Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
11795 results about "Metallic Lead" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lead is a chemical element with symbol Pb (from the Latin plumbum) and atomic number 82. It is a heavy metal that is denser than most common materials. ... Lead is soft and malleable, and has a relatively low melting point.
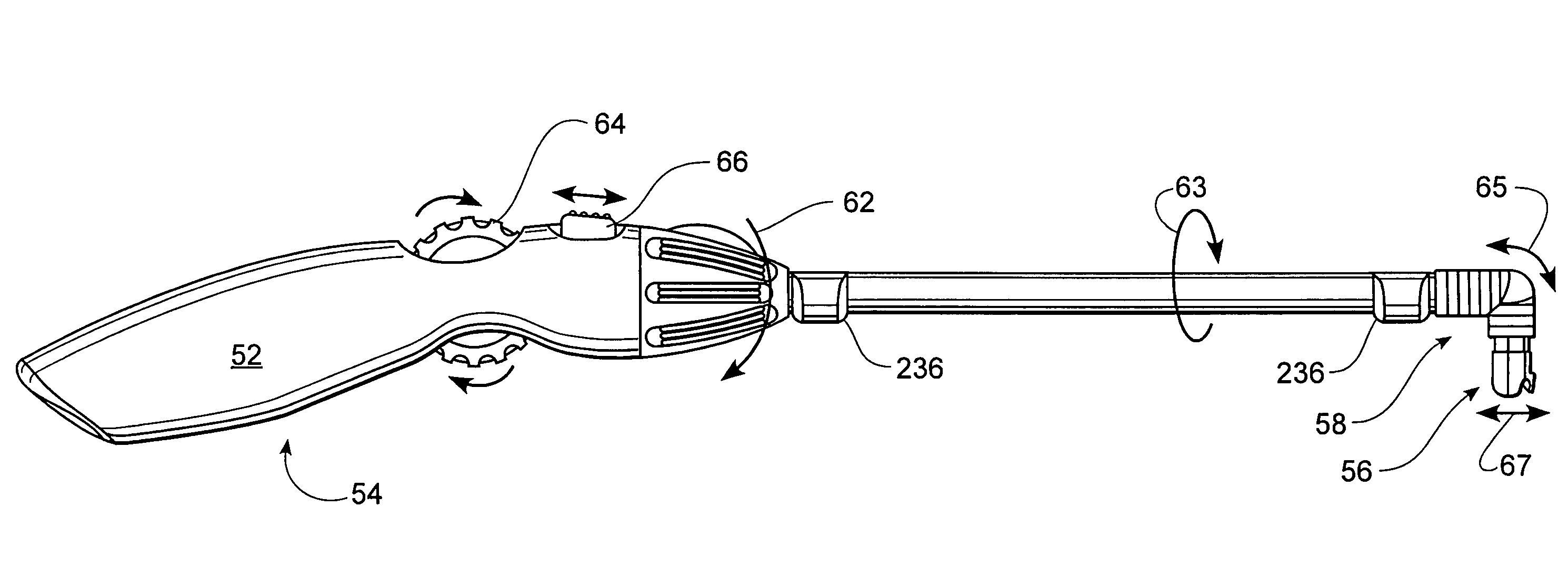
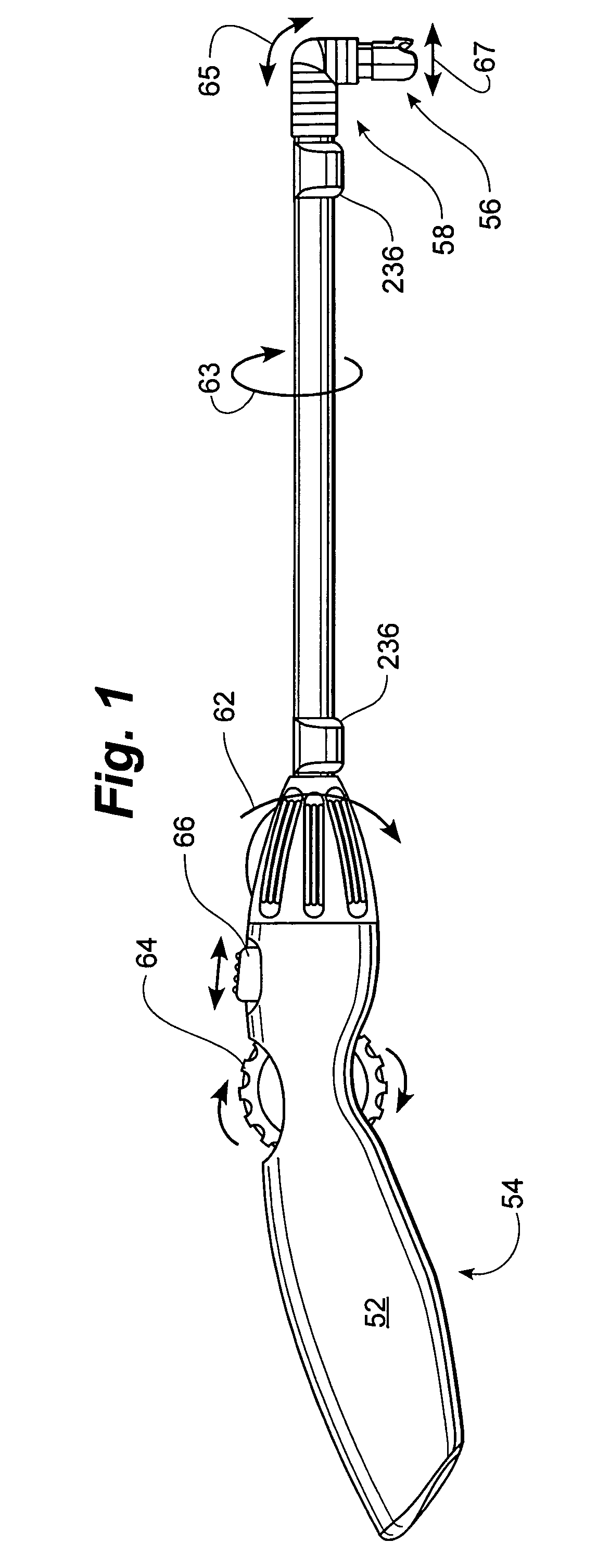
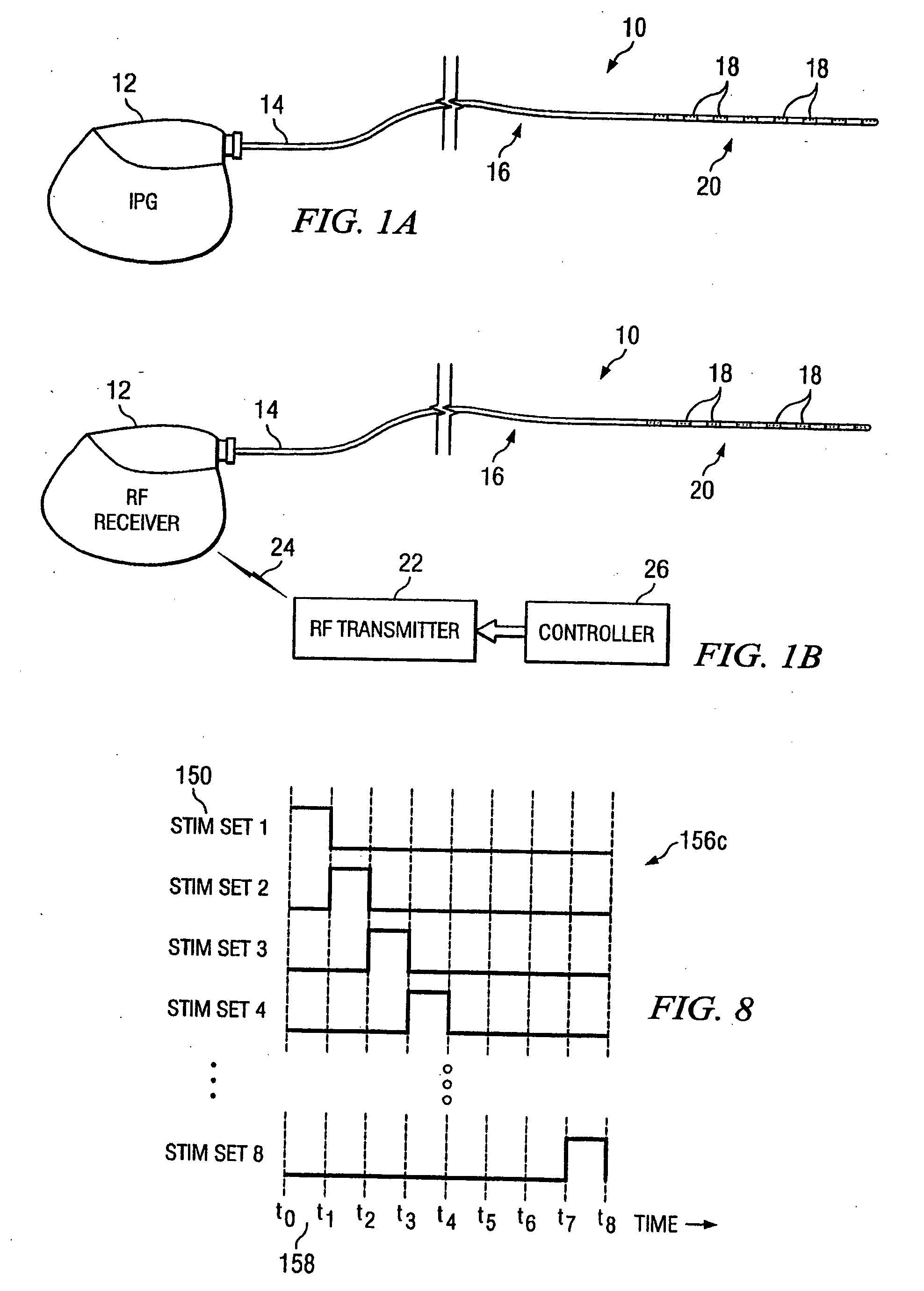
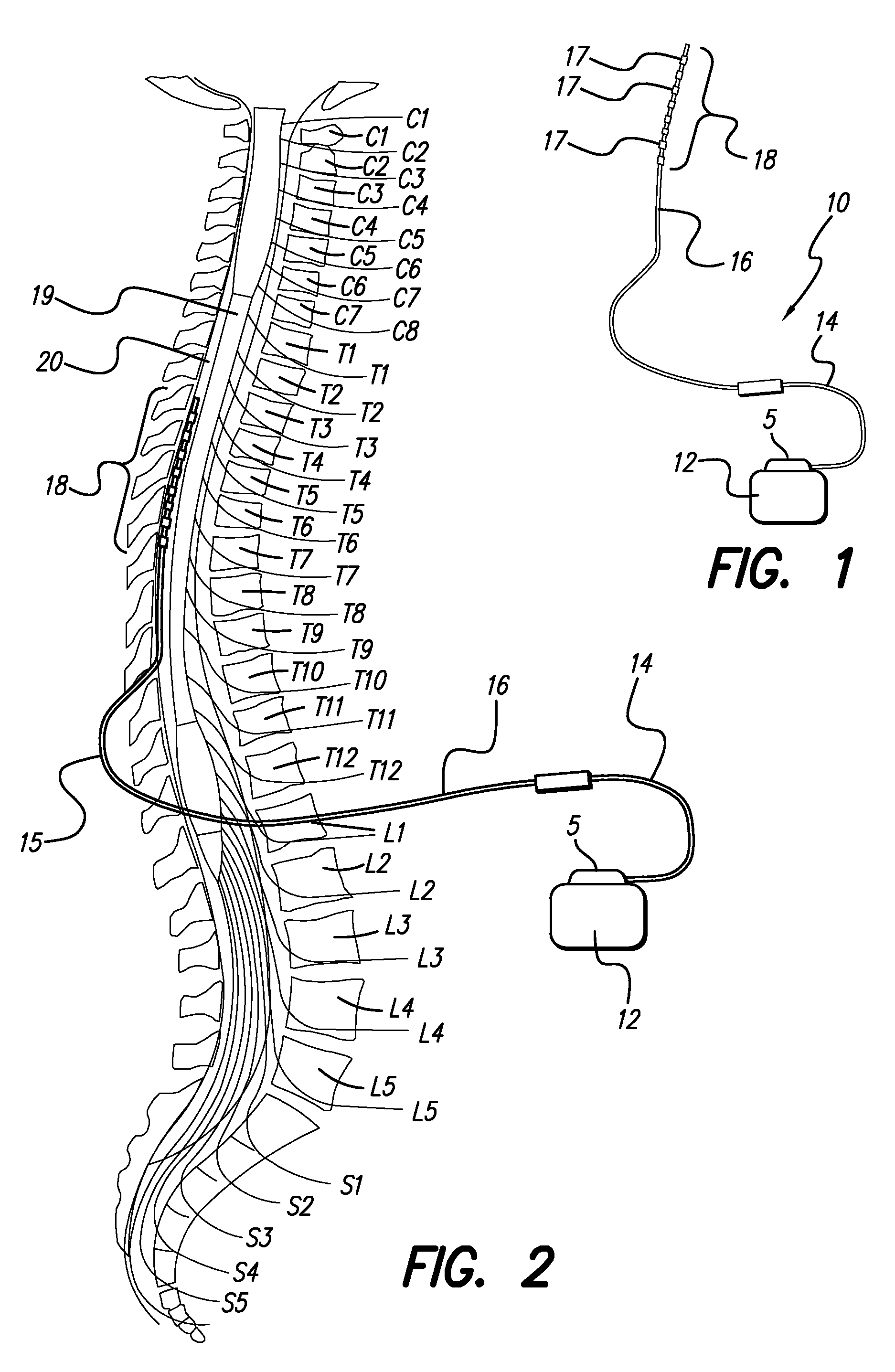
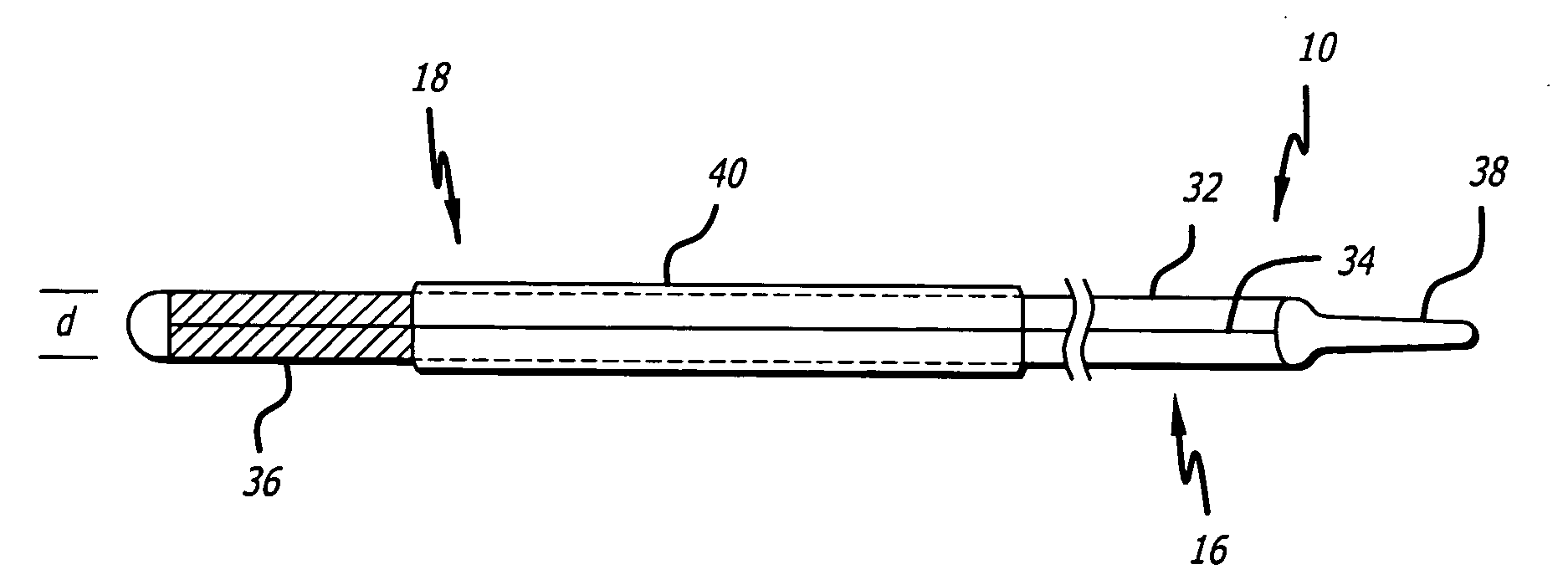
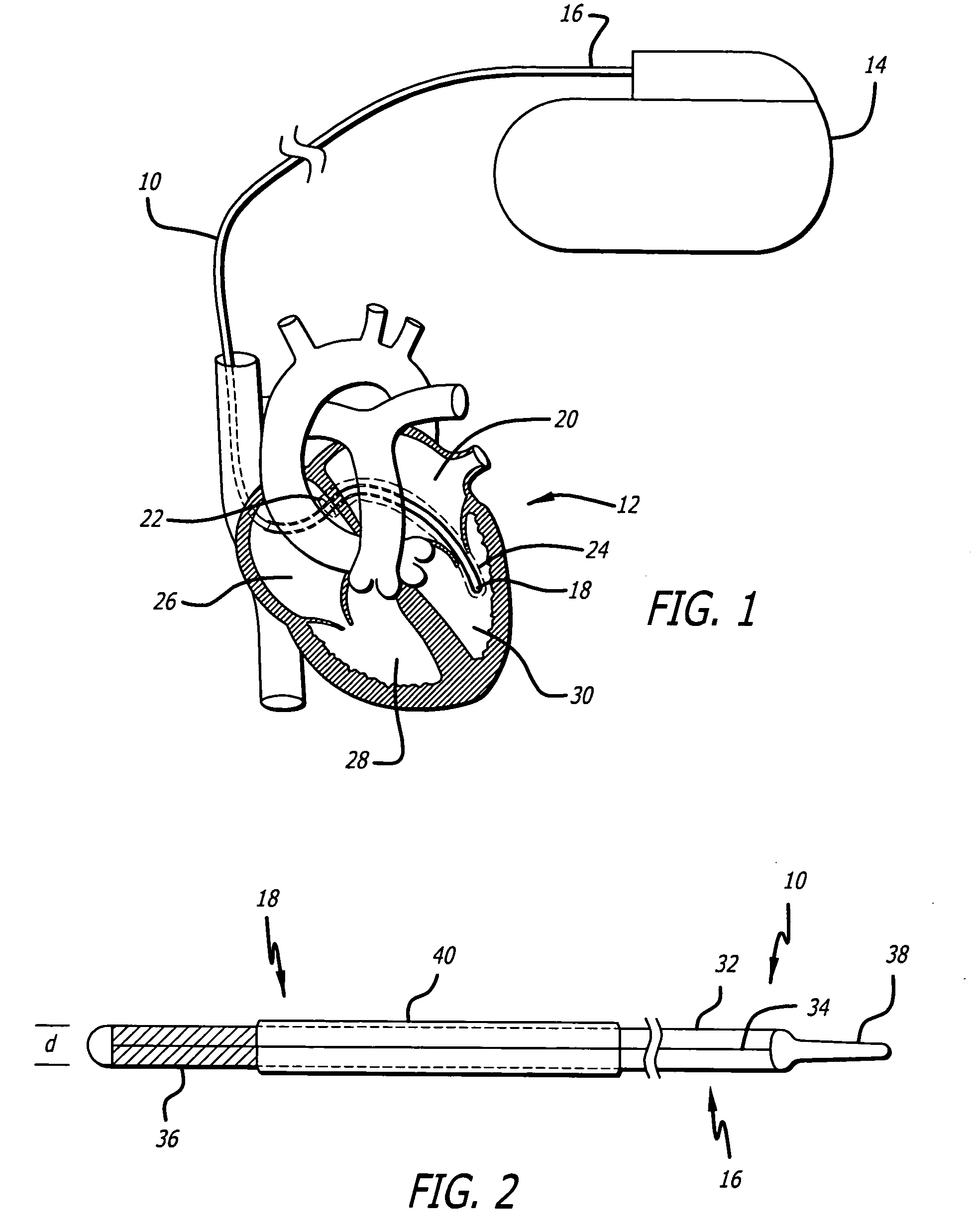
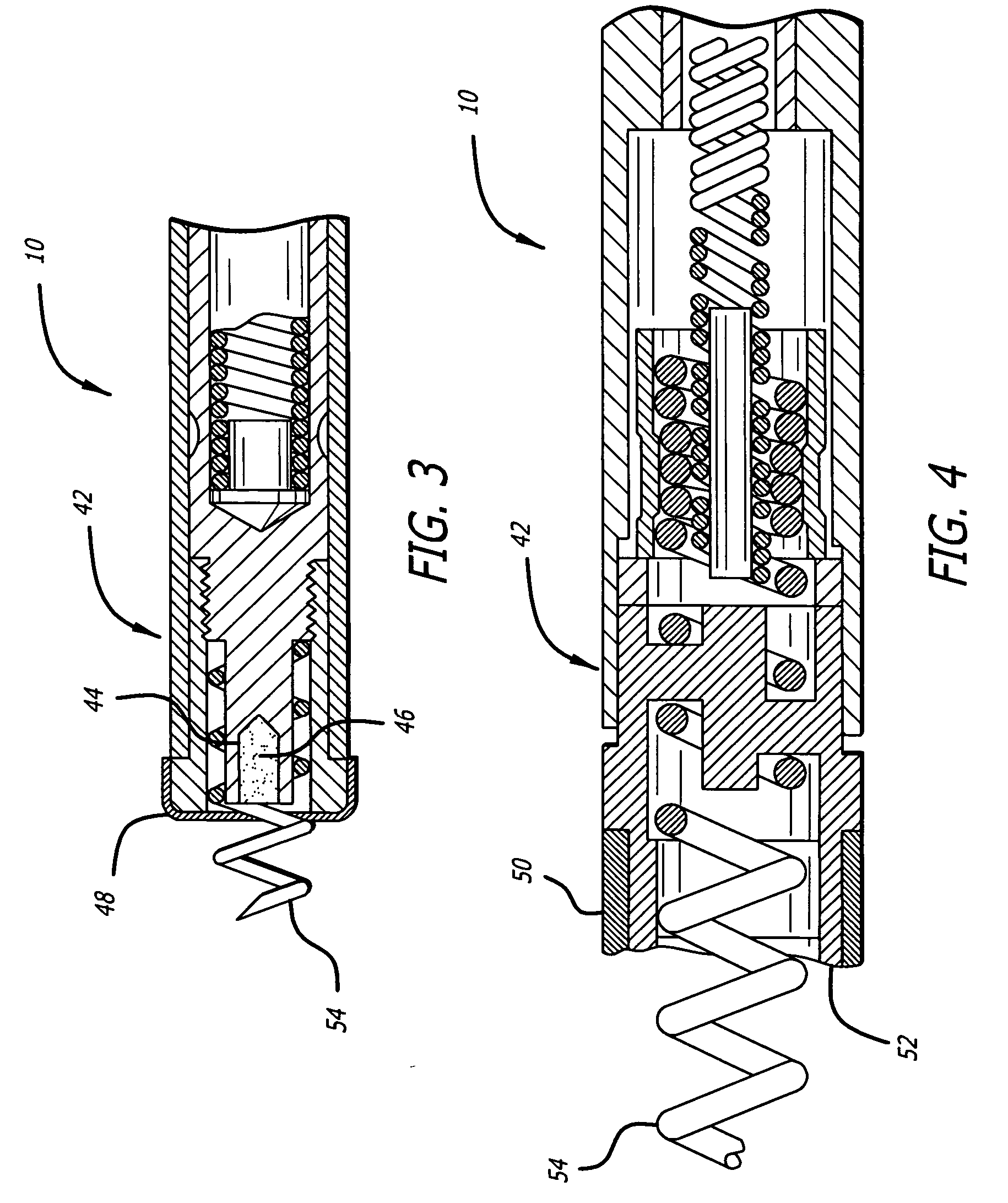
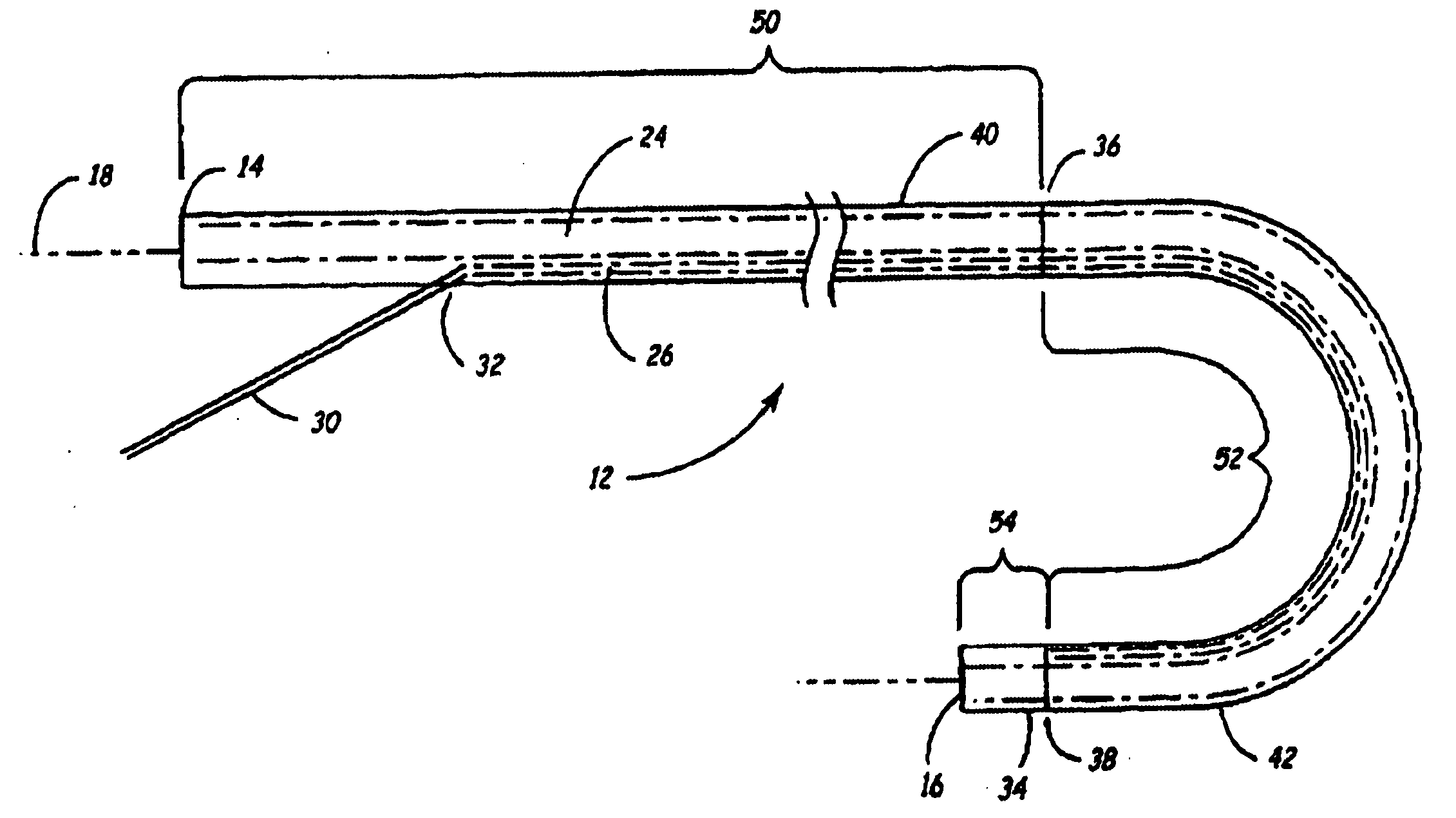
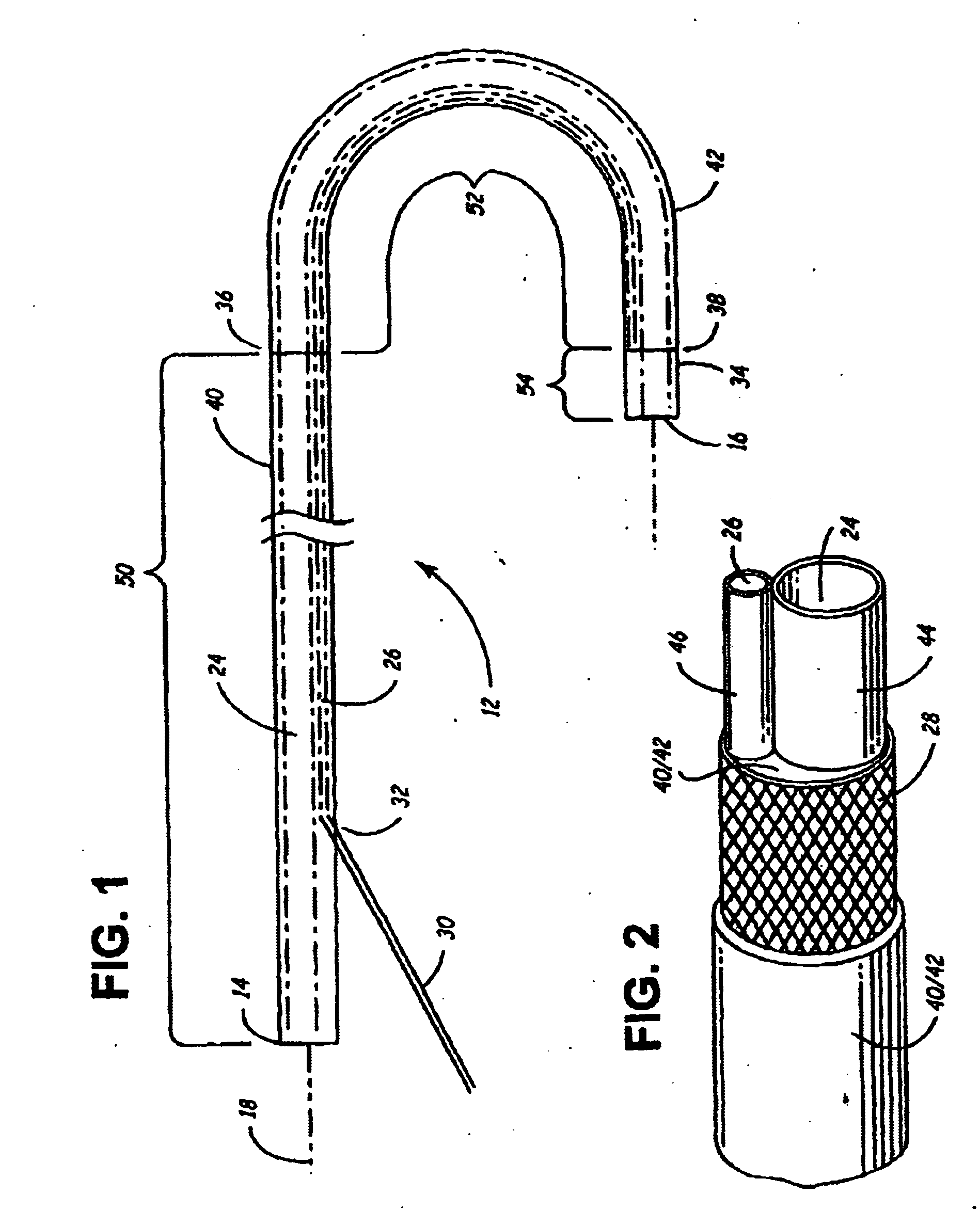
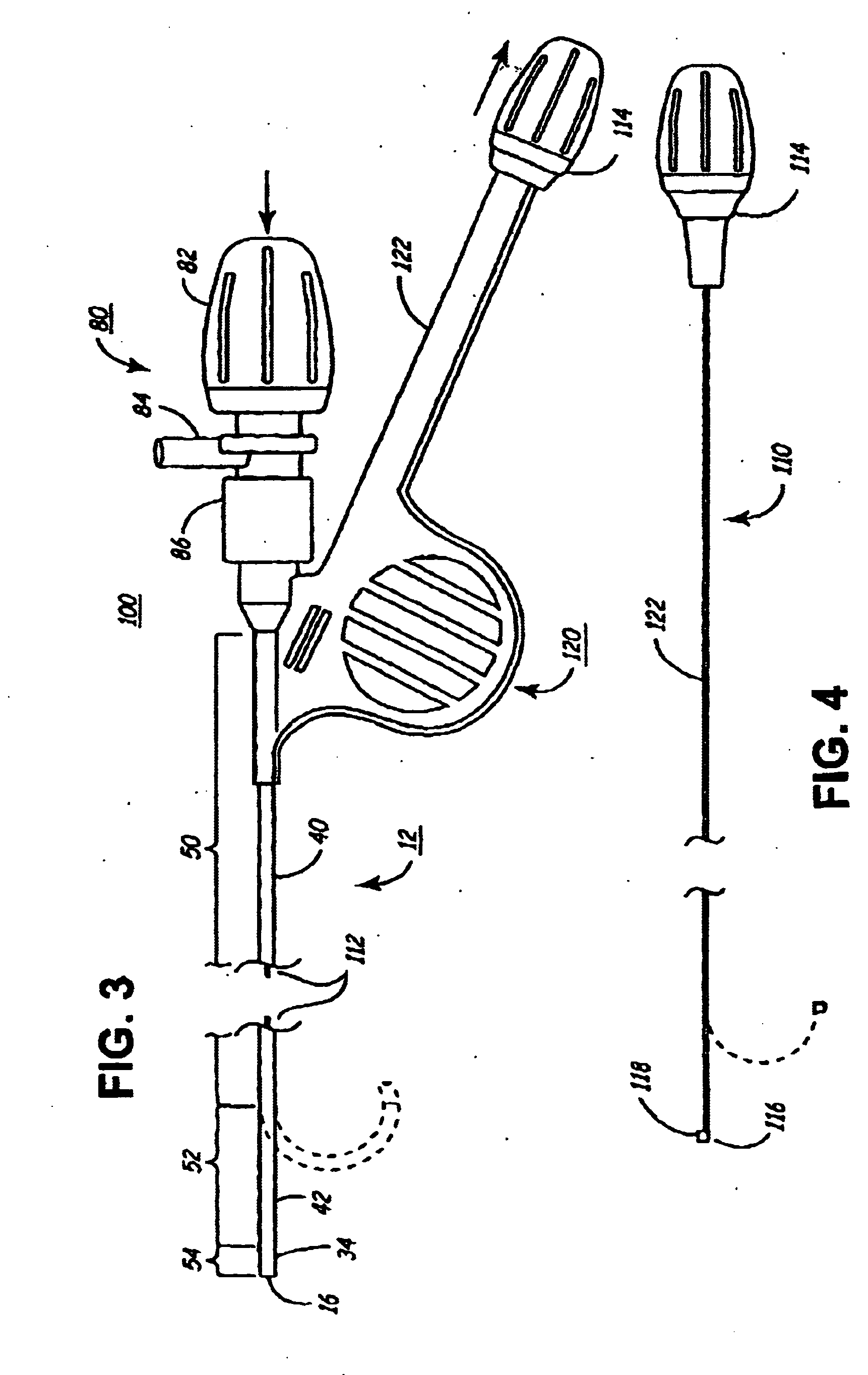
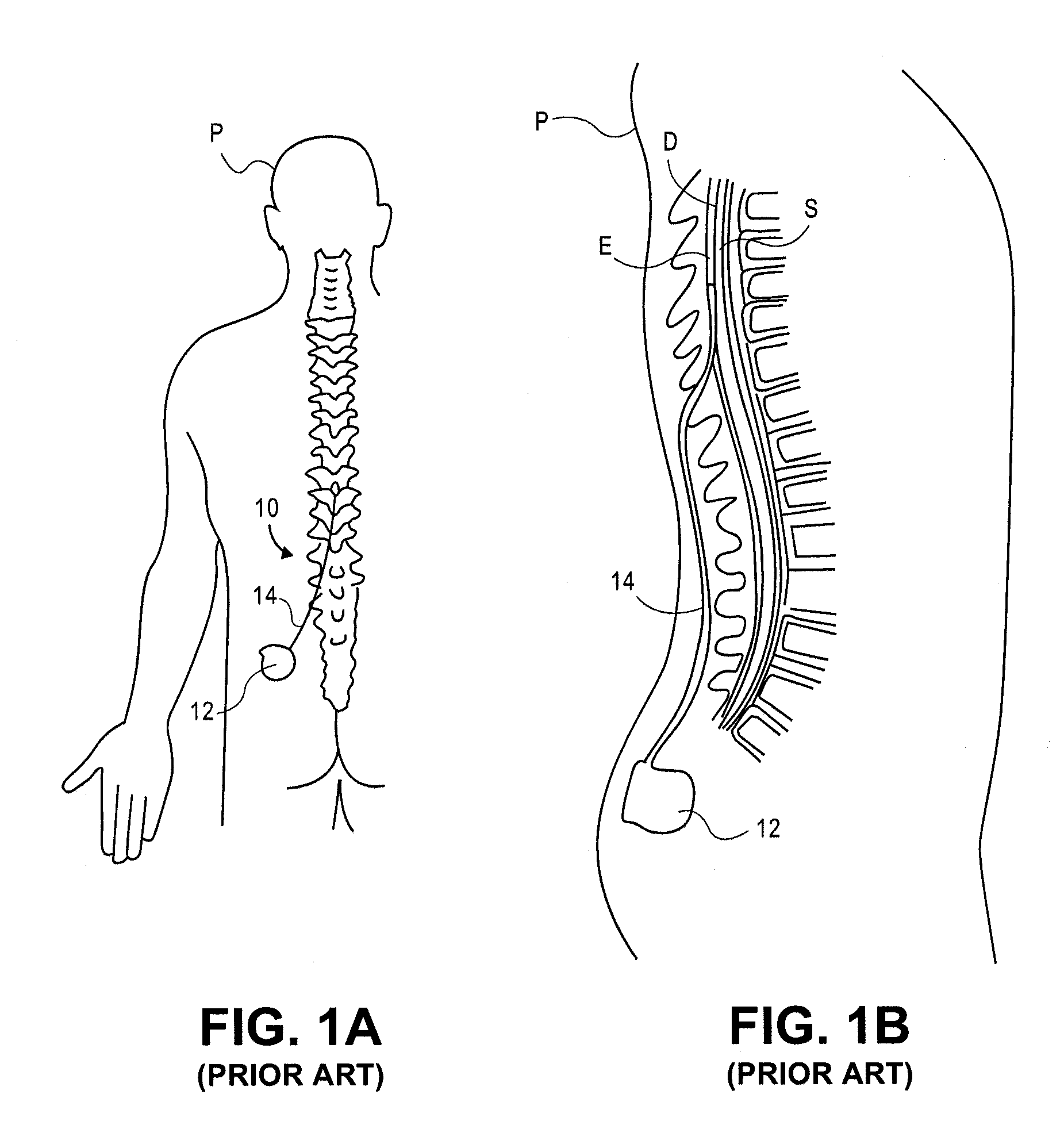
Rotatable lead introducer
Minimally invasive introducers and methods that can be used for rotationally securing devices within the human body. Introducers can include a distal element for releasably engaging a lead head controllable from a proximal control located outside of the body. An inner stem can extend between a proximal portion and a distal portion, and be pivotally and rotatably coupled to the distal lead engagement mechanism. An outer tube can be rotatably disposed over the inner stem and be flexibly coupled over the pivot to rotationally drive the distal element. A helical epicardial-myocardial lead electrode can be secured and oriented straight ahead and introduced through a port or small incision with the introducer in a straight configuration. The introducer can then be bent and rotated to screw the helical electrode into the heart.
