Method of preparing hepatitis A inactivated vaccine
A technology for inactivated vaccines and hepatitis A, applied in biochemical equipment and methods, pharmaceutical formulas, and resistance to vector-borne diseases, etc., can solve the problem that hepatitis A vaccines cannot meet the domestic market demand, and improve vaccine production capacity and increase Virus yield, the effect of achieving large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Human embryonic lung diploid cells (MRC-5) cultured in a monolayer cell factory were taken, the cells were digested with 0.125w / v% trypsin-EDTA, and MEM cell culture medium containing 10% calf serum was added. Wherein, the MEM is 0.03% Glu, 0.08% NaHCO 3 , 0.01% penicillin and 0.01% streptomycin. MEM was purchased from Beijing Qingda Tianyi Biotechnology Co., Ltd., production batch number: 080302.
[0030] Make the cell concentration 1-1.5 million cells per milliliter, add hepatitis A virus to the cell solution at 0.05-0.1 MOI, and carry out mixed adsorption at 20-30°C for 30-90 minutes. Dilute the mixed and adsorbed cell-virus mixture 10 times with MEM cell culture medium containing 10% calf serum, then inoculate it at 0.5 MOI in cell factories on layers 2-40 to proliferate hepatitis A virus, and culture it statically at 35°C±0.5°C for 21 -24 days, during which the cell maintenance solution was changed every 7 days (the cell maintenance solution was MEM cell culture ...
Embodiment 2
[0036]Human embryonic lung diploid cells (MRC-5) cultured in a monolayer cell factory were taken, the cells were digested with 0.125w / v% trypsin-EDTA, and MEM cell culture medium containing 10% calf serum was added. Wherein, the MEM is 0.03% Glu, 0.08% NaHCO 3 , 0.01% penicillin and 0.01% streptomycin. MEM was purchased from Beijing Qingda Tianyi Biotechnology Co., Ltd., production batch number: 080302.
