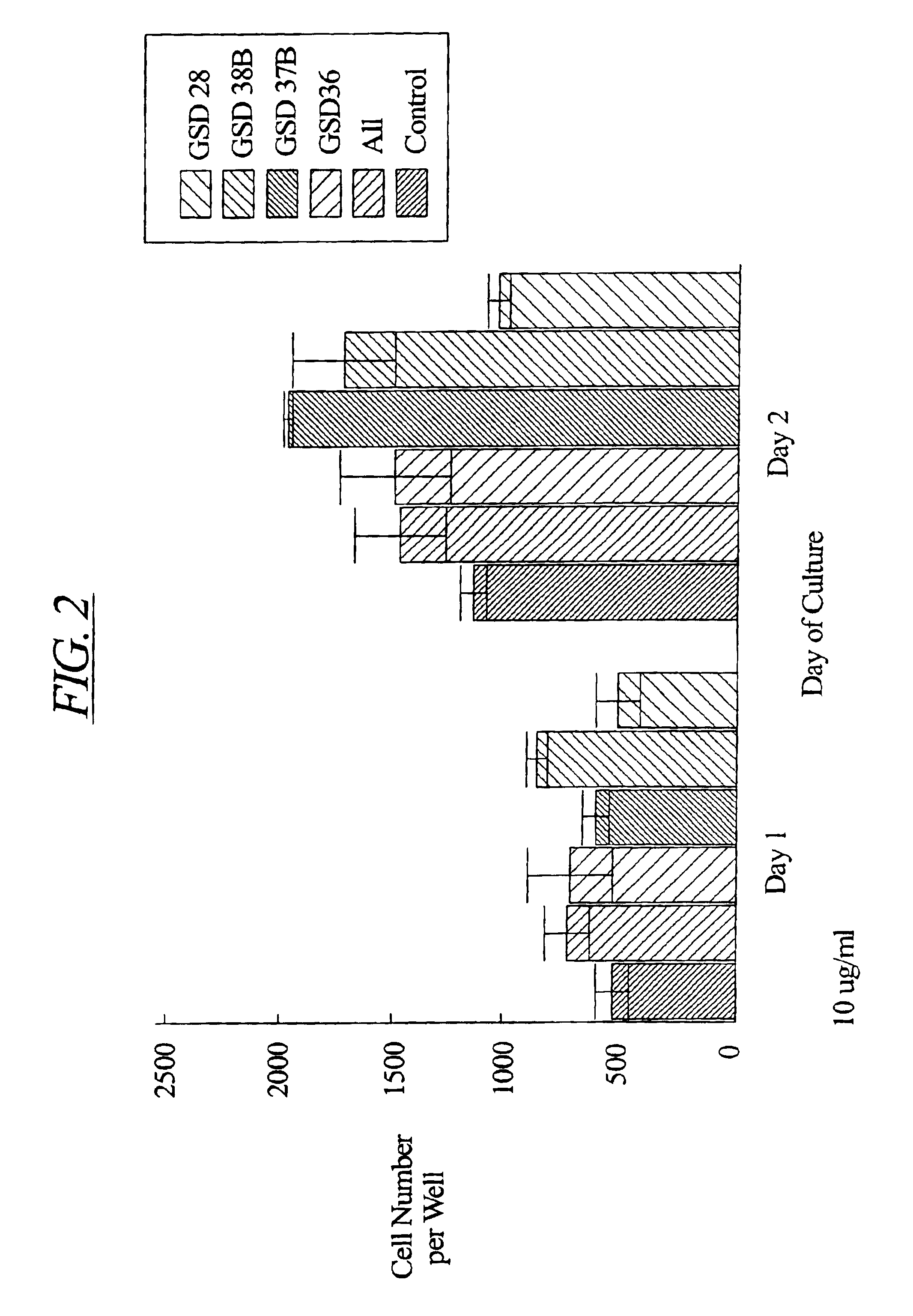
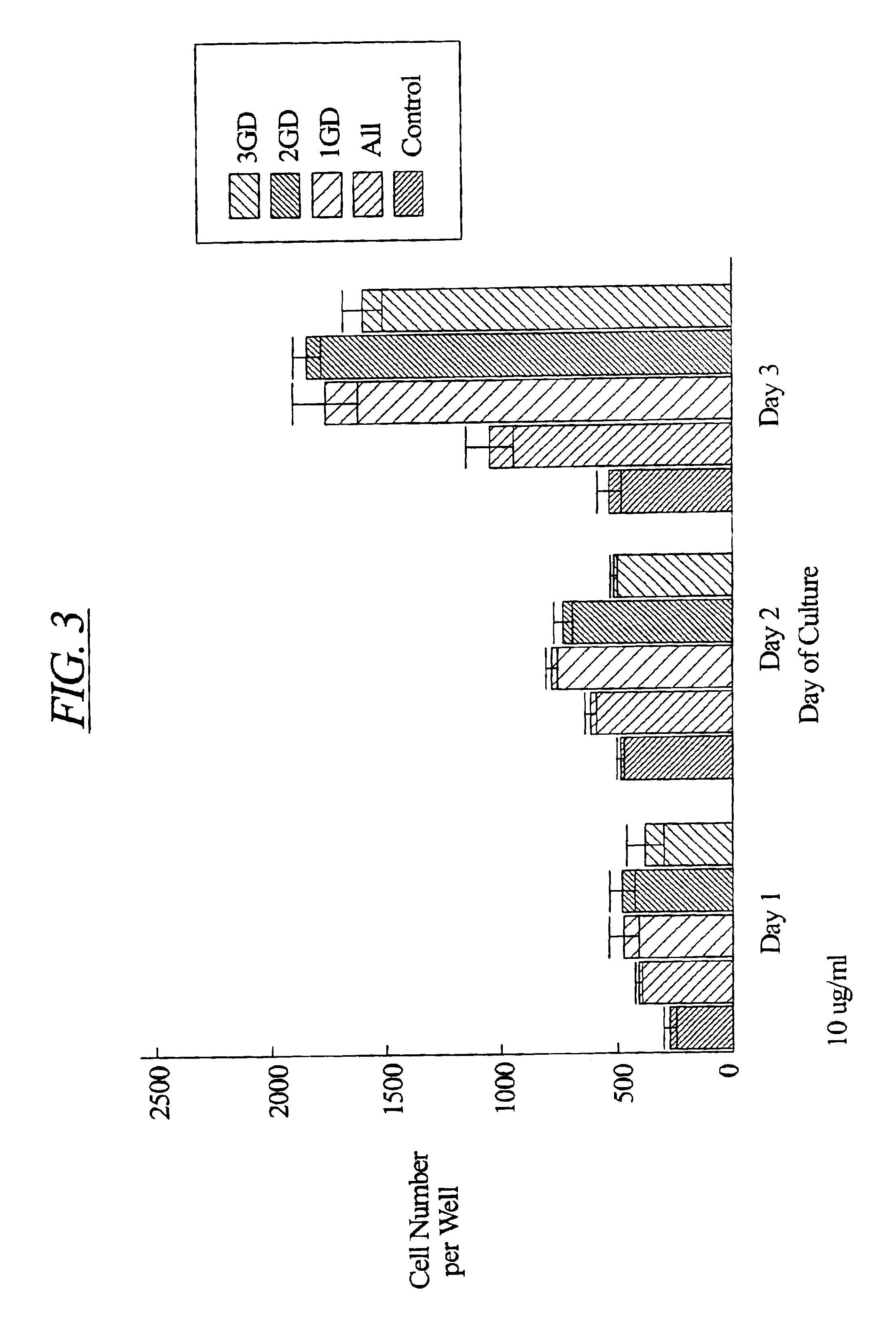
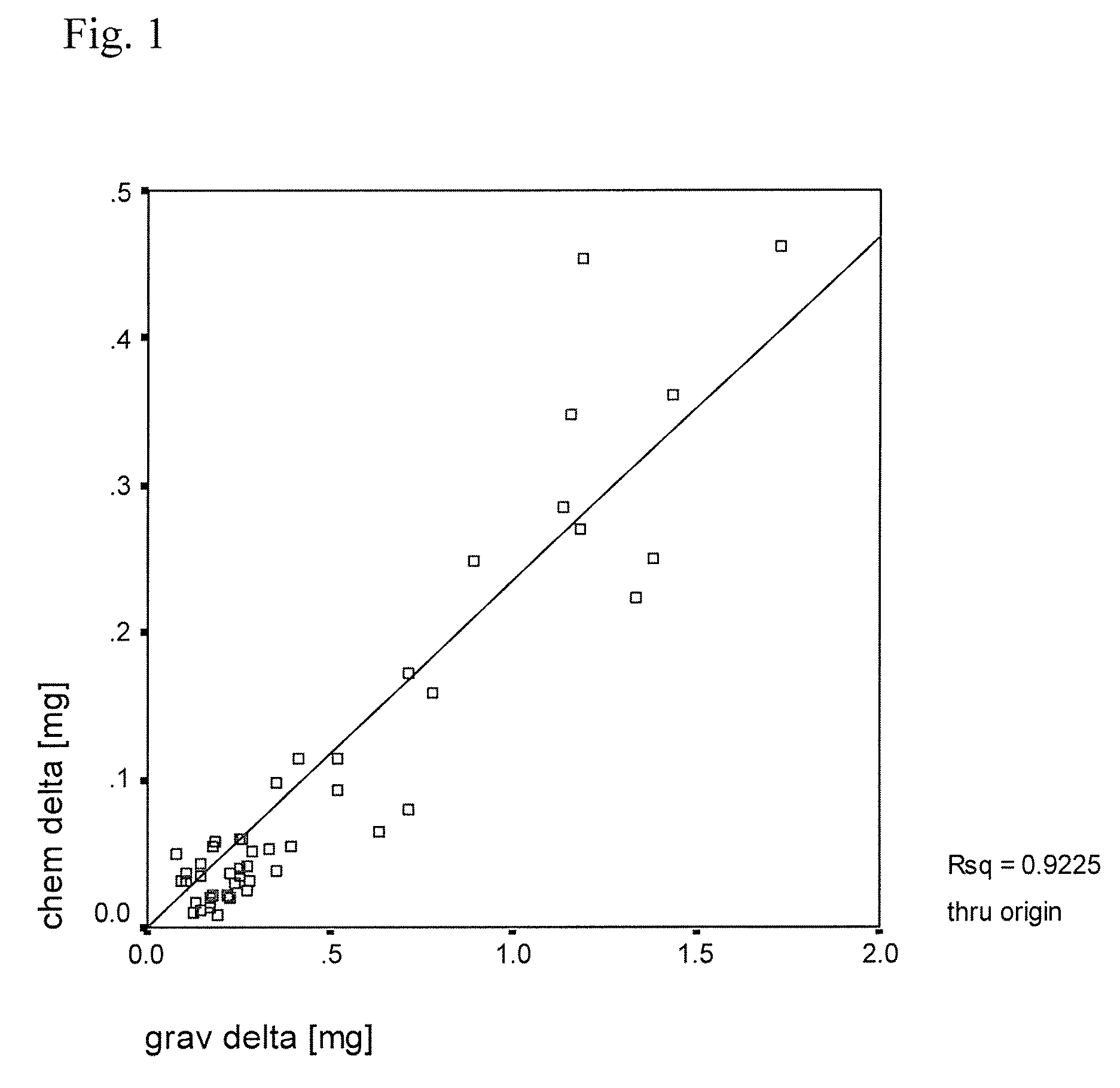
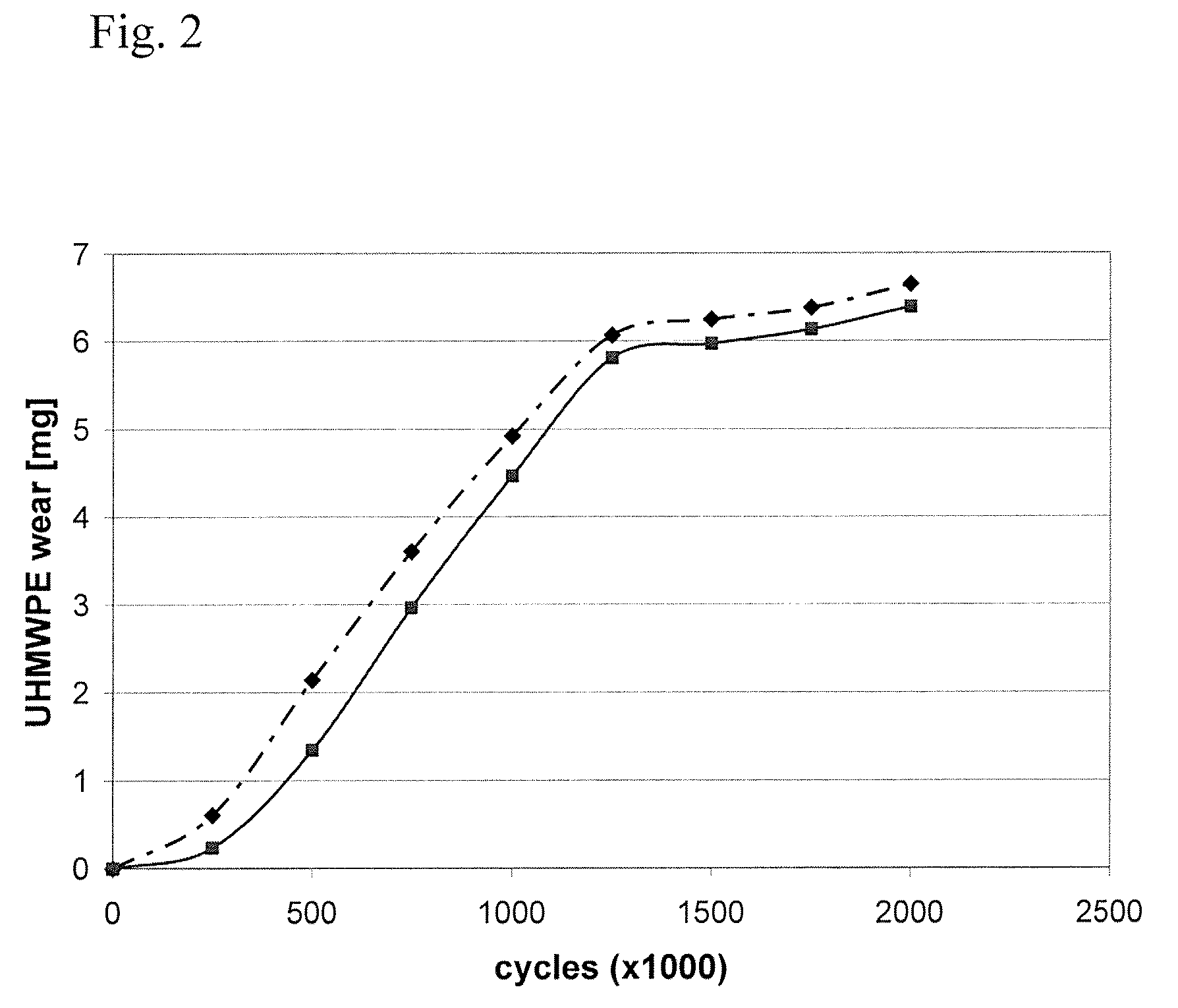
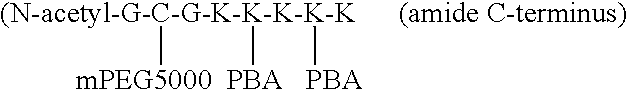Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
201 results about "Periprosthetic" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Periprosthetic in medicine refers to a structure in close relation to an implant.
