Patents
Literature
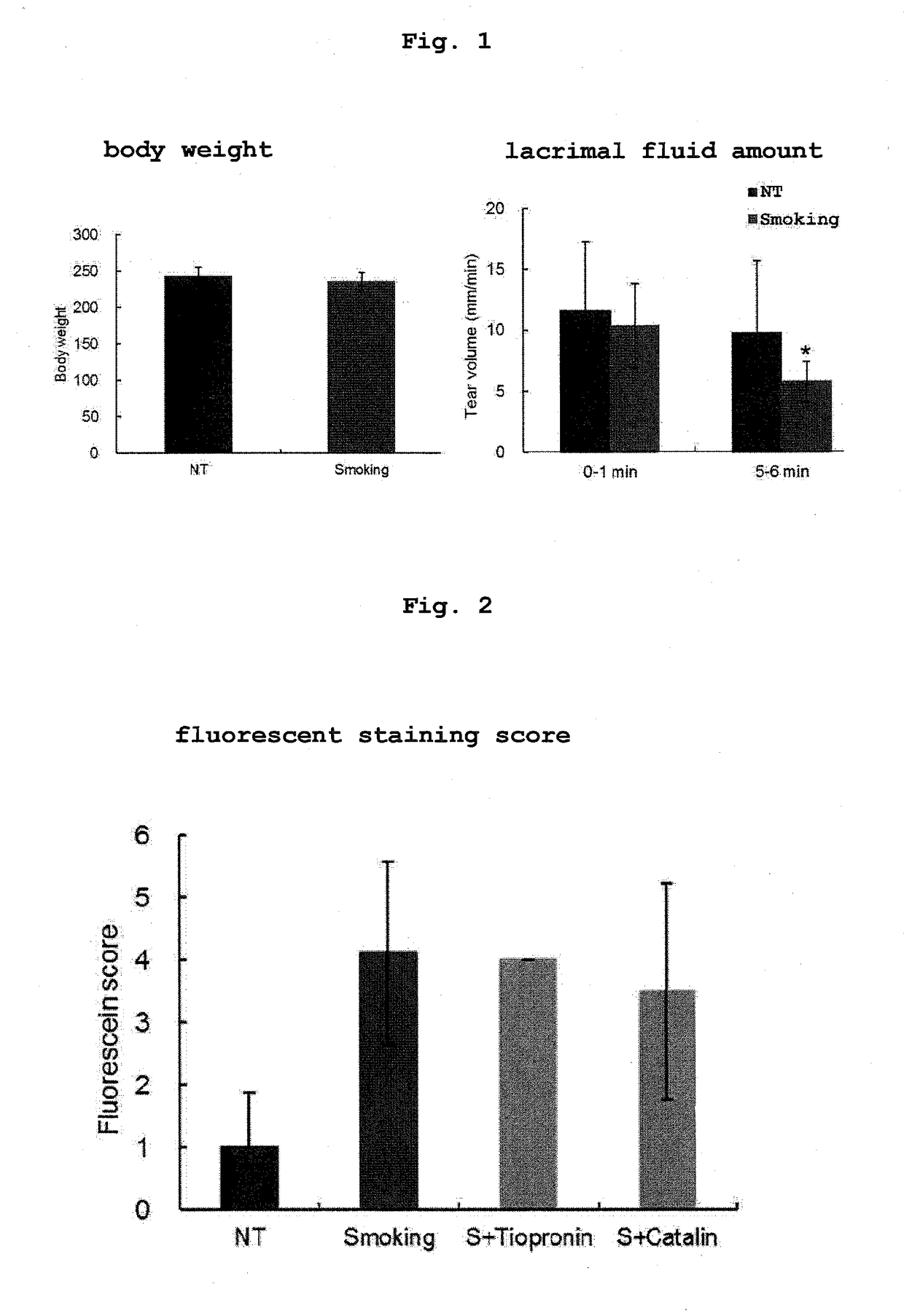
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33 results about "Sulfanyl" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sulfanyl (HS•), also known as the mercapto radical, hydrosulfide radical, or hydridosulfur, is a simple radical molecule consisting of one hydrogen and one sulfur atom. The radical appears in metabolism in organisms as H₂S is detoxified. Sulfanyl is one of the top three sulfur-containing gasses in gas giants such as Jupiter and is very likely to be found in brown dwarfs and cool stars. It was originally discovered by Margaret N. Lewis and John U. White at the University of California in 1939. They observed molecular absorption bands around 325 nm belonging to the system designated by ²Σ⁺ ← ²Πᵢ. They generated the radical by means of a radio frequency discharge in hydrogen sulfide. HS• is formed during the degradation of hydrogen sulfide in the atmosphere of the Earth. This may be a deliberate action to destroy odours or a natural phenomenon.
