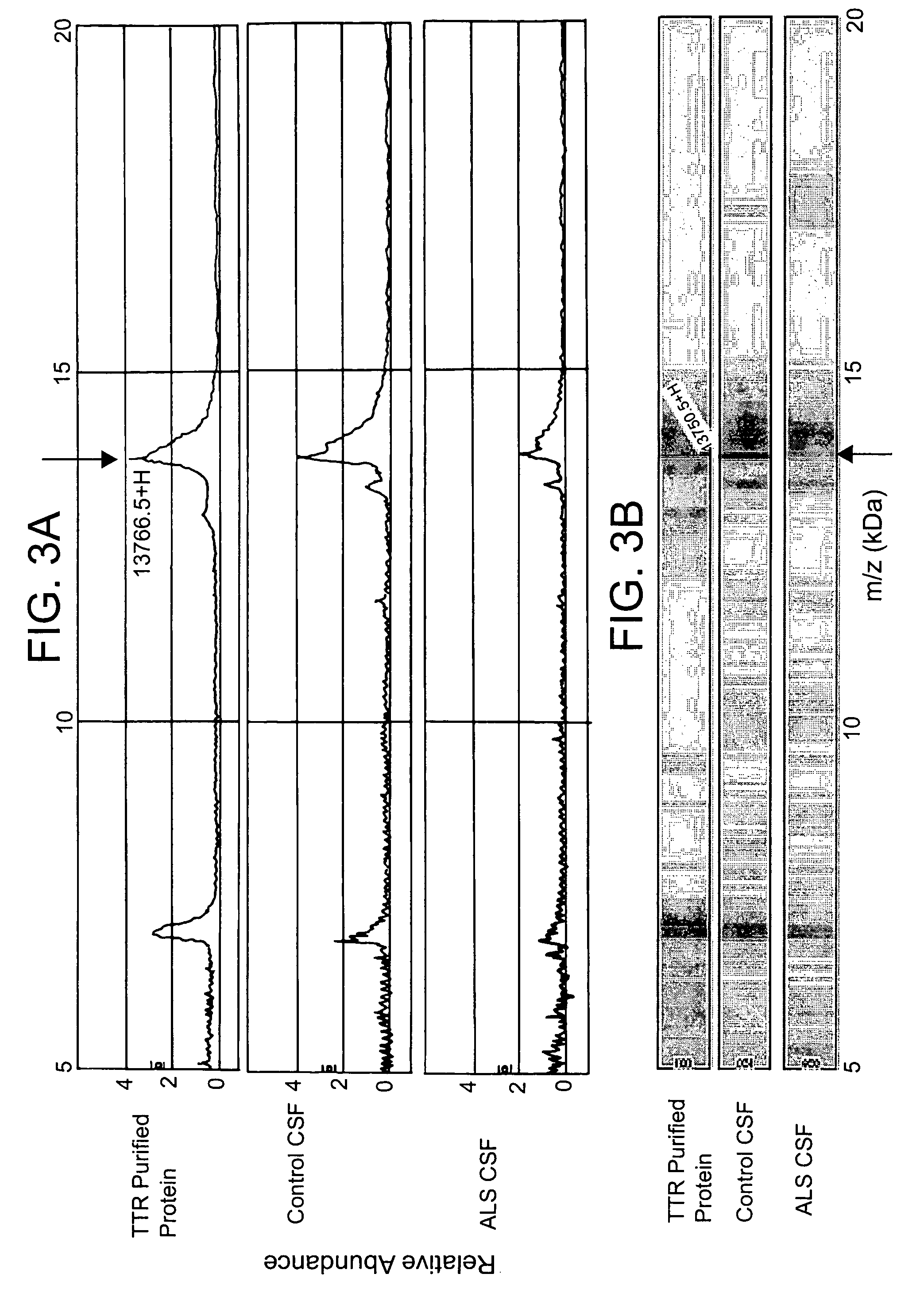
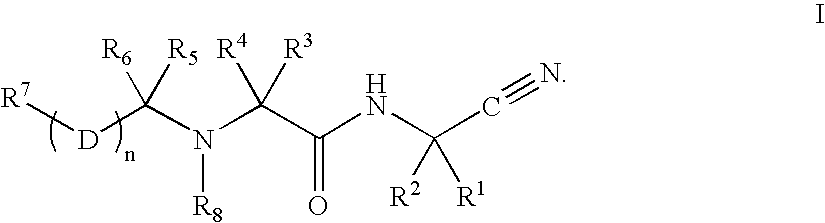
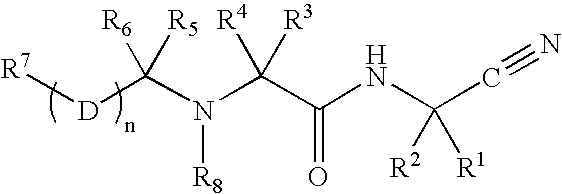
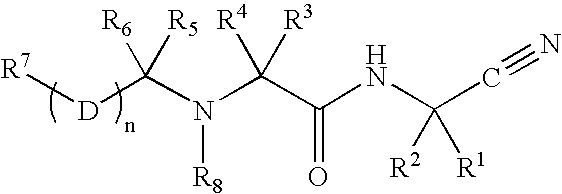
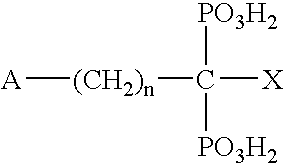Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
345 results about "Pineapple Protease" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
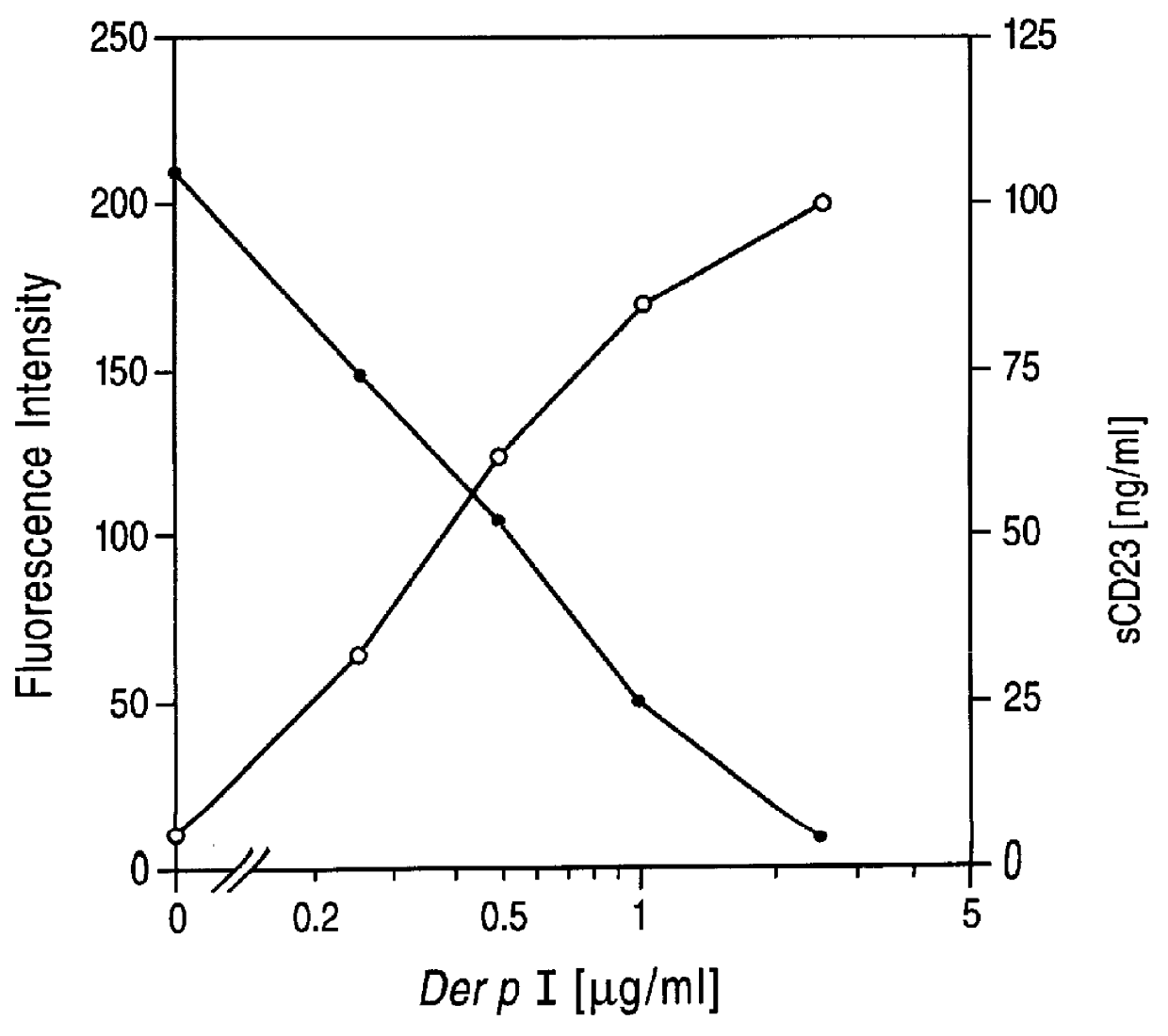
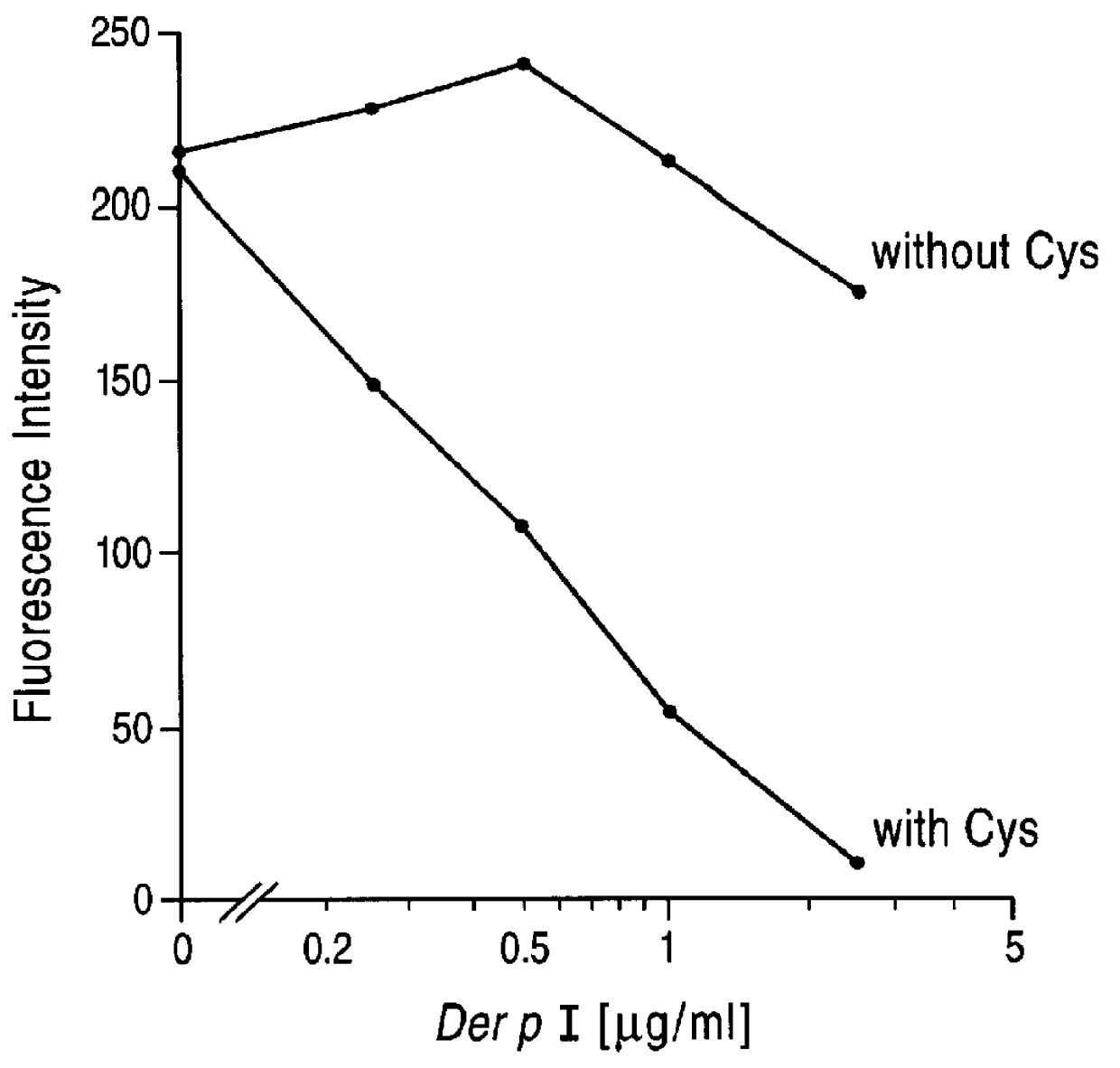
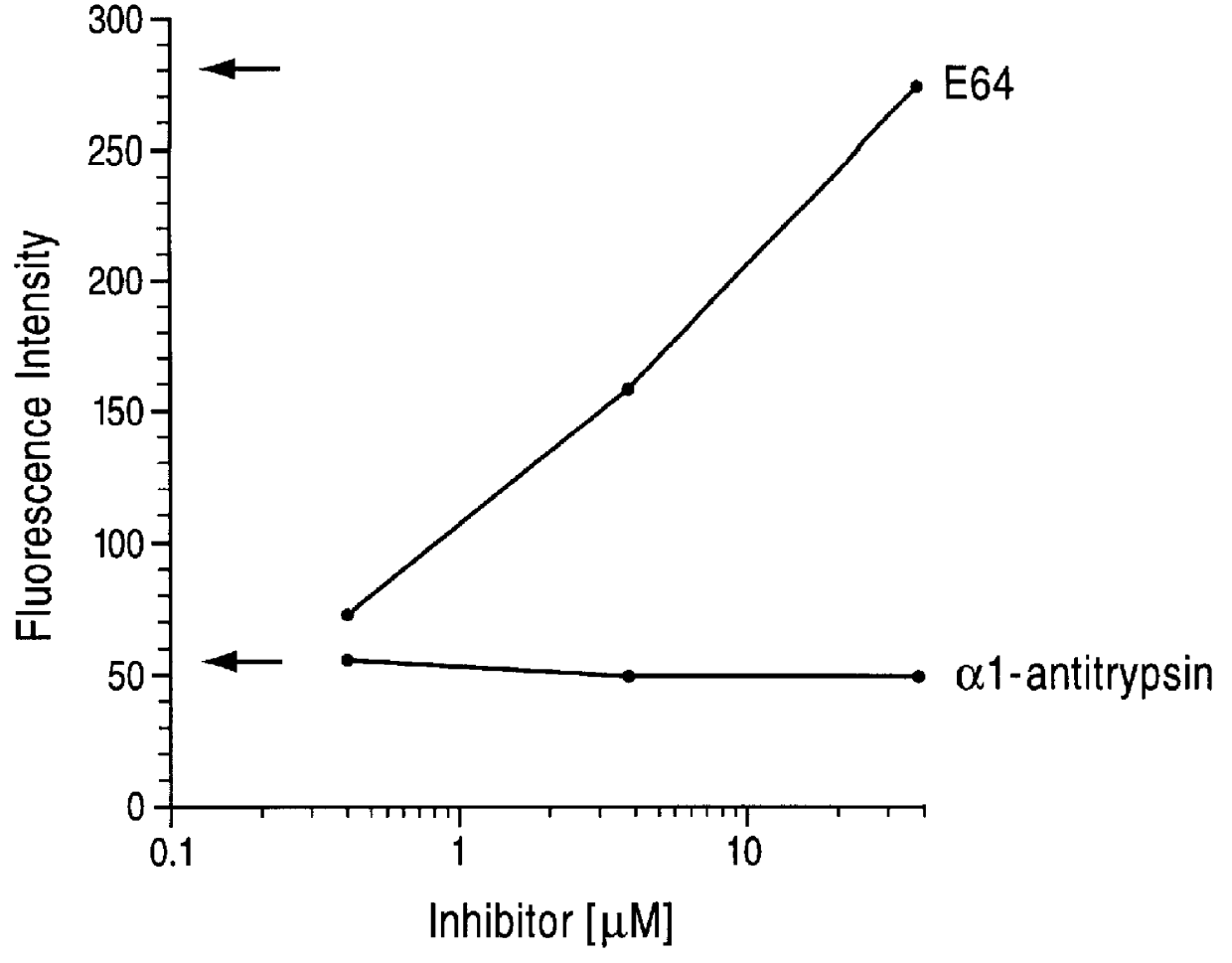
Cysteine proteases are commonly encountered in fruits including the papaya, pineapple, fig and kiwifruit. The proportion of protease tends to be higher when the fruit is unripe.
Novel inhibitors of dipeptidyl peptidase I
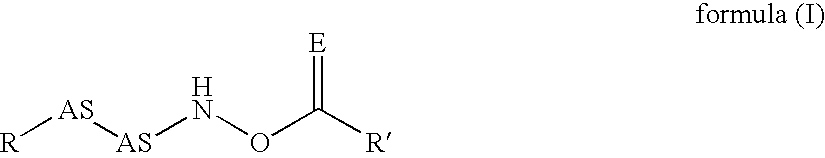
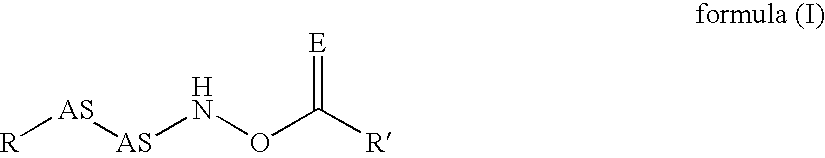
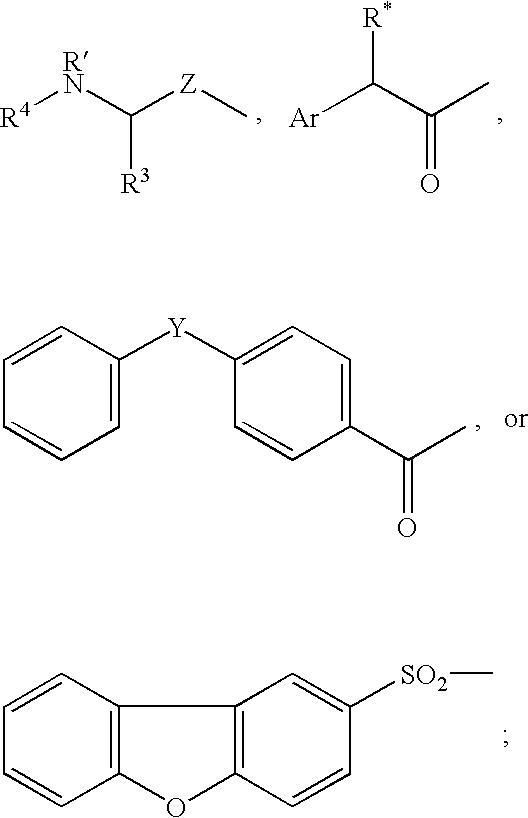
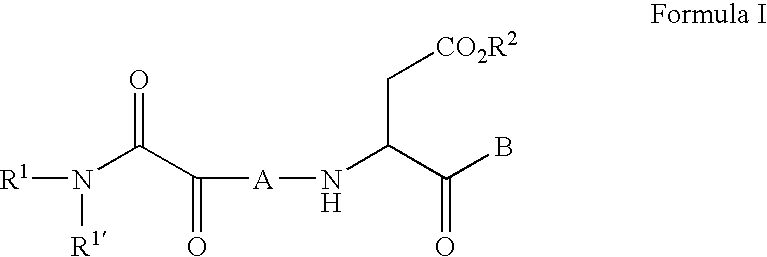
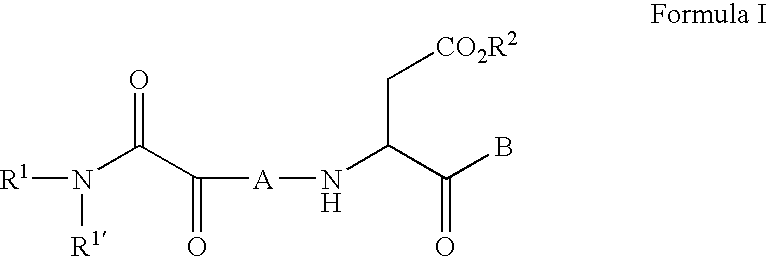
