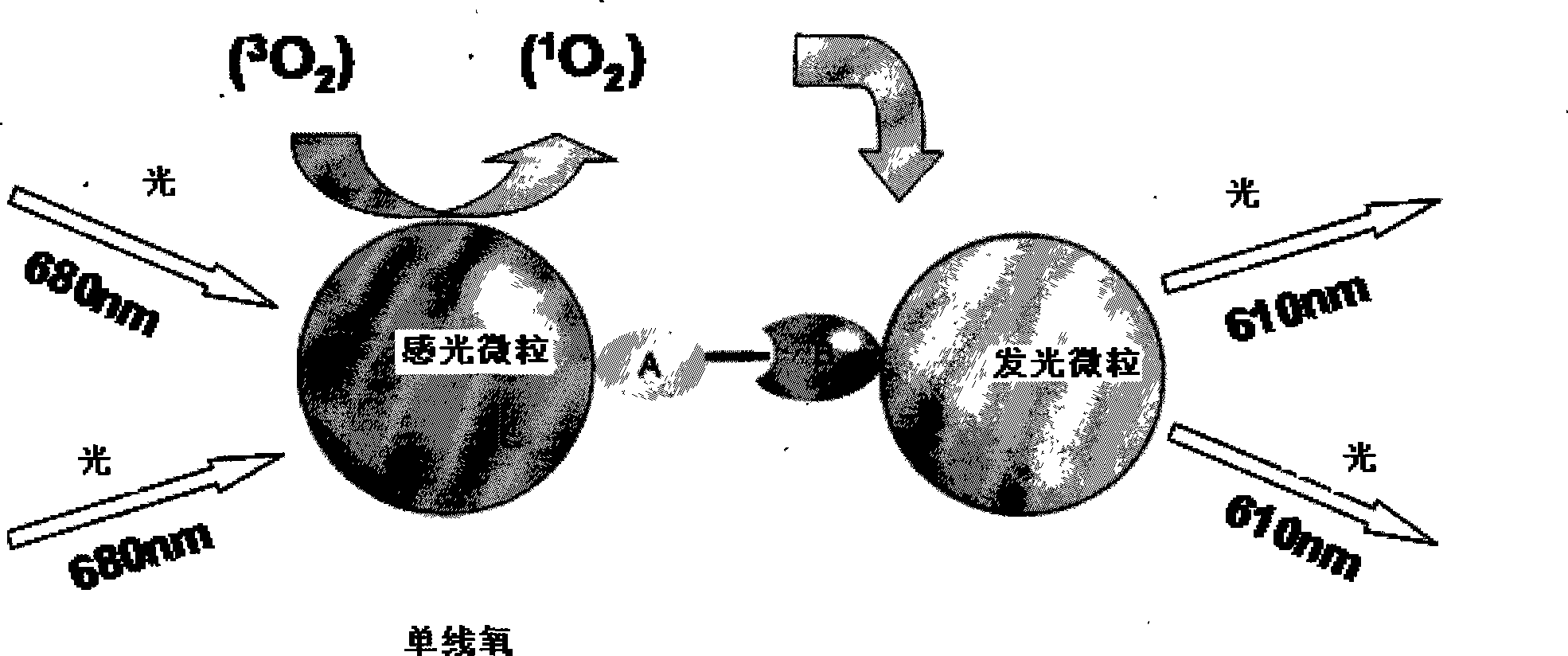Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
189 results about "Vitro diagnostics" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In vitro diagnostics are tests done on samples such as blood or tissue that have been taken from the human body. In vitro diagnostics can detect diseases or other conditions, and can be used to ...
Method for the in vitro diagnosis of alzheimer's disease using a monoclonal antibody
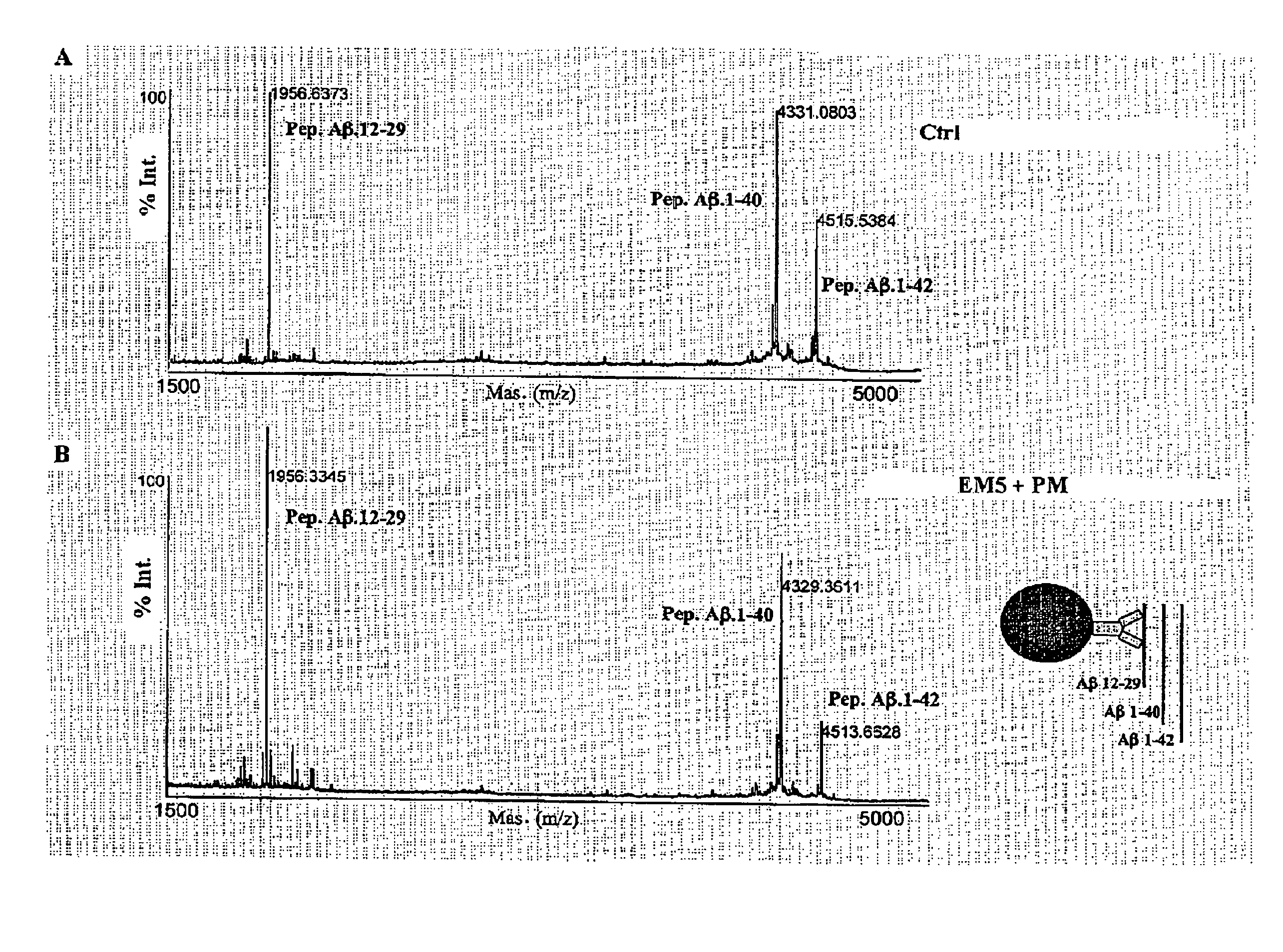
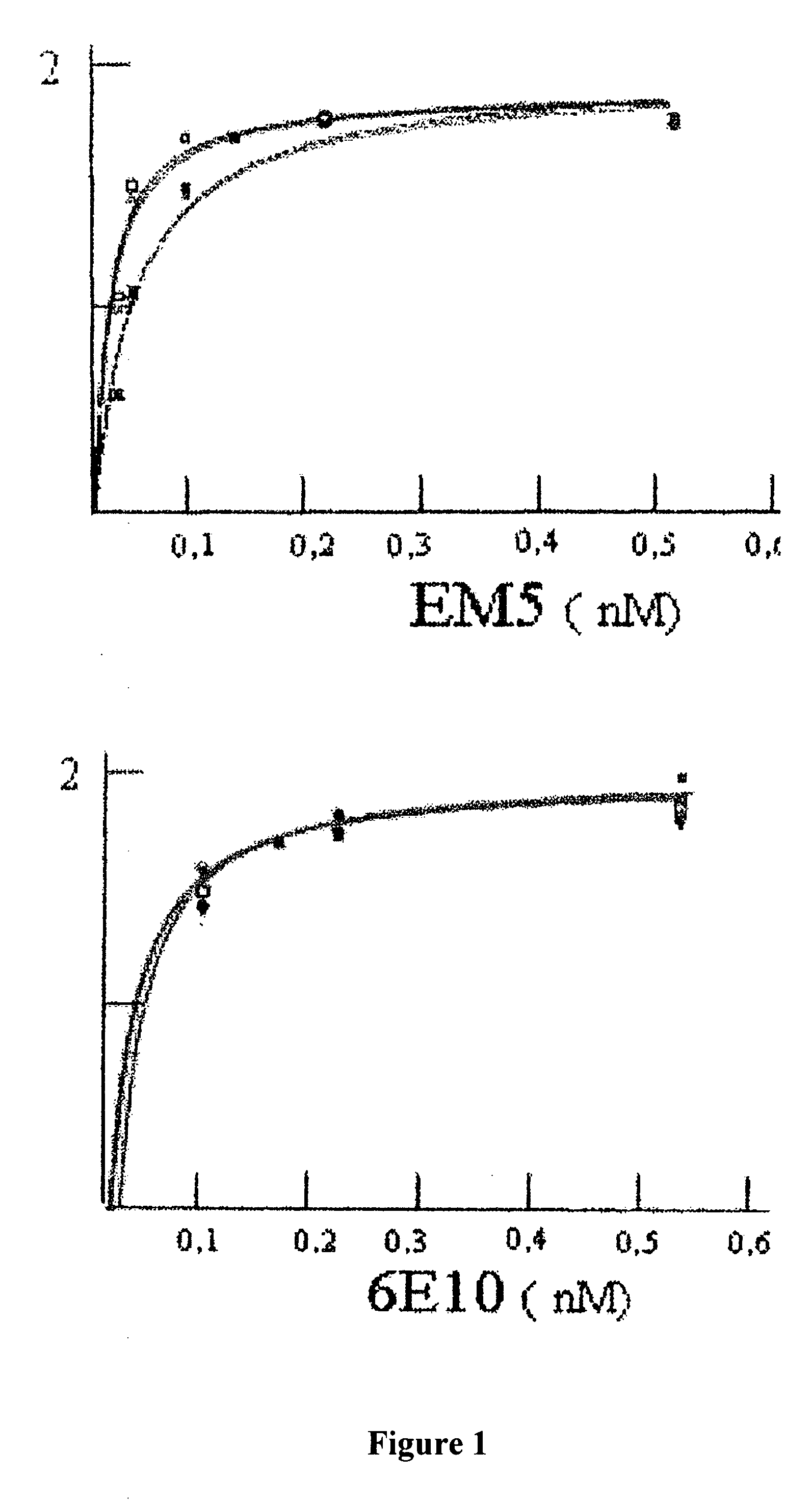
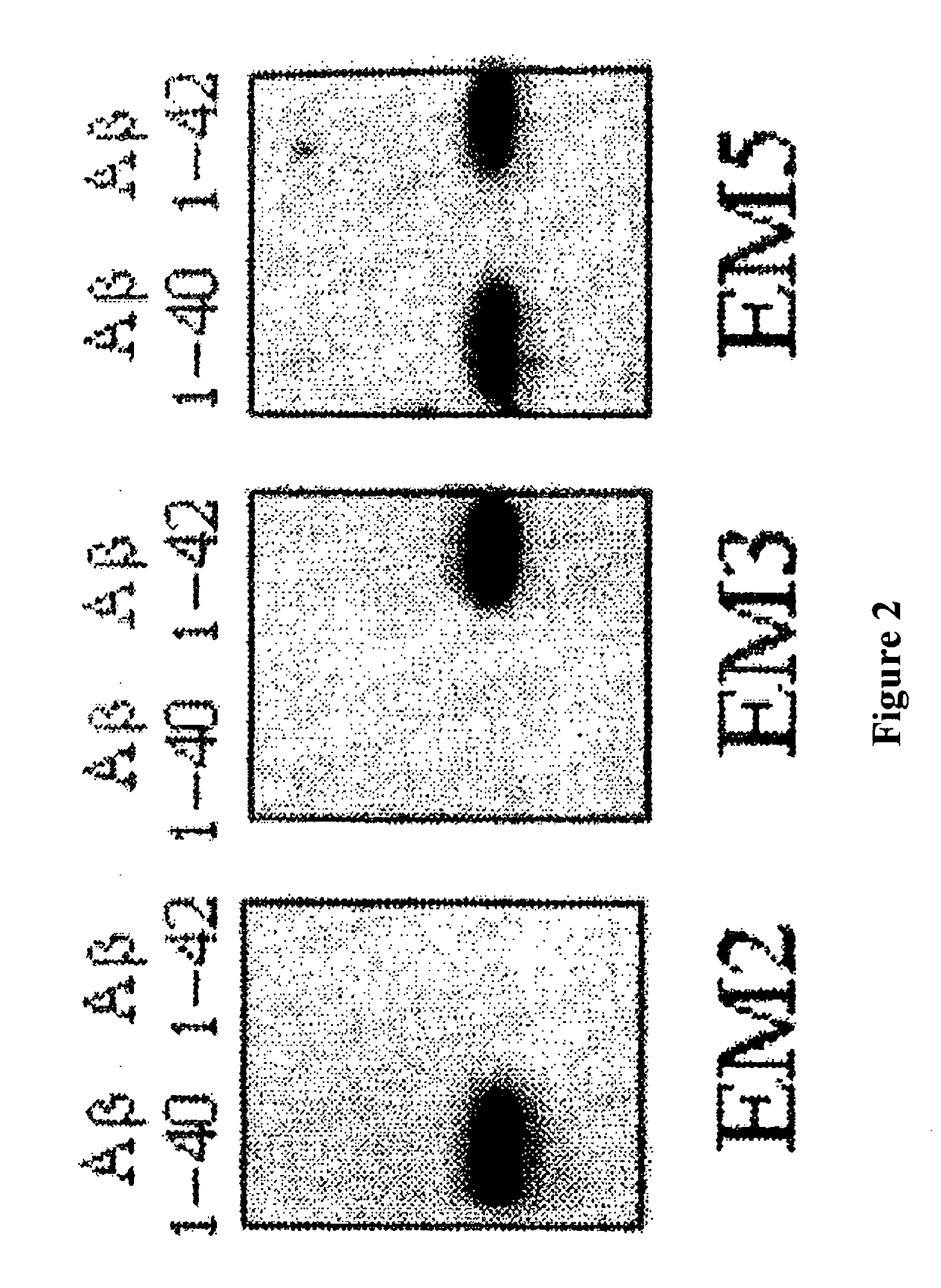
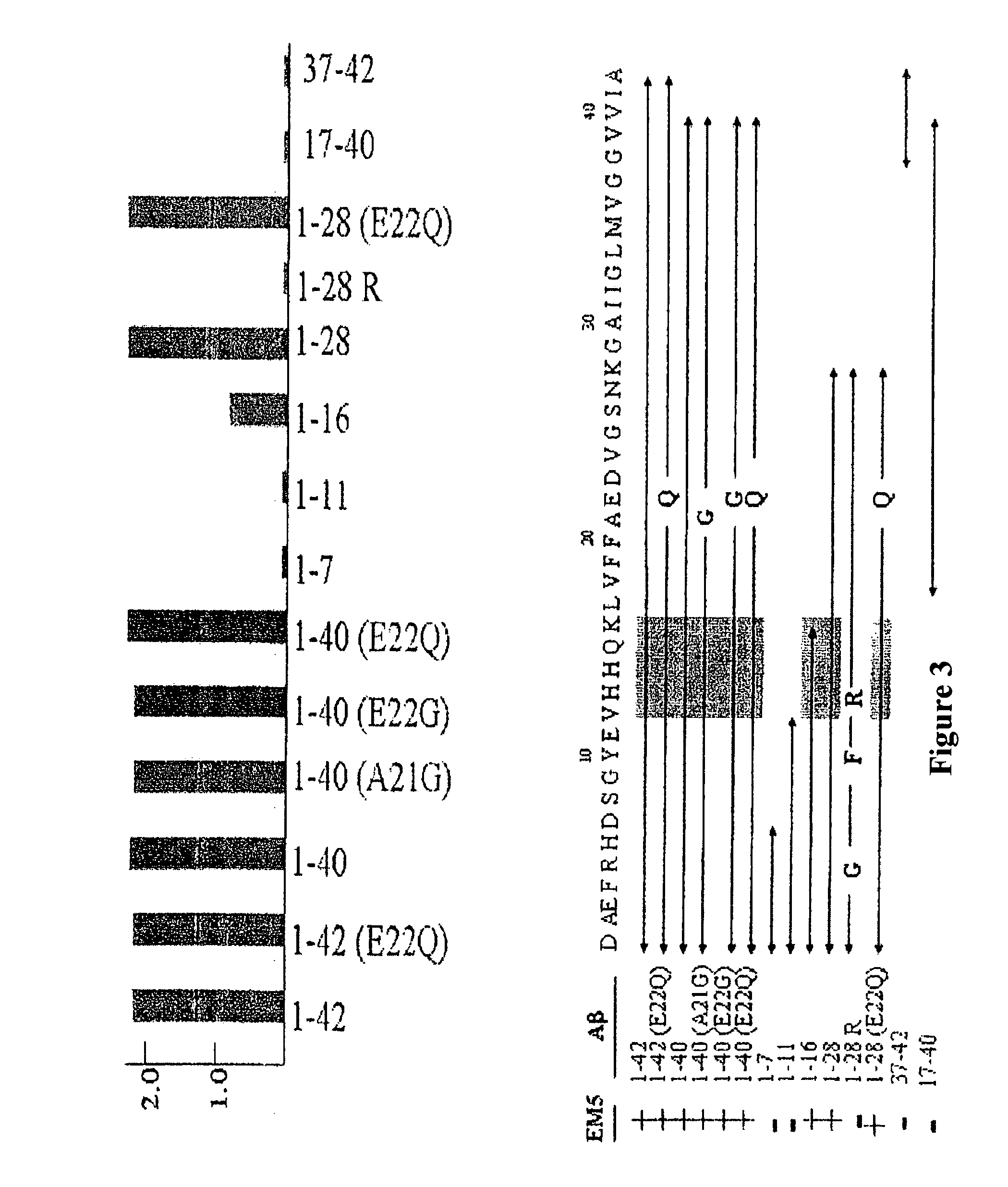
The invention relates to a method for the in vitro diagnosis of Alzheimer's disease using a monoclonal antibody. Said antibody can bind at least to amino acids 12-16 of the β-amyloid peptide, specifically detecting the neuritic plaques which are characteristic of Alzheimer's disease, without detecting diffuse plaques which are not defining characteristics of the disease. Within the neuritic plaques, the monoclonal antibody can detect a different sub-group in the composition of the different deposited isoforms of the β-amyloid peptide, which is associated with the disease progression stage. In addition, the antibody can bind to isoforms of the β-amyloid peptide in biological fluids such as urine. As a result, the inventive monoclonal antibody, the cell lines that produce said antibody and compositions containing same can be used in the in vitro diagnosis of Alzheimer's disease and in determining the disease progression stage.
