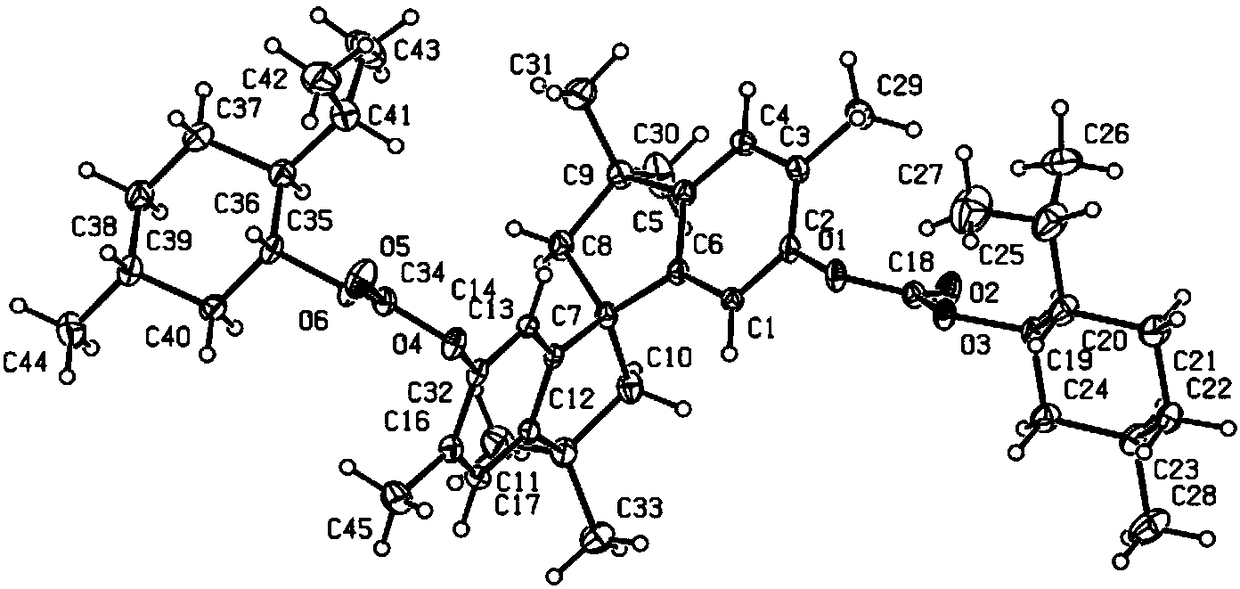
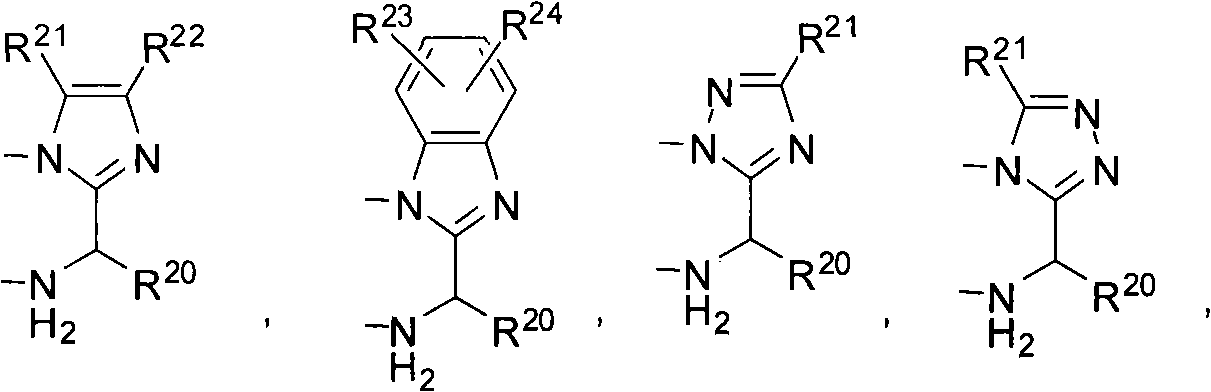
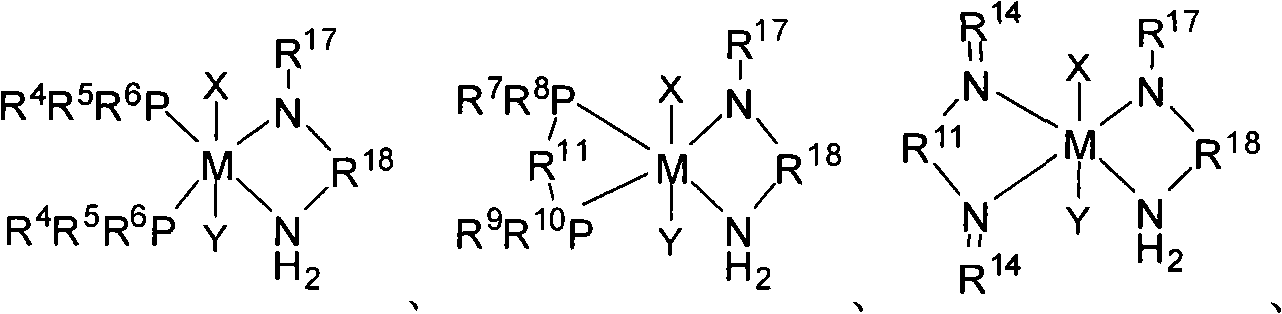
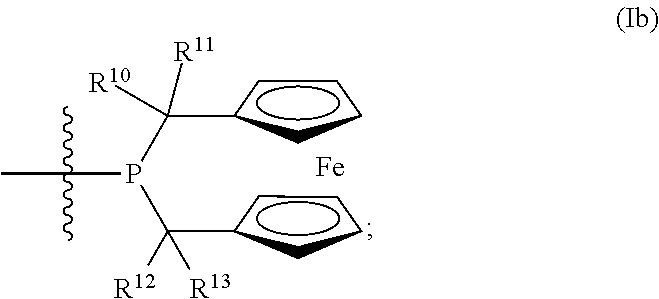
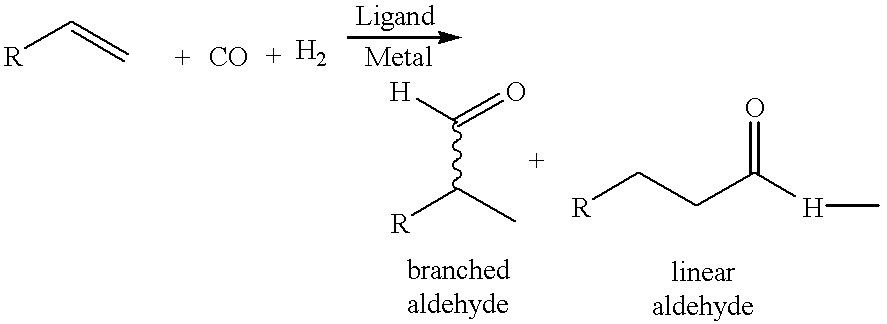
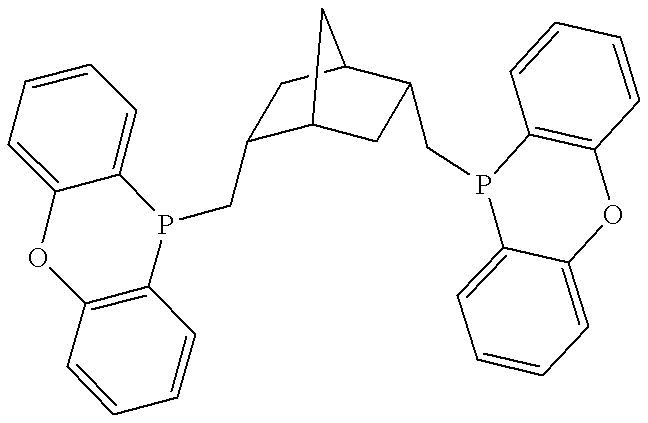
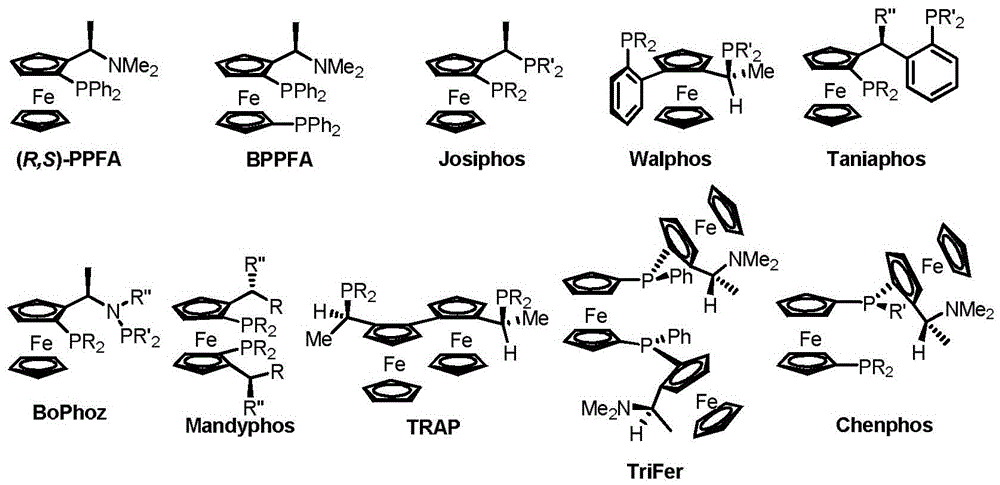
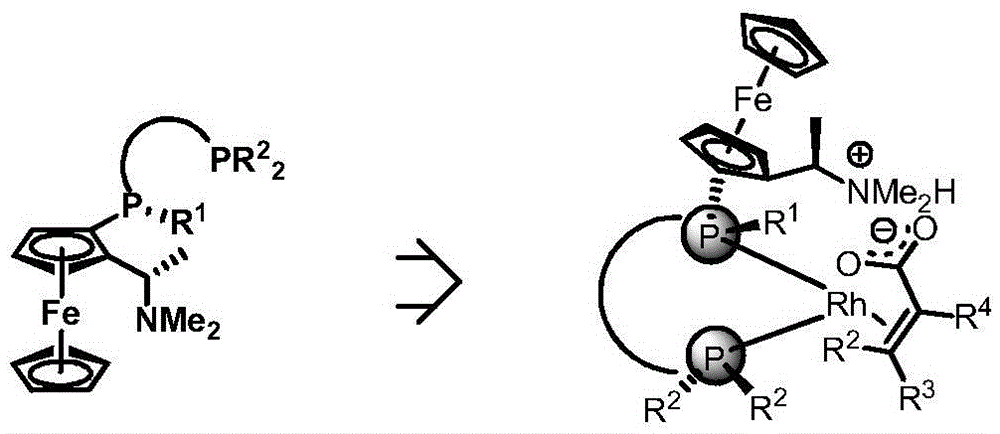
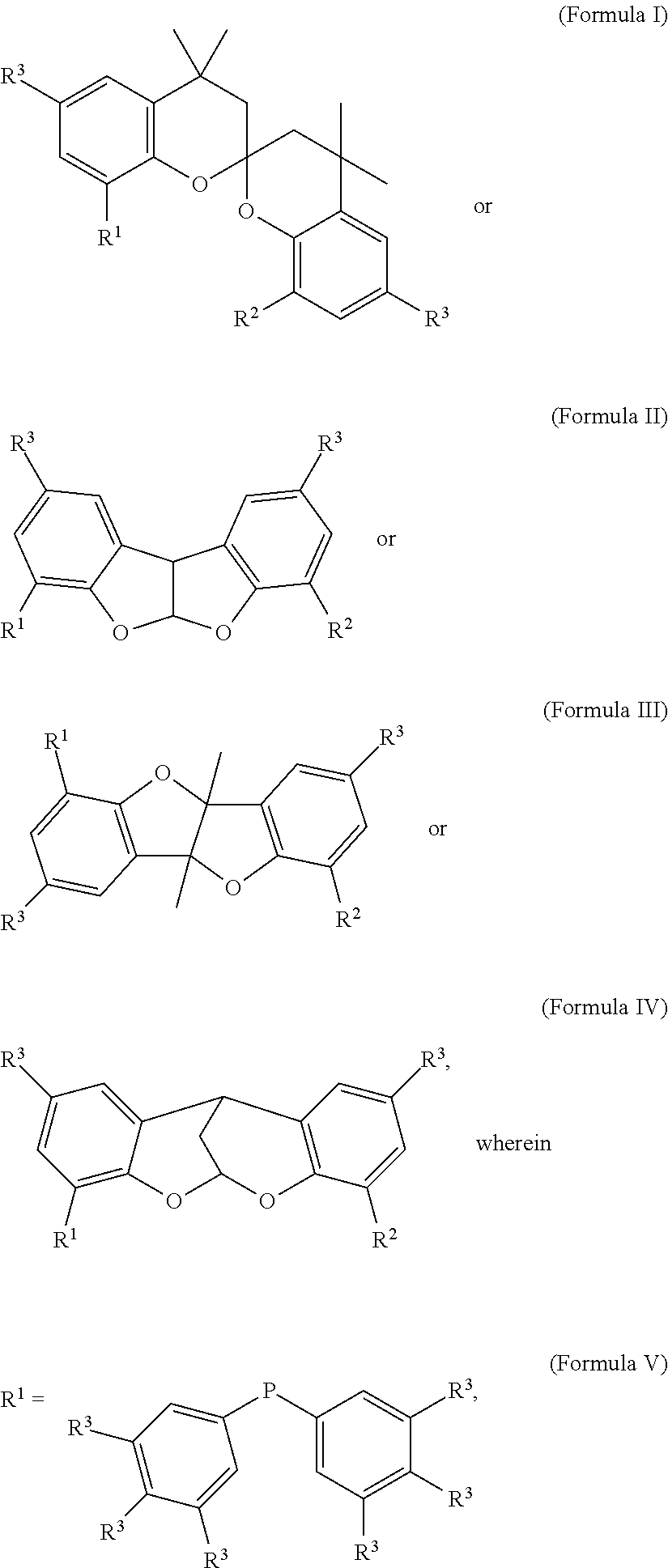
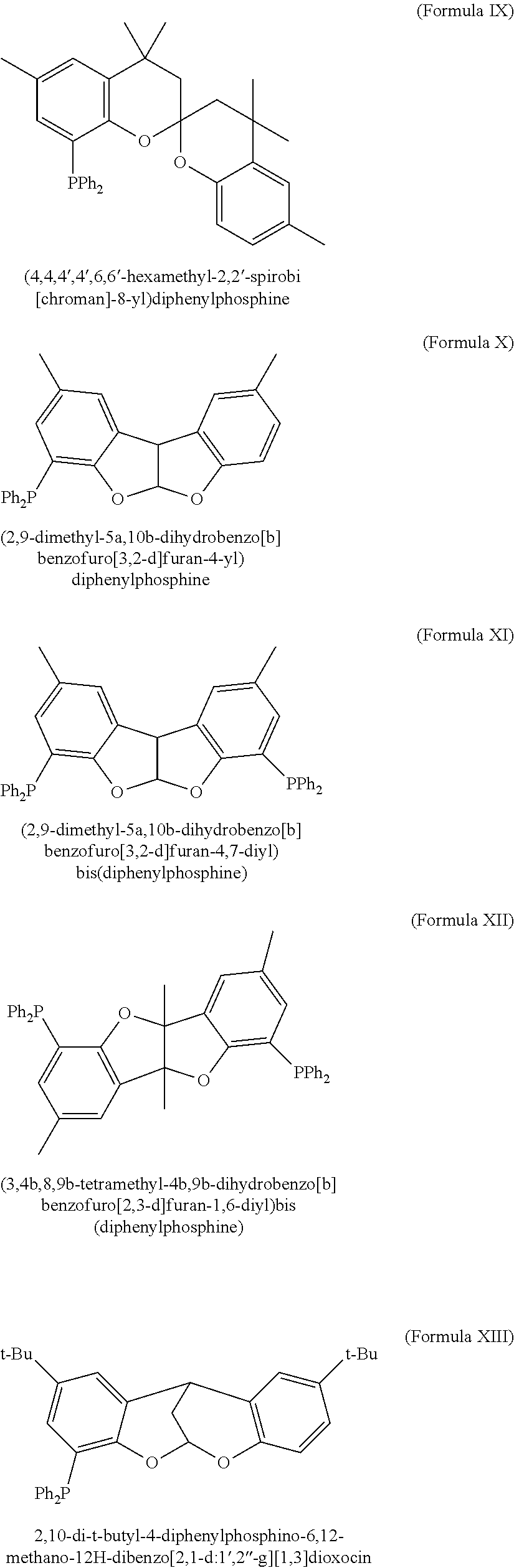
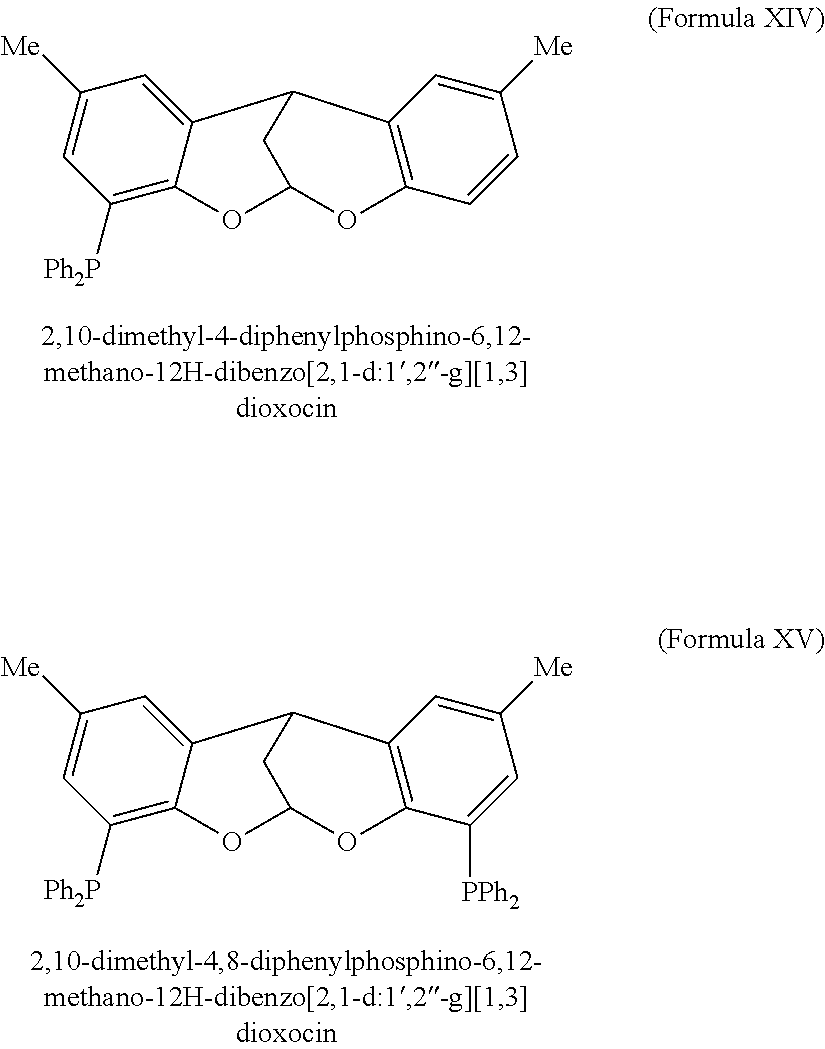
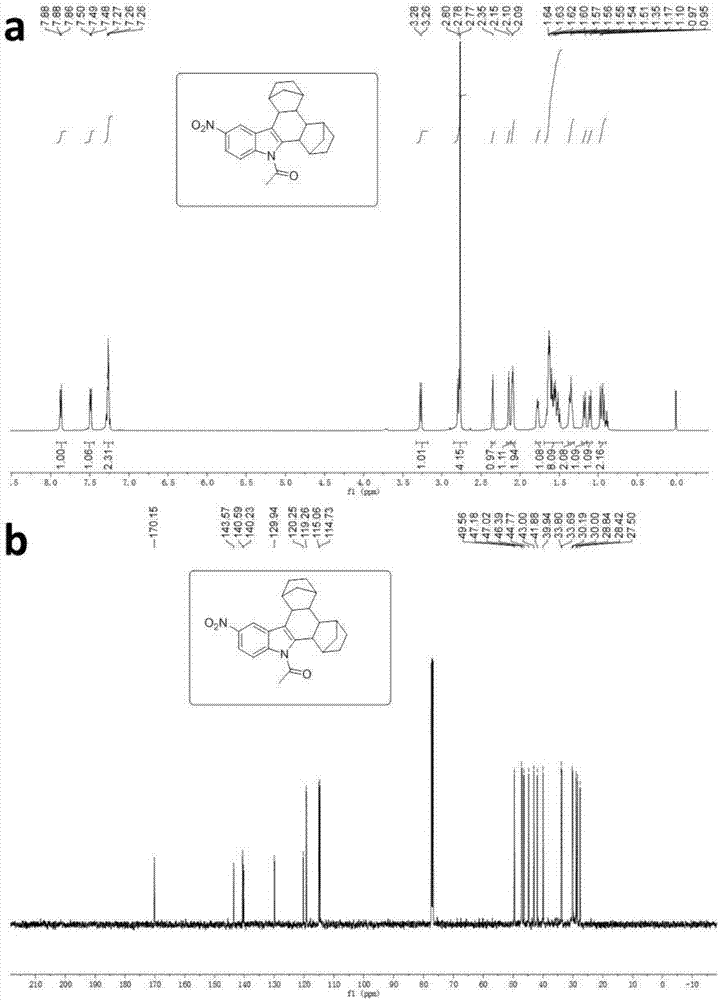
