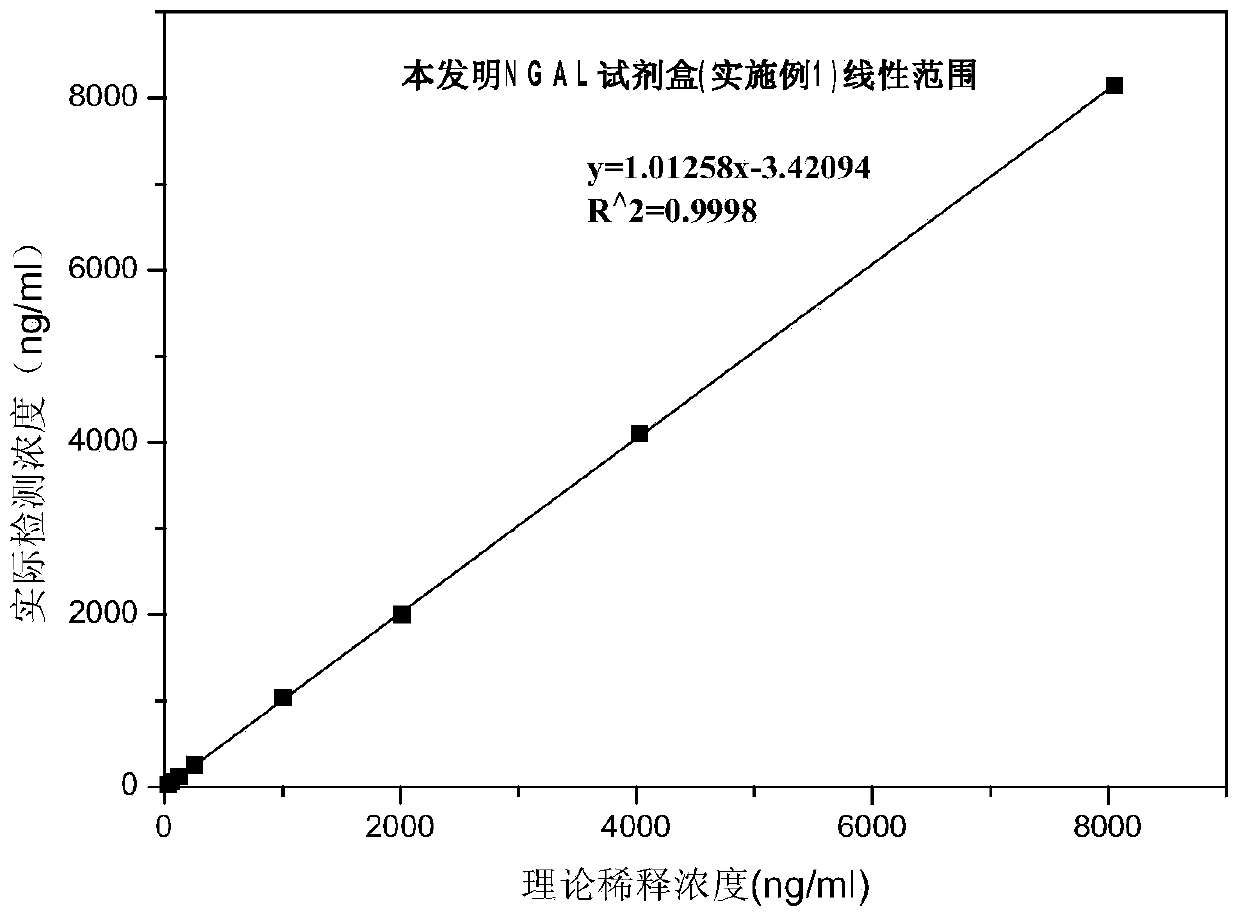
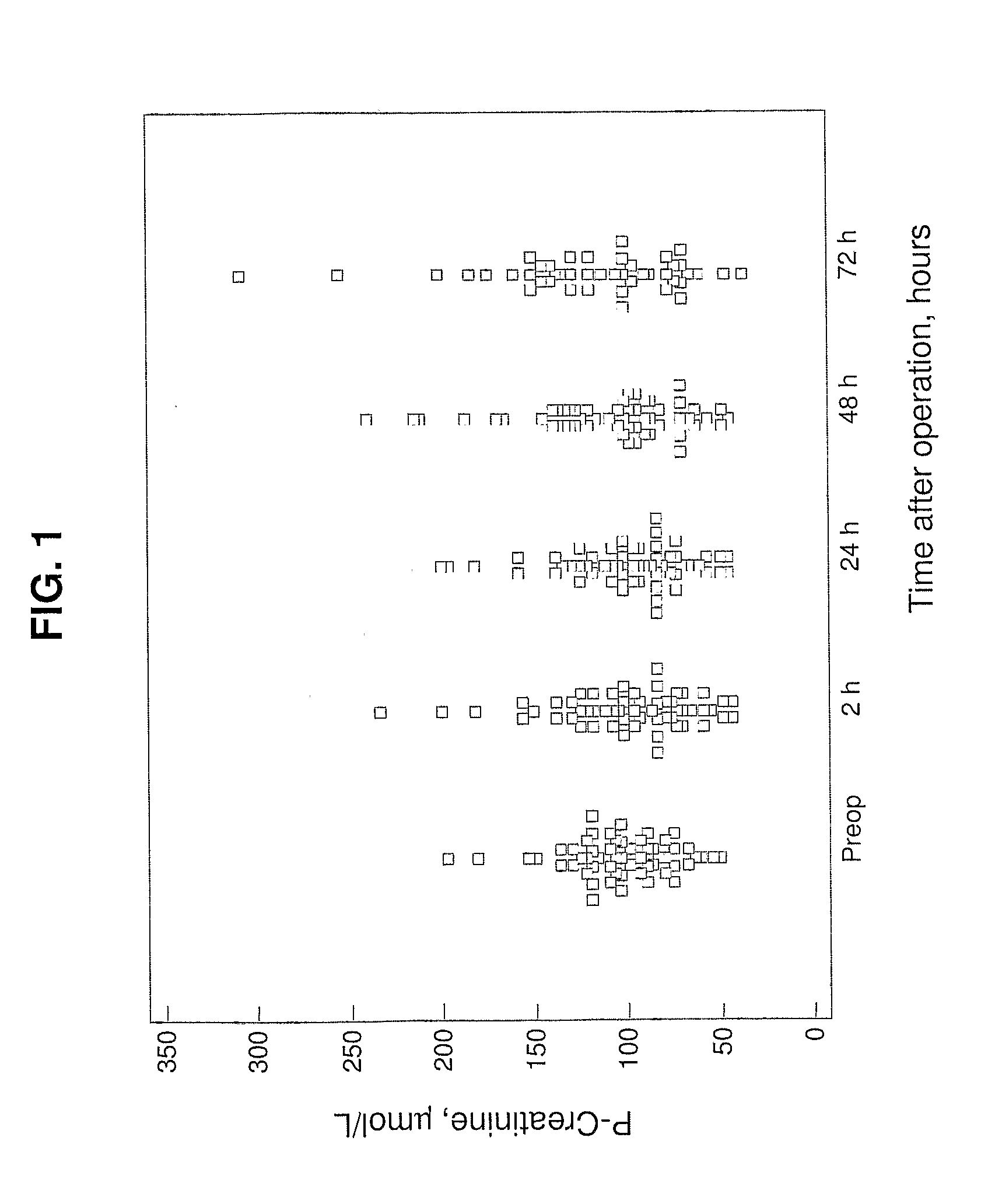
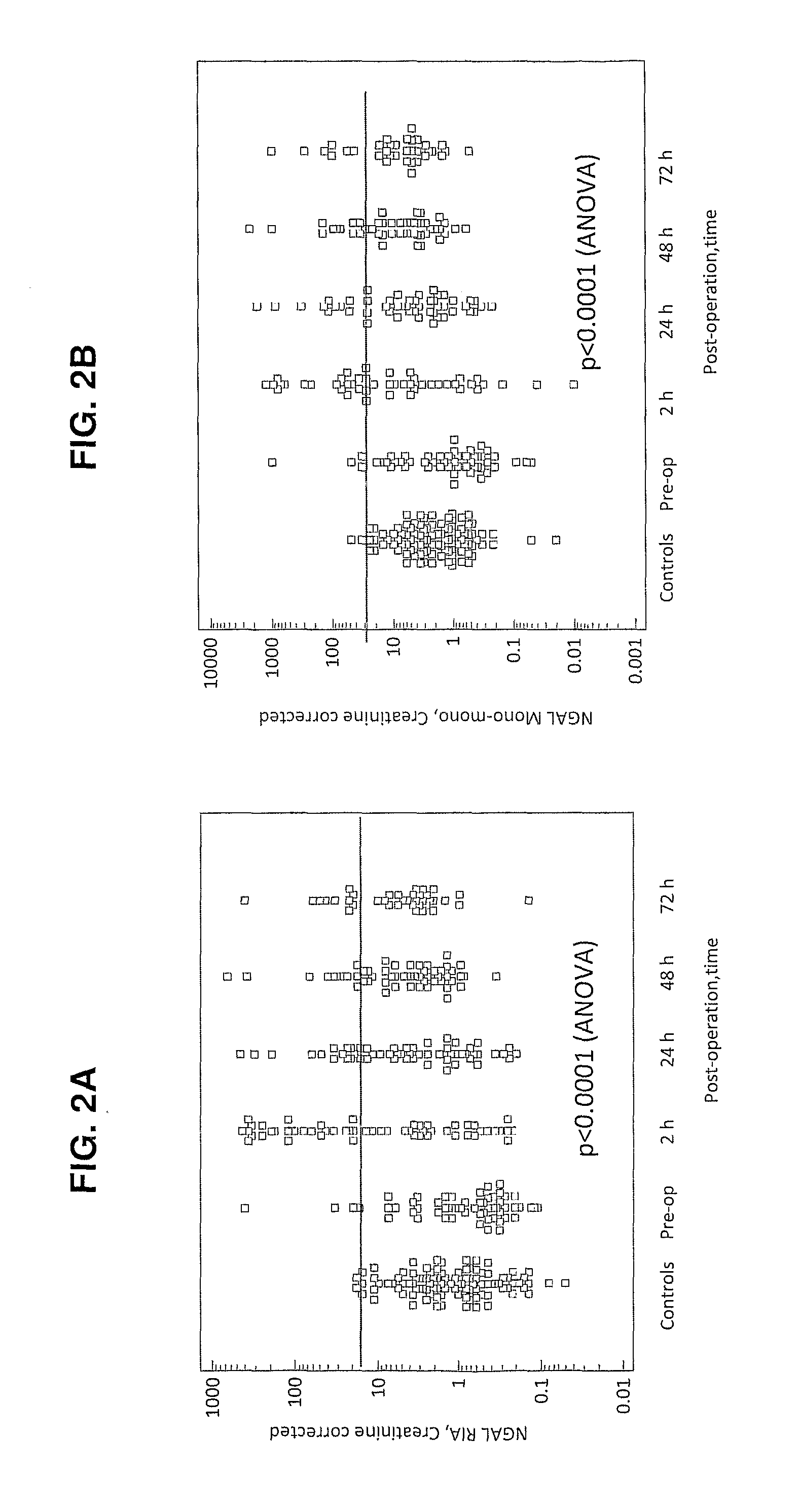
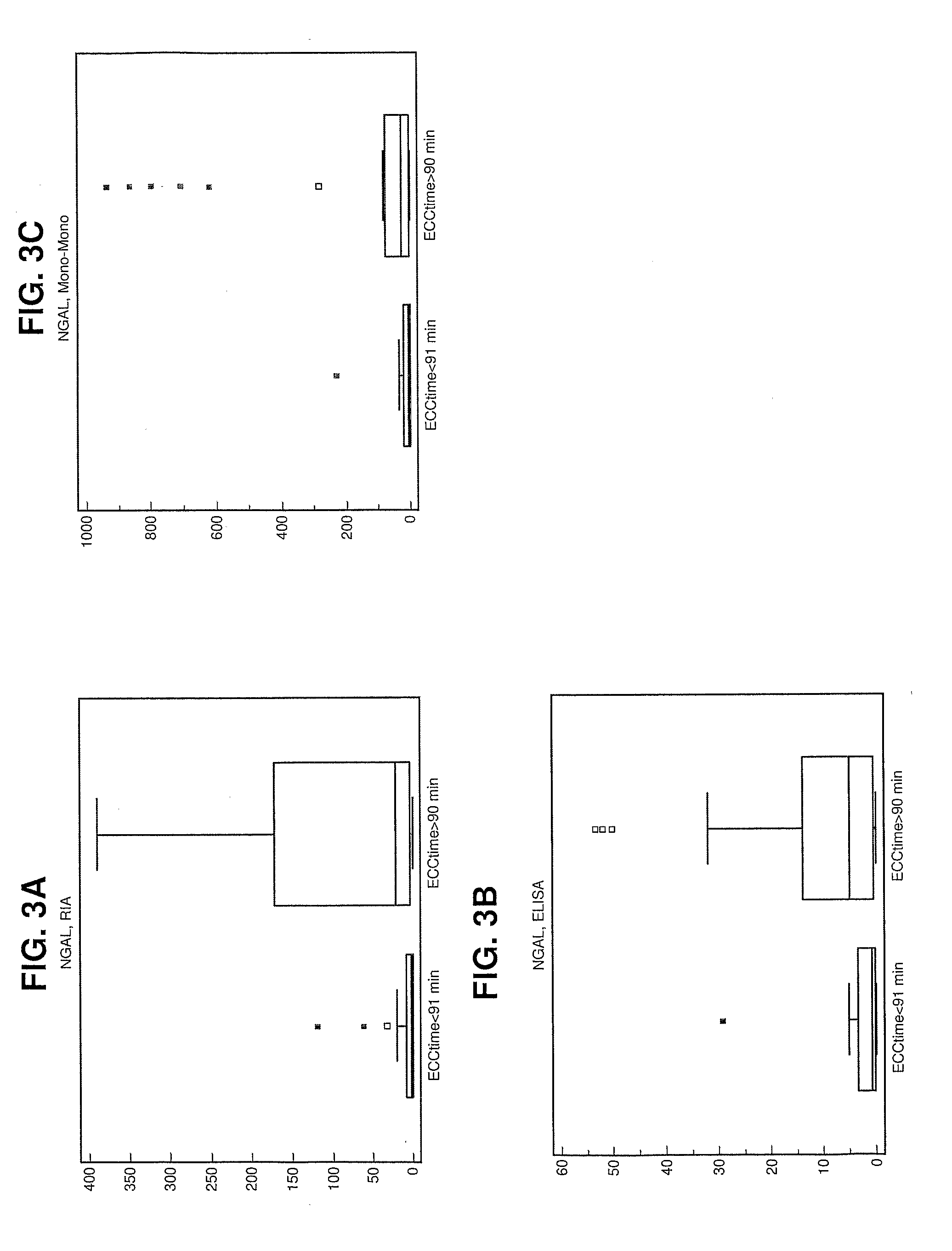
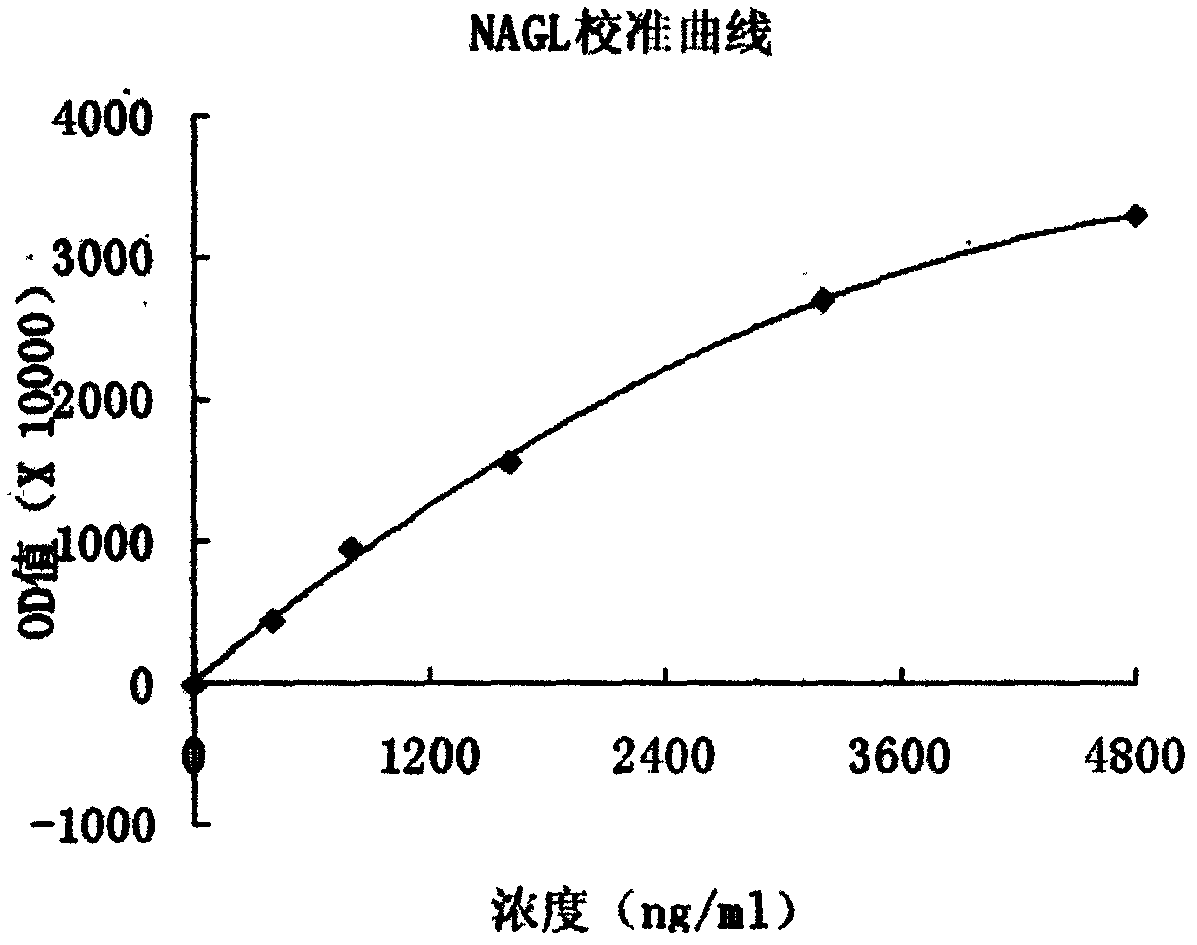
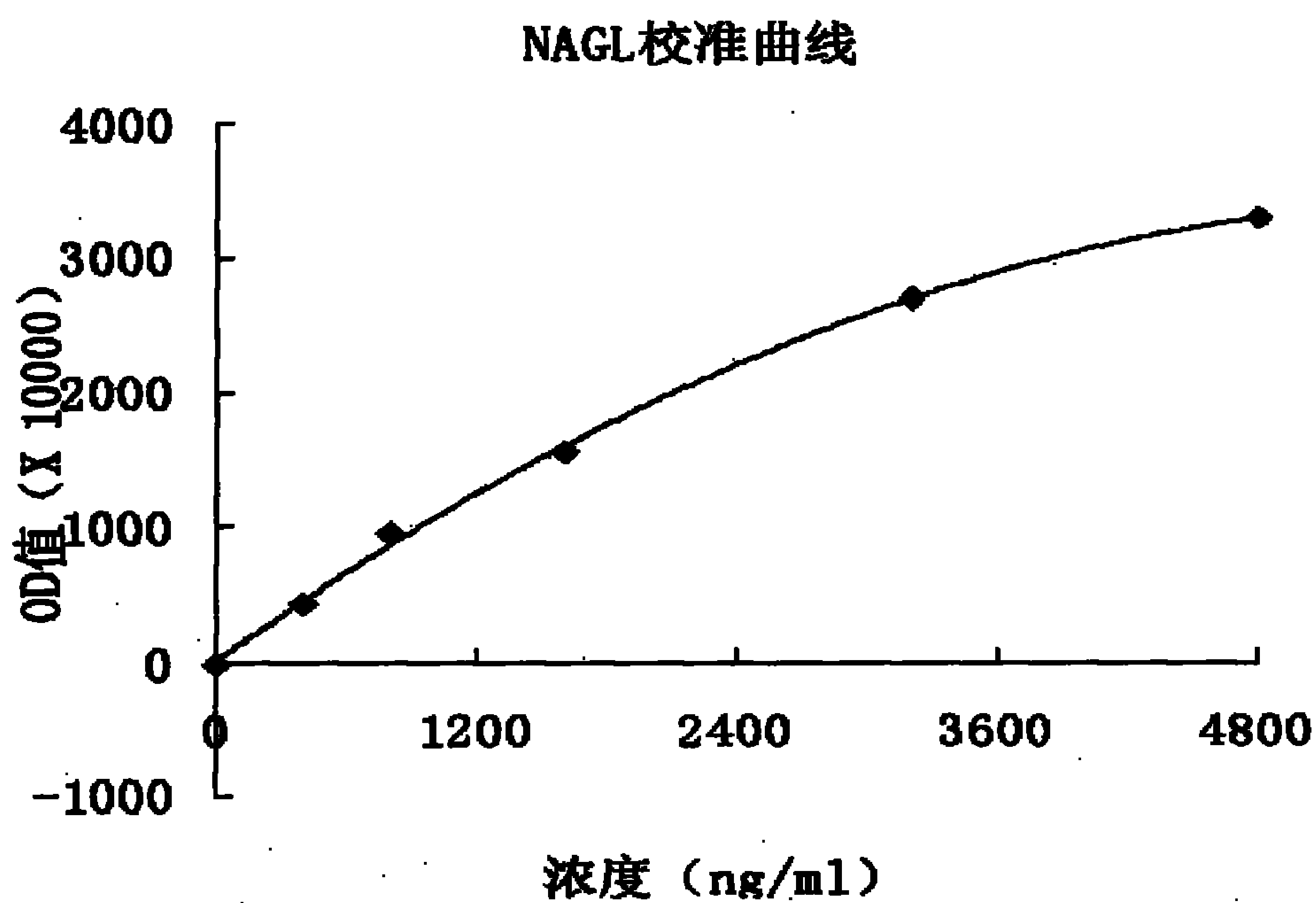
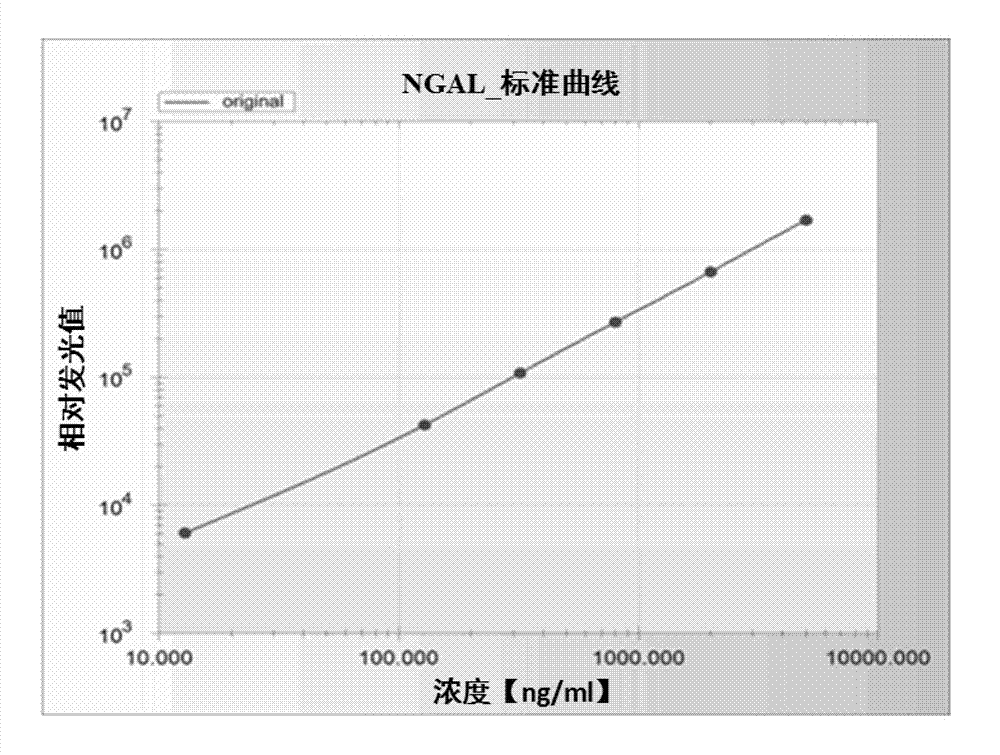
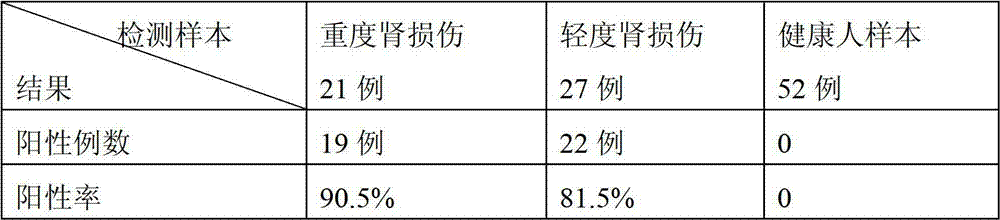
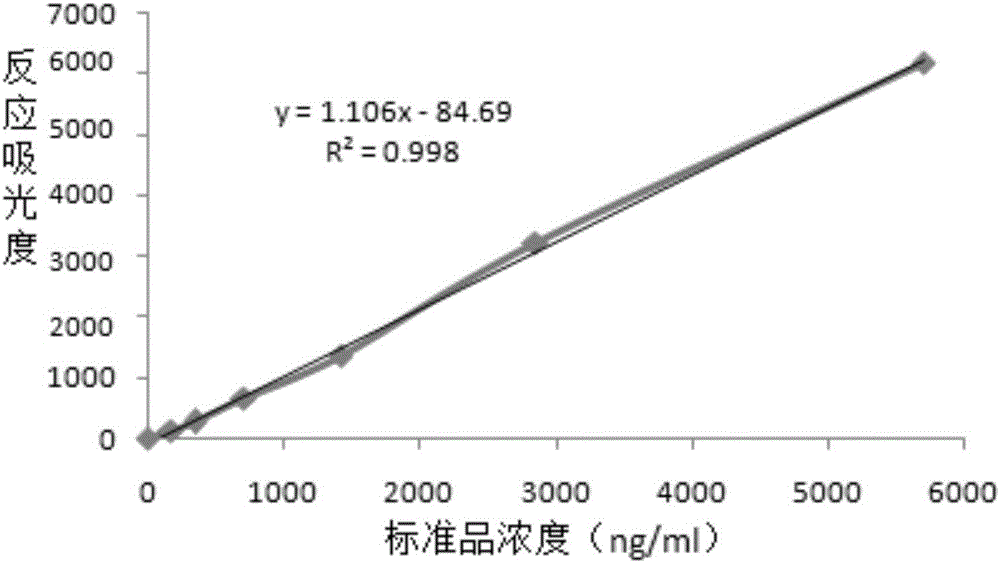
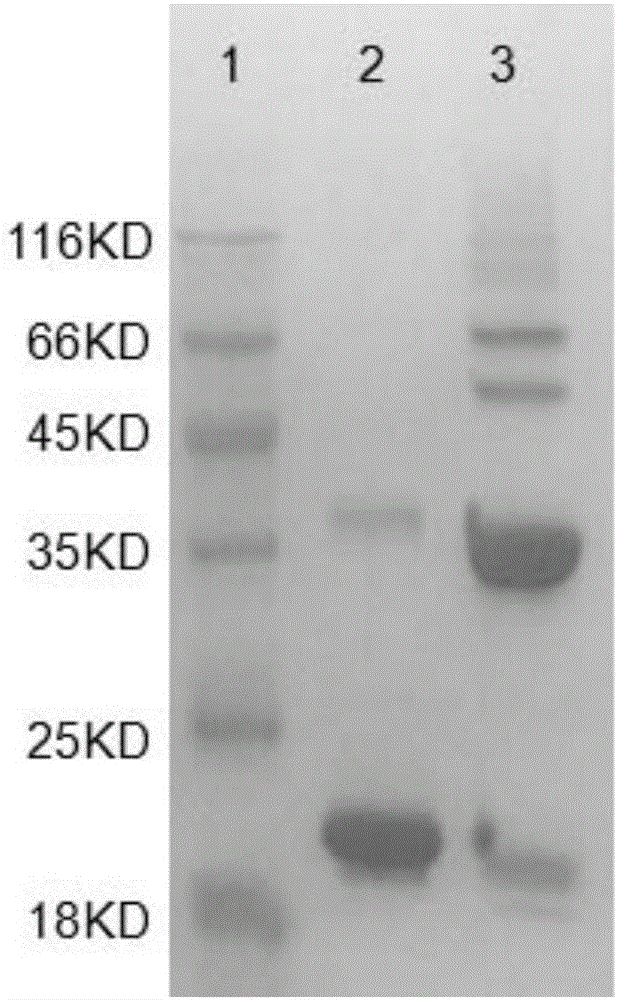
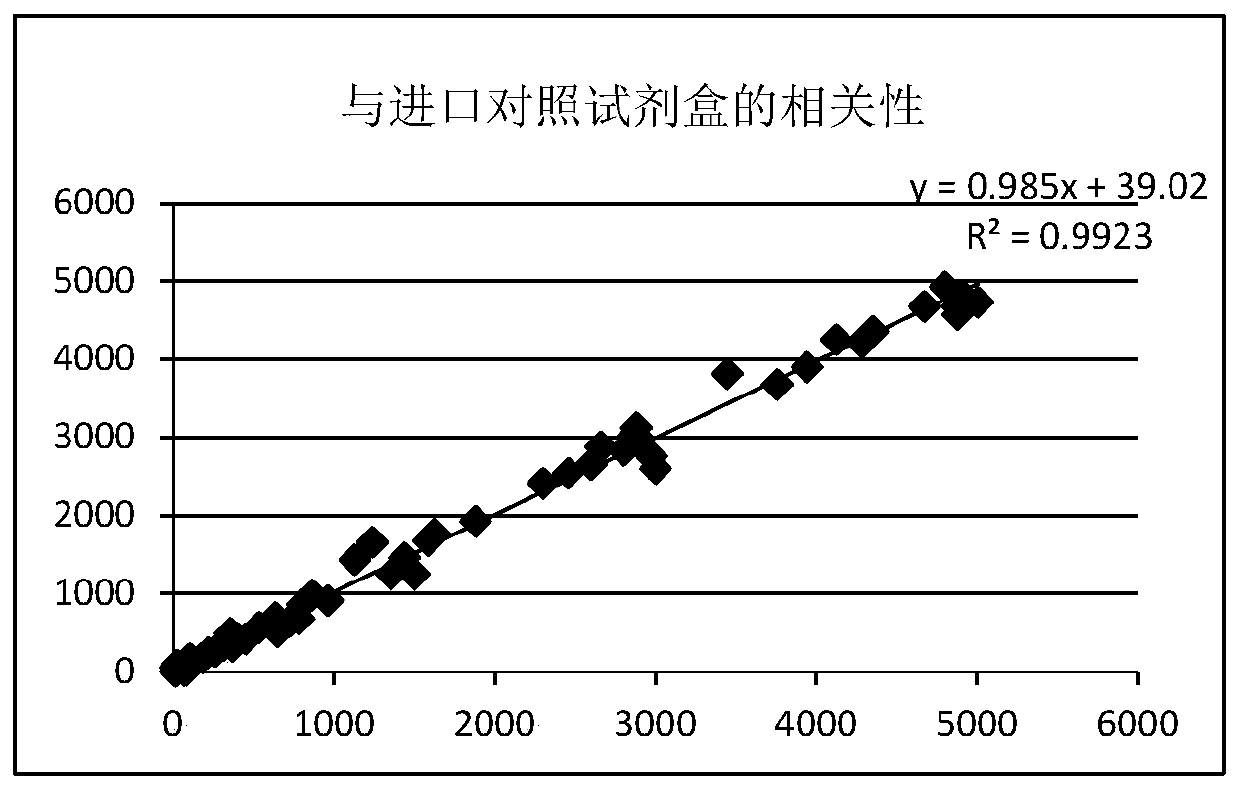
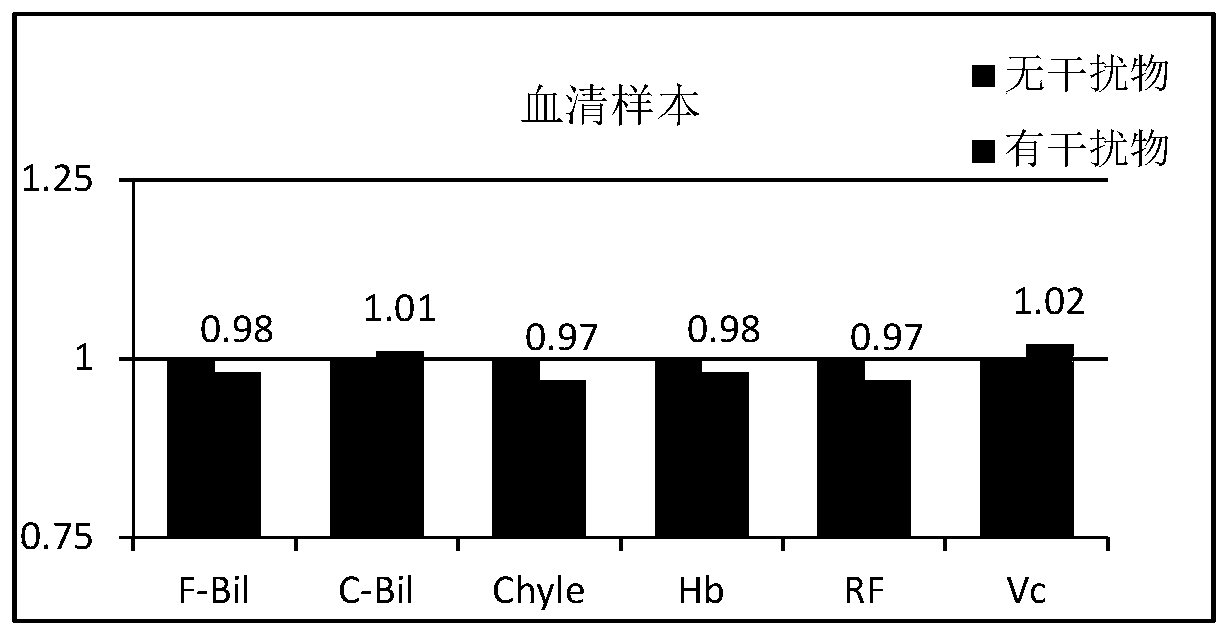
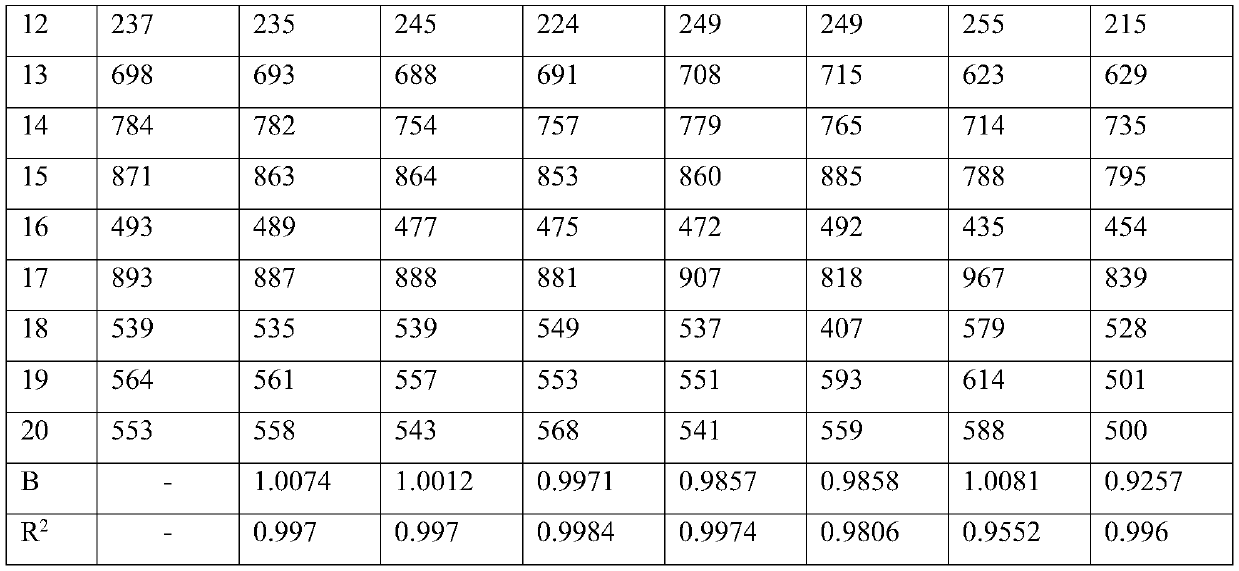
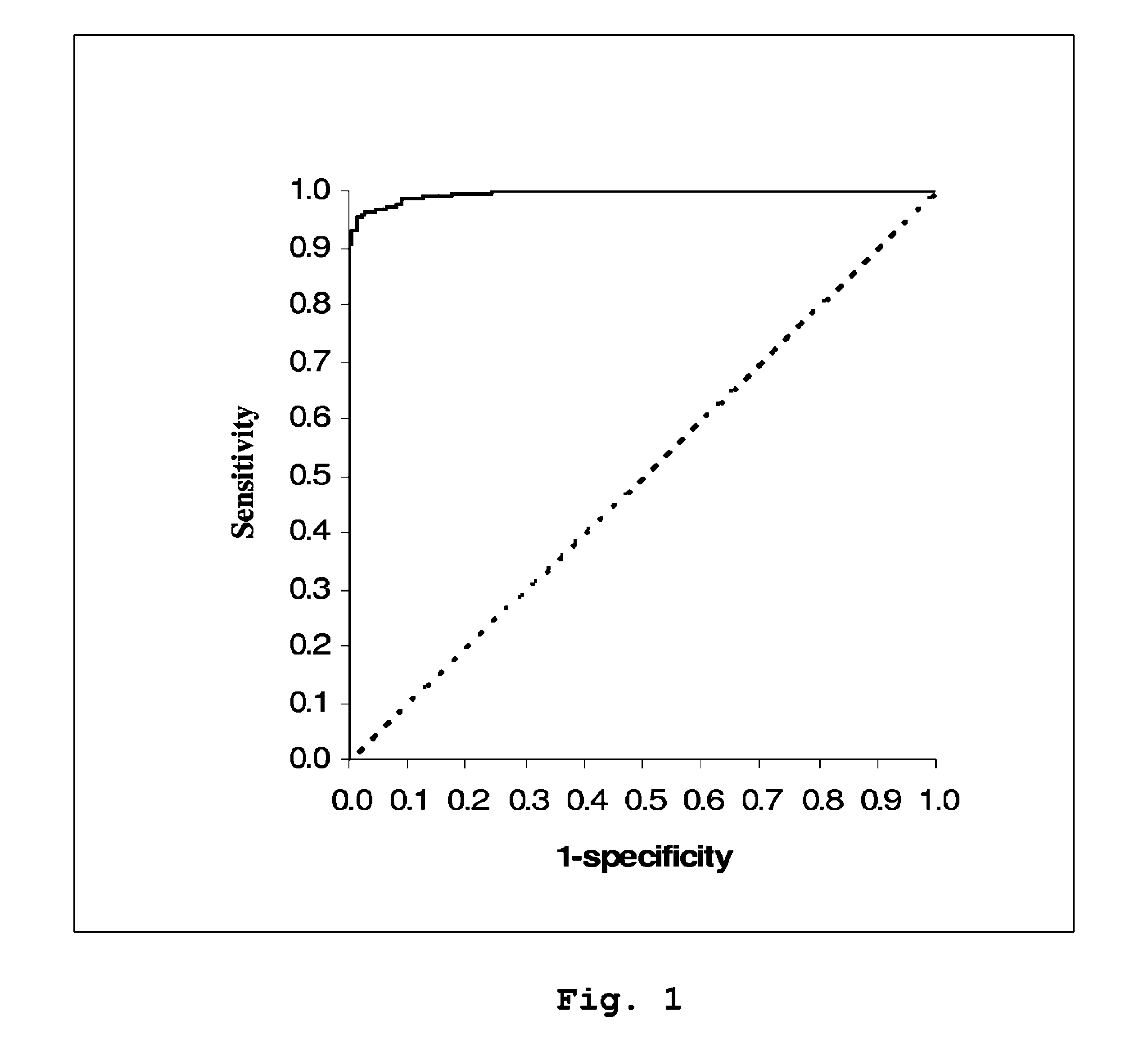
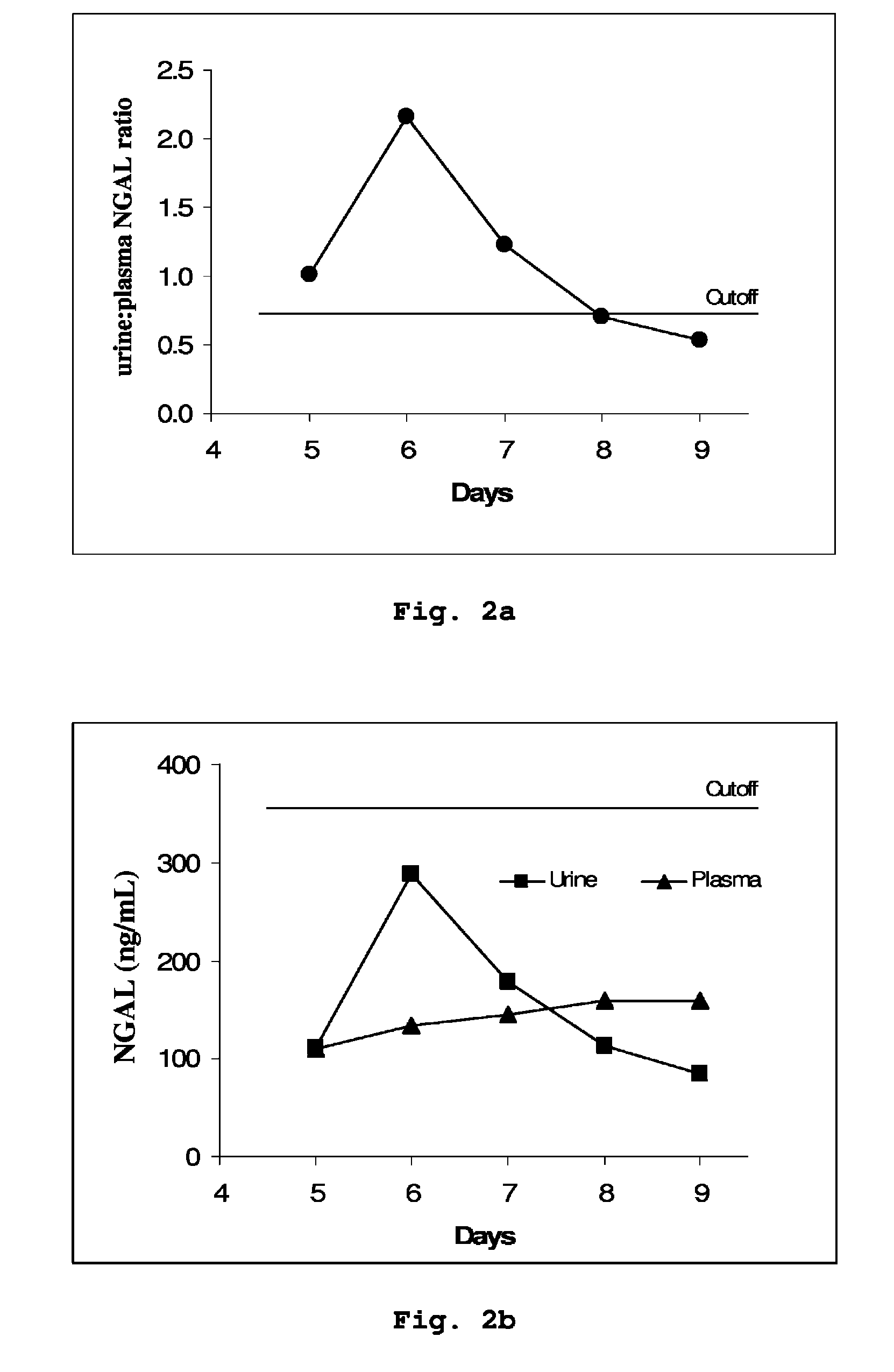
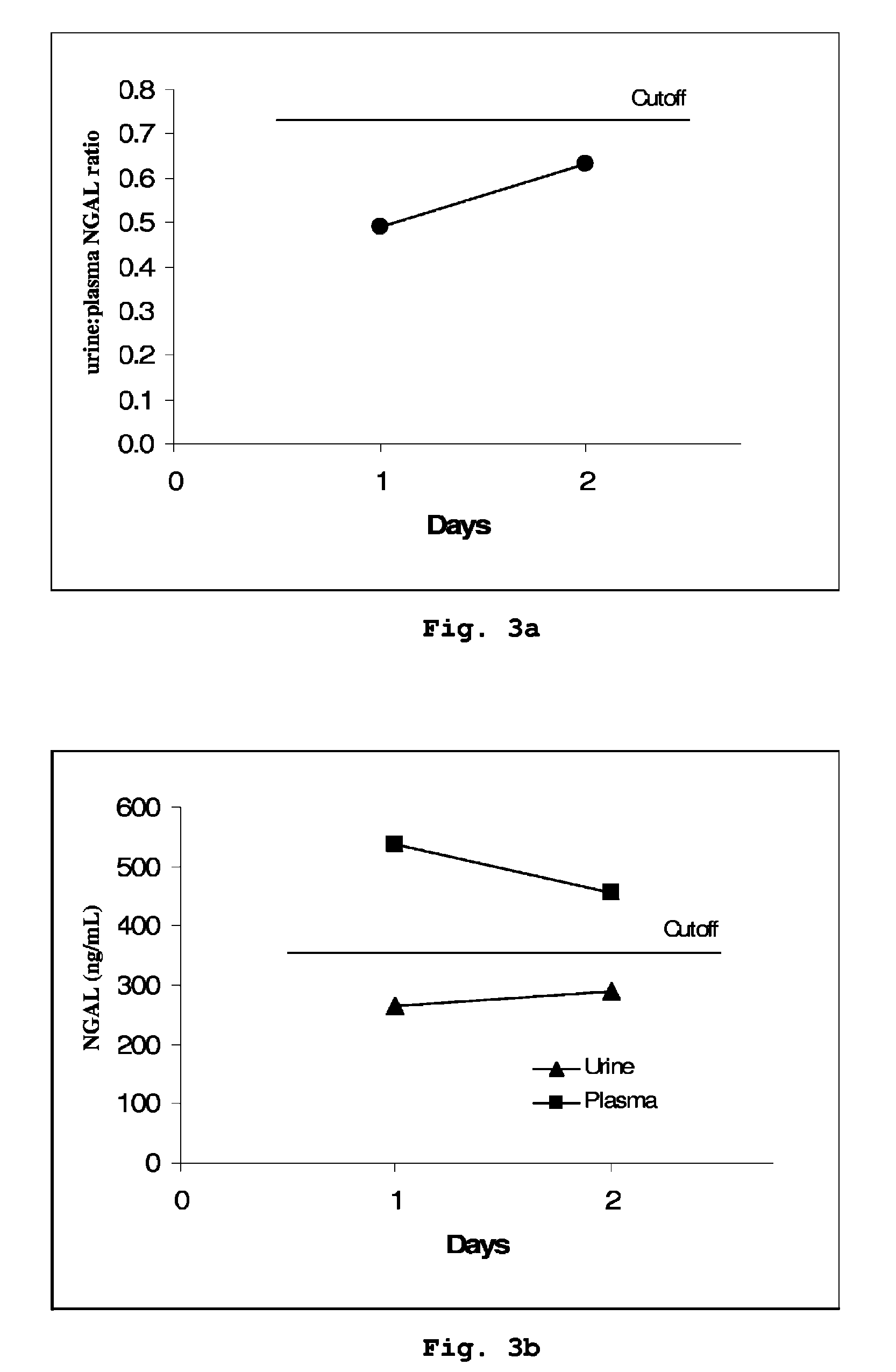
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
47 results about "Neutrophil gelatinase-associated lipocalin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
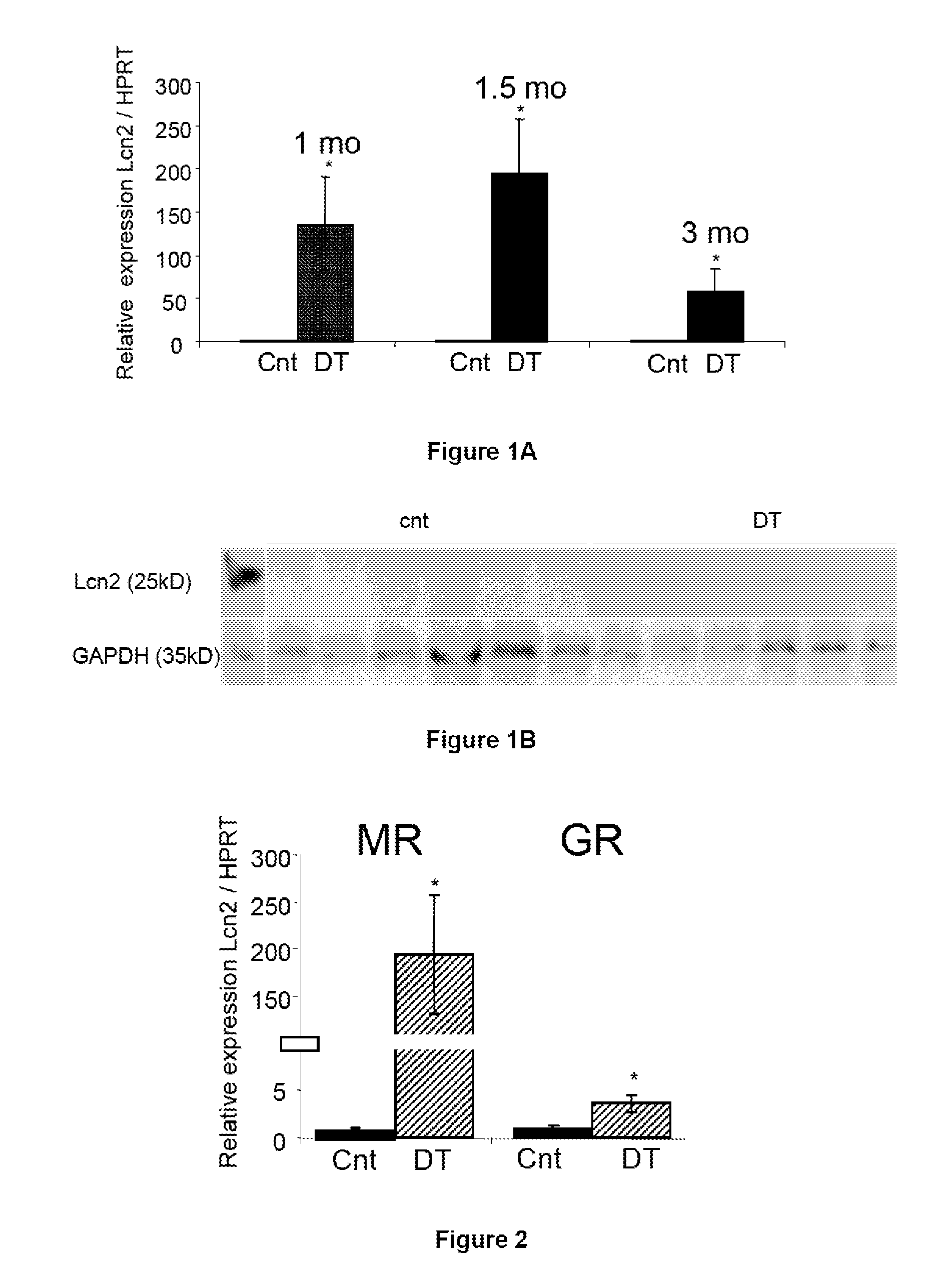
Supavekin et al. identified neutrophil gelatinase-associated lipocalin (Ngal, also known as lcn2) as one of the most upregulated genes in the early post-ischaemic mouse kidney [7,8], a finding that has now been confirmed in several other transcriptome profiling studies following ischaemic and nephrotoxic kidney injuries.
Neutrophil gelatinase-associated lipocalin (NGAL) protein level ELISA kit
ActiveCN106814193ASpeed up dissociationColor/spectral properties measurementsBiological testingElisa kitBinding site
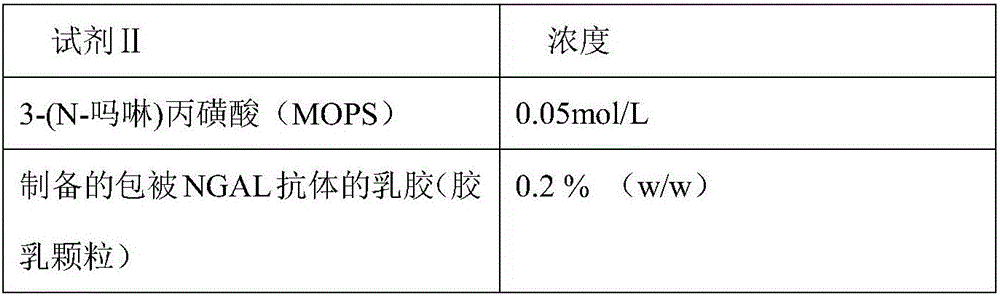
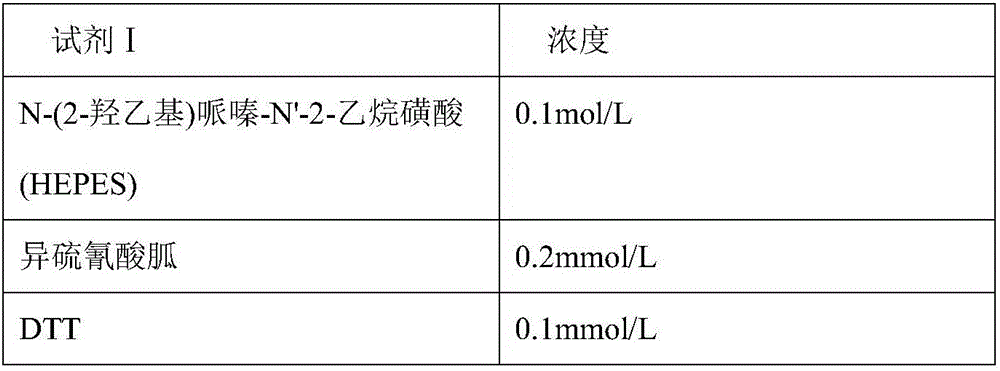
Disclosed is a neutrophil gelatinase-associated lipocalin (NGAL) protein level ELISA kit which is composed of a reagent I and a reagent II. The reagent I comprises a slow-release agent and a denaturant; the reagent II comprises latex particles coated with NGAL antibodies. Aggregated protein is denatured to some extent after being added with the denaturant, physical and (or) chemical binding site is