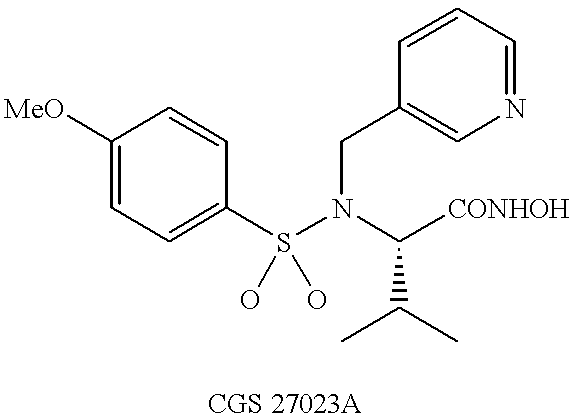
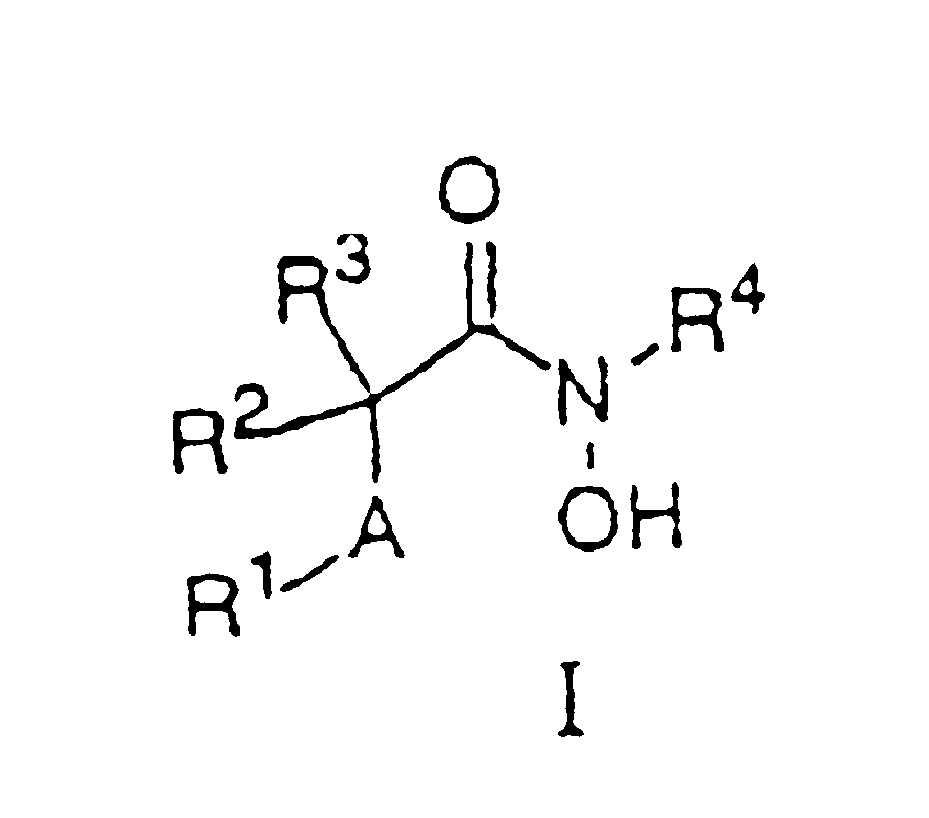
N-hdroxy-2-(alkyl, aryl, or heteroaryl, sulfanyl, sulfinyl or sulfonyl)-3-substituted alkyl, aryl or heteroarylamides as matrix metalloproteinase inhibitors
Matrix metalloproteinases (MMPs) are a group of enzymes that have been implicated in the pathological destruction of connective tissue and basement membranes. These zinc containing endopeptidases consist of several subsets of enzymes including collagenases, stromelysins and gelatinases. TNF-alpha converting enzyme (TACE), a pro-inflammatory cytokine, catalyzes the formation of TNF-alpha from membrane bound TNF-alpha precursor protein. It is expected that small molecule inhibitors of MMPs and TACE therefore have the potential for treating a variety of disease states. The present invention provides low molecular weight, non-peptide inhibitors of matrix metalloproteinases (MMPs) and TNF-alpha converting enzyme (TACE) for the treatment of arthritis, tumor metastasis, tissue ulceration, abnormal wound healing, periodontal disease, bone disease, diabetes (insulin resistance) and HIV infection having the formulawherein R2 and R3 form a heterocyclic ring and A is S, S(O), or S(O)2, and R1 and R4 are defined herein.
Owner:WYETH HOLDINGS CORP
Organic dyes and photoelectric conversion devices
Organic dyes and photoelectric conversion devices are provided. The Organic dye has the structure represented by formula (I), wherein, n is an integral of 2-11; the plurality of X is independent and elected from the group consisting ofand combinations thereof; R, R1, and R2 comprise hydrogen, halogen, C1-18 alkyl group, C1-18 alkoxy group, C3-18 heteroalkyl group, C3-20 aryl group, C3-20 heteroaryl group, C3-20 cycloaliphatic group or C3-20 cycloalkyl group, or R1 is connected to R2 to form a ring having 5-14 members; R3 comprise hydrogen, halogen, nitro group, amino group, C1-18 alkyl group, C1-18 alkoxy group, C1-18 sulfanyl group, C3-18 heteroalkyl group, C3-20 aryl group, C3-20 heteroaryl group, C3-20 cycloaliphatic group or C3-20 cycloalkyl group; and Z is hydrogen, alkali metal, or quaternary ammonium salt.
Owner:IND TECH RES INST
Metal paste and film formation method using the same
InactiveUS20050127332A1Increasing the thicknessLower resistanceConductive materialNon-conductive material with dispersed conductive material2-Ethylhexanoic acidColloidal particle
Owner:TANAKA PRECIOUS METAL IND
Preparation method of high-purity vortioxetine hydrobromide
ActiveCN104725335ARaw materials are easy to getProcess reaction conditions are mildOrganic chemistryChlorobenzene2-Chlorophenol
The invention discloses a preparation method of high-purity vortioxetine hydrobromide. The method comprises the following steps: firstly, synthesizing 2-(2,4-dimethyl phenyl sulfanyl) chlorobenzene from 2-chlorophenol and 2,4-dimethylbenzenethiol; then, adding di(dibenzylideneacetone)palladium, 1,1'-binaphthyl-2,2'-bis(diphenyl phosphine), sodium tert-butoxide, and methylbenzene into a reaction bottle to mix, and adding other materials so as to prepare vortioxetine; and dissolving the prepared vortioxetine by using 14-16 times of ethyl acetate, so that a vortioxetine hydrobromide coarse product is obtained; and finally, purifying the coarse product so as to obtain a vortioxetine hydrobromide fine-product. The method disclosed by the invention is easily-obtained in raw materials, mild in process reaction conditions, high in product yield, high in product purity, and convenient for industrial production. Prepared vortioxetine hydrobromide is white crystalline powder, and the purity is more than 99.5%.
Owner:郑州大明药物科技有限公司
Dual-slurry ammonization granulation compound fertilizer production technology
The invention discloses a dual-slurry ammonization granulation compound fertilizer production technology, which is characterized by high nitrogen content and adjustable available nutrients. The technology comprises the following steps: 1, carrying out secondary dechlorination at low temperature; 2, dissolving phosphorus and mixing slurries; 3, cooling and absorbing; 4, melting and dissolving urea; 5, aminating and granulating; 6, drying and cooling; 7, screening and smashing; and 8, packaging. By using the invention, nitrogen content of sulfanyl compound fertilizers rises to 25% from 13%, recycle ratio reduces to 20-30% from 40-50%, yield of single-pass granulation in a granulator is greatly improved, dechlorination time is shortened to 1h from over 2h by adopting the secondary dechlorination technology, the dechlorination is thorough, and the production efficiency is greatly improved.
Owner:SHANDONG HUAKEN FERTILIZER IMPORT & EXPORT
EBINOL axial chiral compound as well as synthesis method and application thereof
ActiveCN110041174ANovel structureUnique spatial configurationOrganic compound preparationGroup 5/15 element organic compoundsSynthesis methodsPhosphoric acid
The invention belongs to the field of axial chiral compounds, and discloses an EBINOL axial chiral compound. The EBINOL axial chiral compound has the following general formula(the formula is shown inthe description), wherein R<1> and R<2> are respectively and independently selected from hydrogen, alkyl, alkynyl, alkenyl, phenyl, alkoxyl, amine, halogen, trifluoromethyl, cyano group, hydroxyl, aldehyde group, carboxy group, acetyl, ester group, nitro group, amide group, sulfonyl group, sulfonic group, sulfydryl and sulfanyl. The invention further discloses a synthesis method and application ofthe EBINOL axial chiral compound. According to the EBINOL axial chiral compound as well as the synthesis method and application thereof, the axial chiral EBINOL compound with a novel structure is designed, and the skeleton of the EBINOL is constructed by the asymmetric hydrogenation and arylation of chiral acid catalyzed aryl alkyne; the synthesis method has good yield, and excellent E / Z selectivity and enantioselectivity; catalysts, such as chiral phosphoric acid and chiral phosphoramidite, used for asymmetric reactions can be derived from the EBINOL compound.
