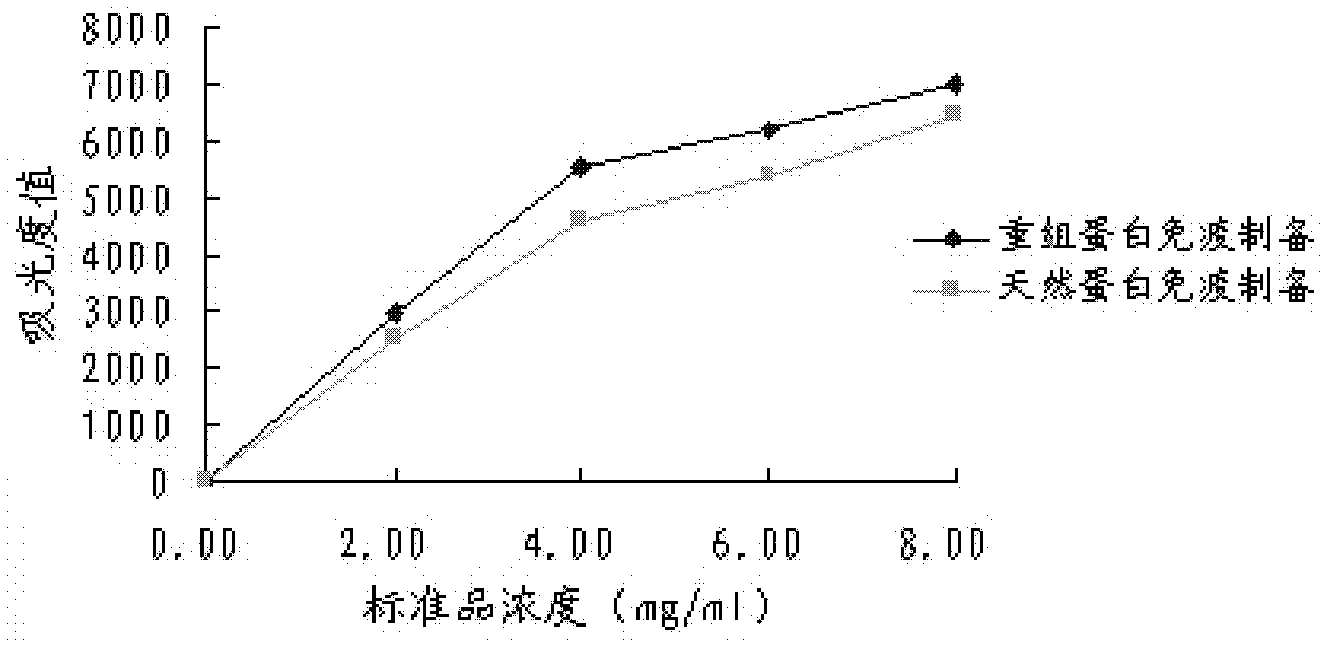
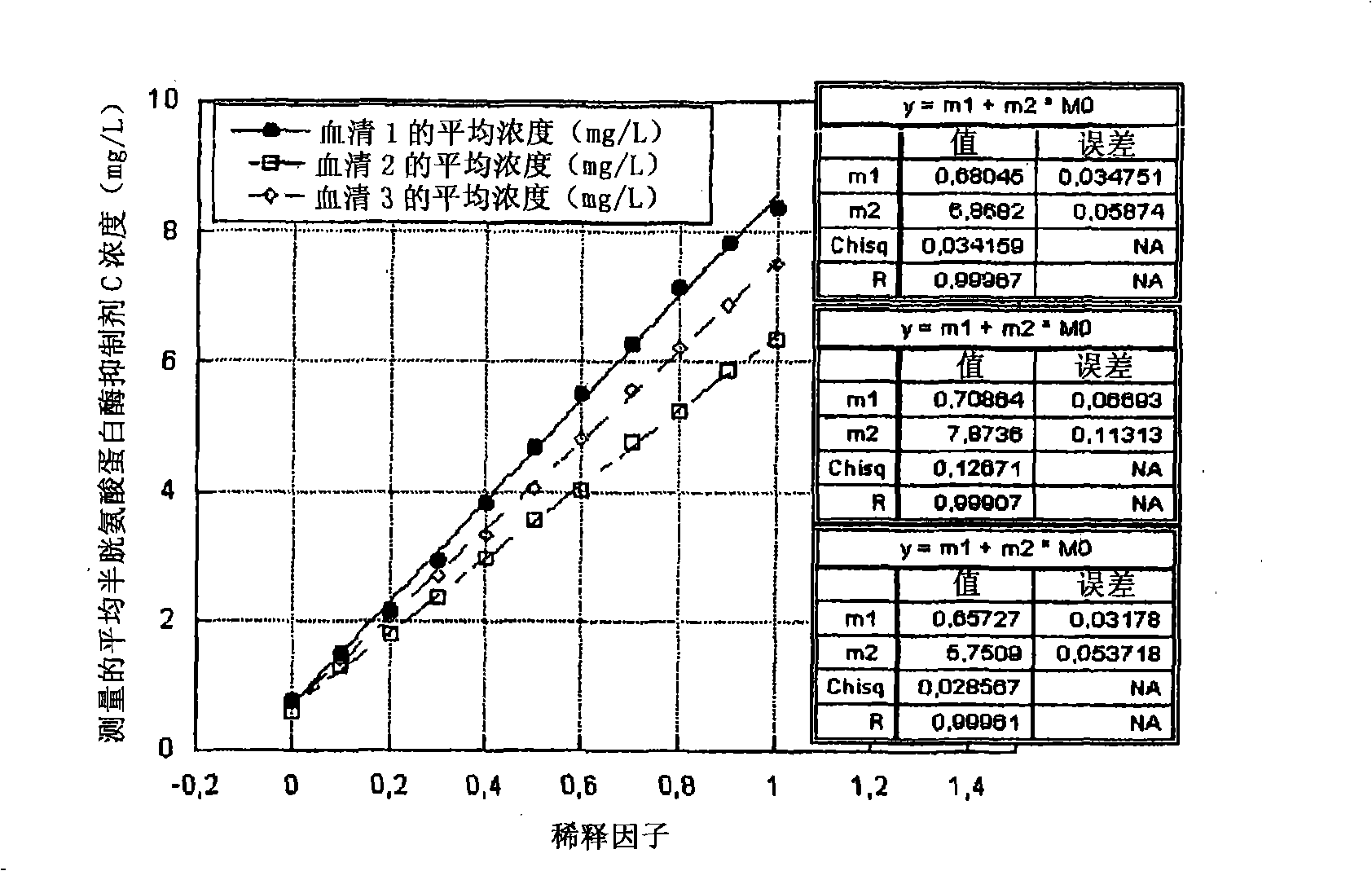
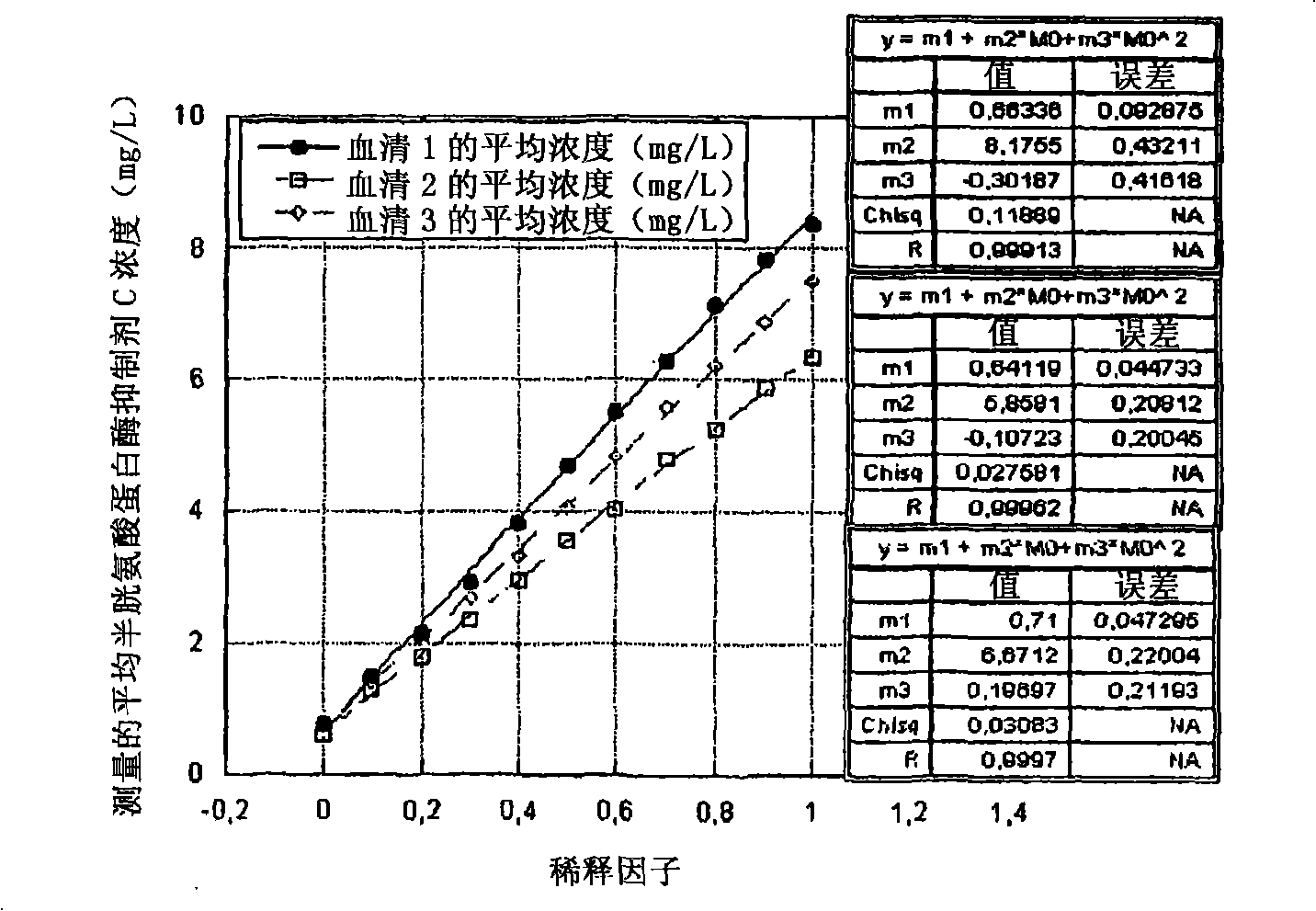
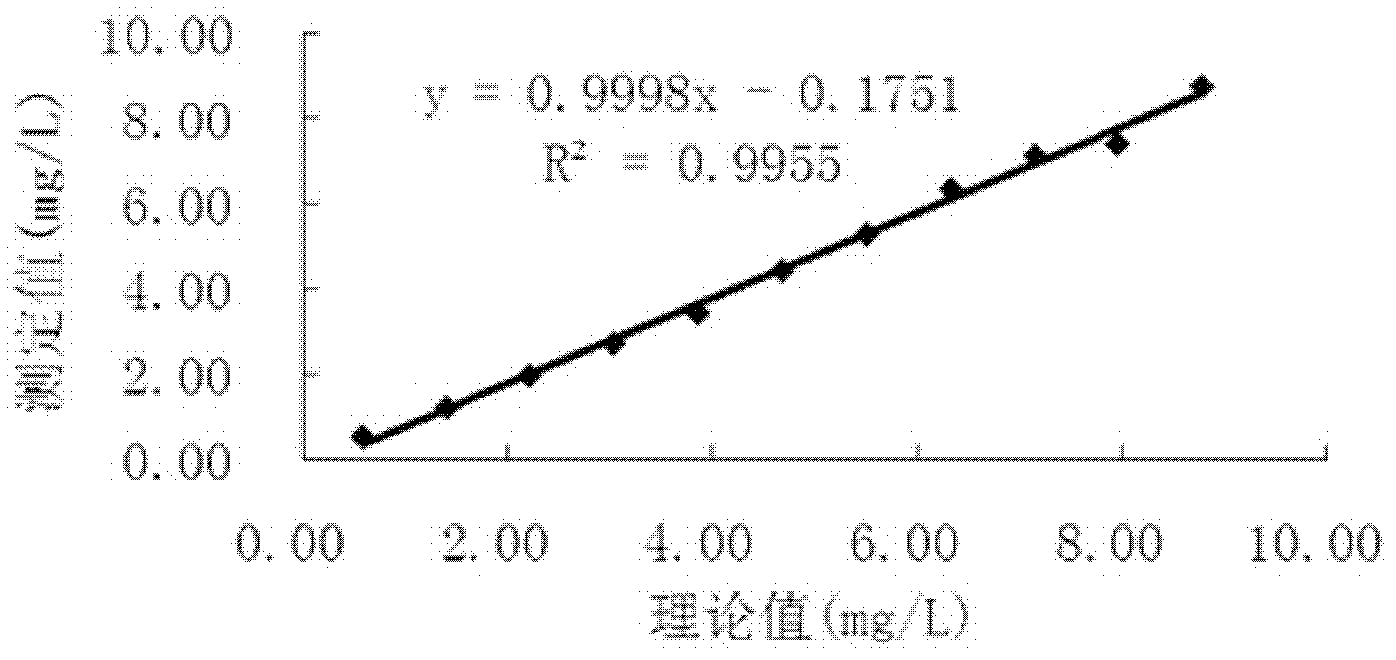Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
133 results about "Cystatin C" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
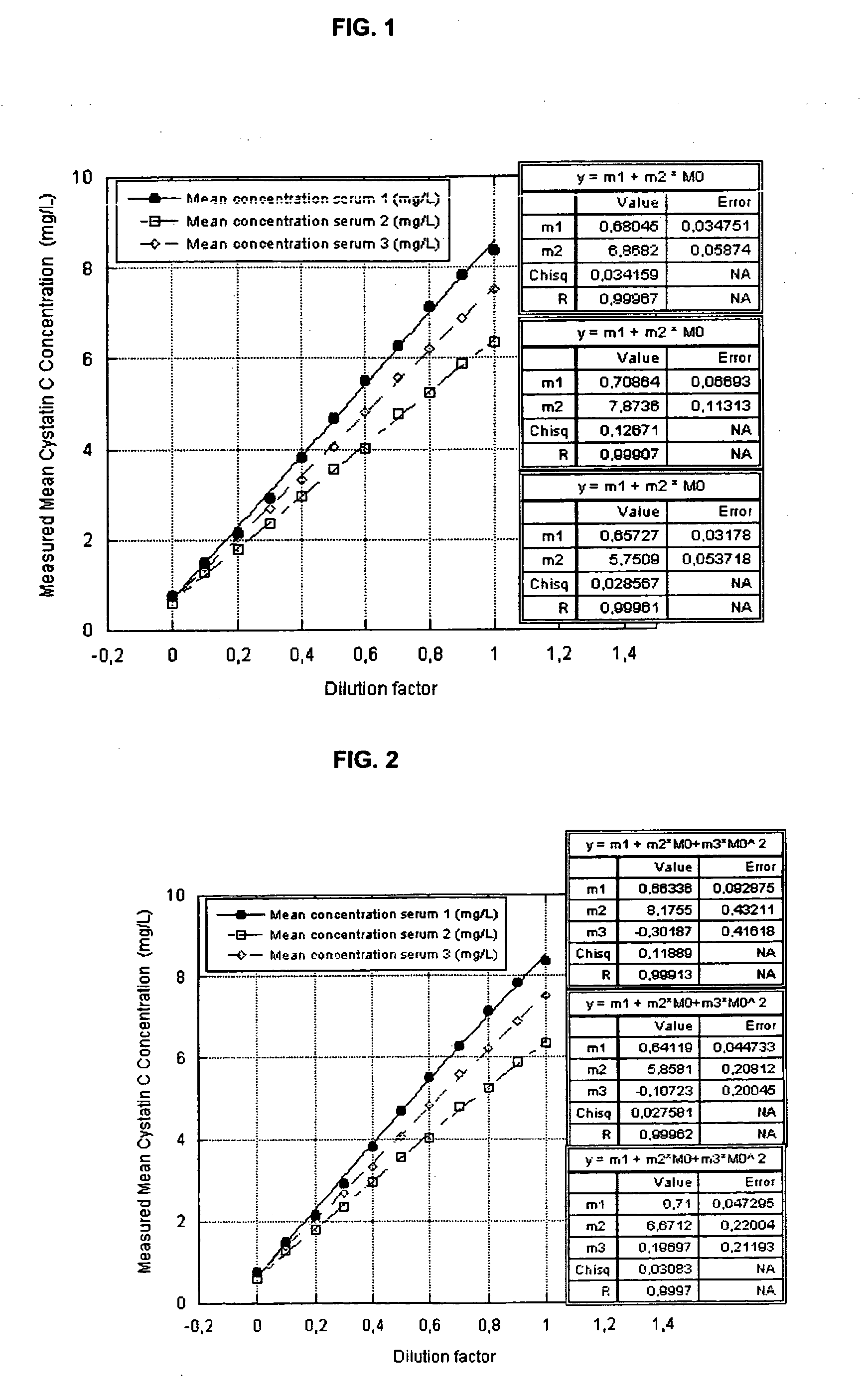
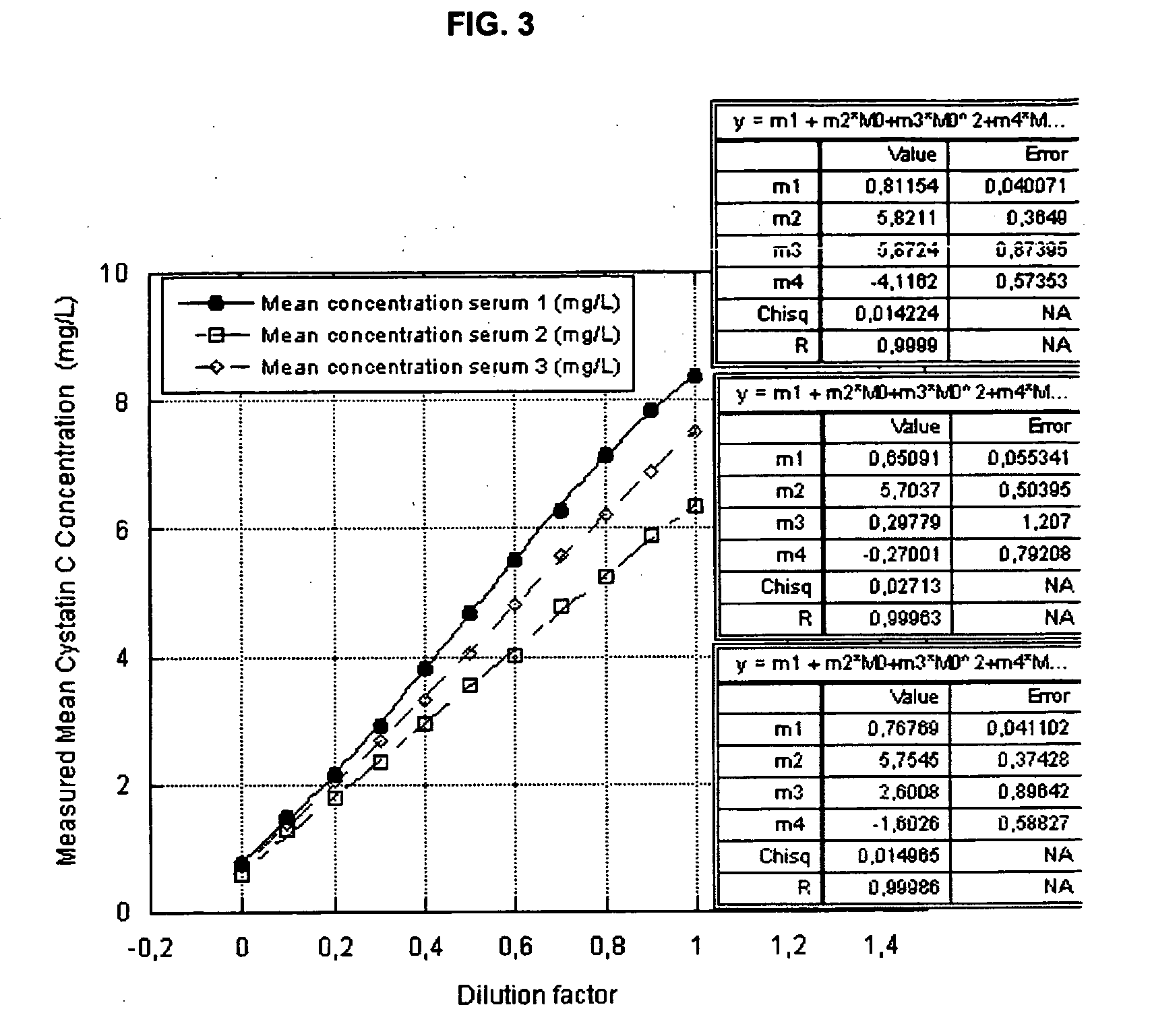
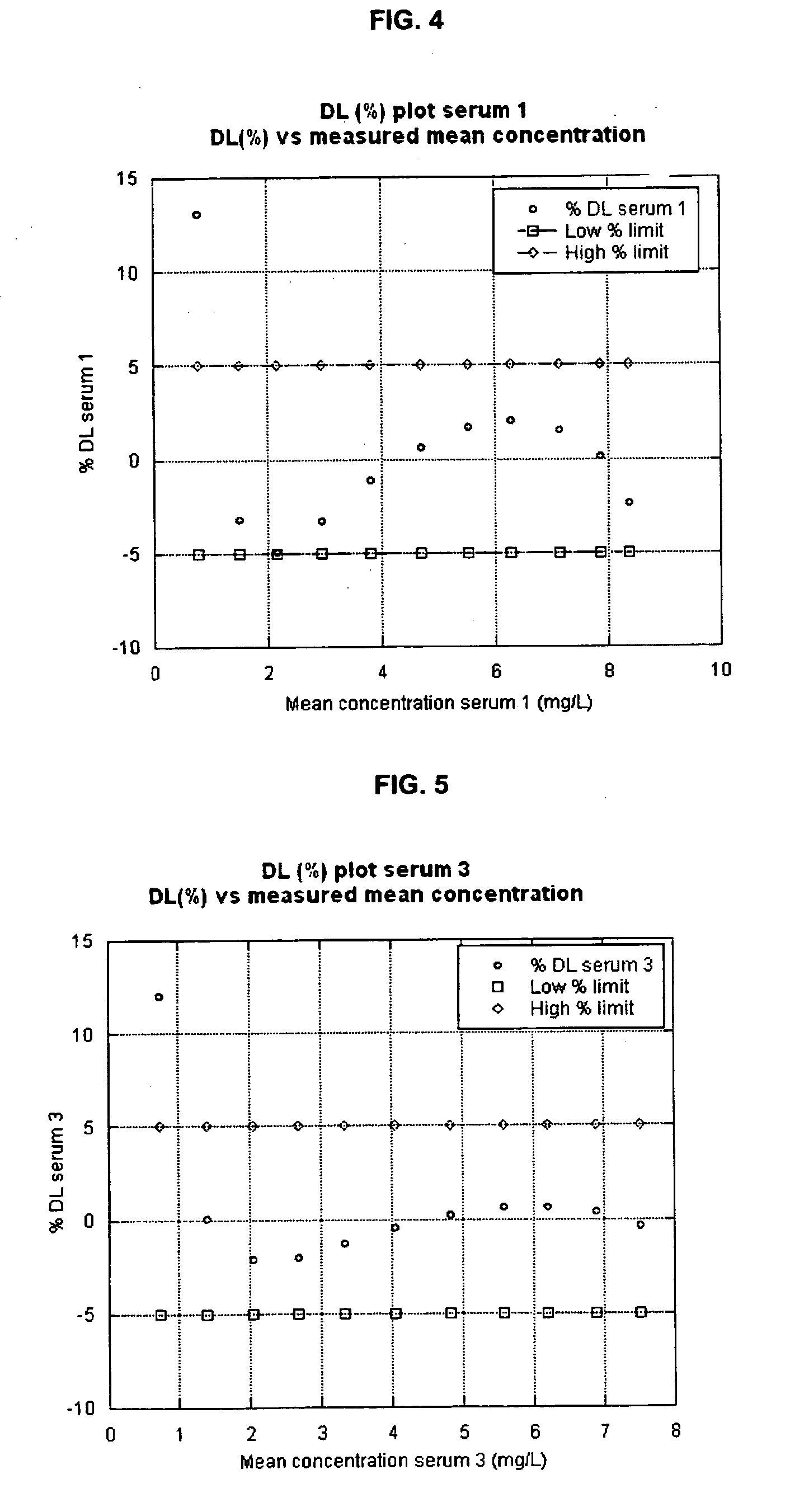
Cystatin C or cystatin 3 (formerly gamma trace, post-gamma-globulin, or neuroendocrine basic polypeptide), a protein encoded by the CST3 gene, is mainly used as a biomarker of kidney function. Recently, it has been studied for its role in predicting new-onset or deteriorating cardiovascular disease. It also seems to play a role in brain disorders involving amyloid (a specific type of protein deposition), such as Alzheimer's disease. In humans, all cells with a nucleus (cell core containing the DNA) produce cystatin C as a chain of 120 amino acids. It is found in virtually all tissues and body fluids. It is a potent inhibitor of lysosomal proteinases (enzymes from a special subunit of the cell that break down proteins) and probably one of the most important extracellular inhibitors of cysteine proteases (it prevents the breakdown of proteins outside the cell by a specific type of protein degrading enzymes). Cystatin C belongs to the type 2 cystatin gene family.