exposed, chemical binding action of the chemical binding site is fractured through sulfydryl dissociation agent , while physical binding action of the physical binding site is dispersed by surface active agent, so that the aggregated protein is disaggregated. Therefore, it is quite important to choose the appropriate types and concentrations of denaturant, sulfydryl dissociation agent, and surface active agent; the aggregate is disaggregated without interference on following immunological detection. According to the method, the the appropriate types and concentrations of denaturant, sulfydryl dissociation agent and surface active agent are determined and chosen specifically, which solves the technical problem above.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Rapid NGAL (Neutrophil Gelatinase Associated Lipocalin) detection kit based on amino acid spacer arm
InactiveCN104198723AReduce binding steric hindranceHigh sensitivityBiological testingGelatinasesMicrosphere
The invention discloses a rapid NGAL (Neutrophil Gelatinase Associated Lipocalin) detection kit based on an amino acid spacer arm and used for quantitatively and rapidly determining NGAL in human serum (plasma) or urine. The kit comprises a reagent 1 (R1) and a reagent 2 (R2), wherein the R1 comprises a biological buffer solution, a surfactant, a stabilizer, a coagulation accelerator and a preservative; the R2 comprises latex particles for enveloping an NGAL antibody, a biological buffer solution, a protective agent, a surfactant and a preservative; in R2, an antibody against human NGAL is connected with a polystyrene latex microsphere through the amino acid spacer arm. The invention also discloses a preparation method of the latex particles for enveloping the antibody against the human NGAL. The kit has high sensitivity and wide linear range; when being used in cooperation with a POCT (Point Of Care Testing) scattering nephelometry analyzer, the kit can achieve the aim of accurately and rapidly determining the NGAL in human serum (plasma) or urine.
