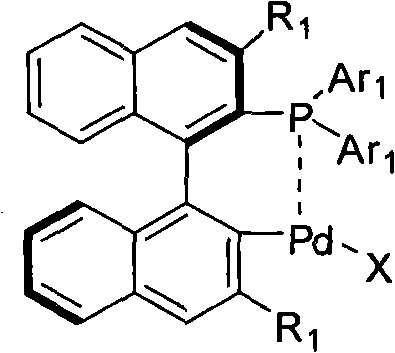
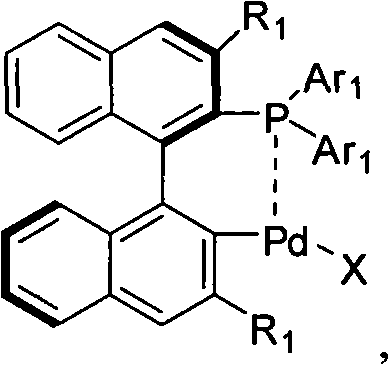
Palladium catalyst of axis chiral dinaphthalene frame phosphine-containing ligand, synthetic method and use thereof
A technology of phosphine ligands and axial chirality, which is applied in the field of cyclopalladium catalysts, synthesis and application of phosphine-containing ligands with axial chiral binaphthyl skeletons, and can solve the limitations of substrates and reagents. Difficult to prepare, unstable zinc reagent and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Synthesis of a cyclopalladium catalyst with an axial chiral binaphthalene skeleton containing a phosphine ligand
[0043]
[0044] Under the protection of argon at 50℃, Pd(OAc) 2 0.30mmol, ligand 0.30mmol, dissolved in 5mL toluene (Tolunene), TLC tracked after 2h until the ligand disappeared. Recrystallized to obtain a yellow-white solid.
[0045] Cat-1 (Ar 1 =Ph): Yield 92%.[a] D 20 =-159°C (c=0.75, CHCl 3 ); M.p.222-228°C; 1 HNMR (300MHz, CDCl 3): δ1.80(s, 3H), 6.45-7.22(m, 11H), 7.23-8.15(m, 11H); 13 CNMR (75MHz, CDCl 3 )δ23.50, 125.01, 126.10, 126.30, 127.20, 128.31, 128.72, 128.84, 131.60, 133.12, 133.70, 134.22, 134.38, 136.01, 136.29, 137.45, 141.30, 176.0 31 P NMR (300MHz, CDCl 3 ): δ35.6; IR(KBr): 3051, 1559, 1436, 1100, 743, 693cm -1 ; MS (ESI) m / z (relative intensity): 625 (M + +Na); HRMS for C 34 h 25 o 2 PPdNa + : theoretical value (Calc) 625.0524; measured value (Found).625.0519.Cat-2 (Ar 1 =p-MeOC 6 h 4 ): Yield 88%.[a] D...
Embodiment 2
[0049] Example 2: Cyclopalladium catalyst asymmetrically catalyzes the ring-opening reaction of organoboron reagents to heteroatom norbornadiene at 0°C.
[0050]
[0051] Add 0.006mmol of cyclopalladium catalyst Cat, 0.2mmol of benzoxa substrate, 0.3mmol of arylboronic acid into the reaction tube, add 0.1mmol of Cs into 3ml of chloroform, and add 0.1mmol of Cs at 0°C 2 CO 3 Aqueous solution; keep stirring at 0°C for 1-24h, then separate by column chromatography, eluent: (V) petroleum ether / (V) ethyl acetate = 10 / 1. The resulting oil was used for further analysis, and the ee% of the product was obtained by HPLC.
[0052] 3aa: oil, 42mg, 95% yield, ee value determined by chiral HPLC. (OD column flow rate, 1.0mL / min, n-hexane / i-PrOH=90 / 10, λ=254nm, t R =8.1min, 12.5min, ee: 79%); 1 H NMR (300MHz, CDCl 3 ): δ3.86-3.92 (m, 1H), 4.90 (t, J = 6.9Hz, 1H), 6.13 (dd, J = 3.9Hz, 9.6Hz, 1H), 6.71 (dd, J = 1.8Hz, 9.9 Hz, 1H), 7.17 (dd, J=1.5Hz, 7.2Hz, 1H), 7.23-7.38 (m, 8H); MS (E...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com