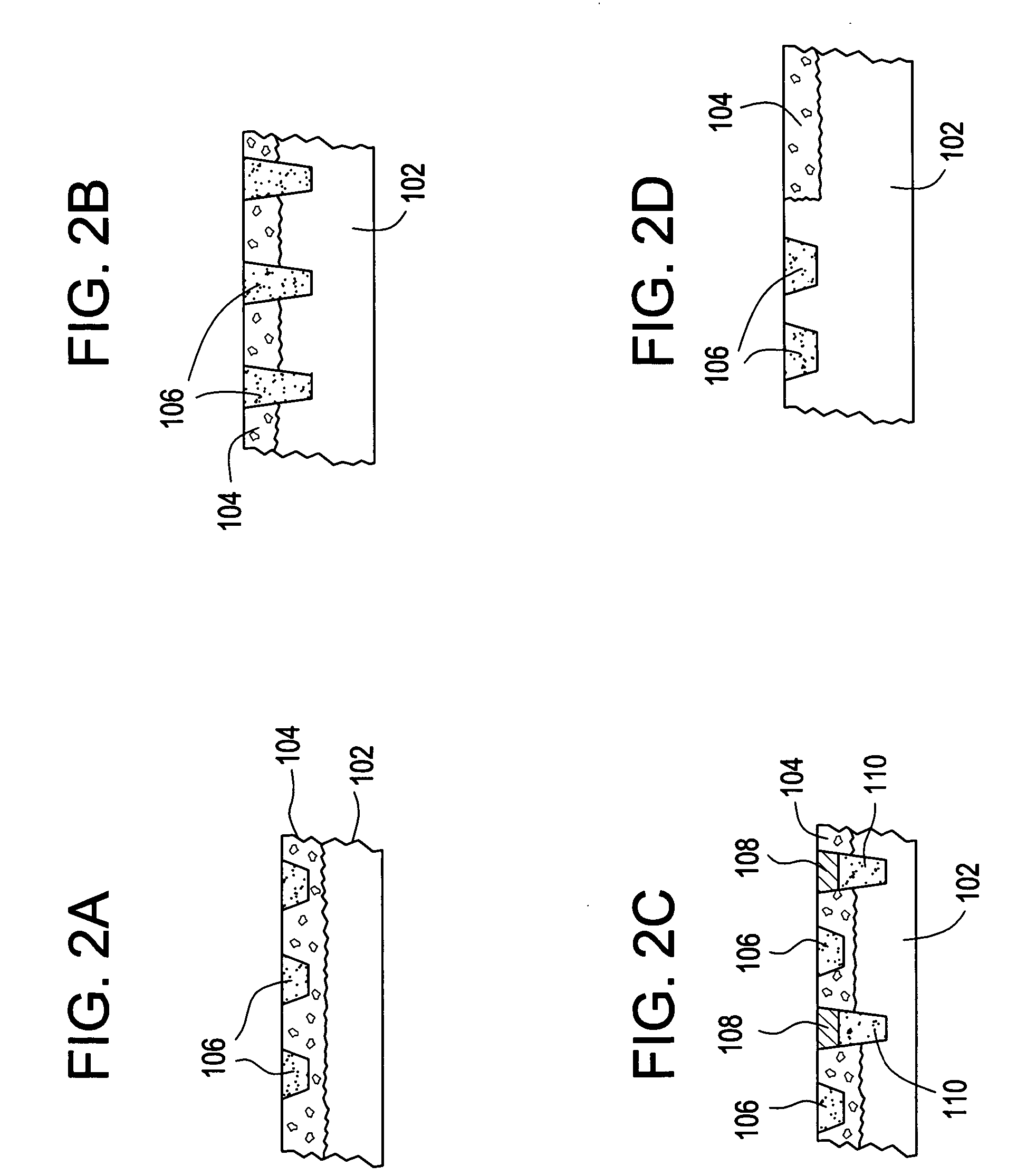
Controlled drug delivery system using the conjugation of drug to biodegradable polyester
The present invention relates to a molecular sustained controlled release system constructed by the conjugation of molecules to be released with biodegradable polyester polymer via covalent bond and method for preparation thereof. In accordance with the present invention, the system may be formulated into microspheres, nanoparticles, or films. The molecular release rate from the above system can be regulated to be proportional to the chemical degradation rate of the biodegradable polyester polymers, resulting in near zero order kinetics profile of release without showing a burst effect, Moreover, a high loading efficiency of hydrophilic drugs can be achieved.
Owner:MOGAM BIOTECH RES INST +1
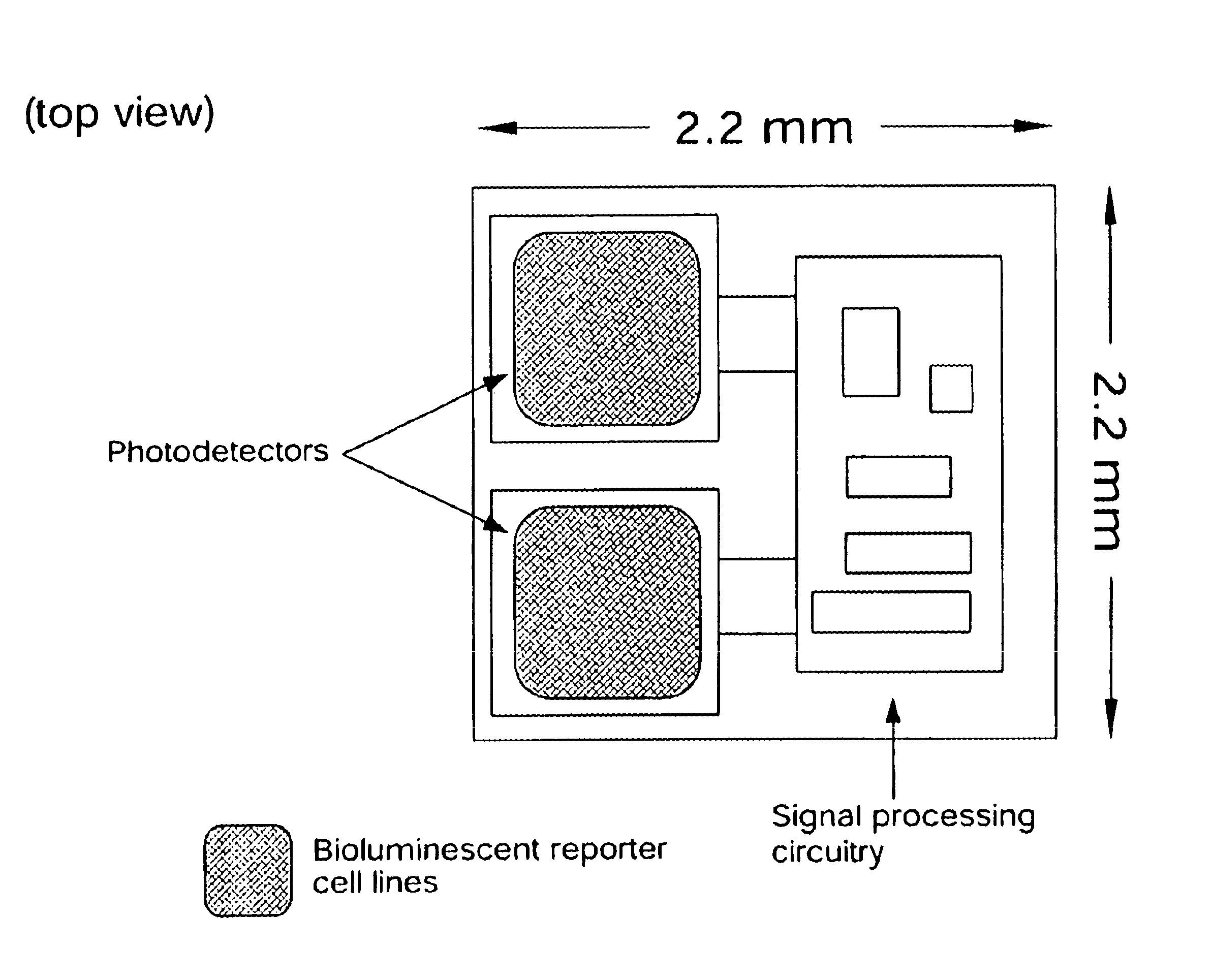
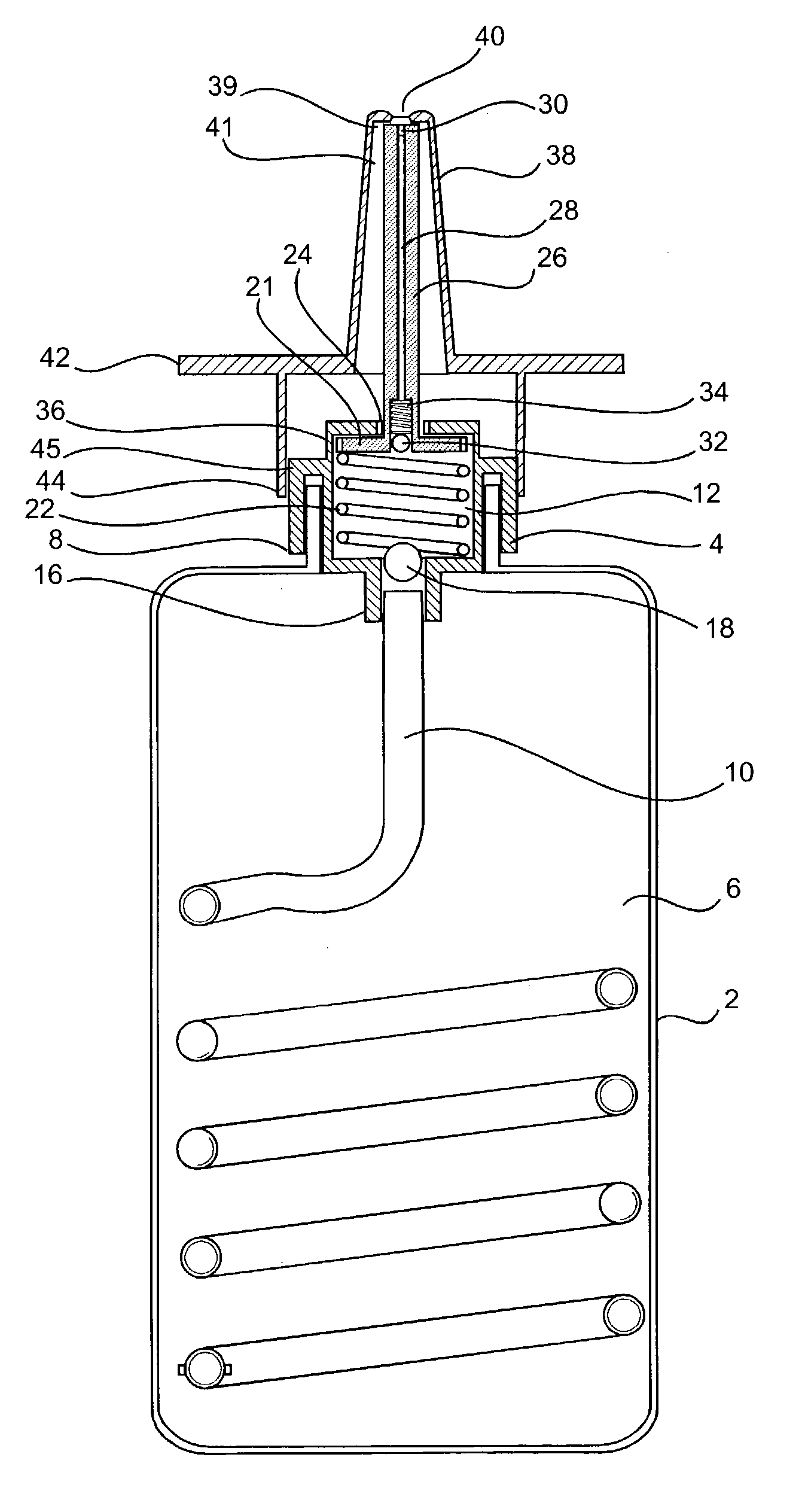
In vivo biosensor apparatus and method of use
InactiveUS6673596B1Less can be administeredCost-effective administration of drugBioreactor/fermenter combinationsBiological substance pretreatmentsIn vivoGenetically engineered
Disclosed are bioluminescent bioreporter integrated circuit devices that detect selected analytes in fluids when implanted in the body of an animal. The device comprises a bioreporter that has been genetically engineered to contain a nucleic acid segment that comprises a cis-activating response element that is responsive to the selected substance operably linked to a gene encoding a bioluminescent reporter polypeptide. In preferred embodiments, the target analyte is glucose, glucagons, or insulin. Exposure of the bioreporter to the target substance causes the response element to up-regulate the nucleic acid sequence encoding the reporter polypeptide to produce a luminescent