Owner:CONSEJO SUPERIOR DE INVESTIGACIONES CIENTIFICAS (CSIC)
Hepatitis B virus e antigen testing corpuscle, preparation and application thereof
ActiveCN101251540AWide detection rangeReduce sensitivityAnalysis by material excitationAntigenAnti hbe
The invention relates to a diagnosing reagent for hepatitis B, disclosing detection grains for e antigens of the hepatitis B virus, which are of the luminous grains coated by anti-HBE antibodies. The invention also discloses preparation and application for the detection grains for e antigens of the hepatitis B virus; moreover, the invention further discloses an outside-body diagnosis reagent box for detecting e antigens of the hepatitis B in a blood serum sample of human beings as well as a method for utilizing the light excitation chemiluminescence principle to quantitatively and qualitatively detect e antigens of the hepatitis B virus. The reagent box of the invention can be jointly used to diagnose the individual acute or chronic hepatitis B together with other blood serums and clinic information, and screen the hepatitis B for women in the perinatal period so as to judge the risk of newborn babies contaminating the hepatitis B.
Owner:BEYOND DIAGNOSTICS (SHANGHAI) CO LTD
Method for in vitro diagnosing a complex disease
InactiveUS20120115138A1Microbiological testing/measurementBiostatisticsIschaemic encephalopathyOncology
The present invention relates to a method and kit for in vitro diagnosing a complex disease such as cancer, in particular, acute myeloid leukemia (AML), colon cancer, kidney cancer, prostate cancer; transient ischemic attack (TIA), ischemia, in particular stroke, hypoxia, hypoxic-ischemic encephalopathy, perinatal brain damage, hypoxic-ischemic encephalopathy of neotatals asphyxia; demyelinating disease, in particular, white-matter disease, periventricular leukoencephalopathy, multiple sclerosis, Alzheimer and Parkinson's disease; in a biological sample. For the diagnosis, use is made of measuring at least two different species of biomolecules and classifying the results by means of suitable classifier algorithms and other statistical procedures. With the present invention, a significant improvement of the reliability of e.g. expression profiles alone, are achieved. In other words, in a defined collective, an up to 100% accurate positive diagnosis could be achieved, which renders the method of the present invention superior over the prior art.
Owner:BIOCRATES LIFE SCIENCES AG
Method for the in vitro diagnosis of alzheimer's disease using a monoclonal antibody
InactiveUS7932048B2Easy extractionEasy to detectNervous disorderImmunoglobulins against animals/humansNeuritisMonoclonal antibody
The invention relates to a method for the in vitro diagnosis of Alzheimer's disease using a monoclonal antibody. Said antibody can bind at least to amino acids 12-16 of the β-amyloid peptide, specifically detecting the neuritic plaques which are characteristic of Alzheimer's disease, without detecting diffuse plaques which are not defining characteristics of the disease. Within the neuritic plaques, the monoclonal antibody can detect a different sub-group in the composition of the different deposited isoforms of the β-amyloid peptide, which is associated with the disease progression stage. In addition, the antibody can bind to isoforms of the β-amyloid peptide in biological fluids such as urine. As a result, the inventive monoclonal antibody, the cell lines that produce said antibody and compositions containing same can be used in the in vitro diagnosis of Alzheimer's disease and in determining the disease progression stage.
Owner:CONSEJO SUPERIOR DE INVESTIGACIONES CIENTIFICAS (CSIC)
Serum or plasma microrna as biomarkers for non-small cell lung cancer
ActiveUS20110117565A1Easy to collectWide detection coverageMicrobiological testing/measurementDiseaseTherapeutic effect
The present invention provides non-small cell lung cancer markers and the use thereof. The non-small cell lung cancer markers in the present invention include at least one of the 26 selected detectable mature microRNAs existing stably in human serum or plasma. The invention also provides a probe combination, kit and biochip for detecting the non-small cell lung cancer markers. The invention further provides a method for detecting microRNAs in the serum of lung cancer patients. By detecting the variations of microRNAs in the serum of lung cancer patients, the disease can be diagnosed in vitro; the progression course of the disease can be predicted; the occurrence of complications, the rate of relapse and the prognosis of the disease can be monitored; the drug efficacy and therapeutic effects can be analyzed. The method in the present invention enables extensive detection spectrum, high sensitivity, low cost, convenient sample taking and preservation. The method can be applied in the general survey of disease, solves problems of the low specificity and sensitivity encountered with previous single markers, and increases significantly the clinical detection rate of diseases, all of which make it an effective means for diagnosing diseases at an early stage.
Owner:MICROMEDMARK BIOTECH CO LTD
Primer in use for in vitro diagnosing GJB2 mutation of deaf gene of autosomal recessive inheritance in non-syndrome
InactiveCN1873027AAbundant sources of supplyLow costMicrobiological testing/measurementForward primerAutosomal recessive inheritance
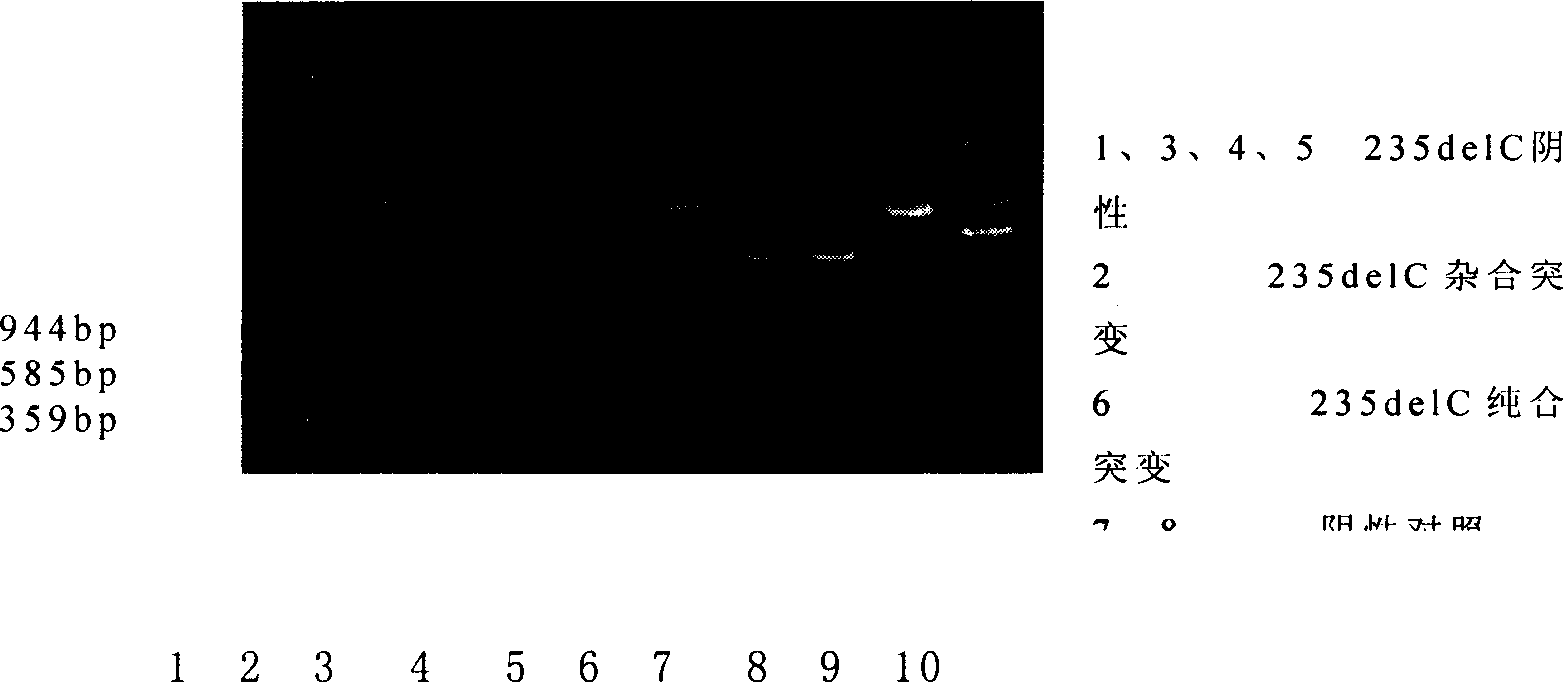
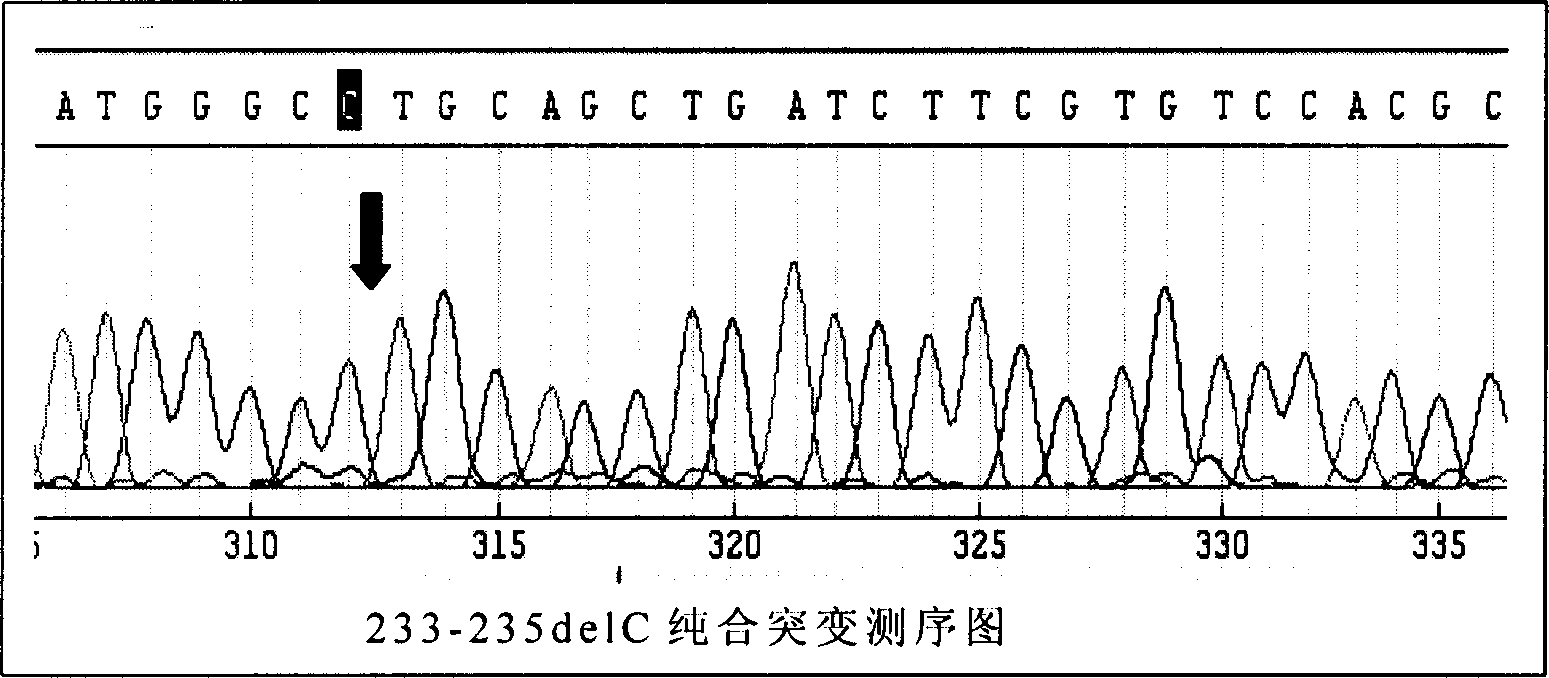
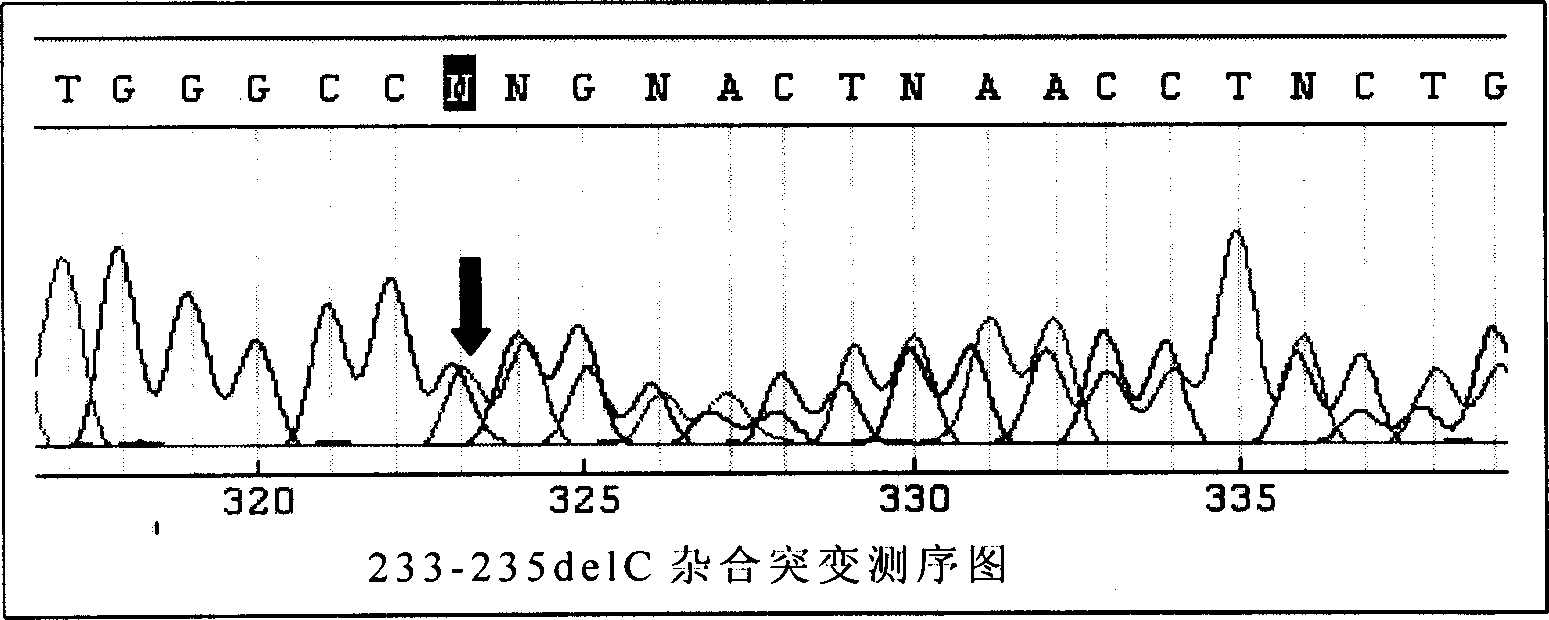
This invention provides primers for in vitro diagnosis of mutation of autosomal recessive nonsyndromic hearing loss gene GJB2. The primers can be used for completely amplifying the coding region and upstream or downstream important splicing sequences of GJB2 gene. This invention also provides a test kit for containing the primers and ApaI restriction endonuclease and their application for in vitro detection of 233-235delC mutation of the complete GJB2 coding region. The test kit can also be used for detecting the mutation sites of the GJB2 coding region through sequencing by the forward primer, and detecting deletion or insertion heterozygous mutation through sequencing by the reverse primer. The test kit is suitable for large-scale screening of autosomal recessive nonsyndromic hearing loss gene GJB2 mutation and genetic counseling.
