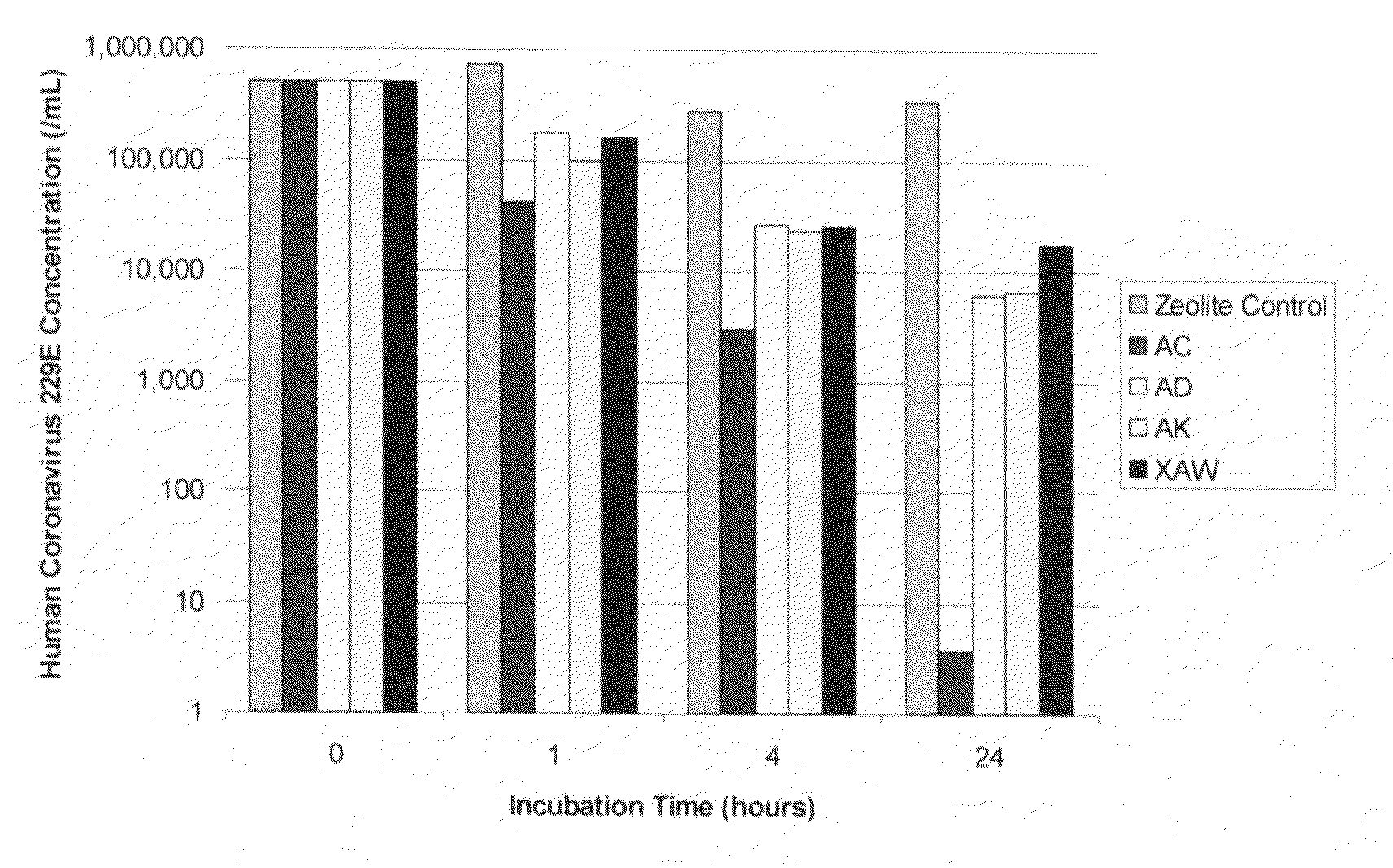
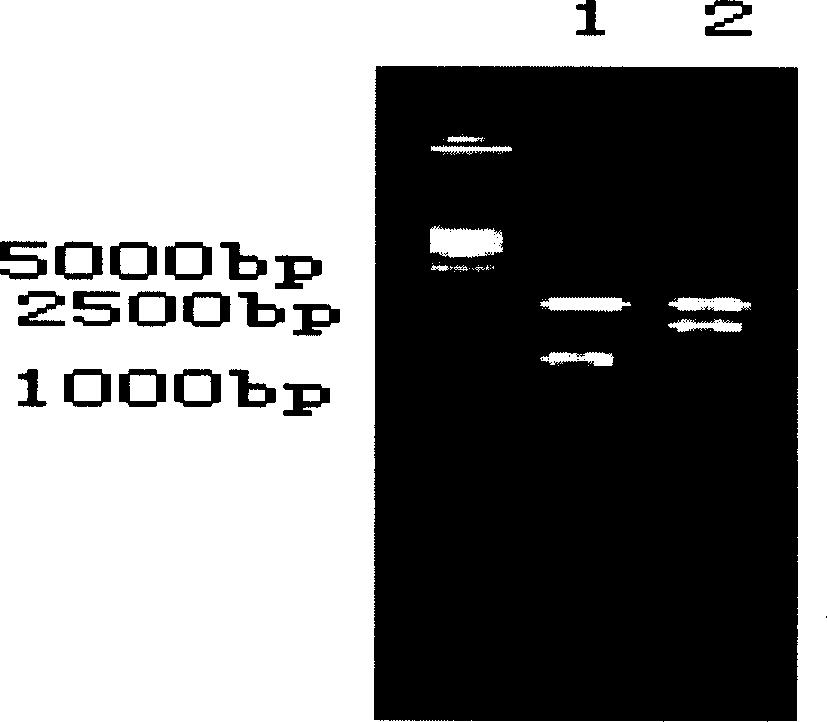Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
121 results about "Viral contamination" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Viral contamination is a potential safety threat common to all animal- and human-derived biologics produced in mammalian cell culture ( 4 ). A viral contamination can arise from a contaminated cell line source or from adventitious introduction of virus during production.
Antiviral Methods
InactiveUS20070243263A1Effectively eradicateProvide protectionBiocideInorganic phosphorous active ingredientsCopperViral infection
Combinations of silver and copper ion sources or a single source of both silver and copper ions are found effective in methods for treating viral infections and for treating surfaces so as to eradicate viral contaminants and / or prevent subsequent contamination of said surfaces with viruses. These methods are particularly applicable in addressing SARS and avian flu viruses.