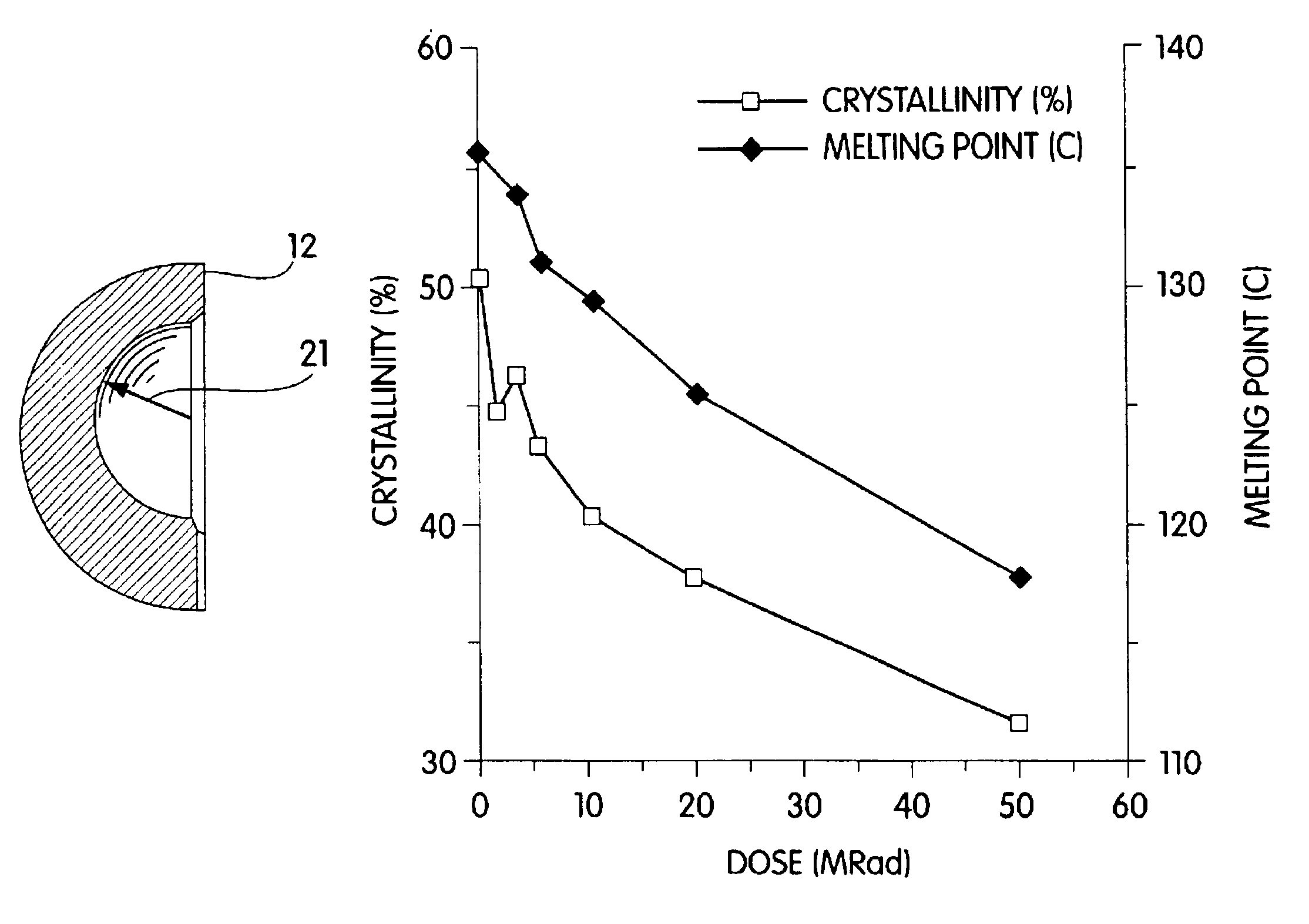
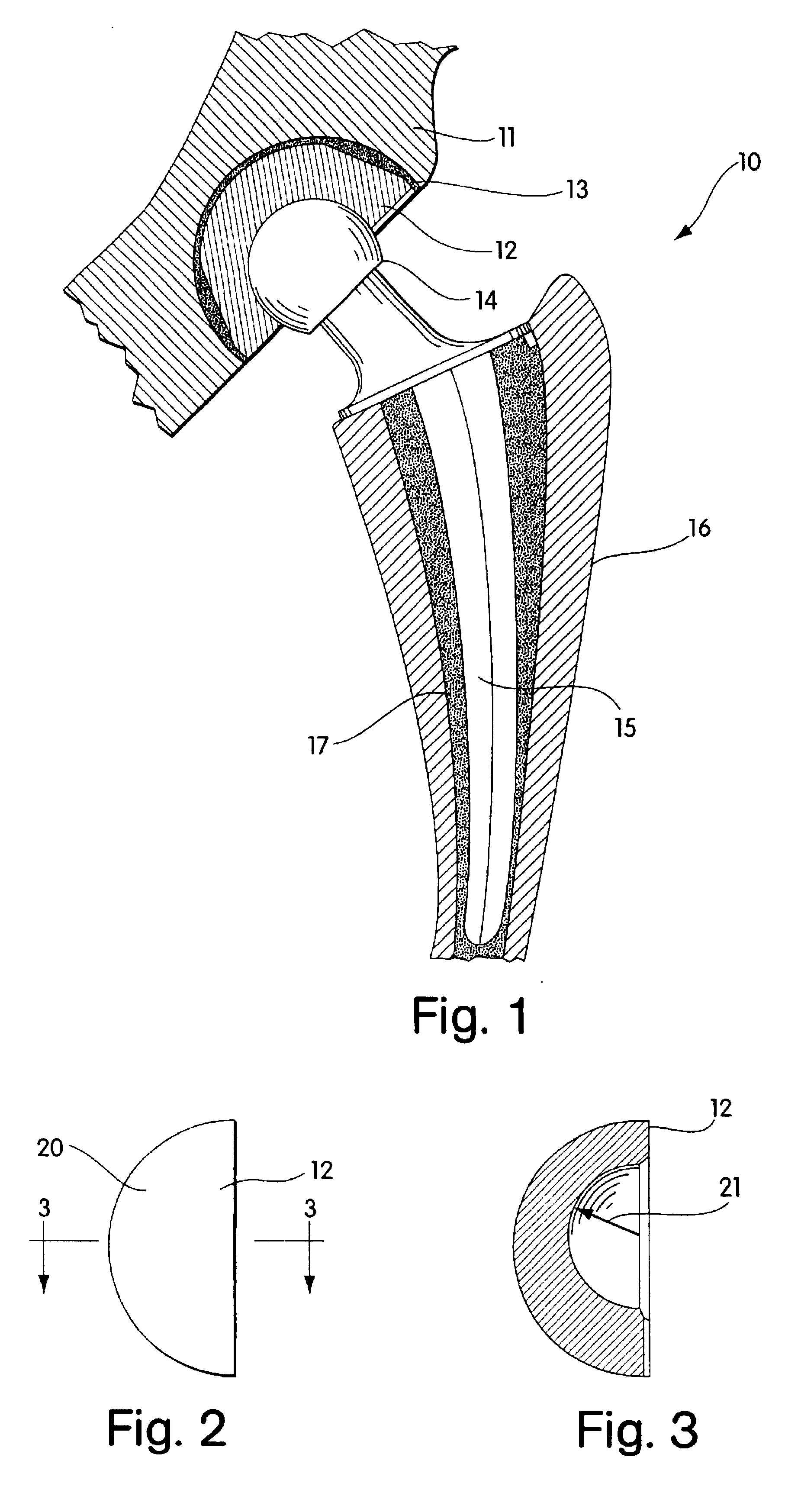
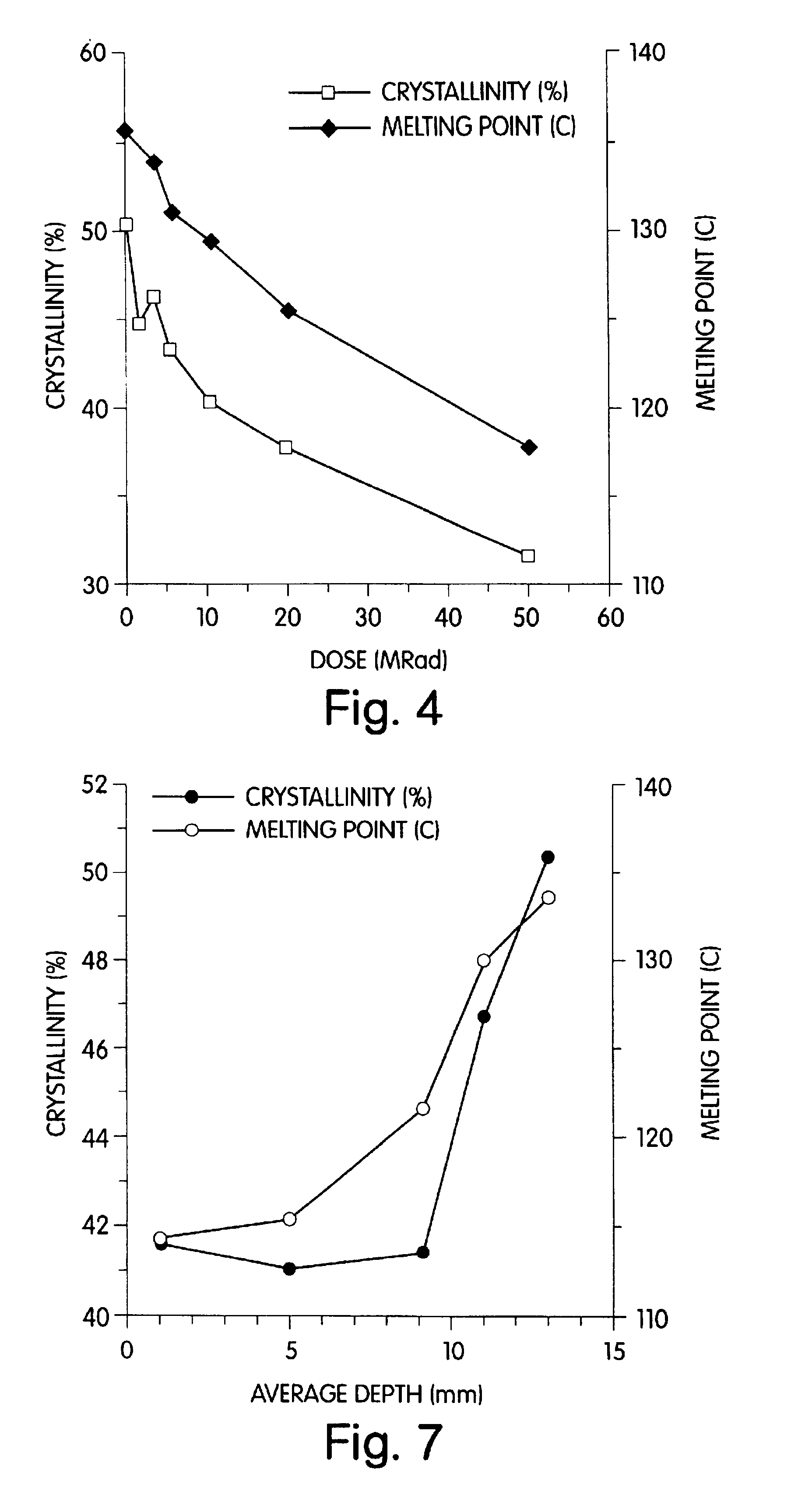
Radiation and melt treated ultra high molecular weight polyethylene prosthetic device and method
InactiveUS6641617B1Reduce productionReduce osteolysis and inflammatory reactionBone implantJoint implantsOxidation resistantPeriprosthetic
A medical prosthesis for use within the body which is formed of radiation treated ultra high molecular weight polyethylene having substantially no detectable free radicals, is described. Preferred prostheses exhibit reduced production of particles from the prosthesis during wear of the prosthesis, and are substantially oxidation resistant. Methods of manufacture of such devices and material used therein are also provided.
Owner:CENTPULSE ORTHOPEDICS +1
Methods and compositions for the prevention and treatment of atherosclerosis, restenosis and related disorders
Methods and compositions for the prevention and treatment of all forms of atherosclerosis are described. Administration of compounds such as thalidomide, its analogs, hydrolysis products, metabolites, derivatives and precursors as well as additional compounds capable of inhibiting tumor necrosis factor alpha (TNF-alpha) are used in the invention. Also disclosed is the coating of prosthetic devices, such as stents, with the compounds of the invention for the prevention and / or treatment of restenosis.
Owner:CELGENE CORP
Bioresorbable hydrogel compositions for implantable prostheses
Crosslinked compositions formed from water-insoluble copolymers are disclosed. These compositions are copolymers having a bioresorbable region, a hydrophilic region and at least two cross-linkable functional groups per polymer chain. Crosslinking of these polymers can be effected in solution in organic solvents or in solvent-free systems. If crosslinking occurs in a humid environment, a hydrogel will form. If crosslinking occurs in a non-humid environment, a xerogel will form which will form a hydrogel when exposed to a humid environment and the resulting crosslinked materials form hydrogels when exposed to humid environments. These hydrogels are useful as components in medical devices such as implantable prostheses. In addition, such hydrogels are useful as delivery vehicles for therapeutic agents and as scaffolding for tissue engineering applications.
Owner:LIFESHIELD SCI
Coated vascular grafts and methods of use
InactiveUS6939377B2Provide strengthProvide compliancePharmaceutical containersPretreated surfacesPorous coatingMedicine
A vascular graft, such as an AAA stent graft, includes a core zone of PET fabric with a non-porous coating comprising a polyurethane, such as Thoralon®, disposed on at least one surface. The coating provides a barrier to prevent short and long term leakage of fluids through the pores of the PET fabric core zone. A method for sealing the pores of a porous PET graft includes the step of coating at least one surface of said graft with at least one polyurethane, or mixtures and combinations thereof. Preferably, the coating is Thoralon®. A method for making a vascular prosthesis includes the steps of providing a core zone of PET fabric, and coating at least one surface of the core zone with at least one polyurethane, or mixtures and combinations thereof, such as Thoralon®. Finally, a method of repairing or treating a defective vessel includes the step of reinforcing or replacing the defective vessel with a graft according to the invention.
Owner:TC1 LLC
Prophylactic bactericidal implant
InactiveUS20060004431A1Prevent microbial infectionSuture equipmentsElectrotherapyProtozoaSurgical operation
A medical implant system is described for inhibiting infection associated with a joint prosthesis implant. An inventive system includes an implant body made of a biocompatible material which has a metal component disposed on an external surface of the implant body. A current is allowed to flow to the metal component, stimulating release of metal ions toxic to microbes, such as bacteria, protozoa, fungi, and viruses. One detailed system is completely surgically implantable in the patient such that no part of the system is external to the patient while the system is in use. In addition, externally controlled devices are provided which allow for modulation of implanted components.
Owner:AIONX ANTIMICROBIAL TECH INC
Silver-containing, sol/gel derived bioglass compositions
Silver-containing, sol-gel derived bioactive glass compositions and methods of preparation and use thereof are disclosed. The compositions can be in the form of particles, fibers and / or coatings, among other possible forms, and can be used, for example, for treating wounds, improving the success of skin grafts, reducing the inflammatory response and providing anti-bacterial treatments to a patient in need thereof. Anti-bacterial properties can be imparted to implanted materials, such as prosthetic implants, sutures, stents, screws, plates, tubes, and the like, by incorporating the compositions into or onto the implanted materials. The compositions can also be used to prepare devices used for in vitro and ex vivo cell culture.