response that is detected and quantitated. In illustrative embodiments, the bioreporter device is encapsulated on an integrated circuit that is capable of detecting the emitted light, processing the resultant signal, and then remotely reporting the results. Also disclosed are controlled drug delivery systems capable of being directly or indirectly controlled by the detection device that provide drugs such as insulin to the animal in reponse to the amount of target analyte present in the body fluids.
Owner:UNIV OF TENNESSEE RES FOUND +1
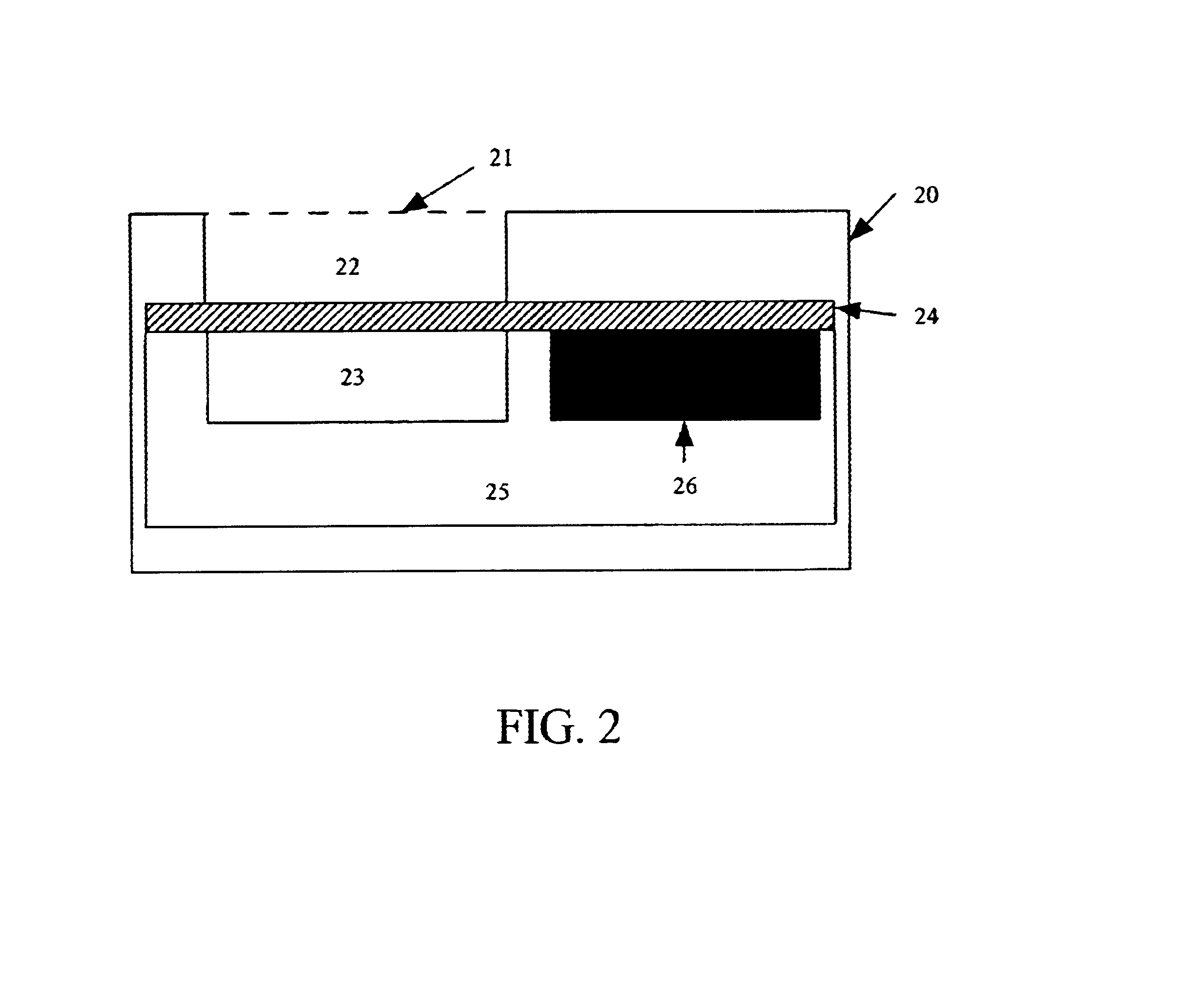
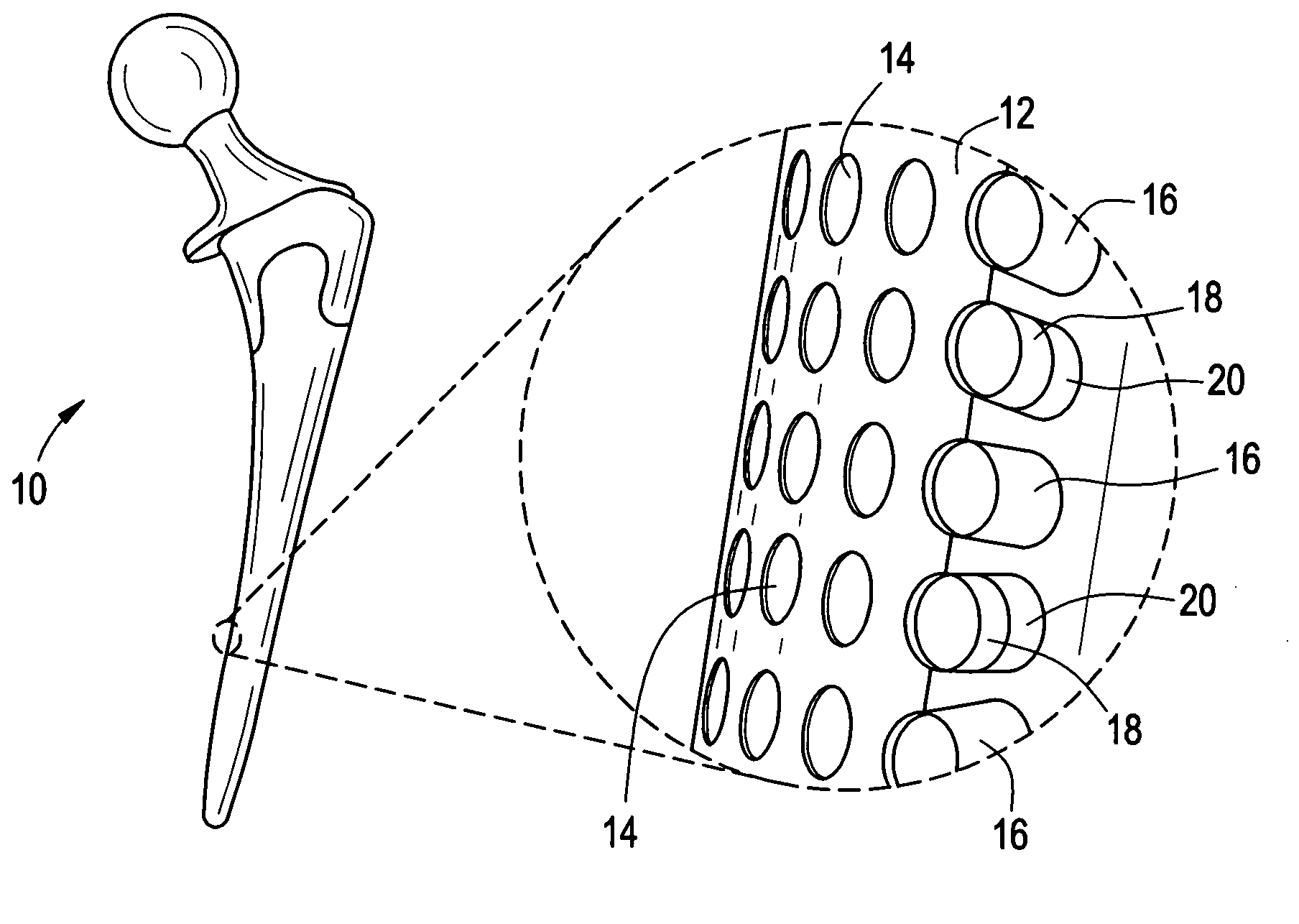
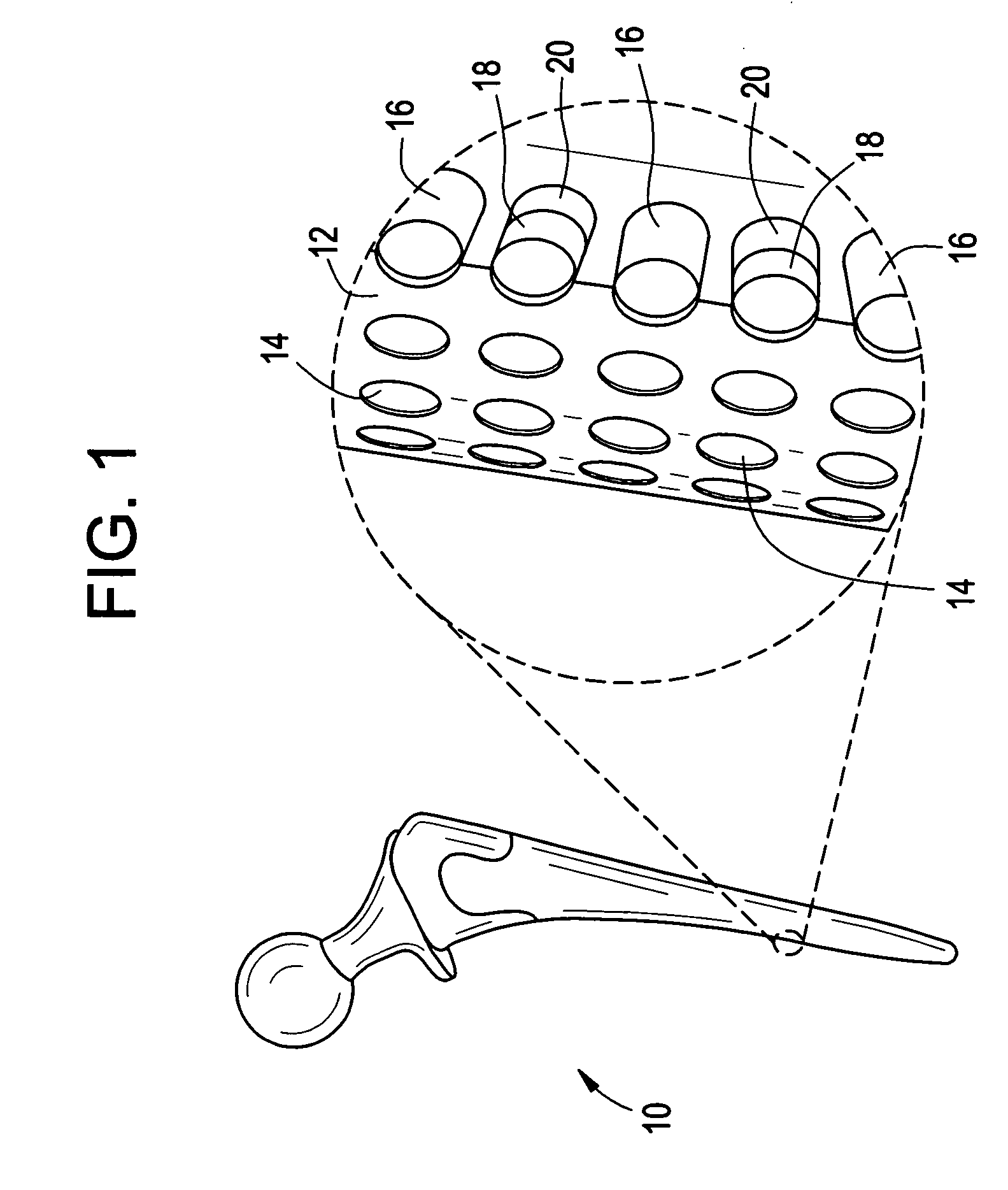
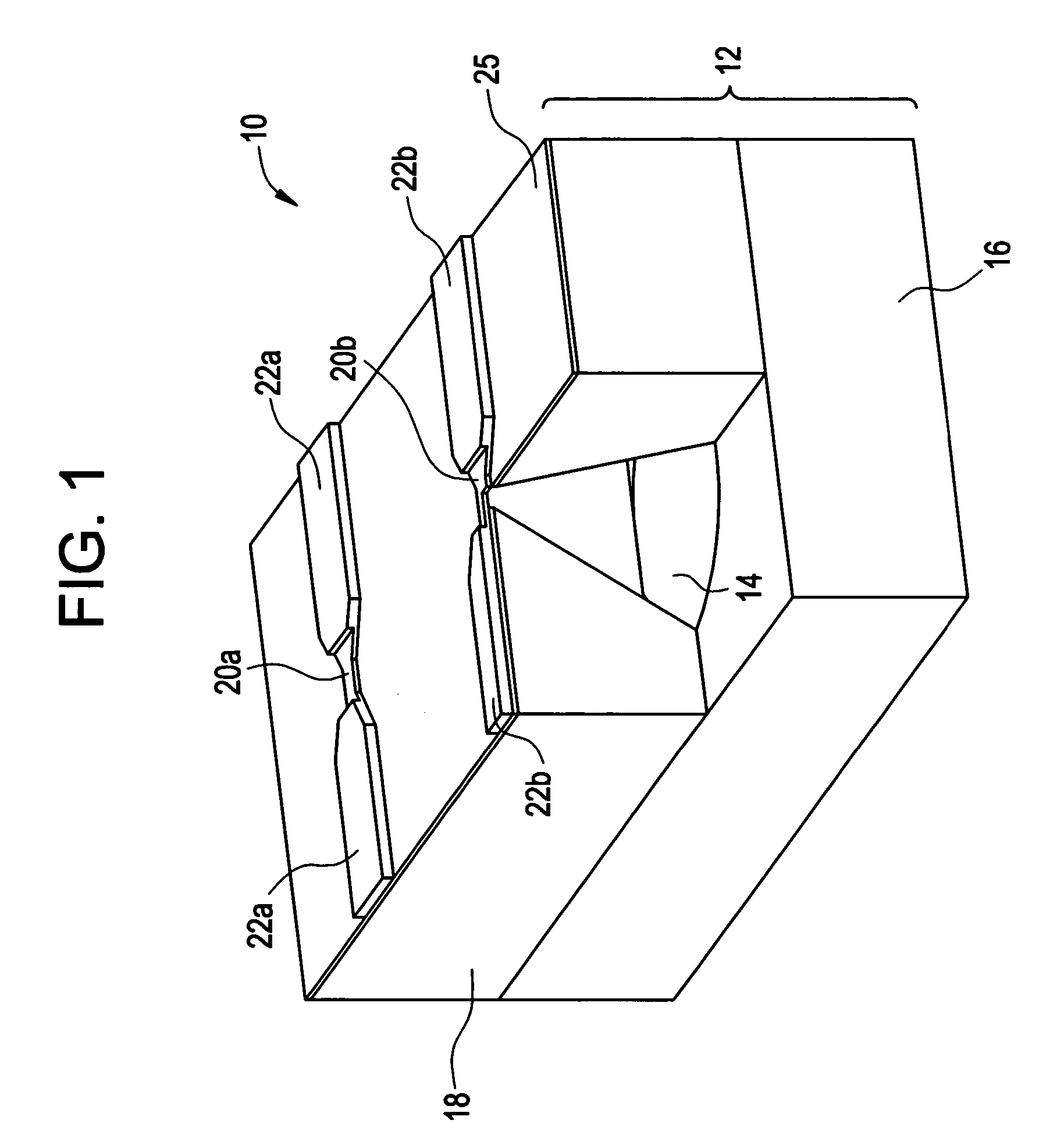
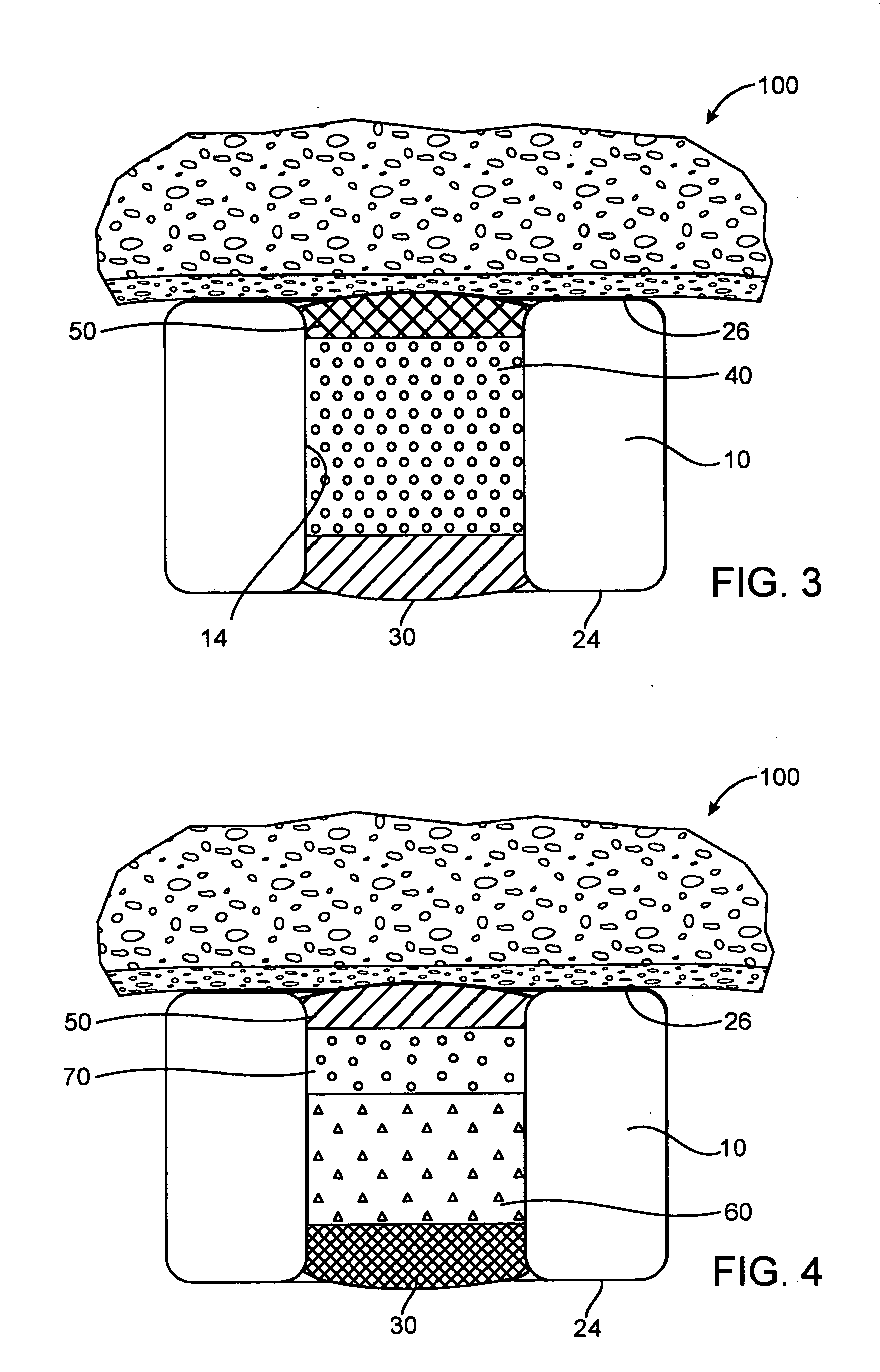
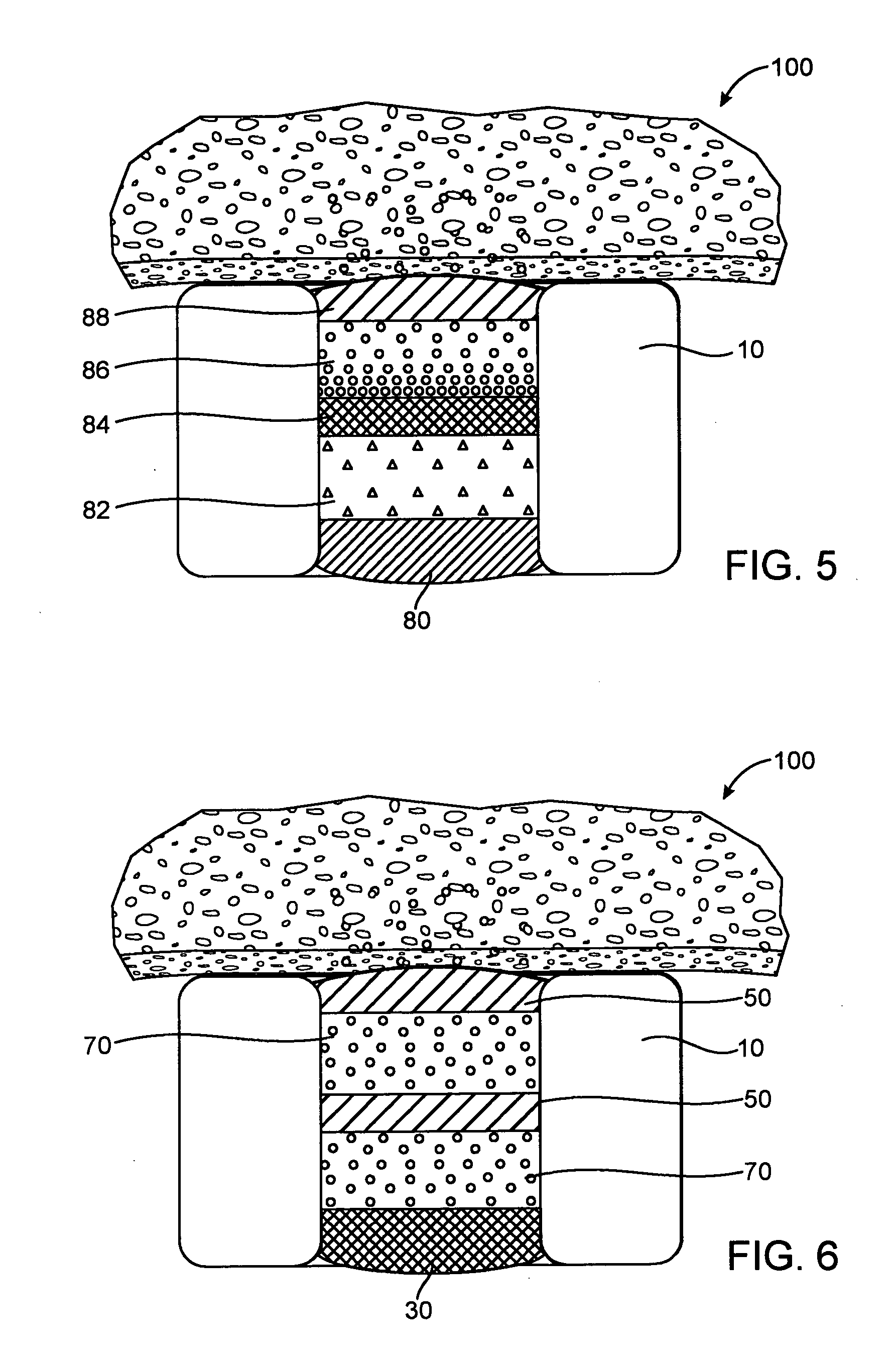
Orthopedic and dental implant devices providing controlled drug delivery
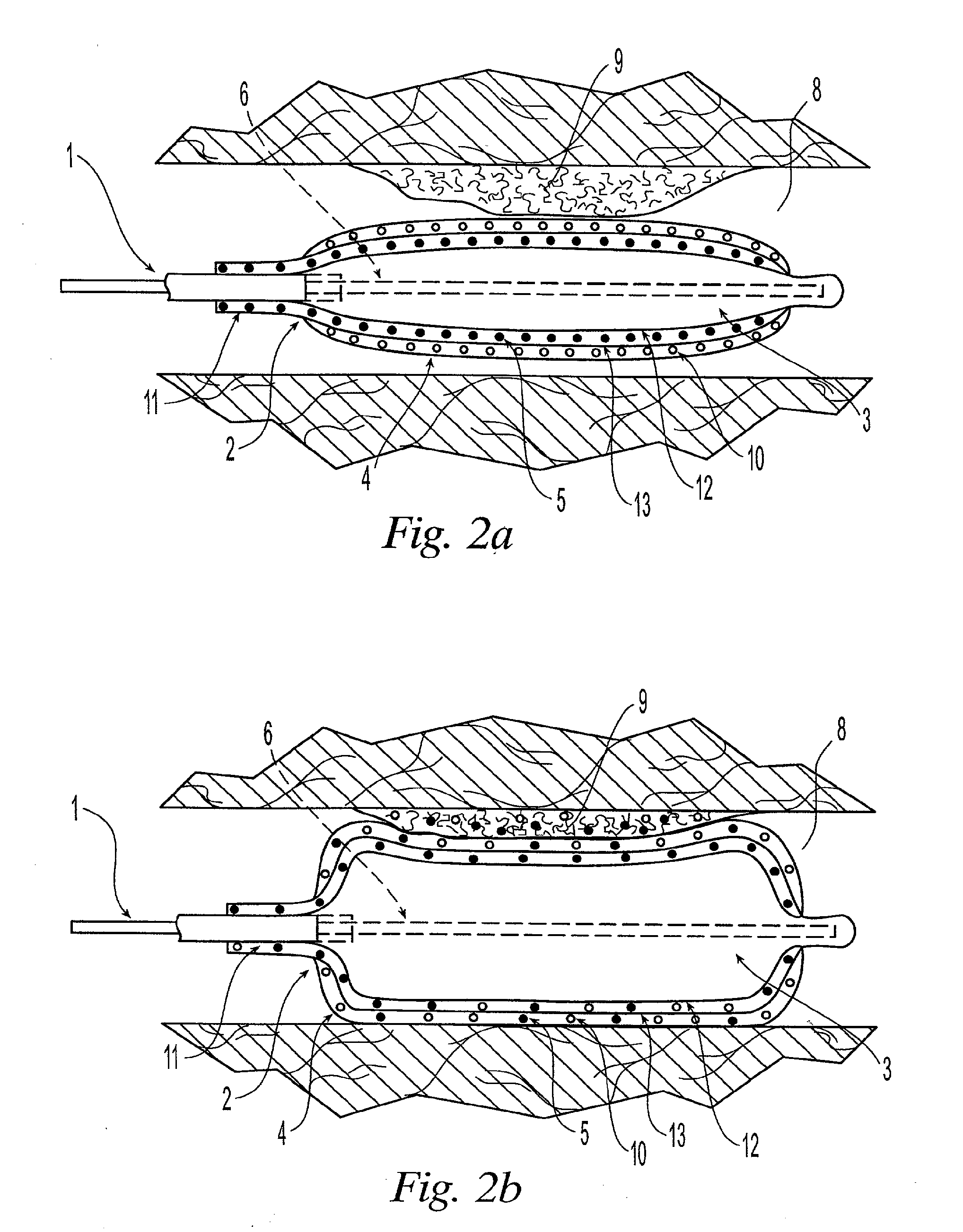
Implantable prosthetic devices are provided for controlled drug delivery, for orthopedic and dental applications. The device may include a prosthetic device body having at least one outer surface area; two or more discrete reservoirs located in spaced apart positions across at least a portion of the outer surface area, the reservoirs formed with an opening at the surface of the device body and extending into the device body; and a release system disposed in the reservoirs which comprises at least one therapeutic or prophylactic agent, wherein following implantation into a patient the therapeutic or prophylactic agent is released in a controlled manner from the reservoirs. The prosthetic device body preferably is a joint prosthesis or part thereof, such as a hip prosthesis, a knee prosthesis, a vertebral or spinal disc prosthesis, or part thereof. Optional reservoir caps may further control release kinetics.