Owner:SCIESSENT LLC
Treatments for reduction of cytotoxicity and viral contamination of implantable medical devices
InactiveUS20060110370A1Reduce pathologic calcificationReduce pollutionBiocideMicrobiological testing/measurementCytotoxicityCalcification
A method for treating biomaterial is provided in which a biological tissue, typically after being cross-linked, is contacted with an anticalcification treatment solution under condition effective to render the biomaterial resistant to in vivo calcification upon implantation in a host animal. The anticalcification treatment solutions comprise higher alcohol solutions, a polyol solutions and / or a polar aprotic organic solvent solutions. Methods of reducing cytotoxicity to host tissue of bioprostheses that comprise fixed animal tissues, and treatments to reduce viral contamination of implantable medical devices are disclosed herein.
Owner:CARBOMEDICS
Methods for Removing Viral Contaminants During Protein Purification
The present invention relates, in general, to methods for removing viral contaminants from therapeutic protein solutions to improve safety of therapeutic proteins administered to patients. Particularly contemplated is the removal of small non-enveloped viruses, such as parvovirus, from therapeutic protein solutions.
Owner:AMGEN INC +1
Virus Filtration of Cell Culture Media
ActiveUS20130344535A1Improve virological safetyImprove securityApparatus sterilizationCell culture mediaFiltrationCell culture media
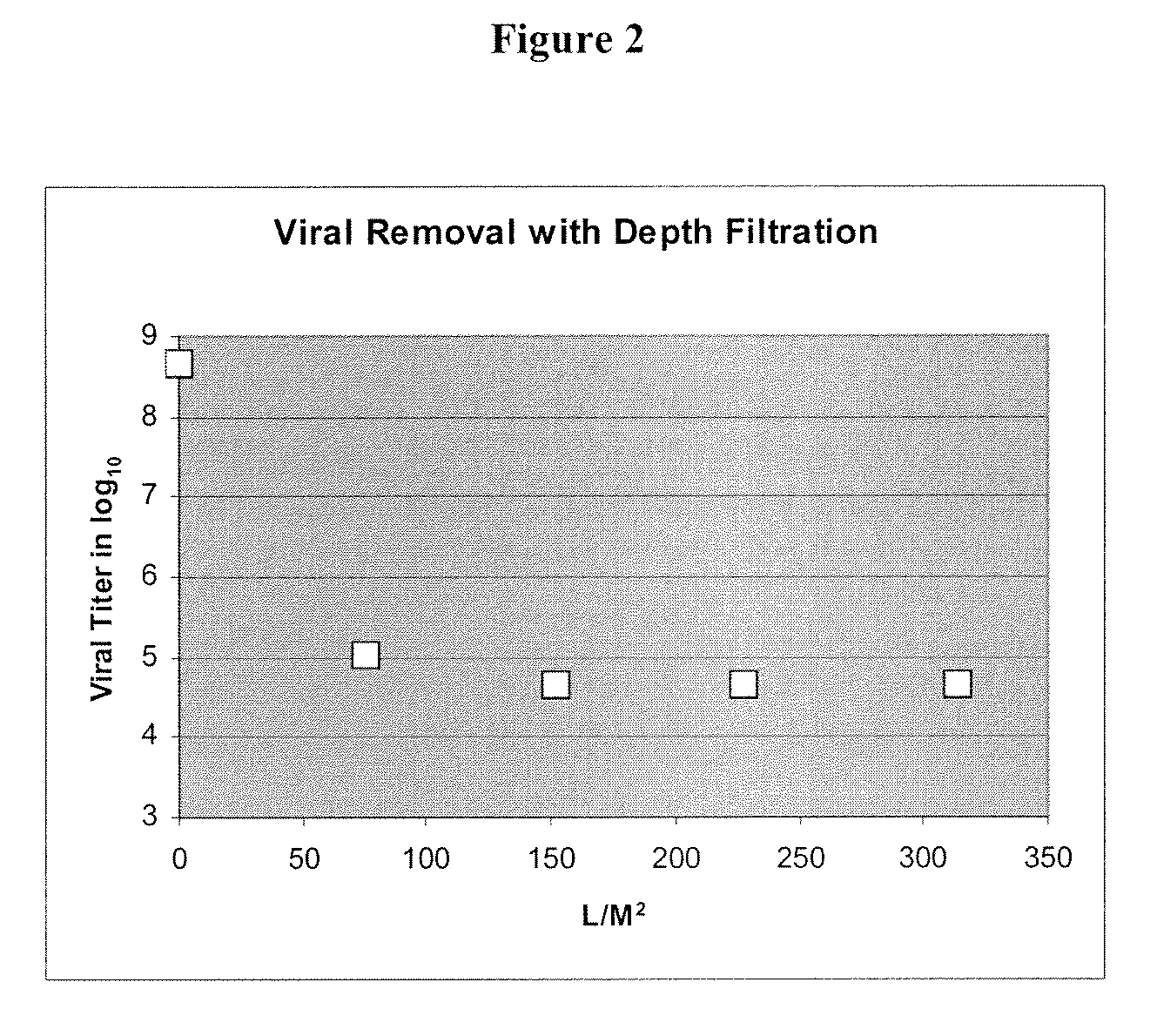
The invention relates to a method for removing a viral contaminant from a preparation, being a cell culture medium or at least a component of a cell culture medium. The method comprises subjecting said preparation to filtration for at least about 24 hours through a virus filter having an effective pore size of maximum about 75 nm. Further, the invention relates to the use of a virus filter in filtration of at least about 24 hours, wherein the virus filter has an effective pore size of maximum about 75 nm for the removal of viral contaminant from a preparation, being a cell culture medium or at least a component of a cell culture medium. In some embodiments the filtration according to the invention operates at a volumetric capacity of at least about 2000 L / m2. Further, the invention relates to the use of a preparation, being a cell culture medium or at least a component of a cell culture medium obtainable according to method of the invention for cell culture; pharmaceutical, diagnostic and / or cosmetic preparations as well as in food preparations.
