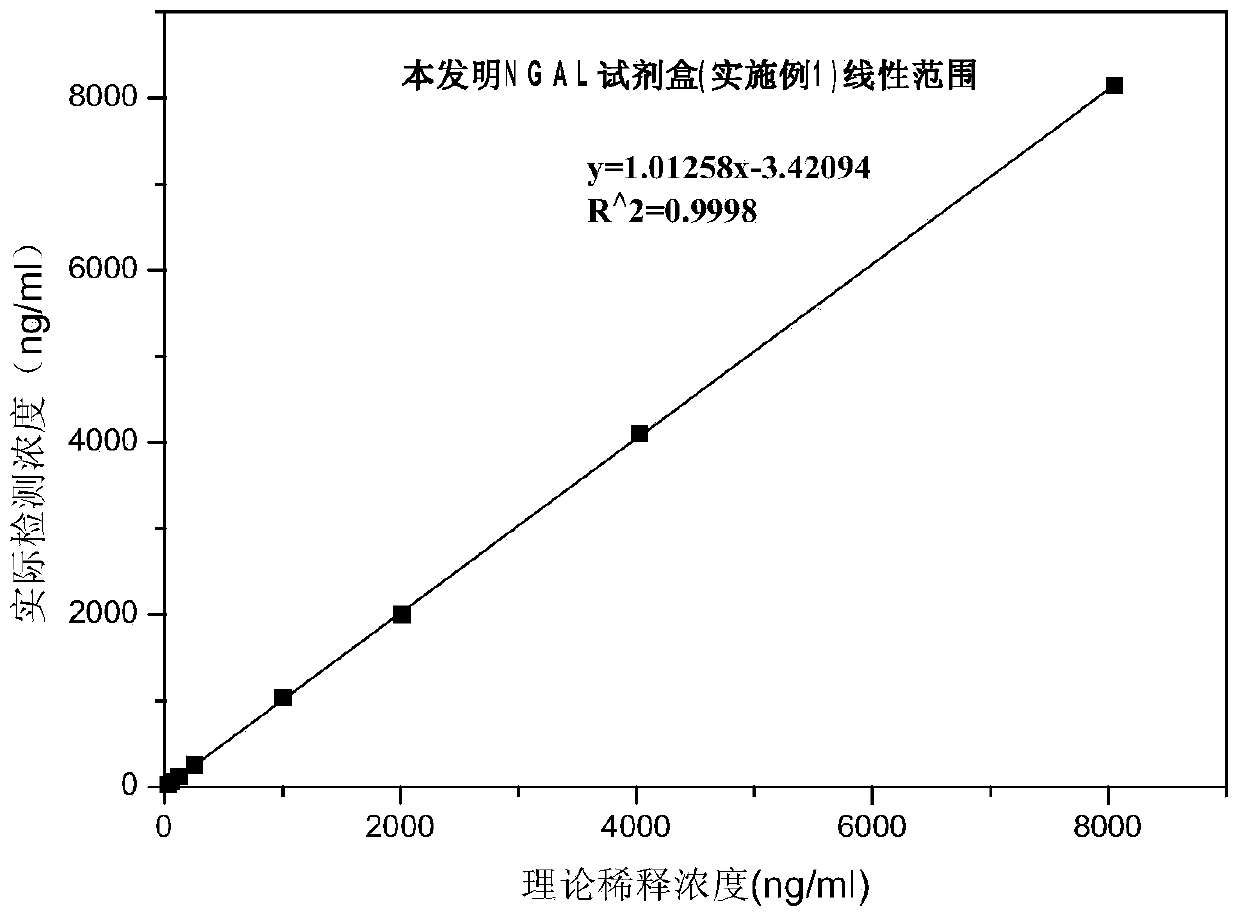
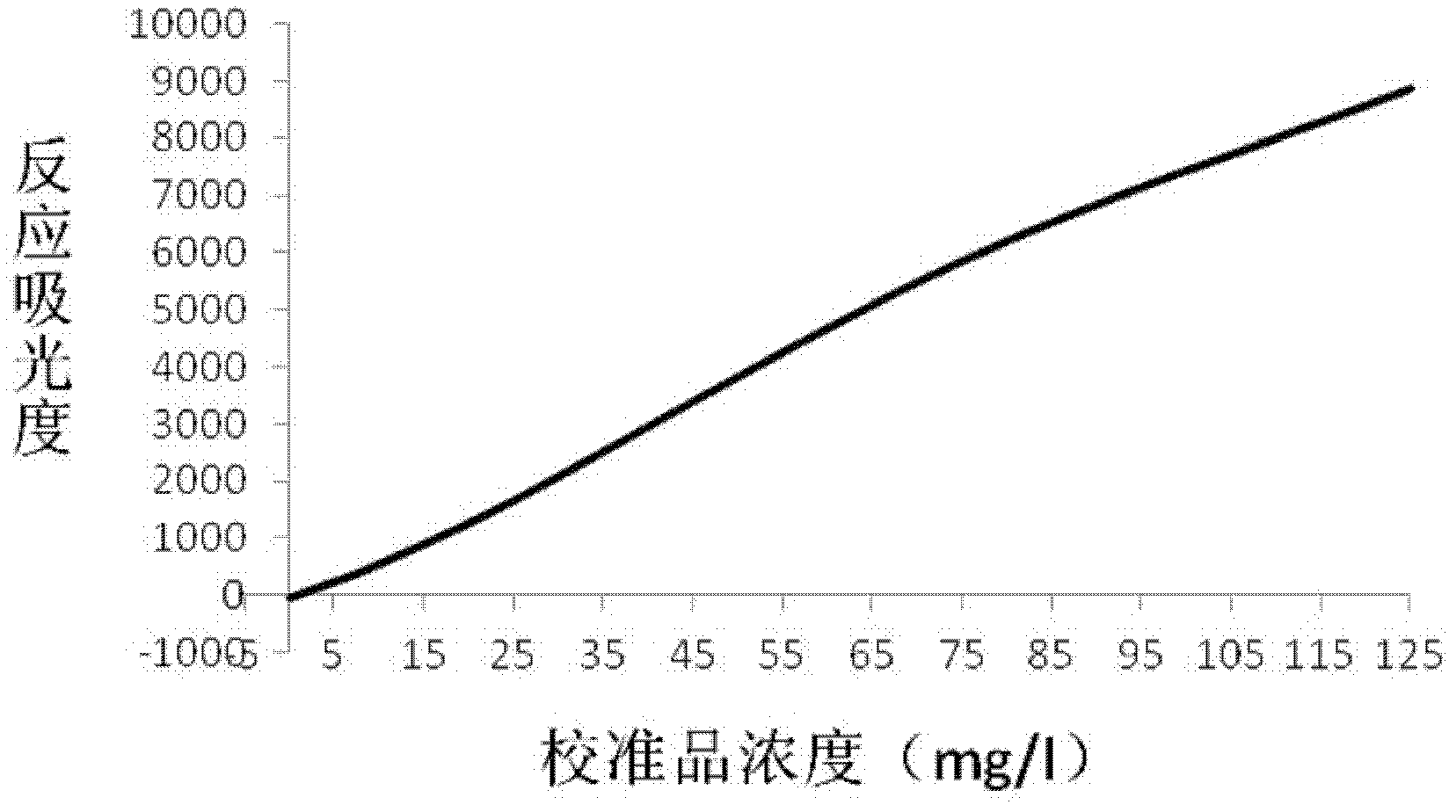
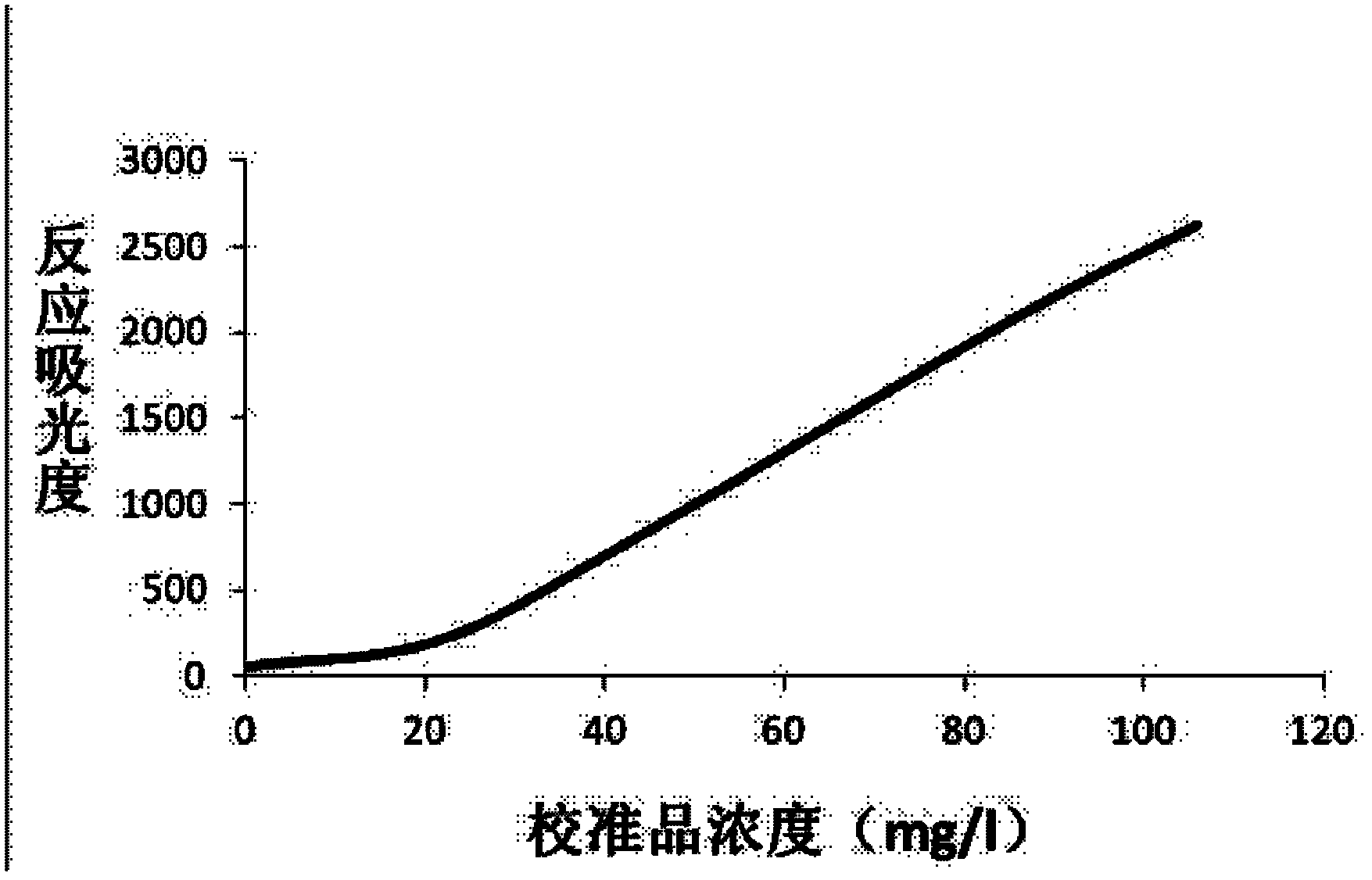
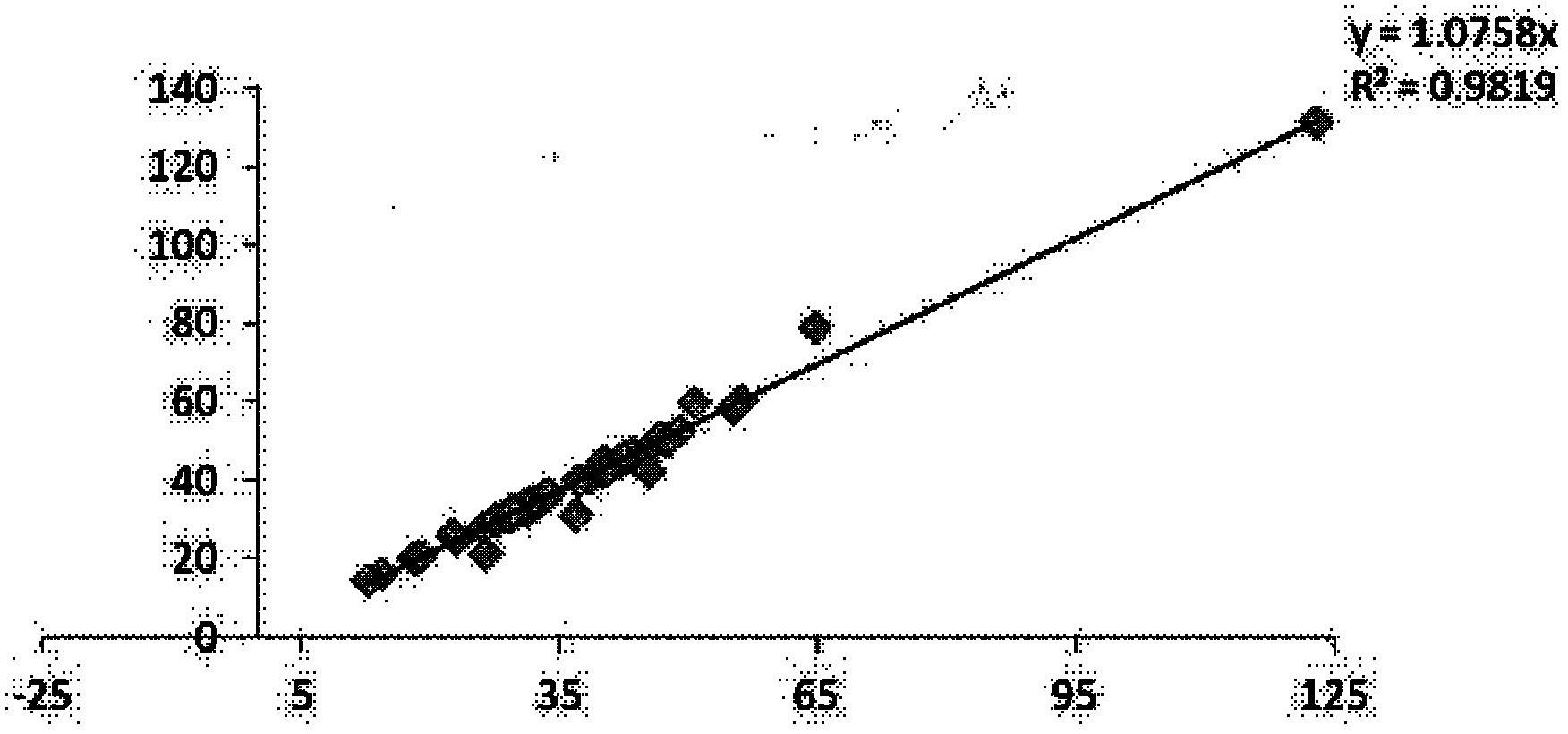
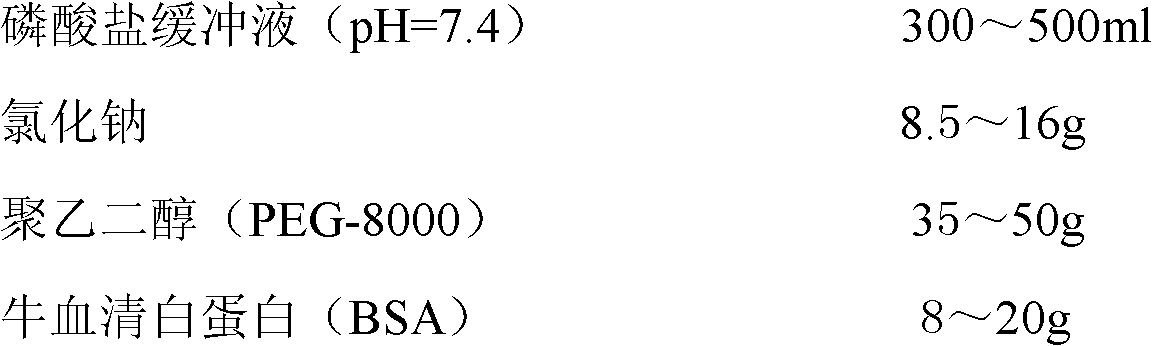Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
173 results about "Polystyrene latex" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
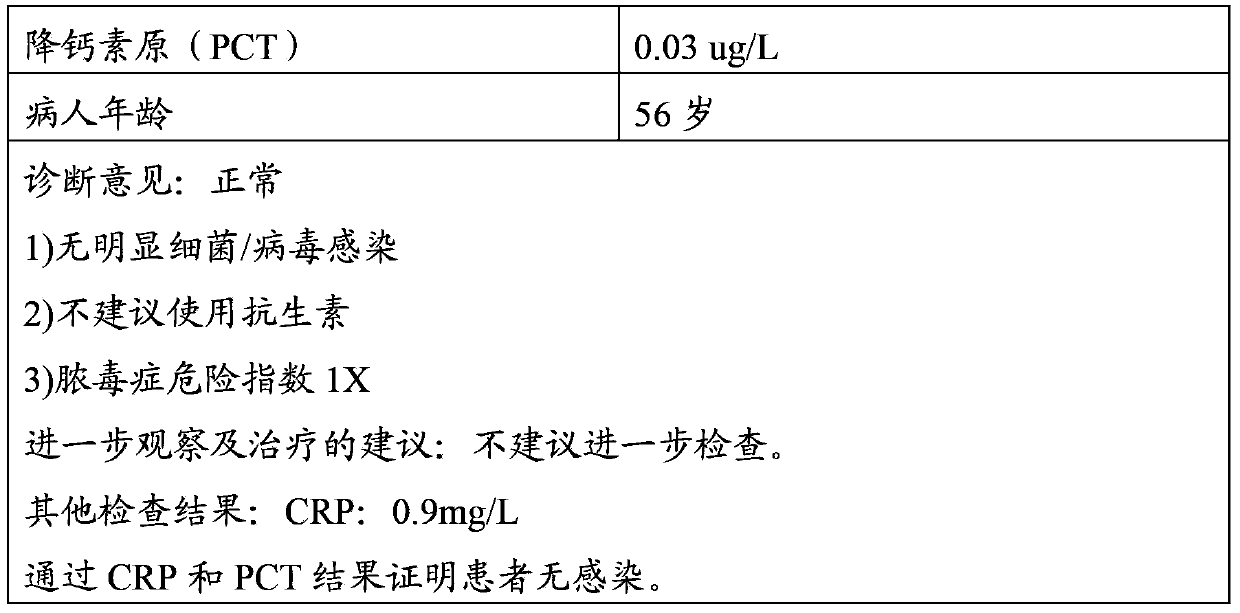
Latex enhanced turbidimetric immunoassay kit of quantitatively detecting procalcitonin PCT
ActiveCN102759631AHigh detection sensitivityMeet the needs of clinical applicationsMaterial analysis by observing effect on chemical indicatorBiotin-streptavidin complexTurbidimetry
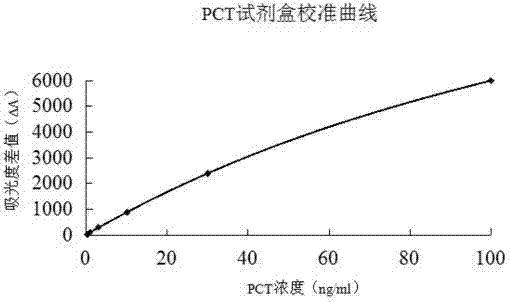
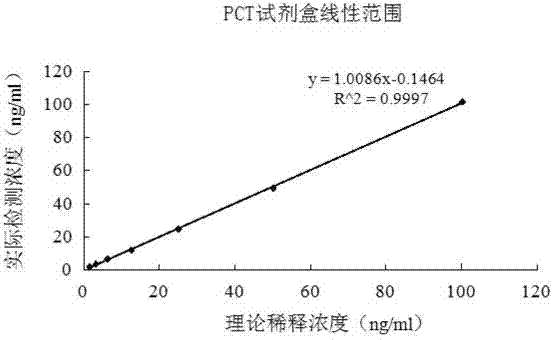
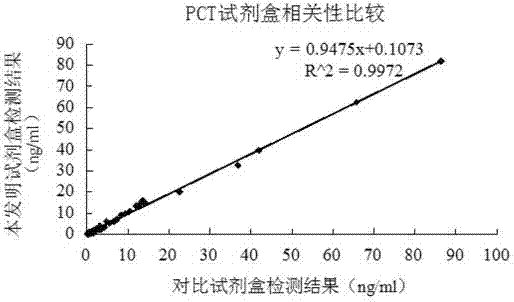
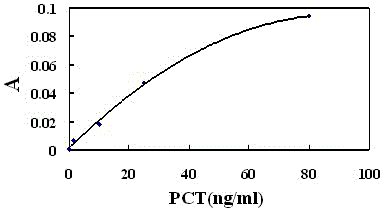
The invention relates to a latex enhanced turbidimetric immunoassay kit of quantitatively detecting procalcitonin PCT. The kit comprises an R1 reagent, an R2 reagent and a calibrator, wherein the R1 reagent comprises a protecting agent, a reaction enhancing agent, a preservative and buffer solution; the R2 reagent comprises a protecting agent, a preservative, buffer solution and anti-human PCT antibody coated sensitization polystyrene latex particles; the calibrator comprises a protecting agent, a preservative, buffer solution and PCT recombinant protein; the human PCT antibody in the R2 reagent is linked with polystyrene latex particles through streptavidin-biotin; and the particle diameter of the latex particles in the R2 reagent is 40-500 nm. The kit can be used on a biochemical analyzer and a scatter turbidimetry analyzer for quantitatively detecting the PCT content in human blood. The invention provides the PCT detection kit which has the advantages of convenience, quickness, high sensitivity, strong specificity and accurate quantification; and the kit has high instrument compatibility, is low in detection cost and meets the requirements on PCT turbidimetric products in clinical use.
