A large-volume electron-rich phosphine ligand catalyst and its preparation method and application
A catalyst and system technology, applied in the field of large-volume electron-rich phosphine ligand catalyst and its preparation, can solve the problems of not obtaining high-yield and high-molecular-weight polymers and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
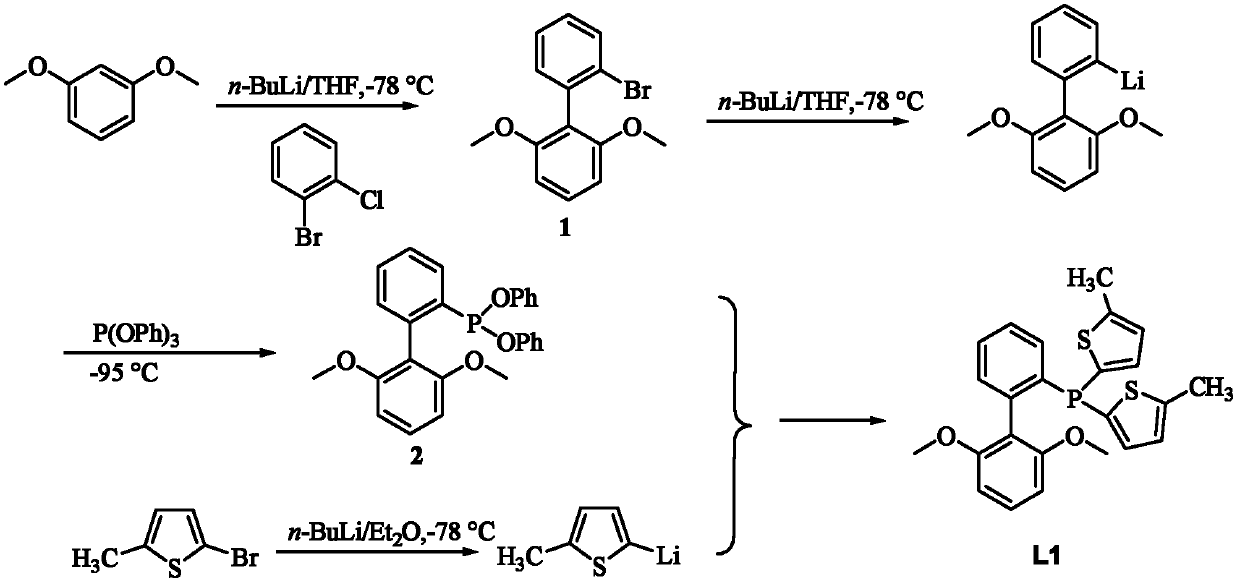
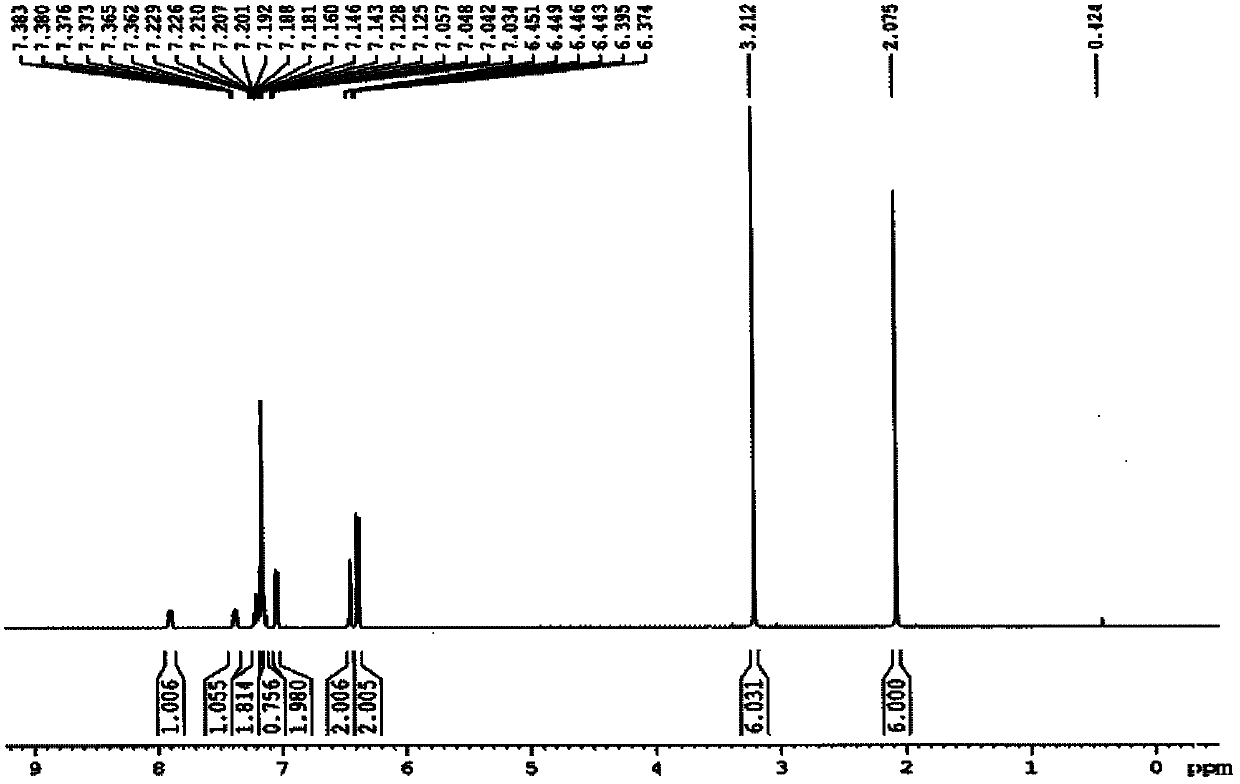
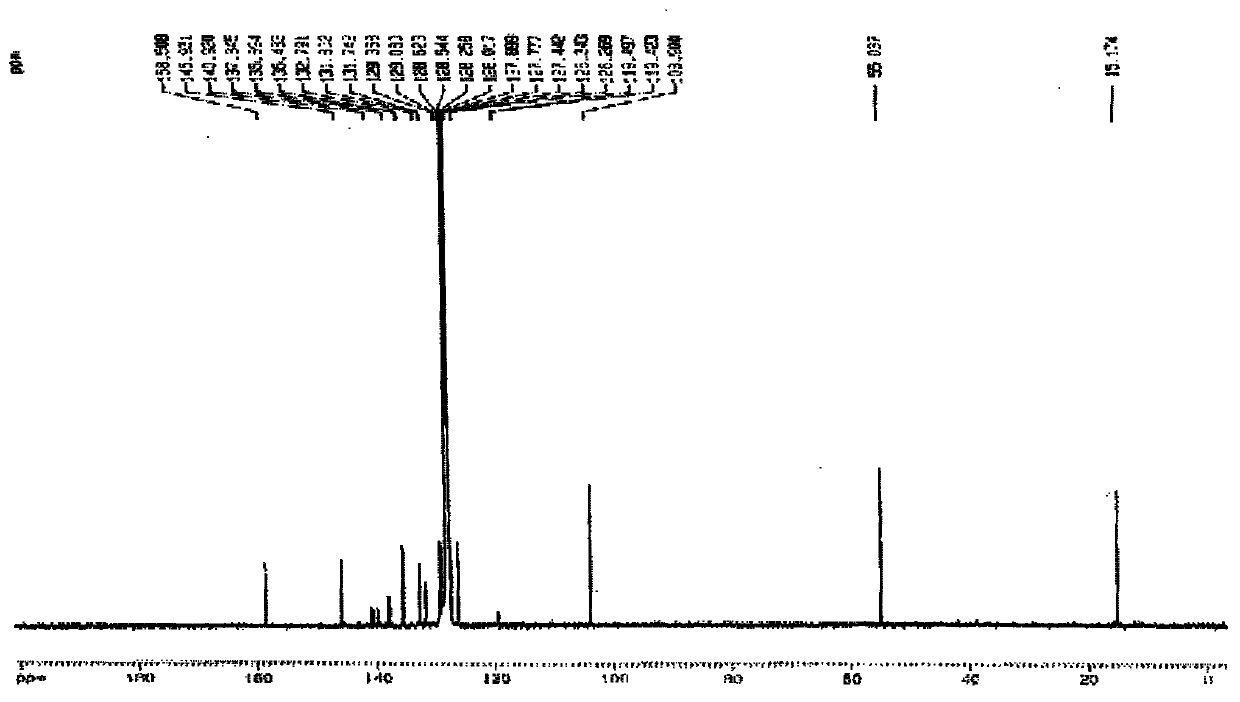
[0035] R in embodiment 1, preparation formula I is CH 3 Compound L1
[0036]
[0037] Compound 1 Compound 2 Compound L1
[0038] 1) Synthesis of compound 1: put 15mmol 1,3-dimethoxybenzene into the reaction flask, add 60ml of dry tetrahydrofuran (THF), and slowly drop into 15mmol n-BuLi (n-butyl base lithium) and then the reaction mixture was raised to room temperature to continue the reaction for 5-10 h, and then the reaction mixture was cooled to -78 ° C, and 14 mmol o-bromochlorobenzene was slowly added dropwise to obtain a light yellow crude product, which was recrystallized from methanol to obtain a white solid. Yield 84%.