Owner:TAKEDA PHARMA CO LTD
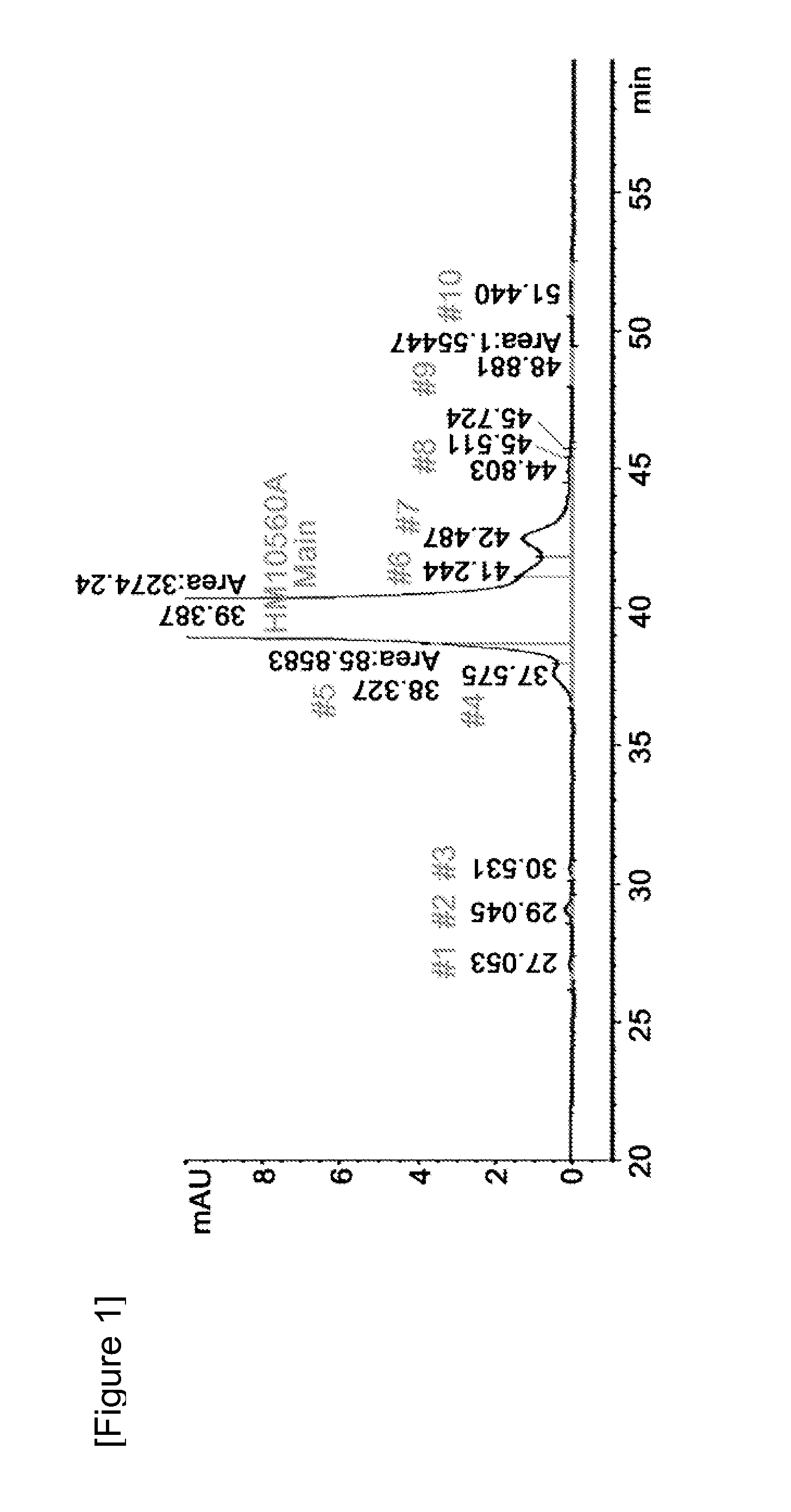
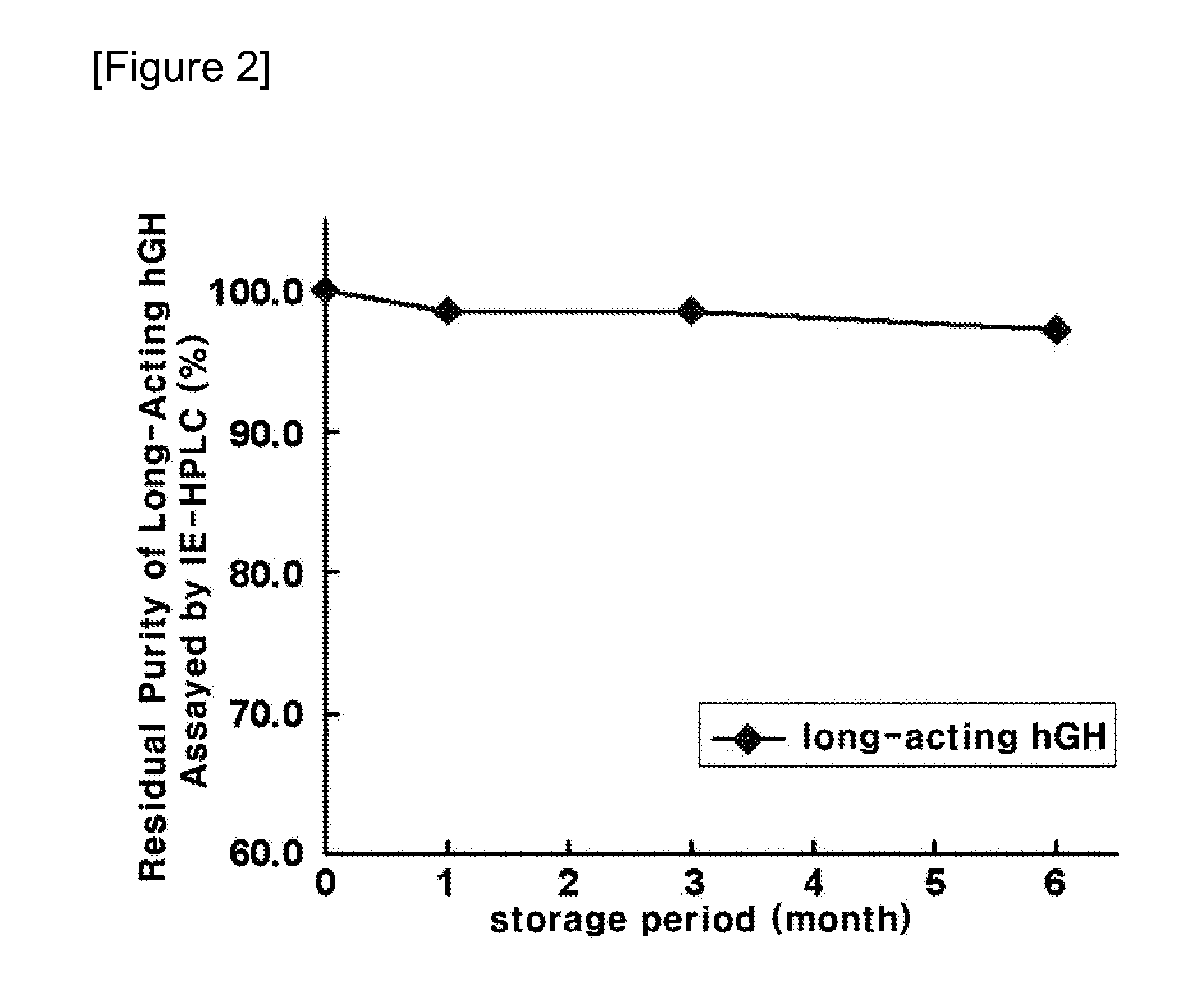
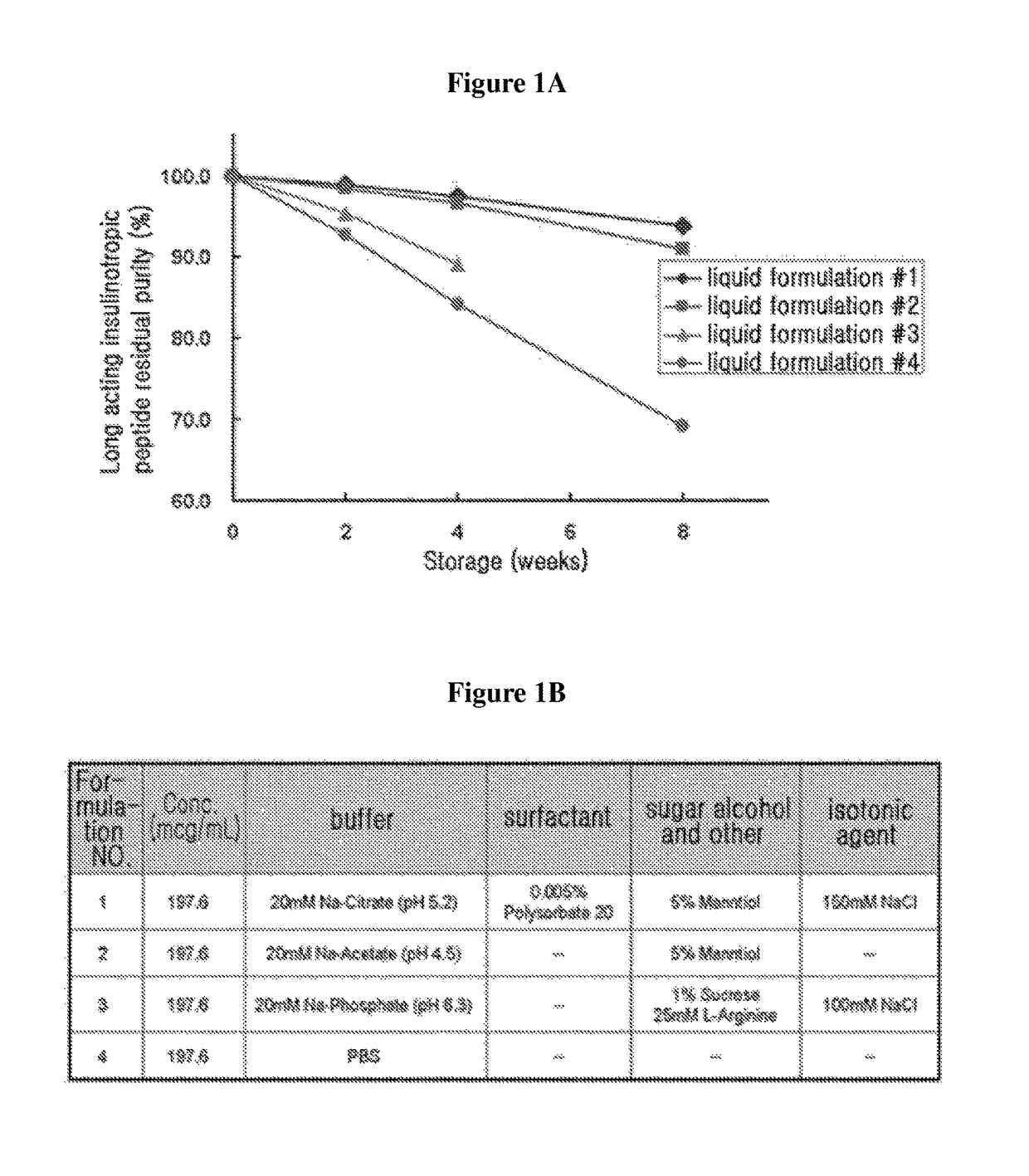
Liquid formulation of long-acting human growth hormone conjugate
InactiveUS20130115231A1Good storage stabilityIncreased durabilityPeptide/protein ingredientsAntibody mimetics/scaffoldsFactor iiAlcohol sugars
Disclosed is a liquid formulation of long-acting human growth hormone (hGH) conjugate, free of albumin, which can guarantee the stability of the long-acting hGH conjugate when stored over a long period of time, wherein the long-acting human growth hormone conjugate includes a human growth hormone linked to an immunoglobulin Fc region, and has a prolonged in vivo stability compared to the native form. The liquid formulation of hGH conjugate including a pH 5.0˜6.0 buffer, a sugar alcohol, a salt and a non-ionic surfactant is free of human serum albumin and other hazardous factors which are potentially contaminated with viruses, and can provide excellent storage stability customized for a long-acting hGH conjugate composed of an hGH polypeptide and an immunoglobulin Fc region which has higher molecular weight and in vivo durability, compared to the native.
Owner:HANMI SCI CO LTD
Virus-like micrograins and process for producing the same
The present invention provides a process for producing virus-like particles, which can produce virus-like particles having the protein of a virus and a lipid bilayer membrane derived from an eukaryotic microorganism as an outer membrane in a short period of time in a form that is not contaminated with a vector virus, and which enables the virus-like particles to be used for various applications. The process of the present invention comprises: (a) incorporating a gene encoding a protein essential for viral particle budding or a fragment thereof into a vector which can be expressed in a eukaryotic microorganism; (b) transfecting the vector into the eukaryotic microorganism; (c) culturing a transformed eukaryotic microorganism; (d) removing a cell wall of the eukaryotic microorganism; and (e) further culturing, followed by collection of a culture supernatant.