Owner:NANJING NORMAN BIOLOGICAL TECH
Microfluidic device with electrode structures
The design, development and fabrication of a DEP microfluidic assembly with an in-built interdigitated microelectrode array is presented. Continuous fractionation of microparticles in a PDMS microfluidic channel is described. Experimental verification of positive and negative DEP of yeast cells and polystyrene latex beads is demonstrated. A microfluidic device with DEP arranged electrodes in a channel has posts extending into the channel for controlling shaping of DEP fields.
Owner:UNIV TECH INT
Method for preparing nanometer gahnite
ActiveCN103449503AReduce pollutionGood quality powderZinc oxides/hydroxidesNanotechnologySucroseGlycerol
A method for preparing nanometer gahnite. The preparation method is as below: dissolving zinc salt in water, adding an aluminum source, stirring for 10-30 min, adding a pore-enlarging agent, stirring, ageing at 20-100 DEG C for 30-60 min, drying, and calcining at 500-1200 DEG C. The molar ratio of the raw materials is as below: Zn:Al:water=1:2:16-35; calculated by the weight of zinc oxide being 100%, the addition amount of the pore-enlarging agent is 0.5-30%; and the pore-enlarging agent is one or more selected from sucrose, glycerol, ammonium carbonate, ammonium hydrogen carbonate, polystyrene latex and polyethylene glycol.
Owner:PETROCHINA CO LTD
Procalcitonin latex enhanced immunoturbidimetry detection kit
The invention discloses a procalcitonin (PCT) latex enhanced immunoturbidimetry detection kit and a preparation method thereof. The procalcitonin latex enhanced immunoturbidimetry detection kit comprises a reagent R1 and a reagent R2, wherein the reagent R1 mainly comprises a buffer solution 1, a stabilizing agent 1, a preservative 1, a coagulation increasing agent, a protective agent 1 and EDTA (Ethylene Diamine Tetraacetic Acid); the reagent R2 mainly comprises a buffer solution 2, a stabilizing agent 2, a preservative 2, a polystyrene latex microballon sphere, a PCT antibody and a protective agent 2; the particle size of the polystyrene latex microballon sphere in the reagent R2 is 100-600nm, the PCT antibody is one or more of a mouse anti-human PCT antibody, a goat anti-human procalcitonin antibody or a rabbit anti-human procalcitonin antibody, and the PCT antibody is connected with the polystyrene latex microballon sphere in a covalent coupling or physical absorption manner. Compared with the prior art, the PCT detection kit provided by the invention has the advantages of low preparation cost, good stability, high detection sensitivity and strong specificity, is easy to store, and is easily popularized in clinical.
Owner:CHONGQING ZHONGYUAN BIOLOGICAL TECH
Beta 2-microglobulin detection kit
The invention provides a beta 2-microglobulin detection kit comprising a reagent R1 and a reagent R2, wherein the reagent R1 comprises a buffer solution, a preservative, a stabilizing agent, an electrolyte, a surfactant and the balance of purified water; and the reagent R2 comprises polystyrene latex grains sensitized by the beta 2-microglobulin antibody, a buffer solution, a preservative, a stabilizing agent and a residual amount of purified water. The polystyrene latex grains sensitized by the beta 2-microglobulin antibody are directionally coupled. A preparation method comprises the following steps of: activating the polystyrene latex grains; oxidizing the beta 2-microglobulin antibody; and coupling the beta 2-microglobulin antibody and the polystyrene latex grains. The kit provided by the invention has the advantages of high sensitivity, good specificity, good accuracy, strong anti-jamming capability and low production cost.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Detection kit for human serum amyloid A protein
InactiveCN105372434AEasy to detectHigh sensitivityBiological material analysisBiological testingSerum igeAnti jamming
The invention discloses a detection kit for human serum amyloid A protein. The detection kit is characterized by comprising a reagent R1 and a reagent R2, the reagent R1 is an appropriate buffer solution, and the reagent R2 is prepared by putting polystyrene latex particles sensitized by a human serum amyloid A protein antibody into the appropriate buffer solution. The detection kit has the advantages of being simple and fast in detection, high in sensitivity, good in accuracy, high in anti-jamming capability and low in production cost.
Owner:ZHEJIANG ZOYUN BIOTECH
Anti-cyclic citrullinated peptide (CCP) antibody detection kit
ActiveCN104198725AHigh refractive indexHigh chemical inertnessBiological testingFluorescence/phosphorescenceAnti ccp antibodiesMicrosphere
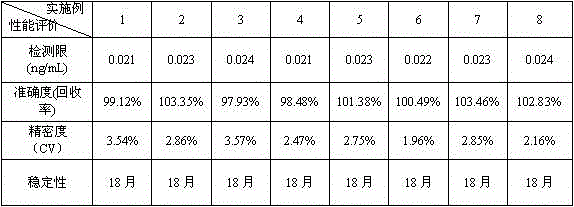
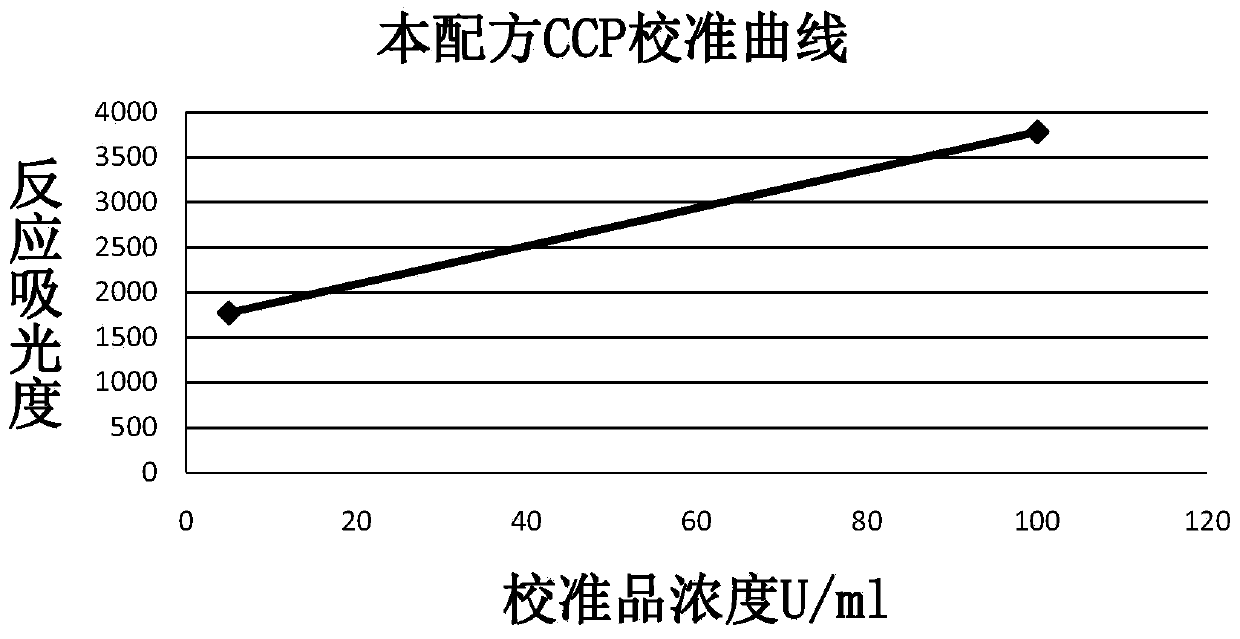
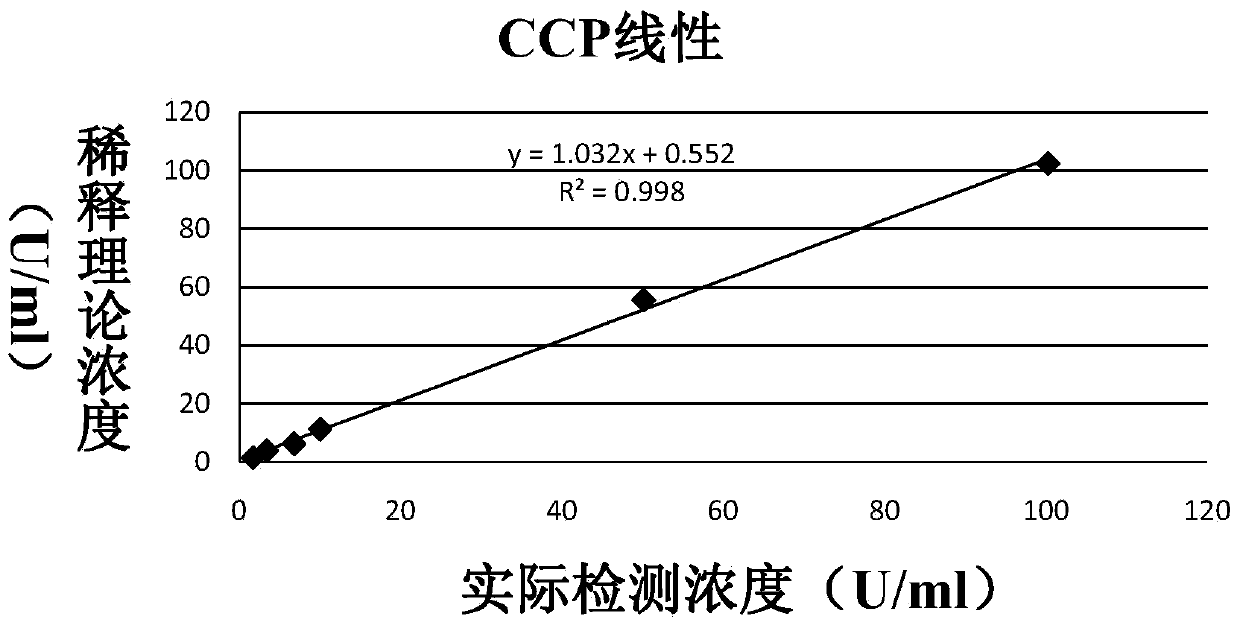
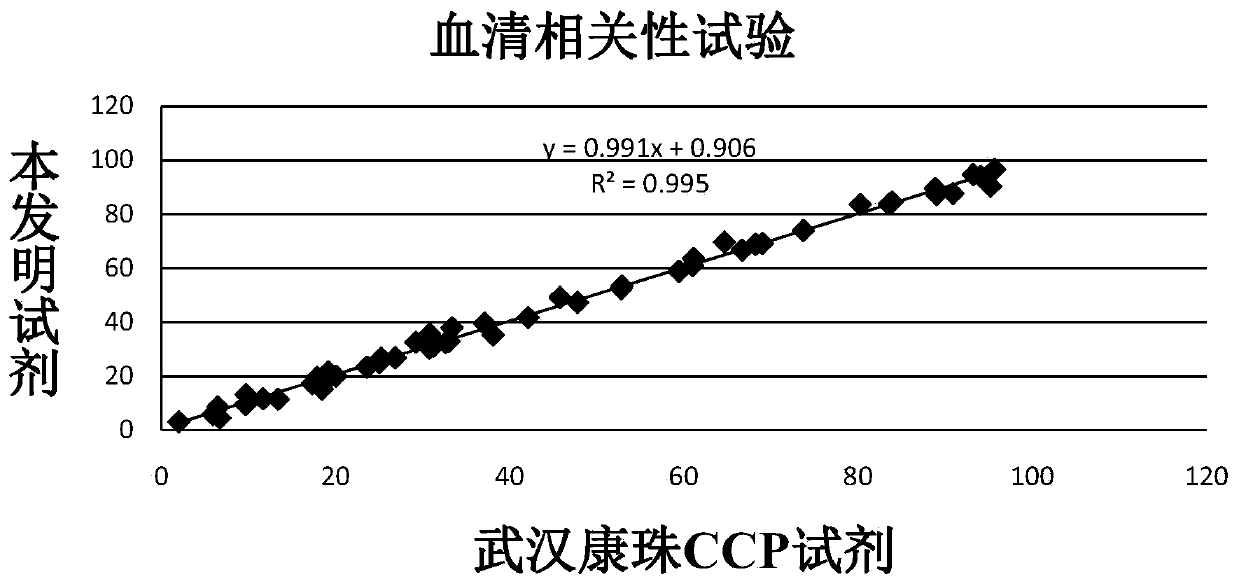
The invention discloses an anti-cyclic citrullinated peptide (CCP) antibody detection kit. The kit comprises a reagent R1, a reagent R2, a standard substance and a standard diluent, wherein the reagent R1 is prepared by washing CCP antigen latex particles by 50mM Tris buffer solution containing 20-500mmol / L first stabilizer and 0.1-1% preservative and then dispersing; and CCP antigens and polystyrene latex microspheres are subjected to chemical crosslinking and then are closed, so that the CCP antigen latex particles are formed. For the kit, the latex-enhanced immunoturbidimetry is adopted for detecting the content of an anti-CCP antibody, the kit has the high sensitivity, the high stability and the high detection speed (the time from determination to result acquisition is only within 5-10min), can detect mass samples on a conventional biochemical analyzer and can greatly improve the detection efficiency.
