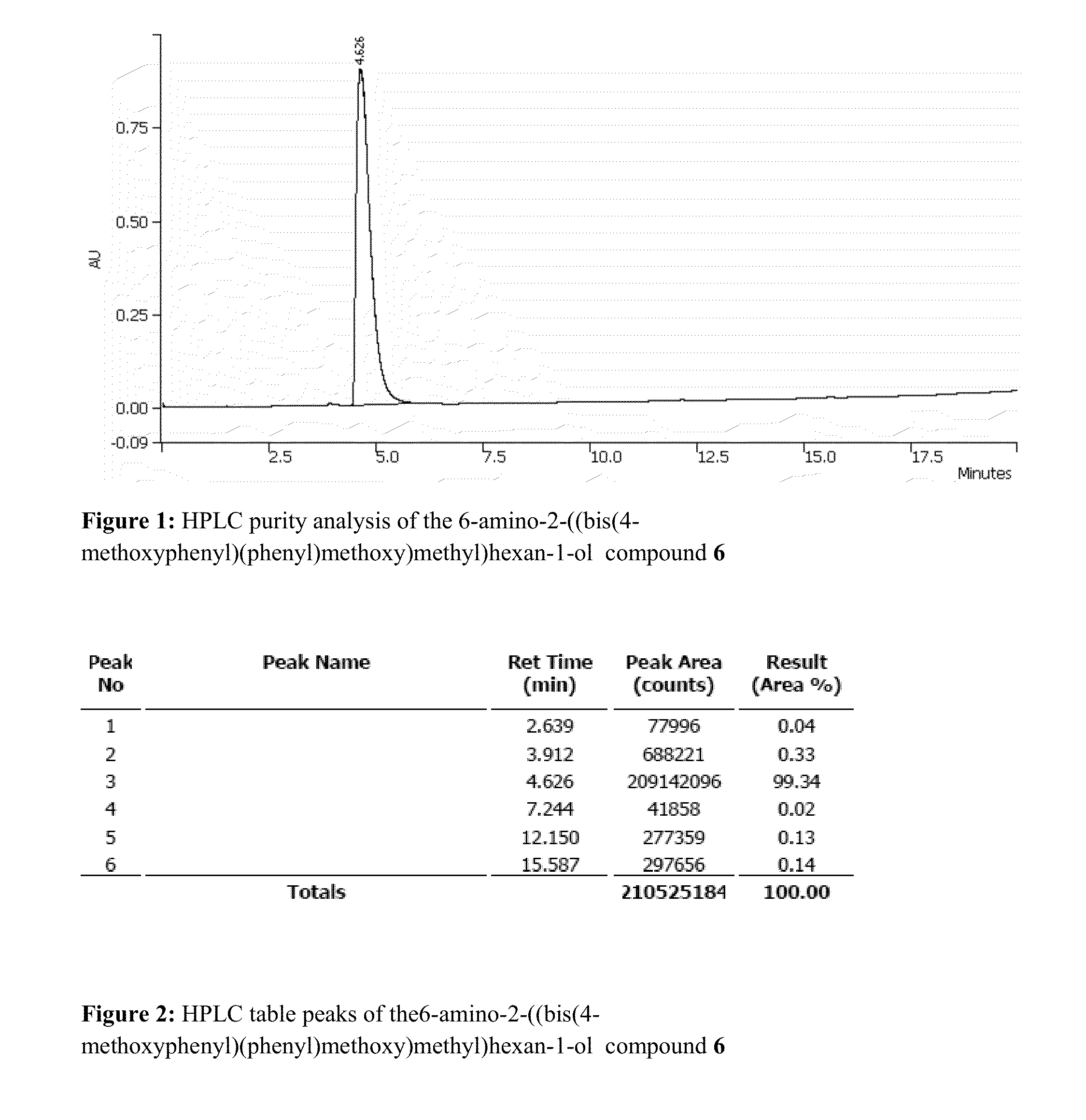
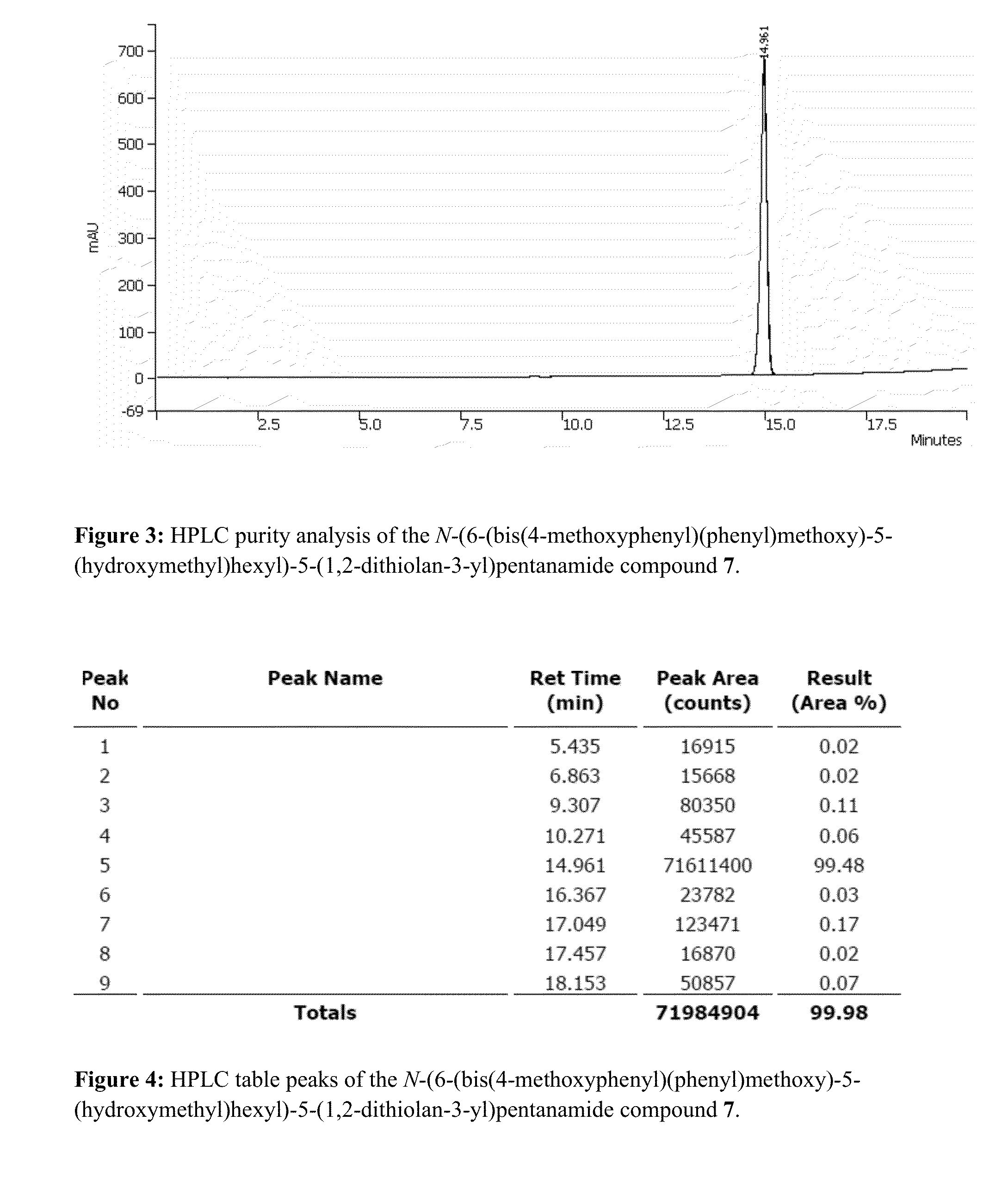
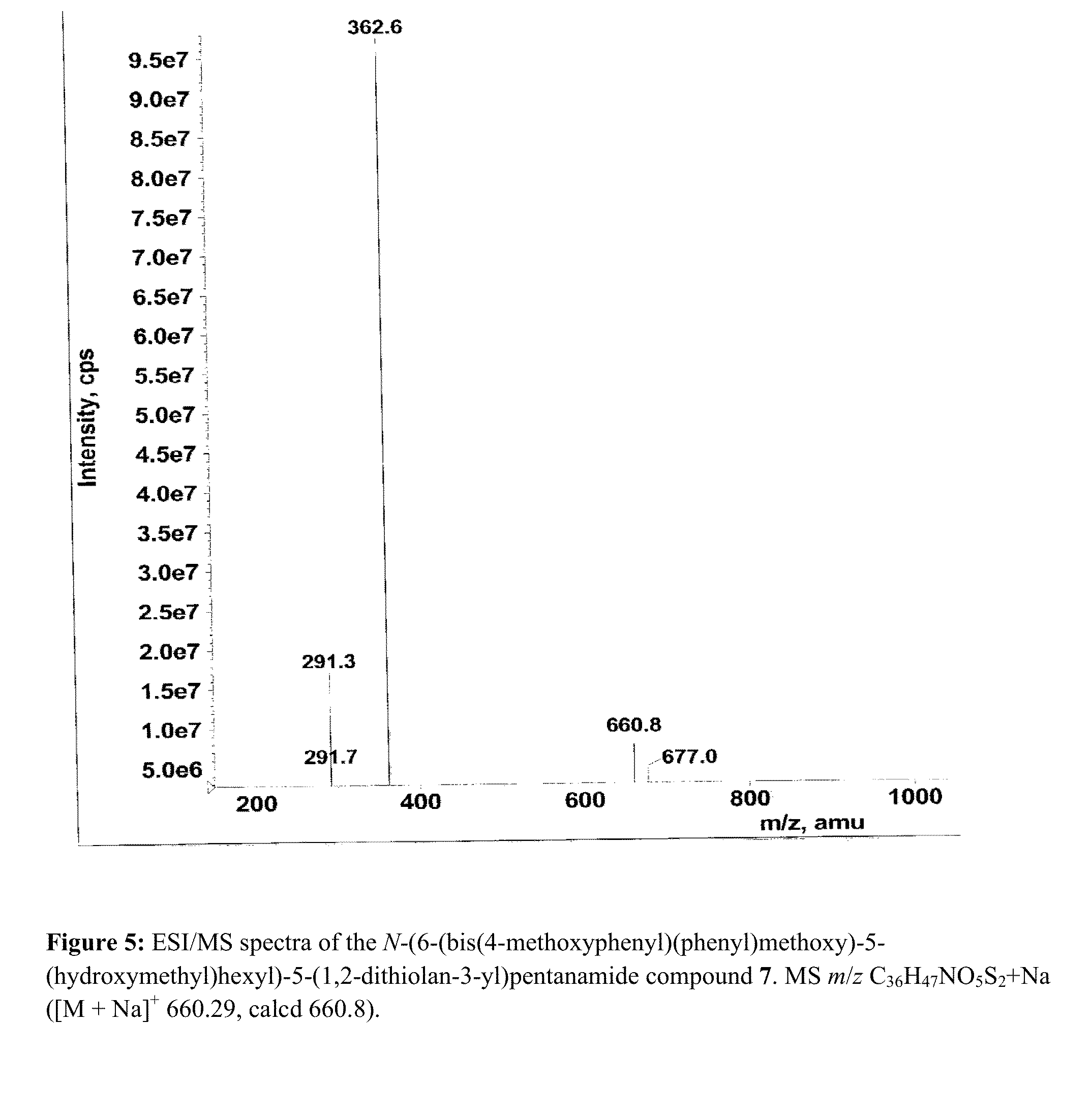
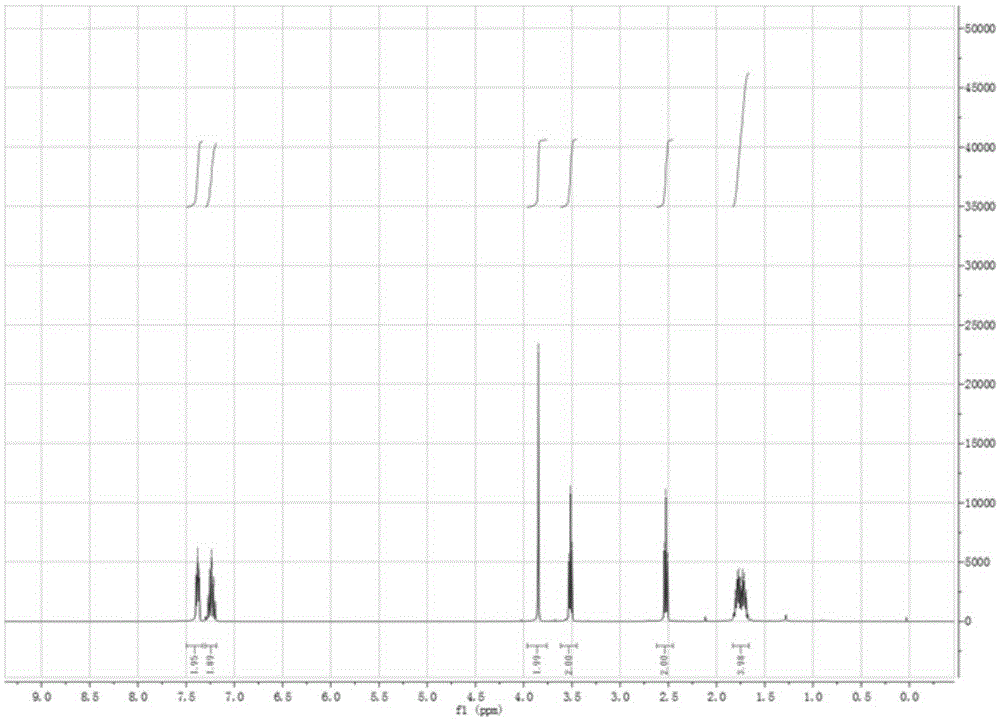
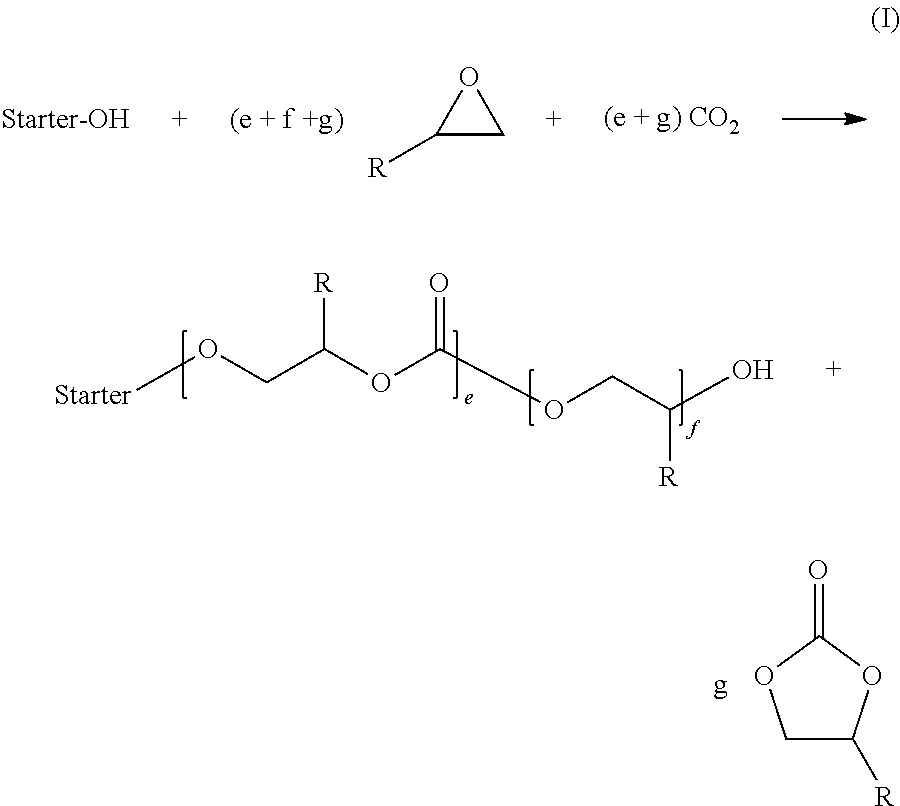
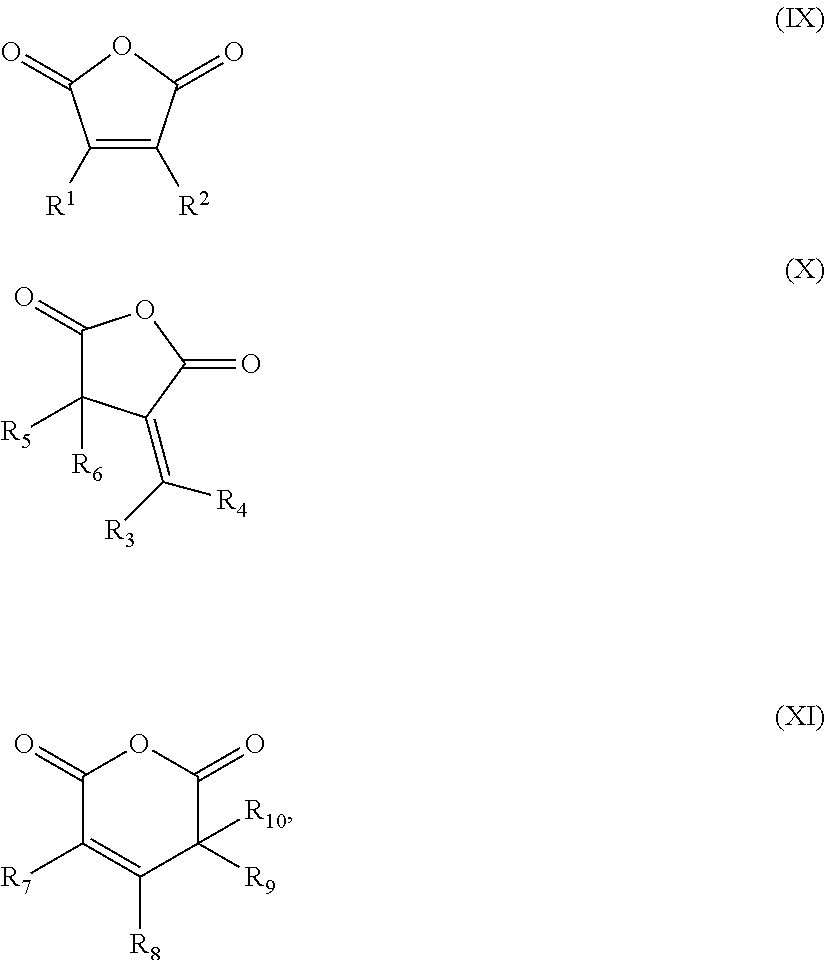
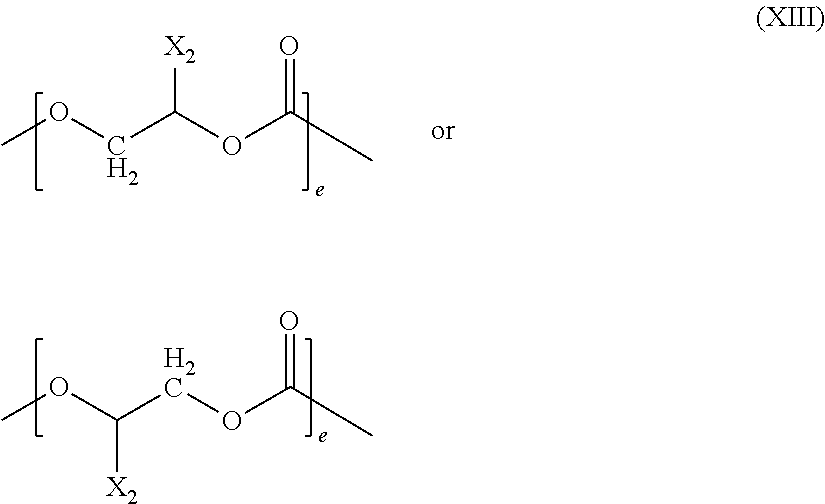
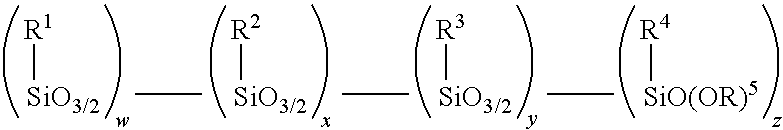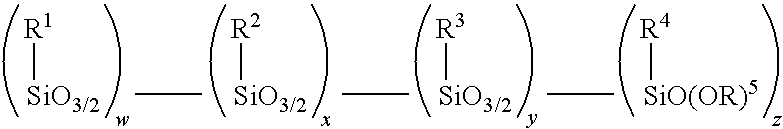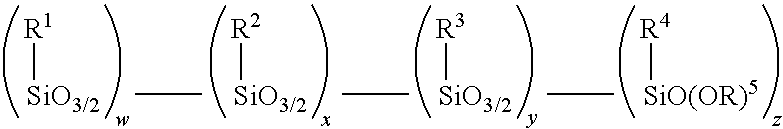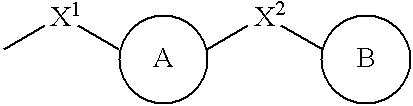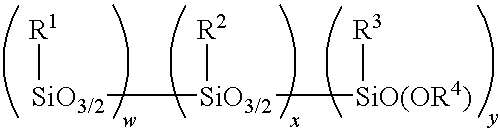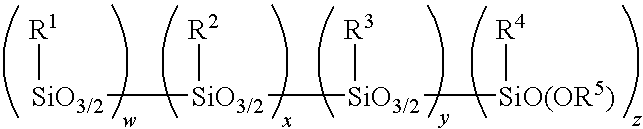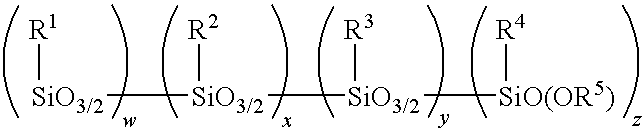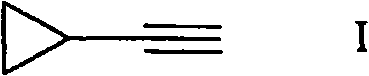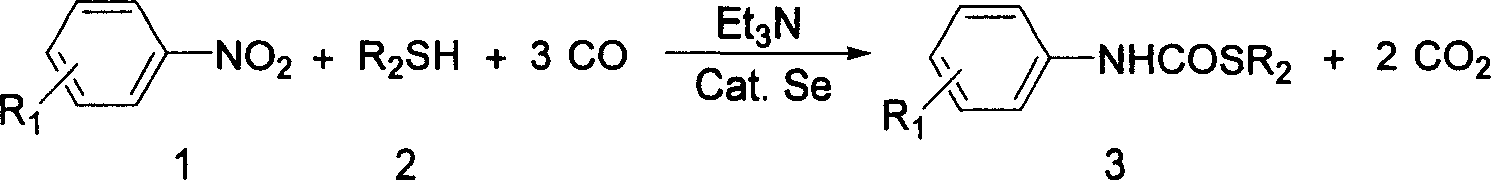Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
43 results about "Thioenol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
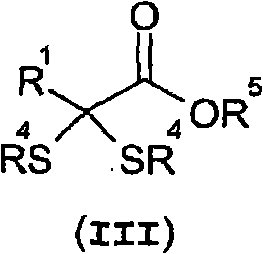
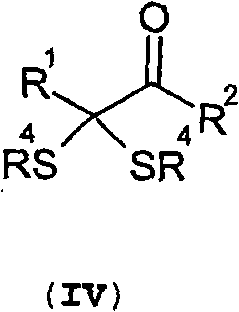
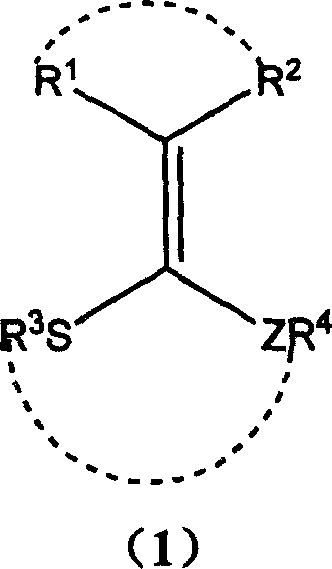
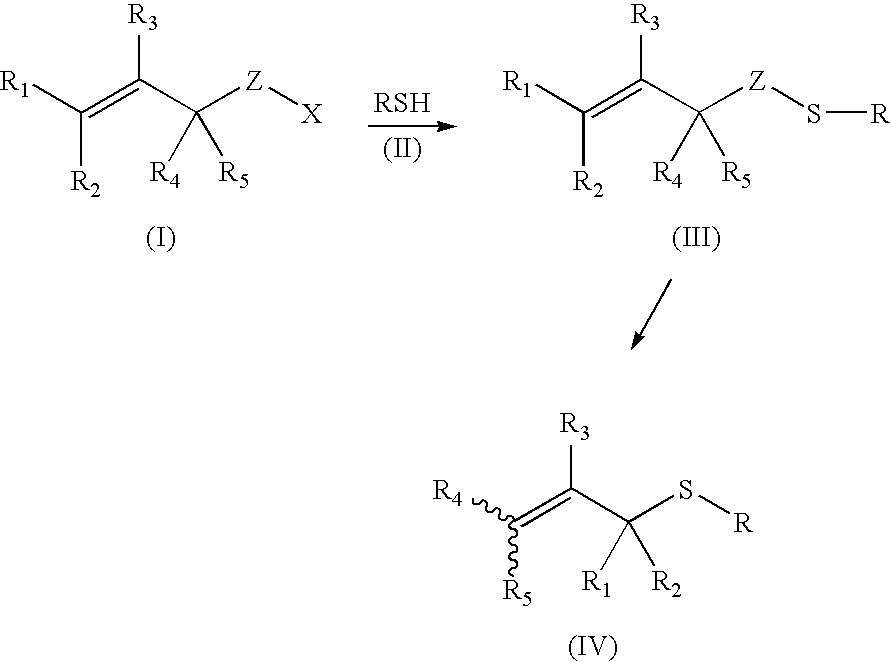
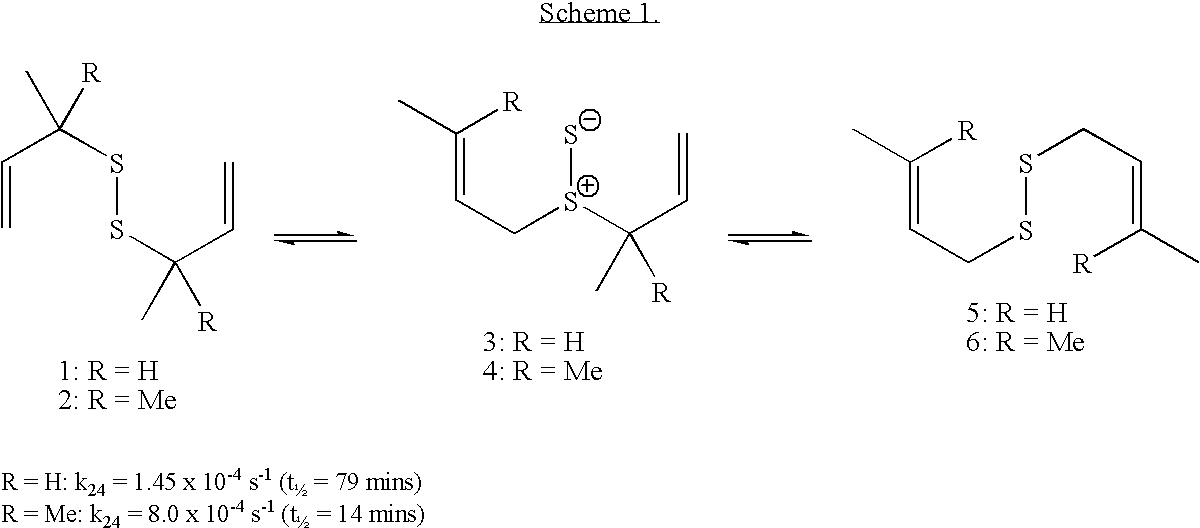
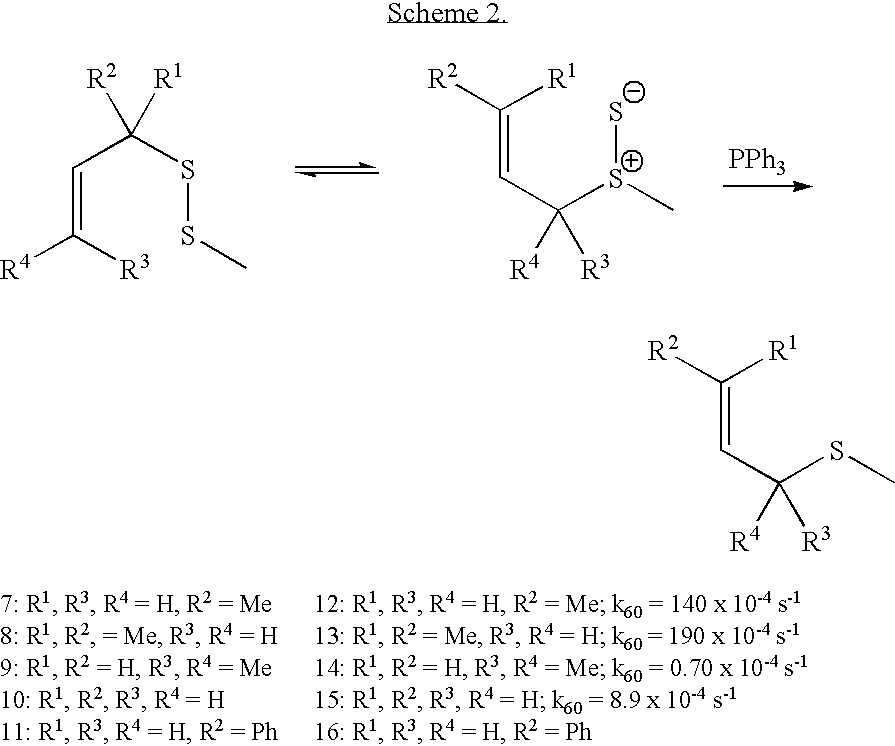
Thioenols (also known as alkenethiols) are alkenes with a thiol group affixed to one of the carbon atoms composing the double bond. They are the sulfur analogs of enols. Alkenes with a thiol group on both atoms of the double bond are called enedithiols. Deprotonated anions of thioenols are called thioenolates.