Owner:SOUTH UNIVERSITY OF SCIENCE AND TECHNOLOGY OF CHINA
Mutated organophosphorus acid anhydrolases and their uses thereof
The invention is directed toward non-wild-type organophosphorus acid anhydrolases having three site mutations, method of production, and method of use to effectively degrade toxic chemical compounds such as (Ethyl({2-[bis(propan-2-yl)amino]ethyl}sulfanyl)(methyl)phosphinate (“VX”).
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Chemically-reactive dyes with sulfanyl and beta-sulfate ethylsulfonyl
InactiveCN101333342AImprove solubilityHigh Alkali SolubilityReactive dyesDyeing processSolubilitySulfate radicals
The invention provides a reactive dye at formula(I)with sulfanyls and beta-sulfate radical ethyl sulphonyls; wherein the definitions of D,R,R1,R2,R3,R4,Q, x and y are detailed in the specification. The reactive dye is of high solubility and high alkali solubility to cellulose base fibre when being used to dye cellulose base fibre and is easy to wash with water; therefore, the reactive dye is applicable in cold pressure dyeing, continuous dyeing, printing and dyeing, and digital printing.
Owner:ETHICAL INT WAREHOUSING TRADING SHANGHAI
Photosensitive monomer, liquid crystal material having the same, liquid crystal panel and method for manufacturing thereof by incorporating the same, and electro-optical device and method for manufacturing thereof by incorporating the same
A photosensitive monomer of formula.“L1”, “L2”, “L3”, “L4”, “L5”, “L6” are selected from hydrogen, fluorine, chlorine, cyano, alkyl, alkylcarbonyl, alkoxycarbonyl, and alkylcarbonyloxy having 1 to 7 carbon atoms, in which one or more hydrogen atoms may be substituted by fluorine or chlorine.“R1”, “R2”, “R3” and “R4” are selected from hydrogen, fluorine, chlorine, cyano, thiocyanato, pentafluoro sulfanyl, nitrite, straight-chained alkyl / branched alkyl, and a “Z-Sp-P” group. At least one of “R1”, “R2”, “R3” and “R4” is “Z-Sp-P” group. “Z” is selected from oxygen, sulfur, methyoxy, carbonyl, caroboxyl, carbamoyl, methylthio, ethenylcarbonyl, carbonylethenyl, and a single bond. “Sp” is selected from straight-chained alkyl or branched alkyl and a single bond. “P” comprises a polymerizable group.
Owner:AU OPTRONICS CORP
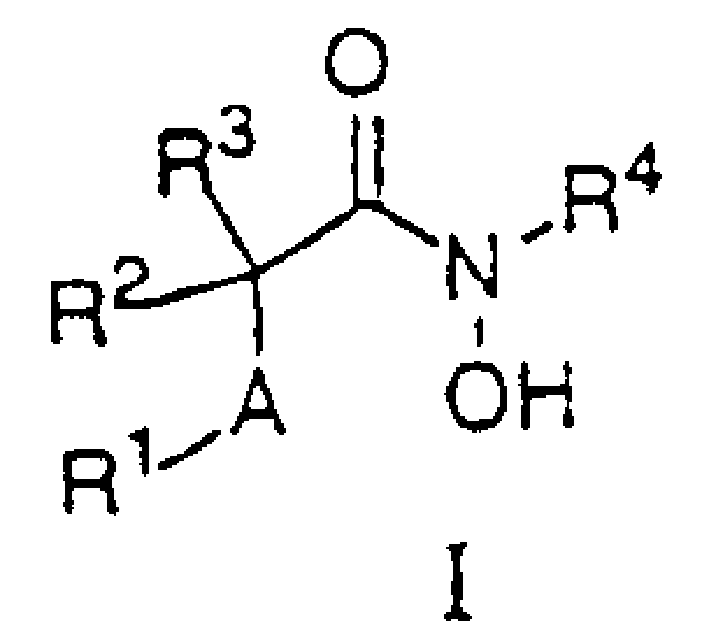
N-hydroxy-2-(alkyl, aryl or heteroaryl sulfanyl, sulfinyl or sulfonyl-3-substituted-alkyl, aryl or heteroarylamides) as matrix metallo protein inhibitors
Matrix metalloproteinases (MMPs) are a group of enzymes that have been implicated in the pathological destruction of connective tissue and basement membranes. These zinc containing endopeptidases consist of several subsets of enzymes including collagenases, stromelysins and gelatinases. TNF-alpha converting enzyme (TACE), a pro-inflammatory cytokine, catalyzes the formation of TNF-alpha from membrane bound TNF-alpha precursor protein. It is expected that small molecule inhibitors of MMPs and TACE therefore have the potential for treating a variety of disease states. The present invention provides low molecular weight, non-peptide inhibitors of matrix metalloproteinases (MMPs) and TNF-alpha converting enzyme (TACE) for the treatment of arthritis, tumor metastasis, tissue ulceration, abnormal wound healing, periodontal disease, bone disease, diabetes (insulin resistance) and HIV infection having the formula wherein R2 and R3 form a heterocyclic ring and A is S, S(O), or S(O)2, and R1 and R4 are defined herein.
Owner:WYETH HOLDINGS CORP
N-hydroxy-2-(alkyl, aryl or heteroaryl sulfanyl, sulfinyl or sulfonyl-3-substituted-alkyl, aryl or heteroarylamides) as matrix metallo protein inhibitors
Matrix metalloproteinases (MMPs) are a group of enzymes that have been implicated in the pathological destruction of connective tissue and basement membranes. These zinc containing endopeptidases consist of several subsets of enzymes including collagenases, stromelysins and gelatinases. TNF-alpha converting enzyme (TACE), a pro-inflammatory cytokine, catalyzes the formation of TNF-alpha from membrane bound TNF-alpha precursor protein. It is expected that small molecule inhibitors of MMPs and TACE therefore have the potential for treating a variety of disease states. The present invention provides low molecular weight, non-peptide inhibitors of matrix metalloproteinases (MMPs) and TNF-alpha converting enzyme (TACE) for the treatment of arthritis, tumor metastasis, tissue ulceration, abnormal wound healing, periodontal disease, bone disease, diabetes (insulin resistance) and HIV infection having the formula wherein R2 and R3 form a heterocyclic ring and A is S, S(O), or S(O)2, and R1 and R4 are defined herein.