Owner:上海睿康生物科技有限公司
Preparation method of cystatin c detection kit
InactiveCN102279269AImmunoglobulins against animals/humansBiological testingAntibody conjugateHybrid antibody
The invention relates to a preparation method of cystatin C antibody and a preparation method of a detection kit. Cystatin C (Cys C) is currently one of the most sensitive diagnostic markers for clinical kidney disease. The antibody preparation of the present invention is extracted and purified from human normal serum, and then a highly sensitive antigen is obtained by artificial modification. Animals were immunized multiple times to obtain hyperimmune serum containing Cys C antibody, and high-purity Cys C antibody was obtained by affinity chromatography. During the preparation process, the loss of antibody activity is small, and it is easy to produce in batches, and the antibody has high affinity and high titer, and does not generate cross-immune reaction. After the antibody is coupled with polystyrene latex balls, the prepared detection kit has higher sensitivity and linear range, good repeatability, low detection limit, easy automatic analysis, fast and simple operation. Due to the high purity of the antibody, the content of heteroantibody is very small, so there is less interference and the matrix is stable.
Owner:王贤俊
Detection kit for cystine protease inhibitor C
ActiveCN102353770AHigh sensitivityImprove accuracyMaterial analysisPolystyrene latexStylosanthes viscosa
The invention discloses a detection kit for a cystine protease inhibitor C. The kit comprises a reagent R1 and a reagent R2, wherein the R1 is a proper buffer, and the reagent R2 is formed through placing polystyrene latex particles sensitized by cystine protease inhibitor C antibodies in a proper buffer. The kit of the present invention has the advantages of high sensitivity, good specificity, good accuracy, strong anti-interference capability, and low production cost.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Latex immune enhancement turbidimetric kit for detecting peptide C quantitatively
ActiveCN103852584AEasy to operateRapid quantitative assayBiological material analysisBiological testingAntiendomysial antibodiesEmergency treatment
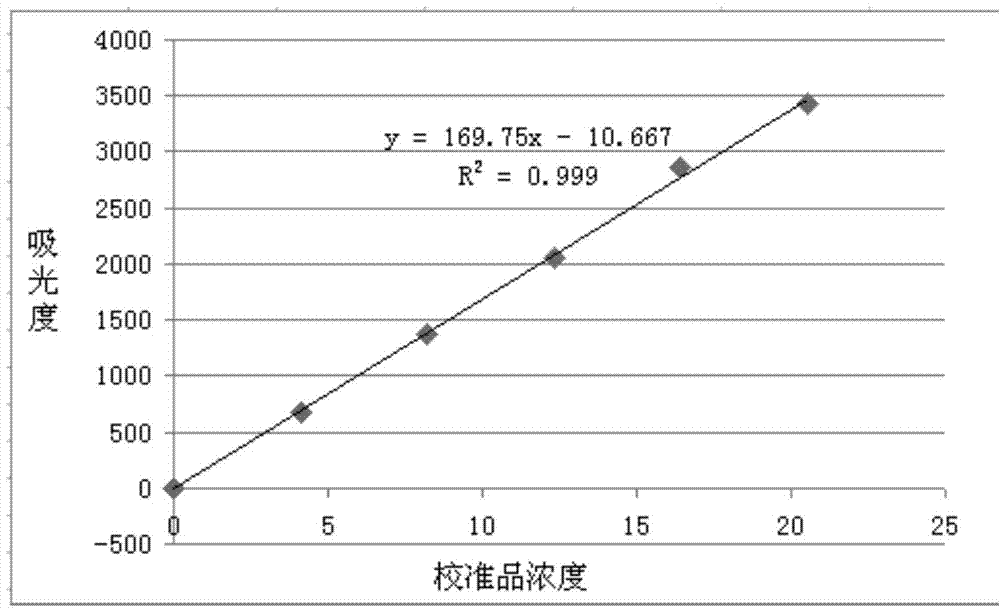
The invention discloses a latex immune enhancement turbidimetric kit for detecting peptide C quantitatively. The latex immune enhancement turbidimetric kit for detecting peptide C quantitatively consists of a peptide C R1 reagent, a peptide C R2 reagent and a peptide C calibration product, wherein the peptide C R1 reagent comprises a buffer liquid, a protective agent I, a reaction enhancer and preservatives; the peptide C R2 reagent comprises a buffer liquid, a protective agent I, preservatives and sensitization polystyrene latex particles coated with anti-human peptide C antibodies; the peptide C calibration product comprises a buffer liquid, a protective agent II, preservatives and peptide C recombinant protein. The kit prepared by the invention is suitable for semi-automatic and full-automatic biochemical analyzers and scattering turbidimetric analyzers, has the advantages of simple and rapid operation, high testing accuracy and high automation degree, is suitable for being used clinically and widely, and is especially suitable for rapid and quantitative determination of emergency treatment.
Owner:CHONGQING ZHONGYUAN BIOLOGICAL TECH
Reagent kit for measuring content of C-reactive protein in whole blood and measuring method thereof
InactiveCN101907628ARelieve painEasy to operateColor/spectral properties measurementsBiological testingBlood collectionAbsorbance
The invention discloses a reagent kit for measuring the content of C-reactive protein in whole blood and a measuring method thereof. The reagent kit comprises a reagent 1 and a reagent 2, wherein the reagent 1 comprises a surfactant and a proper amount of buffer solution, and the content of the surfactant is 0.1-3g / L; and the reagent 2 comprises polystyrene latex adsorbed with a C-reactive protein antibody and a proper amount of buffer solution, and the content of the polystyrene latex adsorbed with the C-reactive protein antibody is 0.5-3g / L. The measuring method comprises the following steps of: collecting a whole blood sample of a subject, uniformly mixing the reagent 1 and the whole blood sample, then adding the reagent 2 for uniformly mixing, detecting the absorbance and the scattering light intensity of a reaction system at 625-800nm, and computing the content of the C-reactive protein in the whole blood by contrasting a standard curve. In the reagent kit and the measuring method, the whole blood of the subject can be used as a measured sample, the operation is simple, and results are accurate. The invention relieves the pain of the subjects, particularly for the child and newborn subjects and the subjects with more difficult vein blood collection, such as patients with large-area burns, and the like.
Owner:武汉中太生物技术有限公司
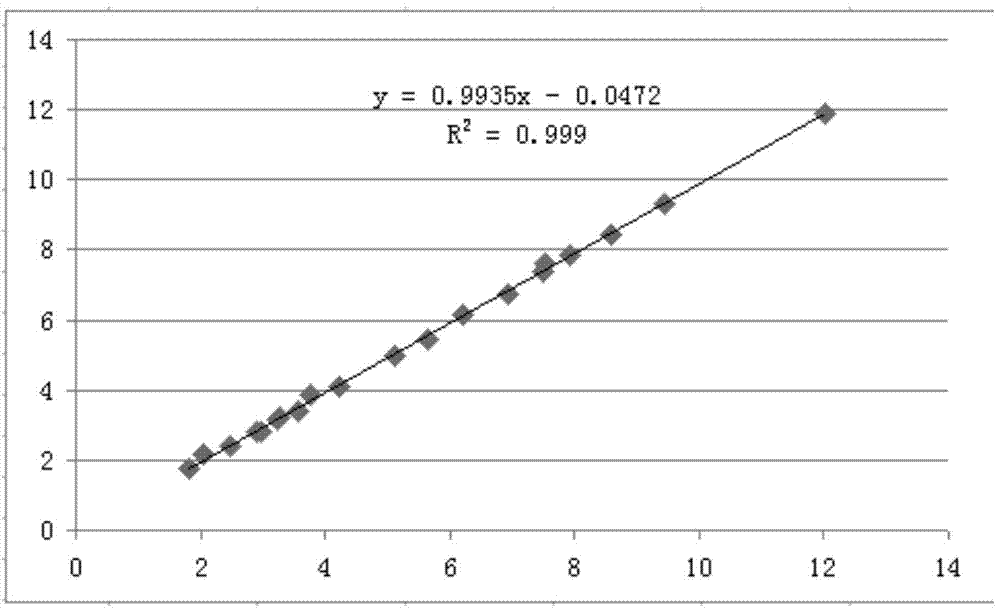
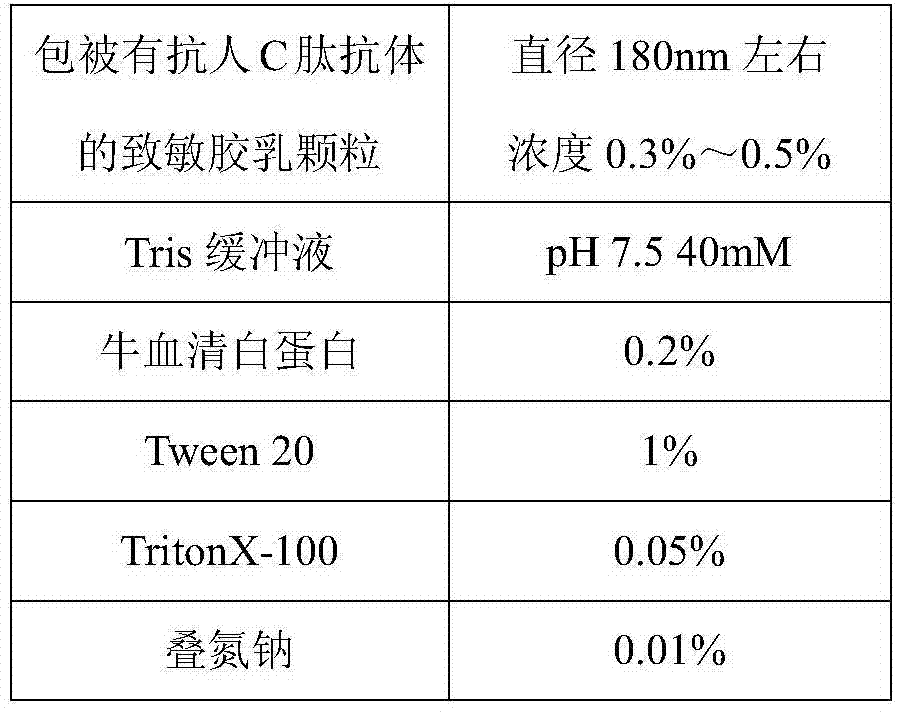
Method for detecting content of C peptide in human serum, kit used by method and preparation method thereof
InactiveCN104749375AConsistent specificitySame sensitivityDisease diagnosisBiological testingC-peptideSurface-active agents
The invention relates to a latex enhanced immunoturbidimetry kit for measuring the content of C peptide in human serum, a preparation method of the kit and a method for detecting the content of C peptide in human serum by use of the kit. The kit comprises a first reagent, a second reagent and a calibrator, wherein the first reagent contains buffer solution, electrolyte, a surface active agent, a stabilizing agent, a reaction enhancer and a preservative; the second reagent contains buffer solution, electrolyte, a surface active agent, a preservative and anti-human C peptide antibody enveloped sensitization polystyrene latex particles; and the calibrator contains buffer solution, a surface active agent, a stabilizing agent, a preservative and human C peptide protein. Compared with the existing kit used clinically, the kit provided by the invention has strong specificity, high sensitivity and high accuracy, the kit is suitable for biochemical analyzers of common detection mechanism, has short detection time, can be operated conveniently, has low detection cost, has no radioactive contamination, and is suitable for popularization and application clinically.