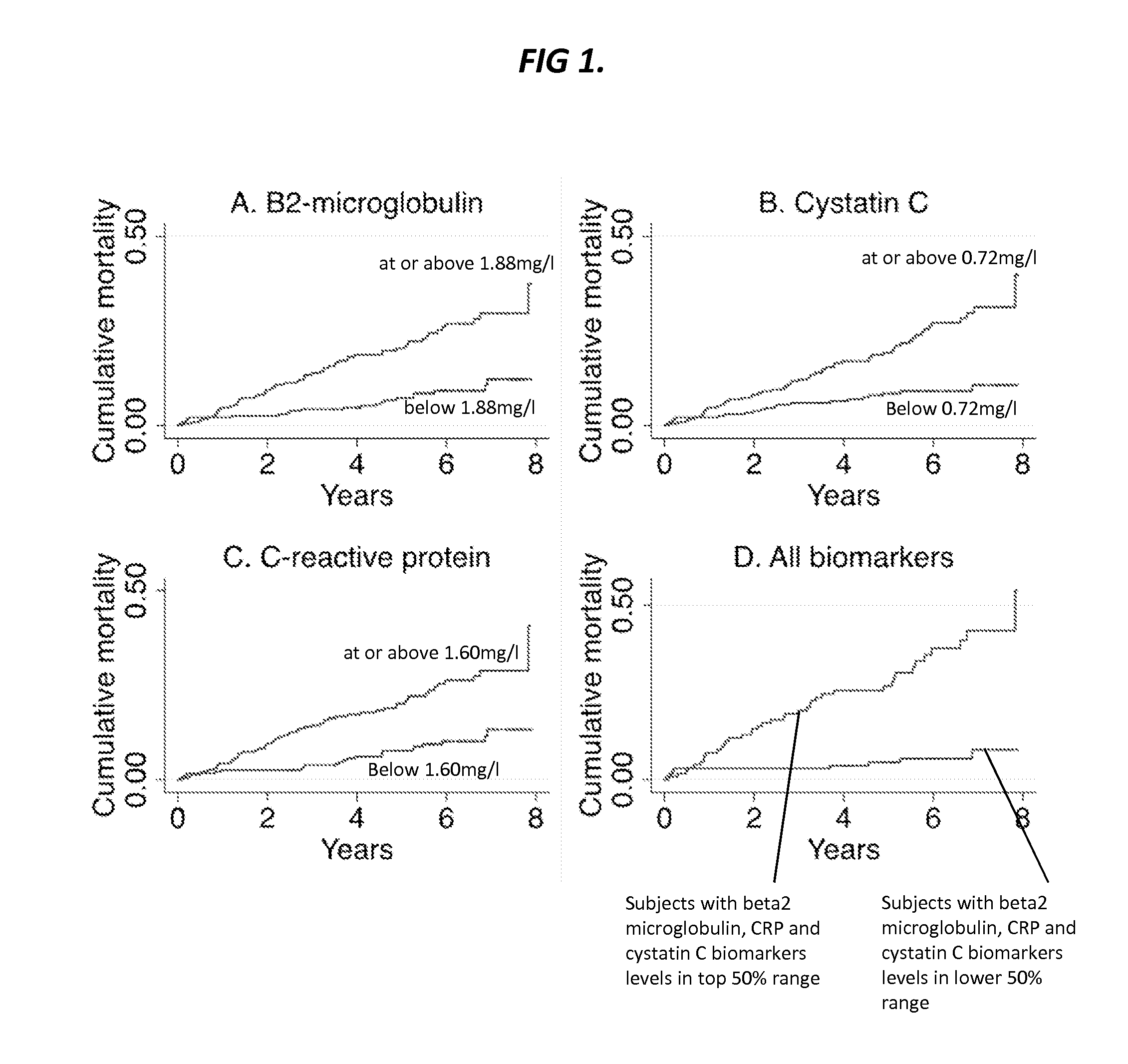
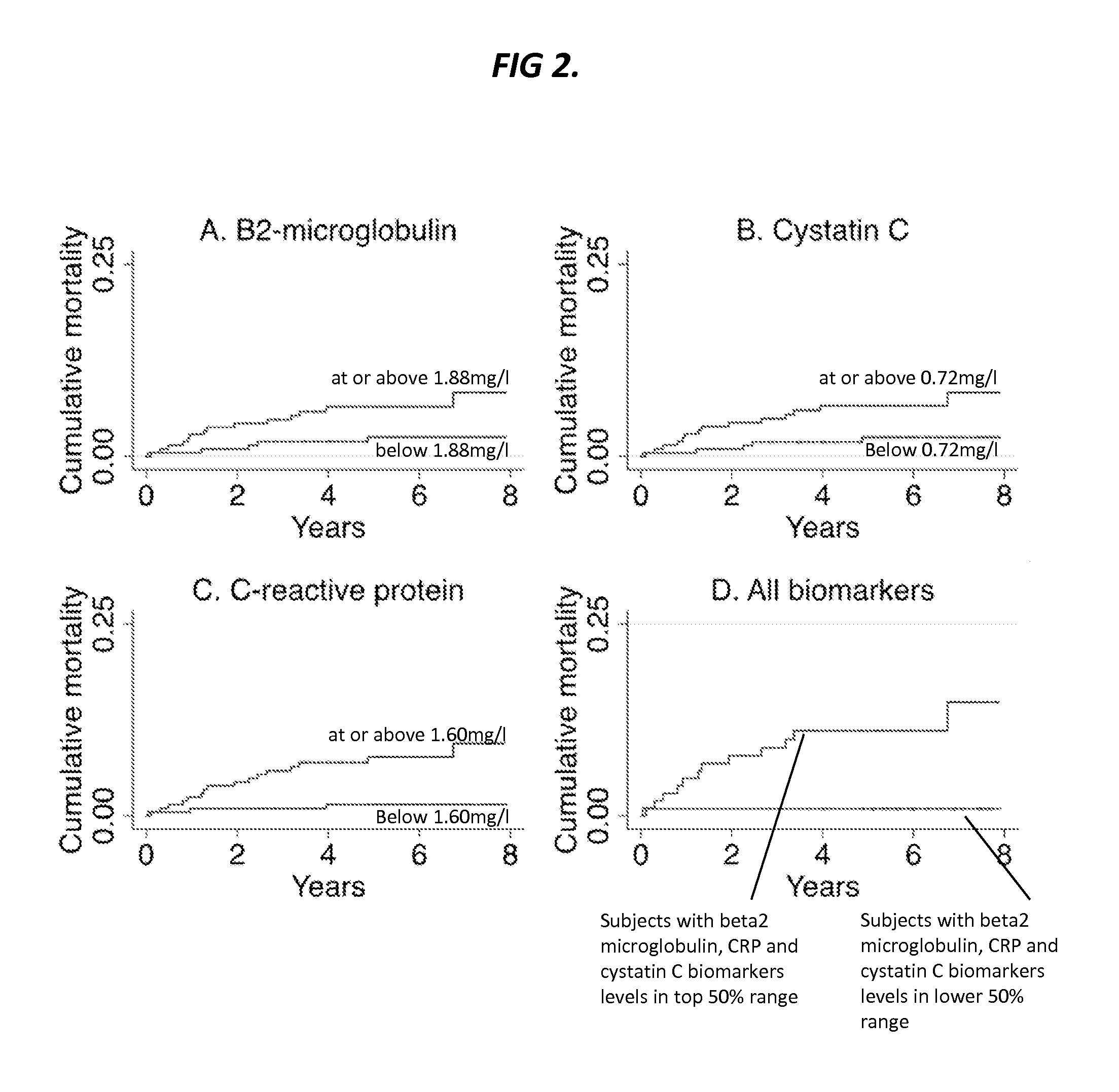
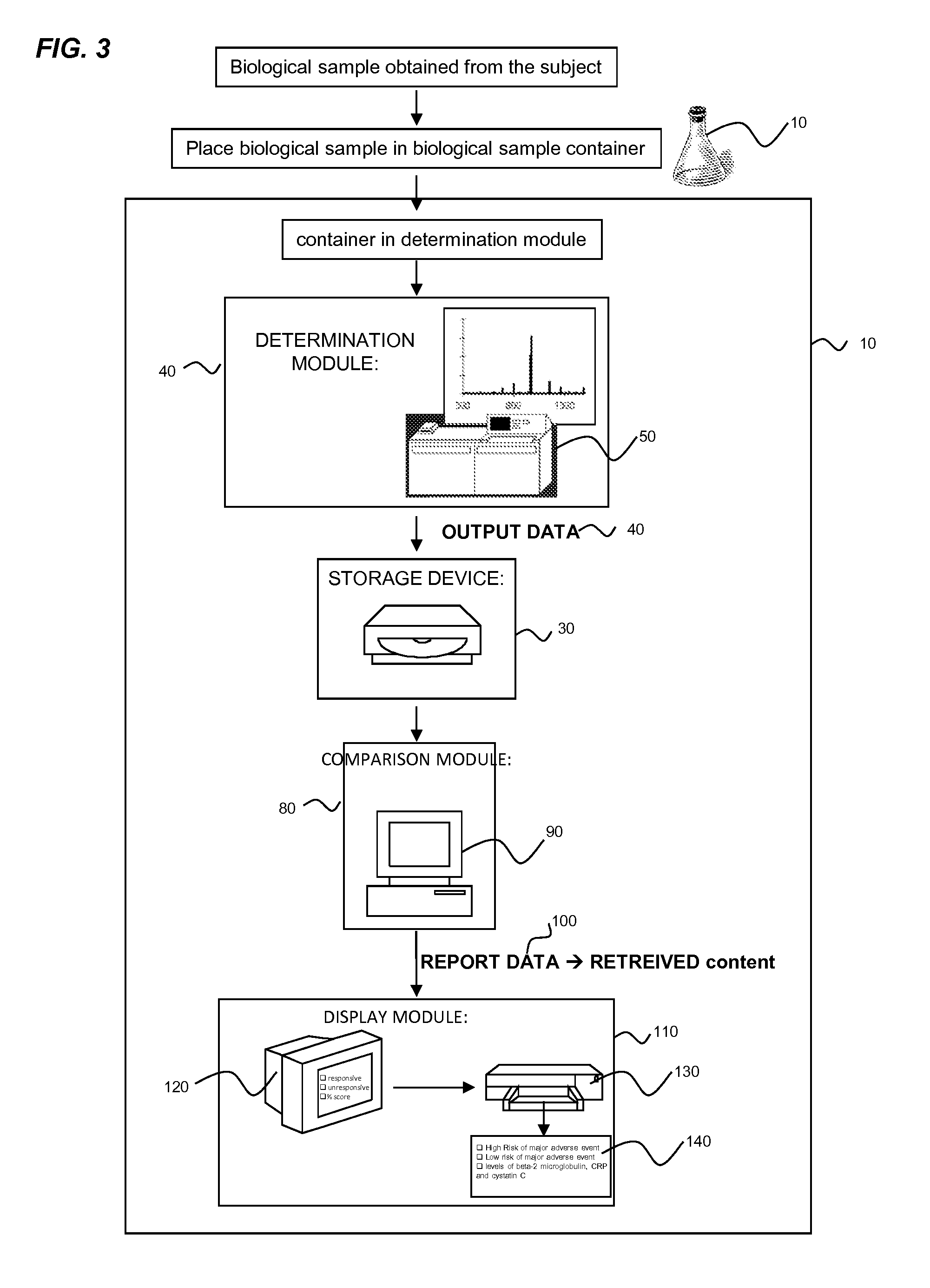
Biomarkers for predicting major adverse events
InactiveUS20140187519A1Improve risk modelingIdentifying riskSalicyclic acid active ingredientsBiocideCvd riskBeta-2 microglobulin
Provided herein are diagnostic markers and uses thereof for predicting if a subject is at risk of a major adverse event. In particular, one aspect provided herein relates to methods to determine if a subject is at risk of having a major adverse effect by measuring at least 2, or at least 3 of the biomarkers beta 2 microglobulin, c-reactive protein and cystatin C.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
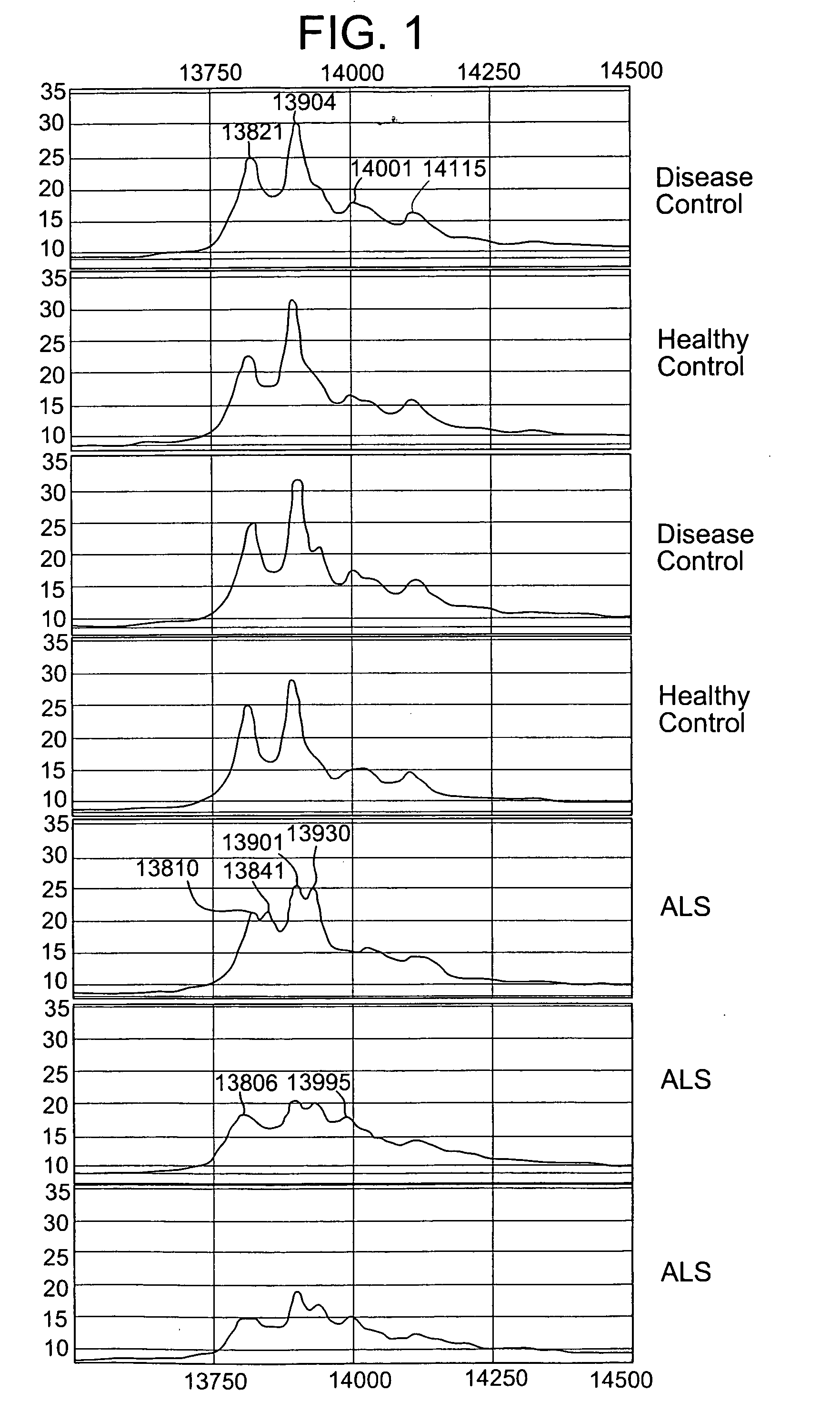
Modulation of the neuroendocrine system as a therapy for motor neuron disease
Owner:UNIVERSITY OF PITTSBURGH
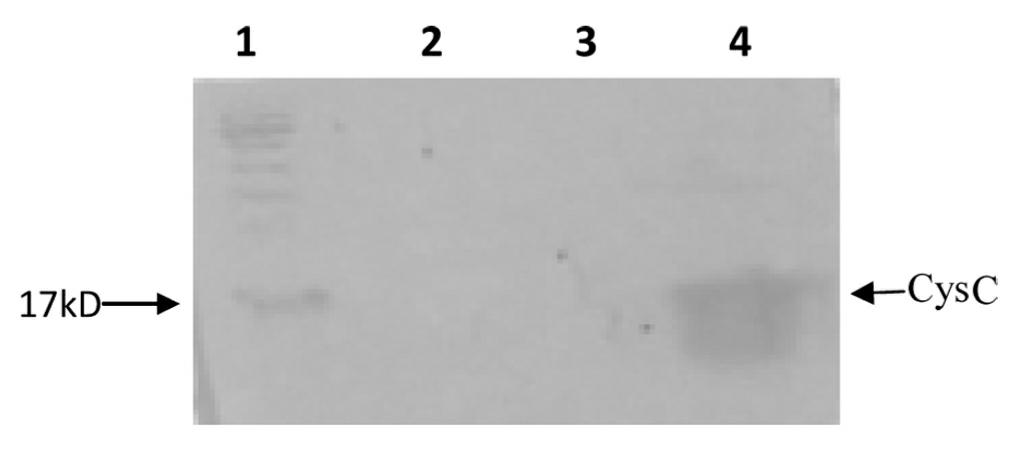
Magnetic particle separation chemiluminescence immunoassay method of human cystatin C
InactiveCN101937000AHigh sensitivityStrong specificityChemiluminescene/bioluminescenceBiological testingFiltrationBlood plasma
The invention provides an in-vitro detection method of human cystatin C. The method adopts a magnetic particle separation chemiluminescence immunoassay technology which is a product integrating an enzyme labeling technology, a magnetic particle separation technology and a chemiluminescence detection technology and has the advantages of high sensitivity, good specificity, good repetitiveness, and the like. The method can be used for measuring the content of cystatin C in the serum, the plasma and the urine of a person, and the indexes are mainly used for clinically evaluating the renal function and mainly used for monitoring the filtration rate of glomerulus, the renal tubular dysfunction and various secondary nephropathies.