Amino alkoxy-modified silsesquioxanes and method of preparation
InactiveUS20090203929A1Low VOC emissionCoating adhesionSilicon organic compoundsCoatingsThioenolThiol
An amino alkoxy-modified silsesquioxane (AMS) comprising one or more compounds selected from the group consisting of an amino AMS, an amino / mercaptan co-AMS, an amino / blocked mercaptan co-AMS, mixtures thereof, and a weak acid-neutralized solid or aqueous solution thereof, and a method of making the amino AMS, are presented. The compounds are useful in compounding, processing, cure and storage of silica-reinforced rubbers because they contain low levels of volatile organic compounds (VOC).
Owner:BRIDGESTONE CORP
Copper-catalyzed formation of carbon-heteroatom and carbon-carbon bonds
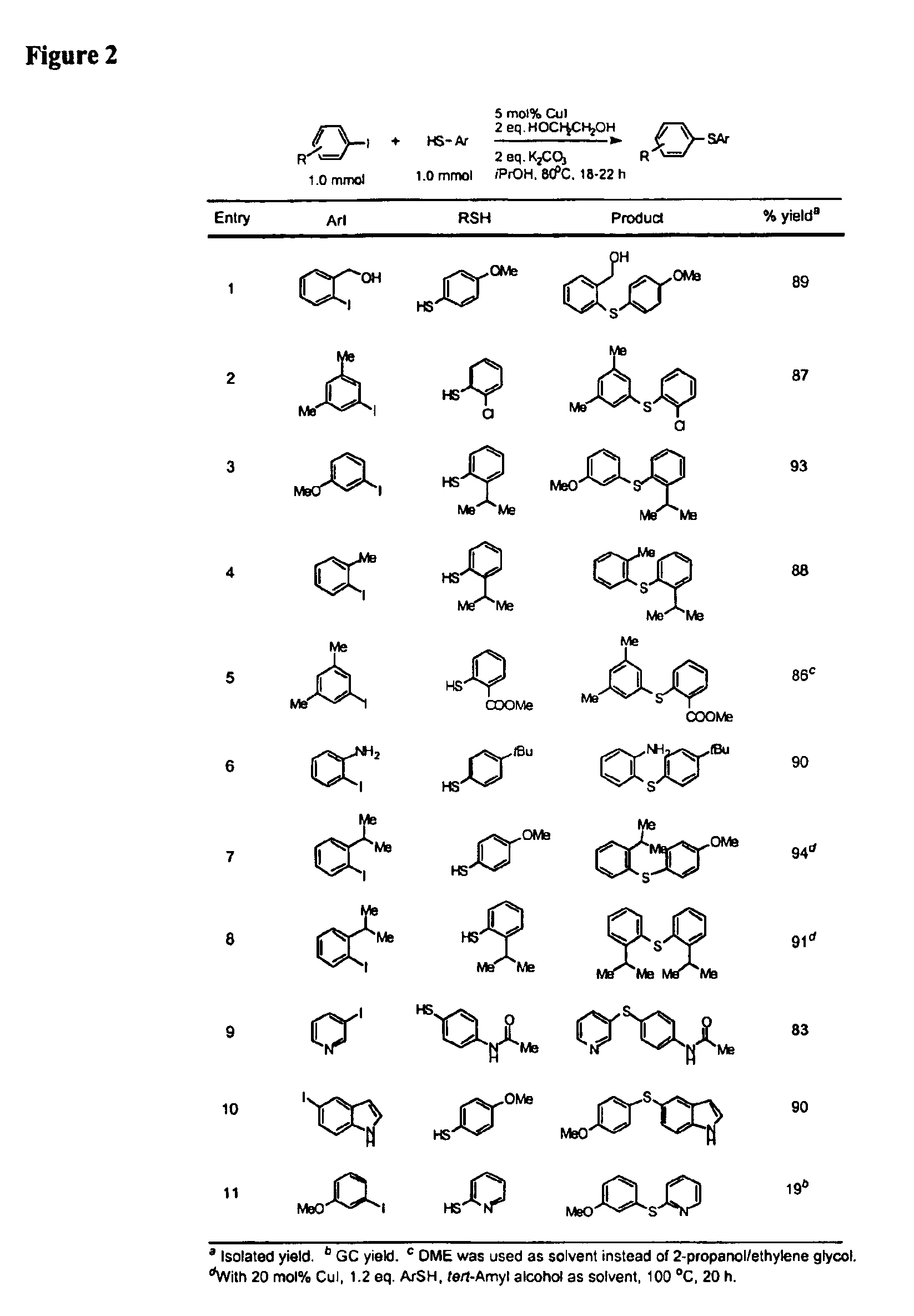
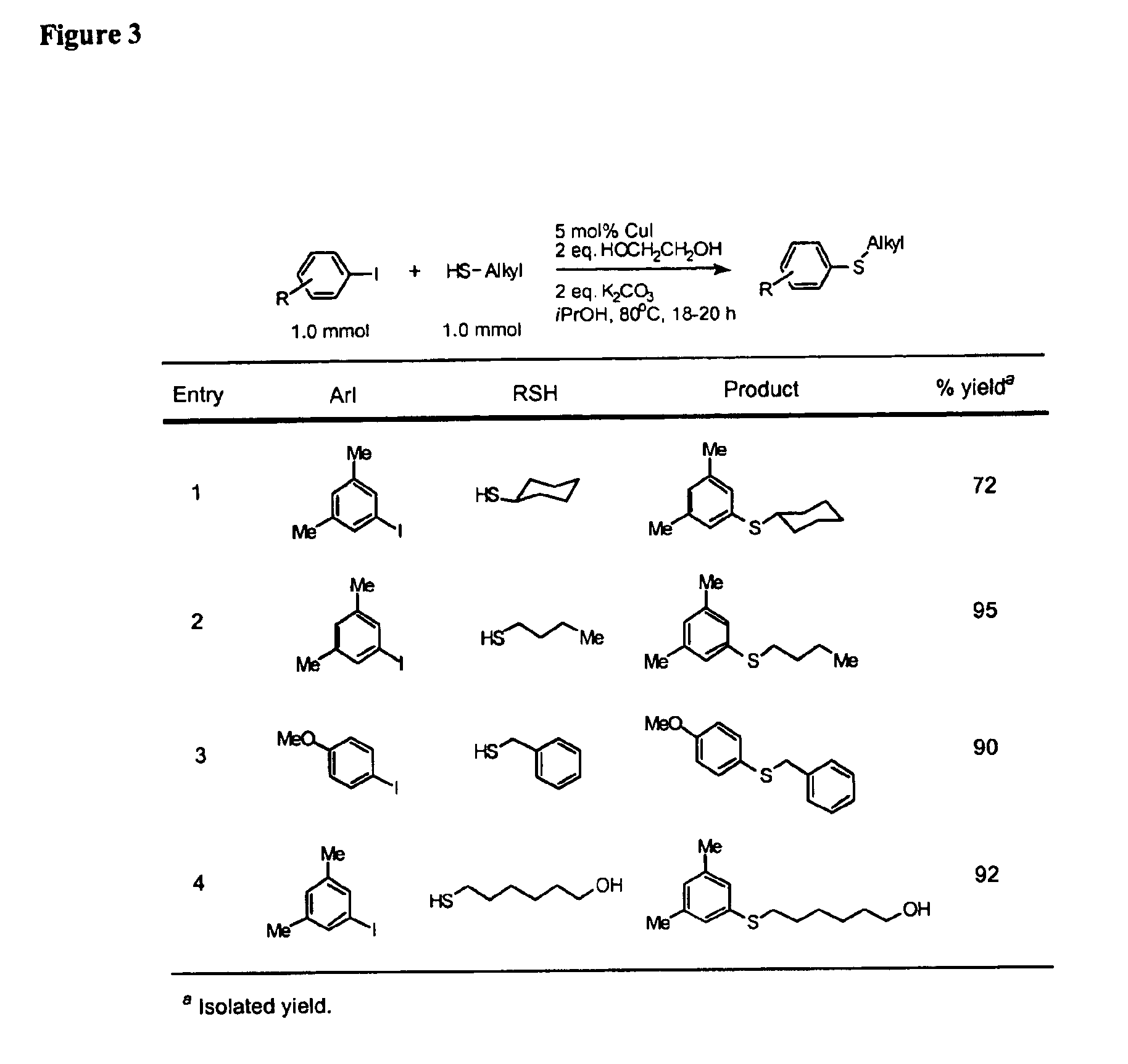
InactiveUS6888032B2Cheap and practicalLow costOrganic compound preparationThiol preparationCarbon–carbon bondSulfide
One aspect of the present invention relates to copper-catalyzed carbon-heteroatom and carbon-carbon bond-forming methods. In certain embodiments, the present invention relates to copper-catalyzed methods of forming a carbon-sulfur bond between the sulfur atom of a thiol moiety and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. In other embodiments, the present invention relates to copper(II)-catalyzed methods of forming a carbon-nitrogen bond between the nitrogen atom of an amide and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. In certain embodiments, the present invention relates to copper-catalyzed methods of forming a carbon-carbon bond between the carbon atom of cyanide ion and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. In another embodiment, the present invention relates to a copper-catalyzed method of transforming an aryl, heteroaryl, or vinyl chloride or bromide into the corresponding aryl, heteroaryl, or vinyl iodide. Yet another embodient of the present invention relates to a tandem method, which may be practiced in a single reaction vessel, wherein the first step of the method involves the copper-catalyzed formation of an aryl, heteroaryl, or vinyl iodide from the corresponding aryl, heteroaryl, or vinyl chloride or bromide; and the second step of the method involves the copper-catalyzed formation of an aryl, heteroaryl, or vinyl nitrile, amide or sulfide from the aryl, heteroaryl, or vinyl iodide formed in the first step.