Owner:SHANGHAI CHUANZHI BIOTECH CO LTD
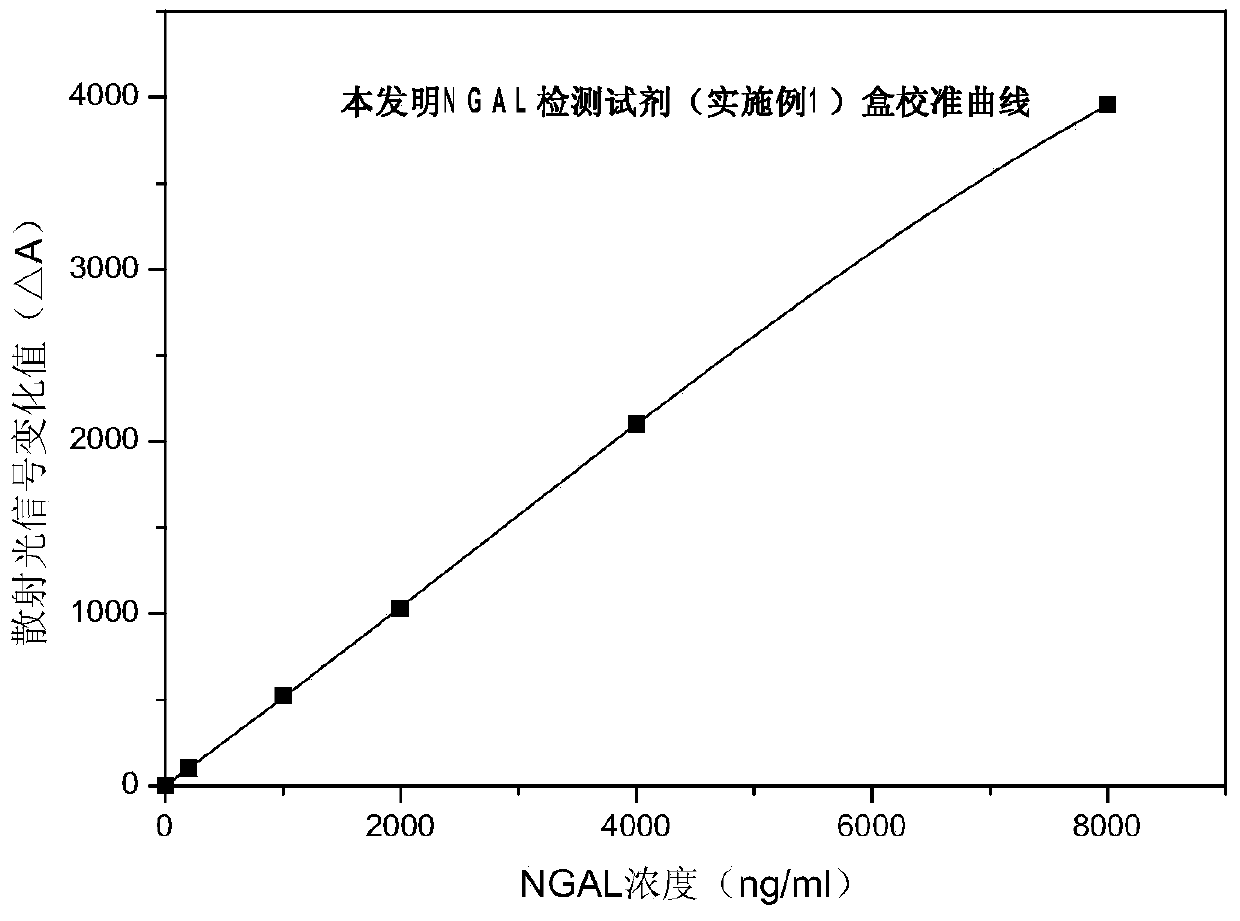
Rapid NGAL (Neutrophil Gelatinase Associated Lipocalin) detection kit based on amino acid spacer arm
InactiveCN104198723AReduce binding steric hindranceHigh sensitivityBiological testingGelatinasesMicrosphere
The invention discloses a rapid NGAL (Neutrophil Gelatinase Associated Lipocalin) detection kit based on an amino acid spacer arm and used for quantitatively and rapidly determining NGAL in human serum (plasma) or urine. The kit comprises a reagent 1 (R1) and a reagent 2 (R2), wherein the R1 comprises a biological buffer solution, a surfactant, a stabilizer, a coagulation accelerator and a preservative; the R2 comprises latex particles for enveloping an NGAL antibody, a biological buffer solution, a protective agent, a surfactant and a preservative; in R2, an antibody against human NGAL is connected with a polystyrene latex microsphere through the amino acid spacer arm. The invention also discloses a preparation method of the latex particles for enveloping the antibody against the human NGAL. The kit has high sensitivity and wide linear range; when being used in cooperation with a POCT (Point Of Care Testing) scattering nephelometry analyzer, the kit can achieve the aim of accurately and rapidly determining the NGAL in human serum (plasma) or urine.
Owner:NANJING PERLONG MEDICAL EQUIP
Improved cystatin C detection kit
The invention provides a latex enhanced turbidimetric immunoassay kit for quantitatively detecting cystatin C. The kit comprises a reagent R1 and a reagent R2 which are independent from each other, wherein the reagent R1 is mainly prepared from a buffer solution 1, a stabilizing agent 1, a preservative 1, EDTA, accelerator and a protective agent 1; the reagent R2 is mainly prepared from polystyrene latex microspheres for crosslinking a cystatin C antibody, a buffer solution 2, a stabilizing 2, a preservative 2 and a protective agent 2, wherein the polystyrene latex microspheres and the cystatin C antibody are connected in a covalent cross-linking mode. The detection kit has the advantages of low preparation cost, good stability, good data repeatability and high detection sensitivity, is easy to store, and can be widely applied to clinical biochemical analyzers.
Owner:ZYBIO INC
Retinol binding protein assay kit based on latex particle coating
The invention relates to the field of medical immonological in vitro diagnosis and in particular relates to a retinol binding protein assay kit based on latex particle coating. The assay kit comprises a reagent R1, a reagent R2 and a calibrator, wherein the reagent R1 is a Tris-HCl buffer system, and comprises a Tris-HCl buffer solution, a polymer and ethylene diamine tetraacetic acid; the reagent R2 comprises goat anti human retinol binding protein polyclonal antibody coated polystyrene latex particle sensitized granules and a phosphate buffer solution; and the reference calibrator comprises bovine serum matrix, as well as 0.2-2.2% of antiseptic and 1-10% of stabilizer according to the volume percentage of the bovine serum matrix. Compared with the prior art, the assay kit can be used for assistant diagnosis of kidney diseases and assistant diagnosis of mal-nutrition, and has high clinical application value.
Owner:AILEX TECH GRP CO LTD +1
Cysteine protease inhibitor C test kit
The invention provides a cysteine protease inhibitor C test kit, wherein the kit comprises a reagent R1 and a reagent R2, the reagent R2 is a reaction solution for promoting specific binding of an antigen and an antibody, the reagent R2 is a polystyrene latex particle mixture coated by an anti-human cystatin C antibody and is put in a proper buffer solution. The polystyrene latex particle mixture in the reagent R2 is a mixture of large-diameter polystyrene latex particles and small-diameter polystyrene latex particles. The kit is high in detection sensitivity, high in accuracy, good in linearity within the test range, good in stability and low in production cost.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Compounded latex particle-enveloped cystatin C detection kit
InactiveCN102636653AHigh sensitivityStrong specificityBiological testingPhosphatePolyethylene glycol
The invention relates to the field of medical immunity in-vitro diagnosis, and in particular relates to a determination kit for detecting cystatin C in blood serum. The kit comprises a reagent R1, a reagent R2 and a calibrator, wherein the reagent R1 is a phosphate buffer system which comprises 30-60mmol / l phosphate buffer solution with PH value of 7.2-7.6, 60-95mmol / l polyethylene glycol 6000-8000 and 6-13mmol / l ethylene diamine tetraacetic acid. The reagent R2 comprises two kinds of polystyrene latex particle sensitization particles with different sizes and enveloped by goat anti-human cystatin C polyclonal antibody; and the reagent R2 also comprises latex diluent and confining liquid. The calibrator is 0-10mg / l bovine serum matrix and also comprises 0.2-2.2% preservative and 1-10% stabilizer. Compared with the prior art, the kit provided by the invention has the characteristics of high sensitivity, good specificity, high speed and accurate and reliable determination result.