Owner:山东三月三基因技术有限公司
Cystatin C detection kit and preparation method therefor
ActiveCN103645323AHigh sensitivityGood precisionBiological material analysisBiological testingMedicinePolystyrene
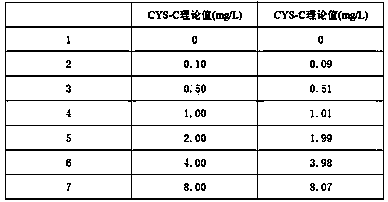
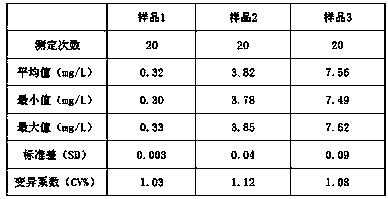
The invention relates to the field of medical immunology in vitro diagnosis, and especially provides a detection kit for detecting cystatin C in serum. The detection kit comprises a reagent R1, a reagent R2 and calibration materials. The ingredients of the reagent R1 are a Tris buffer with a concentration of 0.01-0.05mol / L, PEG 6000 with a concentration of 10-40g / L, NaN3 with a concentration of 0.5-1.5g / L, and sodium chloride with a concentration of12-20g / L, and the pH value is 8.0-8.5. The ingredients of the reagent R2 are a Tris buffer with a concentration of 0.01-0.02mol / L, and polystyrene latex granules coated by goat-anti-human cystatin C polyclonal antibodies with a concentration of 0.5-3.0 g / L. The calibration materials are six gradient standards with cystatin C and the contents of cystatin C are 0.1, 0.5, 1.0, 2.0, 4.0 and 8.0mg / L. The solution is a Tris buffer with a concentration of 0.01mol / L. The kit has simple composition, good stability and high accuracy.
Owner:浙江夸克生物科技有限公司
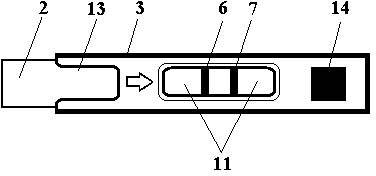
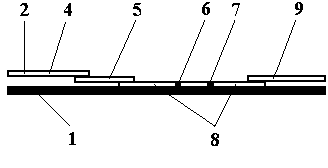
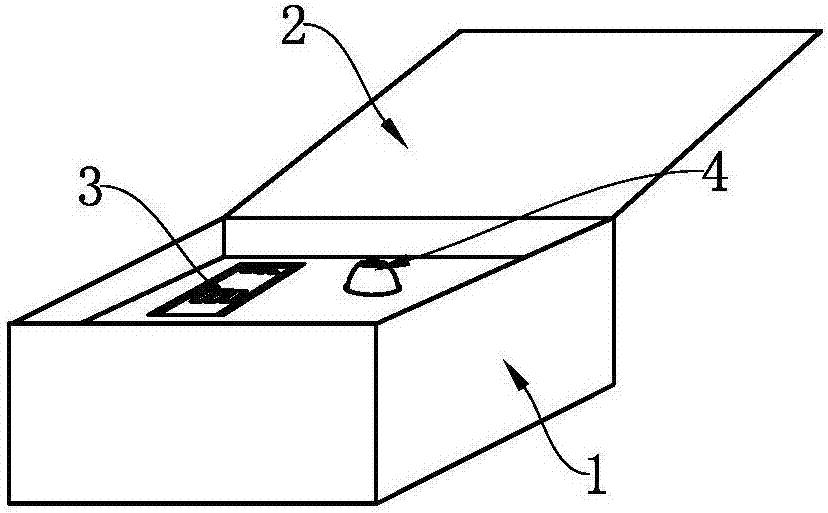
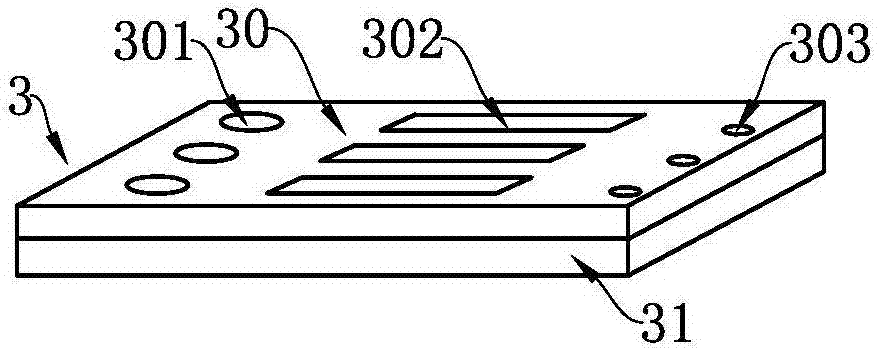
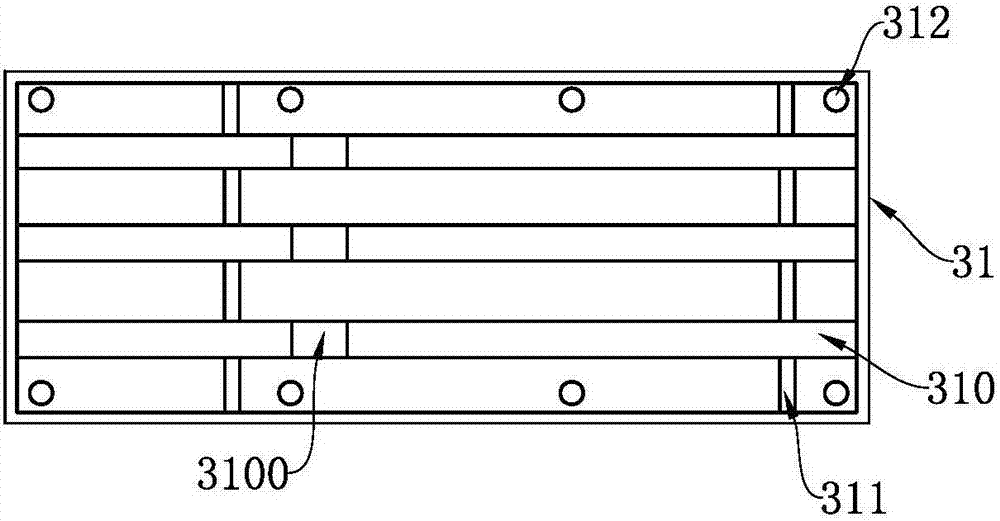
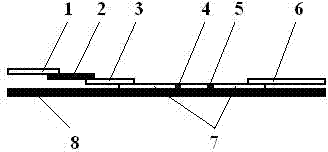
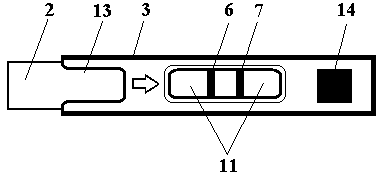
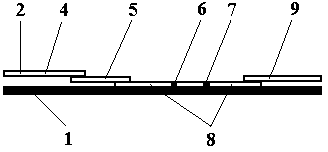
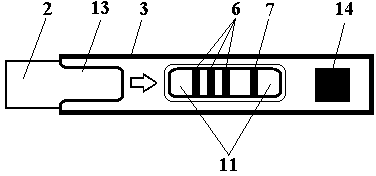
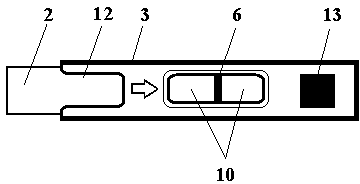
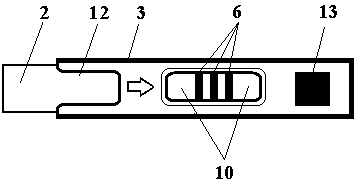
Test strip card
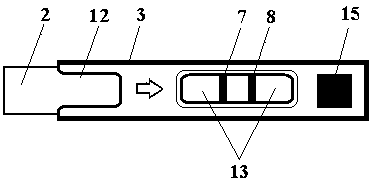
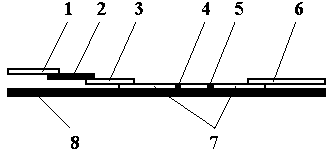
The invention belongs to external diagnosis field, and more specifically relates to a test strip card capable of filtering a sample and having a store medium (15) for storing detection information such as standard curve or coefficient parameter of a detected object. The test strip card comprises a card box (3) and a test strip (2). One end of the card box (3) is opened to form a test strip slot opening (12). The test strip (2) is a separated type plug structure of the card box (3). The store medium (15) is arranged on the card box (3). When the sample is detected, one end of a sample suction pad (4) of the test strip (2) inserted in the card box (3) stretches out of an opening end of the card box (3) for dipping and absorbing the sample, an apparatus having signal detection function collects characteristic signal of a detection strip (7) and a quality control strip (8) of the test strip (2), and combines the standard curve or coefficient parameter of the detected object read through the store medium (15) by the apparatus to obtain the concentration of a sample monocomponent or multiple components. The test strip card for detecting the sample has the characteristics of simple and rapid process, high sensitivity, objective result and flexible usage.
Owner:CHENGDU LINGYU BIOTECH
Kit and method for predicting hepatocarcinogenesis risk
InactiveCN108070655AHigh sensitivityNon-invasive testingMicrobiological testing/measurementCvd riskBiology
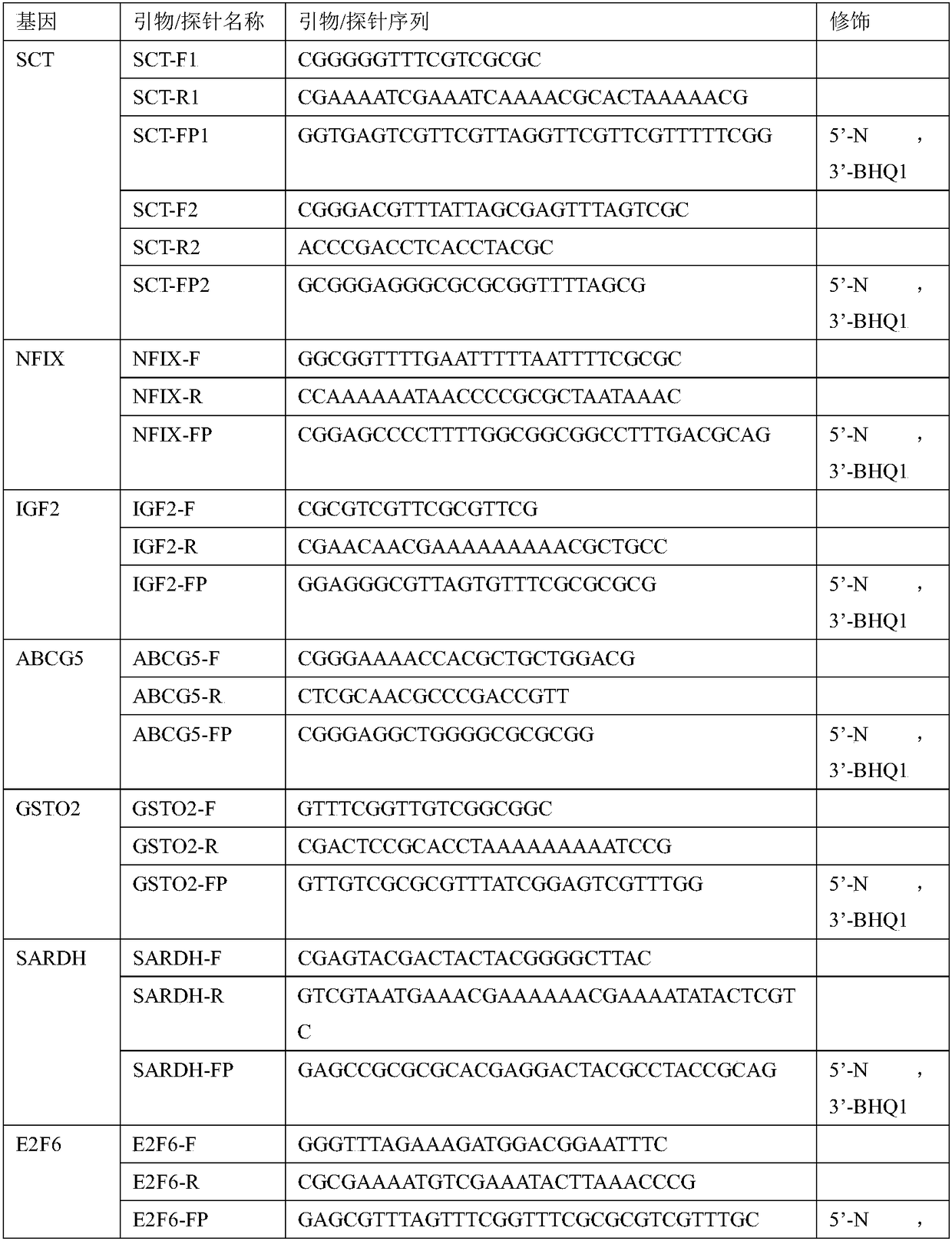
The invention relates to a kit and a method for predicting a hepatocarcinogenesis risk, relating to the technical field of medical in-vitro diagnosis. Particularly, the invention relates to a kit anda method for predicting a hepatocarcinogenesis risk through the CpG island methylation states of single genes or multiple genes in SCT, NFIX, IGF2, ABCG5, GSTO2, SARDH, E2F6, SHANK2, GATA4, OPLAH, NR2F1 and ZFHX3. According to the combined methylation detection of the multiple genes, the sensitivity is greatly improved; the free nucleic acids of saliva, urine, blood and the like are detected, drawing materials from cancer tissue is not required, the detection is noninvasive, and the operation is simple and convenient.
