Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
61 results about "Muscle nerve" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
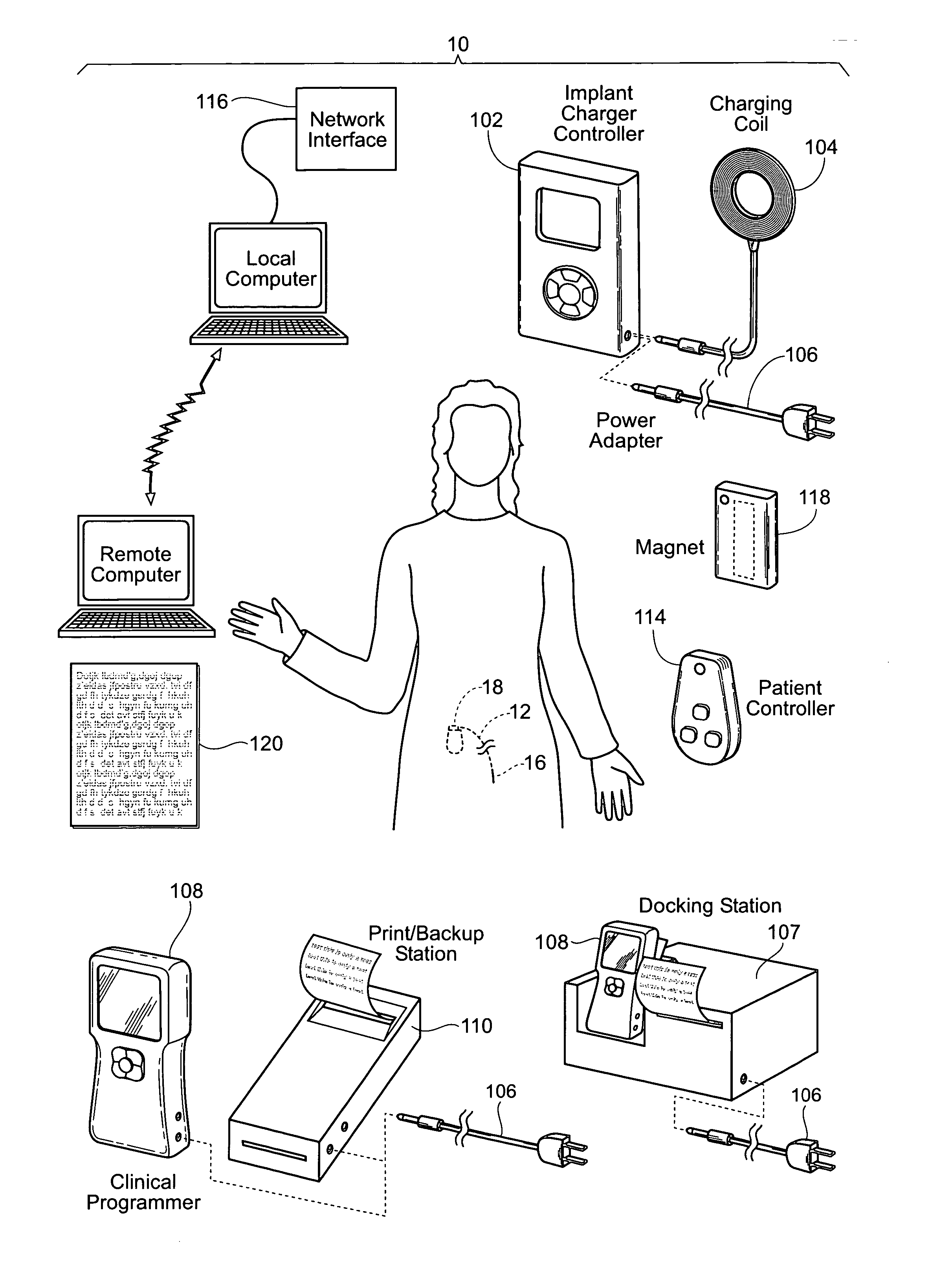
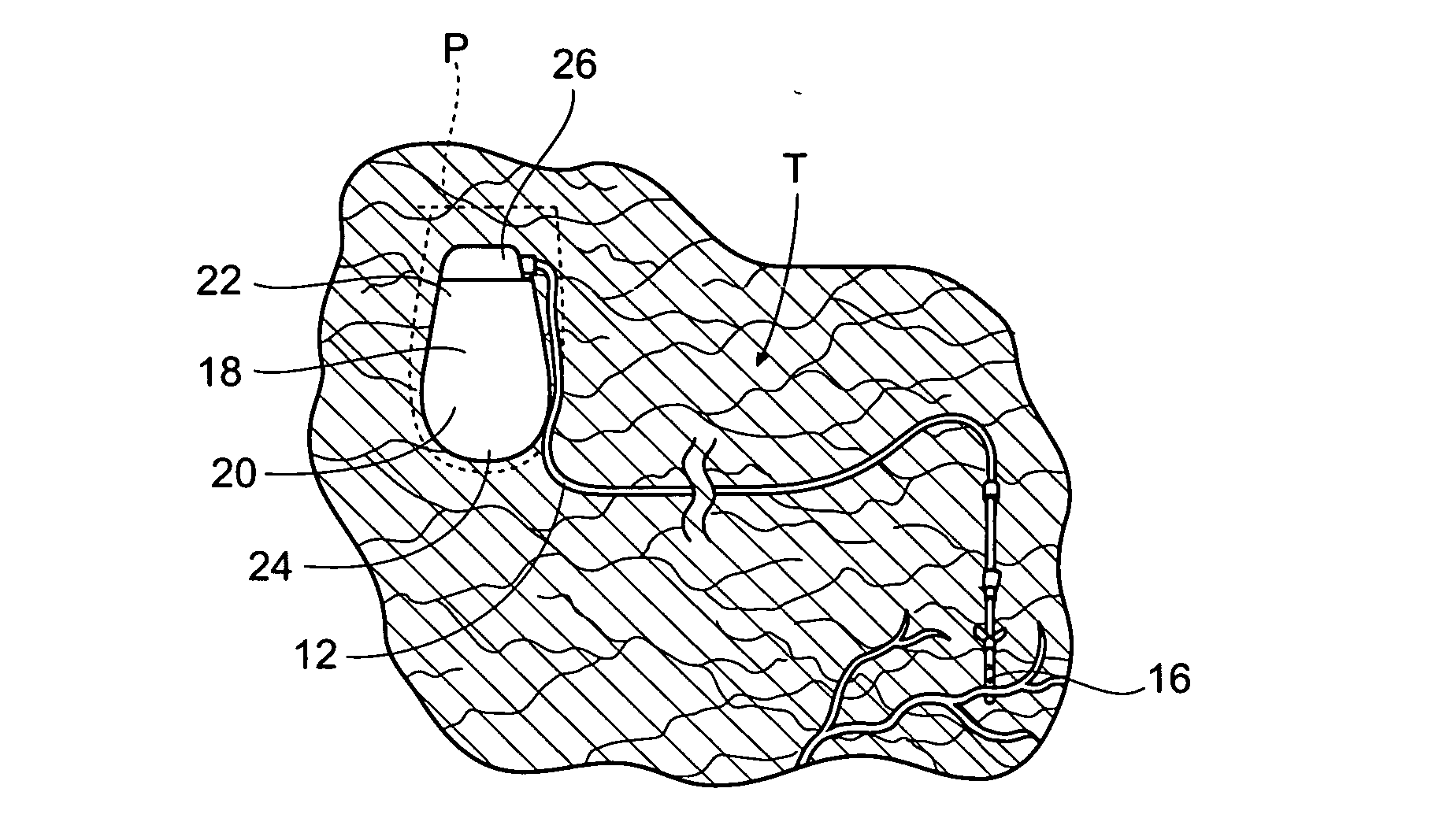
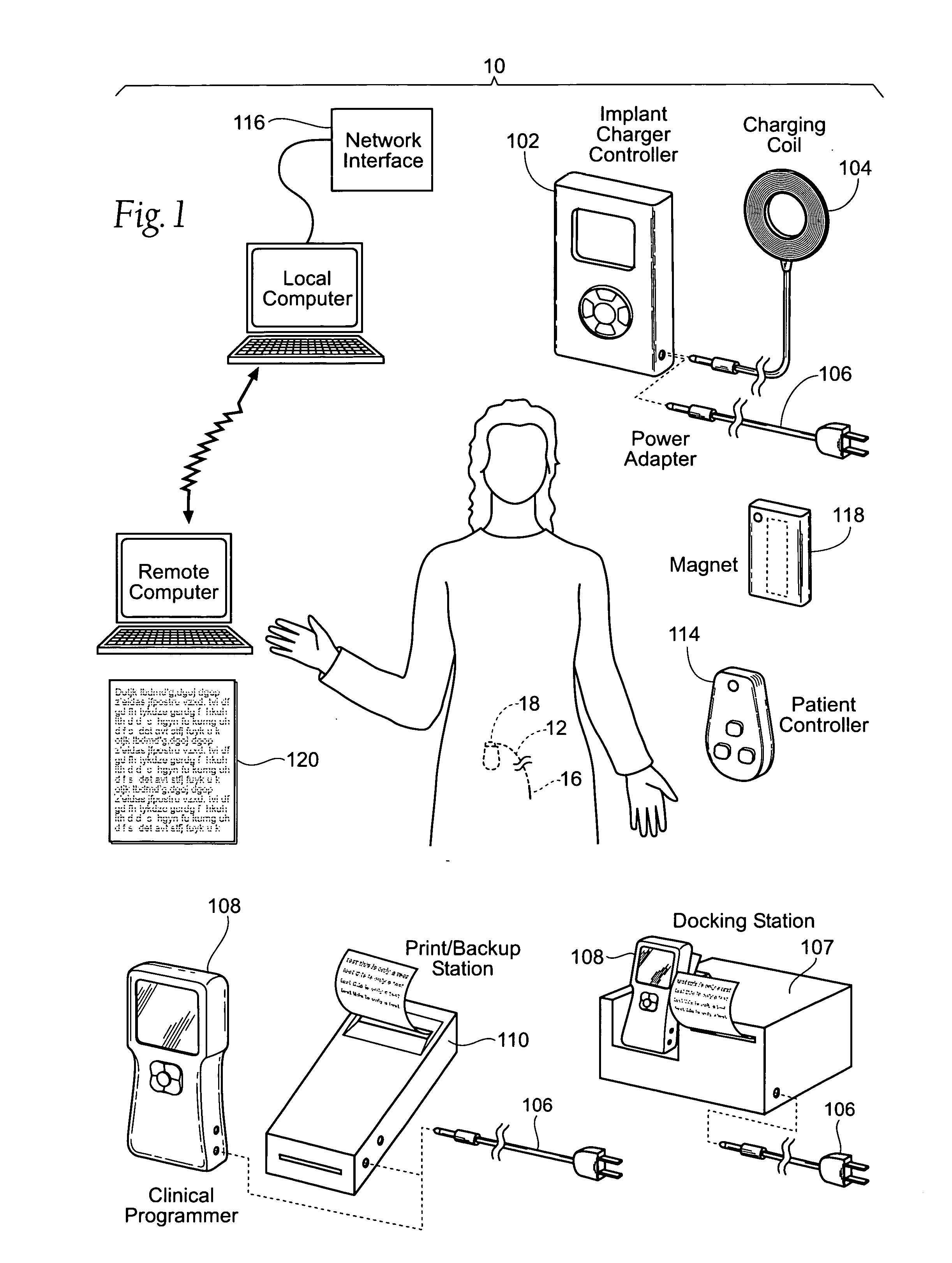
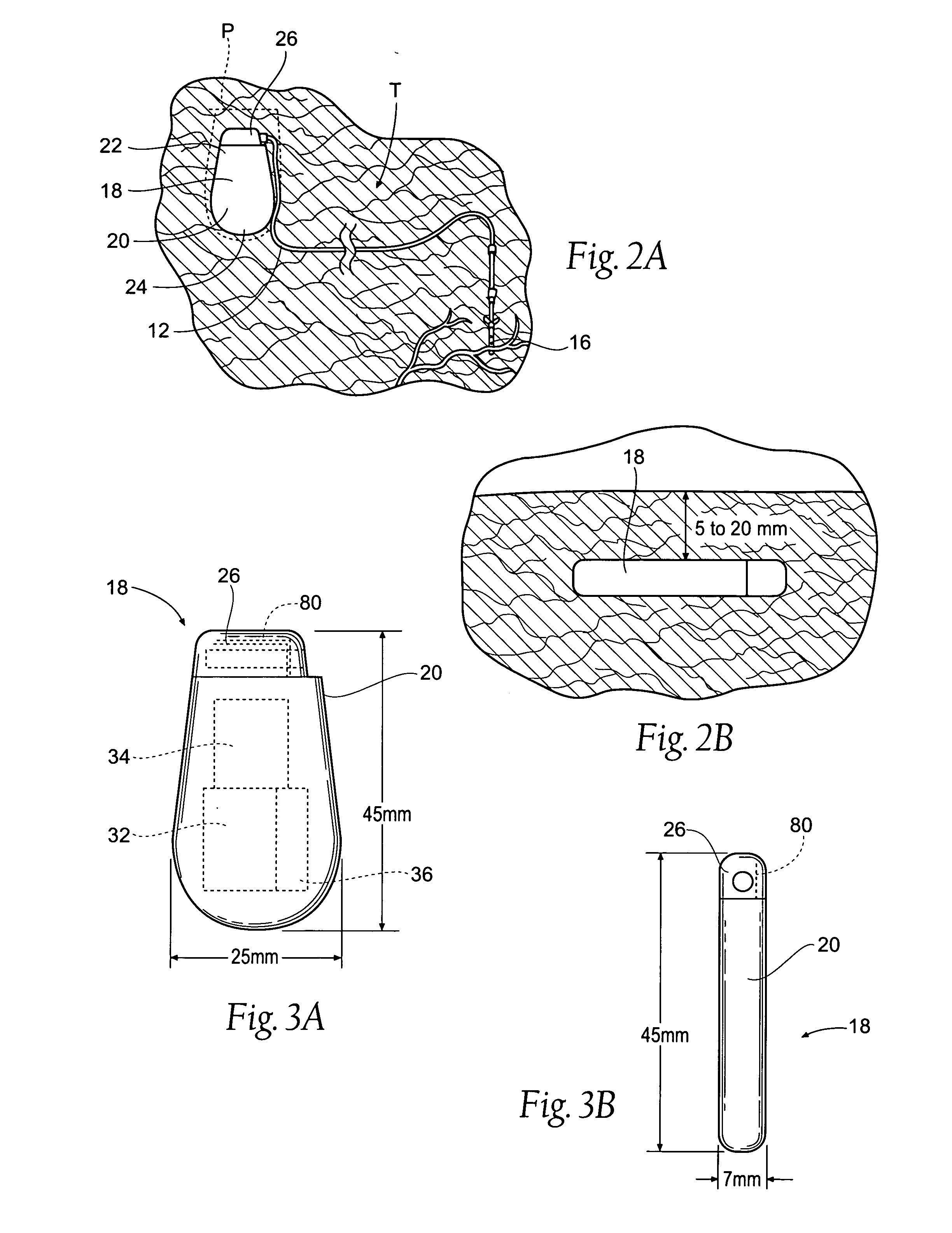
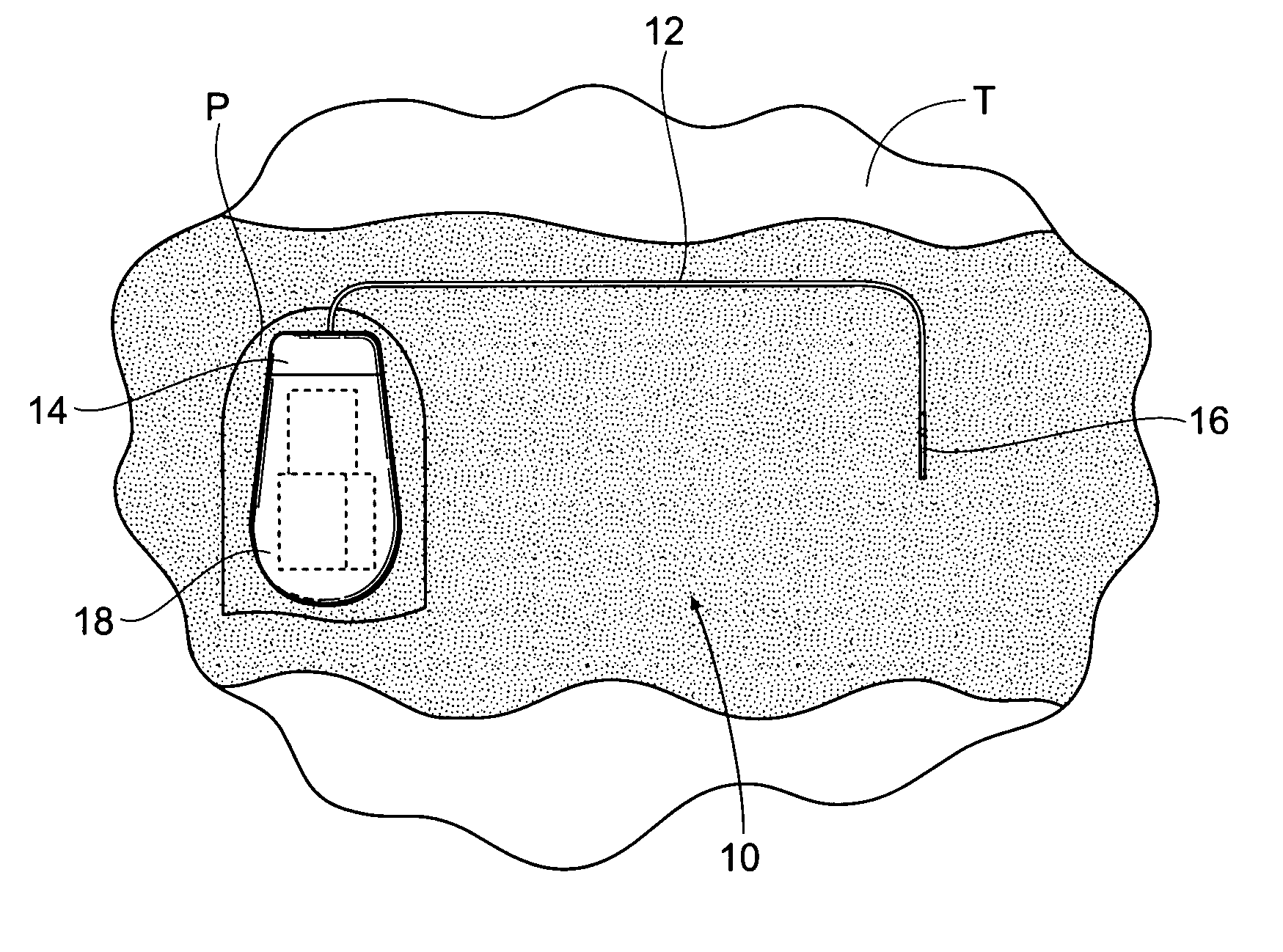
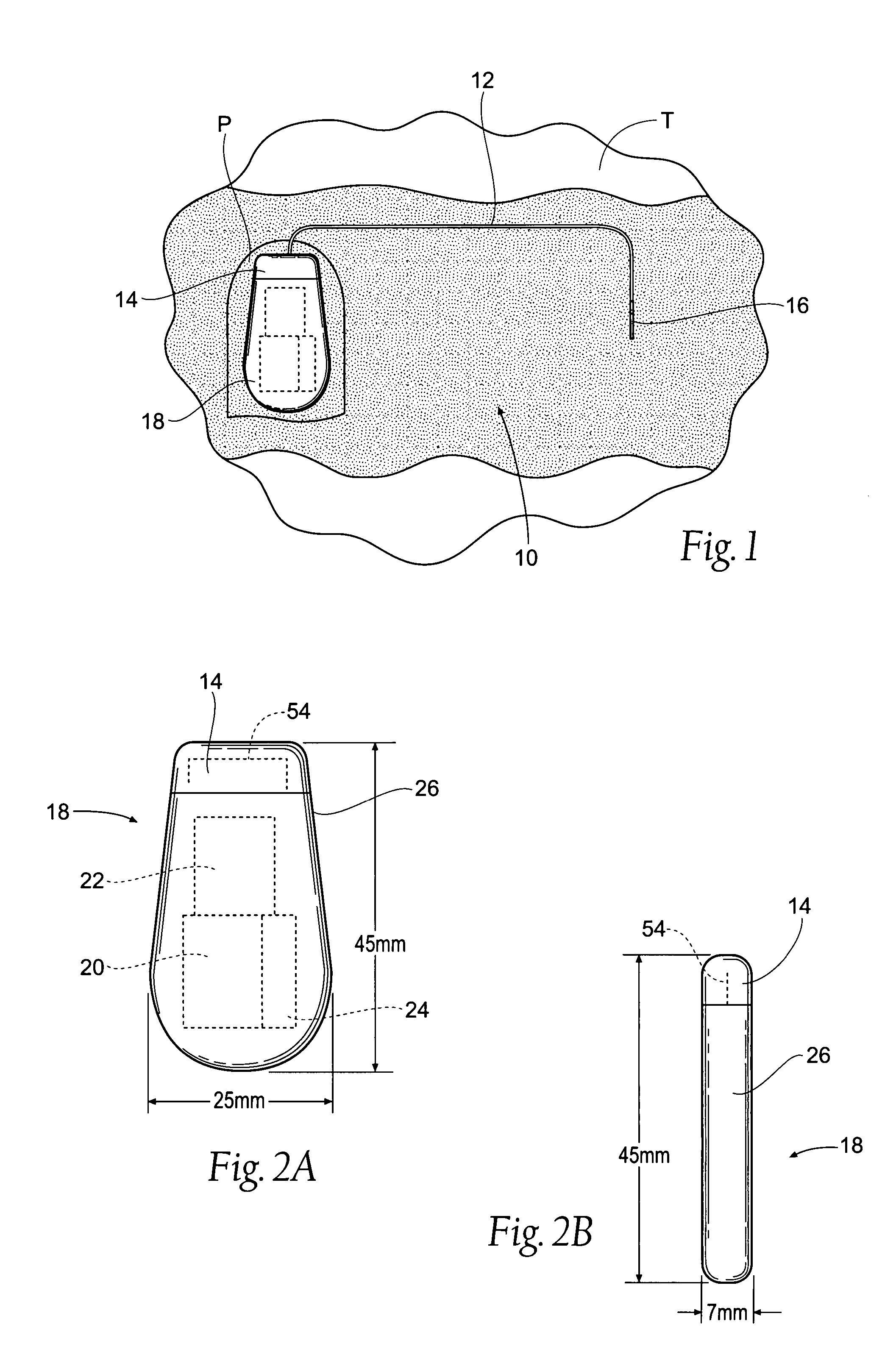
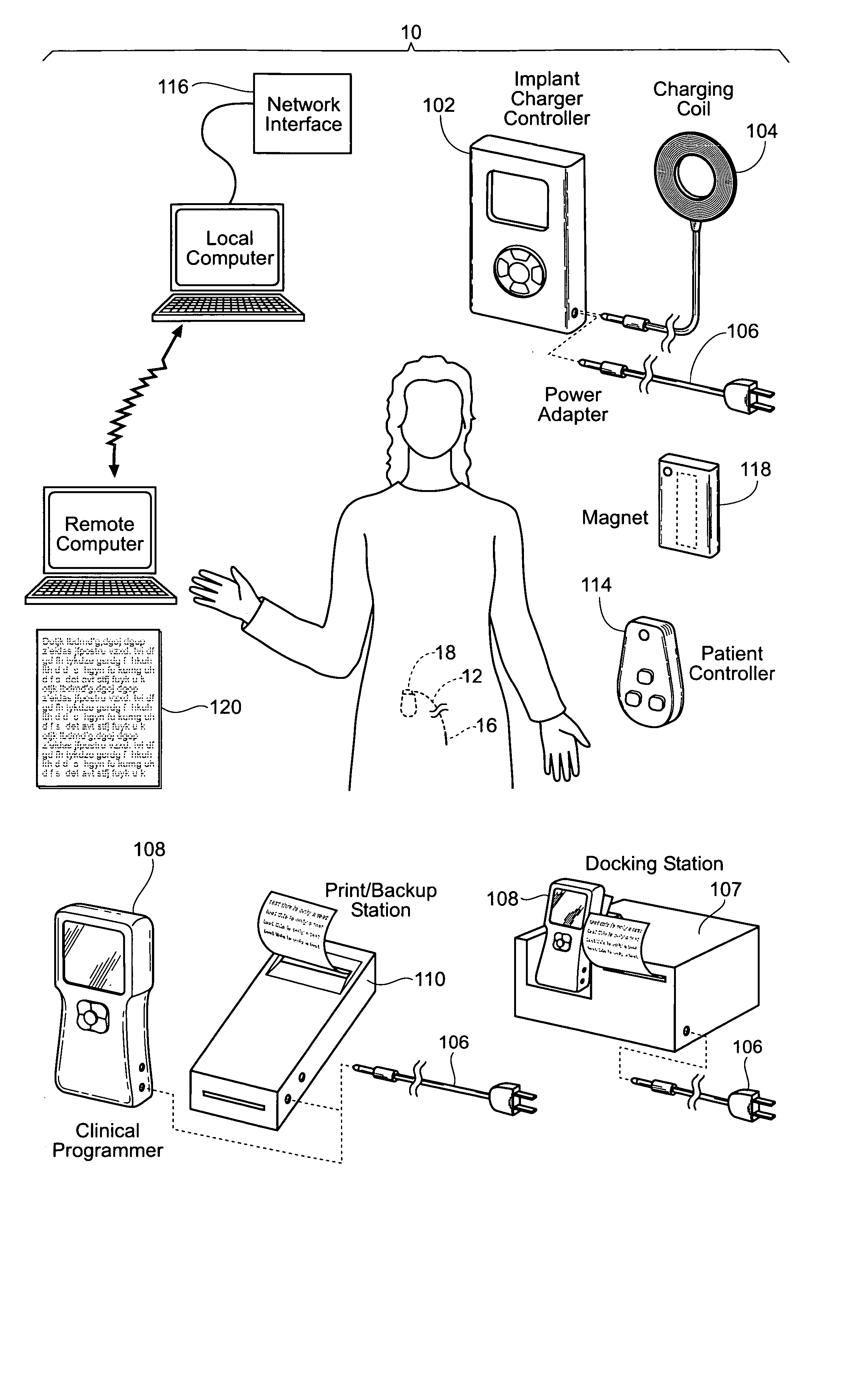
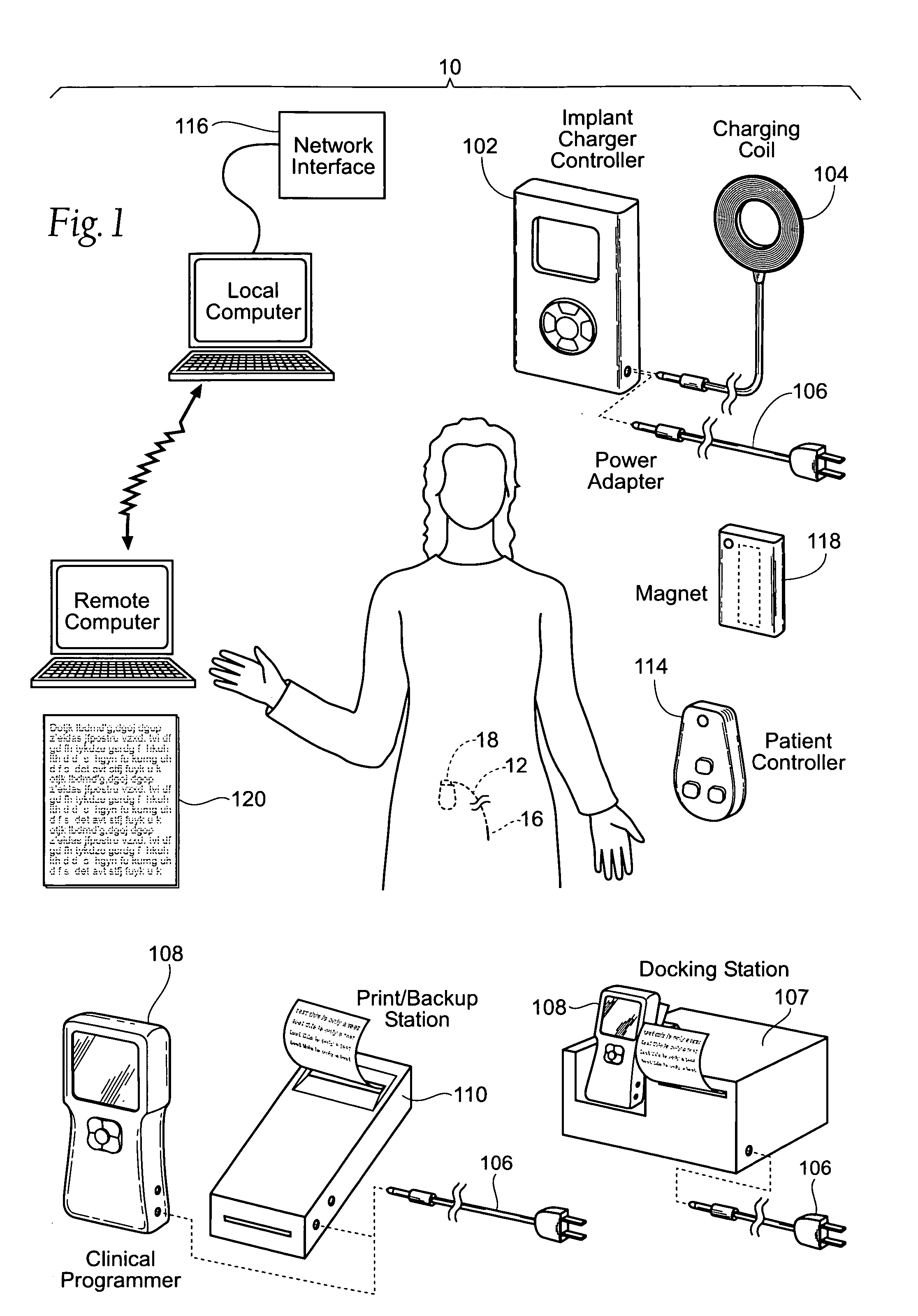
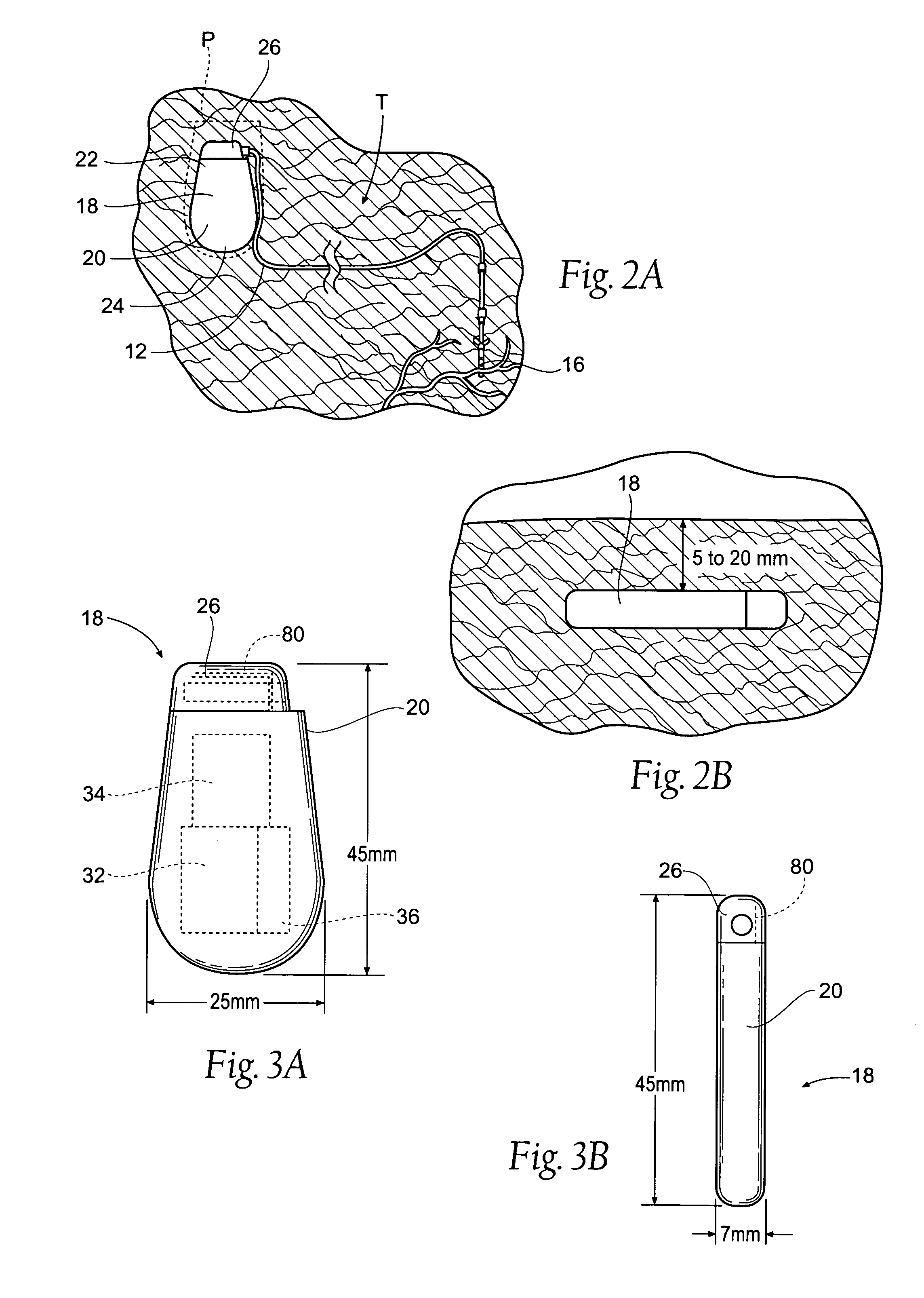
Implantable pulse generator systems and methods for providing functional and/or therapeutic stimulation of muscles and/or nerves and/or central nervous system tissue