Owner:AILEX TECH GRP CO LTD
Method for detecting cardiac troponin I and application thereof
The invention provides a method for detecting cardiac troponin I (cTnI) and a cardiac troponin I detection kit prepared by applying the method. The method comprises the steps of coupling an antibody composition with polystyrene latex particles, then subjecting the coupled material to immunoreaction with corresponding antigen in a sample to be detected to form aggregated particles, and determining turbidity generated by the reactant under a wavelength of 400-700nm to obtain the content of cTnI in the sample to be detected. The antibody composition consists of at least three antibodies, and the antibodies are specific to cTnI without cross reaction with fast skeletal muscle troponin I and slow skeletal muscle troponin I. In addition, if one antibody in the antibody composition is sensitive to a certain factor influencing the accurate determination of cTnI in the sample to be detected, and then at least another antibody is not sensitive to the factor influencing the accurate determination of cTnI. The method of the invention is simple to operate, and has a wide scope of application, high precision, strong capability of resisting disturbance and low production cost.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Creatine kinase isoenzyme detection kit
ActiveCN108548926AMicrobiological testing/measurementBiological material analysisCreatine kinaseCreatine kinase isoenzyme
The invention relates to a creatine kinase isoenzyme detection kit. The kit includes a first reagent and a second reagent. The first reagent comprises a buffer solution, a preservative, a coagulant, asurfactant, a protective agent and a blocker; and the second reagent comprises the buffer solution, a stabilizer, the preservative, the protective agent, polystyrene latex particles and a creatine kinase isozyme antibody. The kit has the advantages of good stability and strong specificity, can be applied to biochemical analyzers in order to carry out large-batch detection, and can replace chemiluminescence products to reduce the detection cost in hospitals.
Owner:BEIJING STRONG BIOTECH INC
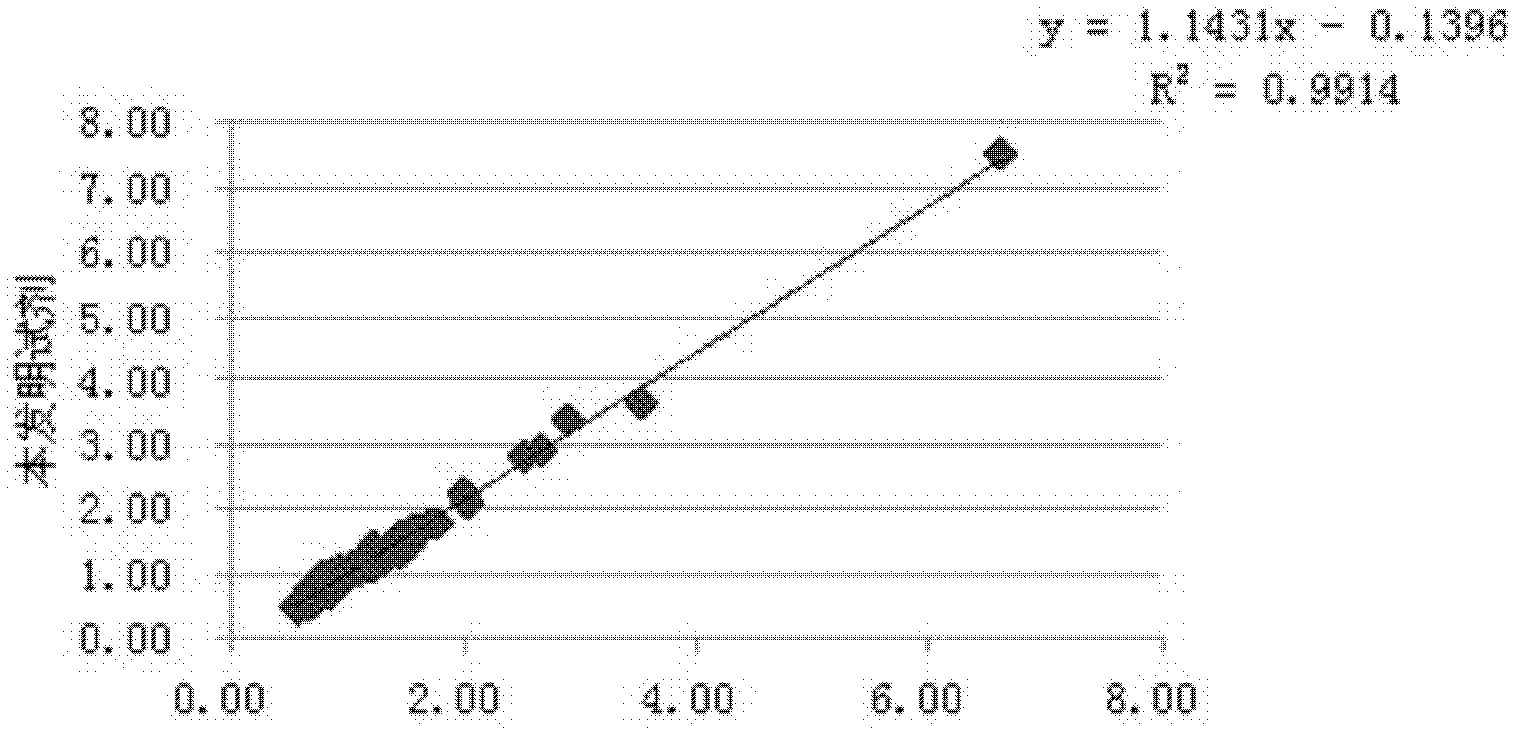
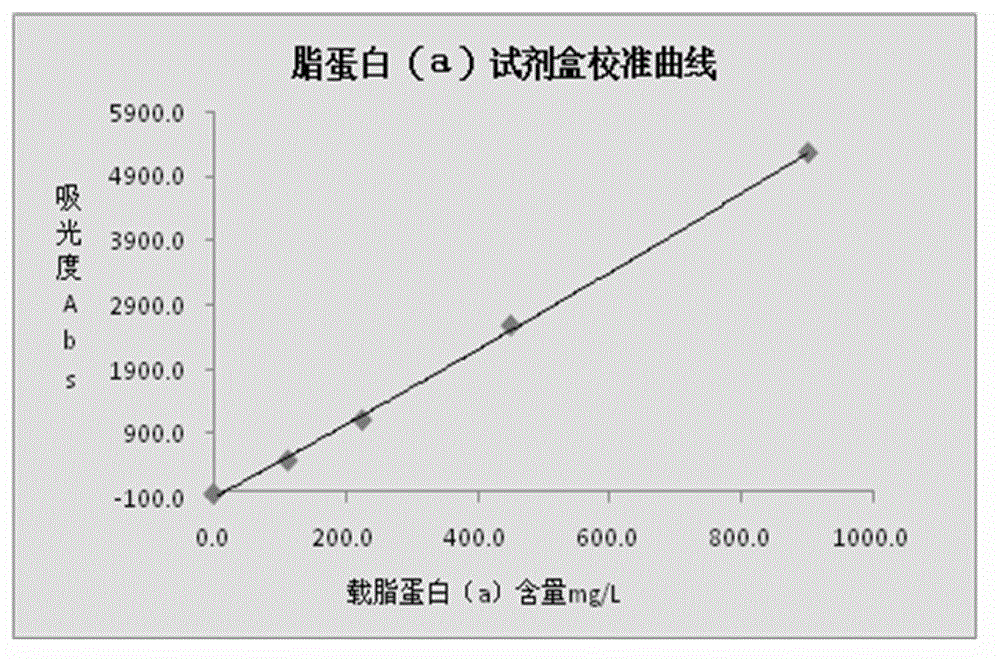
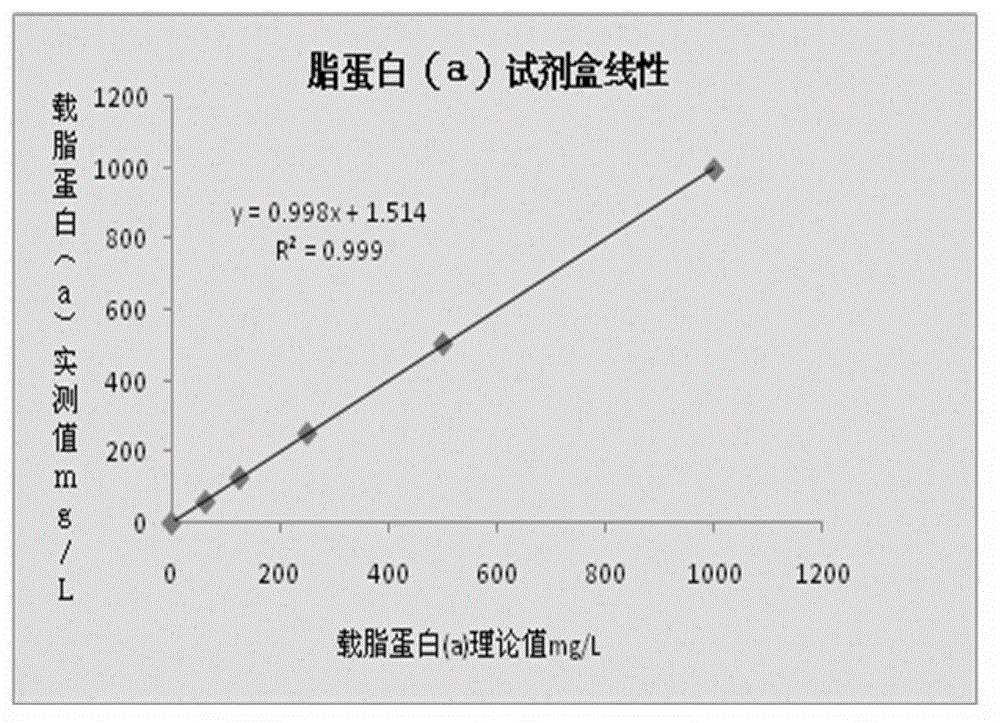
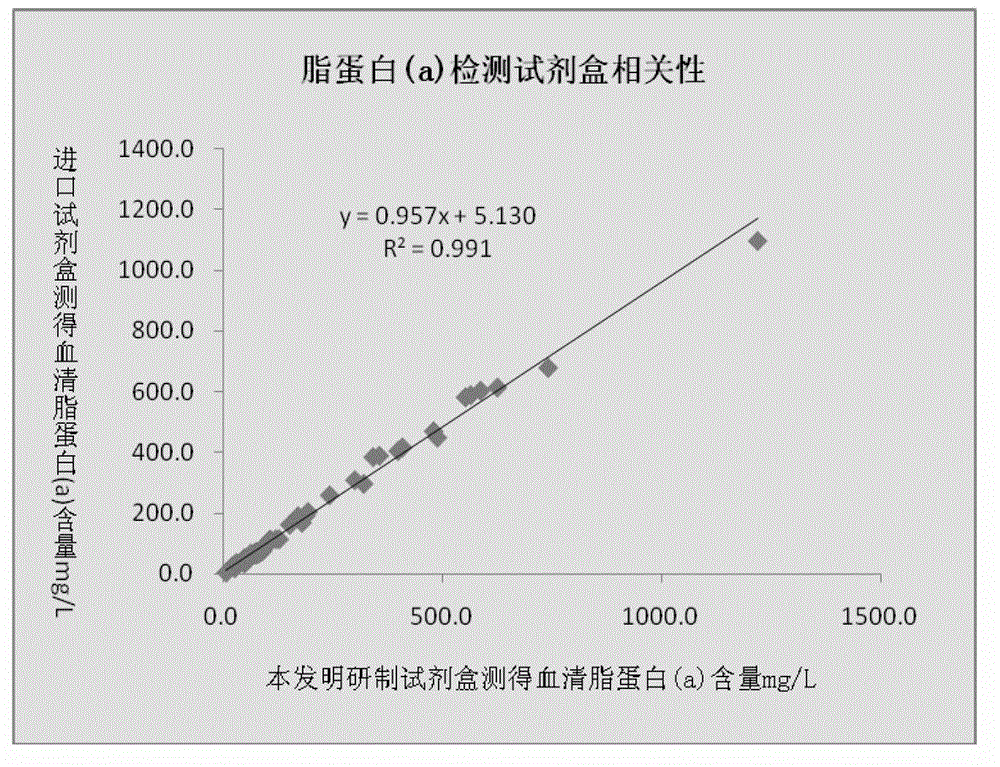
Lipoprotein (a) detection kit
ActiveCN103149370AImprove stabilityImprove bindingBiological testingHuman apolipoproteinA lipoprotein
The invention relates to a lipoprotein (a) detection kit which comprises a reagent R1, a reagent R2 and a reference substance, wherein the reagent R1 is an HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffer system which comprises 0.5-10 g / L HEPES, 2-20 g / L sodium chloride, 0.05-1.0 ml / L Tween-20, 0.1-2 g / L bovine serum albumin, 5-25 g / L polyethyleneglycol-6000, 1-5 g / L EDTA (ethylene diamine tetraacetic acid) and 0.1-2 g / L Proclin 300; the reagent R2 comprises a polystyrene latex particle mixture which is prepared by a chemical crosslinking method, coated with an anti-human-apolipoprotein (a) polyclonal antibody and provided with carboxylic groups on the surface, a 0.5-10 g / L HEPES buffer solution and aspartame, wherein the weight-to-volume ratio of the aspartame to the reagent R2 is (0.01-0.5):100 (g / L); and the reference substance is a human serum or serum-matrix-like liquid with human recombinant apolipoprotein (a). The kit provided by the invention has the advantages of high detection sensitivity, high accuracy, favorable precision, favorable linearity within detection range, high stability, low production cost and low blank absorbance, and has anti-interference performance.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Cystatin C detection kit and preparation method thereof
InactiveCN108107201AIncrease absorbanceHigh blank absorbanceBiological material analysisColor/spectral properties measurementsMicrosphereCentrifugation
The invention provides a cystatin C detection kit and a preparation method thereof. The kit comprises a reagent R1, a reagent R2 and a cystatin C calibration product, wherein the reagent R1 comprisesa preservative, a surfactant, an emulsifier and a buffer solution; the reagent R2 comprises a cystatin C antibody, polystyrene latex microspheres, a latex microsphere activating agent, a sealing agent, an emulsifier, a preservative, a surfactant and a buffer solution. The cystatin C antibody is slowly dropwise added to an activated polystyrene latex microsphere solution, a polystyrene latex microsphere suspension coupled with the cystatin C antibody is obtained after a stirring reaction, sealing is performed, the antibody and latex microspheres are coupled together in a chemical crosslinking manner, and centrifugation, cleaning and ultrasonic resuspension processes in conventional methods are omitted. The production process is simplified, and the kit has the advantages of low blank absorbency, high sensitivity and precision and large linear range.