The present invention relates to new compounds that act as specific inhibitors of the cysteine protease dipeptidyl peptidase I (DP I). Those compounds are represented by the general formula and the pharmaceutical salts thereof, in which R is an acyl-residue including urethane and peptide, or a branched or unbranched C1-C9 alkyl chain, a branched or unbranched C2-C9 alkenyl chain, a branched or unbranched C2-C9 alkynyl chain, a C3-C9 cycloalkyl, C4-C9 carbocyclic, C5-C14 aryl, C3-C9 heteroaryl, C3-C9 heterocyclic, all of the above residues may be optionally substituted, or is H, AS is an amino acid or a peptide mimetic thereof. The amino acid is peptide bound with R and R' is a branched or unbranched C1-C9 alkyl chain, a branched or unbranched C2-C9 alkenyl chain, a branched or unbranched C2-C9 alkynyl chain, a C3-C9 cycloalkyl, C4-C9 cycloalkenyl, C2-C9 heterocycloalkyl, C3-C9 heterocycloalkenyl, C5-C14 aryl, C3-C9 heteroaryl, C3-C9 heterocyclic, whereas the heterocycloalkyl, heterocycloalkenyl, heteroaryl, heterocyclic residue can have up to 6 hetero ring atoms, an amino acid or a peptide mimetic thereof, all of the above residues my be optionally substituted, or is H.
