Protein marker for simultaneously detecting specificity of multiple diseases in blood serum and kit thereof
A technology of protein markers and kits, which is applied in the field of protein markers and kits, can solve the problems of complex, time-consuming, and large sample volume in the pre-processing of sputum samples
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
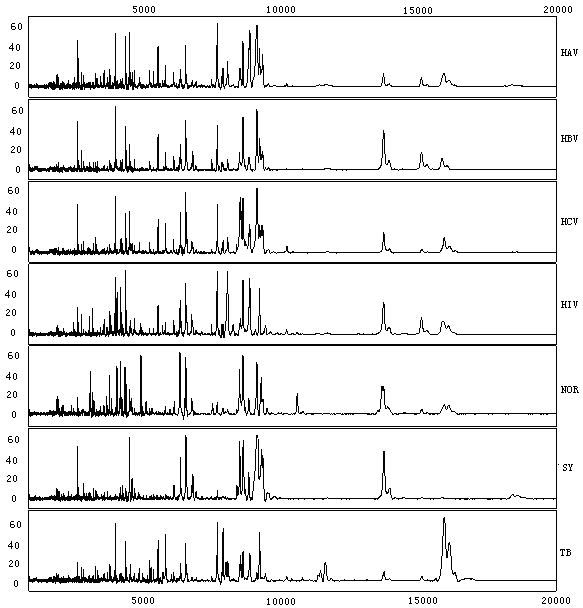
[0121] At the same time, the extraction of protein markers specific to hepatitis A, hepatitis B, hepatitis C, AIDS, syphilis, and tuberculosis in serum is detected.
[0122] Instruments and reagents used: nano-magnetic beads with carboxyl as a ligand: purchased from Bruker Company, product number MBWCX; lysate: 9M Urea, mass ratio of 2% CHAPS, 50mM Tris-HCl, pH9.0 purchased from SIGMA Company , product number U0631, C5070, T2819; buffer: 50mM NaAC, pH4.0 purchased from SIGMA Company, product number S7545; deionized water: purchased from SIGMA Company, product number 27,073-3; sinapinic acid: purchased from SIGMA Company, product number S5019; Trifluoroacetic acid (TFA): purchased from SIGMA, product number T6508; protein reader: purchased from BIORAD company, product number PBSIIc; quality control serum: purchased from BIORAD company, product number StandardS10.
[0123] (1) Serum samples: A total of 230 serum samples were collected from Liaoning International Travel Health Ca...
Embodiment 2
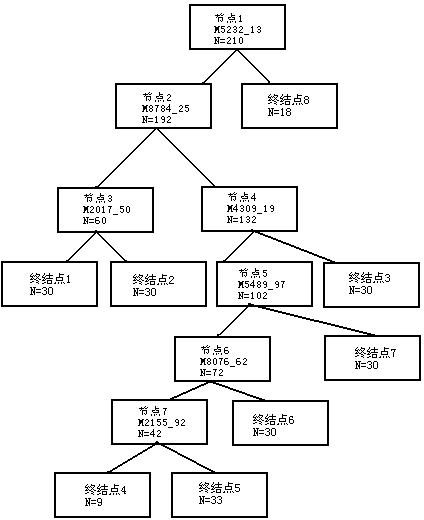
[0135] Simultaneous detection of multiple diseases in serum The composition of the kit:
[0136] 1) Nano magnetic beads with carboxyl as ligand;
[0137] 2) Lysis solution: 9M Urea, 2% CHAPS, 50mM Tris-HCl, pH9.0;
[0138] 3) Buffer: 50mM NaAC, pH4.0;
[0139] 4) Deionized water;
[0140] 5) sinapic acid;
[0141] 6) Trifluoroacetic acid;
[0142] 7) Protein reader;
[0143] 8) Quality control serum.
Embodiment 3
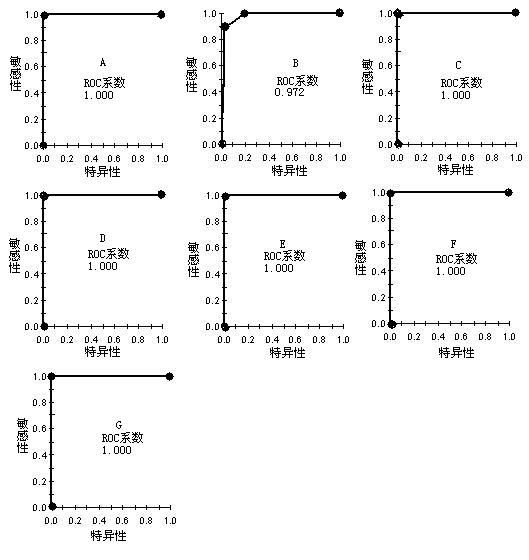
[0145] Simultaneous clinical trial of rapid detection of infectious disease protein marker kit.
[0146] (1) Serum samples: 120 cases of infectious diseases (20 cases each of hepatitis A, hepatitis B, hepatitis C, AIDS, syphilis and tuberculosis) and 30 normal healthy samples.
[0147] (2) Collection of serum samples: Collect 5 mL of whole blood from the patient on an empty stomach in the morning, place it in a vacuum negative pressure tube without anticoagulant, let it stand at room temperature for 1 hour, centrifuge at 2000g for 20 minutes, and take the supernatant. Aliquot into 50μl / tube and store in -80℃ refrigerator.
[0148] (3) Sample preparation: Take out the serum from the -80°C refrigerator and put it on ice to thaw. Centrifuge at 10000rpm for 2min. 10 μl of the supernatant was mixed with 20 μl of U9 lysate (9 mol / L Urea, 20 g / L CHAPS, 10 g / L DTT, 50 mmol Tris-HCl, pH 9.0). After shaking and incubating at 4°C for 30 min, 370 μl of sodium acetate buffer (100 mmol / L s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com