ActiveUS20070060980A1Spinal electrodesImplantable neurostimulatorsElectrical batteryRechargeable cell
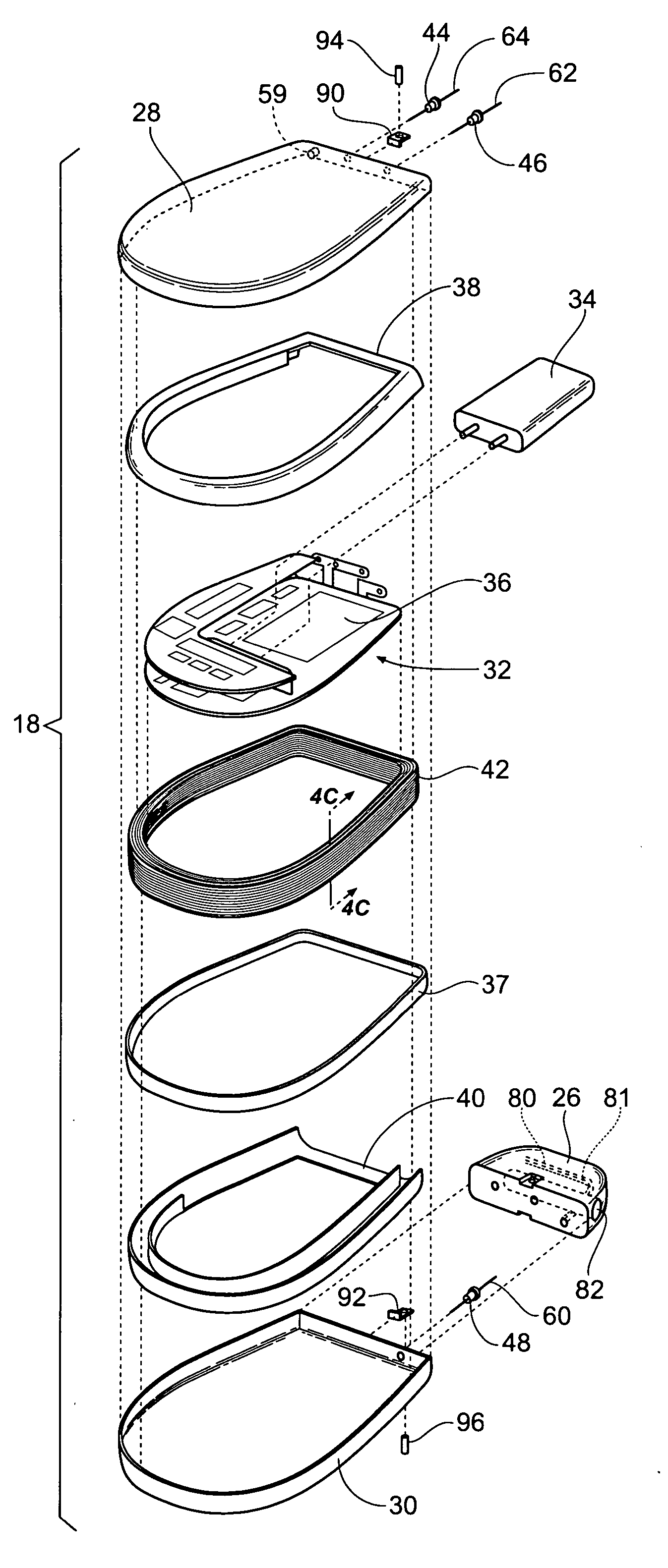
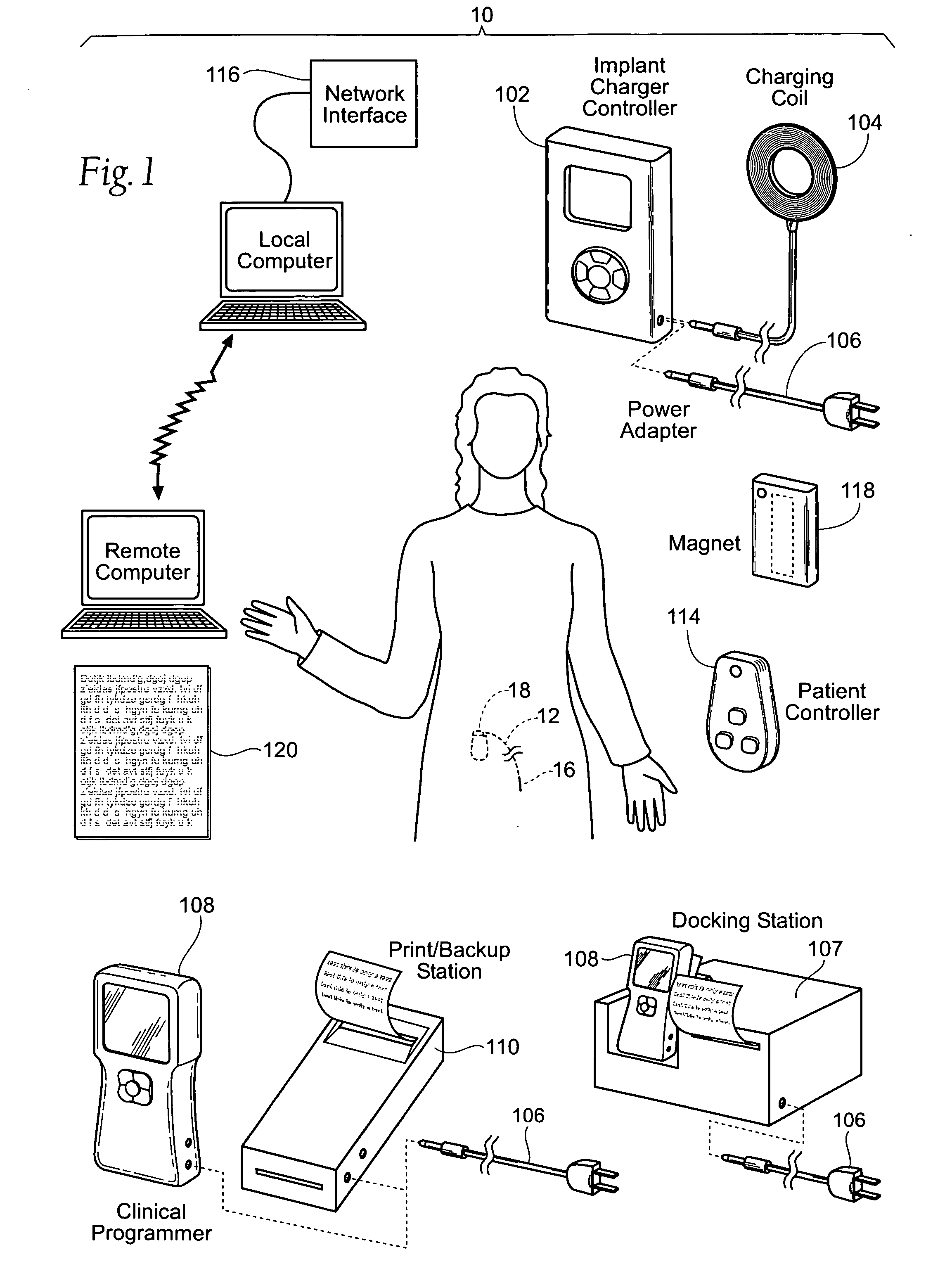
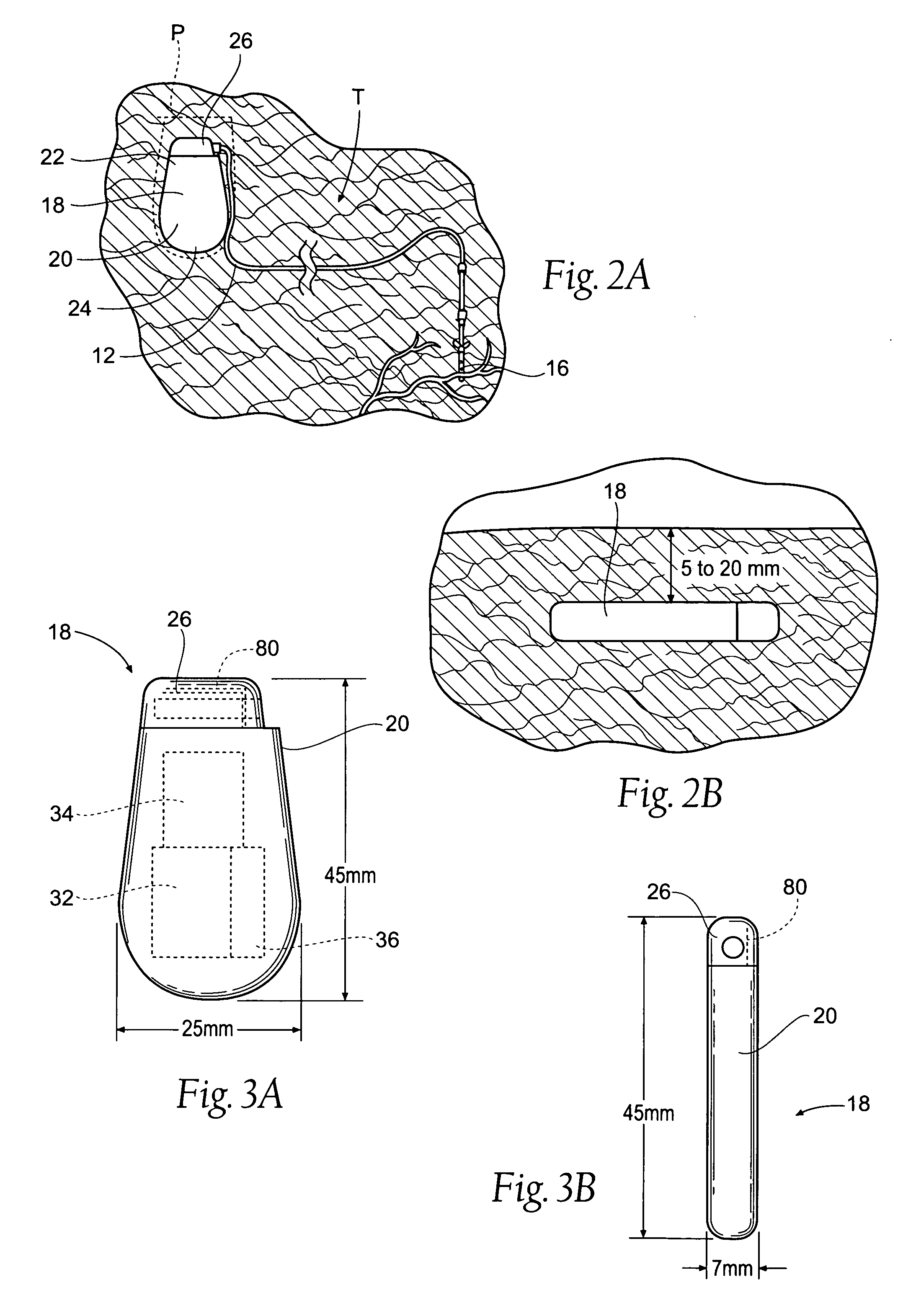
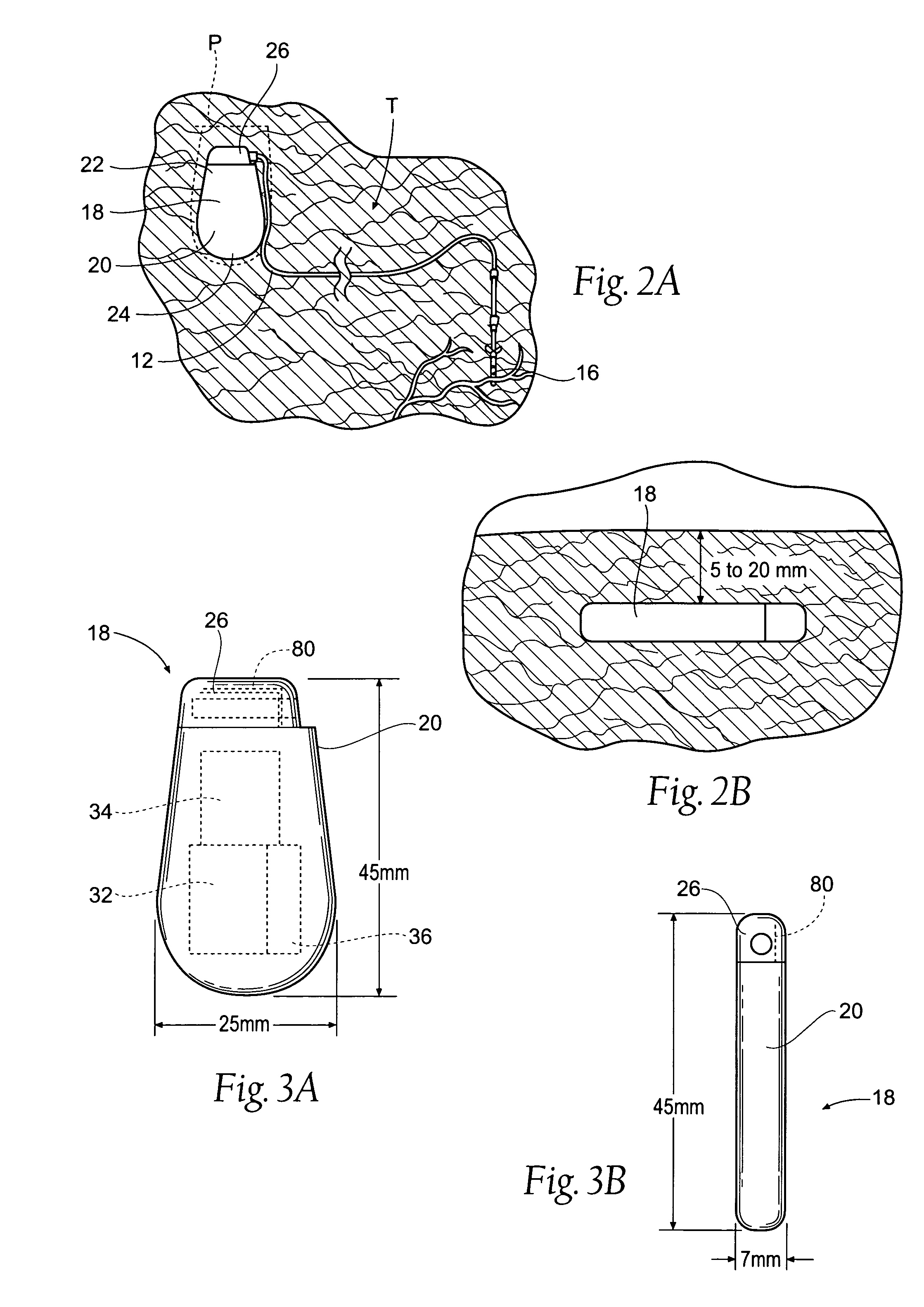
Improved assemblies, systems, and methods provide a stimulation system for prosthetic or therapeutic stimulation of muscles, nerves, or central nervous system tissue, or any combination. The stimulation system includes an implantable pulse generator and a lead sized and configured to be implanted subcutaneously in a tissue region. An external controller includes circuitry adapted for wireless telemetry and a charging coil for generating the radio frequency magnetic field to transcutaneously recharge a rechargeable battery in the pulse generator. Using wireless telemetry, the pulse generator is adapted to transmit status information back to the external controller to allow the external controller to automatically adjust up or down the magnitude of the radio frequency magnetic field and / or to instruct a user to reposition the charging coil, the status information adapted to allow optimal recharging of the pulse generator rechargeable battery.