Owner:VIVORYON THERAPEUTICS NV
Cysteine protease inhibitor
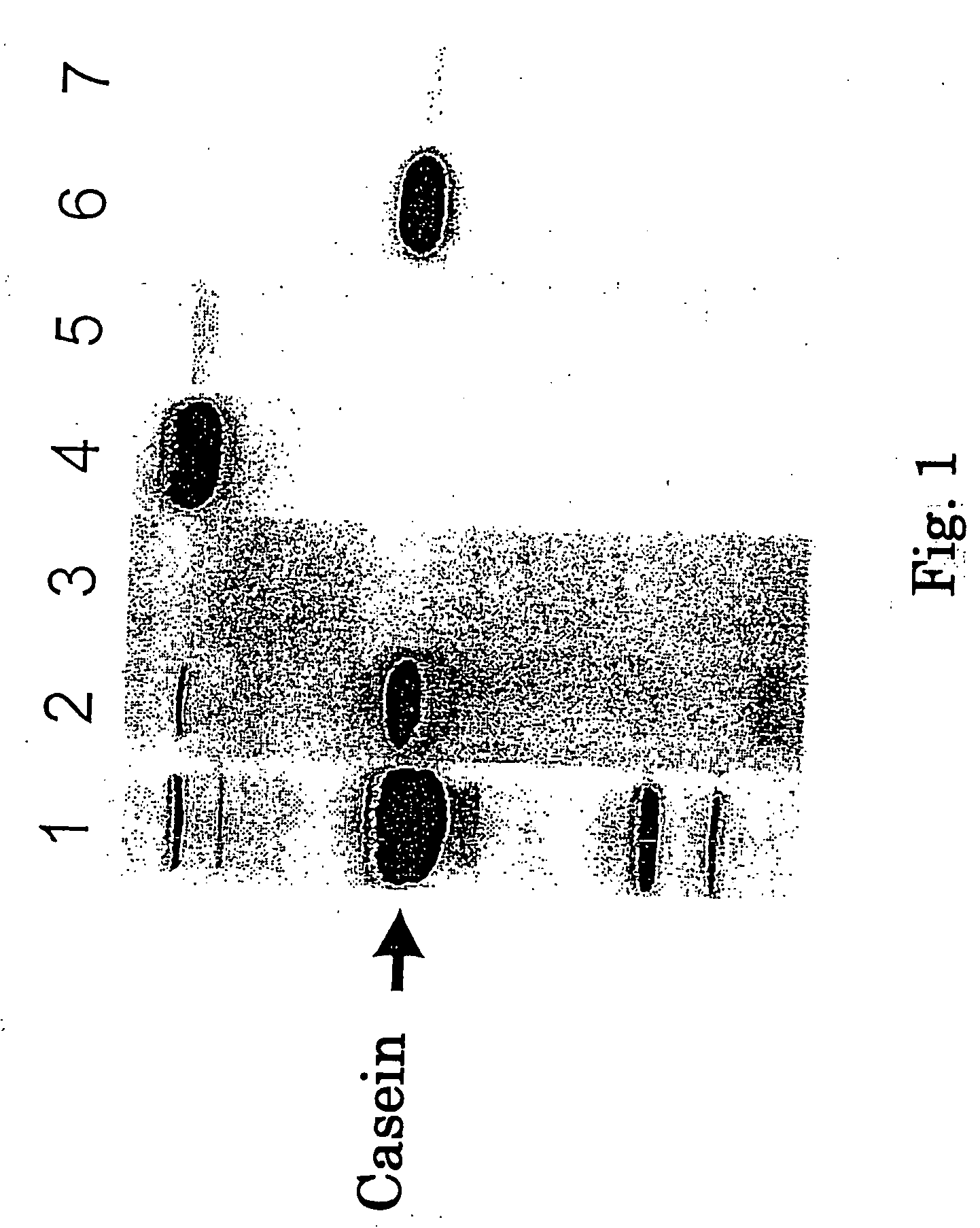
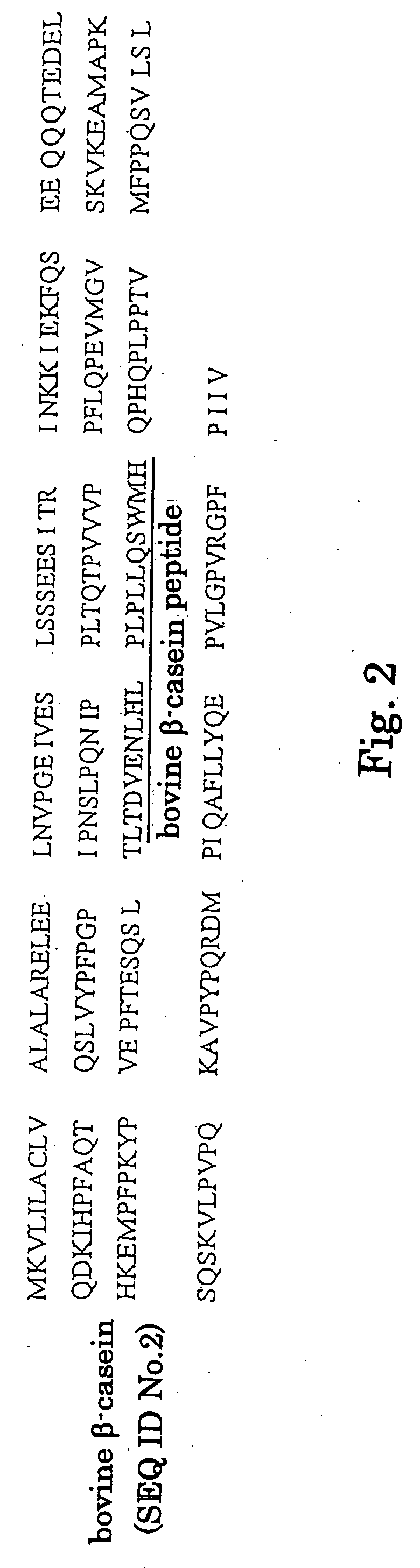
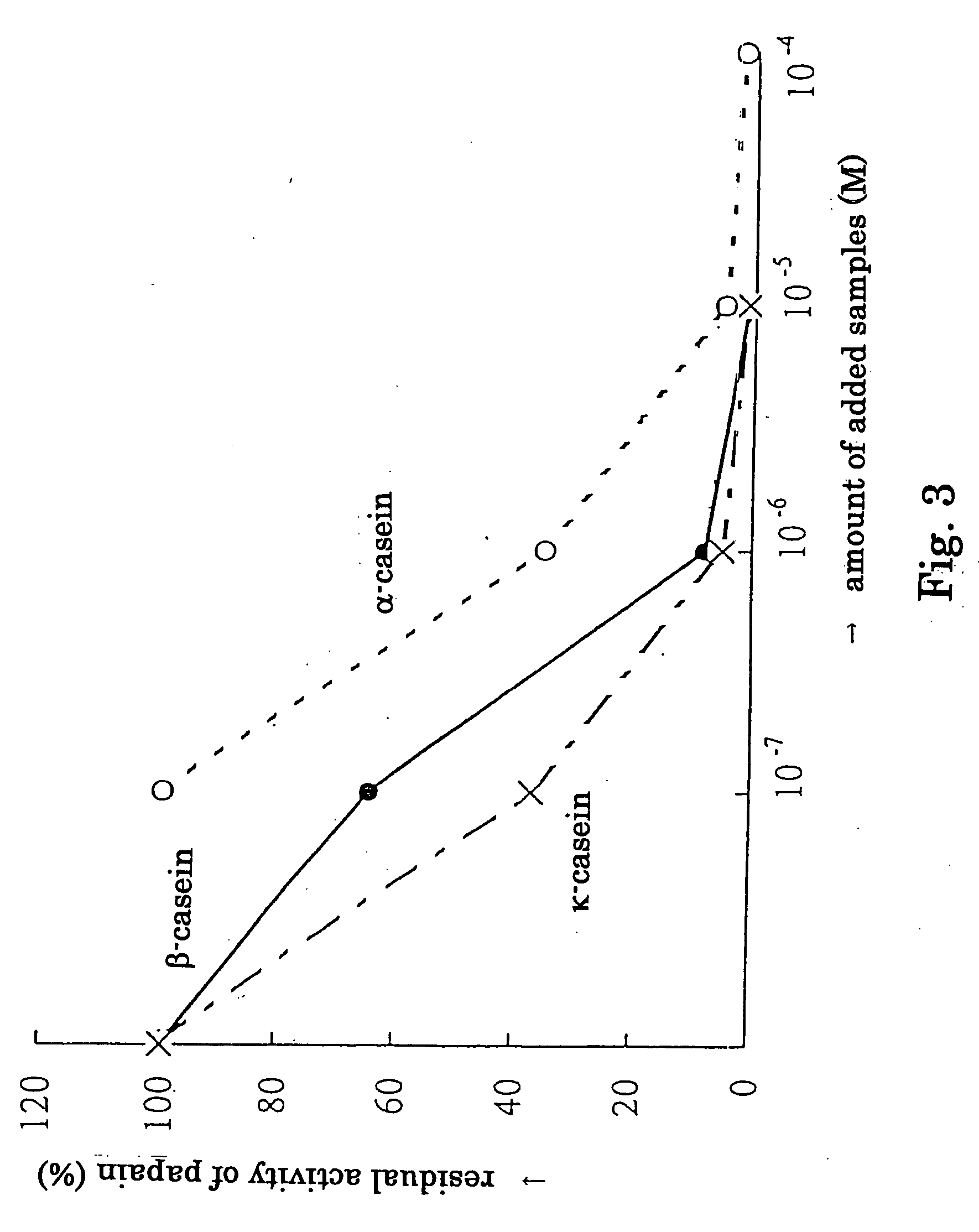
This invention relates to a cysteine protease inhibitor comprising casein which is a protein derived from milk, a partial peptide of casein, and / or hydrolysate of casein as an active ingredient. The cysteine protease inhibitor of the present invention can be used as preventive or therapeutic drug for osteoporosis, malignant hypercalcemia, breast cancer, prostate cancer, periodontitis or bacterial and viral infectious diseases, and food, drink, feed and the like.
