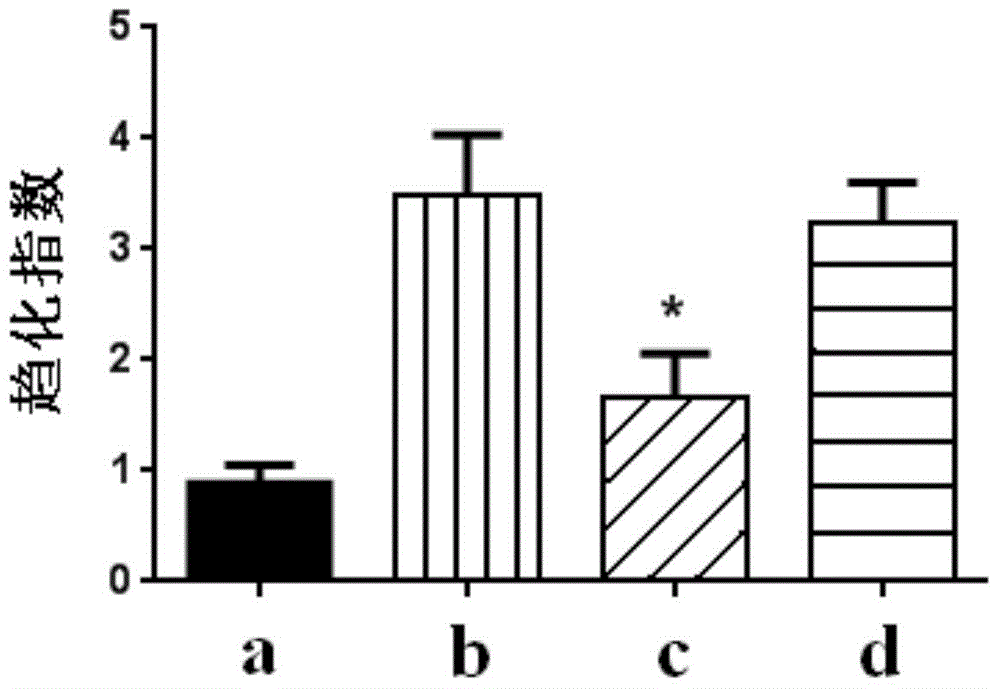
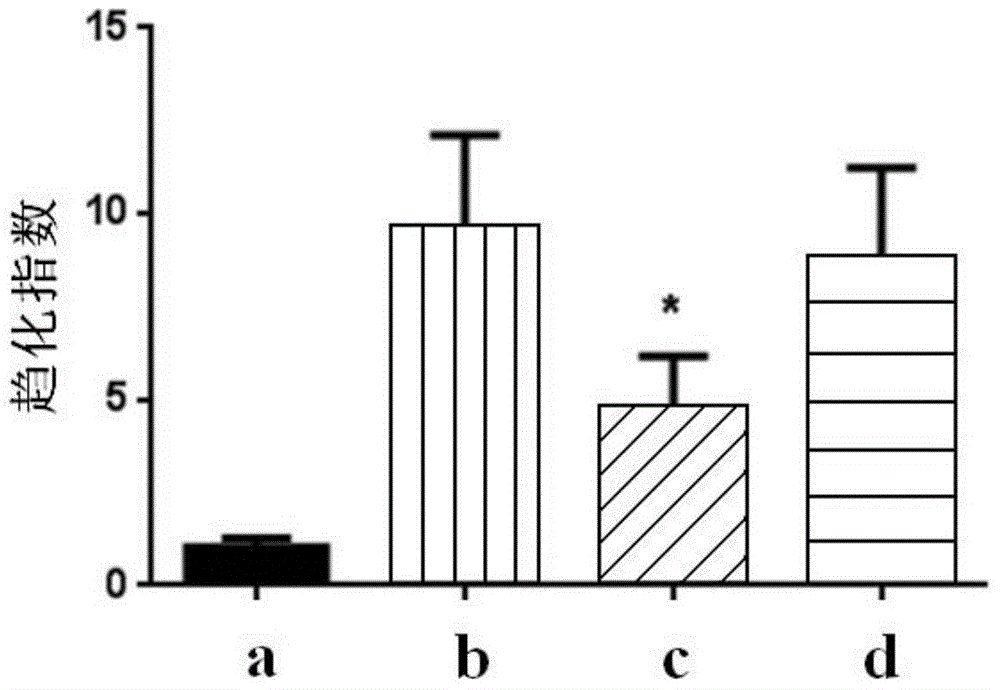
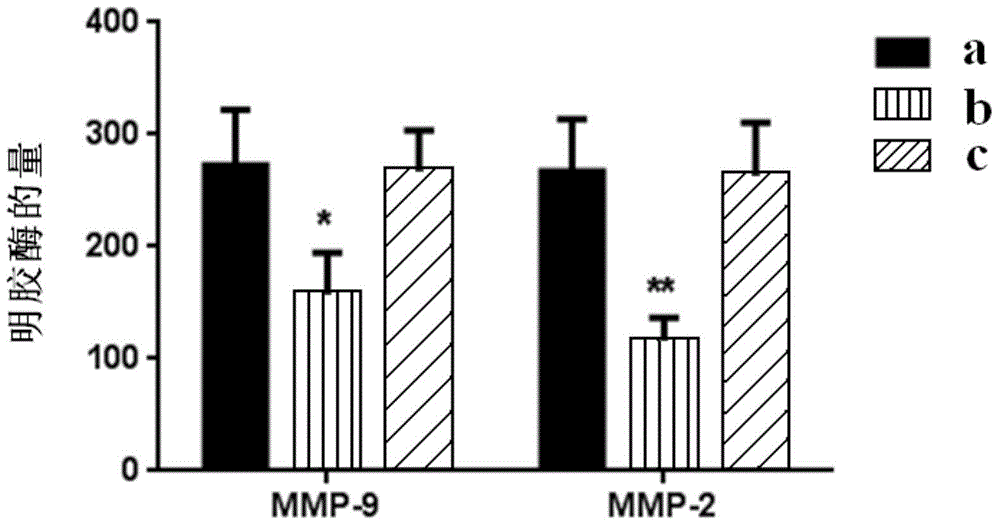
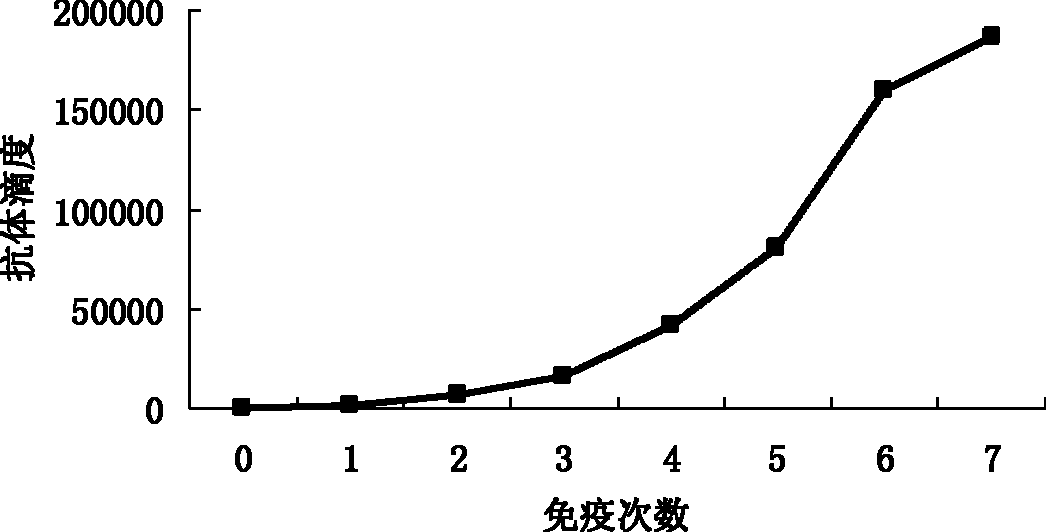
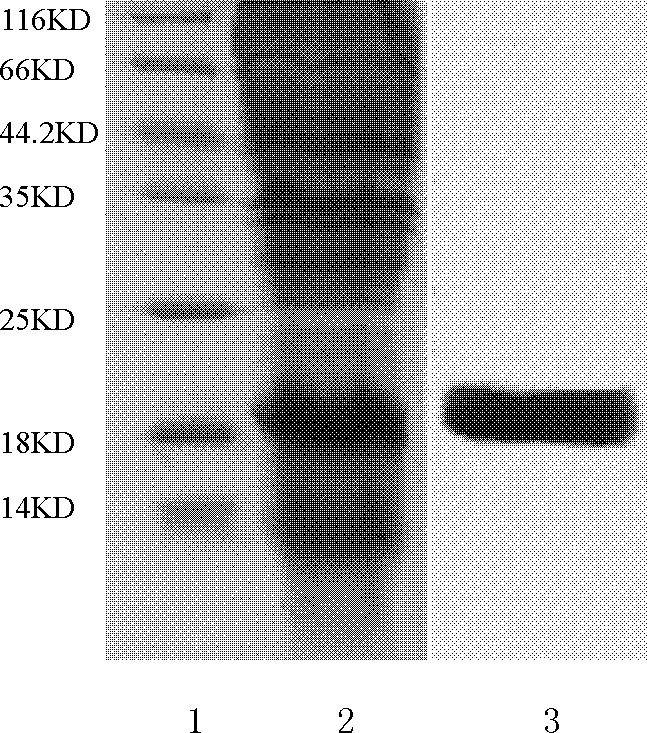Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "Gelatinases" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A class of enzymes that catalyzes the degradation of gelatin by acting on the peptide bonds. EC 3.4.24.-.
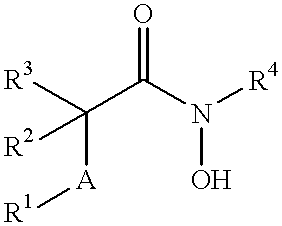
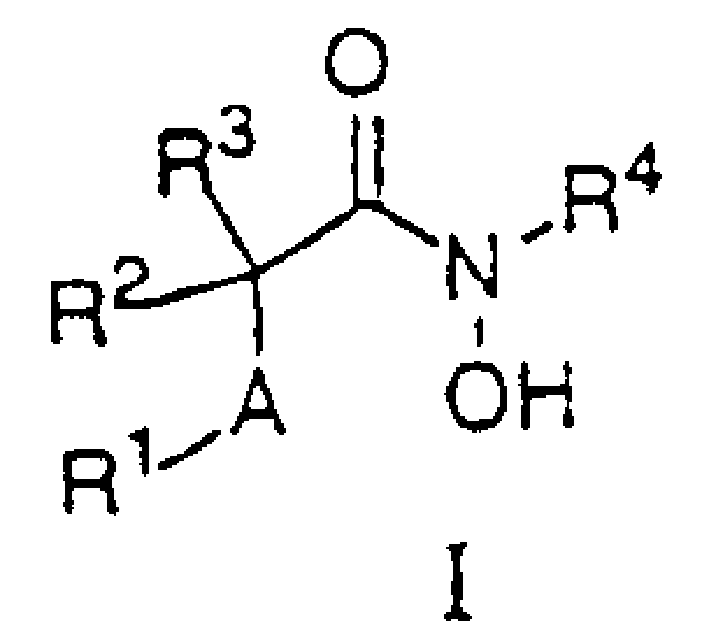
N-hdroxy-2-(alkyl, aryl, or heteroaryl, sulfanyl, sulfinyl or sulfonyl)-3-substituted alkyl, aryl or heteroarylamides as matrix metalloproteinase inhibitors
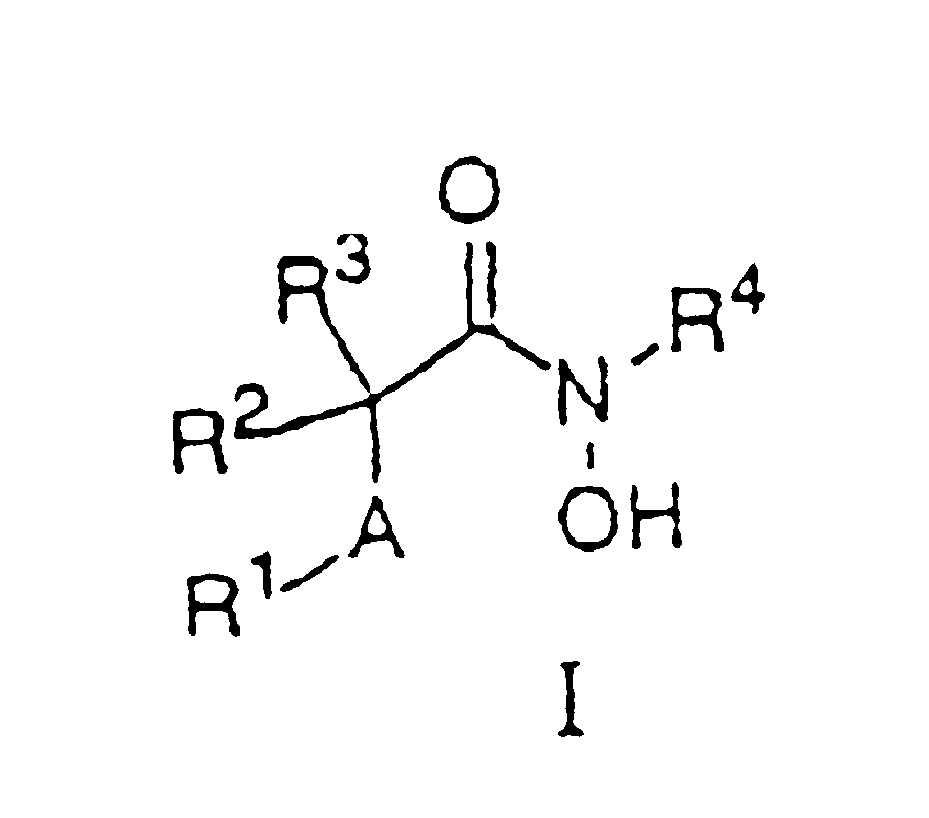
Matrix metalloproteinases (MMPs) are a group of enzymes that have been implicated in the pathological destruction of connective tissue and basement membranes. These zinc containing endopeptidases consist of several subsets of enzymes including collagenases, stromelysins and gelatinases. TNF-alpha converting enzyme (TACE), a pro-inflammatory cytokine, catalyzes the formation of TNF-alpha from membrane bound TNF-alpha precursor protein. It is expected that small molecule inhibitors of MMPs and TACE therefore have the potential for treating a variety of disease states. The present invention provides low molecular weight, non-peptide inhibitors of matrix metalloproteinases (MMPs) and TNF-alpha converting enzyme (TACE) for the treatment of arthritis, tumor metastasis, tissue ulceration, abnormal wound healing, periodontal disease, bone disease, diabetes (insulin resistance) and HIV infection having the formulawherein R2 and R3 form a heterocyclic ring and A is S, S(O), or S(O)2, and R1 and R4 are defined herein.