Owner:WILSON GREATBATCH LTD
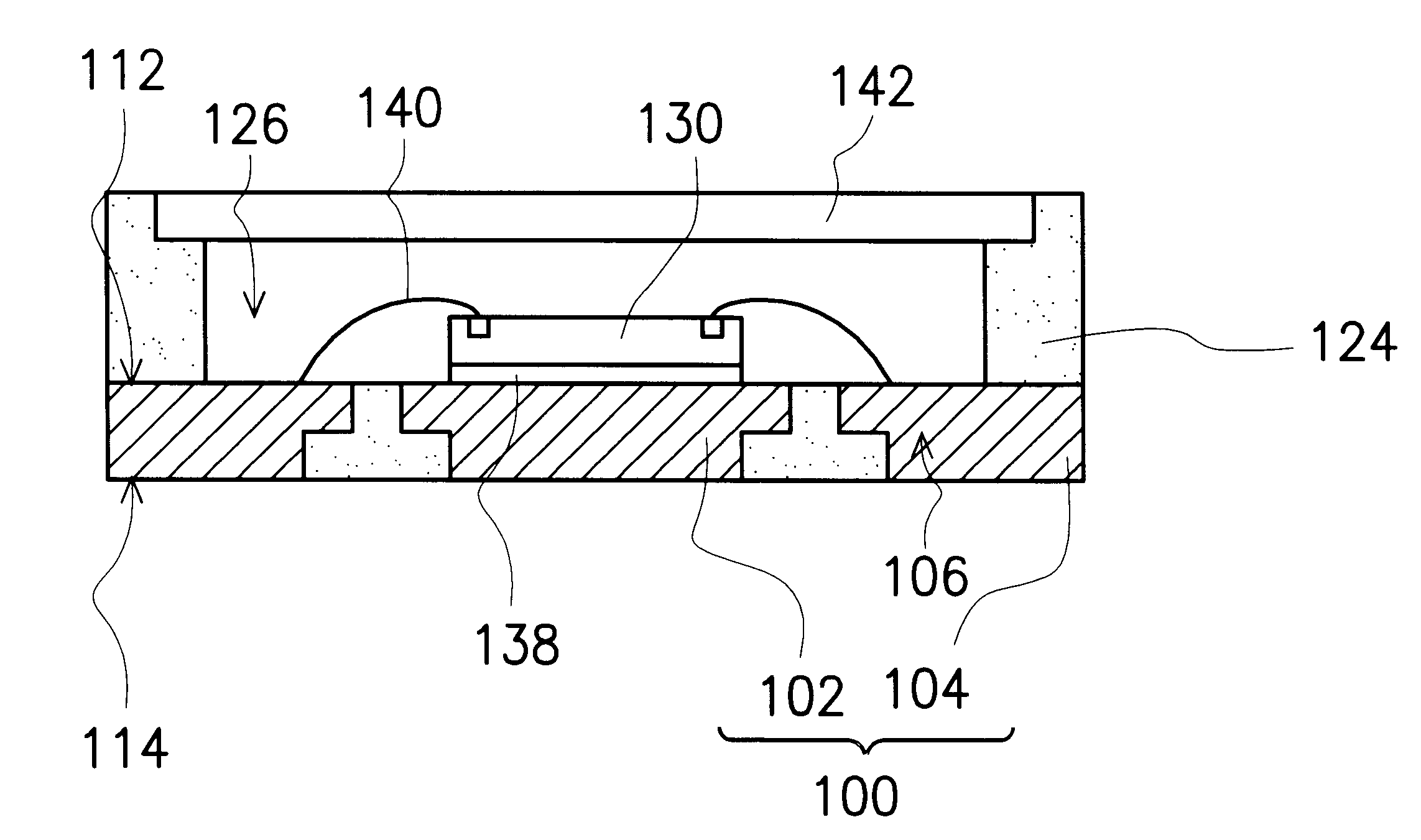
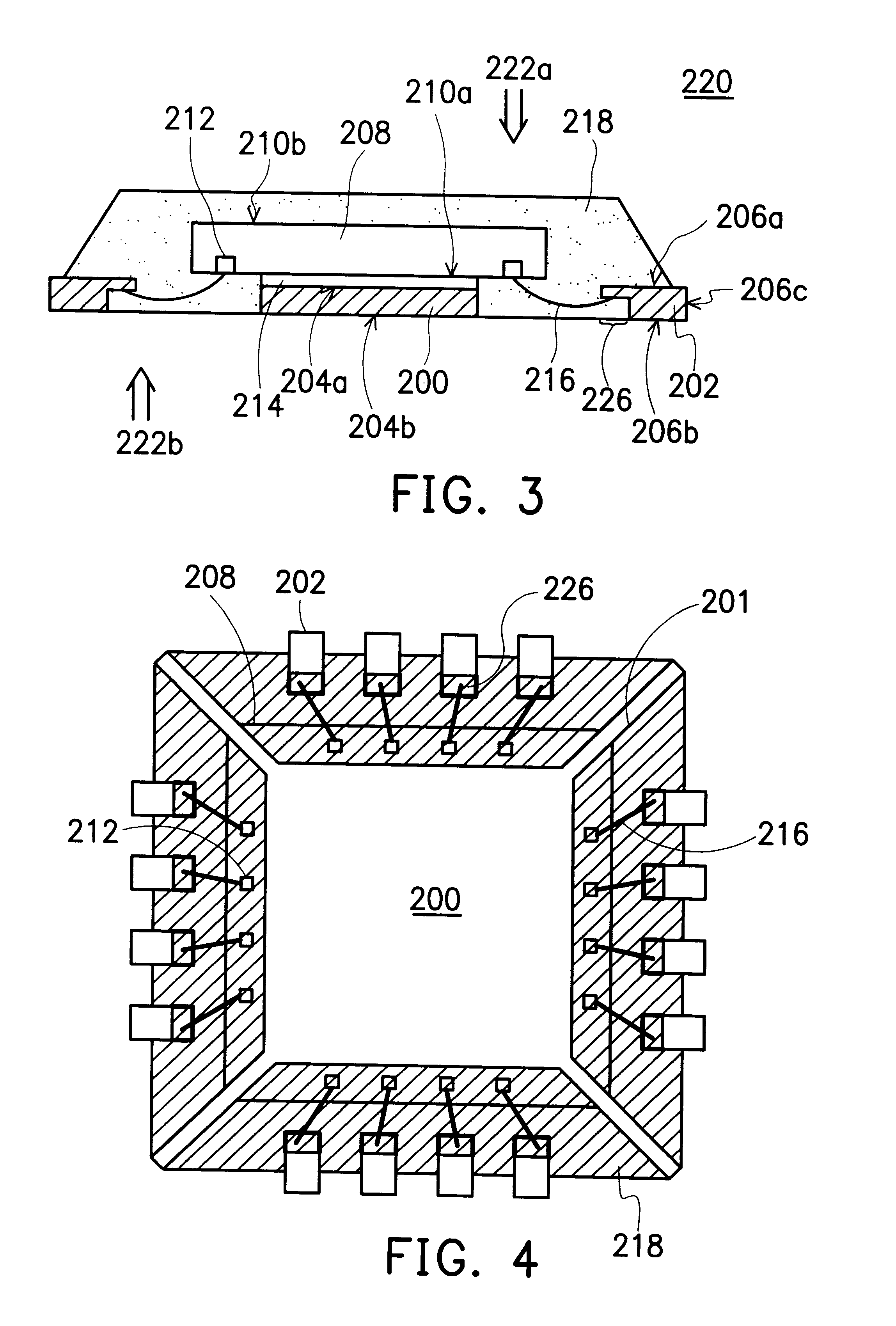
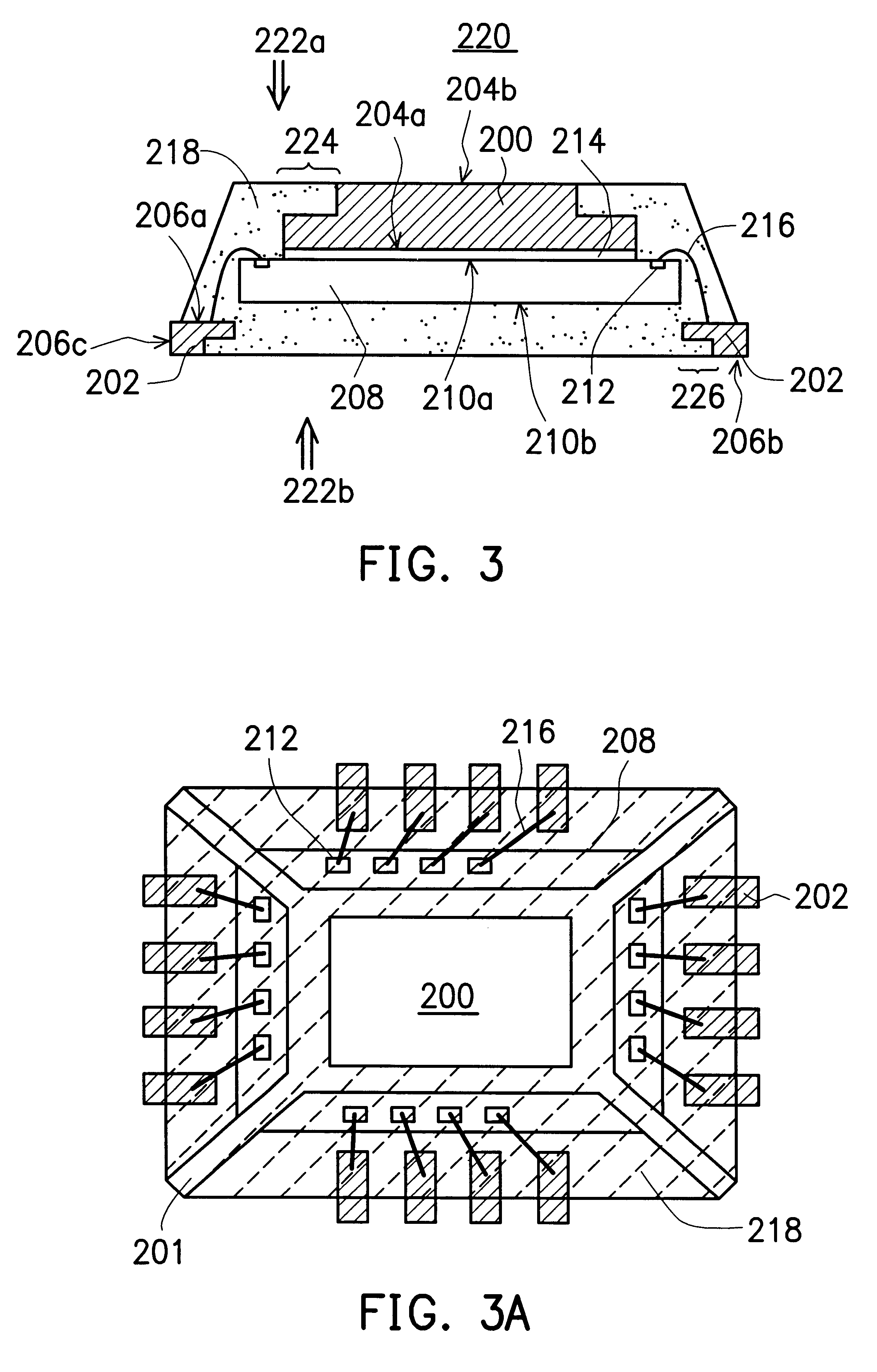
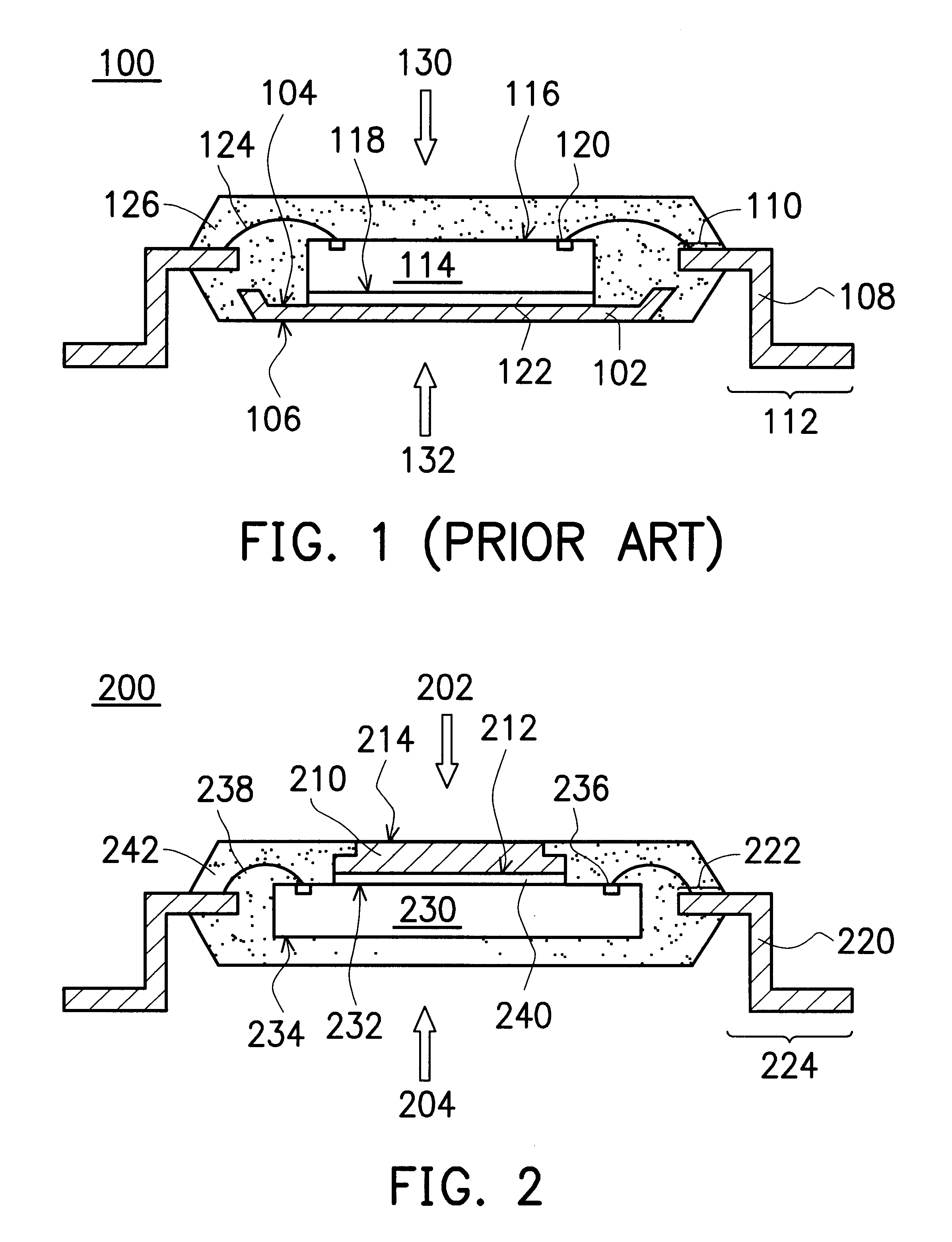
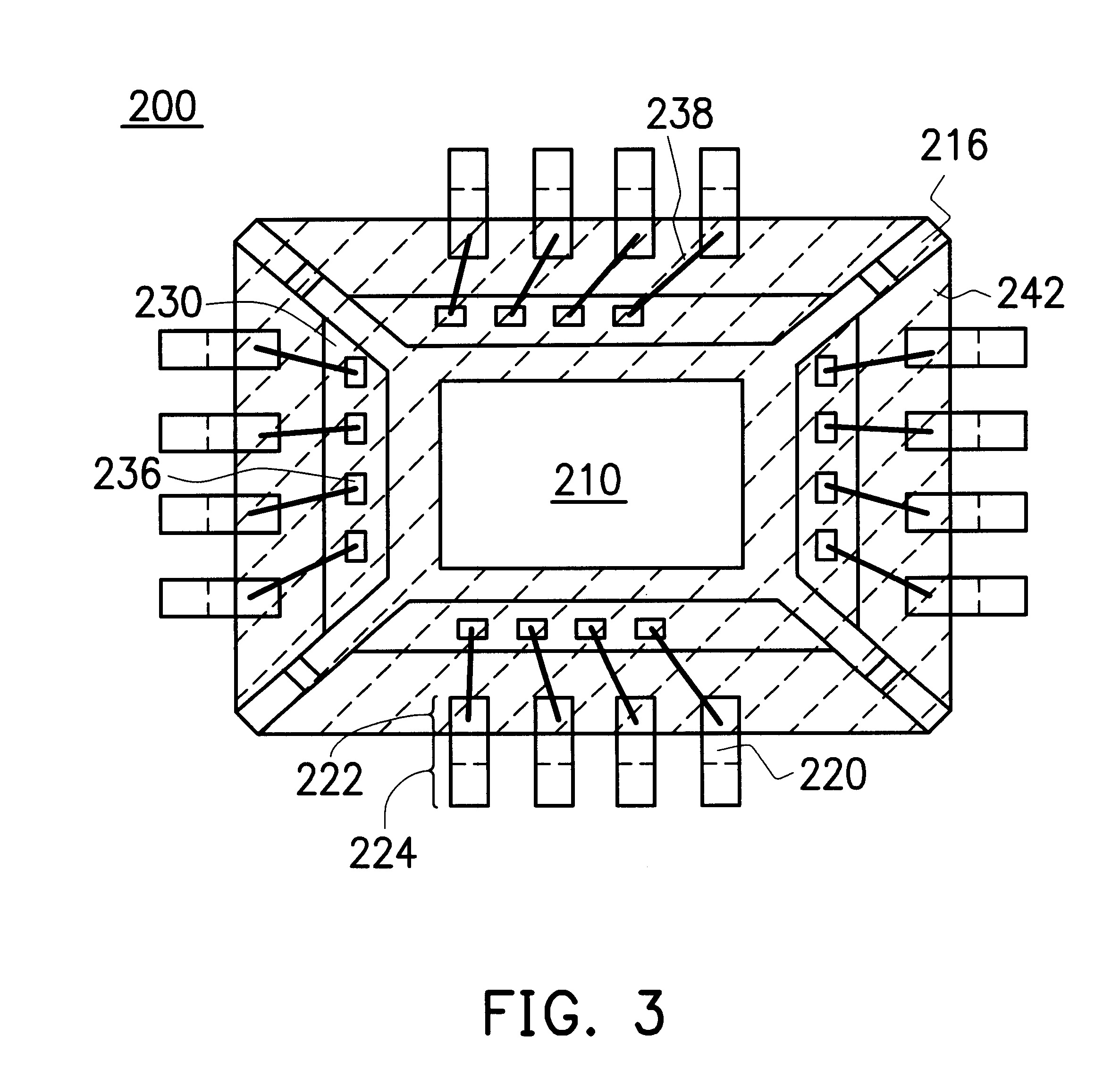
Leadless image sensor package structure and method for making the same
InactiveUS6384472B1Semiconductor/solid-state device detailsSolid-state devicesLead bondingEngineering
A leadless image sensor package constructed on a lead frame includes a die pad and a plurality of leads disposed at the periphery of the die pad. A molding compound, disposed on the top surface of the lead frame and being surrounding the die pad on the periphery of the lead frame, fills the clearance between the die pad and the leads and exposes, on the top surface, the die pad and the wire-bonding portion of the leads. Moreover, the lead frame and the molding compound constitute a "chip containing space" with chip set therein. Further, the chip with its back surface attached to the top surface of the die pad makes use of the wires to electrically connect to the bonding pad and the top surface of the wire-bonding portion, thereafter, a transparent lid is used to cap and seal the "chip containing space".