Owner:MORINAGA MILK IND CO LTD
Cysteine protease inhibitor
InactiveUS6162828ALow toxicitySafety and toxicityBiocideCarbamic acid derivatives preparationHydrogen atomCysteine Proteinase Inhibitors
PCT No. PCT / JP96 / 00840 Sec. 371 Date May 20, 1996 Sec. 102(e) Date May 20, 1996 PCT Filed Mar. 29, 1996 PCT Pub. No. WO96 / 30395 PCT Pub. Date Oct. 3, 1996A pharmaceutical composition for inhibiting cysteine protease which comprises a compound of the formula: wherein R1 is a hydrogen atom or an acyl group; R2, R3 and R4, same or different, are a bond, an amino acid residue or a group of the formula:-Y-R5-in which R5 is a group resulting from imino group removal from an amino acid residue; Y is -O-, -S- or -NR6- in which R6 is a hydrogen atom or a lower alkyl group; A is Z is a hydrogen atom, an acyl group or an optionally substituted hydrocarbon group; n is 1 or 2; provided that when n is 1, then A is and Y is -S- or -NR6-, and, at least one of R2, R3 and R4 is the formula -Y-R5-, provided that when further all Y are -NR6-, at least one of the amino acid residues is not bound to amhydrogen atom at the alpha -carbon thereof but substituted via carbon; provided that when n is 2 and Z is an aldehyde group, then R1 is an acyl group having 6 or more carbon atoms; provided that when n is 2 and A is, then at least one of R2, R3 and R4 is the formula -Y-R5-; or an ester or a salt thereof, and a pharmaceutically acceptable carrier.
Owner:TAKEDA PHARMACEUTICALS CO LTD
Cysteine protease inhimbitors
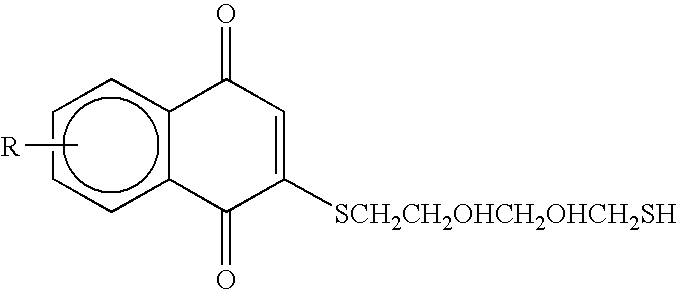
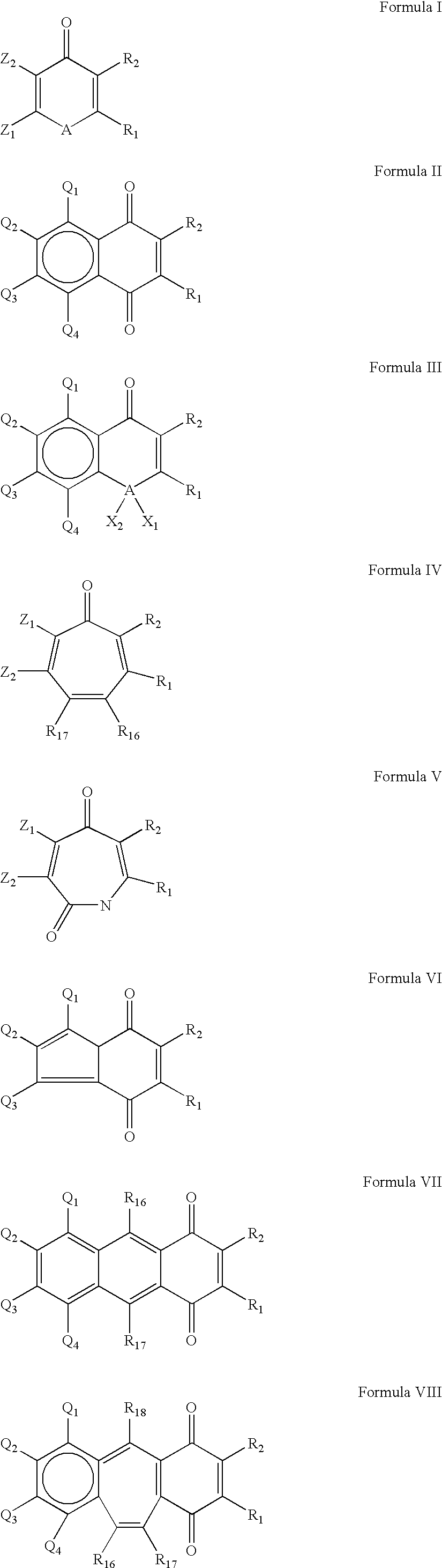
Compounds having quinone and quinone analogs useful for pharmaceutical preparations have now been found which inhibit cysteine proteases, in particular, caspases and 3C-cysteine proteases. The cysteine protease inhibitors of the present invention can be identified by their mode of action in disrupting the ability of cysteine proteases and, in particular, caspases to cleave a peptide chain. These compounds are useful in inhibiting cysteine protease or cysteine protease-like proteins and for treating infections diseases or physiopathological diseases or disorders attributed to the presence of excessive or insufficient levels of cysteine proteases.
Owner:ARAD DORIT +6
Dietary Supplement Cognitive Support System
The present invention relates to a nutritional supplement composition, comprising a therapeutically effective amounts of Vitamin C, Vitamin D3, Thiamin, Riboflavin, Niacin, Vitamin B6, Folic acid, Vitamin B12, Pantothenic acid, Calcium, Magnesium, Zinc, Chromium, Sugar, Protein, Acetyl-L-Carnitine, Dimethylaminoethanol complex, Phosphatidylserine complex, L-Glutamine, N-Acetyl-L-Tyrosine, L-Phenylalanine, Taurine, Methionine, Valine, Isoleucine, 5 Hydroxytryptophan, L-Taurine, N-Acetyl-Tyrosine, N-Acetyl-L-Cysteine, Alpha Lipoic Acid, Alpha Glycerylphosphoricholine complex, Bacopa Monnieri extract, Gingko Biloba extract, Passion flower, Lemon Balm, Gotu Kola, Ashwagandha, Choline Bitartrate complex, Panax Ginseng extract, Turmeric, Organic freeze dried fruit juice blends (concord grape, red raspberry, pineapple, cranberry, acai, pomegranate, acerola cherry, bilberry, lingonberry, black currant, aronia, sour cherry, black raspberry), Organic freeze dried greens blends (barley grass, broccoli, beet, carrot, alfalfa, oat), and Protein digestive enzyme blends (Protease 4.5, peptidase, bromelain, protease 6.0, protease 3.0, L planatrum, B bifidum) in a mixture to provide optimal cognitive function.
Owner:FANTZ DAVID R
Compositions relating to a mutant clostridium difficile toxin and methods thereof
ActiveUS20120269841A1Antibacterial agentsBacterial antigen ingredientsClostridium difficile toxin BNucleotide
In one aspect, the invention relates to an immunogenic composition that includes a mutant Clostridium difficile toxin A and / or a mutant Clostridium difficile toxin B. Each mutant toxin includes a glucosyltransferase domain having at least one mutation and a cysteine protease domain having at least one mutation, relative to the corresponding wild-type C. difficile toxin. The mutant toxins may further include at least one amino acid that is chemically crosslinked. In another aspect, the invention relates to antibodies or binding fragments thereof that binds to said immunogenic compositions. In further aspects, the invention relates to isolated nucleotide sequences that encode any of the foregoing, and methods of use of any of the foregoing compositions.
Owner:WYETH LLC
Cysteine protease inhibitors for use in treatment of IGE mediated allergic diseases
InactiveUS6034066AOrganic active ingredientsGroup 5/15 element organic compoundsOccupational allergensCysteine Proteinase Inhibitors
PCT No. PCT / GB96 / 01707 Sec. 371 Date Feb. 26, 1998 Sec. 102(e) Date Feb. 26, 1998 PCT Filed Jul. 17, 1996 PCT Pub. No. WO97 / 04004 PCT Pub. Date Feb. 6, 1997The invention provides compounds for use in the treatment of allergic diseases including juvenile asthma and eczema. The compounds can inhibit IgE mediated reaction to major environmental and occupational allergens and can also have a prophylactic effect against allergic disease by preventing allergic sensitization to environmental and occupational allergens when administered to at-risk individuals (e.g., those at genetic risk of asthma and those exposed to occupational allergens in the workplace). The compounds are also useful for inactivation or attenuation of the allergenicity of allergens in situ. The invention provides compounds and ligands per se, pharmaceutical compositions containing the compounds, processes for producing the compounds and pharmaceutical compositions, and methods for using the compounds and compositions in treatment or prophylaxis of IgE mediated allergic diseases and in inactivation or attenuation of allergens in situ. The invention also enables the reduction or destruction of the viability of allergy-causing organisms.
Owner:MEDIVIR UK
Inhibitors of dipeptidyl peptidase I
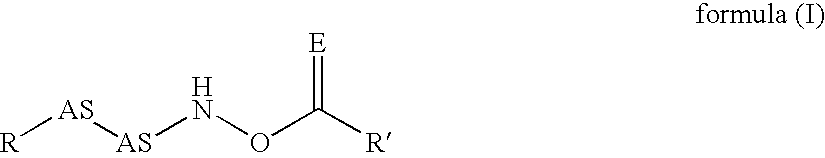
The present invention relates to specific inhibitors of the cysteine protease dipeptidyl peptidase I (DP I), which can be used in the treatment of malignant cell degeneration, immune deseases impaired wound healing and metabolic diseases of humans and are represented by the general formula in which R is a peptide or a branched or unbranched C1-C9 alkyl chain, a branched or unbranched C2-C9 alkenyl chain, a branched or unbranched C2-C9 alkynyl chain, a C3-C9 cycloalkyl, C4-C9 carbocyclic, C5-C14 aryl, C3-C9 heteroaryl, C3-C9 heterocyclic, all of the above residues optionally being substituted, the residue AS—AS is a dipeptide or a mimetic thereof, AS is an amino acid or a peptide mimetic thereof. The amino acid is peptide bound with R and R′ is a branched or unbranched C1-C9 alkyl chain, a branched or unbranched C2-C9 alkenyl chain, a branched or unbranched C2-C9 alkynyl chain, a C3-C9 cycloalkyl, C4-C9 cycloalkenyl, C2-C9 heterocycloalkyl, C3-C9 heterocycloalkenyl, C5-C14 aryl, C3-C9 heteroaryl, C3-C9 heterocyclic, whereas the heterocycloalkyl, heterocycloalkenyl, heteroaryl, heterocyclic residue can have up to 6 hetero ring atoms, an amino acid or a peptide mimetic thereof, all of the above residues may be optionally substituted, or is H.
Owner:VIVORYON THERAPEUTICS NV
Cysteine protease
ActiveUS20180023070A1Good curative effectLow immunogenicityNervous disorderPeptide/protein ingredientsDiseaseCysteine protease activity
Owner:HANSA BIOPHARMA AB
Cathepsin cysteine protease inhibitors
ActiveUS20060111440A1Avoid adjustmentImprove bioavailabilityBiocideSenses disorderDiseaseCathepsin O
This invention relates to a novel class of compounds which are cysteine protease inhibitors, including but not limited to, inhibitors of cathepsins K, L, S and B. These compounds are useful for treating diseases in which inhibition of bone resorption is indicated, such as osteoporosis.