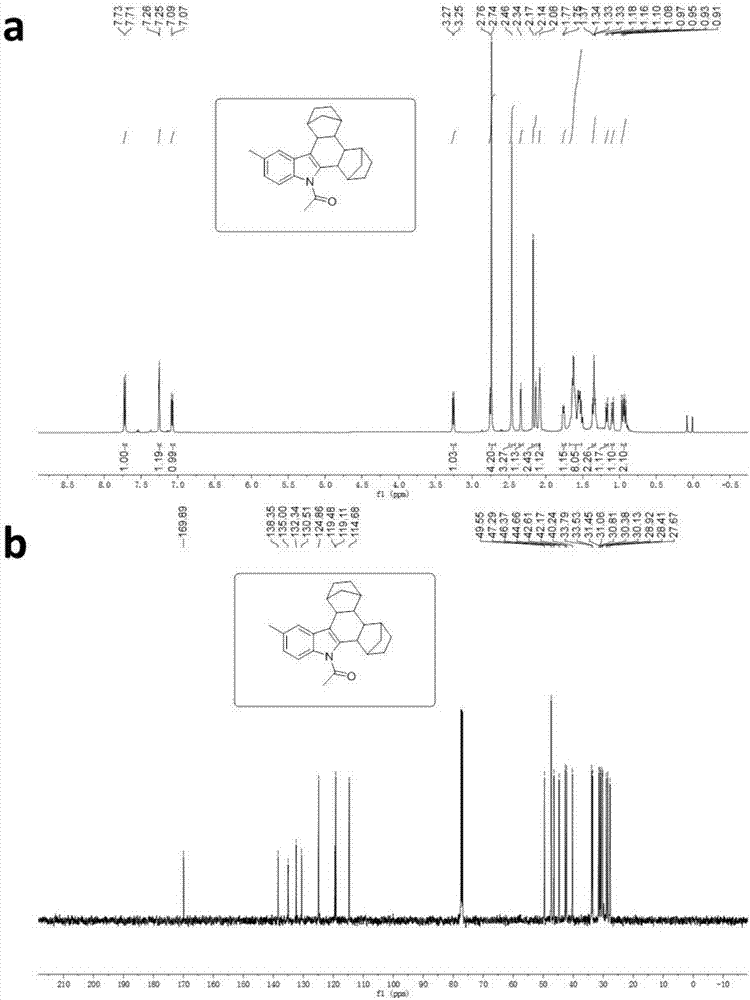
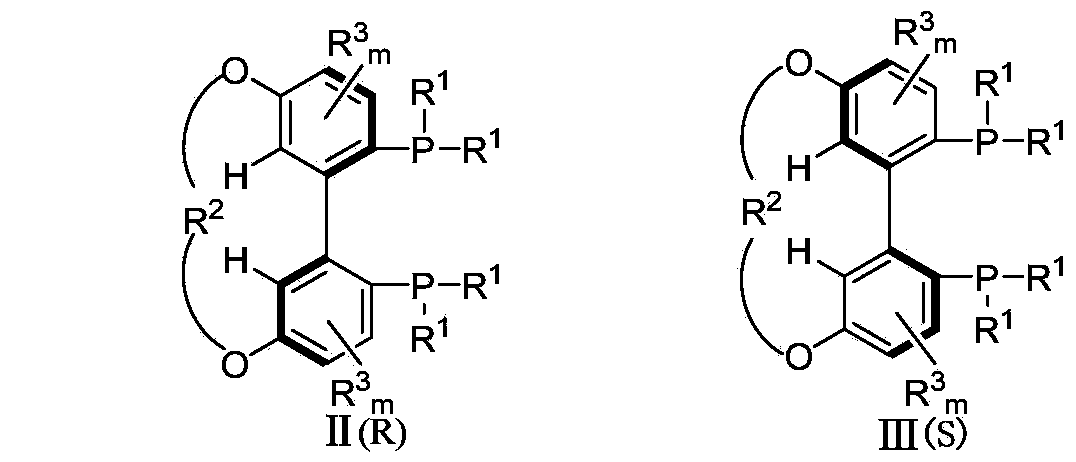
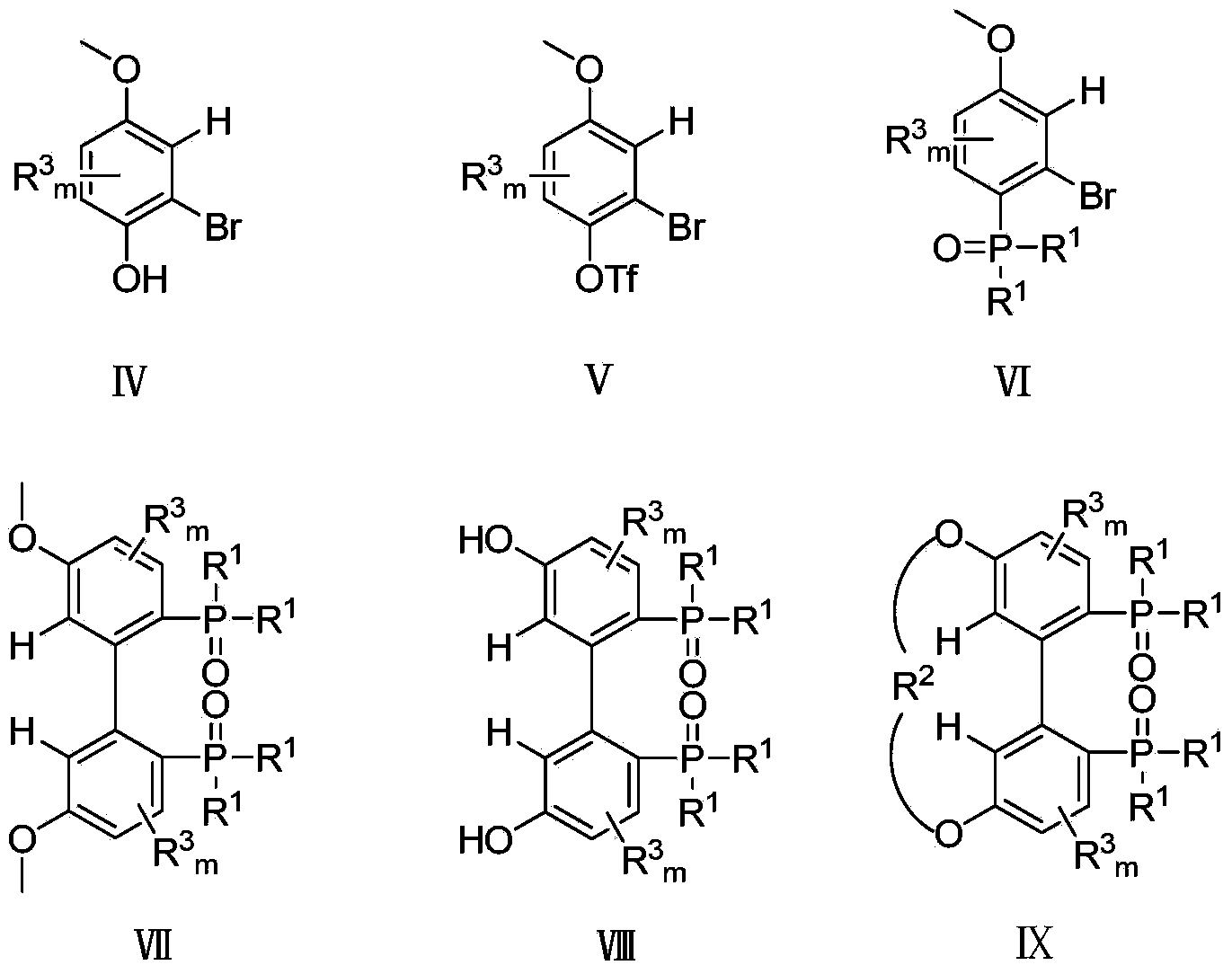
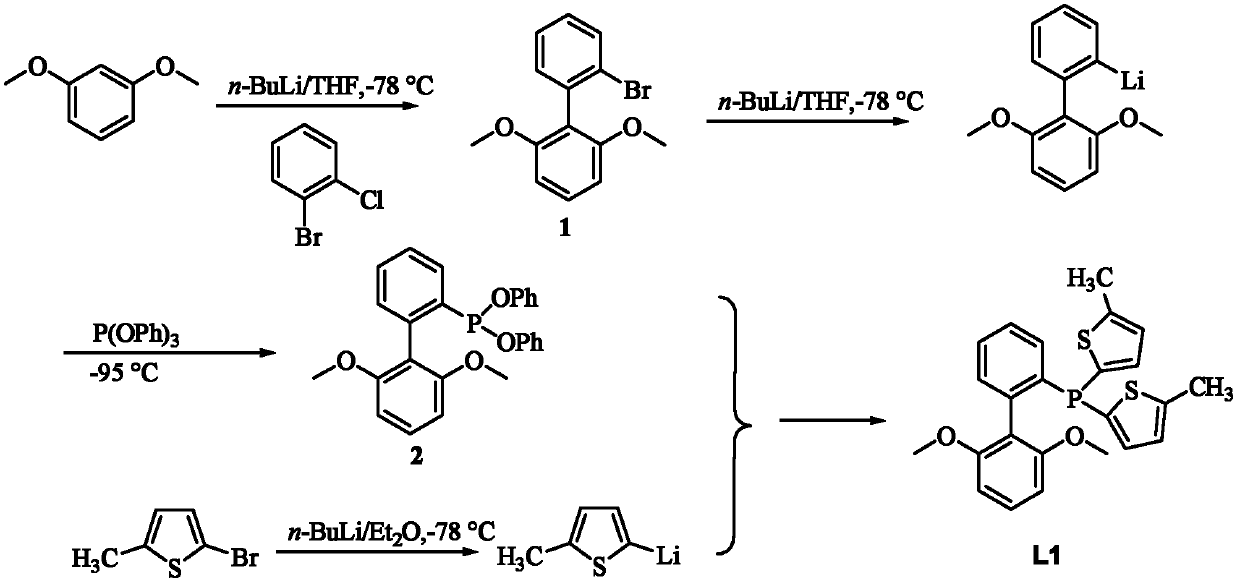
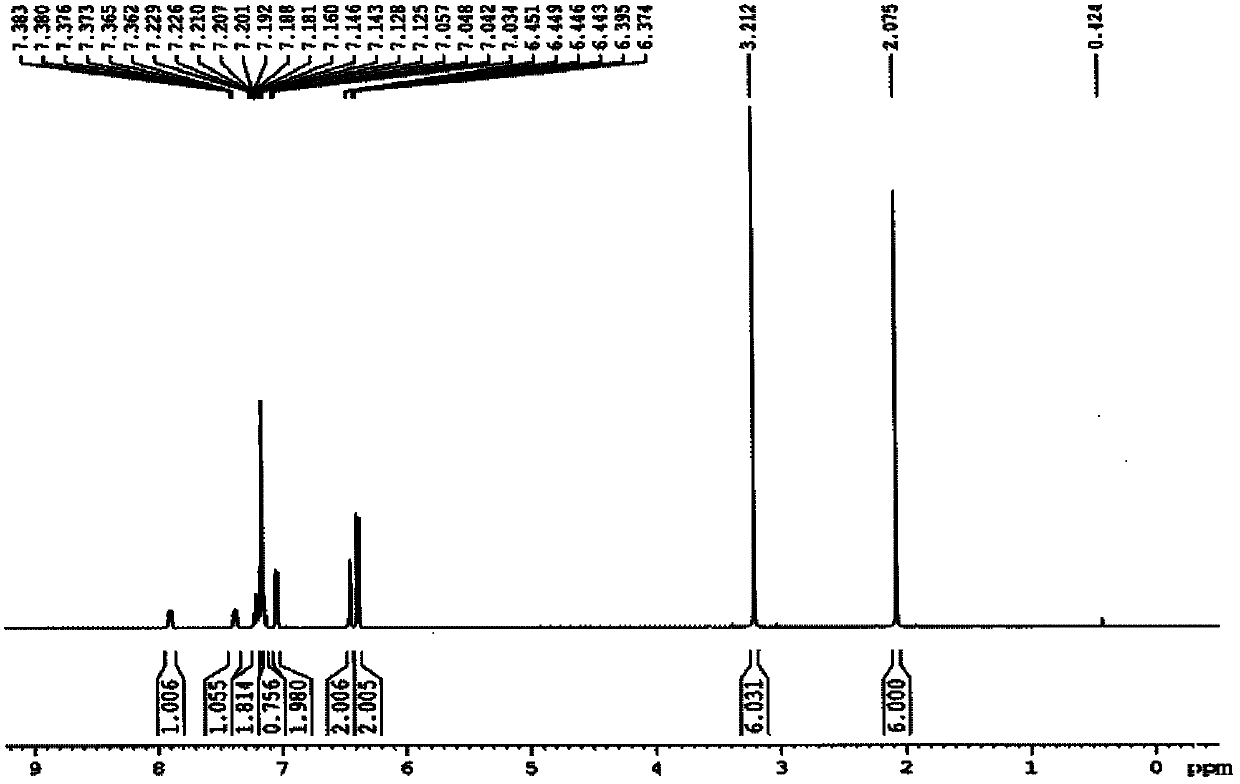
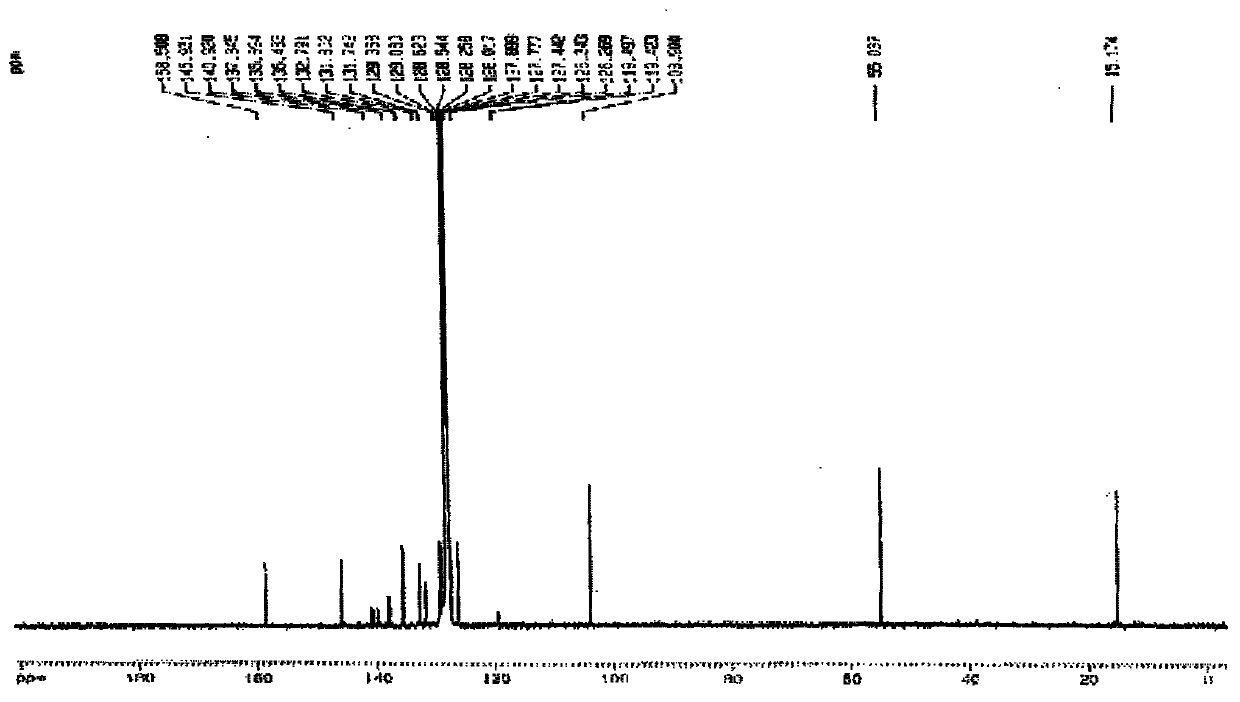
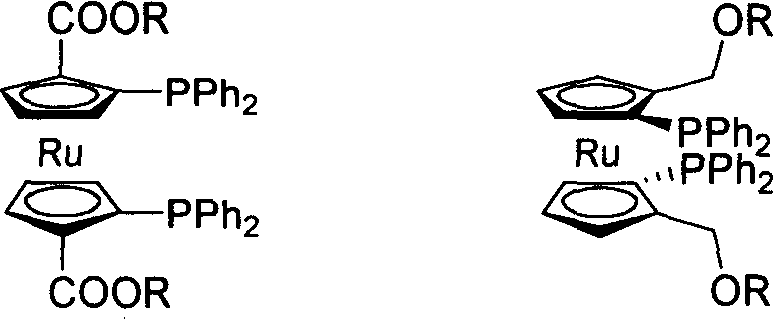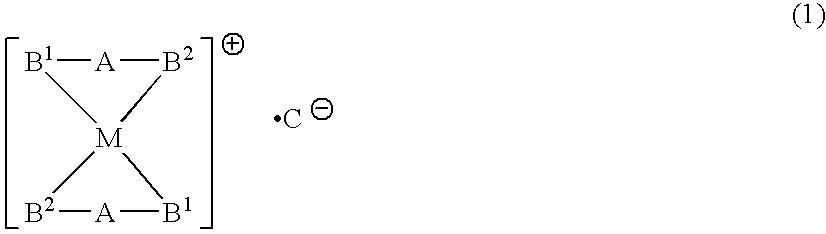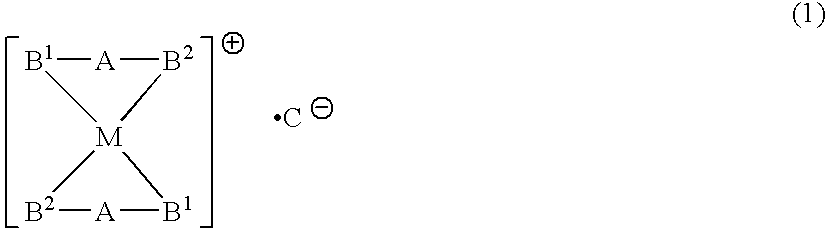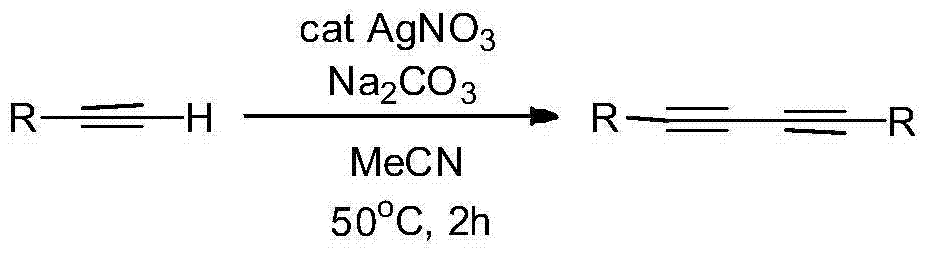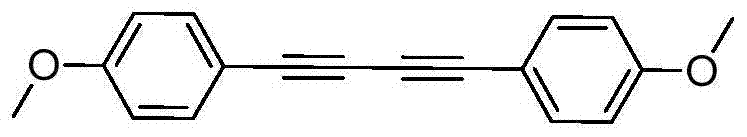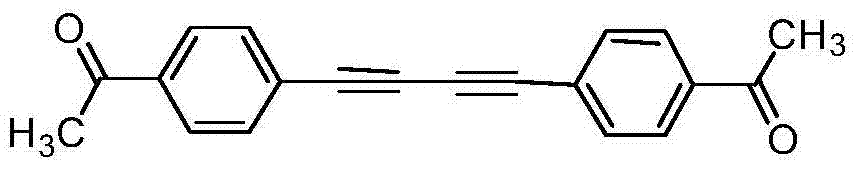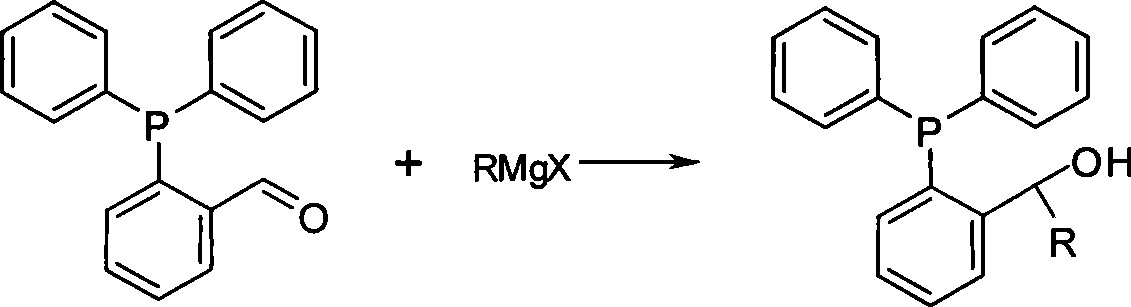