Owner:SILICONWARE PRECISION IND CO LTD
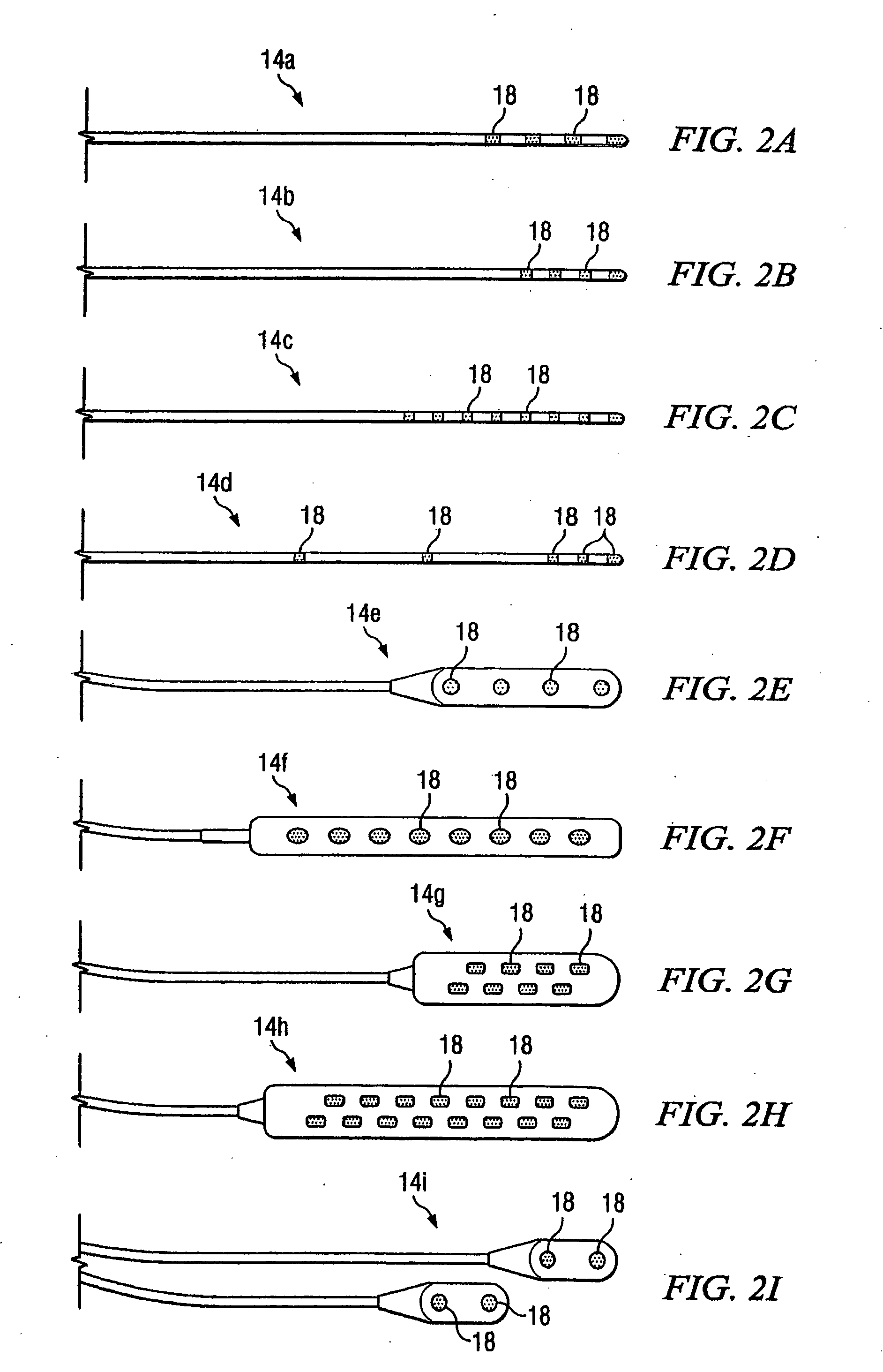
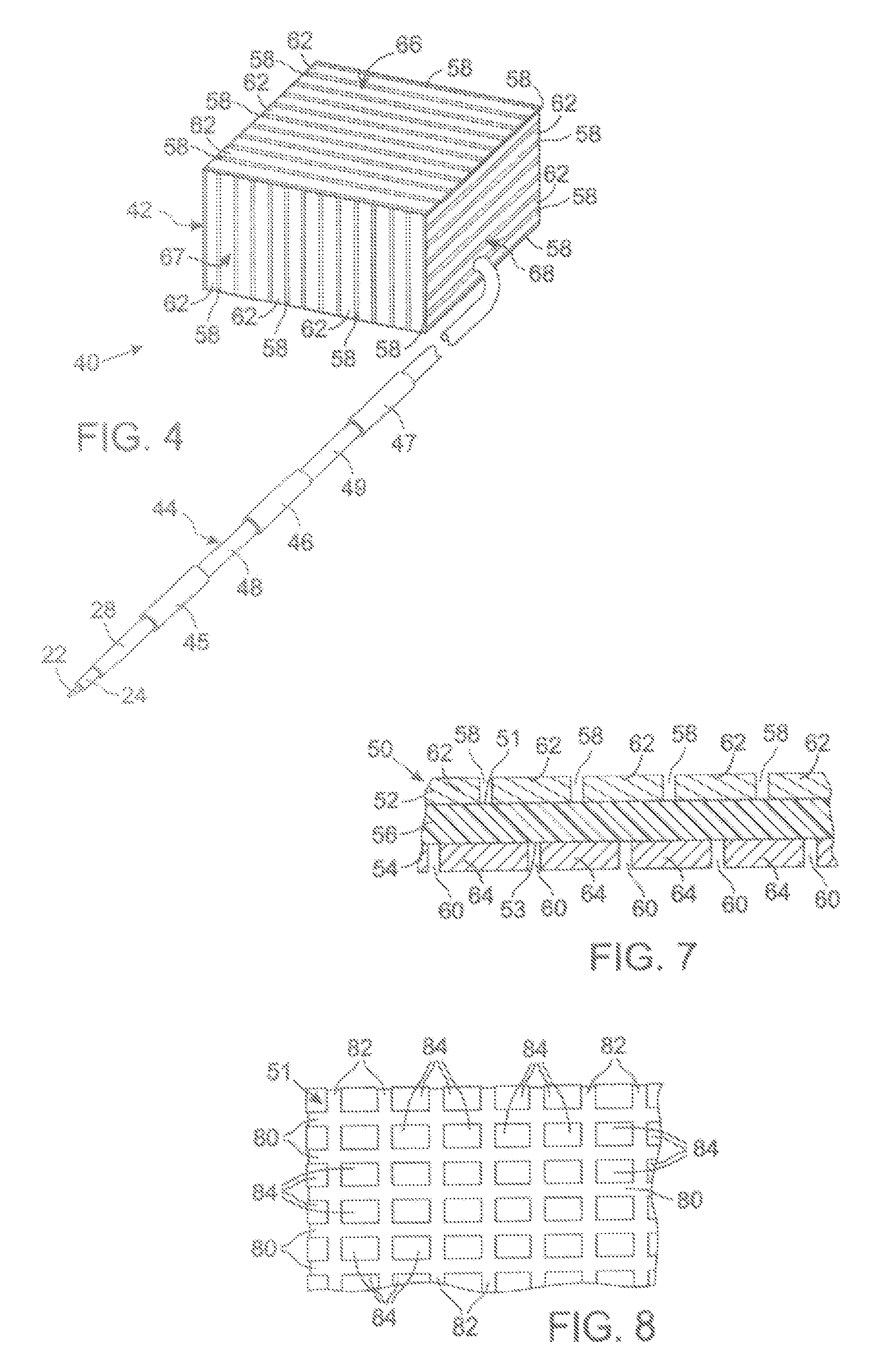
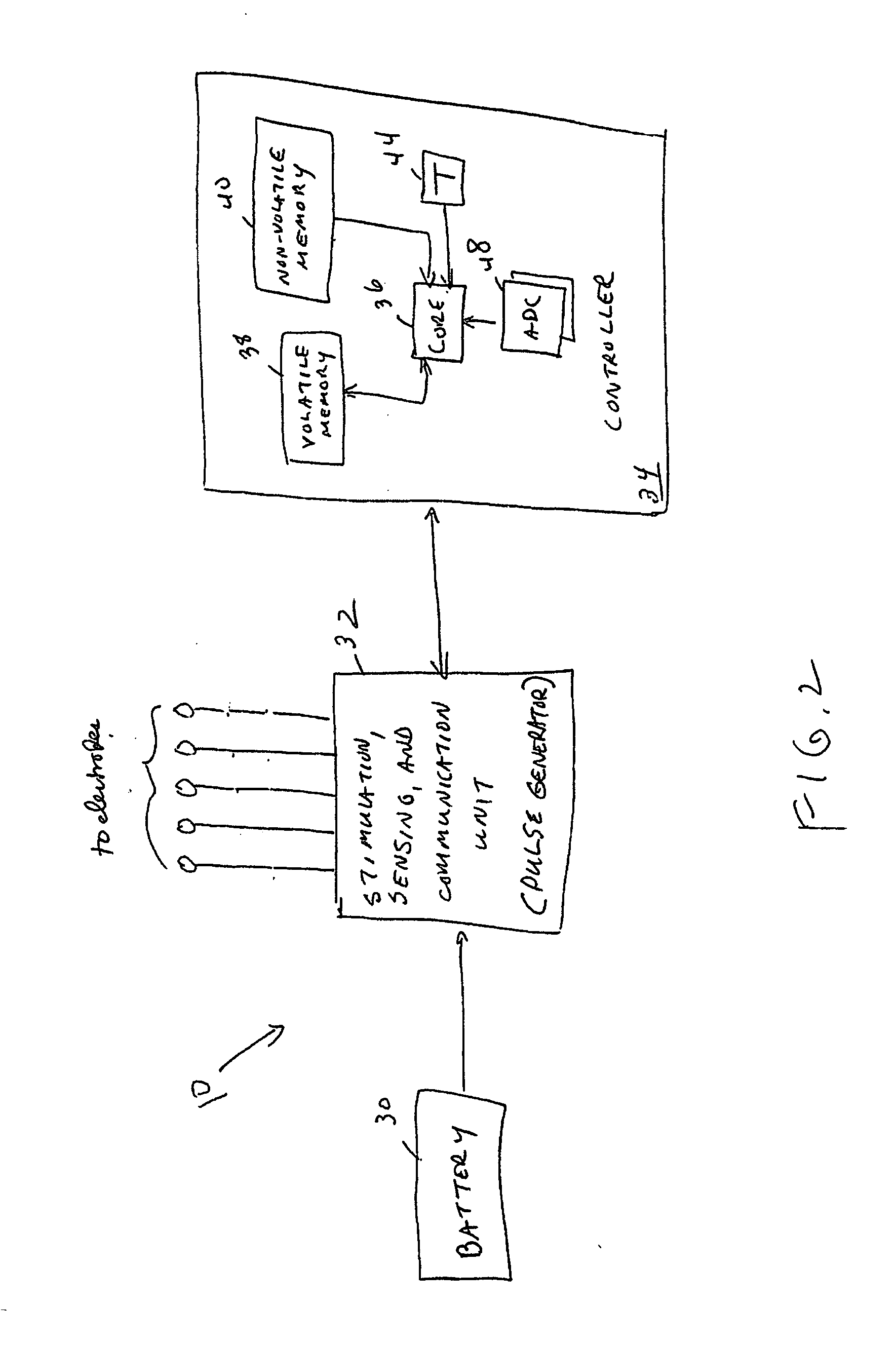
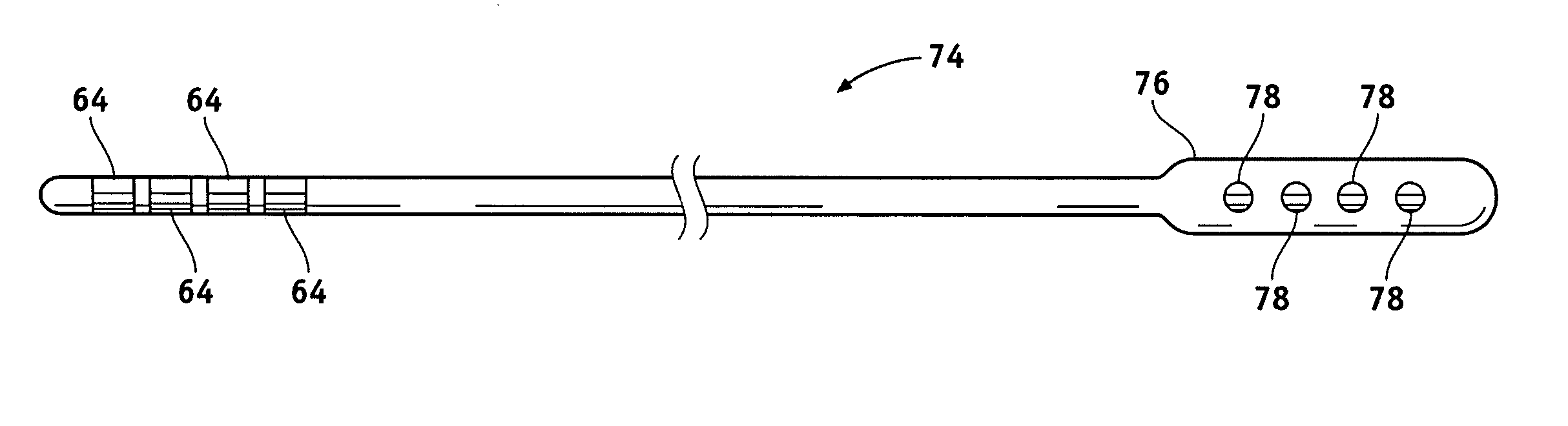
Leads with non-circular-shaped distal ends for brain stimulation systems and methods of making and using
A lead is configured and arranged for brain stimulation. The lead includes a proximal end and a distal end. The proximal end includes a plurality of terminals disposed at the proximal end. The distal end has a non-circular transverse cross-sectional shape and includes a plurality of electrodes disposed at the distal end. A plurality of conductive wires electrically couple at least one of the plurality of electrodes to at least one of the plurality of terminals.
Owner:BOSTON SCI NEUROMODULATION CORP
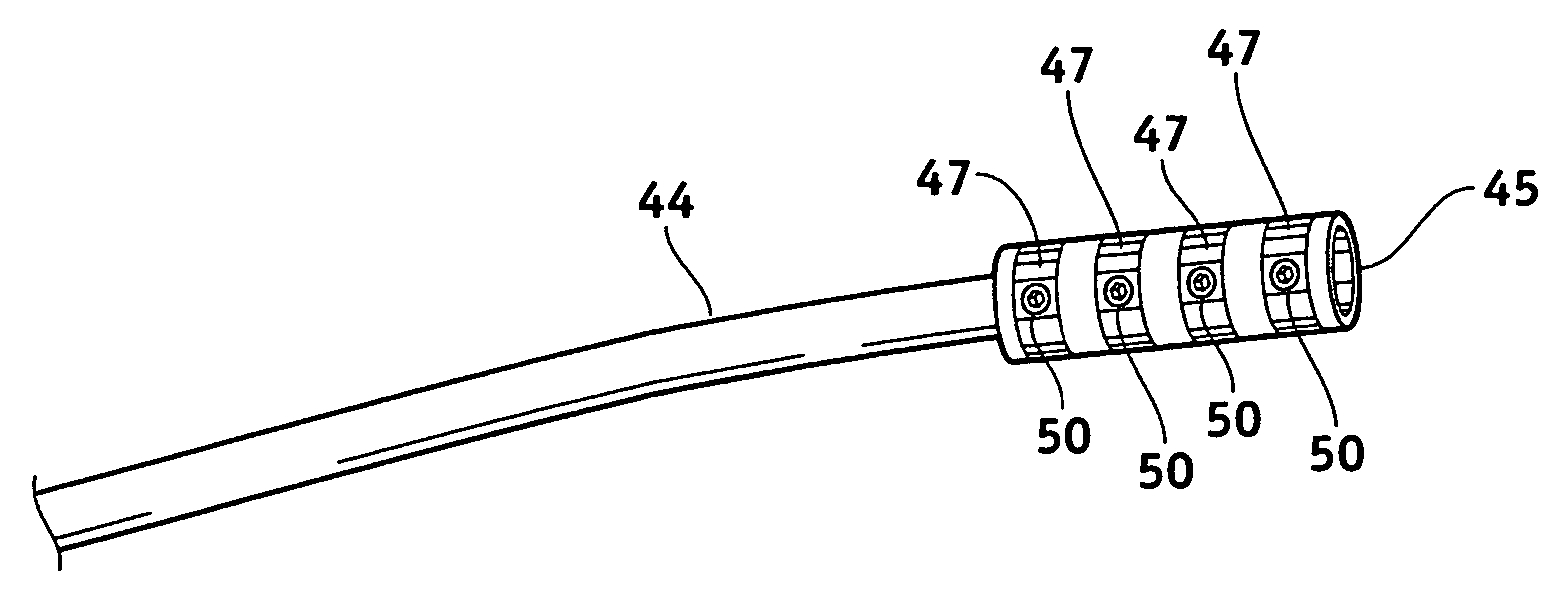
Methods and apparatus for fabricating leads with conductors and related flexible lead configurations
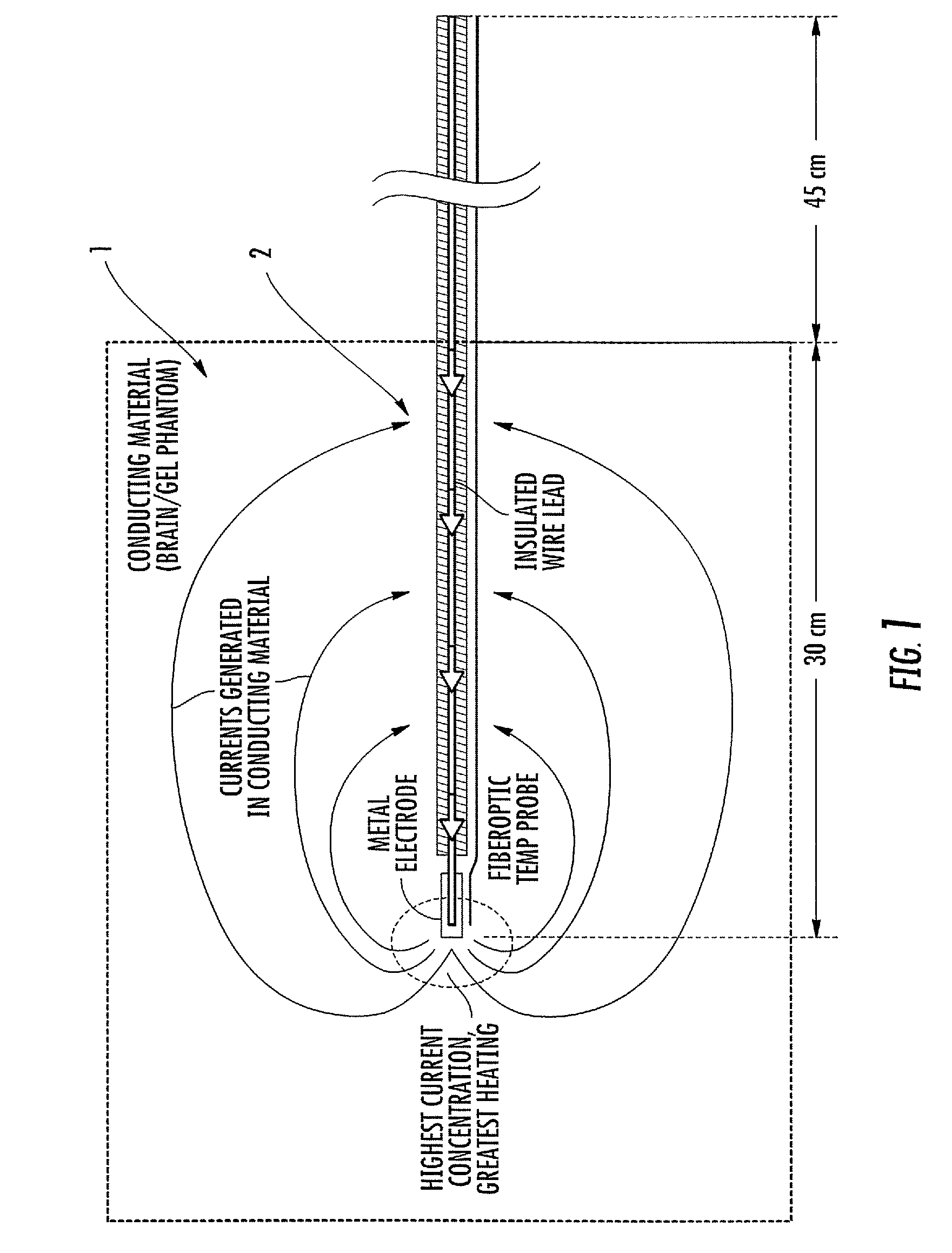
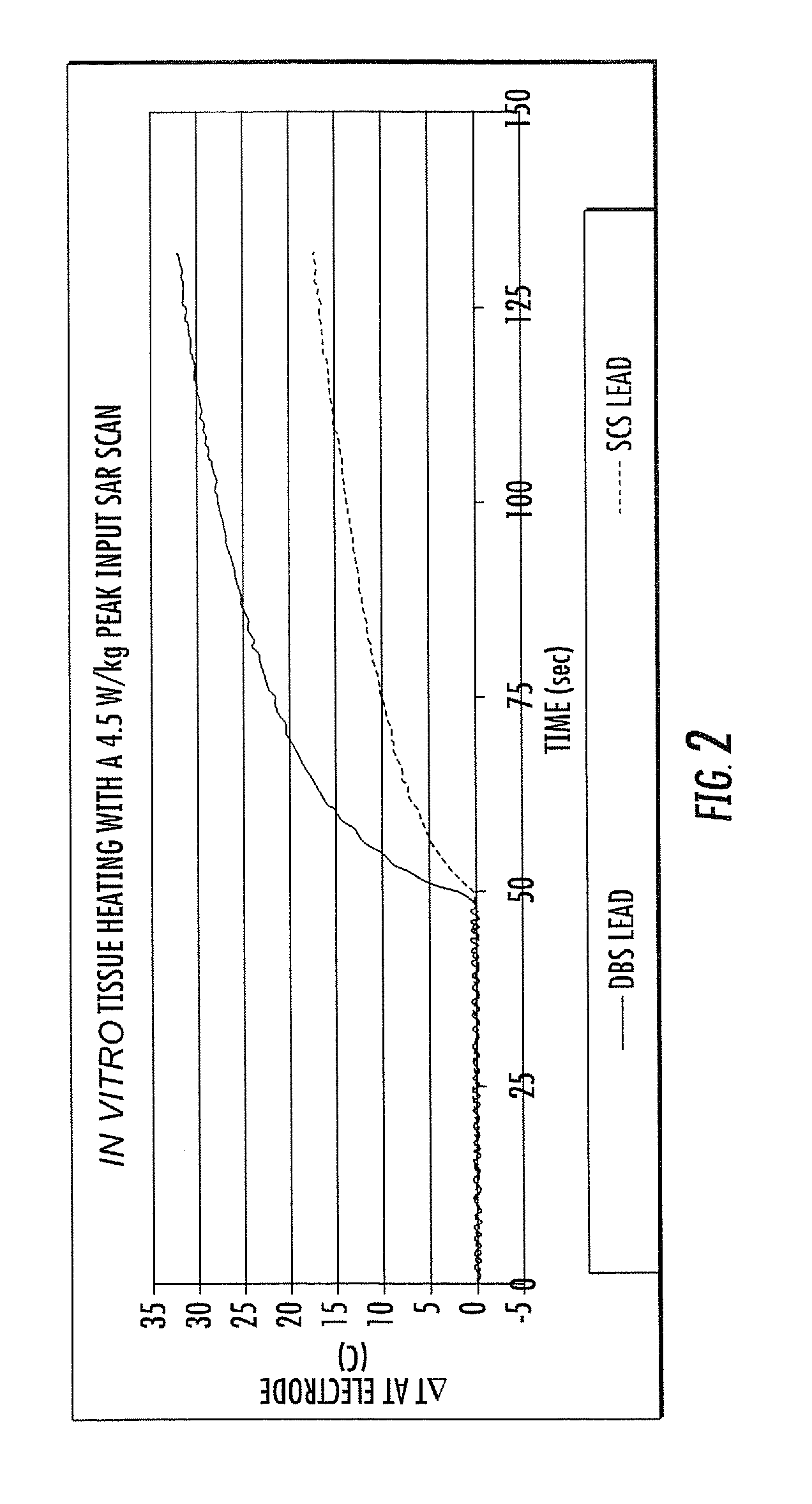
ActiveUS20080262584A1Prevent undesired heatingEasy to useInternal electrodesMaterial strength using steady bending forcesElectrical conductorDegree Celsius
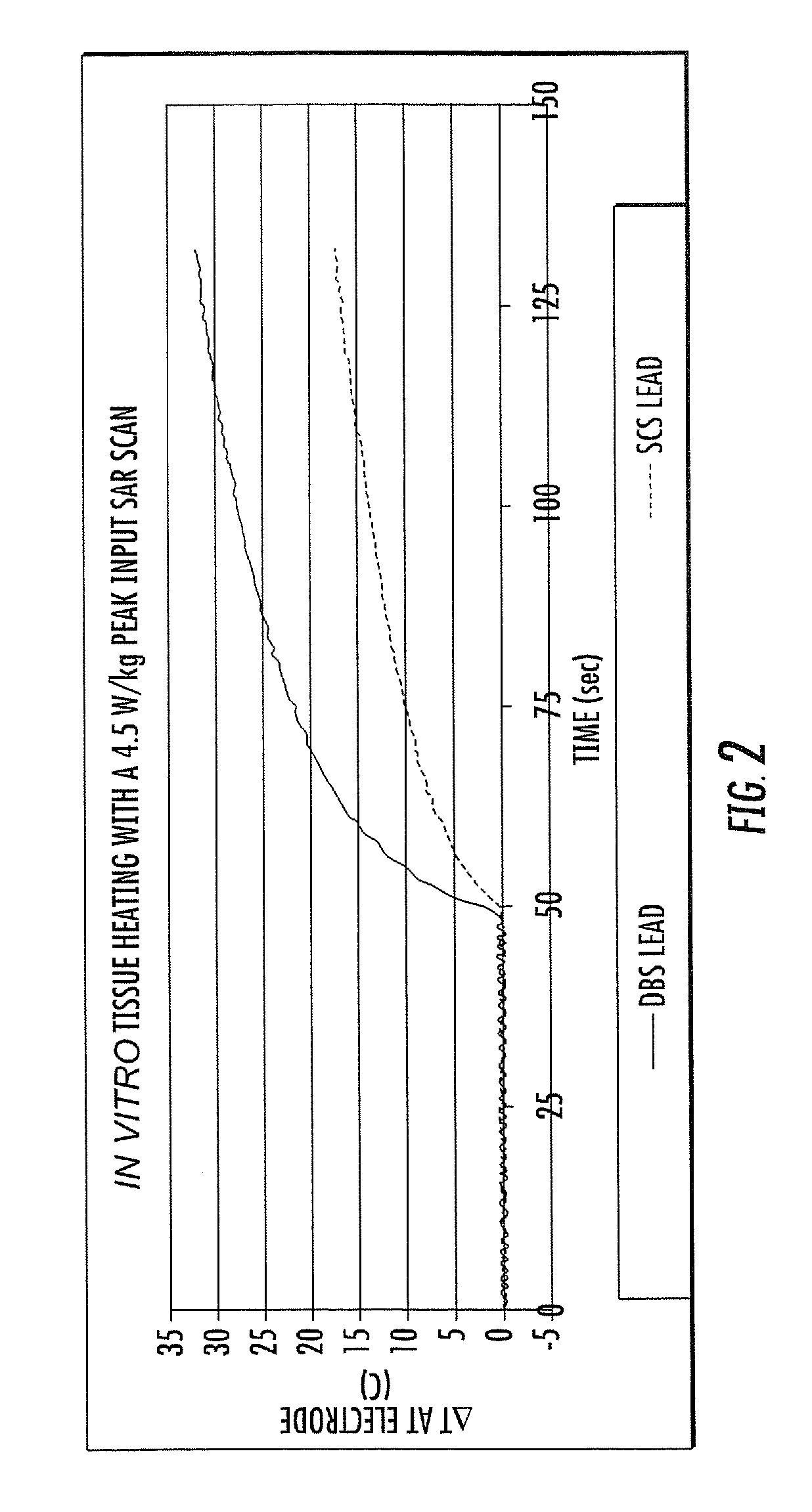
MRI / RF compatible leads include at least one conductor, a respective conductor having at least one segment with a multi-layer stacked coil configuration. The lead can be configured so that the lead heats local tissue less than about 10 degrees Celsius (typically about 5 degrees Celsius or less) or does not heat local tissue when a patient is exposed to target RF frequencies at a peak input SAR of at least about 4 W / kg and / or a whole body average SAR of at least about 2 W / kg. Related leads and methods of fabricating leads are also described.