Owner:WYETH HOLDINGS CORP
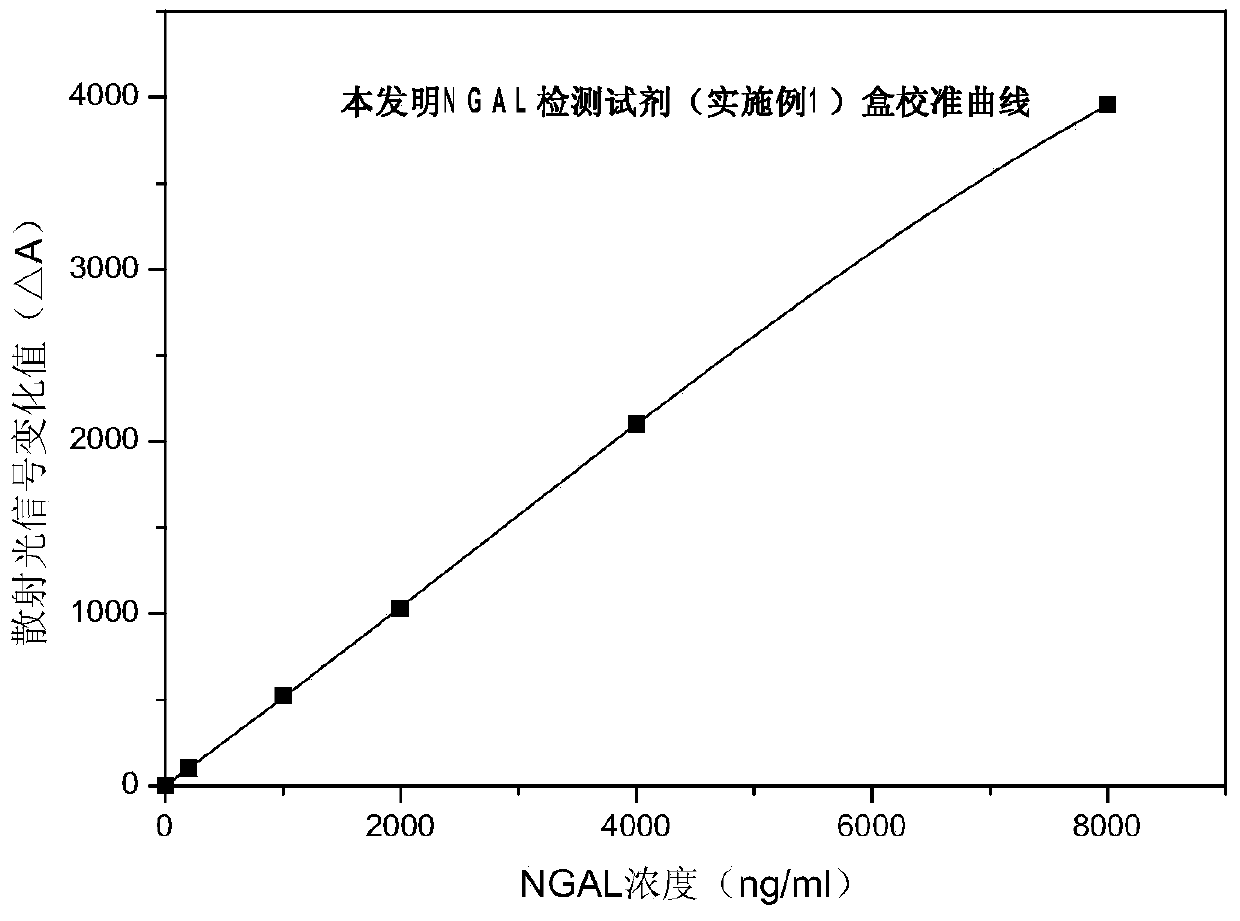
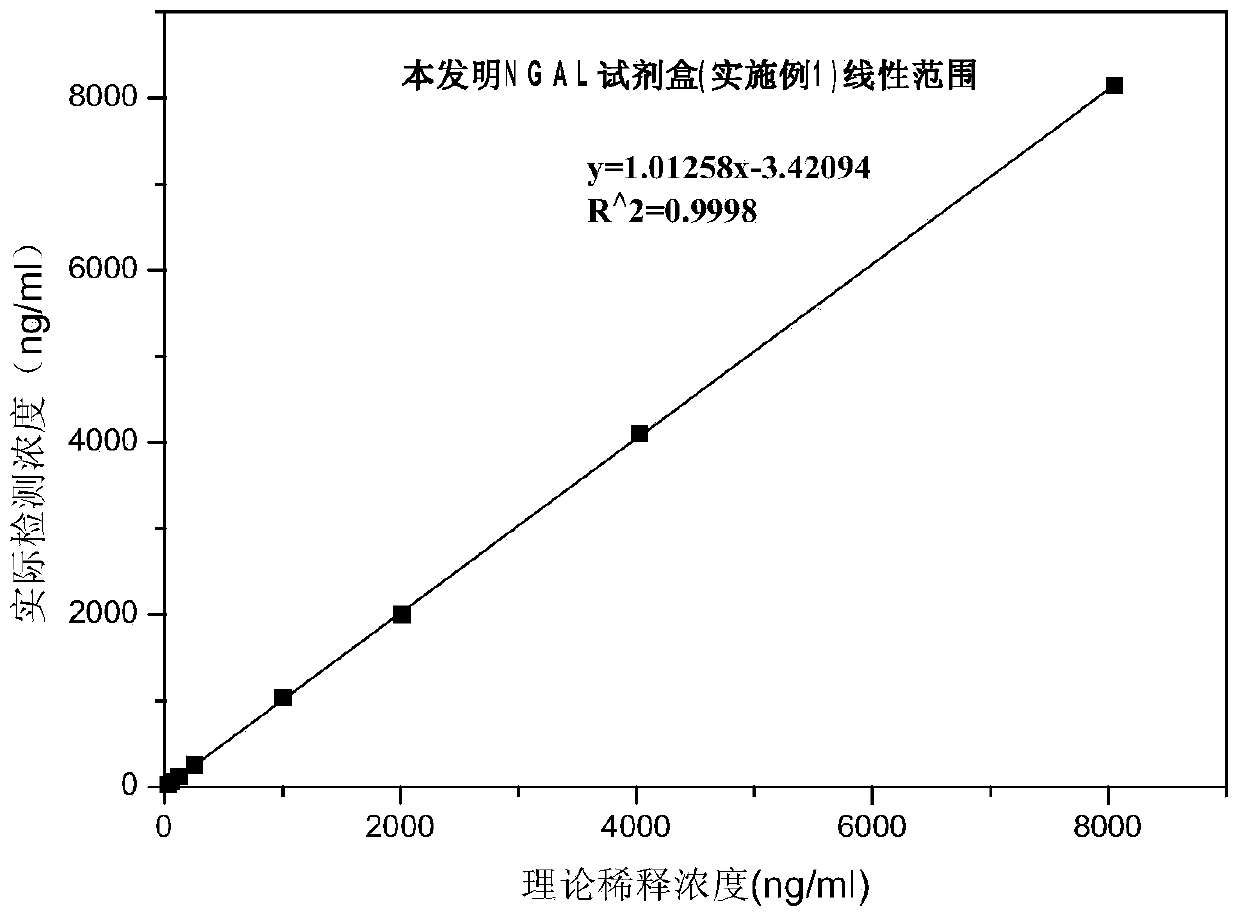
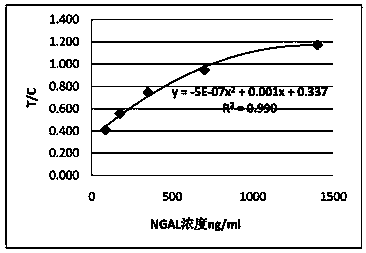
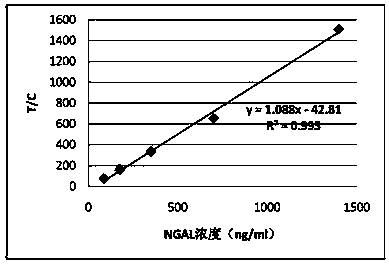
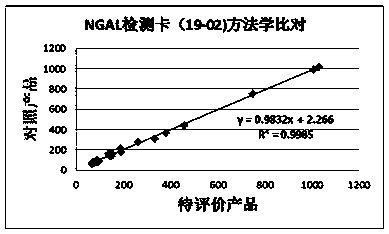
Rapid NGAL (Neutrophil Gelatinase Associated Lipocalin) detection kit based on amino acid spacer arm
InactiveCN104198723AReduce binding steric hindranceHigh sensitivityBiological testingGelatinasesMicrosphere
The invention discloses a rapid NGAL (Neutrophil Gelatinase Associated Lipocalin) detection kit based on an amino acid spacer arm and used for quantitatively and rapidly determining NGAL in human serum (plasma) or urine. The kit comprises a reagent 1 (R1) and a reagent 2 (R2), wherein the R1 comprises a biological buffer solution, a surfactant, a stabilizer, a coagulation accelerator and a preservative; the R2 comprises latex particles for enveloping an NGAL antibody, a biological buffer solution, a protective agent, a surfactant and a preservative; in R2, an antibody against human NGAL is connected with a polystyrene latex microsphere through the amino acid spacer arm. The invention also discloses a preparation method of the latex particles for enveloping the antibody against the human NGAL. The kit has high sensitivity and wide linear range; when being used in cooperation with a POCT (Point Of Care Testing) scattering nephelometry analyzer, the kit can achieve the aim of accurately and rapidly determining the NGAL in human serum (plasma) or urine.
Owner:NANJING PERLONG MEDICAL EQUIP
Latex-enhanced immunoturbidimetric assay kit for NGAL, and preparation method thereof
InactiveCN107942069AGood for three-dimensional structureImprove stabilityDisease diagnosisBiological testingGelatinasesFluorescence
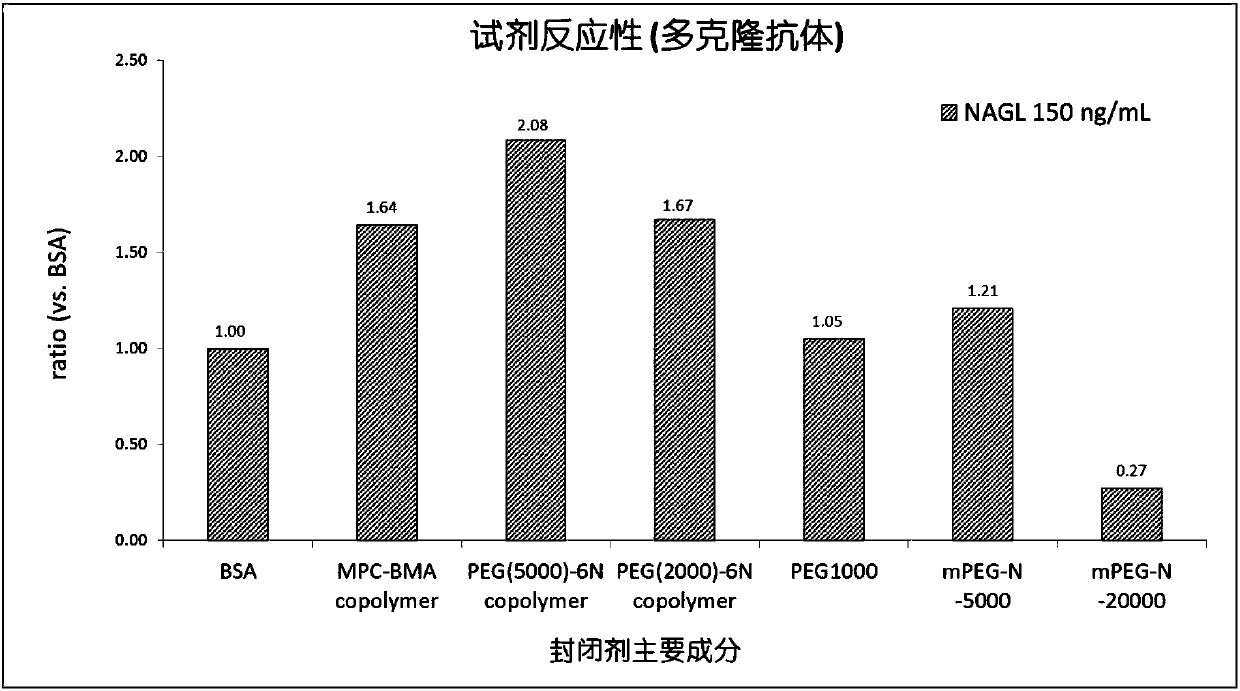
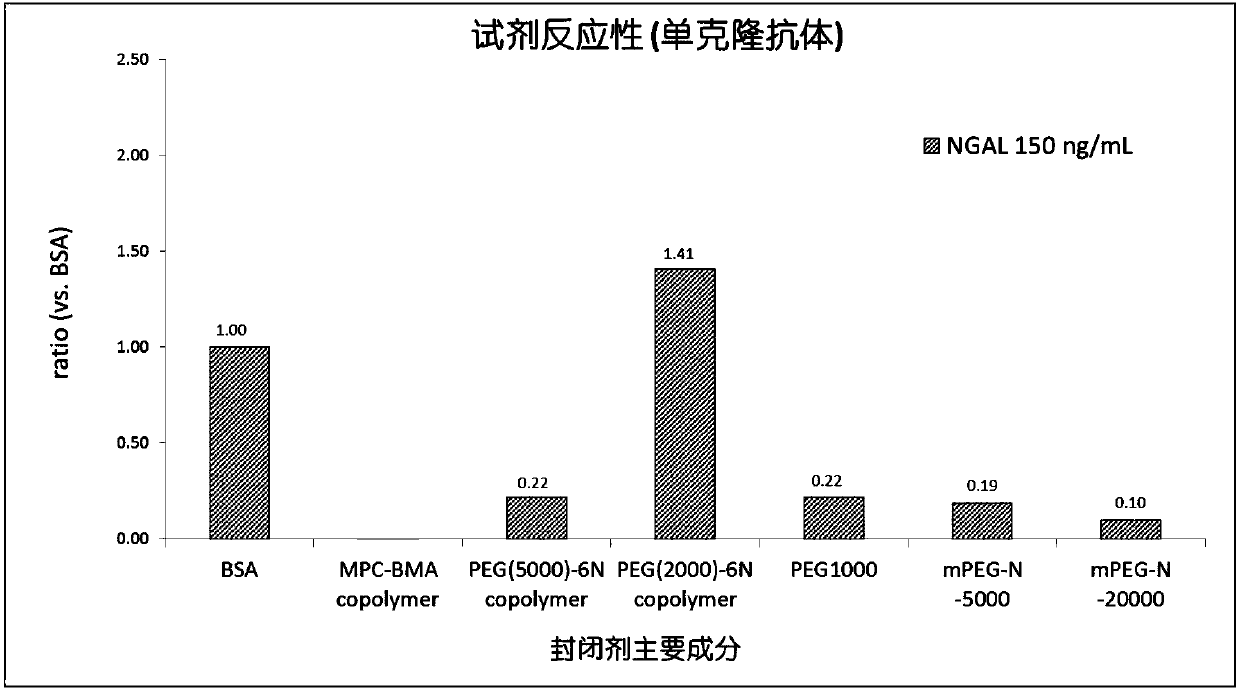
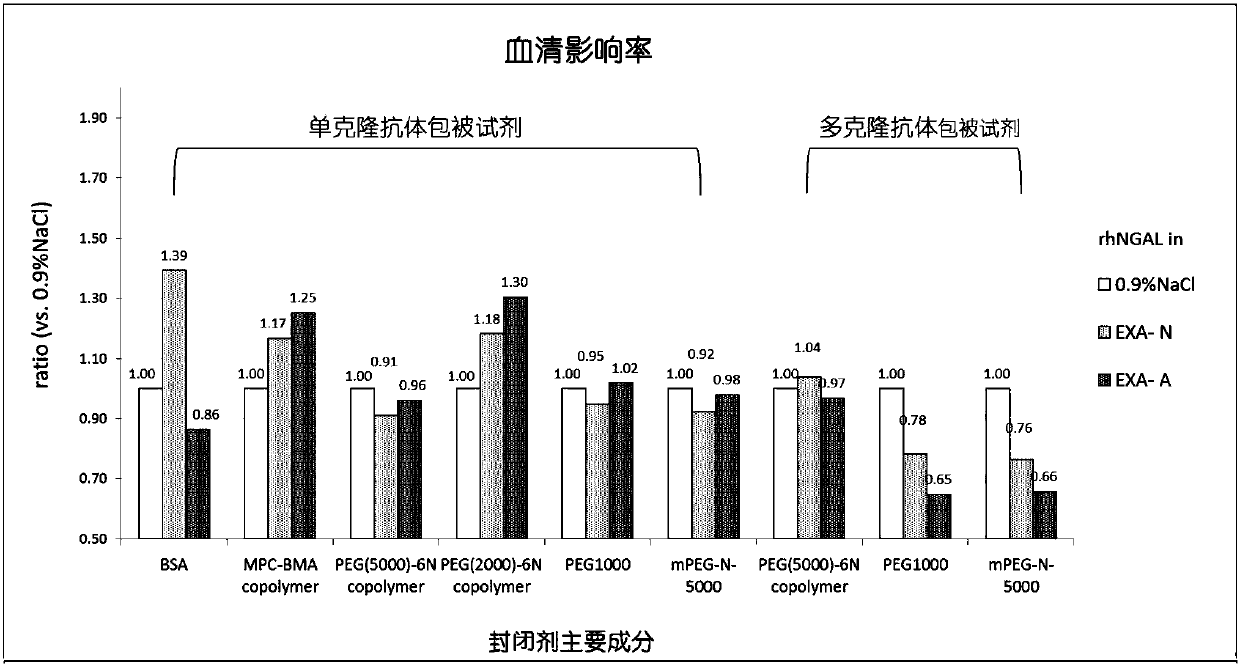
The invention discloses a latex-enhanced immunoturbidimetric assay kit for neutropil gelatinase-associated lipocalin, and a preparation method thereof. Polyethylene glycol hexamine is selected as theblocking agent of an antibody binding latex particle. The kit used for latex-enhanced immunoturbidimetric assay of the NGAL has the advantages of high detection specificity, high sensitivity and goodstability, and can efficiently detect the NGAL content in urine, plasma and serum, and the detection result is well associated with fluorescence immunochromatography and enzyme linked immunosorbent assay.
Owner:捷和泰(北京)生物科技有限公司
Method for diagnosing, evaluating or testing cancer and foreseeing cancer severity
The invention relates to a method for diagnosing, evaluating or testing a cancer and foreseeing the severity of the cancer. The testing method comprises the following steps: an antibody of anti-human tropic neutrophil gelatinase-associated lipocalin (NGAL) is reacted with the human NGAL of a specimen to be tested to form a compound which causes turbidity change; light protection intensity or light scattering intensity is used for presenting the change; the human NGAL content of the specimen to be tested is tested through a standard curve of the concentration of human NGAL standard to relevant light protection intensity or light scattering intensity; and finally the cancer severity is measured based on the tested NGAL content. The method has the advantages of high flexibility, high recovery and good repetitiveness, determines a testing method with great convenience, good repetitiveness and strong suitability for the NGAL in urine or blood plasma of a cancer patient, and provides possibility for the clinical directive function of human NGAL and further research of the functions of the human NGAL.