Owner:NANJING PERLONG MEDICAL EQUIP
Methods, Devices and Kits for Detecting or Monitoring Acute Kidney Injury
ActiveUS20110287455A1High sensitivityEasy to detectPeptide librariesChemiluminescene/bioluminescenceEpitopeGelatinase
Methods for detecting acute kidney injury in an individual comprise (a) contacting a body fluid sample from the individual with an assay device including neutrophil gelatinase-associated lipocalin (NGAL) antibody and a detectable label, to allow complexing of NGAL protein in the sample with NGAL antibody, and determining an amount of complex formed between NGAL protein from the sample and NGAL antibody in the assay device using the detectable label, wherein NGAL antibody in the device has binding capacity with more than two NGAL protein epitopes, and wherein the amount of the formed complex represents a level of acute kidney injury. Methods for determining an origin of NGAL protein in a sample from an individual include the step of determining relative amounts of monomeric, dimeric and heterodimeric forms of NGAL protein in the sample and allow improved diagnosis and therefore better targeted treatment.
Owner:FUTURE MEDICAL DIAGNOSTICS CO LTD
Neutrophil gelatinase-associated lipocalin (NGAL) assay kit (latex-enhanced immunoturbidimetry)
ActiveCN102680698AHigh detection sensitivityEasy to operateBiological testingMicrosphereImmunoturbidimetry
The invention relates to a kit for assaying NGAL in serum, and provides an NGAL assay kit suitable for a full-automatic biochemical analyzer and a special protein analyzer. The technical scheme includes that the NGAL assay kit comprises reagent R1, reagent R2 and a calibrator, wherein