Owner:IMPERIAL INNOVATIONS LTD
Implantable pulse generator for providing functional and/or therapeutic stimulation of muscles and/or nerves and/or central nervous system tissue
ActiveUS20050278000A1Efficient chargingImprove the quality of lifeElectrotherapyElectromyographyMicrocontrollerPrimary cell
Improved assemblies, systems, and methods provide an implantable pulse generator for prosthetic or therapeutic stimulation of muscles, nerves, or central nervous system tissue, or any combination. The implantable pulse generator is sized and configured to be implanted subcutaneously in a tissue region. The implantable pulse generator includes an electrically conductive laser welded titanium case. Control circuitry is located within the case, and includes a primary cell or rechargeable power source, a receive coil for receiving an RF magnetic field to recharge the rechargeable power source, and a microcontroller for control of the implantable pulse generator. Improved assemblies, systems, and methods also provide a stimulation system for prosthetic or therapeutic stimulation of muscles, nerves, or central nervous system tissue, or any combination. The stimulation system provides at least one electrically conductive surface, a lead connected to the electrically conductive surface, and an implantable pulse generator electrically connected to the lead.
Owner:MEDTRONIC URINARY SOLUTIONS
Implantable pulse generator systems and methods for providing functional and/or therapeutic stimulation of muscles and/or nerves and/or central nervous system tissue
Improved assemblies, systems, and methods provide a stimulation system for prosthetic or therapeutic stimulation of muscles, nerves, or central nervous system tissue, or any combination. The stimulation system includes a hermetically sealed implantable pulse generator and methods for assembly and programming.
Owner:MEDTRONIC URINARY SOLUTIONS
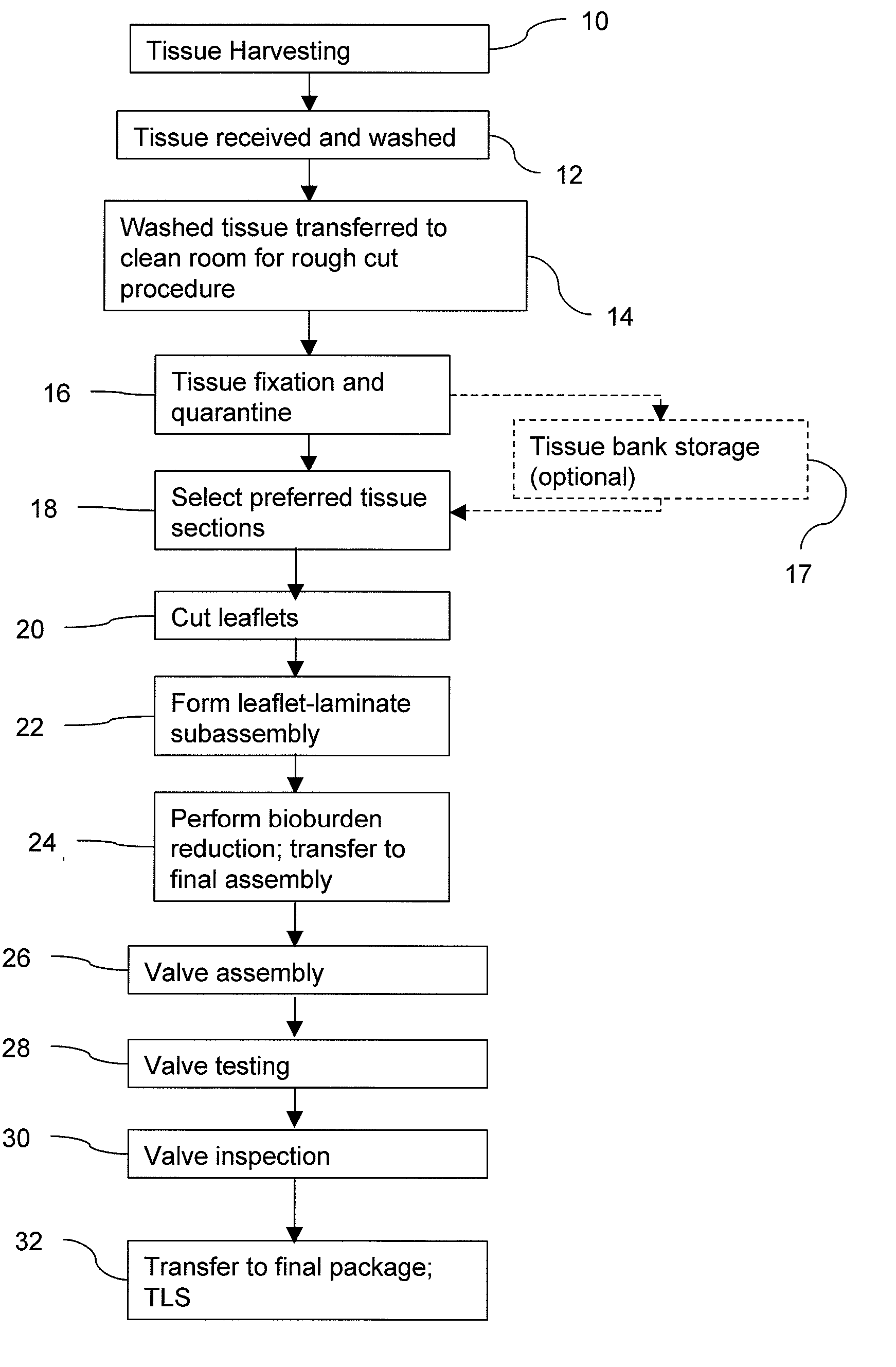
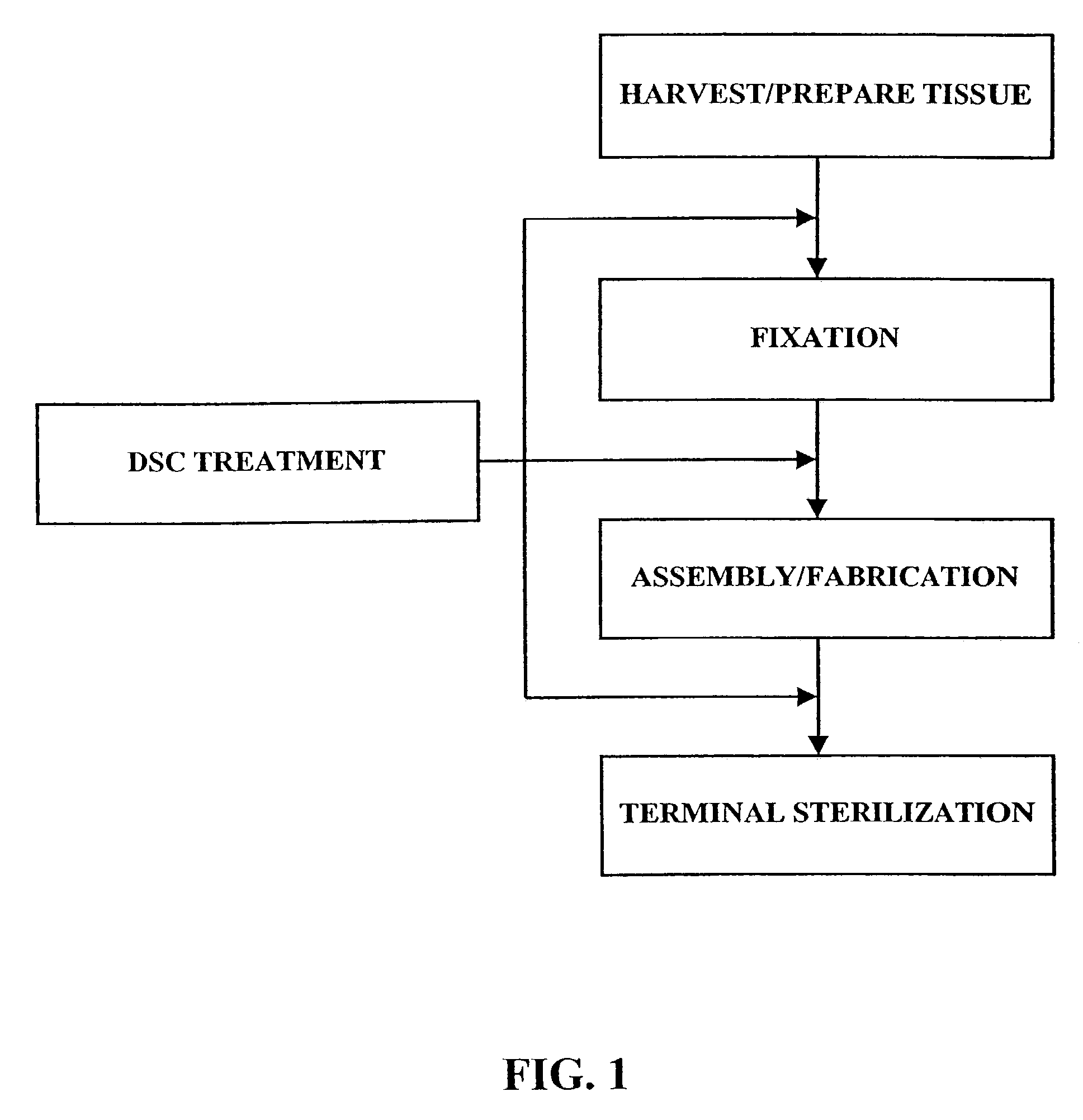
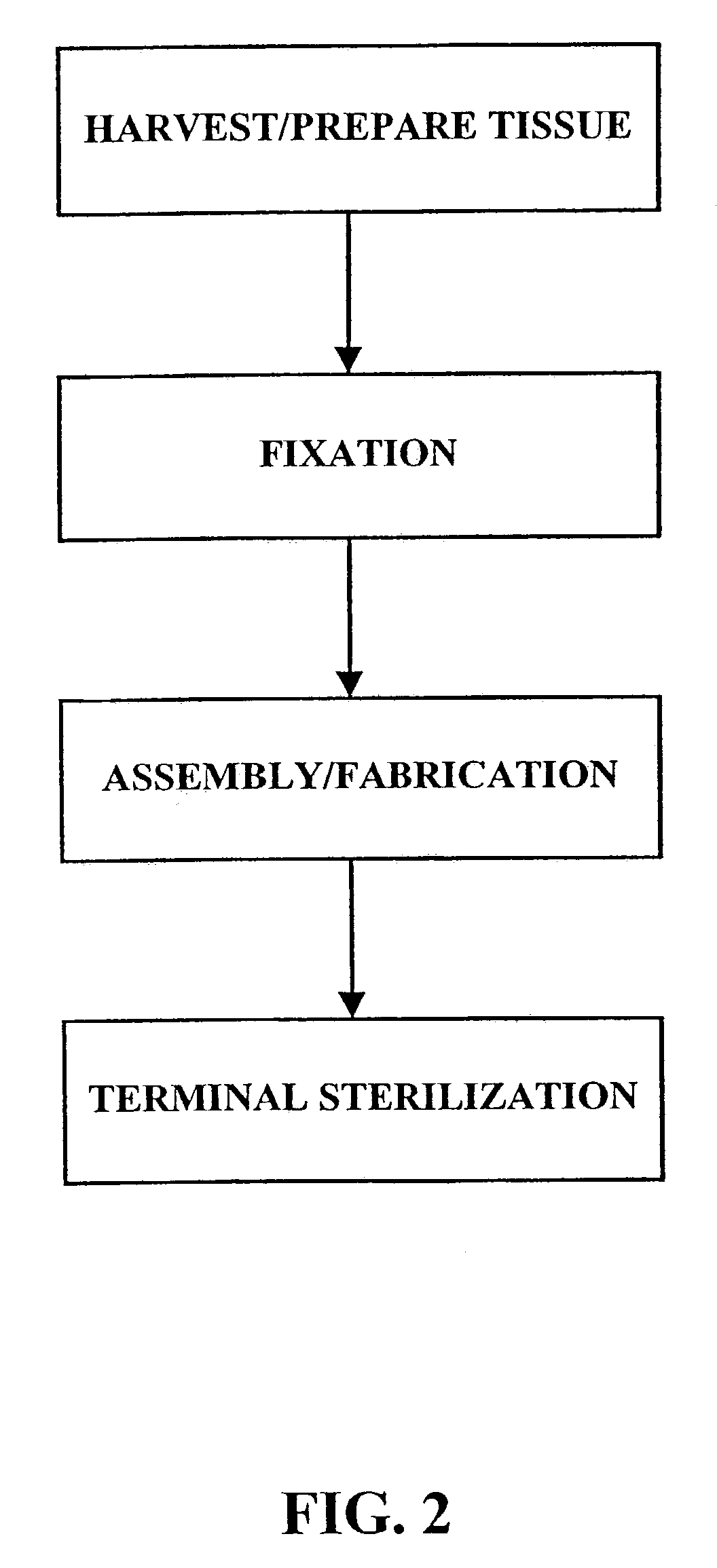
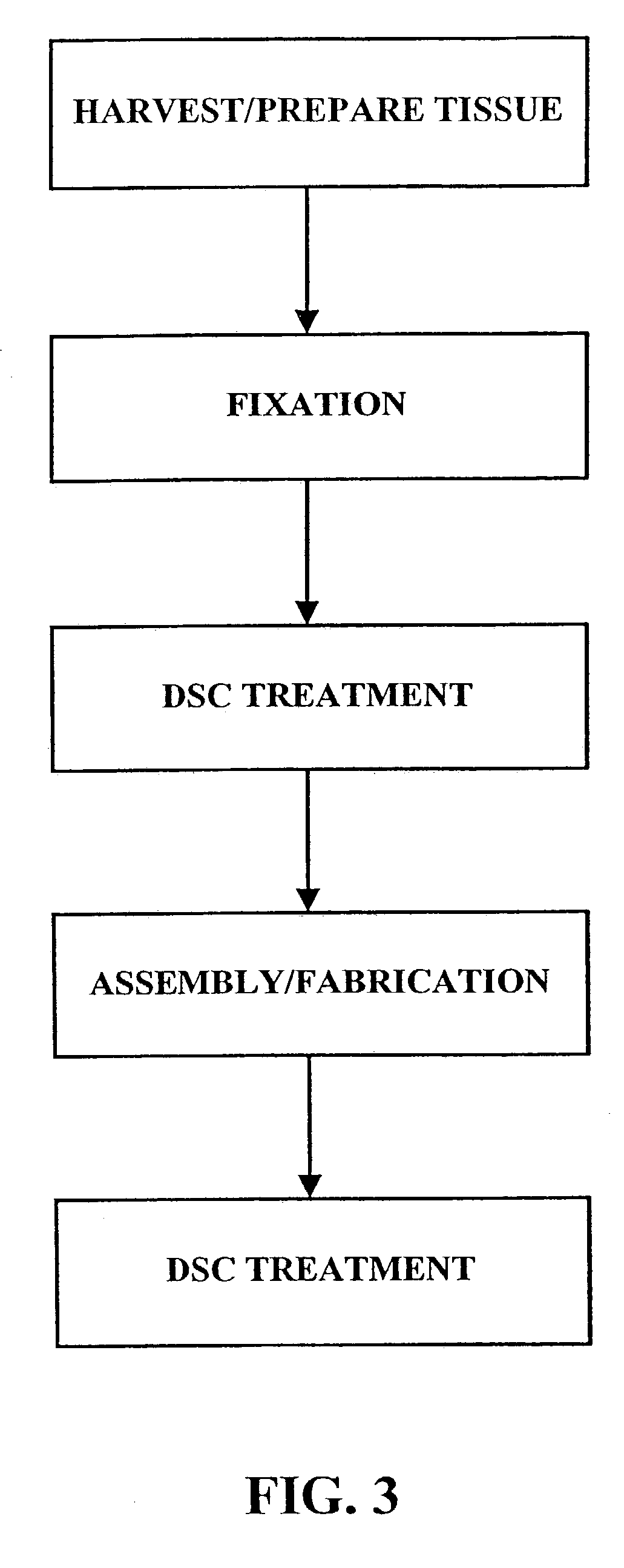
Methods for processing biological tissue
ActiveUS20060207031A1Remove background levelAvoid degradationSuture equipmentsDead animal preservationCalcificationPhospholipid
A method for processing biological tissue used in biological prostheses includes providing a tissue procurement solution formed from a phosphate buffered saline (PBS) solution and a chelating agent. The tissue is transferred from the tissue procurement solution and undergoes chemical fixation. The fixed tissue is then immersed in a series of fresh bioburden reduction process (BRP) solutions to extract phospholipids. The tissue procurement solution reduces the bioburden on the stored tissue and preserves tissue architecture by minimizing tissue swelling. The tissue procurement solution further reduces calcium from the incoming water and / or tissue, and inhibits enzymes that digest the collagen matrix. The serial immersion of the tissue in the fresh bioburden solutions ensures optimal extraction of phospholipids thereby mitigating subsequent calcification of the tissue.
Owner:MEDTRONIC INC
Method for making a porous Polymeric material
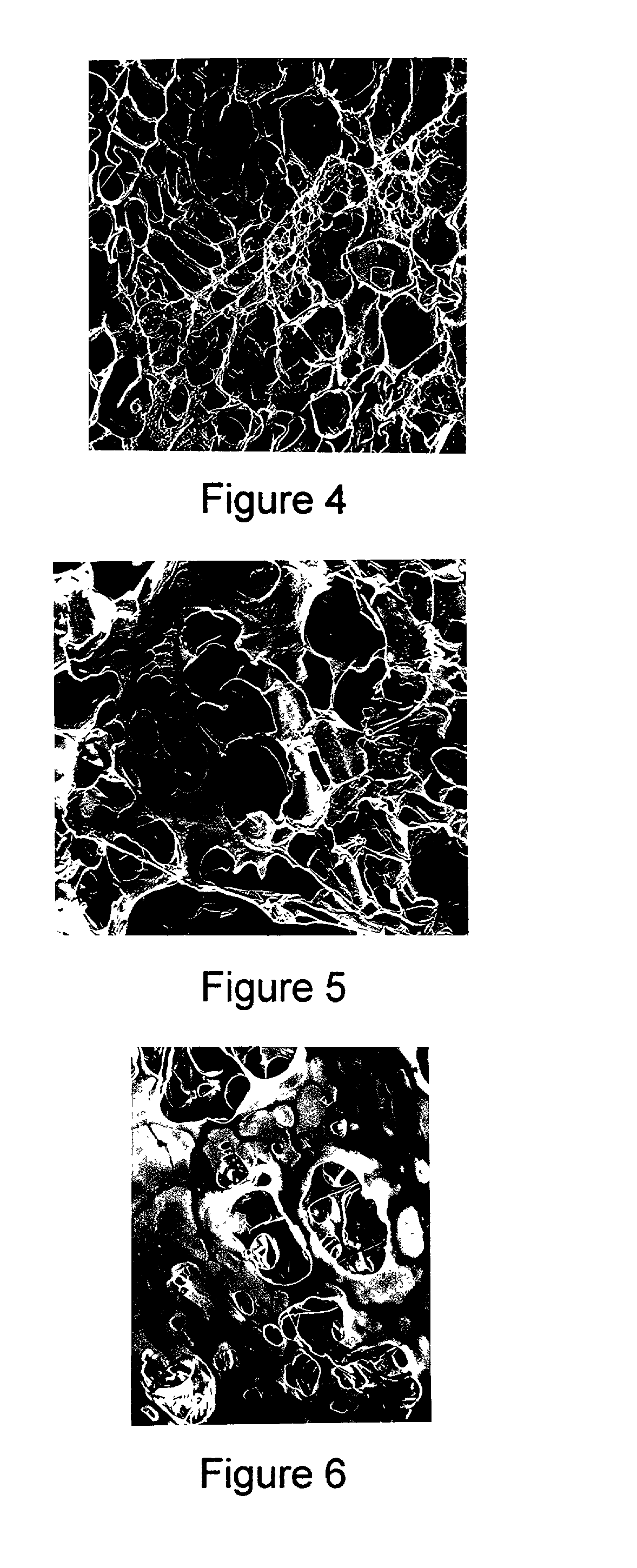
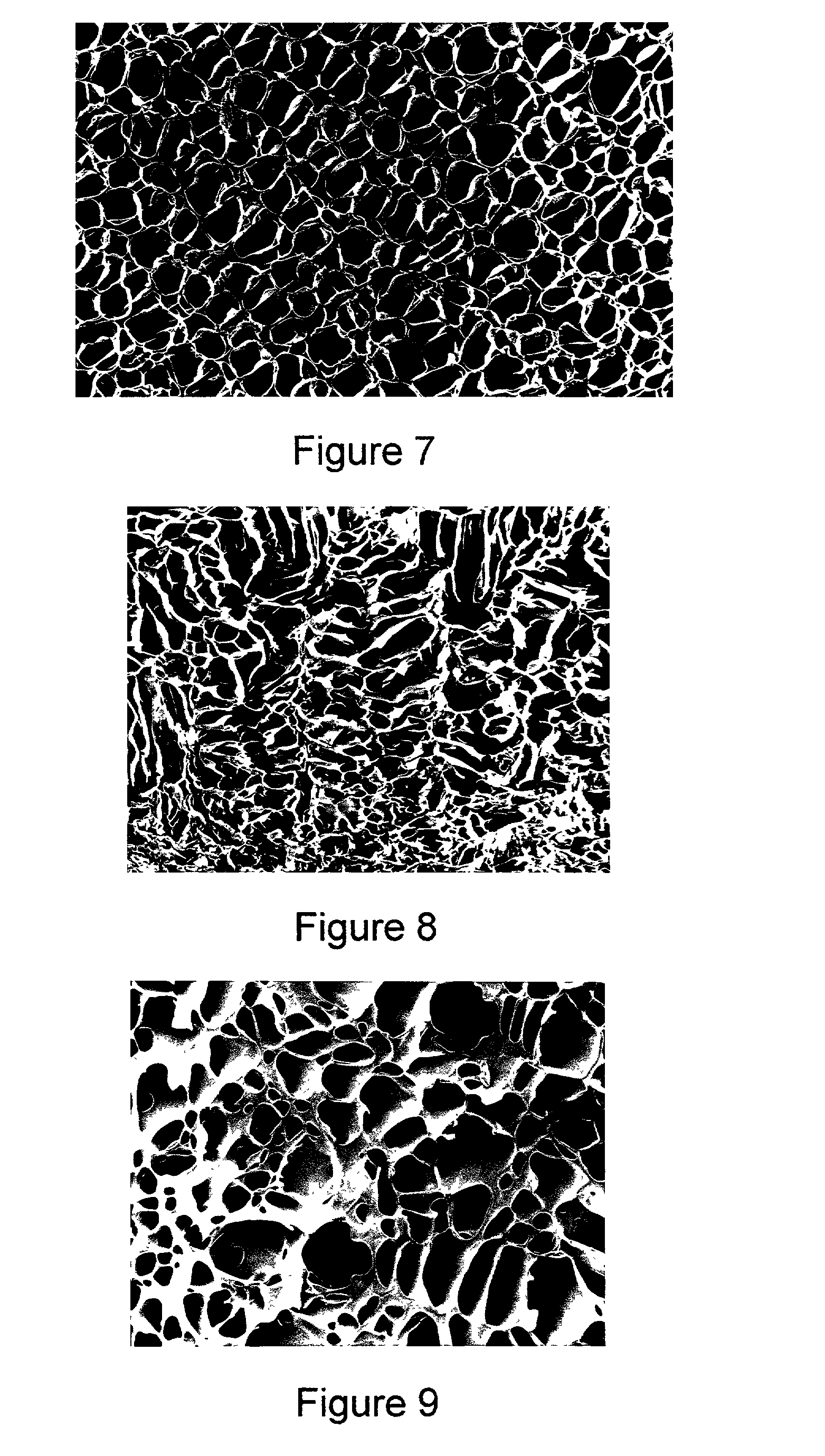
Porous polymers having a plurality of openings or chambers that are highly convoluted, with each chamber being defined by multiple, thin, flat partitions are produced by a new gel enhanced phase separation technique. In a preferred embodiment, a second solvent is added to a polymer solution, the second solvent causing the solution to gel. The gel can then be shaped as needed. Subsequent solvent extraction leaves the porous polymeric body of defined shape. The porous polymers have utility as medical prostheses, the porosity permitting ingrowth of neighboring tissue. The present technique also enhances shape-making capability, for example, of bifurcated vascular grafts, which feature a common entrance region but two or more exit regions.
Owner:KENSEY NASH CORP
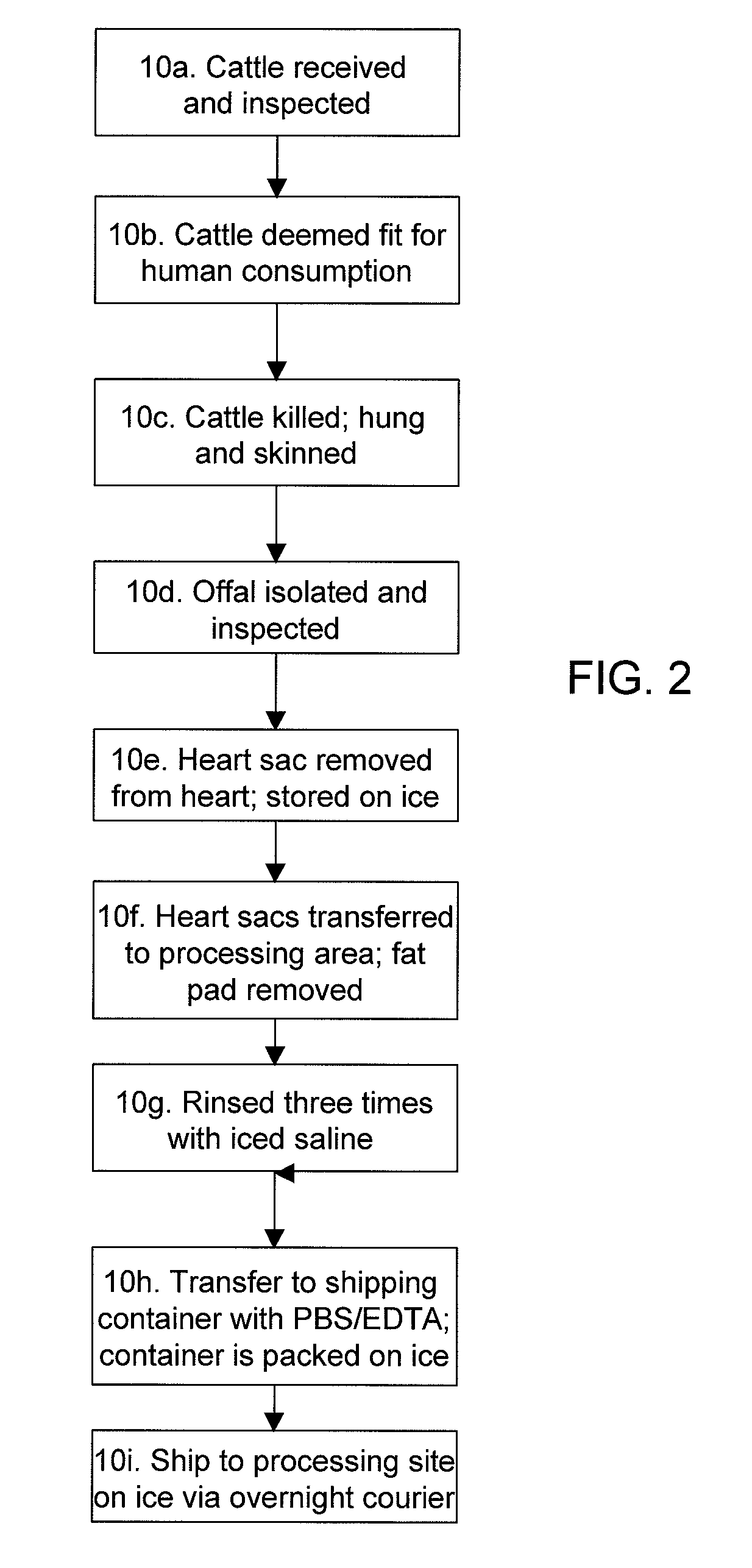
Supercritical fluid extraction process for tissue preparation
ActiveUS7008591B2Penetration thoroughlyShort timeSolvent extractionMammal material medical ingredientsPeriprostheticChemical agent
The present invention provides methods for preparing tissue for incorporation into xenografts and bioprosthetic devices. The methods of the invention make use of supercritical fluids to remove infectious materials and chemical agents from tissues, as well as to permeate a tissue with a chemical agent (e.g. tanning, cross-linking, and bioactive agents).
