Human interferon analogue with long-lasting biological effects
A technology of interferon and human blood, applied in the field of long-acting human interferon analogs, can solve the problems of cytokine disappearance and interferon inactivation, and achieve the effect of easy transportation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Example 1 Brief description of molecular cloning technology
[0093] Conventional molecular cloning techniques include DNA and RNA extraction, agarose gel and polyacrylamide gel electrophoresis, DNA fragment ligation, and restriction enzyme digestion reactions. All refer to the literature (Maniatis et al., "Molecular Cloning Experiment Manual" Cold Spring Published by Port Laboratory, Cold Spring Harbor, New York, 1982). DNA polymerase chain reaction (PCR) (reference Saikiet et al., Science, 230:1350, 1985) used enzymes and PCR machines required for the reaction are all PerkinElmer products. And refer to the manufacturer's operating procedures. The oligonucleotide primers required for DNA sequencing and DNA amplification are completed by specialized agencies. Transgenic Escherichia coli was purchased from GIBCO / BRL. The purification of plasmid DNA and the recovery of DNA fragments are all prepared using commercial Qiagen purification columns. The yeast strains used in the p...
Embodiment 2
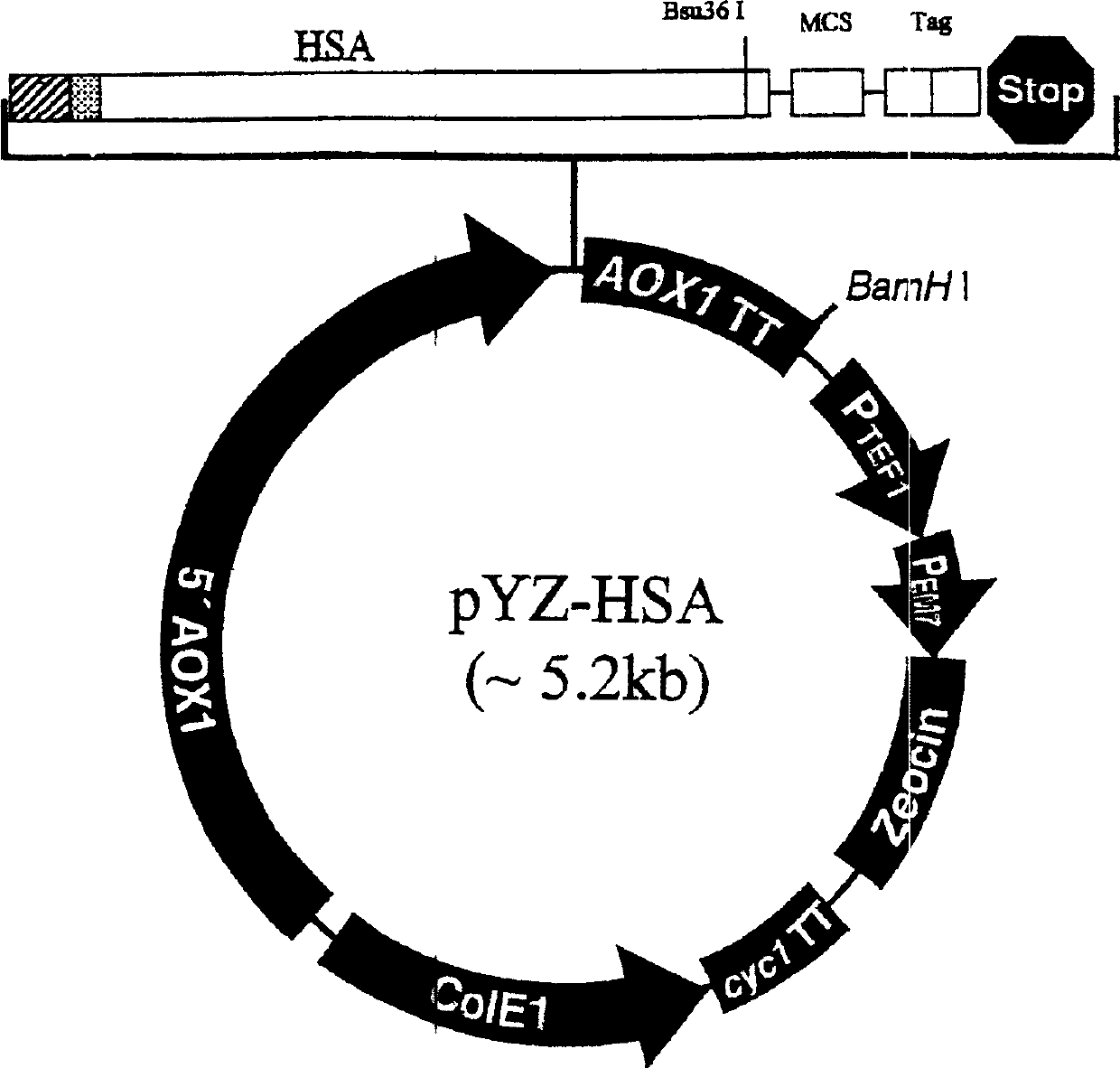
[0094] Example 2 Expression of Human Serum Albumin (HSA) Gene and Construction of Vector Plasmid
[0095] Using total RNA extracted from human fetal liver as a template, the HSA gene was synthesized and amplified by reverse transcription polymerase chain polymerization. 5 micrograms of total RNA are synthesized by reverse transcriptase (purchased from GIBCO / BRL company) to synthesize corresponding DNA strands. The oligonucleotide primers are 18 T+1N (N is random nucleotide). The reaction conditions were 45°C for 20 minutes, then the temperature was raised to 55°C, and the temperature was continued for 40 minutes.