Owner:北京旌准医疗科技有限公司
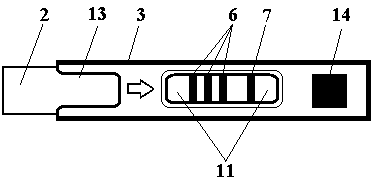
Quantum dot immunochromatographic strip for synchronously quantifying multiple tumor markers and method of quantum dot immunochromatographic strip
InactiveCN104267182ASimultaneous rapid quantitative detectionPrevent passageDisease diagnosisVitro diagnosticsTumor marker
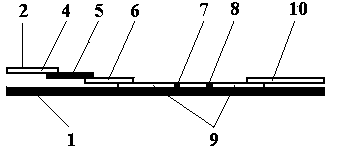
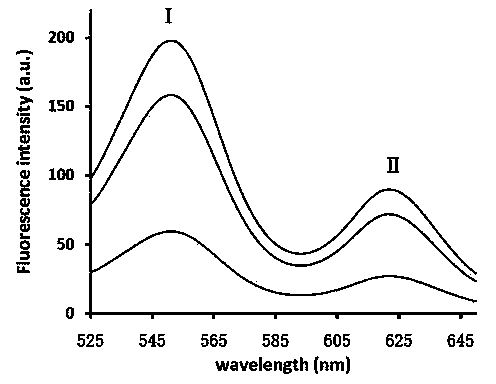
The invention belongs to the field of in-vitro diagnosis and particularly relates to a quantum dot immunochromatographic strip for synchronously quantifying multiple tumor markers and a method of the quantum dot immunochromatographic strip. The quantum dot immunochromatographic strip is characterized in that a marking pad (3) is coated with a mixture of various tumor marker antibodies marked corresponding to quantum dots with different wavelengths, a belt T (4) of an analyzing membrane (7) is coated with a mixture of various tumor marker antibodies, and a belt C (5) of the analyzing membrane (7) is coated with secondary antibodies; a standard curve of each tested object is stored in an electronic tag manner and the electronic tags are mounted on the immunochromatographic strip. According to the quantum dot immunochromatographic strip, a detection instrument having a signal detection function is utilized for reading standard curve data stored in the electronic tags and synchronously and quantitatively detecting concentrations of the various tumor markers in a to-be-detected sample by combining the corresponding fluorescence intensity of the to-be-detected sample measured by the detection instrument.
Owner:CHENGDU LINGYU BIOTECH
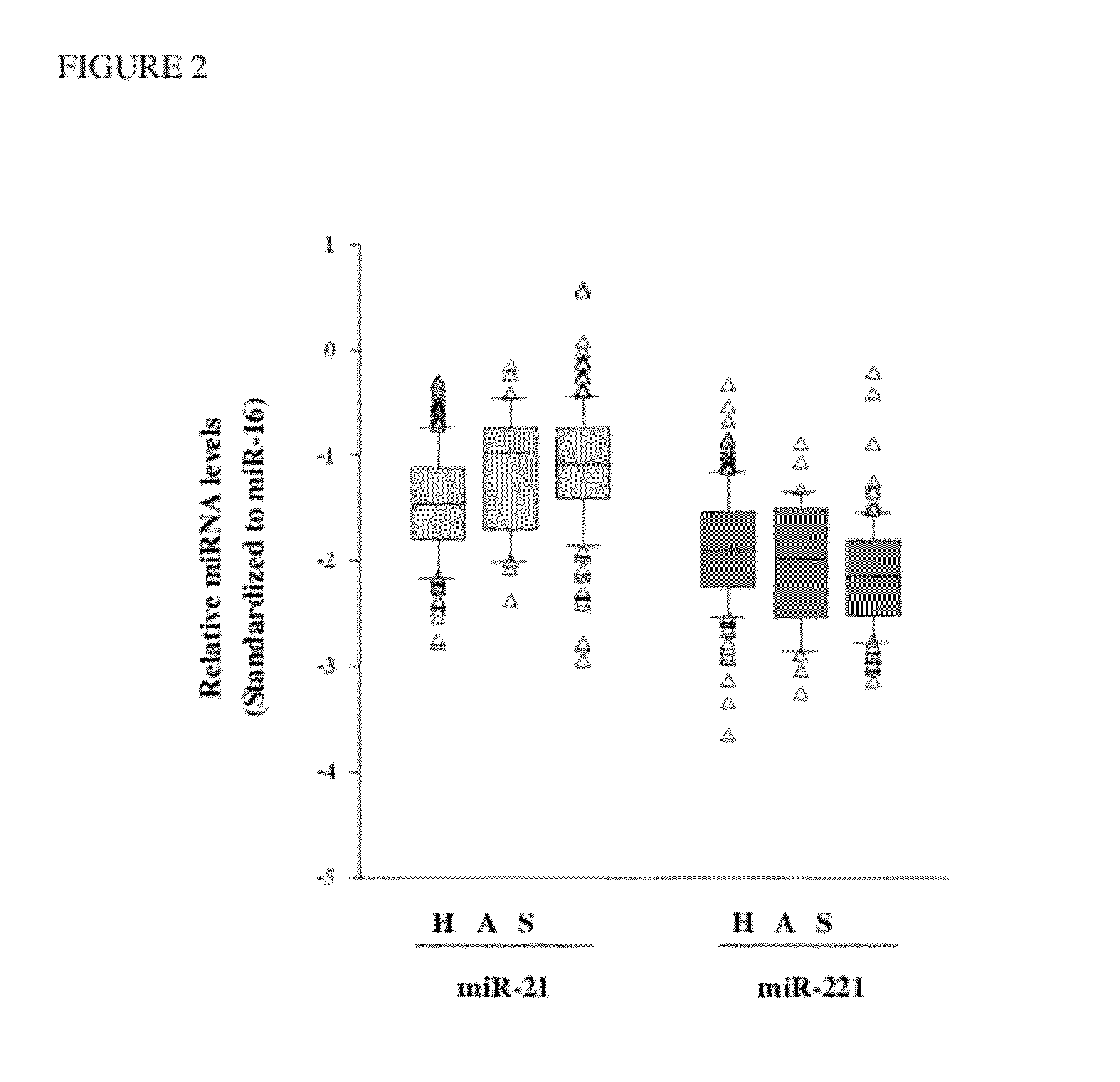
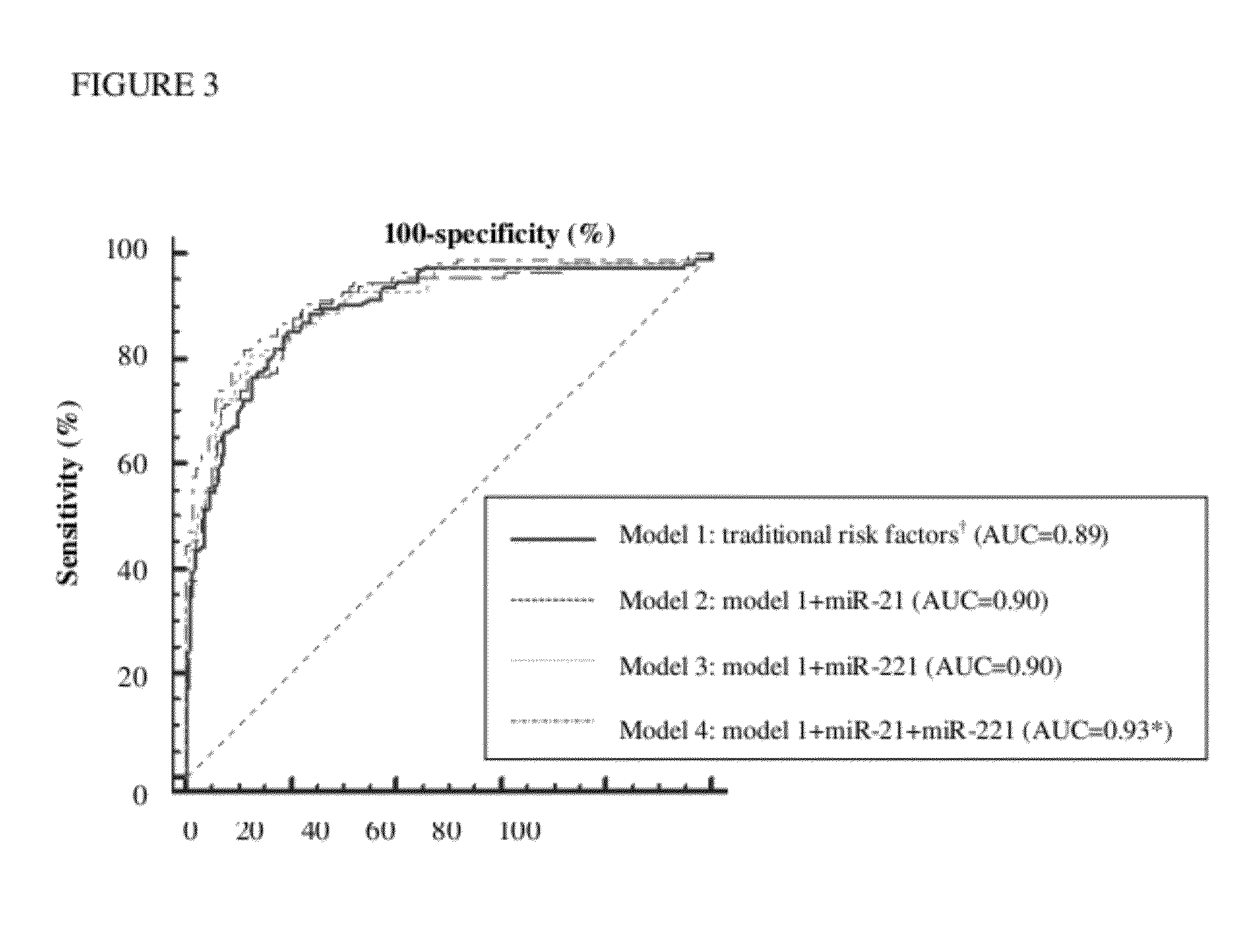
Method and kit for in vitro diagnosis of atherosclerosis
A method for in vitro diagnosis of atherosclerosis, comprising: (a) obtaining a sample from a subject; (b) determining expression levels of one or more microRNAs (miRNAs) as atherosclerotic biomarkers and an internal control RNA; (c) computing the relative expression levels of the one or more miRNAs as atherosclerotic biomarkers; (d) computing a prediction model with one or more variables, wherein the variable includes one or more relative expression levels of the one or more miRNAs as atherosclerotic biomarkers and one or more risk factors of atherosclerosis; and (e) computing a prediction probability by the prediction model, wherein the subject is diagnosed with atherosclerosis if the probability is more than 0.5 is presented. A kit for in vitro diagnosis of atherosclerosis or prognosis of atherosclerosis-inducing diseases is also presented.
Owner:KAOHSIUNG MEDICAL UNIVERSITY
Microglia Microvesicles Contained MicroRNA-Based Methods For The Diagnosis, Prognosis And Treatment Monitoring Of Neurological, Neurodegenerative And Inflammation-Based Diseases
ActiveUS20190249250A1Microbiological testing/measurementDNA/RNA fragmentationMicroRNAVitro diagnostics
The present invention describes a method for the in vitro diagnosis, prognosis and / or treatment monitoring of neurodegenerative, neurological and inflammation-based diseases, wherein the method comprises the steps: a) isolating microglial microvesicles (MVs) from biological fluids obtained from an individual; b) collecting the microRNA (miRNA) contained into said MVs; c) determining the expression profile of a predetermined set of miRNA; d) comparing said expression profile to one or several reference expression profiles, wherein the comparison of said determined expression profile to said one or several reference expression profiles allows for the diagnosis, prognosis and / or treatment monitoring of the disease.
Owner:BRAINDTECH SPA
Test strip card based on quantum dot labeling
InactiveCN103630682ARapid Multi-Component ConcentrationRapid quantification of multicomponent concentrationsMaterial analysisEngineeringVitro diagnostics
The invention relates to the field of in-vitro diagnosis, particularly a test strip card based on quantum dot labeling. The test strip card comprises a card box (3) and a quantum dot labeling test strip (2), wherein one end of the card box (3) is open to form a test strip slot (13), and the test strip (2) is in a card box (3) instant-plug structure; and the card box (3) is provided with a storage medium (14) in which a sample tested object standard curve and other information are stored. When detecting a sample, one end of a shape suction pad (4) of the test strip (2) inserted into the card box (3) excessively protrudes out of the open end of the card box (3) so as to impregnate and absorb the sample, and an instrument with signal detection function acquires characteristic signals of a test strip (2) detection belt (6) and a quality control belt (7) and calculates the sample single component or multicomponent concentration in combination with tested object standard curve or coefficient parameters simultaneously red from the storage medium (14) by the instrument. The test strip card for detecting samples has is simple and quick, and has the characteristics of high sensitivity, objective result, high use flexibility and the like.
