Prophylactic bactericidal implant
a technology of bacterial infection and implant, which is applied in the field of bacterial infection inhibition of surgical implant devices, can solve the problems of increasing the number of fracture fixation devices used around the world, increasing the number of artificial joints, and affecting the healing effect of the patient, so as to achieve the effect of inhibiting bacterial infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
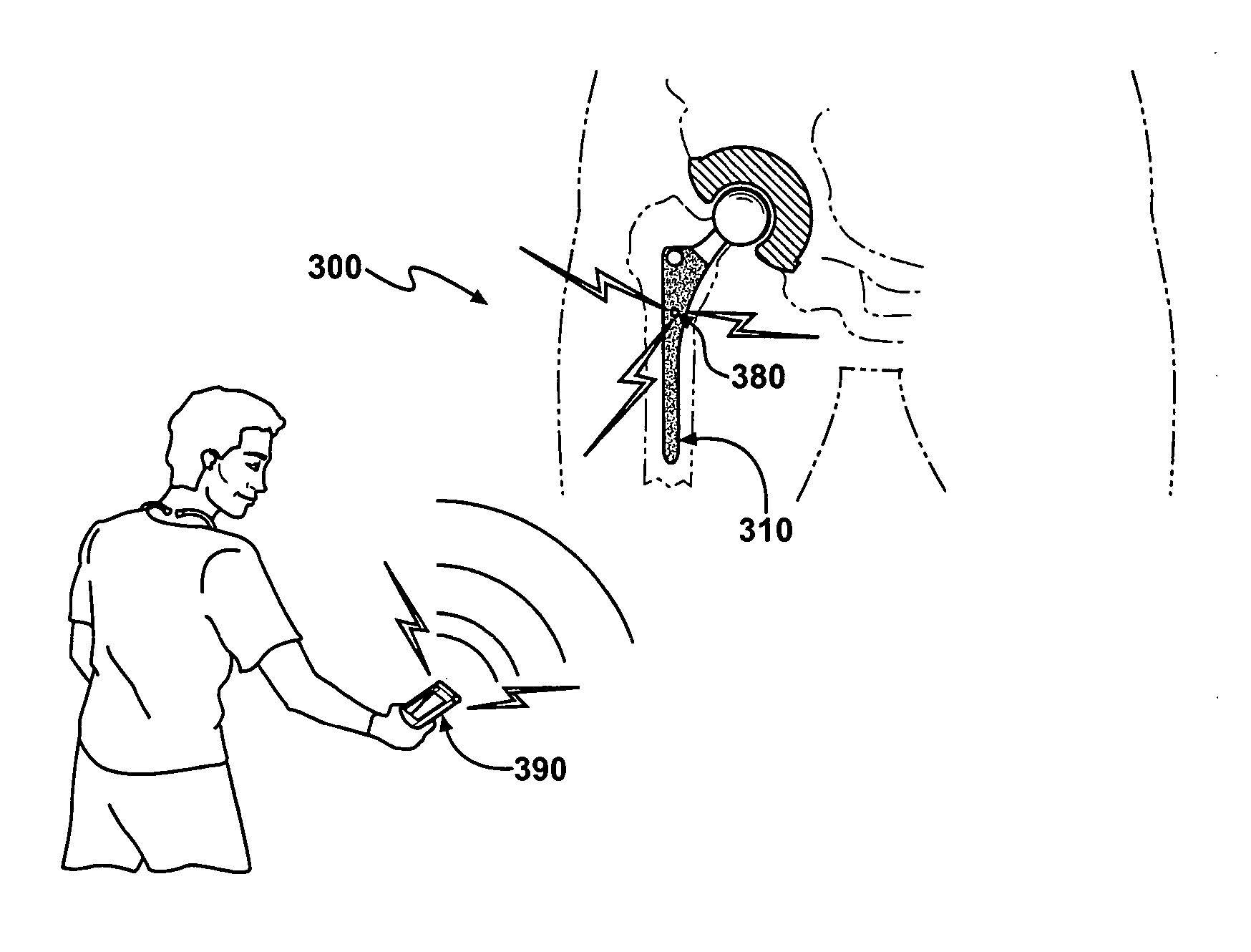
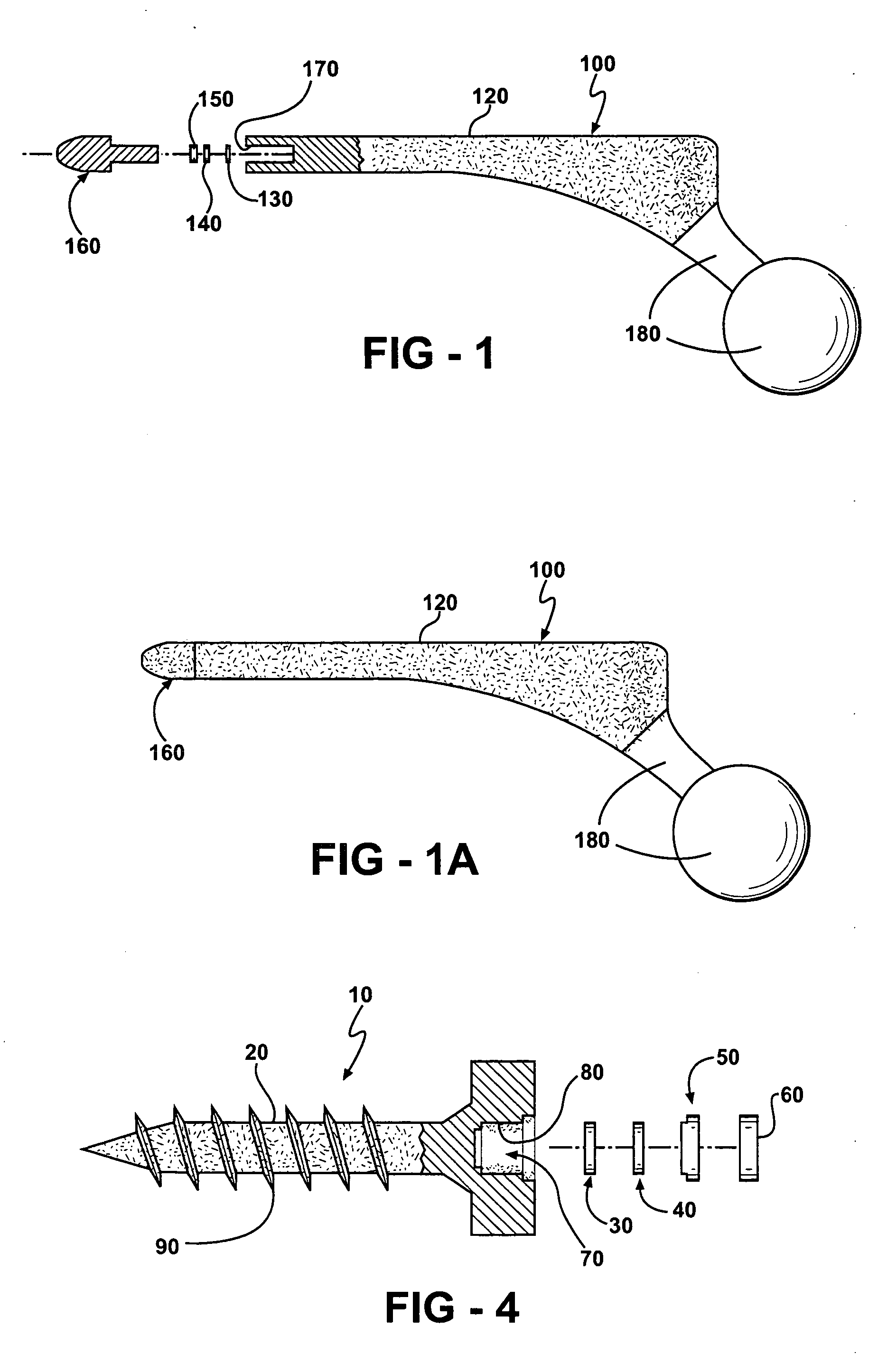
