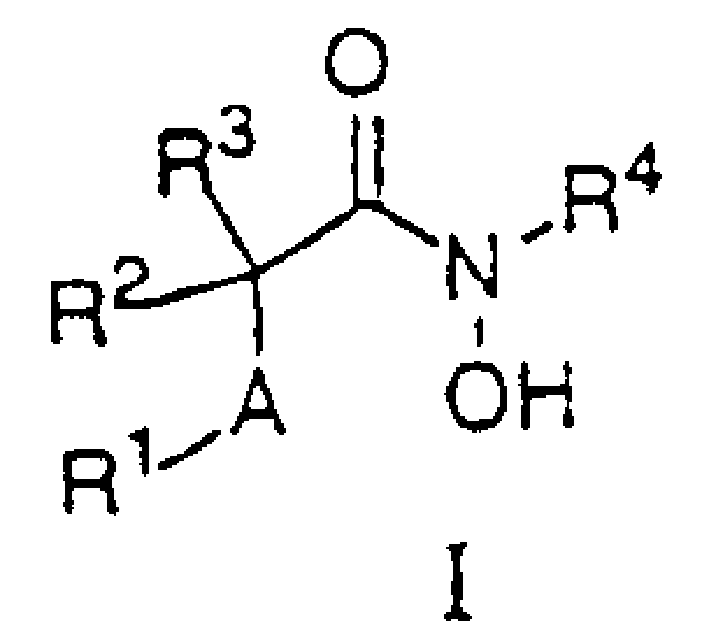
N-hydroxy-2-(alkyl, aryl or heteroaryl sulfanyl, sulfinyl or sulfonyl-3-substituted-alkyl, aryl or heteroarylamides) as matrix metallo protein inhibitors
A heteroaryl, alkyl technology applied to N-hydroxyl-2-(alkyl, aryl or heteroarylsulfanyl, sulfinyl or sulfonyl)-3- as a matrix metalloproteinase inhibitor The field of substituted alkanes, which can solve the problems of bioavailability and pharmacokinetics, limiting clinical effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0225] N-Hydroxy-2-(4-methoxy-phenylsulfanyl)-2-methyl-3-phenyl-propionamide
[0226]To a stirred solution of 4-methoxybenzenethiol (2.8 g, 20 mmol) and anhydrous potassium carbonate (10 g, excess) in anhydrous acetone (100 mL) in a round bottom flask was added 2-bromo-propionic acid ethyl Ester (3.6 g, 20 mmol) and the reaction mixture was heated at reflux for 8 hours with good stirring. Finally, the reaction was cooled and the potassium salt was filtered off, and the above reaction mixture was concentrated. The residue was extracted with chloroform and washed with water and 0.5N sodium hydroxide solution. The organic layer was further washed extensively with water, dried over magnesium sulfate, filtered and concentrated to give ethyl 2-(4-methoxy-phenylsulfanyl)-propionate as a light yellow oil. Yield 4.5 g (94%); MS; 241 (M+H) + .
[0227] To a stirred solution of ethyl 2-(4-methoxy-phenylsulfanyl)-propionate (2.44 g, 10 mmol) in THF (100 ml) at -4°C, bis(trimethyls...
Embodiment 2
[0231] N-Hydroxy-2-(4-methoxy-phenylsulfanyl)-2-phenyl-acetamide
[0232] Ethyl 2-(4-methoxyphenylsulfanyl)-phenylacetate was prepared according to the general procedure described in Example 1. Starting from ethyl [alpha]-bromophenylacetate (7.18 g, 31.4 mmol) and 4-methoxythiophenol (4.4 g, 31.4 mmol), 8.5 g of product were isolated as a pale yellow oil. Yield 90%; MS: 303.1(M+H) + .
[0233] 2-(4-Methoxy-phenylsulfanyl)-phenyl-acetic acid ethyl ester (3.0 g, 10 mmol) was first dissolved in methanol (50 ml) and 10 N sodium hydroxide (20 ml) to prepare 2-( 4-methoxy-phenylsulfanyl)-2-phenylacetic acid. The resulting reaction mixture was worked up as in Example 1. Yield 1.9 g, 70%. Low melting solid. MS: 273(M+H) + .
[0234] Using 2-(4-methoxy-phenylsulfanyl)-2-phenylacetic acid (1.05 g, 3.83 mmol) as raw material, according to the method described in the examples, 154 mg of N-hydroxyl was isolated as a colorless solid -2-(4-Methoxy-phenylsulfanyl)-2-phenyl-ac...
Embodiment 3
[0236] 2-(4-Methoxy-phenylsulfanyl)-2,5-dimethyl-hex-4-enoic acid hydroxyamide
[0237] According to the method in the second paragraph of Example 1, 2-(4-methoxy-phenylsulfanyl)-2,5-dimethyl-hex-4-enoic acid ethyl ester was prepared. Starting from ethyl (4-methoxy-phenylsulfanyl)-propionate (3.5 g, 14.3 mmol) and isoprene bromide (2.25 g, 15 mmol), 2.2 g of product were isolated as an oil . Yield 50%; MS: 310(M+H) + .
[0238] First, ethyl 2-(4-methoxy-phenylsulfanyl)-2,5-dimethyl-hex-4-enoate (2.0 g, 6.4 mmol) was dissolved in methanol (50 ml) and 10N hydrogen 2-(4-Methoxy-phenylsulfanyl)-2,5-dimethyl-hex-4-enoic acid was prepared in sodium oxide (20ml). The resulting reaction mixture was worked up as described in Example 1. Yield of low melting solid 1.9 g, 99%. MS: 28(M+H) + .
[0239] Using 2-(4-methoxy-phenylsulfanyl)-2,5-dimethyl-hex-4-enoic acid (1.67g, 5.8mmol) as raw material, according to the method described in the examples, it was separated to obtain ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com