the reagent R1 is composed of a buffer solution, an accelerator, a surfactant and the balance purified water; the reagent R2 is composed of a buffer solution and latex microspheres combined with anti-human NGAL antibodies; and the calibrator is composed of a buffer solution, a stabilizer, a preservative, a certain amount of pure recombinant human NGAL required by concentration, and the balance purified water. The NGAL content in the serum can be quickly assayed through the reagent combination.
Owner:NANJING NORMAN BIOLOGICAL TECH
Neutrophil gelatinase associated lipocalin (NGAL) chemiluminescence detection kit
InactiveCN102967714AOvercome the greater influence of blood lipid concentrationHigh sensitivityChemiluminescene/bioluminescenceBiological testingElisa methodBiology
The invention discloses a neutrophil gelatinase associated lipocalin (NGAL) chemiluminescence detection kit. The kit comprises the components of a standard substance of NGAL, an NGAL monoclonal antibody labeled by horseradish peroxidase, magnetic particles coated by the NGAL monoclonal antibody, a white non-transparent microwell plate, washing liquid, and chemiluminescence substrates A and B. The kit provided by the invention can be used for detecting the content of the NGAL in blood serum of a patient, and has important guiding significance to the detections on premature kidney diseases and injures. Compared with the conventional ELISA (Enzyme Linked Immunosorbent Assay) method, the NGAL chemiluminescence detection kit provided by the invention has the characteristics of being high in sensitivity and good in specificity and overcoming the large influence of immunoturbidimetry by blood fat concentration, and has the advantages of high stability, reliability and accuracy, security and environment friendliness, simplicity and convenience in operation and the like.