Owner:MASSACHUSETTS INST OF TECH
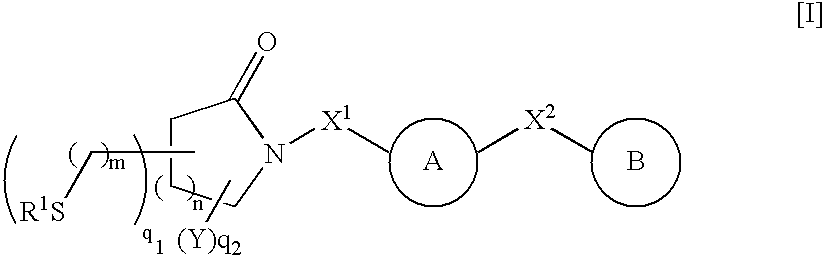
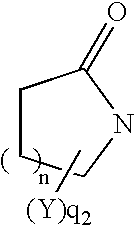
Thiol compounds, their production and use
Compounds represented by general formula (1) or salts thereof which have a matrix metalloprotease inhibitory activity and are useful as drugs, wherein the rings A and B represent each an optionally substituted homocycle or heterocycle, etc.; R1s are the same or different and each represents hydrogen, optionally substituted hydrocarbyl, acyl, etc.; X1 represents a bond, optionally substituted divalent aliphatic hydrocarbyl, etc.; X2 represents a bond, optionally substituted divalent aliphatic hydrocarbyl, -O-, etc.; Ys are the same or different represents hydrogen, optionally substituted hydrocarbyl, oxo, etc.; m is 0 or 1; n is an integer of 1 to 3; q1 is an integer of 1 to 2n+4; and q2 is an integer of 0 to 2n+3, provided that q1+q2 is 2n+4.
Owner:TAKEDA PHARMA CO LTD
Method for making alkoxy-modified silsesquioxanes and amino alkoxy-modified silsesquioxanes
A method is presented for making an amino alkoxy-modified silsesquioxane (amino AMS) comprising one or more compounds selected from the group consisting of an amino-AMS, an amino / mercaptan co-AMS, an amino / blocked mercaptan co-AMS, and mixtures of these. The use of solid strong cationic catalysts in this reaction system is advantageous because the catalyst remains as a solid throughout the reaction, allowing simplified separation of the solid catalyst from the soluble amino AMS or amino co-AMS products, resulting in total or near total recovery of the amino AMS or amino co-AMS products, as well as virtual total recovery of the catalyst for recycling. The use of the solid strong cationic catalysts is advantageous because it results in amino AMS products that are free of, or substantially free of, residual acid catalyst.
Owner:BRIDGESTONE CORP
Dithiolane Based Thiol Modifier For Labeling and Stronger Immobilization of Bio-Molecules On Solid Surfaces
ActiveUS20140142253A1Good adhesionSatisfactory puritySugar derivativesGroup 5/15 element organic compoundsNucleic acid chemistryThioenol
The thiol modified oligonucleotides have vast number of applications in the field of nucleic acid chemistry. The conjugates generated by mono thiol groups are unstable at higher temperature, in high salt concentration buffers and in presence other thiols. There is strong need to develop a novel thiol modifier probes that can generate multiple thiol groups. Described herein are efficient processes and compounds, dithiolane phosphoramidites derivative and dithiolane succinyl supports. The advantage of our cyclic disulfide thiol modifier is multifold a) each incorporation introduces two thiol groups; b) it can be introduced at any desired site of oligonucleotides; c) The symmetrical branching nature of the spacer in the linker arm of dithiolane allows for clean oligo synthesis, where cleavage of the linker arm and thereby of loss of oligo chain is prevented. We have successfully made 20-mer oligonucleotide containing single dithiolane derivative at 3′, and 21-mer oligonucleotides containing single dithiolane derivative at 5′ or in the middle of the mixed base sequence. HPLC and ESI MS analysis of these oligonucleotides indicated satisfactory purity and correct composition of these oligos, respectively.
Owner:CHEMGENES CORP
Method for making alkoxy-modified silsesquioxanes and amino alkoxy-modified silsesquioxanes
InactiveUS8501895B2Easy to separateGroup 4/14 element organic compoundsChemical recyclingThioenolEndcapping
A method is presented for making an amino alkoxy-modified silsesquioxane (amino AMS) comprising one or more compounds selected from the group consisting of an amino-AMS, an amino / mercaptan co-AMS, an amino / blocked mercaptan co-AMS, and mixtures of these. The use of solid strong cationic catalysts in this reaction system is advantageous because the catalyst remains as a solid throughout the reaction, allowing simplified separation of the solid catalyst from the soluble amino AMS or amino co-AMS products, resulting in total or near total recovery of the amino AMS or amino co-AMS products, as well as virtual total recovery of the catalyst for recycling. The use of the solid strong cationic catalysts is advantageous because it results in amino AMS products that are free of, or substantially free of, residual acid catalyst.
Owner:BRIDGESTONE CORP
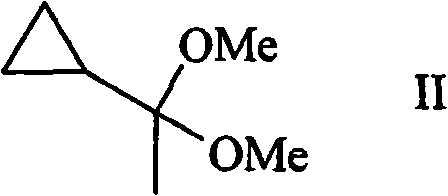
Process for the synthesis of ethynylcyclopropane
Ethynylcyclopropane is prepared in a two-step process by reacting (1,1-dimethoxyethyl)- cyclopropane with a thiol and eliminating the thiol from the intermediate.
Owner:LONZA LTD +1
Quick removal of mercaptans from hydrocarbons
InactiveCN101998985AReduce the amount presentEfficient removalHydrocarbon purification/separationRefining with acid-containing liquidsThioenolScavenger
Mercaptans and / or hydrogen sulfide (H2S) in hydrocarbons, naphthas, gasolines, and the like may be scavenged therefrom by being brought into intimate contact with a mercaptan scavenger formulation containing at least one disubstituted azodicarboxylate of the formula R1OOCN=NCOOR2, where R1 and R2 are independently alky! groups, alkenyl groups and aromatic groups having from 1 to 18 carbon atoms. These scavengers remove mercaptans and / or H2S from hydrocarbons faster than many conventional mercaptan scavengers. An effective scavenging amount of disubstituted azodicarboxylate in the hydrocarbon fluid ranges from about 5 to about 20 parts by weight based on 1 part as sulfur of mercaptan and / or H2S.