Owner:WYETH HOLDINGS CORP
Crystalline form of vortioxetine hydrobromide
ActiveUS9499504B2Suitable for preparationOrganic active ingredientsNervous disorderHydrobromideCrystallography
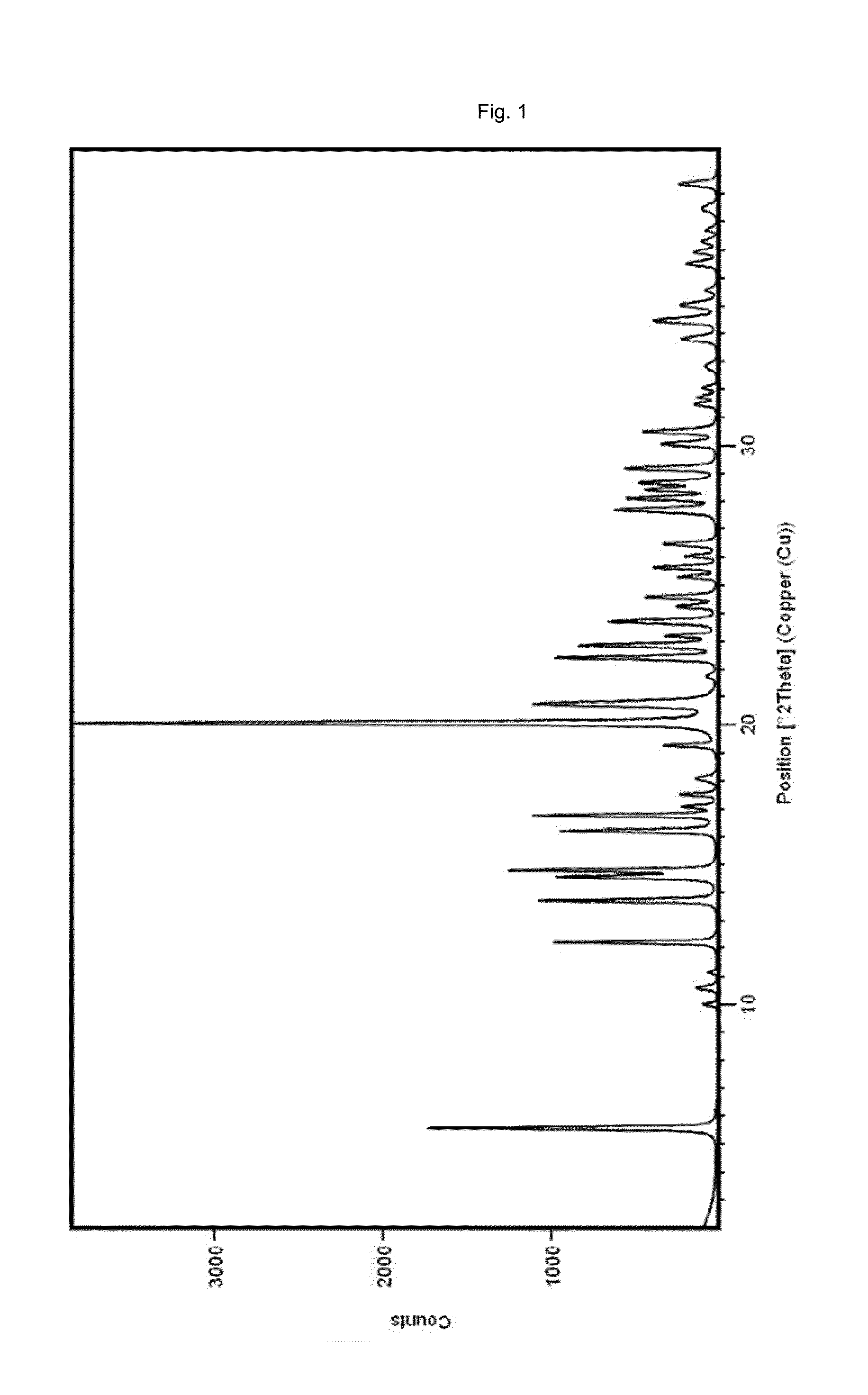
The present invention is directed to a crystalline compound comprising a hydrobromide acid (HBr) salt of a compound of formula (I) (1-{2-[(2,4-dimethylphenyl)sulfanyl]phenyl}piperazine, INN: vortioxetine), having an XRPD pattern with characteristic peaks (expressed in 2θ±0.2° 2θ (CuKα radiation)) at 5.5°, 14.8°, 16.7° and 20.0° and processes for obtaining the same.
Owner:SANDOZ LTD
5-acylsulfanyl-histidine compounds as precursors of the corresponding 5-sulfanylhistidines and their disulfides
The invention relates to a compound of the 5-acylsulfanyl-histidine type and the derivatives thereof, of general formula (I), wherein R1 to R3=H, alkyl, especially CH3; R4=H, alkyl, especially CH3, alkyle(C=0), substituted alkyl (C═O), aryl (C═O); β-alanyl (H2NCH2CH2 (C═O); α-amino-acyl; R5=alkyl, especially methyl, phenyl. The invention also relates to the use of said compound for producing compounds of the 5-sulfanyl-histidine type and the derivatives thereof, in addition to corresponding disulfides; and to the various methods for the production thereof.
Owner:TETRAHEDRON SAS
Method for preparing N-(substituent) benzothiazine-4-ketone without metal participation
The invention belongs to the field of fine chemical engineering, and relates to a method for preparing N-(substituent) benzothiazine-4-ketone without metal participation. Comprising the following steps: sequentially adding N-(substituted)-2-sulfur alkyl benzamide, 1-chloromethyl-4-fluoro-1, 4-diazabicyclo [2.2. 2] octane bis (tetrafluoroborate) salt, sodium iodide and hydroiodic acid into a sealed tube containing a reaction solvent acetonitrile, heating, violently stirring and reacting to obtain the N-(substituted) benzothiazine-4-ketone. The sulfanyl group in the reaction substrate is subjected to C-S bond breakage firstly, then the chloromethyl group in the additive is transferred into the substrate, construction of C-S and C-N is achieved at the same time, and construction of the benzothiazine-4-ketone is achieved. The method has the characteristics of greenness and high efficiency.