Owner:天津市协和医药科技集团有限公司
Detection kit for neutrophil gelatinase-associated lipocalin
The invention discloses a detection kit for neutrophil gelatinase-associated lipocalin. The detection kit comprises a detection liquid and a standard substance, wherein the detection liquid comprises latex particles coupled with an anti-human neutrophil gelatinase-associated lipocalin antibody; the standard substance is naturally configured recombinant neutrophil gelatinase-associated lipocalin. The detection kit measures the content of the neutrophil gelatinase-associated lipocalin through a latex particle intensified immunity turbidity, and compared with the traditional NGAL detection kit, the detection kit has the advantages of high detection sensitivity, high simpleness in operation, good repeatability and short time consumption, does not need sample pretreatment, and can be used for a fully automatic biochemical analyzer.
Owner:FAPON BIOTECH INC
NGAL latex immunoturbidimetric detection kit and preparation method thereof
ActiveCN109738626AGuaranteed responsivenessImprove anti-interference abilityBiological testingImmunonephelometric AssaysMicrosphere
The present invention relates to an NGAL latex immunoturbidimetric detection kit and a preparation method thereof. The kit comprises a reagent R1, a reagent R2, a calibration product, and a quality control product. The reagent R1 comprises a buffer solution, an anti-interference agent, a sensitizer, an electrolyte and a preservative, and the reagent R2 comprises latex microspheres with an NGAL antibody, a buffer solution, a stabilizer and a preservative. Compared with the prior art, according to the technical scheme of the present invention, by using the anti-interference agent in the R1 reagent, the anti-interference ability can be significantly improved, and the applicable population of the neutrophil gelatinase-associated lipocalin assay kit is expanded; and the kit detection provided by the present invention has high sensitivity, high specificity, and good stability of the kit, the NGAL content in urine, plasma and serum can be efficiently detected, and the detection results can bewell correlated with imported reagents.
Owner:SHANGHAI FOSUN LONG MARCH MEDICAL SCI CO LTD
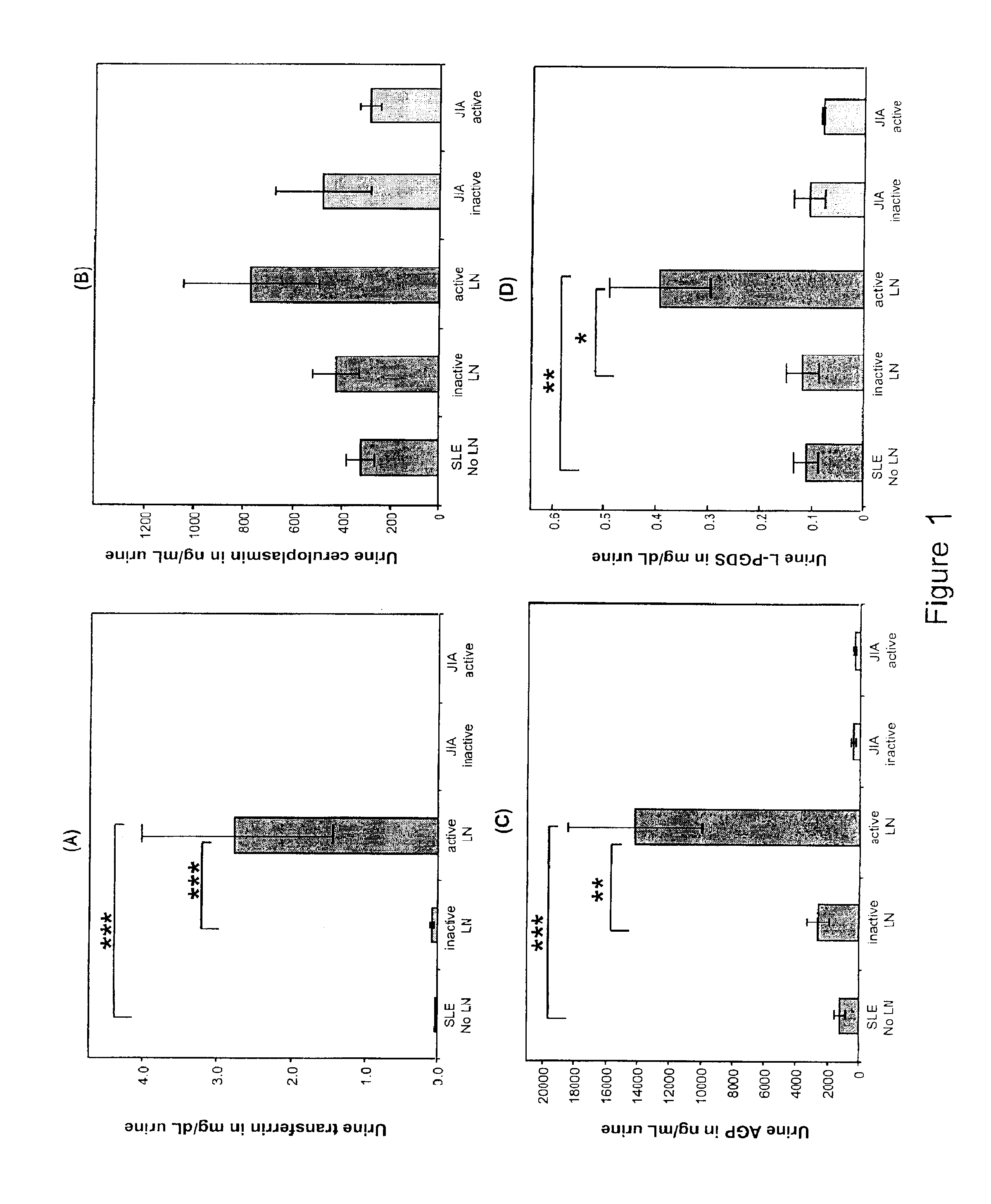
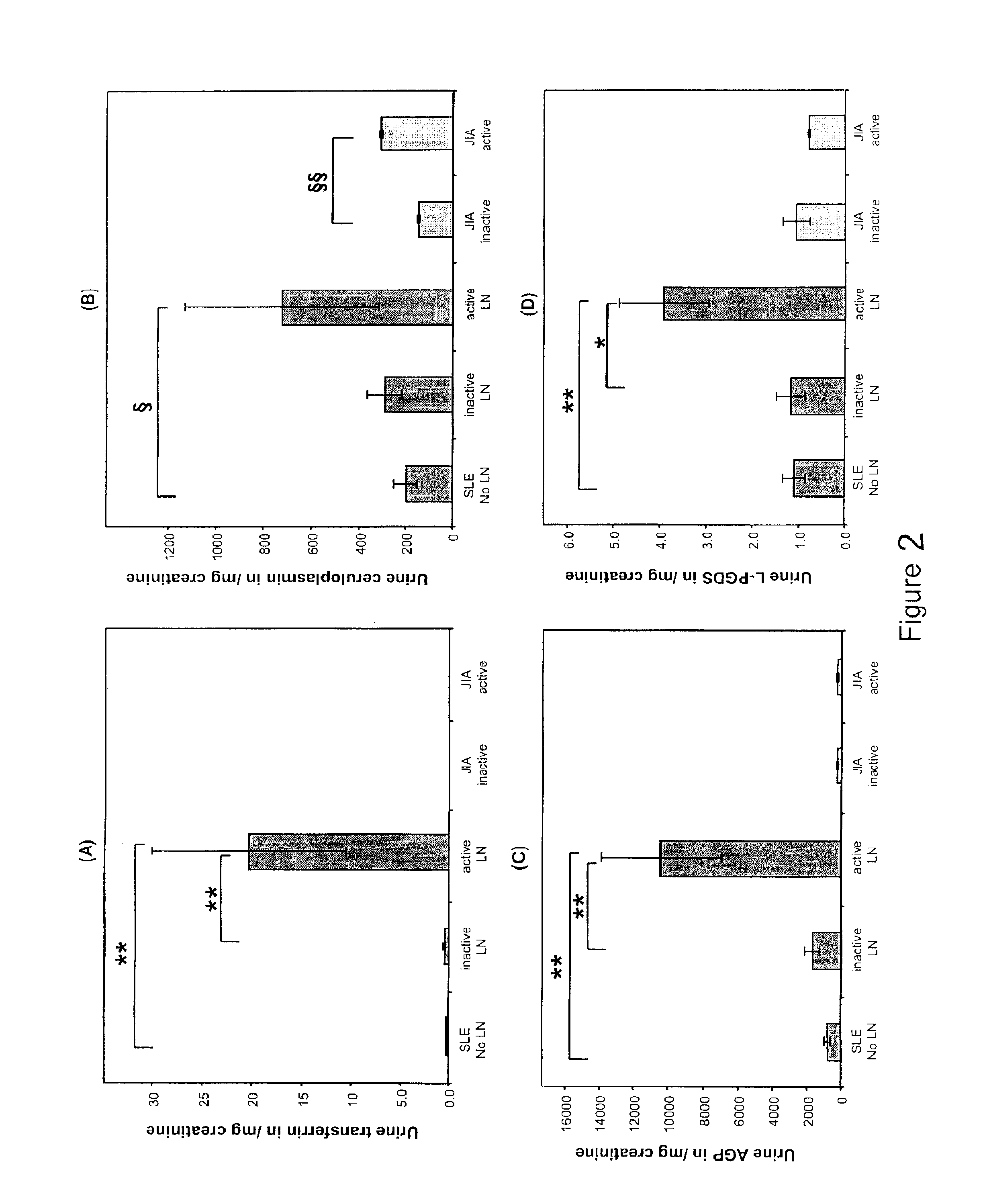
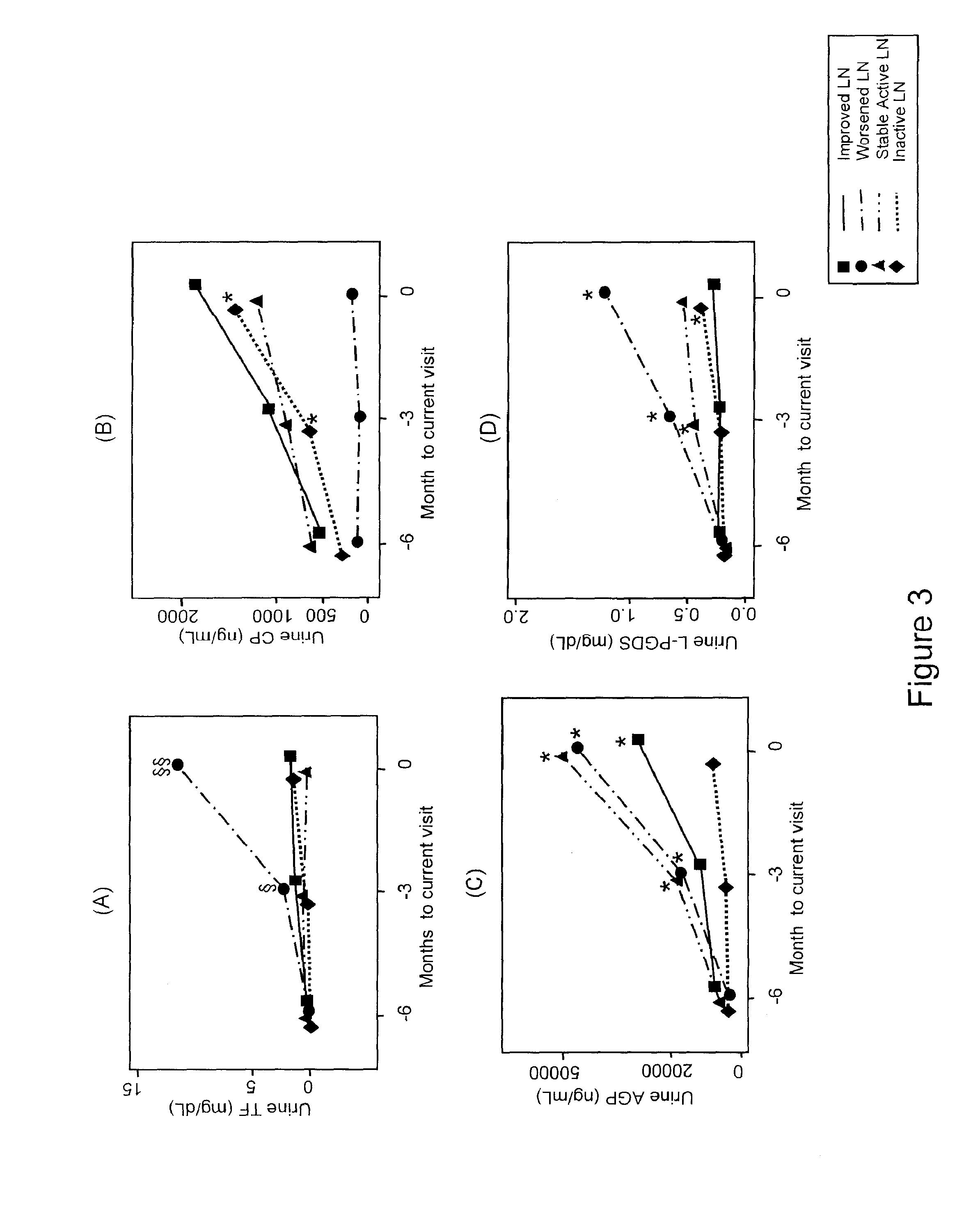
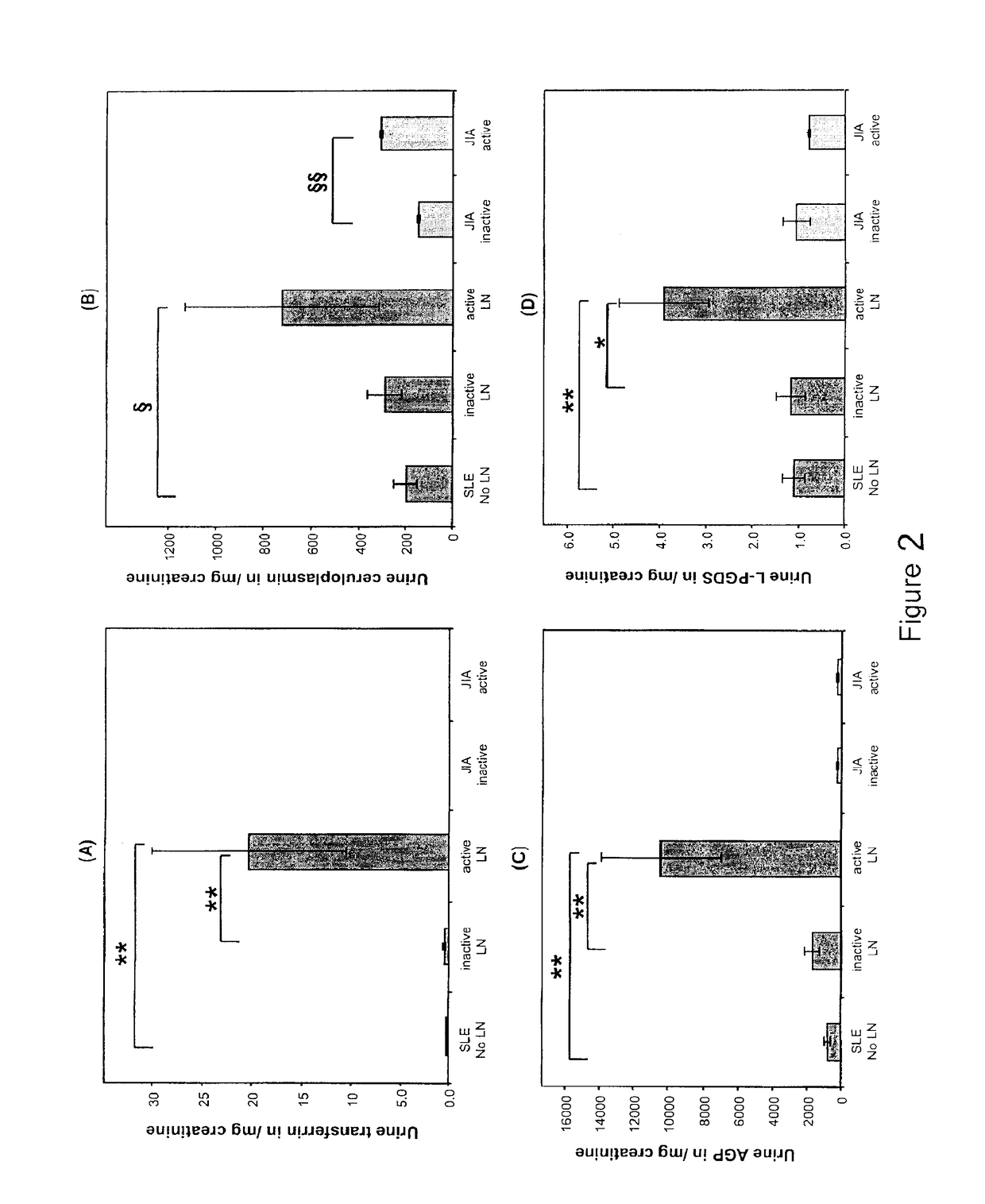
Detection of worsening renal disease in subjects with systemic lupus erythematosus
ActiveUS20100323911A1Worsening renal disease activityAccurate determinationLibrary screeningDisease diagnosisProstaglandins DDisease activity
Methods for the detection of active lupus nephritis (LN) and worsening renal disease activity and / or active LN in patients diagnosed with systemic lupus erythematosus, using a panel of biomarkers including transferrin (Tf), ceruloplasmin (Cp), alpha-1-acid glycoprotein (AGP1), lipocalin-like prostaglandin D synthetase (L-PGDS), and urinary neutrophil gelatinase associated lipocalin (UNGAL).