Owner:BAKER HUGHES HLDG LLC
Introduction of alkyl substituents to aromatic compounds
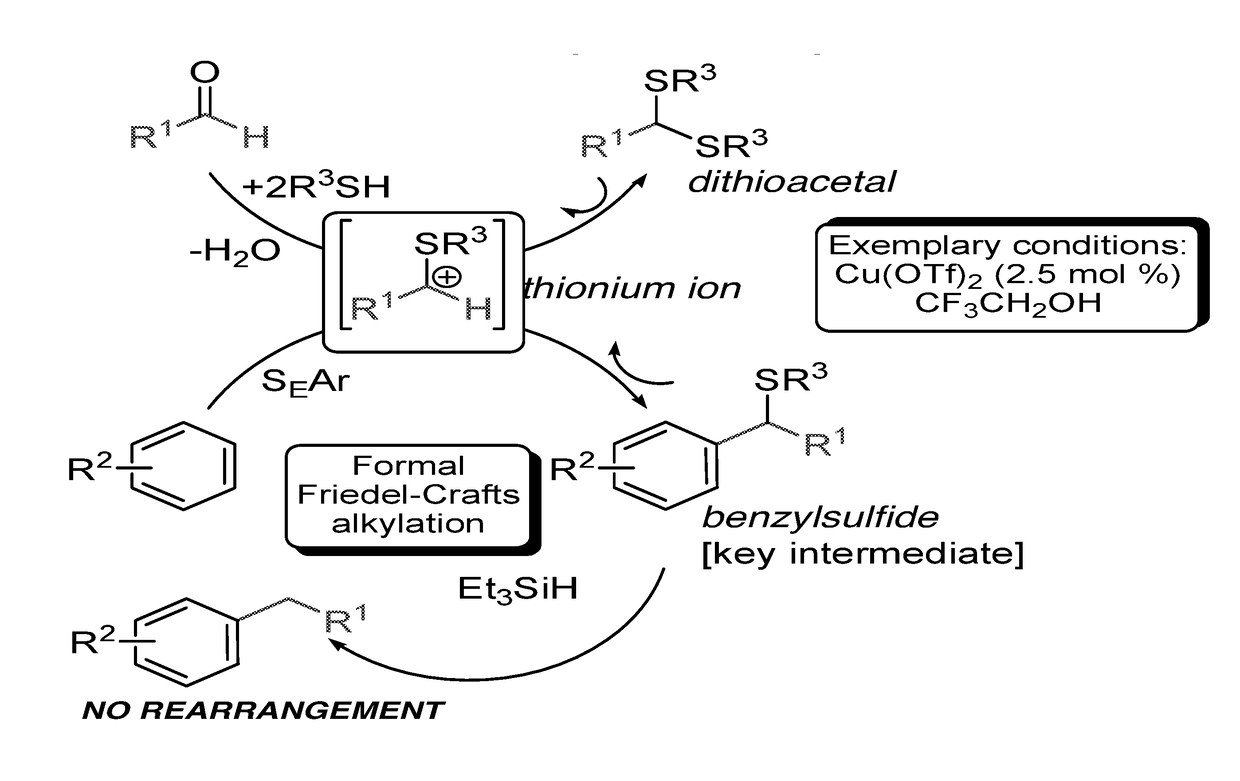
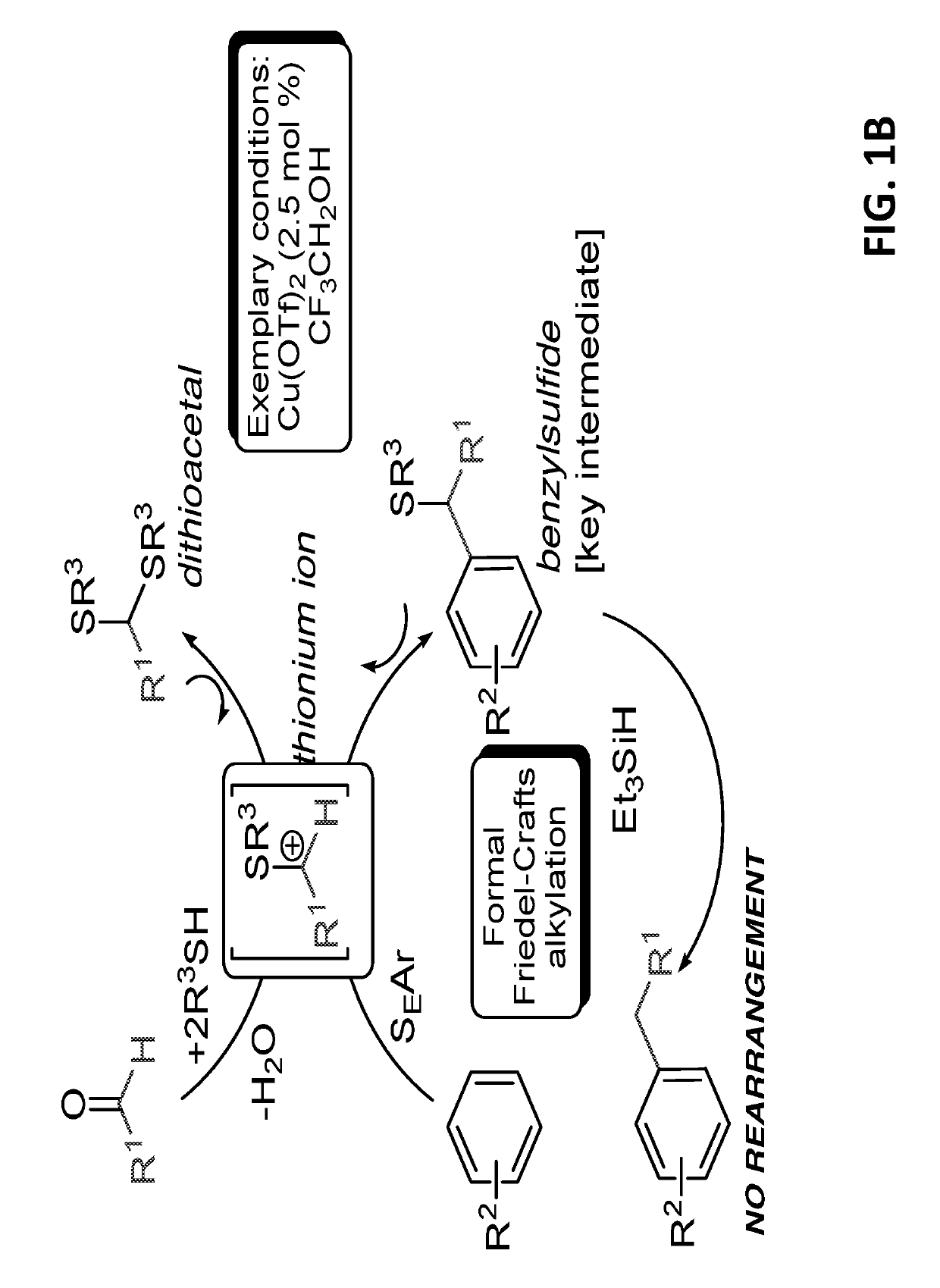
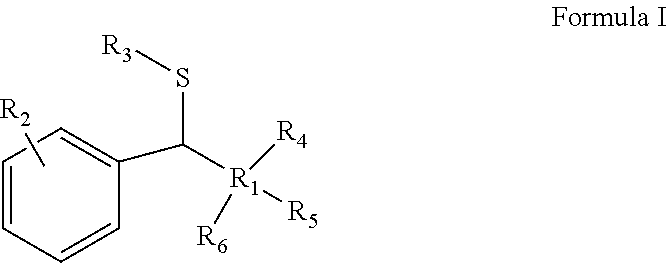
InactiveUS20180065904A1Mercapto/sulfide group formation/introductionOrganic reductionThioenolElectrophilic aromatic substitution
Novel selective synthesis route to introduce primary alkyl groups on aromatic compounds is disclosed. The synthesis route is based on electrophilic aromatic substitutions of thionium ion species that are generated in-situ from aldehydes and thiols, affording benzyl sulfide that can be reduced with triethylsilane.
Owner:B G NEGEV TECH & APPL LTD
Process for synthesizing thiocarbamate
The synthesis process of thiocarbamate is one reaction process between mercaptan or thiophenol and aryl nitro compound in the presence of CO, Se as catalyst and triethylamine as co-catalyst in the condition with organic solvent or without solvent inside sealed high pressure reactor. The nitro benzene has substituent radical R1 in the benzene ring being H or one or several electron donating or electron drawing groups; and the sulfide has R2 capable of being alkyl group or one or several electron donating or electron drawing aryl groups. The molar ratio between mercaptan or thiophenol and aryl nitro compound is 0.1-10; the molar consumption of Se is 0.1-20 % of the reactant with less molar consumption; the molar consumption of triethylamine is 10-200 % of the reactant with less molar consumption; and the molar number of the organic solvent is 1-50 times that of the reactants. The reaction has period of 1-20 hr, temperature of 0-200 deg.c and CO pressure of 0.1-10 MPa.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
New synthesis method for aromatic alpha-hydroxy ketone compounds
InactiveCN104003862AEasy to handleMild reaction conditionsOrganic compound preparationPreparation from heterocyclic compoundsAlkaneSynthesis methods
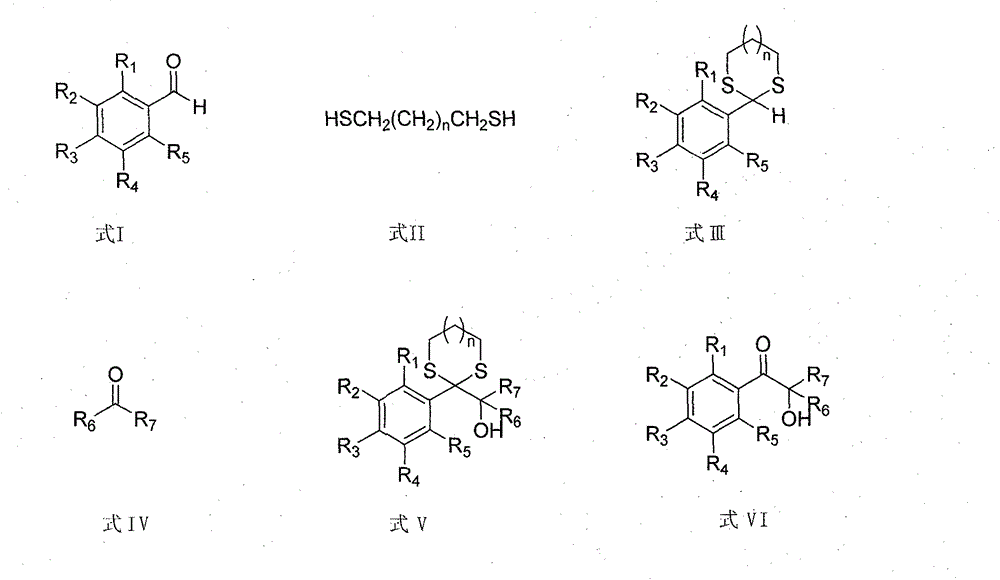
The invention discloses a novel synthesis method for aromatic alpha-hydroxy ketone compounds. The method comprises three steps of reactions of condensation protection, addition and deprotection on aromatic aldehyde which is used as a raw material, i.e., firstly, the aromatic aldehyde reacts with mercaptan to obtain substituted thiophene alkane which reacts with butyl lithium to obtain lithium salt, the lithium salt is subjected to an addition reaction with ketone to obtain hydroxy compounds, and finally, the hydroxy compounds are deprotected under heavy metal catalysis to obtain target products of the aromatic alpha-hydroxy ketone compounds. When the method provided by the invention is compared with a traditional method, the catalyst can be repeatedly used many times, the emission of various wastes is greatly reduced, an oxidation step is omitted, and reaction risks are reduced. The aromatic alpha-hydroxy ketone compounds prepared through reactions can be used as ultraviolet curing photoinitiators for massive use.
Owner:岳阳凯门科技有限公司
Method for the production of ketoacids and their derivatives
InactiveCN101687753AImprove protectionOrganic compound preparationCarboxylic acid esters preparationThioenolThiol
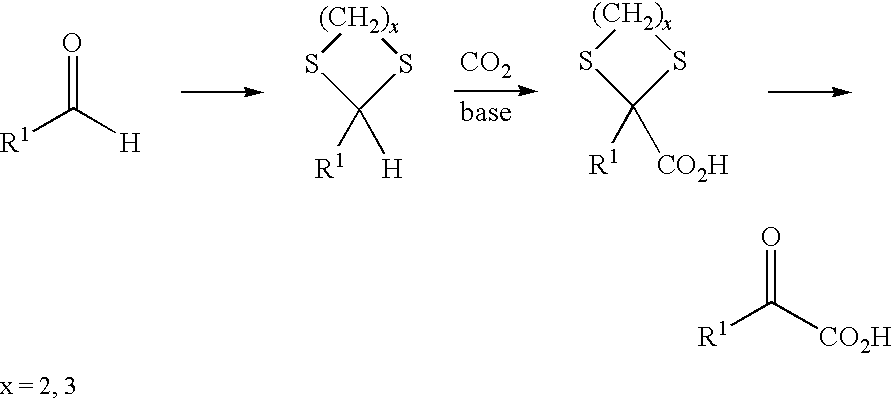
A method for the production of a-ketoacids, in particular of a-ketomethionine, and their derivatives is described, as also is the use of thiols for reversing the polarity of aliphatic or aromatic aldehydes. In this method, a) an aldehyde is reacted with thiols to give the corresponding dithioacetal, and b) the resultant dithioacetal then reacts with an electrophile in the presence of a base and after hydrolysis to give an a,a-(dithio)carboxylic acid and c) the a,a-(dithio)carboxylic acid is then reacted via acid-catalysed solvolysis to give the a-ketoacid or its derivatives, with liberation of thiol.
Owner:EVONIK DEGUSSA GMBH
Thioether allyl isothiocyanate compounds, and preparation method and applications thereof
InactiveCN105237451AHigh anticancer activityLow genotoxicityOrganic chemistryAntineoplastic agentsAlkaneBenzoyl bromide
The invention provides a series of novel thioether allyl isothiocyanate compounds with a general structure disclosed in the invention, and a simple method used for rapid synthesis of the thioether allyl isothiocyanate compounds. According to the method, double-terminal halogen-substituted alkanes are taken as initial raw materials, are reacted with phthalimide potassium so as to introduce N atoms, are reacted with sodium hydrosulfide so as to produce mercaptan, and mercaptan is reacted with benzyl bromide or is reacted with a heterocyclic compound with sulfydryl so as to introduce the thioether structure, an obtained product is reacted with hydrazine hydrate so as to obtain a primary amine, and the primary amine is reacted with an alkali, carbon disulfide, and methylsufonyl chloride so as to prepare the thioether allyl isothiocyanate compounds with heterocyclic nitrogen structures. The method contains few steps; operation is simple; the obtained products can be easily purified; and yield is high. The series of novel thioether allyl isothiocyanate compounds possess obvious killing activity on tumor cells, and possess obvious killing activity on cervical carcinoma cells and lung cancer cells.
Owner:HAINAN NORMAL UNIV
Cross-linking of polyether carbonate polyols containing double-bonds, by adding mercapto-compounds
ActiveUS9708446B2Rapid and easy processabilityGood processing and product propertyAdhesivesPolycarbonate coatingsThioenolThiol
The present invention relates to a process for preparing mercapto-crosslinked polyethercarbonate and sees polyethercarbonate polyols containing double bonds being reacted with polyfunctional mercaptans and / or sulfur with the involvement of initiator compounds.
