Freeze-drying hepatitis A varicella attenuation combined vaccine and producing method thereof
A hepatitis A and combined vaccine technology, applied in freeze-dried delivery, antiviral agents, drug combinations, etc., to prevent hepatitis A and chickenpox, reduce market prices, and reduce adverse reactions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
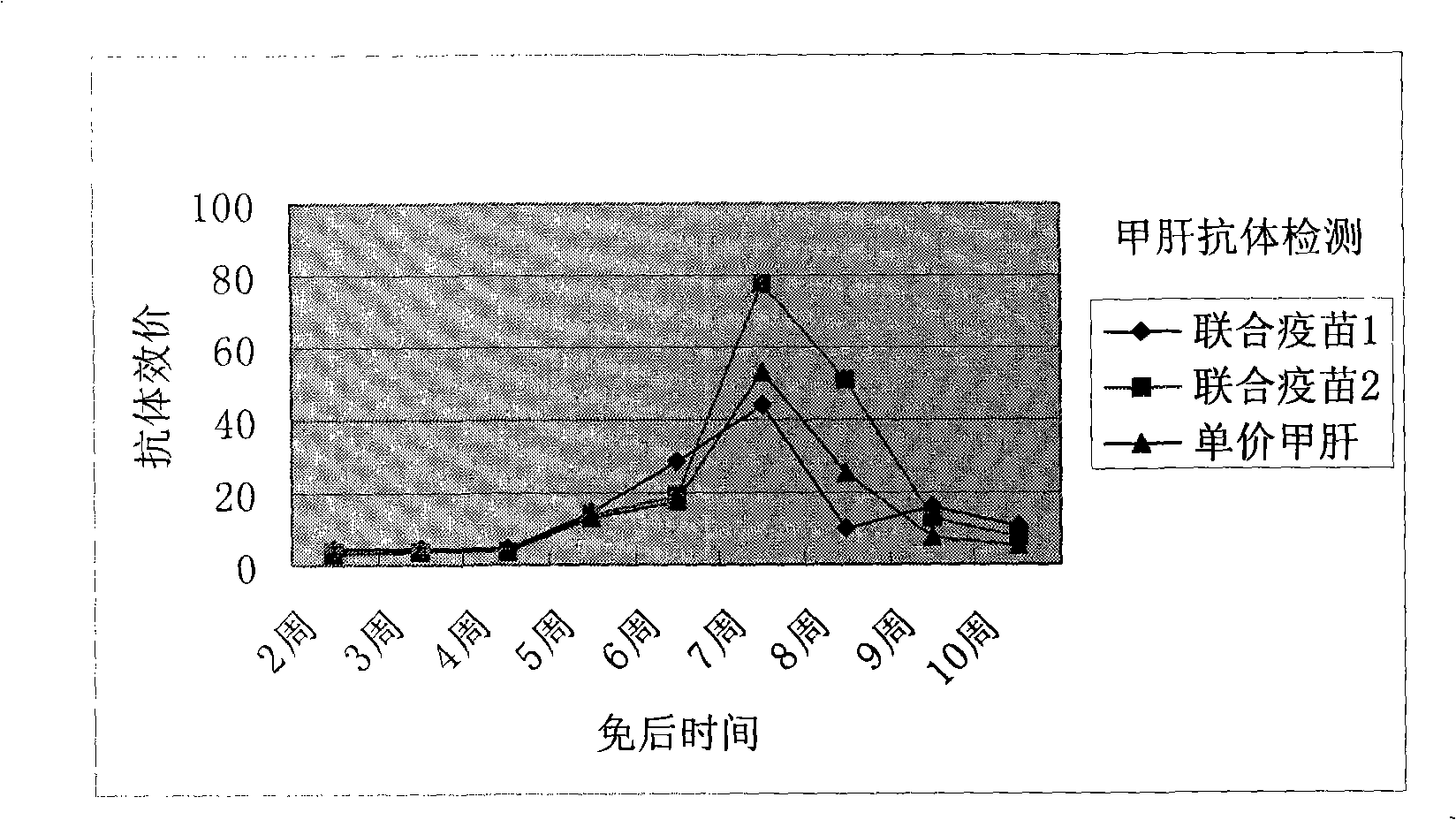
Image
Examples
Embodiment 1
[0051] Preparation of Freeze-dried Hepatitis A-varicella Attenuated Combined Vaccine
[0052] With the stoste of 1.2L hepatitis A vaccine virus (L-A-1 strain, virus titer is 8.0LogCCID 50 / ml) was mixed with the stock solution (6000PFU) of 2.4L varicella vaccine virus, then added the protective agent stock solution prepared as follows: protective agent component A550ml, protective agent component B600ml and protective agent component C450ml. Then, add an appropriate amount of 199 comprehensive solution as the virus vaccine matrix solution to the mixed solution obtained in this way, so that the total volume of the liquid combined vaccine reaches 7200ml, so that the hepatitis A vaccine virus titer is not less than 6.5LogCCID wherein 50 / ml, the virus titer of varicella vaccine is not less than 4000PFU / ml. As a result of such dilution, the concentration (w / v) of each component of the protective agent added in the vaccine solution reached respectively: trehalose 42g / l, dextran 18...
Embodiment 2
[0056] Preparation of Freeze-dried Hepatitis A-varicella Attenuated Combined Vaccine
[0057] With the stoste of 1.2L hepatitis A vaccine virus (L-A-1 strain, virus titer is 8.0LogCCID 50 / ml) mixed with the stock solution (6000PFU / ml) of 2.4L varicella vaccine virus, then add the protective agent stock solution prepared as follows: protective agent component A550ml, protective agent component B900ml and protective agent component C900ml. Then, add an appropriate amount of 199 comprehensive solution as the virus vaccine matrix solution to the mixed solution obtained in this way, so that the total volume of the liquid combined vaccine reaches 7200ml, so that the hepatitis A vaccine virus titer is not less than 6.5LogCCID wherein 50 / ml, the virus titer of varicella vaccine is not less than 4000PFU / ml. As a result of such dilution, the concentration (w / v) of each component of the protective agent added in the vaccine solution reached respectively: trehalose 62.5g / l, dextran 37....
Embodiment 3
[0061] Preparation of Freeze-dried Hepatitis A-varicella Attenuated Combined Vaccine
[0062] With the stoste of 2.4L hepatitis A vaccine virus (L-A-1 strain, virus titer is 8.0LogCCID 50 / ml) mixed with the stock solution (6000PFU / ml) of 2.4L varicella vaccine virus, then add the protective agent stock solution prepared as follows: protective agent component A550ml, protective agent component B900ml and protective agent component C900ml. Then, add an appropriate amount of 199 comprehensive solution as the virus vaccine matrix solution to the mixed solution obtained in this way, so that the total volume of the liquid combined vaccine reaches 7200ml, so that the hepatitis A vaccine virus titer is not less than 6.5LogCCID wherein 50 / ml, the virus titer of varicella vaccine is not less than 4000PFU / ml. As a result of such dilution, the concentration (w / v) of each component of the protective agent added in the vaccine solution reached respectively: trehalose 62.5g / l, dextran 37....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
| Titer | aaaaa | aaaaa |
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com