Owner:MERCK CANADA
Cathepsin cysteine protease inhibitors
ActiveUS20050240023A1Treating and preventing cathepsin dependent conditionBiocideOrganic chemistryCathepsin KCathepsin
This invention relates to a novel class of compounds which are cysteine protease inhibitors, including but not limited to, inhibitors of cathepsins K, L, S and B. These compounds are useful for treating diseases in which inhibition of bone resorption is indicated, such as osteoporosis.
Owner:AXYX PHARMA INC +1
Cyanoalkylamino derivatives as protease inhibitors
The present invention is directed to novel cyanoalkylamino derivatives that are inhibitors of cysteine protease such as cathepsins K, S, B and L, in particular cathepsin K Pharmaceutical composition comprising these compounds, method of treating diseases mediated by unregulated cysteine protease activity, in particular cathepsin K utilizing these compounds and methods of preparing these compounds are also disclosed.
Owner:AXYX PHARMA INC +1
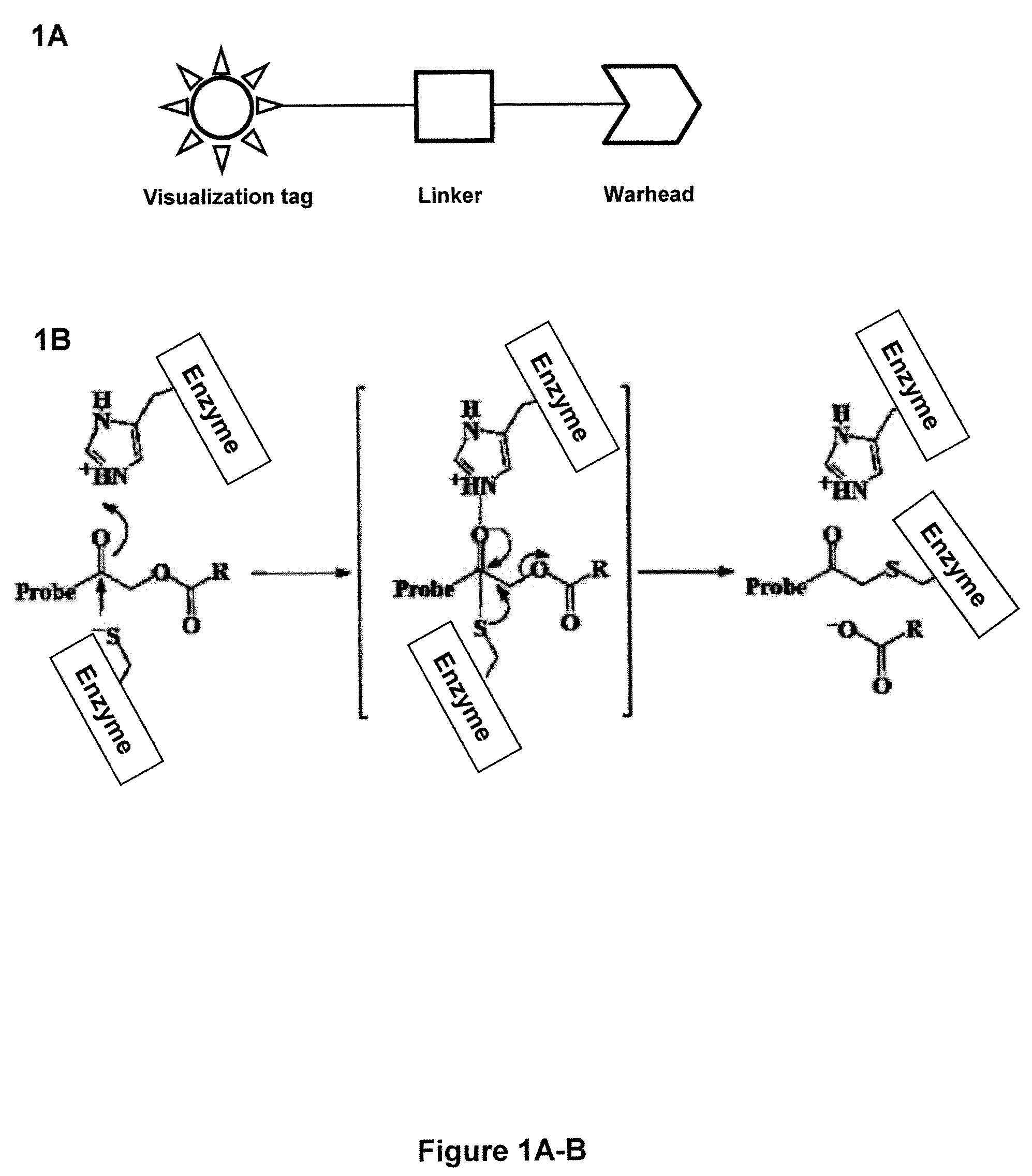
Probes for In Vivo Targeting of Active Cysteine Proteases
ActiveUS20090252677A1Stable labelingSufficient half-lifeRadioactive preparation carriersX-ray constrast preparationsActive enzymeHalf-life
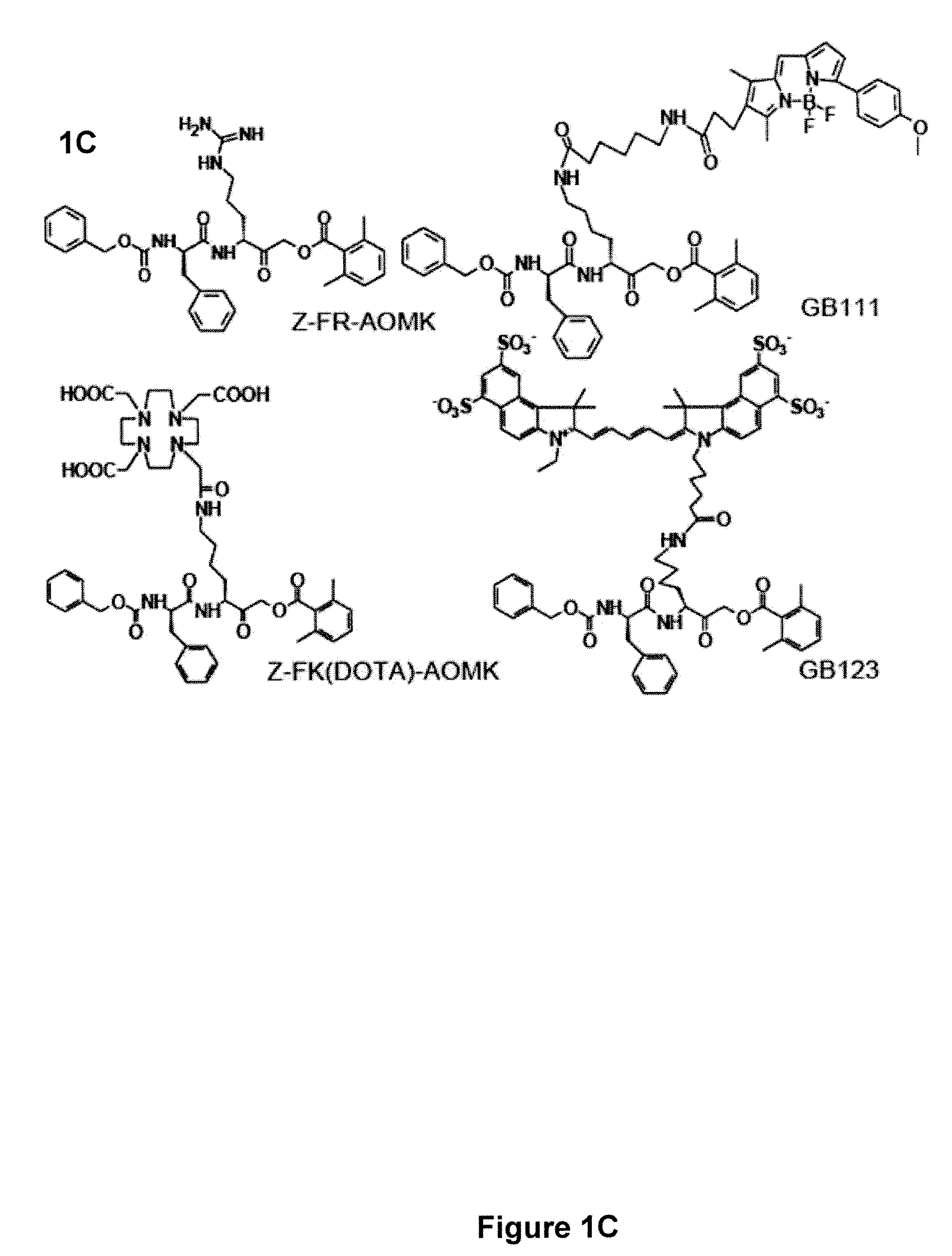
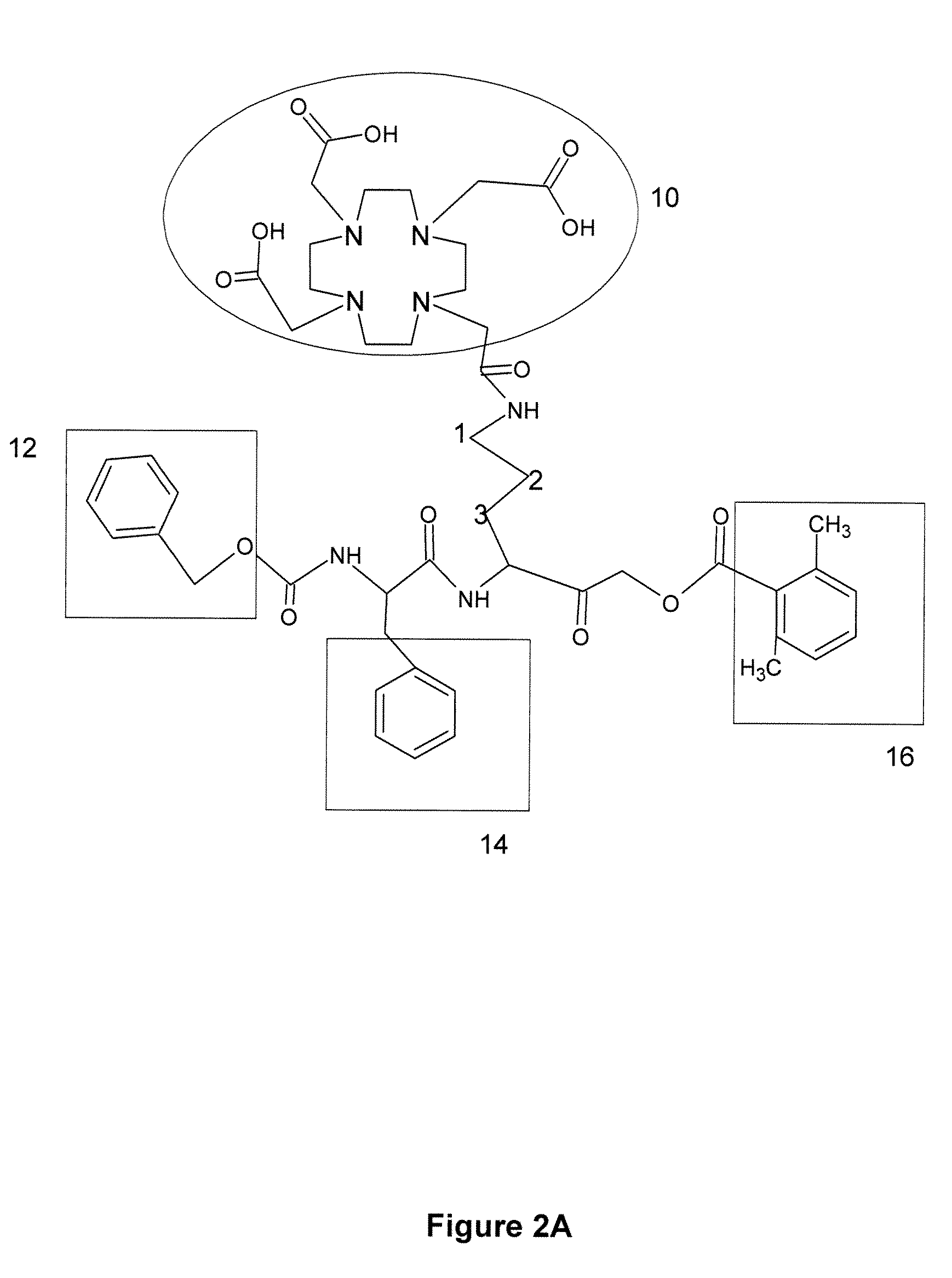
Activity-based probes, which are specific for certain active cysteine proteases (caspase, cathepsin and legumain) and carry radioactive labels, are disclosed. The present probes comprise an acyloxymethyketone (AOMK) “warhead” that binds only to active enzyme. The probes further comprise peptide-like structure that targets the probe to a specific cysteine protease or protease family, and a radiolabel on the probe, which is bound to the targeted enzyme. It has been found that the present probes are stable in vivo and give specific target images distinguishable over background. The preferred probes are labeled with a positron-emitting agent such as 64Cu, 125I (SPECT) and 99mTc (PET). The probes show in vivo half-life and stability well suited for imaging.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Modulation of the neuroendocrine system as a therapy for motor neuron disease
Owner:UNIVERSITY OF PITTSBURGH
Compositions relating to a mutant Clostridium difficile toxin and methods thereof
In one aspect, the invention relates to an immunogenic composition that includes a mutant Clostridium difficile toxin A and / or a mutant Clostridium difficile toxin B. Each mutant toxin includes a glucosyltransferase domain having at least one mutation and a cysteine protease domain having at least one mutation, relative to the corresponding wild-type C. difficile toxin. The mutant toxins may further include at least one amino acid that is chemically crosslinked. In another aspect, the invention relates to antibodies or binding fragments thereof that binds to said immunogenic compositions. In further aspects, the invention relates to isolated nucleotide sequences that encode any of the foregoing, and methods of use of any of the foregoing compositions.