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Neutrophil gelatinase-associated lipocalin determination kit
InactiveCN106093422ARapid responseEliminate distractionsDisease diagnosisBiological testingFully automaticAbsorbance
The invention discloses a neutrophil gelatinase-associated lipocalin determination kit. The kit comprises a reagent R1 and a reagent R2 which are liquid components and are mutually independent, the reagent R1 comprises a buffer solution, inorganic salt ions, an accelerator, a chelating agent, a stabilizer, an antiseptic and an anti-humanr heumatoid factor antibody, and the reagent R2 comprises the buffer solution, the stabilizer, a suspending aid, a surfactant, the antiseptic and a latex coated anti-human neutrophil gelatinase-associated lipocalin. The preparation method comprises the following steps: preparing the reagents according to the component content; mixing a sample to be determined with the reagent R1 and the reagent R2 to fully react the sample to be determined with the reagent R1 and the reagent R2; determining the absorbance difference by using a fully-automatic biochemical analyzer after the reaction; and calculating the concentration of neutrophil gelatinase-associated lipocalin in the sample according to the absorbance change value. The kit has high accuracy.
Owner:ANHUI IPROCOM BIOTECH CO LTD
NGAL (Neutrophil Gelatinase Associated Lipocalin) optical excitation chemiluminescence detection kit and preparation and use methods of kit
ActiveCN103954776AHigh detection sensitivityNot easy to interfereBiological testingAnthraceneMicrosphere
The invention relates to a method for preparing an NGAL (Neutrophil Gelatinase Associated Lipocalin) optical excitation chemiluminescence detection kit. The method comprises the following steps: activating a luminous microsphere, namely activating the luminous microsphere through prepared carbodiimide and Sulfo-NHS solutions in a PBS (Phosphate Buffer Saline) buffer solution, wherein the luminous microsphere is a carboxyl modified luminous microsphere, and the luminous microsphere is coated with dimethylthiophene, anthracene and rubrene and carried with an europium chelate; coupling the luminous microsphere with an anti-NGAL antibody, namely selecting the carboxyl modified luminous microsphere, carrying out mixing reaction on the anti-NGAL monoclonal antibody and the activated carboxyl modified luminous microsphere to couple the anti-NGAL antibody to the luminous microsphere, and adding freshly prepared carbodiimide at the same time while mixing the anti-NGAL antibody and the activated luminous microsphere; labeling the anti-NGAL antibody through biotin; and labeling a photosensitive microsphere through avidin. The invention also relates to preparation and use methods of the NGAL optical excitation chemiluminescence detection kit. The methods disclosed by the invention solve the problems that the sensitivity is low and the detection is easily interfered by the environment in a detection method in the prior art.
Owner:WUHAN LIFE ORIGIN BIOTECH LTD
Novel reagent and kit for renal injury monitoring
ActiveCN102775486AStrong specificityHigh antibody titerImmunoglobulins against animals/humansBiological testingPolyclonal antibodiesRenal injury
The invention relates to a novel reagent and a kit for renal injury monitoring. The inventor identifies critical antigenic determinants from full-length NGAL (neutrophil gelatinase-associated lipocalin). And a short peptide mixture containing the critical antigenic determinants is adopted as an immunogen to obtain a specific polyclonal antibody against NGAL. The antigen fragments and its polyclonal antibody provided in the invention have the advantages of simple preparation method, high titer, strong specificity, and high sensitivity.
Owner:SHANGHAI SUNFORY BIOPHARM INC
Neutrophil gelatinase-associated lipocalin detection kit and preparation
ActiveCN103995128AEasy to cleanExtended service lifeBiological material analysisBiological testingGelatinaseNeutrophil granulocyte
The invention provides a neutrophil gelatinase-associated lipocalin detection kit. Based on colloidal gold immunoturbidimetry, the kit contains a reagent R2 which is a solution containing gold nanoparticles labeled with a neutrophil gelatinase-associated lipocalin antibody. The kit is characterized in that particle size of the gold nanoparticles is 62.2nm-79.1nm; and the mass ratio of the gold nanoparticles to the antibody is 50:20-60. The invention also provides a preparation method of the kit. The kit provided by the invention has characteristics of high sensitivity, high specificity, rapid reaction and good stability. No precipitate is generated after a reaction. It is convenient to clean a biochemical analyzer, and service life of the biochemical analyzer is prolonged.
Owner:BEIJING JIUJIAYI TECH
Kit for content detection of neutrophil gelatinase-associated lipocalin
The invention discloses a kit for content detection of neutrophil gelatinase-associated lipocalin, wherein a reagent R1 comprises Tris, NaCl, BSA, Tween-20, PEG and NaN3; a reagent R2 comprises Tris, NaCl, BSA, Tween-20, NaN3, sucrose and NGAL antibody sensitized latex particles; a NGAL reagent reference standard sample comprises Tris, NaCl, BSA, Tween-20, EDTA, NaN3 and NGAL of different contents; the NGAL antibody sensitized latex particles comprise PS nanometer latex particles and PVN nanometer latex particles, and the particle size of the PS nanometer latex particle is larger than the particle size of the PVN nanometer latex particle. The invention has the advantages of simple reagent composition, good test sensitivity, wide linearity range, good stability, low test cost and high precision, and the kit is convenient for popularization.
Owner:NINGBO RUI BIO TECH
Renal failure rapid detection triple kit and preparation method and application thereof
InactiveCN104865384AStop the disease from getting worseLessen kidney damageDisease diagnosisBiological testingCvd riskSerum cystatin
This invention belongs to the field of biotechnology detection, particularly relates to a triple kit for early warning, treatment process and monitoring after treatment of renal failure, and further discloses a preparation method and application thereof. Serum cystatin (CysC), neutrophil gelatinase-associated lipocalin (NAGL) and C reactive protein (CRP) are used as detecting markers. The triple kit uses NGAL-CysC-CRP triple antibody as the markers, can effectively monitor the risk of renal failure, especially plays an unexpected effect of monitoring of acute renal failure of persons subjected to a traffic accident, and is high in diagnosis sensitivity of acute renal failure, and strong in specificity, the clinical monitoring accuracy is as high as 95%, and the triple kit can make nephropathy self testing and monitoring of persons with diabetes possible, is used for kidney disease early warning, can timely prevent deterioration of the patient's condition, and reduces the patient's degree of kidney damage.
Owner:GOLDBIOMARKERS DIAGNOSTICS
Immunoturbidimetric NGAL detection reagent and method
InactiveCN108089008ASimple compositionSimplify operating proceduresDisease diagnosisBiological testingLatex particleSuspending Agents
The invention discloses a latex-enhanced immunoturbidimetric NGAL (neutrophil gelatinase associated lipocalin) detection reagent. The latex-enhanced immunoturbidimetric NGAL detection reagent is a single reagent, uses NGAL antibody-labeled latex particles as a main component, and further comprises a buffer solution, a surfactant, salt, a stabilizer, a suspending agent and a preservative. The invention further discloses a method for detecting the concentration of NGAL in a blood sample by virtue of a transmitting or scattering turbidimetric principle by using the latex-enhanced immunoturbidimetric NGAL detection reagent. The latex-enhanced immunoturbidimetric NGAL detection reagent adopts the single reagent, is easy to operate, free of need of mixing, high in sensitivity and wide in linearrange, can directly measure various samples such as whole blood, serum and plasma, and can be widely applied to various transmitting or scattering analyzers, including an ordinary biochemical analyzer, a specific protein analyzer and the like.
Owner:SUZHOU KANGHESHUN MEDICAL TECH
Preparation method of human neutrophil gelatinase associated lipocalin (NGAL)
InactiveCN103880948ANo sex change requiredNo renaturationApolipeptidesFermentationPichia pastorisNucleotide
The invention relates to a method of preparing human neutrophil gelatinase associated lipocalin (NGAL) in pichia pastoris. The method comprises the following steps: providing a nucleotide sequence which codes the NGAL; cloning the nucleotide sequence of the NGAL to different yeast expression vectors; converting the yeast expression vectors to one yeast host; inducing the yeast host to express the NGAL with a histidine label at the terminal C; and purifying the treated NGAL in the step 4). The method is high in expression quantity, and the expression product does not need to be subjected to denaturation and renaturation, and the human NGAL with activity can be obtained. The subsequent purifying steps are further simple and convenient. The prepared human NGAL can be used for a NGAL detection kit for detecting acute kidney injury.