Owner:COVESTRO DEUTSCHLAND AG
PROCESS FOR PREPARING a-KETO ACIDS AND DERIVATIVES THEREOF
A method for preparing α-keto acids, especially α-ketomethionine, and / or derivatives thereof, whereby an aldehyde is reacted with thiols to give a corresponding dithioacetal, the dithioacetal formed, is reacted with an electrophile in the presence of a strong base, and the resulting α,α-(dithio)carboxylic acid is solvolyzed with acid-catalysis to release thiol and give the α-keto acid or a derivative thereof. Umpolung of aliphatic or aromatic aldehydes is effected by reaction with thiols.
Owner:EVONIK DEGUSSA GMBH
Hydroxythiol grignard reaction synthesis
InactiveUS6054623AHigh reaction yieldGroup 4/14 element organic compoundsThiol preparationThioenolThiol
A method for the preparation of hydroxythiol compounds by reacting a hydroxyl-protected halide compound having the structure:X-R-OPgwith magnesium in a Grignard-suitable solvent to form a hydroxyl-protected magnesium halide compound, wherein R is selected from substituted or unsubstituted aliphatic radicals, substituted or unsubstituted cyclic aliphatic radicals, substituted or unsubstituted aromatic radicals, substituted or unsubstituted araliphatic radicals and substituted or unsubstituted heterocyclic radicals; Pg is a protecting group; and X is selected from the group consisting of F, Cl, Br and I; then reacting said hydroxyl-protected magnesium halide compound with sulfur in the Grignard-suitable solvent to form a hydroxyl-protected thiomagnesium halide compound; and hydrolyzing the protected hydroxyl group to form a hydroxythiomagnesium halide compound and converting the thiomagnesium halide to a thiol; wherein the protecting group is selected so that species formed by the de-protection of the protecting group are inert toward thiols, or the method further includes the step of removing the protecting group species formed by de-protection of the hydroxyl group from the reaction mixture before converting the thiomagnesium halide to a thiol.
Owner:ALLIEDSIGNAL INC
Process for producing optically active ester
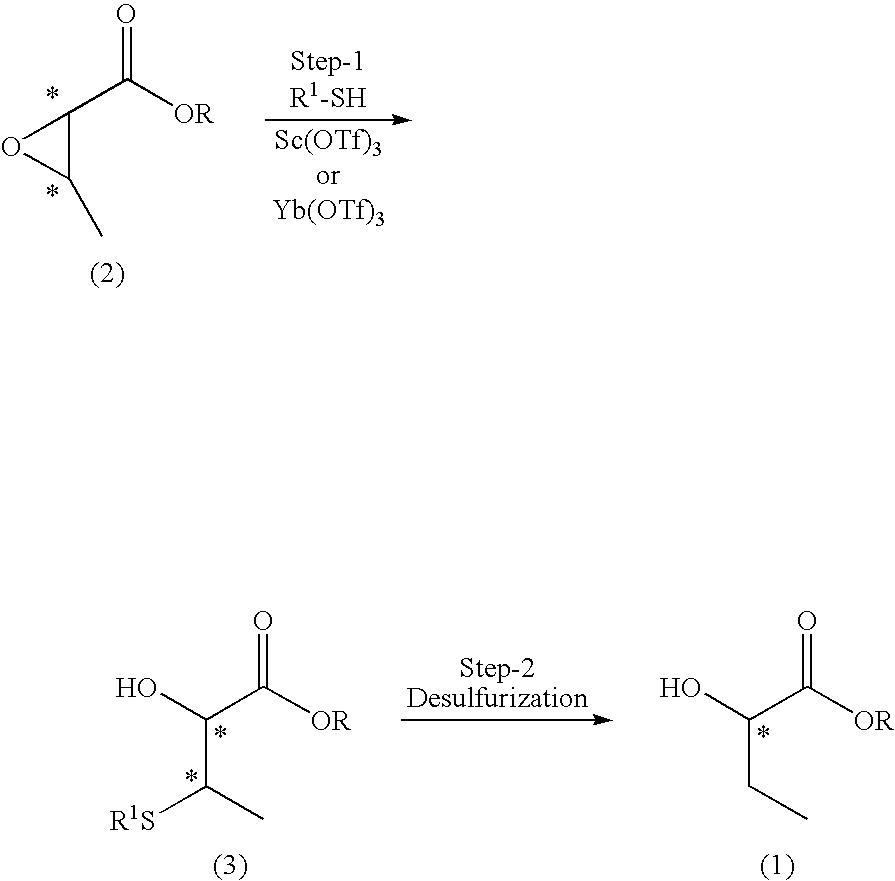
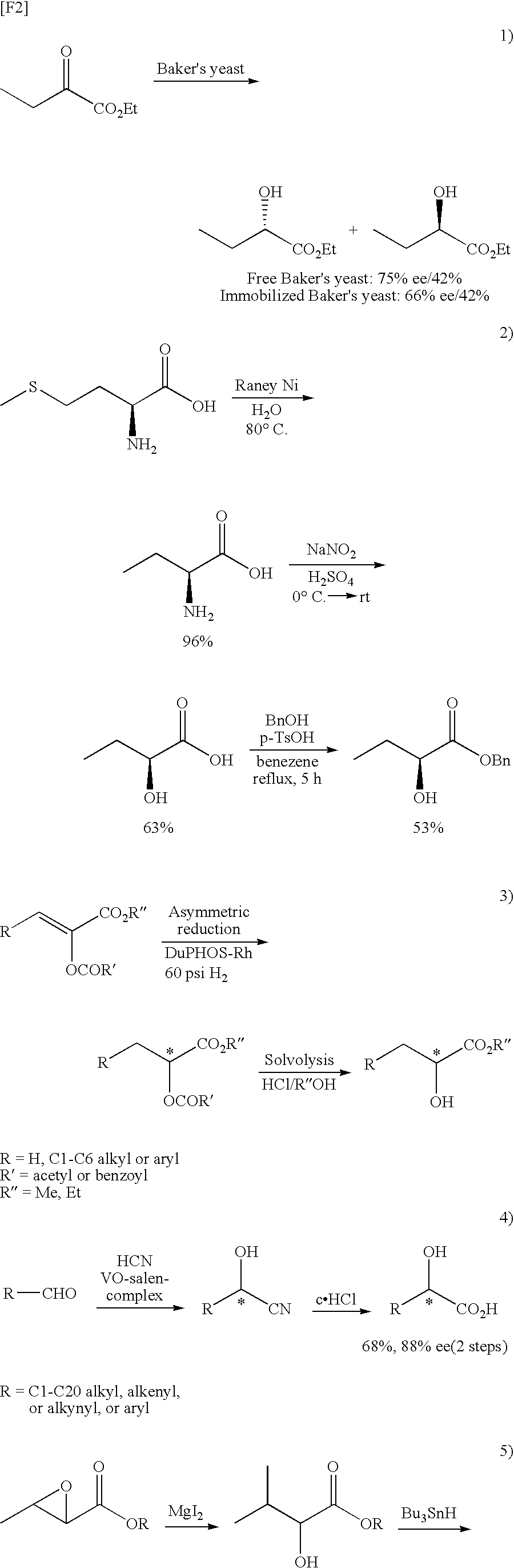
InactiveUS20090118535A1High yieldHigh optical yieldOrganic compound preparationCarboxylic acid esters preparationAbsolute configurationTriflic acid
A process for producing an optically active 2-hydroxybutyric ester (1), characterized by including reacting an optically active 2,3-epoxybutyric ester (2) with a thiol in the presence of scandium trifluoromethanesulfonate or ytterbium trifluoromethanesulfonate, to thereby produce Compound (3), and subjecting Compound (3) to desulfurization reaction:(wherein R represents a C1 to C6 alkyl group or a C7 or C8 aralkyl group; R1 represents a C1 to C12 alkyl group or a phenyl group; and * represents S- or R-absolute configuration).The present invention provides a process for producing an optically active 2-hydroxybutyric ester at high yield and high optical purity.
Owner:KOWA CO LTD
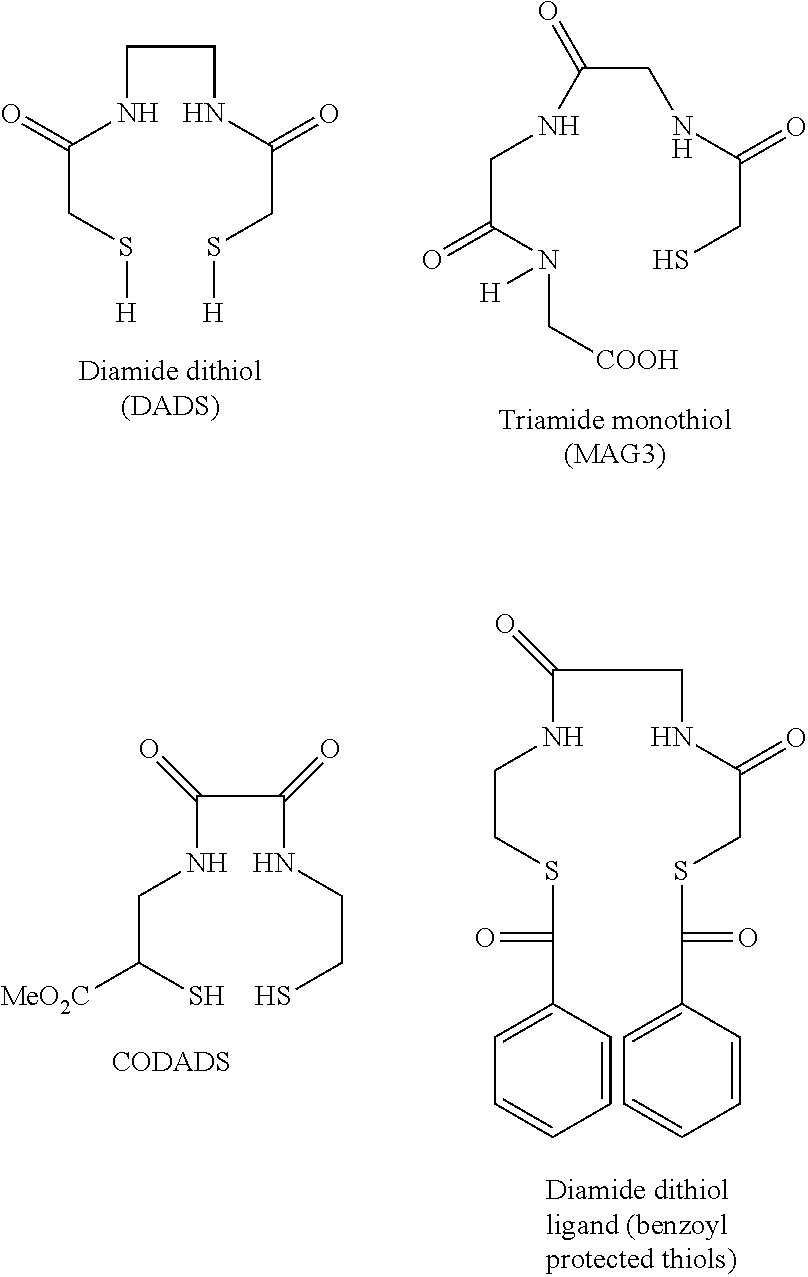
Chelation of metals to thiol groups using in situ reduction of disulfide-containing compounds by phosphines
InactiveUS20120178906A1In-vivo radioactive preparationsPeptide/protein ingredientsThioenolPhosphorine
A method is disclosed for the syntheses of thiol-containing radiopharmaceuticals without the need for purification starting from chelators containing disulfide bonds. This is done by providing a method that reduces disulfide bonds on a precursor molecule or a precursor compound in the presence of phosphine compounds, thus freeing thiols for metal complexation.
Owner:BRACCO IMAGINIG SPA
Kinetic resolutions of chiral 2- and 3-substituted carboxylic acids
InactiveUS7057038B2Preparation rom asymmetrical anhydridesCarbamic acid derivatives preparationCarbamateEnantiomer
One aspect of the present invention relates to a method for the kinetic resolution of racemic and diastereomeric mixtures of chiral compounds. The critical elements of the method are: a non-racemic chiral tertiary-amine-containing catalyst; a racemic or diastereomeric mixture of a chiral substrate, e.g., a cyclic carbonate or cyclic carbamate; and a nucleophile, e.g., an alcohol, amine or thiol. A preferred embodiment of the present invention relates to a method for achieving the kinetic resolution of racemic and diastereomeric mixtures of derivatives of α- and β-amino, hydroxy, and thio carboxylic acids. In certain embodiments, the methods of the present invention achieve dynamic kinetic resolution of a racemic or diastereomeric mixture of a substrate, i.e., a kinetic resolution wherein the yield of the resolved enantiomer or diastereomer, respectively, exceeds the amount present in the original mixture due to the in situ equilibration of the enantiomers or diastereomers under the reaction conditions prior to the resolution step.