Owner:MRI INTERVENTIONS INC +1
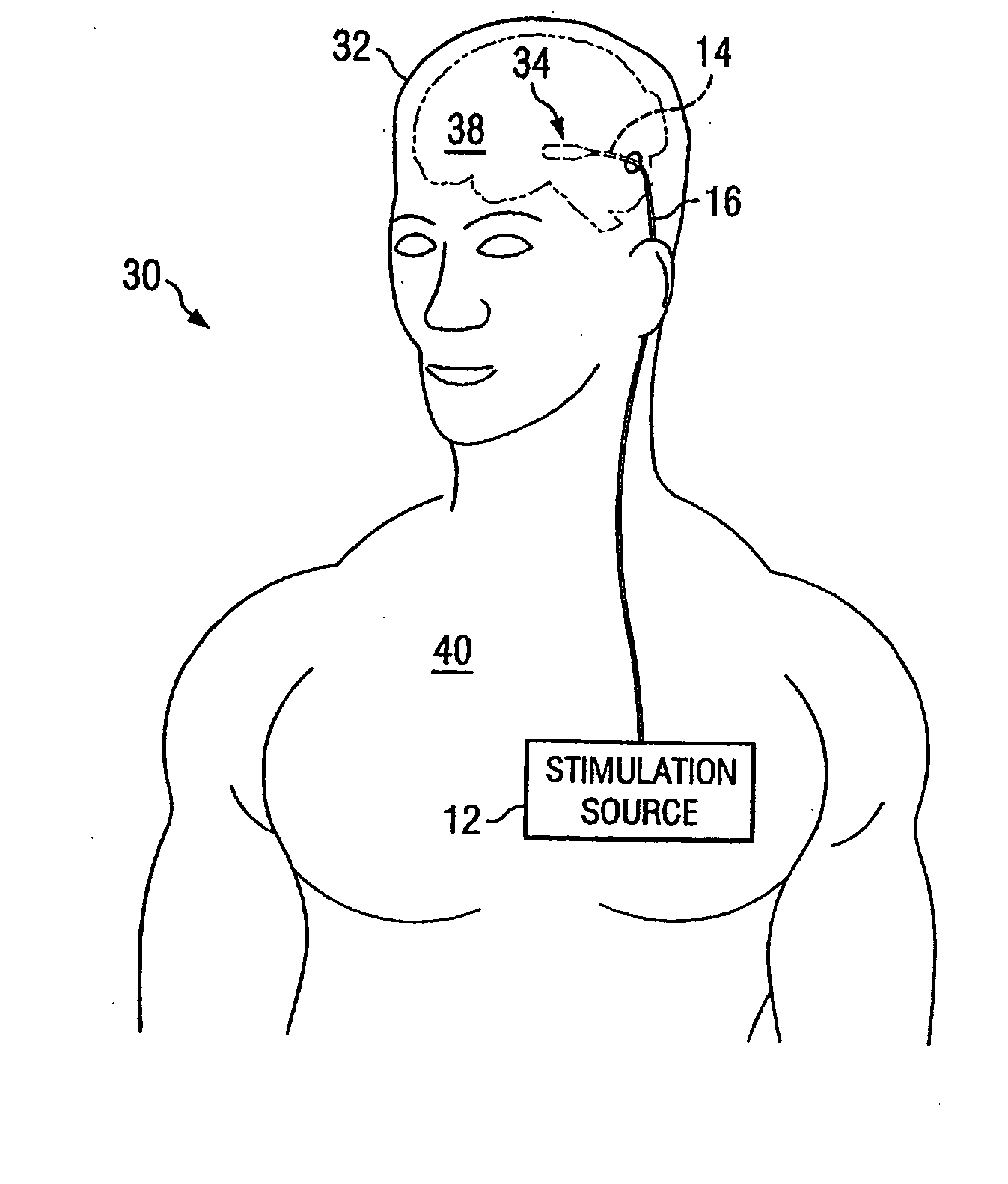
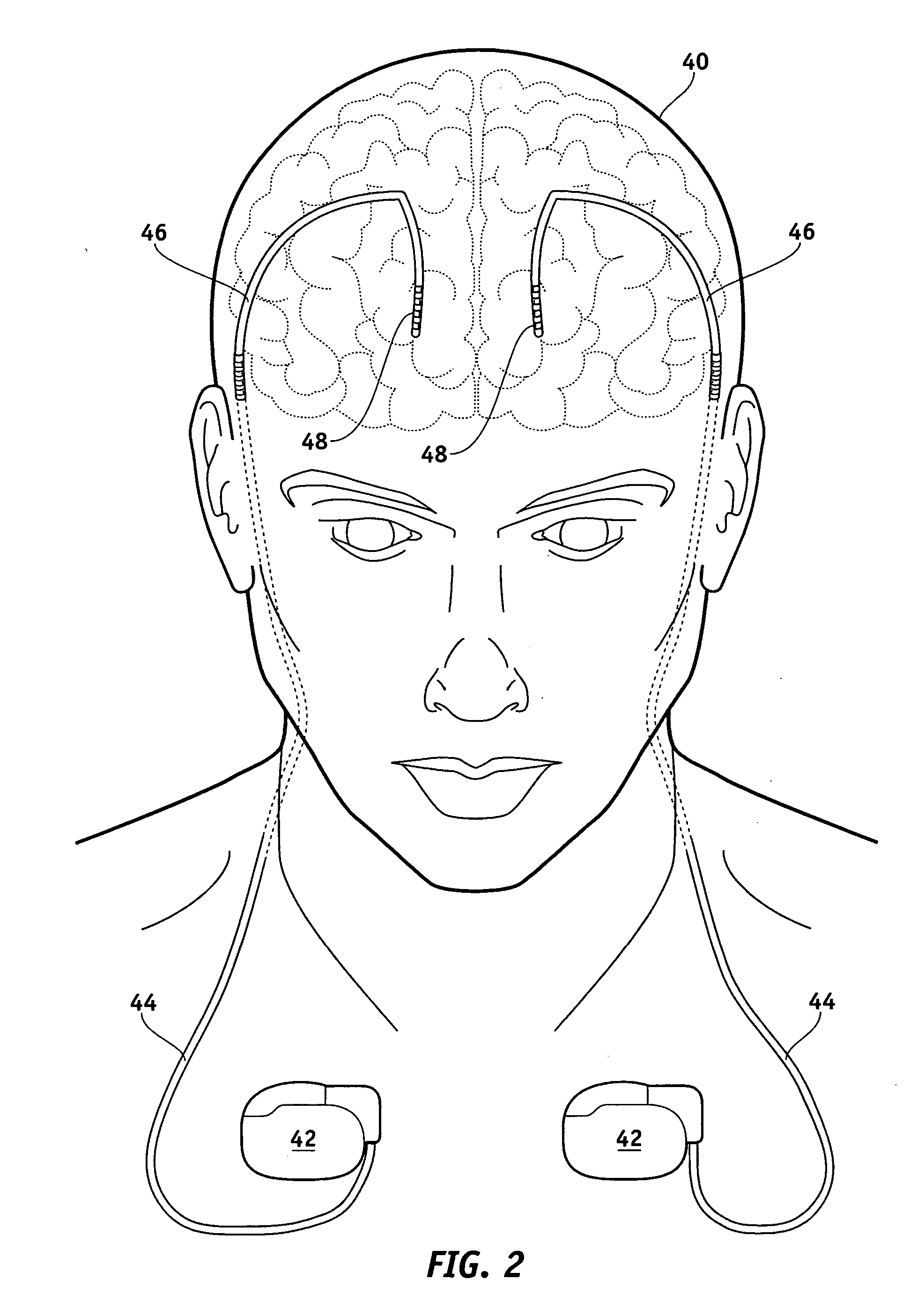
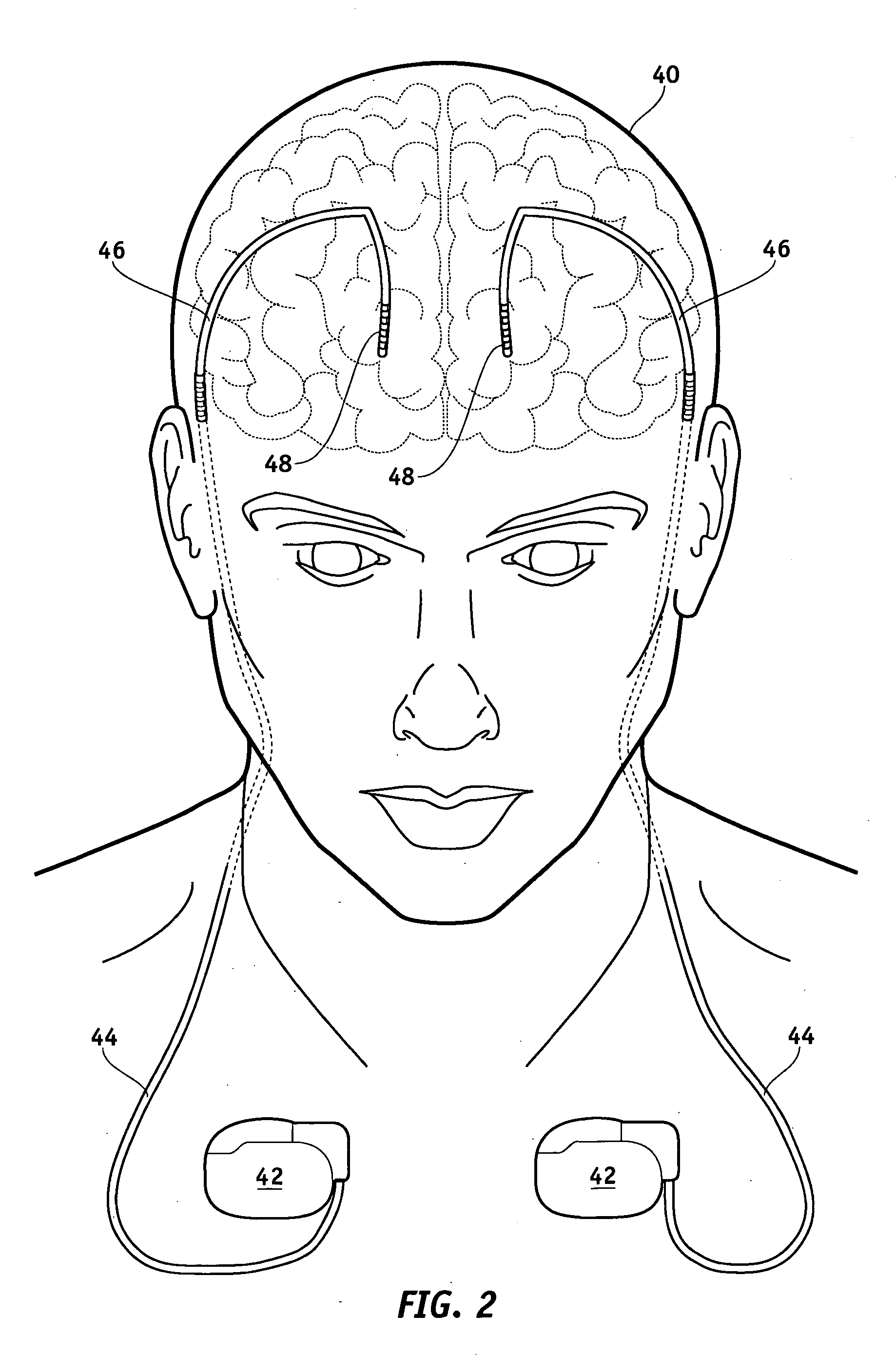
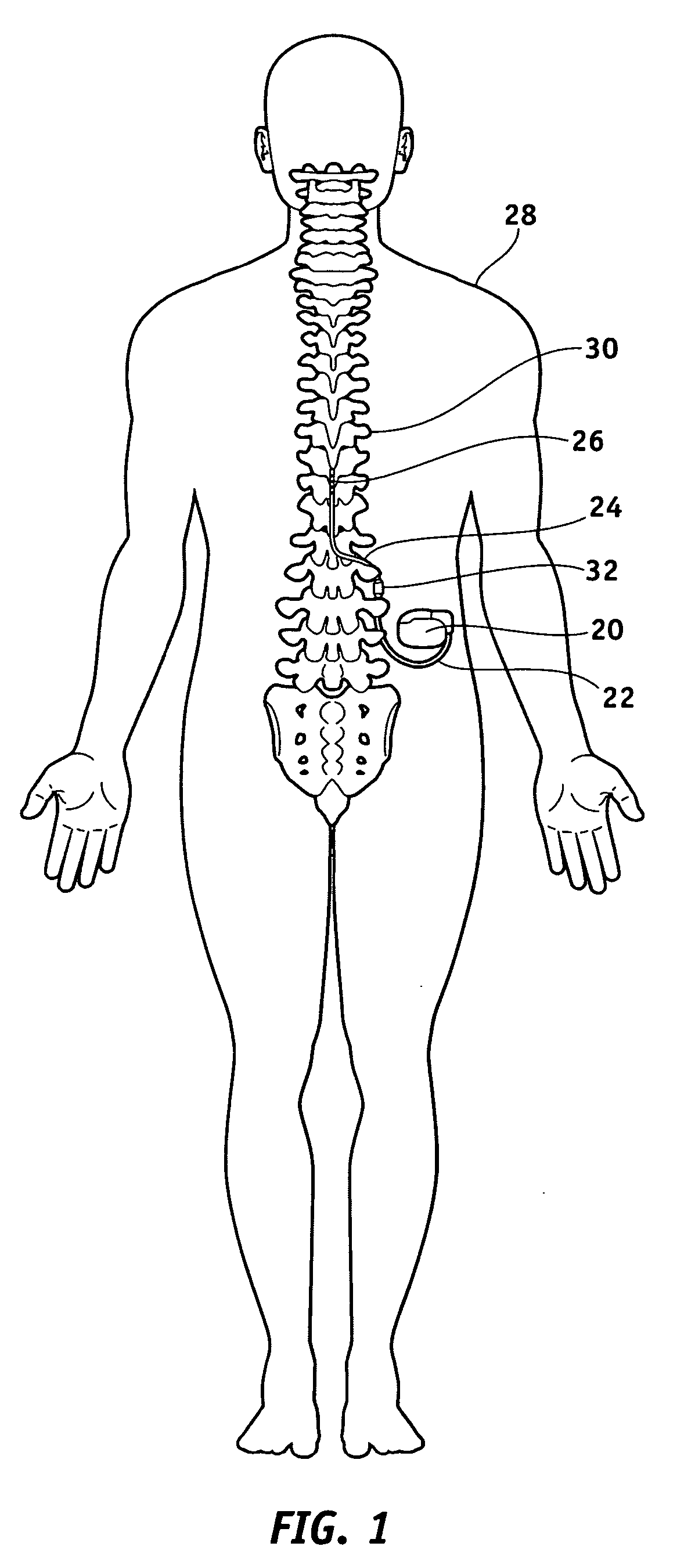
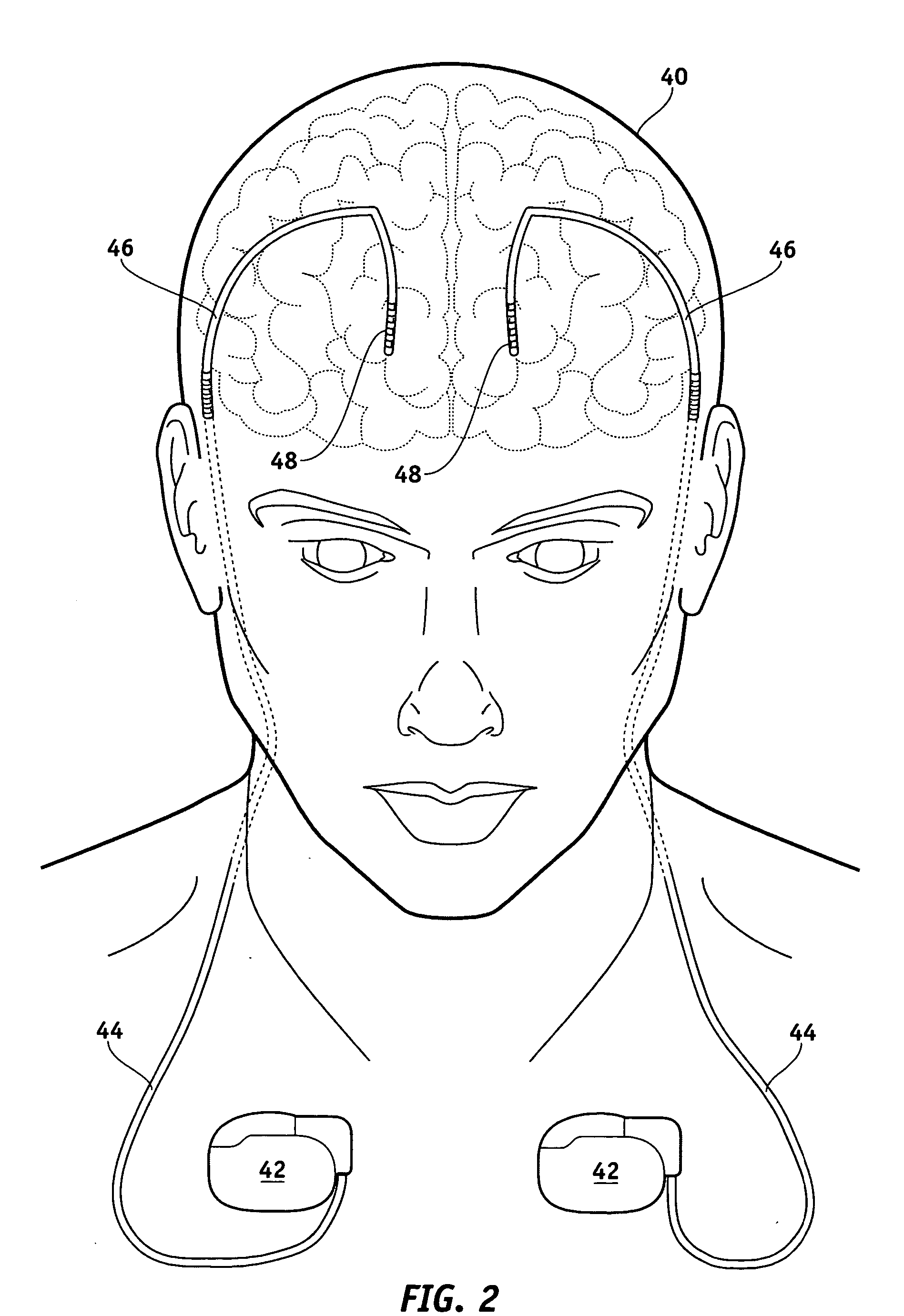
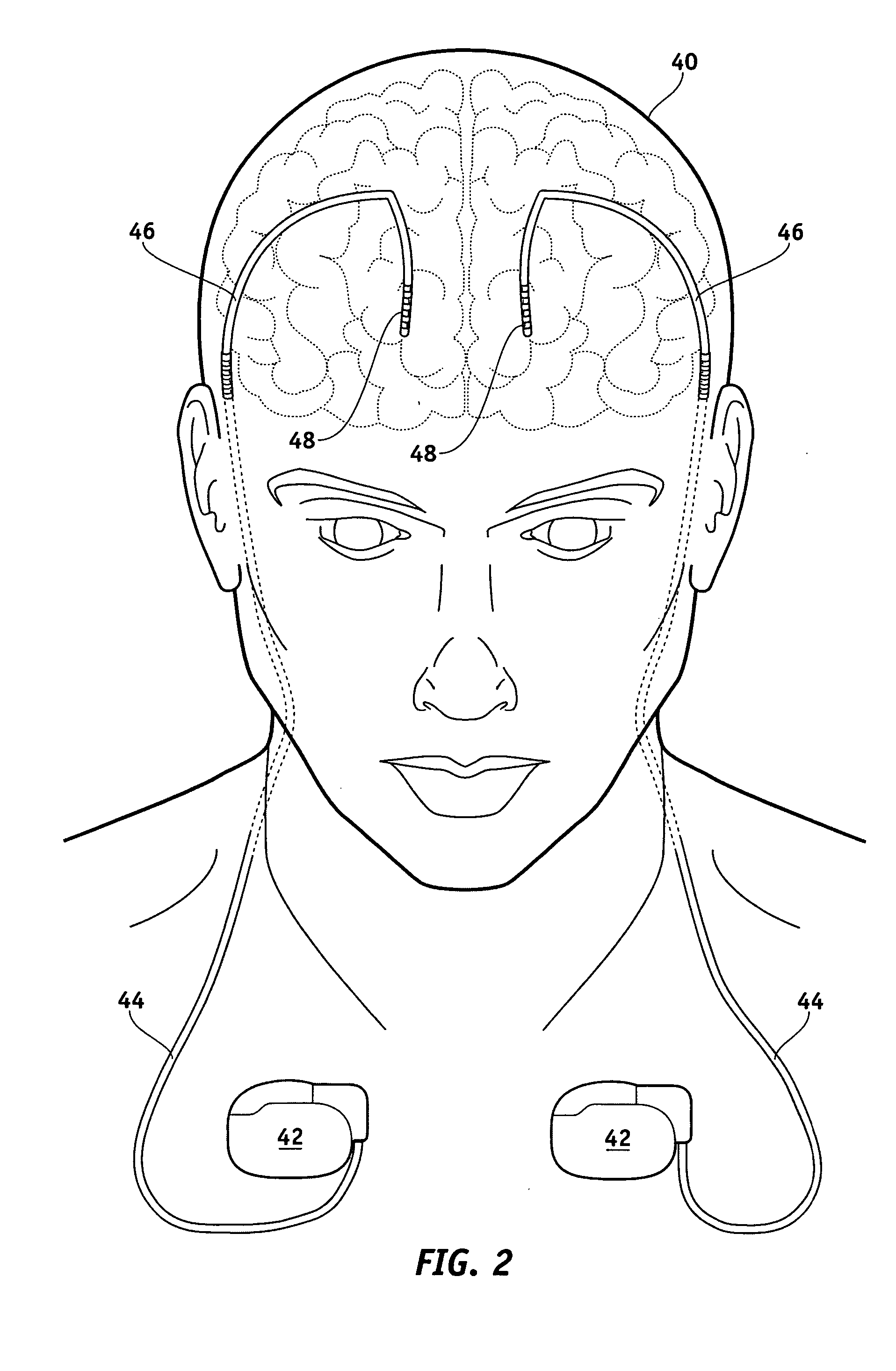
Electrical stimulation system and method for stimulating tissue in the brain to treat a neurological condition
InactiveUS20060004422A1Reduce and eliminate certain problem and disadvantageRemissionSpinal electrodesHead electrodesElectricityMedicine
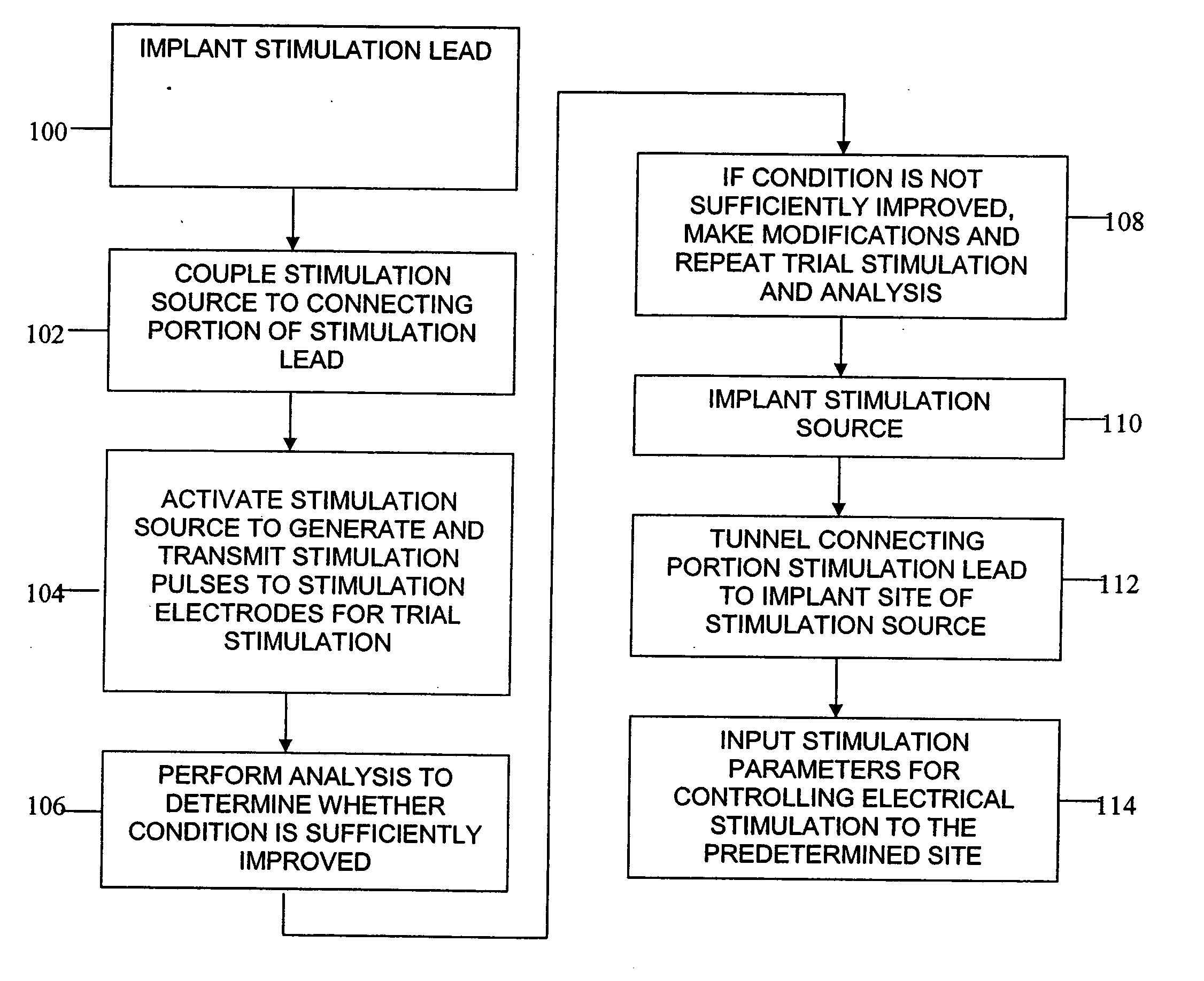
According to one aspect, a stimulation system is provided for electrically stimulating a predetermined site to treat a neurological condition. The system includes an electrical stimulation lead adapted for implantation in communication with a predetermined site, wherein the site is brain tissue site. The stimulation lead includes one or more stimulation electrodes adapted to be positioned in the predetermined site. The system also includes a stimulation source that generates the stimulation pulses for transmission to the one or more stimulation electrodes of the stimulation lead to deliver the stimulation pulses to the predetermined site to treat a neurological disorder or condition.
Owner:ADVANCED NEUROMODULATION SYST INC
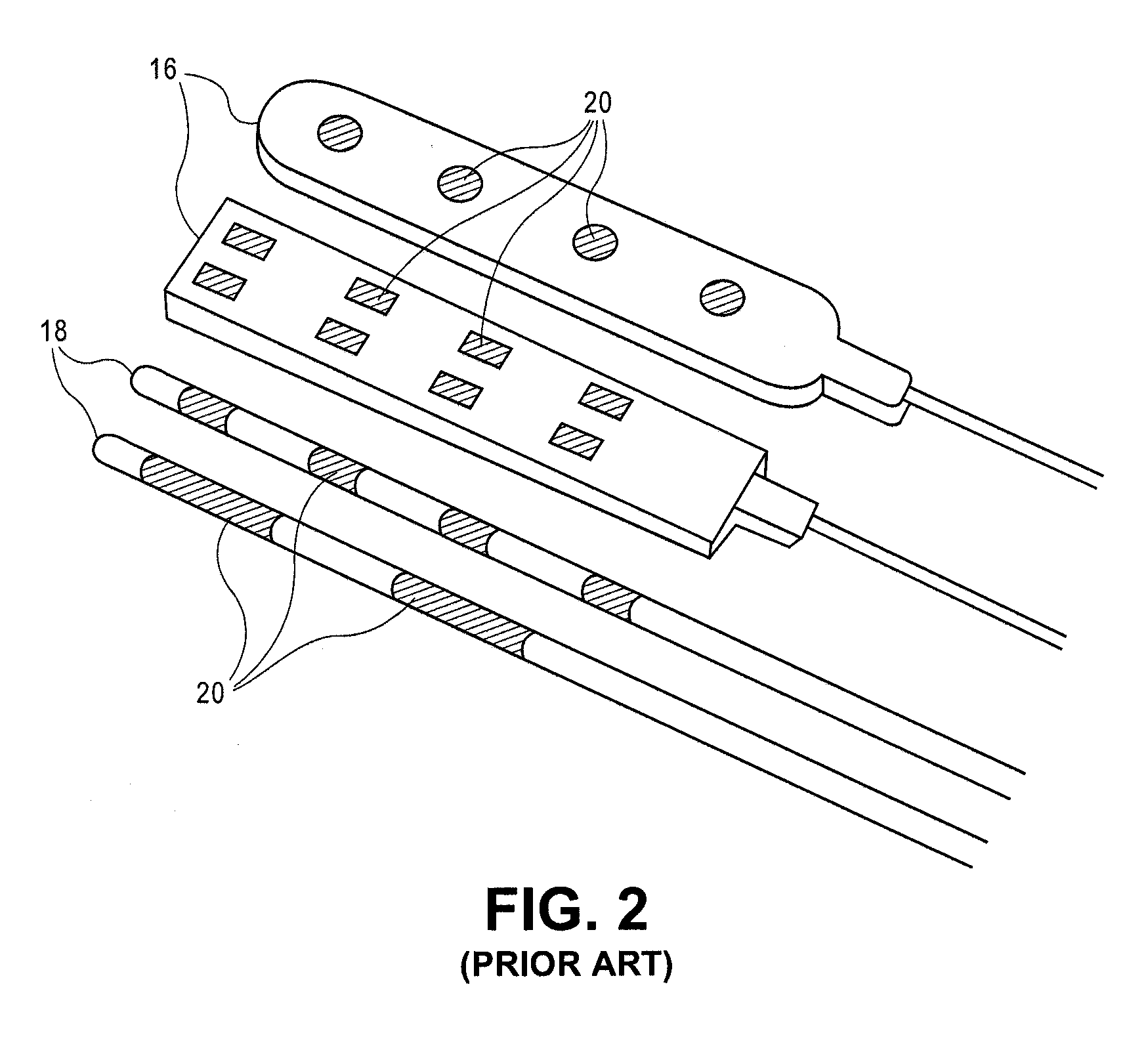
Lead Assembly and Method of Making Same
A lead assembly and a method of making a lead are provided. The lead comprises a terminal, proximal end having a plurality of terminal contacts and material separating the terminal contacts. In one embodiment of the lead, the terminal contacts are separated by a preformed spacer, that may be made from various hard materials such as polyurethane, PEEK and polysulfone. Epoxy may be used to fill spaces at the proximal lead end, including between the spacer and terminal contacts. In one embodiment of the lead, the terminal contacts are separated by epoxy only. The lead may include a plurality of conductor lumens that contain conductors. The lead may also include a stylet lumen for accepting a stylet.
Owner:BOSTON SCI NEUROMODULATION CORP
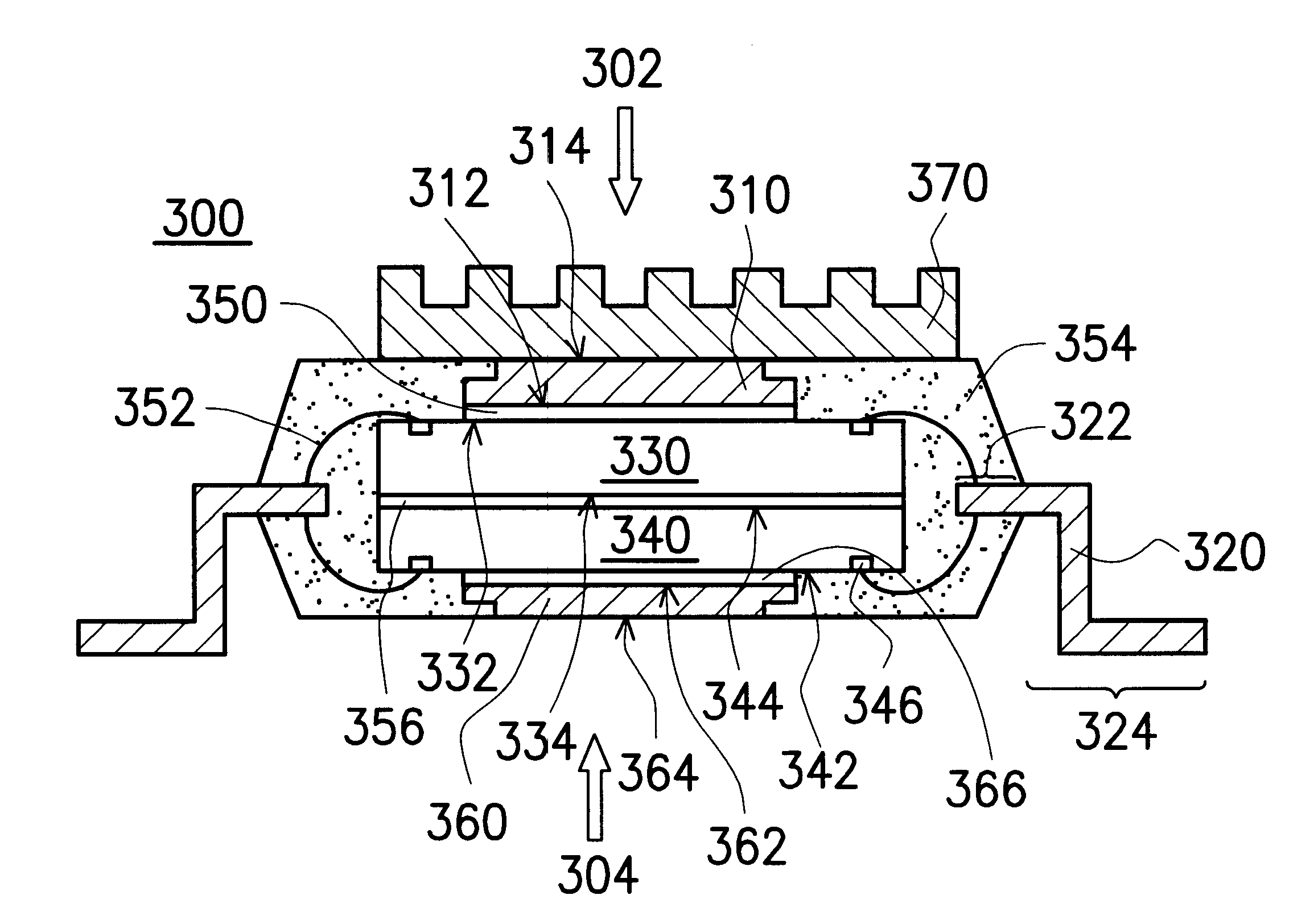
Thermally enhanced quad flat non-lead package of semiconductor
InactiveUS6198171B1Semiconductor/solid-state device detailsSolid-state devicesSemiconductorWire bonding
A thermally enhanced quad flat non-lead package of semiconductor comprises a chip a plurality of leads, and a molding compound. The chip has its active surface bonded to the top surface of the die pad, and the area of the die pad is smaller than that of the chip in order to expose the bonding pads on the active surface of the chip. The leads are disposed at the periphery of the die pad wherein the bottom surface of the lead has a stepped structure with a relatively thin portion to form a wire-bonding protruded zone. A plurality of bonding wires is used to electrically connect the wire-bonding protruded zone of the leads to the bonding pads of the chip. The molding compound encapsulates the chip, bonding wires, the die pad, and a portion of the surface of the leads, but exposes the bottom surface of the die pad. In this way, the encapsulating process makes the side surface of the lead, and the portion excluding the wire-bonding protruded zone of the bottom surface of the lead exposed in order to make the lead become the external connecting points of the package structure.
Owner:SILICONWARE PRECISION IND CO LTD
Lead electrode for use in an MRI-safe implantable medical device
ActiveUS20050222658A1Reduce electromagnetic couplingSpinal electrodesHead electrodesElectromagnetic couplingMedicine
A neurostimulation lead is configured to be implanted into a patient's body and has at least one distal electrode. The lead comprises at least one conductive filer electrically coupled to the distal electrode, a jacket for housing the conductive filer and a shield surrounding at least a portion of the filer for reducing electromagnetic coupling to the filer.