Owner:MICROCHIPS INC
Implantable Drug Delivery Device and Methods for Treatment of the Bladder and Other Body Vesicles or Lumens
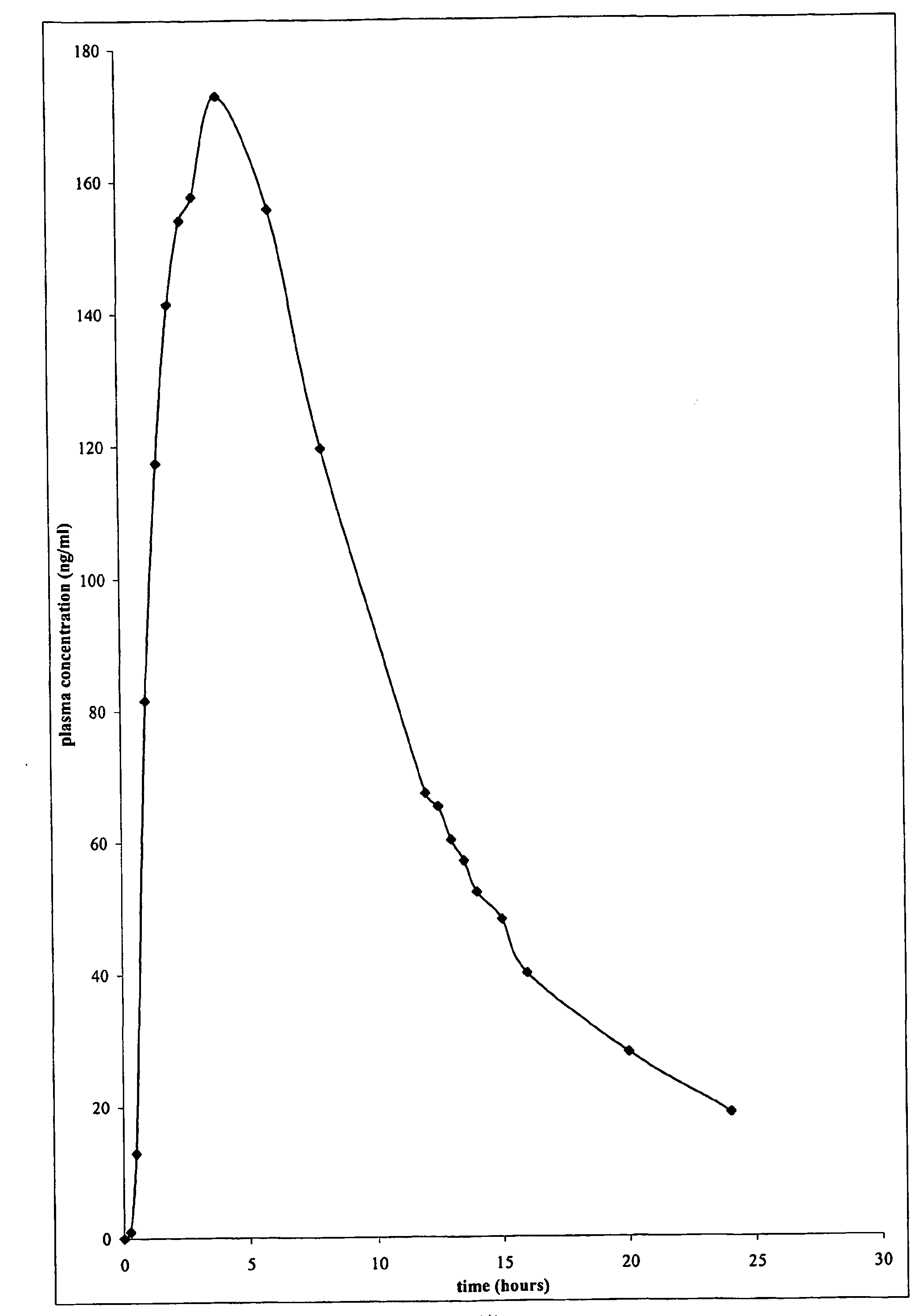
ActiveUS20090149833A1High plasma concentrationMinimize irritationBiocideMedical devicesDrug reservoirControlled drugs
An implantable medical device is provided for controlled drug delivery within the bladder, or other body vesicle. The device may include at least one drug reservoir component comprising a drug; and a vesicle retention frame which comprises an elastic wire having a first end, an opposing second end, and an intermediate region therebetween, wherein the drug reservoir component is attached to the intermediate region of the vesicle retention frame. The retention frame prevents accidental voiding of the device from the bladder, and it preferably has a spring constant selected for the device to effectively stay in the bladder during urination while minimizing the irritation of the bladder.
Owner:MASSACHUSETTS INST OF TECH
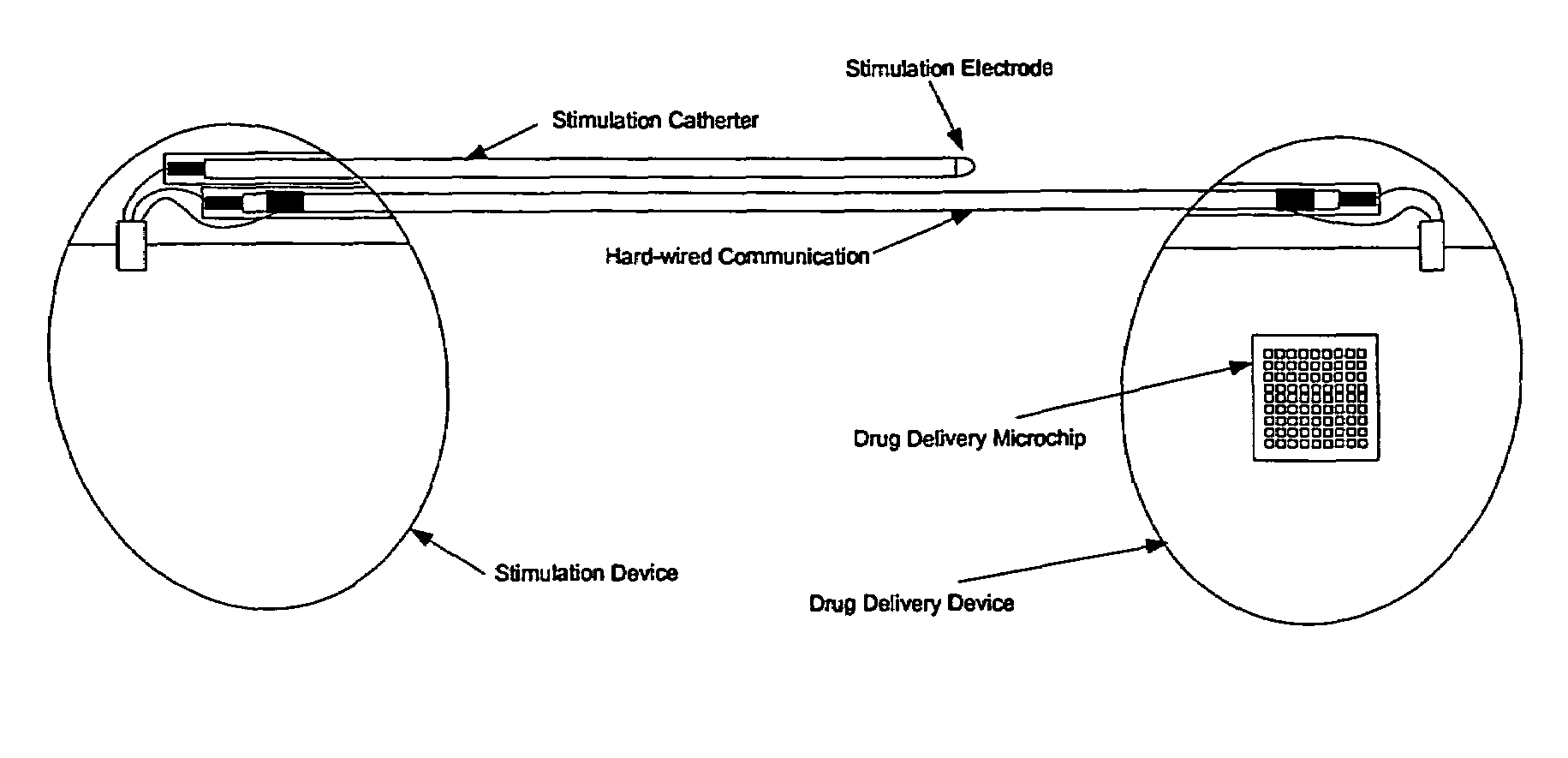
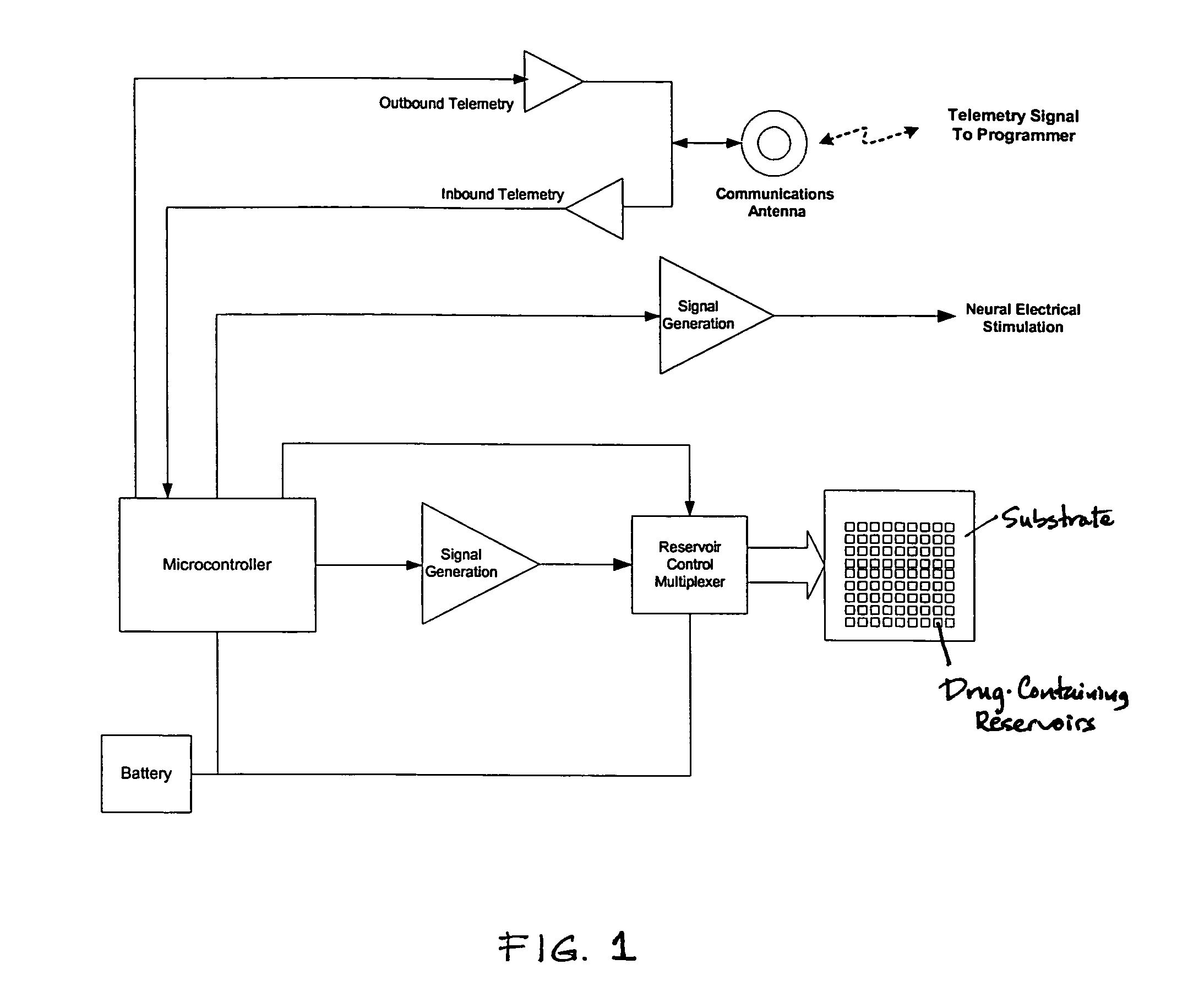
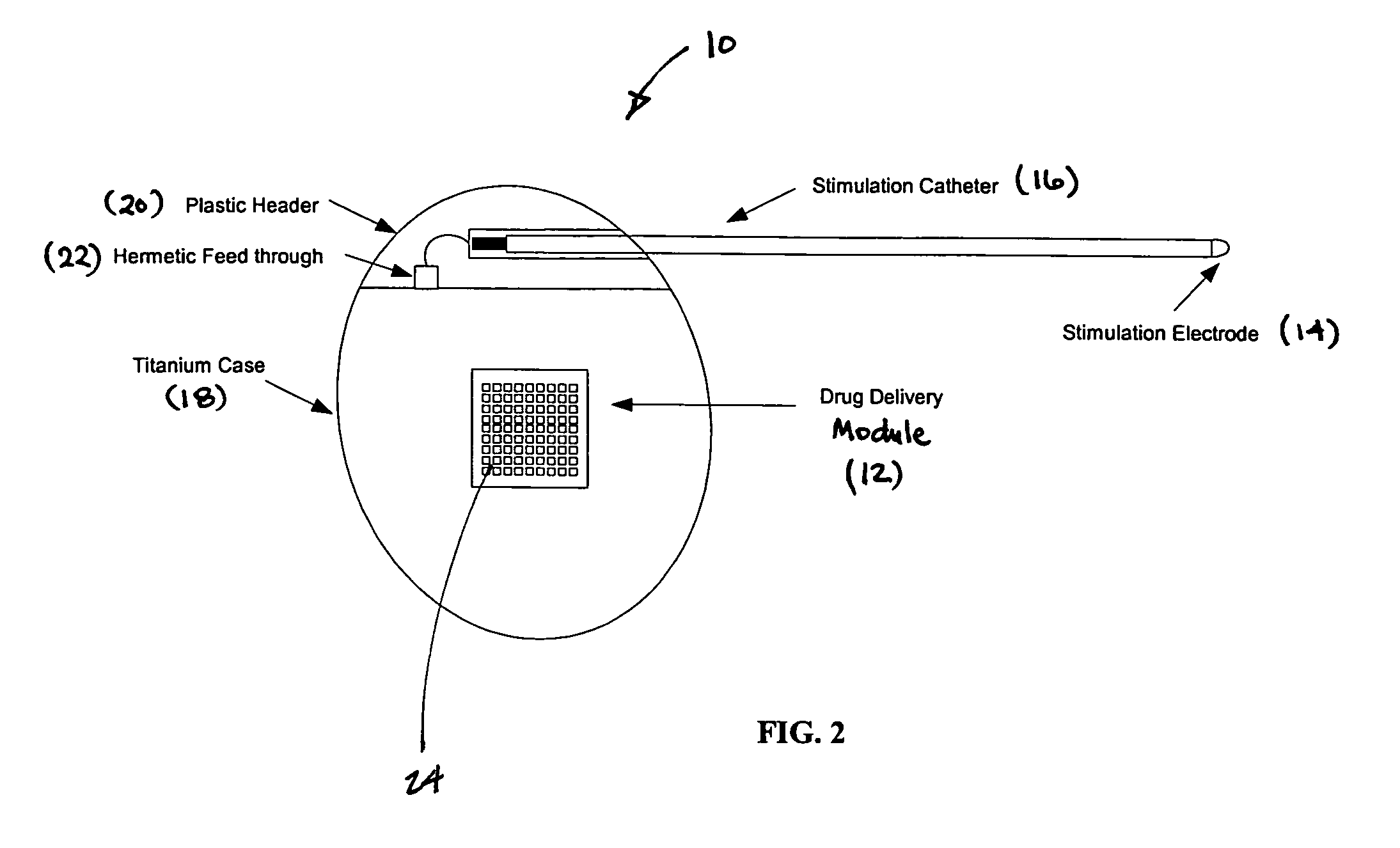
Medical device for neural stimulation and controlled drug delivery
InactiveUS7599737B2Reduce stimulation thresholdReduce stimulationElectrotherapyMicromachined deliveryMicrocontrollerElectrical stimulations
Medical devices and methods are provided for electrical stimulation of neural tissue and controlled drug delivery to a patient. The device includes an implantable drug delivery module which comprises a plurality of reservoirs, a release system comprising at least one drug contained in each of the reservoirs, and control means for selectively releasing a pharmaceutically effective amount of drug from each reservoir; a neural electrical stimulator which comprises a signal generator connected to at least one stimulation electrode for operable engagement with a neural tissue of the patient; and at least one microcontroller for controlling operational interaction of the drug delivery module and the neural electrical stimulator. The microcontroller may control the signal generator and the control means of the drug delivery module. The device may further include a sensor operable to deliver a signal to the microcontroller, for example to indicate when to deliver electrical stimulation, drug, or both.
Owner:DARE MB INC
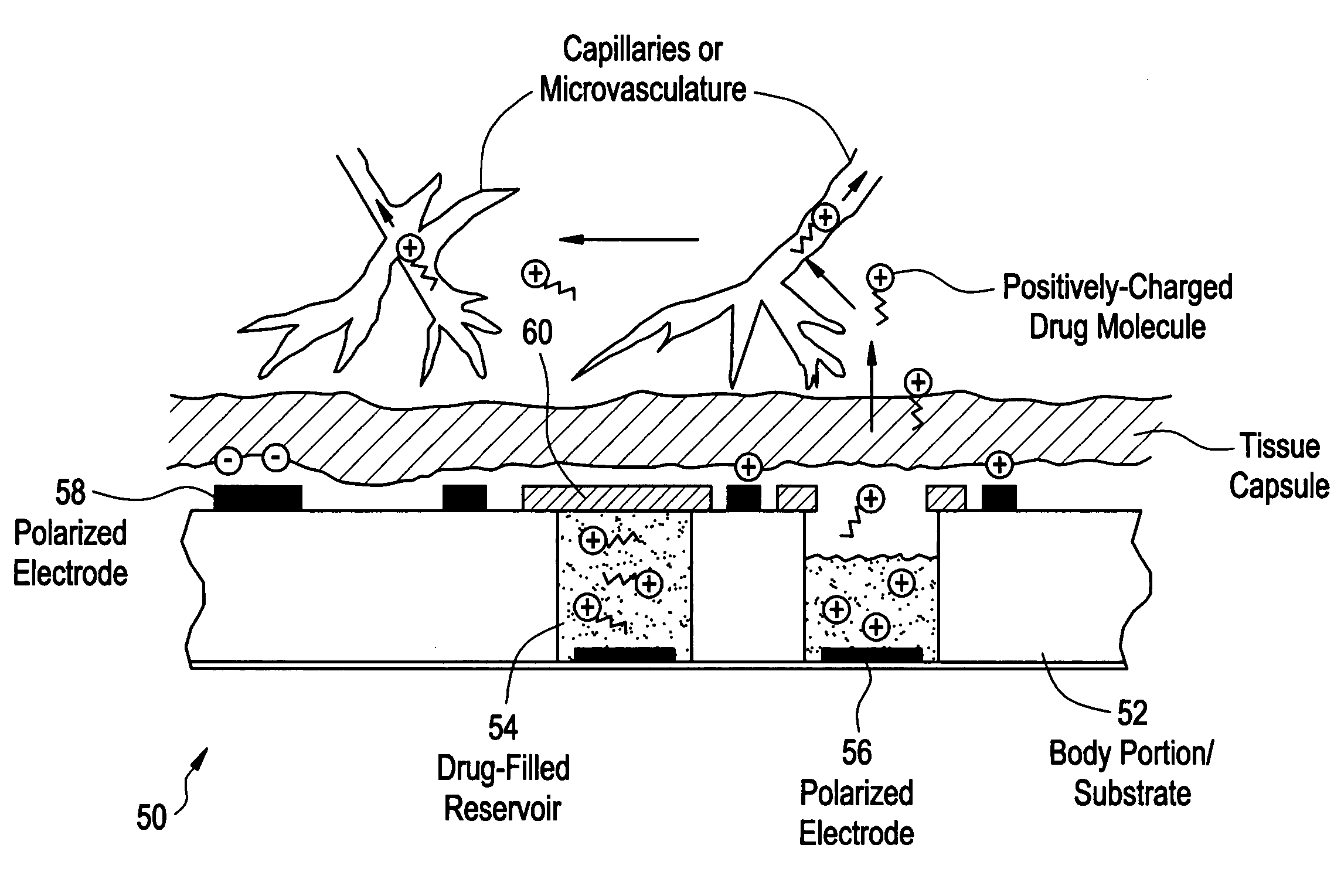
Devices and methods for measuring and enhancing drug or analyte transport to/from medical implant
InactiveUS20050267440A1Easy to transportEfficient propulsionElectrocardiographyAntipyreticControlled drugsAnalyte
Methods and devices are provided for enhancing mass transport through any fibrous tissue capsule that may form around an implanted medical device following implantation. Methods and devices are also provided to enhance vascularization around the implanted device, which also will aid in mass transport to / from the device. The device preferably comprises multiple reservoirs containing (i) a drug formulation for short- or long-term, controlled drug delivery, (ii) sensors for sensing an analyte in the patient, or (iii) a combination thereof.
