Fusion protein, and canine toxoplasma subunit vaccine and vaccine composition thereof
A technology of fusion protein, Toxoplasma gondii, applied in the fields of immunology and biology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: The construction of prokaryotic expression vector of Toxoplasma gondii recombinant protein, according to NCBI (http: / / www.ncbi.nlm.nih.gov) Toxoplasma gondii cyclophilin (cyclophilin, CyP) its accession number: KYF42711.1, altogether Contains 179 amino acids, using it as a reference, after analyzing with DNAstar software, remove the 2nd to 17th amino acids and the 173rd to 179th amino acids, and remove the random coil sequences at both ends that have little influence on the protein domain. It has little effect on the biological function of the protein and can also improve the stability of the protein. Replacing amino acids 116-125 with ENAGVRKAYM can effectively improve the soluble expression efficiency of the protein in Escherichia coli. The final amino acid sequence is Seq ID NO: 2, and a T cell is coupled behind the Toxoplasma gondii cyclophilin mutant Epitope, forming the fusion protein of toxoavidin mutant, its amino acid sequence is Seq ID NO:6. Acc...
Embodiment 2
[0040] Embodiment 2: Expression and purification of Toxoplasma gondii CyP fusion protein
[0041] (1) Soluble expression of Toxoplasma CyP fusion protein
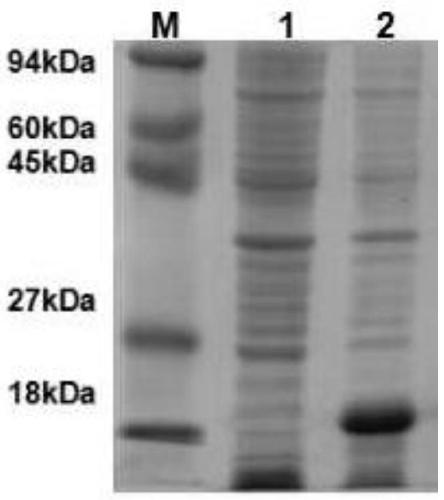
[0042] The single colony of E.coli BL21 (DE3) transformed with the recombinant plasmid pET-28a-CyP was used as the engineering strain (BL21(DE3)-pET-28a-CyP) of the canine Toxoplasma gondii vaccine, and the pET-28a empty vector transformed The single colony of E.coli BL21(DE3) was the blank control strain (BL21(DE3)-pET-28a), and the above two strains were respectively inoculated in 50 μg / mL kanamycin containing LB liquid medium 100mL, cultured at 37°C, 180rpm for 6-8h, to make the bacteria OD 600 Between 0.6-1.0, add IPTG to make the final concentration 0.2mmol / L, continue to cultivate for 5h, collect the cells by centrifugation, suspend the cells with 10mL soluble protein lysate, add 100μL of 0.1M PMSF, freeze and thaw the cells repeatedly 2 After one time (-80°C, 1h / 37°C, 10min), 400w ultrasonic treatment for 30min, ce...
Embodiment 3
[0045] Embodiment 3: western blot identification
[0046] The fusion protein induced and expressed by BL21(DE3)-pET-CyP engineered bacteria was identified by western blot. SDS-PAGE electrophoresis. Membrane transfer: Cut out the PVDF membrane according to the size of the gel (slightly larger than the size of the gel), and immerse the gel together with the membrane transfer buffer to equilibrate for 10 minutes. According to filter paper / gel / PVFD membrane / filter paper, the gel side is placed on the negative side. Calculate the magnitude of the current based on the area of the gel, turn on the power, and take out the PVDF membrane after 1 hour. Wash the PVDF membrane with TBST buffer, 5 minutes each time, shake slowly at room temperature, a total of three times. Transfer the PVDF membrane to blocking buffer and shake gently horizontally for 1 hour at room temperature. Add the PVDF membrane to the diluted Toxoplasma gondii antibody serum, shake slowly at room temperature for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com