Owner:北京倍爱康生物技术有限公司
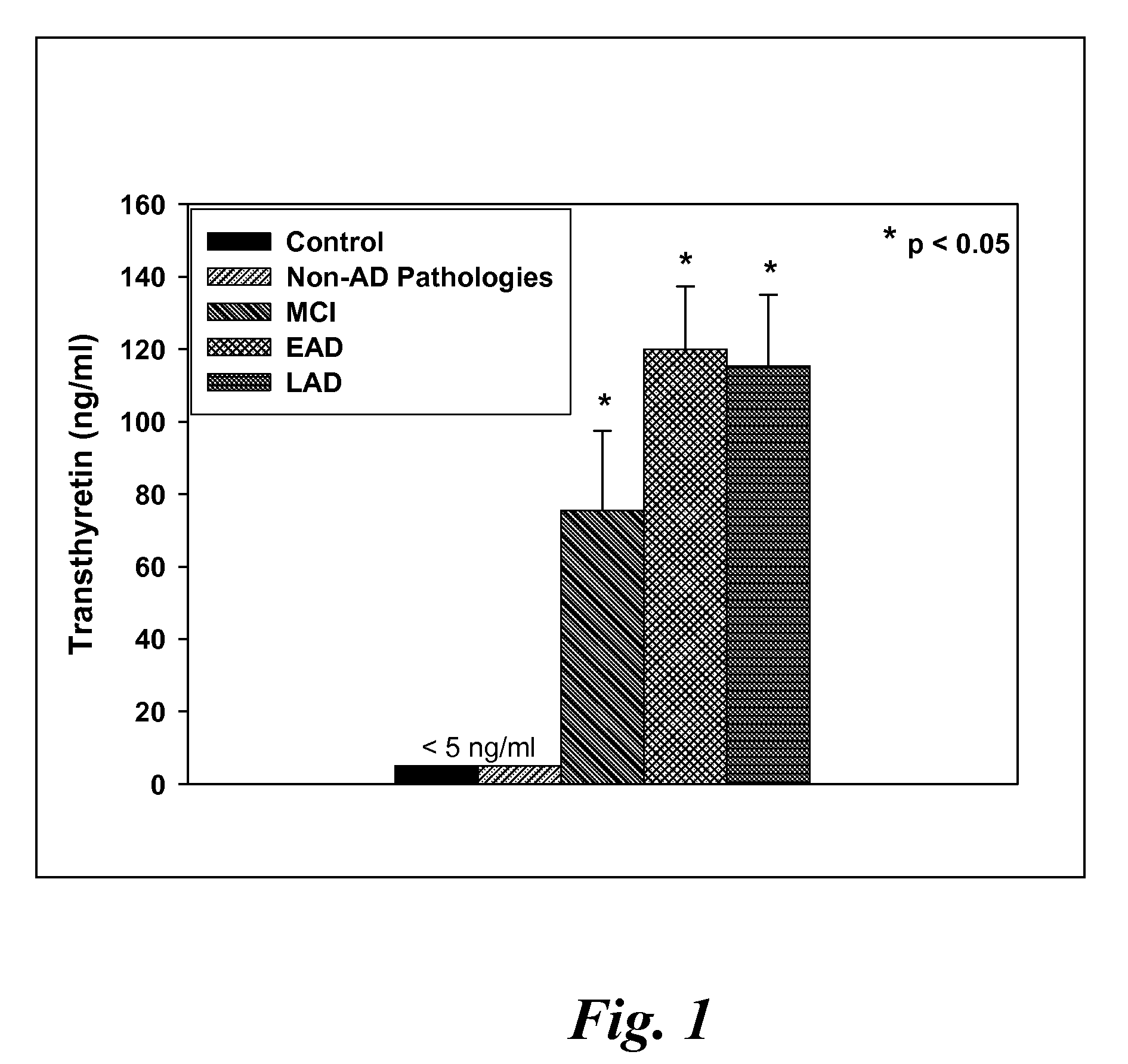
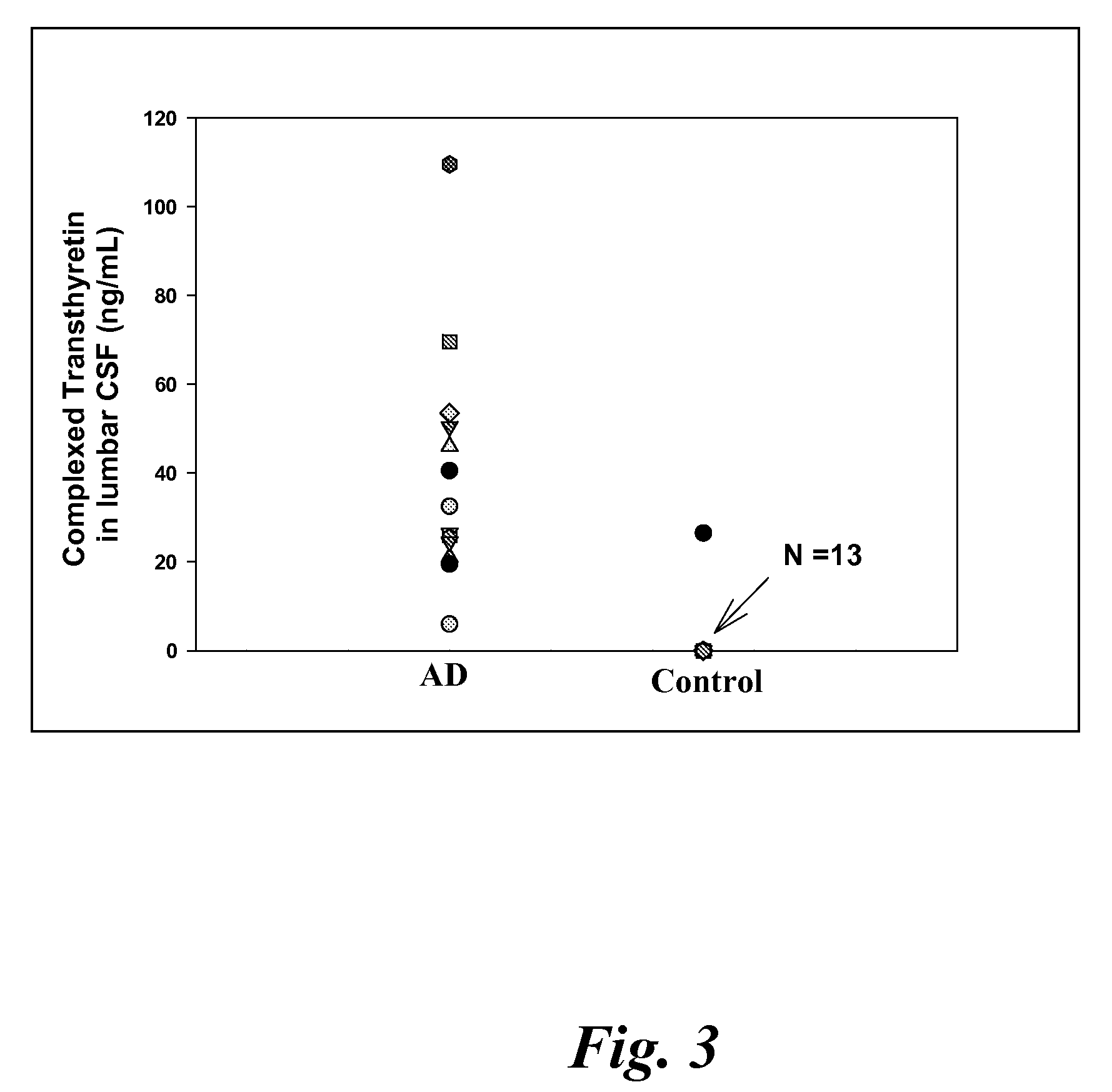
Biomarkers of mild cognitive impairment and alzheimer's disease
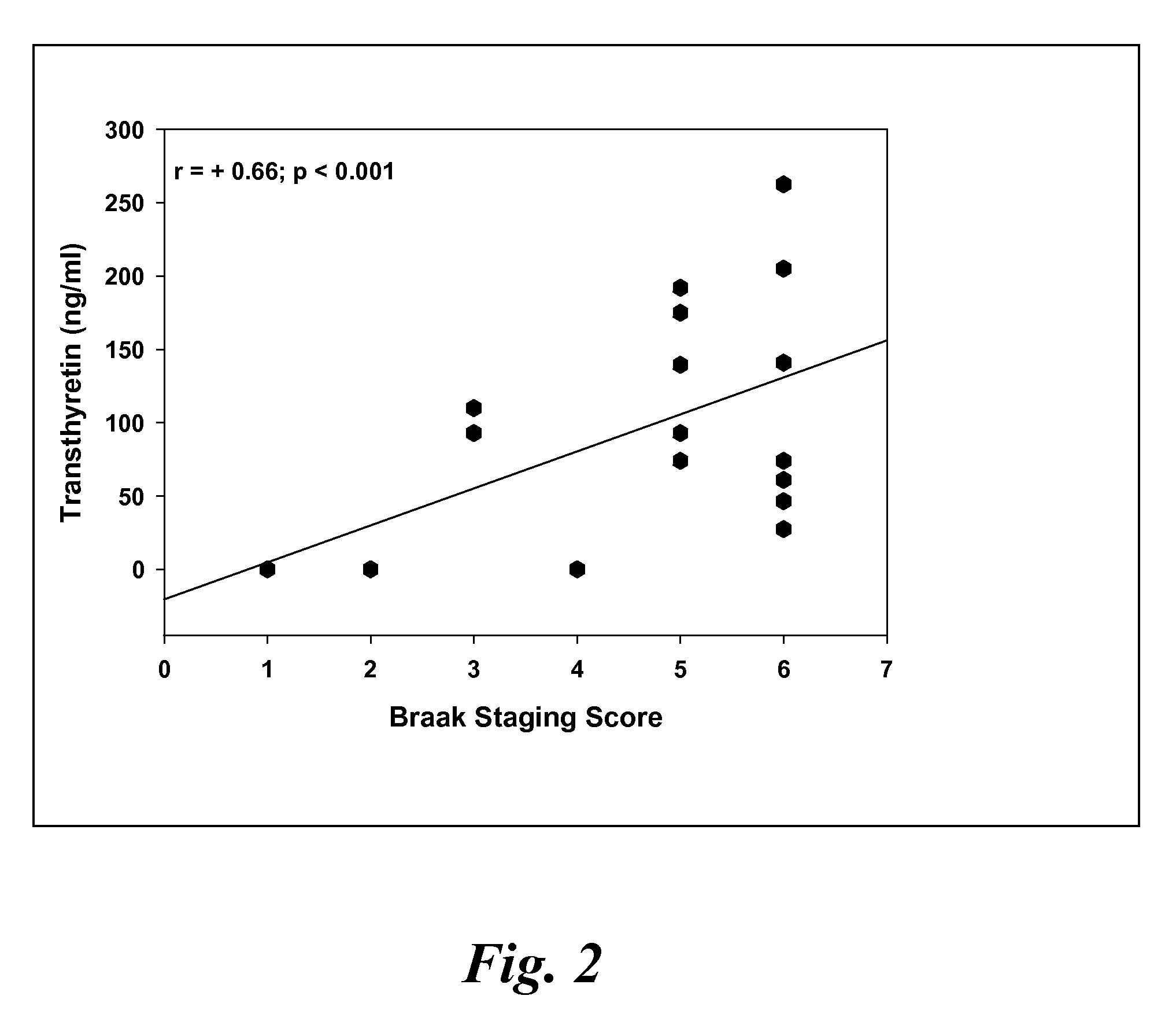
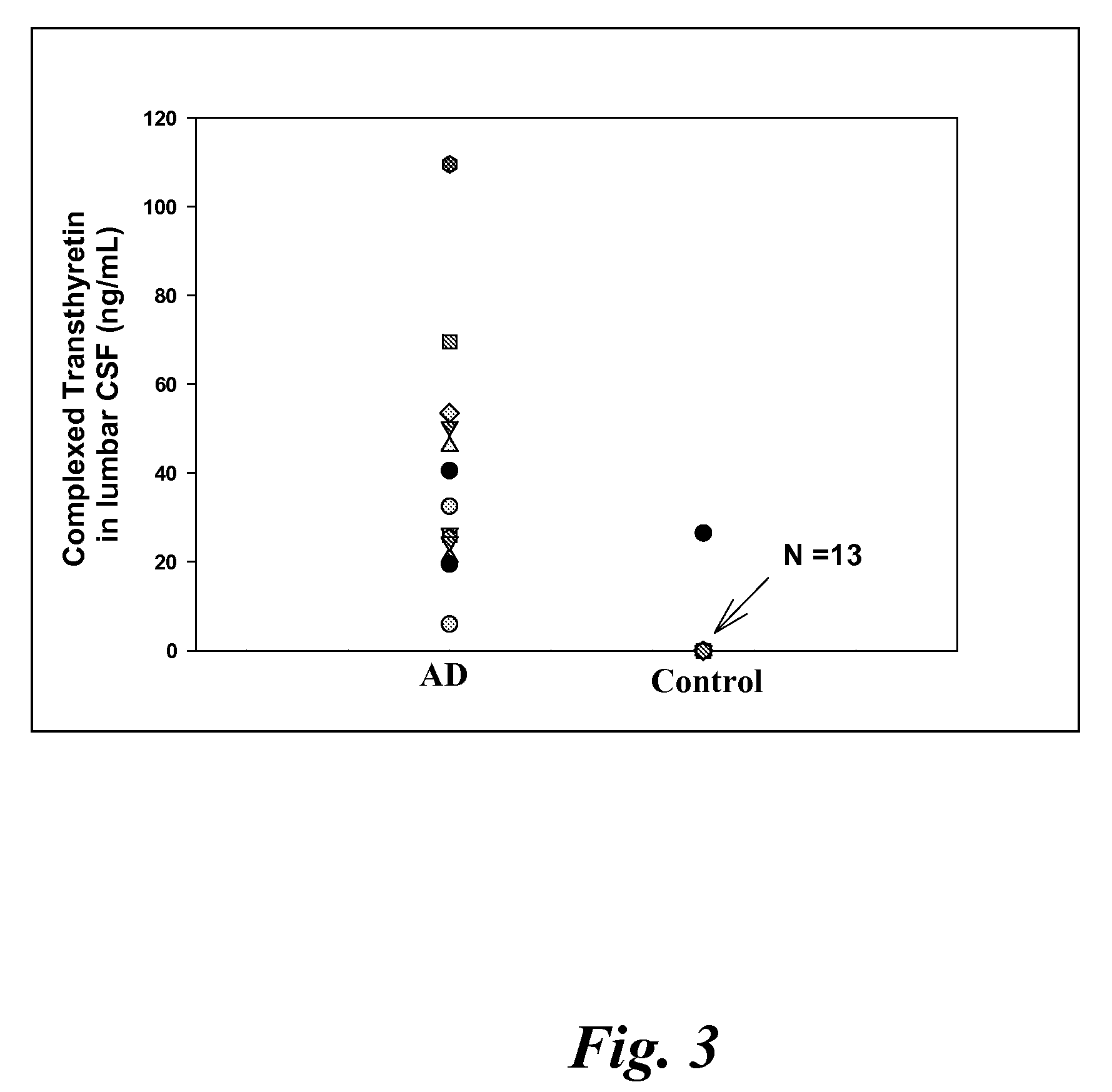
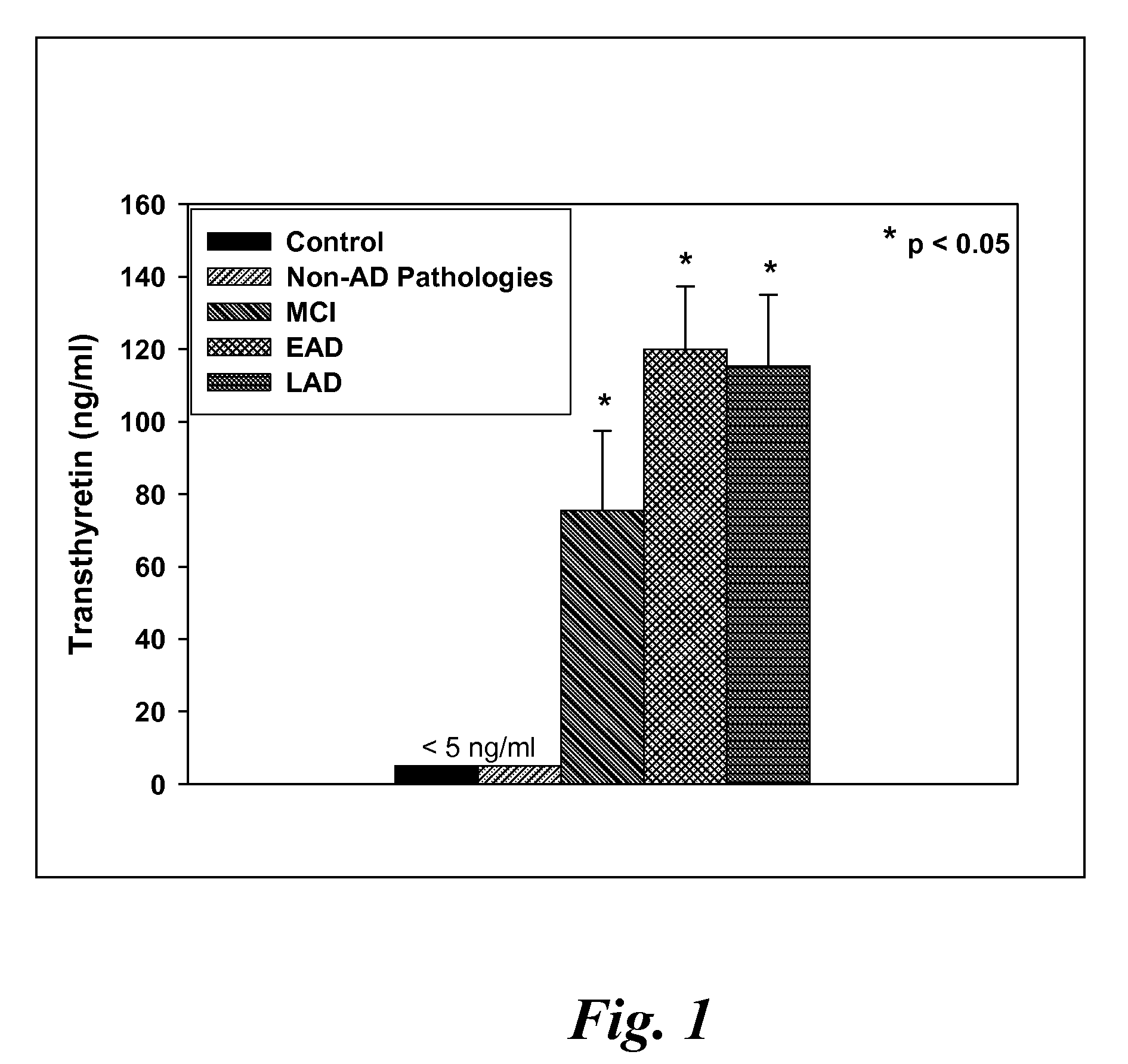
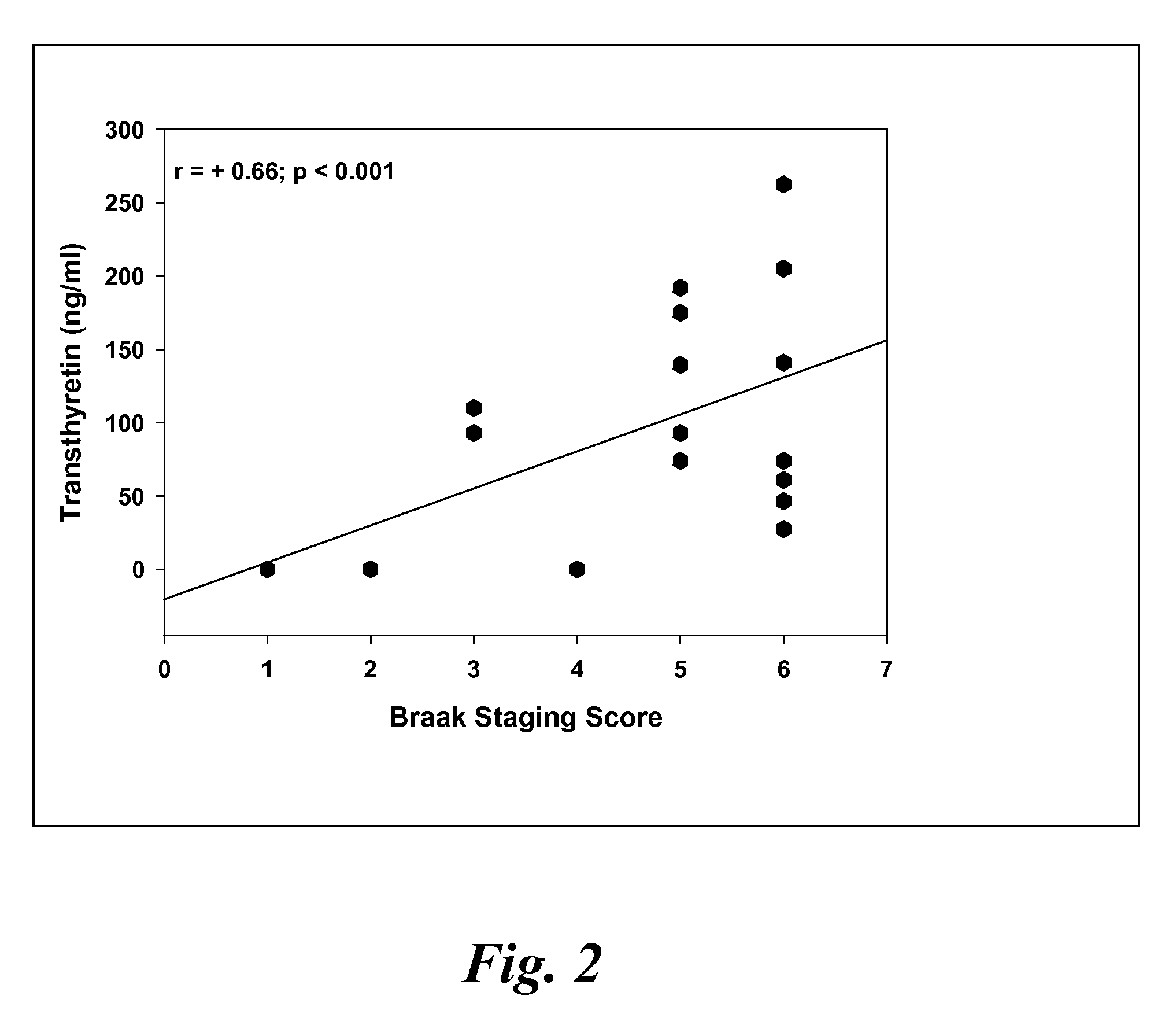
A method for quantifying a neurodegenerative disorder in a patient that includes obtaining a fluid sample from the subject; measuring a protein biomarker complex in said fluid sample and correlating the measurement with mild cognitive impairment or Alzheimer's disease status. The biomarkers include those that comprise at least one of a transthyretin protein and / or a prostaglandin-H2 D-isomerase protein, and at least one second, different protein selected from a transthyretin, prostaglandin-H2 D-isomerase, beta-2-microglobulin, cystatin C, superoxide dismutase [Cu—Zn], plasma retinol-binding protein, phosphatidylethanolamine-binding protein, carbonic anhydrase 2, prostaglandin-H2 D-isomerase, and / or serotransferrin protein.
Owner:UNIV OF KENTUCKY RES FOUND
Biomarkers of mild cognitive impairment and alzheimer's disease
A method for quantifying a neurodegenerative disorder in a patient that includes obtaining a fluid sample from the subject; measuring a protein biomarker complex in said fluid sample and correlating the measurement with mild cognitive impairment or Alzheimer's disease status. The biomarkers include those that comprise at least one of a transthyretin protein and / or a prostaglandin-H2 D-isomerase protein, and at least one second, different protein selected from a transthyretin, prostaglandin-H2 D-isomerase, beta-2-microglobulin, cystatin C, superoxide dismutase [Cu—Zn], plasma retinol-binding protein, phosphatidylethanolamine-binding protein, carbonic anhydrase 2, prostaglandin-H2 D-isomerase, and / or serotransferrin protein;
Owner:UNIV OF KENTUCKY RES FOUND
Improved cystatin C detection kit
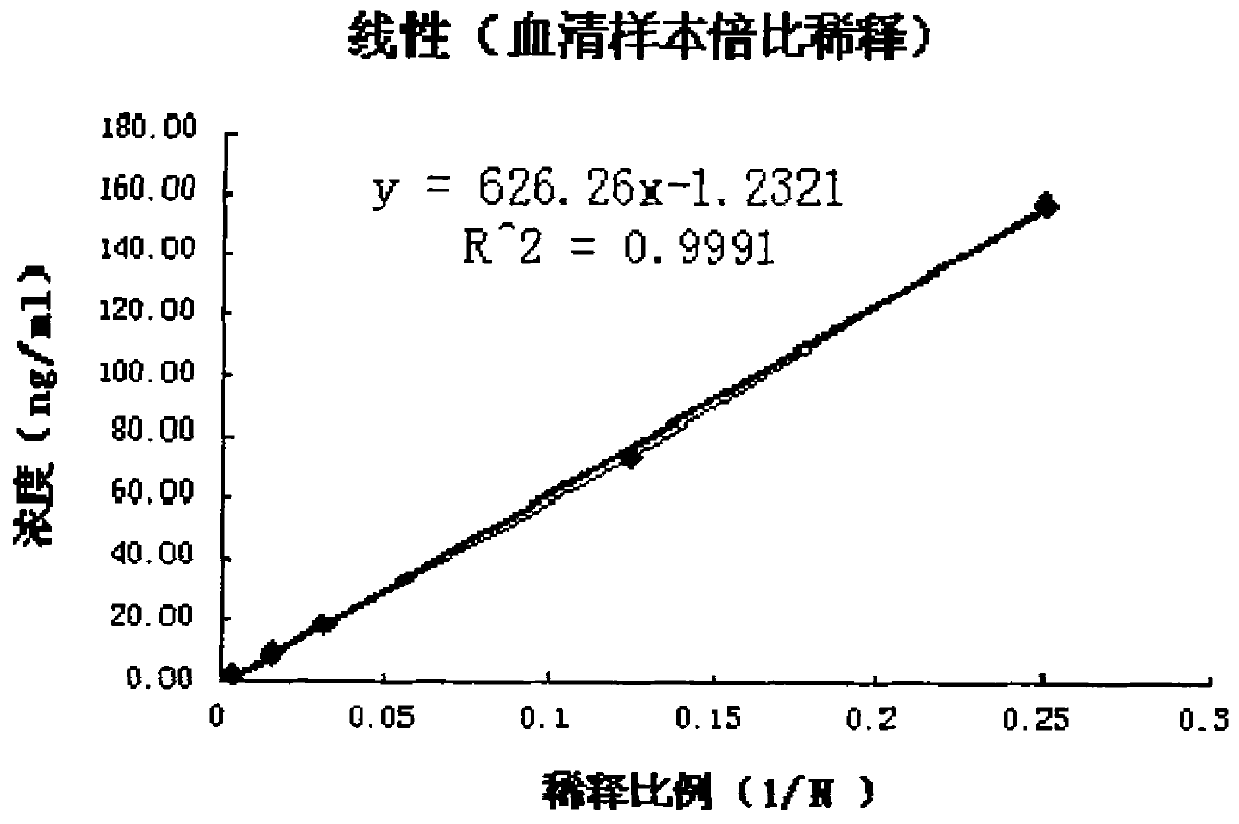
The invention provides a latex enhanced turbidimetric immunoassay kit for quantitatively detecting cystatin C. The kit comprises a reagent R1 and a reagent R2 which are independent from each other, wherein the reagent R1 is mainly prepared from a buffer solution 1, a stabilizing agent 1, a preservative 1, EDTA, accelerator and a protective agent 1; the reagent R2 is mainly prepared from polystyrene latex microspheres for crosslinking a cystatin C antibody, a buffer solution 2, a stabilizing 2, a preservative 2 and a protective agent 2, wherein the polystyrene latex microspheres and the cystatin C antibody are connected in a covalent cross-linking mode. The detection kit has the advantages of low preparation cost, good stability, good data repeatability and high detection sensitivity, is easy to store, and can be widely applied to clinical biochemical analyzers.
Owner:ZYBIO INC
Cysteine protease inhibitor C test kit
The invention provides a cysteine protease inhibitor C test kit, wherein the kit comprises a reagent R1 and a reagent R2, the reagent R2 is a reaction solution for promoting specific binding of an antigen and an antibody, the reagent R2 is a polystyrene latex particle mixture coated by an anti-human cystatin C antibody and is put in a proper buffer solution. The polystyrene latex particle mixture in the reagent R2 is a mixture of large-diameter polystyrene latex particles and small-diameter polystyrene latex particles. The kit is high in detection sensitivity, high in accuracy, good in linearity within the test range, good in stability and low in production cost.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Human cystatin C chemiluminescence quantitative detection method