Owner:MICROCHIPS INC
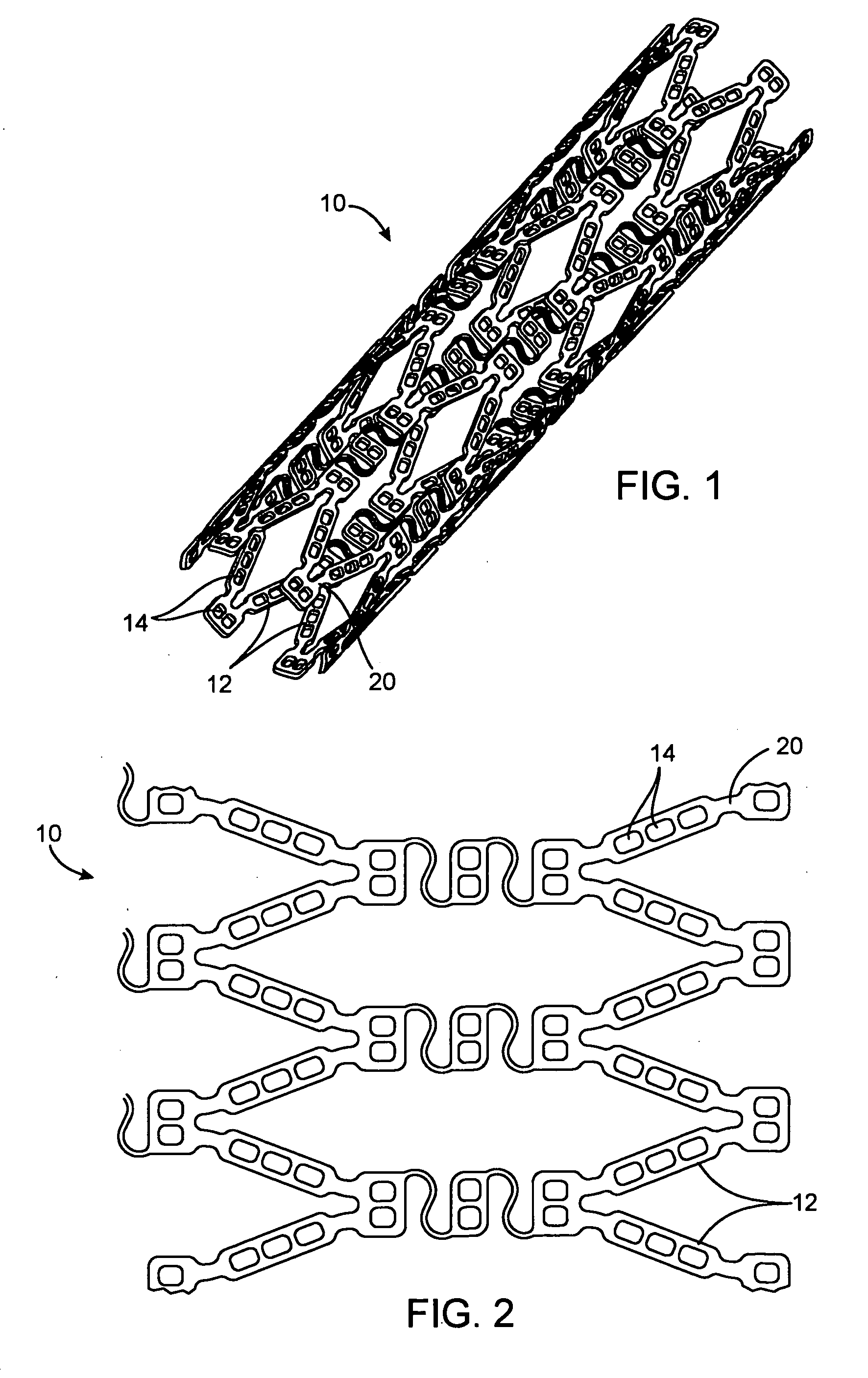
Drug-eluting stent for controlled drug delivery
The present invention provides a stent for delivering drugs to a vessel in a body, including a stent framework with a plurality of reservoirs formed therein, a drug polymer positioned in the reservoirs, and a polymer layer positioned on the drug polymer. The present invention also provides a method of manufacturing a drug-polymer stent and a method of treating a vascular condition using the drug-polymer stent.
Owner:MEDTRONIC VASCULAR INC
Implantable or insertable medical devices for controlled drug delivery
Implantable or insertable medical devices are provided, which comprises: (a) a biocompatible polymer; and (b) at least one therapeutic agent selected from an anti-inflammatory agent, an analgesic agent, an anesthetic agent, and an antispasmodic agent. The medical devices are adapted for implantation or insertion at a site associated with pain or discomfort upon implantation or insertion. In many embodiments, the therapeutic will be selected from at least one of (i) ketorolac and pharmaceutically acceptable salts thereof (e.g., ketorolac tromethamine) and (ii) 4-diethylamino-2-butynylphenylcyclohexyl glycolate and pharmaceutically acceptable salts thereof (e.g., oxybutynin chloride). Also provided are uses for the implantable or insertable medical devices, which uses comprise reducing pain or discomfort accompanying the implantation or insertion of such devices. Further uses may comprise reducing microbial buildup along the device. Methods for manufacturing implantable or insertable medical devices are also provided.
Owner:BOSTON SCI SCIMED INC
Gastric retention controlled drug delivery system
ActiveUS20040180088A1Maintain it physical integrityMaintain physical integrityOrganic active ingredientsNervous disorderDrug deliveryDrug
The present invention provides a gastric retention controlled drug delivery system comprising: (a) a controlled release core comprising a drug, a highly swellable polymer and a gas generating agent, said core being capable of swelling and achieving floatation rapidly while maintaining its physical integrity in gastrointestinal fluids for prolonged periods, and (b) a rapidly releasing coat composition comprising the same drug as in the core and pharmaceutically acceptable excipients, wherein the coating composition surrounds the core such that the system provides a biphasic release of the drug in gastrointestinal fluids.
Owner:SUN PHARMA INDS
Polymeric nanofibers for tissue engineering and drug delivery
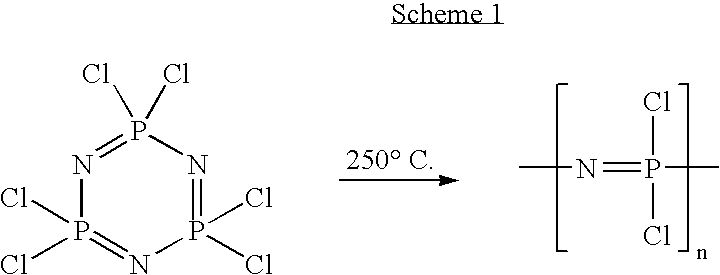
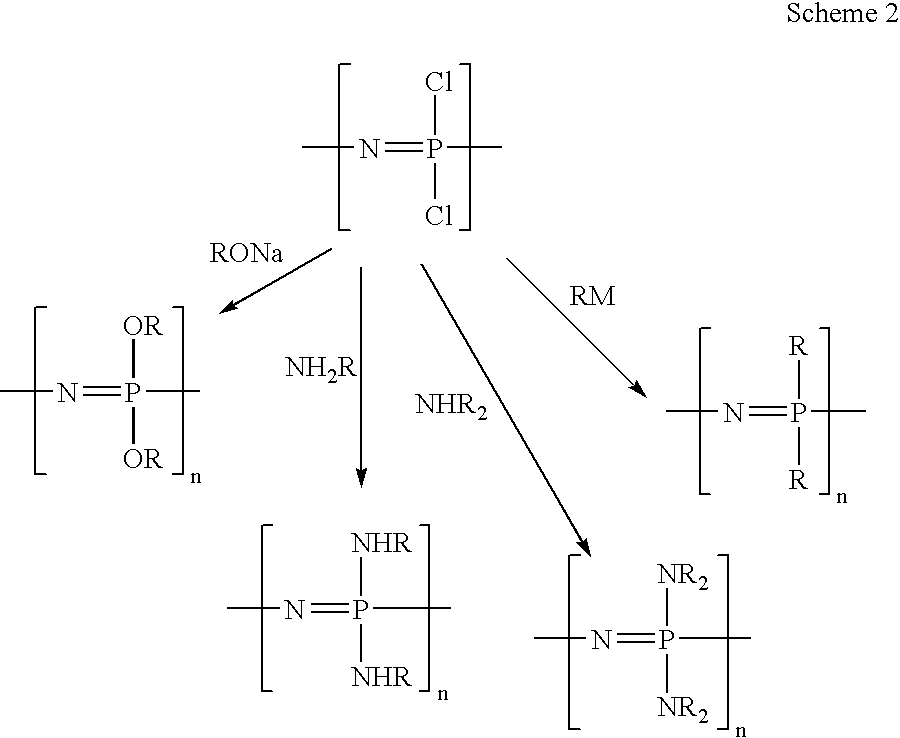
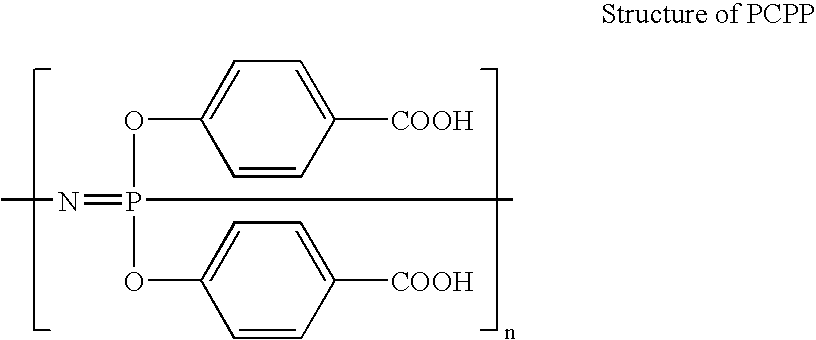
Polymeric nanofibers have been developed which are useful in a variety of medical and other applications, such as filtration devices, medical prosthesis, scaffolds for tissue engineering, wound dressings, controlled drug delivery systems, cosmetic skin masks, and protective clothing. These can be formed of any of a variety of different polymers, either non-degradable or degradable. In a preferred embodiment demonstrated in the following examples, nanofibers are formed of biodegradable and non biodegradable polyphosphazenes, their blends with other polyphosphazenes or with organic, inorganic / organometallic polymers as well as composite nanofibers of polyphosphazenes with nanosized particles such as hydroxyapatites.
Owner:PENN STATE RES FOUND
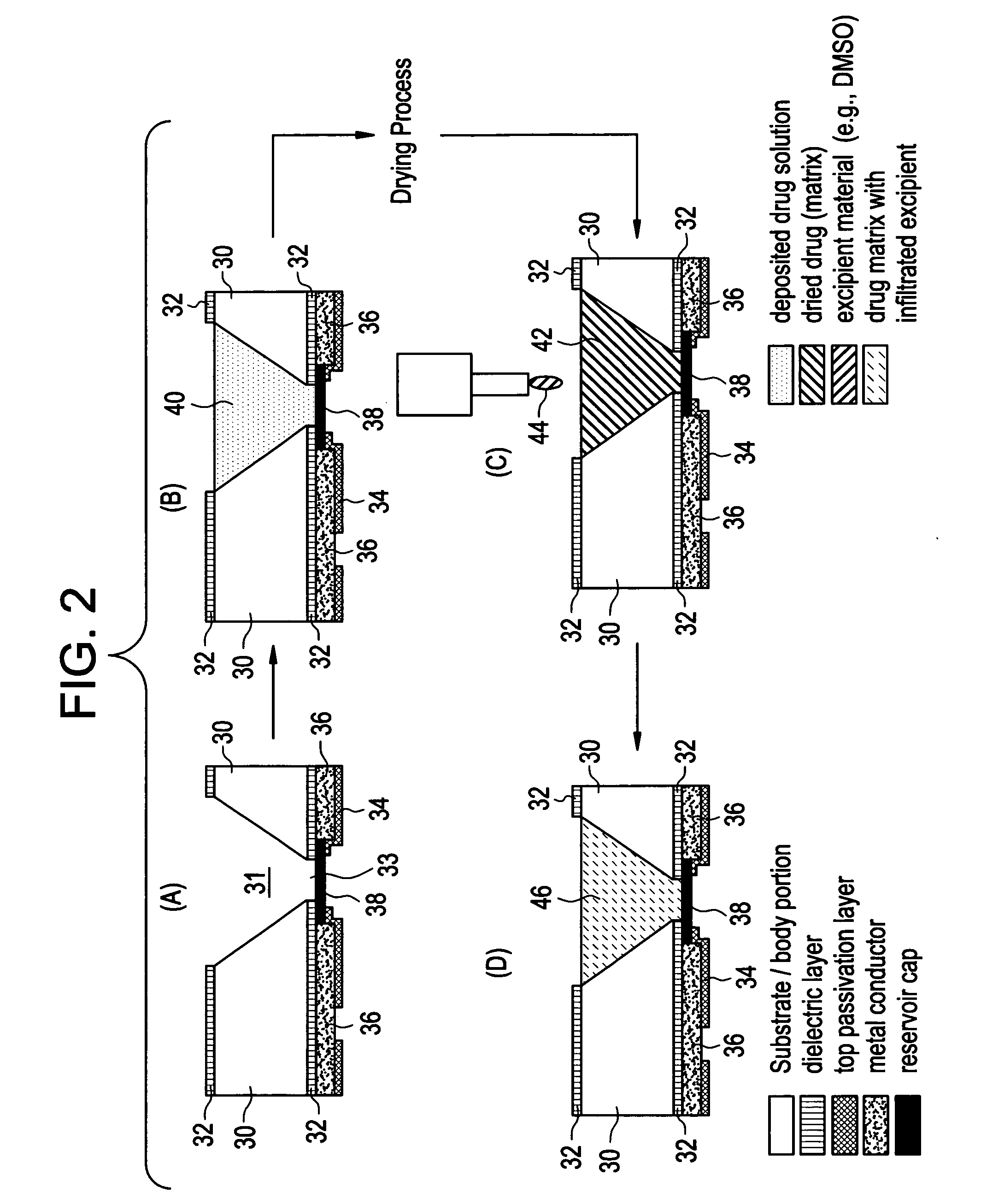
Expandable medical device with beneficial agent matrix formed by a multi solvent system
A multi solvent drug delivery matrix formation method is used to place layers into a reservoir in a stent in a stepwise manner to achieve extended delivery of water soluble, sensitive, or difficult to deliver drugs. The multi solvent matrix formation method allows the formation of a drug reservoir with a layered morphology in which the mixing between layers is limited to allow the different layers to perform different functions in controlling drug delivery. A stent having a drug delivery matrix includes a first beneficial agent layer affixed to the stent by depositing a first solution of a first polymer and a first solvent, and a second beneficial agent layer affixed to the first beneficial agent layer by depositing a second solution of a second polymer and a second solvent. The second solvent is selected so that the first polymer is substantially insoluble in the second solvent to prevent degradation of the first polymer during deposition of the second polymer. A therapeutic agent is provided in the first beneficial agent layer or the second beneficial agent layer to form a drug delivery matrix.
Owner:INNOVATIONAL HLDG LLC
Oral osmotic controlled drug delivery system for a sparingly soluble drug
InactiveUS6534090B2Control swellingIncrease in sizePowder deliveryPill deliveryCelluloseHydrophilic polymers
The present invention is for an oral osmotic controlled drug delivery system for a sparingly soluble drug comprising:a. a core comprising (i) finely particulate anhydrous carbamazepine (ii) a polymeric swelling agent consisting of one or more swellable hydrophilic polymers selected such that the polymeric swelling agent exhibits controlled swelling and the wall does not rupture or burst, (iii) a crystal habit modifier in whose presence, upon contact with an aqueous medium, the anhydrous carbamazepine being transformed into cuboidal or rod-shaped crystals of the dihydrate of carbamazepine, or mixtures thereof, and (iv) water-soluble compounds for inducing osmosis,b. a wall made of acylated cellulose which is impermeable to the components of the core, but permeable to water, andc. a passageway through the wall for releasing the components present in the core to the surrounding environment.
Owner:SUN PHARMA INDS
Patient controlled drug delivery device
InactiveUS20040068222A1Simple and inexpensive to manufactureSimple and inexpensive to and useInfusion devicesMedical devicesControlled drugsCatheter
A delivery device for patient-controlled infusion of a medicament, the delivery device comprising a reservoir for the medicament and a pump having a predetermined delivery dose which is capable of displacing the medicament from the reservoir and delivering it to a patient, wherein the pump comprises a pumping means, a first conduit, capable of restricting flow rate, chosen in conjunction with the delivery dose of the pumping means to define a predetermined maximum dosage rate, said conduit connecting the reservoir to a pumping means, a one-way valve in fluid communication with the first conduit and the pumping means which permits medicament flow into the pumping means but prevents reverse flow, a controlling means, and a second conduit extending from the pumping means and having a distal end through which the medicament may be released, wherein the controlling means: (a) is in fluid communication with the pumping means and the second conduit; (b) opens when pressured within the dose chamber exceeds a predetermined minimum opening pressure for the controlling means; and, (c) is adapted to prevent the reverse flow of medicament and air into the pumping means.
Owner:ONEIL ALEXANDER GEORGE BRIAN & ONEIL CHRISTINE JOINTLY
Method of controlling drug release from a coated medical device through the use of nucleating agents
InactiveUS20060088566A1Reduced nucleation rateSmall sizeSurgeryPharmaceutical delivery mechanismControlled drugsDrug release
A coated medical device have a drug and a nucleating agent thereon. Also provided are methods of increasing or decreasing the size of drug particles on a coated substrate through the use of nucleating agents to thereby increase or decrease the release rate of the drug from the coated substrate.
Owner:BOSTON SCI SCIMED INC
Medical device with sponge coating for controlled drug release
Owner:BOSTON SCI SCIMED INC
Polymeric nanofibers for tissue engineering and drug delivery
Polymeric nanofibers have been developed which are useful in a variety of medical and other applications, such as filtration devices, medical prosthesis, scaffolds for tissue engineering, wound dressings, controlled drug delivery systems, cosmetic skin masks, and protective clothing. These can be formed of any of a variety of different polymers, either non-degradable or degradable. In a preferred embodiment demonstrated in the following examples, nanofibers are formed of biodegradable and non biodegradable polyphosphazenes, their blends with other polyphosphazenes or with organic, inorganic / organometallic polymers as well as composite nanofibers of polyphosphazenes with nanosized particles such as hydroxyapatites.