Owner:CUSABIO TECH LLC
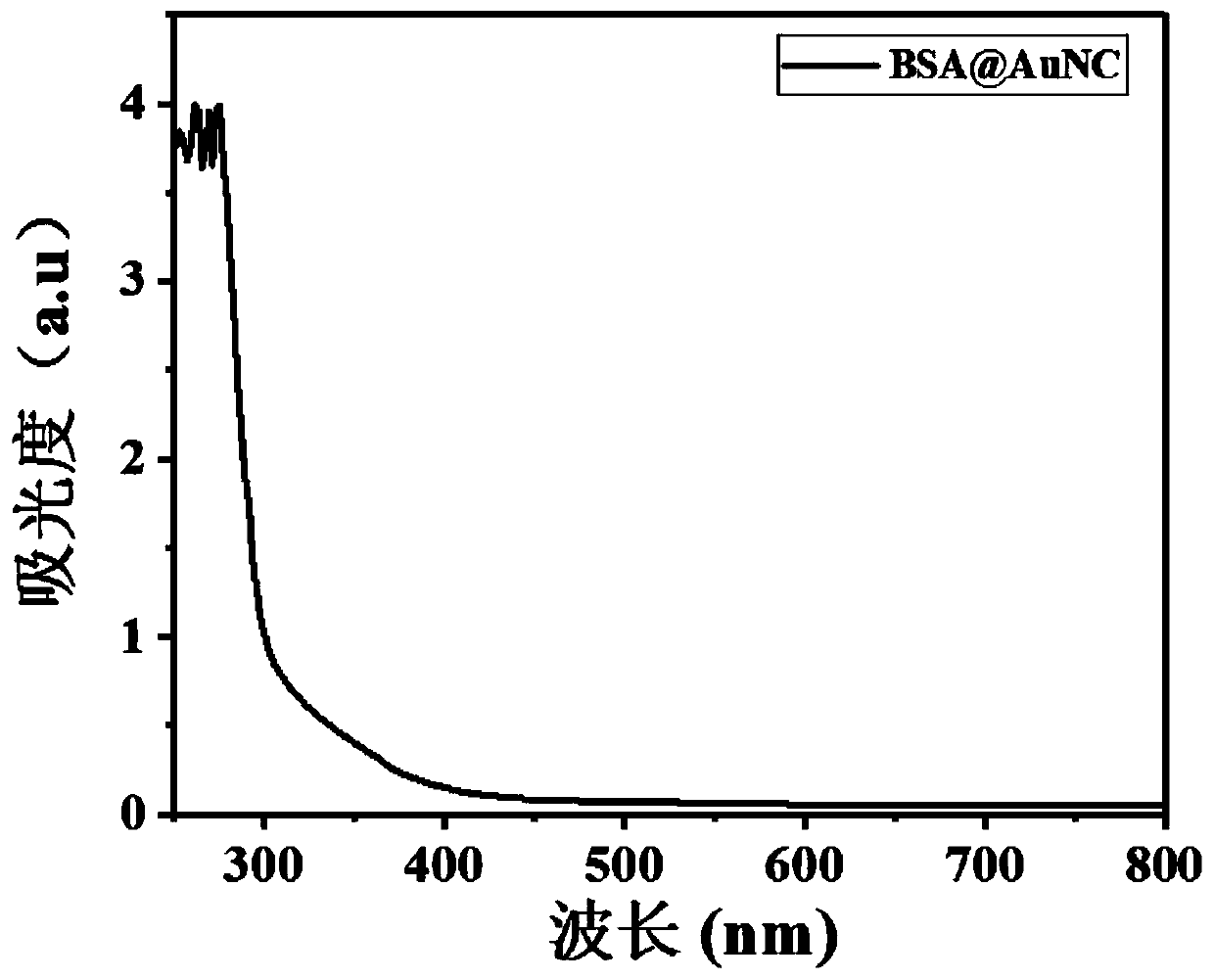
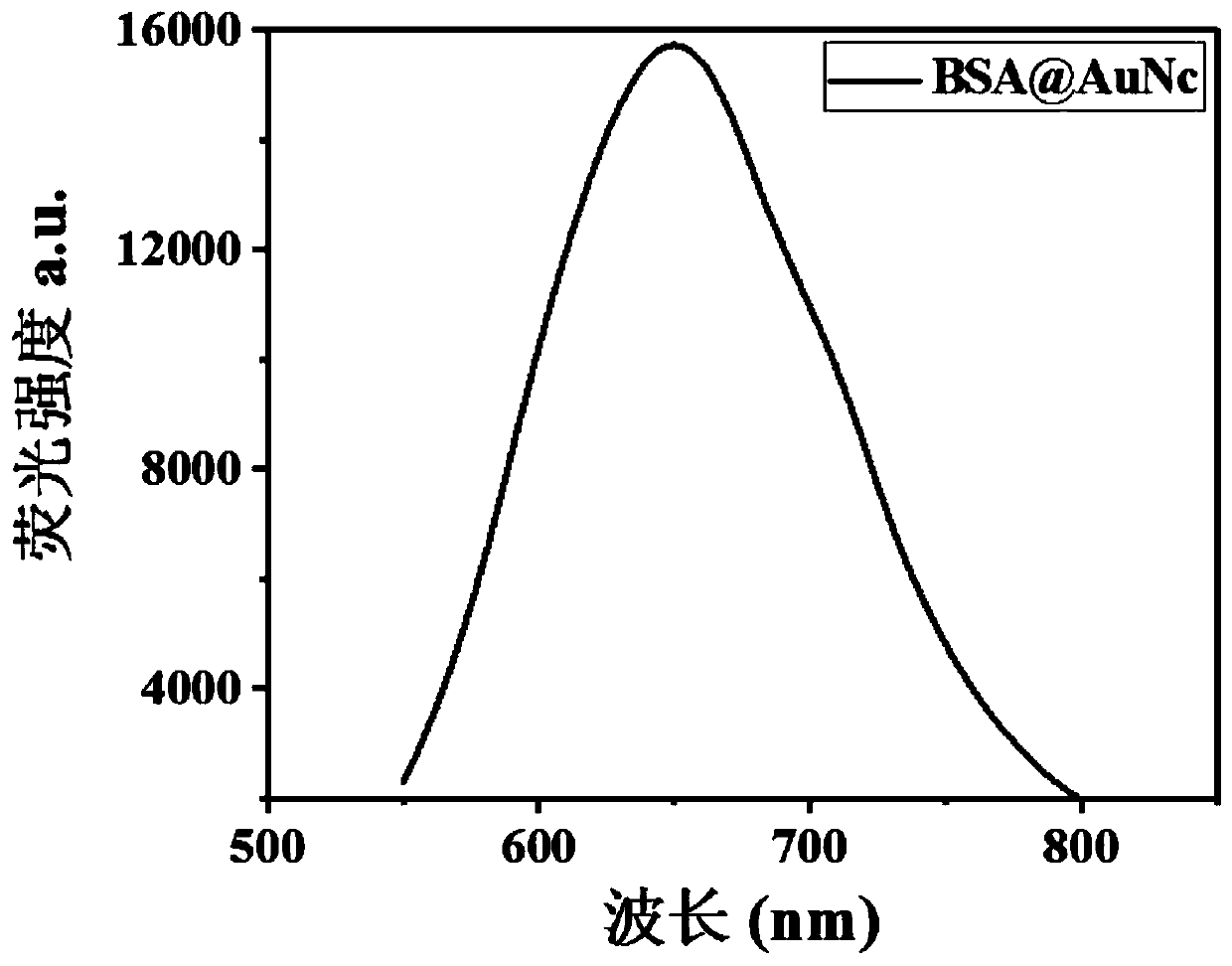
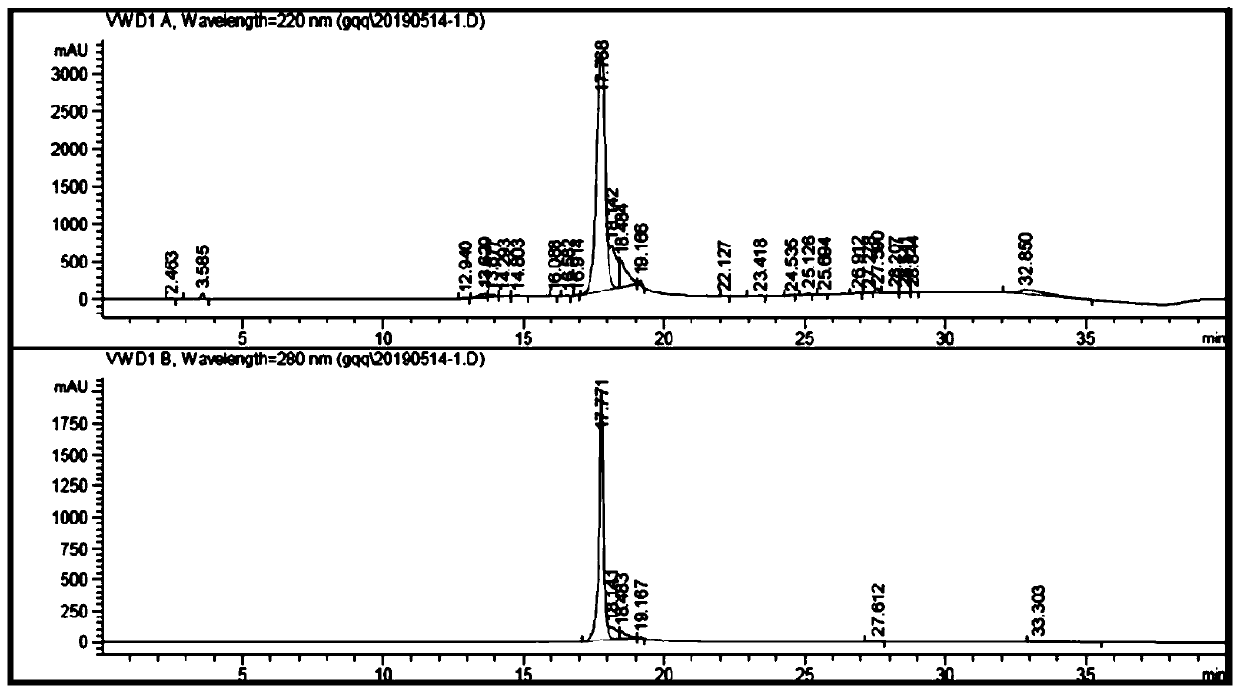
Antibacterial nanoprobe BSA@AuNc-AMP-Ce6 and preparation method and application thereof
InactiveCN110692651AObvious FRET phenomenonObvious disappearance of FRETAntibacterial agentsBiocideGelatinasesEnzyme digestion
The invention belongs to the technical field of metal nanomaterials, and particularly discloses an antibacterial nanoprobe BSA@AuNc-AMP-Ce6 and a preparation method and application thereof. An antibacterial peptide marked by Ce6 is connected to BSA@AuNc through an Au-S bond, and PLGVRG in a polypeptide sequence is a gelatinase enzyme digestion sequence and can be recognized and cut off by gelatinase secreted by S. aureus; the photosensitizer Ce6 is introduced for PDT antibiosis, and the photosensitizer Ce6 generates reactive oxygen species (ROS) under the irradiation of laser, so microbial molecules are oxidized and cells are damaged and die; and the photosensitizer Ce6 and antibacterial peptide (AMP) generate a synergistic effect. The antibacterial nanoprobe BSA@AuNc-AMP-Ce6 prepared by using the preparation method disclosed by the invention has more excellent antibacterial performance than traditional antibacterial peptides, and is good in biocompatibility, small in interference to normal physiological activities of body cells, low in toxicity and high in safety.
Owner:CHANGZHOU UNIV
Colorimetric gelatinase assay
InactiveUS20140004546A1Material analysis by observing effect on chemical indicatorMicrobiological testing/measurementGelatinasesAntiendomysial antibodies
The present invention is drawn toward a lateral-flow, colorimetric, gelatinase assay including a sample pad, a reagent pad, a membrane, and an absorbent pad, in that order, wherein the reagent pad has therein a dried form of gelatin-coated nanoparticles in a salt and sugar matrix, and wherein the membrane portion has a test stripe and a control stripe, such that the test stripe is a solution of electrolyte evaporated into a layer, and the control stripe is a solution of anti-gelatin antibody evaporated into a layer. The present invention is also drawn toward a method of detecting one or more gelatinases in a sample fluid using the assay, and methods of determining treatment for wound healing, cancer, ocular rosacea, periodontal disease and equine arthritis using the assay.
Owner:KERSCHENSTEINER DANIEL A
High-specificity and high-purity tumor cell sorting method based on double-antibody and cell density
InactiveCN105950558AHigh densityImprove screen purityCell dissociation methodsTumor/cancer cellsGelatinasesMicrosphere
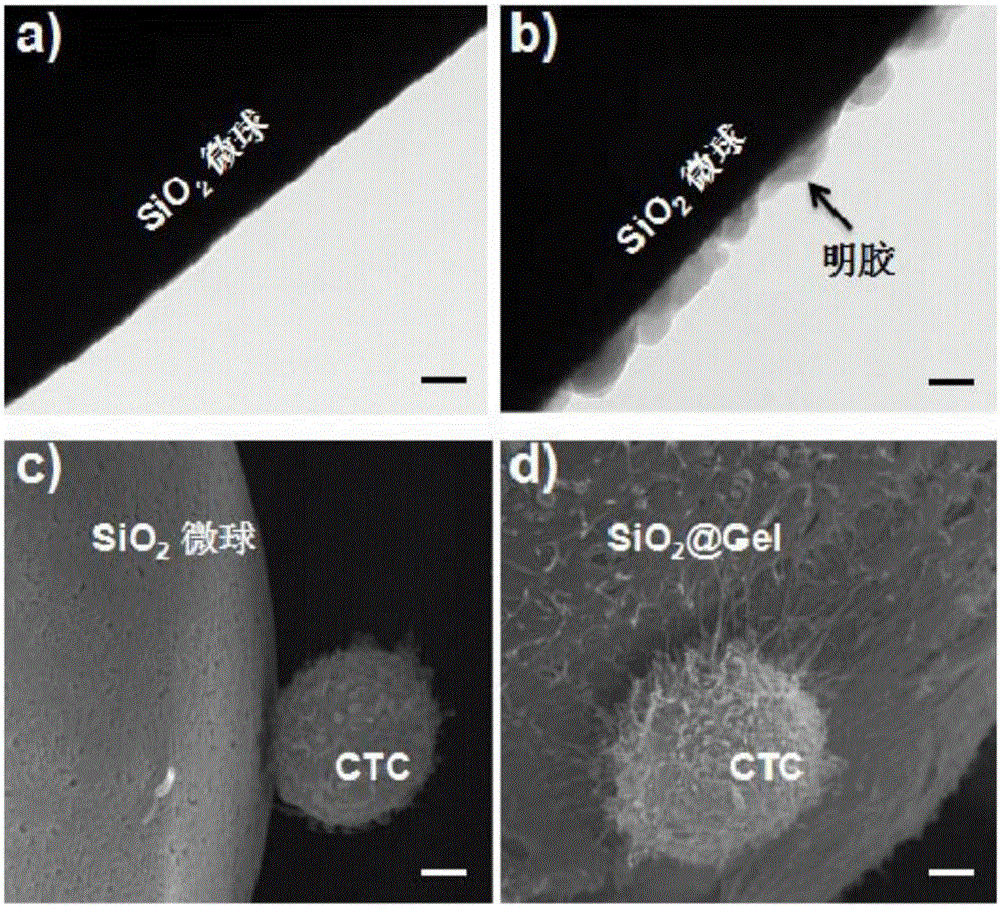
The invention discloses a high-specificity and high-purity tumor cell sorting method based on a double-antibody and cell density. The method comprises the following steps of chemically bonding a layer of gelatin with the thickness of 20 to 100nm on the surface of a hydroxyl microsphere with the grain size of 20 to 100mum and the density of 1.16 to 3g / mL, and obtaining the hydroxyl microsphere coated with the gelatin; modifying the antibodies of anti-EpCAM and anti-CD146 on the surface of the hydroxyl microsphere coated with the gelatin; uniformly mixing a blood sample of a patient and the hydroxyl microsphere with the modified antibodies, incubating to obtain a mixed sample, dropwise adding to an upper layer of 1.15 to 1.20g / mL of cell separation fluid, and after centrifuge separation, and deposing the microsphere acquiring a tumor cell to the bottom part of the cell separation fluid; using a gelatinase on the microsphere acquiring the tumor cell to degrade the gelatin on the surface of the microsphere, and releasing the tumor cell. The high-specificity and high-purity tumor cell sorting method based on the double-antibody and the cell density provided by the invention is higher in sorting efficiency and purity, and the sorted tumor cell can be released and cultured.