Owner:MEDTRONIC URINARY SOLUTIONS
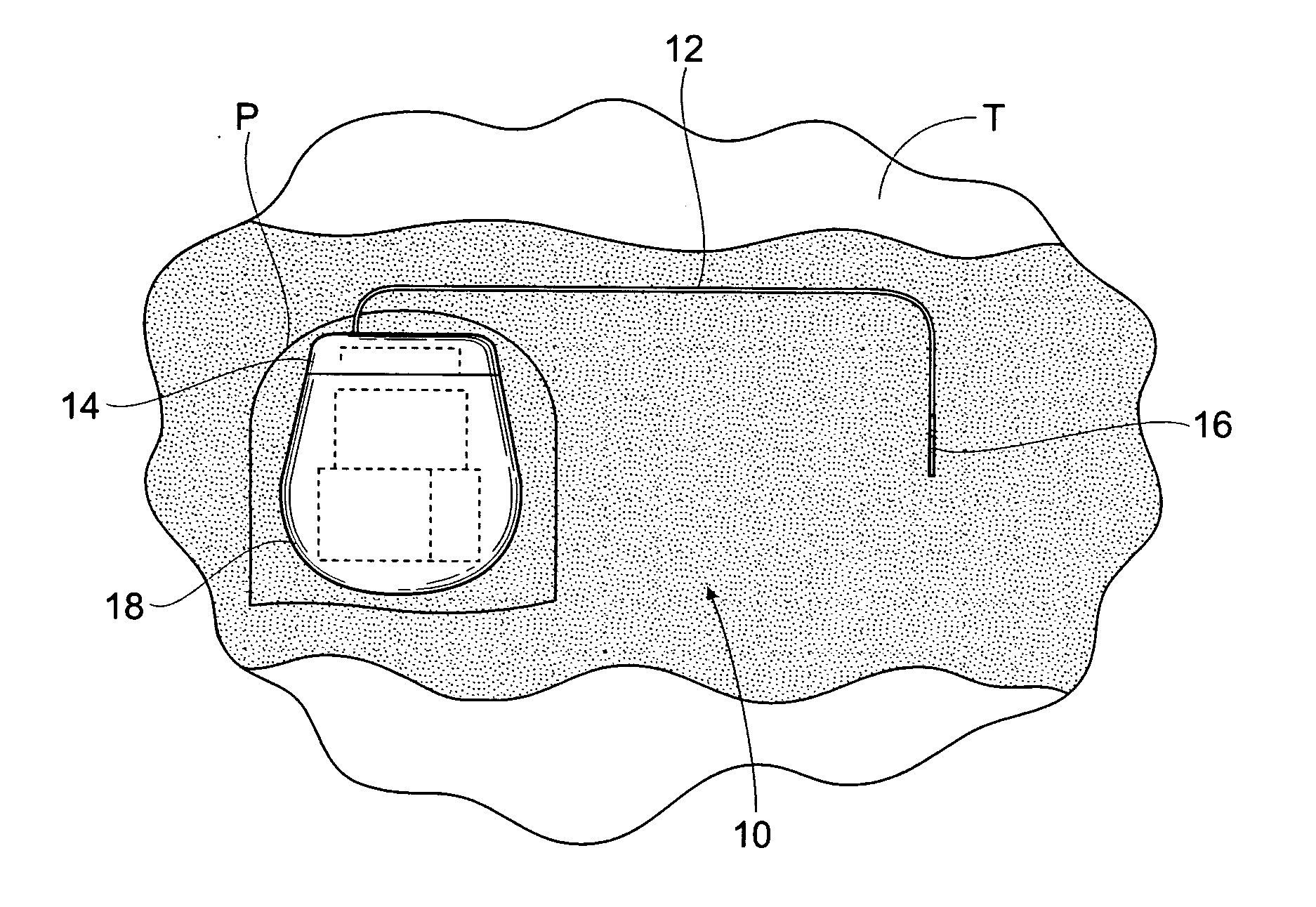
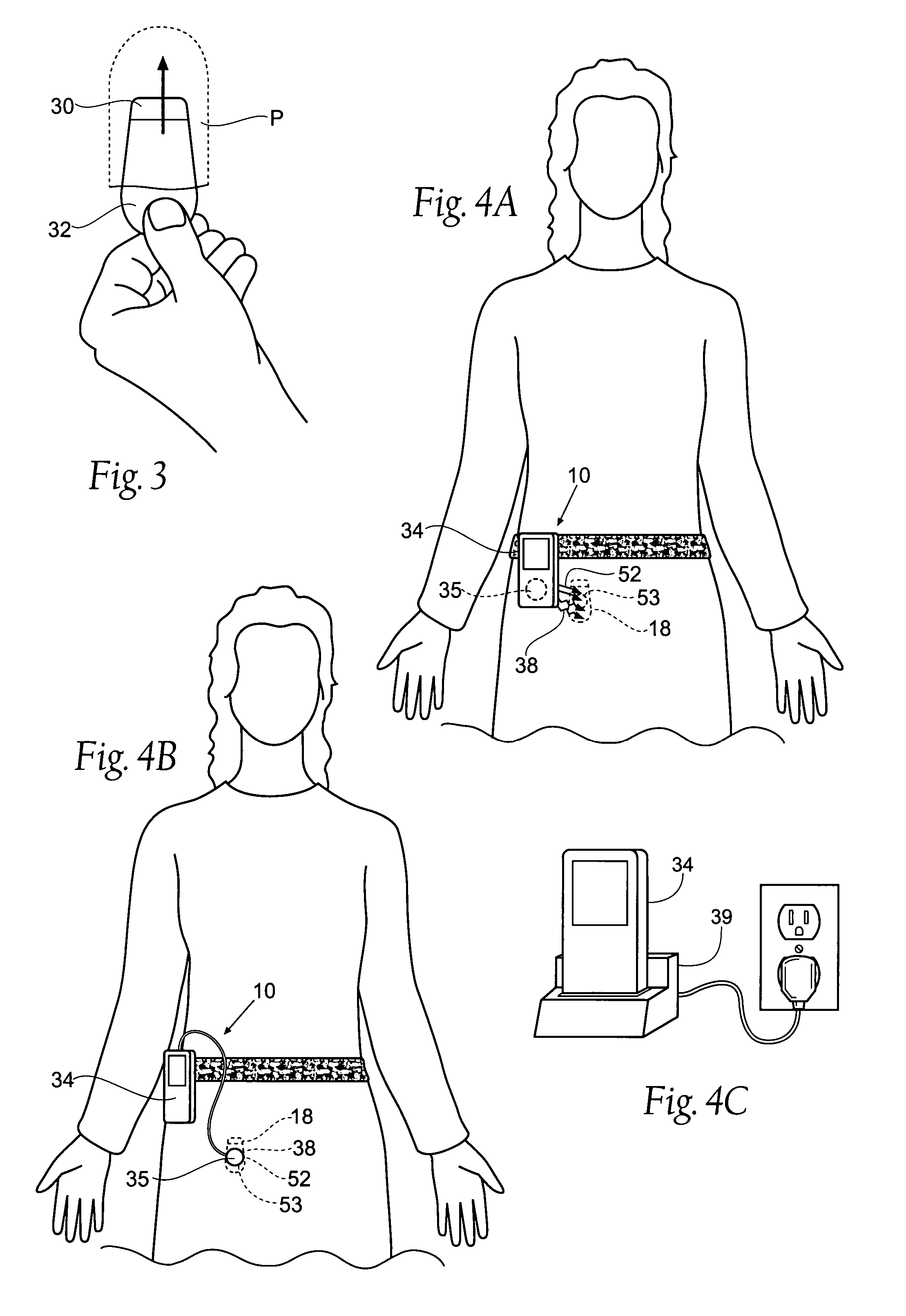
Implantable pulse generator for providing functional and/or therapeutic stimulation of muscles and/or nerves and/or central nervous system tissue
ActiveUS20050278000A1Efficient chargingImprove the quality of lifeElectrotherapyElectromyographyMicrocontrollerPrimary cell
Improved assemblies, systems, and methods provide an implantable pulse generator for prosthetic or therapeutic stimulation of muscles, nerves, or central nervous system tissue, or any combination. The implantable pulse generator is sized and configured to be implanted subcutaneously in a tissue region. The implantable pulse generator includes an electrically conductive laser welded titanium case. Control circuitry is located within the case, and includes a primary cell or rechargeable power source, a receive coil for receiving an RF magnetic field to recharge the rechargeable power source, and a microcontroller for control of the implantable pulse generator. Improved assemblies, systems, and methods also provide a stimulation system for prosthetic or therapeutic stimulation of muscles, nerves, or central nervous system tissue, or any combination. The stimulation system provides at least one electrically conductive surface, a lead connected to the electrically conductive surface, and an implantable pulse generator electrically connected to the lead.
Owner:MEDTRONIC URINARY SOLUTIONS
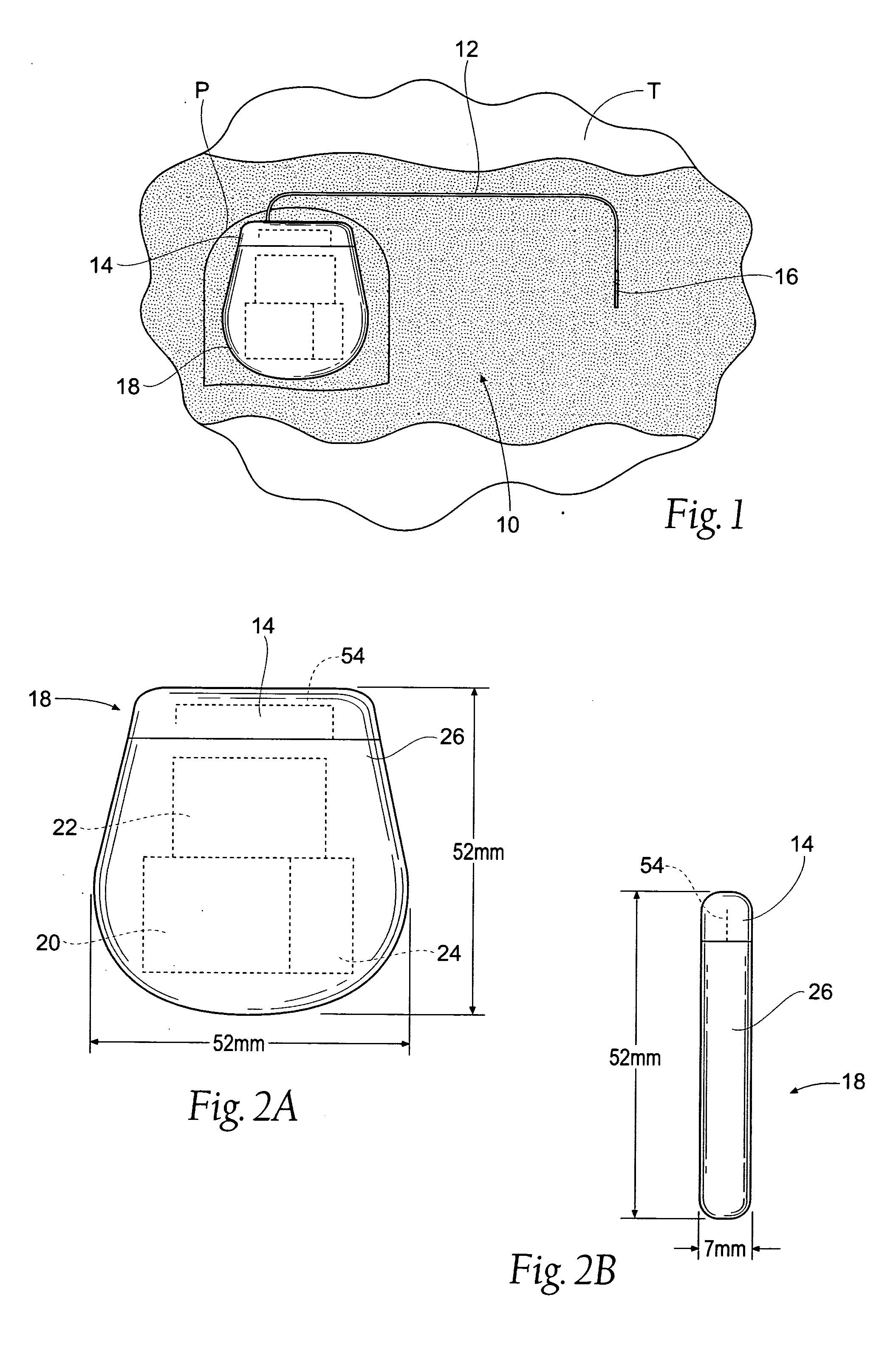
Implantable pulse generator systems and methods for providing functional and/or therapeutic stimulation of muscles and/or nerves and/or central nervous system tissue
Improved assemblies, systems, and methods provide a stimulation system for prosthetic or therapeutic stimulation of muscles, nerves, or central nervous system tissue, or any combination. The stimulation system includes a hermetically sealed implantable pulse generator and methods for assembly and programming.
Owner:MEDTRONIC URINARY SOLUTIONS
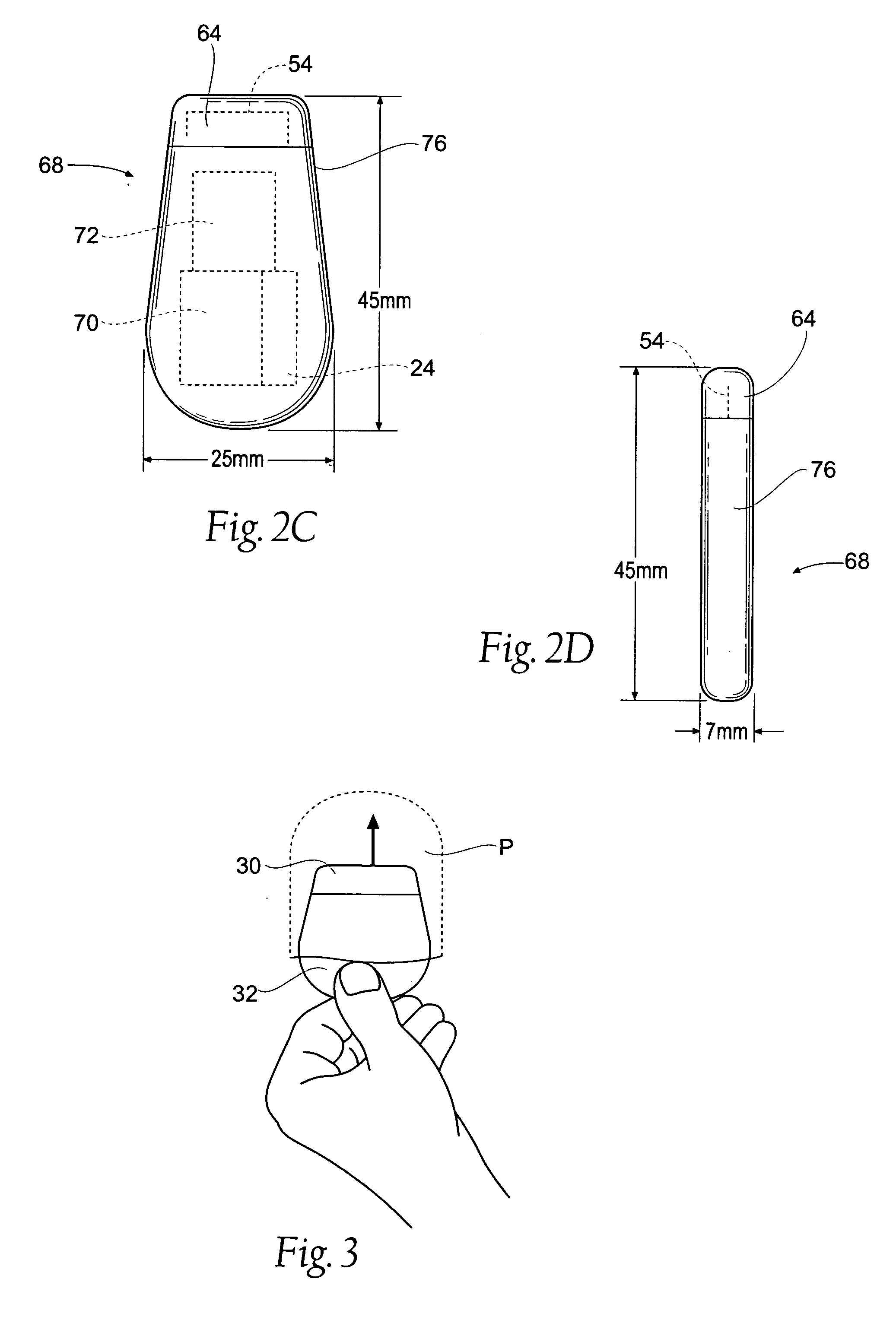
Implantable pulse generator systems and methods for providing functional and /or therapeutic stimulation of muscles and/or nerves and/or central nervous system tissue
Improved assemblies, systems, and methods provide a stimulation system for prosthetic or therapeutic stimulation of muscles, nerves, or central nervous system tissue, or any combination. The stimulation systems and methods include an implantable pulse generator adapted for wireless telemetry and having a unique signature to enable secure communications. An external controller includes circuitry adapted for wireless telemetry. The secure communications includes at least a first communication from the external controller to the pulse generator. The first communication includes the pulse generator's unique signature. A response communication from the pulse generator to the external controller includes data elements that indicate the response communication is a response, and not a command from an external controller.
Owner:MEDTRONIC URINARY SOLUTIONS
Implantable pulse generator systems and methods for providing functional and/or therapeutic stimulation of muscles and/or nerves and/or central nervous system tissue
InactiveUS20070060955A1Spinal electrodesImplantable neurostimulatorsMicrocontrollerElectrical battery
Improved assemblies, systems, and methods provide a stimulation system for prosthetic or therapeutic stimulation of muscles, nerves, or central nervous system tissue, or any combination. The stimulation system may include both external and internal components, with an implantable pulse generator sized and configured to be implanted subcutaneously in a tissue region. The implantable pulse generator includes a welded titanium case. Circuitry and hardware adapted for wireless telemetry is located within the case, and includes a primary cell or rechargeable power source, a microcontroller for control of the implantable pulse generator, and a power receiving coil for receiving an RF magnetic field to recharge the rechargeable power source, the power receiving coil having a maximum outside dimension.
Owner:MEDTRONIC URINARY SOLUTIONS
Implantable pulse generator for providing functional and/or therapeutic stimulation of muscles and/or nerves and/or central nervous system tissue
ActiveUS20070293910A1Efficient chargingImprove the quality of lifeElectrotherapyDiagnosticsElectricityMicrocontroller
Improved assemblies, systems, and methods provide an implantable pulse generator for prosthetic or therapeutic stimulation of muscles, nerves, or central nervous system tissue, or any combination. The implantable pulse generator is sized and configured to be implanted subcutaneous a tissue region. The implantable pulse generator includes an electrically conductive case of a laser welded titanium material. Control circuitry is located within the case, the control circuitry including a rechargeable power source, a receive coil for receiving an RF magnetic field to recharge the power source, and a microcontroller for control of the implantable pulse generator. Improved assemblies, systems, and methods also provide a stimulation system for prosthetic or therapeutic stimulation of muscles, nerves, or central nervous system tissue, or any combination. The stimulation system provides at least one electrically conductive surface, a lead connected to the electrically conductive surface, and an implantable pulse generator electrically connected to the lead.
Owner:MEDTRONIC URINARY SOLUTIONS
Implantable pulse generator systems and methods for providing functional and/or therapeutic stimulation of muscles and/or nerves and/or central nervous system tissue
ActiveUS20070060968A1Low rateSpinal electrodesImplantable neurostimulatorsElectricityElectrical battery
Improved assemblies, systems, and methods provide a stimulation system for prosthetic or therapeutic stimulation of muscles, nerves, or central nervous system tissue, or any combination. The stimulation system includes a pulse generator including a housing sized and configured for implantation in subcutaneous tissue, circuitry carried within the housing, the circuitry operable for generating electrical stimulation pulses, and a rechargeable battery coupled to the circuitry and carried within the housing, the rechargeable battery including a battery capacity. The circuitry is adapted to suspend the generation of electrical stimulation pulses at a first remaining battery capacity, and the circuitry is adapted to enter a dormant mode at a second remaining battery capacity. The first battery remaining capacity may be greater than or equal to the second remaining battery capacity. At the second remaining battery capacity, only a safety margin battery capacity remains.
Owner:MEDTRONIC URINARY SOLUTIONS
Implantable pulse generator for providing functional and/or therapeutic stimulation of muscles and/or nerves and/or central nervous system tissue
InactiveUS20070299483A1Efficient chargingImprove the quality of lifeInternal electrodesExternal electrodesMicrocontrollerElectricity
Improved assemblies, systems, and methods provide an implantable pulse generator for prosthetic or therapeutic stimulation of muscles, nerves, or central nervous system tissue, or any combination. The implantable pulse generator is sized and configured to be implanted subcutaneous a tissue region. The implantable pulse generator includes an electrically conductive case of a laser welded titanium material. Control circuitry is located within the case, the control circuitry including a rechargeable power source, a receive coil for receiving an RF magnetic field to recharge the power source, and a microcontroller for control of the implantable pulse generator. Improved assemblies, systems, and methods also provide a stimulation system for prosthetic or therapeutic stimulation of muscles, nerves, or central nervous system tissue, or any combination. The stimulation system provides at least one electrically conductive surface, a lead connected to the electrically conductive surface, and an implantable pulse generator electrically connected to the lead.