Owner:BRANDEIS UNIV
Application of ketene thioacetal derivatives as thioalcohol substituted reagent
InactiveCN1594245AEasy to useEasy to manufactureFunctional group formation/introductionThioenolThiol
The invention relates to the use of ketene thioacetal derivatives in organic synthesis as thioalcohol substituted environment-friendly type organic reagent, wherein thioalcohol has strong volatility, irritation bad odor, toxicity and carcinogenic action, aiming at solving this problem, the invention employs ketene thioacetal derivatives as thioalcohol substituted reagent to realize the synthesizing of sulfur-containing compounds.
Owner:NORTHEAST NORMAL UNIVERSITY
Photosensitive resin composition and adhesives composition
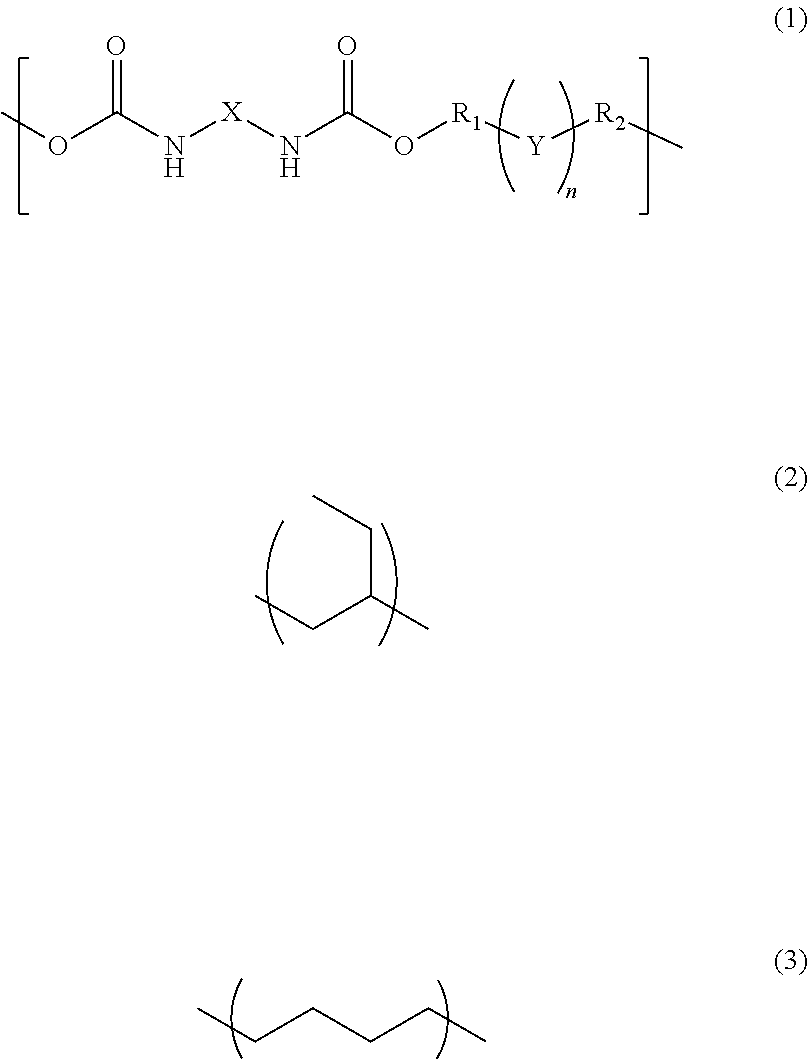
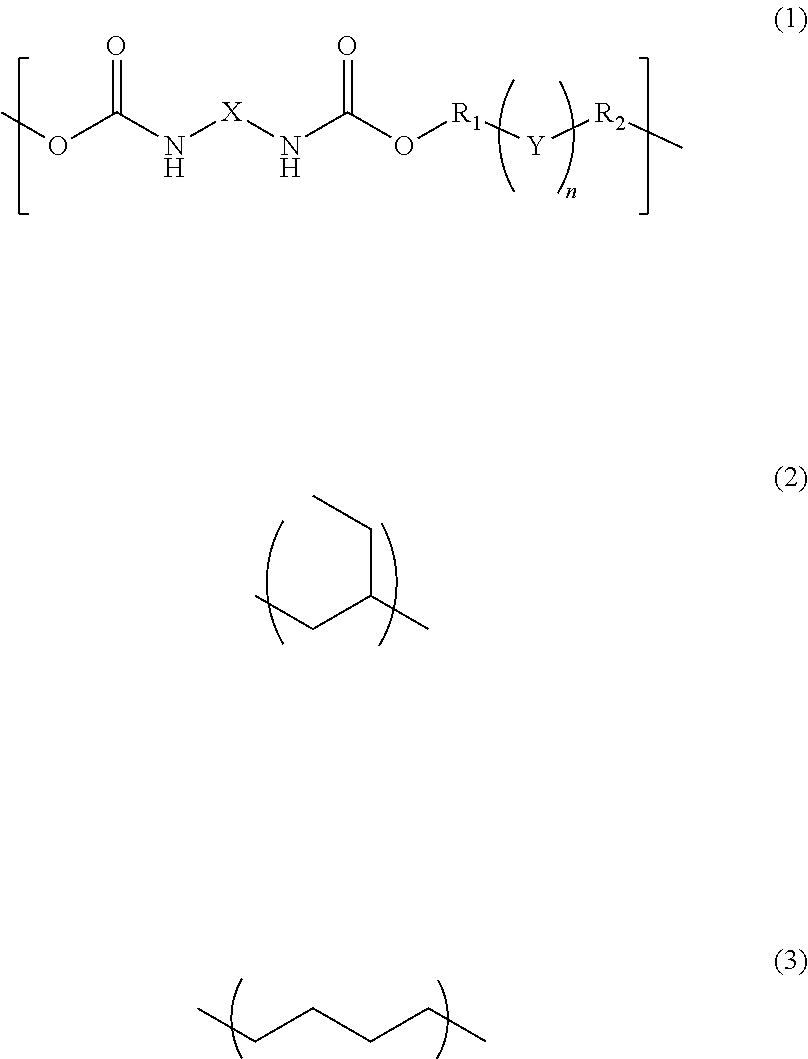
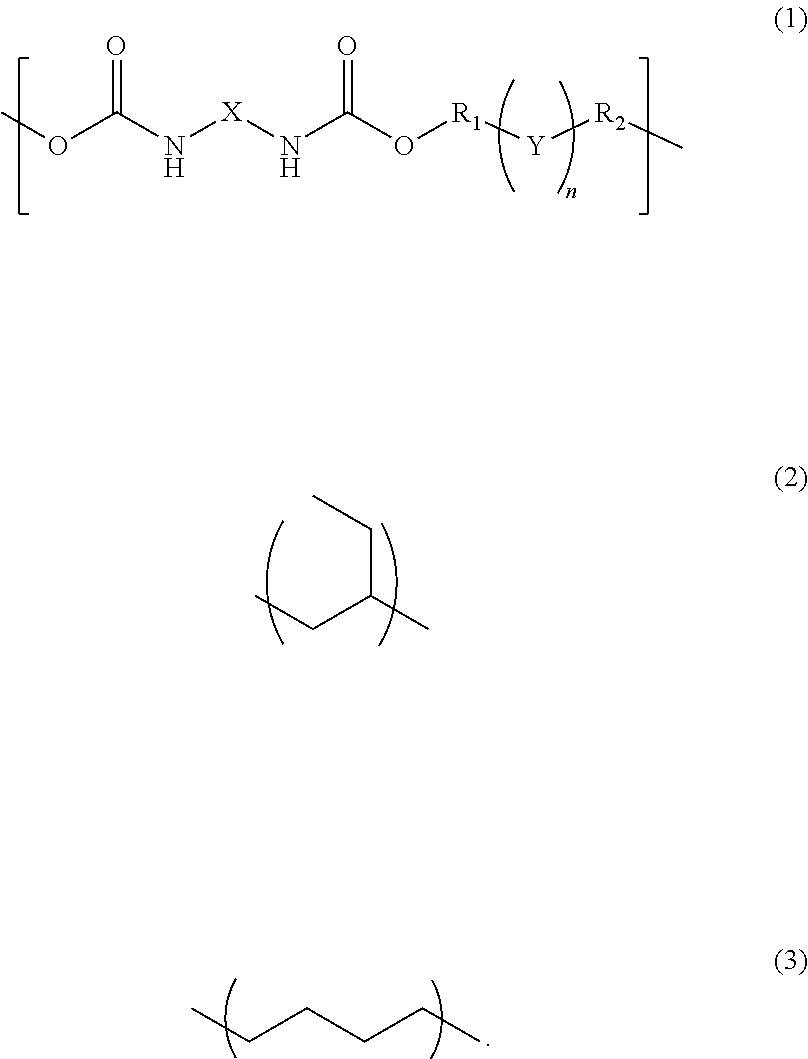
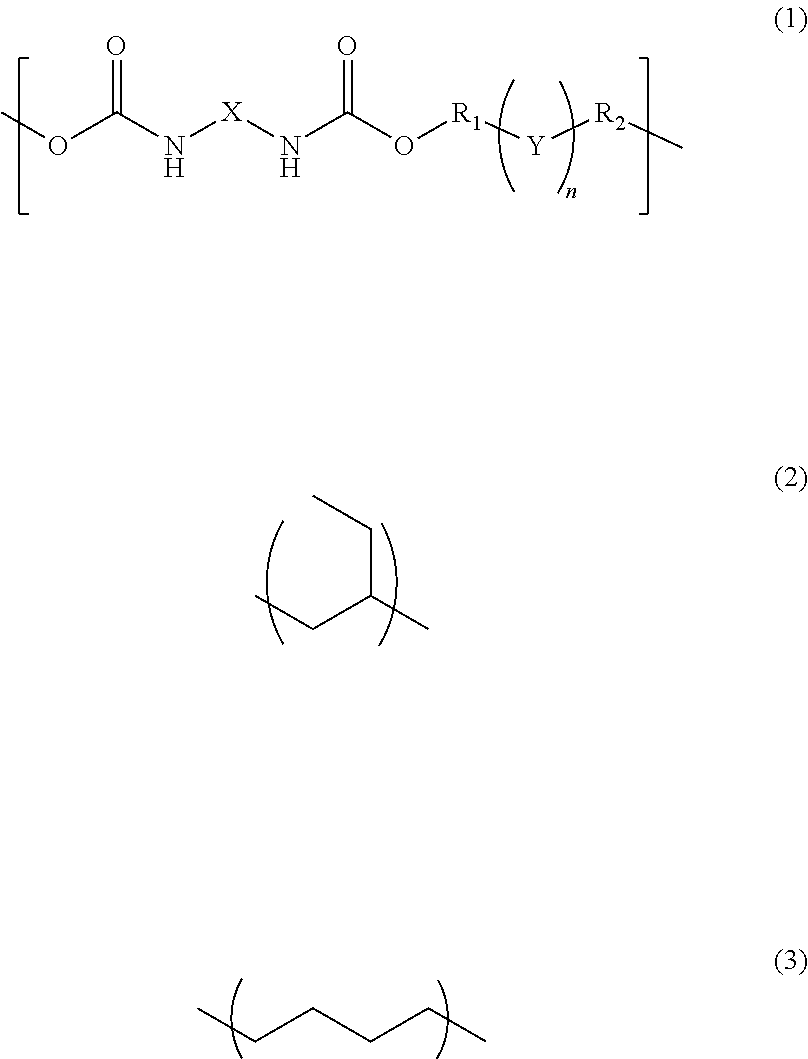
InactiveUS20170342238A1Reduce adhesionPolyureas/polyurethane adhesivesPhotomechanical apparatusAdhesiveAlicyclic Hydrocarbons
A photosensitive resin composition comprises a polymer having a repeating structural unit of the following Formula (1):(wherein R1 and R2 are each independently a single bond, a methylene group, or an ethylene group, Y is an alkylene group of Formula (2) or a combination of the alkylene group of Formula (2) with an alkylene group of Formula (3), n is an integer of 1 to 110, and X is a divalent aliphatic hydrocarbon group, a divalent alicyclic hydrocarbon group, or a divalent aromatic hydrocarbon group) and a (meth)acryloyl group at both ends, a bifunctional (meth)acrylate compound, a polyfunctional thiol compound, a photo-radical generator and an organic solvent, and then the bifunctional (meth)acrylate compound contained in an amount of 5% to 50% by mass and the polyfunctional thiol compound is contained in an amount of 0.1% to 10% by mass, relative to the content of the polymer.