Owner:THE KITASATO INST
Fully human monoclonal antibody against tetanus toxin and derivative thereof, and preparation method and application thereof
ActiveCN105153305AResist attackHigh school and tetanus toxin capacityAntibacterial agentsImmunoglobulins against bacteriaHeavy chainHalf-life
The invention provides a fully human monoclonal antibody against tetanus toxin. The amino acid sequences of a heavy-chain variable region and a light-chain variable region of the monoclonal antibody are SEQ ID No. 3 and SEQ ID No. 4, respectively; preferably, the amino acid sequences of a heavy chain and a light chain of the monoclonal antibody are SEQ ID No. 1 and SEQ ID No. 2, respectively. The invention further provides a preparation method and application of the fully human monoclonal antibody against tetanus toxin and a derivative thereof. The fully human monoclonal antibody against tetanus toxin provided by the invention can eliminate the biological risks of anaphylactic reaction and virus contamination, has a sufficiently long half life and high titer and in-vivo activity, can be applied to large-scale industrial production.
Owner:ANTAGEN BEIJING BIOTECH CO LTD
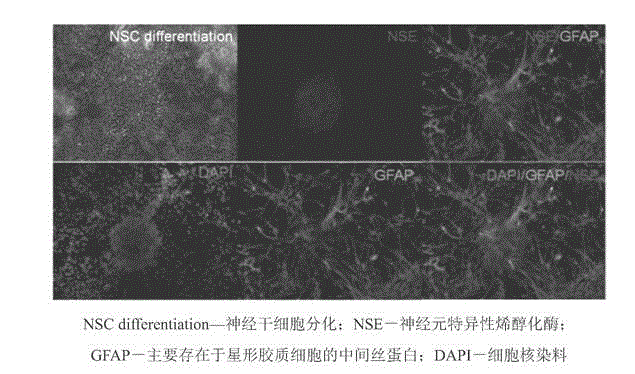
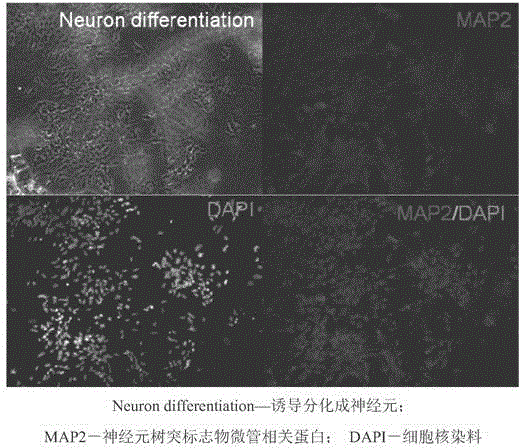
Clinical-grade serum-free medium for adherent culture of human neural stem cells
InactiveCN104560876AImprove antioxidant capacityIncrease profitNervous system cellsLipid formationNutrition
The invention discloses a clinical-grade serum-free medium for adherent culture of human neural stem cells. The medium disclosed by the invention comprises a basic medium, basic nutrition additives, plant-based human serum albumins, saccharides, lipid, hormones, antioxidants and related substances for promoting metabolism; the basic medium is prepared from commercial DMEM / F12 and a commercial neurobasal medium according to a ratio of 1:1; the basic nutrition additives comprise insulin, holo-transferrin, apo-transferrin, putrescine, progesterone and sodium selenite; the hormones comprise biotin, corticosterone, lipoic acid, Ve and Ve acetic ester; the antioxidants comprise human-derived catalase, human-derived superoxide dismutase, glutathione and Vc; the related substances for promoting metabolism comprise carnitine, T3 and ethanol amine. The medium can improve a cell expansion speed by two to three times, well keeps stem properties of the neural stem cells, keeps the cell differentiation potential of the neural stem cells and eliminates potential animal-origin endotoxin and viral pollution.
Owner:广州吉帝生物科技有限公司
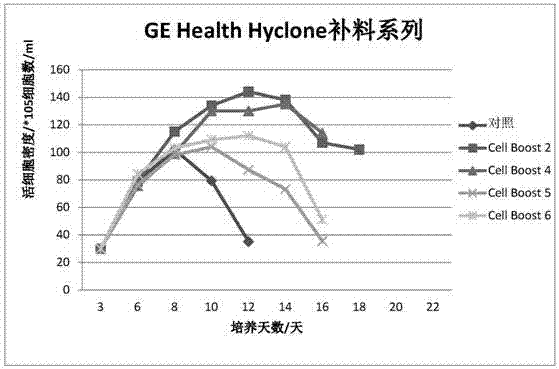
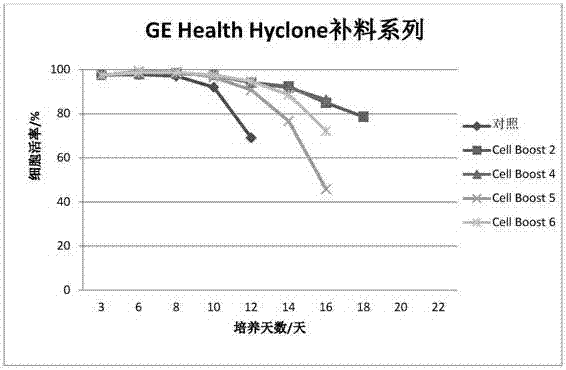
Serum-free protein-free feed culture medium as well as preparation method and application thereof
ActiveCN107460159AReduce the risk of contaminationRich varietyCulture processArtificial cell constructsPollutionCulture mediums
The invention relates to a serum-free protein-free high-efficiency feed culture medium as well as a preparation method and application thereof. The feed culture medium comprises amino acids, inorganic salts, trace elements, vitamins, carbohydrates and other organic matters. Moreover, the culture medium does not contain any glutamine. Since the feed culture medium contains serum-free protein-free animal-source-free components, the viral pollution risk can be greatly reduced, and downstream purification is facilitated. Meanwhile, since the culture medium is full in types of formula components and balanced in proportion, the culture medium can be applicable to high-density culture and high-protein expression of multiple CHO cell strains.
