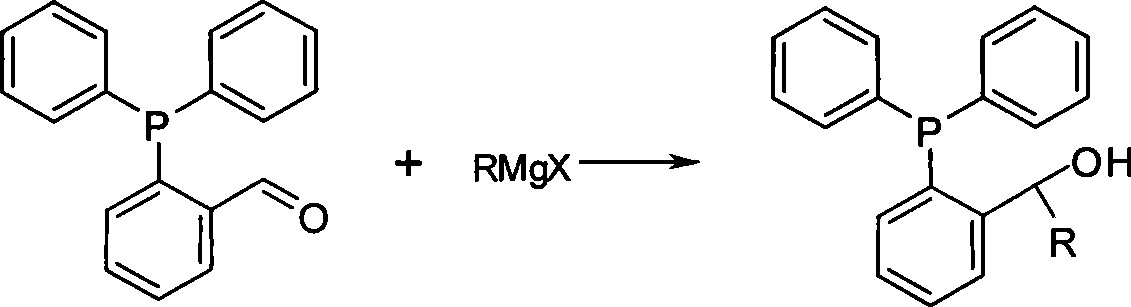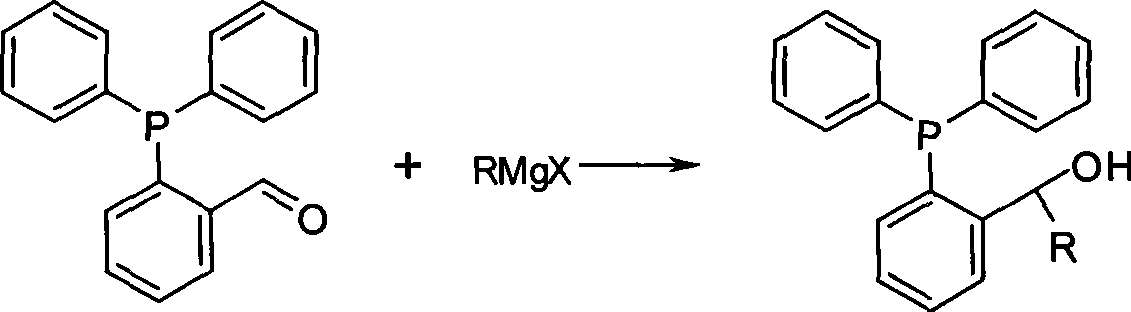Preparation method of phosphine ligand and method for phosphine ligand to catalyze and synthetize biaryl and derivatives thereof
A technology for phosphine ligands and biarenes, which is applied to the preparation method of phosphine ligands and the field of catalyzing synthesis of biarenes and derivatives thereof, can solve the problems of high price, difficulty in synthesizing products, difficulty in recovering catalysts, etc. The effect of reducing activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
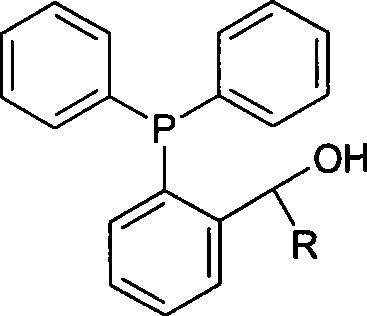
[0027] Preparation of phosphine ligand 1-(2-diphenylphosphino)phenethyl alcohol:
[0028] Under the protection of nitrogen, add 7.6g of 2-diphenylphosphinobenzaldehyde and 100ml of tetrahydrofuran into a 250ml four-necked flask, and add 50ml of anhydrous ether solution of 1M iodomethane Grignard reagent dropwise under stirring at 20°C. After the addition is complete, heat to reflux React for 90 minutes, cool the reaction to room temperature, pour it into cold saturated ammonium chloride aqueous solution, extract the aqueous phase with ether, combine the organic phases, dry with anhydrous sodium sulfate, concentrate, and separate by silica gel column chromatography to obtain the product, NMR The data are consistent with the literature.
[0029] The reaction formula is:
[0030]
[0031] Application of phosphine ligand 1-(2-diphenylphosphino)phenylethanol: preparation of 4-methylbiphenyl
[0032] Under nitrogen protection, in a 50ml Schlenk bottle, add 111mg 4-fluorotoluene...
Embodiment 2
[0036] Preparation of phosphine ligand (2-diphenylphosphinophenyl) benzyl alcohol:
[0037] Under the protection of nitrogen, add 7.6g of 2-diphenylphosphinobenzaldehyde and 100ml of tetrahydrofuran to a 250ml four-necked flask, and add 50ml of bromophenyl Grignard reagent in tetrahydrofuran (1M) dropwise under stirring at 20°C. After the addition is complete, heat to Reflux for 90 minutes, cool the reaction to room temperature, pour into cold saturated ammonium chloride aqueous solution, extract the aqueous phase with ether, combine the organic phases, dry with anhydrous sodium sulfate, concentrate, and separate by silica gel column chromatography to obtain the product.
[0038] The reaction formula is:
[0039]
[0040] Application of phosphine ligand (2-diphenylphosphinophenyl) benzyl alcohol: preparation of 4-phenylpyridine
[0041] Method A: Add 18g p-4-bromopyridine, 2.355g (2-diphenylphosphinophenyl) benzyl alcohol, 0.82g Ni(acac) to a 500ml four-necked flask under ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com