Owner:MEDTRONIC URINARY SOLUTIONS
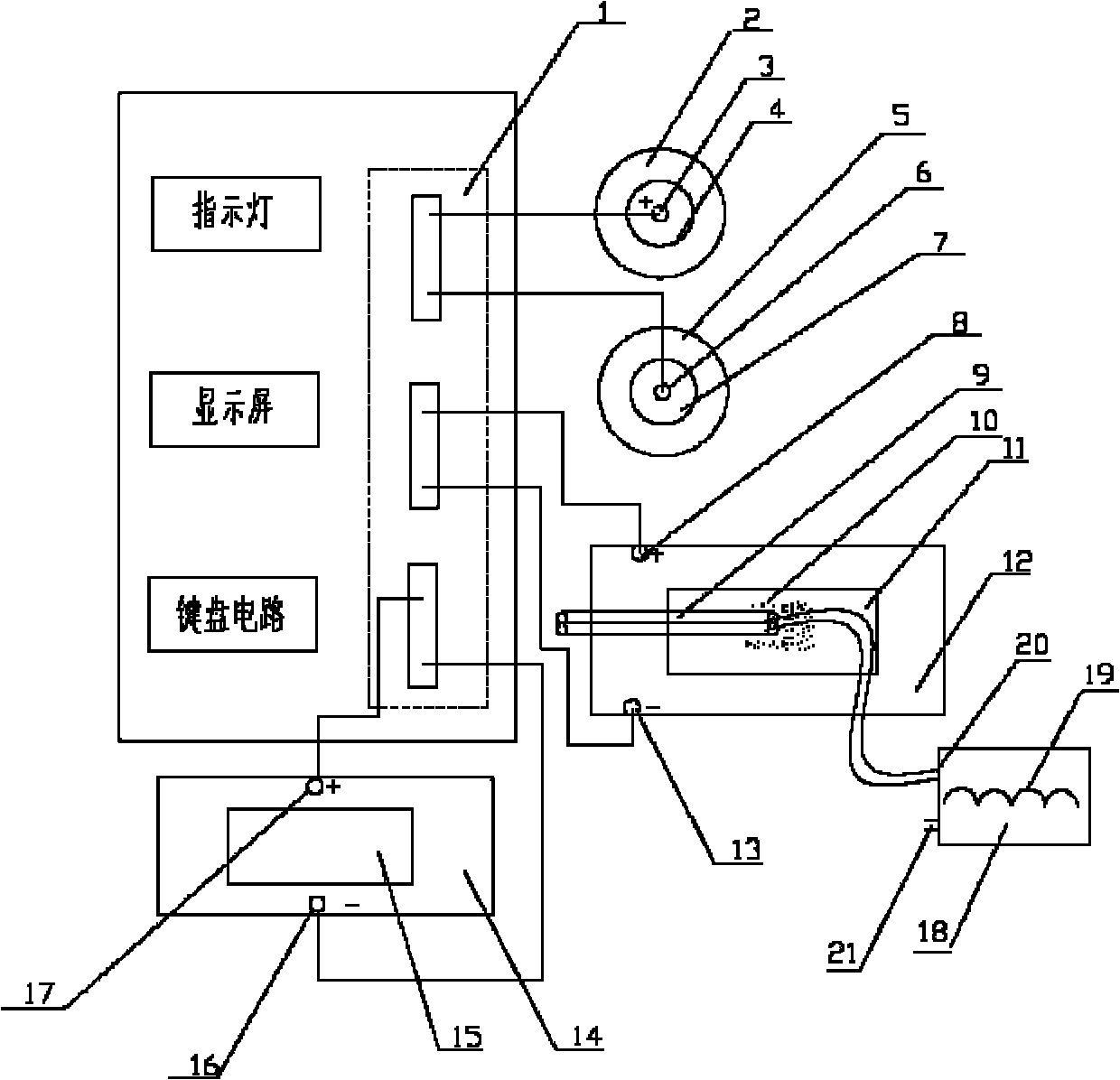
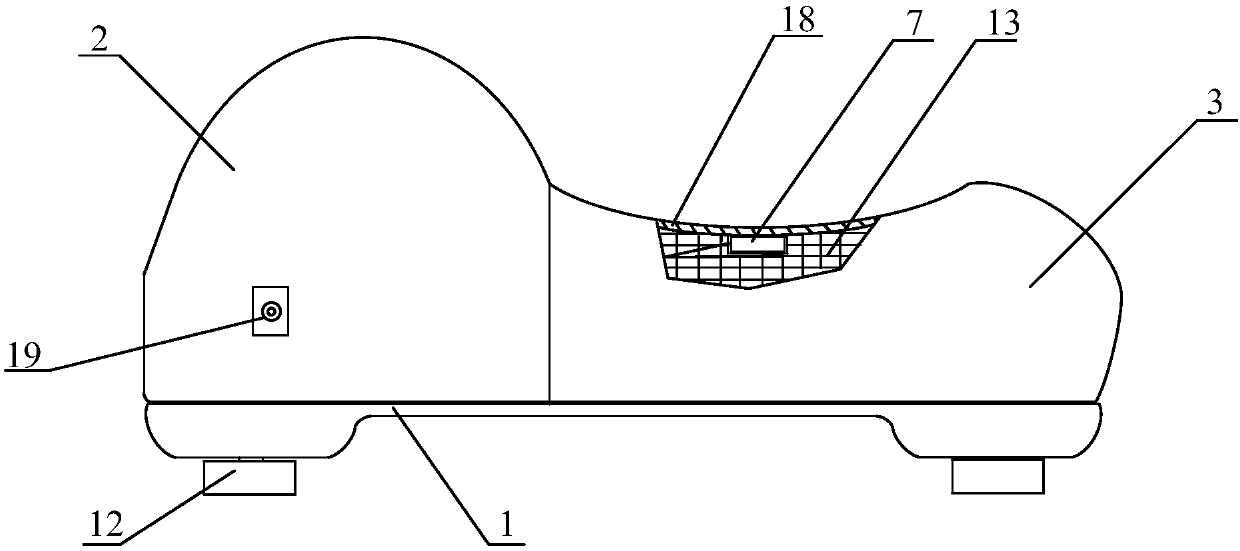
Wound pain quick recovery application instrument
ActiveCN101991484AGood paste performanceImprove conductivityElectrotherapyHydroxy compound active ingredientsMuscle nerveScar tissue
The invention discloses a wound pain quick recovery application instrument, which comprises a body, a controller, a keyboard circuit, an indicator light, a display screen, and three current and voltage regulating circuits, wherein the controller is respectively connected with the keyboard circuit, the display screen, the three current and voltage regulating circuits, a timing circuit and the indicator light; and the output ends of the three current and voltage regulating circuits are respectively connected with three groups of positive and negative electrodes which are respectively connected to three groups of application metal sheets. The wound pain quick recovery application instrument has a reasonable structure, is convenient to use, can form an electric field on the wound surface, accelerates the quick healing of the wound surface, reduces scar tissues, alleviates pain, prevents fat liquoring and postoperative pulmonary embolism, and accelerates the recovery of myoneural junctions.
Owner:龙丹
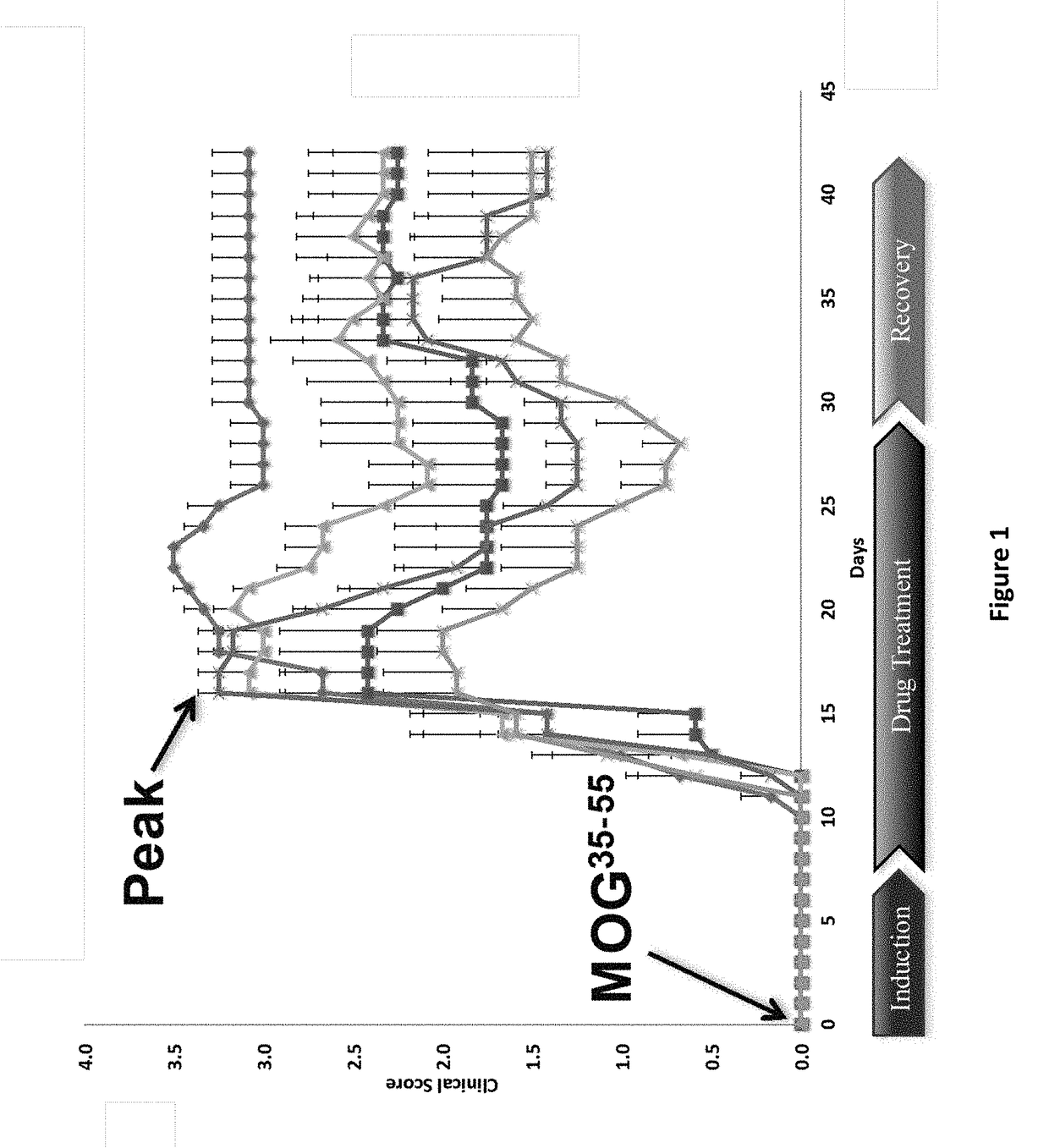
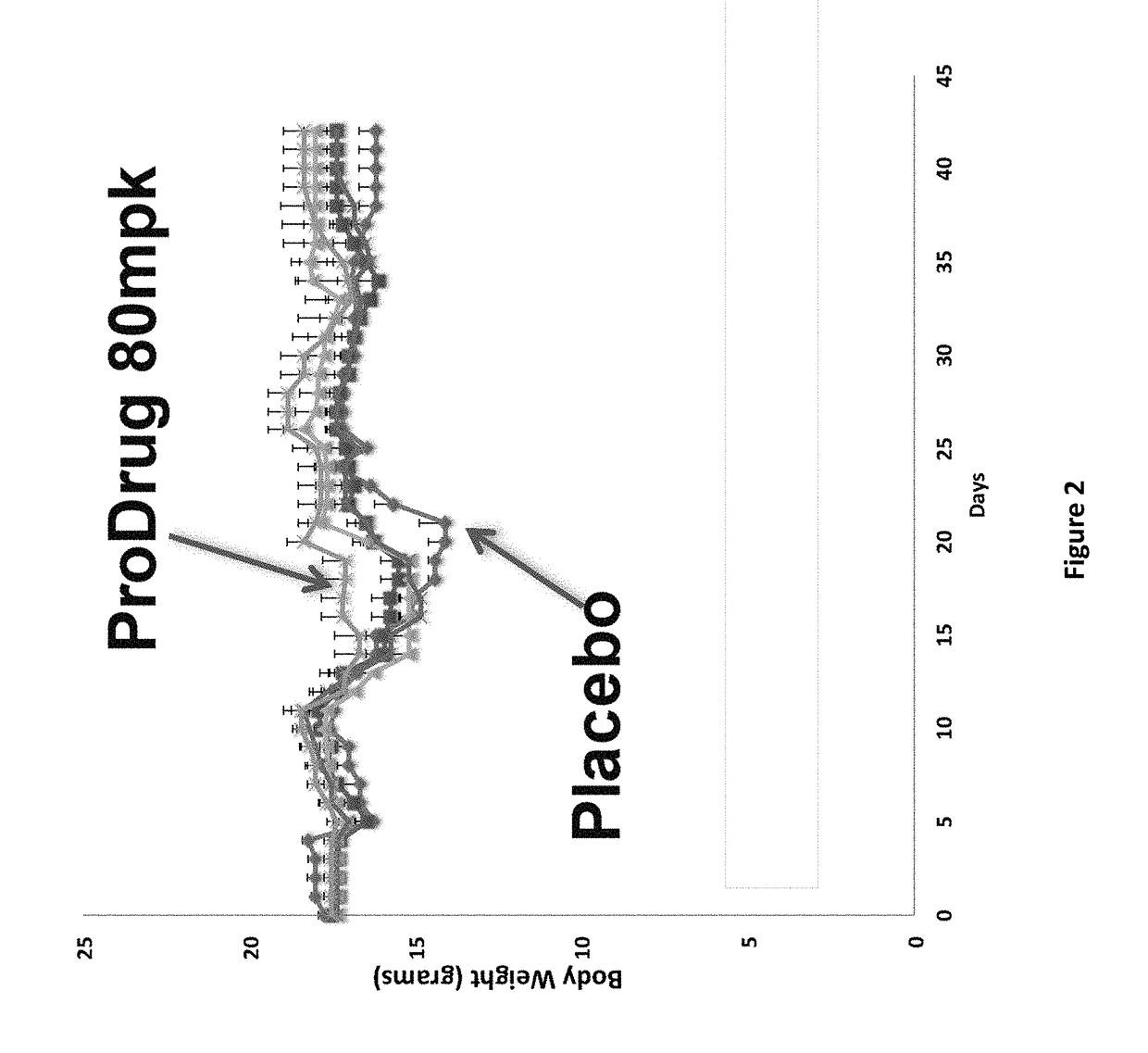
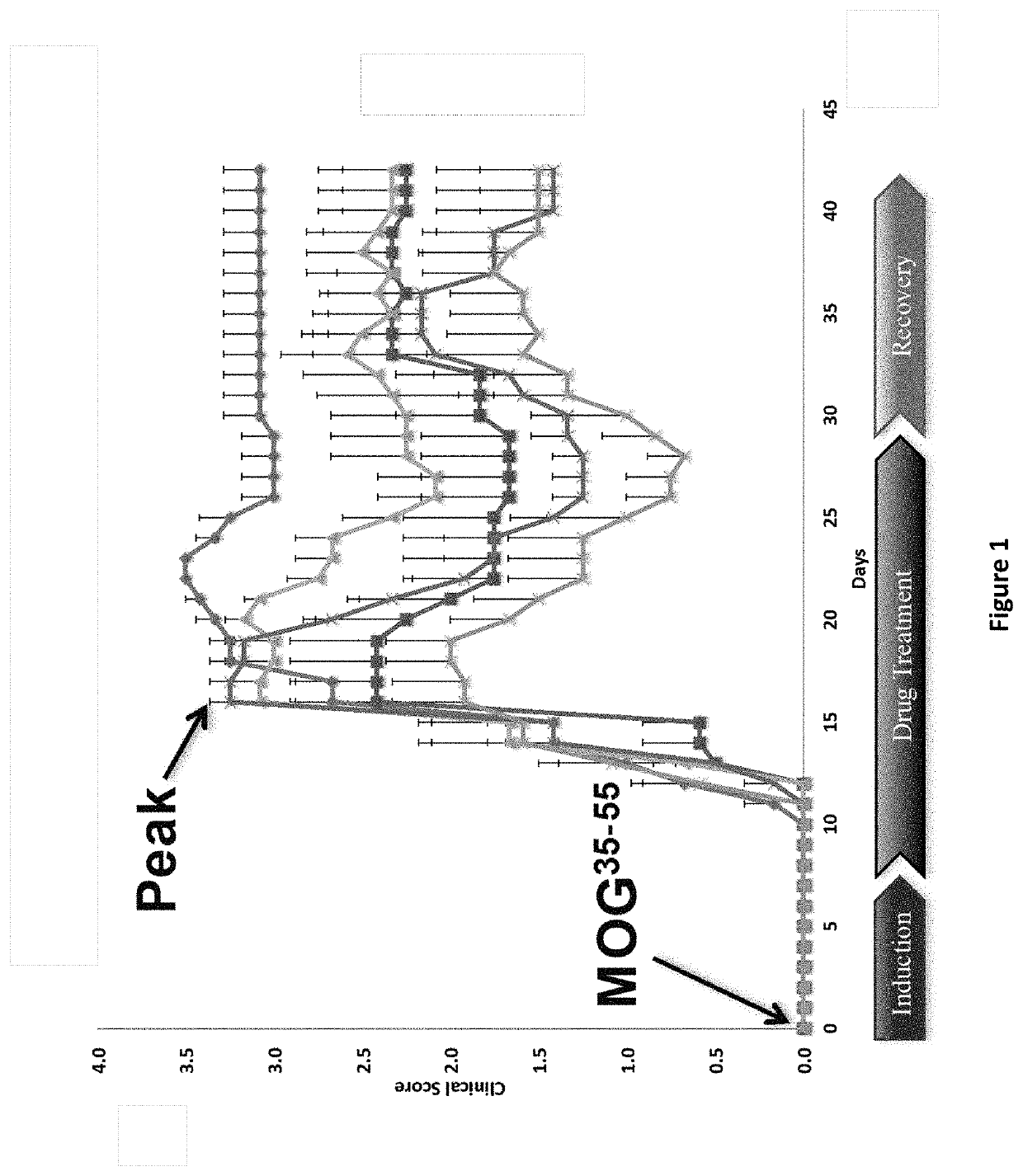
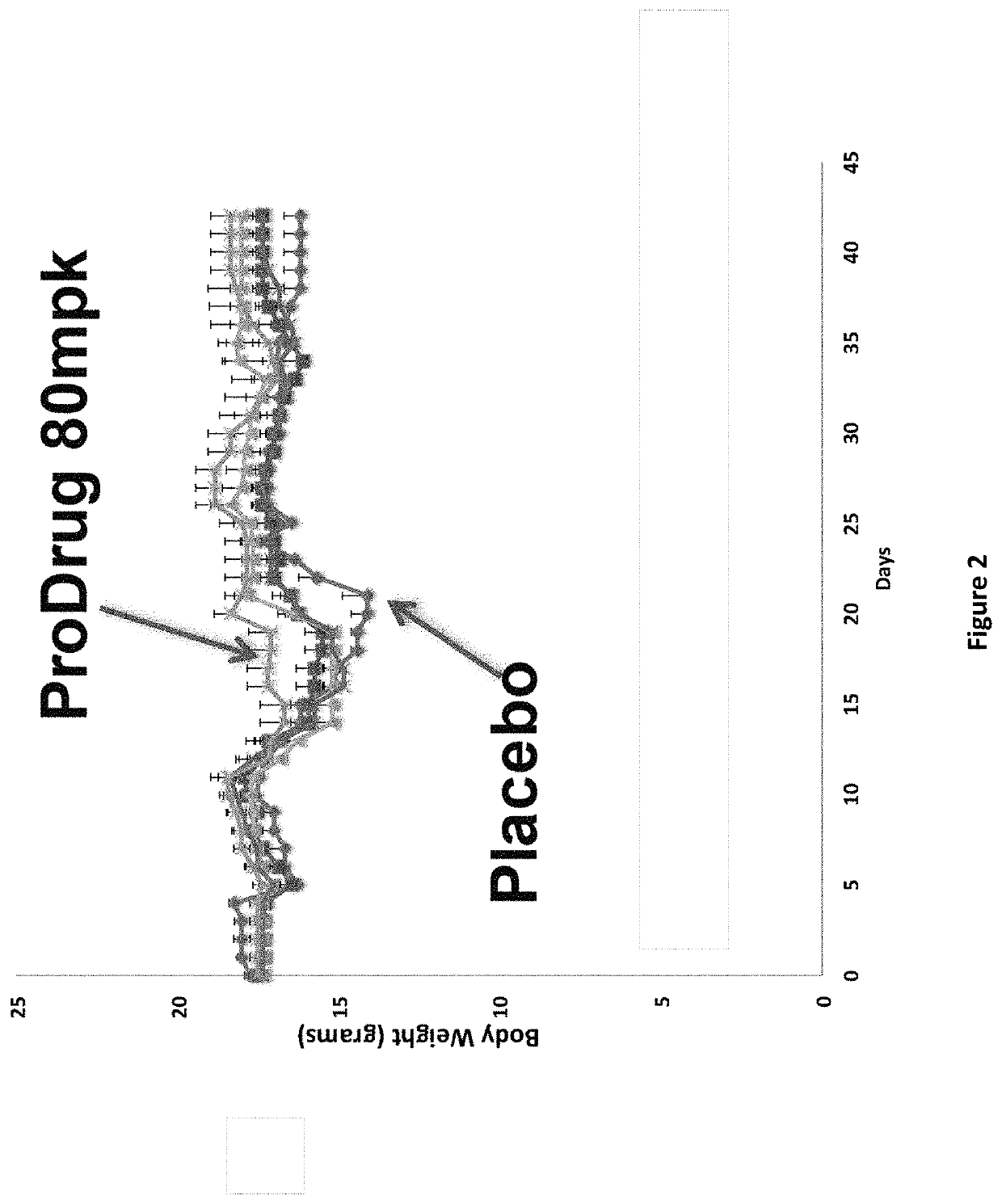
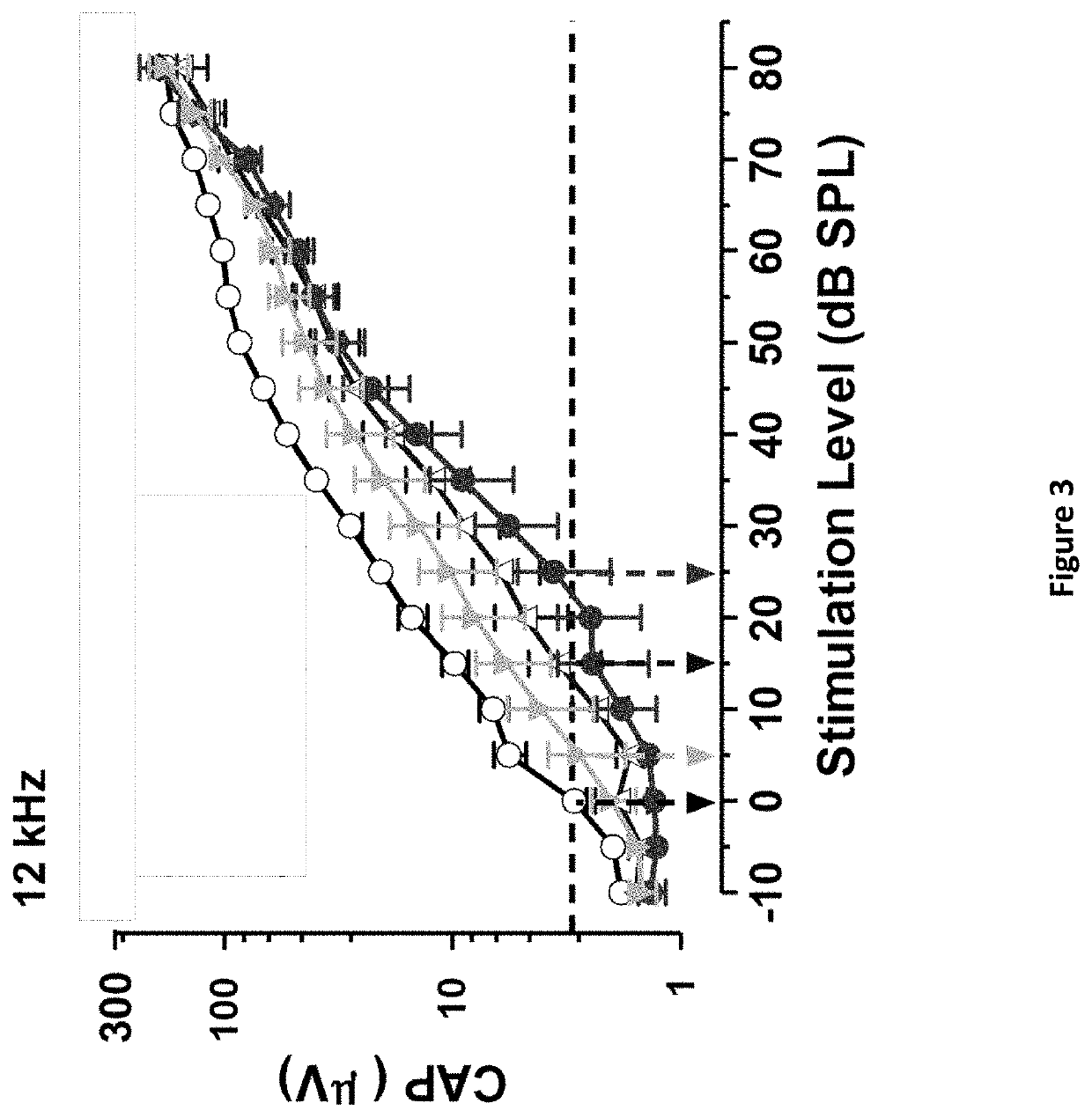
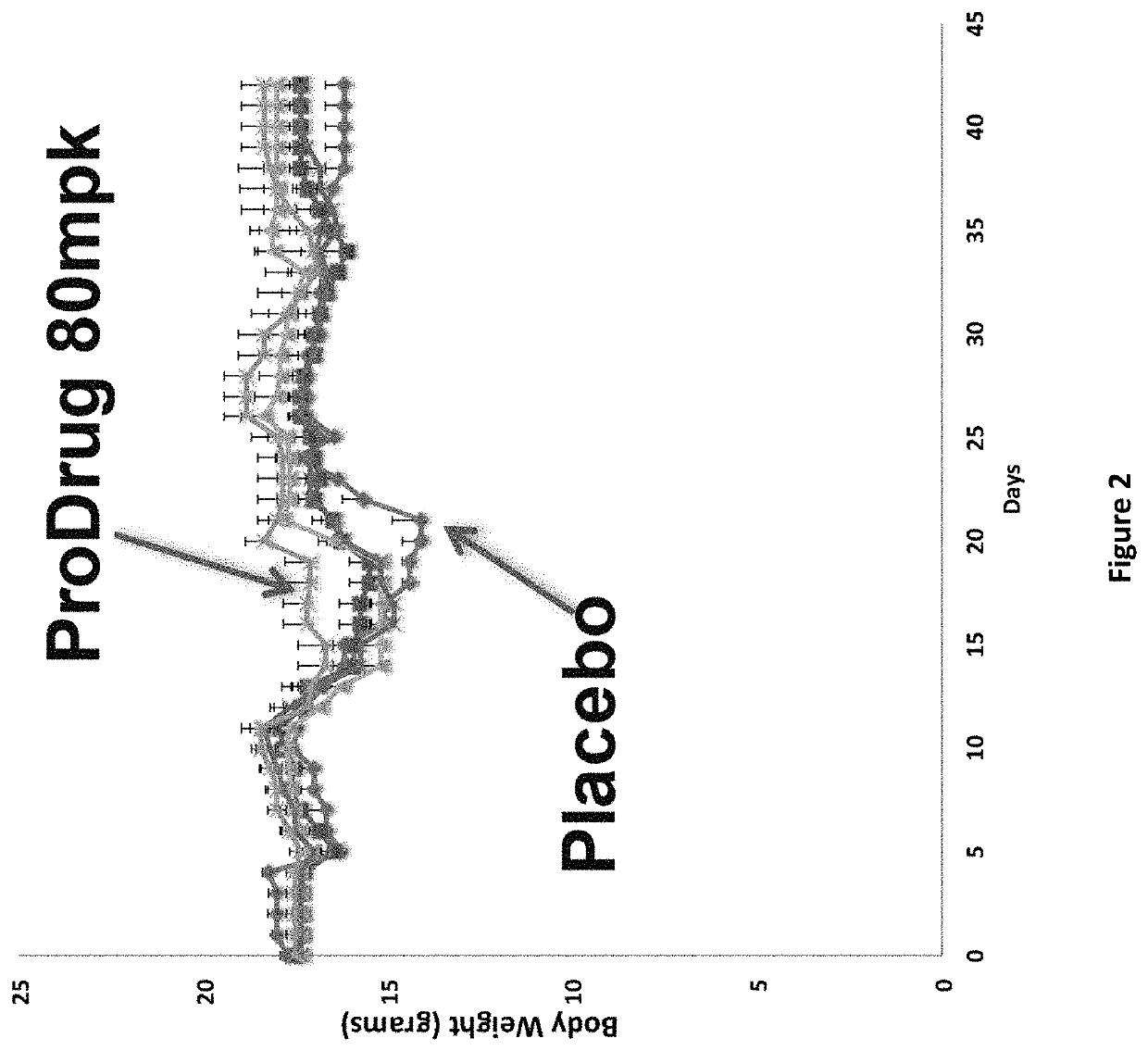
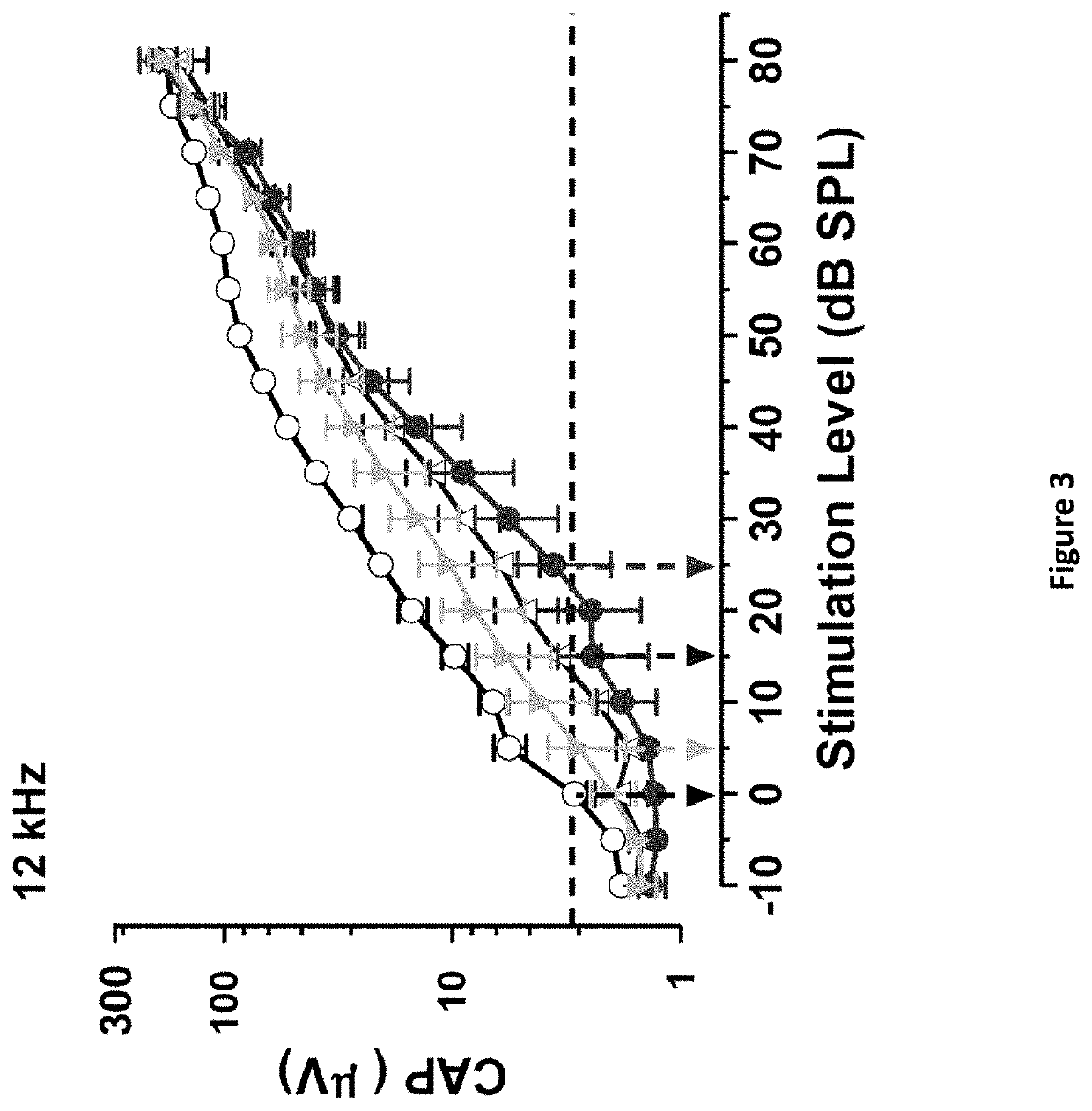
DNP and DNP Prodrug Treatment of Neuromuscular, Neurodegenerative, Autoimmune, Developmental, Traumatic Brain Injury, Concussion, Dry Eye Disease, Hearing Loss and/or Metabolic Diseases
ActiveUS20170252347A1Good blood pressureImprove blood sugar levelsSenses disorderNervous disorderHL - Hearing lossDepressant
A composition and method of treatment of neuromuscular, neuromuscular degenerative, neurodegenerative, autoimmune, developmental, traumatic, hearing loss related, and / or metabolic diseases, including spinal muscular atrophy (SMA) syndrome (SMA1, SMA2, SMA3, and SMA4, also called Type I, II, III and IV), traumatic brain injury (TBI), concussion, keratoconjunctivitis sicca (Dry Eye