Owner:WUHAN UNIV
Assays for measuring matrix metalloproteinase activities
InactiveUS20030229005A1High selectivityHigh sensitivityBiocideOrganic active ingredientsFluorescenceDigestion
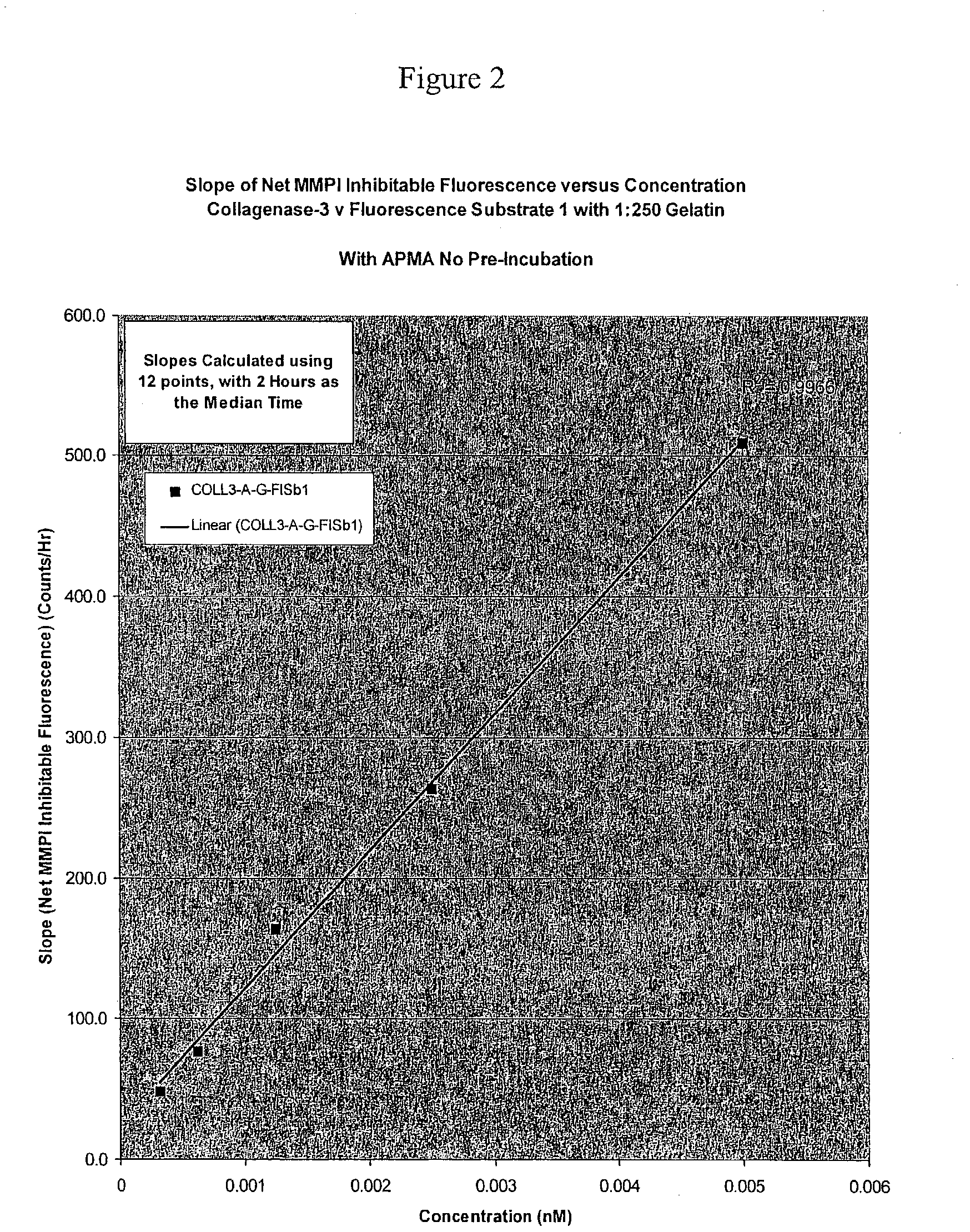
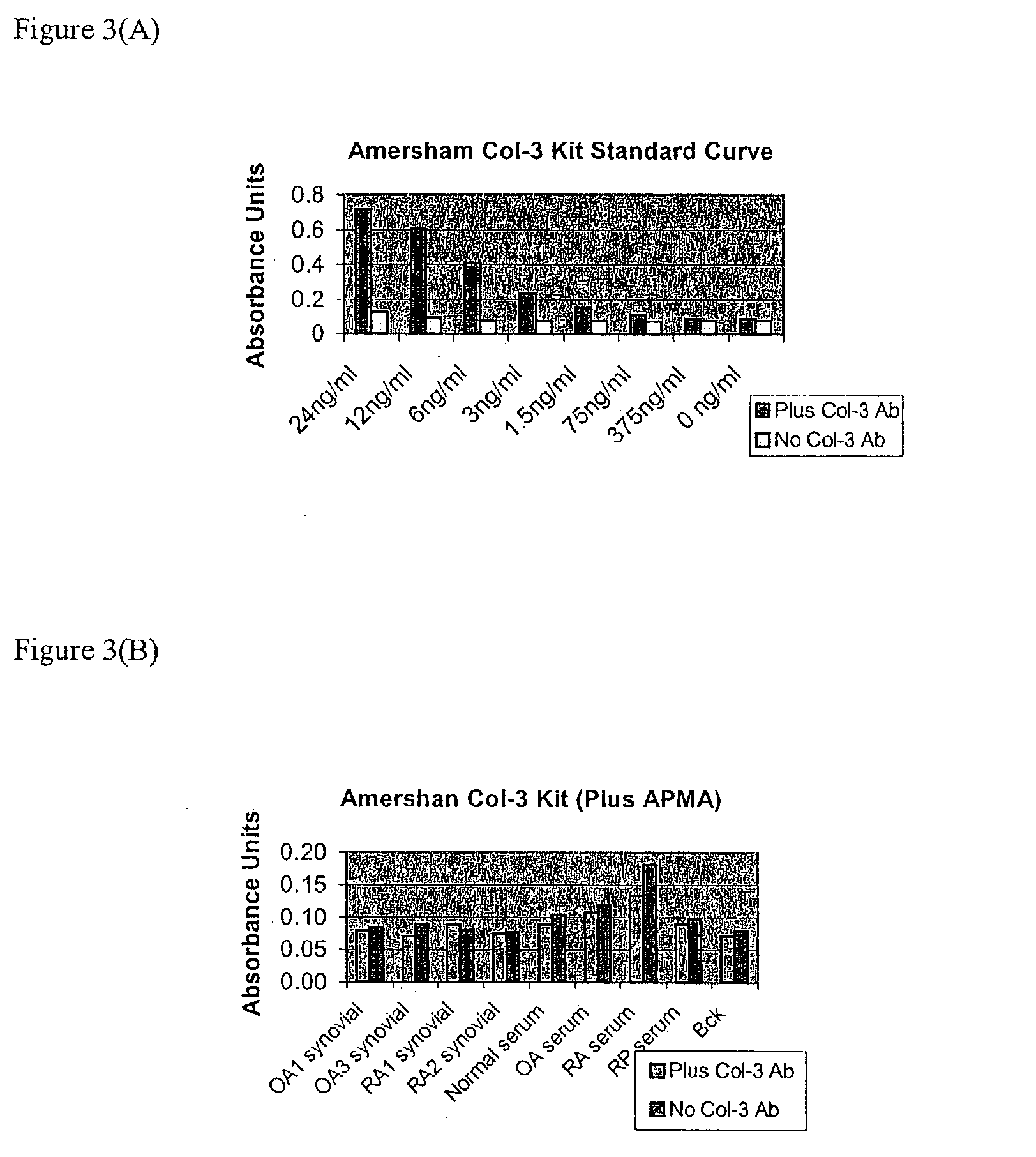
Novel methods to determine matrix metalloproteinase (MMP) activity are described. In a typical embodiment of the invention, the biological fluid sample is from a patient, and the methods of the invention are useful to assess disease severity or progression, diagnose a particular disease, or develop a profile of MMP activity for inflammatory diseases such as arthritis, or cancer. A novel method of the invention involves the use of particular amino acid sequences in substrates that include but are not limited to fluorescence, colorimetric, radiometric and unlabeled substrates. A second method of the invention use neo-epitope antibodies that bind to the cleavage sites generated by collagenase 3 digestion of type IV collagen or biglycan or the peptide substrates disclosed. There is also provided a diagnostic kit for use in determining the amounts of MMP activities in biological samples comprising (a) one or more substrates with different specificities against the MMPs, (b) one or more reagents capable of inhibiting non-metalloproteinase activities, (c) one or more reagents capable of specifically inhibiting metalloproteinase activities, (d) one or more activators of metalloproteinase activities, (e) reagents capable of altering the selectivity ratios for the substrates such as gelatin or type II collagen, and (f) collagenase 3, stromelysin 1, gelatinase A, gelatinase B and / or collagenase 1 as calibration standards.
Owner:BIOZYME
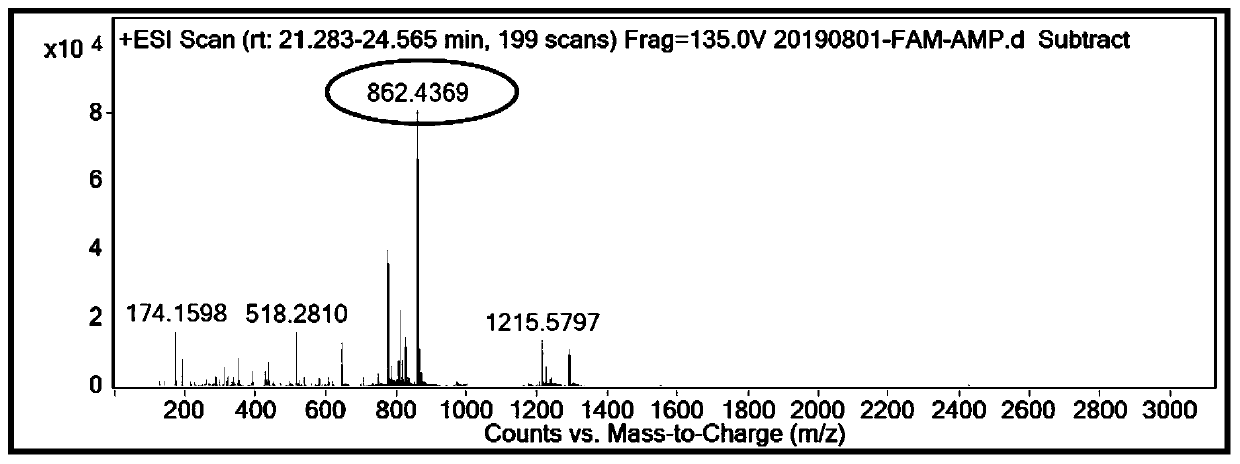
Antimicrobial agent FAM-AMP with gelatinase digestion sequence for detection of S.aureus bacteria
PendingCN110669147AGood antibacterial effectReduce consumptionMicrobiological testing/measurementBiological material analysisGelatinasesAntimicrobial peptides
The invention belongs to the technical field of antimicrobial agents, and discloses an antimicrobial agent FAM-AMP with gelatinase digestion sequence for detection of S.aureus bacteria. GKRWWKWWRR inAMP antimicrobial agent is antimicrobial peptide, PLGVRG is in gelatinase digestion sequence and can be recognized and cut off by the gelatinase secreted by S.aureus bacteria, and the PLGVRG and the gelatinase are coupled through peptide solid phase synthesis method to form new form of antimicrobial agent with gelatinase digestion points. Fluorescein FAM is marked into the antimicrobial agent AMPthrough coupling agents, and the marked FAM-AMP can further detect the concentration of the S.aureus through capillary electrophoresis. The antimicrobial agent FAM-AMP with the gelatinase digestion sequence for the detection of the S.aureus bacteria has the advantages that the concentration of the S.aureus bacteria can be detected, bacterium can be effectively killed, the biocompatibility is good,impact on normal physiological activities of body cells is small, toxicity is low and safety is high.
Owner:CHANGZHOU UNIV
N-hydroxy-2-(alkyl, aryl or heteroaryl sulfanyl, sulfinyl or sulfonyl-3-substituted-alkyl, aryl or heteroarylamides) as matrix metallo protein inhibitors
Matrix metalloproteinases (MMPs) are a group of enzymes that have been implicated in the pathological destruction of connective tissue and basement membranes. These zinc containing endopeptidases consist of several subsets of enzymes including collagenases, stromelysins and gelatinases. TNF-alpha converting enzyme (TACE), a pro-inflammatory cytokine, catalyzes the formation of TNF-alpha from membrane bound TNF-alpha precursor protein. It is expected that small molecule inhibitors of MMPs and TACE therefore have the potential for treating a variety of disease states. The present invention provides low molecular weight, non-peptide inhibitors of matrix metalloproteinases (MMPs) and TNF-alpha converting enzyme (TACE) for the treatment of arthritis, tumor metastasis, tissue ulceration, abnormal wound healing, periodontal disease, bone disease, diabetes (insulin resistance) and HIV infection having the formula wherein R2 and R3 form a heterocyclic ring and A is S, S(O), or S(O)2, and R1 and R4 are defined herein.