Owner:PENN STATE RES FOUND
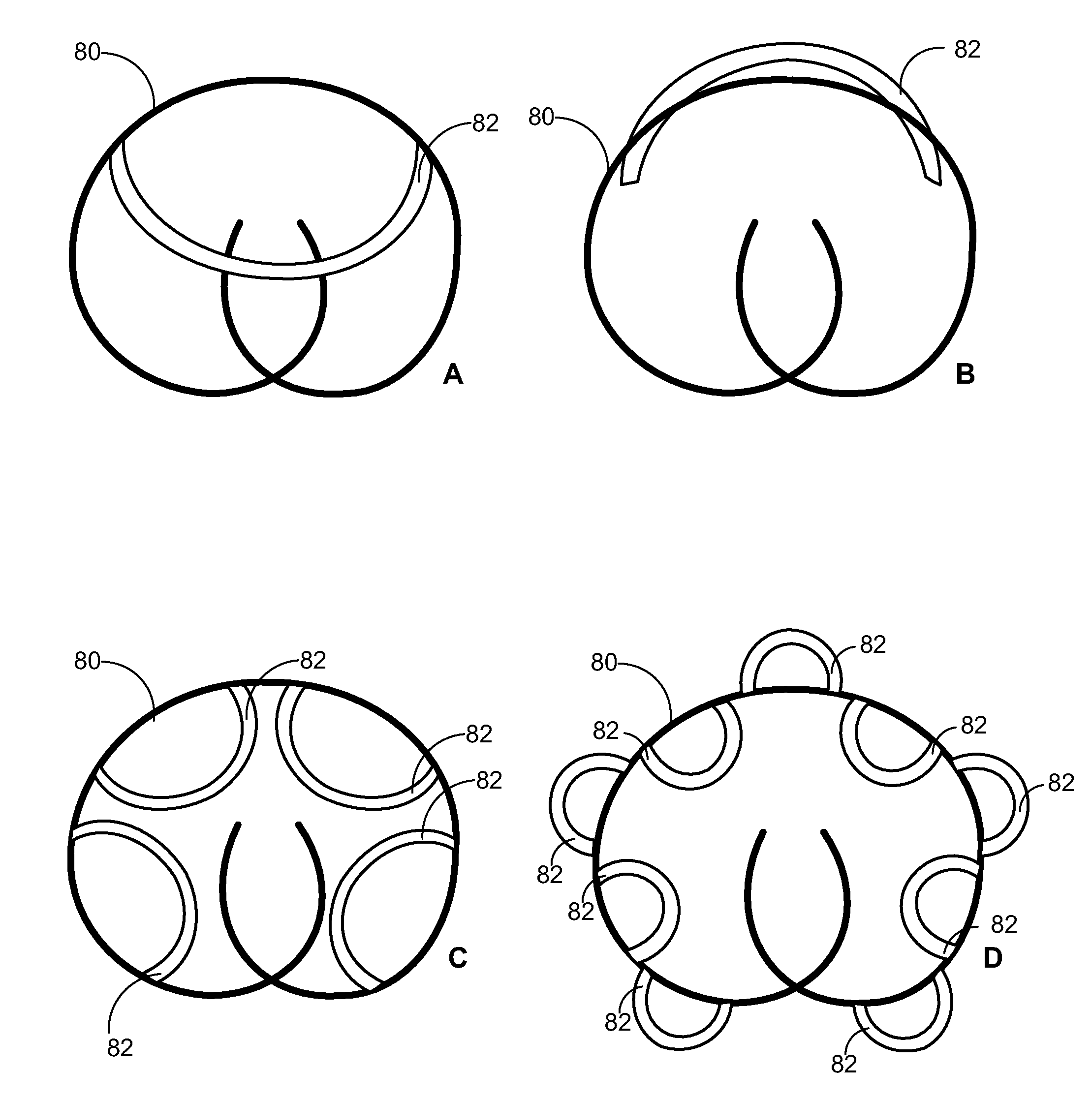
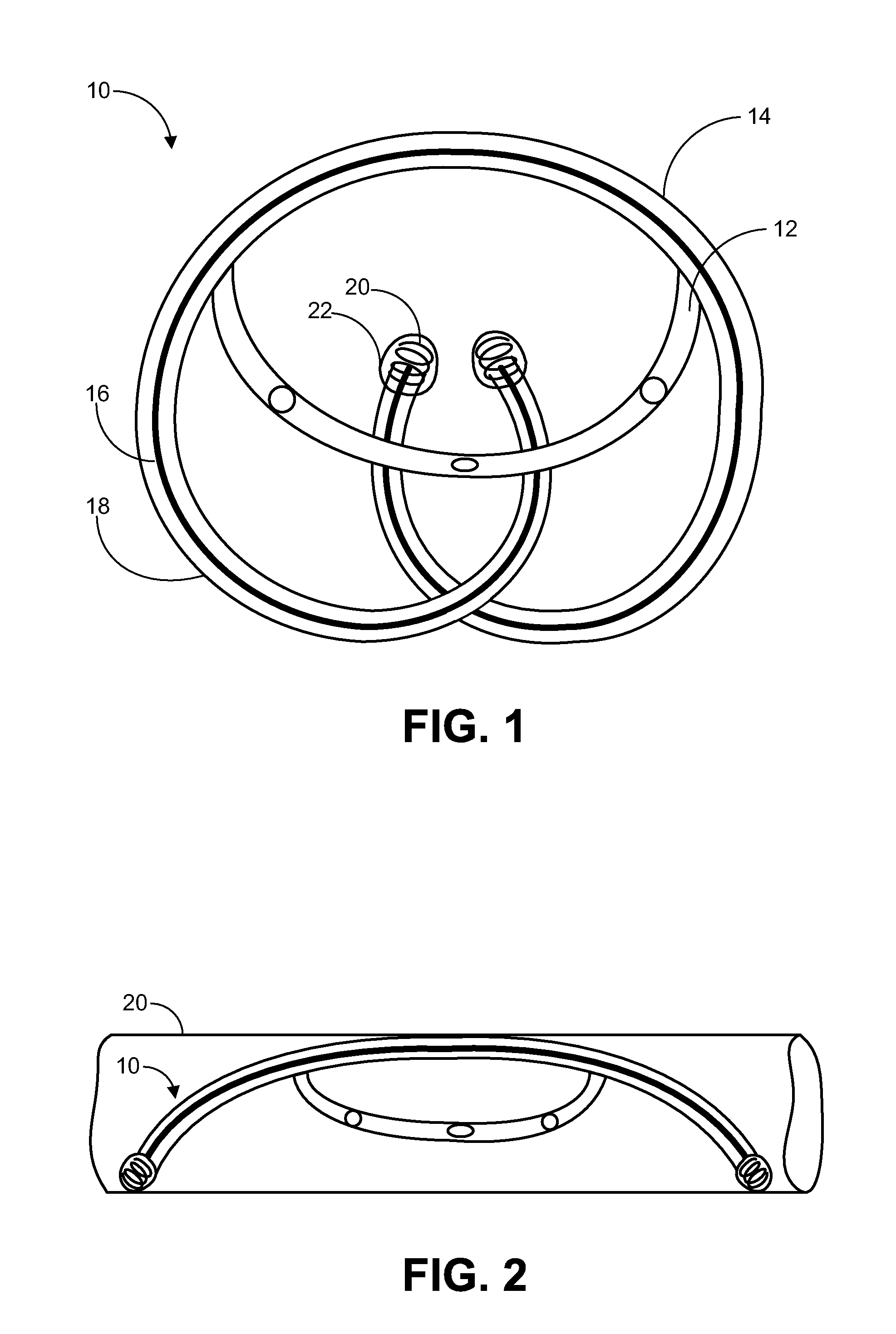
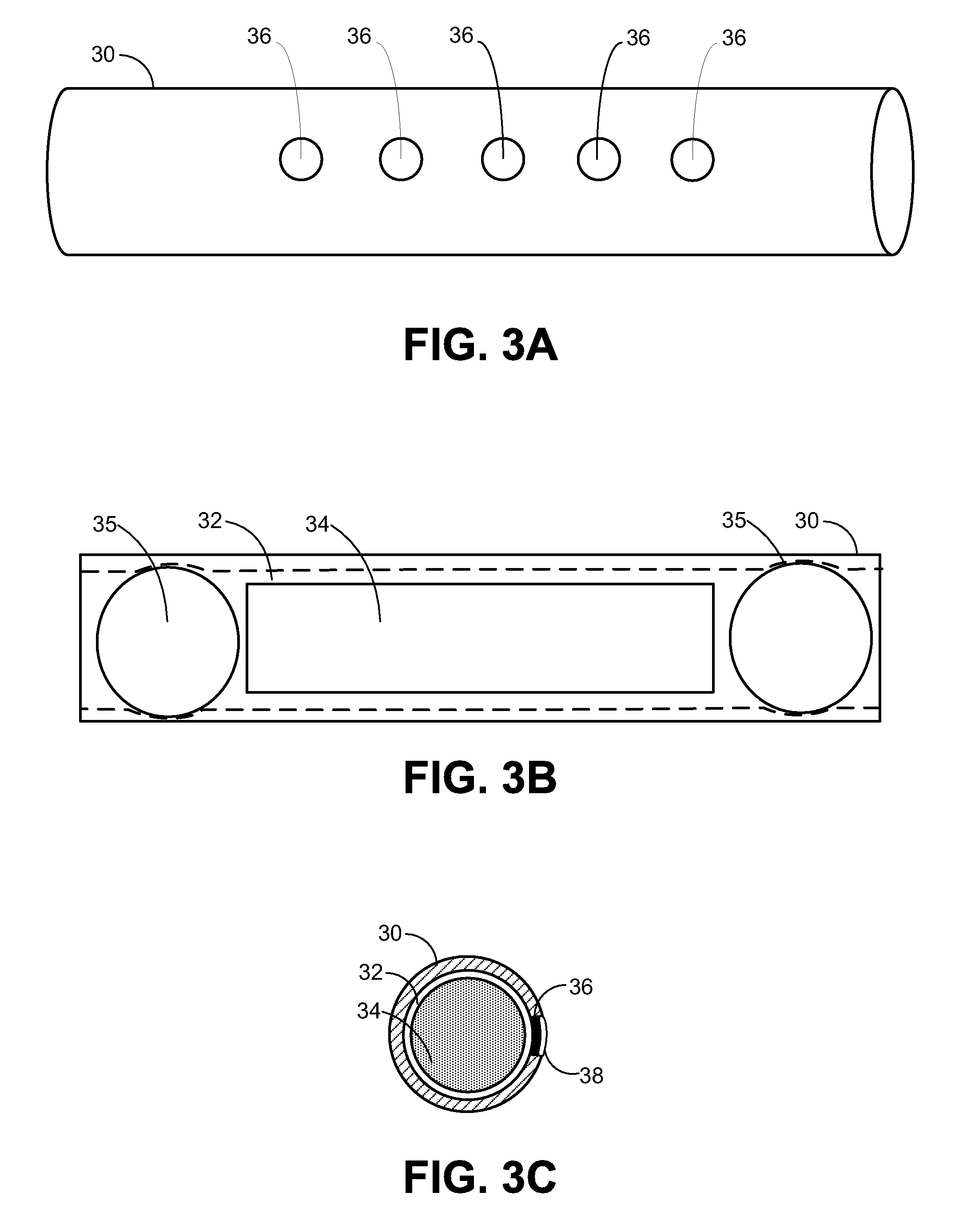
Partially absorbable fiber-reinforced composites for controlled drug delivery
This invention describes a partially absorbable, fiber-reinforced composite in the form of a ring, or a suture-like thread, with modified terminals for use as a controlled delivery system of at least one bioactive agent, wherein said composite comprising an absorbable fiber construct capable of providing time-dependent mechanical properties of a biostable elastomeric matrix containing an absorbable microparticulate ion-exchanger to modulate the release of the bioactive agent(s) for a desired period(s) of time at a specific biological site; this can be a vaginal canal, peritoneal cavity, scrotum, prostate gland, an ear loop or subcutaneous tissue. Such drug delivery systems can be used for the local administration of at least one bioactive agent, including those used as contraceptive, antimicrobial, anti-inflammatory and / or antiviral agents as well as for cancer treatment.
Owner:POLY MED
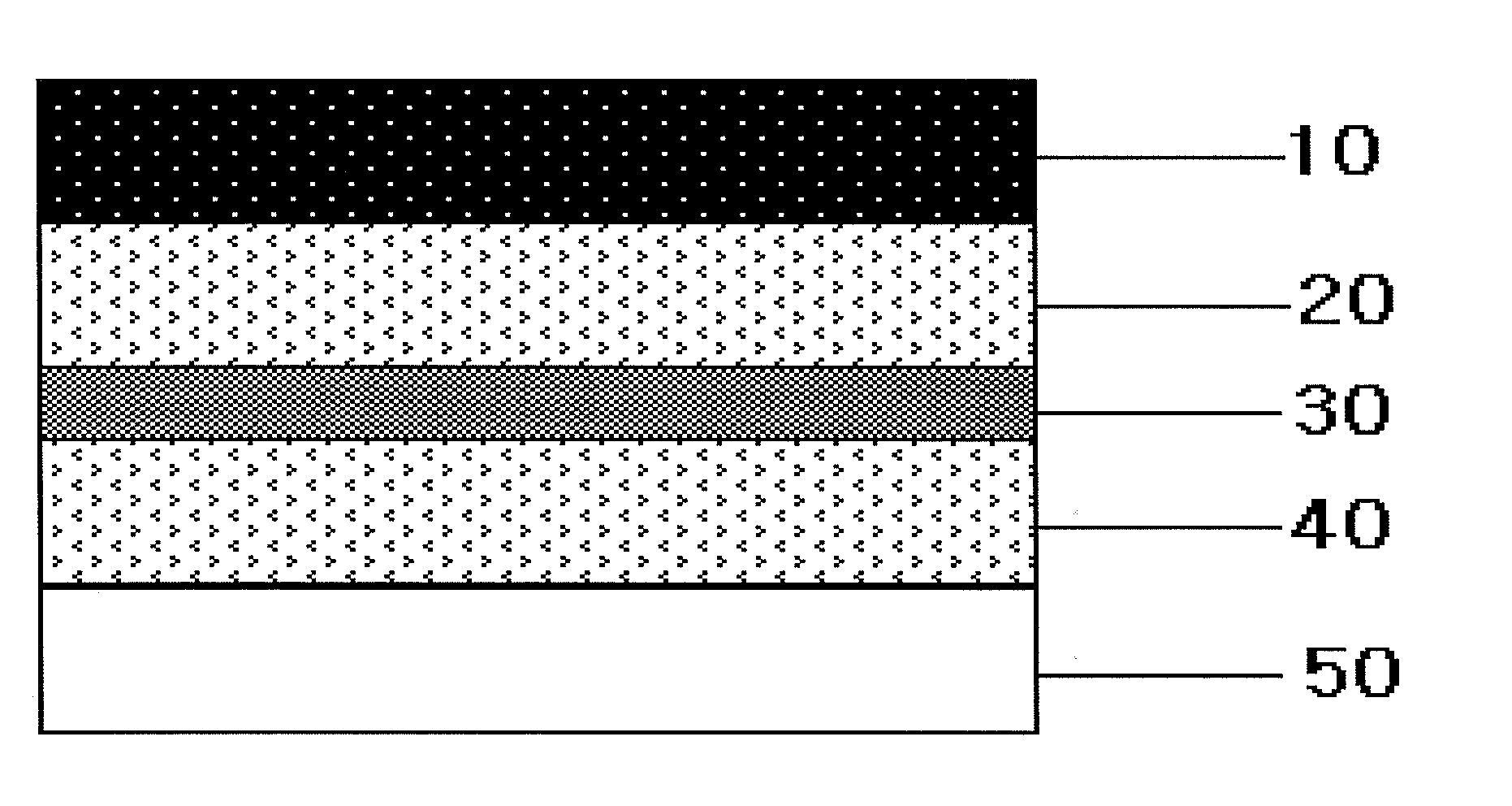
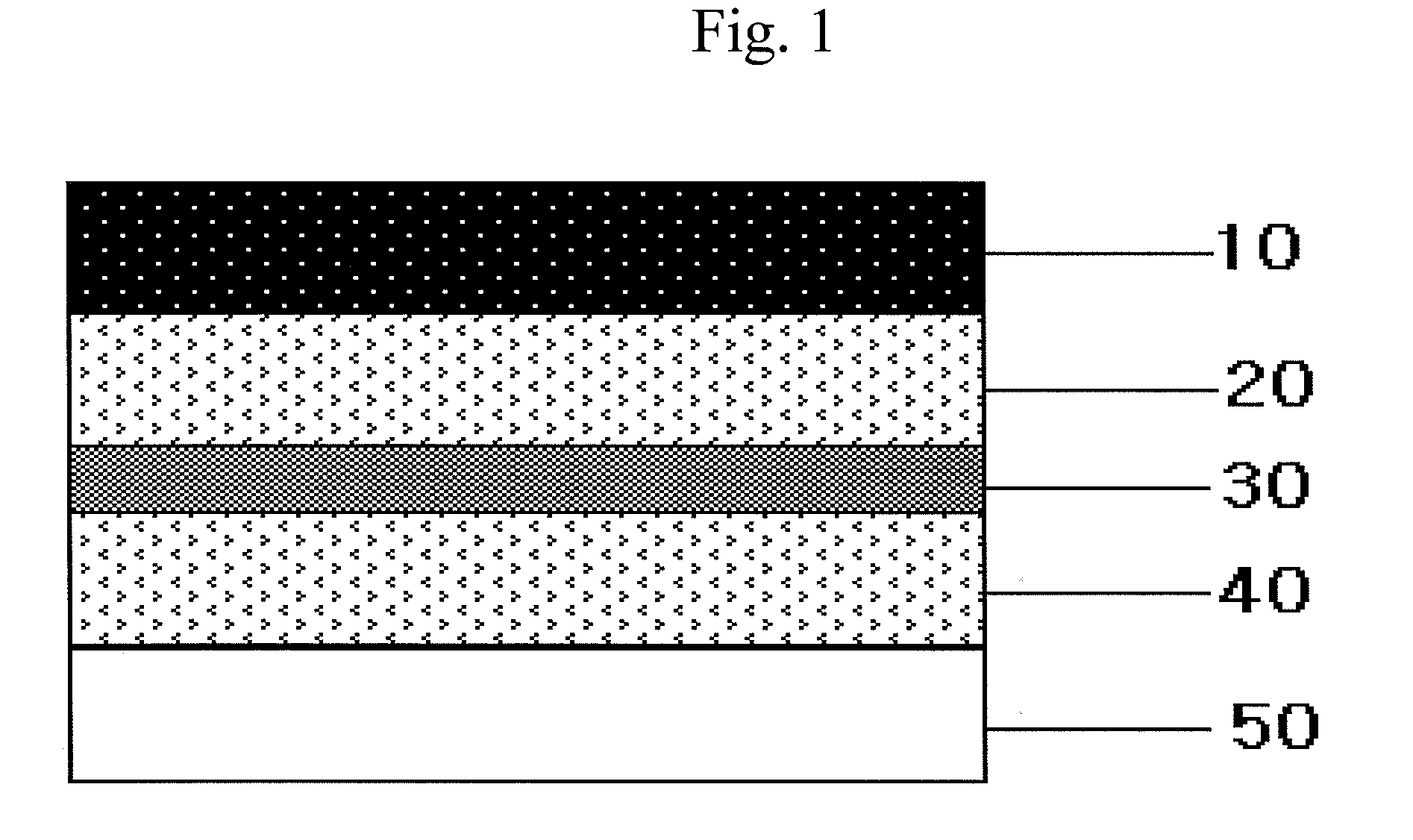
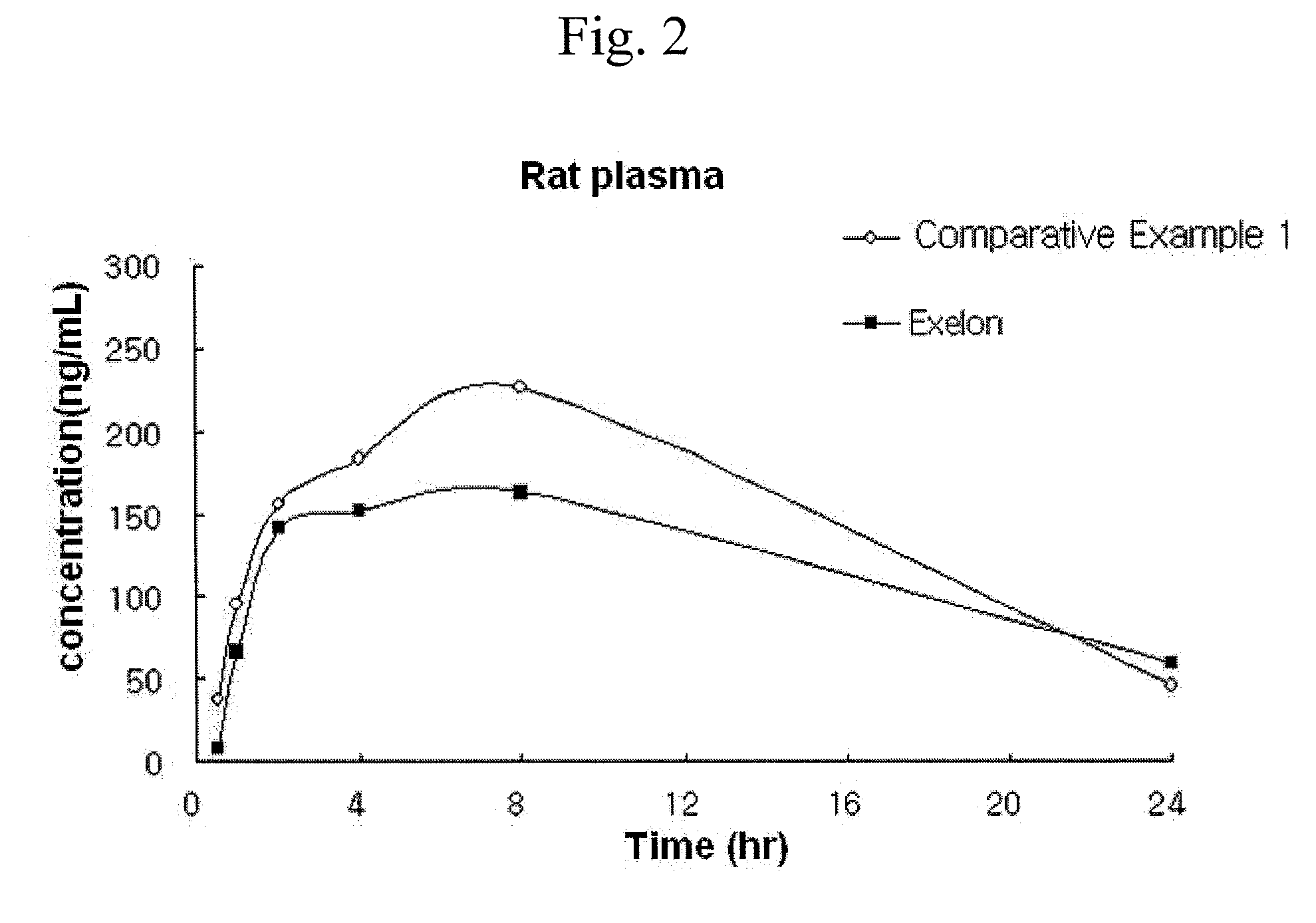
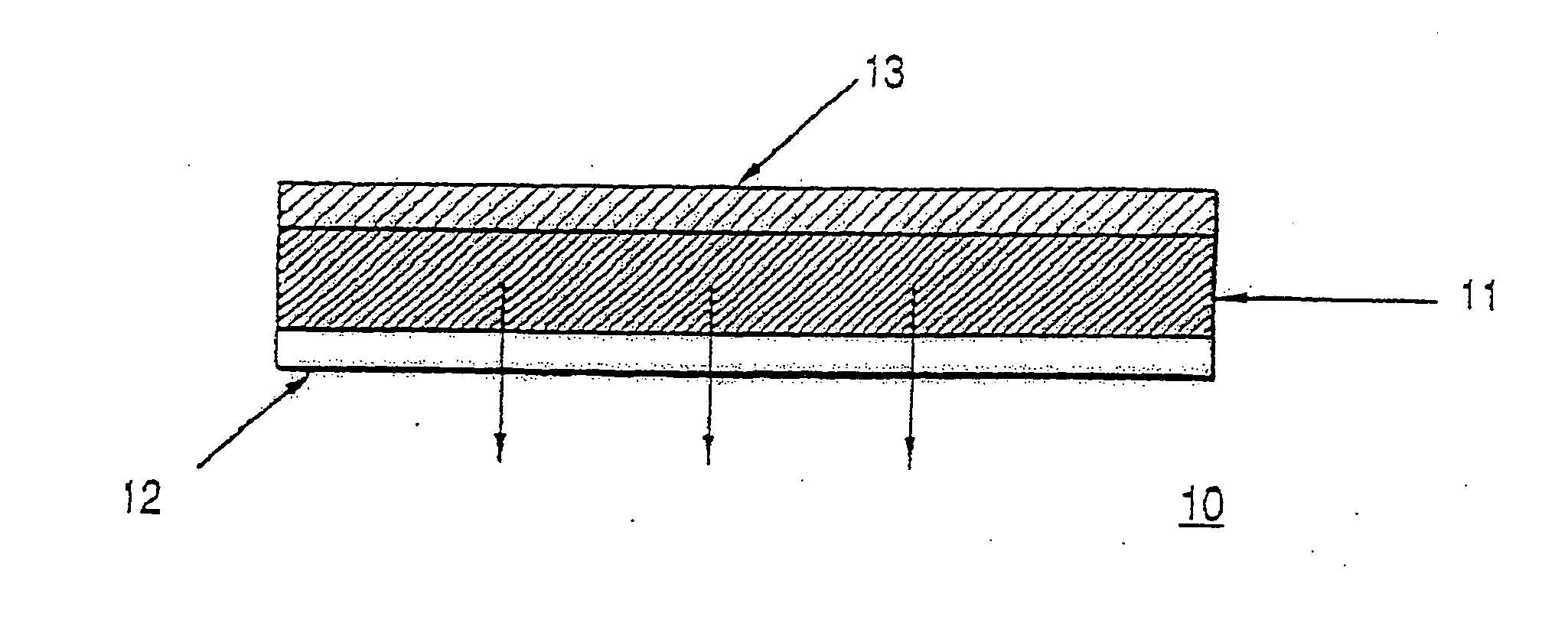
Transdermal delivery system, method for manufacturing the same, and transdermal delivery method using the system
ActiveUS20110066120A1Minimize skin irritationImprove the problemPlastersPretreated surfacesControlled drugsBiomedical engineering
Disclosed is a transdermal delivery system of multiple adhesive layers having a drug-free adhesive layer as an intermediate layer to control drug delivery rate. The transdermal delivery system enhances transdermal delivery rate in the early stage after application on skin and provides sustained control of the drug delivery rate in the intermediate and later stages. Thus, the drug delivery rate effective for treatment can be controlled in a sustained manner.
Owner:SAMYANG HLDG CORP
Composition and method for controlling drug delivery from silicone adhesive blends
InactiveUS20050019385A1Reduce concentrationPrevent/inhibit crystallizationSheet deliveryBandagesControlled drugsSolubility
Compositions and methods for controlling transdermal drug delivery, particularly of amine-functional and basic drugs, comprising a blend of a first silicone-based polymer having a reduced silanol concentration and a second silicone-based polymer have a substantial or high silanol concentration. The blend of such silicone-based polymers, particularly pressure-sensitive silicone adhesives, provides sufficient drug solubility and reduced initial drug delivery onset to permit a prolonged delivery duration at a substantially zero-order rate of delivery.
Owner:NOVEN PHARMA
Electrospun Skin Capable Of Controlling Drug Release Rates And Method
InactiveUS20070087027A1High tensile strengthChange in permeabilityStentsSynthetic resin layered productsFiberDrug release rate