Owner:QINGDAO HIGHTOP BIOTECH
Latex enhanced immuno-nephelometry kit for determining procalcitonin and preparation method and application of latex enhanced immuno-nephelometry kit for determining procalcitonin
ActiveCN104237525AMaterial analysis by observing effect on chemical indicatorBiological testingDiseaseMedicine
The invention discloses a latex enhanced immuno-nephelometry kit for determining procalcitonin and a preparation method and application of the latex enhanced immuno-nephelometry kit for determining procalcitonin, and belongs to the technical field of disease diagnosis and detection. The latex enhanced immuno-nephelometry kit for determining the procalcitonin comprises a diluent, a latex preparation, a blank liquid, a calibrator and a quality control material, wherein the latex preparation contains carboxylated polystyrene latex with different particle sizes and coupled with a PCT monoclonal antibody and a PCT polyclonal antibody respectively. According to the method, the PCT monoclonal antibody and the PCT polyclonal antibody are marked respectively, the latex with the large particle size can improve the detection sensitivity, and the latex with the small particle size can expand the linearity range, and therefore, the composite latex preparation can improve the detection sensitivity, can expand the detection linearity range, and has the advantages of high detection speed, high sensitivity, strong specificity and good accuracy for detection on the procalcitonin.
Owner:BEIJING MOKOBIO LIFE SCI CO LTD
Immunoturbidimetric kit for detecting procalcitonin
InactiveCN107340395AReduce dosageHigh detection sensitivityScattering properties measurementsBiological testingHemolytic AgentsMicrosphere
The present invention relates to the field of medical immunology, particularly to an immunoturbidimetric kit for detecting procalcitonin. The kit comprises a reagent R1 and a reagent R2, wherein the reagent R1 comprises a buffer liquid, a stabilizer, a protection agent, a coagulant, a hemolytic agent and a preservative, the reagent R2 comprises a buffer liquid, a stabilizer, a protection agent, a preservative and a polystyrene latex microsphere-procalcitonin antibody complex, and the polystyrene latex microspheres comprise two types of microspheres such as microspheres having a particle size of 80-150 nm and microspheres having a particle size of 150-400 nm. According to the present invention, the test results show that the kit has advantages of good stability and long storage period, can directly detect the PCT in whole blood, and further has advantages of high detection result accuracy, high sensitivity and wide linear detection range.
Owner:SONOSCAPE MEDICAL CORP
D-dimmer latex-reinforced immunonephelometry detection kit cooperatively adopting ion stabilizer and suspension stabilizer
The invention relates to an in vitro diagnostic kit and in particular relates to a D-dimmer latex-reinforced immunonephelometry detection kit cooperatively adopting an ion stabilizer and a suspension stabilizer. The D-dimmer latex-reinforced immunonephelometry detection kit cooperatively adopting the ion stabilizer and the suspension stabilizer comprises two components, namely a reagent R1 and a reagent R2, wherein the reagent R1 mainly consists of a buffer solution 1, a stabilizer 1, a preservative 1, a coagulation accelerator and a protective agent 1; the reagent R2 mainly consists of a buffer solution 2, two polystyrene latex microspheres crosslinked with different D-dimmer monoclonal antibodies, a stabilizer 2, a protective agent 2 and a preservative 2, and the stabilizer 2 in the reagent R2 adopts the ion stabilizer and the suspension stabilizer which are used cooperatively. Compared with the prior art, the D-dimmer latex-reinforced immunonephelometry detection kit cooperatively adopting the ion stabilizer and the suspension stabilizer has the advantages that a latex-reinforced immunonephelometry method is adopted, and detection can be carried out under the condition that wavelength is 400-800nm, so that the detection is easier and the D-dimmer latex-reinforced immunonephelometry detection kit cooperatively adopting the ion stabilizer and the suspension stabilizer can be applied in clinical more easily.
Owner:ZYBIO INC
Cystatin C latex-particle-enhanced turbidimetry detection reagent kit and application thereof
ActiveCN105738617AEnhanced detection signalHigh detection sensitivityMaterial analysisLatex particleTurbidimetry
The invention provides a cystatin C latex-particle-enhanced turbidimetry detection reagent kit and application thereof.The reagent kit comprises a reagent R1 and a reagent R2.The reagent R1 is prepared from a buffer solution, inorganic salt, a surfactant, a preservative, a stabilizer and interference elimination protein.The reagent R2 is prepared from a buffer solution, inorganic salt, a surfactant, a preservative, a stabilizer and a polystyrene latex particle mixture, and the polystyrene latex particle mixture is interlinked with a cystatin C antibody.The reagent kit based on latex-particle-enhanced turbidimetric immunoassay (PETIA) can be generally applied to analysis of various full-automatic biochemical analyzers and is short in assay time, good in specificity, high in precision and good in accuracy when used.
Owner:WUHAN LIFE ORIGIN BIOTECH LTD
Cystatin C detection kit and preparation method therefor
ActiveCN103645323AHigh sensitivityGood precisionBiological material analysisBiological testingMedicinePolystyrene
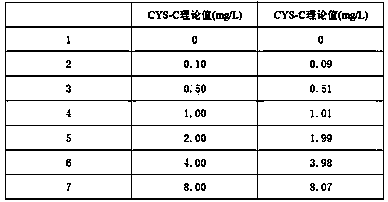
The invention relates to the field of medical immunology in vitro diagnosis, and especially provides a detection kit for detecting cystatin C in serum. The detection kit comprises a reagent R1, a reagent R2 and calibration materials. The ingredients of the reagent R1 are a Tris buffer with a concentration of 0.01-0.05mol / L, PEG 6000 with a concentration of 10-40g / L, NaN3 with a concentration of 0.5-1.5g / L, and sodium chloride with a concentration of12-20g / L, and the pH value is 8.0-8.5. The ingredients of the reagent R2 are a Tris buffer with a concentration of 0.01-0.02mol / L, and polystyrene latex granules coated by goat-anti-human cystatin C polyclonal antibodies with a concentration of 0.5-3.0 g / L. The calibration materials are six gradient standards with cystatin C and the contents of cystatin C are 0.1, 0.5, 1.0, 2.0, 4.0 and 8.0mg / L. The solution is a Tris buffer with a concentration of 0.01mol / L. The kit has simple composition, good stability and high accuracy.