InactiveCN103018465ANo pre-dilution requiredImprove accuracyChemiluminescene/bioluminescenceBiological testingDiseaseImmunonephelometric Assays
The invention provides a human cystatin C quantitative detection method. According to the method, a magnetic micro-particle separation technology and an enzymatic chemiluminescence technology are combined, the principle of a double-antibody sandwich one-step reaction method is adopted, and the method can be used for content determination of human cystatin C in serum, plasma and urine samples and assistance in diagnosis and treatment of various renal dysfunction diseases. The sensitivity of the method is not more than 0.01mu g / L, the quantitative detection range is 0.05-8mu g / L, the precision in analysis is less than 5%, the detection time is 10 minutes, the recovery rate of the added serum and urine is 90-115%, and the correlation analysis R2 with a reagent serum value detected by a latex-enhanced immunoturbidimetric method is 0.9862.
Owner:BEIJING LEADMAN BIOCHEM
Cystatin C detection kit and preparation method thereof
InactiveCN108107201AIncrease absorbanceHigh blank absorbanceBiological material analysisColor/spectral properties measurementsMicrosphereCentrifugation
The invention provides a cystatin C detection kit and a preparation method thereof. The kit comprises a reagent R1, a reagent R2 and a cystatin C calibration product, wherein the reagent R1 comprisesa preservative, a surfactant, an emulsifier and a buffer solution; the reagent R2 comprises a cystatin C antibody, polystyrene latex microspheres, a latex microsphere activating agent, a sealing agent, an emulsifier, a preservative, a surfactant and a buffer solution. The cystatin C antibody is slowly dropwise added to an activated polystyrene latex microsphere solution, a polystyrene latex microsphere suspension coupled with the cystatin C antibody is obtained after a stirring reaction, sealing is performed, the antibody and latex microspheres are coupled together in a chemical crosslinking manner, and centrifugation, cleaning and ultrasonic resuspension processes in conventional methods are omitted. The production process is simplified, and the kit has the advantages of low blank absorbency, high sensitivity and precision and large linear range.
Owner:QINGDAO HIGHTOP BIOTECH
Turbidimetric immunoassay for assessing human cystatin c
ActiveUS20100047922A1Strong and faster signalReduce distractionsDisease diagnosisImmunoglobulinsTurbidityBody fluid
There is a demand for improved turbidimetric immunoassays for human Cystatin C in biological samples, especially in human clinical samples of body fluids. The present invention provides a turbidimetric immunoassay method and reagent set enabling measurement of human Cystatin C by turbidimetric methods, resulting in a surprisingly stronger and faster turbidimetric signal than in the present state of the art. The increased and faster signal is accomplished by the use of new reagents and compositions, and enables shorter assay times and kinetic reading with a stronger signal, improving overall assay speed and quality. Improved robustness to lipid interference and improved linearity is achieved.