Owner:REGENT OF THE UNIVESITY OF MICHIGAN THE +1
EP4 receptor selective agonists in the treatment of osteoporosis
Owner:PFIZER INC
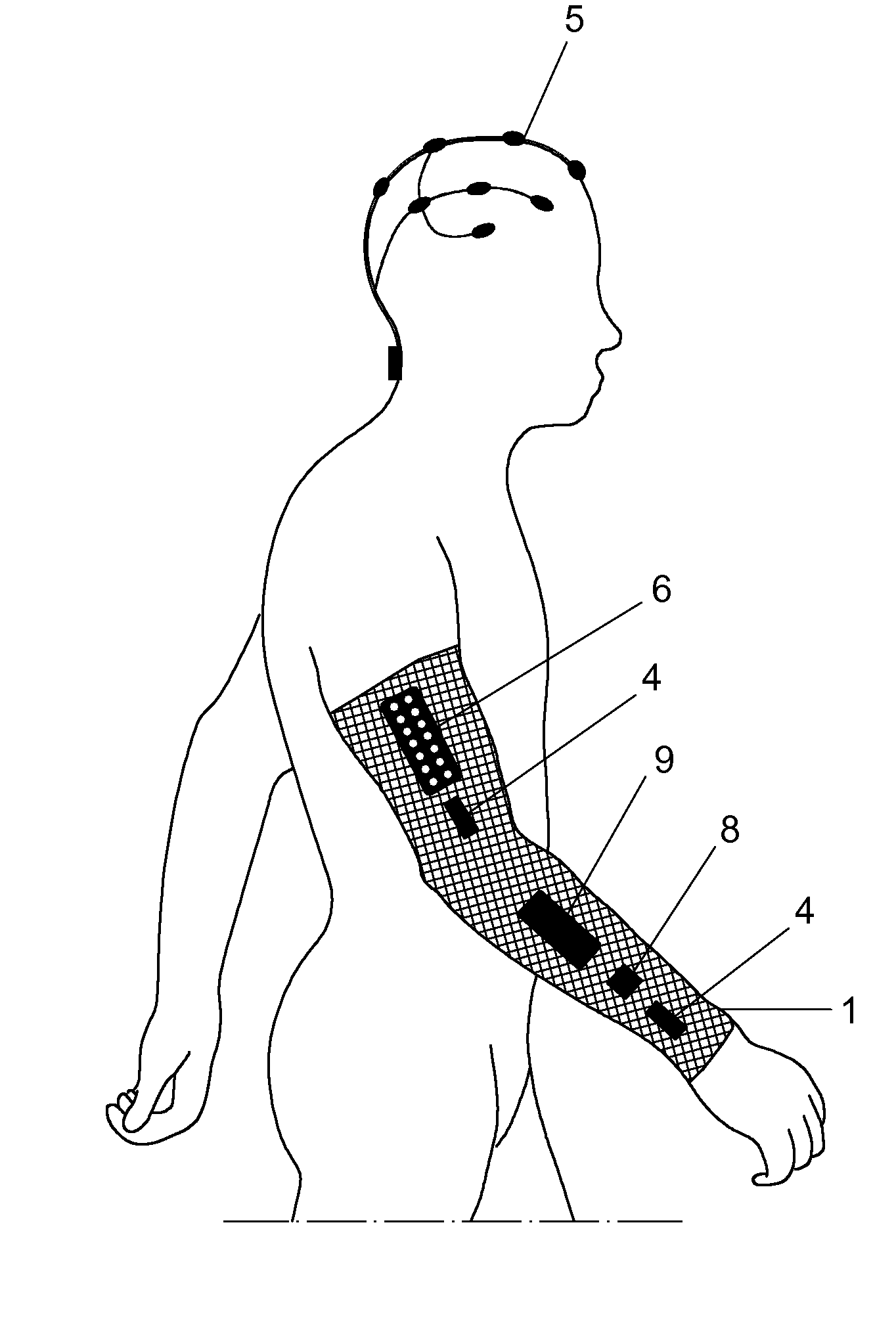
Method and neuroprosthetic device for monitoring and suppression of pathological tremors through neurostimulation of the afferent pathways
PendingUS20140336722A1Reduce tremorReduce severityElectrotherapyArtificial respirationBiomechanicsEngineering
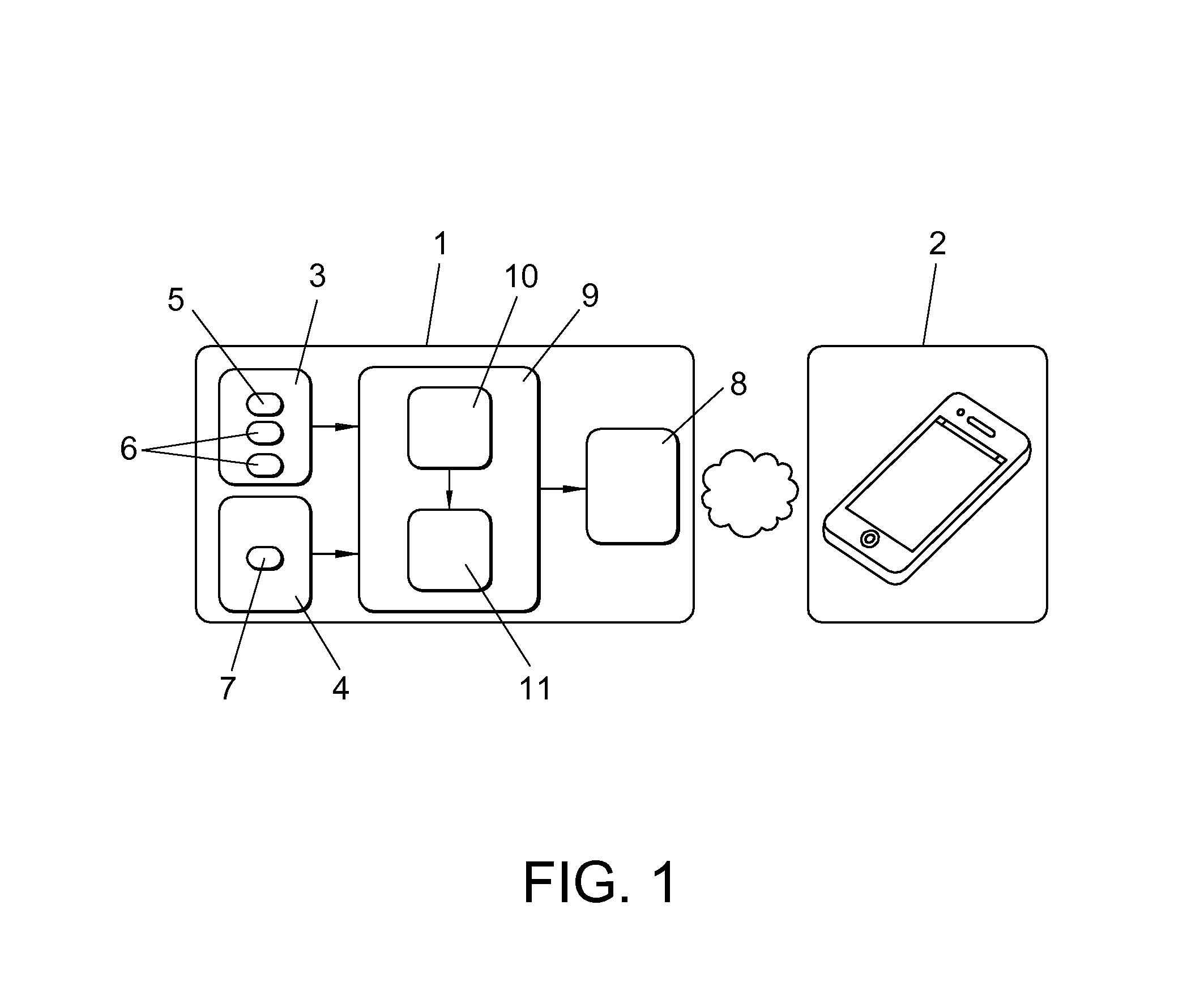
Method and neuroprosthetic device for monitoring and suppression of pathological tremors in a user through the neurostimulation of the afferent pathways to the brain, which comprises wearable elements placed over the parts of the body affected by tremor, wherein said wearable elements (1) have sensors selected from bioelectric sensors (3) generating a bioelectrical signal characterization of tremor, biomechanical sensors (4) generating a biomechanical signal characterization of tremor and a combination thereof, a programmable electronic device (9) comprised of a control, acquisition and processing module of the characterization signals, and a signal generator for the afferent stimulation based on the bioelectrical, biomechanical or combination of both signal characterization and stimulation electrodes (8) which transmit the neuromodulation signals to the afferent pathways projecting into the central nervous system to modulate the activity of the neural structures responsible for tremor generation.
Owner:CONSEJO SUPERIOR DE INVESTIGACIONES CIENTIFICAS (CSIC) +1
Treatment of conditions that present with low bone mass by continuous combination therapy with selective prostaglandin ep4 receptor agonists and an estrogen
InactiveUS20070191319A1High cure rateLong bone extension is enhancedBiocideSkeletal disorderPresent methodCarboxylic acid
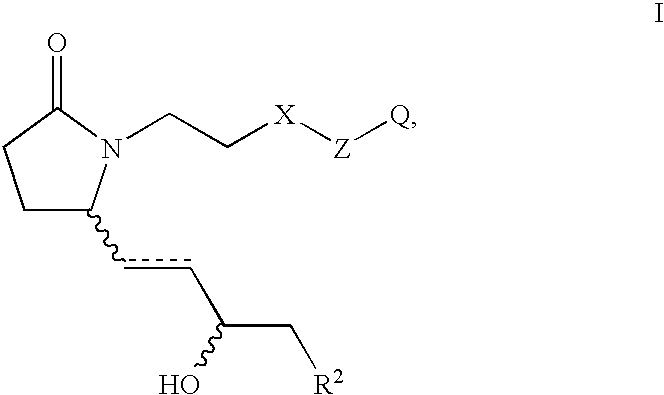
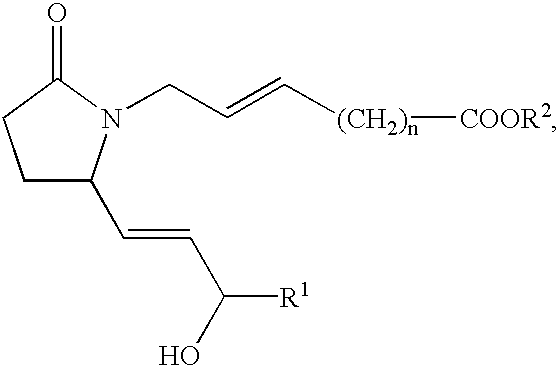
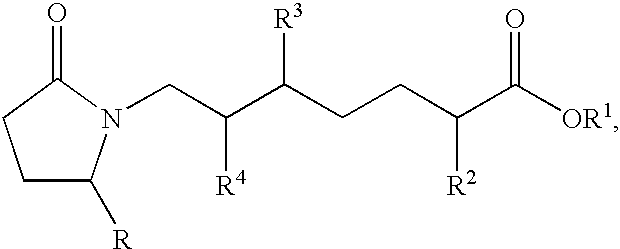
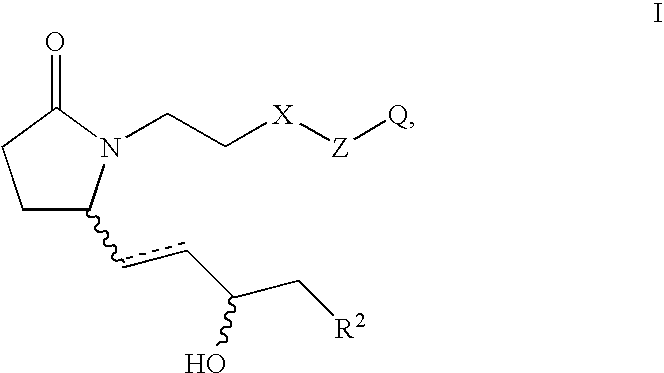
This invention is directed to methods for treating conditions which present with low bone mass in a patient in need thereof using continuous combination therapy with a synergistically effective combination of an EP4 receptor selective agonist or a pharmaceutically acceptable salt thereof, such as 5-(3-{2S-[3R-hydroxy-4-(3-trifluoromethyl-phenyl)-butyl]-5-oxo-pyrrolidin-1-yl}-propyl)-thiophene-2-carboxylic acid or a pharmaceutically acceptable salt thereof; and an estrogen or a pharmaceutically effective salt thereof, The present methods are useful for treating conditions that present with low bone mass including osteoporosis, osteotomy, osteoporotic fracture, childhood idiopathic bone loss, periodontitis and low bone mass and for enhancing bone healing following facial reconstruction, maxillary reconstruction or mandibular reconstruction, inducing vertebral synostosis, enhancing long bone extension, enhancing the healing rate of a bone graft or a long bone fracture or enhancing prosthetic ingrowth in a patient in need thereof.
Owner:PFIZER INC
Method for treatment of biological tissues to mitigate post-implantation calcification and thrombosis
A method of treating a biological tissue including contacting the biological tissue with an aqueous sterilizing solution, and maintaining the aqueous sterilizing solution at a temperature of about 50° C. for a time period of about 1 to 2 days. The method of treating a biological tissue may be utilized as a terminal sterilization step in a method for fixation of biological tissues, and bioprosthetic devices may be prepared by such fixation method. The fixation method may include the steps of A) fixing the tissue, B) treating the tissue with a mixture of i) a denaturant, ii) a surfactant and iii) a crosslinking agent, C) fabricating or forming the bioprosthesis (e.g., forming the tissue and attaching any non-biological components thereto) and D) subjecting the bioprosthesis to the terminal sterilization method. The aqueous sterilizing solution may be glutaraldehyde of about 0.625 weight percent buffered to a pH of about 7.4.
Owner:EDWARDS LIFESCIENCES CORP
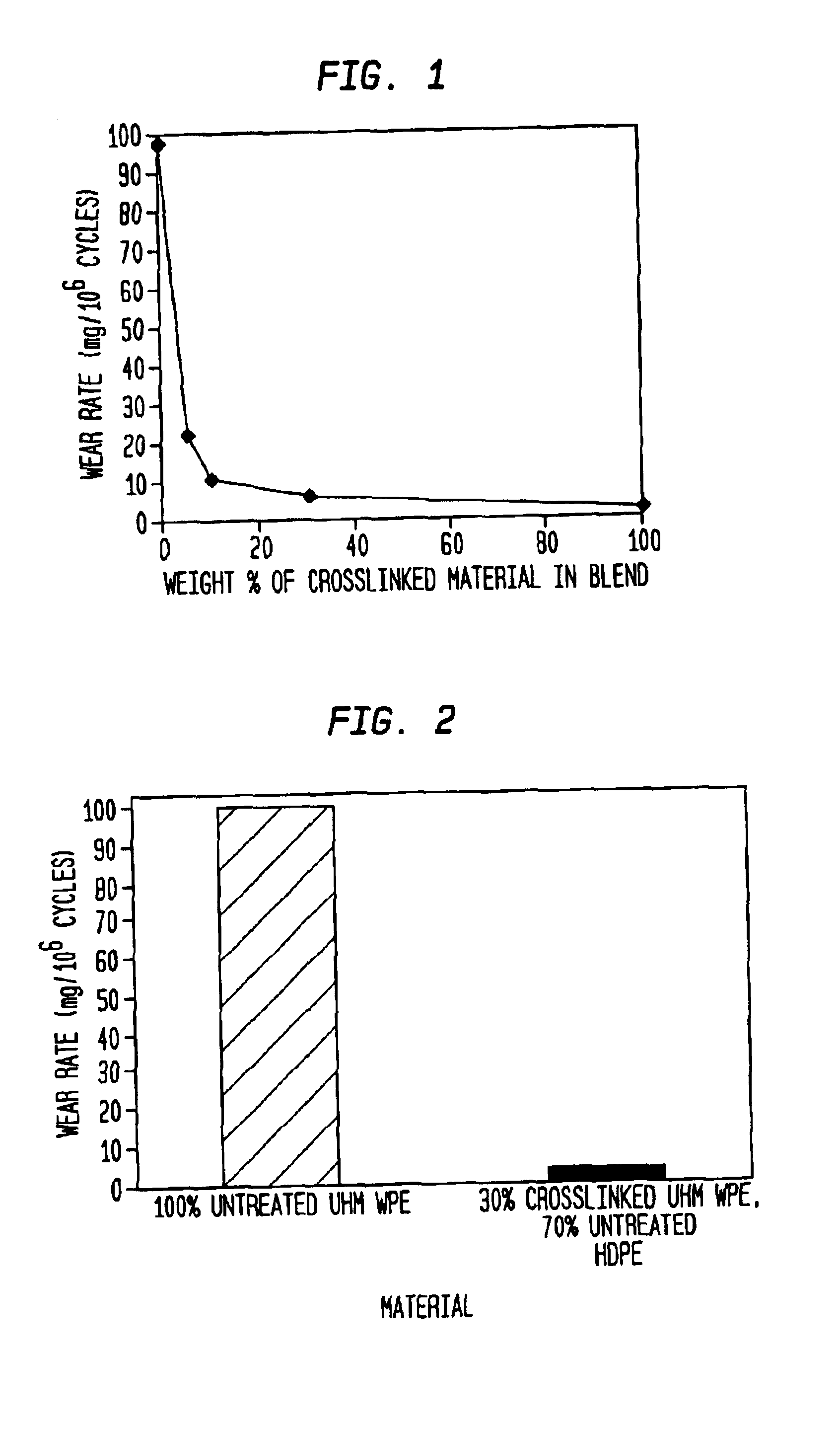
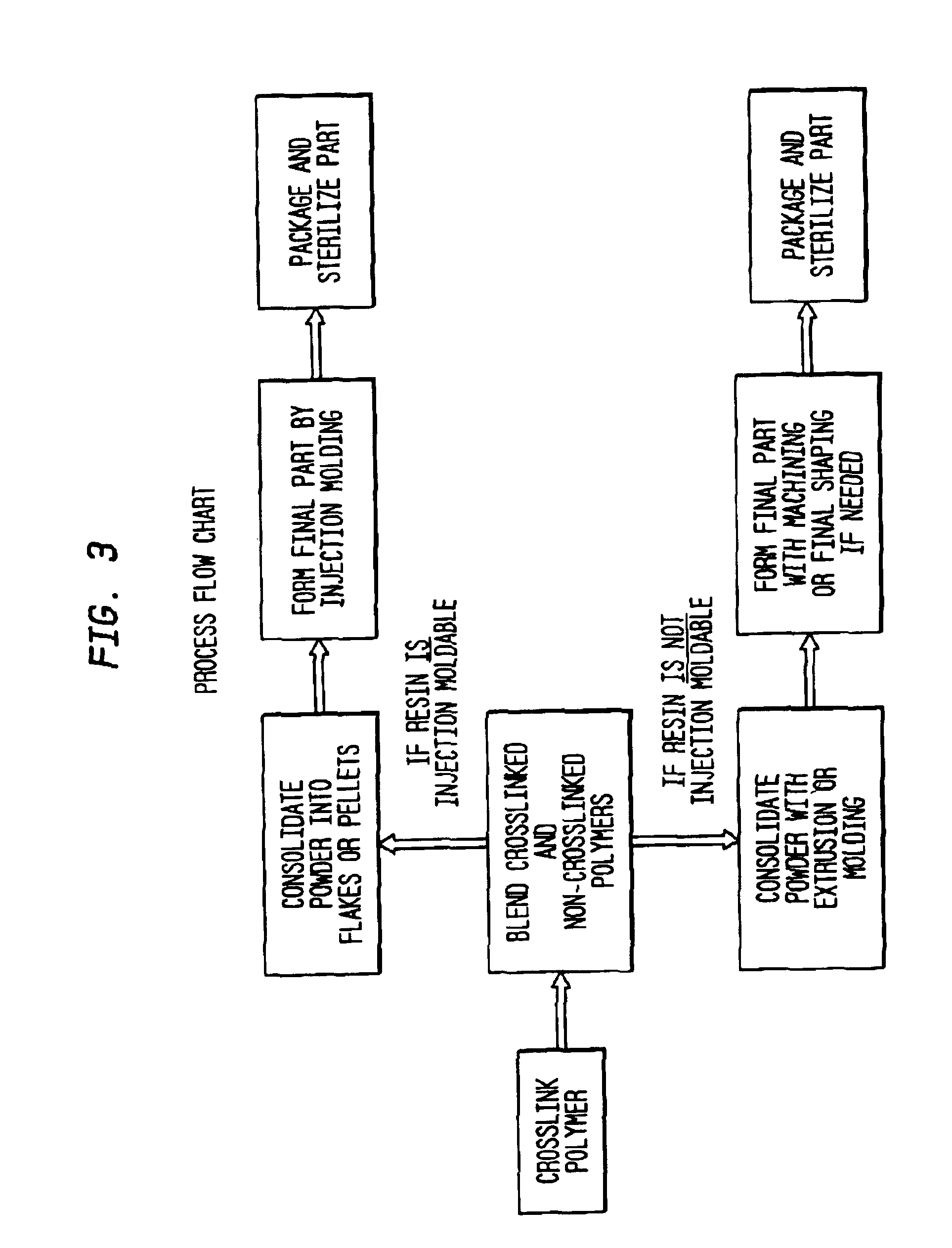
Methods of improving the wear resistance of prosthetic medical devices
InactiveUS6905511B2Avoids and reduces levelWear resistanceImpression capsAnkle jointsCross-linkPolyolefin
A prosthetic medical device exhibiting improved wear resistance is fabricated by irradiating at least one polyolefinic material in the presence of an inert atmosphere to yield a cross-linked irradiated polyolefinic material; blending at least one non-irradiated polyolefinic material with the at least one irradiated polyolefinic material, and forming the prosthetic medical device from the blended material. Selectively cross-linked polymeric compositions may be created by blending a specific amount of cross-linked resins with a specific amount of uncross-linked resins then cured into a polymeric matrix whereby the desired degree or percentage of overall cross-linking is obtained. The polymeric material may then be formed directly into a finished article by injection molding the polymeric material.