Owner:BEIJING AMBITION BIOTECH
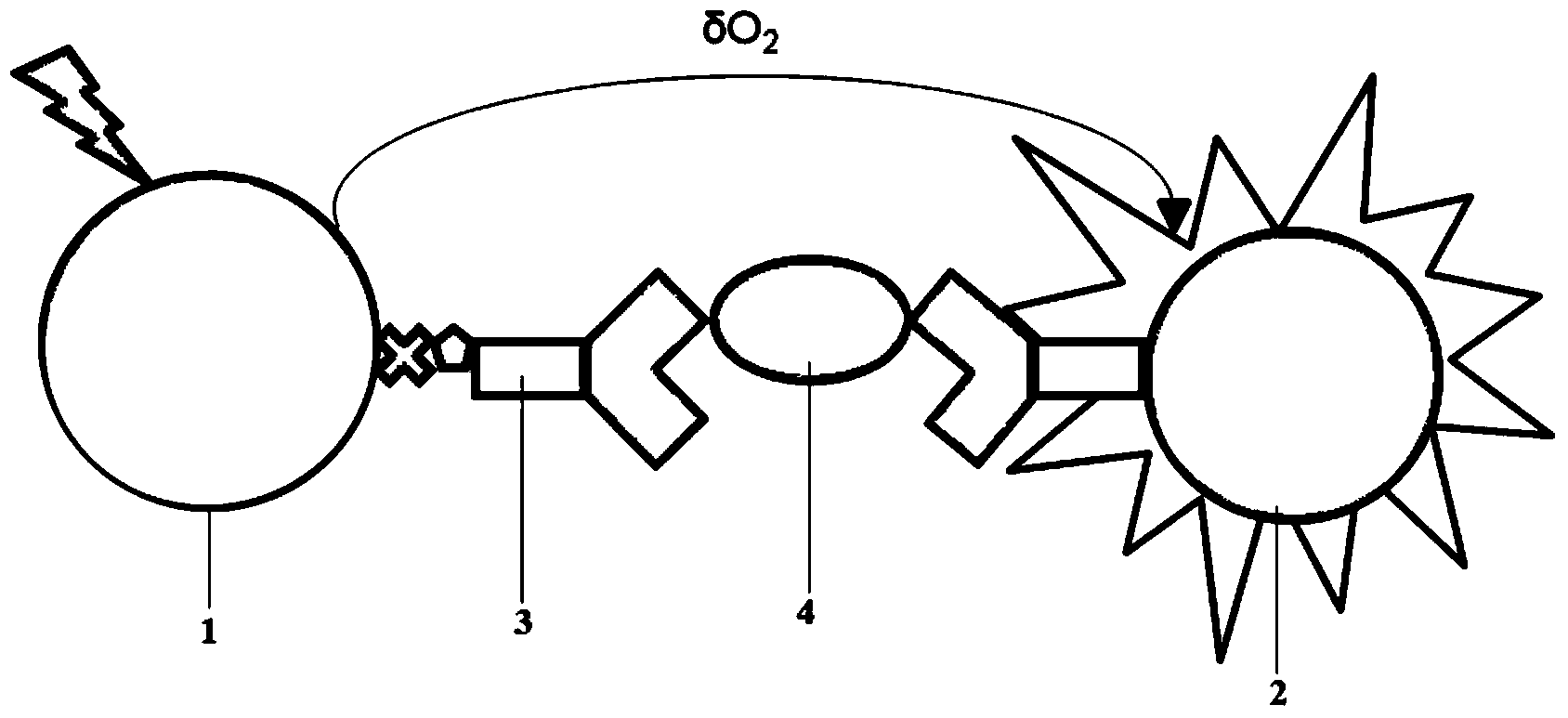
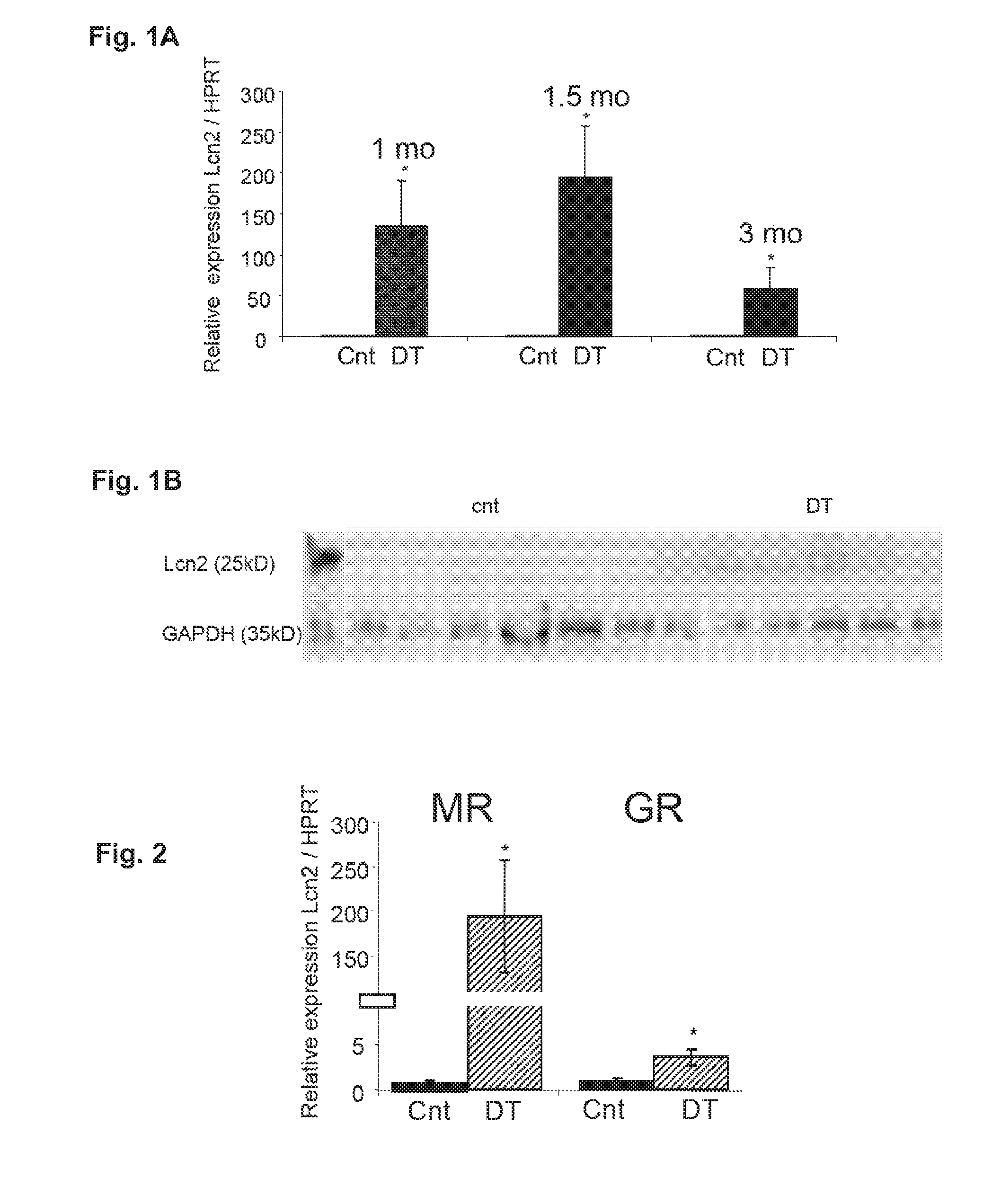
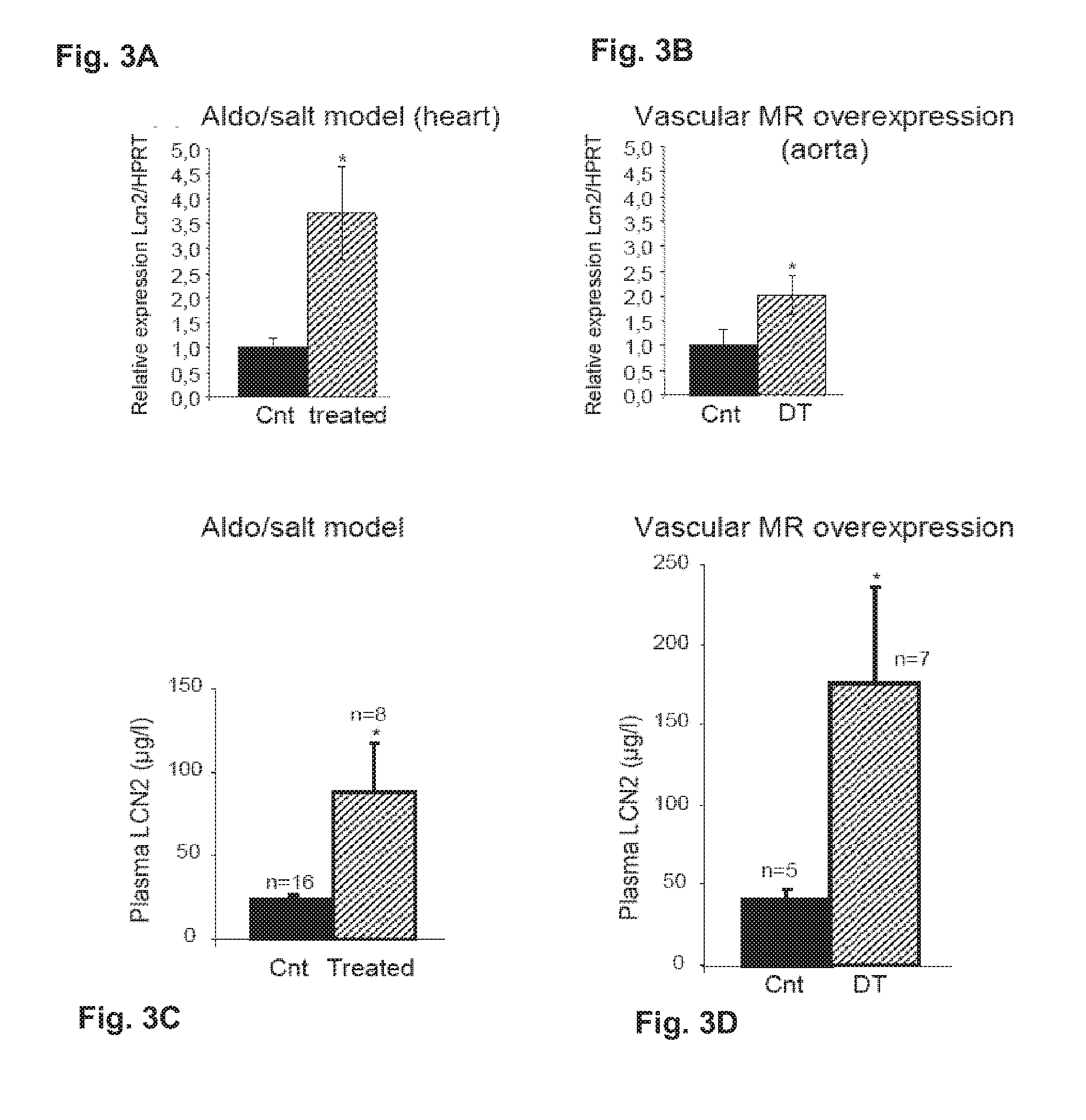
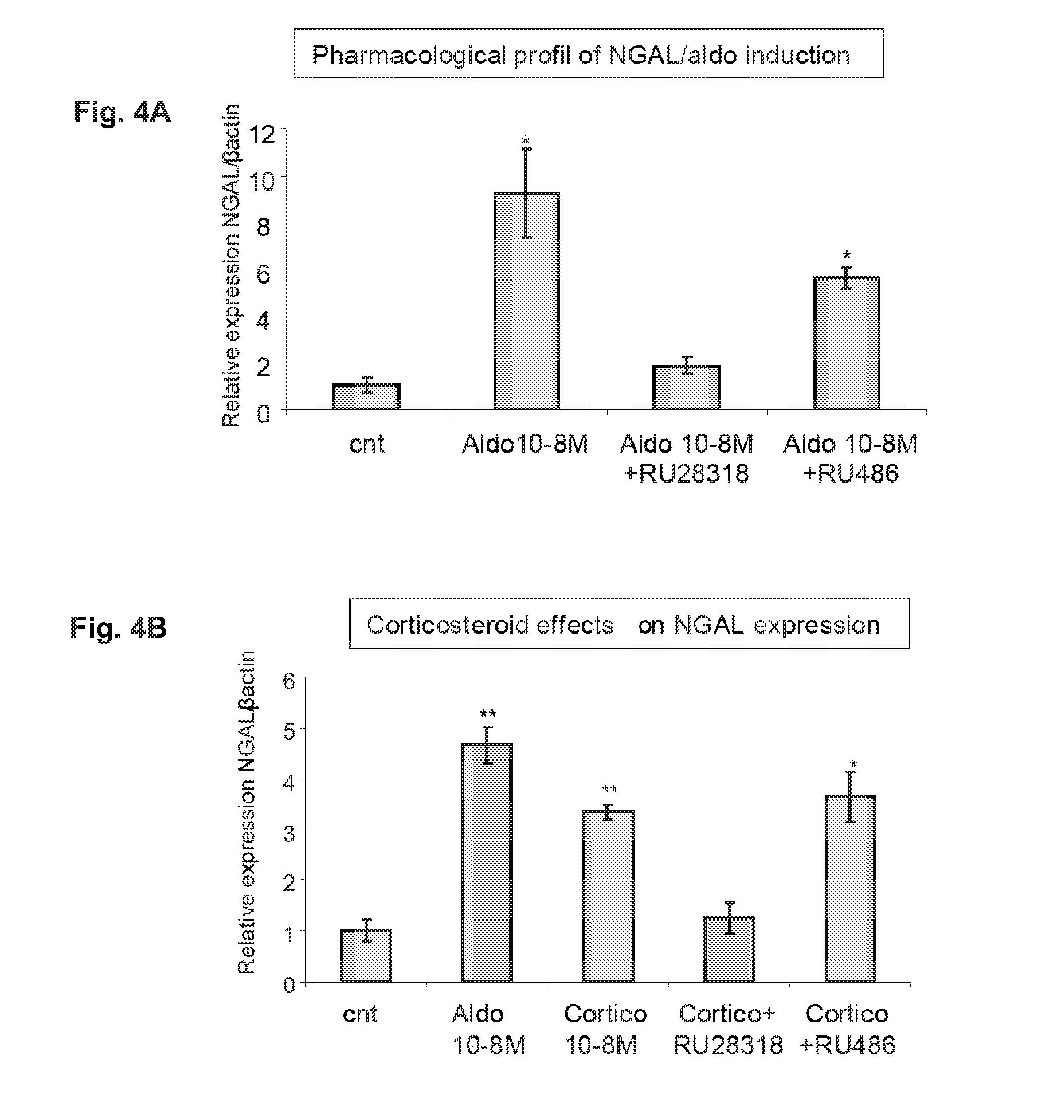
Biomarkers of Mineralocorticoid Receptor Activation
ActiveUS20160362744A1Precision therapyMetabolism disorderMicrobiological testing/measurementGelatinasesAldosterone Synthase Deficiency
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
Neutrophil gelatinase-associated lipocalin detection kit
The present invention relates to the field of biological detection technology, and in particular to a neutrophil gelatinase-associated lipocalin detection kit, the neutrophil gelatinase-associated lipocalin detection kit comprises mutually independent reagent R1 and reagent R2, and the detection kit is characterized in that: the reagent R1 comprises the following substances: 80-120 mmol / L of phosphate buffer solutions; 0.5-1.5mmol / L of glutathione; 0.02-1.5g / L of surfactant; 0.05-2g / L of preservatives; 15-30g / L of bovine serum albumin; 0.02-1g / L of dissociation agents, and the reagent R1 hasa pH of 6.5 to 7.5; and the reagent R2 comprises the following materials: 100-150 mmol / L of phosphate buffer solutions; 8-15 g / L of coagulant; 2-8mg / L of blocking agents; 5-15mg / L of sucrose; 20-50mg / L of nano-microspheres of neutrophil gelatinase-associated lipocalin antibodies; 0.01-0.05g / L of preservatives; wherein component concentration in the reagent R2 is final concentration of the component in the reagent, and pH of the reagent R1 is 6.5 to 7.5. The neutrophil gelatinase-associated lipocalin detection kit has high detection sensitivity.
Owner:芜湖森爱驰生物科技有限公司
Diagnostic test for renal injury
ActiveUS8313919B2Satisfactory linearityStrong specificityMicrobiological testing/measurementDisease diagnosisBacteriuriaBlood plasma
A method is provided of diagnosing and monitoring acute renal injury leading to acute renal failure in a human or mammalian subject by determining the ratio of the concentration of neutrophil gelatinase-associated lipocalin (NGAL) in urine to that in plasma or serum.
Owner:BIOPORTO DIAGNOSTICS AS
Kit for detecting acute kidney injury
InactiveCN107525938AGood correlationHigh correlationDisease diagnosisBiological testingEpitopeTrue positive rate
The invention relates to a kit for detecting acute kidney injury. The kit comprises a first detection liquid and a second detection liquid, wherein a first anti-NGAL (neutrophil gelatinase-associated lipocalin) monoclonal antibody coated with magnetic particles is contained in the first detecting liquid, and a second anti-NGAL monoclonal antibody labeled with a chemiluminescent label is contained in the second detection liquid. The first anti-NGAL monoclonal antibody and the second anti-NGAL monoclonal antibody are used for different NGAL epitopes respectively. The kit for detecting acute kidney injury is used for detection on basis of NGAL serving as a marker for diagnosis detection, can rapidly detect and diagnose the acute kidney injury at the early stage and is higher in sensitivity and specificity of detection.
Owner:FAPON BIOTECH INC +1
Urine special protein composite quality control substance and preparation method thereof
PendingCN110333356AAchieve quality controlReduce the influence of matrix effectsDisease diagnosisBiological testingCreatinine riseProtein detection
The invention relates to the technical field of urine special protein detection, in particular to a urine special protein composite quality control substance and a preparation method thereof, the quality control substance comprises a low-value positive quality control substance and a high-value positive quality control substance, and the low-value positive quality control substance and the high-value positive quality control substance respectively comprise a buffer agent, urea, salts, a preservative, creatinine, PH, a protein protective agent, a stabilizer, human albumin, transferrin, retinolconjugated protein, alpha1-microglobulin, beta2-microglobulin, immune globulin G, neutrophil gelatinase associated lipocalin. The urine special protein composite quality control substance and the preparation method thereof provided by the invention have the advantages of simple operation, instant use after decapping, good stability, low production cost and easiness to achieve the quality control of a urine special protein detection system.