Owner:CHANGZHOU UNIV
Pyridin-2yl sulfanyl acid esters and process for the preparation thereof
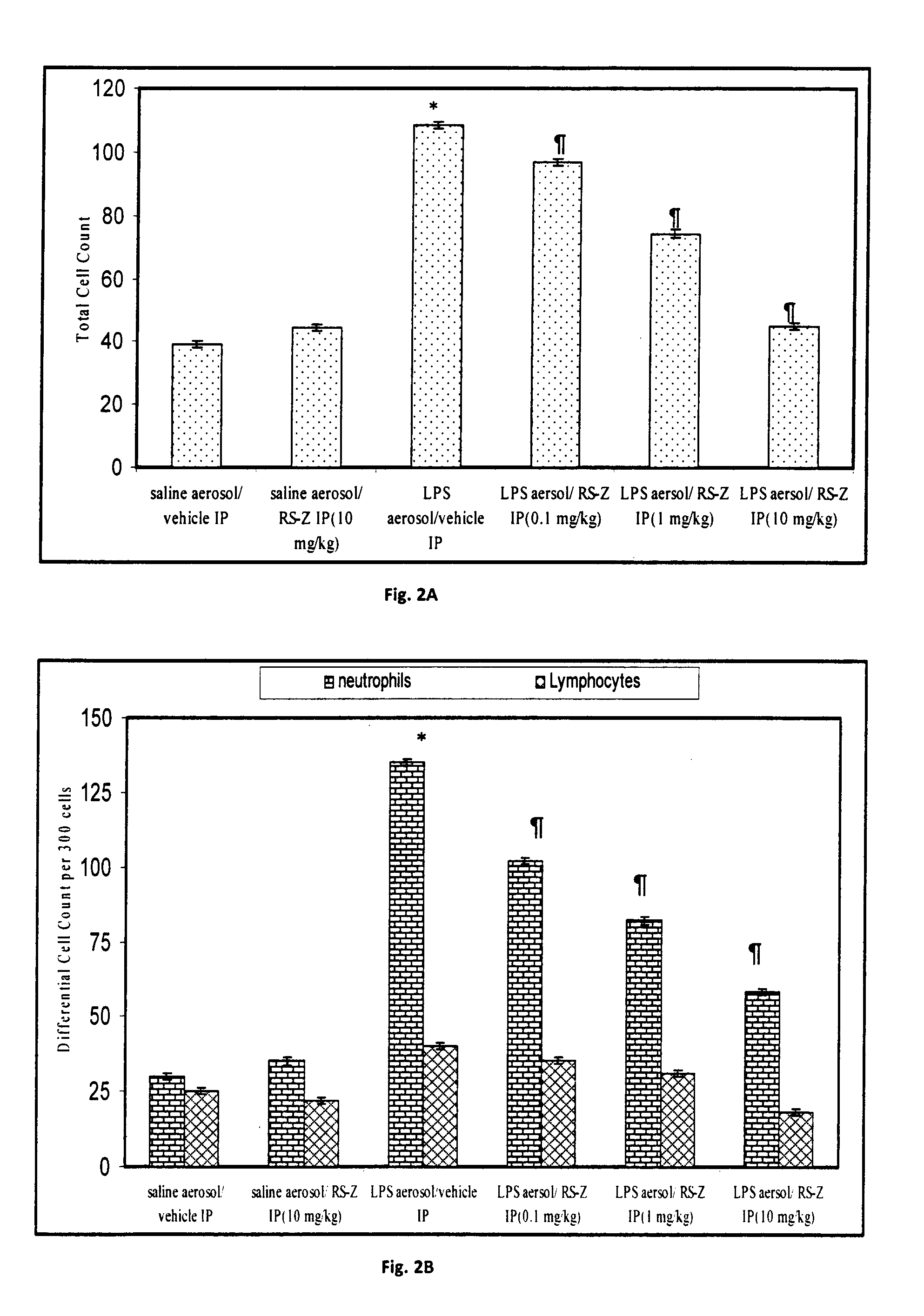
The present invention relates to Pyridin-2-yl sulfanyl acid ester compounds having antiinflammatory properties. The present invention particularly relates to novel anti-inflammatory heterocyclic acid esters of Pyridin-2-yl sulfanyl having the structure of general formula 1 which have been screened for their antiinflammatory activity with respect to inhibition of adhesion of neutrophils, isolated from human peripheral blood, onto the surface of human umbilical vein endothelial cells (HU-VEC) as a result of inhibition of the cytokine-stimulated expression of cell adhesion molecule ICAM-1. The compound RS—Z, 3-(Pyridin-2-yl sulfanyl)-propionic acid pentyl ester (structure 1a, R1=H, R2=H, R3=CH2-COOC5H11) was found to be most effective for ICAM-1 and neutrophil adhesion inhibition and was found to effectively alleviate inflammation mediated by excessive leukocyte infiltration leading to inflammatory disorders or like conditions, such as acute lung injury and acute respiratory distress syndrome in mice.
Owner:COUNCIL OF SCI & IND RES
Functionalized zirconium-based metal organic cage as well as preparation method and application thereof
The invention provides a functionalized zirconium-based metal organic cage as well as a preparation method and application thereof. The preparation method of the functionalized zirconium-based metal organic cage comprises the following steps: S1, dissolving (S, S)-2, 5-bis-(2-hydroxypropyl sulfanyl)-terephthalic acid and zircon salt in an organic solvent to obtain a mixture; s2, the mixture is subjected to a reaction through a solvothermal method, and a reaction mixture is obtained; s3, the reaction mixture is cooled, crystallized, washed and subjected to solvent exchange, and the functionalized zirconium-based metal organic cage is obtained. The functionalized zirconium-based metal organic cage is simple in preparation method, good in stability, high in detection efficiency, high in sensitivity and strong in anti-interference performance, can realize specific recognition of various environmental pollutants including sulfonamides, nitrofurans and toxic heavy metals under different concentrations, and has good universality.
Owner:GUANGZHOU UNIVERSITY
Pyridin-2yl sulfanyl acid esters and process for the preparation thereof
InactiveUS20130131350A1Reduction of neutrophil influxOrganic chemistryAntipyreticPropanoic acidCell adhesion
The present invention relates to Pyridin-2-yl sulfanyl acid ester compounds having antiinflammatory properties. The present invention particularly relates to novel anti-inflammatory heterocyclic acid esters of Pyridin-2-yl sulfanyl having the structure of general formula 1 which have been screened for their antiinflammatory activity with respect to inhibition of adhesion of neutrophils, isolated from human peripheral blood, onto the surface of human umbilical vein endothelial cells (HU-VEC) as a result of inhibition of the cytokine-stimulated expression of cell adhesion molecule ICAM-1. The compound RS—Z, 3-(Pyridin-2-yl sulfanyl)-propionic acid pentyl ester (structure 1a, R1=H, R2=H, R3=CH2-COOC5H11) was found to be most effective for ICAM-1 and neutrophil adhesion inhibition and was found to effectively alleviate inflammation mediated by excessive leukocyte infiltration leading to inflammatory disorders or like conditions, such as acute lung injury and acute respiratory distress syndrome in mice.