Disease), glaucoma, Sjogren's syndrome, rheumatoid arthritis, post-LASIK surgery, anti-depressants use, Wolfram Syndrome, and Wolcott-Rallison syndrome. The composition is selected from the group consisting of 2,3-DNP, 2,4-DNP, 2,5-DNP, 2,6-DNP, 3,4-DNP, or 3,5-DNP, bipartite 2,3-dinitrophenol, 2,4-dinitrophenol, 2,5-dinitrophenol, 2,6-dinitrophenol, 3,4-dinitrophenol, or 3,5-dinitrophenol (2,3-DNP, 2,4-DNP, 2,5-DNP, 2,6-DNP, 3,4-DNP, or 3,5-DNP) prodrugs; Gemini prodrugs, bioprecursor molecules, and combinations thereof. A dose of the composition for treatment of neurodegenerative diseases may be from about 0.01 mg / kg of body weight to about 50 mg / kg of body weight of the patient in need of treatment. A dose of the composition for treatment of metabolic diseases may be from about 1 mg / 70 kg of body weight to about 100 mg / 70 kg of body weight of the patient in need of treatment, and a maximum dose per day is about 200 mg / 70 kg of body weight of the patient in need of treatment.
Owner:MITOCHON PHARMA INC +1
Medical painless chest drainage pipe
InactiveCN105031800AReduce local painReduce painBalloon catheterMedical devicesMuscle nerveThoracic cavity
The invention discloses a medical painless chest drainage pipe. The medical painless chest drainage pipe comprises a drainage pipe body, a membranous anther sac, an infusion tube and a connector thereof and a marker line, wherein the drainage pipe body is sleeved with the membranous anther sac of a special structure, and the infusion tube and the connector thereof are connected with the anther sac. In the practical application process of the drainage pipe, the anther sac structure is left in a muscle nerve tissue area under human skin, meanwhile, local anesthetic is injected to the anther sac through the infusion tube with a microsyringe, by means of the anther sac of the special structure, the local anesthetic is overflowed into soft tissue under skin, nerve endings are narcotized, and the effect of local analgesia is achieved. After a cardiac and thoracic surgery or an abdominal surgery is conducted, by means of the cooperation of the painless chest drainage pipe, the muscle nerve tissue of the drainage area is subjected to local anaesthesia, and the local pain on account of the drainage pipe of a patient can be reduced.