[0037] Make the cell concentration 1-1.5 million cells per milliliter, add hepatitis A virus to the cell solution at 0.05-0.1 MOI, and carry out mixed adsorption at 20-30°C for 30-90 minutes. Dilute the mixed and adsorbed cell-virus mixture 10 times with MEM cell culture medium containing 10% calf serum, then inoculate it at 0.5 MOI in cell factories on layers 2-40 to proliferate hepatitis A virus, and culture it statically at 35°C±0.5°C for 21 -24 days, during which the cell maintenance solution was changed every 7 days (the cell maintenance solution was MEM cell culture s...
experiment example 3
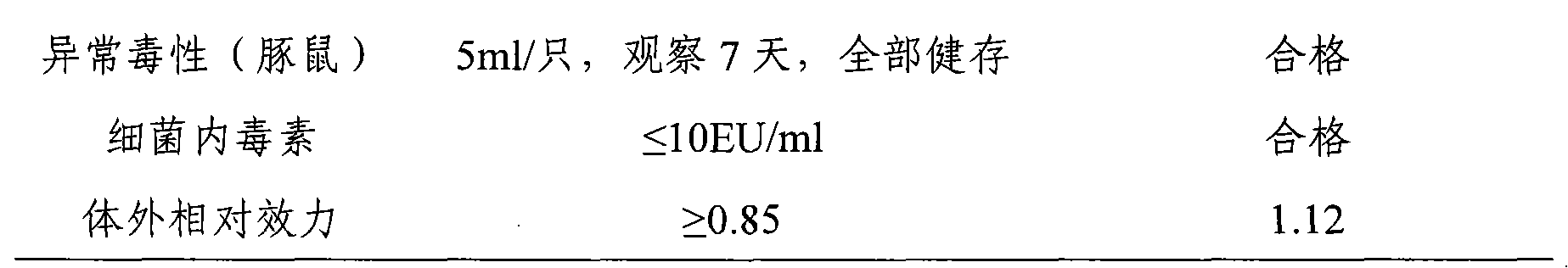
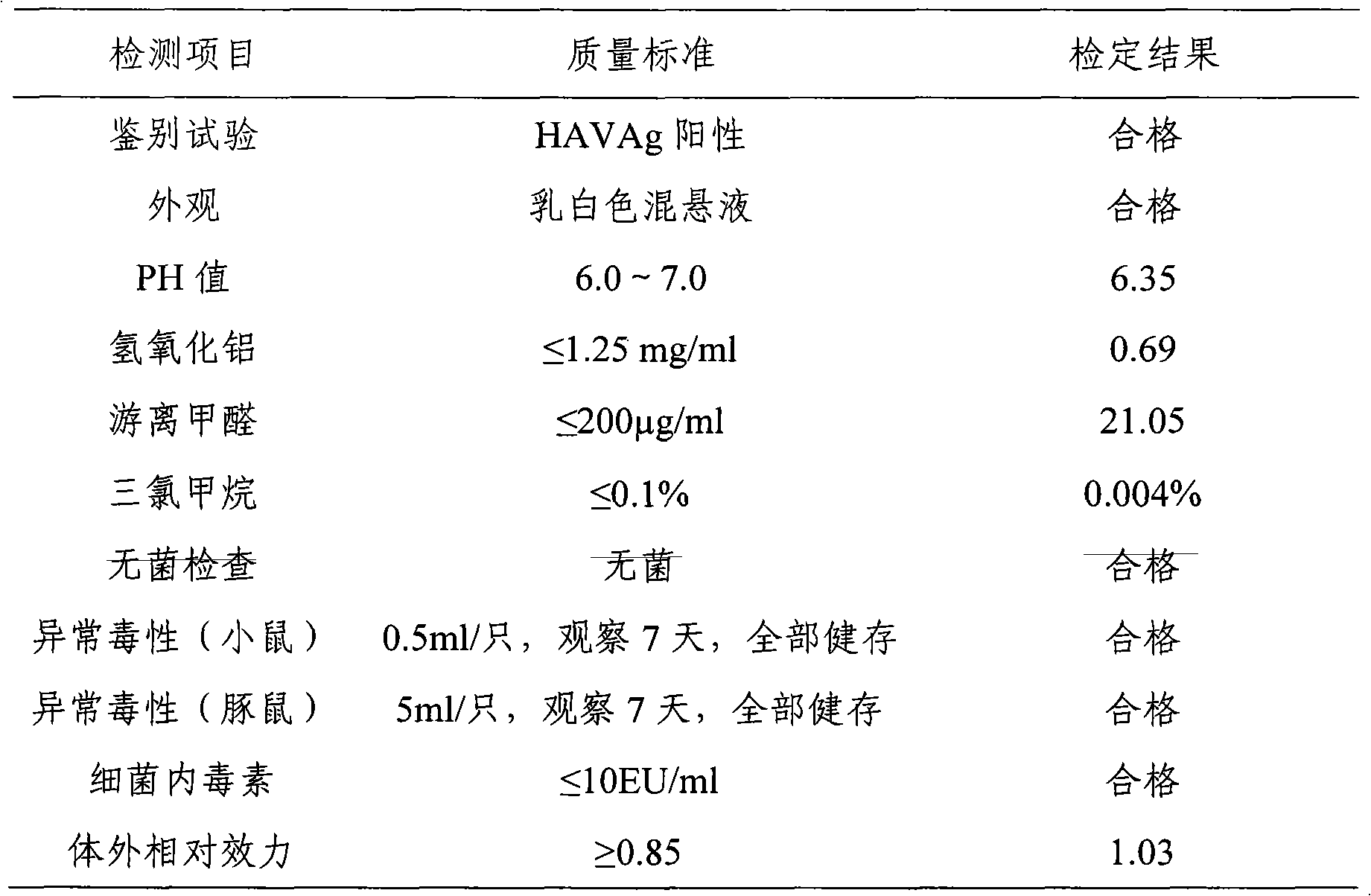
[0042] The Hepatitis A vaccine prepared in Example 1 and Example 2 of the present invention was subjected to an in vivo efficacy test in mice, the endpoint dilution was calculated, and the immunogenicity was observed.
[0043] km mice (purchased from the Institute of Pathogenic Microbiology, Chinese Academy of Military Medical Sciences), male and female, weighing 16-18 g, were randomly divided into groups. Test is divided into 12 groups, every group is 10, and test vaccine 8 groups: inject respectively the vaccine that embodiment 1 and embodiment 2 prepare; , Ai Basu: Product batch number: 3001424.02). The test vaccine and the reference vaccine were serially diluted 4 times with the vaccine diluent, totaling 4 dilutions: original times, 1:4, 1:16, 1:64. Each mouse was intraperitoneally injected with 1.0ml, and after 4 weeks of immunization, blood was collected from the inner canthus of the eye, the serum was separated by centrifugation, and stored at -20°C for future use. Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com