Owner:浙江夸克生物科技有限公司
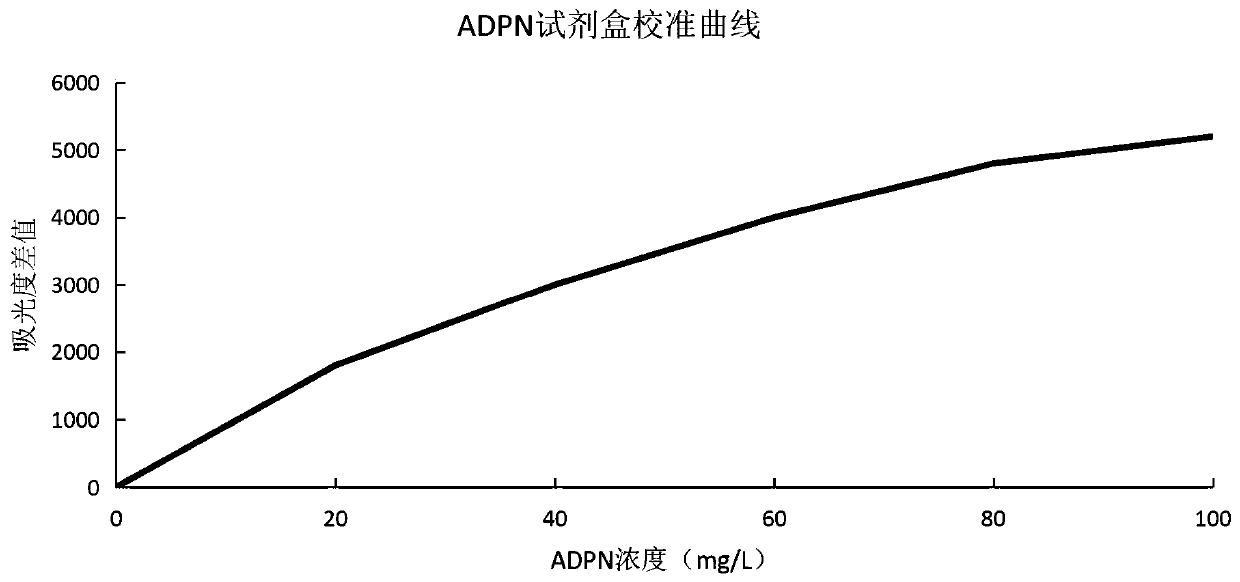
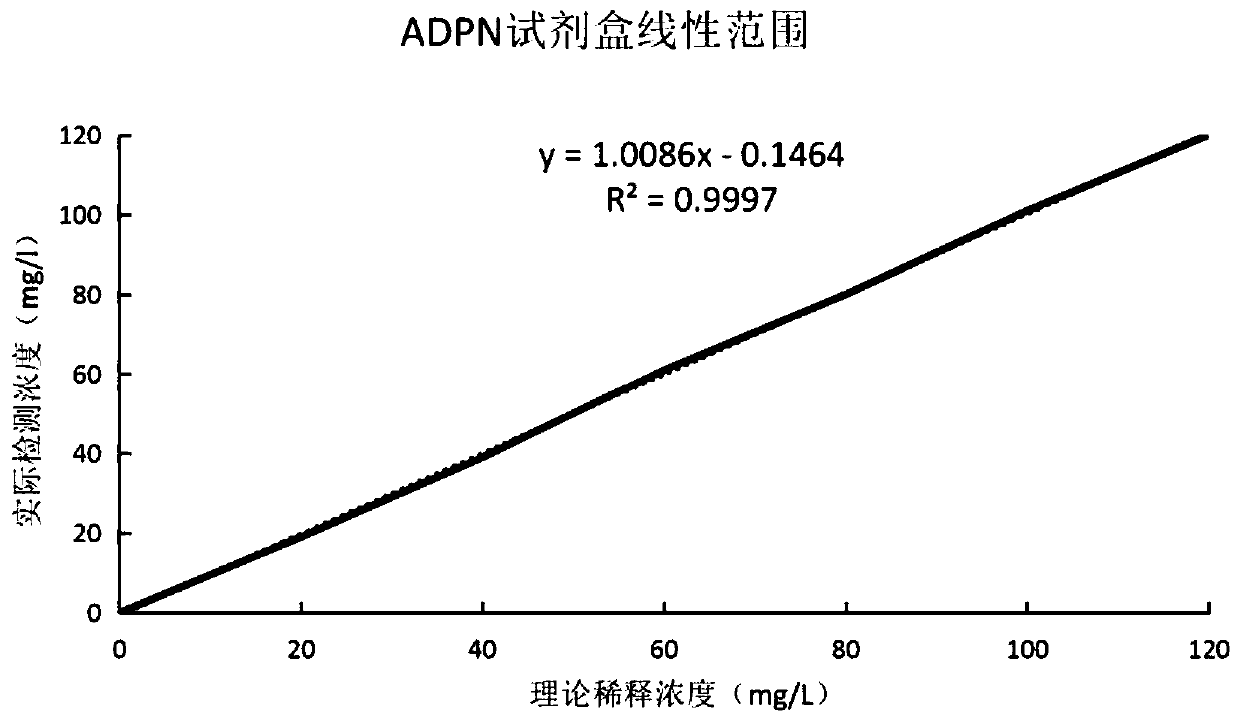
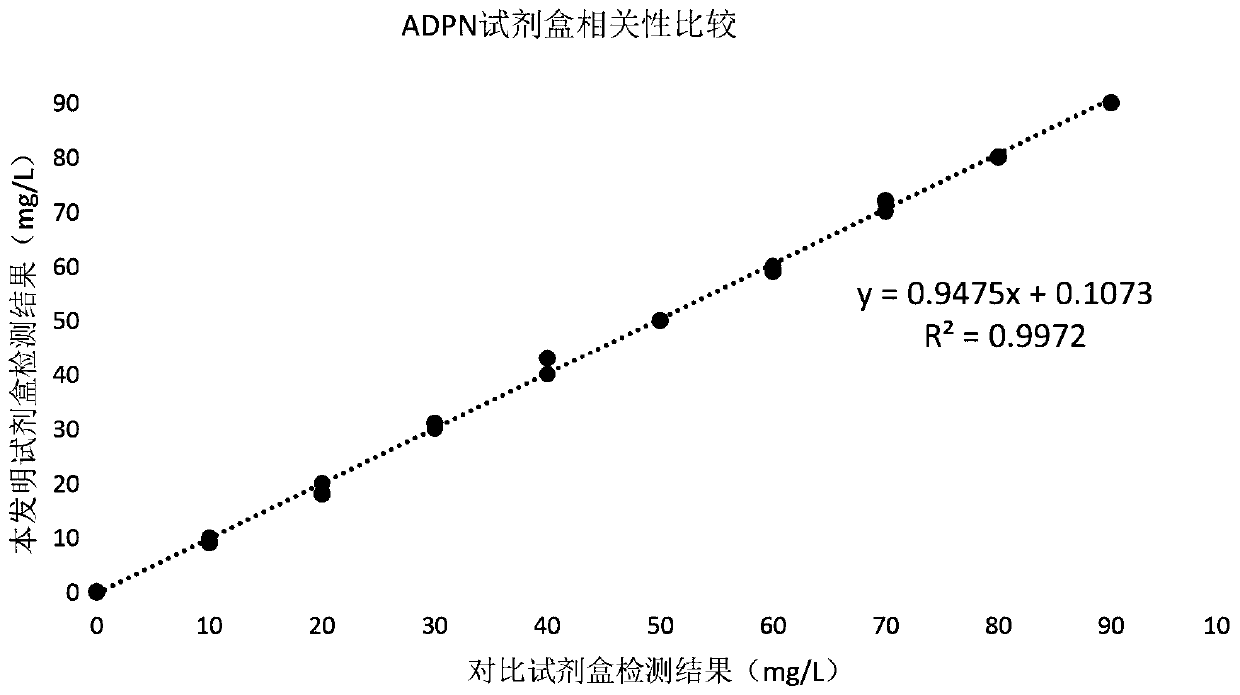
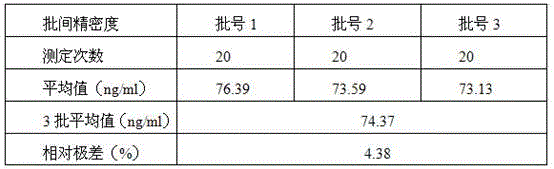
Latex-enhanced turbidimetric immunoassay kit for quantitatively detecting adiponectin ADPN and preparation and application methods thereof
PendingCN111239421AHigh sensitivityStrong specificityDisease diagnosisBiological testingAntiendomysial antibodiesLatex particle
The invention provides a latex-enhanced turbidimetric immunoassay kit for quantitatively detecting adiponectin ADPN. The latex-enhanced turbidimetric immunoassay kit comprises an adiponectin R1 reagent and an adiponectin R2 reagent, wherein the adiponectin R1 reagent comprises a buffer solution A, a protective agent A, a reaction enhancer and a preservative; the adiponectin R2 reagent comprises abuffer solution B, a protective agent B, a preservative and sensitized polystyrene latex particles coated with an anti-human adiponectin antibody; the anti-human adiponectin antibody in the sensitizedpolystyrene latex particle coated with the anti-human adiponectin antibody is connected with the sensitized polystyrene latex particle through a streptavidin-biotin system. The invention also provides a preparation method and an application method of the latex-enhanced turbidimetric immunoassay kit for quantitatively detecting adiponectin ADPN. The kit has the advantages that the kit is convenient, rapid, high in sensitivity and specificity and capable of quantitatively and accurately detecting ADPN; and the kit is strong in instrument compatibility and low in detection cost, and makes up theclinical requirements on ADPN turbidimetric products.
Owner:ANHUI DAQIAN BIO ENG LIMITED
Preparation method for polystyrene latex with carboxyl on surface and for immunoassay technology
ActiveCN101775094AHigh carboxyl contentImprove the measurement effectMaterial analysisPotassium persulfateTurbidity
The invention discloses a preparation method for polystyrene latex with carboxyl on the surface and for immunoassay technology; the preparation method comprises the following steps that: styrene, methyl methacrylate and water are added into a three-neck flask to be stirred for 10 to 30min; the molar ratio of the methyl methacrylate to the styrene is 0.1 to 0.2:1; after stirring, N2 is fed into the reaction system; and then potassium persulfate and the aqueous solution of sodium chloride are added in, so that the concentration of sodium chloride in the reaction system is 0.064mol / L, and the concentration of the potassium persulfate is 7.00*10-4 to 25.0*10-3mol / L; N2 is continued to be fed in; the temperature goes up to 60 to 80DEG C, and the reaction is carried out for 12 to 24h; and after the reaction, the temperature is cooled to room temperature, and the target product of the invention is prepared. The latex prepared by the invention has the advantages of uniform grain size, good monodispersity and strong stability; and simultaneously, the surface of the latex has higher carboxyl content, and the preparation method is applicable to a latex immune turbidity determination method.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Lipoprotein phospholipase A2 detection kit
InactiveCN105717292AHigh sensitivityImprove accuracyBiological material analysisSurface-active agentsBiological activation
The invention provides a lipoprotein phospholipase A2 detection kit which comprises reagent R1 and reagent R2, the reagent comprises a buffer solution, a stabilizing agent, electrolyte, a surface active agent and preservative; the reagent R2 comprises polystyrene latex particles coupled to an antibody resisting to the lipoprotein phospholipase A2, a buffer solution, a stabilizing agent, a surface active agent and preservative. A directional coupling method is adopted for the polystyrene latex particles sensitized by the antibody resisting to the lipoprotein phospholipase A2, and the preparation steps include activation of the polystyrene latex particles; oxidation of the antibody resisting to the lipoprotein phospholipase A2; coupling of the antibody resisting to the lipoprotein phospholipase A2 to the polystyrene latex particles. The kit is high in detection sensitivity, wide in detection range, good in accuracy and high in interference resistance.
Owner:HANGZHOU WEIXIN BIOTECH CO LTD
D dimer latex-enhanced immunoturbidimetric assay kit utilizing surface functional group
The invention relates to an in vitro diagnostic kit, and in particular relates to a D dimer latex-enhanced immunoturbidimetric assay kit utilizing a surface functional group. The D dimer latex-enhanced immunoturbidimetric assay kit utilizing the surface functional group consists of two components, namely a reagent R1 and a reagent R2, wherein the reagent R1 mainly consists of a buffer solution 1, a stabilizer 1, a preservative 1, a gelling agent and a protective agent 1; the reagent R2 mainly consists of a buffer solution 2, two polystyrene latex microspheres cross-linked to different D dimer monoclonal antibodies, a stabilizer 2, a protective agent 2 and a preservative 2; the polystyrene latex microspheres are connected to the D dimer antibodies in a manner of covalent cross-linking or physical adsorption; and the surface functional group of the polystyrene latex microsphere in the reagent R2 is an amino group, a carboxyl group, hydrazide, an aldehyde group or an epoxy group. Compared with the prior art, by virtue of the latex-enhanced immunoturbidimetric assay, the kit can be used for detecting by wavelength of 400-800nm, so that the kit is more convenient to detect and is easy in clinical application.
Owner:ZYBIO INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com