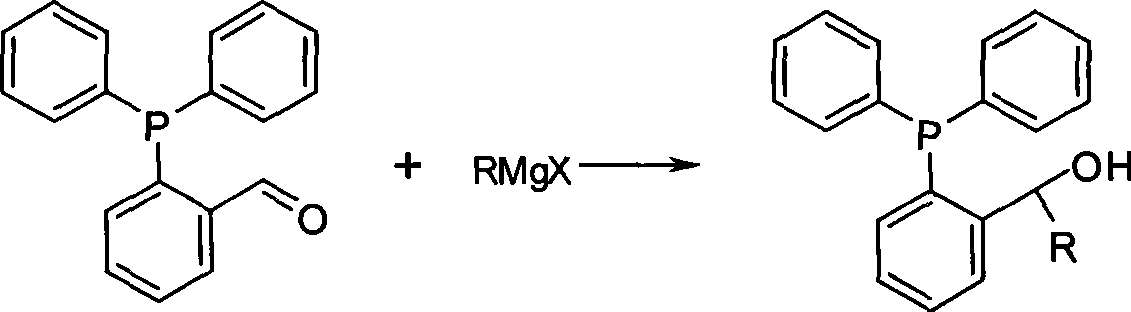Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
68 results about "Phosphorine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
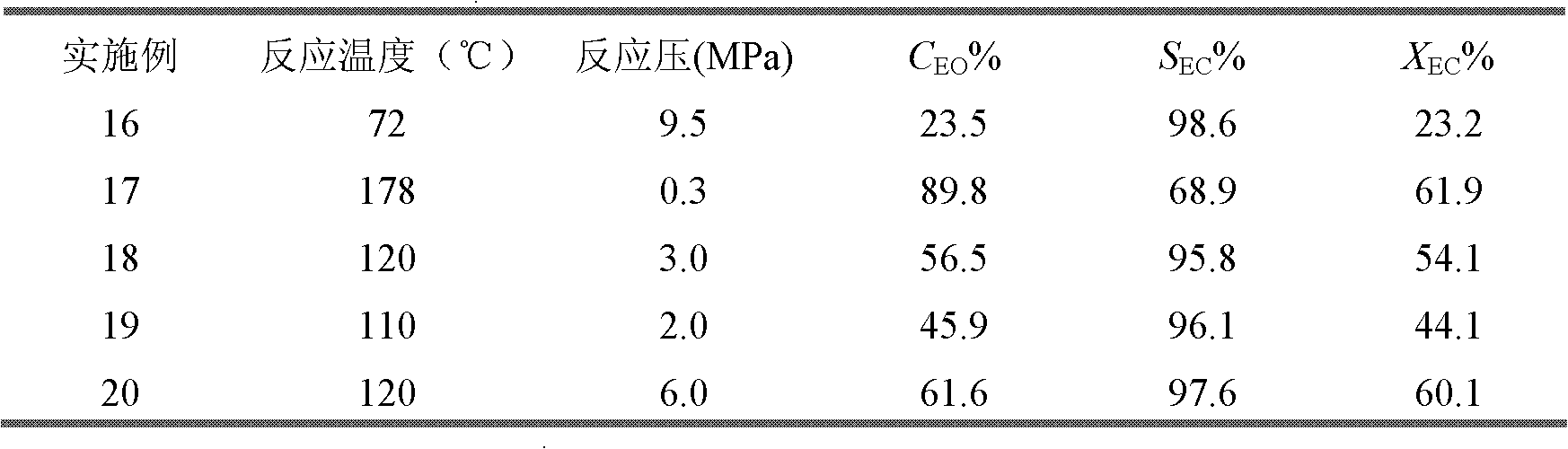
Phosphorine (IUPAC name: phosphinine) is a heavier element analog of pyridine, containing a phosphorus atom instead of an aza- moiety. It is also called phosphabenzene and belongs to the phosphaalkene class. It is a colorless liquid that is mainly of interest in research.