Owner:WYETH HOLDINGS CORP
Tricyclic sulfonamides and their derivatives as inhibitors of matrix metalloproteinases
Tricyclic sulfonamide compounds and derivatives are described as well as methods for the preparation and pharmaceutical compositions of same, which are useful as inhibitors of matrix metalloproteinases, particularly gelatinase A, collagenase-3, and stromelysin-1 and for the treatment of multiple sclerosis, atherosclerotic plaque rupture, aortic aneurysm, heart failure, left ventricular dilation, restenosis, periodontal disease, corneal ulceration, treatment of burns, decubital ulcers, wound healing, cancer, inflammation, pain, arthritis, osteoporosis, renal disease, or other autoimmune or inflammatory disorders dependent upon tissue invasion by leukocytes or other activated migrating cells, acute and chronic neurodegenerative disorders including stroke, head trauma, spinal cord injury, Alzheimer's disease, amyotrophic lateral sclerosis, cerebral amyloid angiopathy, AIDS, Parkinson's disease, Huntington's disease, prion diseases, myasthenia gravis, and Duchenne's muscular dystrophy.
Owner:WARNER-LAMBERT CO
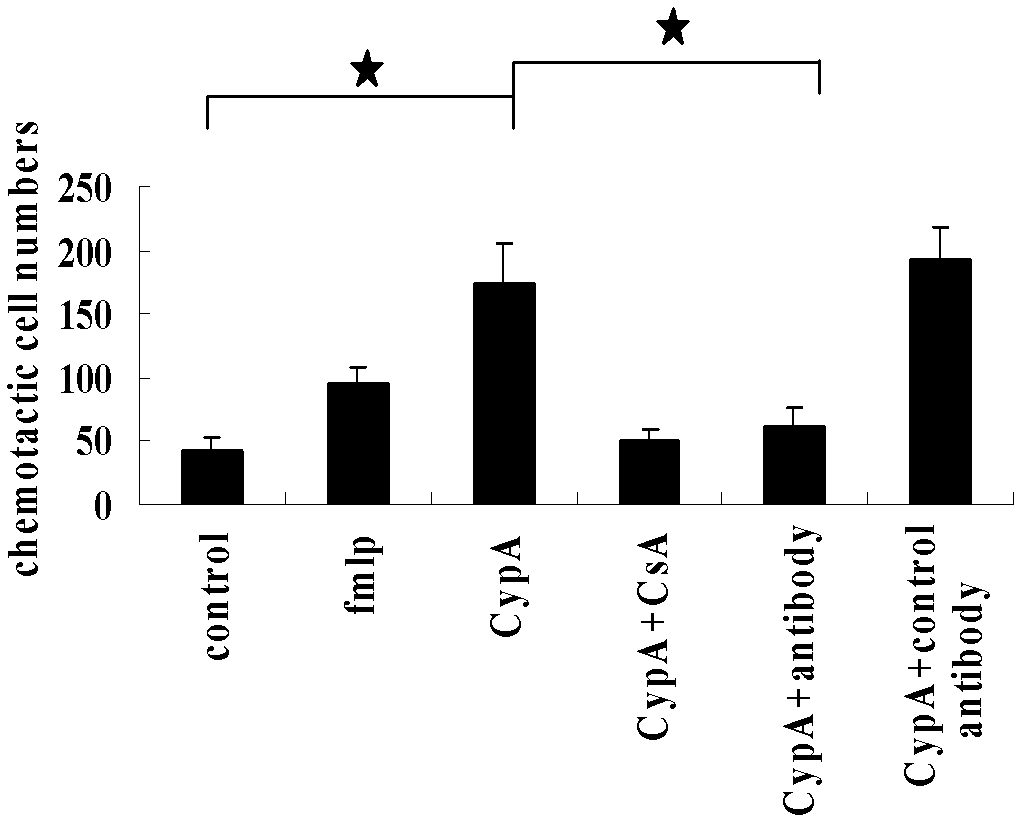
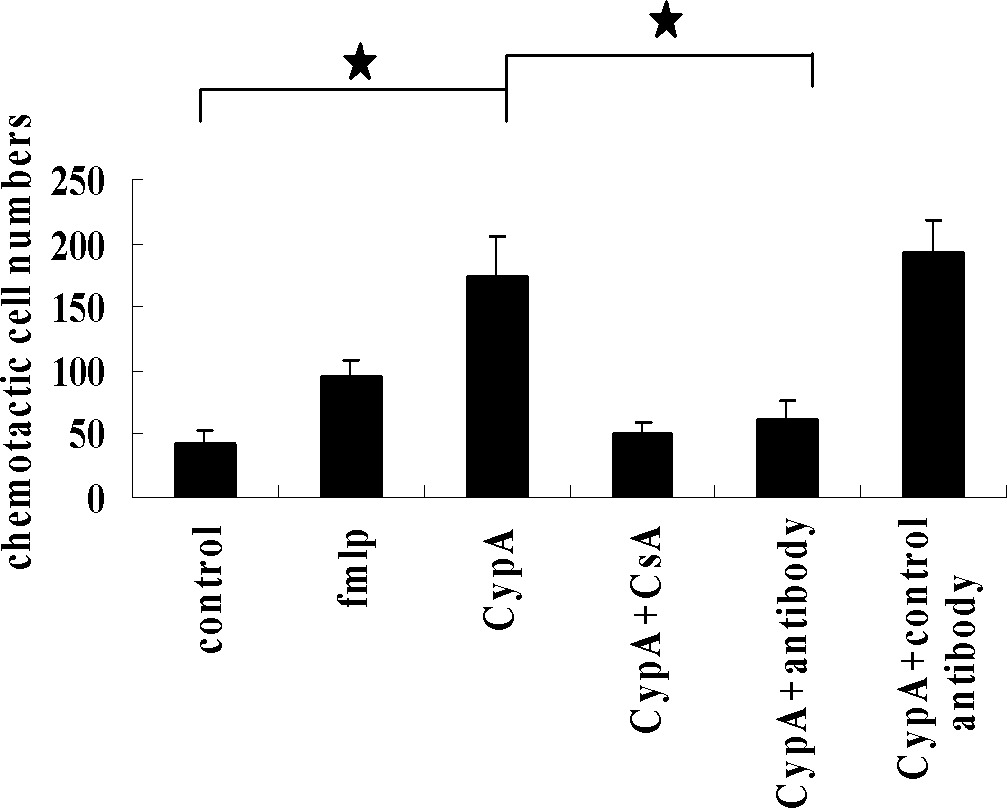
VHH (variable domain of heavy chain of heavy-chain antibody) antibody gene derived from anti-CyPA (CyclophilinA) animal of family Camelidae, encoded polypeptide, and application thereof
ActiveCN102660551AInhibition of chemotaxisInhibition of secretionAntipyreticGenetic material ingredientsAntigenDisease
The invention relates to VHH (variable domain of heavy chain of heavy-chain antibody) antibody gene derived from anti-CyPA (CyclophilinA) animal of family Camelidae, encoded polypeptide, and application of the gene and the polypeptide in preparing drugs for treating inflammatory diseases such as rheumatoid arthritis. The VHH antibody gene has gene sequence shown in sequence table (400)1, the encoded polypeptide has amino acid sequence shown in sequence table (400)2. Lama pacos as immune animal is used, and phage display techniques (PDT) are adopted to successfully obtain an anti-CyPA VHH antibody capable of specifically combining with expressed CyPA antigen. The antibody can inhibit monocytes THP-1 chemotaxis and gelatinase secretion induced by CyPA. Dimer or polymer can be constructed by gene engineering method, to be applied in diagnosis or research on drugs for treating inflammatory diseases. The gene and the polypeptide establish foundation for further research on related drugs for treating rheumatoid arthritis.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
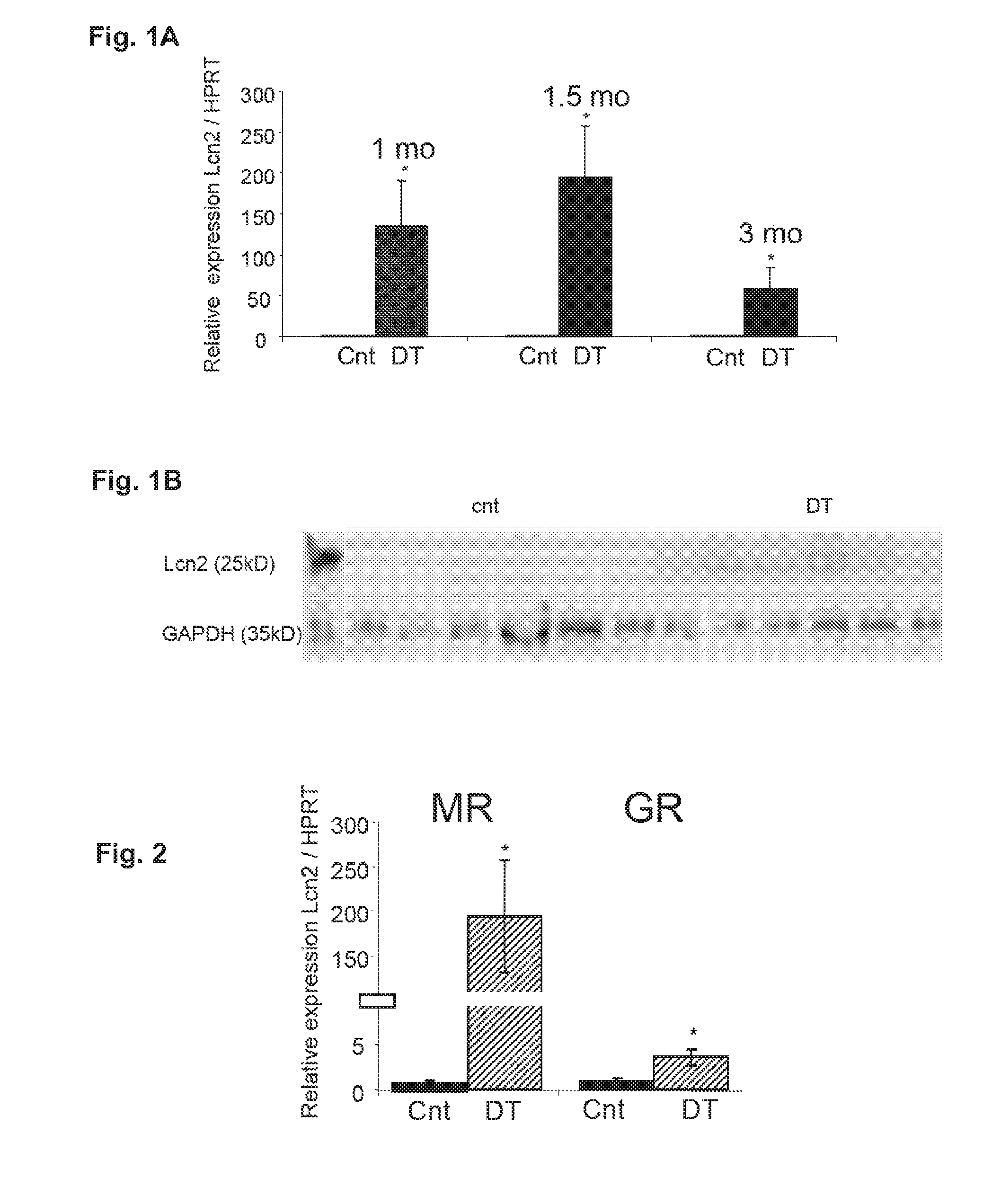
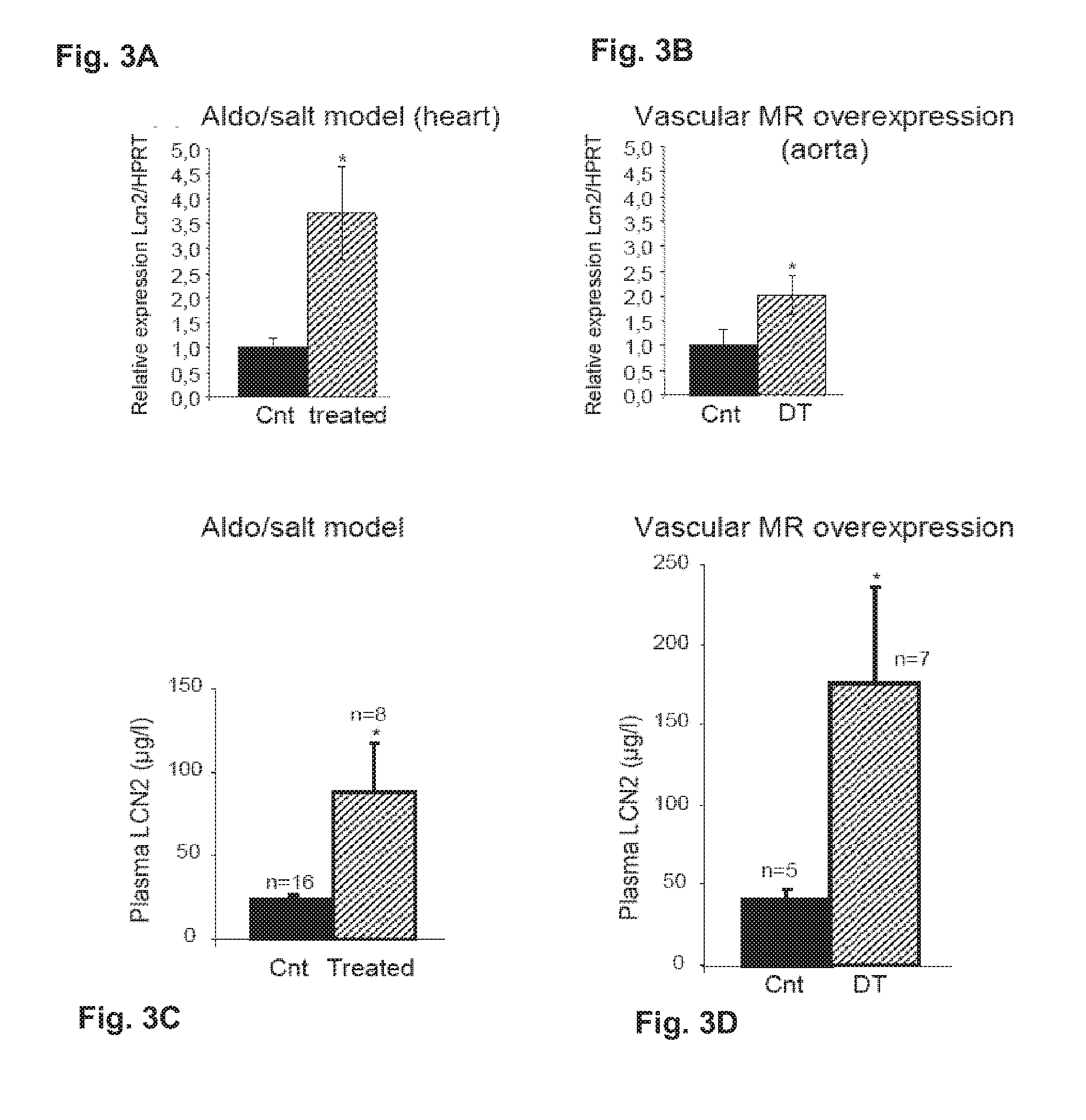
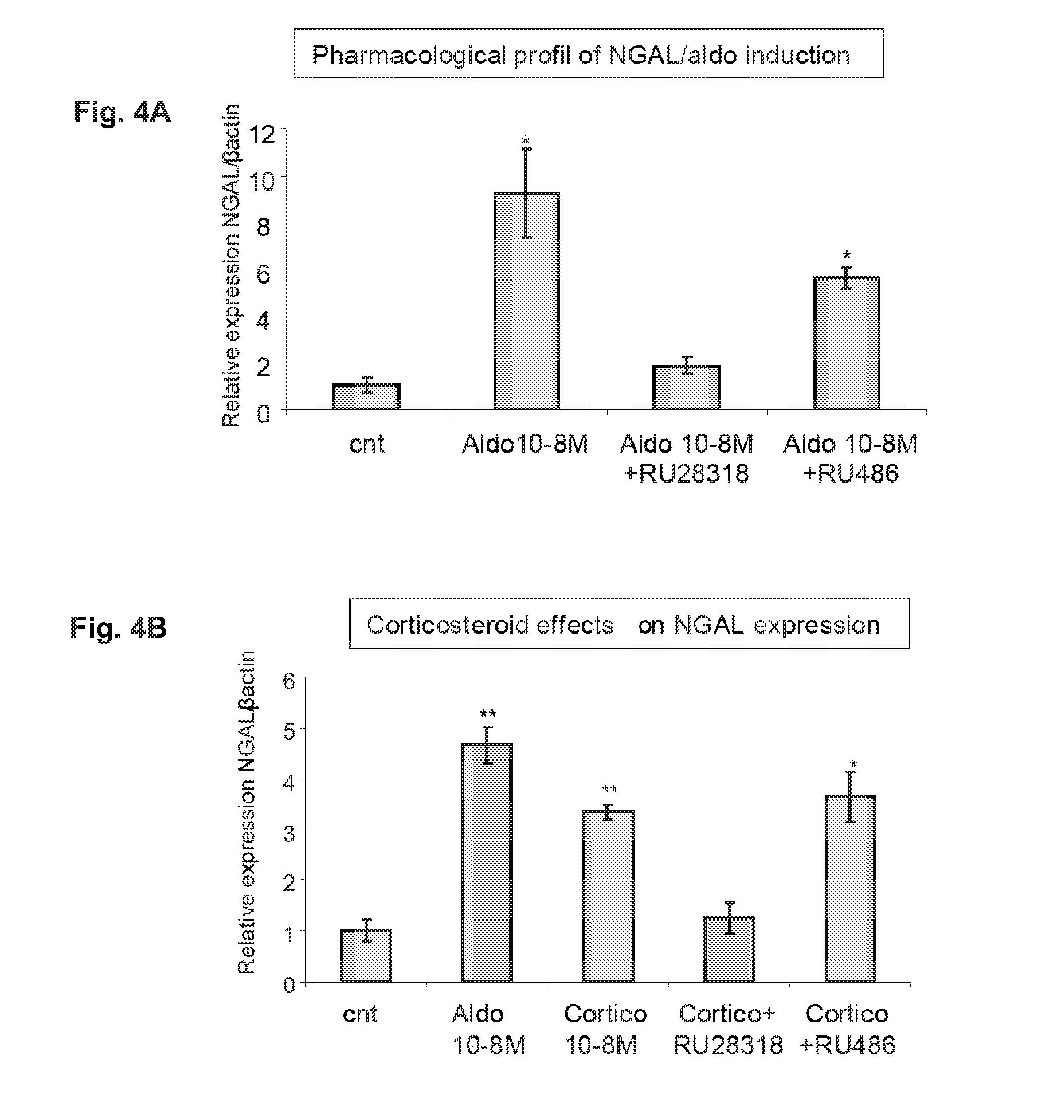
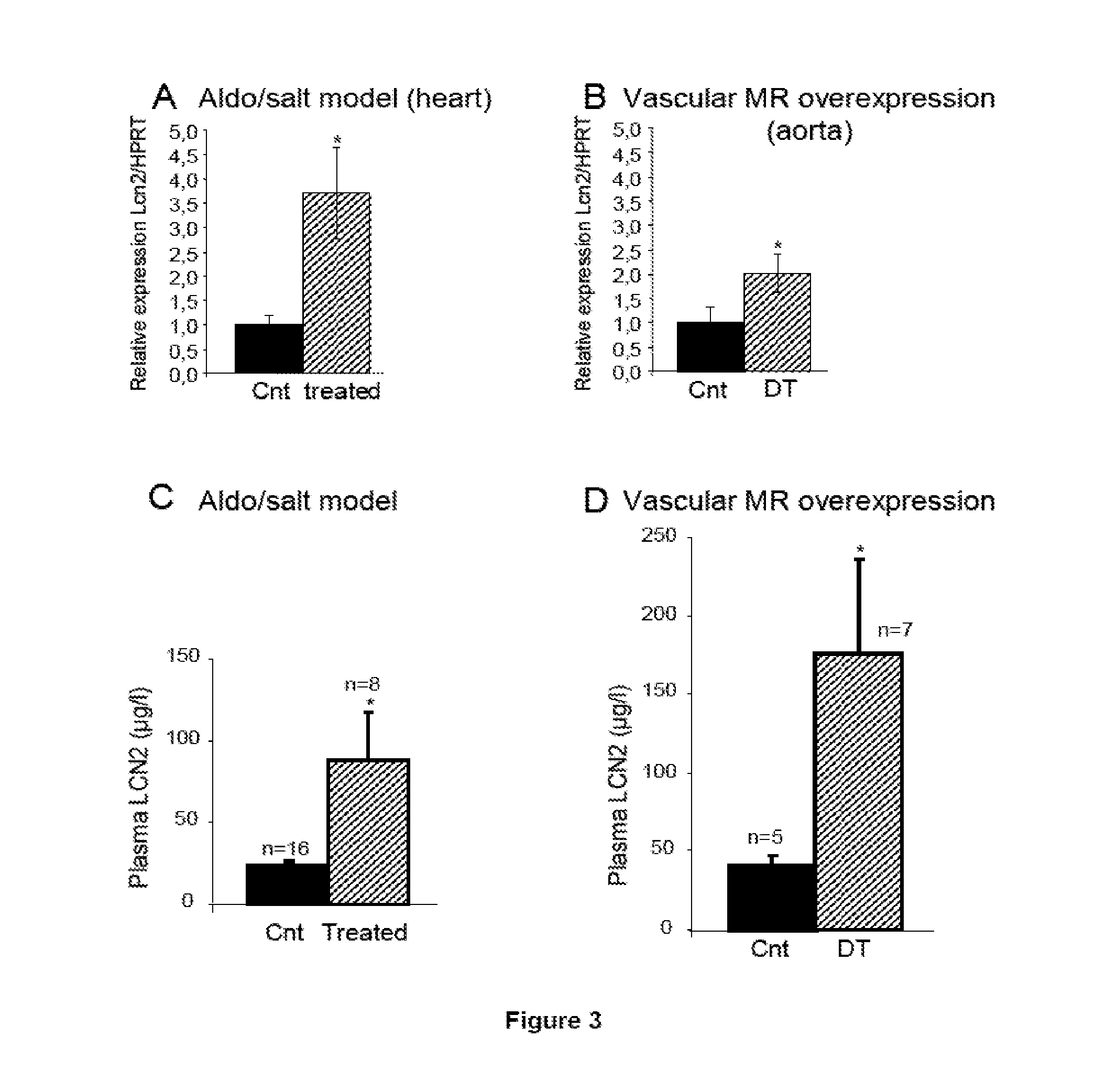
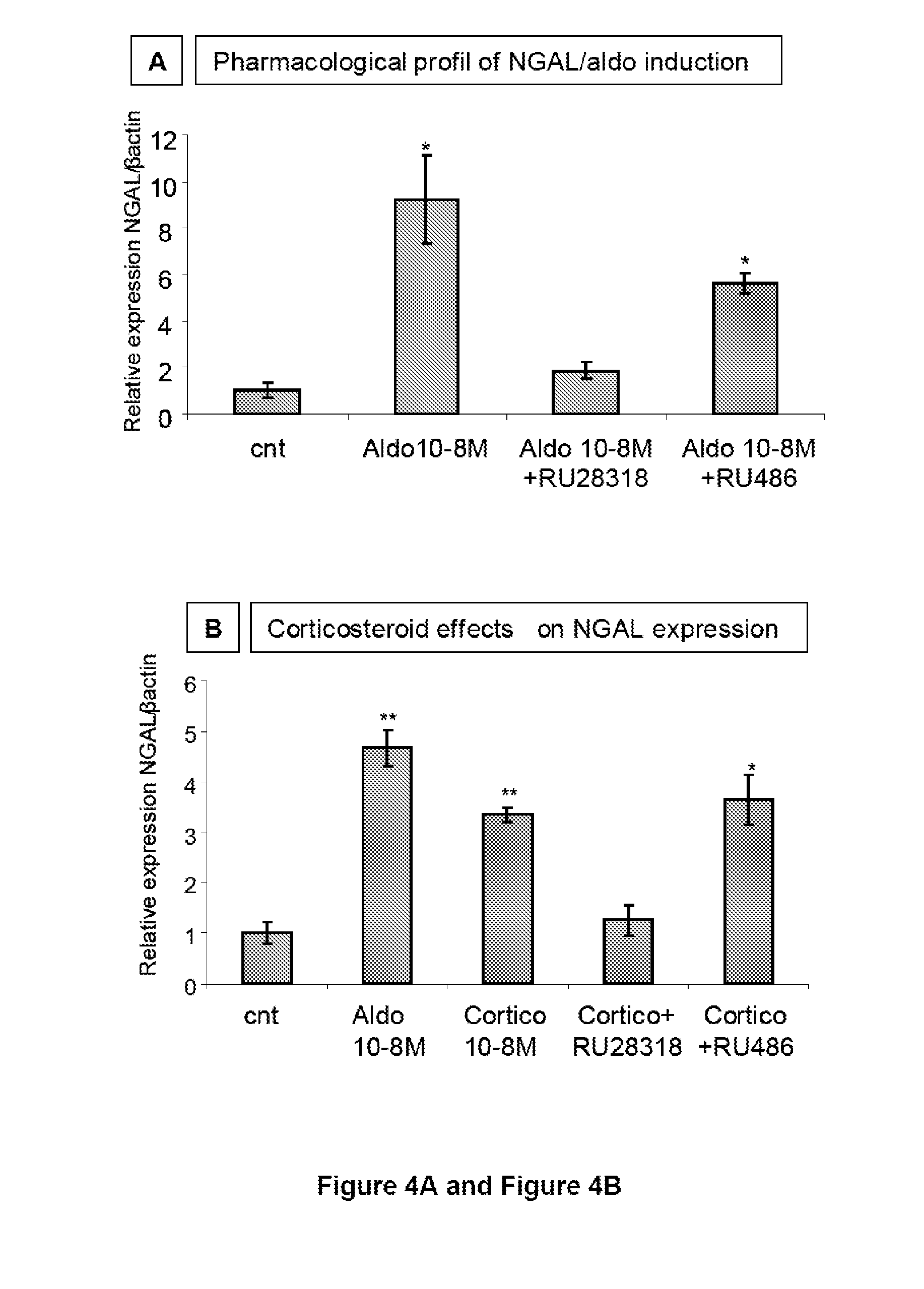
Biomarkers of Mineralocorticoid Receptor Activation
ActiveUS20160362744A1Precision therapyMetabolism disorderMicrobiological testing/measurementGelatinasesAldosterone Synthase Deficiency
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
N-hydroxy-2-(alkyl, aryl or heteroaryl sulfanyl, sulfinyl or sulfonyl-3-substituted-alkyl, aryl or heteroarylamides) as matrix metallo protein inhibitors
Matrix metalloproteinases (MMPs) are a group of enzymes that have been implicated in the pathological destruction of connective tissue and basement membranes. These zinc containing endopeptidases consist of several subsets of enzymes including collagenases, stromelysins and gelatinases. TNF-alpha converting enzyme (TACE), a pro-inflammatory cytokine, catalyzes the formation of TNF-alpha from membrane bound TNF-alpha precursor protein. It is expected that small molecule inhibitors of MMPs and TACE therefore have the potential for treating a variety of disease states. The present invention provides low molecular weight, non-peptide inhibitors of matrix metalloproteinases (MMPs) and TNF-alpha converting enzyme (TACE) for the treatment of arthritis, tumor metastasis, tissue ulceration, abnormal wound healing, periodontal disease, bone disease, diabetes (insulin resistance) and HIV infection having the formula wherein R2 and R3 form a heterocyclic ring and A is S, S(O), or S(O)2, and R1 and R4 are defined herein.