Owner:MEDTRONIC INC
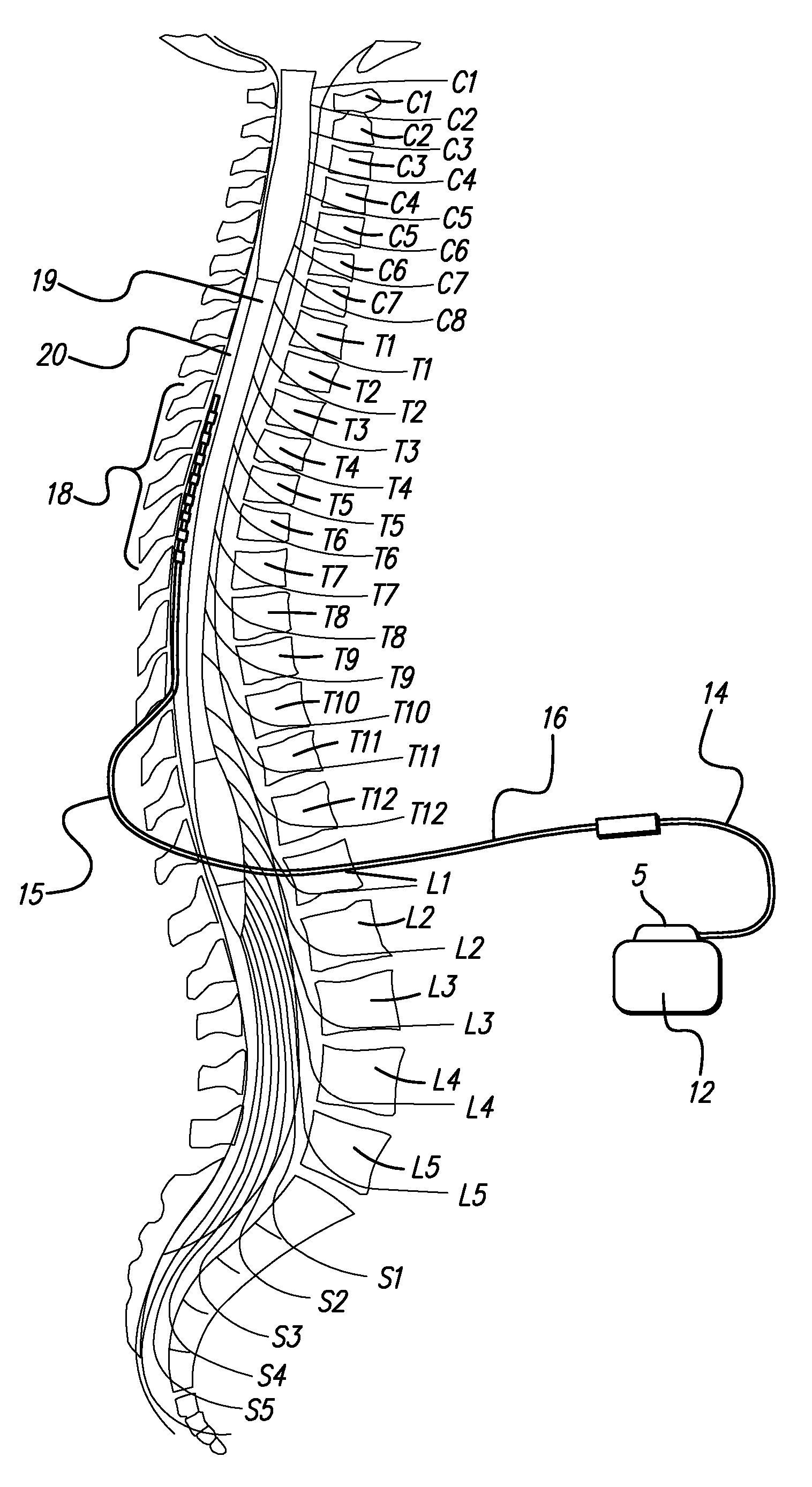
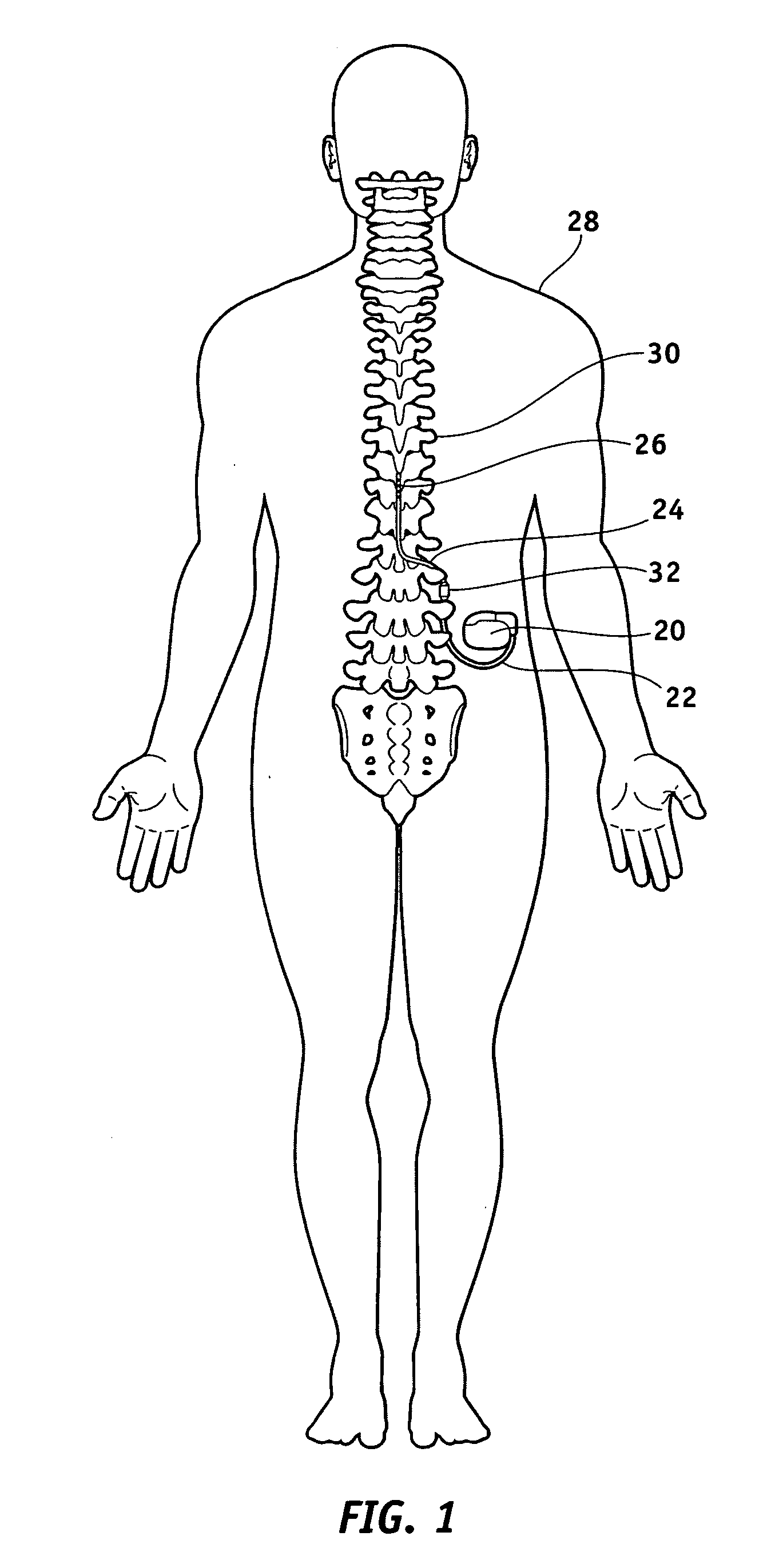
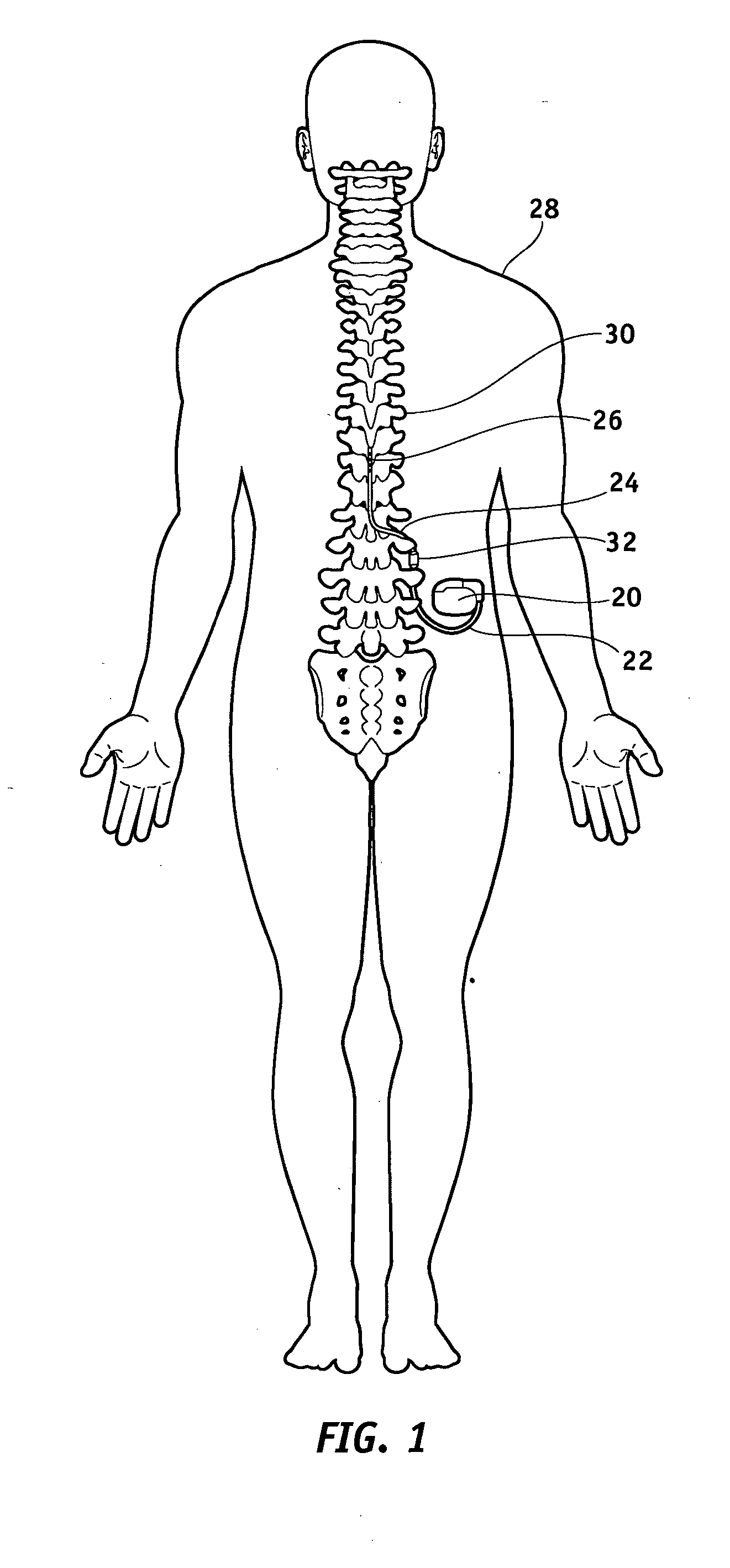
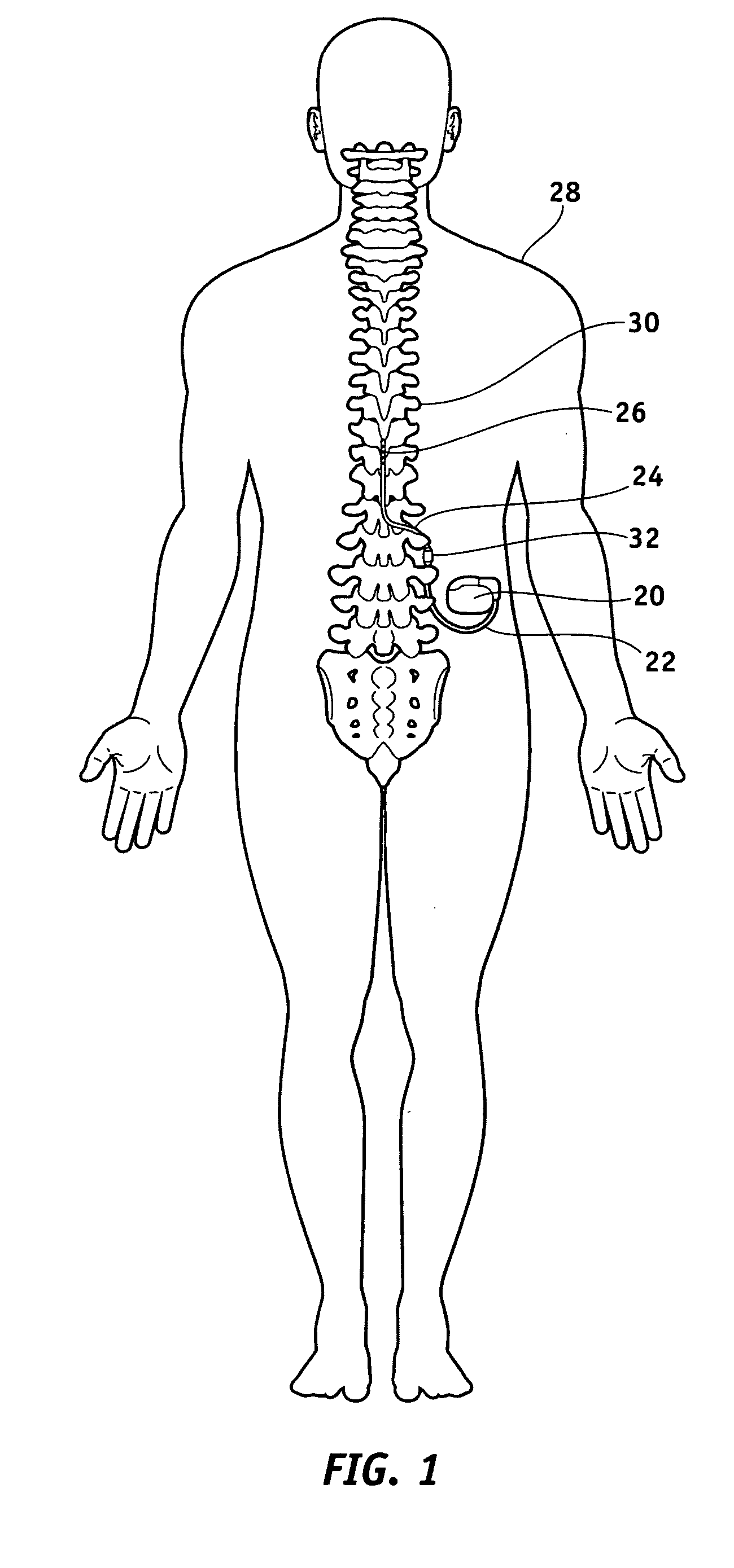
Method of using spinal cord stimulation to treat neurological disorders or conditions
InactiveUS20070060954A1Improve the quality of lifeEffectively treat lack of coordinationSpinal electrodesImplantable neurostimulatorsMedicineElectrical stimulations
The present invention involves methods and systems for using electrical stimulation to treat neurological disorders. More particularly, the method comprises surgically implanting an electrical stimulation lead that is in communication with spinal nervous tissue associated with a first, second, or third cervical vertebral segment to result in spinal nervous tissue stimulation, thus treating a wide variety of neurological disorders.
Owner:ADVANCED NEUROMODULATION SYST INC
Compartmentalised screening by microfluidic control
InactiveUS20050221339A1Rapid and high-throughput screeningLow costCompound screeningSequential/parallel process reactionsCompound (substance)Drug development
The invention describes a method for the identification of compounds which bind to a target component of a biochemical system or modulate the activity of the target, comprising the steps of: a) compartmentalising the compounds into microcapsules together with the target, such that only a subset of the repertoire is represented in multiple copies in any one microcapsule; and b) identifying the compound which binds to or modulates the activity of the target; wherein at least one step is performed under microfluidic control. The invention enables the screening of large repertoires of molecules which can serve as leads for drug development.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE +1
Quad flat non-lead package of semiconductor
InactiveUS6414385B1Semiconductor/solid-state device detailsSolid-state devicesLead bondingSemiconductor package
A Quad Flat Non-Lead package of semiconductor comprises a chip, a plurality of leads, and a molding compound. The chip has its active surface bonded to the die pad, and the area of the die pad is smaller than that of the chip in order to expose the bonding pad on the active surface of the chip. The leads are disposed at the periphery of the die pad. A plurality of bonding wires is used to electrically connect the top surface of the leads to the bonding pads. The molding compound encapsulates the chip, the die pad, the bonding wires, and a portion of the surface of the leads. In this way, the encapsulating process make the side surface of the lead, and the portion excluding the wire-bonding protruded zone of the bottom surface of the lead exposed in order to make the leads become the external connections of the package structure.
Owner:SILICONWARE PRECISION IND CO LTD
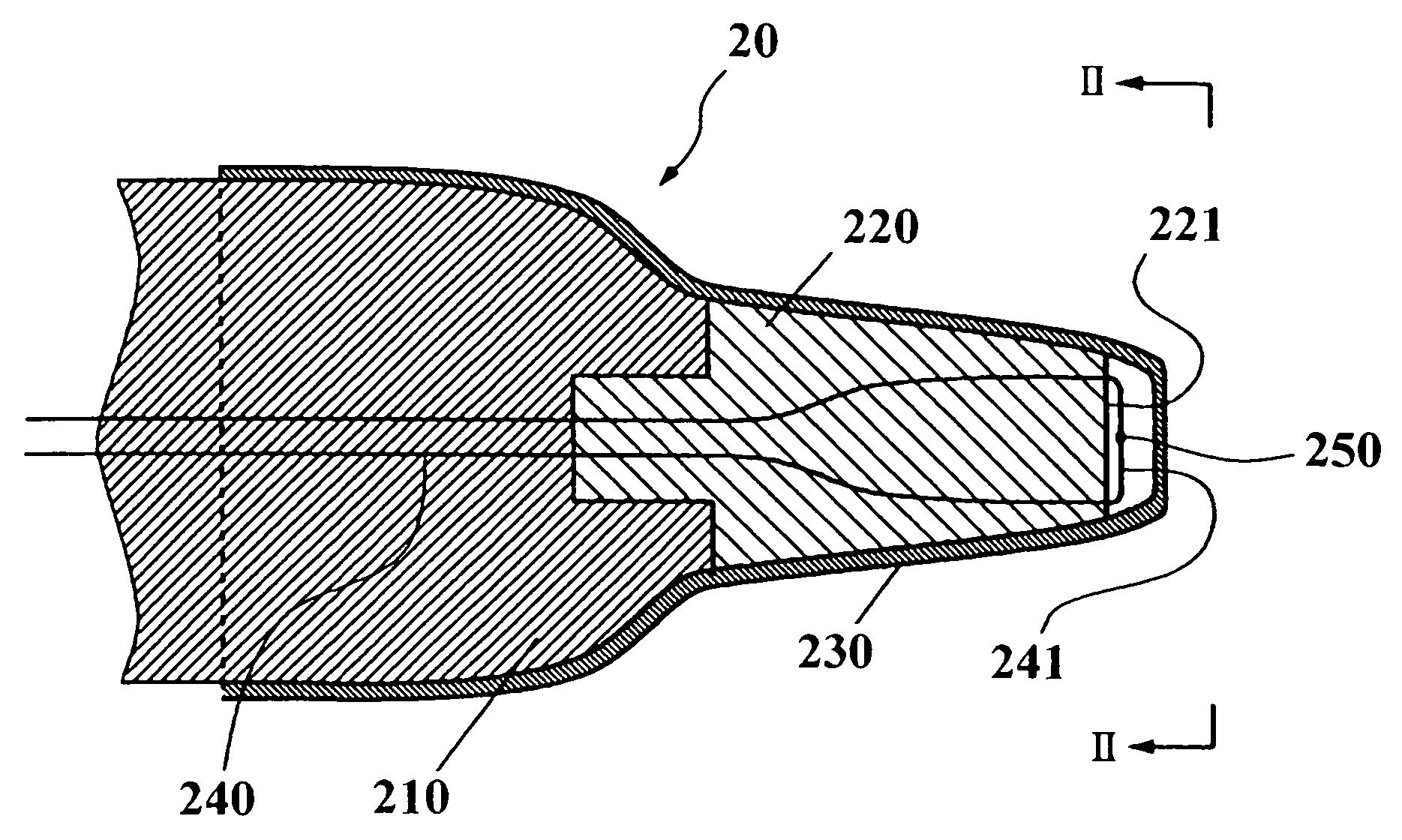
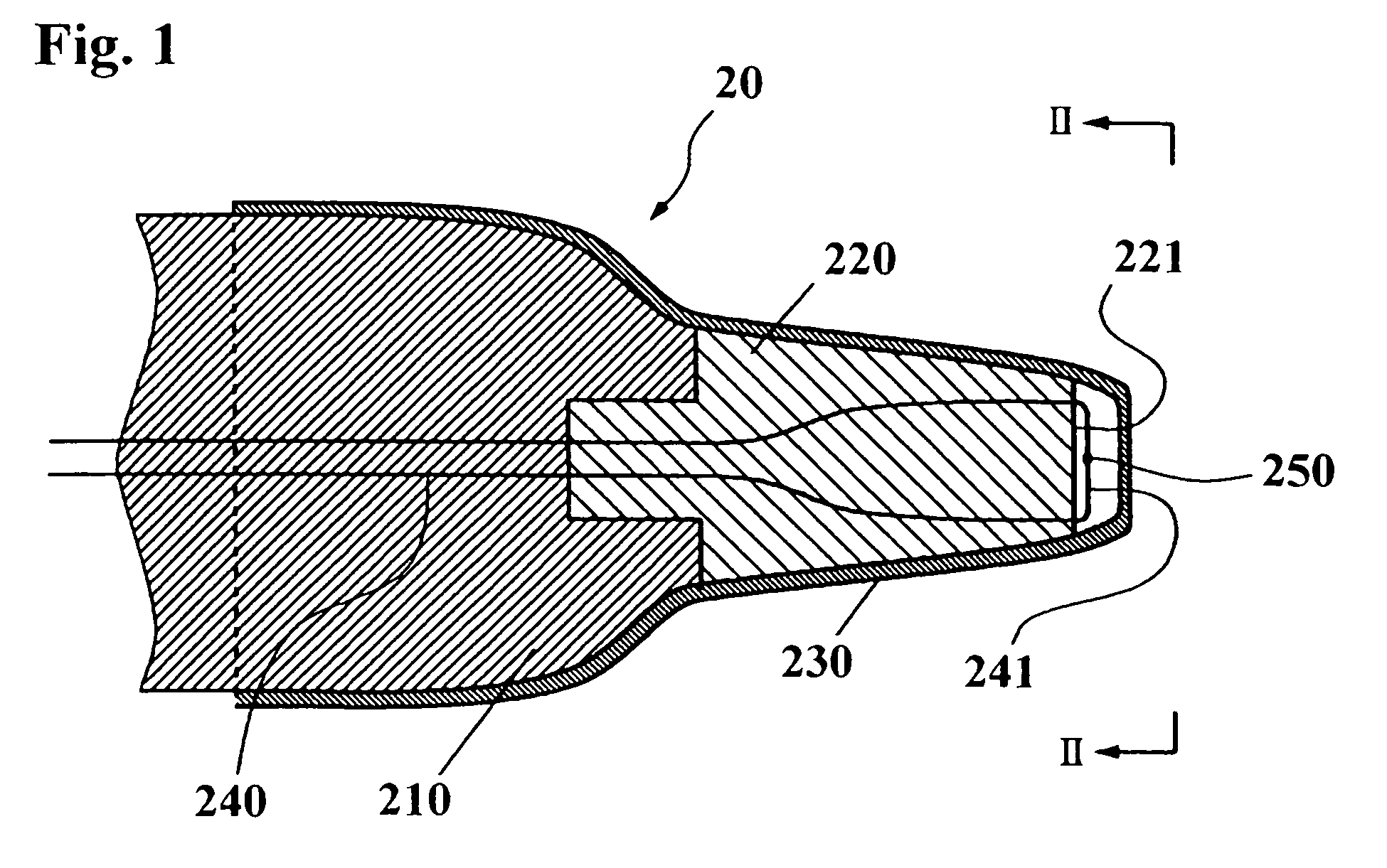
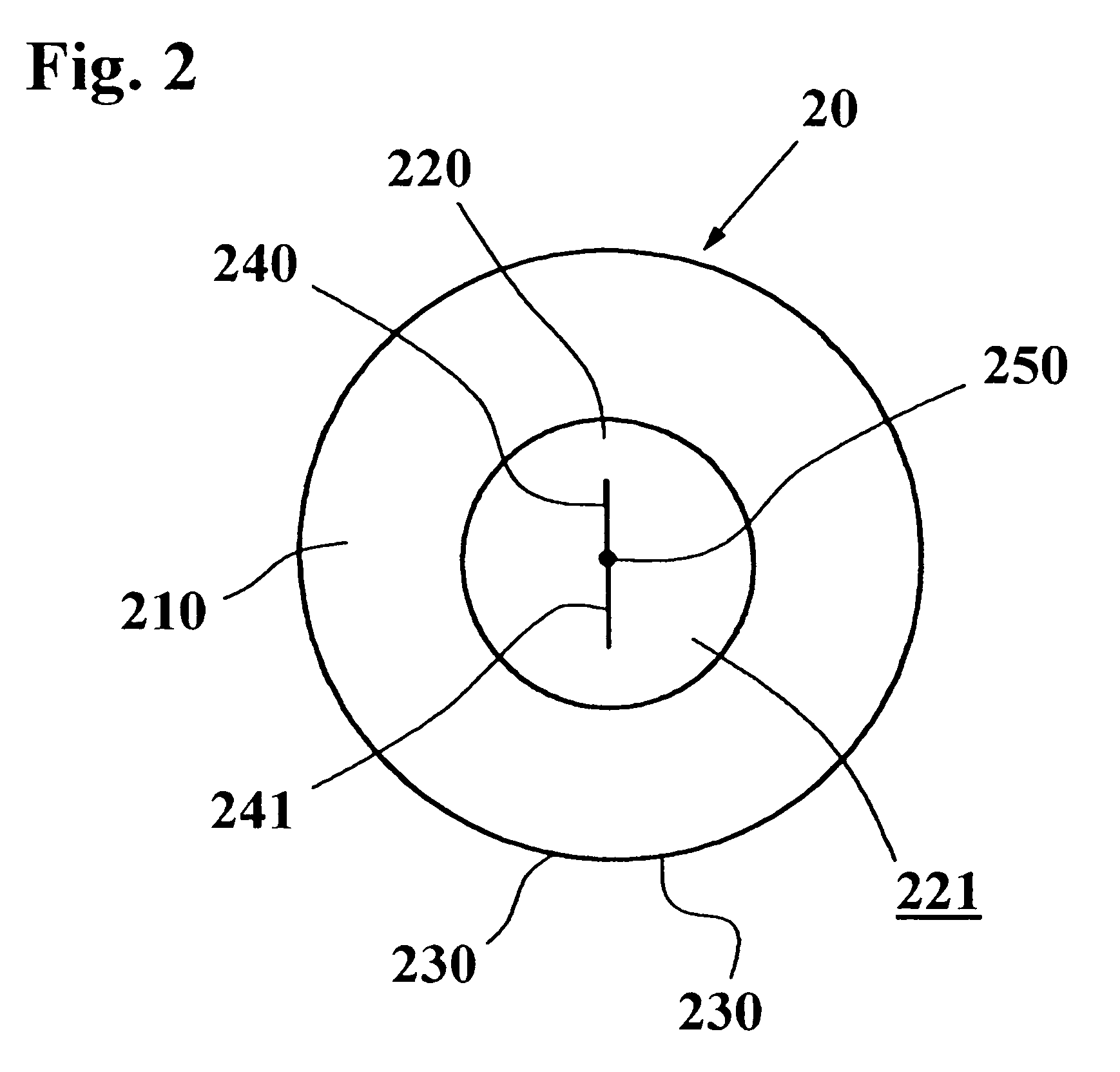
Ear-type clinical thermometer
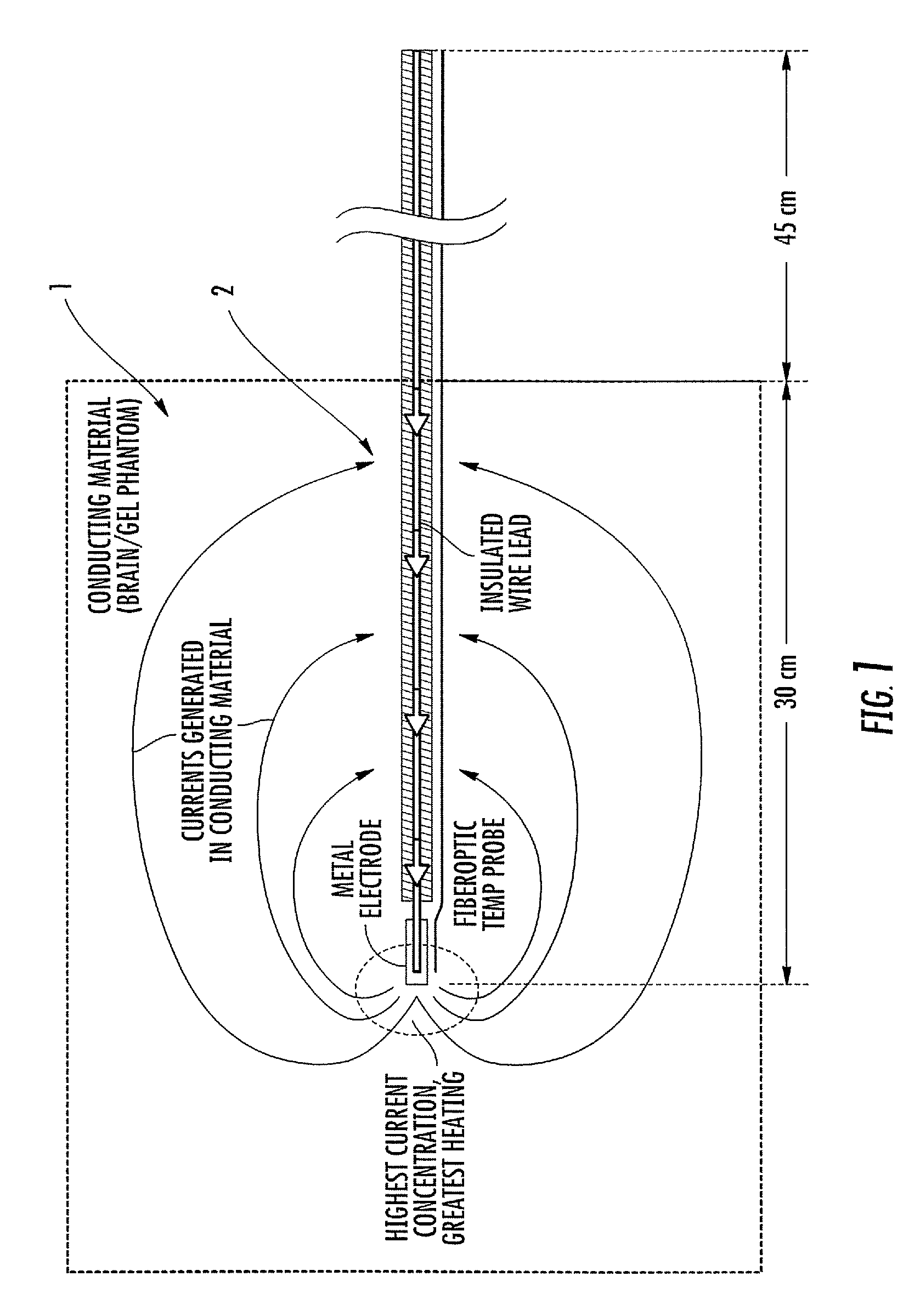
ActiveUS7410290B2Shorten the time periodConvenience to workThermometer detailsThermometers using electric/magnetic elementsCouplingResponsivity
A probe of an ear-type clinical thermometer 20 comprises a first heat insulation member 210 made of a resin material and a second high heat insulation member 220 made of a resin material that is connected to a distal end of the first heat insulation member 210 by conventional coupling means. The second high heat insulation member 220 is tapered forwardly and is provided on the distal end with a concave surface 221. A protection cover 230 sheathes the first heat insulation member 210 and second high heat insulation member 220. A thermistor fine lead wire 240 is embedded in the first heat insulation member 210 and second high heat insulation member 220 so that a turning end portion 241 of the wire 240 is bridged over the concave surface 221 of the second high heat insulation member 220 to be exposed above the concave surface 221. An ultrafast responsivity thermistor 250 is mounted substantially on a center of the turning end portion 241 of the thermistor fine lead wire 240.