Owner:CHENGDU LINGYU BIOTECH
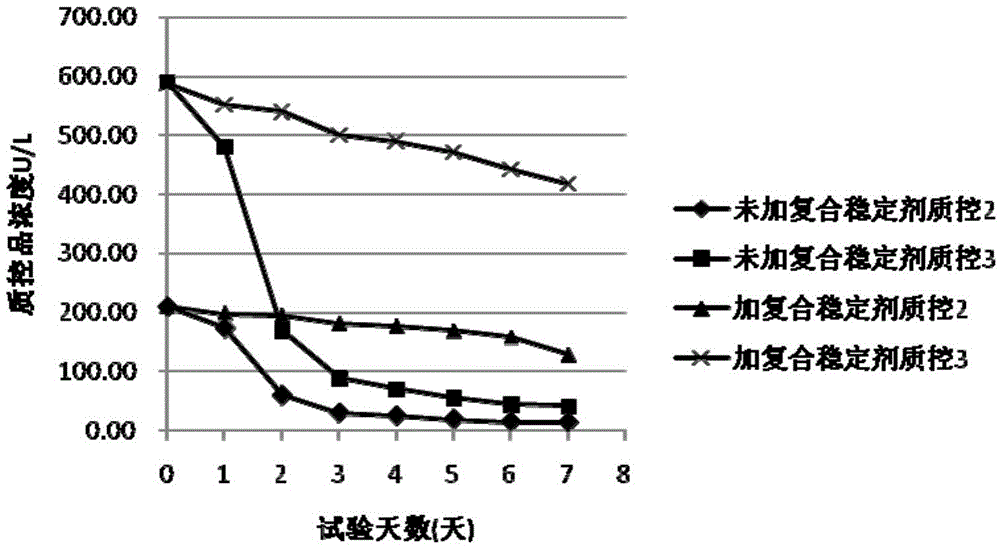
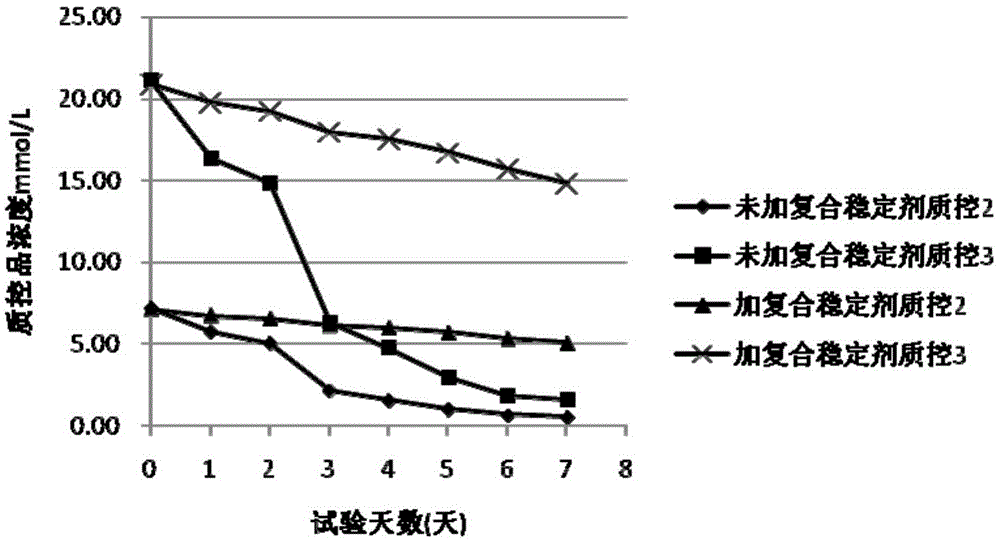
Compound stabilizer and kit for in vitro diagnosis reagents
ActiveCN105603049AEasy to useImprove stabilityMicrobiological testing/measurementBiotechnologyPreservative
The invention belongs to the technical field of biology, and particularly relates to a compound stabilizer and a kit for in vitro diagnosis reagents. The compound stabilizer comprises a sealing agent, a surfactant, a micromolecule organic reductant, a nucleic acid ingredient stabilizer, a biological active substance protecting agent and a biological preservative. The compound stabilizer can be universally applied to various in vitro diagnosis reagents and has better reagent stabilizing effect, and validity period of the reagents is prolonged effectively.
Owner:SHENYANG BAICHUANGTE BIOTECH
Test strip card based on quantum dot labeling
InactiveCN103675267ARapid Multi-Component ConcentrationRapid quantification of multicomponent concentrationsDisease diagnosisBiological testingEngineeringVitro diagnostics
The invention relates to the field of in-vitro diagnosis, particularly a test strip card based on quantum dot labeling. The test strip card comprises a card box (3) and a quantum dot labeling test strip (2), wherein one end of the card box (3) is open to form a test strip slot (12), and the test strip (2) is in a card box (3) instant-plug structure; and the card box (3) is provided with a storage medium (13) in which a sample tested object standard curve and other information are stored. When detecting a sample, one end of a sample suction pad (4) of the test strip (2) inserted into the card box (3) excessively protrudes out of the open end of the card box (3) so as to impregnate and absorb the sample, and an instrument with signal detection function acquires characteristic signals of a test strip (2) detection belt (6) and calculates the sample single component or multicomponent concentration in combination with tested object standard curve or coefficient parameters simultaneously read from the storage medium (13) by the instrument. The test strip card for detecting samples has is simple and quick, and has the characteristics of high sensitivity, objective result, high use flexibility and the like.
Owner:CHENGDU LINGYU BIOTECH
Breast tumor prognosis biomarker LncRNA detection method and clinical application thereof
InactiveCN105238782AWide detection rangeHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationPrognosis biomarkerScreening method
The invention discloses a breast tumor prognosis biomarker LncRNA detection method and clinical application thereof, provides a group of new LncRNA in sequence shown as SEQ ID No1, SEQ ID No2, SEQ ID No3, SEQ ID No4 and SEQ ID No5 and relates to a method of taking specific LncRNA in samples (tissues, serum, urine, body fluid and the like) of patients suffering from breast tumors and corresponding juxtacancerous samples or normal samples as detection markers and biomarkers for prognostic auxiliary detection of breast tumors, related kits and a high-throughput sequencing and screening method. In-vitro diagnosis and judgment of canceration and process of breast tumors are realized by identification of differences of LncRNA expression quantity in the breast tumor samples and the corresponding juxtacancerous samples or normal samples. The invention further provides usage of the LncRNA as a breast tumor marker and probes and primers for LncRNA detection.
Owner:杭州壹锋生物科技有限公司 +1
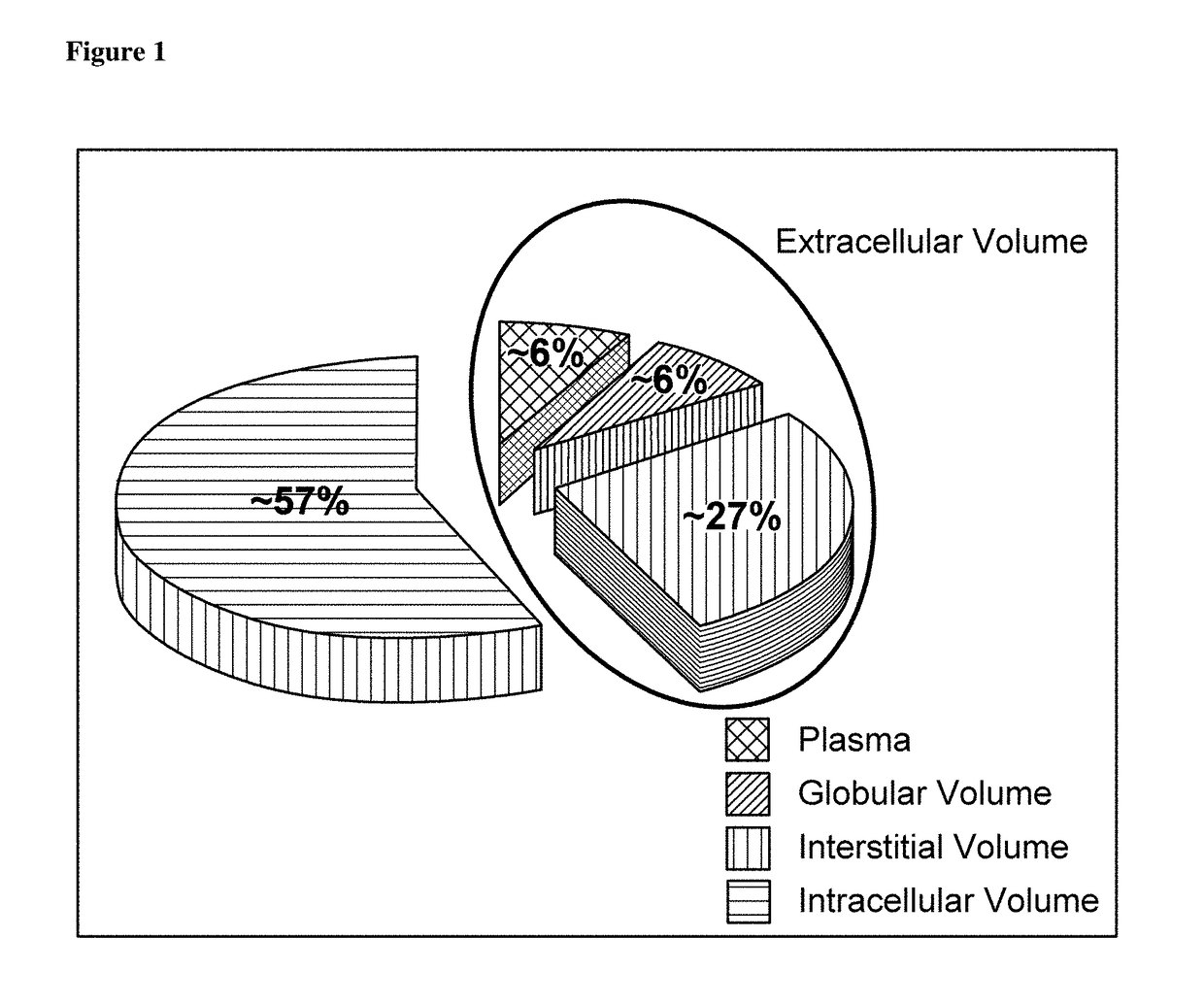
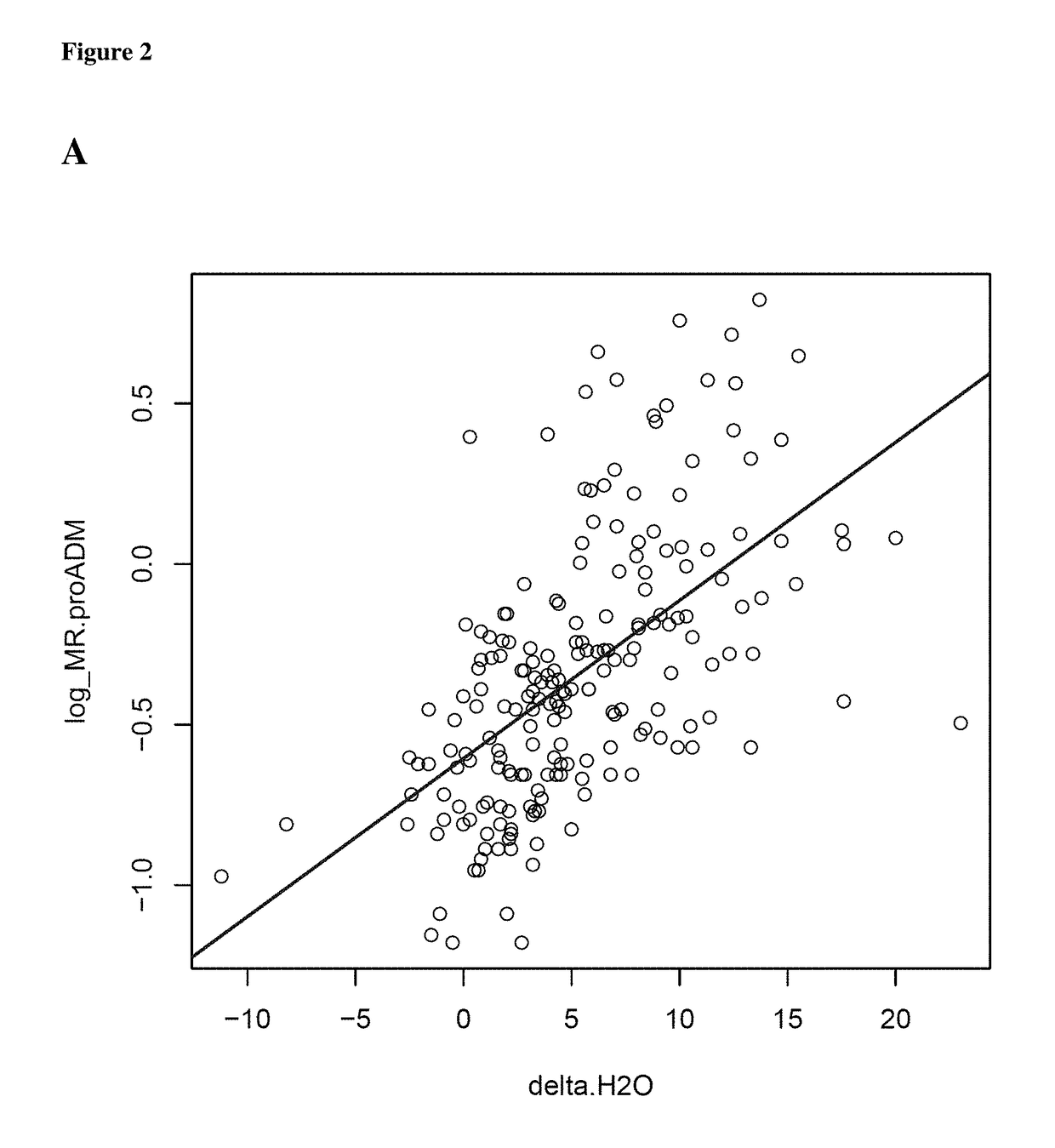
MR-proADM as marker for the extracellular volume status of a subject
PendingUS20180348235A1High medical potentialImprove the level ofBiological material analysisBiological testingFluid balanceVitro diagnostics
The present invention relates to a method for determining the extracellular volume status of a subject. The method comprises determining in a sample obtained from a subject the level of the marker proadrenomedullin (proADM) or a fragment thereof, preferably MR-proADM. Further, based on the level of proADM or a fragment thereof, the fluid balance is determined and wherein said fluid balance determines the extracellular volume status. Further, based on the level of proADM or a fragment thereof, the salt balance is determined and wherein said salt balance determines the extracellular volume status and salt retention. Further, the invention relates to a method for in vitro diagnosis, risk stratification, therapy control and / or operative control of a disorder or medical condition in a subject, wherein said extracellular volume status and salt retention of said subject is determined by the herein provided method. Further, the invention relates to a kit and / or a diagnostic device for carrying out the herein provided method.
Owner:BRAHMS GMBH
Multiple-detection kit for vaginitis, and preparation method thereof
PendingCN107389940AImprove hydrophilicityImprove reagent sensitivityMaterial analysisGardnerella vaginalis DNABiology
The invention relates to the technical field of in vitro diagnosis, and concretely relates to a multiple-detection kit for vaginitis. The kit comprises a kit body and a kit cover, a detection card and a sample processing solution bottle are arranged in kit body, the detection card comprises a card cover and a card body, a Gardnerell avaginalis test strip, a Candida albicans test strip and a Trichomonas vaginalis test strip are arranged in the card body, and each of the Gardnerell avaginalis test strip, the Candida albicans test strip and the Trichomonas vaginalis test strip is provided with a first sample pad layer and a second sample pad layer treated with the sample pad treatment fluid. The kit can decompose and filter out interfering substances in vaginal secretions, so the sensitivity of the kit is improved.