Owner:WYETH HOLDINGS CORP
Anti-human neutrophil gelatinase-related lipid carrier protein antibody and application thereof in detection test paper card
ActiveCN109553682AMeet the needs of large-scale clinical applicationsEase of mass productionImmunoglobulins against animals/humansBiological testingGelatinasesTrue positive rate
The invention relates to an anti-human neutrophil gelatinase-related lipid carrier protein antibody and an application thereof in a detection test paper card. A variety of antibodies are prepared andsubjected to paired screening, and a group of antibody combination (NG02 and NG19) with sensitivity and specificity that can meet the needs is obtained; at the same time, the antibodies are prone to mass production and can meet the needs of large-scale clinical application in the future. The antibody combination is used for debugging and optimizing the detection system, and a colloidal gold immunochromatographic quantitative detection card for human neutrophil gelatinase-related lipid carrier proteins is obtained, wherein the detection card is simple to operate and has sensitivity, specificityand relevant detection performance meeting the detection of human blood or urine samples.
Owner:ZONHON BIOPHARMA INST
Anti-inflammatory application of small molecule compound AG-4
ActiveCN103655556AInhibit biological activityInhibition of secretionOrganic active ingredientsAntipyreticDiseaseGelatinases
The invention discloses anti-inflammatory application of a small molecule compound AG-4, and application in preparing anti-inflammatory drug. The small molecule compound AG-4 is screened through a candidate drug screening platform combining structural biology, information biology and biological activity determination. The small molecule compound AG-4 can realize specific binding with CD147 protein to inhibit inflammatory cell chemotaxis and secretion of chemotaxis; the invention is also suitable for preparing medicines for treating CD147 protein high expression related diseases (such as rheumatoid arthritis).
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Biomarkers of Mineralocorticoid Receptor Activation
InactiveUS20110257140A1Precision therapyOrganic active ingredientsMetabolism disorderGelatinasesAldosterone Synthase Deficiency
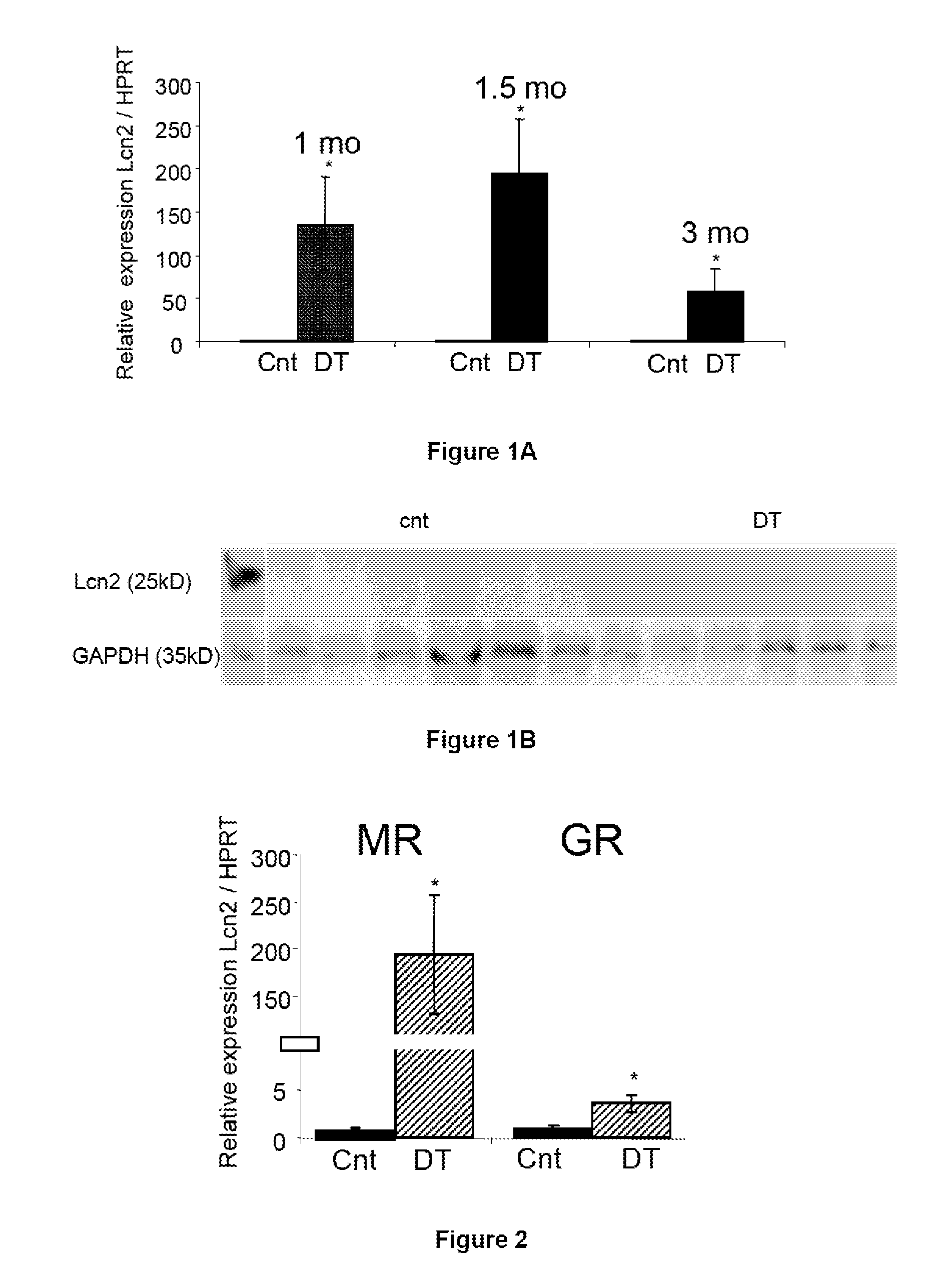
The present invention relates to the use of Neutrophil Gelatinase-Associated Lipocalin (NGAL) and / or SERPINA3 as biomarkers of the Mineralocorticoid Receptor (MR) activation in a patient. More particularly, the present invention relates to a method for predicting the responsiveness of a patient to a treatment with a MR antagonist or an aldosterone synthase inhibitor, said method comprising determining in a biological sample obtained from said patient the expression level of the NGAL gene and / or of the SERPINA3 gene.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
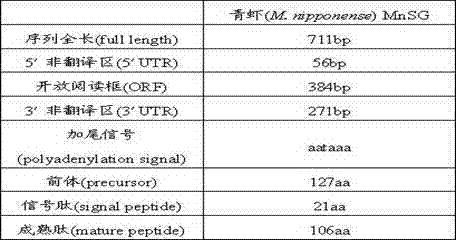
Freshwater shrimp MnSG (Macrobrachium nipponense Sperm Gelatinase) gene, and amplification method and amplification primer group thereof
The invention relates to the field of gene cloning, and particularly relates to a freshwater shrimp MnSG (Macrobrachium nipponense Sperm Gelatinase) gene, and an amplification method and amplification primer group thereof. By utilizing the primer group provided by the invention, a full-length cDNA sequence of the MnSG gene is cloned from a freshwater shrimp spermary tissue, thereby laying a foundation for the research on the function of the freshwater shrimp MnSG gene, and simultaneously accumulating background data for the research on the procreation of shellfish.
Owner:FRESHWATER FISHERIES RES CENT OF CHINESE ACAD OF FISHERY SCI
Anti-wrinkle essence containing collagen
InactiveCN112972285AReduce generationEnhanced anti-wrinkle effectCosmetic preparationsToilet preparationsGelatinasesPolymer science
The invention discloses anti-wrinkle essence containing collagen. The anti-wrinkle essence is prepared from the following raw materials in parts by weight: 3 to 5 parts of xanthan gum, 5 to 8 parts of glycerol, 50 to 60 parts of deionized water, 2 to 5 parts of glyceryl stearate, 1 to 3 parts of squalane, 2 to 3 parts of collagen, 1 to 1.5 parts of capric triglyceride, 2 to 5 parts of butanediol, 1.5 to 2 parts of avocado ester, 0.5 to 1.5 parts of carbomer, 2 to 3 parts of humectant and 5 to 8 parts of anti-wrinkle micro-capsules, the anti-wrinkle microcapsule contains rich polyphenol substances, the molecular structure of a phenolic compound contains some phenolic hydroxyl groups capable of releasing hydrogen, and the hydrogen and free radicals are condensed together to terminate chain reaction caused by the free radicals, so that the anti-wrinkle microcapsule has high free radical scavenging capacity, the anti-oxidation effect of cells is better. The curcumin can well inhibit the activity of the white gelatinase, so that the anti-wrinkle effect of the essence is improved, and the effective time of the essence is prolonged.
Owner:吴秀琴
Method for pre-judging gelatin viscosity in gelatin production and application thereof
PendingCN113049444AComparableInstructiveGlue/gelatin preparationDirect flow property measurementGelatinasesPolymer science
The invention relates to a method for quickly pre-judging the viscosity of gelatin in advance. The method comprises the following specific steps: preparing a dilute glue solution with the concentration of 2% from a primary glue solution obtained in gelatin production, and measuring the viscosity. The method provided by the invention is helpful for finding out links which destroy viscosity or even destroy more viscosity in the gelatin production process so as to control in the production process, so that the viscosity is protected from being destroyed, and the viscosity of the product is controllable. The method is suitable for the production process of various types of gelatin such as acid-process gelatin, alkaline-process gelatin and enzyme-process gelatin, and is suitable for the production process of various types of gelatin such as skin gelatin, bone gelatin and fish skin gelatin.
Owner:NINGXIA XINHAOYUAN BIOLOGICAL POLYTRON TECH INC
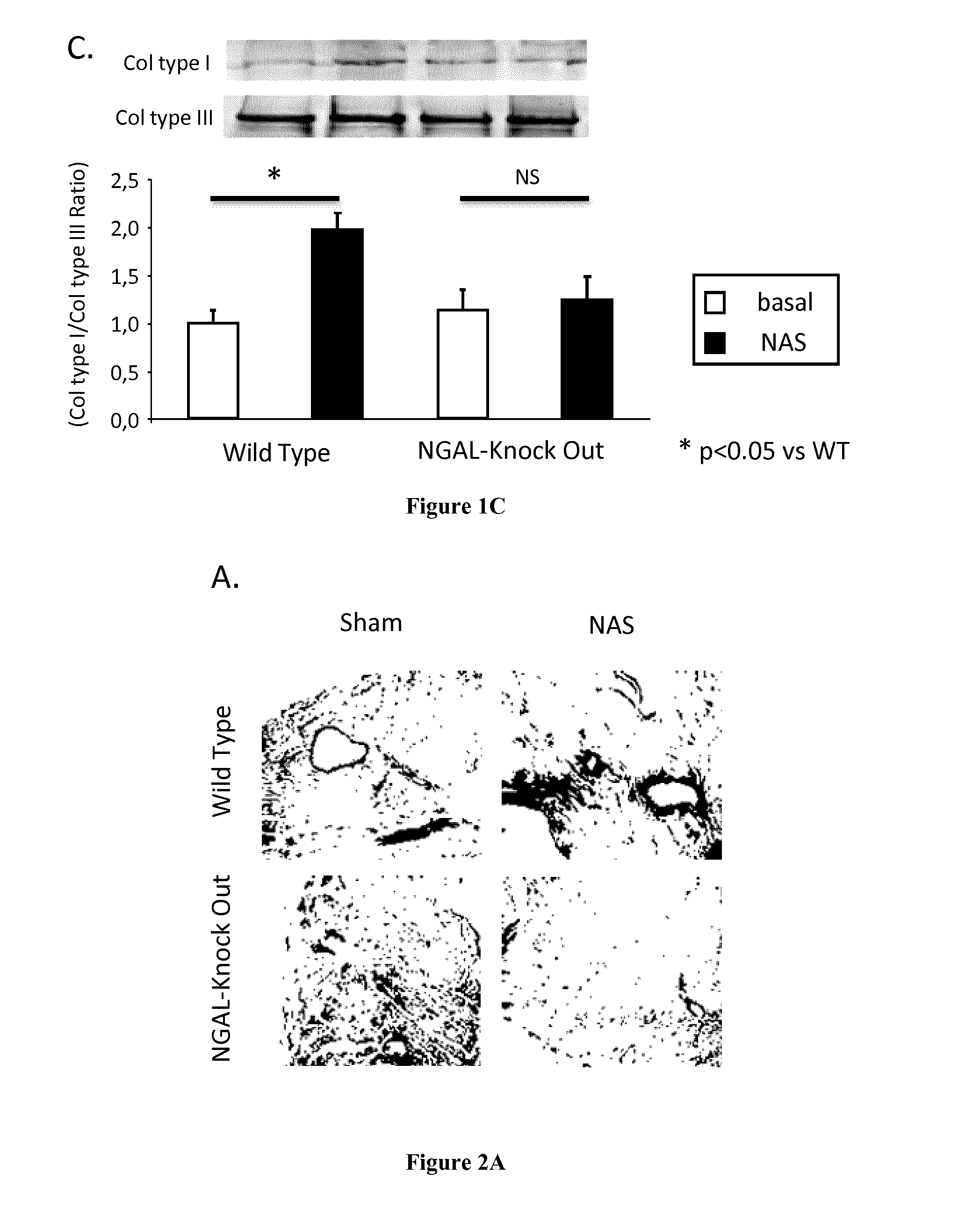
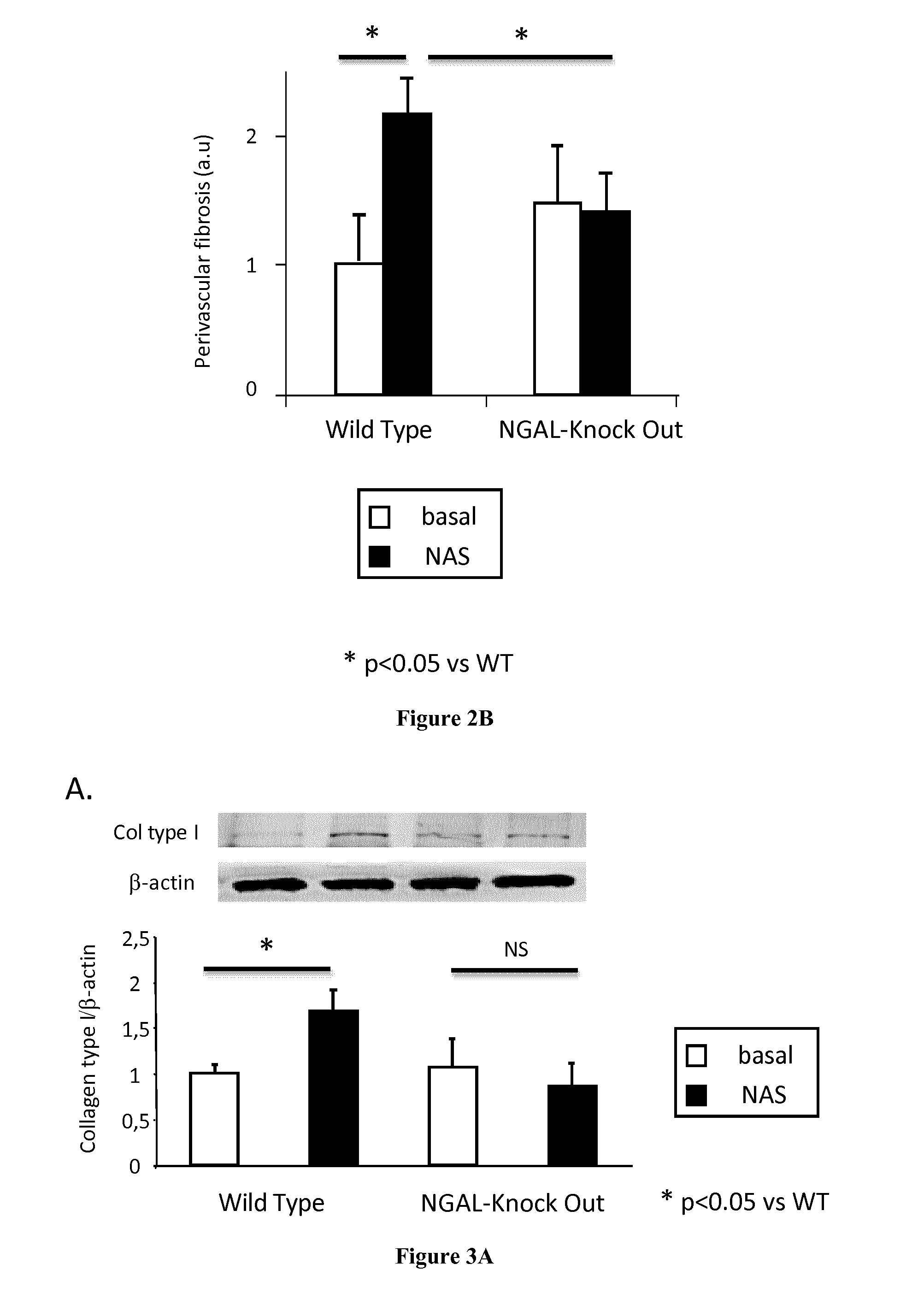
Methods and Pharmaceutical Compositions for the Treatment of Cardiovascular Fibrosis
InactiveUS20150247855A1Avoid difficult choicesHigh affinityOrganic active ingredientsCompound screeningGelatinasesVascular fibrosis
The present invention relates to methods and pharmaceutical compositions for the treatment of cardiovascular fibrosis. In particular, the present invention relates to an inhibitor of Neutrophil Gelatinase-Associated Lipocalin (NGAL) activity or expression for use in a method for treating or preventing cardiovascular fibrosis in a subject in need thereof.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +4
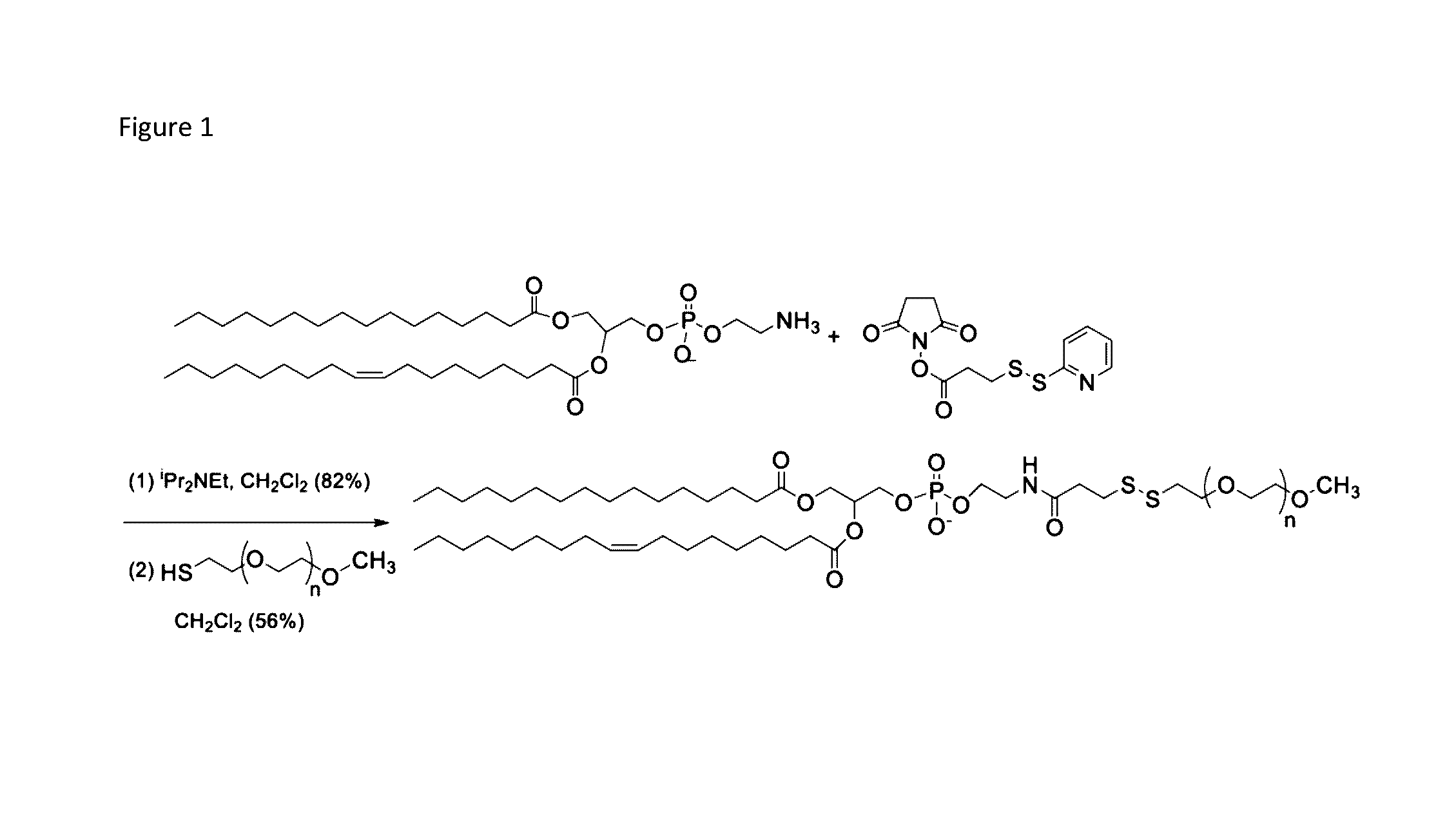
Controlled release nanoparticles and methods of use
Provided herein are nanoparticles that include a lipid layer and a compartment surrounded by the lipid layer. The lipid layer may include a lipid and a lipoprotein. The lipid may include a POPE lipid covalently attached to a hydrophilic polymer by a disulfide bond. The lipoprotein may include a trigger protein. The concentration of the first lipid may be between 1 mol % and 30 mol %. The disulfide bond of the first lipid is stable under conditions that include 10% human serum and is broken under conditions that include 50 micromolar glutathione. The hydrophilic polymer may include a PEG molecule. The trigger protein may include an amino acid repeat region, such as (GPX)n. The trigger protein may include a peptide bond that is cleaved by a gelatinase (e.g., gelatinase-B protease), or a member of the ADAM family of proteases (e.g., ADAM10 protease). Also provided are methods of using the nanoparticles.