A versatile covering process enabled through the identification and manipulation of a plurality of variables present in the electrospinning method of the present invention. By manipulating and controlling various identified variables, it is possible to use electrospinning to predictably produce thin materials having desirable characteristics. The fibers created by the electrospinning process have diameters averaging less than 100 micrometers. Proper manipulation of the identified variables ensures that these fibers are still wet upon contacting a target surface, thereby adhering with each other to form a cloth-like material and, if desired, adhering to the target surface to form a covering thereon. The extremely small size of these fibers, and the resulting interstices therebetween, provides an effective vehicle for drug and radiation delivery, and forms an effective membrane for use in fuel cells.
Owner:GREENHALGH SKOTT E +1
Apparatus for drug delivery in association with medical or surgical procedures
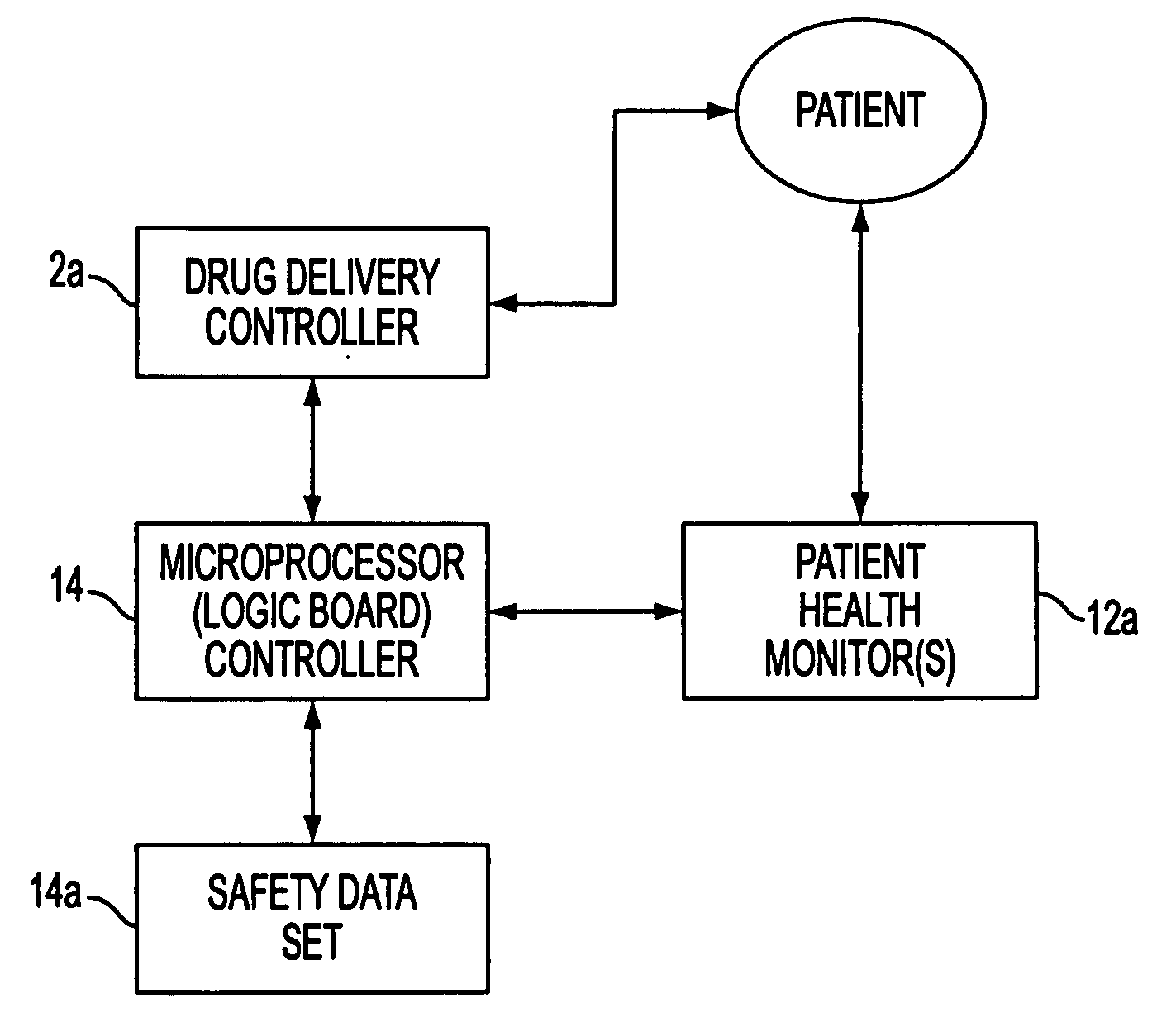
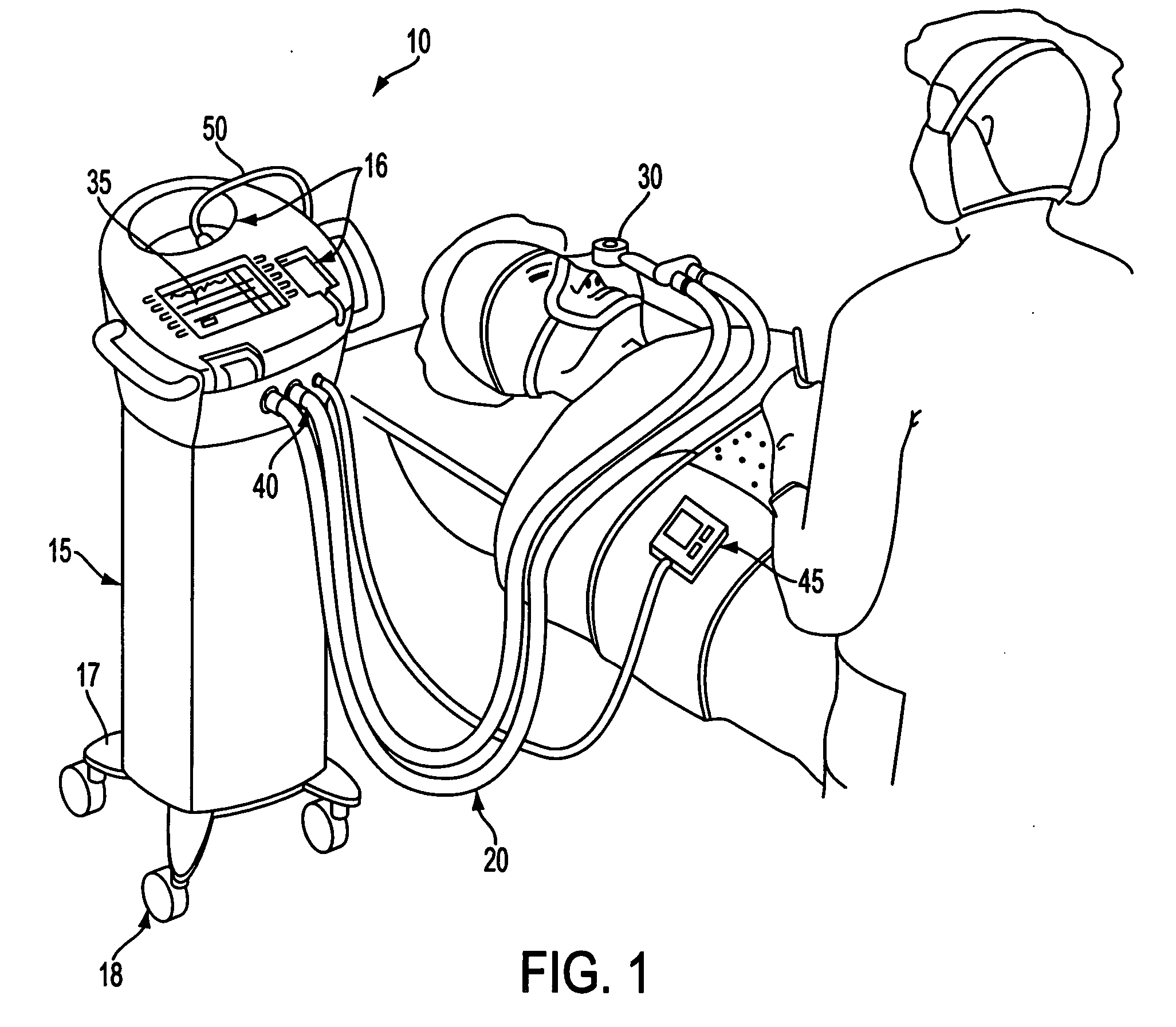
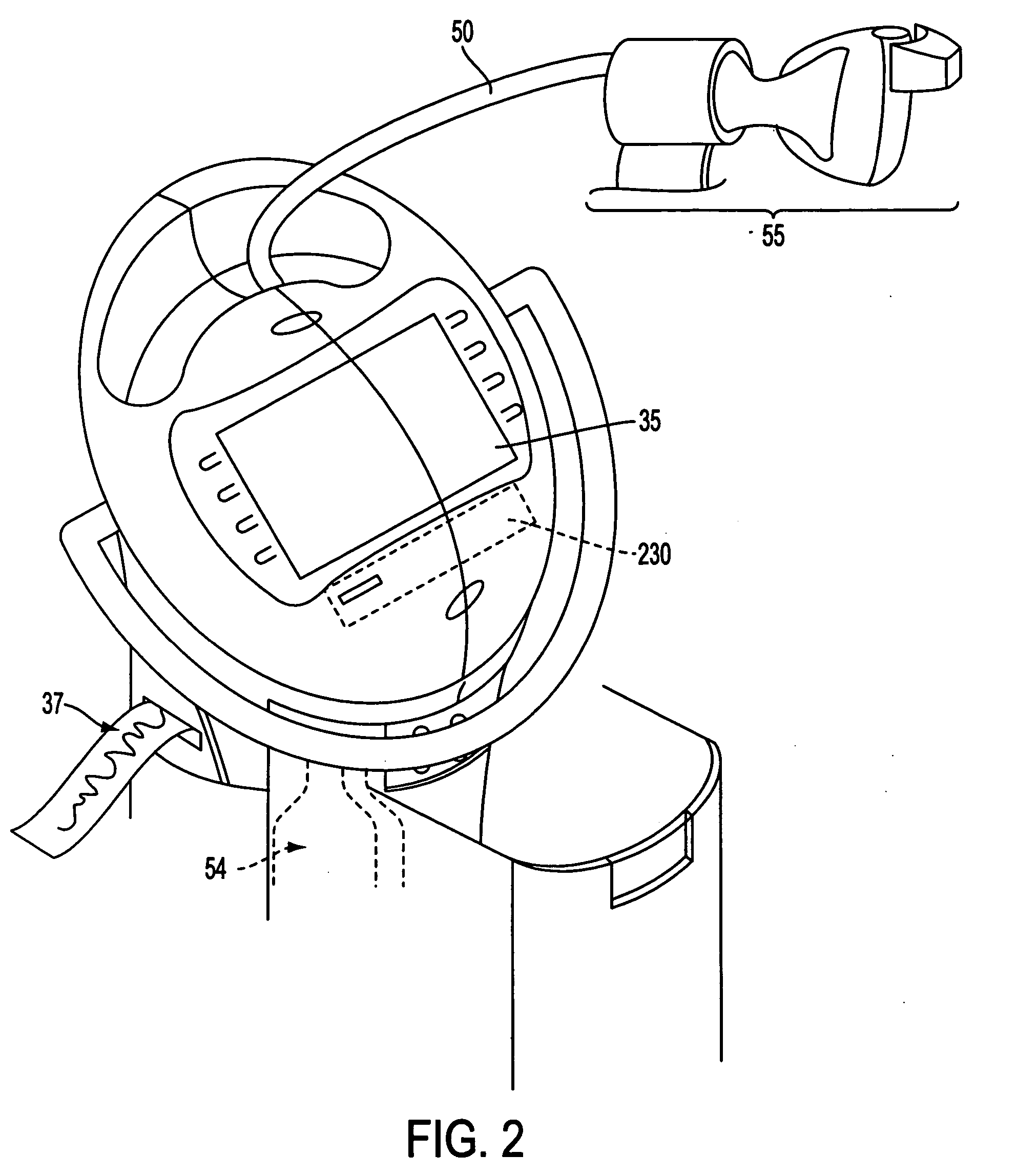
InactiveUS20050010166A1Safe and effectiveRelieve painDrug and medicationsSurgeryData setConsciousness monitoring
A care system and associated methods are provided for alleviating patient pain, anxiety and discomfort associated with medical or surgical procedures, the system comprising: at least one patient health monitor device coupled to a patient and generating a signal reflecting at least one physiological condition of the patient; a drug delivery controller supplying one or more drugs to the patient; a memory device storing a safety data set reflecting parameters of the at least one patient physiological condition; and an electronic controller interconnected between the patient health monitor, the drug delivery controller and the safety data set; wherein said electronic controller manages the application of the drugs in accord with the safety data set. In another aspect of the invention, the care system facilitates a procedural physician's safely and efficaciously providing conscious sedation to a patient by additionally providing a consciousness monitoring system which monitors the consciousness of the patient and generates a value representing the level of patient consciousness. Methods for alleviating patient pain and anxiety in accordance with the invention comprise connecting a drug delivery device to a patient, such device having a drug delivery controller supplying one or more drugs and being coupled to an electronic controller; attaching at least one patient health monitor device to the patient; accessing a memory device which stores a safety data set reflecting parameters of at least one patient physiological condition; and delivering the drugs to the patient in accord with the safety data set. In further aspects of the invention, the consciousness monitoring system is an automated consciousness monitoring system which includes patient query and response devices. Additional embodiments of the system and methods are directed to alleviating patient pain or discomfort while enabling safe patient controlled drug delivery in correlation with the monitoring of patient health conditions.