Owner:BIO ECHO NET
Internet-enabled lead generation
InactiveUS6868389B1Special data processing applicationsMarket data gatheringEmail addressThe Internet
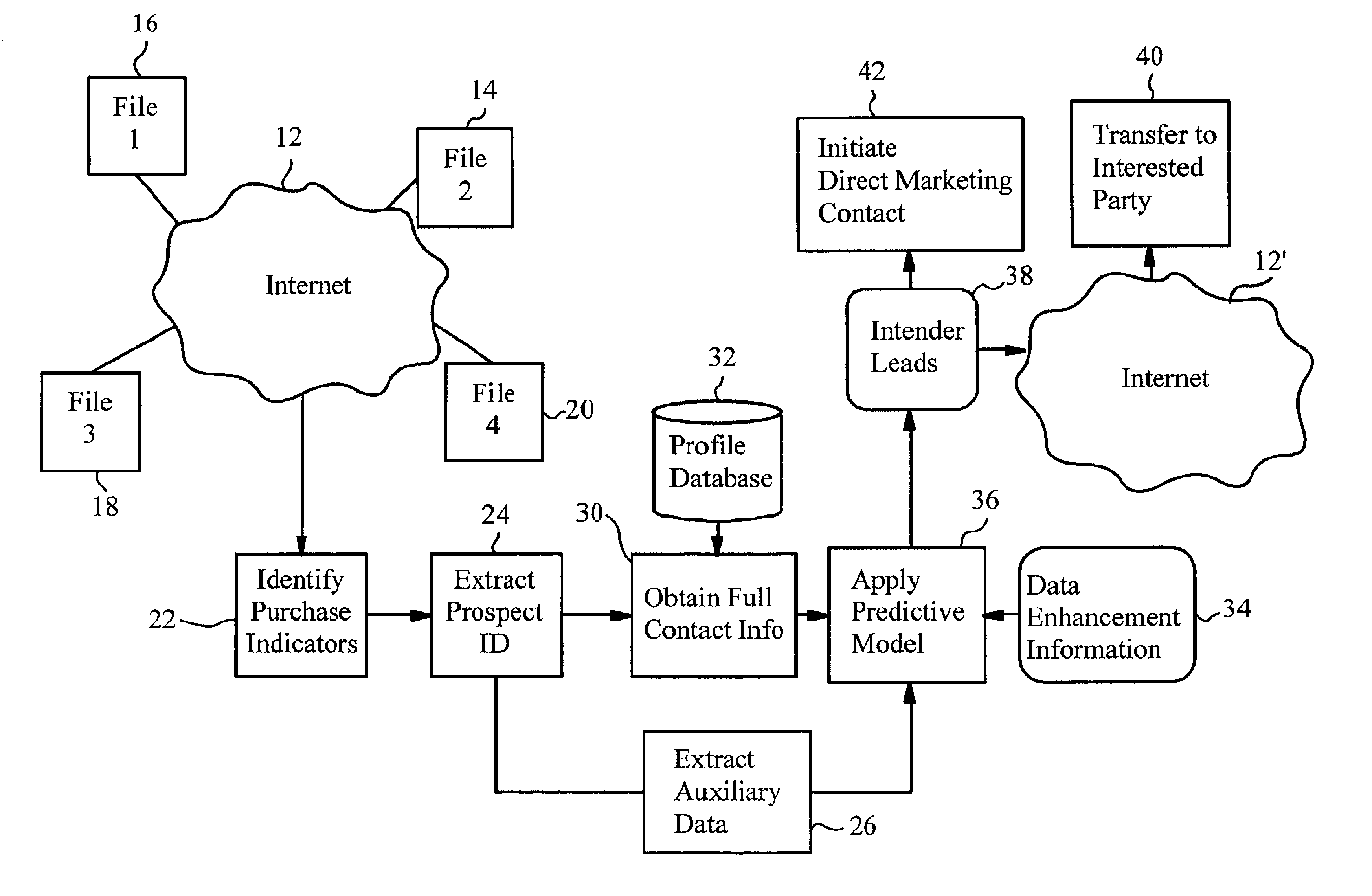
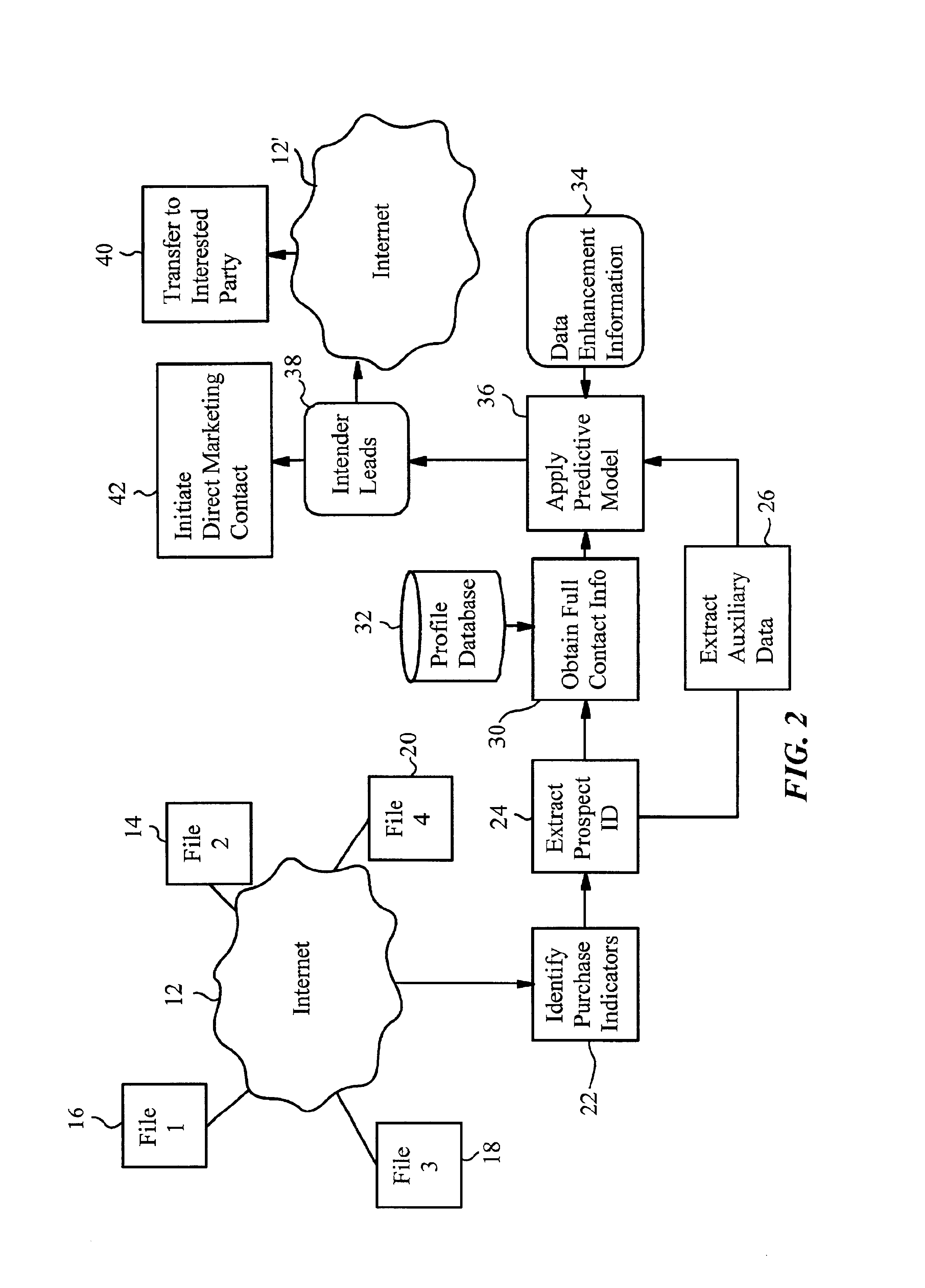
A method of generating intender leads in a distributed computer system includes the steps of identifying purchase indicators and extracting prospect identifiers from the purchase indicators. Purchase indicators are pieces of data that represent a potential future purchase by a prospect. For example, an online classified advertisement selling an automobile is a purchase indicator for a potential future purchase of a new car by the old car seller. The prospect identifier, such as a telephone number or email address, uniquely identifies the prospect likely to make the future purchase. Preferably, the method also contains the steps of obtaining full contact information for the prospect from a profile database, applying a predictive model to the prospects to select intender leads, and transferring the intender leads to an interested party, such as a direct marketing service or sales force. An intender lead is a lead for a person intending to make a purchase of a particular product or service within a given time period. Only some of the prospects are actual intenders. Preferably, the method also includes the steps of extracting auxiliary data that is independent of the prospect from the purchase indicator, and obtaining data enhancement information about the prospect from data enhancement databases. The predictive model is preferably also applied to the data enhancement information and auxiliary data. The method is particularly well-suited for the Internet, which is a large source of publicly-available purchase indicators that is constantly updated. The intender leads are preferably transferred over the Internet, e.g. by email, so that they arrive at the sales force when they are still “hot.”
Owner:MEDIA DIRECT
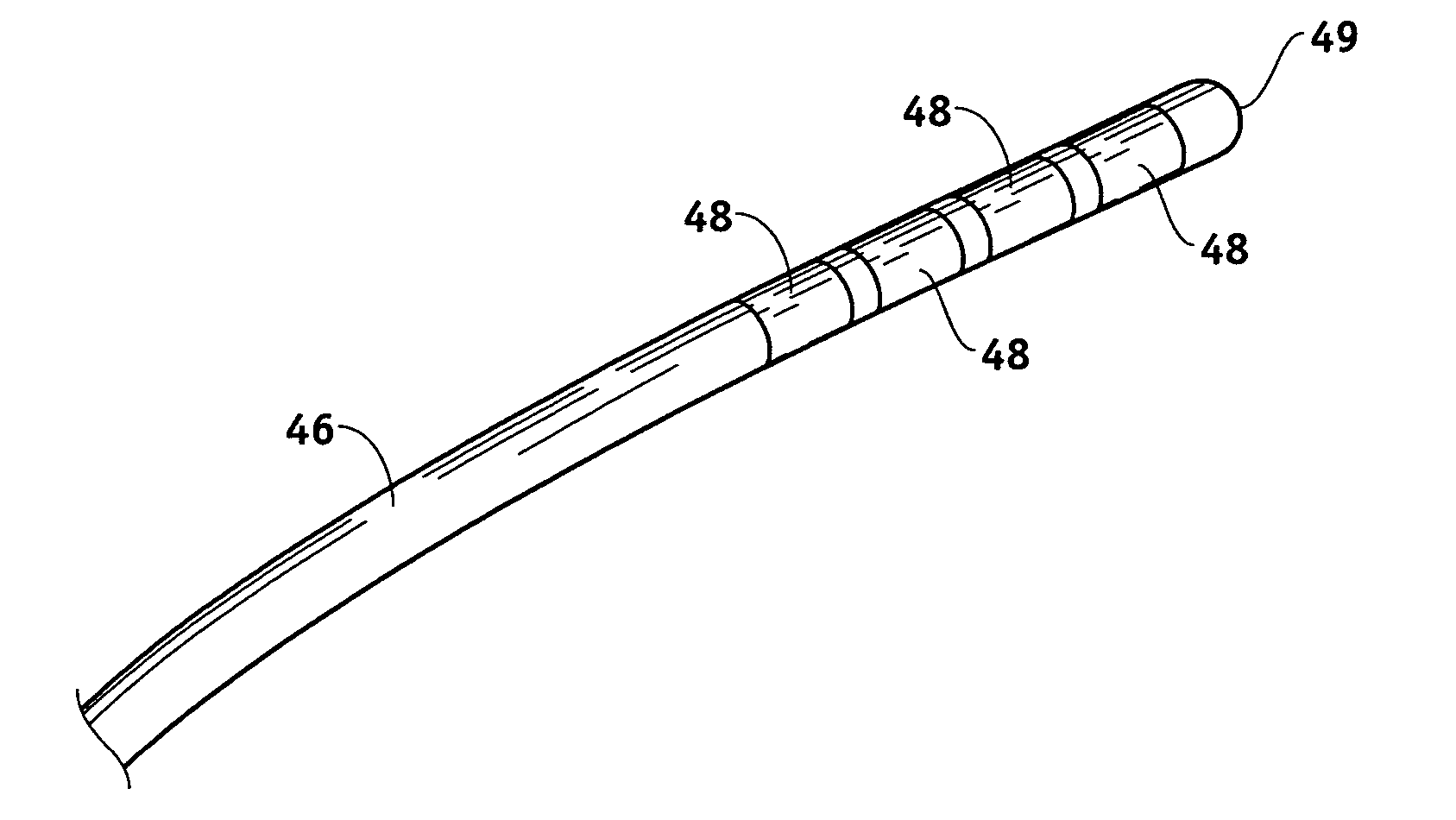
MRI and RF compatible leads and related methods of operating and fabricating leads
ActiveUS20080243218A1Prevent undesired heatingEasy to useLine/current collector detailsInternal electrodesElectricityCelsius Degree
RF / MRI compatible leads include at least one conductor that turns back on itself at least twice in a lengthwise direction, and can turn back on itself at least twice at multiple locations along its length. The at least one electrical lead can be configured so that the lead heats local tissue less than about 10 degrees Celsius (typically about 5 degrees Celsius or less) or does not heat local tissue when a patient is exposed to target RF frequencies at a peak input SAR of at least about 4 W / kg and / or a whole body average SAR of at least about 2 W / kg. Related devices and methods of fabricating leads are also described.
Owner:MRI INTERVENTIONS INC +1
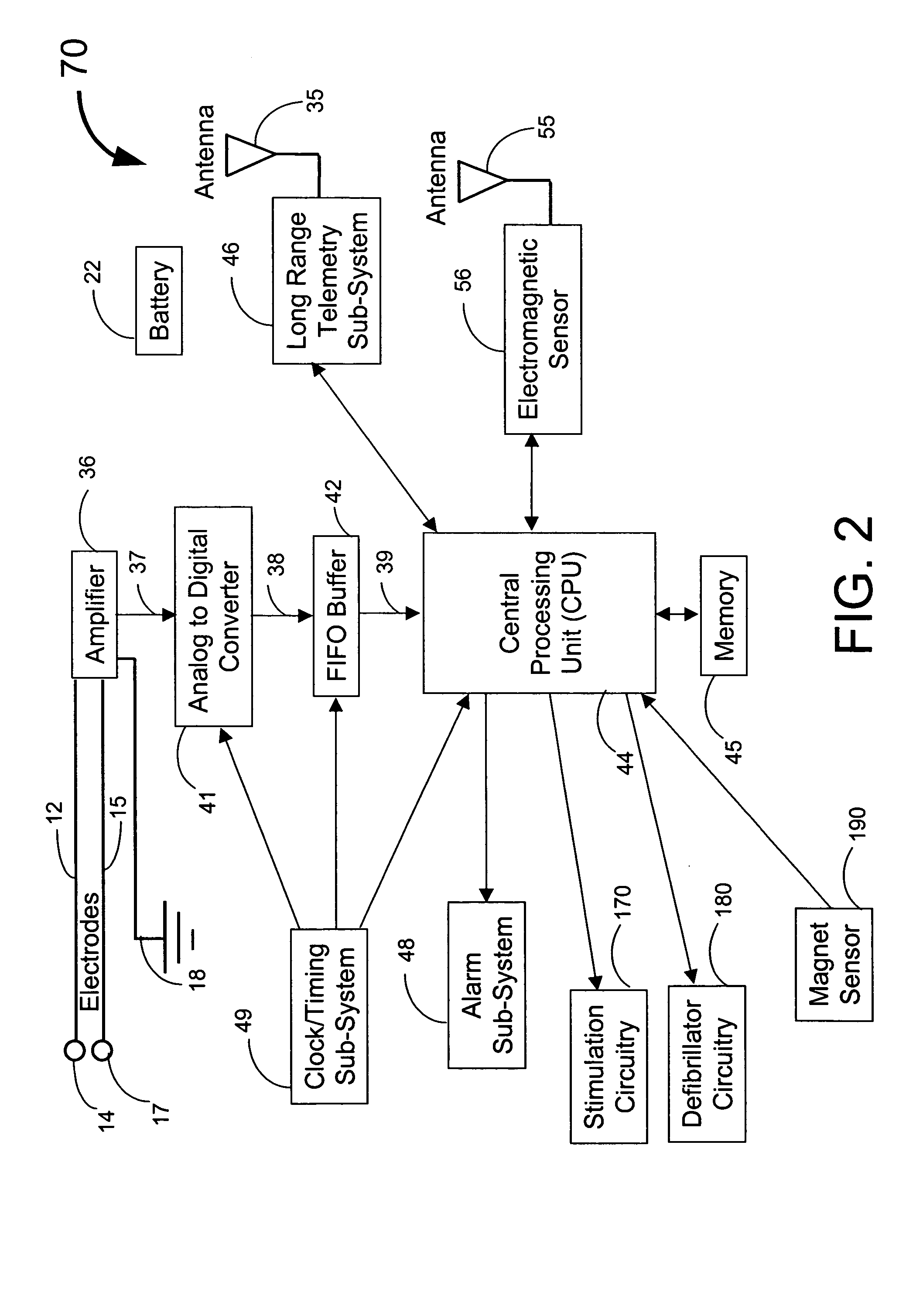
Implantable medical system with long range telemetry
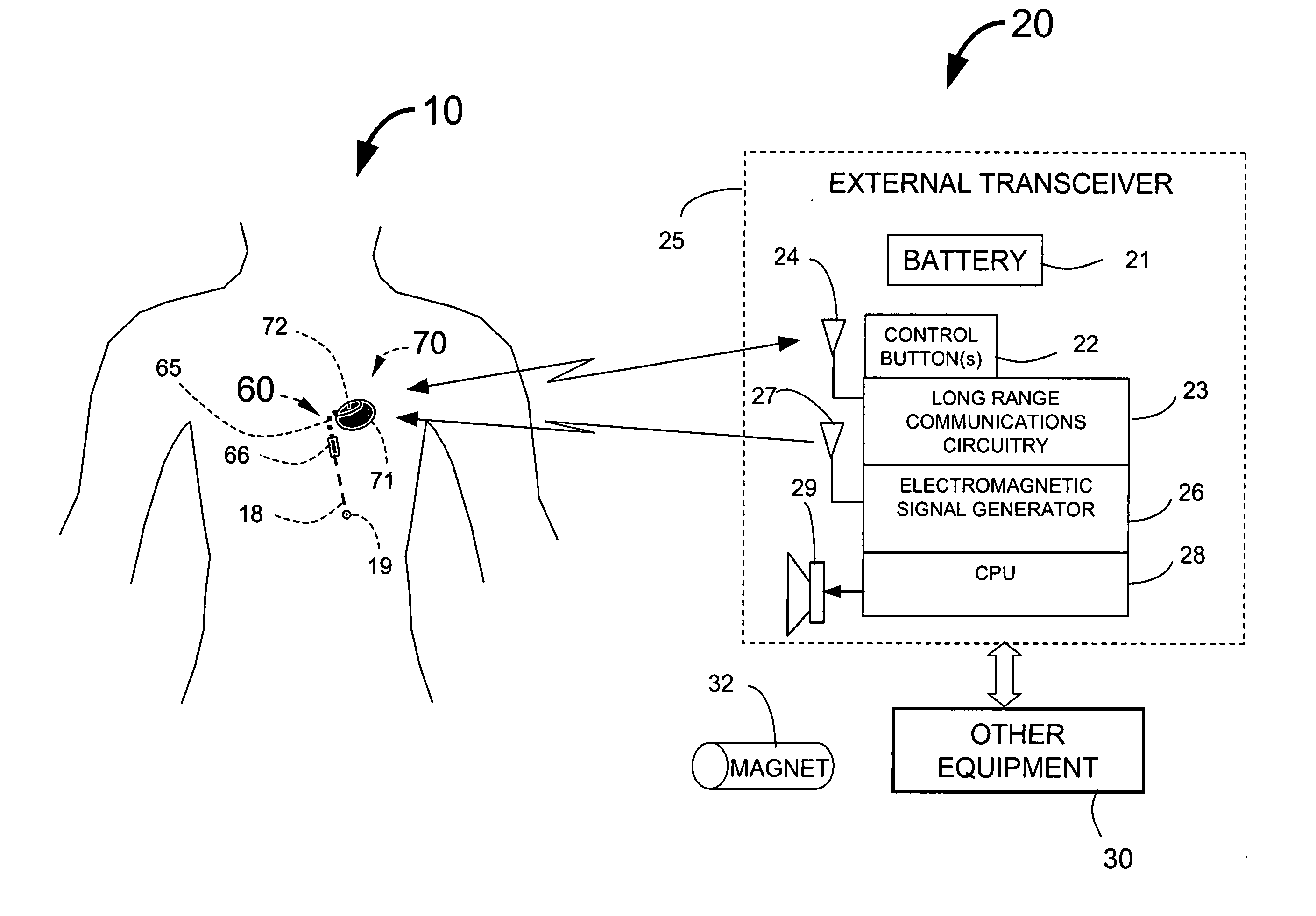
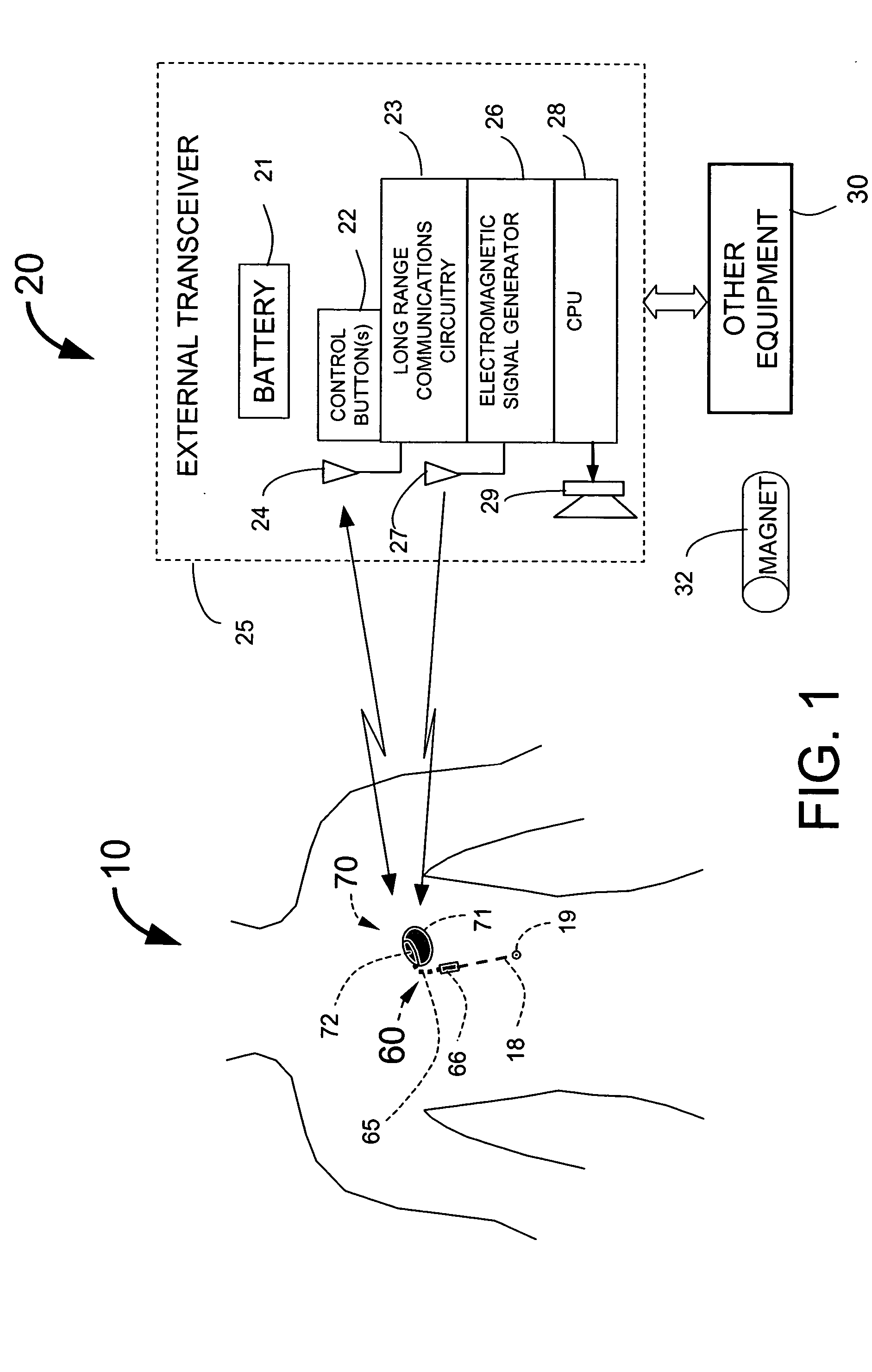
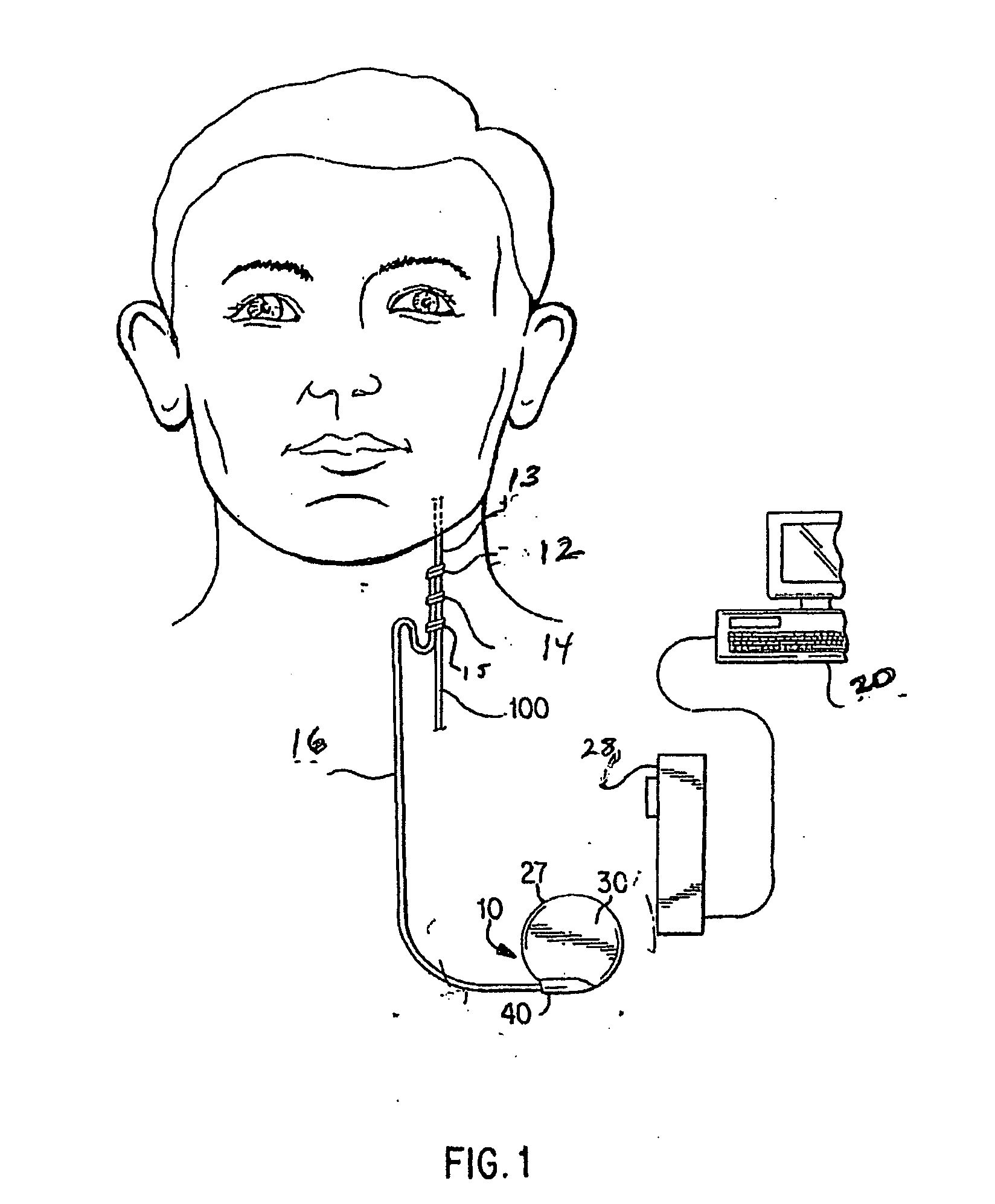
InactiveUS20050113886A1Minimize and eliminate chanceStrong enoughTransvascular endocardial electrodesExternal electrodesImplanted deviceTelemetry Equipment
An implantable medical system for implantation within the body of a patient is provided. The system includes an implanted device having an implant casing and a long range telemetry sub-system housed therein. The system also includes an implantable lead operationally coupled to the implanted device and an antenna coupled to the implant casing to extend therefrom. The antenna is operationally coupled to the long-range telemetry sub-system to enable wireless bi-directional communication between the long range telemetry sub-system and predetermined external equipment disposed outside the body of the patient.
Owner:ANGEL MEDICAL SYST +1
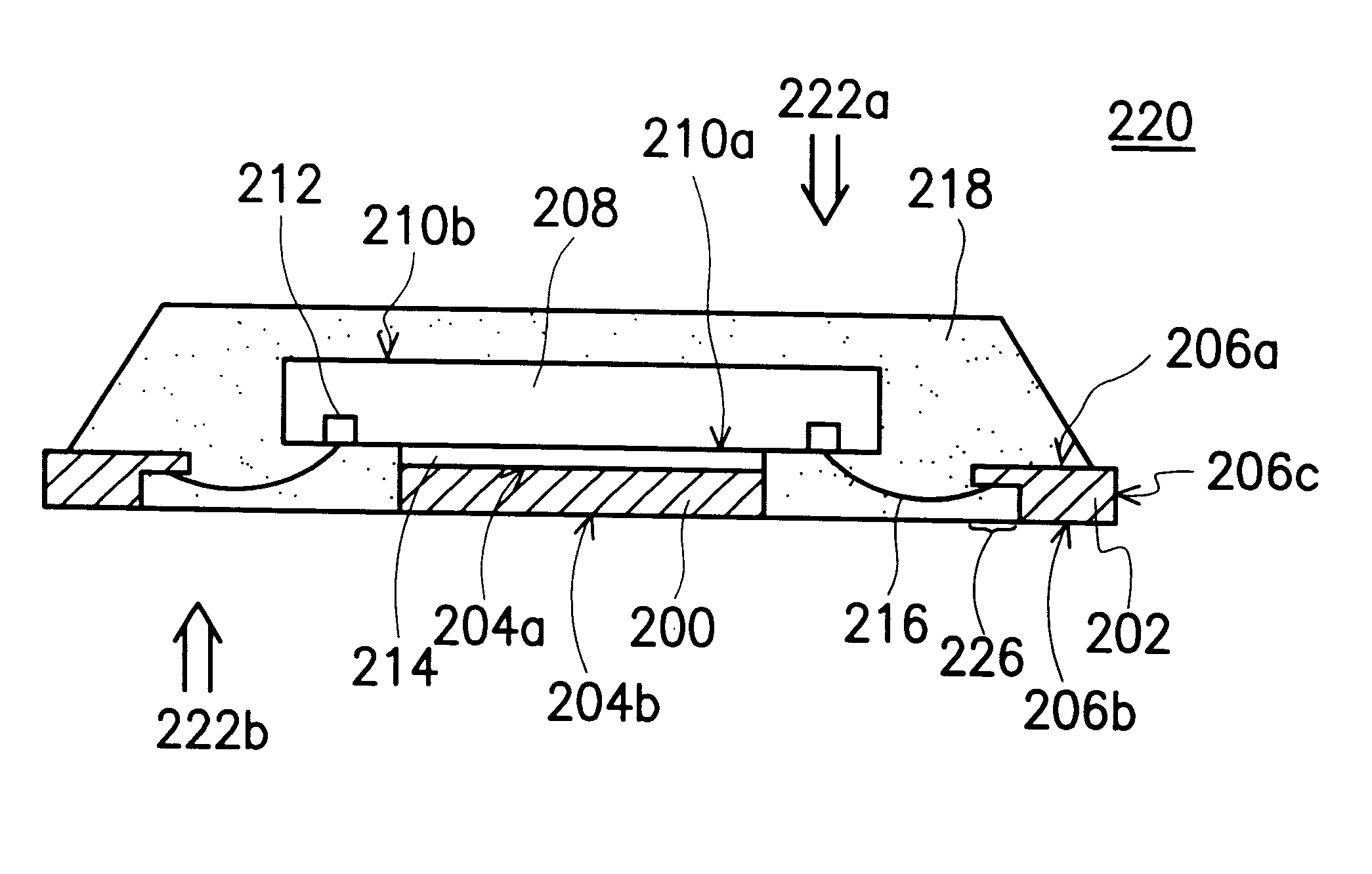
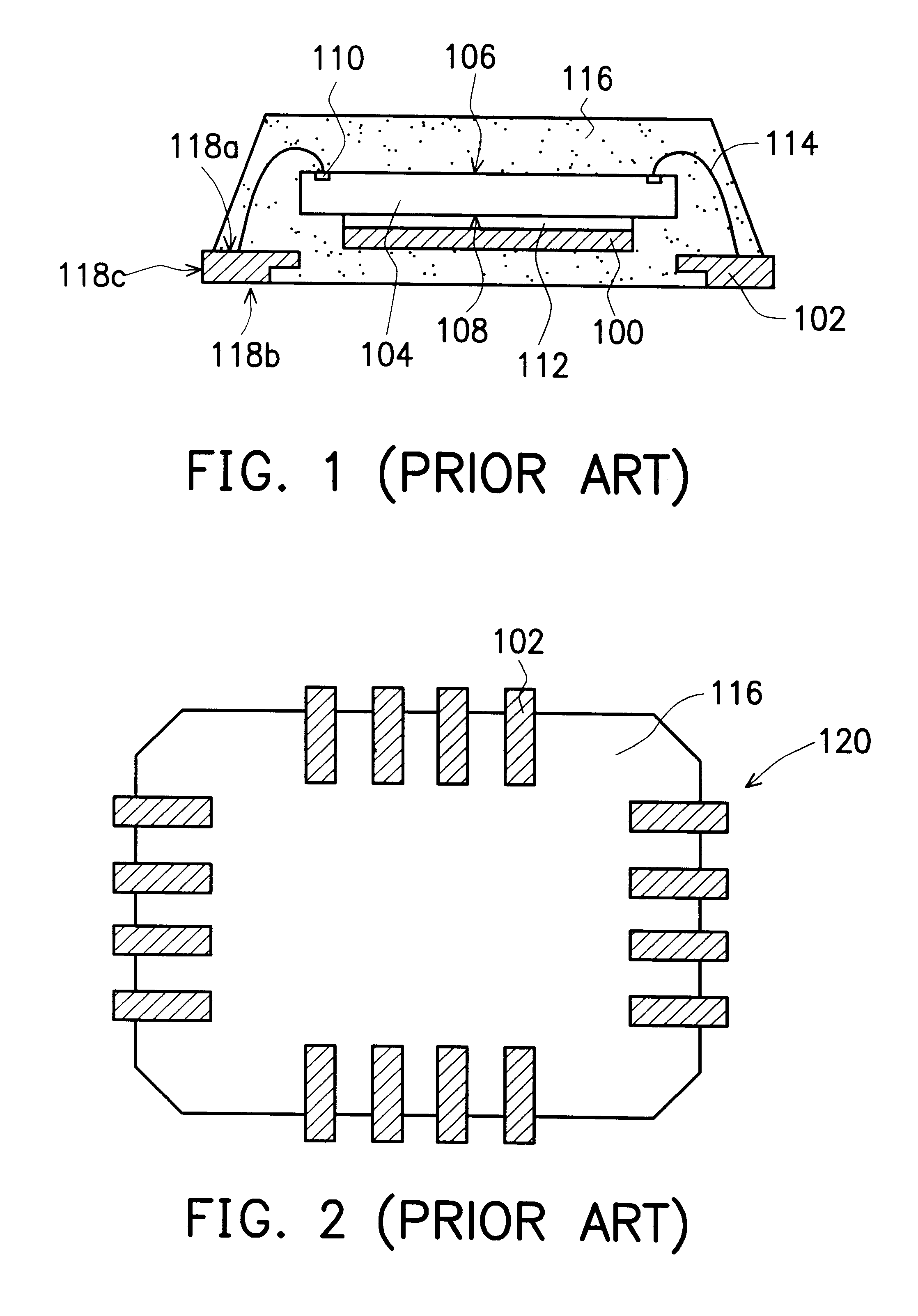
Semiconductor package having heat sink at the outer surface
InactiveUS6559525B2Easy to addImprove packaging efficiencySemiconductor/solid-state device detailsSolid-state devicesSemiconductor packageLead frame
A semiconductor package having heat sink at the outer surface is constructed on a lead frame. The package comprises a chip, a die pad, a plurality of leads, a plurality of bonding wires, and a molding compound. The die pad has a first surface and a second surface, and the chip has its active surface bonded to the first surface of the die pad. The area of the die pad is smaller than the area of the chip in order to expose the bonding pads on the active surface of the chip. The leads having an inner lead portions and an outer lead portions are disposed at the periphery of the die pad, and the inner lead portions are electrically connected to the bonding pads by a plurality of bonding wires. The molding compound encapsulates the chip, the die pad, the inner lead portions of the leads, and the bonding wires. The second surface of the die pad is exposed on the top surface of the package structure while the outer lead portion of the leads is exposed at the side edge of the package structure.