Owner:HOWMEDICA OSTEONICS CORP
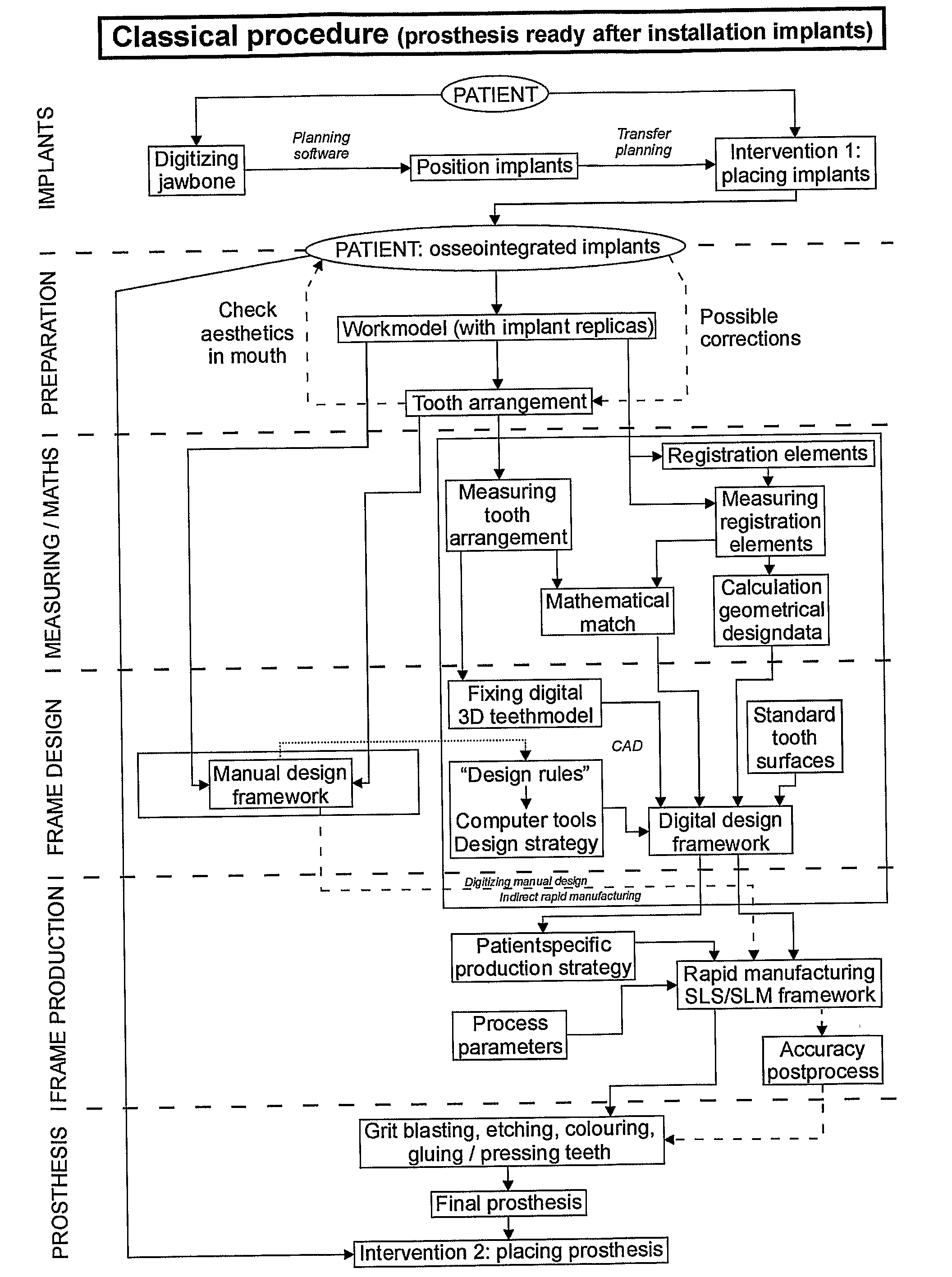
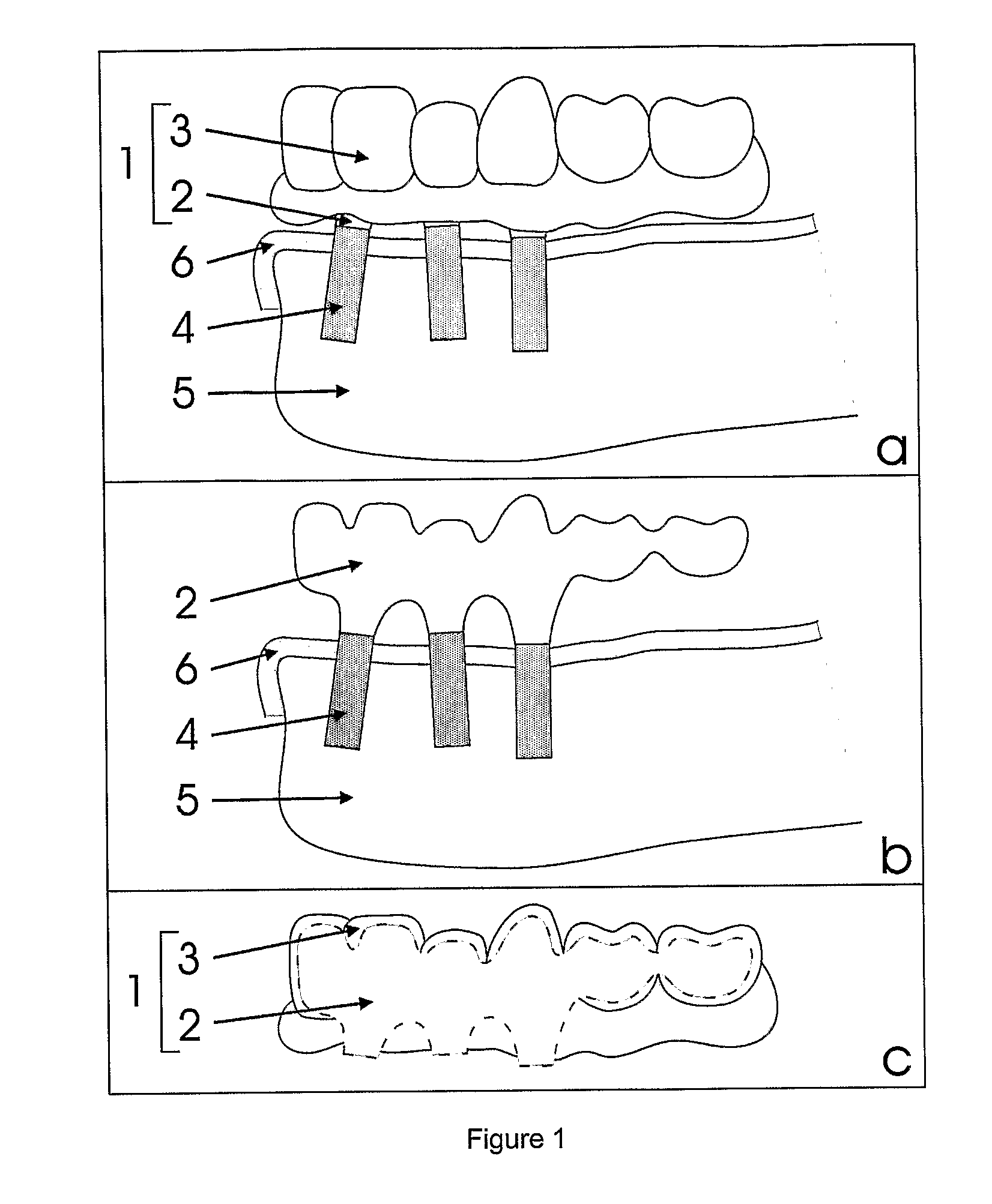
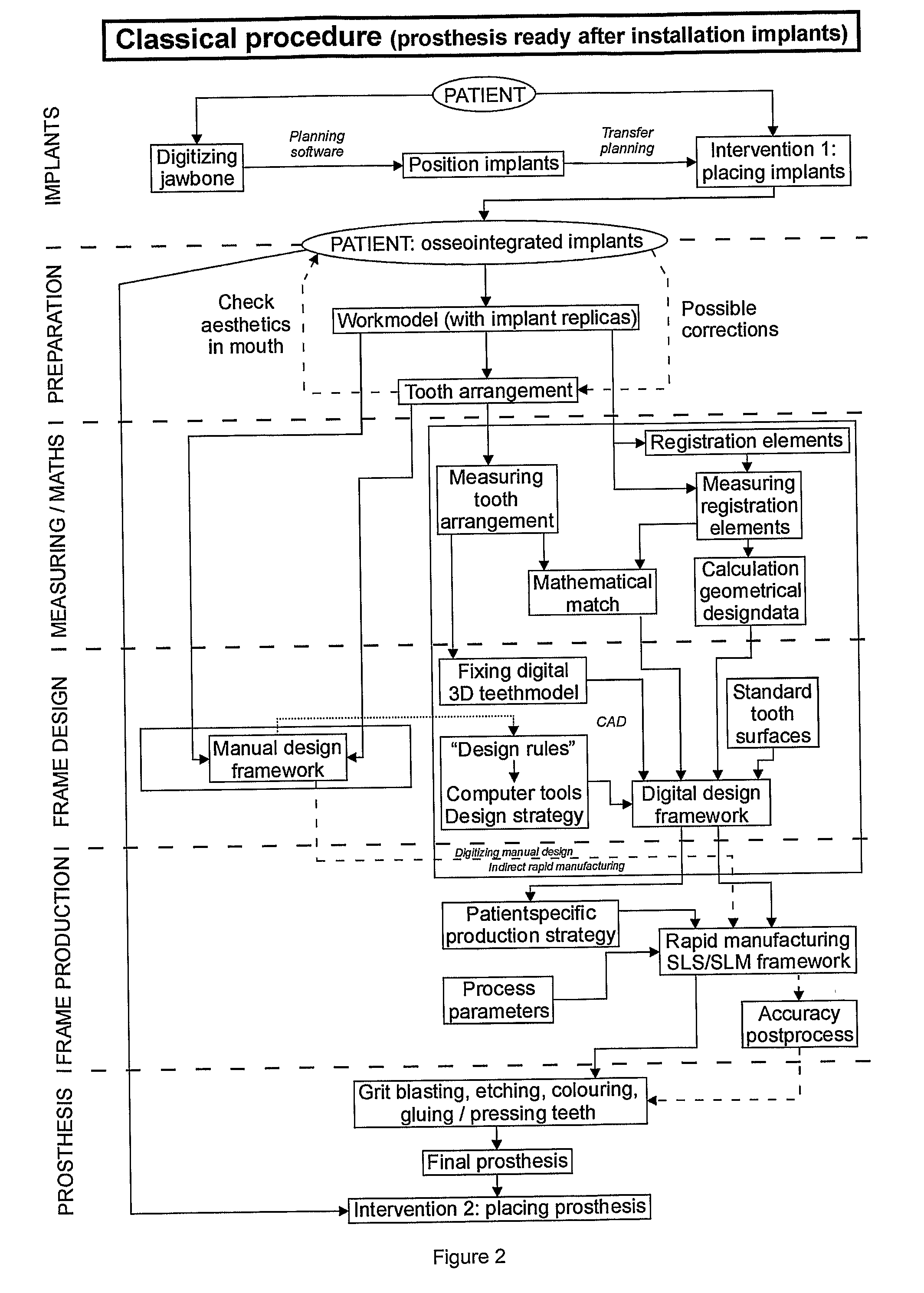
Procedure for Design and Production of Implant-Based Frameworks for Complex Dental Prostheses
ActiveUS20080206710A1High densityDental implantsMechanical/radiation/invasive therapiesMedicineMechanical property
The present invention provides methods allowing the use of selective laser powder processing techniques for the production of medically acceptable prosthetic dental frameworks. The frameworks produced according to the present invention have high grade mechanical properties as well as a high accuracy.
Owner:LAYERWISE
Methods and compositions to prevent formation of adhesions in biological tissues
InactiveUS7029688B2Decrease extent of metastasisAvoid problemsNanotechAntipyreticPhenylboronic acidPolyethylene glycol
The invention discloses materials that adsorb readily to the surfaces of body tissues in situ and provide a steric barrier between such tissues, so that tissue adhesions, which typically form following surgical procedures, are minimized. These materials contain a polymer of hydrophilic molecules such as polyethylene glycol (PEG) bound to a polymer that spontaneously adsorbs to biological tissue such as phenylboronic acid (PBA). The PEG-PBA co-polymer can be formed in a variety of geometries. The materials can also be used to coat prosthetics and other implants.