Owner:WYETH LLC
Cathepsin cysteine protease inhibitors
Owner:AXYX PHARMA INC +1
Method and product for cleaning and/or whitening of teeth
A method and a product for cleaning and / or whitening of teeth. Natural human cysteine proteinases are employed for cleaning and whitening purposes and this activity can be blocked by natural cysteine protease inhibitors, which are released secondarily from the product at a later stage. The use of natural cysteine proteinases and their inhibitors provides the advantage that they are man's own proteins, and therefore the risk of allegorization is minimized. In addition, their enzyme kinetics are will known.
Owner:RINNE ARI
Inhibitors of cysteine protease
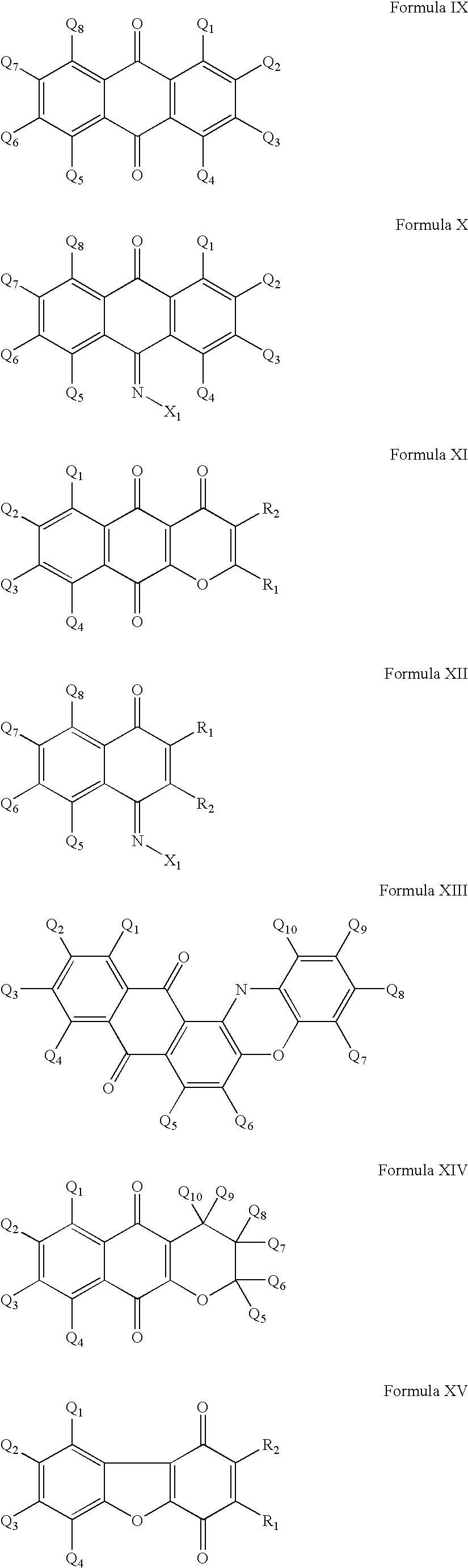
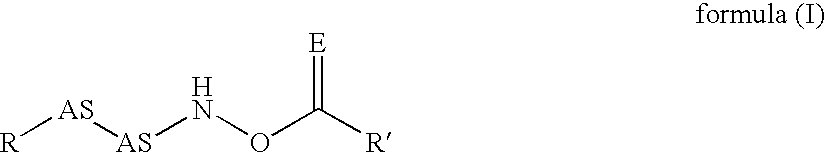
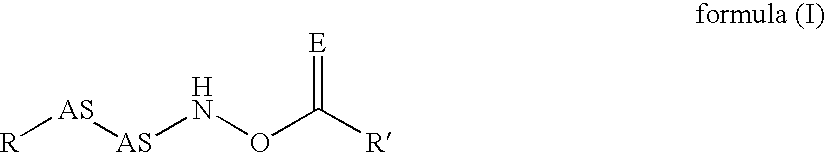
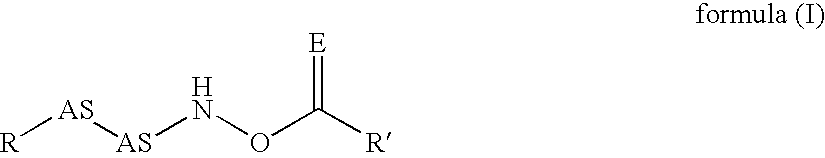
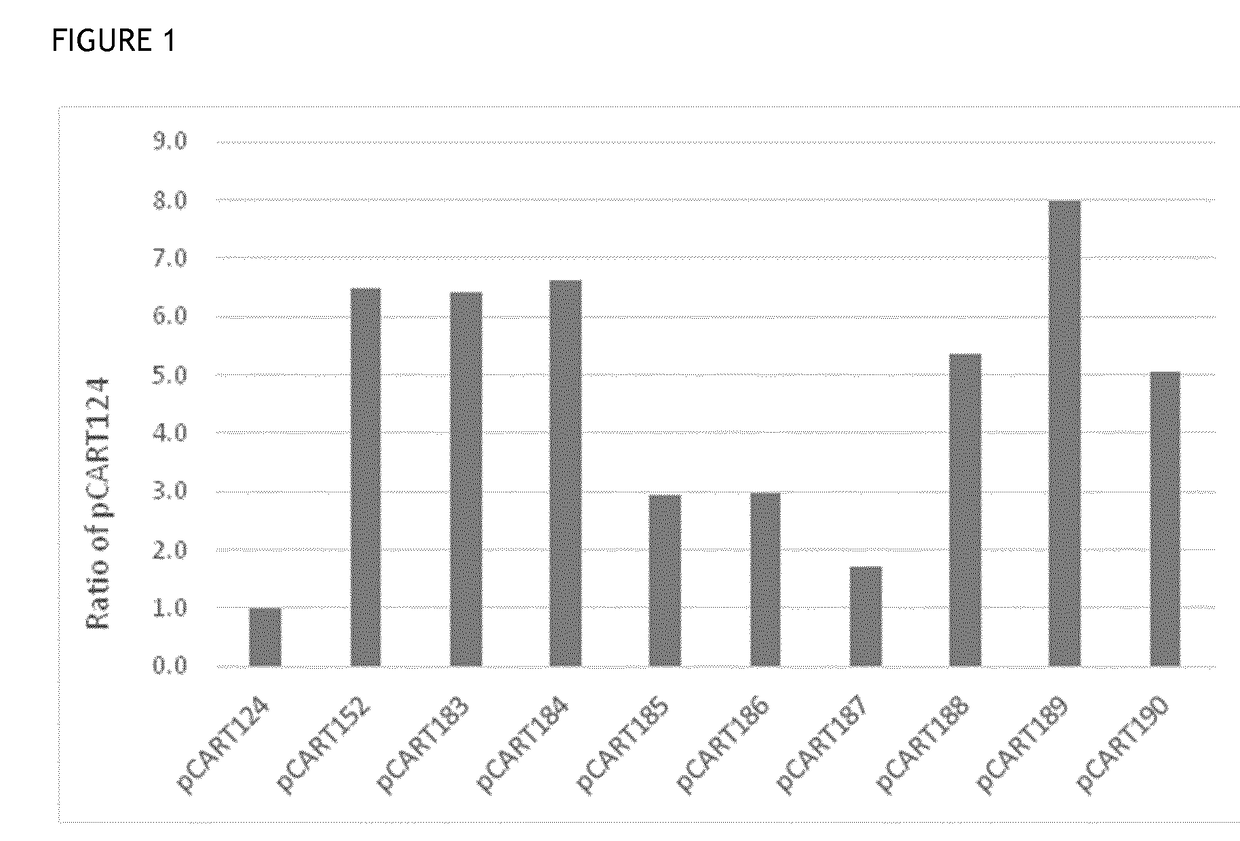
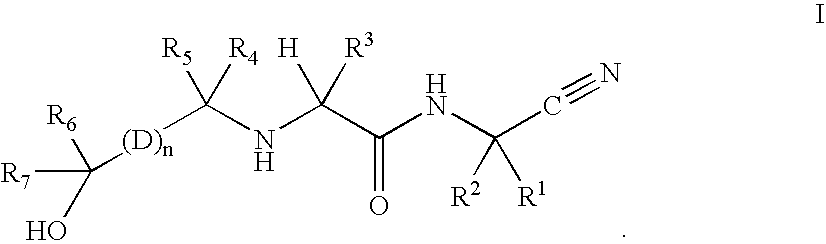
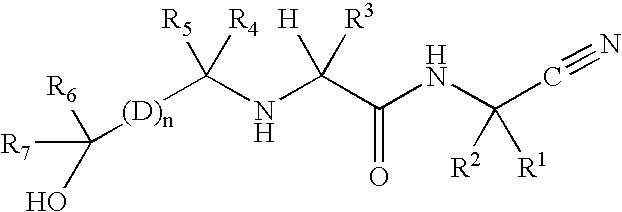
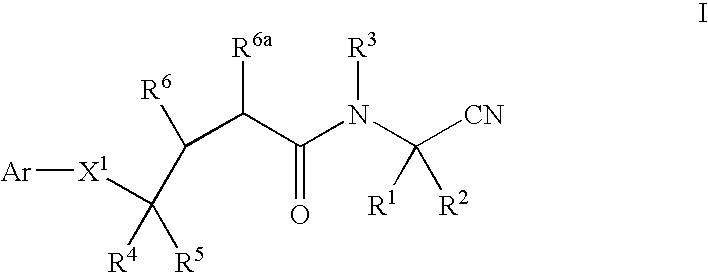
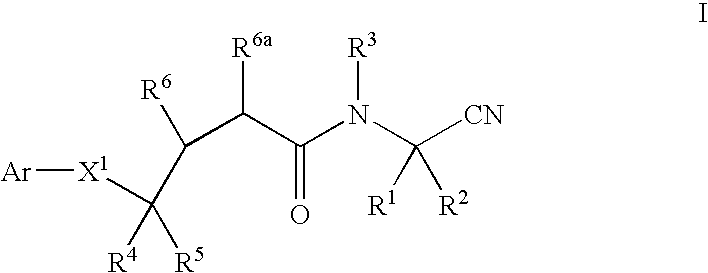
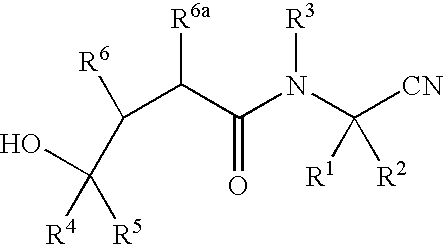
InactiveUS20020128476A1Excessive degradationOrganic chemistryHydrolasesStereochemistryPineapple Protease
This invention relates to compounds of formula (I): 1 wherein: A is C(O) or CH(OH); R.sup.1 is 2 R.sup.2 is H, C.sub.1-6alkyl, C.sub.3-6cycloalkyl-C.sub.0-6alkyl, Ar-C.sub.0-6alkyl, Het-C.sub.0-6alkyl, R.sup.5C(O)--, R.sup.5C(S)--, R.sup.5S0.sub.2--, R.sup.5OC(O)--, R.sup.5R'NC(O)--, R.sup.5R'NC(S)--, adamantyl-C(O)--, or 3 R" is H, C.sub.1-6alkyl, Ar-C.sub.0-6alkyl, or Het-C.sub.0-6alkyl; R'" is H, C.sub.1-6alkyl, C.sub.3-6cycloalkyl-C.sub.0-6alkyl, Ar-C.sub.0-6alkyl, or Het-C.sub.0-6alkyl;
Owner:SMITHKLINE BECKMAN CORP
Extraction method of ganoderma lucidum polysaccharide
The invention belongs to the technical field of polysaccharide extraction and particularly relates to an extraction method of a ganoderma lucidum polysaccharide. The extraction method of the ganoderma lucidum polysaccharide comprises the following steps: selecting air-dried ganoderma lucidum; grinding the ganoderma lucidum, and screening by a 40-80-mesh sieve to obtain ganoderma lucidum powder; adding water into the ganoderma lucidum powder and uniformly stirring, wherein the weight ratio of the ganoderma lucidum powder to the water is 1: (5-10); soaking the ganoderma lucidum powder in the water for 8 to 16 hours; adding cellulase for enzymolysis; then adding alkaline protease and bromelain; carrying out enzymolysis under the microwave condition; after enzymolysis, concentrating and decolorizing; centrifuging; taking supernate to carry out concentration and alcohol precipitation; centrifuging again; washing precipitates with ethanol; after freeze-drying, grinding to obtain the ganoderma lucidum polysaccharide. When the method disclosed by the invention is adopted to extract the polysaccharide from the ganoderma lucidum, the action condition is mild due to the adoption of enzymes, fat and cellulose in the ganoderma lucidum are subjected to enzymolysis by adopting various enzymes to extract the polysaccharide from the ganoderma lucidum, and thus, the utilization rate of the ganoderma lucidum is increased. The polysaccharide obtained by adopting the method provided by the invention not only has high yield, but also has high purity.
Owner:牛睦元