Owner:SILICONWARE PRECISION IND CO LTD
Method and apparatus for a semiconductor package for vertical surface mounting
InactiveUS6291894B1Electrically conductive connectionsDigital data processing detailsDevice materialSurface mounting
A method for packaging a semiconductor device includes connecting a plurality of wire leads to a corresponding plurality of electrical connection pads on the semiconductor device, covering at least a portion of the semiconductor device and at least a portion of each of the wire leads with an encapsulating material, and removing a portion of the encapsulating material and a portion of each of the wire leads to form a packaged semiconductor device wherein each of the wire leads has an exposed portion only at an end. The invention also includes a packaged semiconductor device having an integrated circuit device with a plurality of electrical connection pads, a plurality of wire leads coupled to the plurality of electrical connection pads, and a covering of encapsulating material covering at least a portion of the integrated circuit device and covering each of the wire leads, wherein each of the wire leads has an exposed end. The present invention contemplates wire bonding and encapsulation of individual die as well as multiple die on a single wafer.
Owner:MICRON TECH INC
Lead assembly for implantable microstimulator
InactiveUS20050165465A1Contact member manufacturingCoupling device detailsElectrical conductorMicro devices
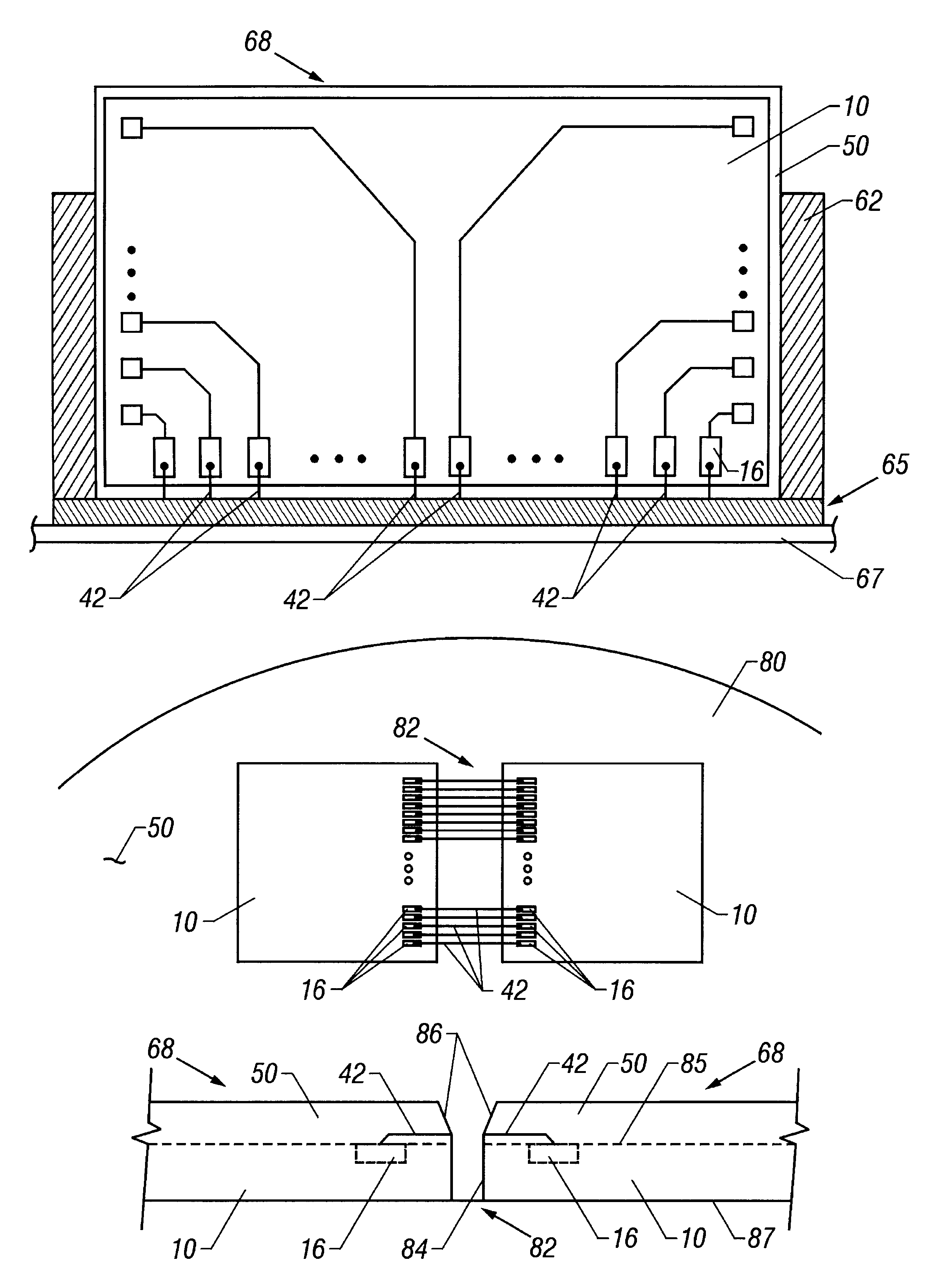
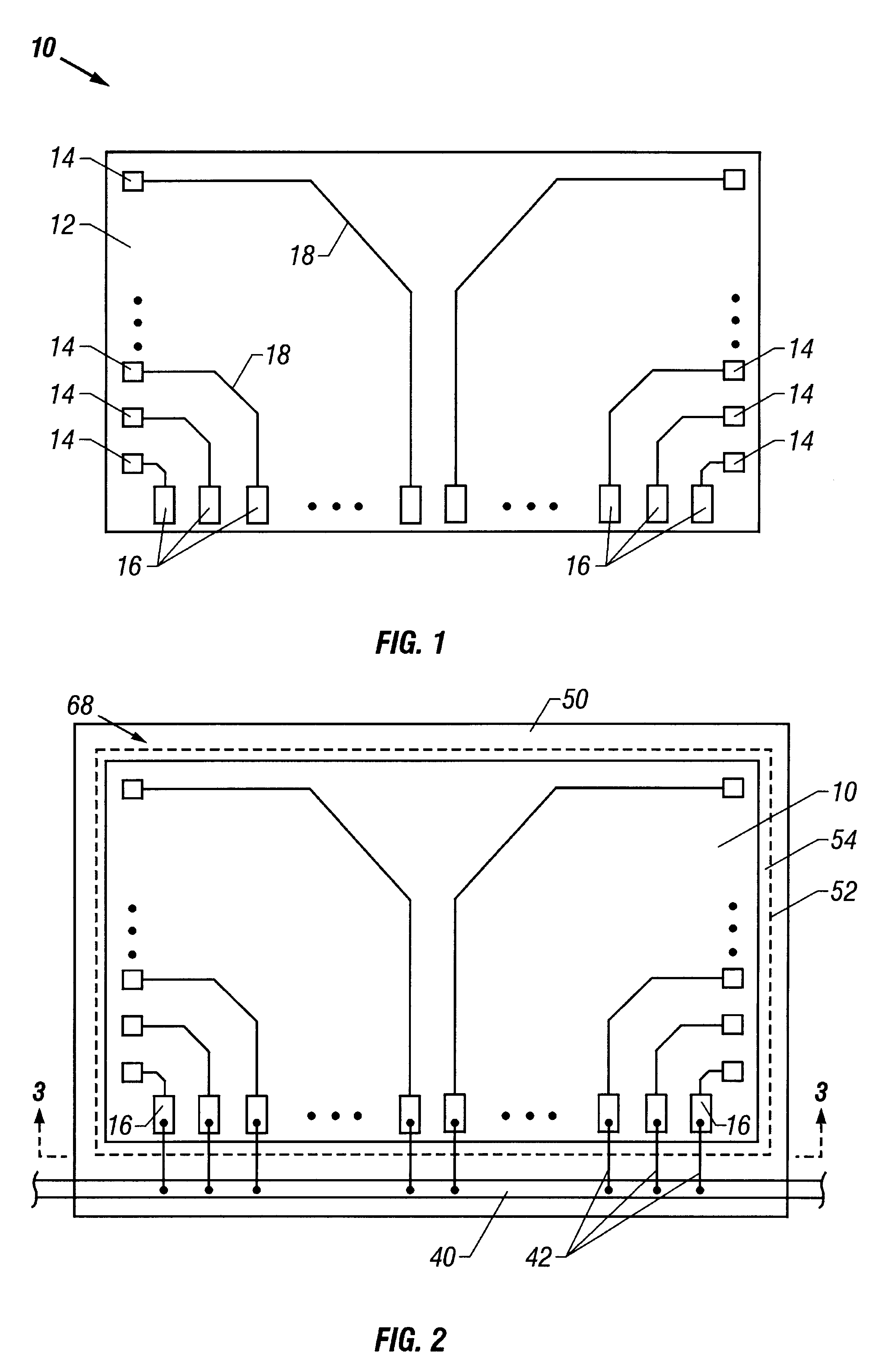
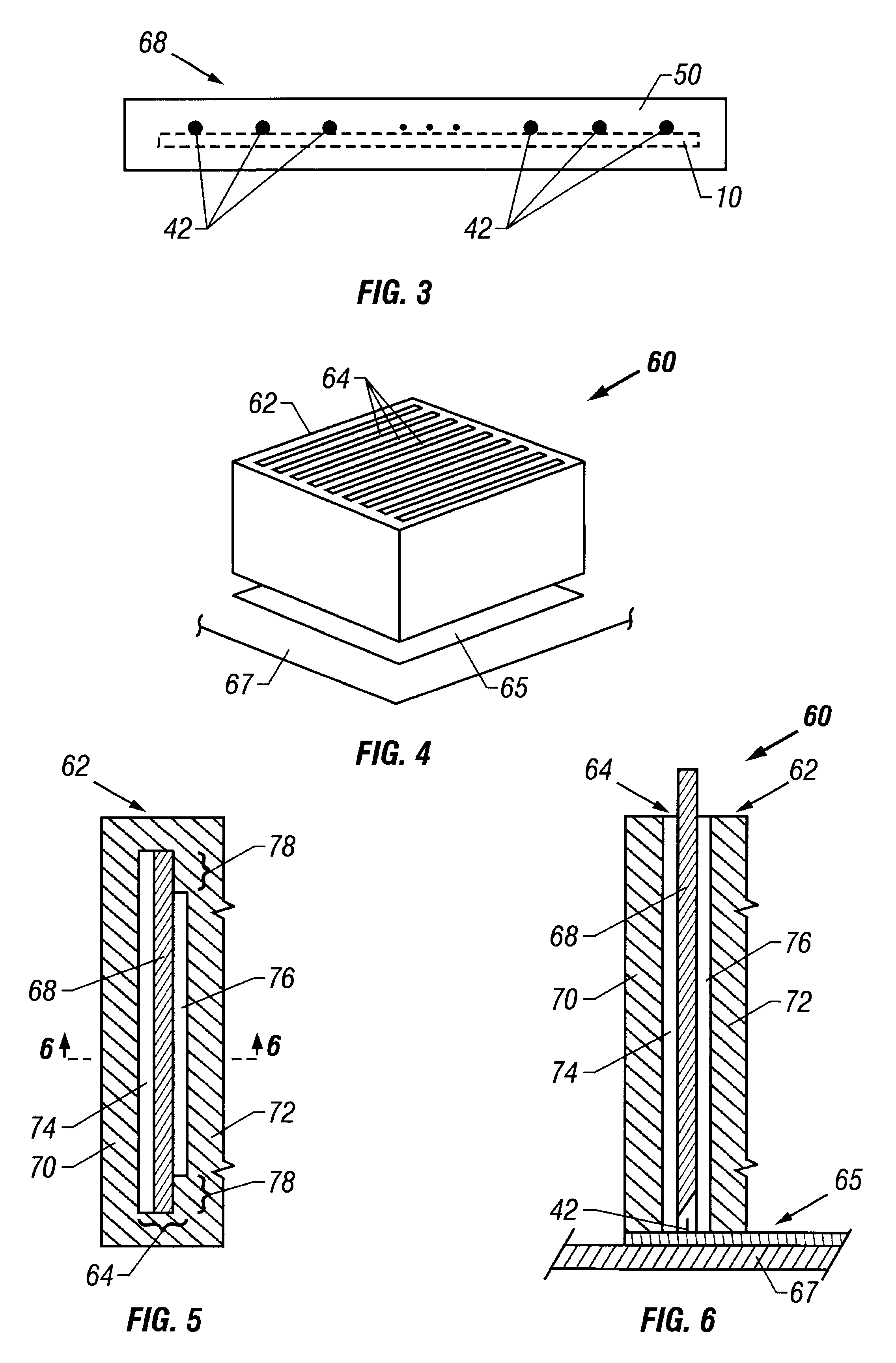
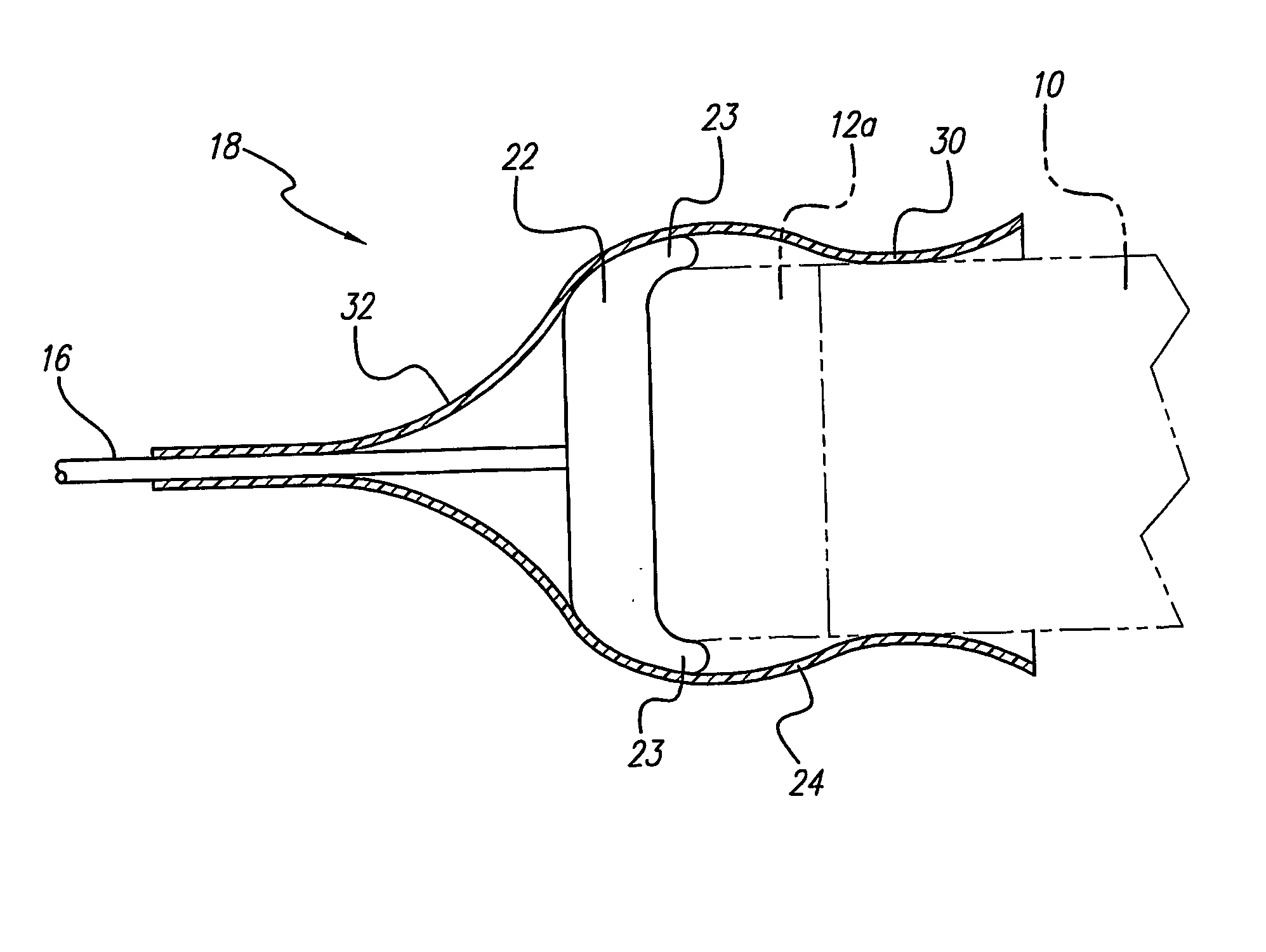
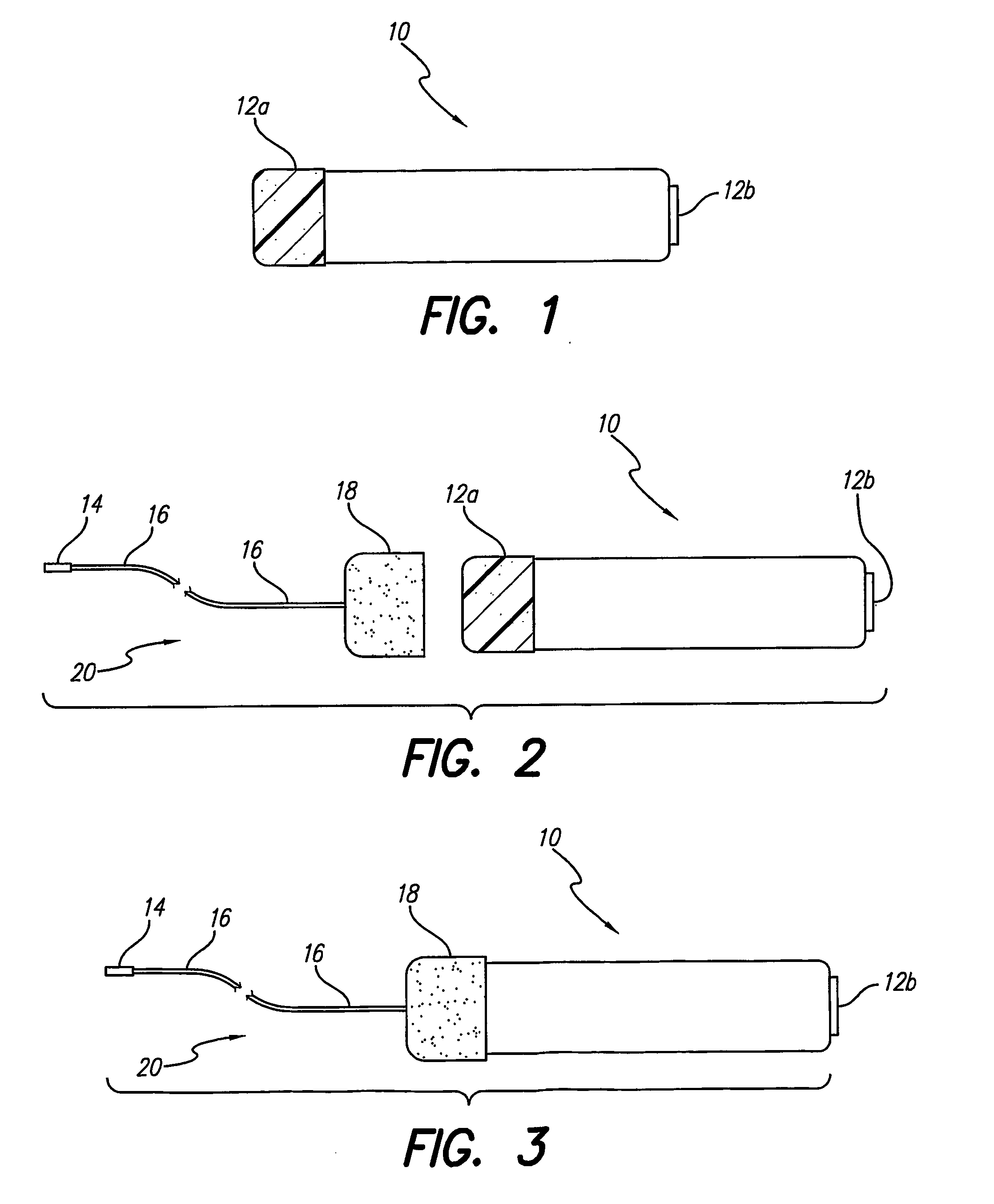
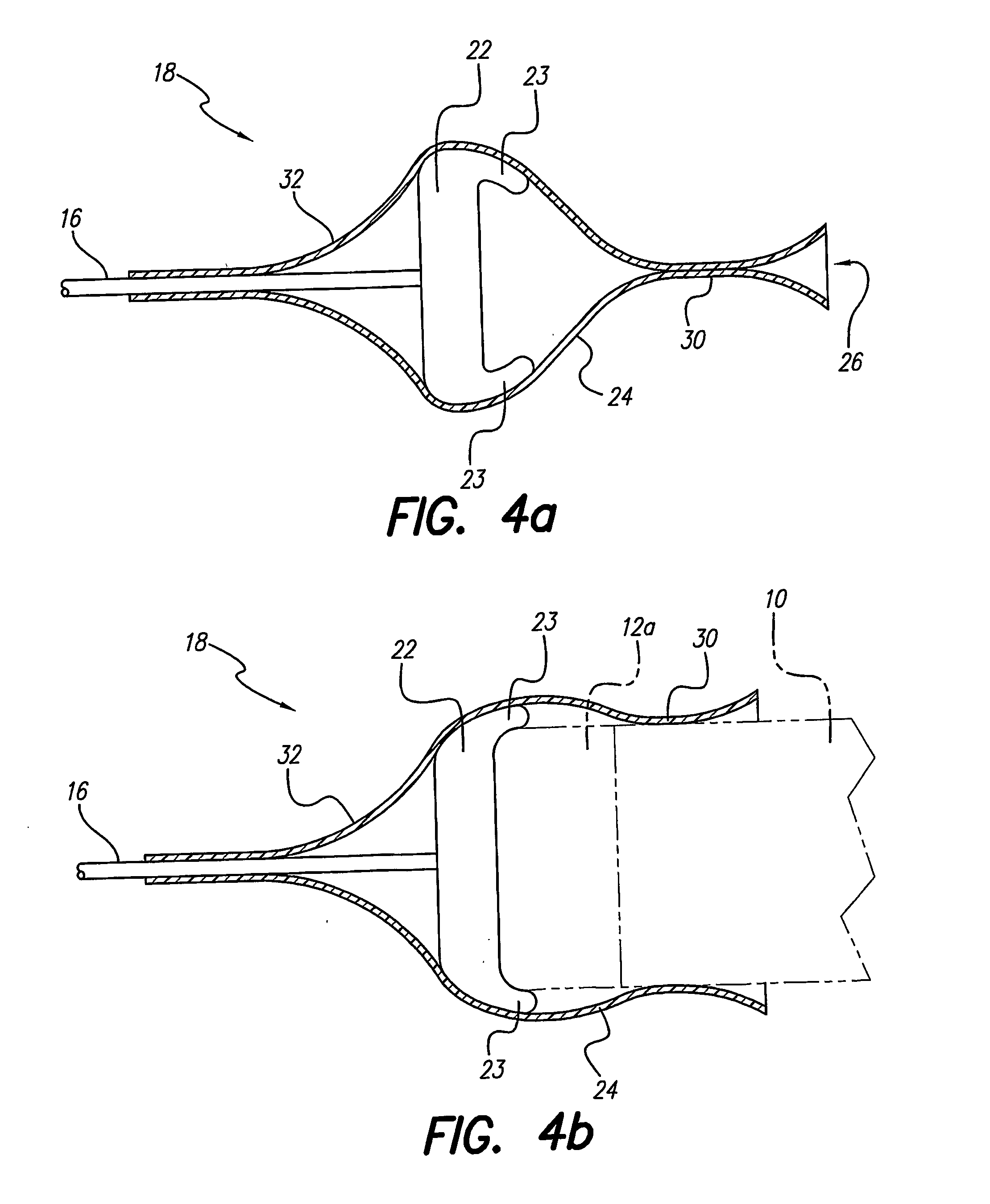
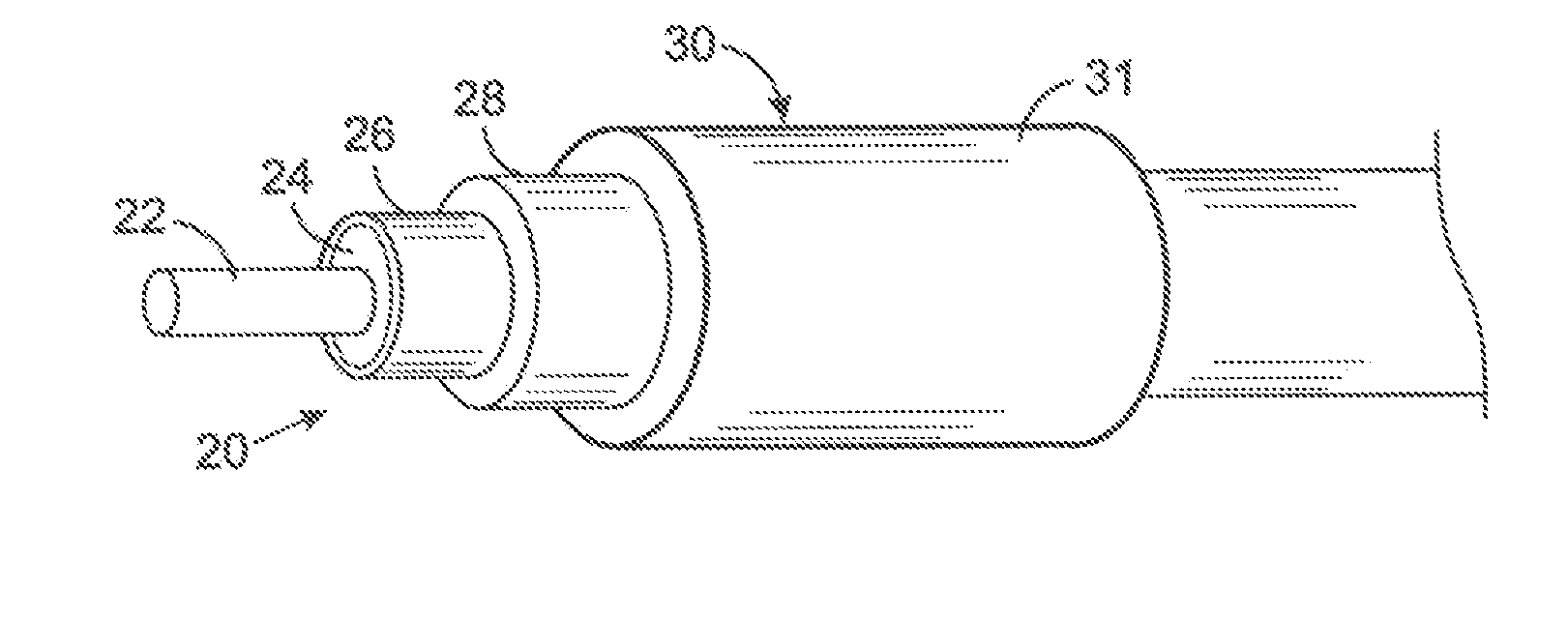
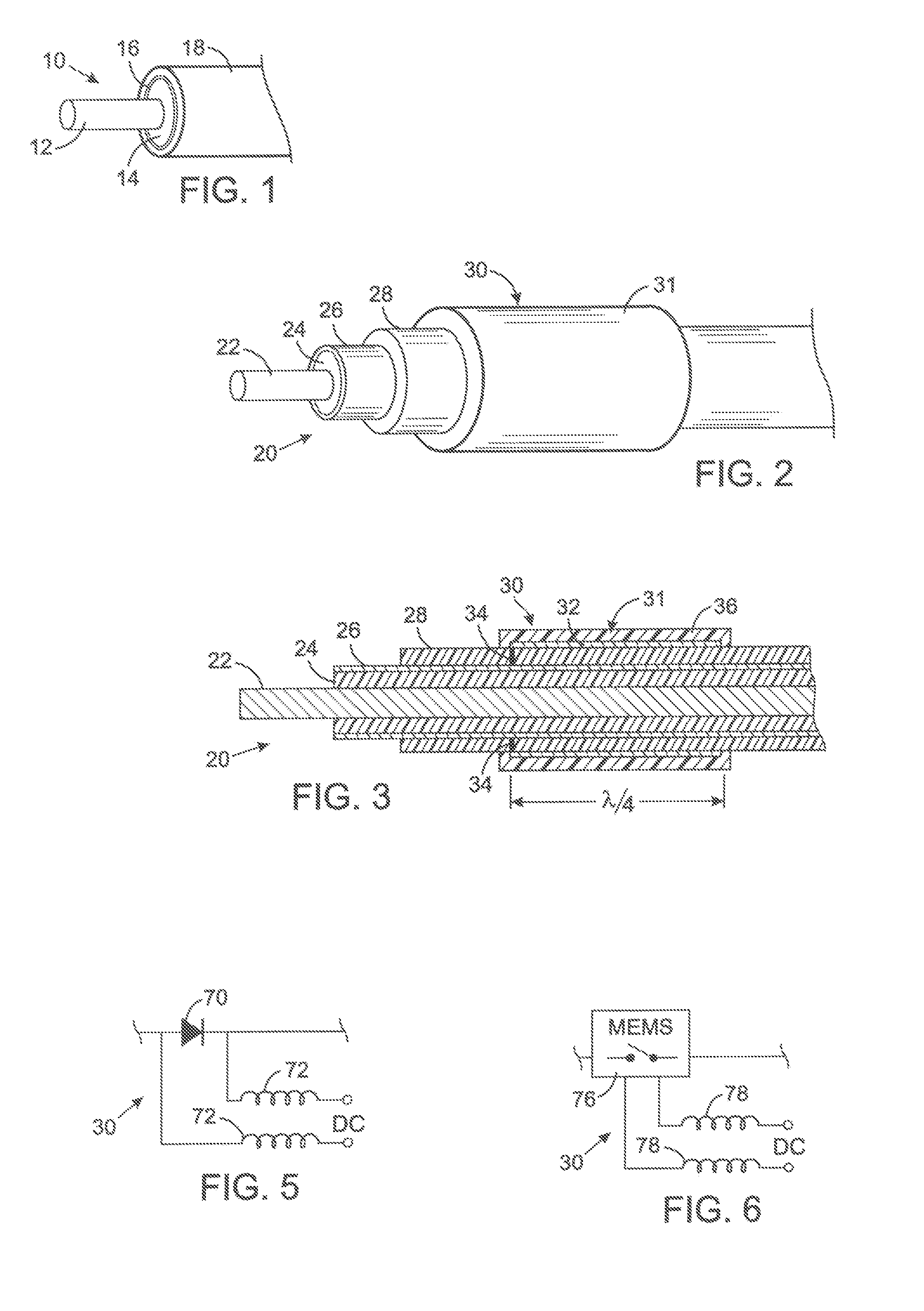
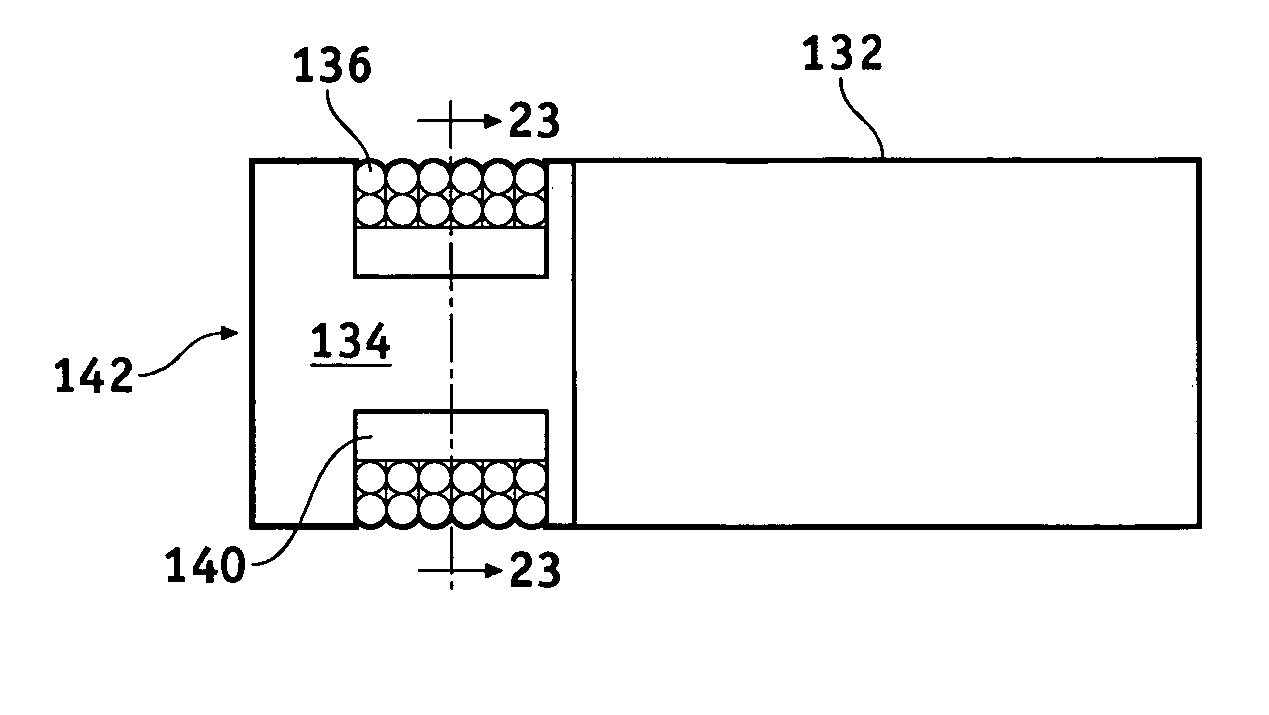
A lead assembly (20) for a small implantable medical device (10) (a.k.a., microdevice (10) provides means to attach a remote electrode (14) to microdevice (10), which means inhibit fluid ingress when microdevice (10) is not attached to lead assembly (20). Microdevices may provide either or both tissue stimulation and sensing. Known microdevices (10) include spaced apart electrodes (12a, 12b) on the outer surface of the microdevice. Lead assembly (20) includes an insulated lead (16) including a proximal end and a distal end, with at least one conductor therebetween; at least one electrode (14) at the distal end of the lead and electrically connected to the at least one conductor, and a connector (18) attached to the proximal end of the lead and adapted to be removably connectable to microdevice (10). Connector (18) includes at least one contact (22) to electrically connect at least one device electrode (12a) on microdevice (10) to at least one conductor, thereby electrically connecting at least electrode (12a) and the at least one electrode (14) at the distal end of lead (16). Lead assembly (20) is configured to inhibit fluid ingress into the connector (18). A number of embodiments of the invention, capable of inhibiting fluid ingress into connector (18), are taught.