Owner:上海多宁生物科技股份有限公司
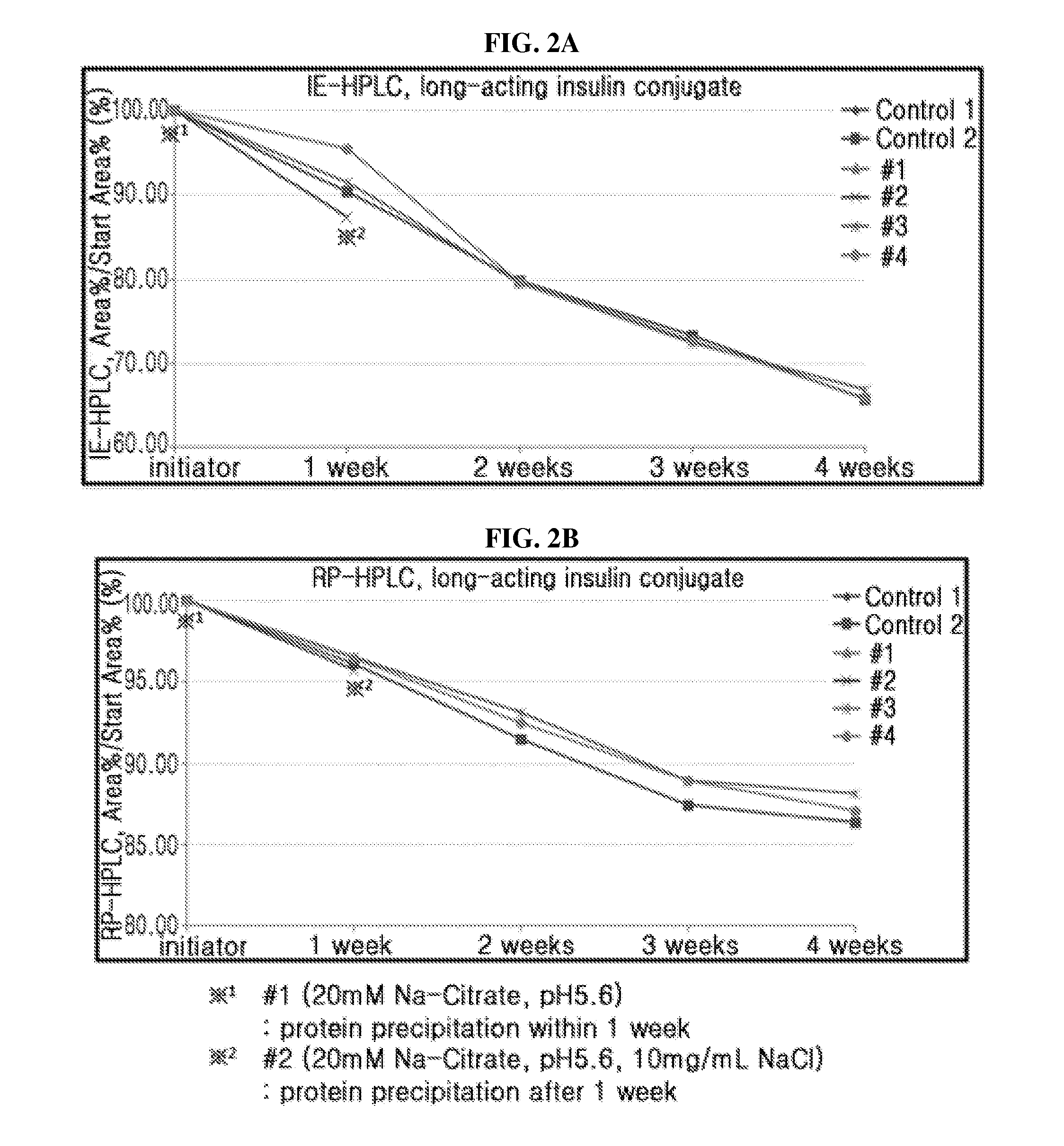
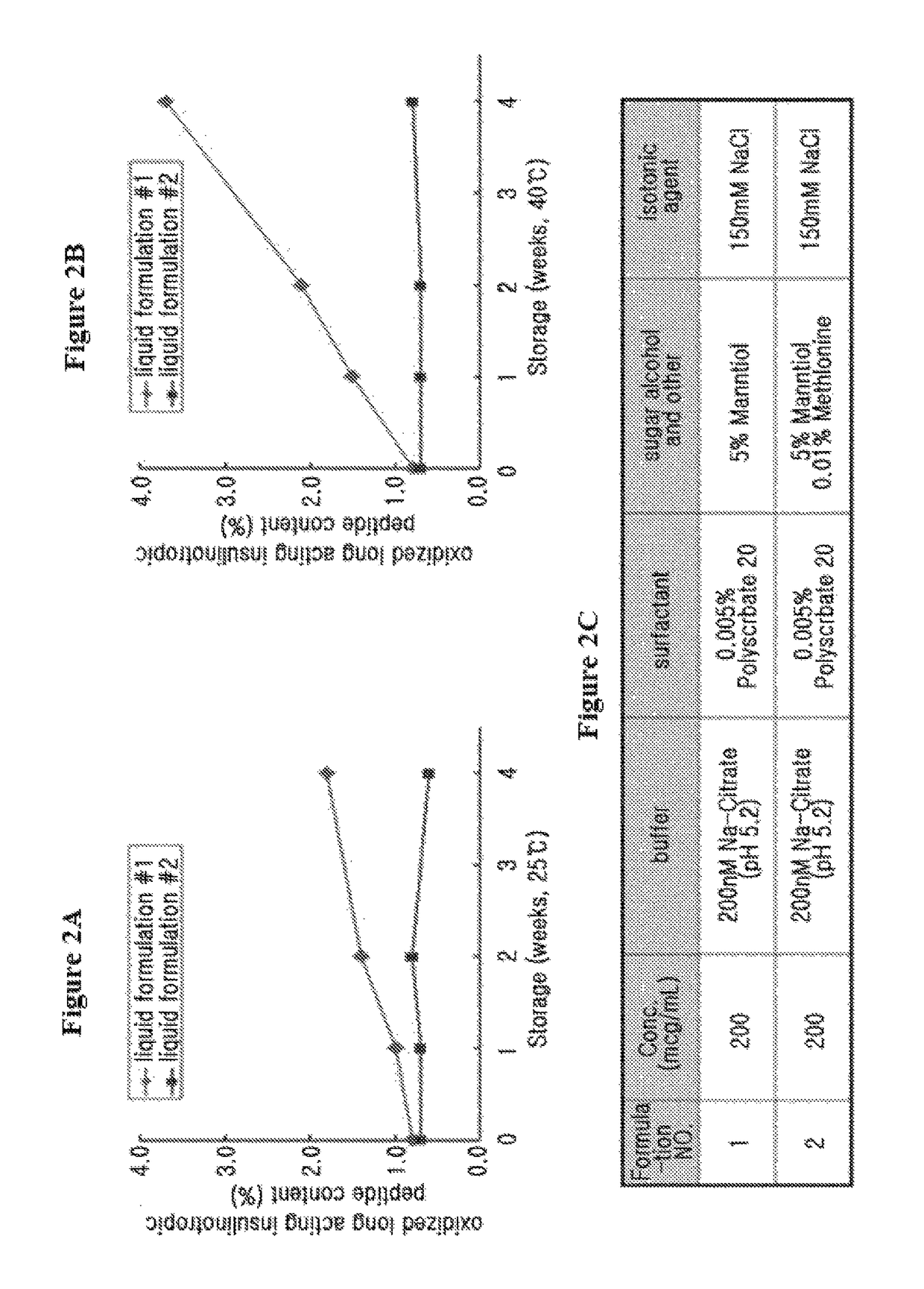
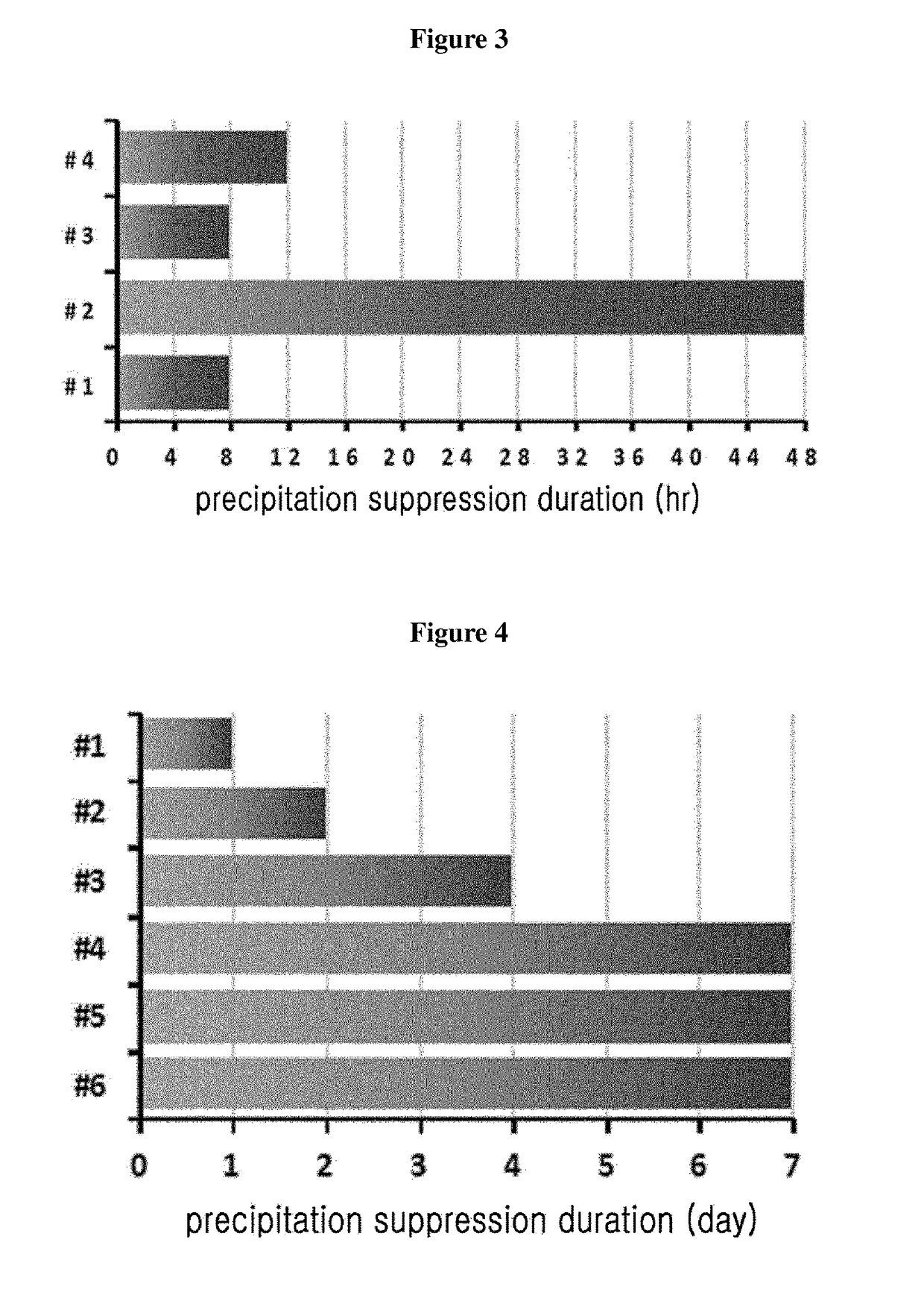