Owner:JIANGSU PROVINCE HOSPITAL
Covalent Small Molecule DCN1 Inhibitors and Therapeutic Methods Using the Same
ActiveUS20180289677A1Inhibit activityAvoid interactionOrganic active ingredientsOrganic chemistryDiseaseNerve degeneration
Small molecule covalent inhibitors of DCN1 and compositions containing the same are disclosed. Methods of using the DCN1 covalent inhibitors in the treatment of diseases and conditions wherein inhibition of DCN1 provides a benefit, like oxidative stress-related diseases and conditions, neurodegenerative diseases and conditions, metabolic disorders, and muscular nerve degeneration, also are disclosed.
Owner:THE RGT OF THE UNIV OF MICHIGAN
Catheter with Seal Layer
A catheter assembly for locating an object of interest (e.g., an anatomical region such as a muscle, nerve bundle, etc.) is provided. The catheter assembly includes a catheter having a catheter body defining a proximal end and a distal end; a transducer located at the distal end of the catheter; one or more transducer wires extending from a proximal end of the transducer towards the proximal end of the catheter; and a length of tubing surrounding the catheter, the transducer, and the one or more transducer wires, wherein the length of tubing has a proximal portion and a distal portion, wherein the proximal portion surrounds the one or more transducer wires and the distal portion surrounds the transducer, wherein the proximal portion includes a conductive filler.
Owner:AVENT INC
Combined correcting and repairing device used after scoliosis operation
InactiveCN111529158AImprove the phenomenon of postoperative nerve damagePromote postoperative repairElectrotherapyMedical devicesSpinal columnPhysical medicine and rehabilitation
The invention discloses a combined correcting and repairing device used after a scoliosis operation. The device comprises a correction unit, a physiotherapy unit and a micro-control unit, the correction unit comprises a correction belt composed of shoulder belts, back belts and a waist belt and a correction instrument which is detachably connected to the correction belt, and the correction instrument comprises a supporting plate, a soft wrapping layer, a telescopic sleeve frame and a miniature electric push rod. According to the invention, an extrusion block matched with spine joint is arranged on the soft wrapping layer, each extrusion block is provided with the miniature electric push rod as a rear support, and precise strength support can be provided for a patient under detection feedback of a flexible pressure sensor. A physiotherapy patch is further provided, not only can be used as a dressing, but also can perform pulse activation on muscle nerves around the back of the patient through a motor column, and can effectively improve the phenomenon that nerves of the patient are damaged after an operation. In conclusion, the correcting and repairing device is convenient to use andgood in supporting and correcting effect, and well helps to perform postoperative nerve repair.
Owner:WEIFANG UNIV OF SCI & TECH
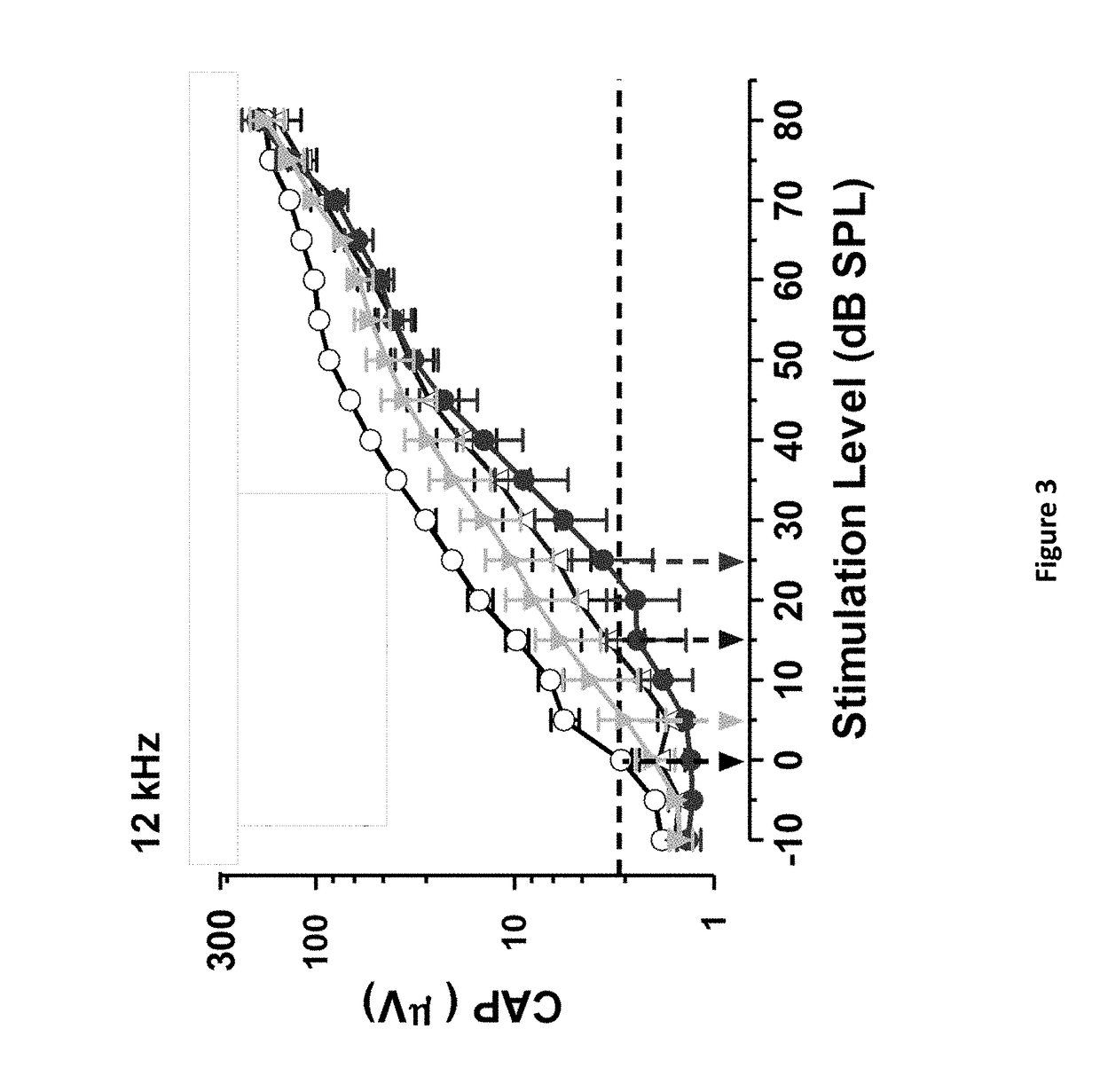
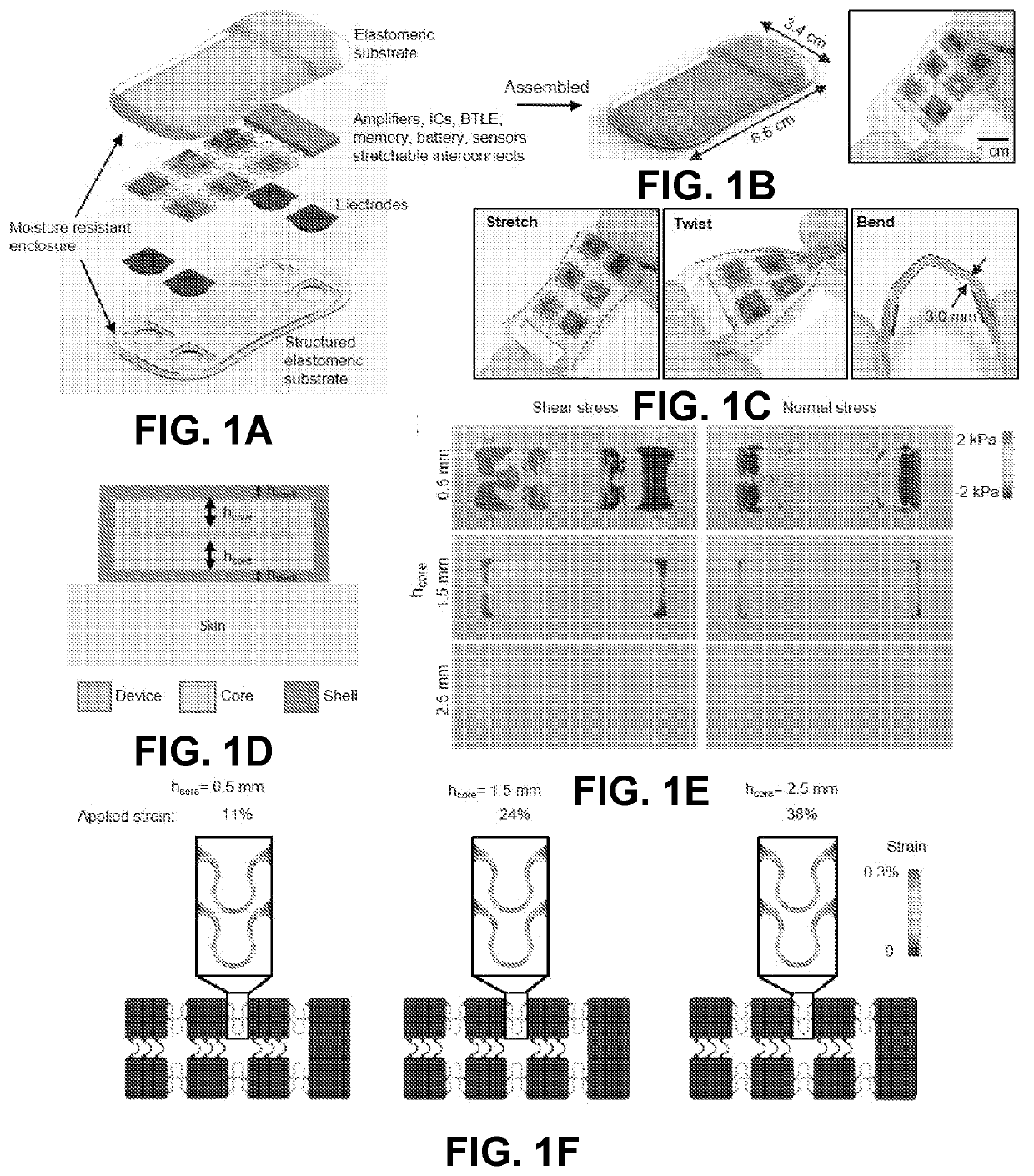
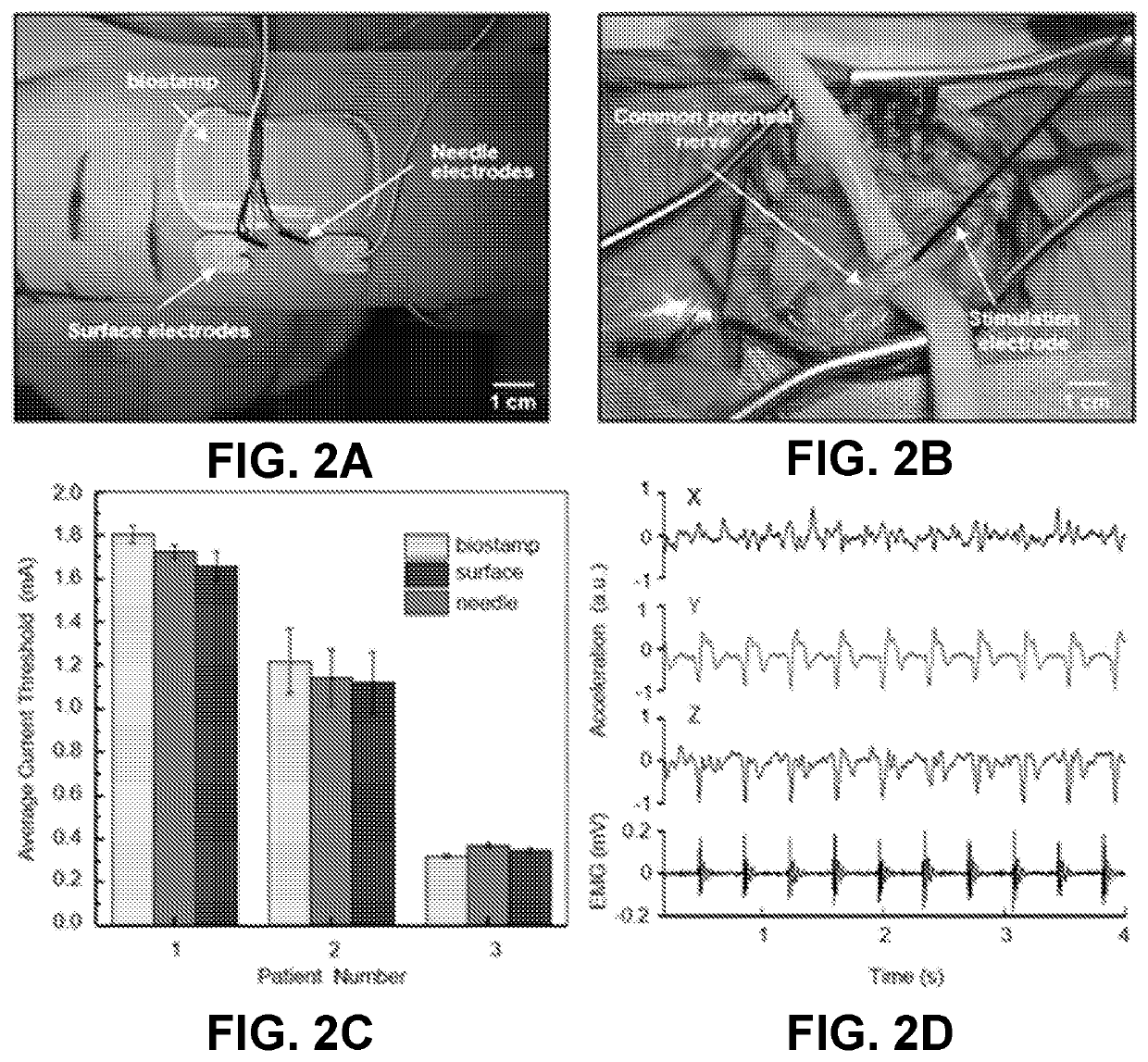
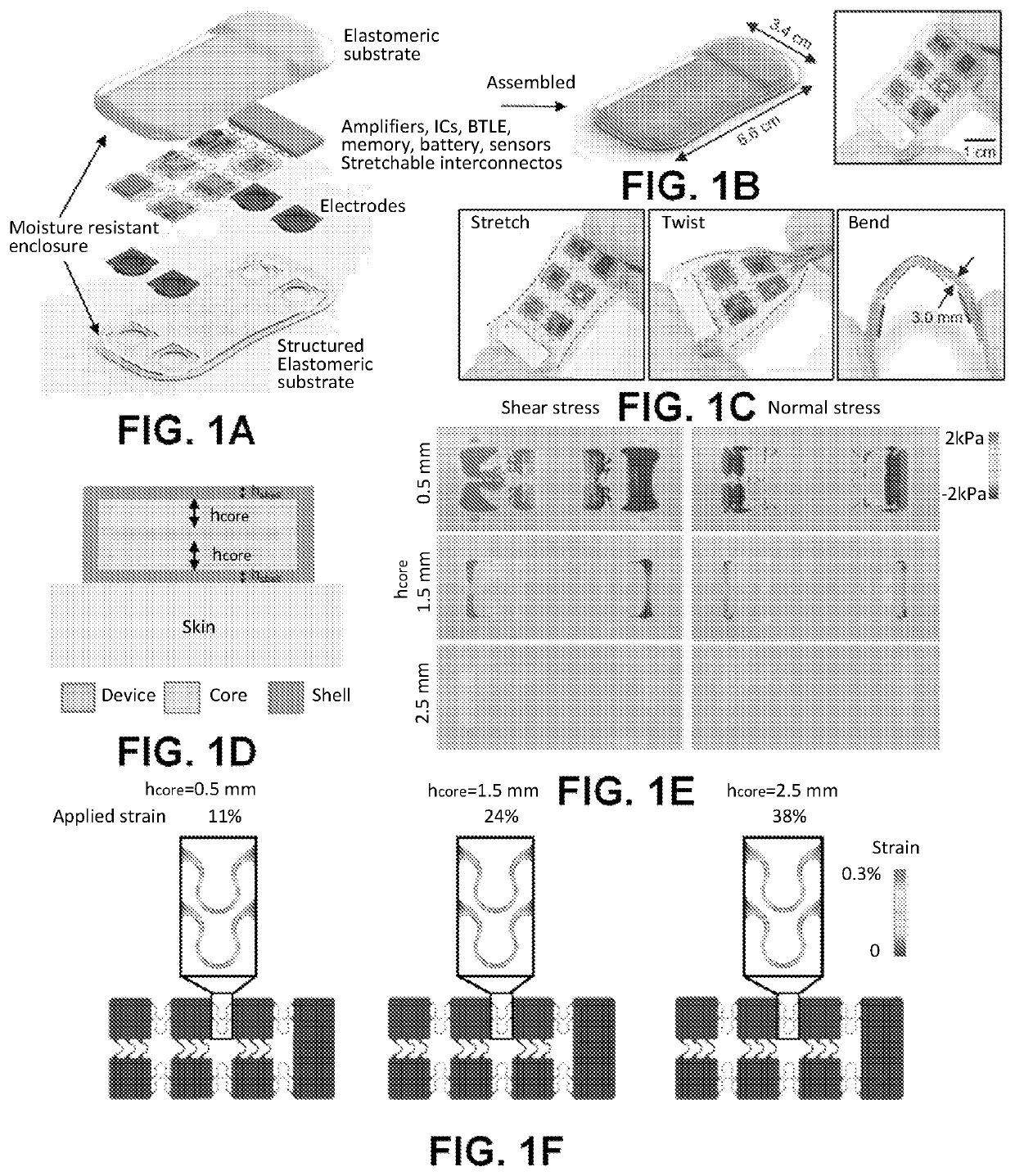
Intraoperative monitoring of neuromuscular function with soft, tissue-mounted wireless devices
ActiveUS20200397326A1Small sizeEliminate needElectrocardiographyElectro-oculographyNerve muscleMuscle nerve
The provided systems, methods and devices describe lightweight, wireless tissue monitoring devices that are capable of establishing conformal contact due to the flexibility or bendability of the device. The described systems and devices are useful, for example, for skin-mounted intraoperative monitoring of nerve-muscle activity. The present systems and methods are versatile and may be used for a variety of tissues (e.g. skin, organs, muscles, nerves, etc.) to measure a variety of different parameterps (e.g. electric signals, electric potentials, electromyography, movement, vibration, acoustic signals, response to various stimuli, etc.).