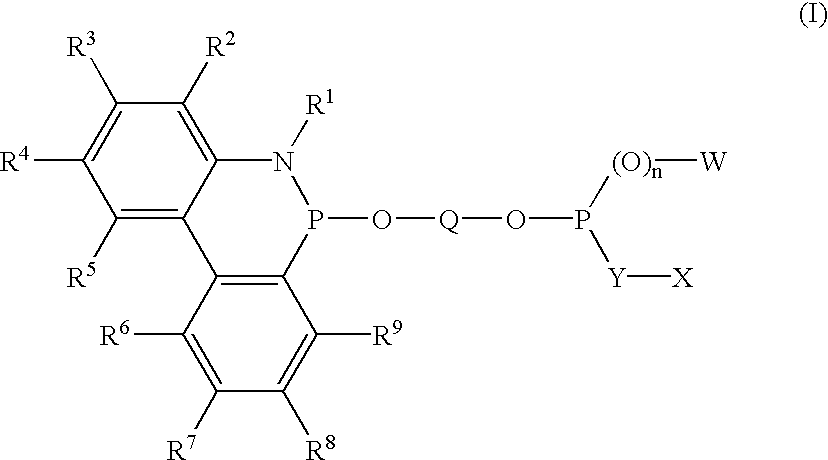
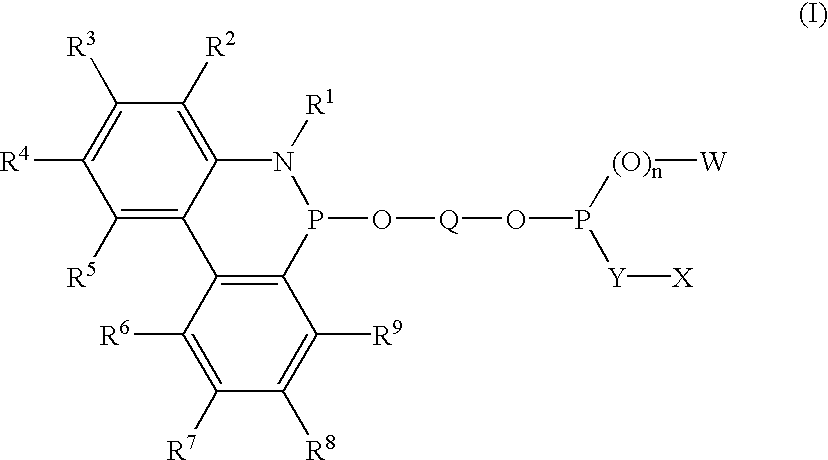
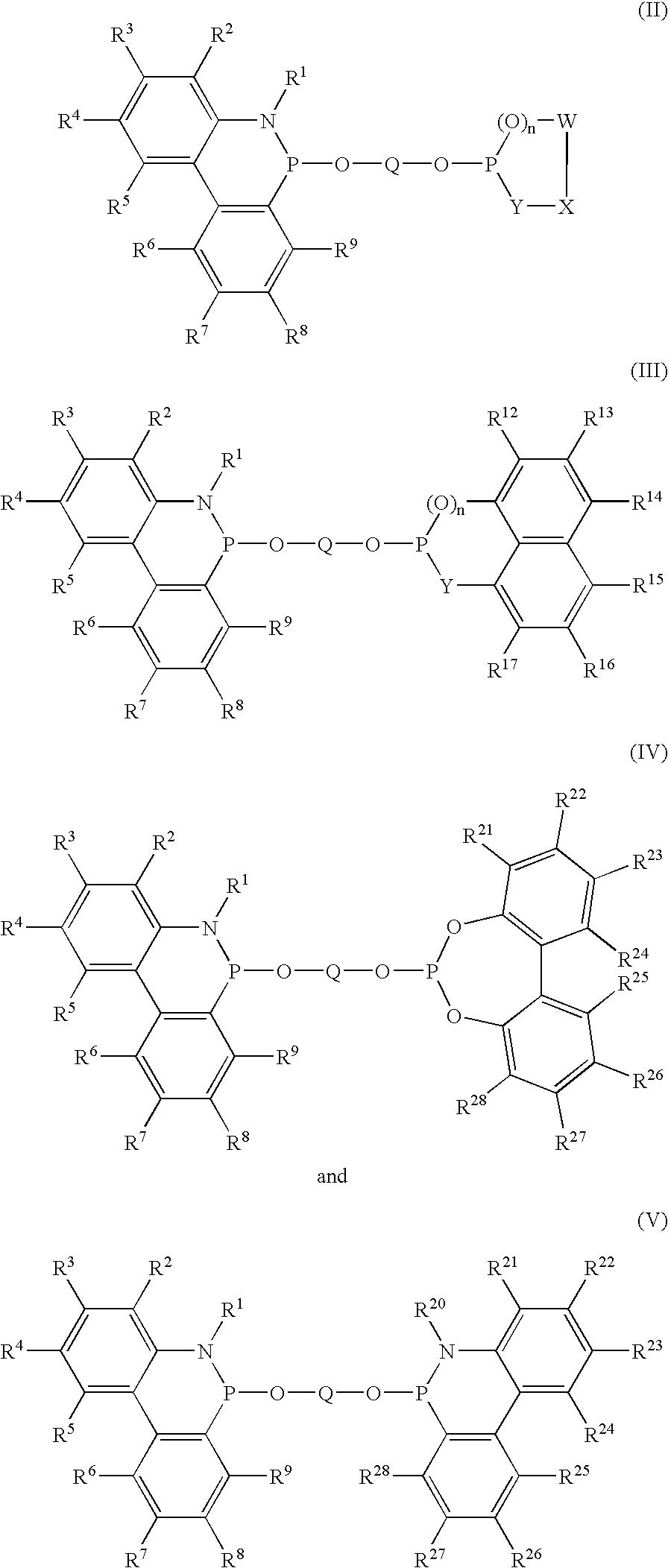
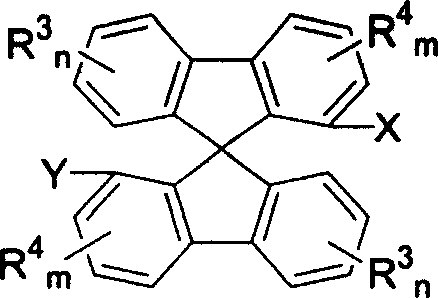
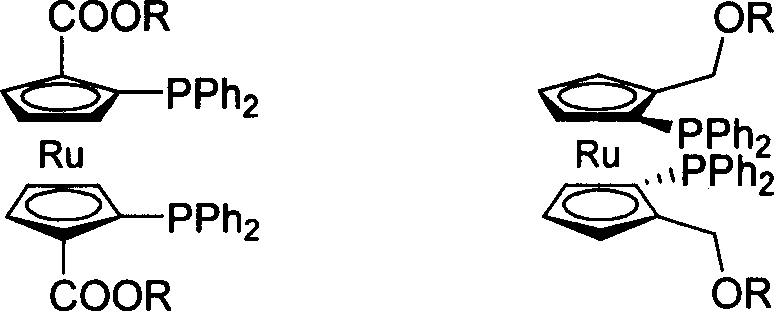
Phosphinine compounds and metal complexes thereof
InactiveUS6818770B2Ruthenium organic compoundsOrganic compound preparationPhosphorineCoordination complex
Phosphinines of formula (I) can be combined with metal salts to prepare hydroformylation catalysts. The phosphinine complexes have two phosphorus centers that may be substituted with a variety of hetero atoms or alkyl substituents to modify the ligand characteristics of the phosphinine. Phosphinine metal complexes are employed under normal hydroformylation reaction conditions. The preparatory routes to the phosphinine ligands of formula (I) allow for their convenient synthesis.
Owner:EVONIK OXENO GMBH (DE)
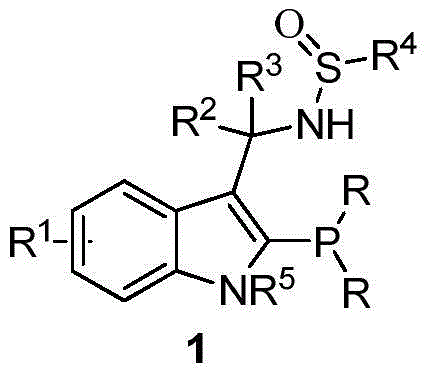
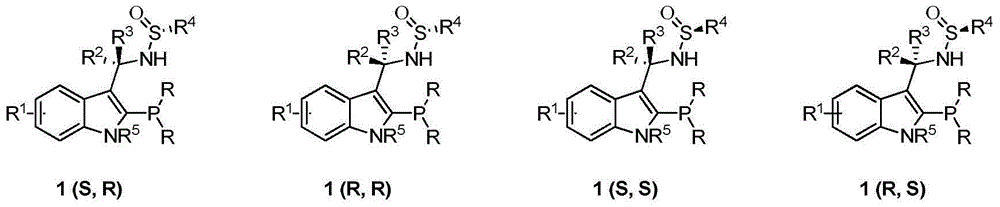
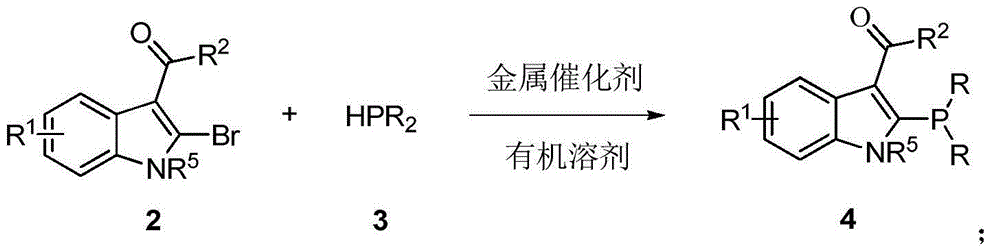
Indole framework based center chirality sulfonamides monophosphine ligand and preparation method
ActiveCN104610355ASave raw materialsEfficient synthesisGroup 5/15 element organic compoundsKetonePhosphorine
The invention discloses an indole framework based center chirality sulfonamides monophosphine ligand and a preparation method thereof. The monophosphine ligand adopts the structure of a formula I, and is prepared through adopting 2-halogenated indole formaldehyde (ketone) to obtain 2-disubstituted phosphine indole formaldehyde (ketone), and taking the 2-disubstituted phosphine indole formaldehyde (ketone) and chirality sulfonamide as raw materials to react with a nucleophilic reagent or a reducing reagent to prepare the compound of the formula I; optical pure compounds of four configurations of (R,R), (R,S), (S,S) and (S,R) can be obtained conveniently through adopting different chirality sulfonamides and different nucleophilic reagents. The monophosphine ligand is simple in framework, convenient to synthesize, easy to transform, can be applied to various metal catalyzed asymmetric reactions as well as the tertiary phosphine catalyzed reaction, and has good application prospect.(The formula I is shown in the description).