Owner:NISSAN CHEM IND LTD
Method for purifying antioxidant 2,4-di(n-octyl sulfur methylene)-6-methylphenol
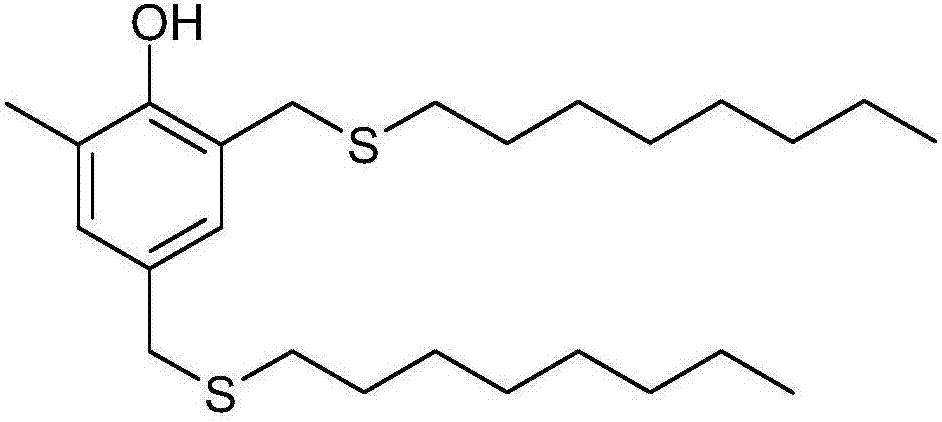
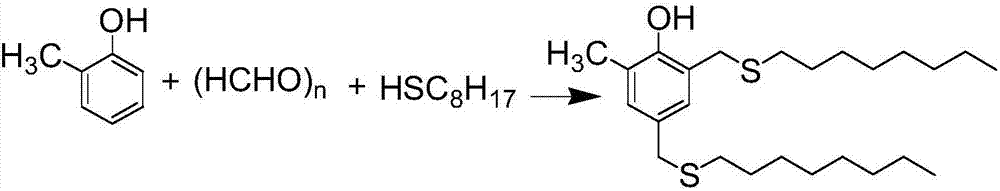
InactiveCN107473997AImprove qualitySimple and fast operationOrganic chemistryOrganic compound preparationThioenolThiol
The invention relates to a method for purifying antioxidant 2,4-di(n-octyl sulfur methylene)-6-methylphenol, that is, a crude product of 2,4-di(n-octyl sulfur methylene)-6-methylphenol of which components with low melting points are removed is decolored and deodorized to remove mercaptan odor by using a molecular distillation device, and then a product which is colorless and transparent and free of odor of n-octyl mercaptan is prepared.
Owner:CHANGZHOU UNIV
Process for the addition of thiolates to ?,?-unsaturated carbonyl or sulfonyl compounds
ActiveUS20100048939A1Easy to prepareOrganic compound preparationCarbonyl compound preparationThioenolThiol
Alkylthio substituted aldehydes, ketones, esters and sulfones are prepared by reacting α,β-unsaturated carbonyl and sulfonyl compounds with a sodium or potassium thiolate in the presence of a alkane carboxylic acid and water.
Owner:CORTEVA AGRISCIENCE LLC
Dechalcogenative methods for the preparation of allylic sulfides
InactiveUS20100222549A1Mercapto/sulfide group formation/introductionConnective tissue peptidesSolventPhosphine
A dechalcogenative method for the preparation of an allylic sulfide comprises contacting an activated chalcogenide of Formula (I) with a thiol of Formula (II) for a period of time sufficient to form an intermediate of Formula (III), and supplying sufficient activation energy to the intermediate of Formula (III), in a suitable solvent, preferably in the absence of a phosphine or other thiophile, to induce a [2,3]-sigmatropic rearrangement therein to form an allylic sulfide of Formula (IV), with concomitant loss of chalcogen Z, as set forth in the following reaction scheme, wherein X is an activating group selected from the group consisting of CN, S-pyridyl, S-heteroaryl, SO2-aryl, and SO3Y; Y is an alkali metal ion; Z is Se or S; R1, R2, R3, R4, and R5 are each independently H or a hydrocarbon moiety; and R is an organic moiety.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
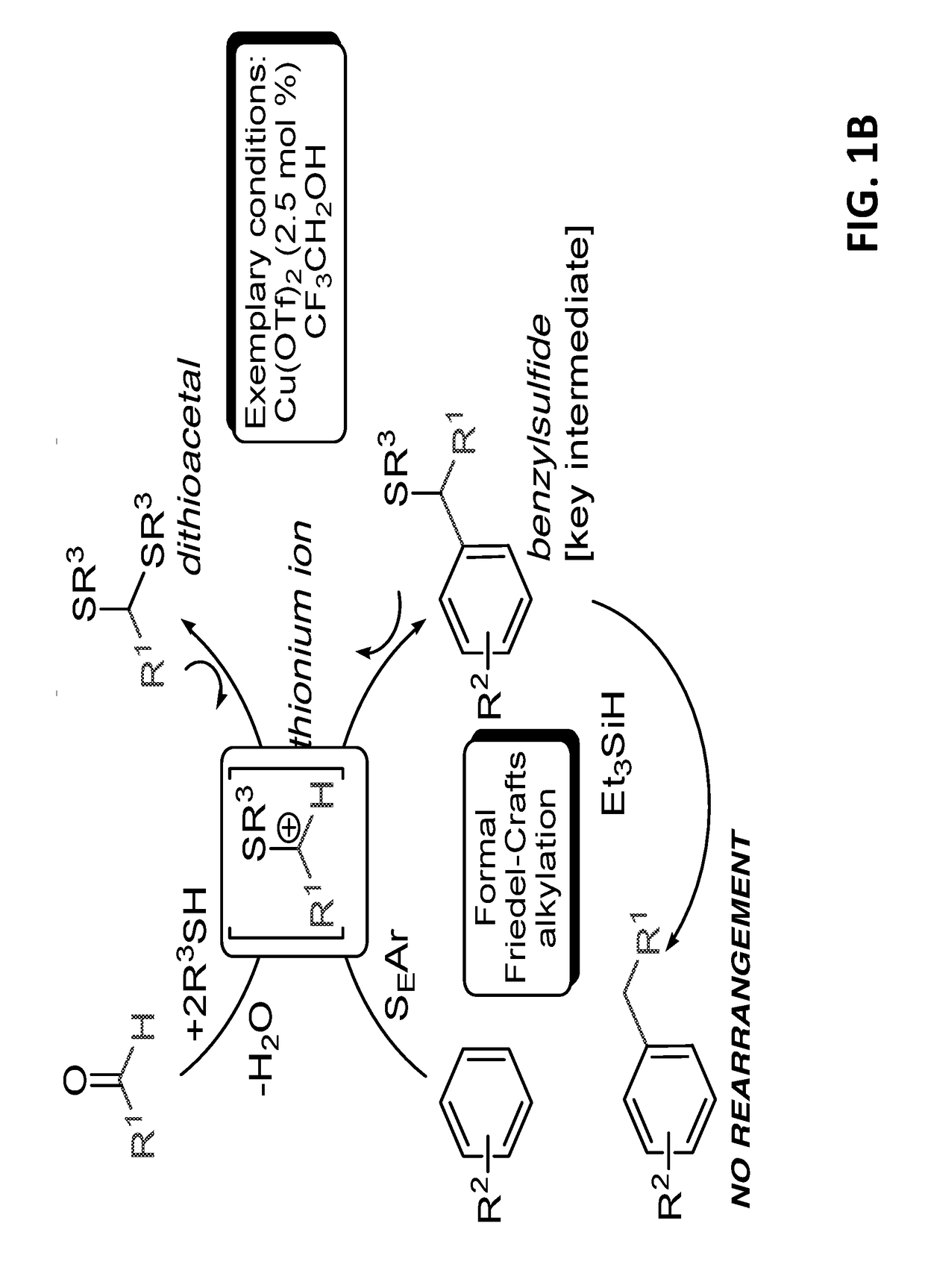
Introduction of alkyl substituents to aromatic compounds
InactiveUS10351492B2Mercapto/sulfide group formation/introductionOrganic reductionThioenolElectrophilic aromatic substitution
Novel selective synthesis route to introduce primary alkyl groups on aromatic compounds is disclosed. The synthesis route is based on electrophilic aromatic substitutions of thionium ion species that are generated in-situ from aldehydes and thiols, affording benzyl sulfide that can be reduced with triethylsilane.
Owner:B G NEGEV TECH & APPL LTD
Photosensitive resin composition and adhesives composition
InactiveUS10208184B2Reduce adhesionPolyureas/polyurethane adhesivesPhotomechanical apparatusAdhesiveAlicyclic Hydrocarbons
A photosensitive resin composition comprises a polymer having a repeating structural unit of the following Formula (1):(wherein R1 and R2 are each independently a single bond, a methylene group, or an ethylene group, Y is an alkylene group of Formula (2) or a combination of the alkylene group of Formula (2) with an alkylene group of Formula (3), n is an integer of 1 to 110, and X is a divalent aliphatic hydrocarbon group, a divalent alicyclic hydrocarbon group, or a divalent aromatic hydrocarbon group) and a (meth)acryloyl group at both ends, a bifunctional (meth)acrylate compound, a polyfunctional thiol compound, a photo-radical generator and an organic solvent, and then the bifunctional (meth)acrylate compound contained in an amount of 5% to 50% by mass and the polyfunctional thiol compound is contained in an amount of 0.1% to 10% by mass, relative to the content of the polymer.
Owner:NISSAN CHEM IND LTD
Methyltetrazole sulfides and sulfones
The present invention relates to a process for the preparation of a 1-methyl-1H-tetrazole-5-thio derivative comprising reaction of a halomethyl substrate with 1-methyl-H-tetrazole-5-thiol to obtain a thio-ether compound, and oxidizing the thio-ether compound to the corresponding sulfone. In case of a chiral halomethyl substrate, the resulting chiral diol sulfone derivative is suitable as a building block for statin type compounds.
Owner:DSM SINOCHEM PHARMA NETHERLANDS
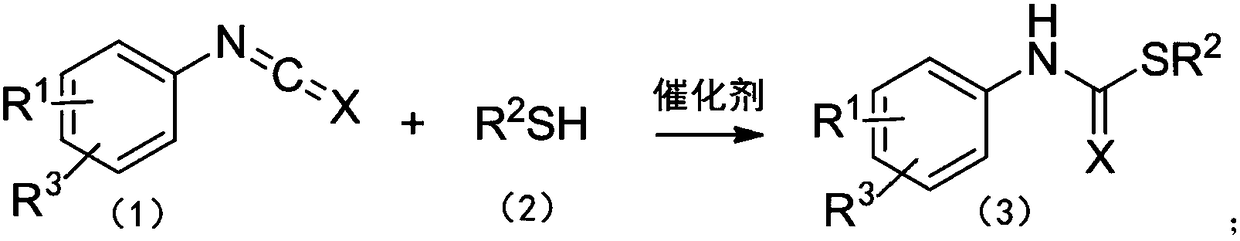
Method for catalyzing addition reaction of phenyl isocyanate or phenyl isothiocyanate with thiol
The invention relates to a method for catalyzing addition reaction of phenyl isocyanate or phenyl isothiocyanate with thiol. The method for catalyzing the addition reaction of the phenyl isocyanate orthe phenyl isothiocyanate with the thiol includes the steps of allowing the phenyl isocyanate or phenyl isothiocyanate shown in a formula (1) and the thiol shown in a formula (2) to react at the temperature of 10-75 DEG C under the catalysis of a catalyst of rare earth amination and obtaining a thiocarbamate compound shown in a formula (3). The reaction route is as follows, wherein R1 and R3 areindependently selected from C1-C6 alkyl, halogen, C1-C6 alkoxy, nitro or trifluoromethyl; X is an oxygen atom or a sulfur atom; R2 is substituted benzyl, furanmethyl, cyclohexyl or trityl; a substituent group on the substituted benzyl is a C1-C6 alkyl, a halogen or a C1-C6 alkoxy; the molecular formula of the rare earth amination is RE[N(SiMe3)2]3; Re is a rare earth metal element. The method forcatalyzing the addition reaction of the phenyl isocyanate or the phenyl isothiocyanate with the thiol has the advantages that raw materials are simple and easy to get; operation is easy; reaction conditions are mild; yield is high; substrates have a wide range of applications.
Owner:SUZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com