Owner:SCOTT LAB
Durable Biocompatible Controlled Drug Release Polymeric Coatings for Medical Devices
Disclosed herein are biocompatible durable controlled drug releasing polymeric coatings for medical devices. The drug release rates of the polymers are controlled by adjusting monomer ratios and glass transition temperatures Tgs. The polymers are durable; they do not delaminate from the coated medical device.
Owner:MEDTRONIC VASCULAR INC
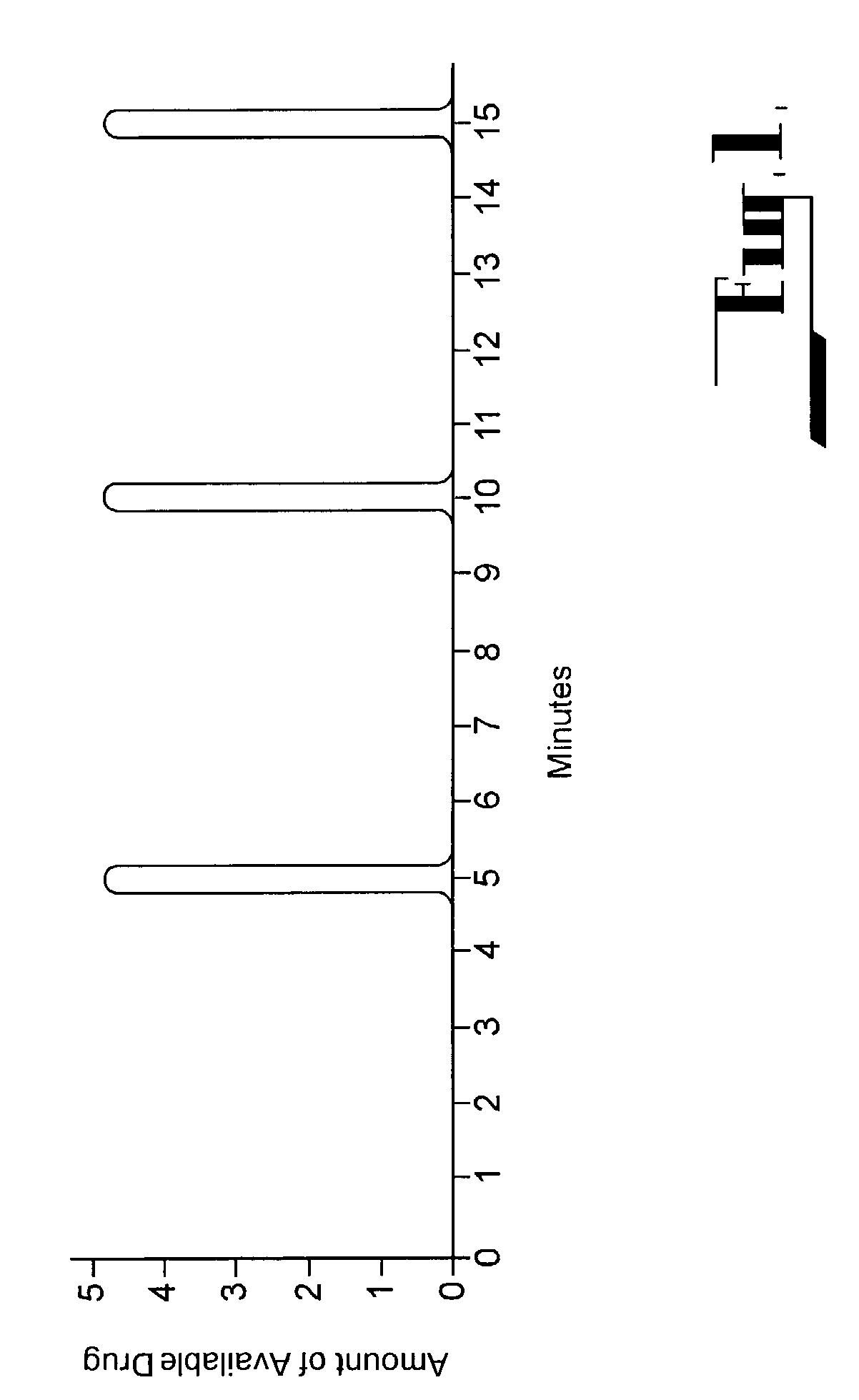
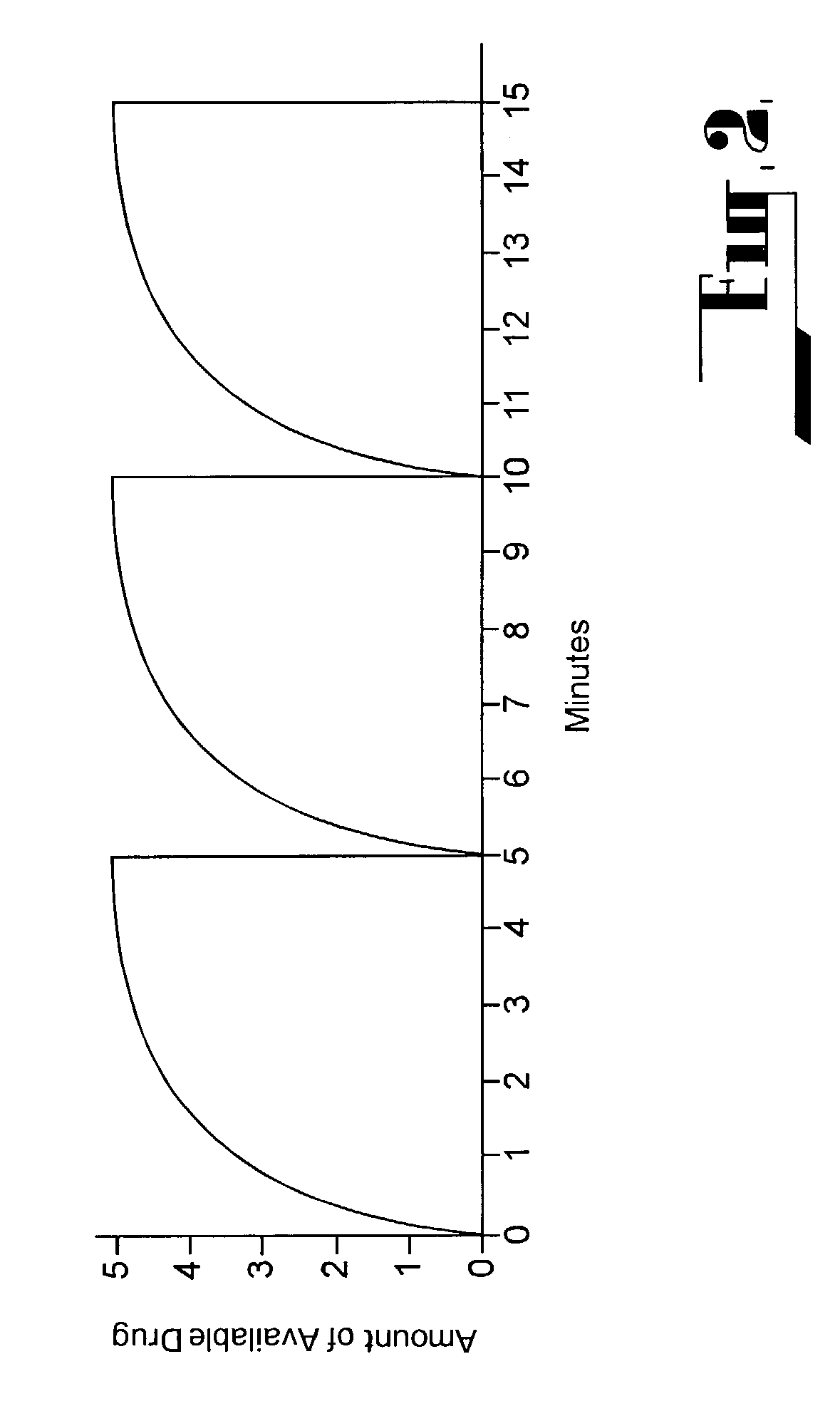
Mixed drug aerosol compositions
InactiveUS20080299048A1Overcome Manufacturing ComplexityOvercomes shelf-life stability issuePowder deliveryNervous disorderControlled drugsDrug aerosol
The present invention pertains to aerosols which comprise a first compound which is physiologically active and a second compound which is different from the first compound. Such aerosols may be produced “on demand” and can be used to control drug release, to improve vaporizability, or to reduce, modify or eliminate undesirable taste associated with a drug aerosol. The present invention also pertains to methods for producing such aerosols.
Owner:ALEXZA PHARMA INC
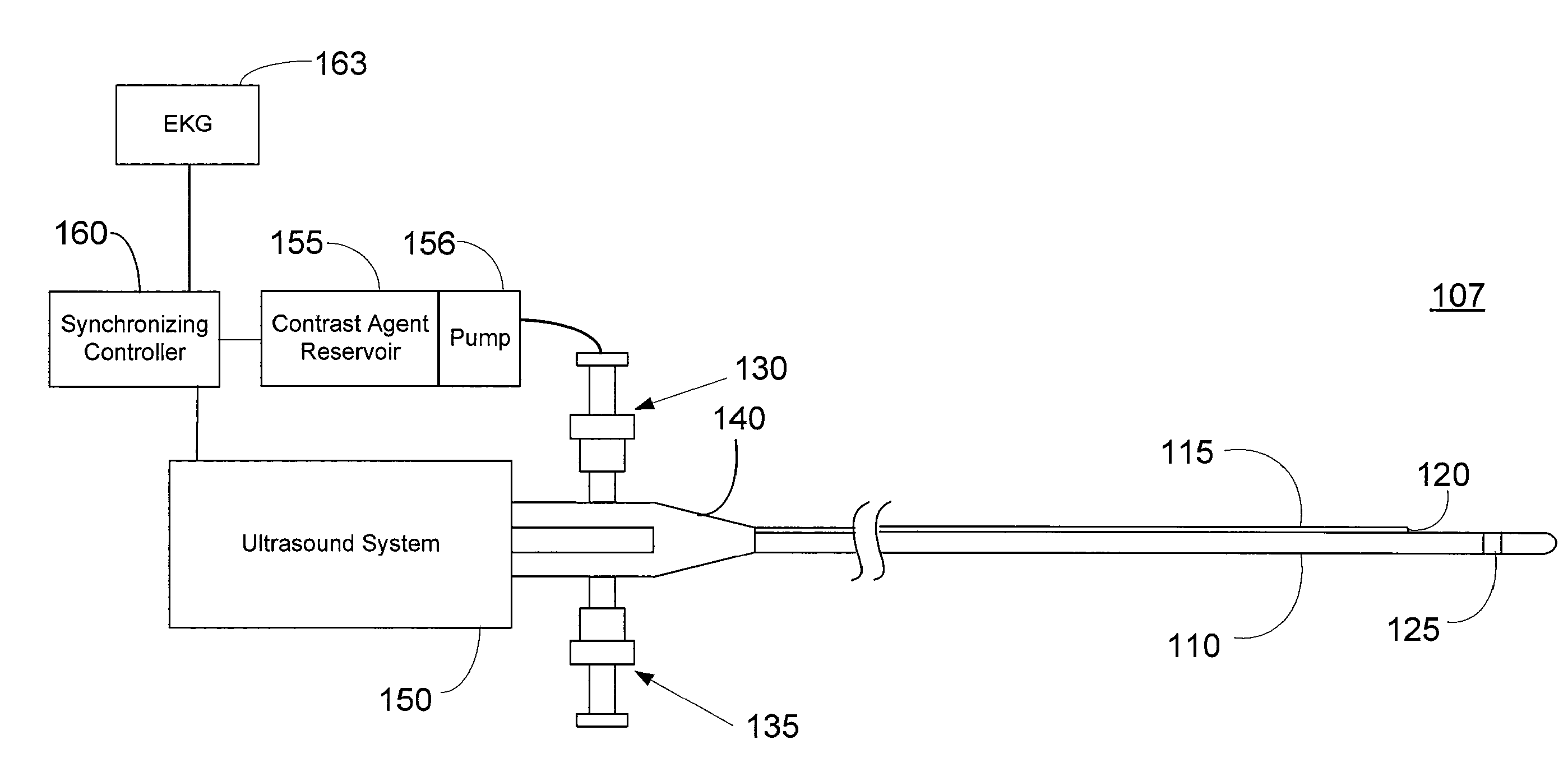
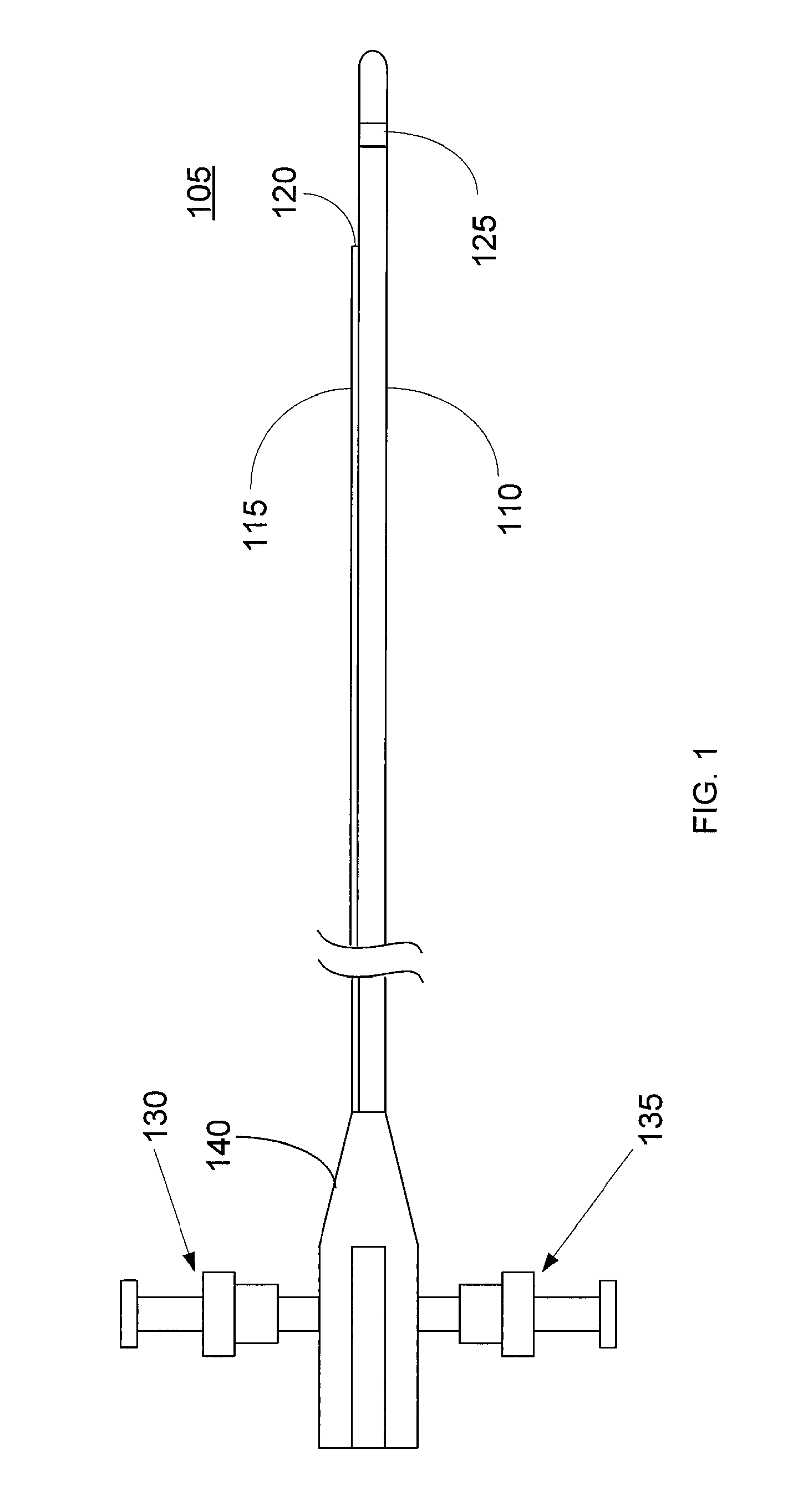
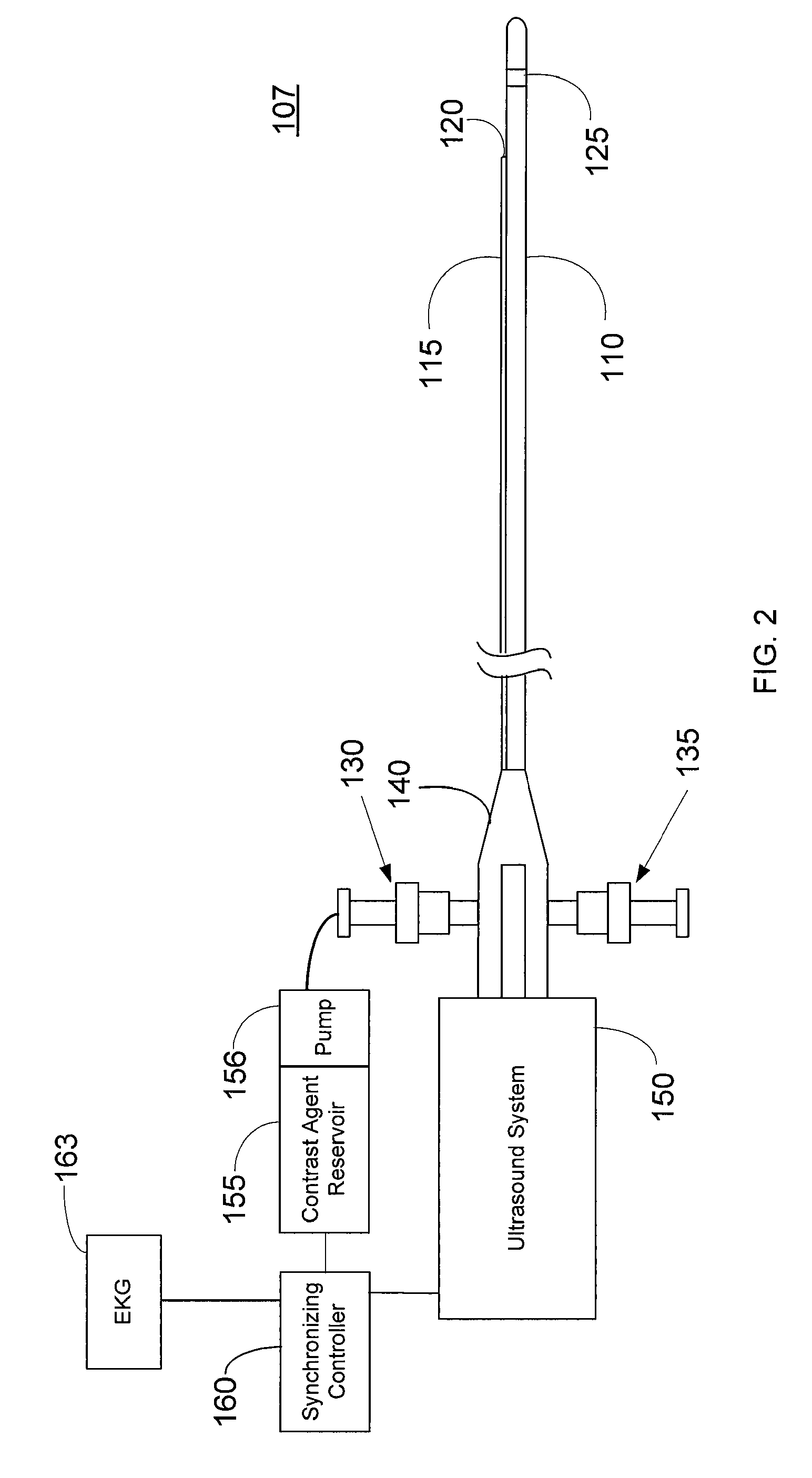
Imaging Catheter With Integrated Contrast Agent Injector
InactiveUS20090234231A1Lower the volumeSynchronization is simpleUltrasound therapyMulti-lumen catheterUltrasound imagingControlled drugs
Described herein are systems and methods that integrate the injection of contrast agents with imaging catheters. In an embodiment, an imaging catheter comprises a catheter sheath and an imager, e.g., ultrasound transducer. The imaging catheter further comprises a contrast lumen having one or more exit ports for injecting contrast agent into the patient. The contrast lumen extends along the catheter sheath and may be external to or integrated into the catheter sheath. Preferably, the exit port of the contrast lumen is positioned along the catheter sheath at a relatively short known distance from the imager. The catheter may include multiple contrast lumens for injecting different types of contrast agents. In an embodiment, a synchronizing controller is provided to automatically synchronize the injection of contrast agent with imaging. In another embodiment, drug-filled microbubbles in combination with ultrasound imaging are used to deliver a controlled drug dose to a specific treatment site.