Owner:苏州凯若利新材料科技有限公司
Phosphine ligand compounds based on tetramethylspirobiindane skeleton, intermediate of compounds, and preparation method and application of compounds
The invention discloses phosphine ligand compounds based on a tetramethylspirobiindane skeleton, an intermediate of the compounds, and a preparation method and application of the compounds. The phosphine ligand compounds are compounds having a structure represented by a general formula I or a general formula II shown in the description, or an enantiomer, a despinner or a diastereomer of the compounds. The phosphine ligands are obtained by a preparation route using a cheap and easily-available tetramethylspirobiindanediol as a raw material, and compounds represented by a general formula III shown in the description as a key intermediate. The method disclosed by the invention develops the novel phosphine ligands, the phosphine ligands can be used for catalyzing organic reactions, in particular used as chiral phosphine ligands to be widely used in a plurality of asymmetric catalytic reactions such as asymmetric hydrogenation and asymmetric allyl alkylation, and have economical practicability and industrial application prospects.
Owner:ZHEJIANG UNIV
Transient metal complex compound, synthetic method and use thereof
InactiveCN101323630AThe synthesis method is simpleOrganic reductionOrganic compound preparationKetoneAcetophenone
The invention relates to a metal ruthenium complex with the general formula MXYLnL', other transition metal complexes, a synthetic method thereof and an application thereof. Dinitrogen ligand with active hydrogen reacts with transition metal under the action of alkali to generate a transition metal complex which has novel structure, contains phosphine and nitrogen and can be used for catalytic hydrogenation reaction with asymmetric transferring and also for the catalytic hydrogenation reaction, especially used for the catalytic asymmetric hydrogenation reaction of hypnone and derivatives, benzophenone and derivatives, Beta-N, N-dimethylamino-Alpha-hypnone and derivatives, and other ketone chemical compounds.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Phosphine ligands for catalytic reactions
InactiveUS20120022252A1Urea derivatives preparationCarboxylic acid nitrile preparationPhosphazenePhosphorine
Owner:ABBVIE INC
Method of synthesizing tricyclic decane dimethyl carbinol
InactiveCN103396293ALow priceEasy to makePreparation by oxo-reaction and reductionOrganic-compounds/hydrides/coordination-complexes catalystsCobalt(II,III) oxideDecane
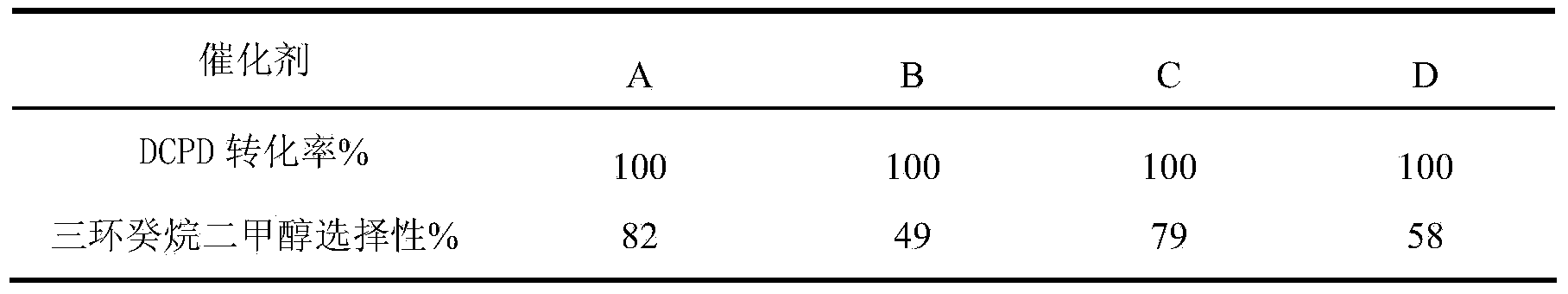
The invention relates to a method of synthesizing tricyclic decane dimethyl carbinol. The method comprises the following steps of: preparing a cobaltosic oxide-supported nanogold catalyst, and modifying the obtained catalyst by a phosphine ligand; and catalyzing dicyclopentadiene to synthesize tricyclic decane dimethyl carbinol by a one-step method under the relatively low temperature and pressure, wherein the conversion rate of the dicyclopentadiene (DCPD) by the method is 99% or higher, and the selectivity of the tricyclic decane dimethyl carbinol can reach 80% or higher.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
Carbonyl sulphide insecticide
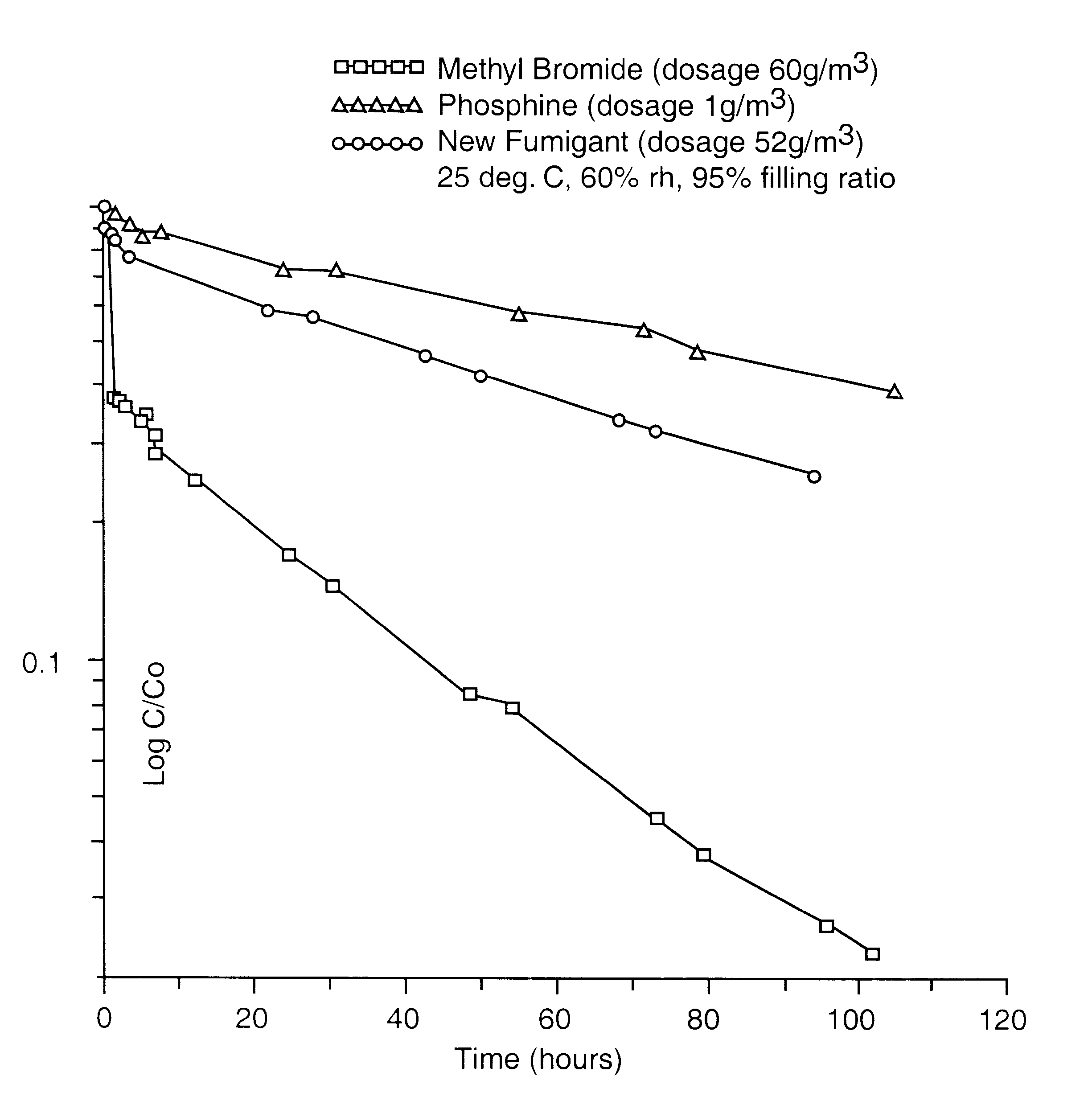
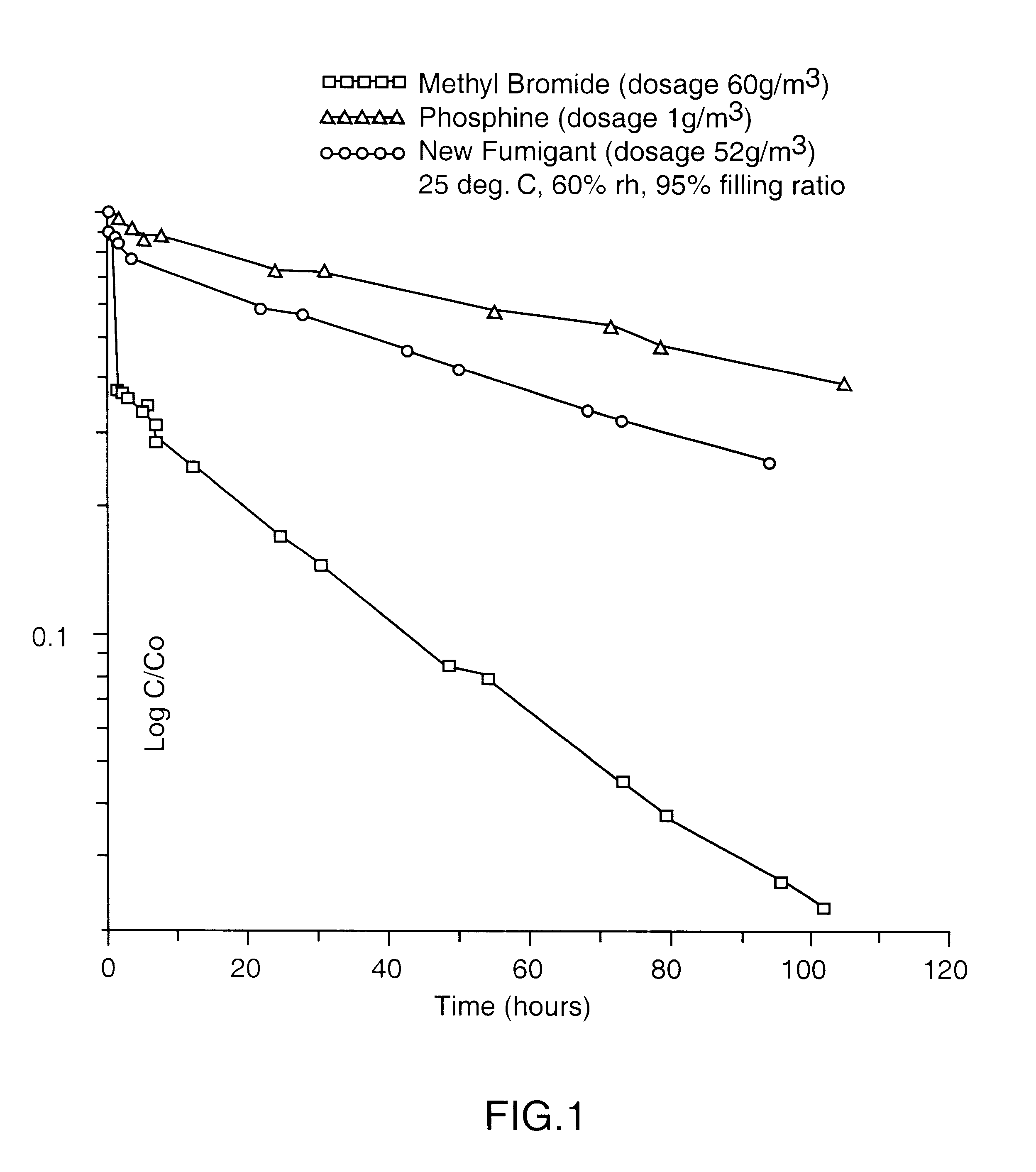
The gaseous chemical compound, carbonyl sulphide, has hitherto been unknown as a fumigant for the control of insects and mites. Experiments have shown conclusively that carbonyl sulphide can be used as such a fumigant, with fumigation properties comparable to those of phosphine and methyl bromide. The effectiveness of carbonyl sulphide against insects (both adult and immature stages), mites, termites and moulds is demonstrated. In addition, its low absorbtion by grain, lower flammability than phosphine, lack of influence on seed germination, and apparent environmental safety make carbonyl sulphide particularly beneficial as a fumigant of stored grain. It may also be used to fumigate other stored produce (including perishable foodstuff), soil, timber and spaces (such as buildings) and any material likely to be infested by insects or mites, or act as a source of such infestation.
Owner:COMMONWEALTH SCI & IND RES ORG
New type spirocyclic diphosphine ligand, and application in asymmetric catalytic hydrogenation
InactiveCN1562926AWide range of usesOrganic-compounds/hydrides/coordination-complexes catalystsGroup 5/15 element organic compoundsAsymmetric hydrogenationRuthenium
The invention relates to a new type chirality spiro Hannane and the key midbody spiro diphenol, the composing method and application of ruthenium complex acetate of said chirality spiro Hannane in Alpha, Beta-unsaturated carboxylic acid. The compound can be used as chirality part for asymmetrical catalysis hydrogenating reaction-especialy the Alpha, Beta-unsaturated carboxylic acid has high stereoselectivity, the highest ee value reach 98 percent and has high reaction activity and high transform quantity (S / C=10000).