Owner:GENTIAN AS
Determination of Exosomel Biomarkers for Predicting Cardiovascular Events
InactiveUS20120309041A1Reduce stepsComplicate to detectMicrobiological testing/measurementImmunoglobulins against animals/humansAmniotic fluidNGAL Protein
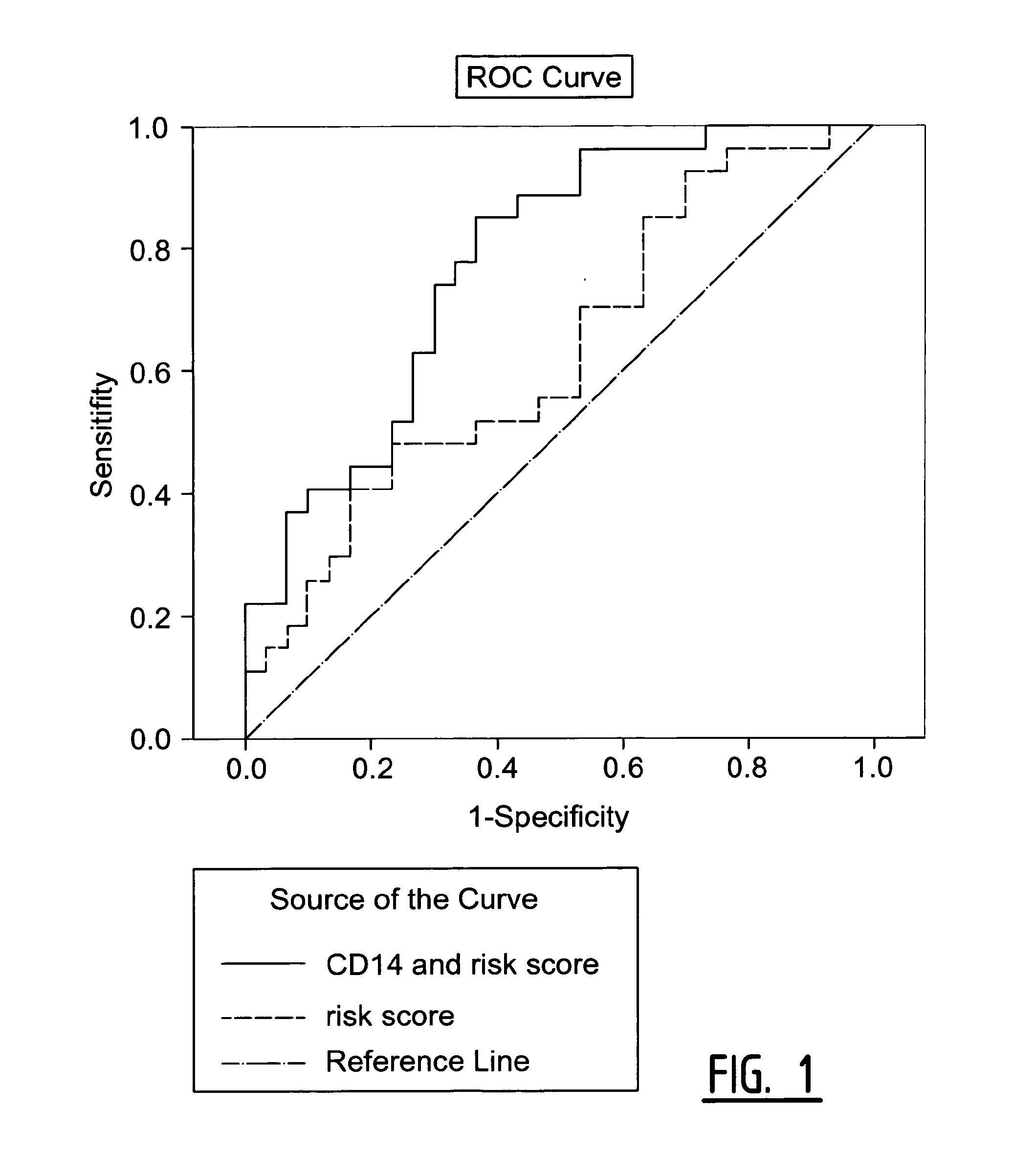
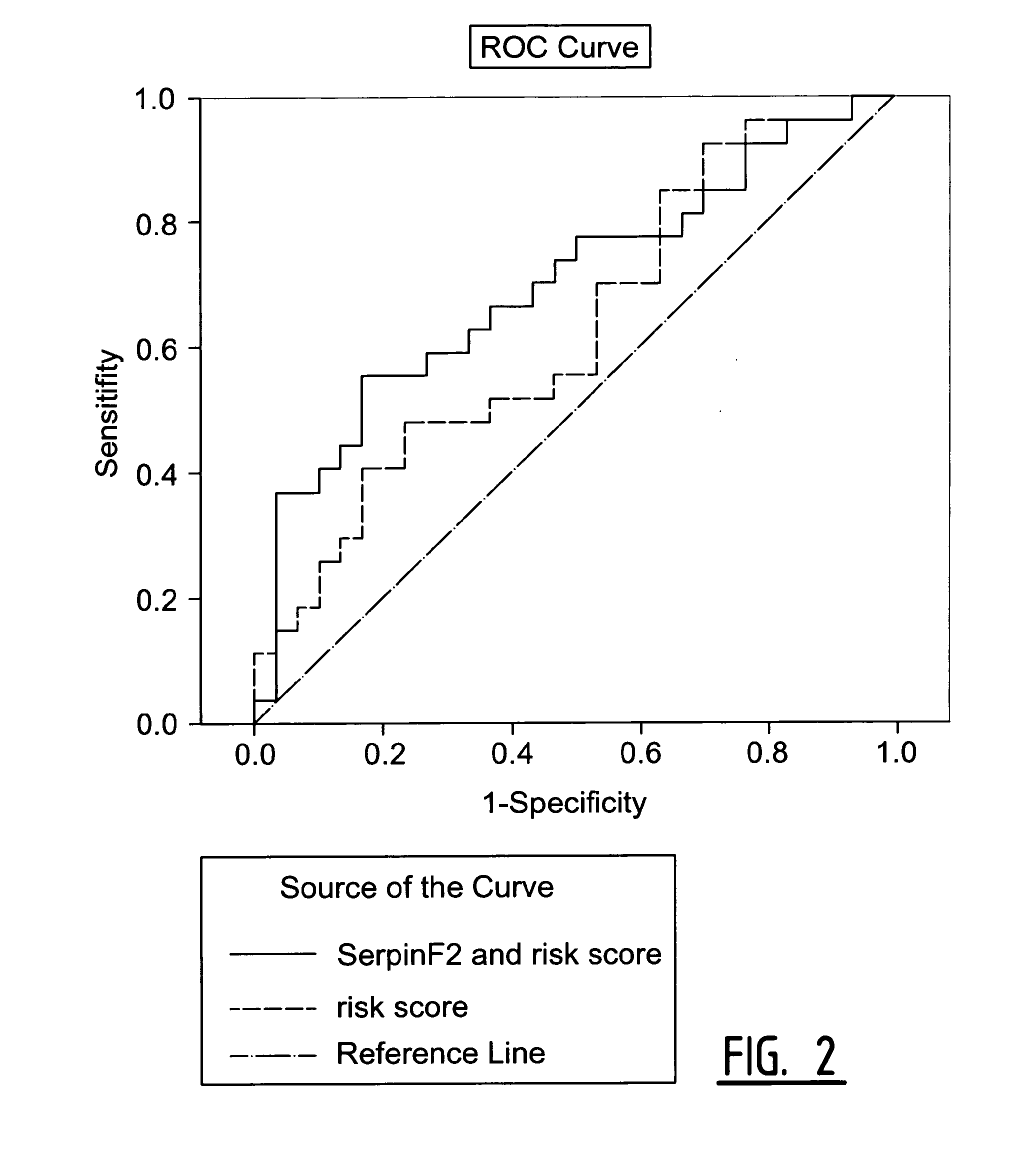
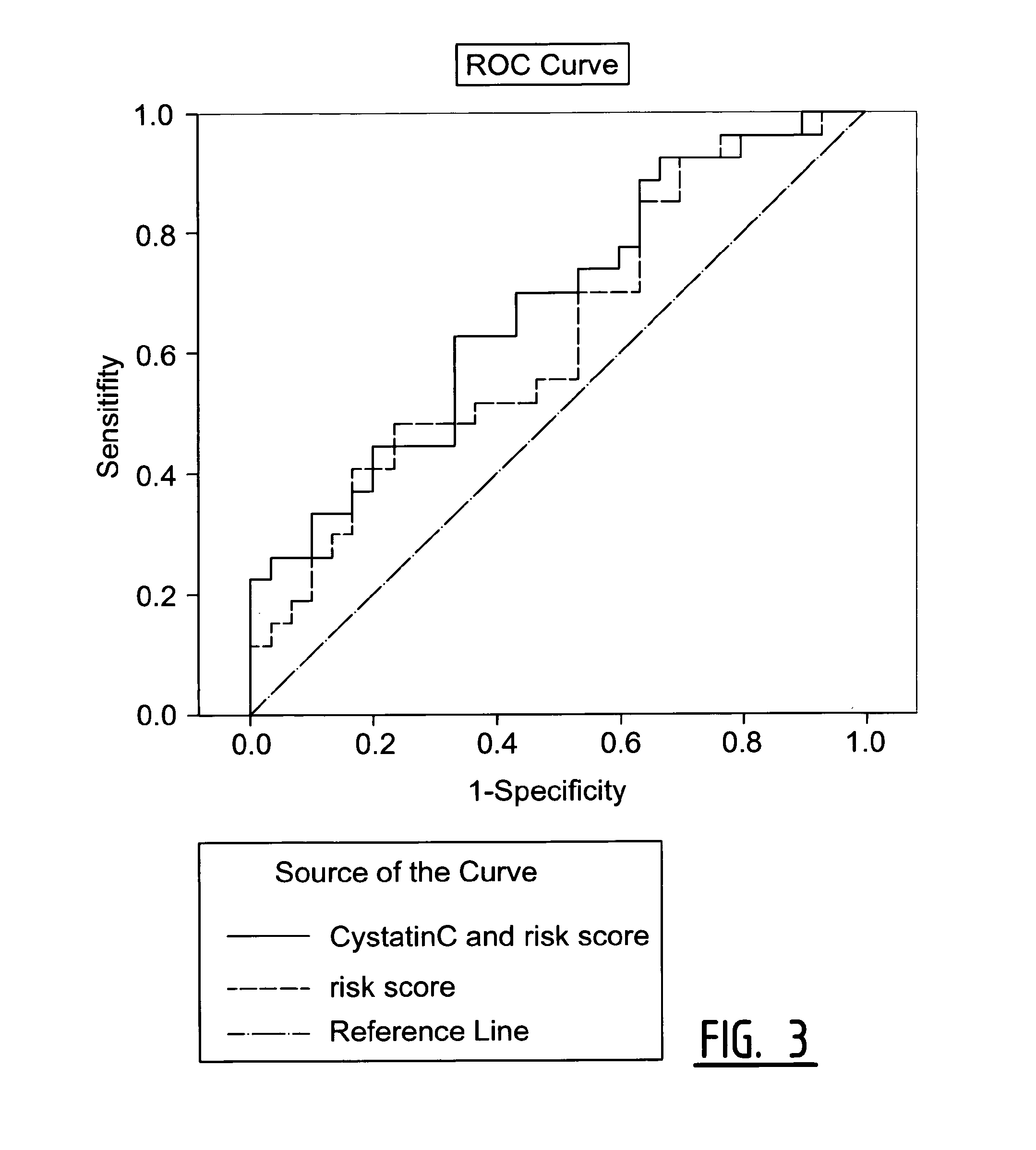
The present invention relates to a method of predicting the risk of a subject developing a cardiovascular event, comprising determining the presence of a biomarker that is indicative of the risk of developing a cardiovascular event in exosomes from the subject. The exosomes are suitably isolated from a body fluid selected from serum, plasma, blood, urine, amniotic fluid, malignant ascites, bronchoalveolar lavage fluid, synovial fluid, breast milk, saliva, in particular serum. The biomarker is selected from the proteins Vitronectin, Serpin F2, CD14, Cystatin C, Plasminogen, Nidogen 2 or any combination of two or more of these proteins.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
Cystatin C fluorescent microsphere immunochromatographic quantitative test strip, and test card thereof
InactiveCN108398562AQuick checkSimple production processBiological testingMonoclonal antibodyPolyclonal antibodies
The invention relates to a cystatin C fluorescent microsphere immunochromatographic quantitative test strip, and a test card including the test strip. The test strip comprises a base plate, a sample pad, a binding pad, a nitrocellulose membrane and an absorbent paper, the binding pad is provided with a fluorescent microsphere-labeled cystatin C monoclonal antibody, and the nitrocellulose membraneis coated with a detection line formed by a cystatin C polyclonal antibody or a monoclonal antibody and a quality control line formed by a sheep anti-mouse IgG polyclonal antibody. The test strip realizes the rapid and quantitative detection and analysis of cystatin C, has the advantages of high sensitivity, good accuracy, wide linear range, short detection time and convenience in use, and can meet bedside emergency and onsite rapid detection demands.
Owner:河南省生物工程技术研究中心
Cystatin C latex-particle-enhanced turbidimetry detection reagent kit and application thereof
ActiveCN105738617AEnhanced detection signalHigh detection sensitivityMaterial analysisLatex particleTurbidimetry
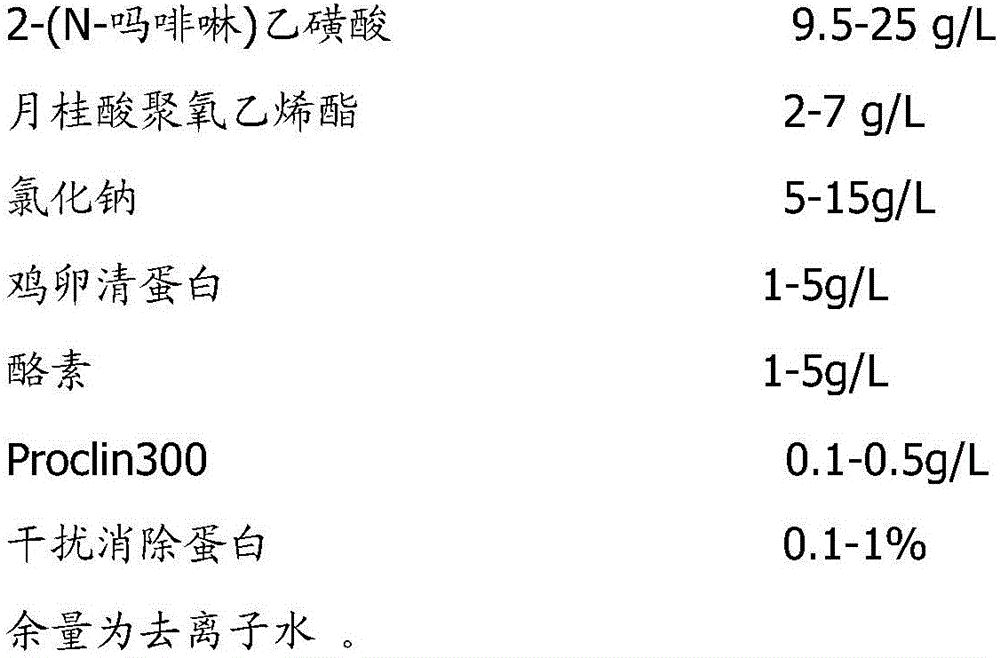
The invention provides a cystatin C latex-particle-enhanced turbidimetry detection reagent kit and application thereof.The reagent kit comprises a reagent R1 and a reagent R2.The reagent R1 is prepared from a buffer solution, inorganic salt, a surfactant, a preservative, a stabilizer and interference elimination protein.The reagent R2 is prepared from a buffer solution, inorganic salt, a surfactant, a preservative, a stabilizer and a polystyrene latex particle mixture, and the polystyrene latex particle mixture is interlinked with a cystatin C antibody.The reagent kit based on latex-particle-enhanced turbidimetric immunoassay (PETIA) can be generally applied to analysis of various full-automatic biochemical analyzers and is short in assay time, good in specificity, high in precision and good in accuracy when used.
Owner:WUHAN LIFE ORIGIN BIOTECH LTD
Cystatin C detection kit and preparation method therefor
ActiveCN103645323AHigh sensitivityGood precisionBiological material analysisBiological testingMedicinePolystyrene
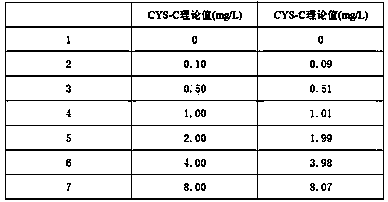
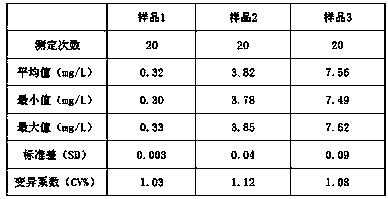
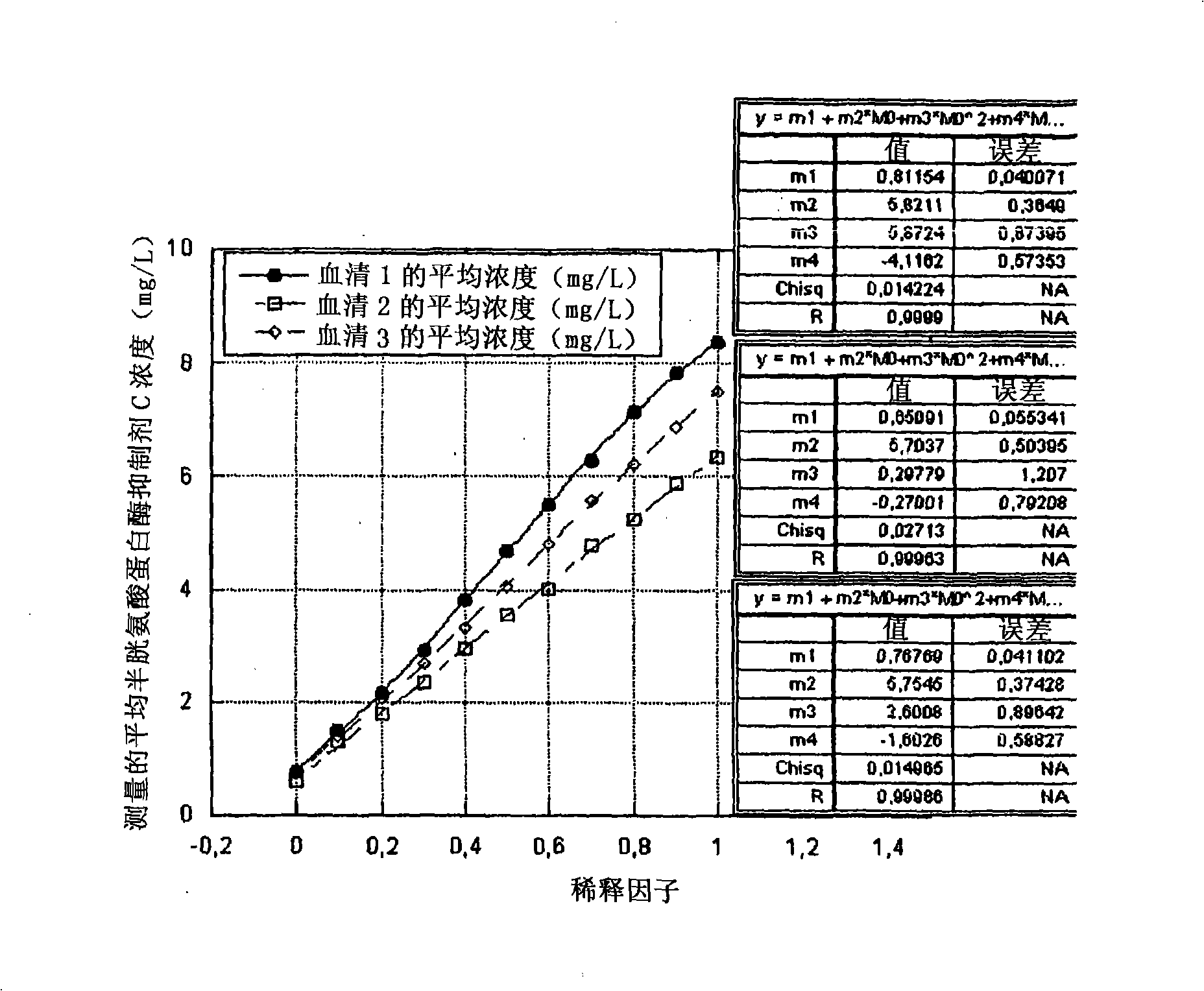
The invention relates to the field of medical immunology in vitro diagnosis, and especially provides a detection kit for detecting cystatin C in serum. The detection kit comprises a reagent R1, a reagent R2 and calibration materials. The ingredients of the reagent R1 are a Tris buffer with a concentration of 0.01-0.05mol / L, PEG 6000 with a concentration of 10-40g / L, NaN3 with a concentration of 0.5-1.5g / L, and sodium chloride with a concentration of12-20g / L, and the pH value is 8.0-8.5. The ingredients of the reagent R2 are a Tris buffer with a concentration of 0.01-0.02mol / L, and polystyrene latex granules coated by goat-anti-human cystatin C polyclonal antibodies with a concentration of 0.5-3.0 g / L. The calibration materials are six gradient standards with cystatin C and the contents of cystatin C are 0.1, 0.5, 1.0, 2.0, 4.0 and 8.0mg / L. The solution is a Tris buffer with a concentration of 0.01mol / L. The kit has simple composition, good stability and high accuracy.