[0039] 2) Synthesis of compound 2: Add 1 mmol of compound 1 to the reaction flask, add 100 ml of tetrahydrofuran, under nitrogen atmosphere, control the temperature at -78 ° C, slowly drop 1.2 mmol of n-BuLi, and continue stirring at this temperature for 20 min. The reaction mixture was cooled to -95°C, and 0.95 mmol P(OPh) was added by syringe ...
Embodiment 2
[0041] Embodiment 2, monomer G4 and M2 are in catalyst Pd2 (dba) 3 Polymerization under / L1 catalysis
[0042]
[0043] (Compound G4) (Compound M2)
[0044] PD 2 (dba) 3 Purchased from Shanghai Darui Fine Chemicals Co., Ltd., CAS code 51364-51-3 (52409-22-0).
[0045] Compound G4 was prepared with reference to the literature method, and the specific preparation method was as follows: (1) under nitrogen atmosphere, 1mol 1,4-dibromo-2-(bromomethyl)-5-toluene and 1mol phlorotriphenol were mixed, acetone was used as solvent, Then add 5mol of salt of wormwood, heat and reflux reaction, generate compound 5-(2,5-dibromo-4-methyl benzyloxy)-1,3-benzenediol;
[0046] (2) Under a nitrogen atmosphere, 1mol compound 5-(2,5-dibromo-4-methylbenzyloxy)-1,3-benzenediol is mixed with 2mol dimethyl (1,1-dimethylbenyloxy) Base) chlorosilane reacts to generate compound 3;
[0047] (3) Under nitrogen atmosphere, mix 1mol of 5-(bromomethyl)-1,3-benzenediol with 2mol of bromine, acetone as ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com