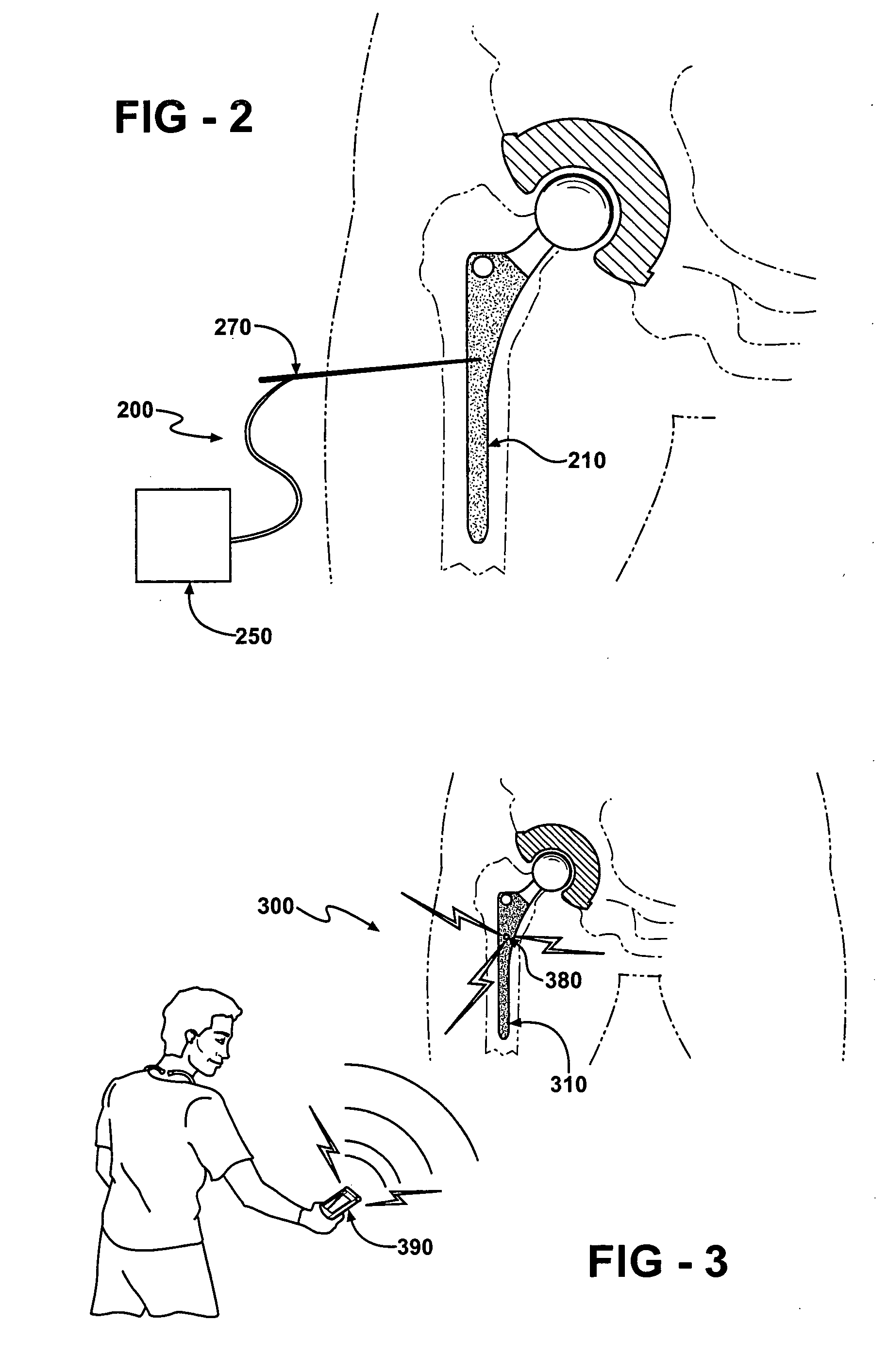
[0088] An implant body is manufactured by obtaining a hip replacement prosthesis similar to a DePuy SUMMIT Tapered Hip System designed to include an internal cavity, about 10 millimeters in length and about 5 millimeters in width and a cap to close the opening of the cavity as described herein. Articular surfaces of the implant body are masked and the remaining external surfaces are coated with a silver metal film about 1 micron in thickness. A battery, resistor and switch are chosen to fit in the cavity. A portion of the cavity wall adjacent to the external surface of the implant body is also coated with silver metal to a depth adjacent the positive terminal of the battery.
[0089] A battery with the desired profile is currently in production by many battery manufacturers. The Energizer battery number 337 satisfies all of the required size characteristics needed for implementation within a bactericidal hip implant. When examining the Energizer 337 battery one can see that the small ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| current | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com