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS +1
Intelligent massaging pillow
PendingCN107744303AEnsure natural stretchRealize automatic controlPillowsChiropractic devicesHuman bodyPhysical medicine and rehabilitation
The invention discloses an intelligent massaging pillow. The intelligent massaging pillow is characterized by mainly consisting of a base (1), a neck body (2), a head body (3), a motor (9), a controller (6) and a pressure sensor (7) arranged on the head body (3). It can be ensured that the cervical vertebra of a user naturally stretches by combining the neck body and the head body of the intelligent massaging pillow, a massaging assembly arranged on the neck body can conduct frictional massage on the cervical vertebra of the human body under the driving of the motor so that the muscles and nerves of the cervical vertebra can be effectively relaxed, and a human body cervical vertebra traction effect of the neck body is improved. In addition, the controller and the pressure sensor of the intelligent massaging pillow are combined so that the motor driving the massaging assembly can be automatically started up and shut down, namely automatic control of the working states of the massaging assembly is achieved. Accordingly, the intelligent massaging pillow has a good cervical vertebra traction effect and very well achieves automatic startup and shutdown.
Owner:广东佳风科技有限公司
DNP and DNP Prodrug Treatment of Neuromuscular, Neurodegenerative, Autoimmune, Developmental, Traumatic Brain Injury, Concussion, Dry Eye Disease, Hearing Loss and/or Metabolic Diseases
A composition and method of treatment of neuromuscular, neuromuscular degenerative, neurodegenerative, autoimmune, developmental, traumatic, hearing loss related, and / or metabolic diseases, including spinal muscular atrophy (SMA) syndrome (SMA1, SMA2, SMA3, and SMA4, also called Type I, II, III and IV), traumatic brain injury (TBI), concussion, keratoconjunctivitis sicca (Dry Eye Disease), glaucoma, Sjogren's syndrome, rheumatoid arthritis, post-LASIK surgery, anti-depressants use, Wolfram Syndrome, and Wolcott-Rallison syndrome. The composition is selected from the group consisting of 2,3-DNP, 2,4-DNP, 2,5-DNP, 2,6-DNP, 3,4-DNP, or 3,5-DNP, bipartite 2,3-dinitrophenol, 2,4-dinitrophenol, 2,5-dinitrophenol, 2,6-dinitrophenol, 3,4-dinitrophenol, or 3,5-dinitrophenol (2,3-DNP, 2,4-DNP, 2,5-DNP, 2,6-DNP, 3,4-DNP, or 3,5-DNP) prodrugs; Gemini prodrugs, bioprecursor molecules, and combinations thereof. A dose of the composition for treatment of neurodegenerative diseases may be from about 0.01 mg / kg of body weight to about 50 mg / kg of body weight of the patient in need of treatment. A dose of the composition for treatment of metabolic diseases may be from about 1 mg / 70 kg of body weight to about 100 mg / 70 kg of body weight of the patient in need of treatment, and a maximum dose per day is about 200 mg / 70 kg of body weight of the patient in need of treatment.
Owner:BIOVENTURES LLC +1
Drill bit for total hip replacement minimally invasive surgery
The invention discloses a drill bit for a total hip replacement minimally invasive surgery. A flexible shaft is formed by a spring with certain rigidity and certain flexibility; a handle part and a cutting head are respectively welded on two ends of the flexible shaft; a stainless steel isolation sleeve is arranged in a puncture hole near a surgical incision; the cutting head at the lower end of the flexible shaft is placed in along an inner cavity of the isolation sleeve; a limiting guide sleeve is placed in the surgical incision through an operating handle; the cutting head is driven by theflexible shaft to conduct downward limiting drilling along the limiting guide sleeve; muscle nerves and other important structures of a patient cannot be damaged during high-speed rotation; and the limiting guide sleeve can prevent a drilled hole from entering a pelvic cavity too deep. The cutting head is made of 316L stainless steel, the serious consequence that a drill bit is broken in an acetabular bone and cannot be taken out during bent drilling can be prevented, after drilling is completed, a screw with a proper length is screwed in along an inner cavity of the isolation sleeve through areversible screwdriver, so that an metal part connecting acetabulum can be fixedly, and the drill bit is safe and reliable, and convenient to operate.
Owner:谢军
Intraoperative monitoring of neuromuscular function with soft, tissue-mounted wireless devices
ActiveUS11246522B2Small sizeReduce rigidityInertial sensorsExternal electrodesNerve muscleMuscle nerve
The provided systems, methods and devices describe lightweight, wireless tissue monitoring devices that are capable of establishing conformal contact due to the flexibility or bendability of the device. The described systems and devices are useful, for example, for skin-mounted intraoperative monitoring of nerve-muscle activity. The present systems and methods are versatile and may be used for a variety of tissues (e.g. skin, organs, muscles, nerves, etc.) to measure a variety of different parameterps (e.g. electric signals, electric potentials, electromyography, movement, vibration, acoustic signals, response to various stimuli, etc.).
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS +1
Adjustable spinal back orthopedic lumbar vertebra fixator
ActiveCN112294515APromote circulationReduce or relieve painElectrotherapyMedical devicesHuman bodyInfrared lamp
The invention discloses an adjustable spinal back orthopedic lumbar vertebra fixator, relates to the technical field of medical instruments, and aims to solve the problem in that the spine of a patient is subjected to physiotherapy and restoration while being fixed and supported. The fixator specifically comprises two pads; a controller is fixed to the outer wall of one side of a fixing assembly through bolts; adjusting mechanisms are arranged on the outer walls of the two sides of each pad; three or more infrared lamps are fixed to the inner wall of one side of each pad through bolts; a heating mechanism is arranged on the wall of each pad; and a temperature sensor is bonded to the outer wall of one side of the top of one pad. According to the invention, weak current is released through the controller and is transmitted to a massage neodymium stone through a wire, so that the massage neodymium stone starts to act on the human body in a low-frequency mode; and muscles and muscle internal nerve ending sensory receptors are stimulated through electrode plates, so that the injured muscles and nerves are relaxed, and pain is alleviated or relieved.
Owner:JIANGSU AIAIJIA HEALTH TECH CO LTD
Bracelet device with treatment function
InactiveCN110841191APromote circulationReduce or relieve painElectrotherapyVibration massageMuscle nerveWeak current
The invention discloses a bracelet device with a treatment function. The device comprises a glove body and a smart bracelet. Weak current acts on a hand in a low-frequency mode by the aid of various pulse patterns, muscles and nerve ending sensory receptors in the muscles are directly stimulated by the aid of electrode slices, the muscles can unconsciously contract, so that the muscles and nervesaching due to exhaustion or injury are relaxed, local blood circulation is promoted, and pain can be relieved or soothed.
Owner:朱建国
Cornea shaping cushion and cornea shaping corrector
PendingCN110338971AReduce riskComfortable to wearElectrotherapyEye-masksCorneal sculptingPressure transmission
The invention provides a cornea shaping cushion and a cornea shaping corrector, and belongs to the technical field of myopia correction. The cornea shaping cushion is provided with an arc part with the opposite curvature direction as the cornea, the end, away from the arc end, of the arc part extends to form a cylindrical part, the arc part and the cylindrical part are both of a hollow structure,wherein a magnetic plate is horizontally arranged inside the cylindrical part in the radial direction. The cornea shaping corrector comprises a heating base plate and a cornea shaping cushion. The heating base plate comprises a first shaping cotton cloth layer, a heating layer and a second shaping cotton cloth layer which are vertically stacked, wherein the heating layer comprises a graphene heating piece. Pressure applied to the palpebra superior through the cornea shaping cushion is transmitted to the cornea, and therefore the eye axis is controlled to be shortened; the graphene heating piece is arranged inside the heating base plate of the cornea shaping corrector, generated infrared rays heat and stimulates the eye skin muscle-nerve peripheral part, blood circulation of the eye peripheral acupuncture points is improved, and the correcting effect is improved.
Owner:上海欧利文实业有限公司
DNP and DNP prodrug treatment of neuromuscular, neurodegenerative, autoimmune, developmental, traumatic brain injury, concussion, dry eye disease, hearing loss and/or metabolic diseases
A composition and method of treatment of neuromuscular, neuromuscular degenerative, neurodegenerative, autoimmune, developmental, traumatic, hearing loss related, and / or metabolic diseases, including spinal muscular atrophy (SMA) syndrome (SMA1, SMA2, SMA3, and SMA4, also called Type I, II, III and IV), traumatic brain injury (TBI), concussion, keratoconjunctivitis sicca (Dry Eye Disease), glaucoma, Sjogren's syndrome, rheumatoid arthritis, post-LASIK surgery, anti-depressants use, Wolfram Syndrome, and Wolcott-Rallison syndrome. The composition is selected from the group consisting of 2,3-DNP, 2,4-DNP, 2,5-DNP, 2,6-DNP, 3,4-DNP, or 3,5-DNP, bipartite 2,3-dinitrophenol, 2,4-dinitrophenol, 2,5-dinitrophenol, 2,6-dinitrophenol, 3,4-dinitrophenol, or 3,5-dinitrophenol (2,3-DNP, 2,4-DNP, 2,5-DNP, 2,6-DNP, 3,4-DNP, or 3,5-DNP) prodrugs; Gemini prodrugs, bioprecursor molecules, and combinations thereof. A dose of the composition for treatment of neurodegenerative diseases may be from about 0.01 mg / kg of body weight to about 50 mg / kg of body weight of the patient in need of treatment. A dose of the composition for treatment of metabolic diseases may be from about 1 mg / 70 kg of body weight to about 100 mg / 70 kg of body weight of the patient in need of treatment, and a maximum dose per day is about 200 mg / 70 kg of body weight of the patient in need of treatment.
Owner:MITOCHON PHARMA INC +1
Ointment for treating genuine myopia and preparation method of ointment
InactiveCN108653640ASufficient blood supplyPromote blood circulationSenses disorderHydroxy compound active ingredientsAdditive ingredientMedicine
The invention discloses ointment for treating genuine myopia. The ointment comprises vaseline, edible oil and ointment. The invention further provides a preparation method of the ointment for treatinggenuine myopia. The preparation method of the ointment for treating genuine myopia comprises the following steps: (1) extracting the medicinal materials; (2) refining medicinal oil; (3) feeding vaseline for forming ointment; (4) releasing fire-toxins; and (5) spreading the ointment. The ingredients can dredge the blocked meridian points of the eyes, so that the local blood circulation is promoted, the muscle nerve and the optic nerve are nourished and invigorated, the supply of the blood and qi for the eyes is sufficient, the recovery is complete, the spasm is controlled, the muscle activityis invigorated, the atrophic optic nerve is activated, the purpose of eradicating the myopia with the symptoms and the root cause completely treated is achieved, and the relapse and sequelae of othertreatment means are avoided.
Owner:马锡磊
Surface electromyography detecting device applied to spinal rehabilitation system
ActiveCN109288521ARehabilitation is effectiveRecovery safetyOrganic active ingredientsElectrotherapyDiseaseHuman body
Disclosed is a surface electromyography detecting device applied to a spinal rehabilitation system. The spinal rehabilitation system aims at treating muscle slight contracture and muscle tension whichare caused by unhealthy living habits, diseases and the like of modern people. Various drugs which promote bone formation and avoid heterotopic osteogenesis are involved, selectively released in theinterstitial space through special coating layers and enriched in the affected part through the arrangement of a magnetic field. In order to improve the activity of the drugs, a device for forming ganglion interference, an artificial weakly-alkaline environment and the like is additionally arranged. According to the surface electromyography detecting device applied to the spinal rehabilitation system, electricity discharge is evoked through muscle tension-related ganglions of the human body, the muscle and nerve health indexes are effectively improved, and injury healing is promoted; meanwhile, in combination with electromyography detection, self-adaptive adjustment is made in real time to obtain a better therapeutic effect. In the electromagnetic therapy process, in order to seize a better participation time, an instructing device reminds a patient to perform abdominal breathing to cooperate with the magnetic therapy under the management of a control device, and the interference between evoked potentials and normal human electrocardio is avoided.
Owner:NORTH CHINA UNIVERSITY OF SCIENCE AND TECHNOLOGY
The Method of Making Four-piece Specimens by Splitting the Same Body
The present invention proposes a method for making a four-piece specimen set by dissecting the same body, which includes the following steps: selecting objects; peeling off the skin; dissecting; peeling off muscles, nerves and blood vessels; taking out internal organs and dissecting them; , degreasing, resin infiltration; skin specimen preparation; muscle specimen preparation; bone and its ligament specimen preparation; internal organ specimen preparation; restoration, curing and cleaning. The present invention splits the same body into four sets of specimens, saves raw materials, and is convenient for storage, and each split specimen can independently display its specific anatomical characteristics and various survival action morphological characteristics, satisfying the requirements of the same body vertebrate The complexity of the hierarchical structure of anatomical plastination specimens and the diversity of living forms.
Owner:DALIAN HOFFEN BIO TECHN
Composition for resisting cardiac muscle tremor
ActiveCN114470133ASuppress tremorReduce tensionMuscular disorderNeuromuscular disorderButter cocoaPharmaceutical Substances
The invention relates to the technical field of medicines, health care products and foods, in particular to a composition for resisting cardiac muscle tremor, which is prepared from the following raw materials in parts by weight: 1-35 parts of turmeric, 1-35 parts of polygonum cuspidatum, 1-35 parts of mint, 1-35 parts of fructus cnidii, 1-35 parts of pyrola, 1-35 parts of hematoxylon, 1-35 parts of lignum dalbergiae odoriferae, 1-35 parts of inula flower and 1-50 parts of cocoa butter. The traditional Chinese medicine composition has the effects of antagonizing cardiac muscle nerve abnormal excitement, reducing muscle tension, inhibiting non-autonomous movement of muscles and resisting muscle tremor, has no inhibiting effect on normal nerves, cannot cause muscle relaxation, can effectively reduce muscle energy consumption, and can be used for preparing the traditional Chinese medicine composition for preventing muscle tremor. The muscle strain, the muscle pain and the inflammatory hyperplasia of the muscle interstitial substance caused by continuous tremor of the muscle are reduced.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
How to make a fur specimen
ActiveCN108711352BAvoid deformationNon-volatileEducational modelsHuman bodyPhysical medicine and rehabilitation
Owner:大连鸿峰文化发展有限公司
Formula of vagina tightening antibacterial liquid for preventing gynecological diseases and tightening vaginae
PendingCN113069536AEasy to prepareHigh activityPeptide/protein ingredientsHydroxy compound active ingredientsSomatotropic hormonePerilla oil
The invention discloses a formula of vagina tightening antibacterial liquid for preventing gynecological diseases and tightening vaginae. The formula of the vagina tightening antibacterial liquid comprises the following components in parts by weight: 200-280 parts of traditional Chinese medicine extract, 35-40 parts of growth hormone, 35-40 parts of ICE deep sea mineral crystal ions, 150-160 parts of glycerol, 80-90 parts of perilla oil, 50-60 parts of collagen, 40-50 parts of natural pectin and 650-700 parts of activated water. According to the formula, the traditional Chinese medicine extract, the growth hormone, the ICE deep sea mineral crystal ions, the glycerol, the perilla oil, the collagen, the natural pectin and the activated water are uniformly mixed together through a scientific and reasonable proportion to prepare the vagina tightening antibacterial liquid, and the preparation method is simple; and the vagina tightening antibacterial liquid can stimulate release of pudendum muscle nerve information, improve muscle cell activity, improve the relaxed state of the pudendum and enhance elasticity, so that the vitality of cells in the vagina can be quickly recovered, cell regeneration in the vagina is stimulated, and the inner wall of the vagina is lustrous and elastic.
Owner:郑州世纪亿佰商贸有限公司
A magnetic nanoparticle sphere mixture for spinal rehabilitation system
ActiveCN109289119BHigh activityImprove bindingOrganic active ingredientsElectrotherapySpinal columnDisease
A magnetic nanoparticle sphere mixture for use in spinal rehabilitation systems. The spinal rehabilitation system treats the slight muscle contracture and muscle tension caused by modern people's bad living habits and diseases. It contains a variety of drugs that promote bone formation and avoid ectopic bone formation. The drugs are selectively released in the tissue gap through a special coating layer and enriched in the affected area through a magnetic field setting. In order to improve the activity of the drug, the present invention also adds devices for forming ganglion interference and artificial weak alkaline environment. The present invention can effectively improve muscle and nerve health indicators and promote injury healing by inducing discharges from ganglia related to muscle tension in the human body; at the same time, self-adaptive adjustments can be made in real time in combination with myoelectric detection to obtain better therapeutic effects. In order to achieve a better cut-in timing in the above electromagnetic therapy process, under the management of the control device, the indicating device prompts the patient to perform abdominal breathing to cooperate with the magnetic therapy, so as to avoid interference between the evoked potential and the normal human ECG.
Owner:NORTH CHINA UNIVERSITY OF SCIENCE AND TECHNOLOGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com