Owner:山东康华生物医疗科技股份有限公司
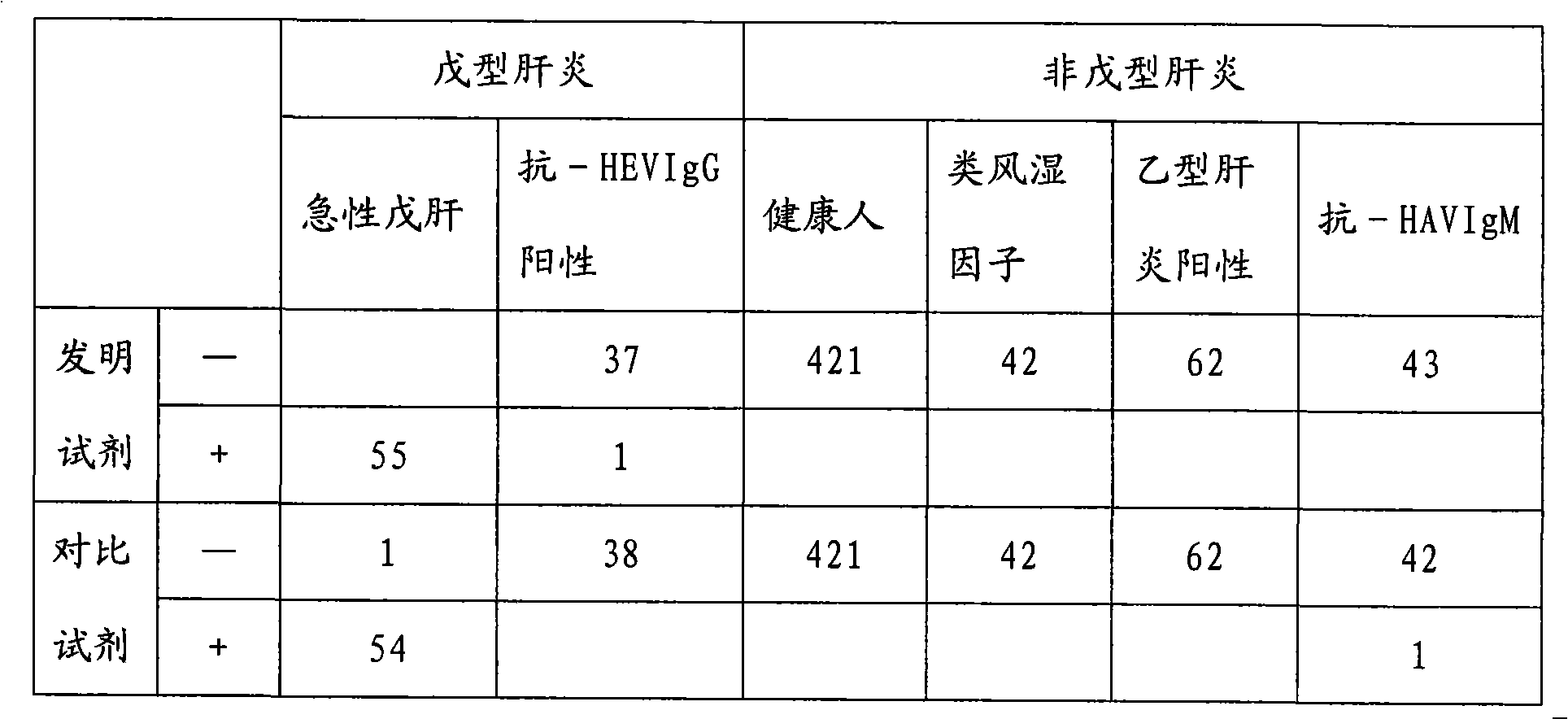
Chemiluminescence immunoassay kit of hepatitis E virus IgM antibody and preparation method thereof
InactiveCN101551395AEfficient use ofGuaranteed SensitivityChemiluminescene/bioluminescenceIgm antibodyPerformance index
The invention relates to a chemiluminescence immunoassay kit of a hepatitis E virus IgM antibody and a preparation method thereof, which belongs to the technical field of clinical in-vitro diagnosis immunoassay. The preparation method of the kit comprises the following steps: the reference substance is prepared according to the negative and the positive serums of the hepatitis E virus IgM antibody; a solid phase carrier is coated by an anti-human-mu chain anti-body (monoclonal antibody or polyclonal antibody); the recombinant antigen of the hepatitis E virus is labeled by enzyme; a chemiluminescent substrate solution acted by the enzyme is prepared; a concentrated cleaning solution is prepared; the negative reference substance, the positive reference substance, the labeled combination, the chemiluminescent substrate and the concentrated cleaning solution of the hepatitis E virus IgM antibody are subpackaged; and all the components are assembled into finished products. The prepared kit has stable performance indexes (specificity, sensitivity and stability) and can be applied to the early diagnosis of the hepatitis E.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
In-vitro diagnosis analyzer and reagent card
ActiveCN111257548AReliable on-off controlFix the leakLaboratory glasswaresBiological testingMedicineVitro diagnostics
The invention discloses an in-vitro diagnosis analyzer and a reagent card. The reagent card comprises a reagent card body and a mounting body, wherein the mounting body comprises a mounting hole for sleeving a sample tube, a hollow needle arranged in the mounting hole, a sealing part arranged in the mounting hole, and an air inlet channel; one end of the hollow needle can be inserted into the sample tube, the sealing part is in sealing fit with an outer wall of the sample tube, the air inlet channel comprises an air outlet hole, an air inlet hole formed in a surface of the reagent card body and a first flow stopping structure arranged between the air outlet hole and the air inlet hole, and the air outlet hole is used for being communicated with the sample tube arranged on the mounting hole; wherein the reagent card body comprises a sample injection channel communicated with a liquid outlet end of the hollow needle, a detection cavity and a gas receiving end, and the sample injection channel and the gas receiving end are communicated with the detection cavity. The reagent card adopts a new sample liquid on-off control scheme, and can solve the hidden danger that the sample liquid leaks and cannot be extracted. When the in-vitro diagnosis analyzer is used, the reliability of detection can be improved.
Owner:GUANGZHOU WONDFO BIOTECH
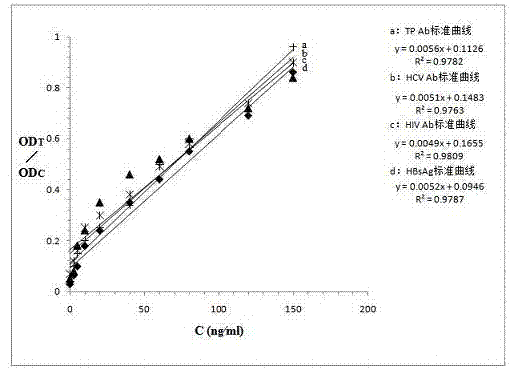
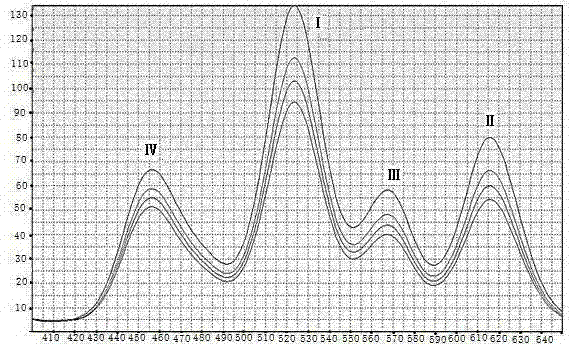
Quantum-dot marking test strip capable of quantitatively determining multiple indexes of blood infectious diseases and preparation method and quantitative determination method thereof
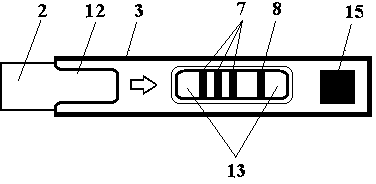
The invention belongs to the field of in-vitro diagnosis, and concretely relates to a quantum-dot marking test strip capable of quantitatively determining multiple indexes of blood infectious diseases and a preparation method and a quantitative determination method. A marking pad (3) of the test strip is coated with a quantum-dot marked mixture of HBsAg monoclonal antibody, HIVAg, HCVAg and TPAg, a T band (4) of an analysis membrane (7) is coated with a mixture of HBsAg monoclonal antibody, HIVAg, mouse anti-human antibody and TPAg, and a C band (5) of the analysis membrane (7) is coated with secondary antibody. The standard curves of all detected substances are stored and installed on the test strip by employing electron labels. The test strip employs a detector with signal detection function to read the standard curves stored in the electron labels and combines with the to-be-tested sample corresponding fluorescence intensity measured by the detector, so as to synchronously quantitatively determine the concentration of HBsAg, HIVAb, HCVAb and TPAb in the samples.
Owner:CHENGDU LINGYU BIOTECH
Test strip card
InactiveCN103630685ARapid quantitationAccurate quantitative detectionDisease diagnosisEngineeringVitro diagnostics
The invention belongs to the field of in-vitro diagnosis, and particularly relates to a test strip card which is provided with a storage medium (14) for storing a tested object standard curve, coefficient parameters or other detection information. The test strip card comprises a card box (3) and a test strip (2), wherein one end of the card box (3) is open to form a test strip slot (13), and the test strip (2) is in a card box (3) instant-plug structure; and a storage medium (14) is installed on the card box (3). When detecting a sample, one end of a shape suction pad (4) of the test strip (2) inserted into the card box (3) excessively protrudes out of the open end of the card box (3) so as to impregnate and absorb the sample, and an instrument with signal detection function acquires characteristic signals of a test strip (2) detection belt (6) and a quality control belt (7) and calculates the sample single component or multicomponent concentration in combination with the tested object standard curve or coefficient parameters simultaneously red from the storage medium (14) by the instrument. The test strip card for detecting samples has is simple and quick, and has the characteristics of high sensitivity, objective result, high use flexibility and the like.
Owner:CHENGDU LINGYU BIOTECH
Method for rapidly detecting human leucocyte antigen B27 (HLA-B27) and kit thereof
ActiveCN102443625AStrong specificityIncreased sensitivityMicrobiological testing/measurementAntigenFluorescence
The invention relates to a method for rapidly detecting a human leucocyte antigen B27 (HLA-B27) and an in-vitro diagnosis kit, which belong to the technical field of medical biology. In the kit, a pair of HLA-B27 specific primers and a specific fluorescent probe are adopted for rapidly detecting the HLA-B27. In the kit, the pair of HLA-B27 specific primer and the specific fluorescent probe can beused for rapidly and accurately detecting HLA-B27 genes in samples such as human peripheral blood and the like, and the kit has the advantages of high specificity, high sensitivity, time saving, labor saving, low cost, high flux and the like, and can be applied to auxiliary diagnosis of multiple spondyloarthropathies which severely endanger human health such as clinical ankylosing spondylitis, Reiter syndrome, psoriatic arthritis, ulcerative colitis accompanying arthropathy, acute anterior tract uveal, and the like.
Owner:浙江夸克生物科技有限公司
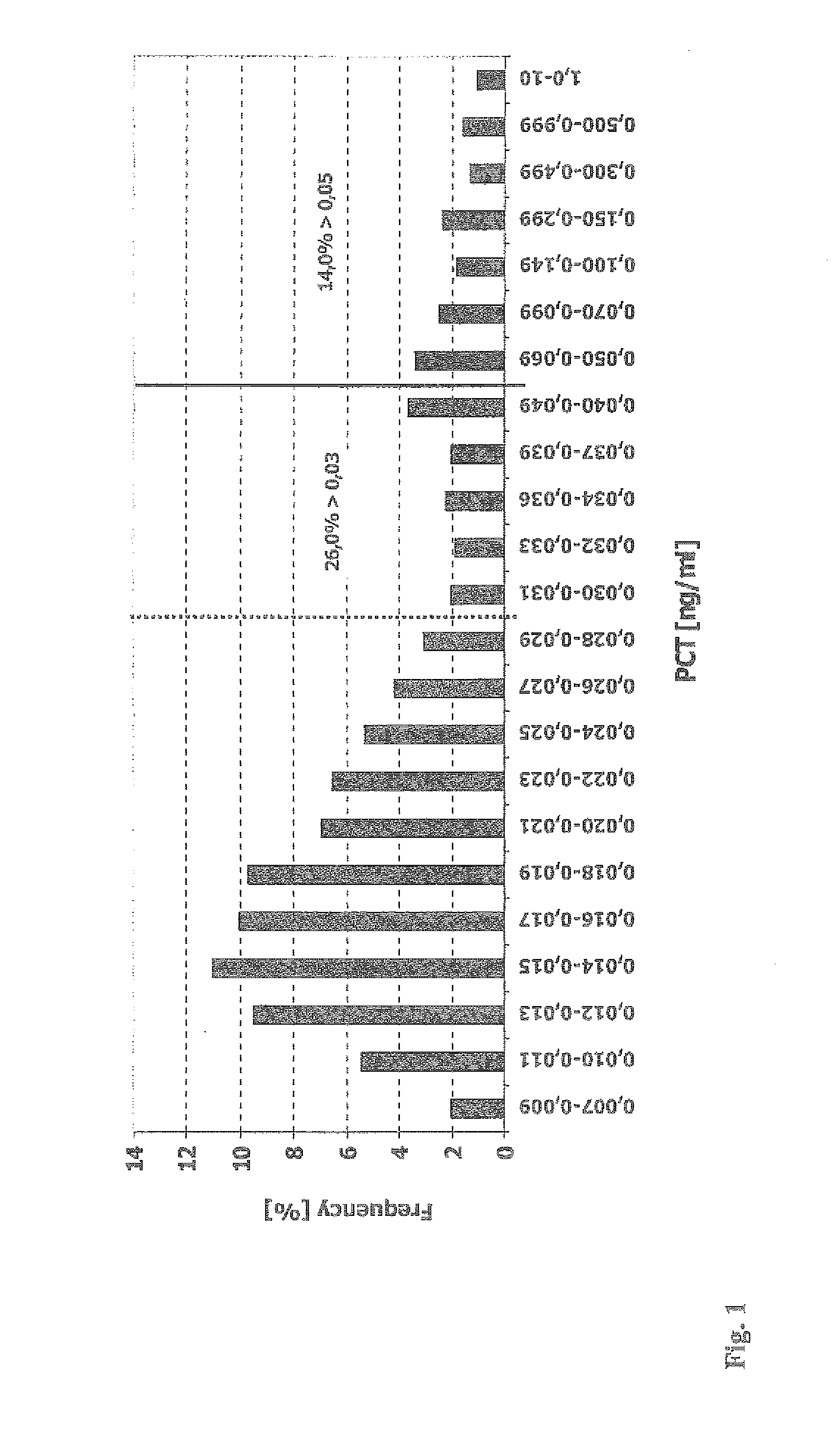
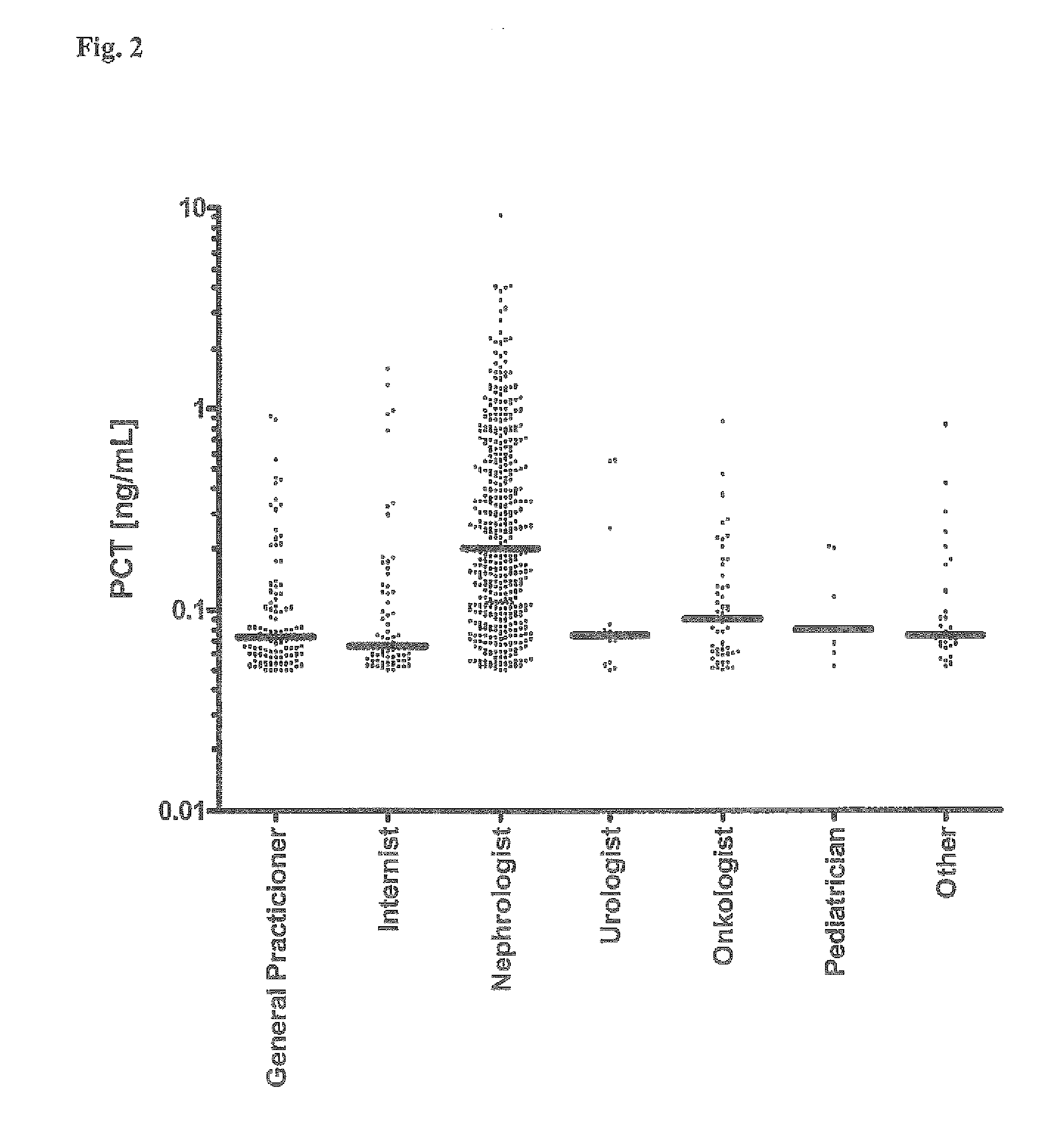
Use of Procalcitonin (PCT) in Risk Stratification and Prognosis of Patients with a Primary, Non-Infectious Disease
ActiveUS20110152170A1Facilitate accelerate enhance riskImprove the level ofAntibacterial agentsOrganic active ingredientsVitro diagnosticsCvd risk
Subject of the present invention are assays and in vitro methods for the in vitro diagnosis, prognosis and risk stratification of a patient having a primary, non-infectious disease, whereby the level of Procalcitonin (PCT) in a sample of a body fluid of the patient is indicative for the risk of the patient to contract a further disease or medical condition.