[0096] The oligonucleotide primer sequence is:
[0097] Seq.ID NO.23: 5’- GAATTC ATGAAGTGGGTAACCTTTATTTCC-3’, and
[0098] Seq.ID NO.24: 5’- GAATTC TTATAAGCCTAAGGCAGCTTGACTT GC-3’
[0099] The oligonucleotide primers used to amplify HSA are designed according to the DNA sequence of V00494 in GenBank.
[0100] Two EcoRI recognition sites were added to both ends of the ...
Embodiment 3
[0104] Example 3. Molecular cloning of human interferon
[0105] 3.1. Molecular cloning of human interferon-α-1b
[0106] The molecular cloning of human interferon-α-1b was amplified from the total RNA preparation of human leukocytes (monocytes / macrophages / B lymphocytes) using the RT-PCR method similar to that described in Example 2. The oligonucleotide primers are:
[0107] SEQ ID NO. 25: 5'- CATATG TGTGATCTCCCTGAGACCC-3’
[0108] SEQ ID NO. 26: 5'- GGATCC TTACTTCCTCCTTAATCTTTC-3’
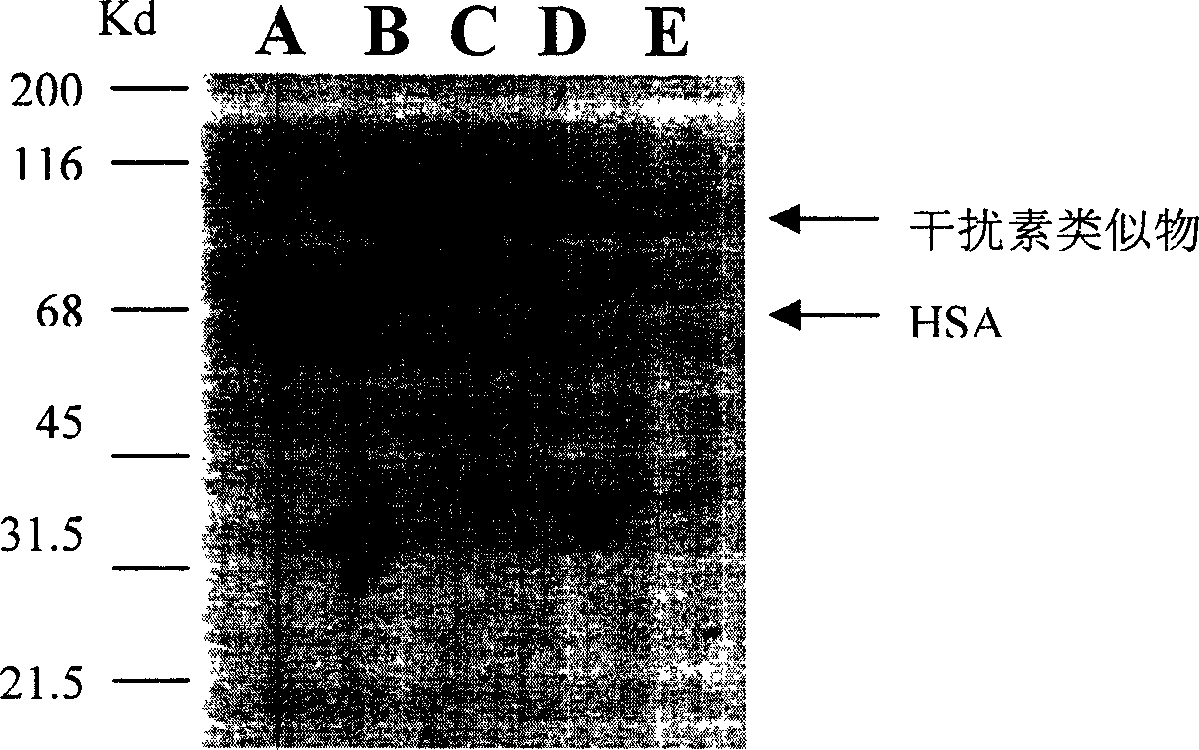
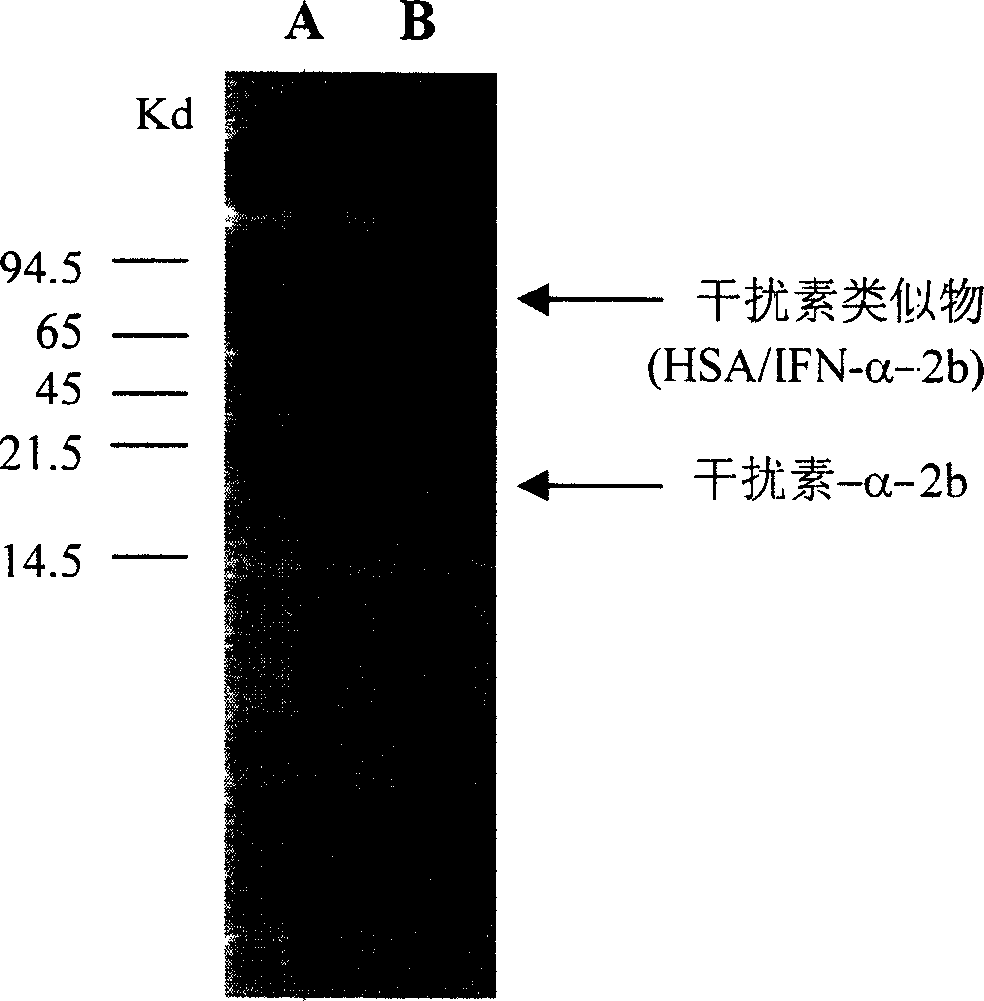
[0109] The polynucleotide has 579 base pairs, amplified by RT-PCR, and subcloned into Invitrogen's pCRIITA vector. DNA sequencing analysis confirmed the reading frame of human interferon-α-1b. The Nde I restriction enzyme site forms the 5'end, and the Bam HI restriction enzyme site forms the 3'end (the line part). The artificial start codon of interferon-α-1b is also included in this site (the line part of SEQ ID NO. 25). Figure 1 shows the DNA sequence (SEQ ID NO. 13) of human interferon α-1b and i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com