Owner:浙江夸克生物科技有限公司
Recombined human cystatin-C protein with natural activity and preparation method thereof
ActiveCN103014047AImprove solubilitySolve the key problems of independent research and developmentMicroorganism based processesProtease inhibitorsBiotechnologyProteinase activity
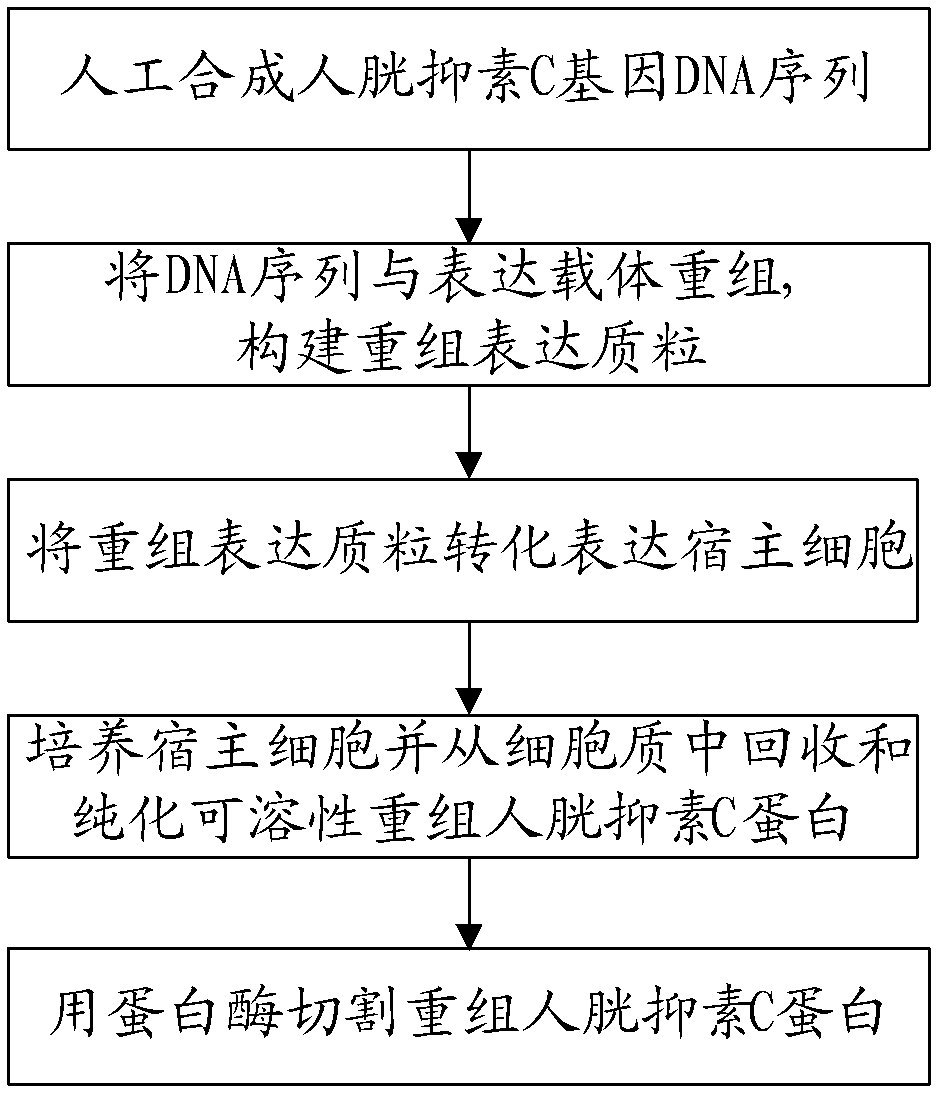
The invention discloses a recombined human cystatin-C protein with natural activity and a preparation method thereof. According to the preparation method, lots of stable recombined human cystatin-C proteins are expressed and purified by employing a gene engineering technique, and the recombined human cystatin-C proteins are cut by utilizing the protease, so that the recombined human cystatin-C protein has high natural activity. The recombined human cystatin-C protein can be used for immune preparation of monoclonal antibodies and multi-antibody blood serum, and excellent raw materials are provided for preparing stable cystatin-C detection agents and quality control products thereof.
Owner:深圳前海菲鹏基因科技有限公司 +1
Turbidimetric immunoassay for assessing human cysteine proteinase inhibitor C
There is a demand for improved turbidimetric immunoassays for human Cystatin C in biological samples, especially in human clinical samples of body fluids. The present invention provides a turbidimetric immunoassay method and reagent set enabling measurement of human Cystatin C by turbidimetric methods, resulting in a surprisingly stronger and faster turbidimetric signal than in the present state of the art. The increased and faster signal is accomplished by the use of new reagents and compositions, and enables shorter assay times and kinetic reading with a stronger signal, improving overall assay speed and quality. Improved robustness to lipid interference and improved linearity is achieved.
Owner:根田股份有限公司
Nanometer antibodies to human cystatin C and application thereof
InactiveCN103509115AEasy to manufactureLow costTissue cultureViruses/bacteriophagesEpitopeMonoclonal antibody
The invention relates to nanometer antibodies to human cystatin C and high-sensitivity quantitative-detection kits thereof. The invention reveals nanometer antibodies and monoclonal antibodies to different epitopes of cystatin C, and the antibodies have good compatibility with cystatin C. Through usage of the nanometer antibodies and the monoclonal antibodies, a series of kits capable of conveniently, rapidly and accurately detecting cystatin C can be prepared. The invention provides the nanometer antibodies which can be used for matched detection of cystatin C. With the kits provided by the invention, cystatin C antigens can be sensitively detected, and good repeatability and stable results are obtained; the kits can realize rapid detection and are convenient and fast to use, and the nanometer antibodies need low production cost and are easily available.
Owner:上海方恩医疗用品有限公司
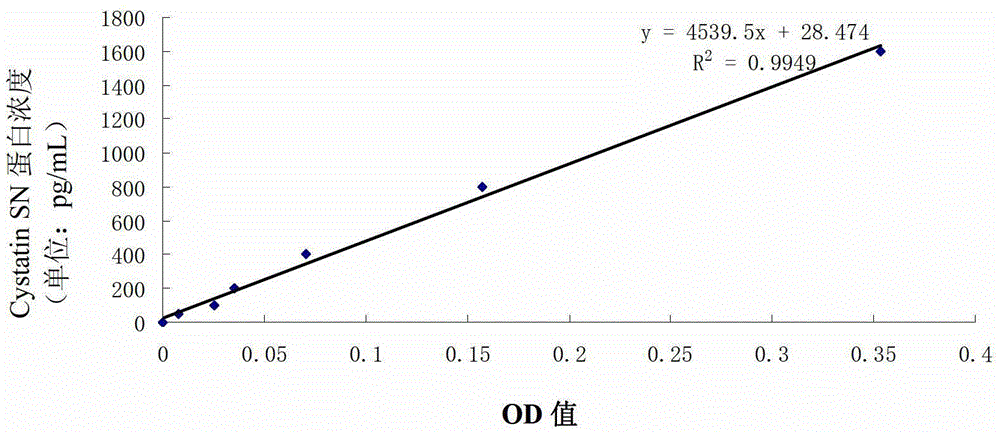
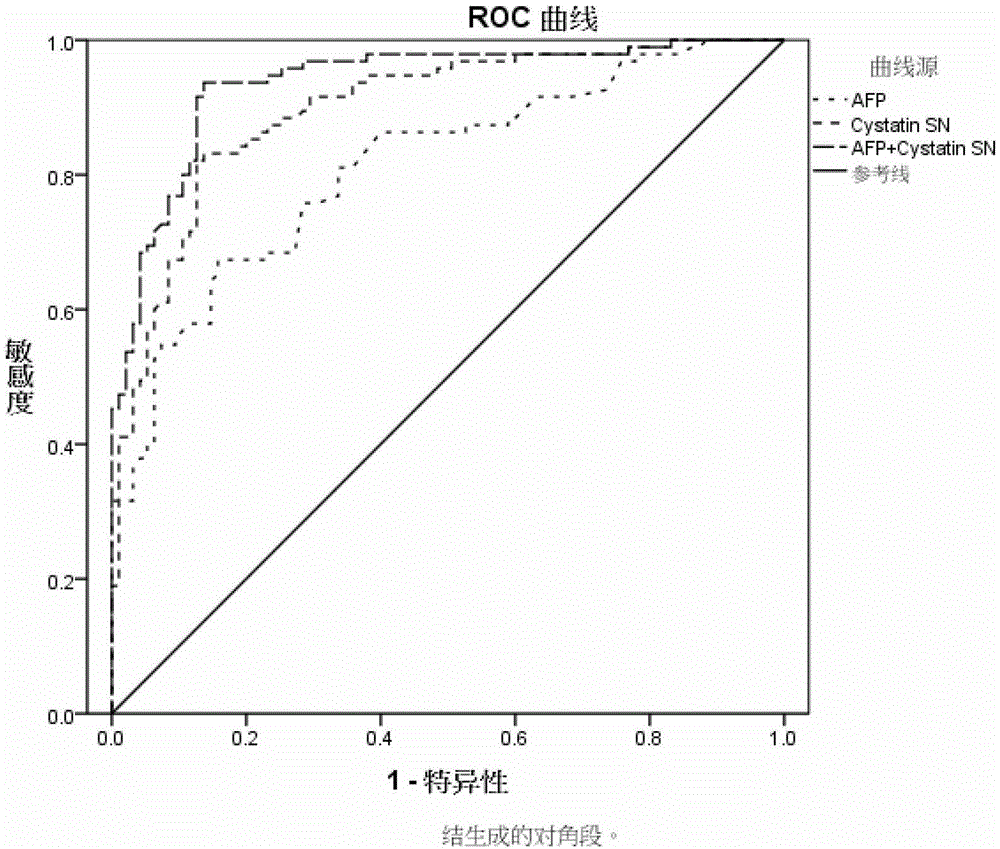
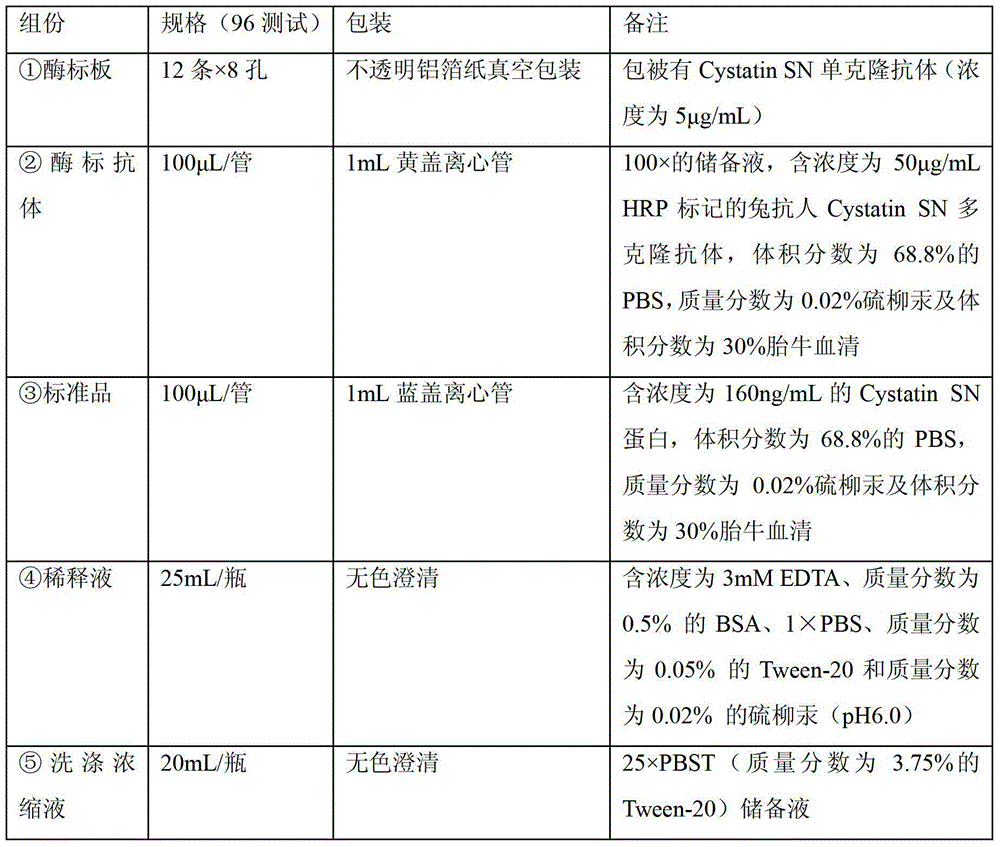
Application of cystatin SN and AFP in preparation of marker for diagnosis and predicting of liver cancer
ActiveCN103940996AHigh sensitivityImprove featuresDisease diagnosisBiological testingDiagnosis earlyLymphatic Spread
The invention discloses a combined application of a cystatin SN and an alpha-fetoprotein (AFP), and particularly discloses the application of the cystatin SN and the AFP in preparation of a marker for diagnosis and predicting of liver cancer. The invention also discloses a capture agent of the liver cancer marker. A liver cancer detection kit is formed by combining the capture agent with conventional reagents. The obtained kit has the advantages of good specificity, high sensitivity and the like, can be used for early diagnosis of liver cancer, therapeutic effect evaluation in a therapeutic process and monitoring of metastasis and recurrence after therapy, and has test results sooner than clinical symptoms.
Owner:SHANGHAI LIANGRUN BIOMEDICINE TECH CO LTD
BIOMARKERS FOR IgA NEPHROPATHY AND APPLICATIONS THEREOF
InactiveUS20110183349A1Microbiological testing/measurementDisease diagnosisSecreted Phosphoprotein 1Butyrophilin
The present invention provides a method for diagnosis or prognosis of IgA nephropathy in a subject based on detection of the expression level of one or more biomarker genes selected from the group consisting of thymosin β4 (Tmsb4), serine or cysteine proteinase inhibitor clade E member 2 (Serpine2), secreted phosphoprotein 1 (OPN), butyrophilin-like-2 (BTNL2), S100 calcium binding protein A8 (S100A8), Cystatin C (CysC), and any combination thereof.
Owner:NAT DEFENSE MEDICAL CENT
Optimal cystatin C detection kit and preparation method thereof
InactiveCN106932594AGuaranteed stabilityLong validity periodDisease diagnosisColor/spectral properties measurementsCross-linkInorganic salts
The invention relates to a cystatin C detection kit and a preparation method thereof. The kit includes reagents R1 and R2 which are independent of each other. The reagent R1 mainly consists of a buffer solution 1, an inorganic salt ion 1, a stabilizer 1, Preservative 1, solubilizer 1 and accelerator 1; the reagent R2 is mainly composed of polystyrene microspheres of cross-linked cystatin C antibody, buffer 2, stabilizer 2, solubilizer 2, preservative 2 and protection Reagent 2, the polystyrene microspheres are connected to the cystatin C antibody through physical adsorption or chemical cross-linking, and are encapsulated by β-cyclodextrin. The cystatin C detection kit of the invention has the advantages of low preparation cost, good stability, easy storage, good data accuracy and high detection sensitivity, and can be widely used in clinical biochemical instruments.
Owner:吉林省富生医疗器械有限公司
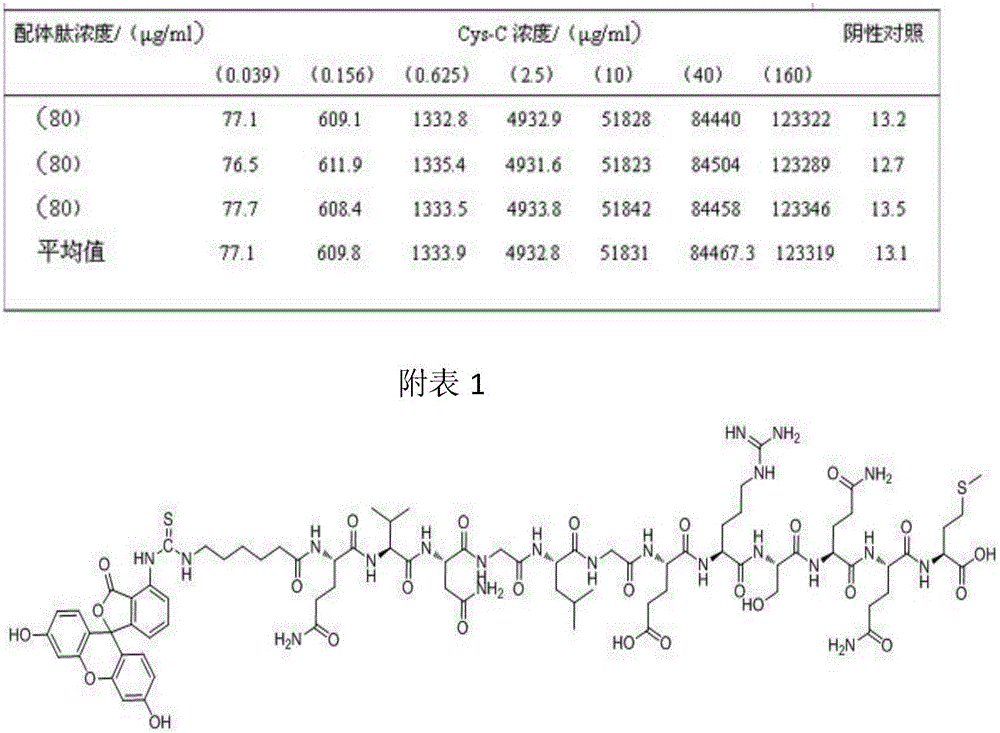
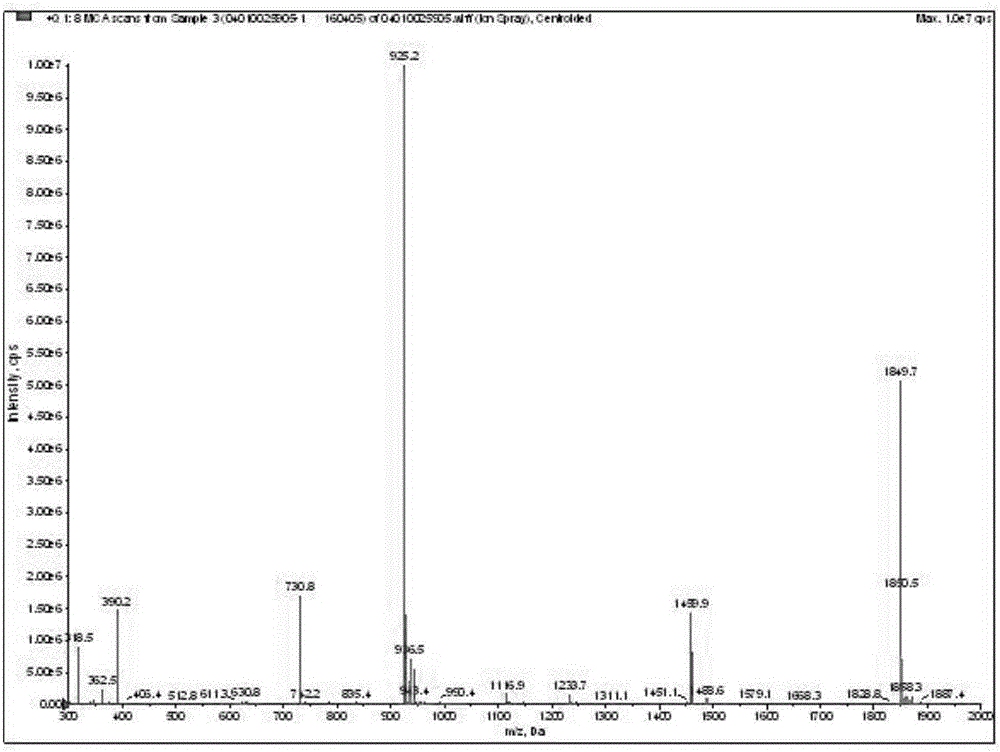
Fluorescent probe for detection of cystatin C and construction method thereof
The invention discloses a fluorescent probe for detection of cystatin C and a construction method thereof, relates to the molecular biological and microbiological technical fields, and is characterized in that the construction method comprises the steps: with Cys-C as a target, screening to obtain a Cys-C specific-binding recombinant phage by using a phage surface random displayed 12-mer peptide library, extracting single stranded DNA of the recombinant phage, carrying out sequencing and sequence comparison, to obtain a sequence Gln-Val-Asn-Gly-Leu-Gly-Glu-Arg-Ser-Gln-Gln-Met of a Cys-C specific affinity ligand having the molecular weight of 1346.5, then carrying out solid phase polypeptide synthesis and fluorescence labeling to obtain the fluorescent probe FITC-Acp-Gln-Val-Asn-Gly-Leu-Gly-Glu-Arg-Ser-Gln-Gln-Met having the molecular weight of 1848.7 and specifically bond with the Cys-C. The fluorescent probe and the construction method thereof have the beneficial effects: 1, a brand new 'tool' is provided for specific identification and content detection of the Cys-C; and 2, the fluorescent ligand peptide probe not only can qualitatively identify the Cys-C, but also can quantitatively detect the content of the Cys-C in samples.