Owner:NORTH DAKOTA STATE UNIV RES FOUND
Anti-inflammatory application of small molecule compound AG-4
ActiveCN103655556BInhibit biological activityInhibition of secretionOrganic active ingredientsAntipyreticDiseaseGelatinases
The invention discloses anti-inflammatory application of a small molecule compound AG-4, and application in preparing anti-inflammatory drug. The small molecule compound AG-4 is screened through a candidate drug screening platform combining structural biology, information biology and biological activity determination. The small molecule compound AG-4 can realize specific binding with CD147 protein to inhibit inflammatory cell chemotaxis and secretion of chemotaxis; the invention is also suitable for preparing medicines for treating CD147 protein high expression related diseases (such as rheumatoid arthritis).
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
VHH (variable domain of heavy chain of heavy-chain antibody) antibody gene derived from anti-CyPA (CyclophilinA) animal of family Camelidae, encoded polypeptide, and application thereof
ActiveCN102660551BInhibition of chemotaxisInhibition of secretionAntipyreticGenetic material ingredientsDiseaseAntigen
The invention relates to VHH (variable domain of heavy chain of heavy-chain antibody) antibody gene derived from anti-CyPA (CyclophilinA) animal of family Camelidae, encoded polypeptide, and application of the gene and the polypeptide in preparing drugs for treating inflammatory diseases such as rheumatoid arthritis. The VHH antibody gene has gene sequence shown in sequence table (400)1, the encoded polypeptide has amino acid sequence shown in sequence table (400)2. Lama pacos as immune animal is used, and phage display techniques (PDT) are adopted to successfully obtain an anti-CyPA VHH antibody capable of specifically combining with expressed CyPA antigen. The antibody can inhibit monocytes THP-1 chemotaxis and gelatinase secretion induced by CyPA. Dimer or polymer can be constructed by gene engineering method, to be applied in diagnosis or research on drugs for treating inflammatory diseases. The gene and the polypeptide establish foundation for further research on related drugs for treating rheumatoid arthritis.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
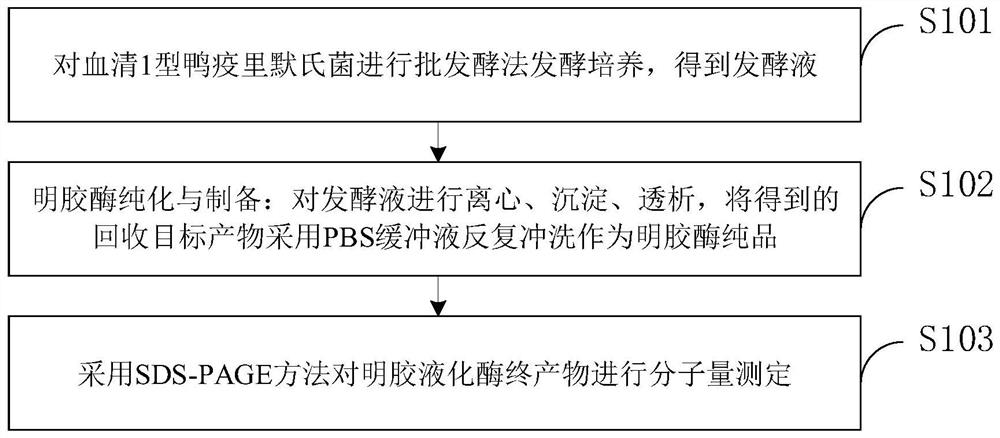
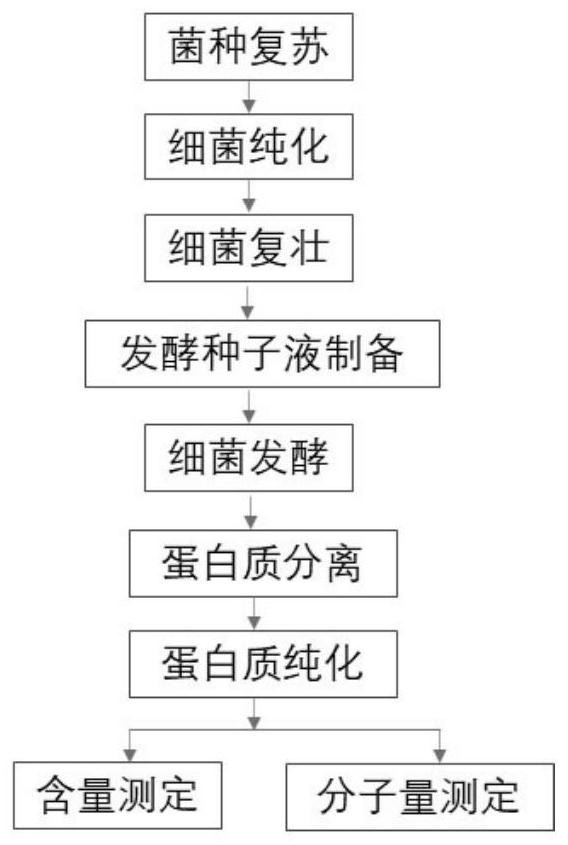
Method for increasing yield of riemerella anatipestifer gelatin liquefying enzyme through batch fermentation
The invention belongs to the technical field of microbial fermentation culture, and discloses a method for increasing the yield of riemerella anatipestifer RA1 gelatin liquefying enzyme by batch fermentation, which comprises the following steps: performing fermentation culture on serum type 1 riemerella anatipestifer by a batch fermentation method to obtain fermentation liquor; centrifuging, precipitating and dialyzing the fermentation liquid, repeatedly washing the recovered target product with a PBS buffer solution to obtain a gelatinase pure product, and verifying that the gelatinase pure product is positive through a gelatin liquefying enzyme liquefaction experiment, so as to obtain a gelatin liquefying enzyme final product; and carrying out molecular weight determination on the gelatin liquefying enzyme end product by adopting an SDS-PAGE method. The fermentation liquor culture specifically comprises the following steps: strain recovery: melting serum type 1 riemerella anatipestifer RA1 stored in a refrigerator, taking bacterial sludge by using an inoculating loop through a sterile operation method, coating a freshly prepared blood plate with the bacterial sludge, and carrying out streak culture to obtain a single colony; rA1 amplification culture: coating a blood plate with RA1 single colonies, and culturing overnight after a large number of bacterial clusters grow on the plate; and culturing RA1 by a batch fermentation method.
Owner:重庆轻工职业学院
Drug-loaded nano-particles with core-shell structure as well as preparation method and application of drug-loaded nano-particles
ActiveCN114699387AImprove physiological activityPromote healingAntibacterial agentsPeptide/protein ingredientsGelatinasesComposite nanoparticles
The invention belongs to the field of biological medicine, and particularly relates to a drug-loaded nanoparticle with a core-shell structure and a preparation method and application thereof.The nanoparticle is of a shell-core structure composed of gelatin nanoparticles, Cypate and fluorescently-labeled antibacterial peptide, A-type gelatin and a photothermal agent Cypate coupled with the A-type gelatin jointly form a nano shell, and Cy3-labeled antibacterial peptide (AMP-Cy3) serves as an embedded core. When the antibacterial peptide exists in a gelatinase microenvironment of an infected part, a gelatin shell is degraded, and the internal fluorescent antibacterial peptide is released in a responsive manner, so that the non-target toxicity of the antibacterial peptide is reduced. In addition, heat generated by the photothermal agent Cypate irradiated by near-infrared light can also provide a good bactericidal synergistic effect for the antibacterial peptide, so that the purpose of selectively and rapidly eradicating bacteria at an infected part is achieved. The composite nanoparticles are simple and convenient to synthesize, high in biocompatibility and excellent in bactericidal effect, and have potential clinical conversion value as an antibacterial agent.