Owner:COUNCIL OF SCI & IND RES
Alkyl/sulfanyl N-heterocyclic terminal D (A-Ar) type 2 conjugate compound as well as preparation method and application thereof
ActiveCN110003245AImprove solubilityHigh carrier mobilityOrganic chemistrySolid-state devicesHeterojunctionOrganic solar cell
The invention belongs to the technical field of organic photoelectric materials and particularly relates to an alkyl / sulfanyl N-heterocyclic terminal D (A-Ar) type 2 conjugate compound as well as a preparation method and application thereof. A conjugate organic small molecule photovoltaic donor material with a D (A-Ar) type 2 structure is obtained by taking 3,3'-difluoro-2,2'-bisthiophene donor unit as a center core and carrying out alkylation reaction, electrophilic substitution bromination reaction and stille coupling reaction. The D (A-Ar) type 2 conjugate compound disclosed by the invention has the advantages of good solubility and stability, wide spectral absorption range, high light absorption capacity and proper electrochemical energy level and capability of being expected to be used in donor materials of organic solar cells. The maximum energy conversion efficiency and short-circuit current of a single-layer device body heterojunction solar cell with Fullerene PC71BM as a receptor respectively reach 8.91 percent and 16.75mA cm<-2>.
Owner:CHANGZHOU UNIV
Novel Crystalline Form Of Vortioxetine Hydrobromide
ActiveUS20150266841A1Suitable for preparationOrganic active ingredientsNervous disorderHydrobromideCrystallography
The present invention is directed to a crystalline compound comprising a hydrobromide acid (HBr) salt of a compound of formula (I) (1-{2[2,4-dimethylphenyl)sulfanyl]phenyl}piperazine, INN: vortioxetine), having an XRPD pattern with characteristic peaks (expressed in 2θ±0.2° 2θ (CuKα radiation)) at 5.5°, 14.8°, 16.7° and 20.0° and processes for obtaining the same.
Owner:SANDOZ AG
Phacosclerosis inhibitor
InactiveUS20170246176A1Effective treatmentOrganic active ingredientsSenses disorderHalogenAlkoxy group
The present invention provides a crystalline lens hardening inhibitor, containing a compound represented by the formula (I):wherein R1-R4 are the same or different and each is a hydrogen atom, a halogen atom, a hydroxy group, a sulfanyl group, a lower alkyl group, a lower acyl group, a lower alkoxy group, a carboxyl group, a carbamoyl group or a carbonylamino acid group, or a salt thereof, and / or a compound represented by the formula (II):wherein R is a hydrogen atom, or a lower alkyl group optionally substituted by an amino group, a hydroxy group, a sulfanyl group or a carboxyl group, and A is a lower alkylene group, or a salt thereof.
Owner:TSUBOTA LAB
Benzodiazaborole derivative and organic light-emitting diodes using the same
InactiveCN109867690AImprove thermal stabilityGood optical effectSolid-state devicesSemiconductor/solid-state device manufacturingHydrogen atomAlkoxy group
Owner:YUAN ZE UNIV
Method for producing 4-pentafluoride-sulfanyl-benzoylguanidines
The invention relates to a method for producing 4-pentafluoride-sulfanyl-benzoylguanidines of formula (I) wherein groups from R1 to R4 correspond to meanings given in claims. The compounds of the formula (I) constitute NHE1 inhibitors and can be used for curing cardiovascular diseases.
Owner:SANOFI AVENTIS DEUT GMBH
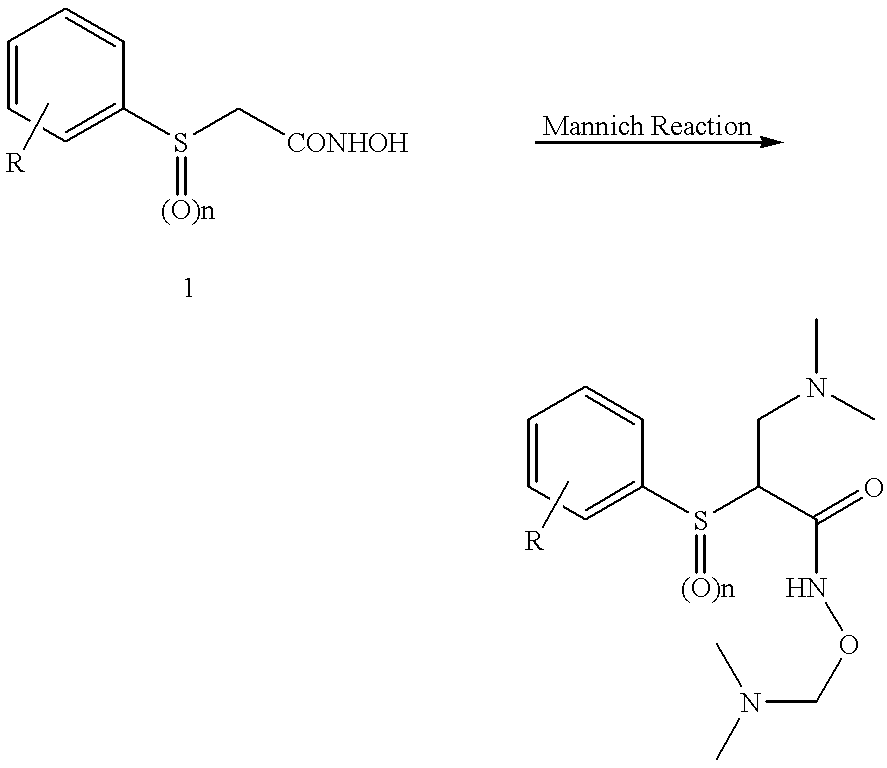
Method of producing n-[(2S)-sulfanyl-4-(1,5,5-trimethylhydantoinyl)butanoyl]-L-leucyl-L-tert-leucine N-methylamide and intermediate thereof
InactiveUS6960567B2Increase productionReduce presencePeptide/protein ingredientsPeptide sourcesPhenacylL-tert-leucine
Process for producing N-[(2S)-sulfanyl-4-(1,5,5-trimethylhydantoinyl) butanoyl]-L-leucyl-L-tert-leucine N-methylamide by forming an intermediate compound N-[(2S)-thiobenzoyl-4-(1,5,5-trimethylhydantoinyl)butanoyl]-L-leucyl-L-tert-leucine N-methylamide by an improved process that substantially reduces the presence of contaminants in the reaction mixture.