Owner:BOSTON SCI NEUROMODULATION CORP
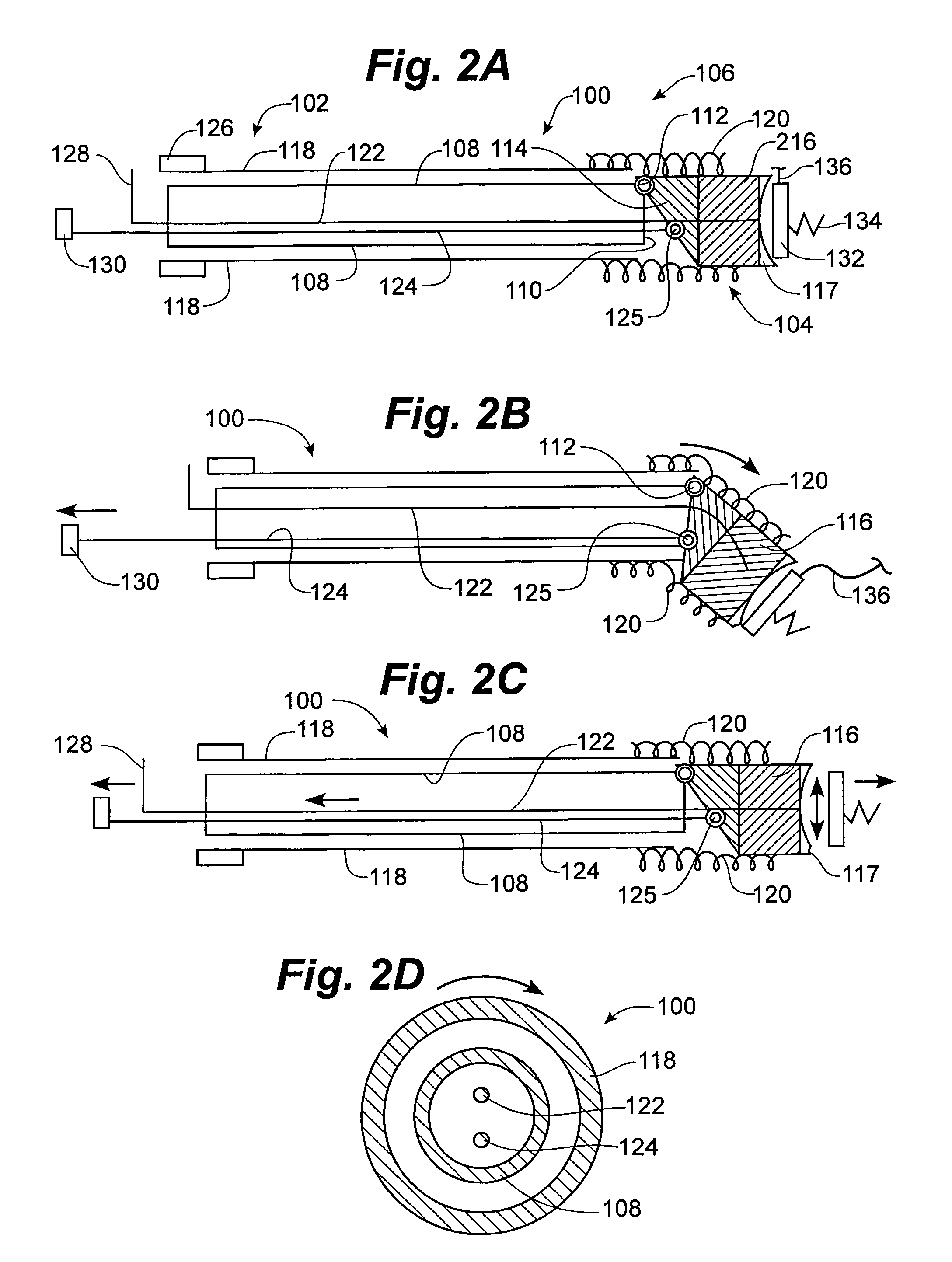
MRI compatible implanted electronic medical device and lead
An implantable biocompatible lead that is also compatible with a magnetic resonance imaging scanner for the purpose of diagnostic quality imaging is described. The implantable electrical lead comprises a plurality of coiled insulated conducting wires wound in a first direction forming a first structure of an outer layer of conductors of a first total length with a first number of turns per unit length and a plurality of coiled insulated conducting wires wound in a second direction forming a second structure of an inner layer of conductors of a second total length with a second number of turns per unit length. The first and the second structures are separated by a distance with a layer of dielectric material. The distance and dielectric material are chosen based on the field strength of the MRI scanner. The lead may further comprise a conducting layer formed by coating a material consisting of medium conducting particles in physical contact with each other and a mechanically flexible, biocompatible layer forming an external layer of lead and in contact with body tissue or body fluids.
Owner:KENERGY INC
Lead electrode for use in an MRI-safe implantable medical device
A lead configured to be implanted into a patient's body comprises a lead body and a conductive filer positioned within the lead body and having a distal portion. An electrode is electrically coupled to the lead body and comprises a stimulation portion, a bobbin, and at least one coil of wire wound on the bobbin and electrically coupled between the stimulation portion and the distal end region to form an inductor between the distal end region and the stimulation portion.
Owner:MEDTRONIC INC
Drug eluting implants to prevent cardiac apoptosis
Implantable devices are configured to be positioned in or near the heart and to carry and deliver an anti-apoptotic drug to a treatment site in or near the heart. The implantable devices include, but are not limited to, leads, stents, heart valves, atrial septal defect devices, cardiac patches and ventricular restraint devices. Depending on the composition of the device, the drug may be carried by the device through a coating applied to the device, or may be included in the device during the device manufacturing process. The drug may also be included in microparticles, such a microspheres, that are delivered locally through a conduit, such as a catheter.
Owner:CARDIAC PACEMAKERS INC
Methods and systems for accessing the pericardial space
Methods and systems for transvenously accessing the pericardial space via the vascular system and atrial wall, particularly through the superior vena cava and right atrial wall, to deliver treatment in the pericardial space are disclosed. A steerable instrument is advanced transvenously into the right atrium of the heart, and a distal segment is deflected into the right atrial appendage. A fixation catheter is advanced employing the steerable instrument to affix a distal fixation mechanism to the atrial wall. A distal segment of an elongated medical device, e.g., a therapeutic catheter or an electrical medical lead, is advanced through the fixation catheter lumen, through the atrial wall, and into the pericardial space. The steerable guide catheter is removed, and the elongated medical device is coupled to an implantable medical device subcutaneously implanted in the thoracic region. The fixation catheter may be left in place.
Owner:MEDTRONIC INC
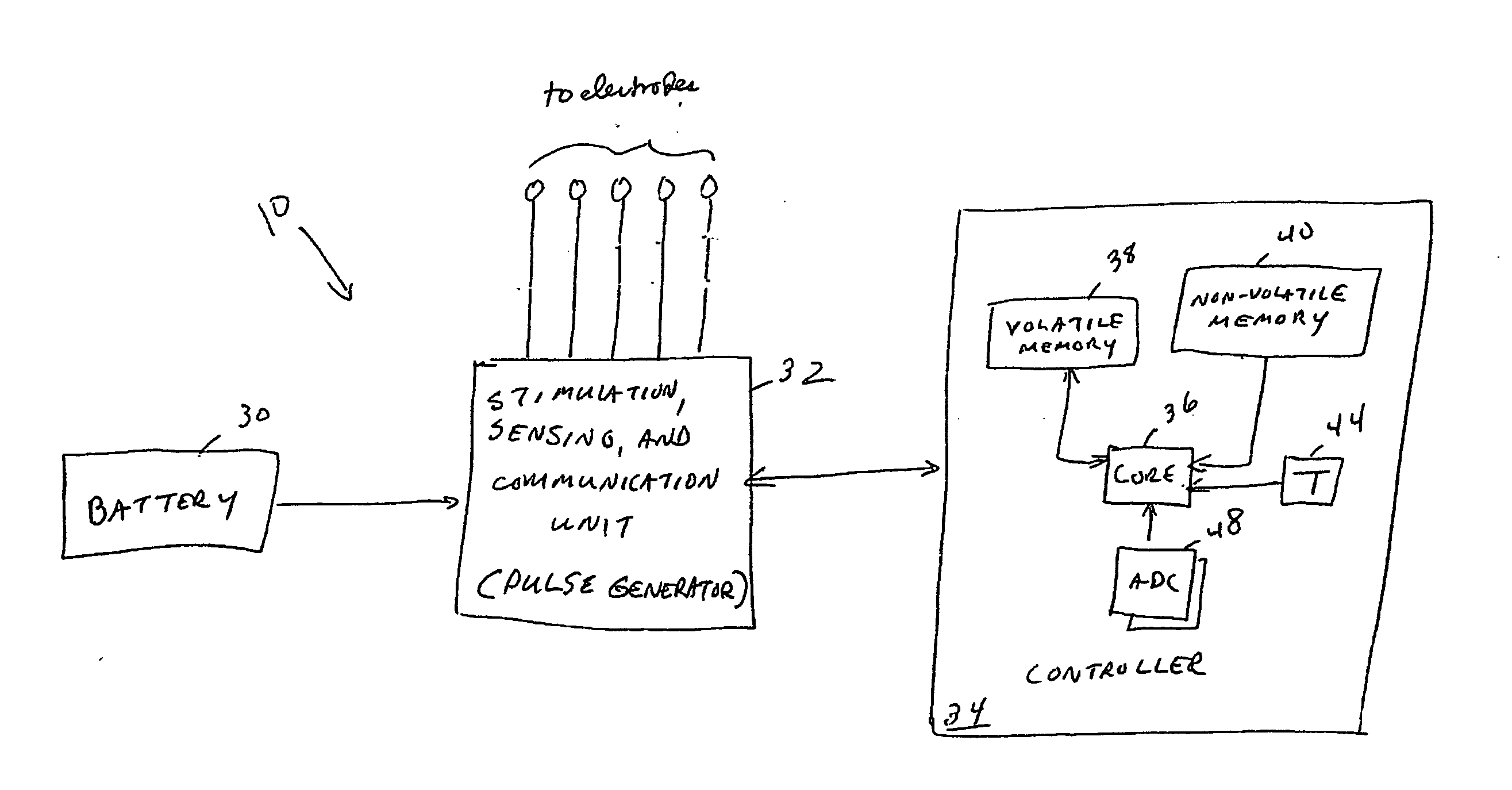
Implantable medical device having multiple electrode/sensor capability and stimulation based on sensed intrinsic activity
ActiveUS20060167497A1BlockingGood curative effectElectrotherapyDiagnostic recording/measuringElectricityIntrinsic activity
In one embodiment, an implantable neurostimulator comprises a pulse generator that generates an electrical pulse signal to stimulate a neural structure in a patient, a stimulation lead assembly coupled to the pulse generator for delivering the electrical pulse signal to the neural structure, a plurality of sensors coupled to the pulse generator, and sensor select logic. Each sensor is individually selectable and the sensor select logic selects any two or more of the plurality of sensors for sensing a voltage difference between the selected sensors. In other embodiments, two or more physiologic parameters are sensed. In yet another embodiment, a method comprises sensing intrinsic electrical activity on a person's nerve and stimulating the nerve based on the sensed intrinsic electrical activity of the nerve.
Owner:LIVANOVA USA INC
MRI-safe implantable medical device
ActiveUS20050222656A1Spinal electrodesHead electrodesElectrical resistance and conductanceElectricity
A medical lead is provided for use in a pulse stimulation system of the type which includes a pulse generator for producing electrical stimulation therapy. The lead comprises an elongate insulating body and at least one electrical conductor within the insulating body. The conductor has a proximal end configured to be electrically coupled to the pulse generator and has a DC resistance in the range of 375-2000 ohms. At least one distal electrode is coupled to the conductor.
Owner:MEDTRONIC INC
MRI-safe implantable lead
A stimulation lead is configured to be implanted into a patient's body and includes at least one distal stimulation electrode and at least one conductive filer electrically coupled to the distal stimulation electrode. A jacket is provided for housing the conductive filer and providing a path distributed along at least a portion of the length of the lead for conducting induced RF energy from the filer to the patient's body.
Owner:MEDTRONIC INC
Compartmentalised screening by microfluidic control
InactiveUS20070092914A1Rapid and high-throughput screeningLow costCompound screeningSequential/parallel process reactionsCompound (substance)Drug development
The invention describes a method for the identification of compounds which bind to a target component of a biochemical system or modulate the activity of the target, comprising the steps of: a) compartmentalising the compounds into microcapsules together with the target, such that only a subset of the repertoire is represented in multiple copies in any one microcapsule; and b) identifying the compound which binds to or modulates the activity of the target; wherein at least one step is performed under microfluidic control. The invention enables the screening of large repertoires of molecules which can serve as leads for drug development.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE +1
RFID detection and identification system for implantable medical lead systems
A system for identifying active implantable medical devices (AIMD) and lead systems implanted in a patient using a radio frequency identification (RFID) tag having retrievable information relating to the AIMD, lead system and / or patient. The RFID tag may store information about the AIMD manufacturer, model number, serial number; lead wire system placement information and manufacturer information; MRI compatibility due to the incorporation of bandstop filters; patient information, and physician and / or hospital information and other relevant information. The RFID tag may be affixed or disposed within the AIMD or lead wires of the lead system, or surgically implanted within a patient adjacent to the AIMD or lead wire system.
Owner:WILSON GREATBATCH LTD
Combination Electrical Stimulating and Infusion Medical Device and Method
InactiveUS20080009927A1Prevent inadvertent bucklingAvoid displacementSpinal electrodesSurgical needlesElectricityChemical stimuli
A combined electrical and chemical stimulation lead is especially adapted for providing treatment to the spine and nervous system. The stimulation lead includes electrodes that may be selectively positioned along various portions of the stimulation lead in order to precisely direct electrical energy to ablate or electrically stimulate the target tissue. Embodiments of the stimulation lead include single or multiple lead elements. The multiple lead element embodiments can be selectively deployed to cover a targeted area. The lead may also includes central infusion passageway(s) or lumen(s) that communicates with various infusion ports spaced at selected locations along the lead to thereby direct the infusion of nutrients / chemicals to the target tissue. Some embodiments utilize a disposable sheath in combination with a reusable stimulation lead.
Owner:VILIMS BRADLEY D
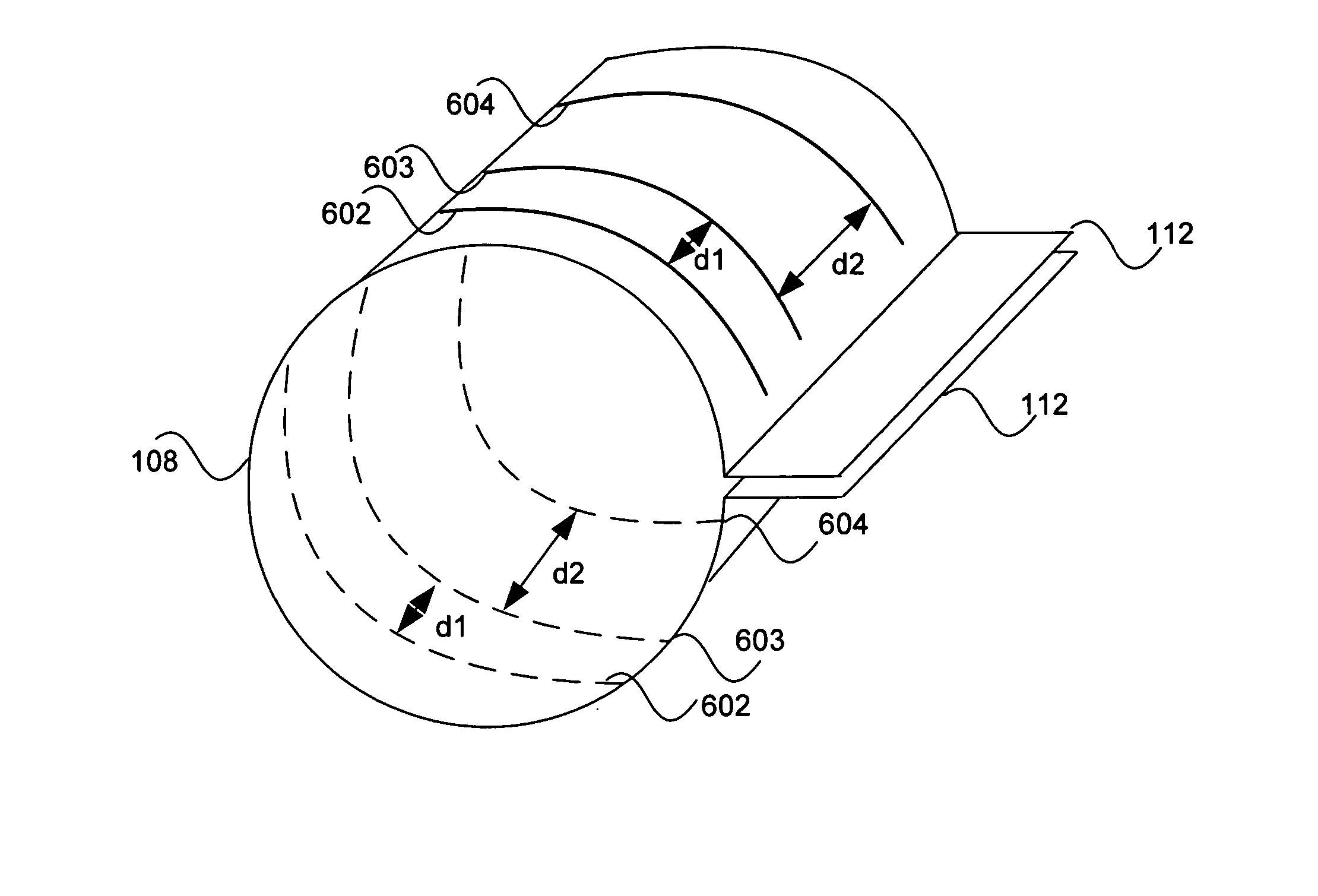
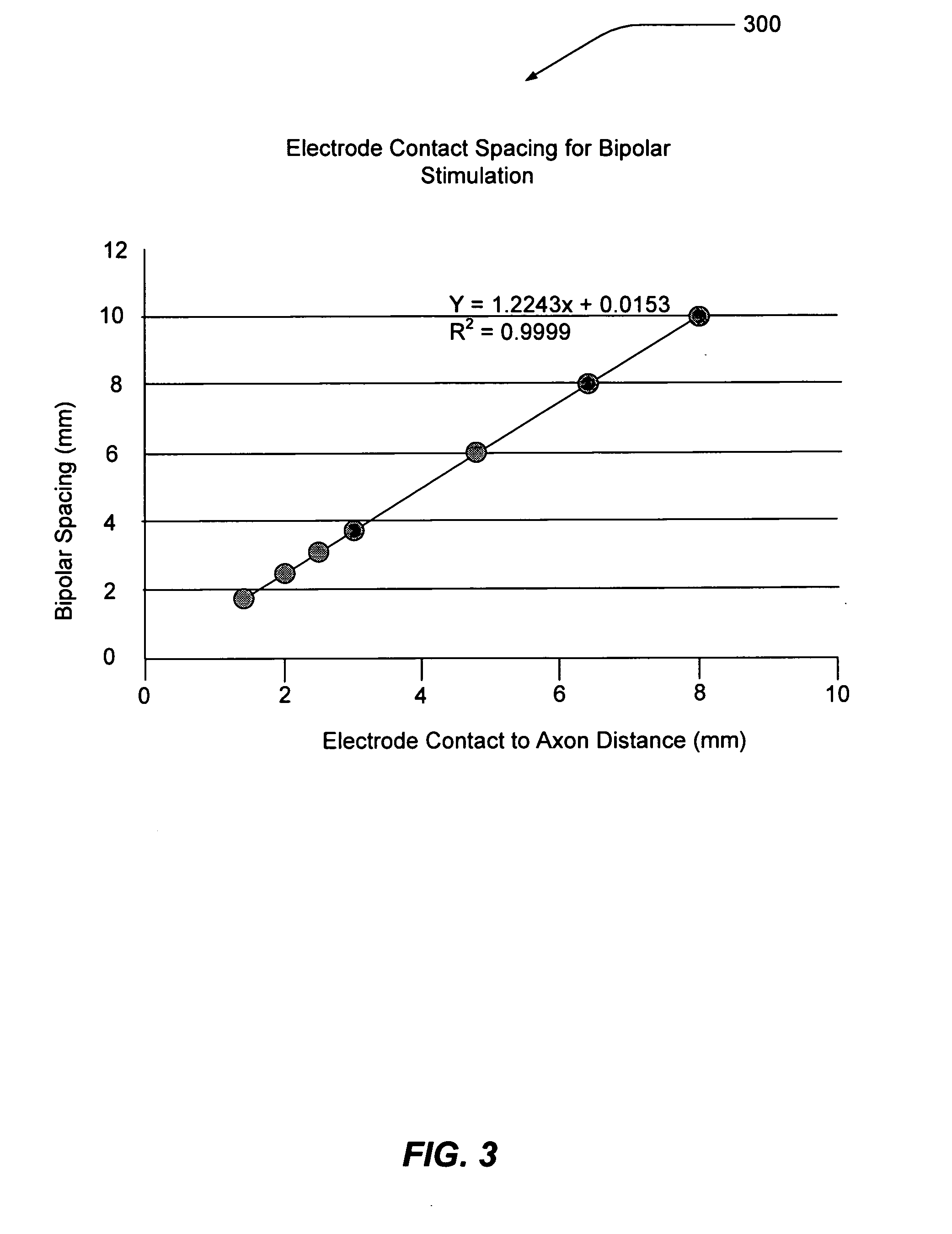
Electrode contact configurations for cuff leads
A stimulation system is disclosed that may include a stimulator unit coupled to electrode contacts on a cuff. In one embodiment, the cuff may be placed at least partially around a nerve. The stimulation system may include at least two electrode contacts disposed on the cuff such that a distance between the at least two electrode contacts various along a length of the electrode contacts. In another embodiment, a plurality of electrode contacts are disposed on the cuff such that distances between at least one electrode contact within the plurality of electrode contacts and each electrode contact immediately adjacent to the at least one electrode contact are different. The stimulator unit may also be implantable.
Owner:BOSTON SCI NEUROMODULATION CORP
Implantable flexible circuit leads and methods of use
InactiveUS20080140152A1Easy to manufactureStrong specificitySpinal electrodesExternal electrodesDielectricFlexible circuits
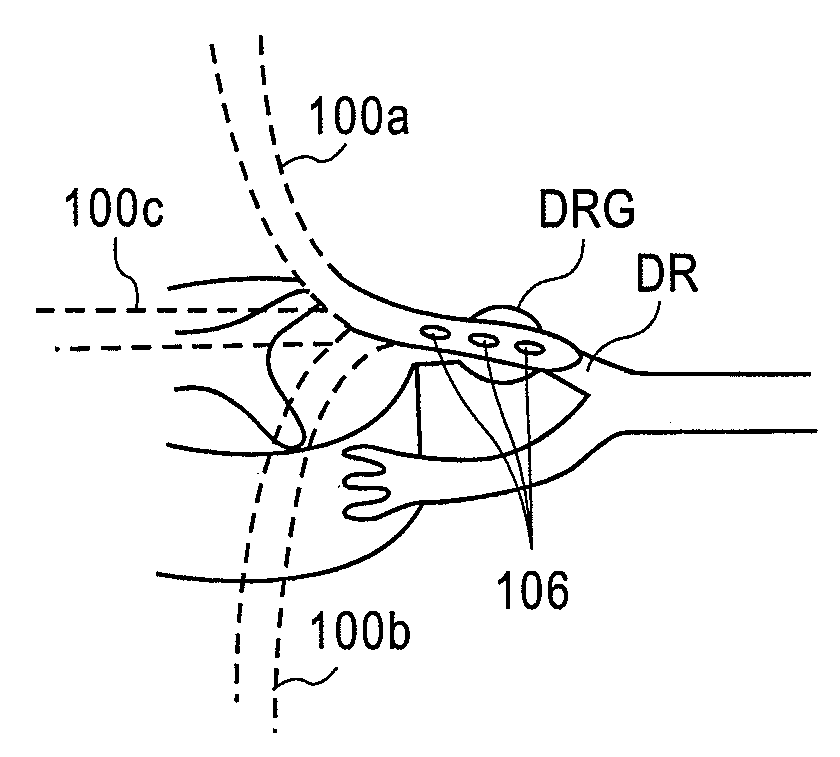
Devices, systems and methods are provided for stimulation of tissues and structures within a body of a patient. In particular, implantable leads are provided which are comprised of a flexible circuit. Typically, the flexible circuit includes an array of conductors bonded to a thin dielectric film. Example dielectric films include polyimide, polyvinylidene fluoride (PVDF) or other biocompatible materials to name a few. Such leads are particularly suitable for stimulation of the spinal anatomy, more particularly suitable for stimulation of specific nerve anatomies, such as the dorsal root (optionally including the dorsal root ganglion).
Owner:ST JUDE MEDICAL LUXEMBOURG HLDG SMI S A R L SJM LUX SMI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com