Kit for quantitatively determining NGAL (neutrophil gelatinase-associated lipocalin) in urine or blood
A quantitative detection and kit technology, applied in the field of biomedicine, can solve the problems of high consumption of kits, high cost, and inability to freely replace reagent units, so as to improve detection sensitivity, easy to use and operate, and meet the needs of clinical testing.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
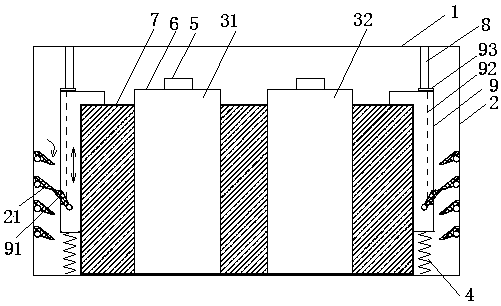
[0015] as attached figure 1 , 2 As shown, in this embodiment, the kit for quantitatively detecting NGAL in urine or blood includes a box cover 1, a box body 2, and a first reagent unit 3-1 and a second reagent unit 3-2 placed in the box body, Both the first reagent unit and the second reagent unit are composed of a bottle cap 5 and a bottle body 6, the outside of the first reagent unit and the second reagent unit are independent of each other, and the first reagent unit and the second reagent unit are connected by a ring-shaped external bracket 7 The surroundings of the two reagent units connected by brackets are filled with foam plastics or sponges and other fixtures. The reagents contained in the first reagent unit include phosphate buffer 0.1mol / L, PH7.5, Triton X-100 0.1g / 100ml , polyethylene glycol 6000 1g / 100ml, sodium azide 0.05g / 100ml, the reagent contained in the second reagent unit contains 0.1g / 100ml of latex particles modified by mouse anti-human NGAL monoclonal a...
Embodiment 2
[0021] The difference between this example and Example 1 is that the reagent contained in the first reagent unit contains phosphate buffer 0.05mol / L, pH7.5, Triton X-100 0.1g / 100ml, polyethylene glycol 6000 1g / 100ml, sodium azide 0.1g / 100ml. At the same time, the reagents contained in the second reagent unit include 0.1g / 100ml of latex particles modified by mouse anti-human NGAL monoclonal antibody, 0.25g / 100ml of latex particles modified by goat anti-human NGAL polyclonal antibody, 0.05g / 100ml of casein, Sodium nitrogen 0.05g / 100ml.
[0022] The reagent was evaluated on an automatic biochemical analyzer, and the results showed that the minimum detection limit was 12ng / ml, and the linear range was 20ng / ml~4000ng / ml.
Embodiment 3
[0024] In this embodiment, the reagent contained in the first reagent unit contains phosphate buffer 0.1mol / L, pH7.5, Triton X-100 0.2g / 100ml, polyethylene glycol 6000 1g / 100ml, sodium azide 0.05 g / 100ml. At the same time, the reagents contained in the second reagent unit include 0.15g / 100ml of latex particles modified by mouse anti-human NGAL monoclonal antibody, 0.25g / 100ml of latex particles modified by goat anti-human NGAL polyclonal antibody, 0.05g / 100ml of casein, Sodium nitrogen 0.05g / 100ml.
[0025] The reagent was evaluated on an automatic biochemical analyzer, and the results showed that the minimum detection limit was 8ng / ml, and the linear range was 20ng / ml~4000ng / ml.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com