Method for treatment of cardiovascular and metabolic diseases and detecting the risk of the same
InactiveUS20070072798A1Peptide/protein ingredientsMetabolism disorderCysteine thiolateCoronary heart disease
This invention relates to the therapeutic, diagnostic and pharmacogenetic use of nucleic acids and proteins involved in human proteolytical system such as serine and cysteine proteases and their inhibitors and pharmaceutical agents and other therapies affecting these. This invention discloses methods for the treatment and prevention of cardiovascular diseases such as coronary heart disease (CHD), acute myocardial infarction (AMI), chronic CHD, arterial hypertension (HT) and cerebrovascular stroke and metabolic disorders such as the metabolic syndrome (MBO) and obesity and methods for detecting or diagnosing a risk of, or predisposition to the said diseases in a subject, for selecting treatment in a subject and for selecting subjects for studies testing cardiovascular, anti-diabetic and anti-obesity drugs, as well as to transgenic animals.
Owner:JURILAB
Novel compounds and compositions as protease inhibitors
InactiveUS20030092634A1Urea derivatives preparationOrganic active ingredientsProteinase activityCysteine Proteinase Inhibitors
Owner:AXYX PHARMA INC
Oral compositions for prevention and reduction of bacterial adhesion to oral surfaces
ActiveUS20060134018A1Reduce interactionCosmetic preparationsToilet preparationsAnti-Adhesion AgentAdditive ingredient
The present invention encompasses an oral composition containing an anti-adhesion agent, preferably a cysteine protease and most preferably ficin. In another aspect, the cysteine protease is in combination with one or more ingredients, such as antibacterial agent and surfactant. The anti-adhesion agent mitigates interaction between a subject oral cavity and plaque-forming materials.
Owner:COLGATE PALMOLIVE CO
Cysteine protease inhibitor C test kit
The invention provides a cysteine protease inhibitor C test kit, wherein the kit comprises a reagent R1 and a reagent R2, the reagent R2 is a reaction solution for promoting specific binding of an antigen and an antibody, the reagent R2 is a polystyrene latex particle mixture coated by an anti-human cystatin C antibody and is put in a proper buffer solution. The polystyrene latex particle mixture in the reagent R2 is a mixture of large-diameter polystyrene latex particles and small-diameter polystyrene latex particles. The kit is high in detection sensitivity, high in accuracy, good in linearity within the test range, good in stability and low in production cost.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
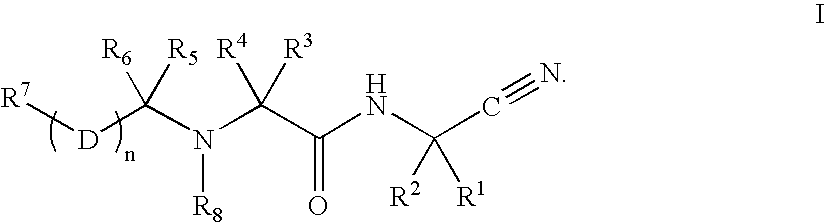
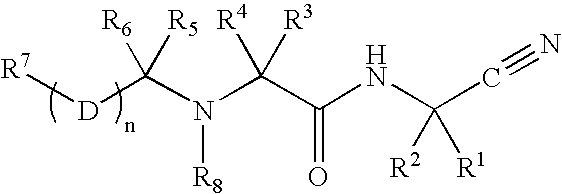
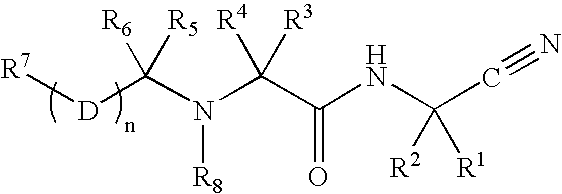
C-terminal modified oxamyl dipeptides as inhibitors of the ICE/ced-3 family of cysteine proteases
InactiveUS7183260B2Improve propertiesImprove permeabilityAntibacterial agentsOrganic active ingredientsDiseaseDipeptide
This invention is directed to novel oxamyl dipeptide ICE / ced-3 family inhibitor compounds. The invention is also directed to pharmaceutical compositions containing these compounds, as well as to the use of such compositions in the treatment of patients suffering inflammatory, autoimmune and neurodegenerative diseases, for the prevention of ischemic injury, and for the preservation of organs that are to undergo a transplantation procedure.