Owner:BRAHMS GMBH
Test strip card
InactiveCN103645313ADoes not involve active inactivationFor long-term storageDisease diagnosisEngineeringVitro diagnostics
Belonging to the in vitro diagnosis field, the invention in particularly relates to a test strip card with a storage medium (13) for storing a detected object standard curve or coefficient parameter and other information for the detection. The test strip card includes a card box (3) and a test strip (2). One end of the card box (3) is opened to form a test strip slot opening (12). The test strip (2) is a detachment plug structure of the card box (3). The storage medium (13) is mounted on the card box (3). During sample detection, one end of a sample absorbing pad (4) in the test strip (2) inserted into the card box (3) interferes and extends out of the opening end of the card box (3) to soak and absorb the sample. Then an instrument with a signal detection function collects the characteristic signal of a detection band (6) in the test strip (2). And by combining the instrument, the detected object standard curve or coefficient parameter are read at the same time from the storage medium (13), so that a sample single-component or multicomponent concentration can be calculated. The test strip card for sample detection provided by the invention has the characteristics of simplicity, rapidity, high sensitivity, subjective result, and flexible application, etc.
Owner:CHENGDU LINGYU BIOTECH
Latex enhanced immunoturbidimetric detection kit for retinol binding protein and clinical application thereof
ActiveCN109721651AHigh expressionShort cycleBacteriaImmunoglobulins against animals/humansSerum igeAntigen
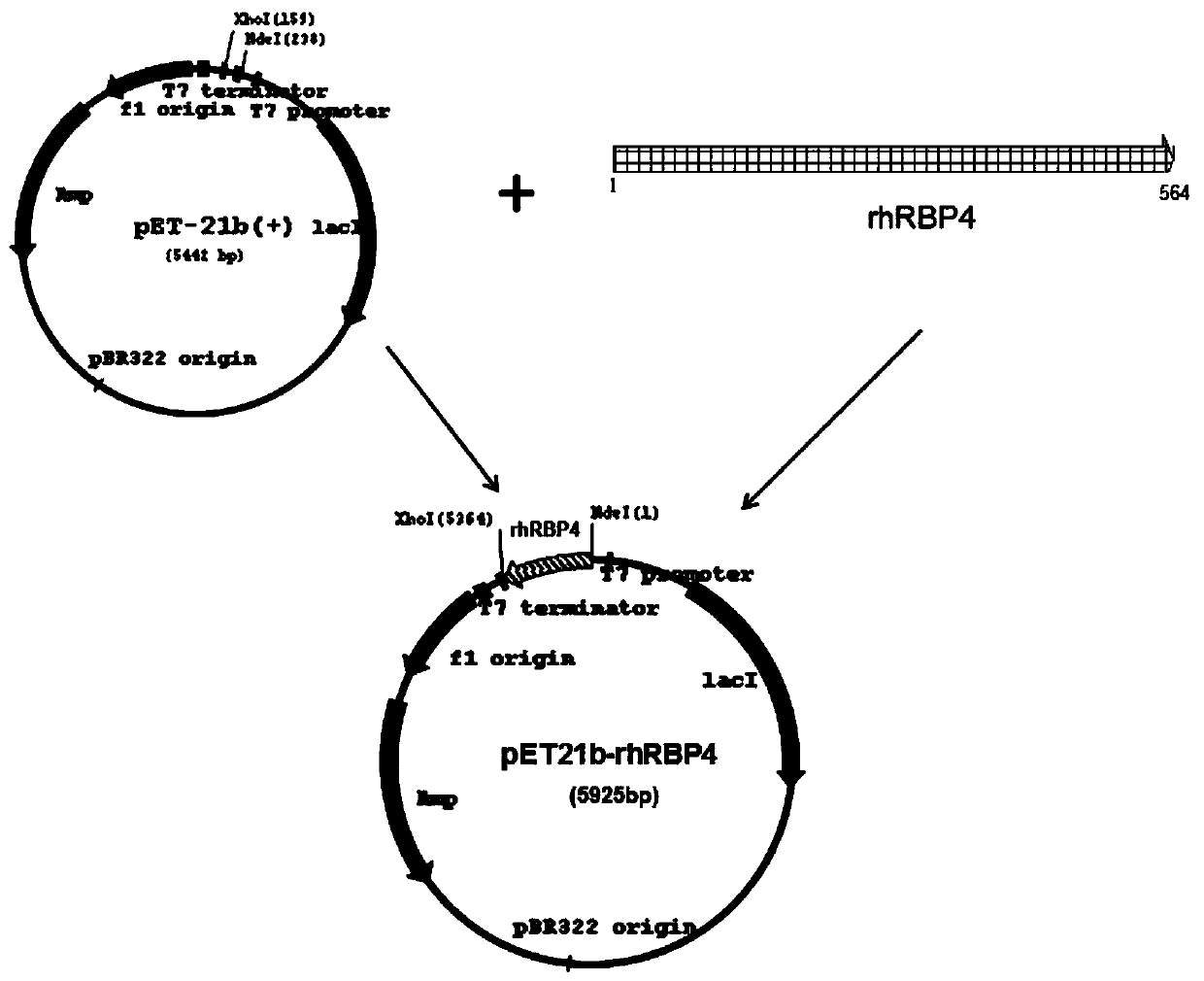
The invention relates to a latex enhanced immunoturbidimetric detection kit for retinol binding protein and an application thereof in clinical detection of content of retinol binding protein (RBP4) inserum, and belongs to the field of in vitro diagnosis of medical immunology. The RBP latex enhanced immunoturbidimetric detection kit provided by the invention adopts recombinant expressed rhRBP4 asa calibration product, a quality control product and related rabbit polyantibody immune antigens, and the rabbit polyantibody, the rhRBP4 calibration product and the quality control product are used for preparing the latex enhanced immunoturbidimetric detection kit, and the content of RBP4 in blood can be accurately detected. Compared with commercial products, the kit has better specificity and higher accuracy, and shows better clinical application prospect. Besides, the recombinant expression method of the rhRBP has the characteristics of short period, large expression amount and low cost, and improves the quality uniformity of related products to a great extent.
Owner:ZONHON BIOPHARMA INST
Methods and products for in vitro diagnosis, in vitro prognosis and the development of drugs against invasive carcinomas
ActiveUS9702879B2Microbiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsPharmacy medicineVitro diagnostics
The present invention relates to in vitro methods and products for detecting the presence of an invasive carcinoma in an individual, for determining and / or predicting the stage and / or invasiveness of said carcinoma in an individual, or for monitoring the effect of the therapy administered to an individual who has said carcinoma based on col11a1 gene and proCOL11A1 protein expression. The invention also relates to the search for, identification, development and evaluation of the efficacy of compounds for therapy for said carcinoma, for the purpose of developing new medicinal products. The invention also relates to agents inhibiting proCOL11A1 protein expression and / or activity, and / or the effects of this expression.
Owner:ONCOMATRYX BIOPHARMA
Kit for early diagnosis of bladder cancer and preparation method of kit
ActiveCN103018461BHigh sensitivitySensitive assayChemiluminescene/bioluminescenceBladder cancerBacteriuria
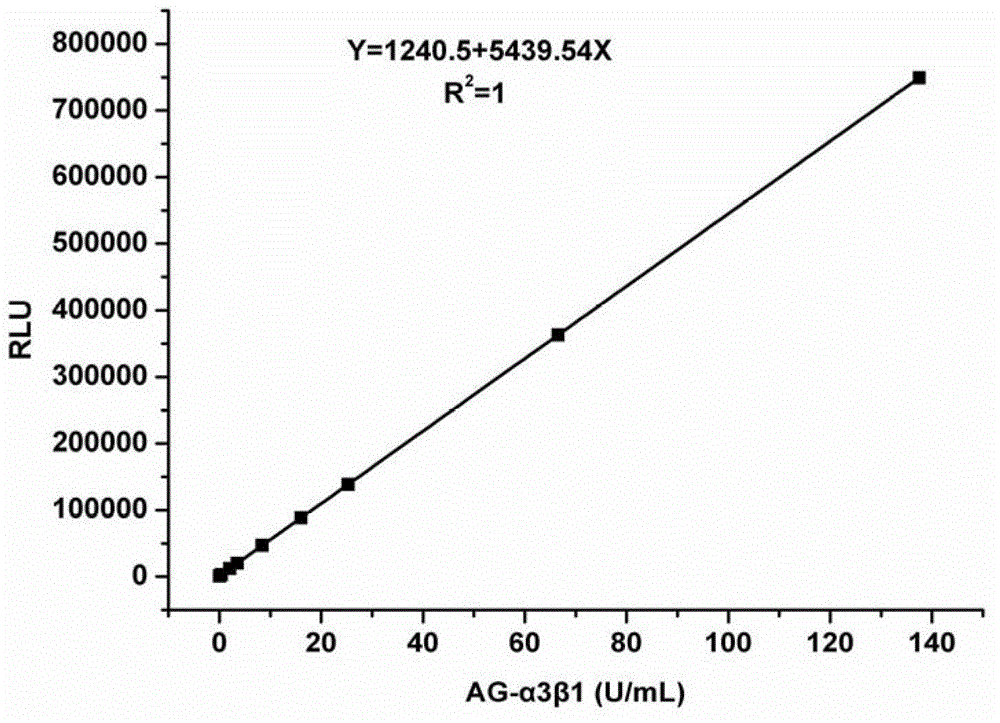
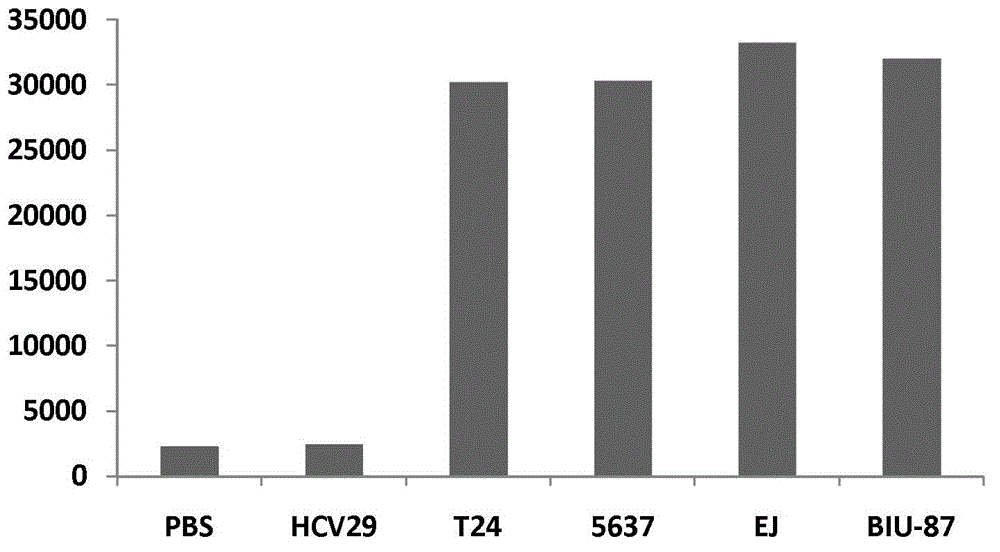
The invention discloses a kit for early diagnosis of bladder cancer and a method for preparing the kit, and relates to the field of biomedical immune analysis. The detecting kit, which is fast, sensitive, highly specific in the early diagnosis of the bladder cancer is created by detecting a tumor marker of the bladder cancer tumor marker, namely, abnormally glycosylated integrin AG-alpha3beta, in human urine by means of microplate chemiluminescence immunoassay. The kit provided by the invention has the advantages of simplicity, convenience, quickness, sensitivity, high specificity, stability and the like, can be applied to early screening of the bladder cancer in a routine examination and to tracking observation and prognostic evaluation of the bladder cancer, and can satisfy the need that the existing in-vitro diagnosis of the bladder cancer is lack in specific diagnosis method.
Owner:INSITUTE OF BIOPHYSICS CHINESE ACADEMY OF SCIENCES
Inter-batch quality control method for external diagnostic reagent
The invention provides an inter-batch quality control method for external diagnostic reagents. The inter-batch quality control method comprises the following steps of printing bar codes including information about external diagnostic reagent kinds and production batch numbers on boxes containing solid or semisolid materials, such as external liquid diagnostic reagents or test paper strips and sheets, and comparing the information carried by the external diagnostic reagents with immediately updated dates of manufacture, batch numbers, correction factors, calculation formulas and formula key coefficients in a storage medium to be corrected. The storage medium comprises a built-in memory disc, a USB flash disk, a mobile hard disk drive or a self-control storage medium. According to the inter-batch quality control method for the external diagnostic reagents provided by the invention, a system can find out relevant information of correction factors and the like to accomplish the marking in the detection process without resetting marks when products of different batches appear, thus the cumbersome work of remarking due to the exchange of the product batch numbers is avoided.