Owner:NANKAI UNIV
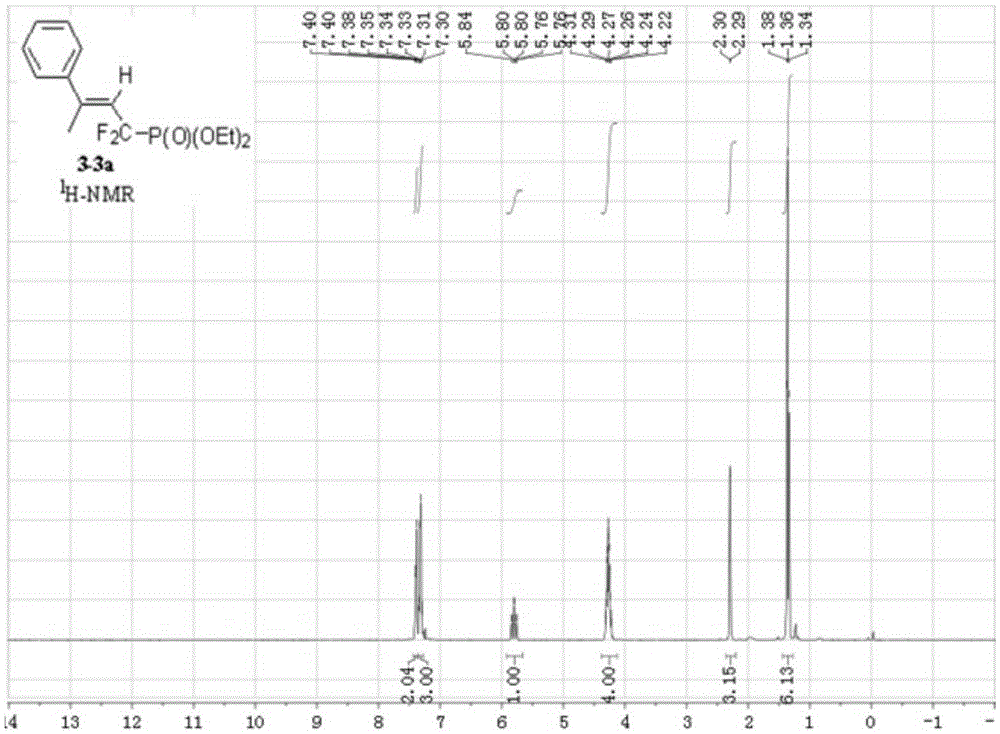
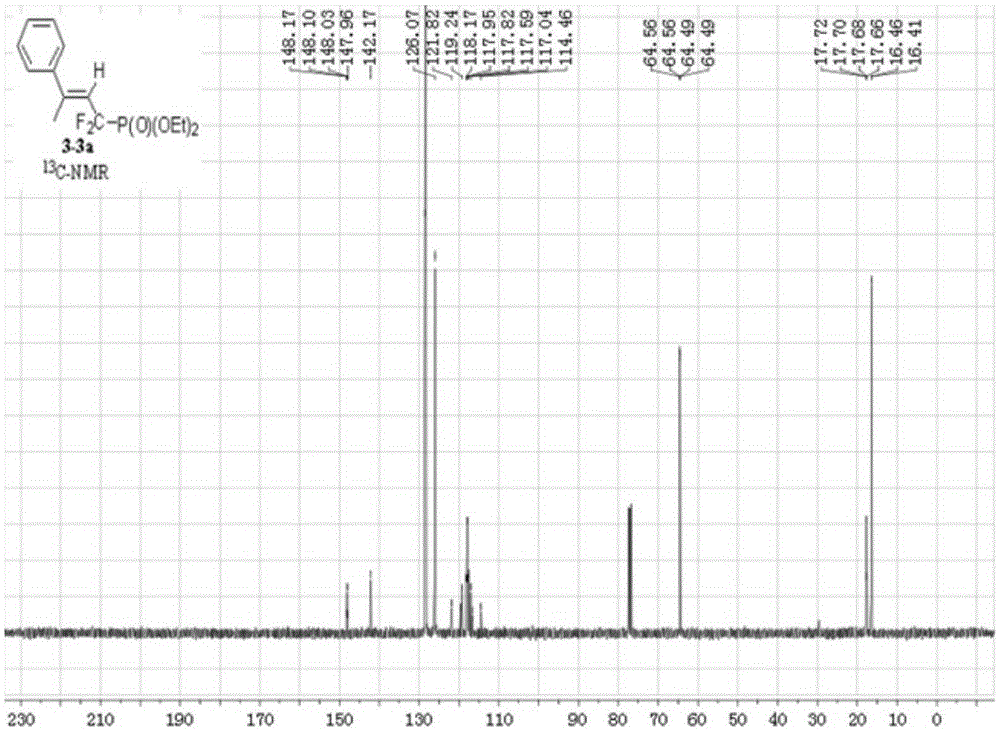
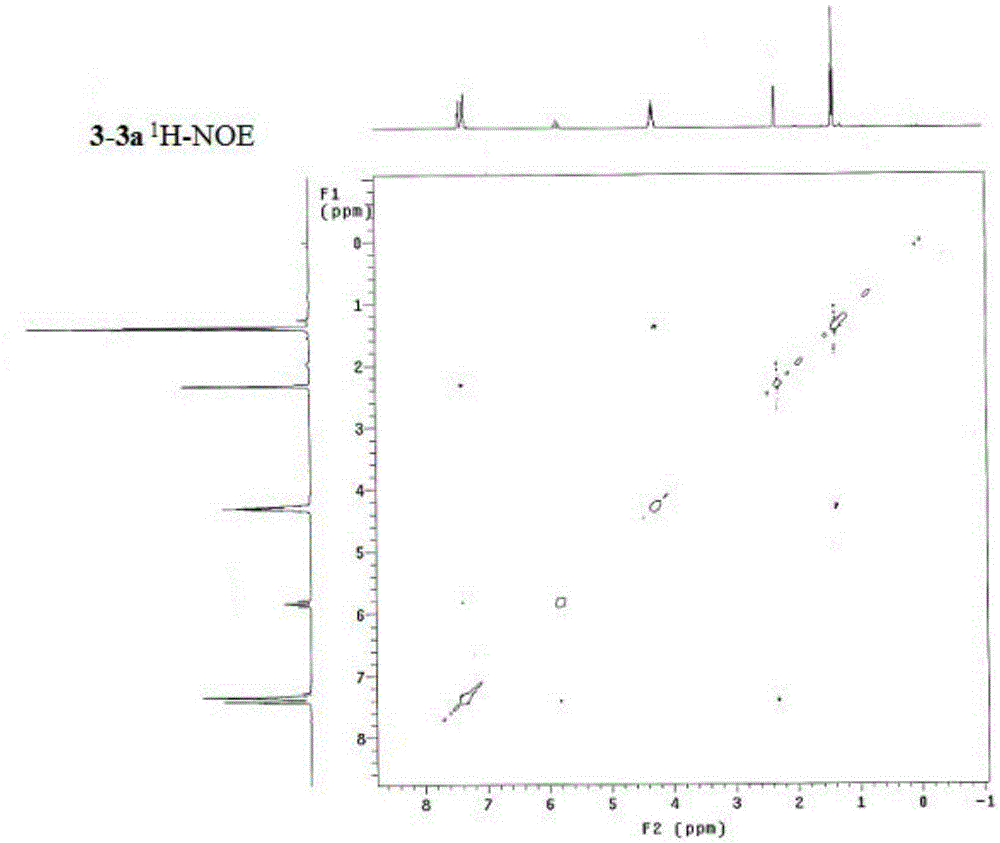
Synthesis method of alpha,alpha-difluoromethylene vinyl phosphonate
InactiveCN105348321AHigh stereoselectivityHigh selectivityGroup 5/15 element organic compoundsSynthesis methodsAllene
A synthesis method of alpha,alpha-difluoromethylene vinyl phosphonate having a structure represented by the formula I and having high position and high stereoselectivity is characterized by comprising the steps: with alpha,alpha-difluoromethylene-beta-allene phosphonate as a raw material, adding aryl or aromatic vinyl boric acid RB(OH)2, and under the action of a catalyst palladium acetate Pd(OAc)2 and a phosphine ligand, undergoing an aryl / vinyl hydrogenation reaction, to obtain alpha,alpha-difluoromethylene vinyl phosphonate having the E structure, wherein a substituent R is aromatic vinyl, aryl or heteroaryl. The synthesis method is simple and convenient, mild in conditions, and high in productive rate, the yield of the target fluorine-containing vinyl phosphonate can reach 71%-94%, and the E structure has high selectivity.
Owner:NANJING NORMAL UNIVERSITY
Valeraldehyde and process for its preparation
InactiveUS6342605B1High selectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPhosphorinePhosphine
Owner:CELANESE CHEM EURO GMBH
Chiral diphosphine ligand and application thereof to asymmetric hydrogenation and correlated reactions
ActiveCN105481909AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAsymmetric hydrogenationDiphosphines
The invention discloses a chiral diphosphine ligand based on a ferrocene framework and application thereof to asymmetric hydrogenation and correlated reactions. The novel chiral diphosphine ligand possesses a structure shown as a general formula I. In the general formula I, R1 is methyl, phenyl, t-butyl, hydroxyl and the like, R2 is ethyl, phenyl, cyclohexyl, p-mehtylphenyl, t-butyl, 3,5-dimethylphenyl, 3,5-di-t-butylphenyl, 3,5-di-t-butyl-4-methoxyphenyl, 2,6-dimethoxyphenyl, 2,6-dimethylphenyl and anthryl, and the bridging group between two phosphorus atoms is phenyl, naphthyl, alkyl and the like. Also, the invention discloses synthesis of the novel chiral diphosphine ligand and application of the chiral diphosphine ligand to prepare chiral medicines ibuprofen, naproxen and the like.
Owner:WUHAN CATALYS TECH CO LTD
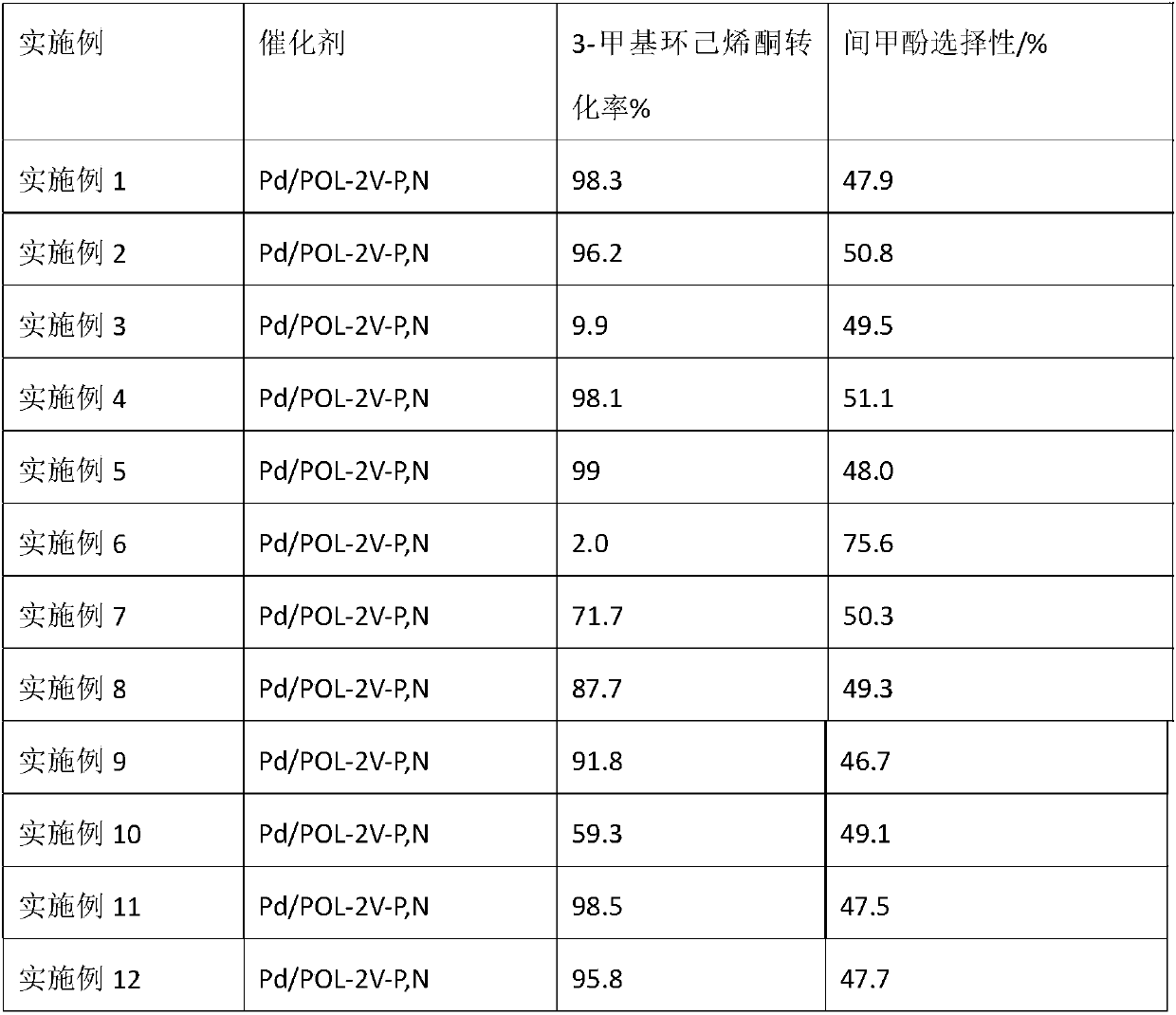
Method for synthesizing m-cresol by 3-methylcyclohexenone dehydrogenation
The invention relates to a method for synthesizing m-cresol by 3-methylcyclohexenone dehydrogenation. With 3-methylcyclohexenone as a reaction material, under the condition that reaction temperature is 393K to 423K and pressure is normal pressure, multifunctional phosphine-and-nitrogen-containing polymer-supported palladium-based catalyst Pd / POL-2V-P,N is adopted to catalyze 3-methylcyclohexenonedehydrogenation, so that m-cresol is synthesized. With porous phosphine-and-nitrogen-containing polymer as a carrier, the method adopts an impregnation method to complex and support palladium to prepare the porous polymer-supported palladium-based catalyst. The method applies the supported palladium-based catalyst Pd / POL-2V-P,N to dehydrogenate 3-methylcyclohexenone on a slurry bed to prepare m-cresol. Under the conditions of normal pressure and no oxygen, high activity is shown, and the catalyst is easy to separate and recycle.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
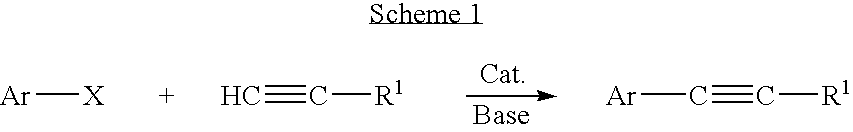
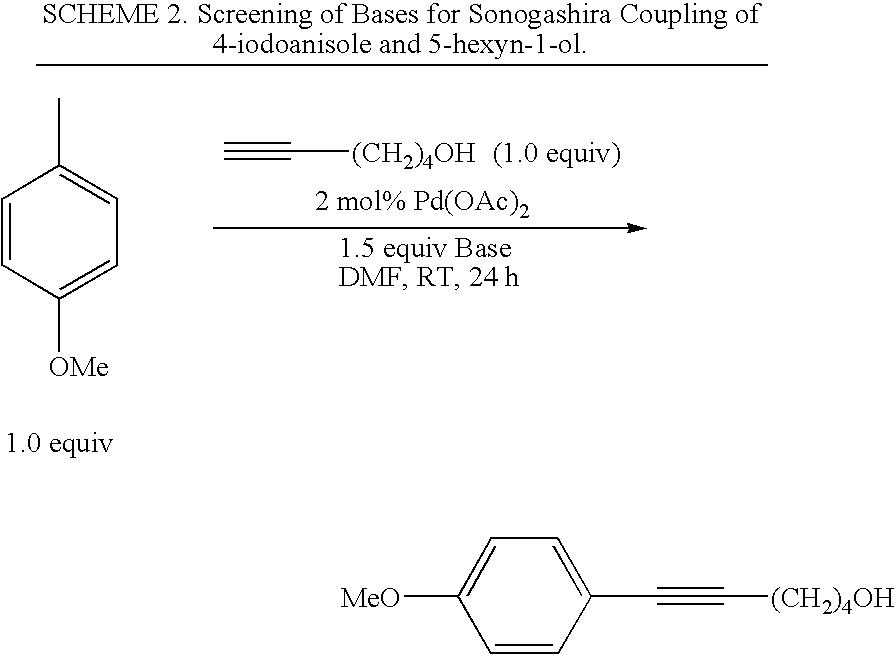
Palladium-catalyzed coupling of aryl halides with alkynes
A method is provided to couple an aryl halide to an alkyne comprising reacting a compound of the formula ArX, wherein Ar is a substituted or unsubstituted aryl group and X is I or Br, with a compound of the formula HC≡C—R1 wherein R1 is a substituted or unsubstituted organic group, in the presence of an effective amount of a phosphine-free, oxime-free palladium catalyst; (C1-C4)alkyl N+(−OAc) or an alkali metal carbonate, to yield a compound of the formula Ar—C≡C—R1, wherein the reaction is carried out in the absence of an organic amine or copper(I).