Owner:CALIFORNIA INST OF TECH
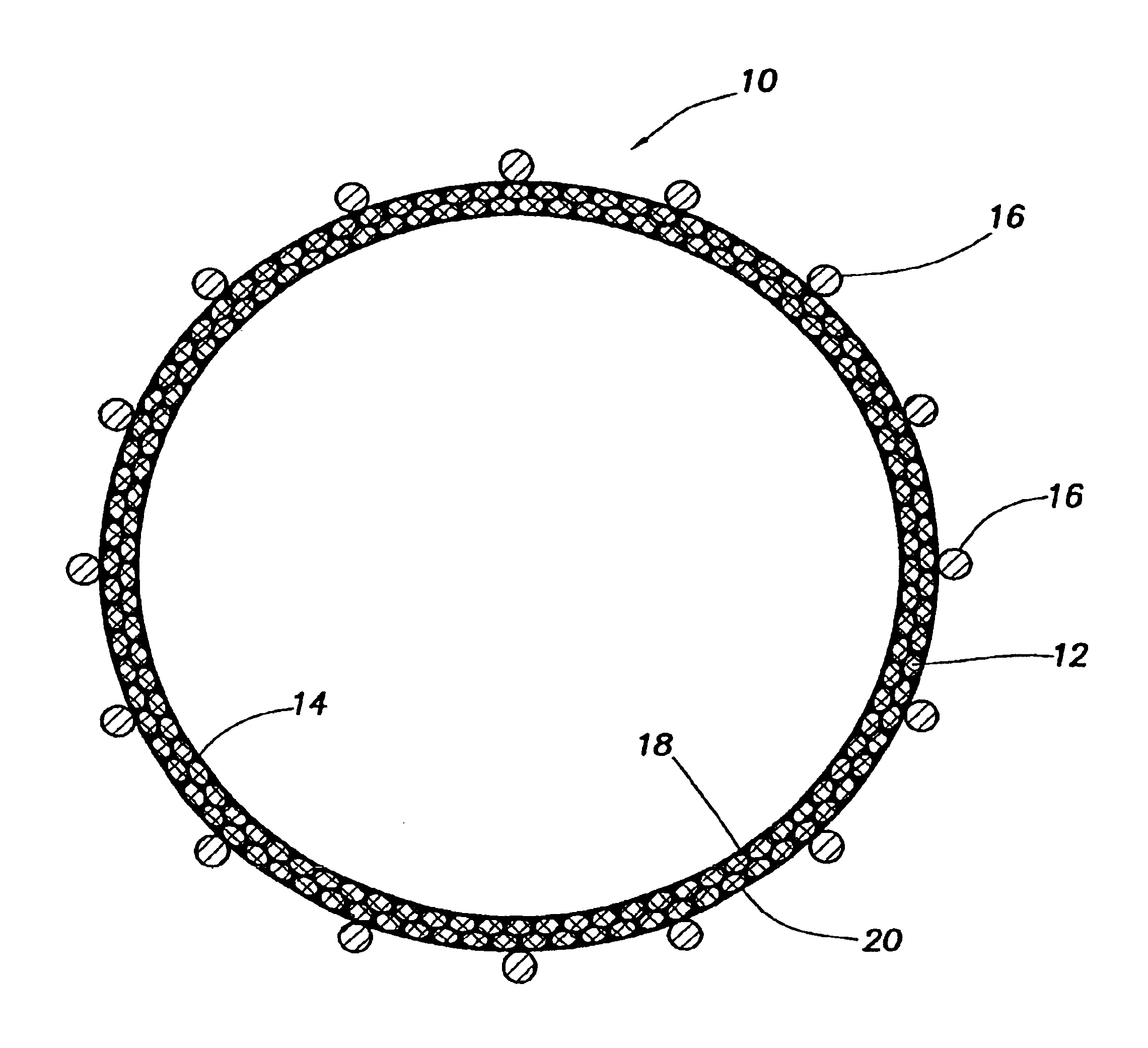
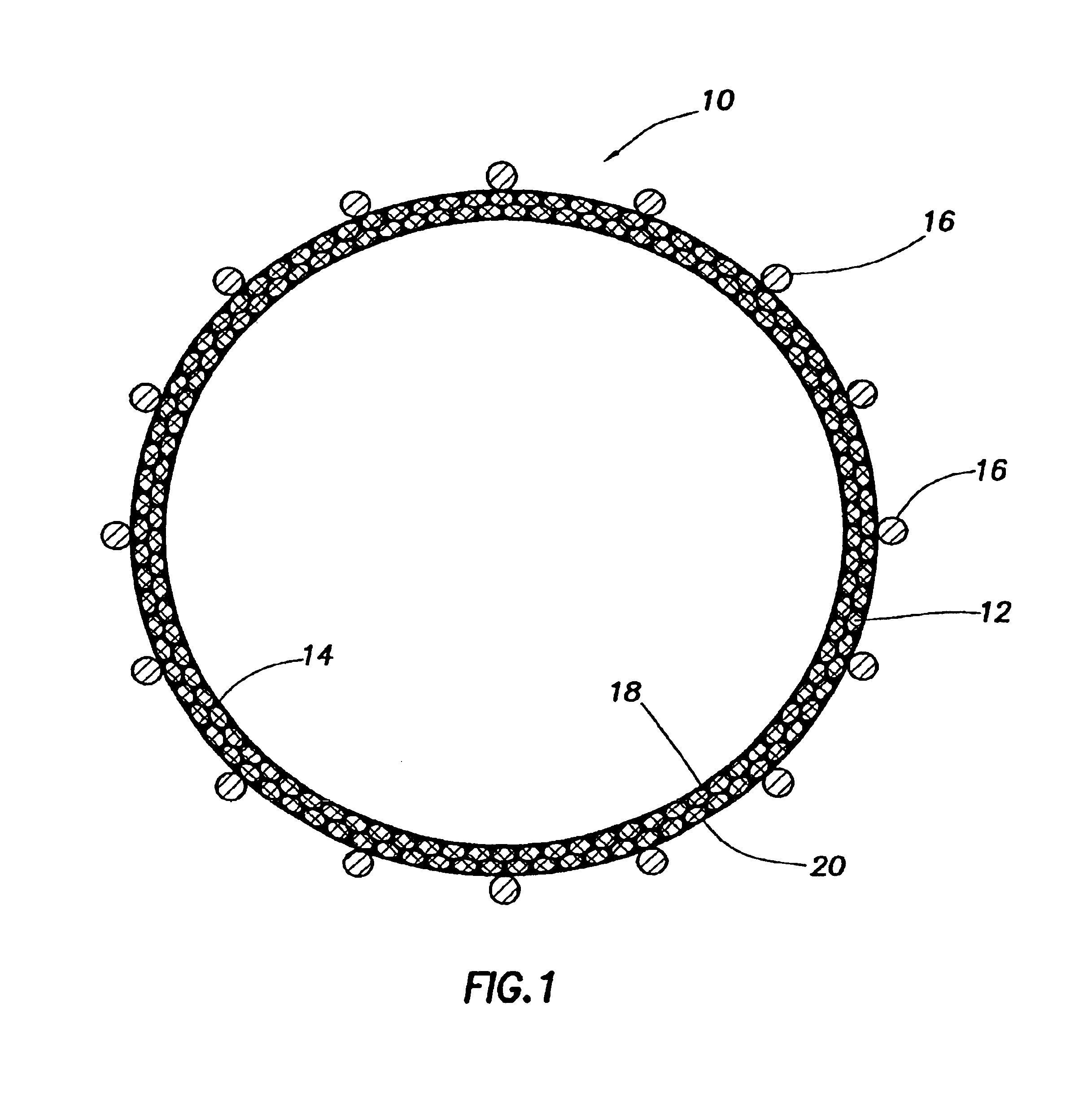
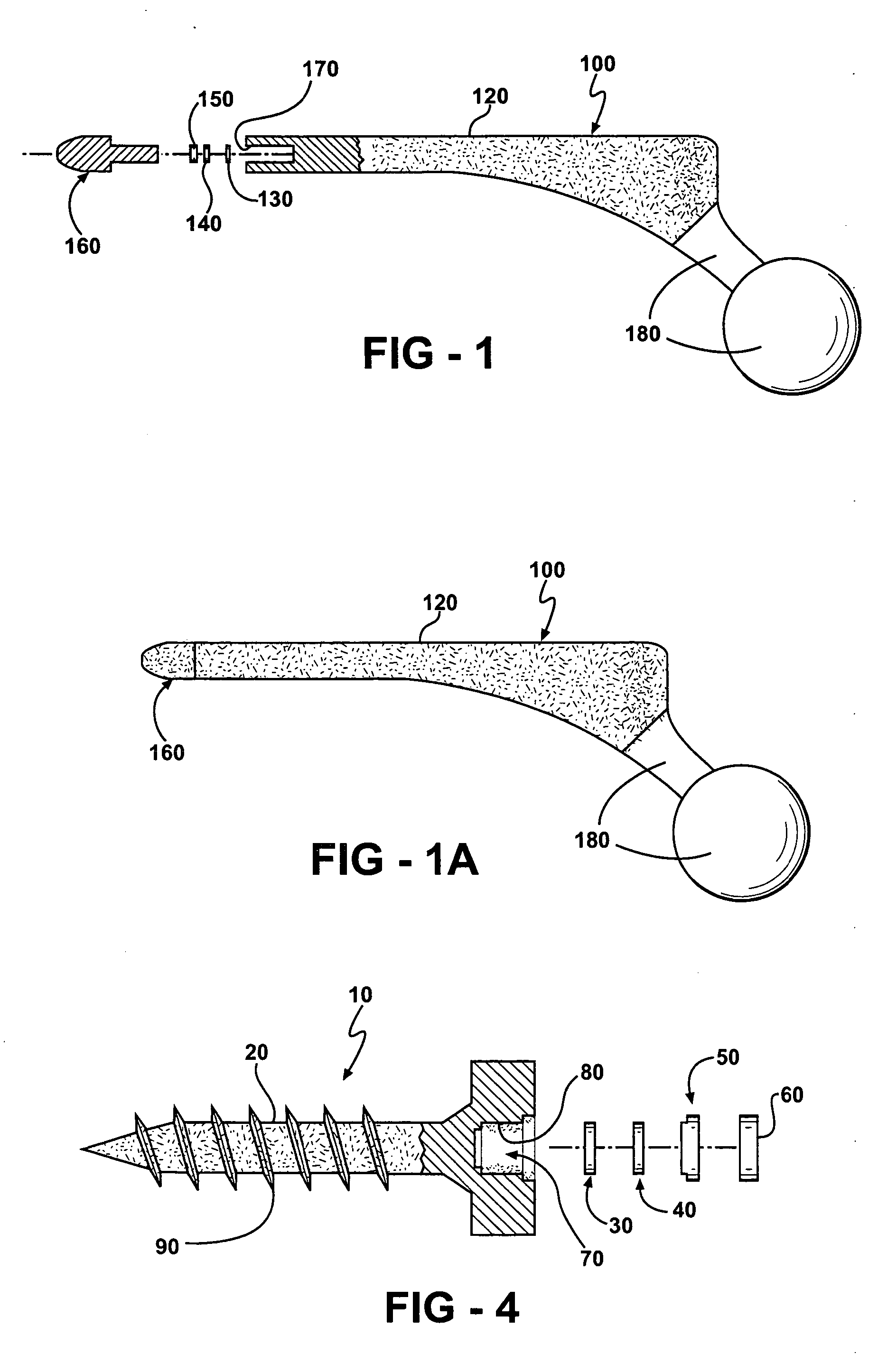
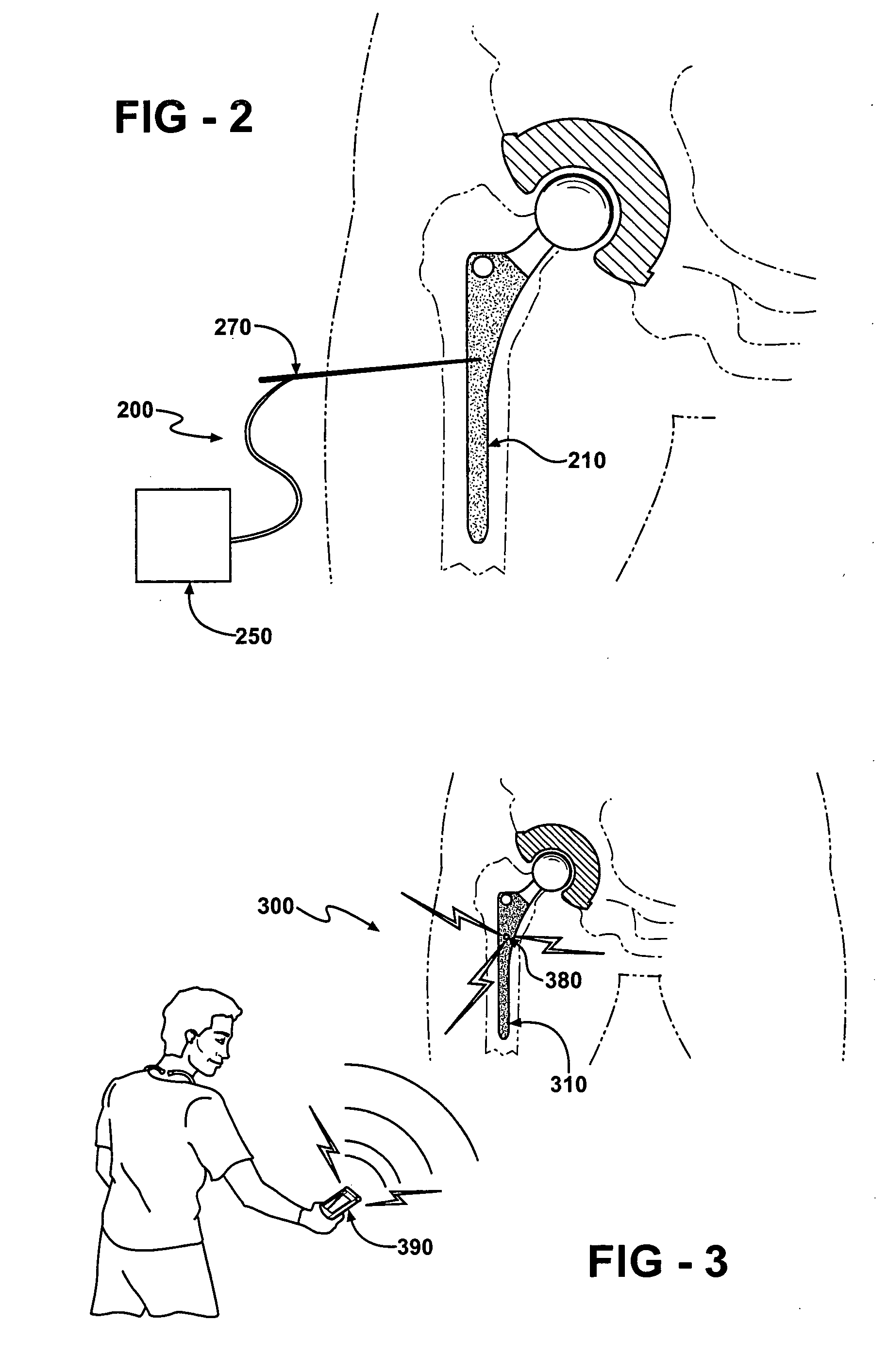
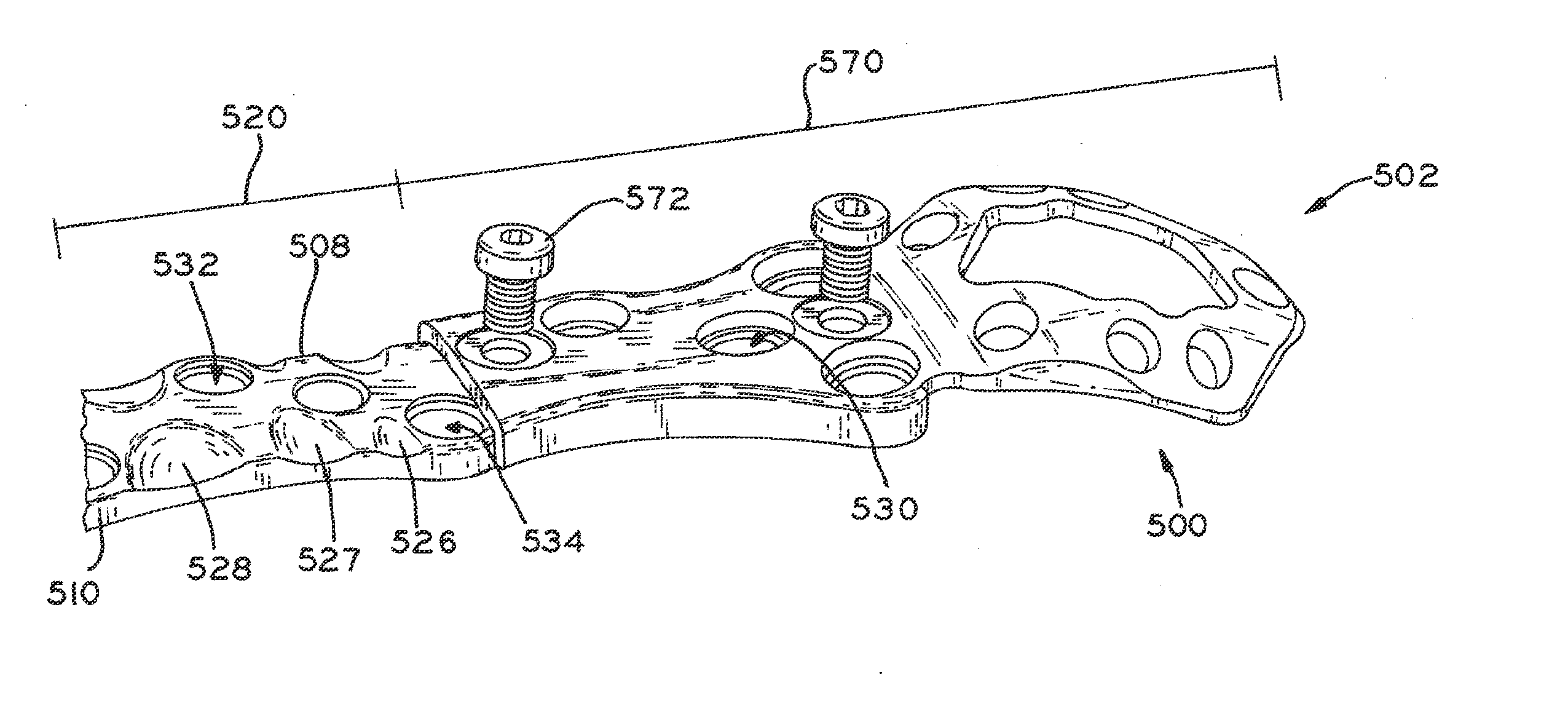
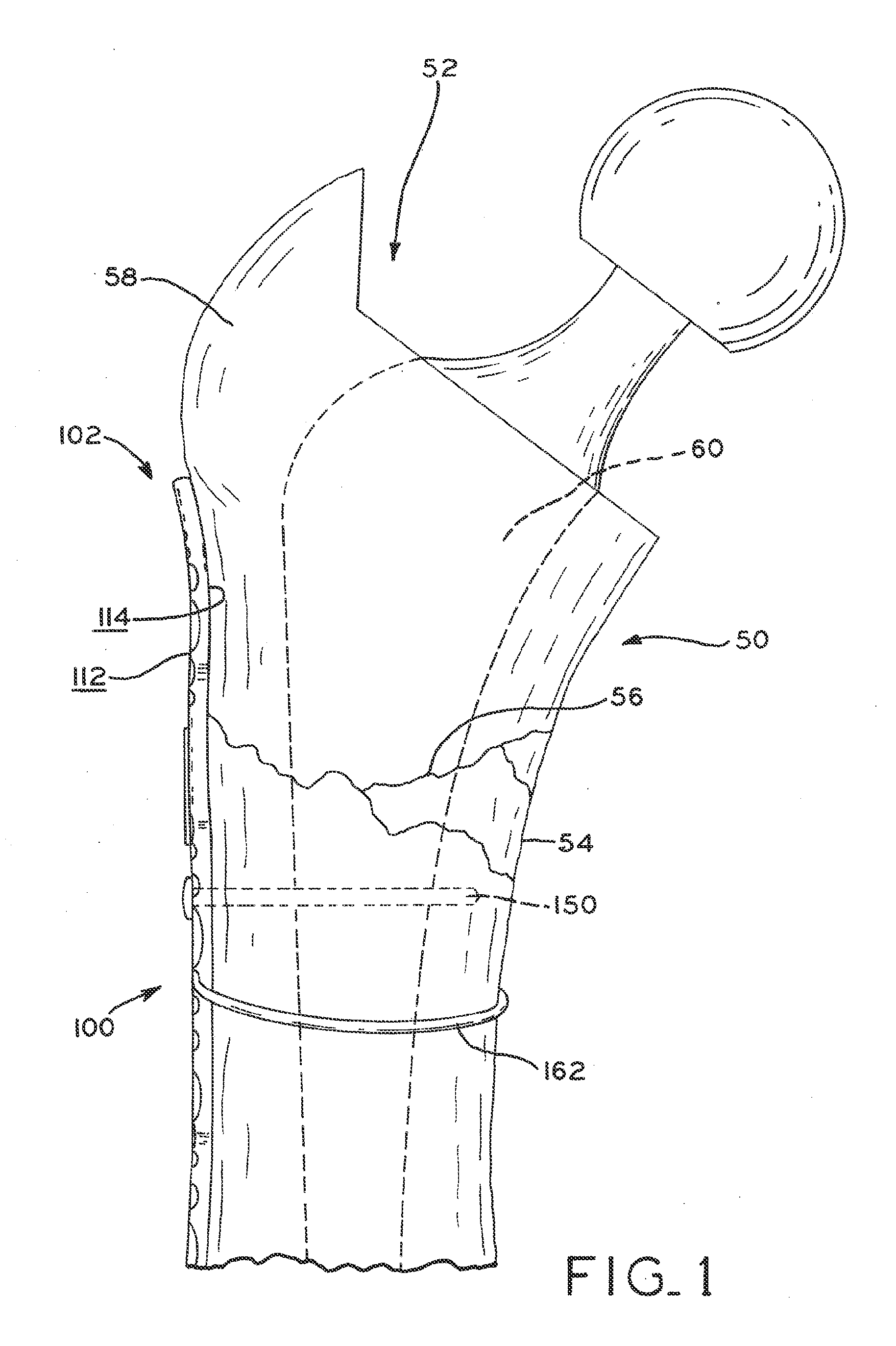
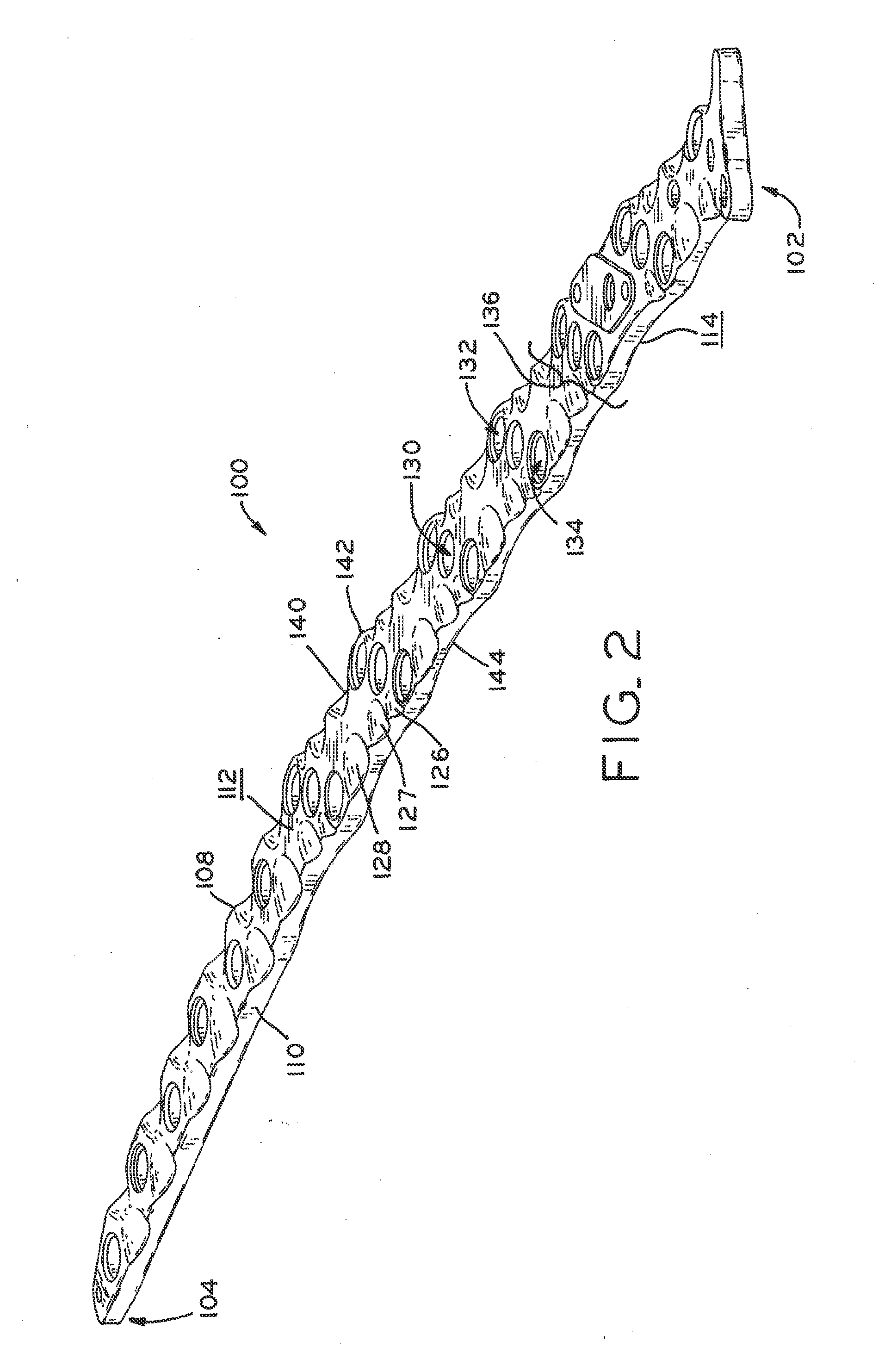
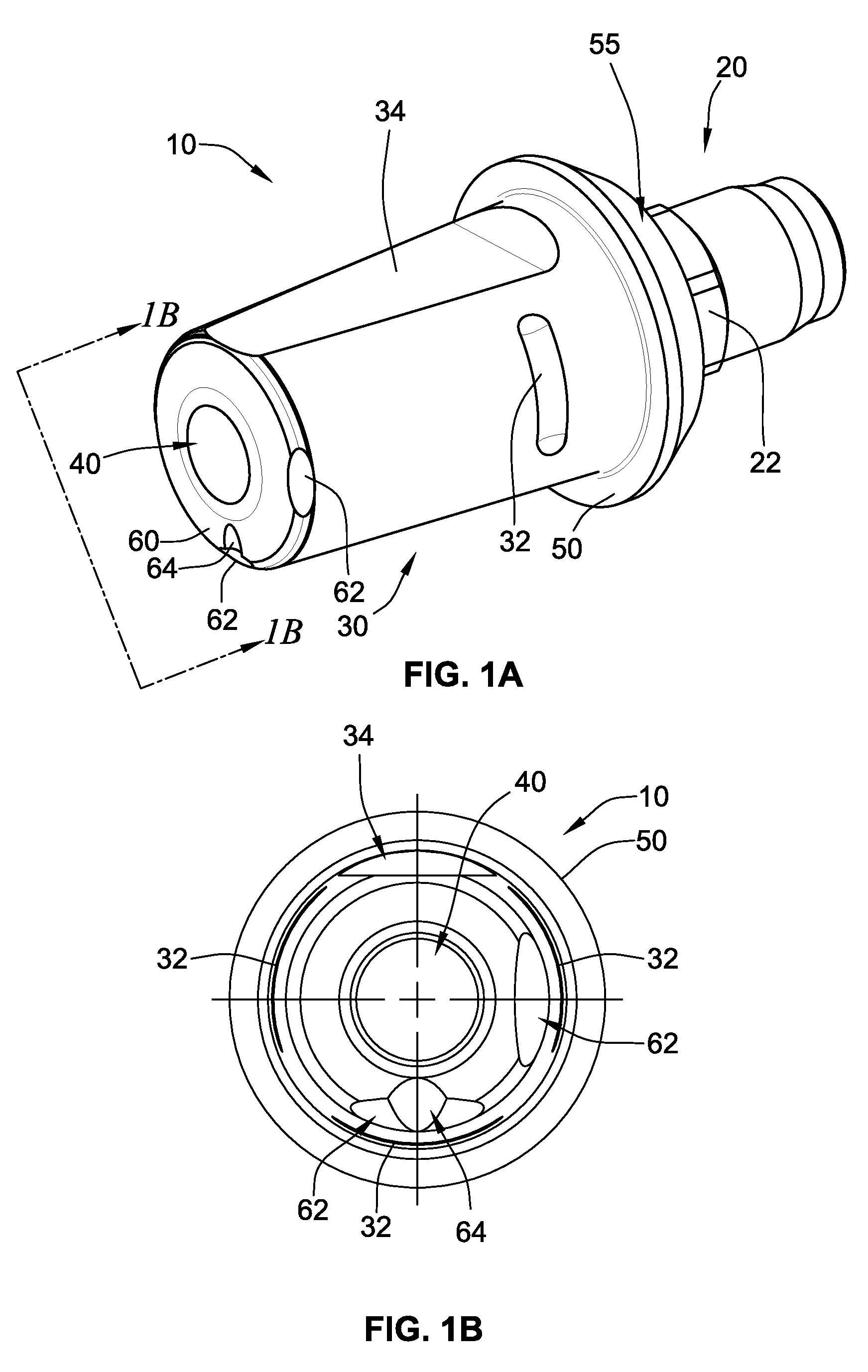
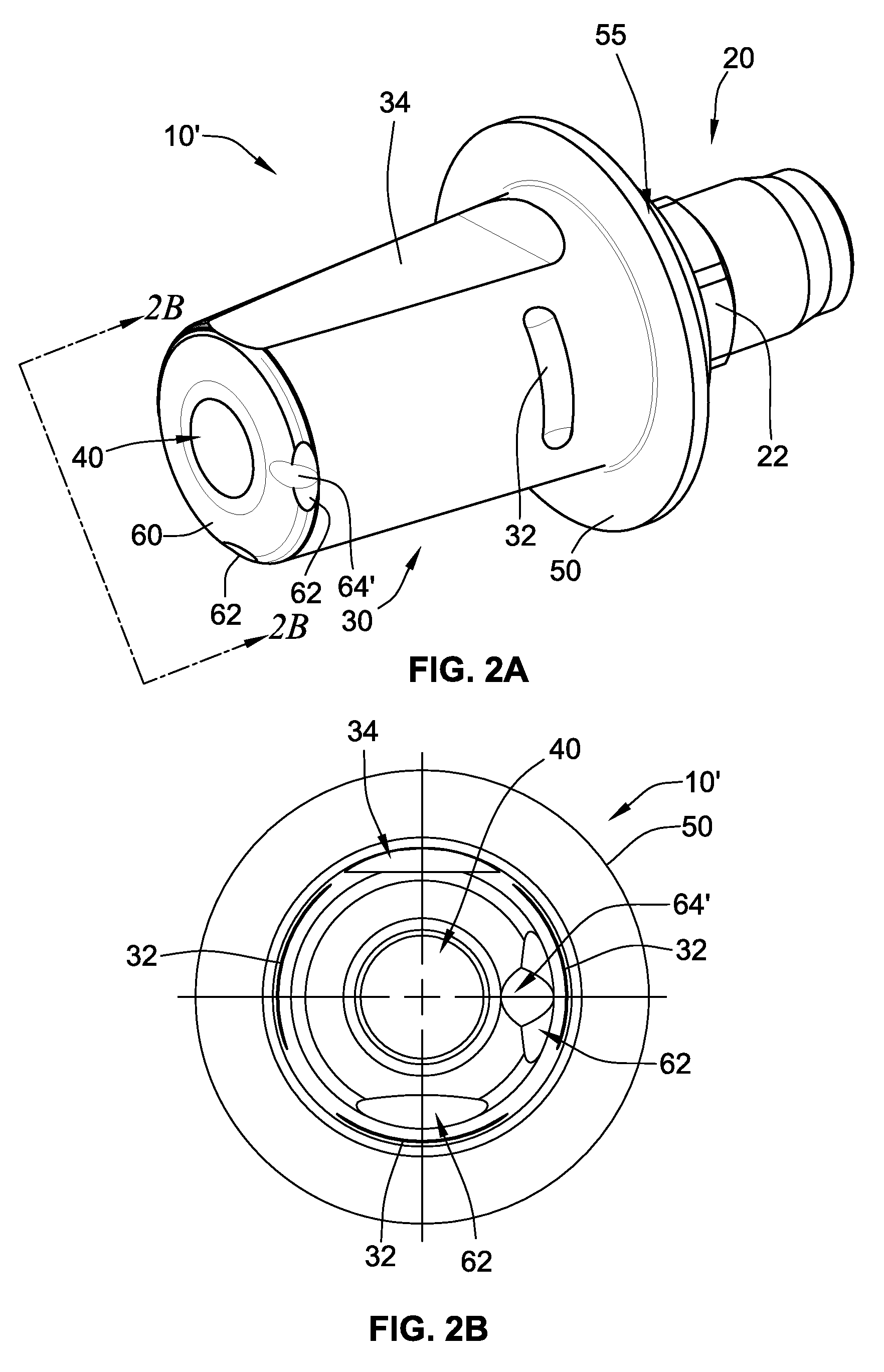
Periprosthetic bone plates
The present disclosure relates to bone plates that are configured for use with bones having periprosthetic fractures. For example, in the event that a proximal femur is fractured in an area that is adjacent to a prosthetic component, such as a femoral stem used in a hip replacement, the periprosthetic bone plates of the present invention may be used. In one exemplary embodiment, the periprosthetic bone plates include a periprosthetic zone having a plurality of central apertures and a plurality of outer apertures that are offset from the central apertures. The periprosthetic zone may further include a plurality of indentations, each indentation extending longitudinally between adjacent outer apertures to narrow a width of the bone plate.