Owner:URIT MEDICAL ELECTRONICS CO LTD
Kit for quantitatively determining NGAL (neutrophil gelatinase-associated lipocalin) in urine or blood
InactiveCN107656078ARapid Quantitative DetectionAccurate quantitative detectionDisease diagnosisBiological testingLatex particlePolyethylene glycol
The invention relates to a kit for quantitatively determining the NGAL (neutrophil gelatinase-associated lipocalin) in urine or blood. The kit comprises a kit cover, a kit body, a first reagent unit and a second reagent unit, wherein the first reagent unit and the second reagent unit are arranged in the kit body; the outside of the first reagent unit and the outside of the second reagent unit aremutually independent; the first reagent unit and the second reagent unit are connected through a support frame; fixing articles are filled around the two reagent units connected through the support frame; reagents contained by the first reagent unit include phosphate buffer solution, triton, polyethylene glycol and sodium azide; reagents contained by the second reagent unit include mouse anti-human NGAL monoclonal antibody modified latex particles, goat anti-human NGAL polyclonal antibody modified latex particles, casein and sodium azide. The kit has the advantages that the use and the operation are simple and convenient; the NGAL in the urine or blood can be fast, accurately and quantitatively determined; the detection sensitivity is improved; the linear measurement range is increased; the clinical detection requirements are fully met.
Owner:SHANDONG ZHUSHITANG MEDICAL TREATMENT INSTR CO LTD
A kit for detecting the content of neutrophil gelatinase-associated lipocalin
ActiveCN104198732BHigh sensitivityImprove stabilityBiological testingLatex particlePolyethylene glycol
Owner:NINGBO RUI BIO TECH
Biomarkers of Mineralocorticoid Receptor Activation
InactiveUS20110257140A1Precision therapyOrganic active ingredientsMetabolism disorderGelatinasesAldosterone Synthase Deficiency
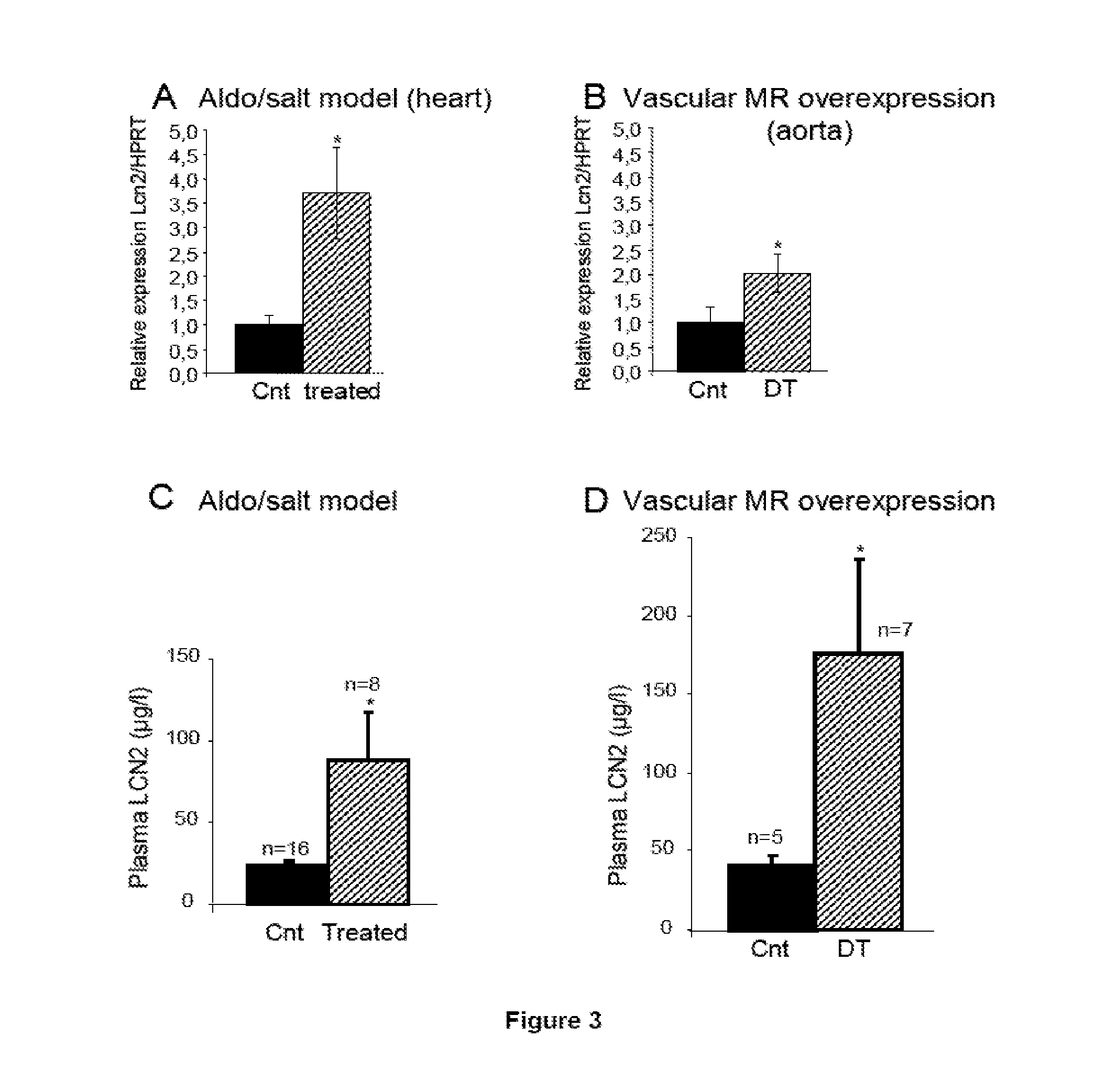
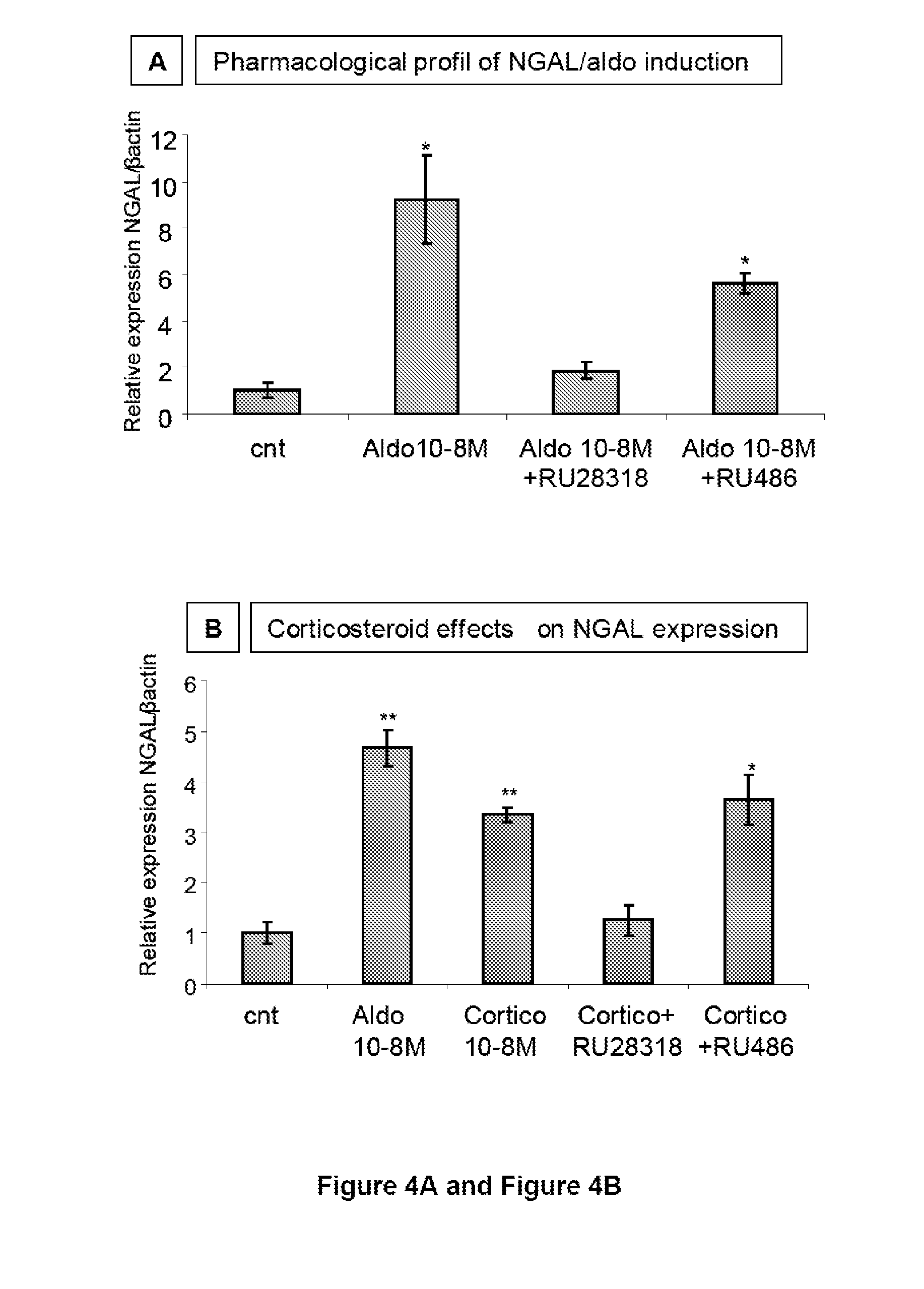
The present invention relates to the use of Neutrophil Gelatinase-Associated Lipocalin (NGAL) and / or SERPINA3 as biomarkers of the Mineralocorticoid Receptor (MR) activation in a patient. More particularly, the present invention relates to a method for predicting the responsiveness of a patient to a treatment with a MR antagonist or an aldosterone synthase inhibitor, said method comprising determining in a biological sample obtained from said patient the expression level of the NGAL gene and / or of the SERPINA3 gene.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
Application of synovial fluid neutrophil gelatinase-associated lipocalin in detection and diagnostic kit for prosthetic joint infection
InactiveCN108593923AImprove anti-interference abilityImprove stabilityBiological material analysisBiological testingFluorescenceSevere complication
The invention relates to an application of synovial fluid neutrophil gelatinase-associated lipocalin (NGAL) in detection and a diagnostic kit for prosthetic joint infection. Joint replacement is an effective treatment mode for treating joint disease at a terminal stage, and periprosthetic joint infection is a severe complication after joint replacement. The synovial fluid NGAL has high sensitivityand specificity for diagnosing the prosthetic joint infection. According to the invention, fluorescence particles are taken as a maker, and the diagnostic kit (a test paper strip) for the synovial fluid NGAL is prepared through an immunochromatography method. By using an immunofluorescence quantitative analyzer, quantitative detection and instant detection on a synovial fluid specimen can be carried out, and diagnosis of the prosthetic joint infection is realized. A synovial fluid NGAL detection reagent and the diagnostic kit have the advantages of simple usage, sensitive reaction, and high diagnosis accuracy.
Owner:THE FIRST AFFILIATED HOSPITAL OF FUJIAN MEDICAL UNIV
Method, reagent and kit for quantitative determination of NGAL content in human serum
The invention relates to a reagent for the quantitative determination of NGAL (Neutrophil Gelatinase-Associated Lipocalin) content in human serum. The reagent comprises a reagent I and a reagent II which are placed separately, wherein the reagent I contains T phosphate buffer and polyethylene glycol-6000; the reagent II contains mouse anti-human NGAL monoclonal antibody latex particles and an antibody stabilizer. A kit and a detection method adopted in the invention only require a few microlitres of serum, need no centrifugation or electrophoresis and other separation treatments, are easy to operate, can meet the requirements of automatic analysis, and are applicable to the timely and accurate detection of large-scale samples.
Owner:ZHEJIANG LANSEN BIOTECH
Neutrophil gelatinase-associated lipocalin determination kit
ActiveCN107918020ALow detection limitHigh detection sensitivityDisease diagnosisBiological testingPreservativeGelatinase
The invention belongs to the technical field of medicine and biochemistry and in particular relates to a neutrophil gelatinase-associated lipocalin determination kit. The kit is composed of a reagentR1 and a reagent R2, wherein the reagent R1 contains biological buffer, a coagulation accelerator, a surfactant, an osmotic pressure regulator and water; and the reagent R2 contains latex particles coating a neutrophil gelatinase-associated lipocalin antibody, biological buffer, a sealing agent, a preservative and water. The neutrophil gelatinase-associated lipocalin detection kit performs determination by adopting particle-enhanced turbidimetric immunoassay, and compared with the traditional neutrophil gelatinase-associated lipocalin (NGAL) detection kit, the detection kit disclosed by the invention has the advantages of being rapid, sensitive, excellent in accuracy, high in specificity, excellent in stability and the like.
Owner:浙江夸克生物科技有限公司
Application of NGAL (Neutrophil Gelatinase-Associated Lipocalin) to preparation of early diagnostic kit for canine acute kidney injury
InactiveCN109521205ADisease diagnosisBiological testingStandard solutionNeutrophil gelatinase-associated lipocalin
The invention belongs to the technical field of application of biological markers, and particularly relates to application of NGAL (Neutrophil Gelatinase-Associated Lipocalin) to the preparation of anearly diagnostic kit for canine acute kidney injury. The biological marker NGAL is made into a standard solution with a concentration of 46.96 ng / mL. The NGAL standard solution made in the inventionhas the sensitivity of 0.957 and the specificity of 0.96, and has important meaning to the research and development of the early diagnostic kit for the canine acute kidney injury.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Detection of worsening renal disease in subjects with systemic lupus erythematosus
ActiveUS9880165B2Accurate determinationAccurate diagnosisLibrary screeningDisease diagnosisProstaglandins DDisease activity
Methods for the detection of active lupus nephritis (LN) and worsening renal disease activity and / or active LN in patients diagnosed with systemic lupus erythematosus, using a panel of biomarkers including transferrin (Tf), ceruloplasmin (Cp), alpha-1-acid glycoprotein (AGP1), lipocalin-like prostaglandin D synthetase (L-PGDS), and urinary neutrophil gelatinase associated lipocalin (UNGAL).
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Colloidal gold immunocolorimetry kit for detecting neutrophil gelatinase associated lipocalin (NGAL) and preparation method of kit
InactiveCN105891503AIncrease or decrease the ratioHigh sensitivityBiological testingAntigenGold particles
The invention provides a colloidal gold immunocolorimetry kit for detecting neutrophil gelatinase associated lipocalin (NGAL) and a preparation method of the kit and belongs to the field of in-vitro diagnostic reagents. The kit is used for judging whether the situation of individual acute kidney injury occurs or not. According to the method, colloidal gold particles are adopted as a carrier, a neutrophil gelatinase associated lipocalin (NGAL for short) antibody and the colloidal gold particles are coupled, NGAL protein in a sample makes contact with the coupled gold particles in a corresponding detection device, an antigen-antibody-gold compound is formed, the light absorbance of the gold particles under detection wavelength is changed, the change amount of the light absorbance is positively correlated with the concentration of NGAL protein, and then the purpose of detecting NGAL protein in the sample is achieved. The kit has high analysis sensitivity.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com