Owner:CHANGZHOU UNIV
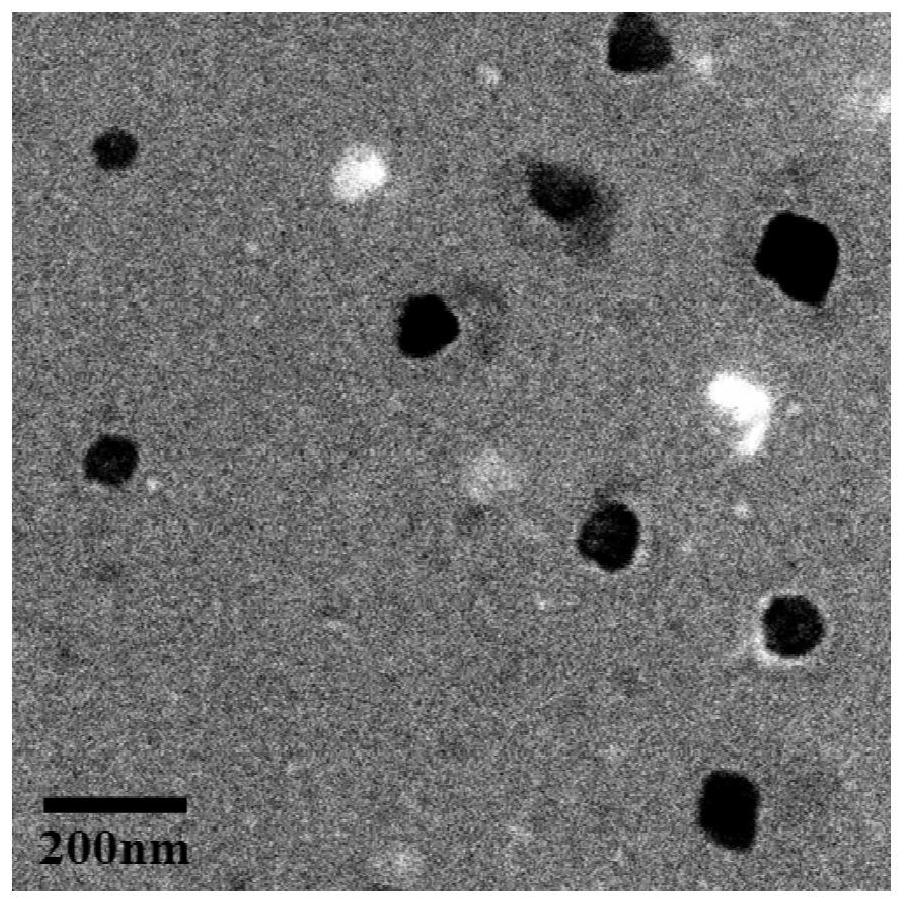
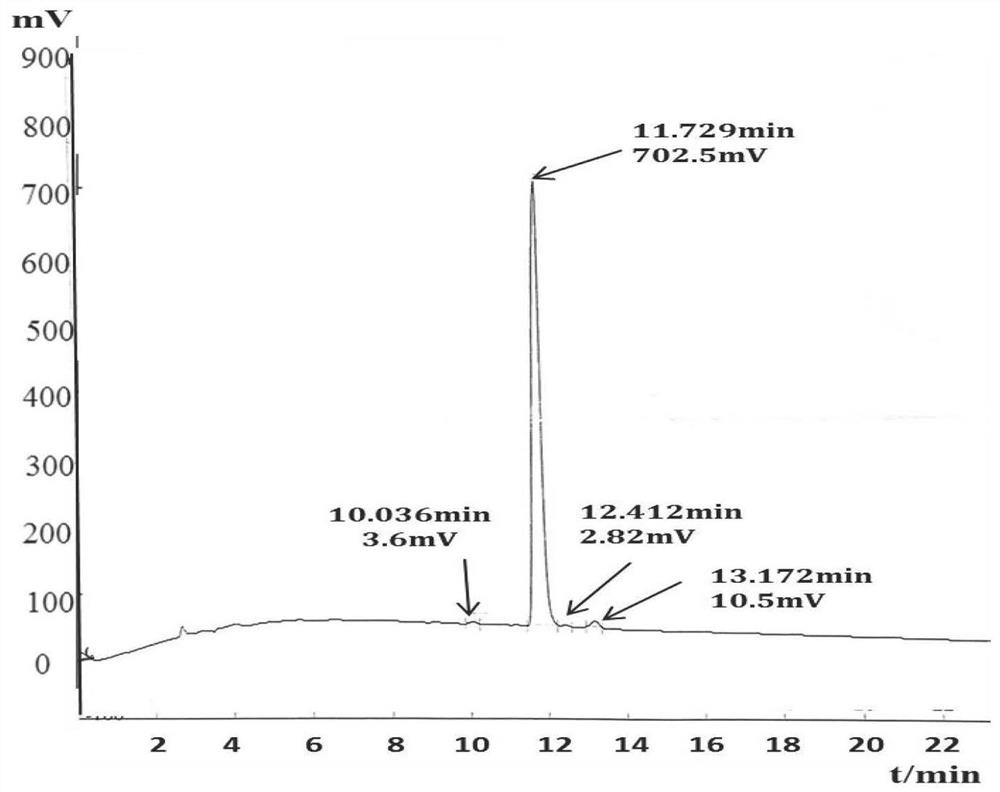
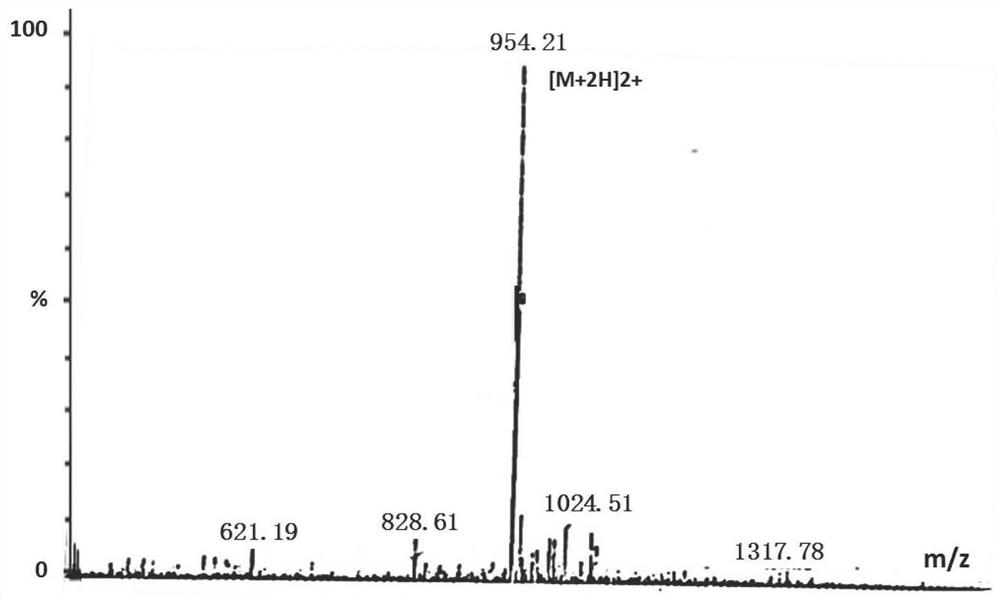
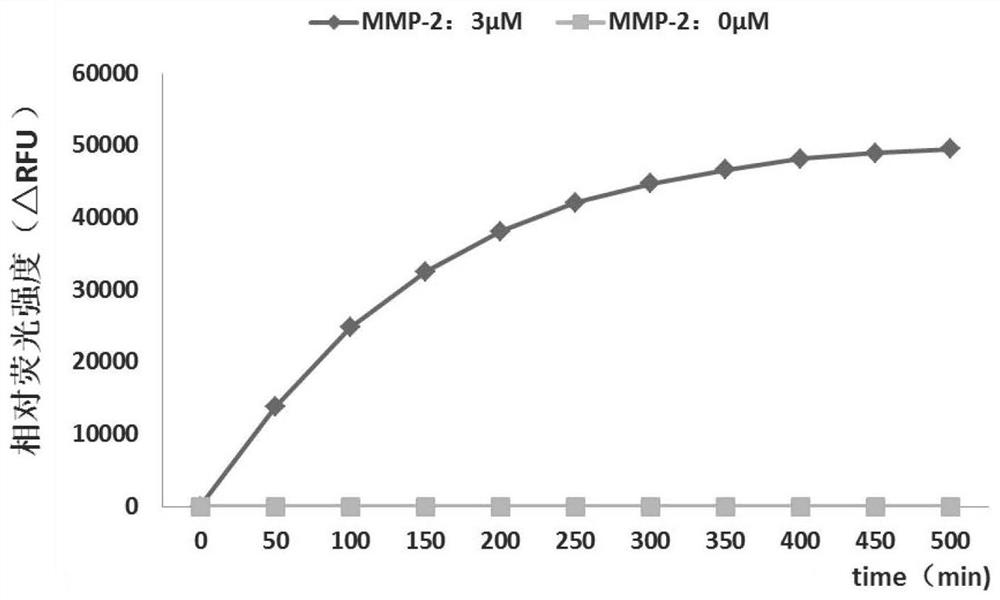
A fluorescent polypeptide substrate for detecting human gelatinase mmp-2 and its application
ActiveCN109750082BImprove stabilityLong storage timeMicrobiological testing/measurementFluorescence/phosphorescenceGelatinasesPeptide substrate
Owner:FIRST AFFILIATED HOSPITAL OF KUNMING MEDICAL UNIV
Prostate-specific antigen isomer, coding gene and application thereof
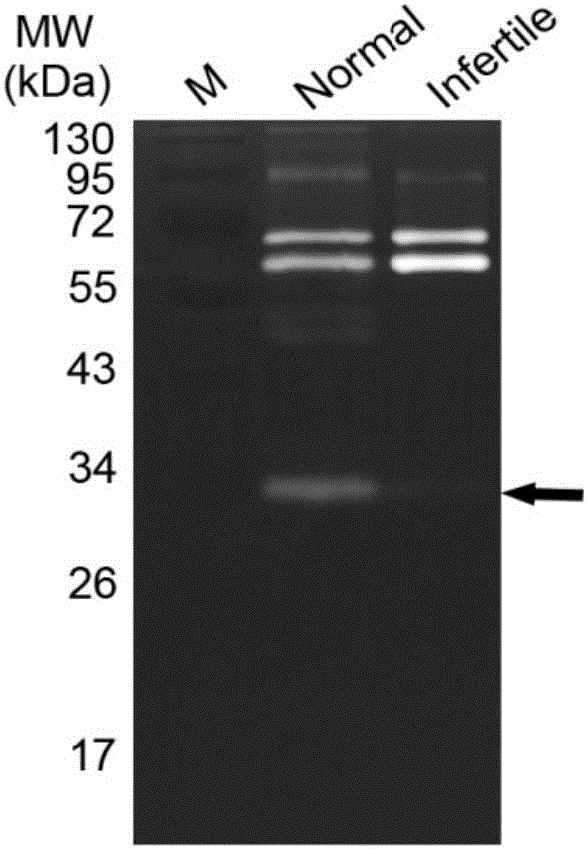
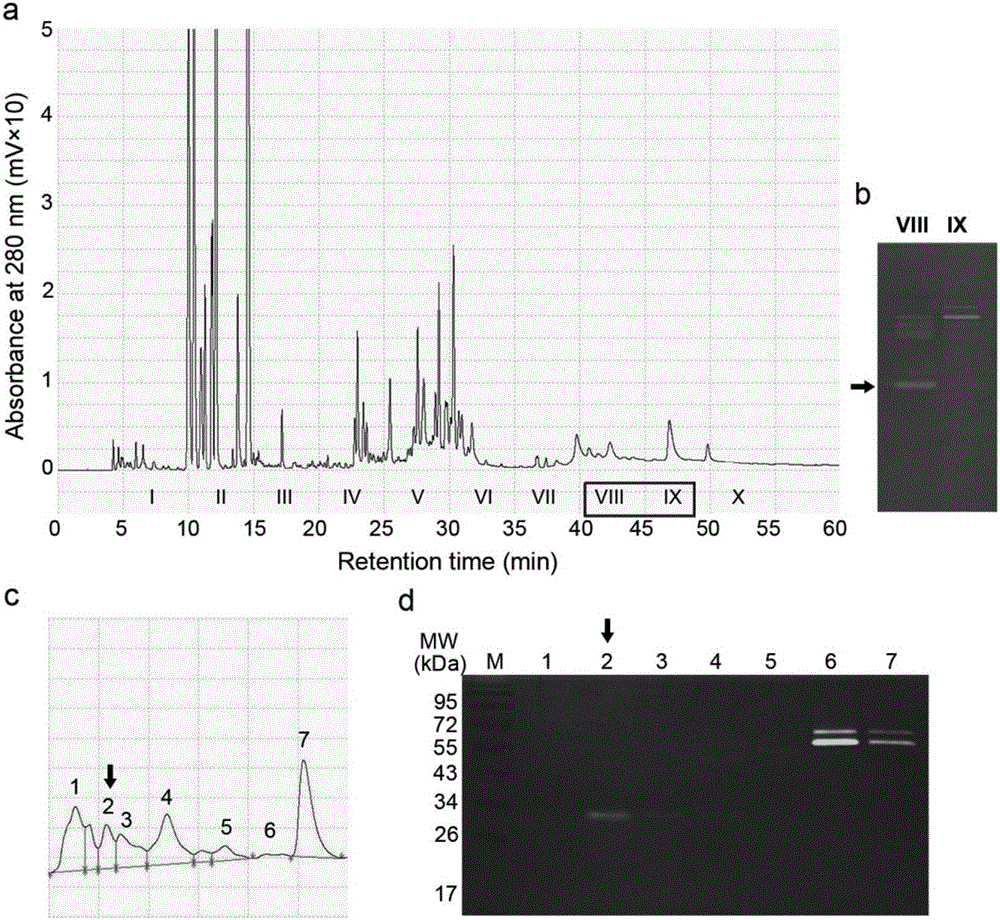
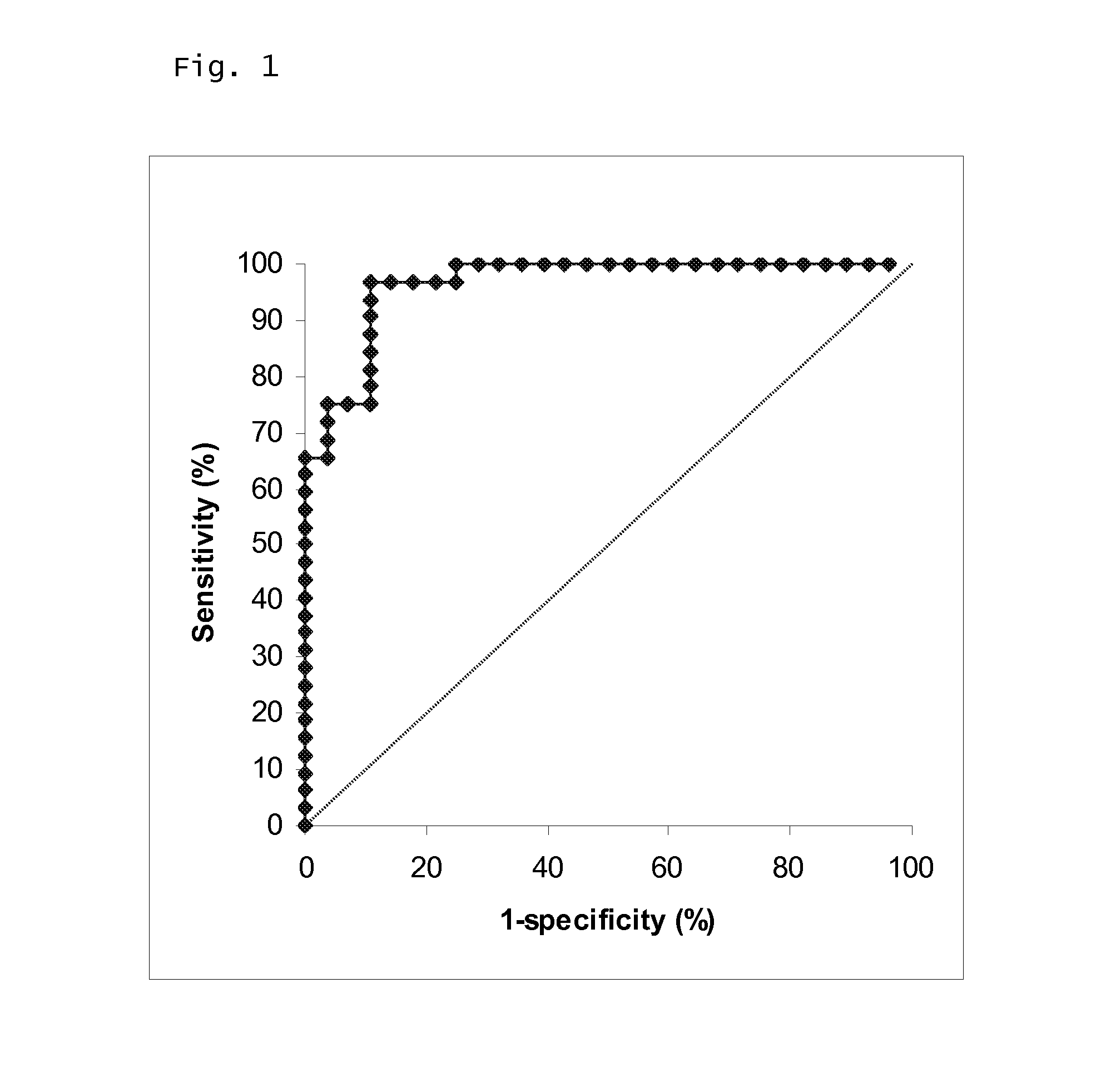
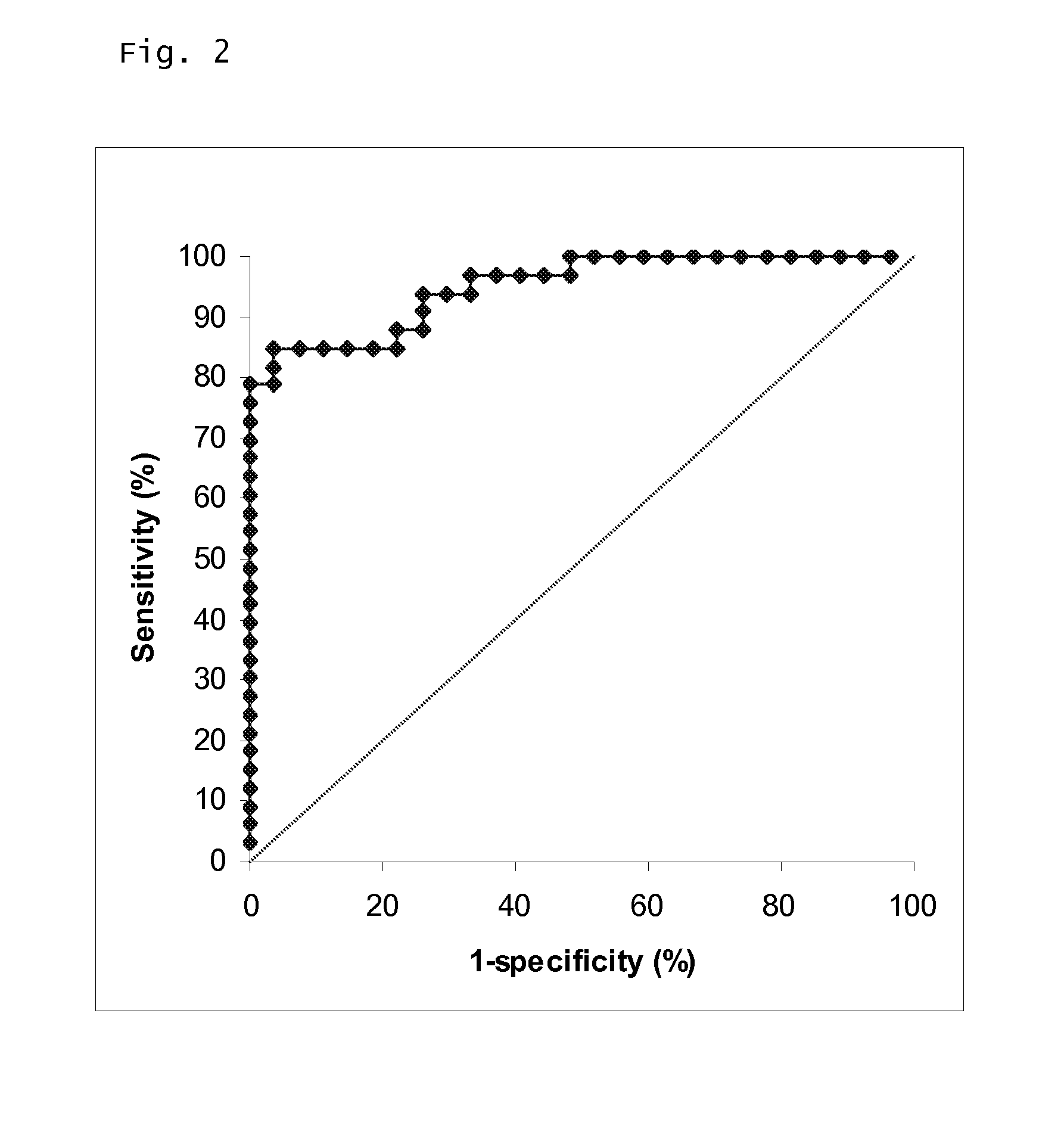
The invention discloses a prostate-specific antigen isomer, a coding gene and application thereof. The protein has obvious gelatinase activity in vitro; and the deficiency and decrease of the protein are key factors for male infertility, and therefore, the protein can be used as a new index for male infertility. The protein-deficient sperm is capable of completing fertilization, and thus, the protein can also be used as an index for selecting artificially assisted reproduction IVF and ICSI technologies. Besides, the protein is also an important drug screening target molecule for male infertility.
Owner:NINGBO BOFENG BIOTECH
Application of oleaginous yeast in biological feed product
PendingCN114766586ALow equipment requirementsEasy to operateAnimal feeding stuffAccessory food factorsBiotechnologyMicrobial oil
The invention relates to the field of biological feeds, in particular to application of oleaginous yeast in a biological feed product, the main components of the application of the oleaginous yeast in the biological feed product comprise an oleaginous yeast culture solution and a catalytic enzyme solution, the oleaginous yeast culture solution is obtained by inoculating oleaginous yeast into a culture solution with the pH value of 3.4-3.9 for culture, the initial concentration of the oleaginous saccharomycetes in the culture solution is (1.2-1.5) * 100 cells / mL, the catalytic enzyme solution is composed of a protease solution and distilled water, the protease solution is acid protease, the PH value is 2-4, and the protease solution is bacillus subtilis secreted gelatinase and casein and can hydrolyze gelatin and casein. The microbial oil is prepared in the culture solution through the oleaginous saccharomycetes, synchronous purification and oil production of the feed by the oleaginous saccharomycetes can be realized, sterile conditions are not required to be created at high temperature and high pressure, and the method has the advantages of low equipment requirement, simplicity in operation and short fermentation reaction time.
Owner:江苏普方生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com