Owner:IOWA STATE UNIV RES FOUND
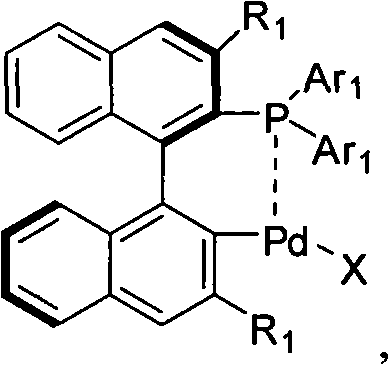
Palladium catalyst of axis chiral dinaphthalene frame phosphine-containing ligand, synthetic method and use thereof
InactiveCN101306388AOrganic-compounds/hydrides/coordination-complexes catalystsAsymmetric synthesesPalladium catalystPhosphorine
The invention comprises a cyclopalladated catalyst with axial chiral binaphthyl backbone containing phosphine ligand as well as the synthesis thereof, and the application of the catalyst with cyclopalladated catalyst with axial chiral binaphthyl backbone containing phosphine ligand with a similar structure to the hetero atom norbornadiene ring cleavage reaction through an asymmetric catalytic organic boron agent. The cyclopalladated catalyst containing axial chiral binaphthyl backbone has the following structural formula. The complex is applied to the ring cleavage reaction of various organic boron agents to the hetero atom norbornadiene.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Method for preparing 2-benzyl pyridine compound
InactiveCN101863826AAvoid dependenceImprove compatibilityOrganic chemistryOrganic synthesisPalladium catalyst
The invention discloses a method for preparing 2-benzyl pyridine compounds, which comprises the following step of performing a decarboxylation coupling reaction of a decarboxylation coupling reagent and an electrophilic substrate in an organic solvent in the presence of a palladium catalyst and phosphine ligand to obtain 2-benzyl pyridine compounds. Through the method, the aim of synthesizing an important intermediate, which is functionalized 2-benzyl pyridine, and derivatives thereof in the organic synthesis or the synthesis of medicinal intermediates is realized, and the method provides an effective solution for the synthesis of the compounds in the organic synthetic chemistry and the synthesis of the medicinal intermediates. The method has the advantages of avoidance of dependency on alkali, low cost, safety, stability, low toxicity, convenient operation, a few by-products, atomic economy higher than that of the prior art, green chemistry properties, little catalytic amount, high catalytic efficiency, easy separation and high conversion rate and yield, and has industrial and synthetic values.
Owner:UNIV OF SCI & TECH OF CHINA
C2-symmetrical bis ruthenium Diphosphine Ligand only with surface chirality
InactiveCN1876668AHigh reactivityHigh stereoselectivityGroup 5/15 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsRutheniumDiphosphines
The invention relates the synthesis of C2-bis ruthenium doublephosphine ligand. The ligand can be used in metallic catalysis asymmetric reaction, and the ligand has the good reaction active and stereoselectivity. The R is -Me, -Et.
Owner:SHANGHAI JIAO TONG UNIV
Phosphine transition metal complex, method for producing the same, and antitumor agent containing the same
InactiveUS7655810B2Good antitumor activityEliminate side effectsBiocidePlatinum organic compoundsPlatinumCopper
A phosphine transition metal complex is expressed by general formula (1):wherein A represents a groups selected from among alkylene, phenylene, and cis-vinylene; M represents an atom selected from the group consisting of gold, silver, copper, and platinum; B1 and B2 each represent a substituted or unsubstituted heterocyclic group containing a trivalent phosphorus atom forming a covalent bond with A and coordinating with M; and C represents an anionic atom.
Owner:NIPPON CHECMICAL IND CO LTD
Phosphine-based catalysts useful for the telomerization of butadiene
InactiveUS8779164B2Organic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsButadiene Dioxide1-Octene
A phosphine-based catalyst composition suitable for the telomerization of butadiene to produce 1-octene includes palladium and one of a class of novel phosphine ligands characterized by two potentially functionalized phenyl rings and cyclized 5- or 6-member alkoxy groups that, together, bridge the two potentially functionalized phenyl rings. In these groups the alkoxy moiety of each is located ortho to at least one functionalizing moiety, if any, on the phenyl rings. The catalysts including this class of phosphine ligands may exhibit higher catalytic activity and selectivity, and may be used at lower temperatures, than many other phosphine ligand catalysts, reducing costs. Palladium precipitation may also be reduced.
Owner:DOW GLOBAL TECH LLC
Preparation method of symmetric 1,4-disubstituted-1,3-diacetylene
InactiveCN104710254AHigh yieldImprove practicalityOrganic compound preparationHydrocarbon by hydrocarbon condensationDiacetyleneRoom temperature
The invention discloses a preparation method of symmetric 1,4-disubstituted-1,3-diacetylene. Homocoupling of alkyne is carried out in a solvent in the presence of an alkali with silver as a catalyst to obtain the symmetric 1,4-disubstituted-1,3-diacetylene. The method has the advantages of simple operation, obtaining of corresponding diacetylene compounds at room temperature without air isolation or addition of a phosphine ligand or a precious metal, simple post-treatment, and very good practicality and economic values.
Owner:SHAOXING UNIVERSITY
Catalyst for cross-coupling reaction, and process for production of aromatic compound using the same
InactiveUS20110152523A1Proceed efficientlyHigh yieldOrganic-compounds/hydrides/coordination-complexes catalystsIron group organic compounds without C-metal linkagesCarbon atomHalide
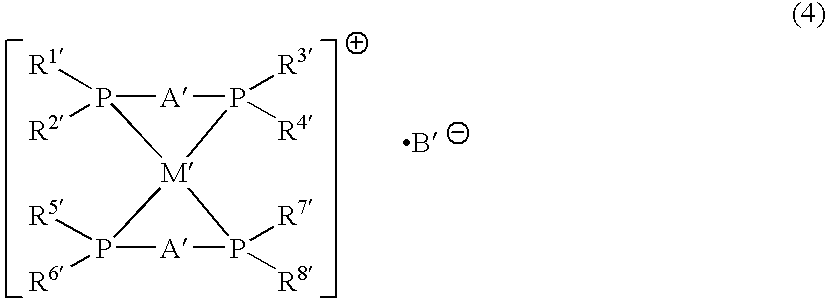
The present invention provides a process for efficiently producing an alkylated aromatic compound in good yield, by a cross-coupling reaction between an alkyl halide and an aromatic magnesium reagent. A process for producing an aromatic compound represented by Formula (1):R—Ar′ (1)wherein R is a hydrocarbon group, and Ar′ is an aryl group;the process comprising:reacting a compound represented by Formula (2):R—X (2)wherein X is a halogen atom, and R is as defined above, with a magnesium reagent represented by Formula (3):Ar′—MgY (3)wherein Y is a halogen atom, and Ar′ is as defined above, in the presence of a catalyst for cross-coupling reactions comprising an iron compound and a bisphosphine compound represented by Formula (4):wherein Q is a divalent group derived from an aromatic ring by removing two hydrogen (H) atoms on adjacent carbon atoms; and each Ar is independently an aryl group.
Owner:KYOTO UNIV
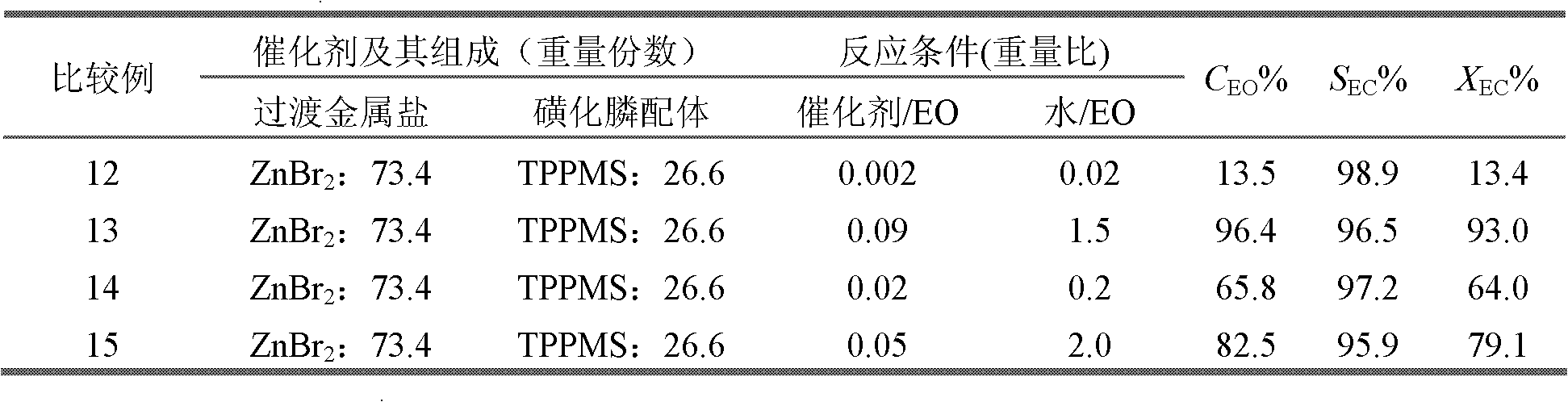
Method for preparing vinyl carbonate by epoxy ethane and carbon dioxide
ActiveCN103030624AHigh activityHigh selectivityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsEpoxyReaction temperature
The invention relates to a method for preparing vinyl carbonate by epoxy ethane and carbon dioxide, which mainly solves the problem of the prior art that the activity of a catalyst is quickly reduced when water exists. Aiming at well solving the problem, the method adopts the technical scheme that the epoxy ethane and the carbon dioxide are used as raw materials, and the reaction raw materials come into contact with the catalyst to generate the vinyl carbonate when the mass ratio of the epoxy ethane to the water is (0.01-5):1, the reaction temperature is 50 to 200 DEG C, the pressure of the carbon dioxide is 0.1 to 10.0 MPa, and the mass ratio of the catalyst to the epoxy ethane is (0.001-0.1):1; and the catalyst comprises the following components in parts by weight: (a) 10 to 80 parts of transition metal salt, and (b) 20 to 90 parts of phosphine ligand containing sulfonic acid groups, wherein the structural formula of the phosphine ligand containing the sulfonic acid groups is CaHbPc(SO3R)d, a is 14 to 30, b is 12 to 32, c is 1 to 2, d is 1 to 3, and R is H, Na, K or Cs. The method can be used for the industrial production of the vinyl carbonate prepared by the epoxy ethane and the carbon dioxide.
Owner:CHINA PETROLEUM & CHEM CORP +1
Water-soluble cyclic palladium hydrate mono-phosphine salt compound, and preparation method and application thereof
InactiveCN106674287AEasy to synthesizeReduce dosageOrganic-compounds/hydrides/coordination-complexes catalystsPalladium organic compoundsSolventPhosphine
The invention relates to a water-soluble cyclic palladium hydrate mono-phosphine salt compound, and a preparation method and an application thereof. The compound has a formula shown in the specification, wherein L is a sulfo group-containing mono-phosphine ligand, a phosphine atom and a palladium coordinate; the hydroxy benzyl groups can be singly or simultaneously located on a pyridine ring and a benzene ring; and the hydroxy benzyl groups can be located on any positions on the two rings. According to the invention, the cyclic palladium dimer containing the hydroxy benzyl groups and the sodium salt of the sulfo group-containing mono-phosphine ligand react with each other in an acetone solvent, so that the corresponding water-soluble cyclic palladium hydrate mono-phosphine salt compound can be conveniently synthesized. When the compound is used as a metal catalyst, the dosage of the catalyst is small; clean water used as the solvent and low-cost weak base can be utilized to effectively catalyze the coupled reaction between aryl halide and pyrazole, so as to synthesize and prepare 1-aryl pyrazole. The method has the advantages that the scope of the reaction substrate is wide, the reaction condition is mild, the yield is high, the method is economical and efficient and the method has an important application value.