Owner:CONATUS PHARMA
Anti-neoplastic compounds, compositions and methods
ActiveUS20120114765A1Easy to manageEven doseBiocideHeavy metal active ingredientsBiochemistryWilms' tumor
Owner:PROGENRA INC
Epoxide inhibitors of cysteine proteases
Provided herein are novel epoxide inhibitors of cysteine proteases, compositions comprising the epoxide inhibitors, and packaged pharmaceuticals. Also provided are methods of inhibiting a papain-family cysteine protease and methods of treating or preventing a disease by administering a composition containing an epoxide inhibitor of the invention. The compositions may be administered in combination with another therapeutic agent.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
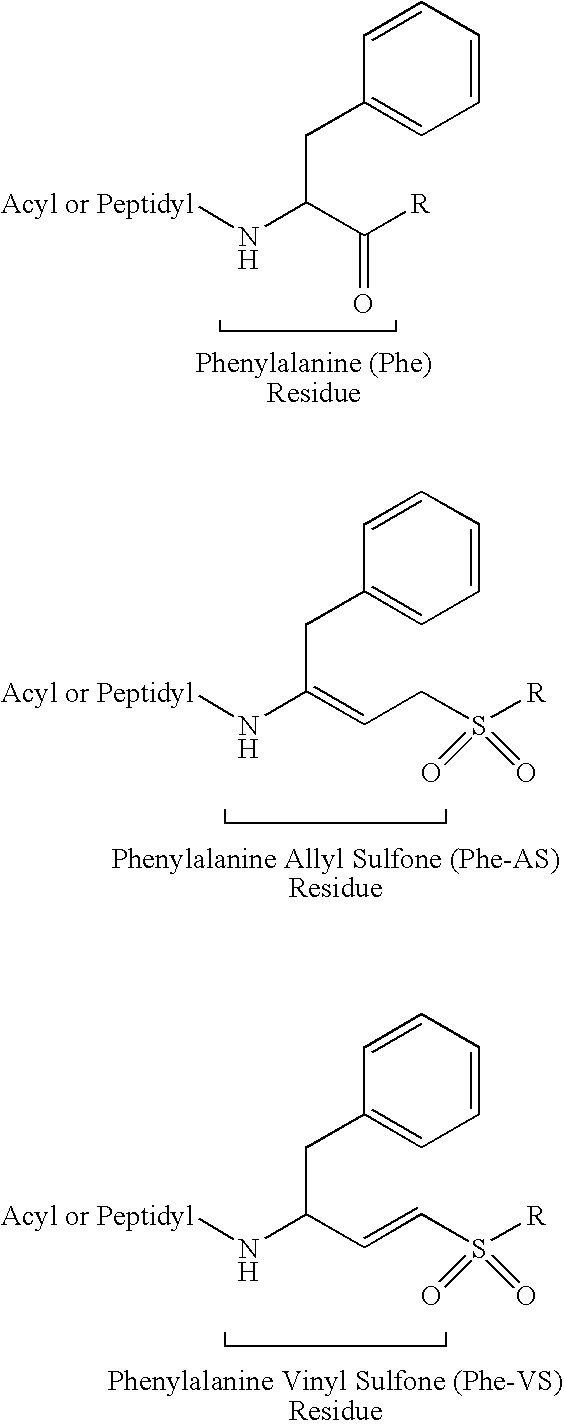
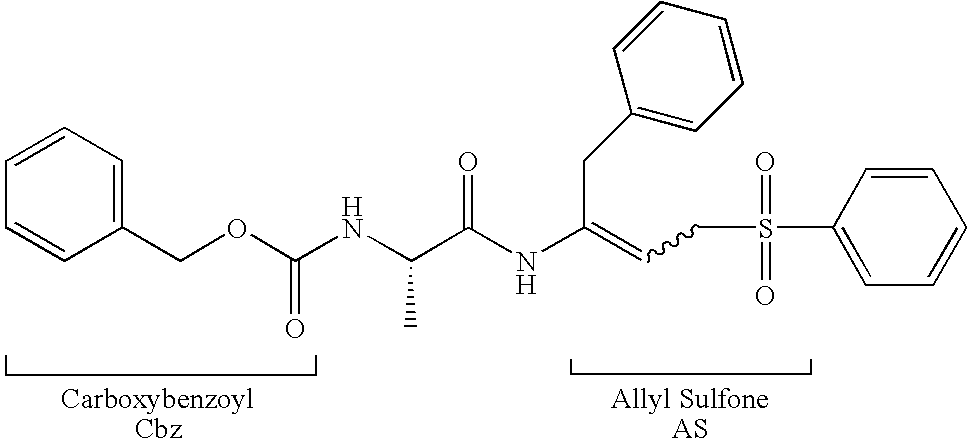
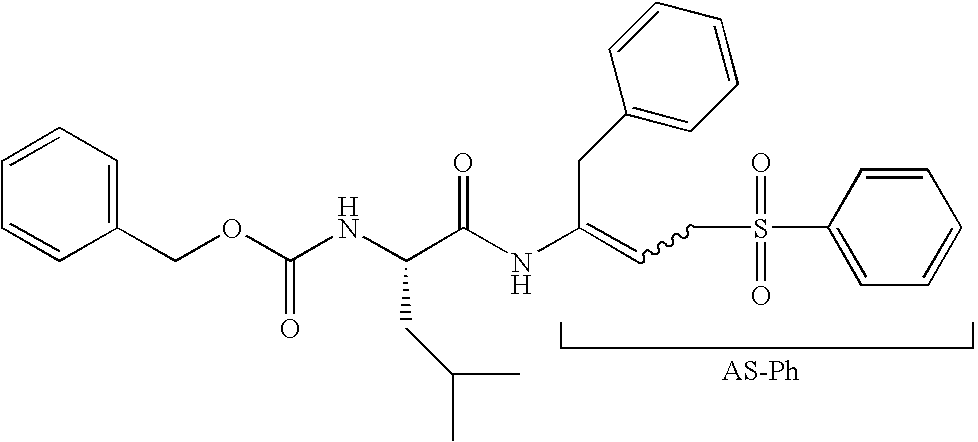
Peptidyl allyl sulfones
The present disclosure provides compositions for inhibiting proteases, methods for synthesizing the compositions, and methods of using the disclosed protease inhibitors. Aspects of the disclosure include peptidyl allyl sulfone compositions that inhibit proteases, for example cysteine proteases, either in vivo or in vitro.
Owner:GEORGIA TECH RES CORP
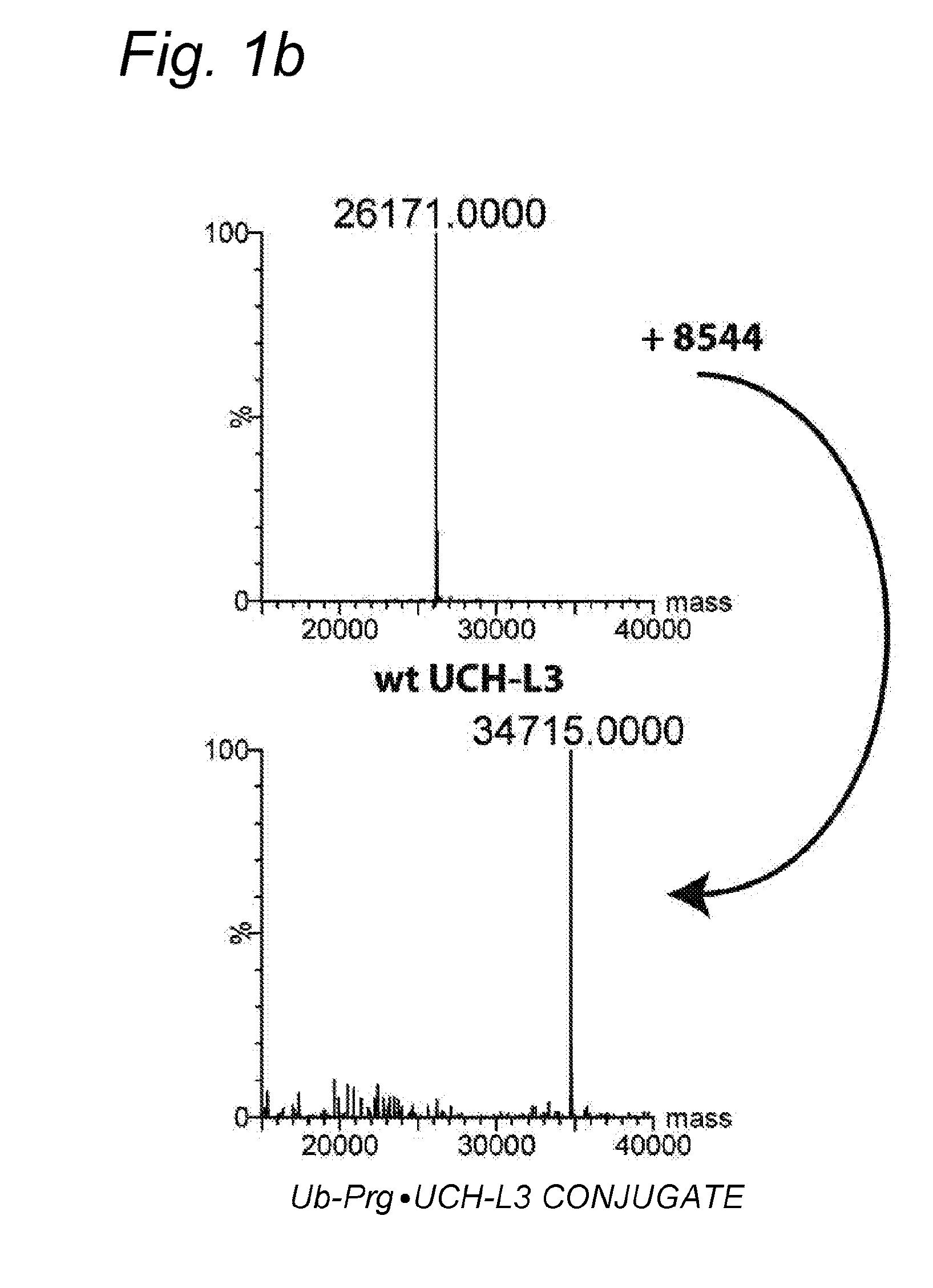
Cysteine protease capturing agents
The invention concerns cysteine protease capturing agents capable of highly selective and irreversible binding of the corresponding cysteine protease. Such compounds may have utility in fundamental biological research and diagnostics, e.g. involving labeled or immobilized versions of such compounds, and they may also have potential utility in therapy, based on competitive inhibition of the cysteine protease, as will be readily apparent to those skilled in the art. The present inventors have discovered that such capturing agents can be obtained by modification of a cleavage fragment of a ‘natural’ substrate for the cysteine protease of interest, said modification involving the introduction of a propargyl moiety in such a way that the terminal alkyne group is positioned to allow for interaction with the free thiol group of the cysteine residue at the active site of the protease.
Owner:NETHERLANDS CANCER INST
Macrocyclic Cysteine Protease Inhibitors and Compositions Thereof
InactiveUS20110021434A1Reduced activitySenses disorderNervous disorderCalpain activityCysteine protease activity
The present invention provides a novel class of macrocyclic compounds, which are useful as cysteine protease inhibitors. Also provided are novel intermediates and methods of preparing the compounds. The invention also provides pharmaceutical compositions comprising the compounds. The compounds and compositions are useful in methods of treating or preventing one or more diseases associated with cysteine protease activity, particularly those associated with calpain activity.
Owner:UNIVERSITY OF CANTERBURY +2
Cysteine protease inhibitors containing heterocyclic leaving groups
A class of cysteine protease inhibitors which inactivate a cysteine protease by covalently bonding to the protease and releasing a heterocyclic leaving group is presented. The cysteine protease inhibitors of the present invention comprise a first portion which targets a desired cysteine protease and positions the inhibitor near the thiolate anion portion of the active site of the protease, and a second portion which covalently bonds to the cysteine protease and irreversibly deactivates that protease by providing a carbonyl or carbonyl-equivalent which is attacked by the thiolate anion of the active site of the cysteine protease to sequentially cleave a heterocyclic leaving group. The heterocyclic leaving group of the protease inhibitor is of the formula: -O-Het, where Het is a heterocycle having 4-7 atoms in the ring, with at least one of the heterocycle atoms being N, O or S.
Owner:PROTOTEK
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com