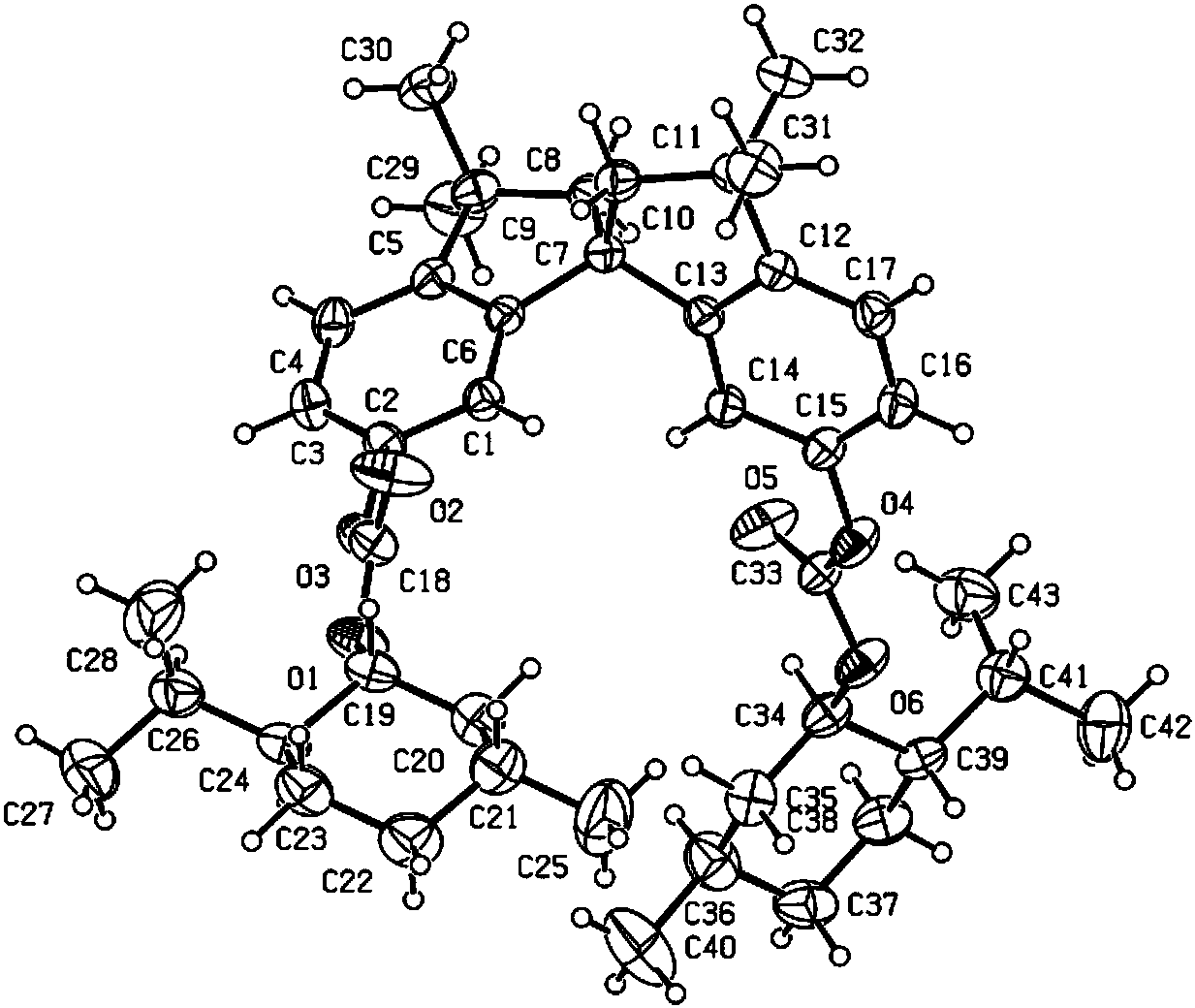
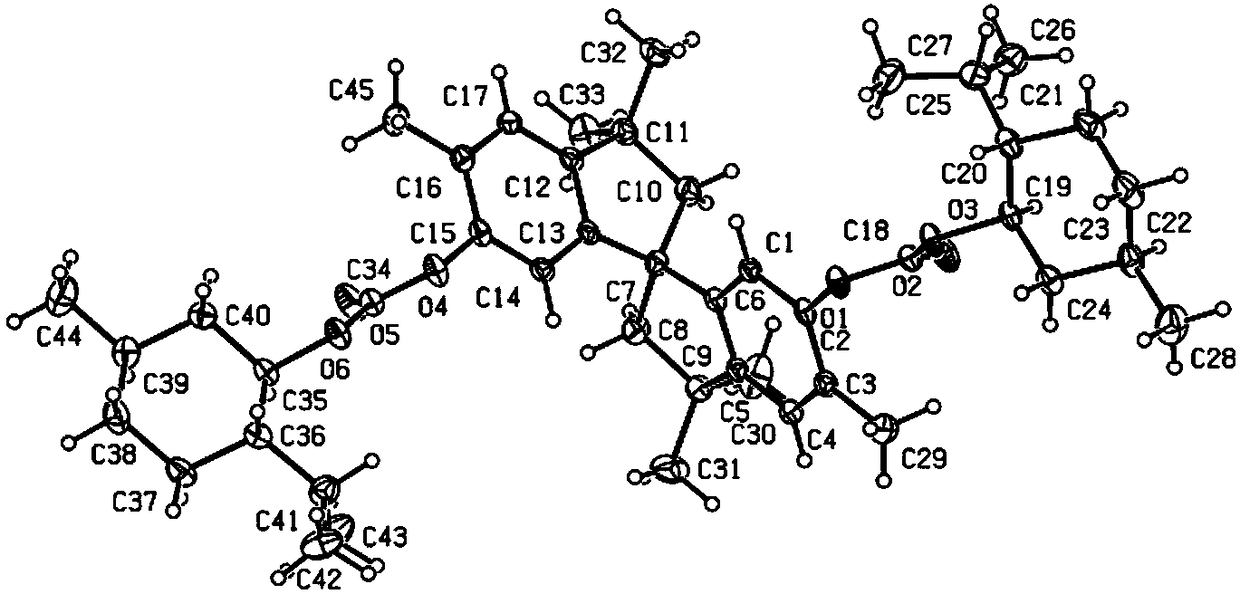
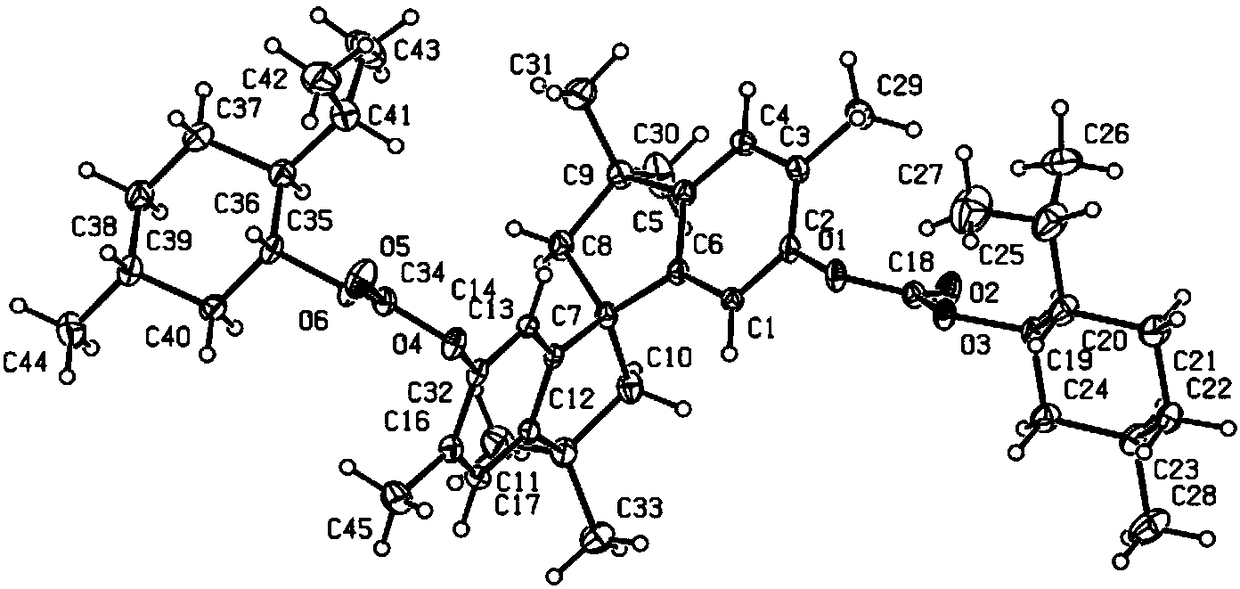
Phosphine ligand compounds based on tetramethylspirobiindane skeleton, intermediate of compounds, and preparation method and application of compounds
A technology of tetramethylspirodihydroindane and compounds, which is applied in the field of phosphine ligand compounds and their intermediates and preparation, and can solve problems such as the preparation or application of useless ligands
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Synthesis of 3,3,3',3'-tetramethyl-1,1'-spiroindane-6,6'-diol (MSPINOL)
[0058]
[0059] Add 100g of bisphenol A and 500mL of methanesulfonic acid into the reaction flask, stir and dissolve to obtain a dark red solution. After stirring and reacting at room temperature for 96 hours, the reaction solution was poured into 600 mL of water, cooled and filtered with suction, and the obtained solid was washed with water. Dissolve the solid with ethanol under reflux, add 50°C hot water until no solid precipitates, filter while hot, and wash the filter cake with hot water. Dry to get 45g of near-white flocculent solid, namely 3,3,3',3'-tetramethyl-1,1'-spirodihydroindane-6,6'-diol (MSPINOL), yield Greater than 99%.