Owner:ZIMMER GMBH
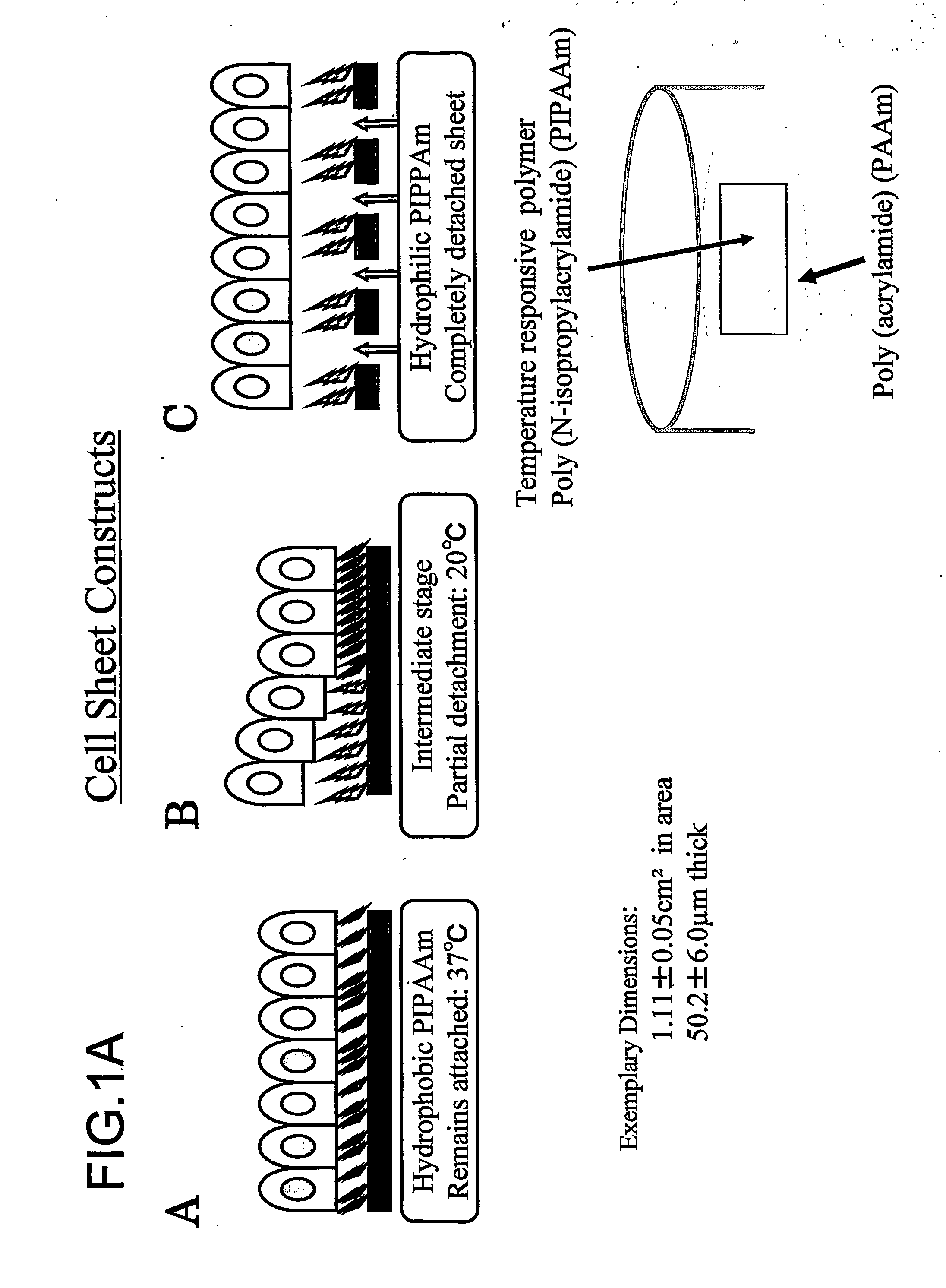
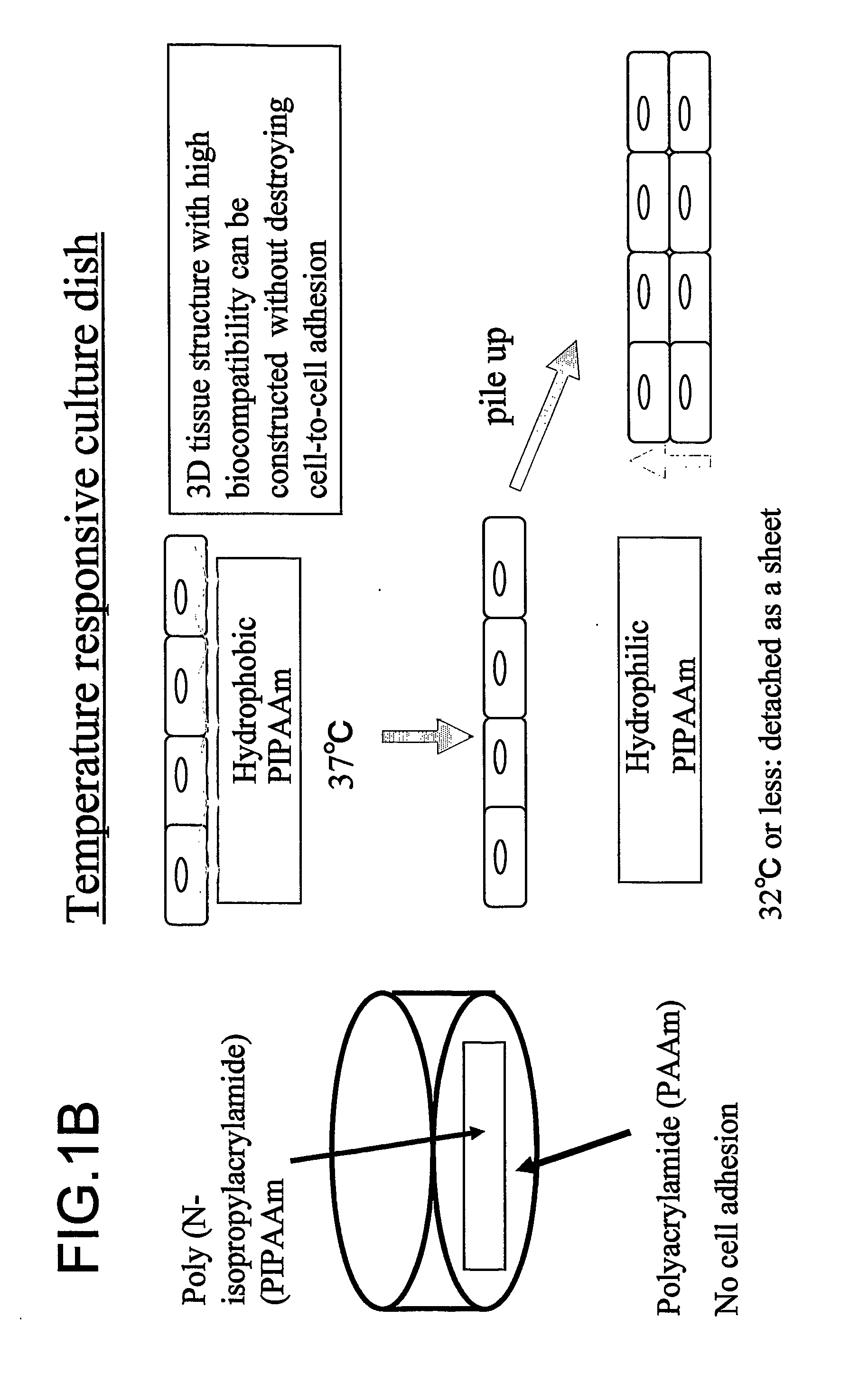
Three-dimentional tissue structure
InactiveUS20070092492A1Easy to identifySmall sizeBiocideMammal material medical ingredientsCulture cellCardiac muscle
The present invention provides a prosthetic tissue or sheet capable of withstanding implantation operations, which can be used in actual operation and can be produced by culture. The present invention also provides a novel therapy which can substitute for cell therapy. Particularly, the present invention provides a method for producing a prosthetic tissue comprising a cell derived from a part other than myocardium and capable of withstanding implantation operation. The above-described objects of the present invention were partially achieved by finding that by culturing cells under specific culture conditions, the cells are unexpectedly organized into a tissue, and the resultant prosthetic tissue is capable of being detached from culture dishes. The present invention also provides a three-dimensional structure applicable to heart, comprising a cell derived from a part other than the myocardium of an adult.
Owner:CELLSEED
Hydrogel providing cell-specific ingrowth
InactiveUS20050119762A1Facilitate cell-specific ingrowthEncourage adherenceSurgeryPharmaceutical containersCross-linkCell specific
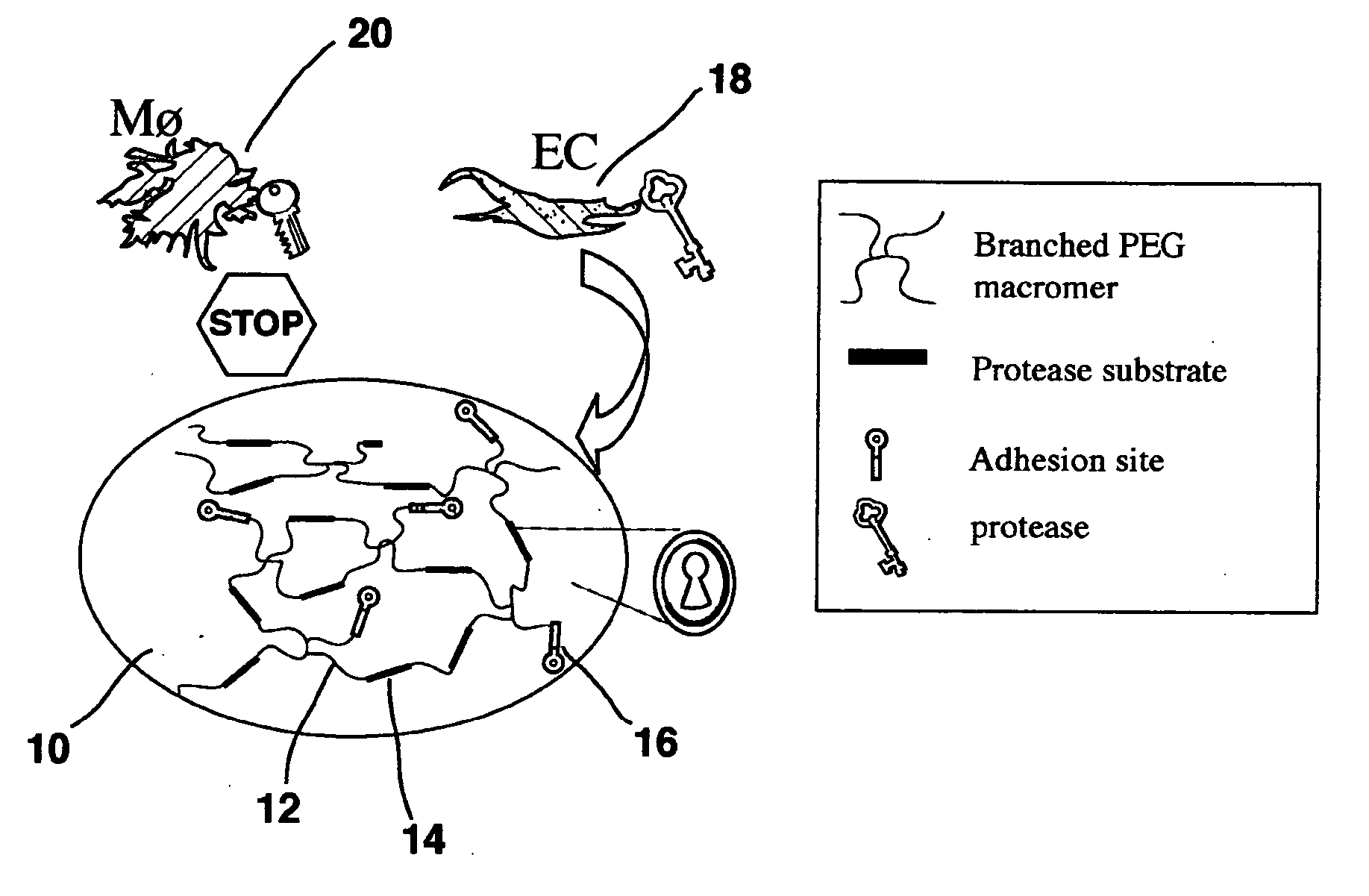
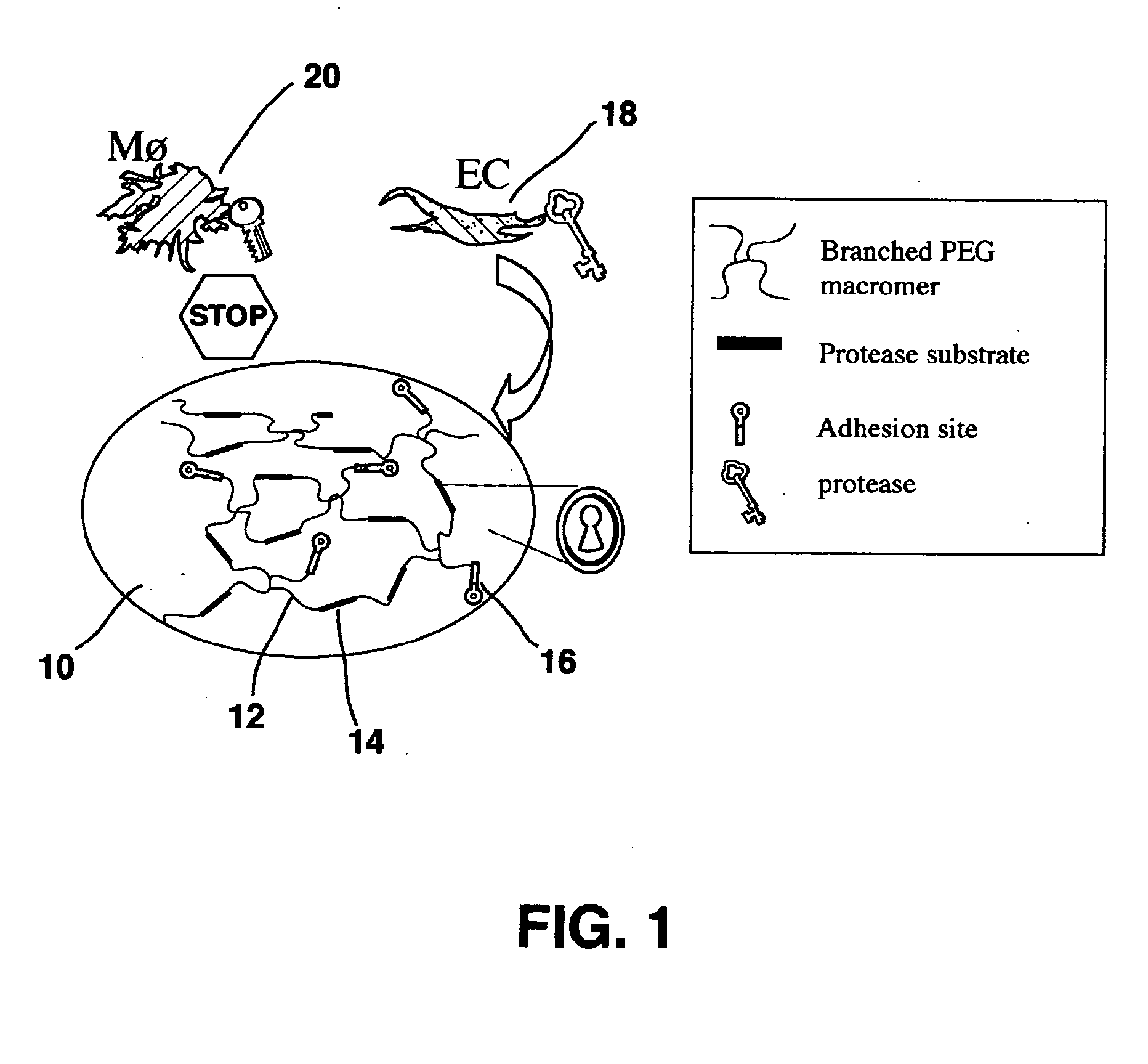
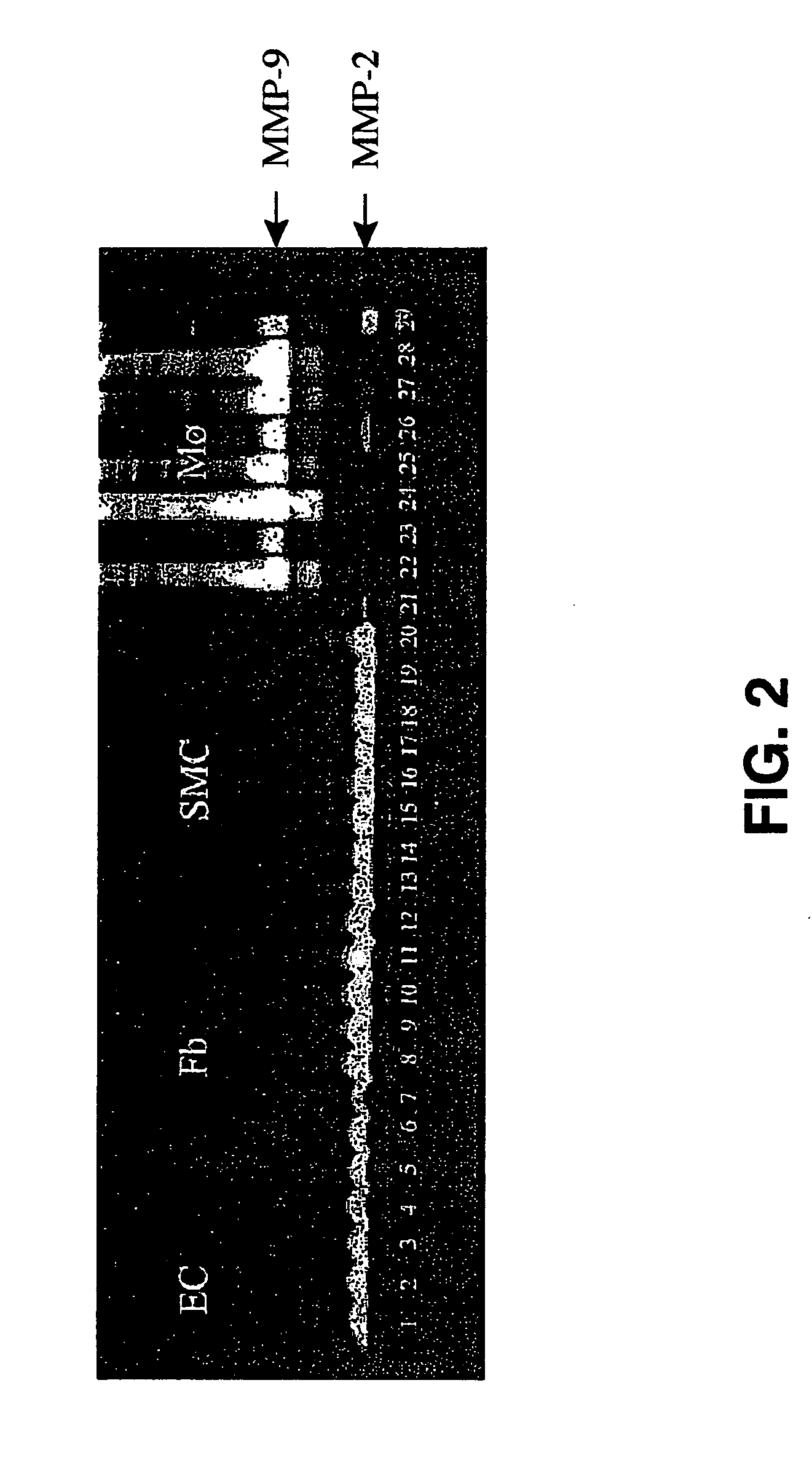
A polymeric biomaterial that facilitates cell-specific ingrowth. The polymeric biomaterial encourages the ingrowth of cell types while reducing the ingrowth of undesirable cell types. This activity encourages proper integration of prosthetic implants or scaffolds utilizing this biomaterial by discouraging encapsulation or the accumulation of inflammatory cells such as macrophages, while encouraging infiltration by desirable cells such as endothelial or smooth muscle cells. Short peptide sequences are included in a polymeric biomaterial that result in complementary activities. Peptide sequences that are specifically cleaved by proteases found within preferred cells are used to cross-link the biomaterial and lead to degradation by those cells. Peptide sequences taken from proteins involved in cell adhesion can also be attached to the biomaterial to encourage adhesion by preferred cells. Combined use of both peptides in the polymeric biomaterial provides both specific adhesion and selective ingrowth.