Owner:SUZHOU INST OF BIOMEDICAL ENG & TECH
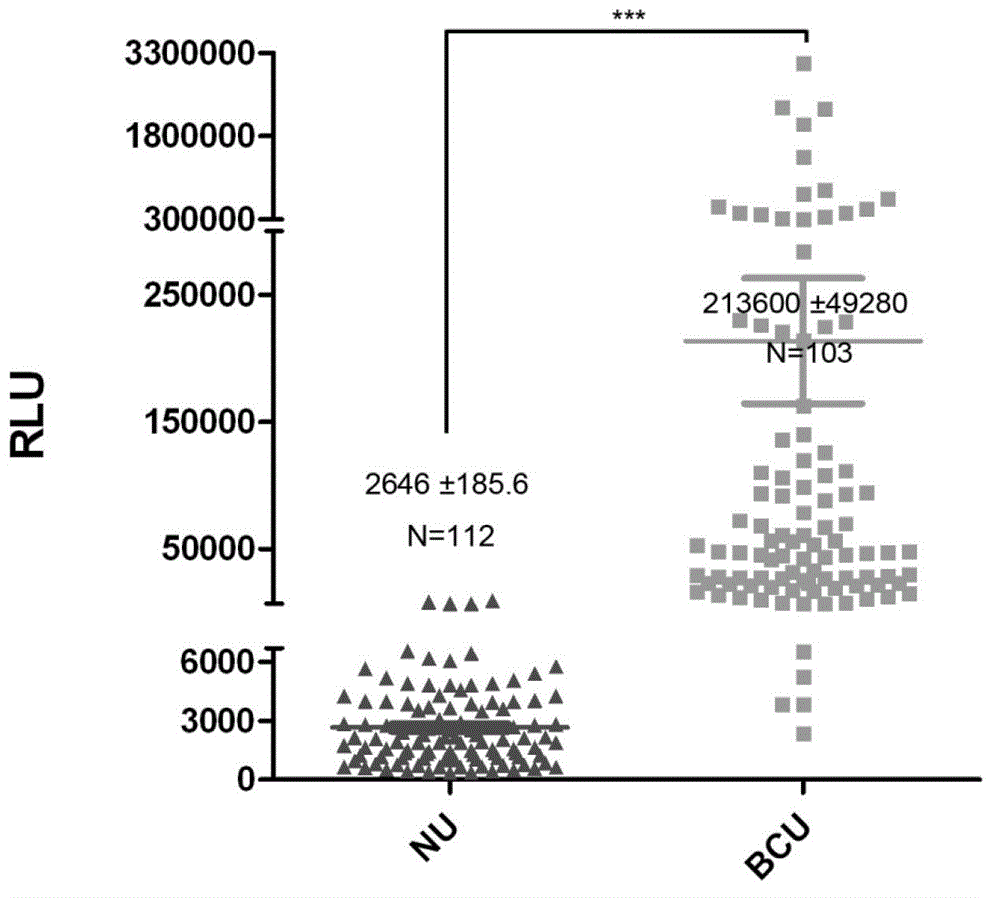
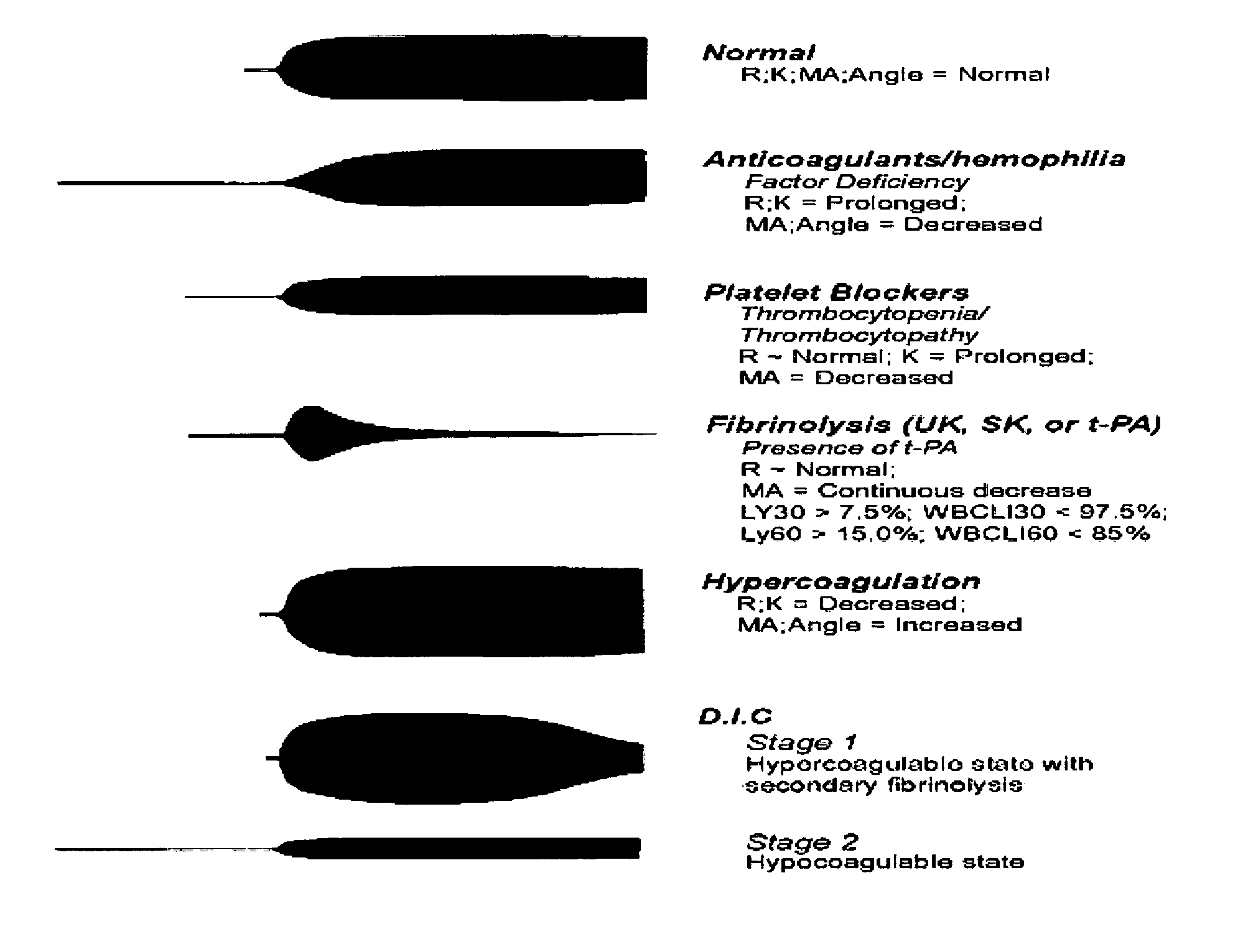
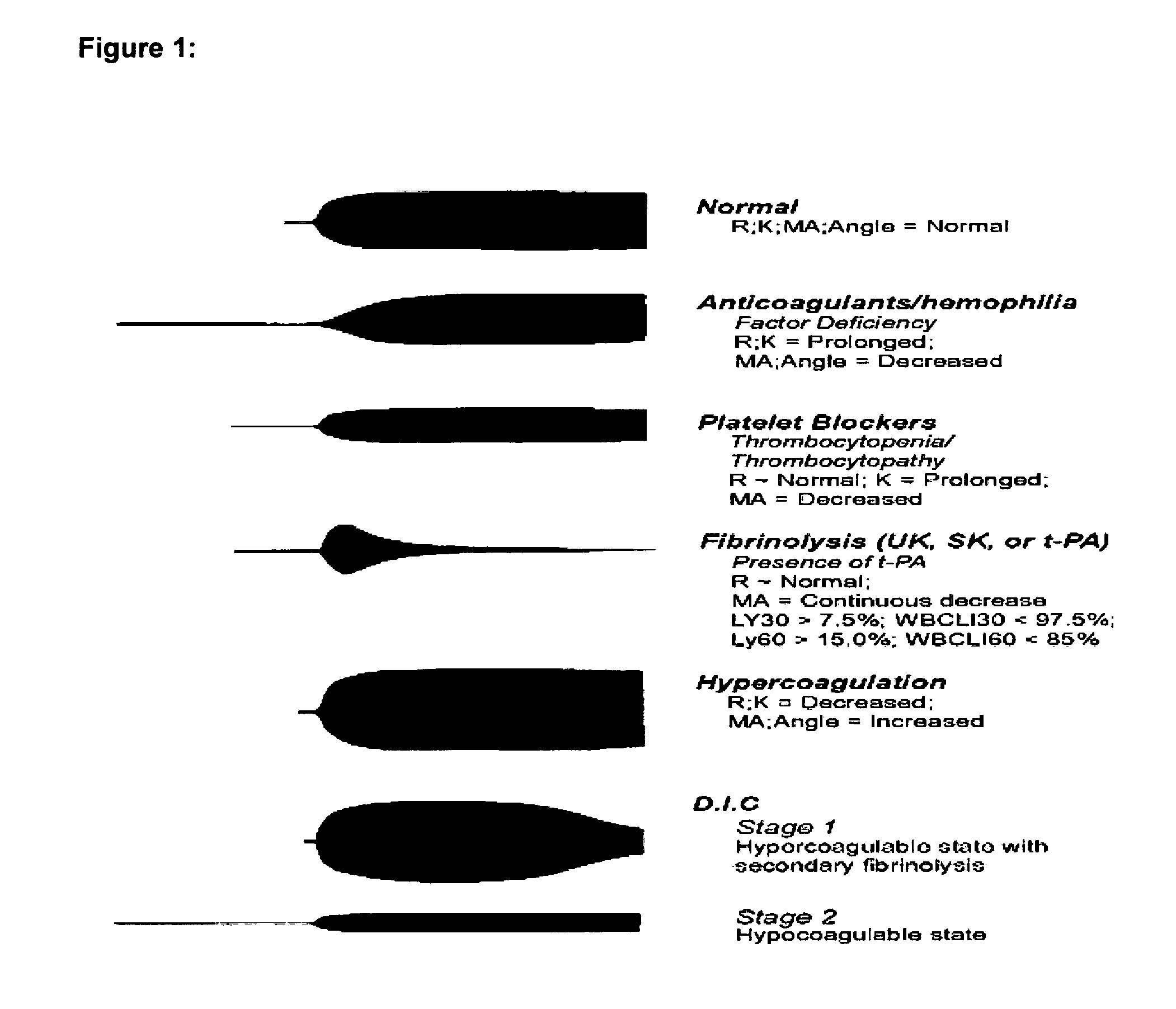
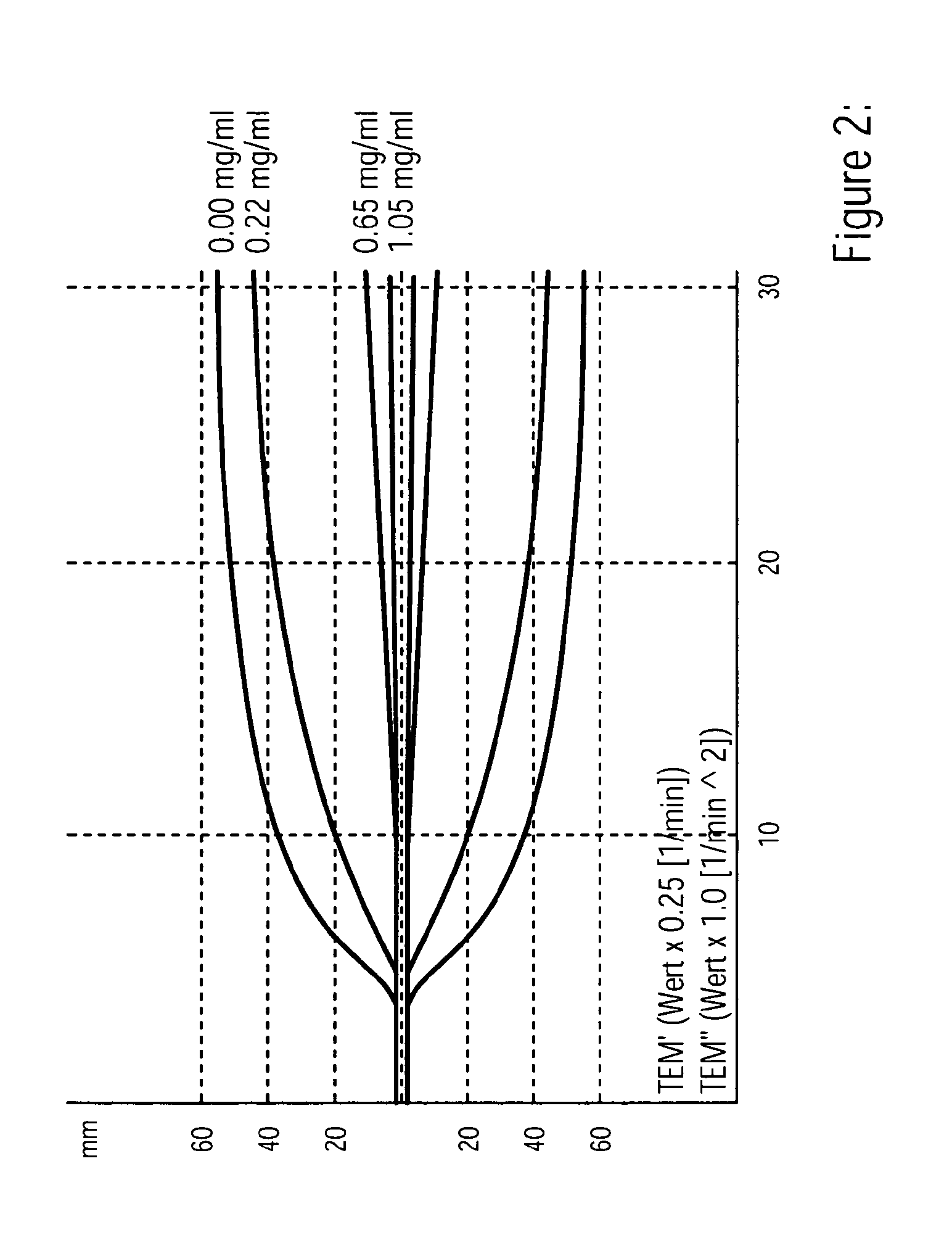
Diagnostic in vitro method for assessing von willebrand disease and increased bleeding risk associated with von willebrand disease and acquired or congenital disorders of platelet function
InactiveUS20100273206A1Superior in predicting bleeding riskMicrobiological testing/measurementDisease diagnosisPoint of careFactor ii
The invention relates to an in-vitro method for diagnosing Von Willebrand Disease (VWD) and an increased bleeding risk associated with Von Willebrand Disease and / or acquired or congenital platelet function defects that reduce the interactions of Von Willebrand Factor (VWF) with platelets. The in-vitro method of the invention may also be used to diagnose further bleeding risks. The test is suitable for use as a screening test based on whole blood and has the additional benefit of being suitable as a point of care test. The method involves the incubation of a sample containing platelets and hemostasis factors with an activator of platelet aggregation and the measurement of the viscoelastic change after inducing coagulation, e.g., by means of thromboelastography (TEG).
Owner:CSL BEHRING GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com