[0060] The resolution process of racemate MSPINOL:
[0061]
[0062] 23 g of racemic MSPINOL, 26 ml of triethylamine and 0.22 g of 4-(N,N-dimethylamino)pyridine (DMAP) were dissolved in 200 ml of dichloromethane, cooled in an ice bath, and added within 30 ...
Embodiment 2
[0069] Synthesis of 3,3,5,3',3',5'-hexamethyl-1,1'-spiroindane-6,6'-diol (HMSPINOL)
[0070]
[0071] Add 50g of bisphenol C and 250mL of methanesulfonic acid into a 500mL round bottom flask, stir and react at room temperature for 3 days, then add 100mL of methanesulfonic acid, continue the reaction for 1 day, then stop the reaction. The reaction solution was poured into a beaker filled with 300 mL crushed ice, filtered with suction, and the filter cake was washed successively with saturated sodium bicarbonate solution and water. After washing, transfer the obtained crude product to a 500mL single-necked flask, add an appropriate amount of ethanol to make the product just dissolve at the reflux temperature, add water until solids are obviously precipitated, stir well and cool, a large amount of solids are precipitated, filter with suction, and wash , the filter cake can be dried to obtain 20g of white powdery solid, which is 3,3,5,3',3',5'-hexamethyl-1,1'-spirodihydroindane...
Embodiment 3
[0087] Synthesis of (R)-3,3,3',3'-tetramethyl-7,7'-dibromo-1,1'-spiroindane ((R)-III-a)
[0088]
[0089] In the reaction flask, add 15.4g compound (R)-MSPINOL (molecular weight: 308, 0.05 mole), 15mL tert-butanol (0.156 mole), and 180mL dichloromethane. After stirring evenly (suspension), add 27mL methanesulfonic acid (0.41 mol) dropwise under ice-water bath cooling, and the turbidity gradually disappears; after the dropwise addition is completed, the reaction solution will become turbid again after removing the ice-water bath, and continue to stir for reaction 2 After 1 hour, 100 mL of ice water was added to quench the reaction. The reaction solution was evaporated under reduced pressure to remove dichloromethane, and then more than 200 mL of ethyl acetate was added under stirring to dissolve all the precipitated solids; the liquid was separated, the aqueous phase was extracted with ethyl acetate, and the organic phase was combined; the organic phase was washed with satur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com