Owner:MEDTRONIC INC
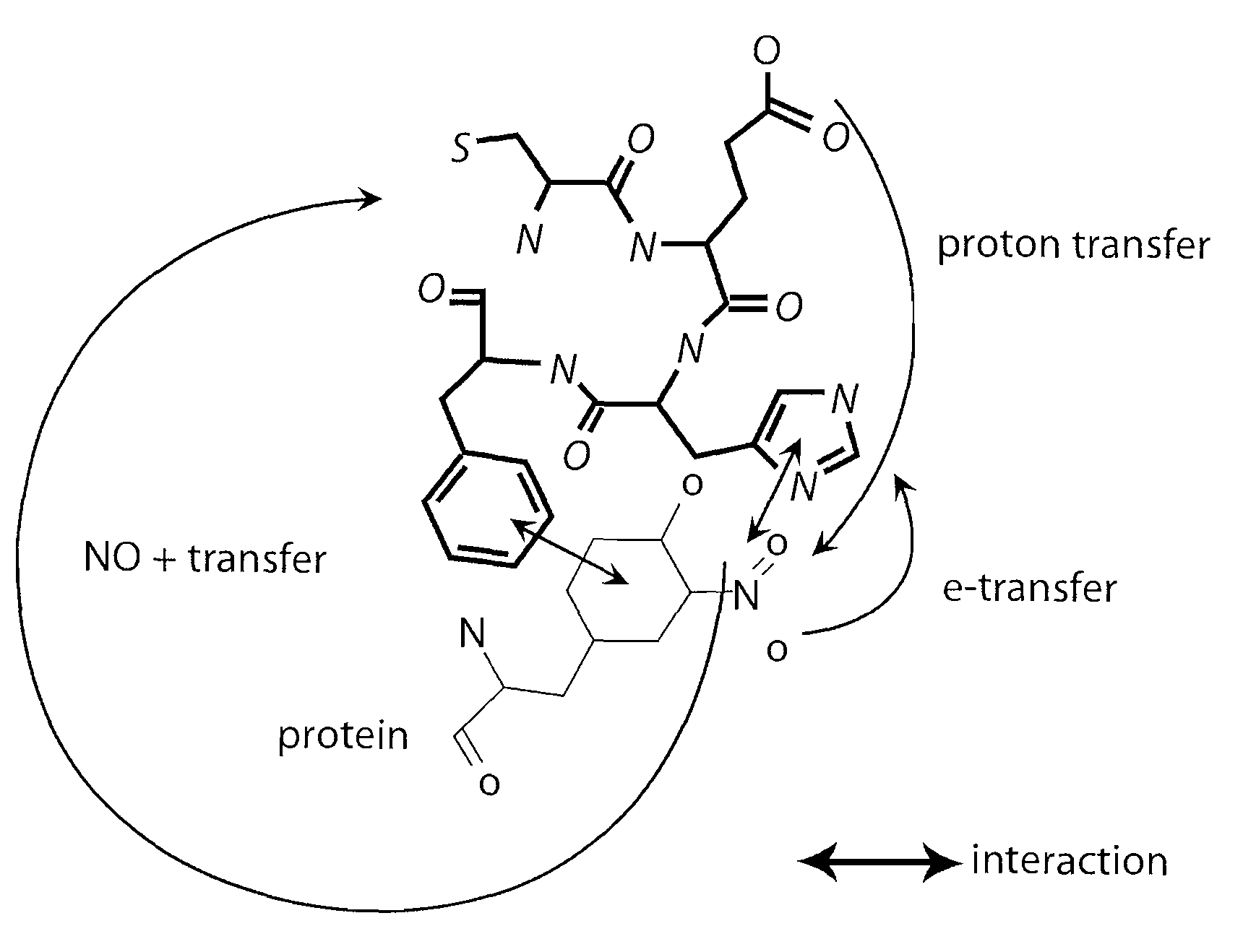
Short peptides useful for treatment of ischemia/reperfusion injury and other tissue damage conditions associated with nitric oxide and its reactive species
ActiveUS20080182797A1Avoid tissue damageLevel of protectionNervous disorderTetrapeptide ingredientsPregnancyAllograft rejection
This invention discloses isolated short peptides comprising the amino acid sequence Cys-Glu-Phe-His (CEFH) and analogs thereof as well as compositions comprising CEFH peptides and analogs thereof. The CEFH peptides disclosed herein are effective in mediating the denitration of 3-nitrotyrosines (3-NT) in cellular proteins thereby preventing tissue damage associated with excess nitric oxide (NO) and its reactive species. The CEFH peptides disclosed herein are useful in the treatment of ischemia / reperfusion (I / R) injury of various tissues (e.g., I / R injury of heart muscle associated with heart attack or cardiac surgery, I / R injury of brain tissue associated with stroke, I / R injury of liver tissue, skeletal muscles, etc.), septic shock, anaphylactic shock, neurodegenerative diseases (e.g., Alzheimer's and Parkinson's diseases), neuronal injury, atherosclerosis, diabetes, multiple sclerosis, autoimmune uveitis, pulmonary fibrosis, oobliterative bronchiolitis, bronchopulmonary dysplasia (BPD), amyotrophic lateral sclerosis (ALS), sepsis, inflammatory bowel disease, arthritis, allograft rejection, autoimmune myocarditis, myocardial inflammation, pulmonary granulomatous inflammation, influenza- or HSV-induced pneumonia, chronic cerebral vasospasm, allergic encephalomyelitis, central nervous system (CNS) inflammation, Heliobacterium pylori gastritis, necrotizing entrerocolitis, celliac disease, peritonitis, early prosthesis failure, inclusion body myositis, preeclamptic pregnancies, skin lesions with anaphylactoid purpura, nephrosclerosis, ileitis, leishmaniasis, cancer, and related disorders.
Owner:NEW YORK UNIVERSITY
Temporary abutment with combination of scanning features and provisionalization features
Owner:BIOMET 3I LLC
Treatments for reduction of cytotoxicity and viral contamination of implantable medical devices
InactiveUS20060110370A1Reduce pathologic calcificationReduce pollutionBiocideMicrobiological testing/measurementCytotoxicityCalcification
A method for treating biomaterial is provided in which a biological tissue, typically after being cross-linked, is contacted with an anticalcification treatment solution under condition effective to render the biomaterial resistant to in vivo calcification upon implantation in a host animal. The anticalcification treatment solutions comprise higher alcohol solutions, a polyol solutions and / or a polar aprotic organic solvent solutions. Methods of reducing cytotoxicity to host tissue of bioprostheses that comprise fixed animal tissues, and treatments to reduce viral contamination of implantable medical devices are disclosed herein.
Owner:CARBOMEDICS
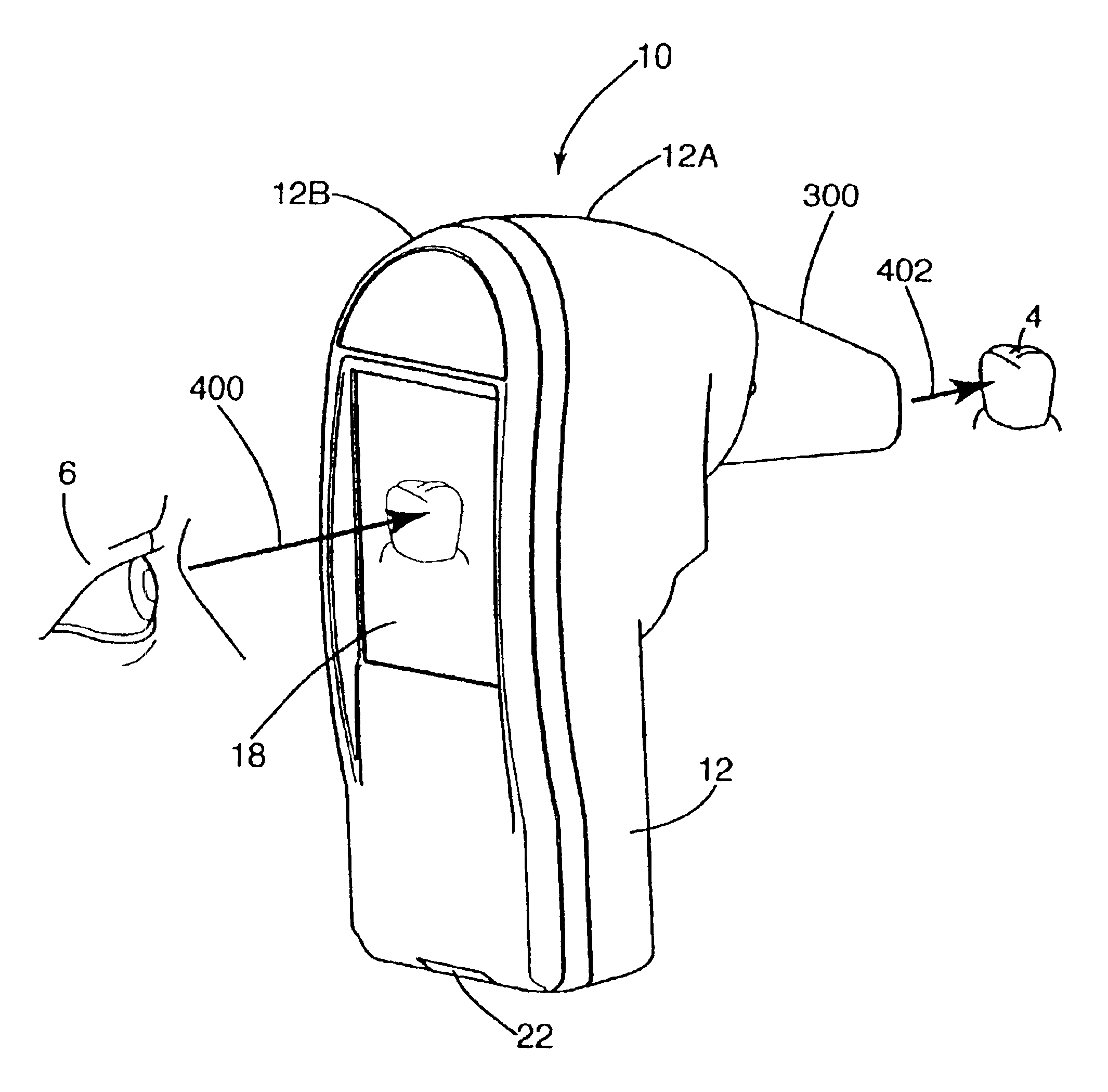
Optical measurement device and related process
InactiveUS6867864B2Accurate representationPoor color fidelityRadiation pyrometrySurgeryColor imageOptical property
An instrument and related process for measuring color, shade, gloss, shape and / or translucence of a tooth. First, the instrument uses searchlight illumination to illuminate a tooth with constant irradiance. Second, the instrument uses colorimetric imaging to collect time-separated frames of different wavelengths of light reflected from a tooth and to combine those frames into a color image. Third, the instrument includes a sanitary shield to establish a reference color and a predetermined distance to a target tooth. Fourth, the instrument provides line-of-sight viewing so an operator may simultaneously view a display of the image on the instrument and the object being measured. Fifth, the instrument is impervious to pollutants because it incorporates a sealed measurement window. Sixth, optical measurements of a tooth taken by a dentist are compared to optical measurements of a prosthetic restoration for that tooth to confirm satisfactory matching of optical characteristics of the tooth and restoration.
Owner:X RITE INC
Implant and Method for Producing a Degradation-Inhibiting Layer on the Surface of an Implant Body
A method for manufacturing a degradation-inhibiting first layer on the surface of an implant body, in particular an intraluminal endoprosthesis, whereby the body has at least one metallic material, which is at least largely biodegradable, comprising the following steps: preparing the body of the implant, and applying the first layer to at least a portion of the body surface, whereby the first layer contains magnesium stearate. An implant obtainable by such a method.
Owner:BIOTRONIK AG
Polymeric Prosthetic Liner With Controlled Stretch Characteristics
A controlled-stretch prosthetic liner comprising a polymeric material covered with at least a stretch-controlling fabric. Certain liner types, such as below knee (BK) liners, may also include a panel of more stretchable fabric so as to prevent any interference with knee flexion. The polymeric material of the liner may also be used to control liner stretch, such as by adding Kevlar pulp or other materials that increase the strength and reduce the elasticity of the polymeric material and / or by reducing the amount of plasticizer(s) present therein. The stretch-controlling fabric, possibly in conjunction with the polymeric material, acts to limit the longitudinal stretch of the liner while not adversely affecting the radial stretch thereof.
Owner:WILLOWWOOD GLOBAL LLC
Method and system for improving the effectiveness of artificial joints by the application of gas cluster ion beam technology
InactiveUS6863786B2Minimize impactMinimize wear debrisCellsElectric discharge tubesSurface patternGas cluster ion beam
Regardless of the materials used in an artificial joint component design, the present invention applies gas cluster ion beam (GCIB) technology in order to modify the component's surface(s) so as to increase lubrication between contact surfaces, thereby substantially reducing wear debris, osteolysis complications, and accelerated wear failure. The approach of the surface modification comprises an atomic level surface patterning utilizing GCIB to apply a predetermined pattern to the surface(s) of the joint implant to reduce frictional wear at the interface of the surfaces. A reduction in wear debris by GCIB patterning on any surface(s) of a joint prosthesis reduces accelerated failure due to wear and osteolysis and results in a substantial cost savings to the healthcare system, and reduces patient pain and suffering.
Owner:EXOGENESIS CORP +1
Methods for accelerating bone and cartilage growth and repair
InactiveUS6916783B2Strengthen bonesEnhancing cartilage repairAngiotensinsPeptide/protein ingredientsProsthesis ImplantationAgonist
The present invention provides improved methods, kits, and compositions for enhancing bone, cartilage and cartilage repair, bone and prosthesis implantation, and attachment and fixation of cartilage and cartilage to bone or other tissues, and chondrocyte proliferation composing the administration of an effective amount of angiotensinogen, angiotensin I (AI), AI analogues, AI fragments and analogues thereof, angiotensin II (AII), AII analogues, AII fragments or analogues thereof or AII AT2 type 2 receptor agonists.
Owner:UNIV OF SOUTHERN CALIFORNIA
Plastic implant impregnated with a degradation protector
ActiveUS7615075B2Extended service lifeEasily and accurately diagnose degradationBone implantJoint implantsRare earth metal compoundsPlastic implant
A plastic implant device for a mammal that contains a rare earth metal compound tracer and a method for detecting degradation such as wear of the implanted device are disclosed. The tracer can also be present with a separate antioxidant or the tracer compound can be can be the salt of a C6-C22 unsaturated carboxylic acid. The rare earth metal compound tracer is released when the prosthetic is worn down or otherwise degraded in the mammalian body in which it was implanted. The presence and amount of released tracer present in a body fluid or tissue sample measured and is proportional to the degree of degradation of the implant.
Owner:RUSH UNIV MEDICAL CENT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com