Owner:长春技特生物技术有限公司
Cystatin C latex-enhanced turbidimetry detection kit and preparation and application methods thereof
ActiveCN109406771ADetection signal multiple amplificationHigh detection sensitivityDisease diagnosisFermentationSingle-Chain AntibodiesMicrosphere
The invention provides a cystatin C latex-enhanced turbidimetry detection kit. The cystatin C latex-enhanced turbidimetry detection kit comprises double liquid components, namely, a reagent R1and a reagent R2, wherein the reagent R1 is prepared from 10 to 100 mM / L buffer solution, 5 to 50g / L stabilizer and 0.1 to 1g / L preservative, wherein purified water is taken as the solvent; the reagent R2 isprepared from 10 to 100mM / L buffer solution, 0.1 to 1 percent surfactant, 5 to 50g / L stabilizer, 0.1 to 1g / L preservative, 1 to 10 percent latex microspheres crosslinking with a recombinant antihumanCys-C single-chain antibody, and 2 to 15 percent of latex microspheres crosslinking with a goat antihuman Cys-C polyclonal antibody, wherein purified water is taken as the solvent. The invention further provides preparation and application methods of the cystatin C latex-enhanced turbidimetry detection kit. By adopting the detection kit, the advantages of a polyclonal antibody detection kit and amonoclonal antibody detection kit are combined, and the specificity and sensitivity of detection can be enhanced simultaneously.
Owner:ANHUI DAQIAN BIO ENG LIMITED
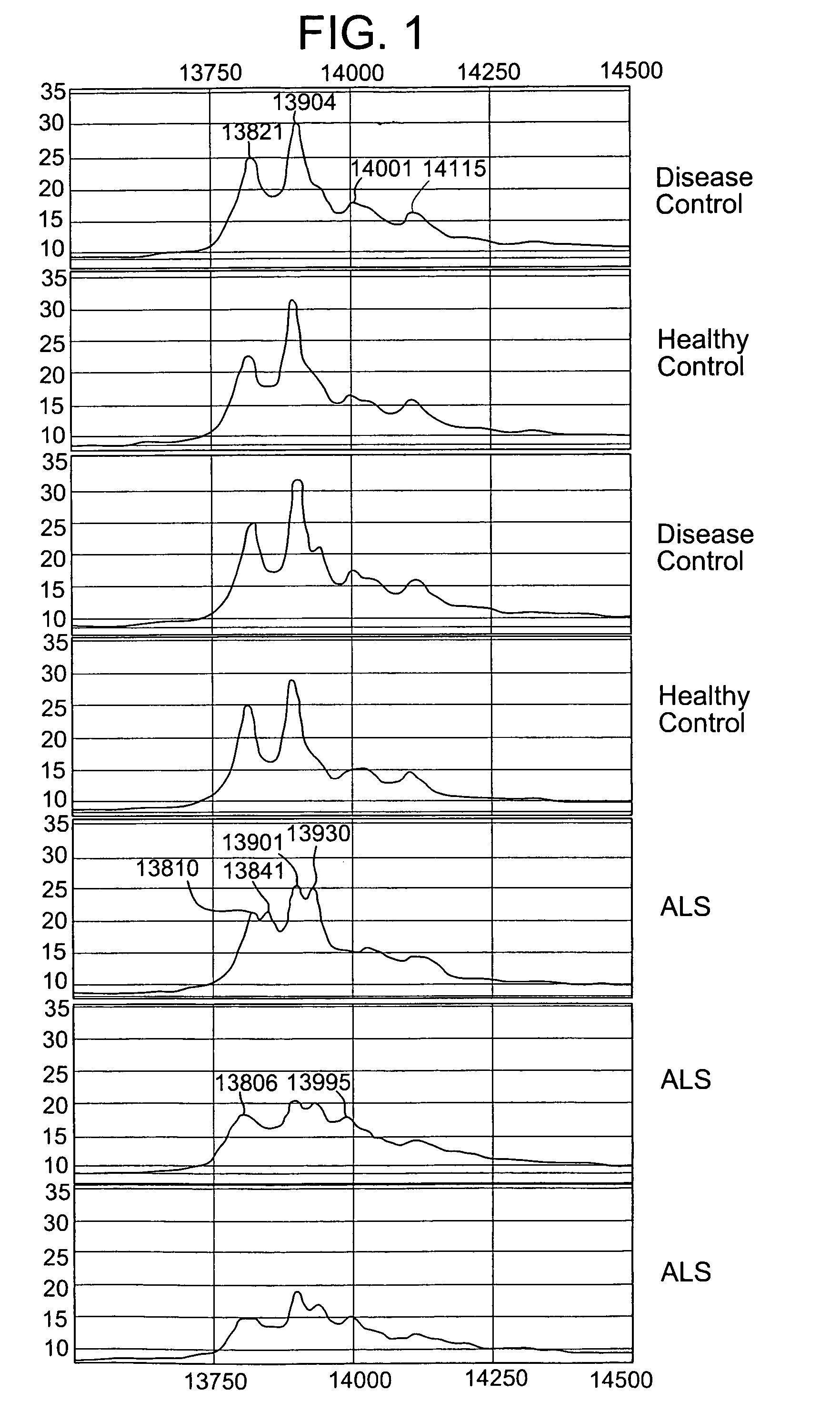
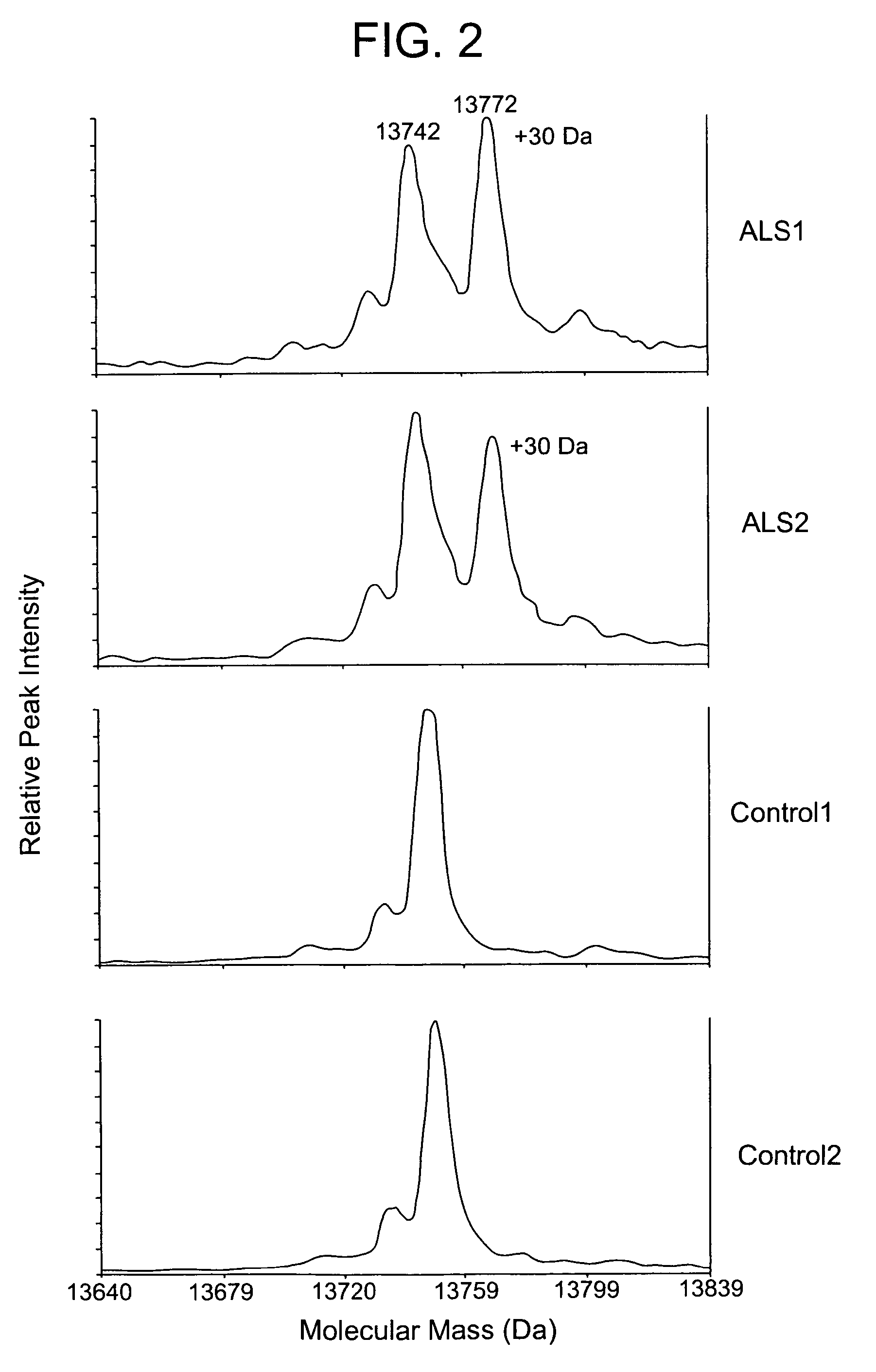
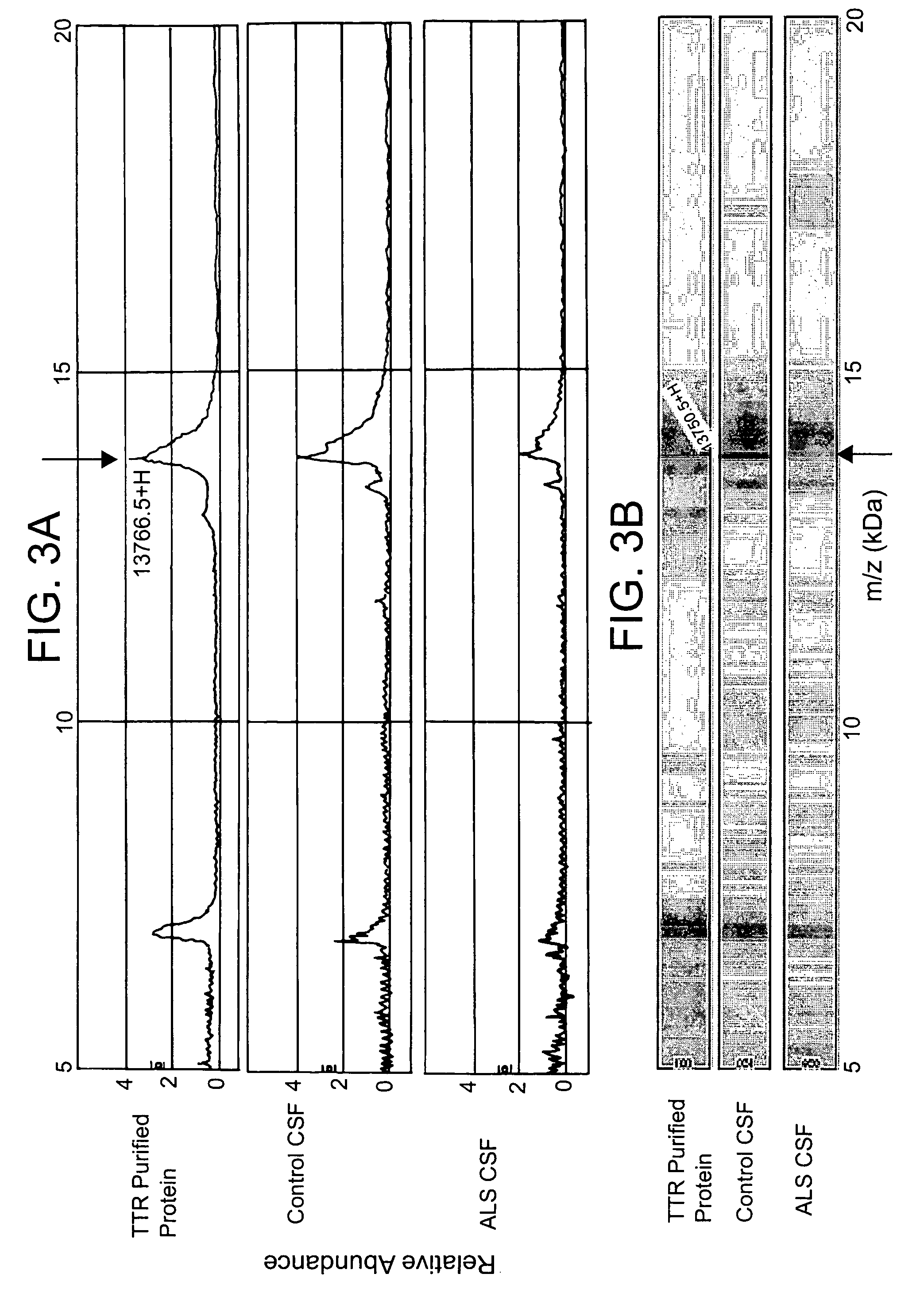
Modulation of the neuroendoctrine system as a therapy for motor neuron disease
ActiveUS20060178306A1Level of in subjectImprove the level ofNervous disorderSamplingNervous systemPrimary motor neuron
The invention provides a method for treating amyotrophic lateral sclerosis (ALS) in a subject. The method comprises administering to the nervous system of the subject a composition comprising a thyroxine protein or a therapeutic fragment or pharmacologic mimic thereof and a pharmaceutically acceptable carrier. The invention also provides a method for treating ALS in a subject that comprises administering to the subject a transthyretin protein, 7B2 protein, a cystatin C protein, a neuroendocrine protein, a cysteine protease inhibitor, or an inhibitor of an enzyme that processes a 7B2 protein. In addition, the invention provides methods for determining the susceptibility of a subject to developing ALS and for determining the progression of ALS in a subject.
Owner:UNIVERSITY OF PITTSBURGH
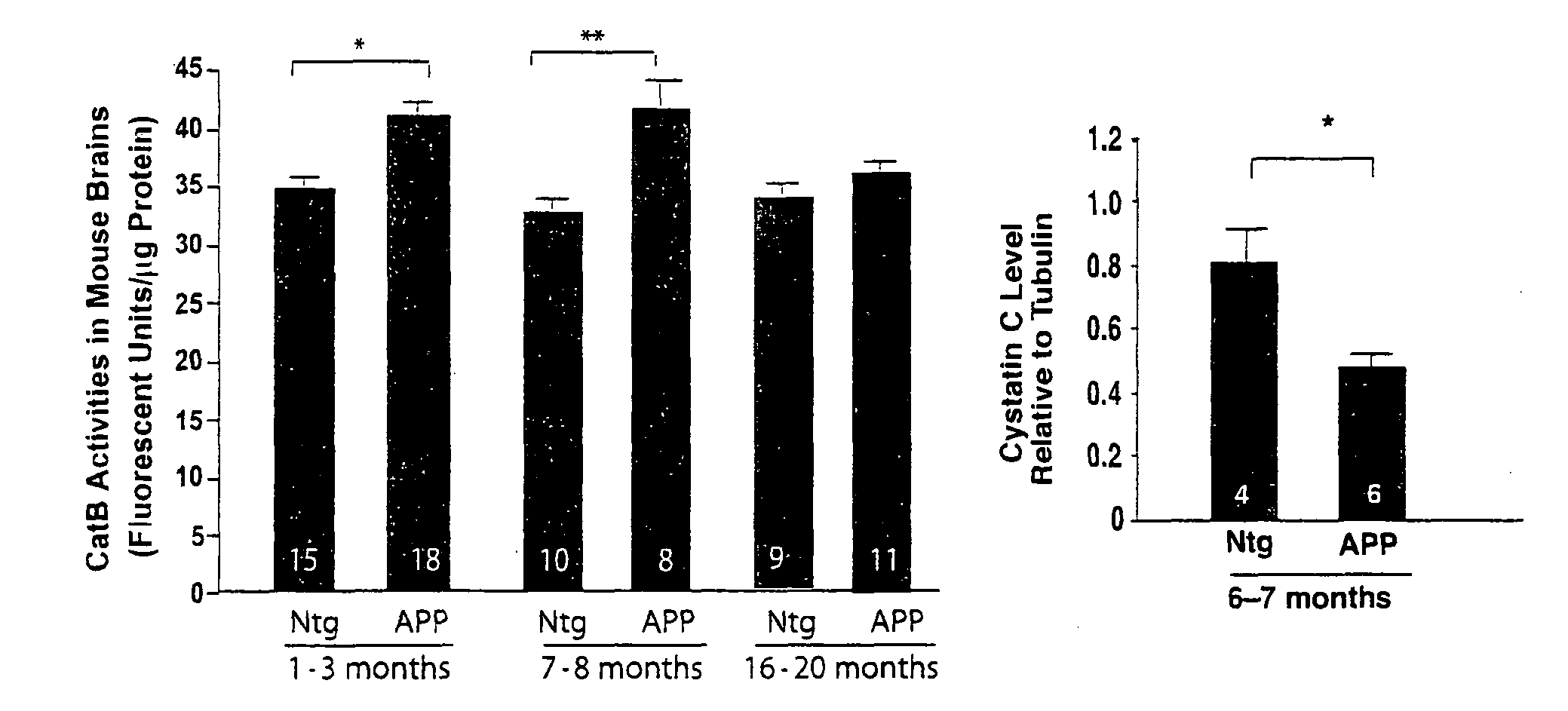
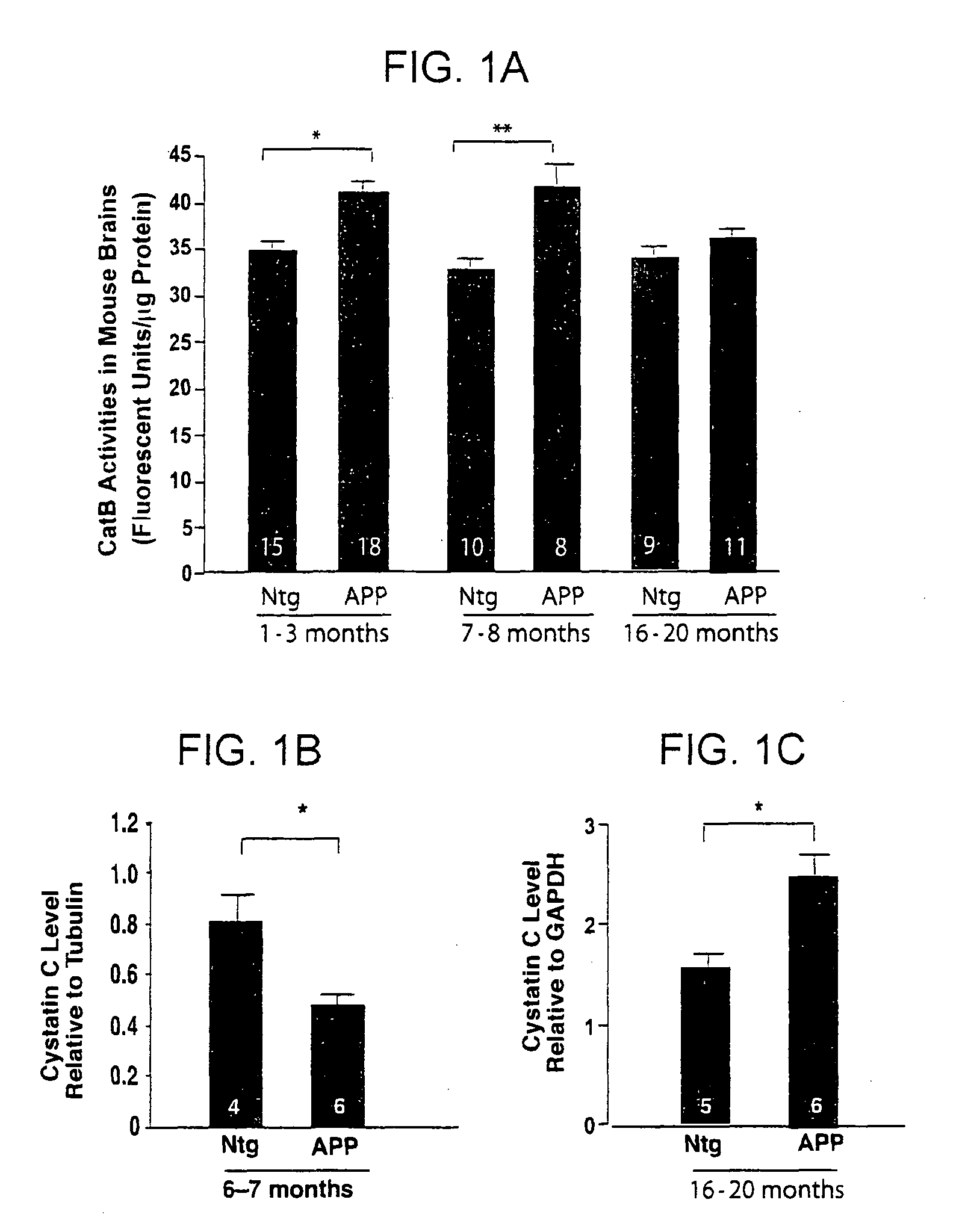
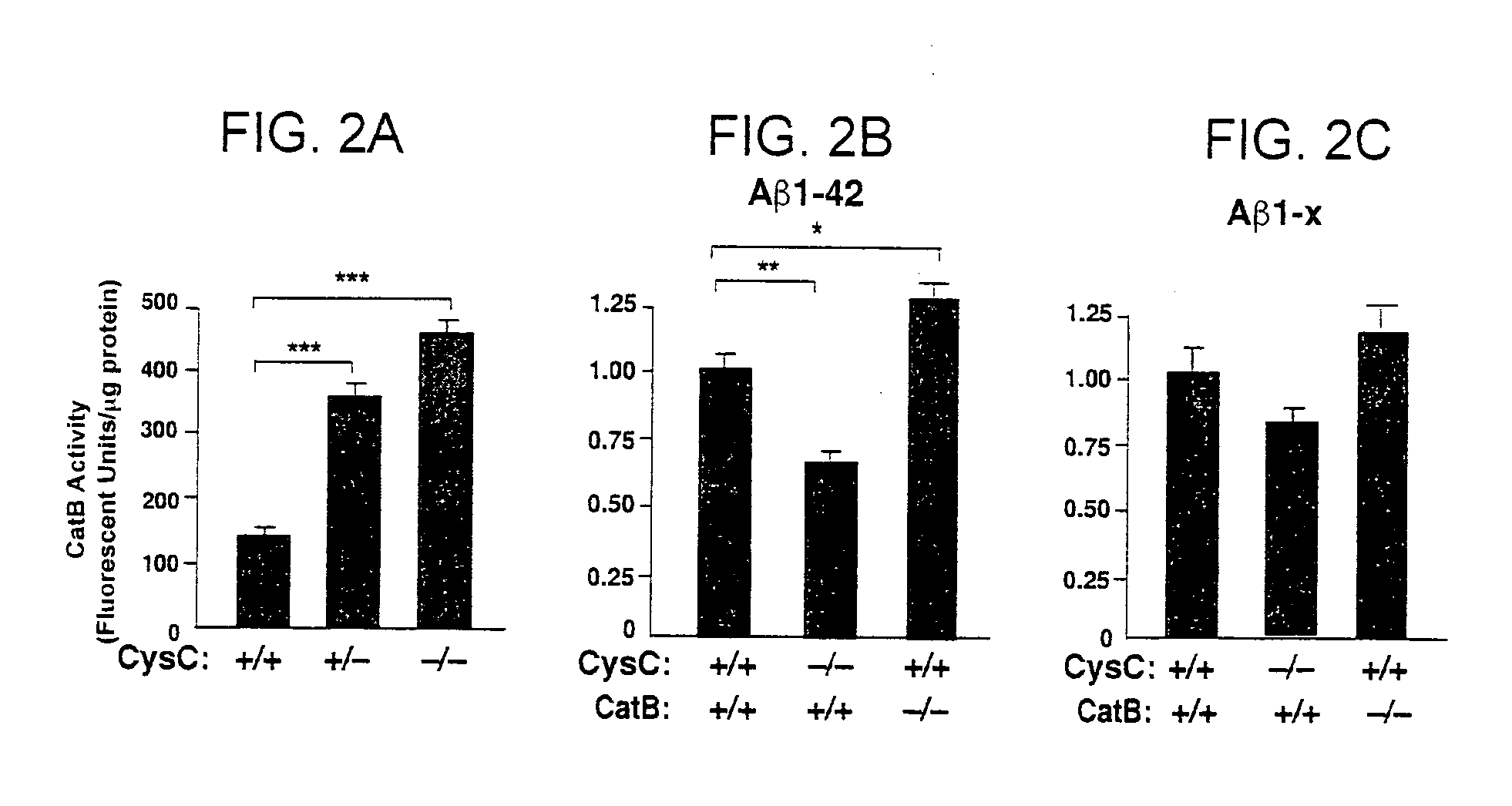
Methods and compositions for reducing amyloid beta levels
The present invention provides methods for reducing the level of amyloid beta protein in a cell or tissue, the methods generally involving contacting the cell or tissue with an agent that reduces cystatin C levels and / or activity. The present invention provides methods for treating Alzheimer's disease (AD), and methods for treating cerebral angiopathy, in an individual, the methods generally involving administering to an individual having AD a therapeutically effective amount of an agent that reduces cystatin C levels and / or activity. The present invention further provides methods for identifying an agent that reduces cystatin C levels and / or activity.