Owner:LUOYANG NORMAL UNIV
Phosphine-ionic liquid organic porous copolymer-containing heterogeneous catalyst, preparation method and applications thereof
ActiveCN107537562AHigh specific surface area porous structureImprove utilization efficiencyOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsEpoxyFixed bed
The present invention provides a phosphine-ionic liquid organic porous copolymer-containing heterogeneous catalyst, a preparation method and applications thereof, wherein an olefin-functionalized organic phosphine ligand and an olefin-functionalized ion liquid are co-polymerized to form a phosphine-ionic liquid organic porous copolymer, a Lewis acid metal salt is added or is not added, and when the Lewis acid metal salt is added, the metal ions in the metal salt and the naked phosphine in the organic porous copolymer form a coordination bond so as to finally obtain the heterogeneous catalyst.According to the present invention, the catalyst can be used in fixed beds, slurry beds, tank reactors, trickle beds and other reactors; with the application of the catalyst of the present invention in the coupling reaction process of epoxy compounds and carbon dioxide, the respective advantages of the ion liquid (as the ring-opening nucleophilic reagent) and the phosphine-metal coordination compound (activated epoxy compound) are integrated; and by adjusting and changing the copolymerization monomer proportion and the metal loading amount, the obtained catalyst has the double advantages of extremely high activity and high stability.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Phosphine compound of possessing plane chirality cyclophane alkyl, synthetic method, and appliction
InactiveCN101003549AOrganic-compounds/hydrides/coordination-complexes catalystsGroup 5/15 element organic compoundsPlanar chiralityChirality
This invention discloses a method for synthesizing planar chiral cycloaralkyl phosphine compound and its application. The chirality of the compound is derived from the planar chirality of cycloaralkyl framework. The method comprises: deriving from [2.2] dicycloaralkyl to obtain the compound, performing chiral resolution with chiral annulate Pd, separating the diastereoisomers by column chromatography to obtain planar chiral cycloaralkyl phosphine compound, and recovering annulate Pd. The compound, when used as catalyst in allyl amination and alkylation, has high reactivity and high antipode selectivity. Besides, the compound can be used as a ligand in asymmetric catalytic hydrogenation and aldehyde allylation. The compound has such advantages as high stability and simple synthesis.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Preparation method of phosphine ligand and method for phosphine ligand to catalyze and synthetize biaryl and derivatives thereof
ActiveCN101445518AHigh catalytic activityEasy to manufactureOrganic-compounds/hydrides/coordination-complexes catalystsGroup 5/15 element organic compoundsNickel catalystAryl
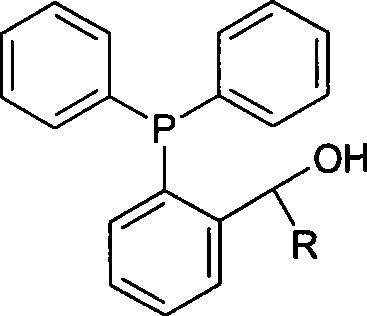
The invention relates to a preparation method of phosphine ligand and a method for the phosphine ligand to catalyze and synthetize biaryl and derivatives thereof. The preparation method of the phosphine ligand comprises the following steps: 2-diphenyl phosphono benzaldehyde is used to react with a Grignard reagent, and the phosphine ligand is acquired; X in the Grignard reagent is halogen, and R is C1-C8 alkyl, aryl or substituted aryl; wherein, the substituted aryl is that one or multiple positions in number 2 to 6 positions of aromatic ring are substituted; and substituent is one or varieties in fluorine, chlorine, phenyl, C1-C8 alkyl and C1-C8 alkoxy. The invention discloses the method of using the phosphine ligand to jointly catalyze and synthetize the biaryl and the derivatives thereof with a nickel catalyst. The invention uses high catalytic activity of the phosphine ligand, TON is usually higher than 2500, and the phosphine ligand is easy to be prepared and suitable for industrial production. The phosphine ligand can be produced by the Grignard reagent and 2-diphenyl phosphono-containing benzaldehyde in the field without separation, and directly catalyze a coupling reaction; and furthermore, the reactivity of the phosphine ligand is not obviously reduced.
Owner:上海立科化学科技有限公司
Synthetic method of indol norborneol alkanes and derivative thereof
InactiveCN107501166ASynthetic conditions are mildOne-step double substitutionOrganic chemistrySolventEthyl Chloride
The invention discloses a synthetic method of indol norborneol alkanes and a derivative thereof. The indol norborneol alkanes and the derivative thereof are shown as formula I in the description structurally. The synthetic method comprises the following steps: firstly taking an indol compound as a raw material, under the alkaline condition of sodium hydroxide, generating 3-iodine-N-acetyl indole or a derivative compound thereof through a reaction of indole or substituted indole and iodine and acetyl chloride, then under the condition of alkaline cesium carbonate in the 3-iodine-N-acetyl indole or the derivative compound therefore, using 1,4-dioxane as a solvent, using palladium acetate as a catalyst, using tri(2-furyl) phosphine as a ligand, and carrying out a heating reaction with norbornene to generate the indol norborneol alkanes and the derivative thereof. The preparation method is mild in condition and simple in operation, has relatively high atom economy, and the synthesized indol norborneol alkanes and derivative thereof are of relatively high potential value and wide application prospect in the field of medicinal chemistry.
Owner:NANJING UNIV OF SCI & TECH
Method for promoting CuCl to catalyze Sonogashira cross-coupling reaction by diindolylmethane derivative
InactiveCN105130723AEasy to deriveEasy to retouchOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAlkynePhosphorine
The invention discloses a method for promoting CuCl to catalyze Sonogashira cross-coupling reaction by a diindolylmethane derivative. According to the method, by taking the diindolylmethane derivative which are stable in water and air and are cheap and are easily available as a ligand, CuCl is promoted to catalyze Sonogashira cross-coupling reaction of aryl halide and terminal alkyne, so that not only is the use level of CuCl reduced, but also a conventional phosphine ligand needs not to be used. The method disclosed by the invention has good primer applicability, is suitable for aryl alkyne and alkyl terminal alkyne, and is high in yield of target products. The method is an efficient, low-consumption and safe Sonogashira cross-coupling reaction system which is wide in applicability and can be widely applied to constructing Csp-Csp2 bonds.
Owner:SHAANXI NORMAL UNIV
5,5'-connected 1,1'-biphenyl axially chiral 2,2'-diphosphine ligand and preparation method thereof
ActiveCN103524557AWide range of usesImprove catalytic performanceGroup 5/15 element organic compoundsChemical industryDiphosphines
The invention relates to a 5,5'-connected 1,1'-biphenyl axially chiral 2,2'-diphosphine ligand and a preparation method thereof, and belongs to the technical field of chemical industry. The 5,5'-connected 1,1'-biphenyl axially chiral 2,2'-diphosphine ligand is a compound I with an (R) configuration and an (S) configuration axially or optically pure compounds II and III with (R) and (S) configurations axially. The preparation method comprises the following steps: converting substituted 4-metoxyphenol into a compound IV in an organic solution; converting the compound IV into a compound V; reacting the compound V with di(R<1>)phosphine oxyhydrogen to convert the compound V into a compound VI; converting the compound VI into a compound VII; reacting the compound VII with boron tribromide to prepare a compound VIII; reacting the compound VIII with dihalogenated hydrocarbon to prepare a 5,5'-connected compound IX with mixed (R) and (S) configurations, and performing splitting by chiral HPLC (High Performance Liquid Chromatography) to obtain compounds X and XI with single configuration of (R) and (S), thereby preparing the compound I or the compounds II and III. The 5,5'-connected 1,1'-biphenyl axially chiral 2,2'-diphosphine ligand can be synthesized into a catalyst by complexation with divalent metal of palladium, and high catalytic performance and high enantioselectivity can be obtained.
Owner:SHANGHAI JIAO TONG UNIV +1
A large-volume electron-rich phosphine ligand catalyst and its preparation method and application
InactiveCN102268041AHigh activityExtend your lifeGroup 5/15 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsAlkaneLone electron pair
The invention discloses a bulky electron-rich phosphine ligand compound and a preparation method thereof. The structural formula of the phosphine ligand is shown in formula I; wherein, R represents an alkyl group, an alkenyl group, an alkynyl group, an alkoxy group or an aryl group substituted by an alkane. The method for preparing the compound of formula I comprises the following steps: reacting the compound shown in formula II with the compound shown in formula III at -90°C to -60°C for 2-5h, and then reacting at room temperature for 5-15 hours, namely have to. The lone electron pair on the methoxyl oxygen in the large-volume thiophene-containing ligand synthesized by the present invention can increase the electron cloud density of the molecule, which is beneficial to stabilizing the Pd intermediate in the reaction process, preventing cyclization, and increasing steric hindrance. Improve catalyst life. In addition, the thiophene ring is a group with a stronger electron supply than aryl or alkyl, which is beneficial to increase the electron cloud density on the phosphine and improve the activity of the ligand. The ligand of the present invention is coordinated with the palladium catalyst and then applied to Suzuki coupling or polymerization reaction to synthesize photoelectric polymer materials with high molecular weight and high yield.
Owner:INST OF CHEM CHINESE ACAD OF SCI
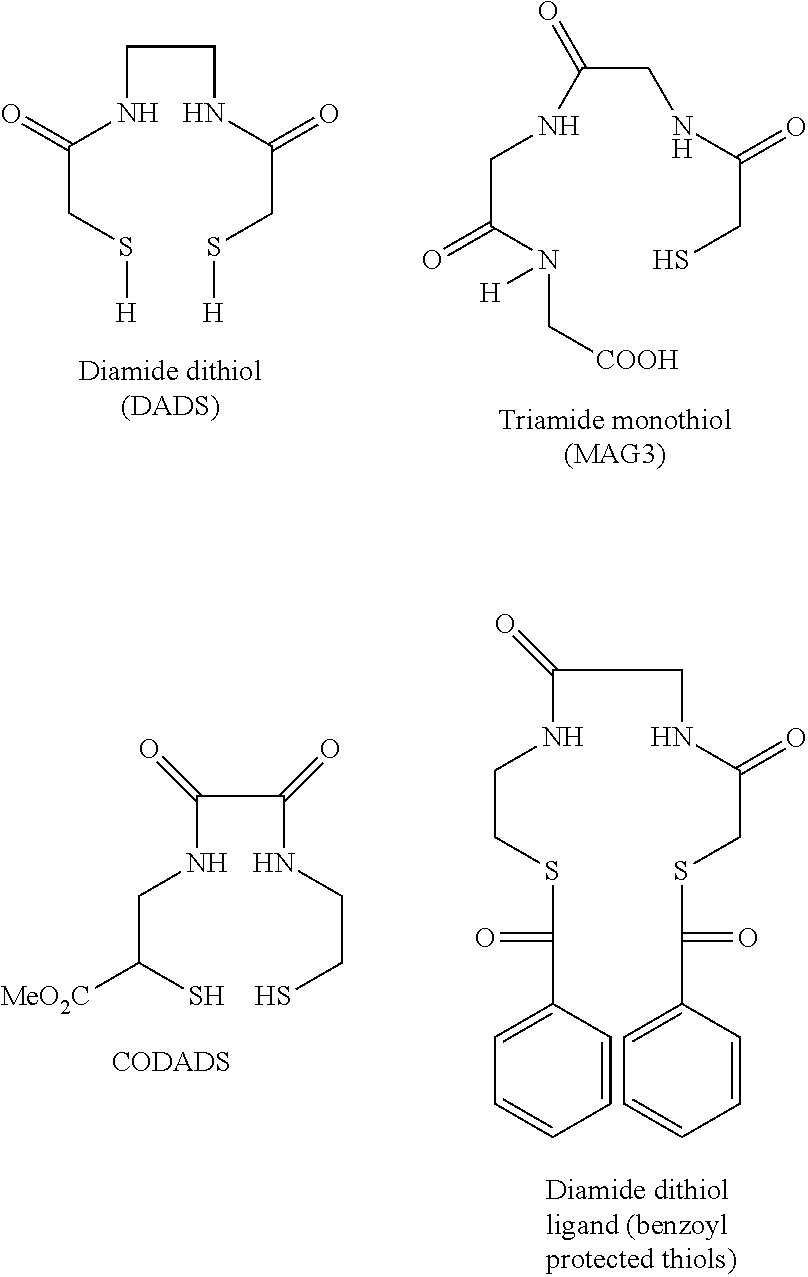
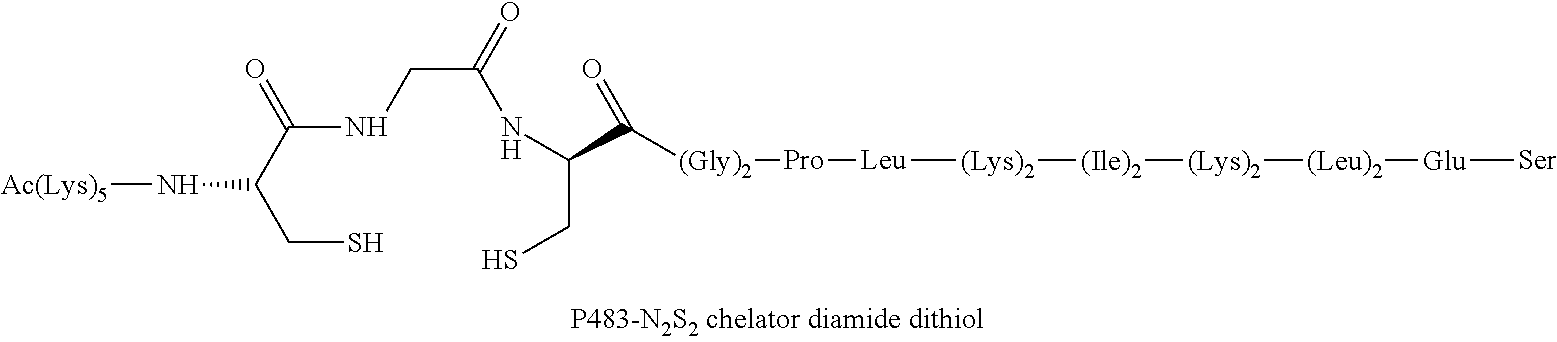
Chelation of metals to thiol groups using in situ reduction of disulfide-containing compounds by phosphines
InactiveUS20120178906A1In-vivo radioactive preparationsPeptide/protein ingredientsThioenolPhosphorine
A method is disclosed for the syntheses of thiol-containing radiopharmaceuticals without the need for purification starting from chelators containing disulfide bonds. This is done by providing a method that reduces disulfide bonds on a precursor molecule or a precursor compound in the presence of phosphine compounds, thus freeing thiols for metal complexation.
Owner:BRACCO IMAGINIG SPA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com