Owner:BRISTOL MYERS SQUIBB CO
Method for producing 4-pentafluoride-sulfanyl-benzoylguanidines
Owner:SANOFI AVENTIS DEUT GMBH
A Class of Alkyl/Sulfanyl Aza-Heteroaromatic Terminations of d(a-ar) 2 Type conjugated compound and its preparation method and application
ActiveCN110003245BImprove solubilityHigh carrier mobilityOrganic chemistrySolid-state devicesOrganic solar cellHeterojunction
The invention belongs to the technical field of organic optoelectronic materials, and in particular relates to a D(A-Ar) at the end of a class of alkyl / sulfanyl nitrogen heteroaromatic rings 2 Type conjugated compound and preparation method and application thereof. Taking 3,3'-difluoro-2,2'-bisthiophene donor unit as the central nucleus, through alkylation reaction, electrophilic substitution on bromine reaction and stille coupling reaction, D(A-Ar) was obtained 2 Conjugated Organic Small Molecule Photovoltaic Donor Materials with Type Structure. D(A-Ar) according to the present invention 2 The type conjugated compounds have good solubility and stability, wide spectral absorption range, strong light absorption ability and suitable electrochemical energy level, and are expected to be used as donor materials for organic solar cells. with fullerene PC 71 The maximum energy conversion efficiency and short-circuit current of single-layer device bulk heterojunction solar cells with BM as acceptor are as high as 8.91% and 16.75 mA cm, respectively ‑2 .
Owner:CHANGZHOU UNIV
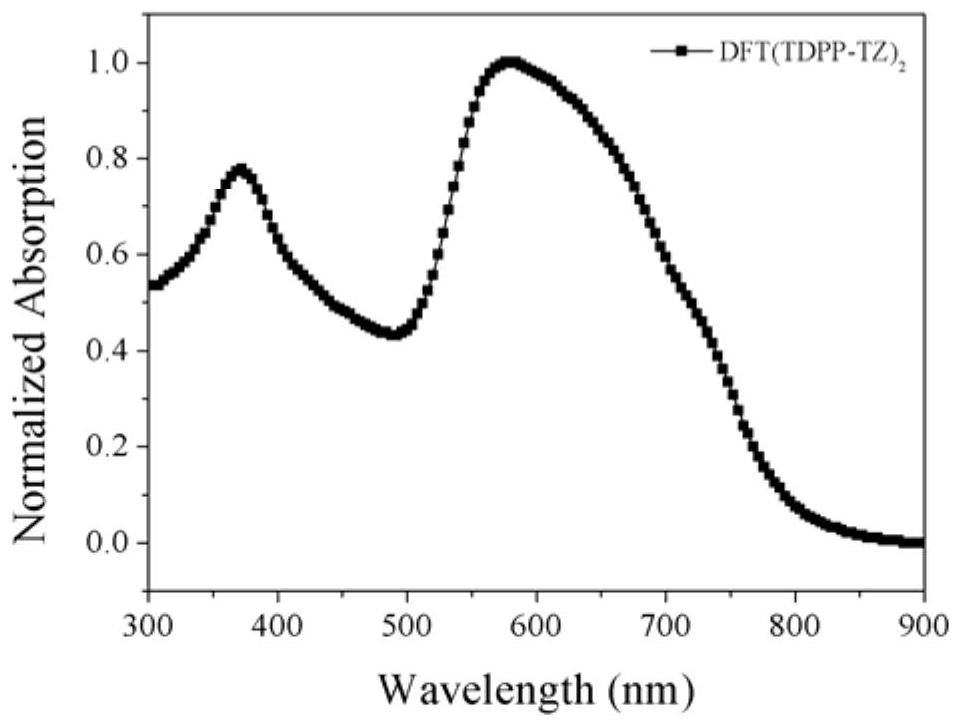
Process for the preparation of sodium salt of 1-(((1(r)-(3-(2-(7-chloro-2-quinolinyl)-ethenyl)phenyl)-3-(2-(1-hydroxy-1-methylethyl)phenyl)propyl)sulfanyl)methyl)cyclopropaneacetic acid
InactiveUS20100069641A1Reduction in yieldReduce process yieldOrganic chemistryAcetic acidTert-Butylamine
A novel process for the preparation of a sodium salt of 1-(((1(R)-(3-(2-(7-chloro-2-quinolinyl)-ethenyl)phenyl)-3-(2-(1-hydroxy-1-methyl-ethyl)phenyl)propyl)sulfanyl)methyl)cyclopropaneacetic acid, comprising: a) reacting a compound of Formula 1 with methanesulfonyl chloride in the presence of a tertiary amine to yield a crude solution of a compound of Formula 2; b) filtering the crude solution of compound of Formula 2 obtained in a) to remove solid amine hydrochloride, reacting the filtrate without isolation or further purification with a compound of Formula 3, and isolating 1-(((1(R)-(3-(2-(7-chloro-2-quinolinyl)-ethenyl)phenyl)-3-(2-(1-hydroxy-1-methylethyl)phenyl)propyl)sulfanyl)methyl)cyclopropaneacetic acid; c) reacting the 1-(((1(R)-(3-(2-(7-chloro-2-quinolinyl)-ethenyl)phenyl)-3-(2-(1-hydroxy-1-methylethyl)phenyl)propyl)sulfanyl)methyl)cyclopropaneacetic acid isolated in b) with tert-butylamine to yield a compound of formula 4; d) isolating and purifying the compound of formula 4; and e) converting the compound of formula 4 to a compound of formula 5.