Owner:THE J DAVID GLADSTONE INST A TESTAMENTARY TRUST ESTABLISHED UNDER THE WILL OF J DAVID GLADS
Cystatin C as an antagonist of TGF-beta and methods related thereto
Disclosed are Cystatin C (CysC) homologues, including CystC homologues that act as antagonists or inhibitors of transforming growth factor-β (TGF-β). Also disclosed are methods to identify CystC homologues that are antagonists or inhibitors of TGF-β and compositions and therapeutic methods using CystC and homologues thereof to regulate the activity of TGF-β, and TGF-β-mediated tumor malignancy and invasion and other TGF-β-mediated fibrotic or proliferative conditions and diseases.
Owner:NAT JEWISH HEALTH
Recombinant human cystatin C coding gene and expression method
InactiveCN102676533AEasy to operateImprove expression efficiencyFermentationVector-based foreign material introductionEscherichia coliCloning Site
The invention discloses a recombinant human cystatin C coding gene and an expression method. The nucleotide sequence of the coding gene is shown in SEQ ID NO:1, and the expression method of recombinant human cystatin C protein comprises the following steps that the recombinant human cystatin C coding gene is inserted into a polyclone locus of a prokaryotic expression carrier, a recombinant expression plasmid is constructed, the recombinant expression plasmid is converted to an expression host bacterium escherichia coli, and positive clone bacteria are screened out to obtain engineering bacteria; and the engineering bacteria are fermented, induced, separated and purified to obtain the recombinant human cystatin C protein. The recombinant human cystatin C coding gene provided by the invention can express the human cystatin C protein, the operation is convenient, the purity of the human cystatin C protein expressed by the coding gene reaches more than 90%, the expression efficiency is high, and no higher expression efficiency exists currently.
Owner:GUANGZHOU GENERAL HOSPITAL OF GUANGZHOU MILITARY COMMAND
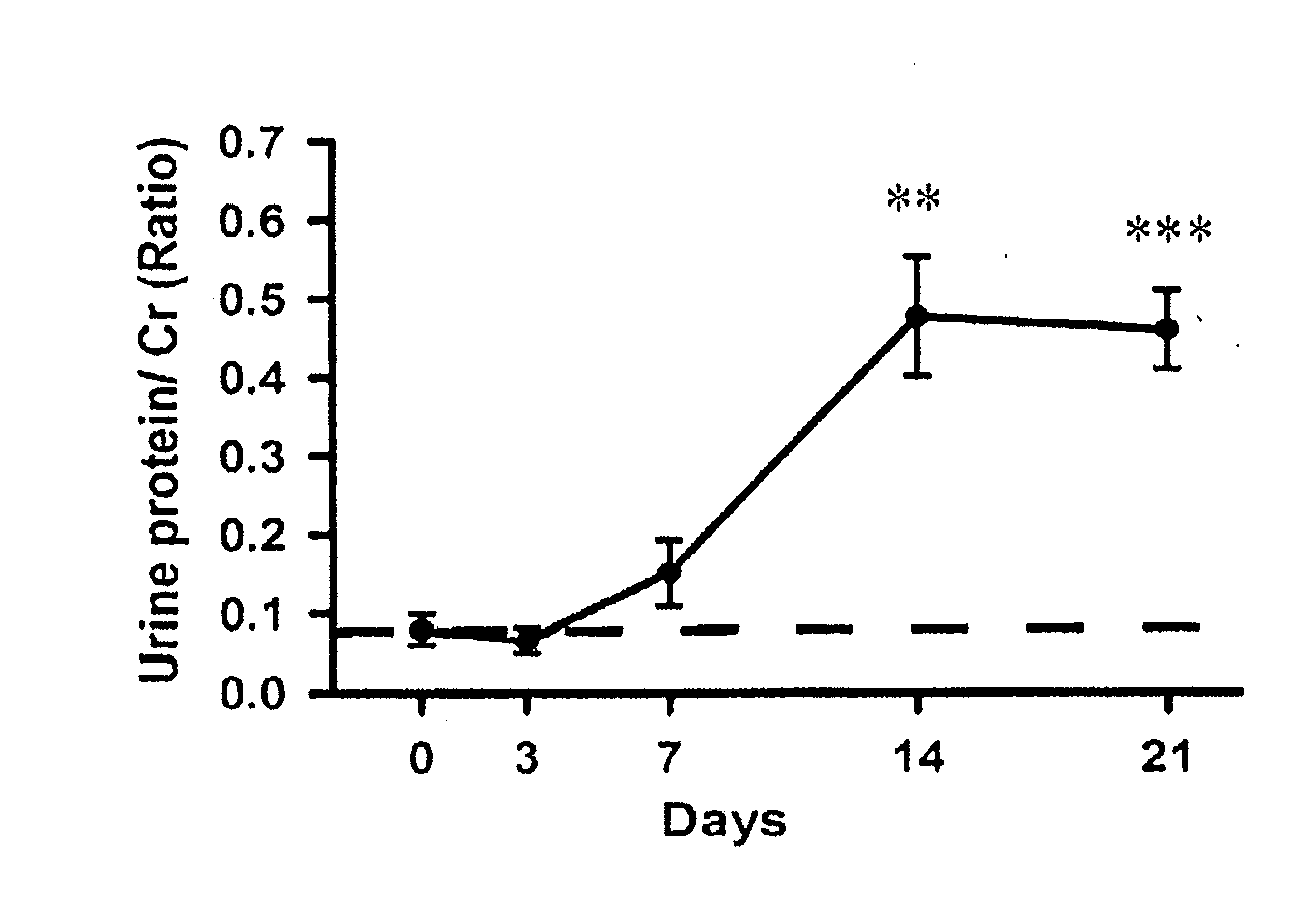
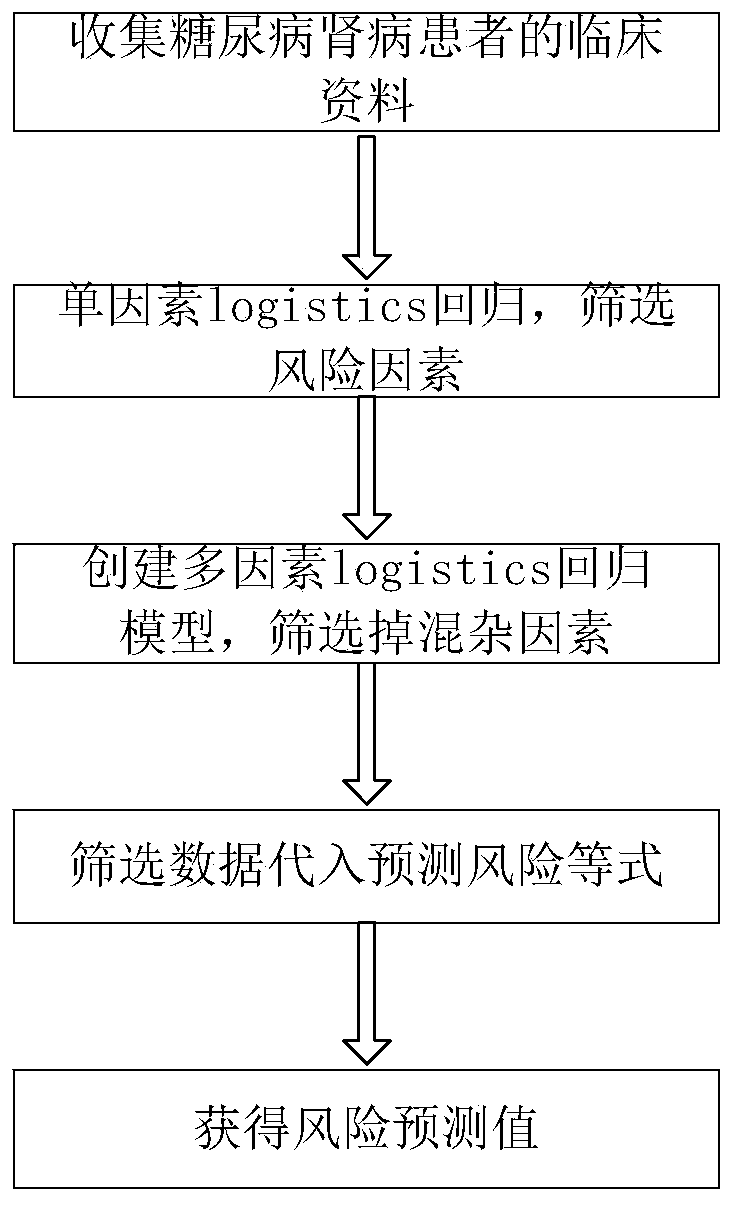
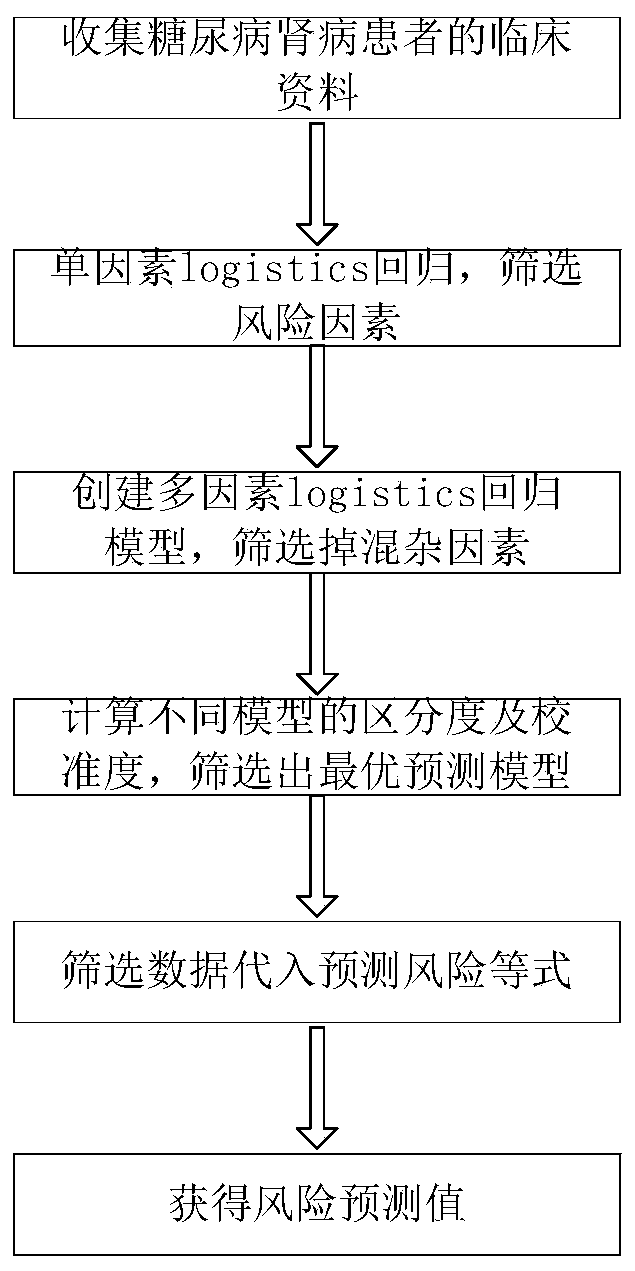
Method for predicting risk of end-stage renal disease in patient diagnosed with diabetic nephropathy through renal biopsy within three years
InactiveCN110491512ADelay progressImprove targetingMedical simulationMedical data miningPuncture BiopsyDiabetic nephropathy screening
The invention relates to a method for predicting the risk of the end-stage renal disease in a patient diagnosed with diabetic nephropathy through renal puncture biopsy within three years. The method comprises the following steps that clinical data of a large number of patients who are diagnosed with the diabetic nephropathy through the renal puncture biopsy pathology and do not suffer from the end-stage renal disease is collected, whether or not the patients suffer from the end-stage renal disease is judged after 3 years of follow-up visit; according to the follow-up visit results, diabetic nephropathy pathological grades, cystatin C and 3GFR data of the patients diagnosed with the diabetic nephropathy through the renal puncture biopsy are collected, and a predictive risk equation of a prediction model is obtained. Accordingly, the risk of the patients suffering from the end-stage renal disease is helped to be judged according to initial detection data, early intervention is achieved,and the progress of the renal disease is delayed. According to the risk prediction method, early clinical risk prediction and reasonable systematic management can be provided for more patients definitely diagnosed with the diabetic nephropathy.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
Turbidimetric immunoassay for assessing human cystatin c
ActiveUS20110033950A1Strong and faster signalReduce distractionsDisease diagnosisBiological testingHuman bodyAssay
There is a demand for improved turbidimetric immunoassays for human Cystatin C in biological samples, especially in human clinical samples of body fluids. The present invention provides a turbidimetric immunoassay method and reagent set enabling measurement of human Cystatin C by turbidimetric methods, resulting in a surprisingly stronger and faster turbidimetric signal than in the present state of the art. The increased and faster signal is accomplished by the use of new reagents and compositions, and enables shorter assay times and kinetic reading with a stronger signal, improving overall assay speed and quality. Improved robustness to lipid interference and improved linearity is achieved.
Owner:GENTIAN AS
Cystatin C detection reagent and method
ActiveCN107228940AEasy to operateImprove stabilityColor/spectral properties measurementsSucroseMicrosphere
The invention relates to the technical field of in-vitro diagnostic reagents and particularly relates to a cystatin C detection reagent and method. The detection reagent is composed of a reagent 1 and a reagent 2. The reagent 1 comprises ammonium chloride, polyoxyethylene lauryl ether, sodium chloride, PEG 6000, a preservative and water. The reagent 2 comprises latex microspheres coated with a cystatin C monoclonal antibody, a Tris-HCl buffer solution, sucrose, gelatin, NP30, a preservative and water. The detection reagent has high detection result accuracy, a good precision, a wide linear range, good specificity, good stability and strong anti-interference ability, can be conveniently detected on a full-automatic biochemical analyzer, has a wide application range and is easy to promote.
Owner:吉林省汇酉生物技术股份有限公司
Construction, expression and purification of human cystatin C eucaryon expression vector
The invention discloses a method for expressing and purifying recombinant human cystatin C proteins by using a eukaryon expression system. Because posttranslational modification and other mechanisms are lacked in a pronucleus expression system, the expressed and purified proteins have the defects of low activity and large structural difference as compared with the natural proteins, which results in that a cystatin C detection agent is difficult in the establishment process by using a monoclonal antibody prepared from Escherichia coli source recombinant cystatin C proteins. In the method, the defects of the pronucleus expression are overcome by utilizing the eukaryon expression system so as to obtain the high-activity recombinant human cystatin C protein, and a powerful basis is provided for obtaining a high-quality antibody.
Owner:WUHAN LIFE ORIGIN BIOTECH LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com