Owner:BOSTON SCI SCIMED INC
Patient controlled drug delivery device
InactiveUS7335186B2Simple and inexpensive to manufactureSimple and inexpensive to and useInfusion devicesMedical devicesControlled drugsCatheter
Owner:ONEIL ALEXANDER GEORGE BRIAN & ONEIL CHRISTINE JOINTLY
Medical devices having porous polymeric regions for controlled drug delivery and regulated biocompatibility
Owner:BOSTON SCI SCIMED INC
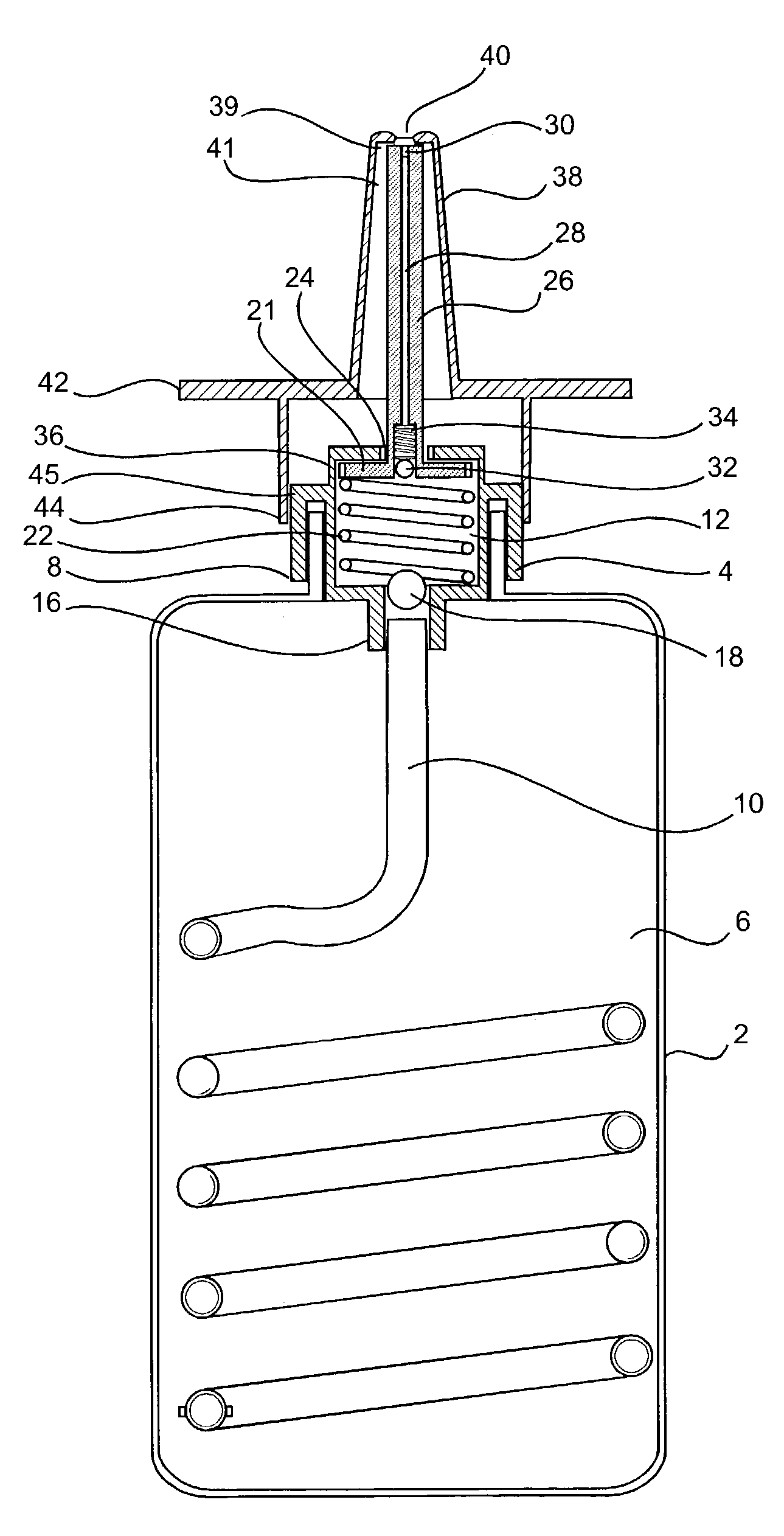
Ceramic structures for controlled release of drugs
The present invention provides compositions for controlled drug delivery, dosage forms, and processes for producing dosage forms. In a composition aspect of the present invention, a composition including a drug and a ceramic structure is provided. The ceramic structure has either a hollow portion wherein the drug is included in the hollow portion or is a collection of smaller particles bound together.
Owner:SPECTRUM PHARMA INC
Vaginal matrices: nanofibers for contraception and prevention of HIV infection
Described are drug delivery systems incorporating electrospun fibers that comprise and deliver physicochemically diverse drug compounds. Such fibers provide significant advantages in drug agent release, such as adaptability for solid dosage delivery to mucosal tissues. This is in addition to allowing for controlled drug release. Systems and methods for large-scale electrospinning productivity are described, including novel microarchitectures allowing for variable pharmacokinetics in drug release.
Owner:UNIV OF WASHINGTON CENT FOR COMMERICIALIZATION
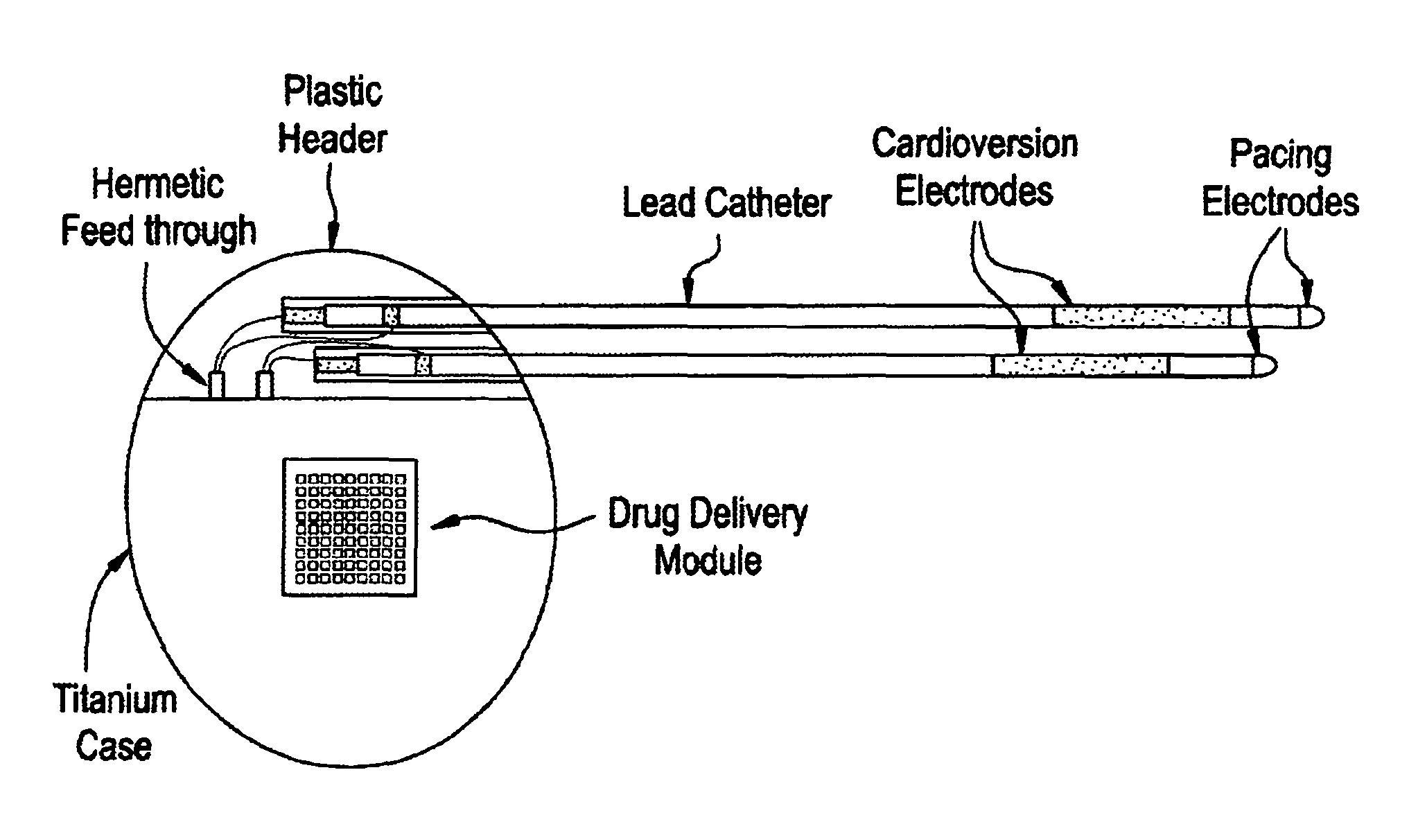
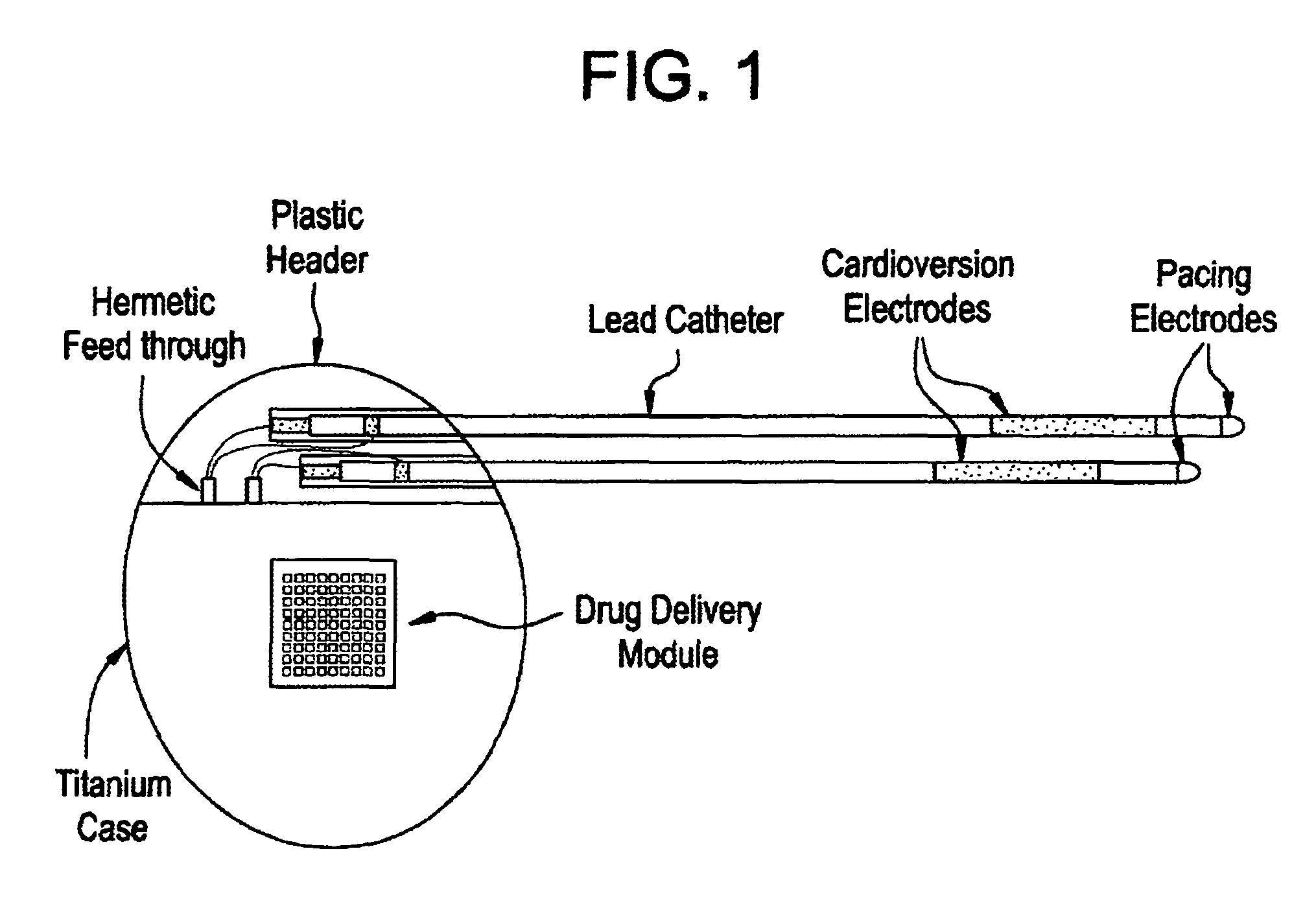
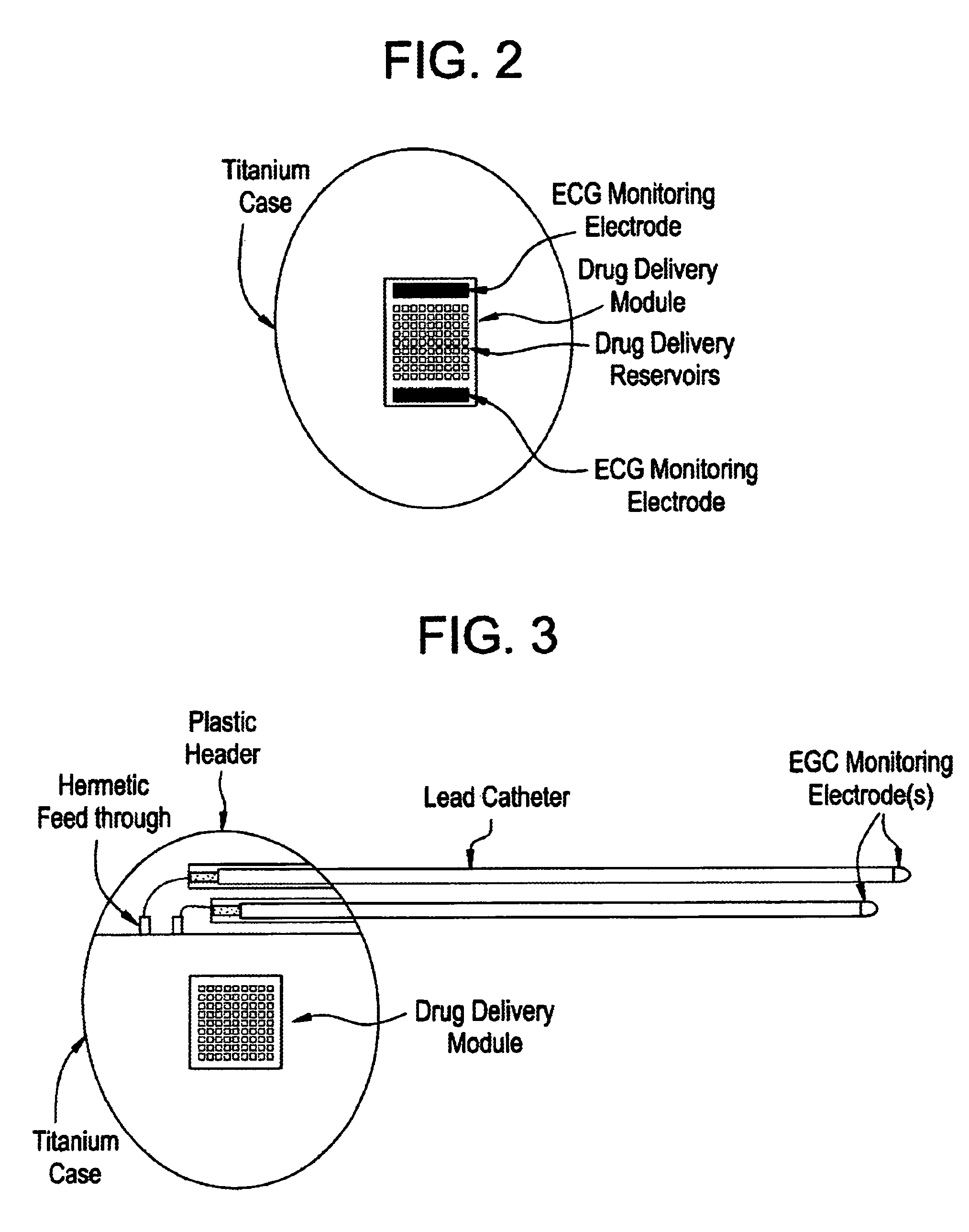
Medical device for controlled drug delivery and cardiac monitoring and/or stimulation
InactiveUS7917208B2Reduce frequencyHigh sensitivityElectrocardiographyHeart defibrillatorsMicrocontrollerCardioversions
Medical device and methods are provided for controlled drug delivery in a cardiac patient. The device includes an implantable drug delivery module comprising reservoirs containing a drug and a control means for selectively releasing an effective amount of drug from each reservoir; one or more electrodes or sensors for cardiac monitoring, stimulation, or both; and a microcontroller for controlling operational interaction of the drug delivery module and the cardiac electrode. The electrodes may comprise ECG monitoring, cardioversion, or cardiac pacing electrodes. A medical device also is provided for controlled delivery of drug to a patient having congestive heart failure, which includes an implantable drug delivery module comprising a natriuretic peptide and a release mechanism for selectively releasing a pharmaceutically effective amount of the natriuretic peptide into the patient; and a microcontroller for controlling the release mechanism, for example, in response to one or more monitored patient parameters.
Owner:MICROCHIPS BIOTECH INC
Medical device with sponge coating for controlled drug release
The medical devices of the invention comprise an expandable portion which is covered with a sponge coating for release of at least one biologically active material. The sponge coating is made of a non-hydrogel polymer having a plurality of voids. The device can further include means for infusing or expelling the biologically active material or drug into the voids. The drug is delivered to the body lumen of a patient by expelling the drug and inflating or expanding the expandable portion of the catheter or device.
Owner:BOSTON SCI SCIMED INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com