Owner:ZAKLADY FARMACEUTYCZNE POLPHARMA SA
Pyrrolidine derivatives as oxytocin antagonists
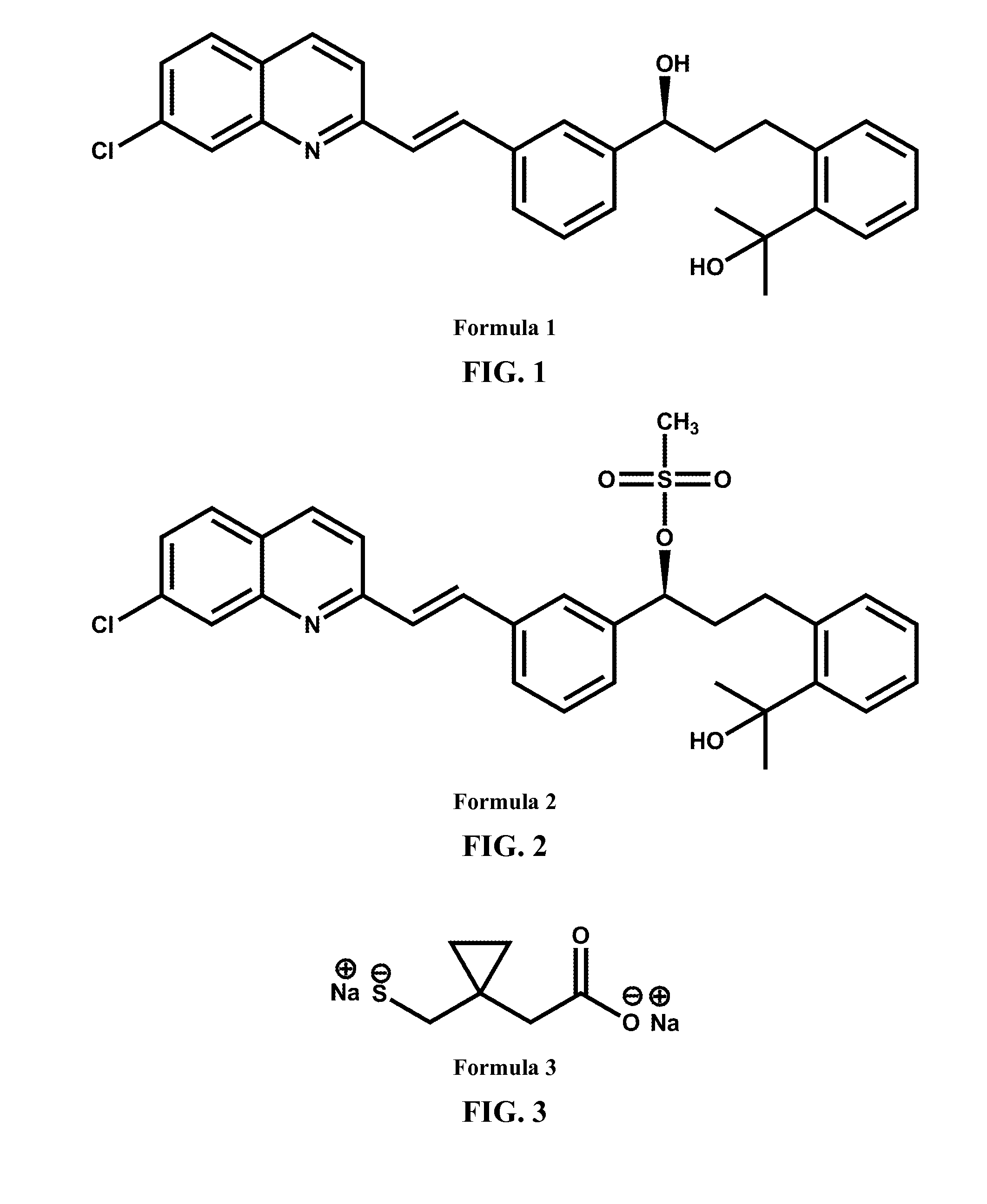
The present invention relates to novel pyrrolidine derivatives of Formula (I), its geometrical isomers, its optically active forms as enantiomers, diastercomers, mixtures of these and its racemate forms, as well as salts thereof, wherein: R1is selected from the group comprising or consisting of H and C1-C6-alkyl; R2 is selected from the group comprising or consisting of hydrogen, C1-C6-alkyl, C1-C6 alkyl aryl, heteroaryl, C1-C6 alkyl heteroaryl, C2-C6-alkenyl, C2-C6-alkenyl aryl, C2-C6 alkenyl heteroaryl, C2-C6-alkynyl, C2-C6-alkynyl aryl, C2C6-alkynyl heteroaryl, C3-C8 cycloalkyl, heterocycloalkyl, CI-C6-alkyl cycloallcyl, CI-C6-alkyl heterocycloalkyl, CIC6 alkyl carboxy, acyl, C1-C6-alkyl acyl, C1-C6-alkyl acyloxy, C1-C6-alkyl alkoxy, alkoxycarbonyl, C1-C6-alkyl alkoxycarbonyl, aminocarbonyl, C1-C6-alkyl aminocarbonyl, C1-C6-alkyl acylarnino, C1-C6-alkyl ureido, amino, C1-C6-alkyl amino, sulfonyloxy, C1-C6 alkyl sulfonyloxy, sulfonyl, C1-C6-alkyl sulfonyl, sulfinyl, C1-C6-alkyl sulfinyl, C1-C6alkyl sulfanyl, C1-C6-alkyl sulfonylamino.
Owner:MERCK SERONO SA
Dual-slurry ammonization granulation compound fertilizer production technology
The invention discloses a dual-slurry ammonization granulation compound fertilizer production technology, which is characterized by high nitrogen content and adjustable available nutrients. The technology comprises the following steps: 1, carrying out secondary dechlorination at low temperature; 2, dissolving phosphorus and mixing slurries; 3, cooling and absorbing; 4, melting and dissolving urea; 5, aminating and granulating; 6, drying and cooling; 7, screening and smashing; and 8, packaging. By using the invention, nitrogen content of sulfanyl compound fertilizers rises to 25% from 13%, recycle ratio reduces to 20-30% from 40-50%, yield of single-pass granulation in a granulator is greatly improved, dechlorination time is shortened to 1h from over 2h by adopting the secondary dechlorination technology, the dechlorination is thorough, and the production efficiency is greatly improved.
Owner:SHANDONG HUAKEN FERTILIZER IMPORT & EXPORT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com















































![Method of producing n-[(2S)-sulfanyl-4-(1,5,5-trimethylhydantoinyl)butanoyl]-L-leucyl-L-tert-leucine N-methylamide and intermediate thereof Method of producing n-[(2S)-sulfanyl-4-(1,5,5-trimethylhydantoinyl)butanoyl]-L-leucyl-L-tert-leucine N-methylamide and intermediate thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/148bc1e9-1982-46da-9368-ff4d189dc242/US06960567-20051101-C00001.png)
![Method of producing n-[(2S)-sulfanyl-4-(1,5,5-trimethylhydantoinyl)butanoyl]-L-leucyl-L-tert-leucine N-methylamide and intermediate thereof Method of producing n-[(2S)-sulfanyl-4-(1,5,5-trimethylhydantoinyl)butanoyl]-L-leucyl-L-tert-leucine N-methylamide and intermediate thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/148bc1e9-1982-46da-9368-ff4d189dc242/US06960567-20051101-C00002.png)
![Method of producing n-[(2S)-sulfanyl-4-(1,5,5-trimethylhydantoinyl)butanoyl]-L-leucyl-L-tert-leucine N-methylamide and intermediate thereof Method of producing n-[(2S)-sulfanyl-4-(1,5,5-trimethylhydantoinyl)butanoyl]-L-leucyl-L-tert-leucine N-methylamide and intermediate thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/148bc1e9-1982-46da-9368-ff4d189dc242/US06960567-20051101-C00003.png)