Liquid formulation of long-acting insulin and insulinotropic peptide
ActiveUS20150190528A1Good storage stabilityMaintain activityPeptide/protein ingredientsMetabolism disorderHigh concentrationAlcohol sugars
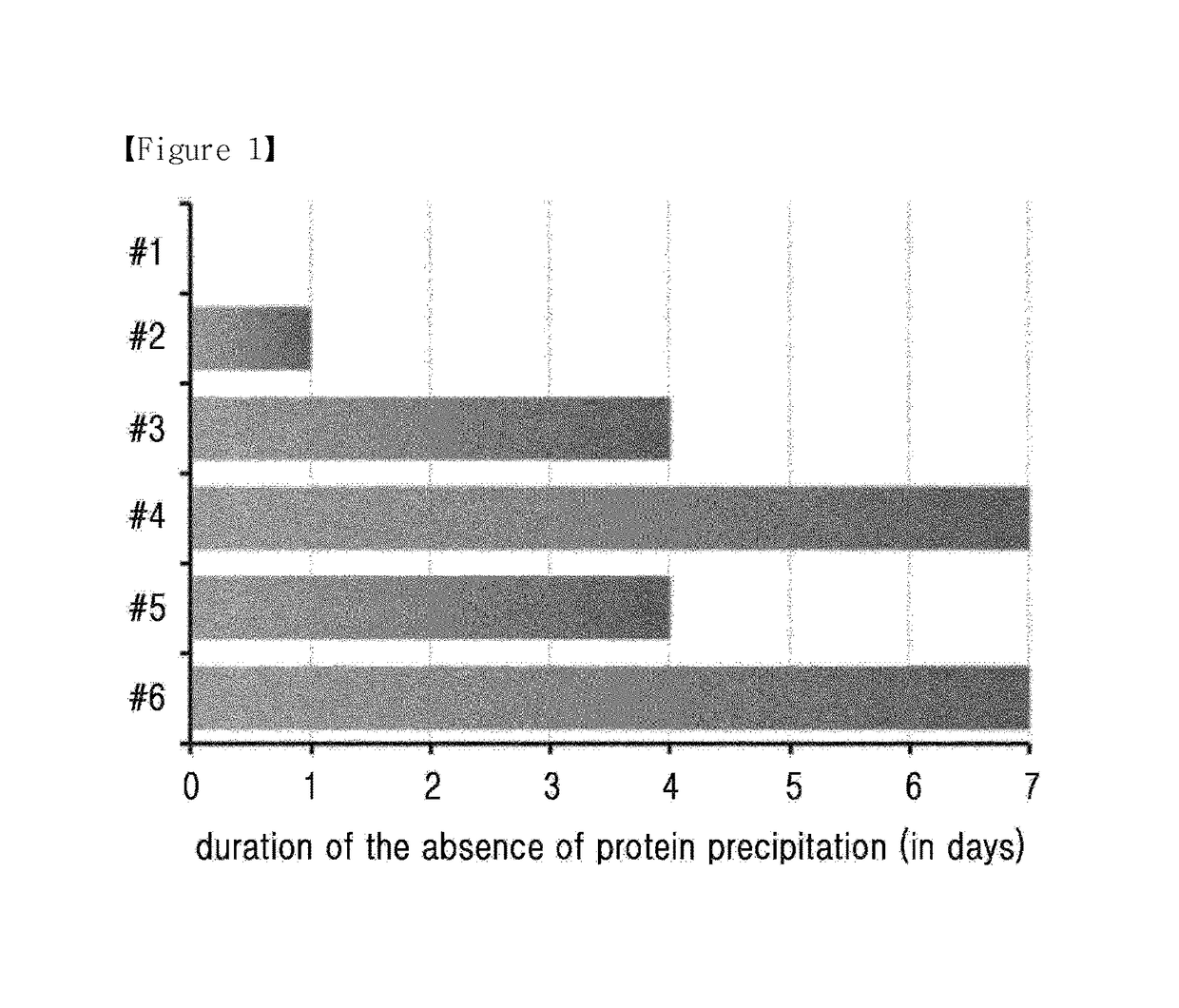
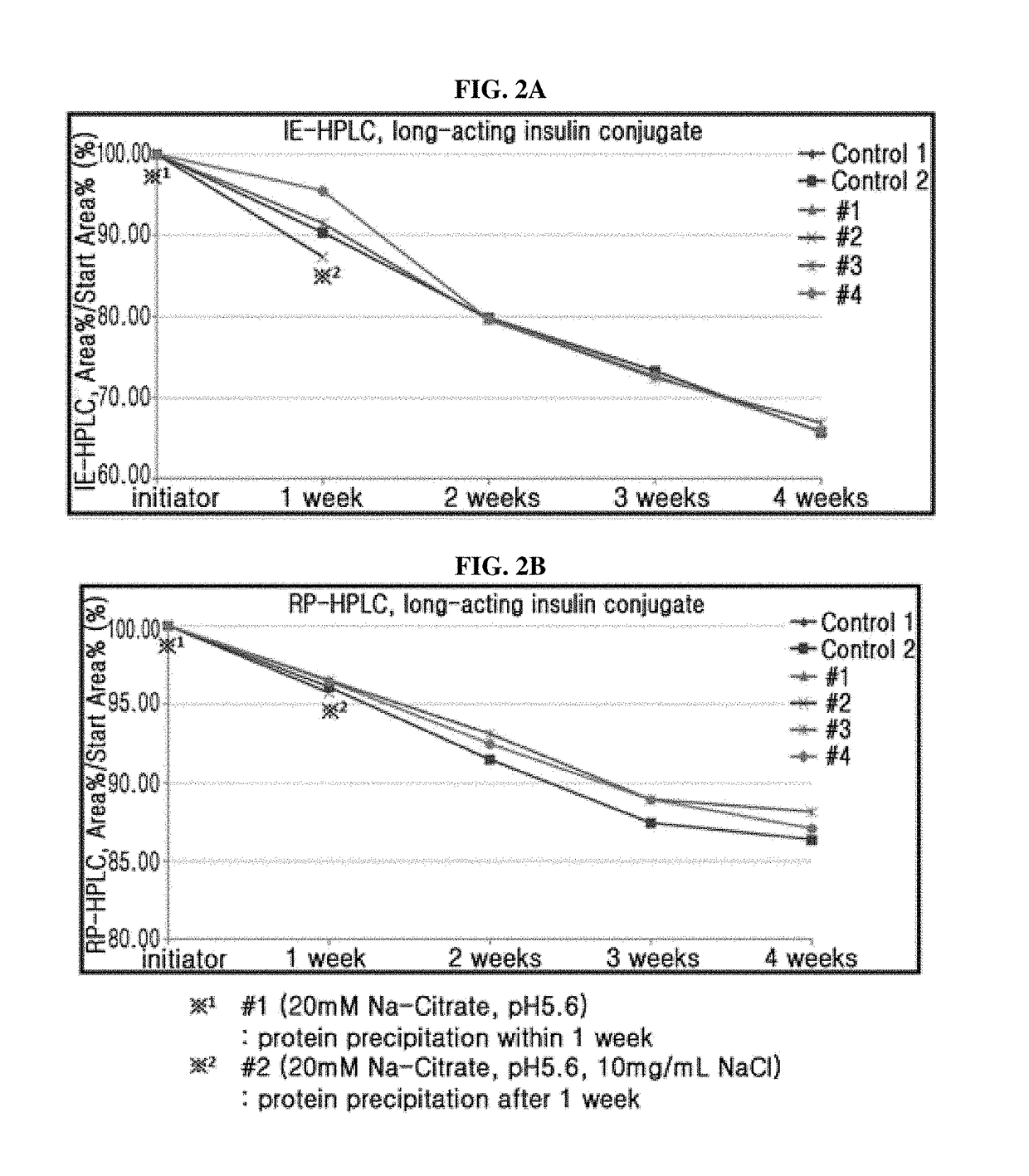
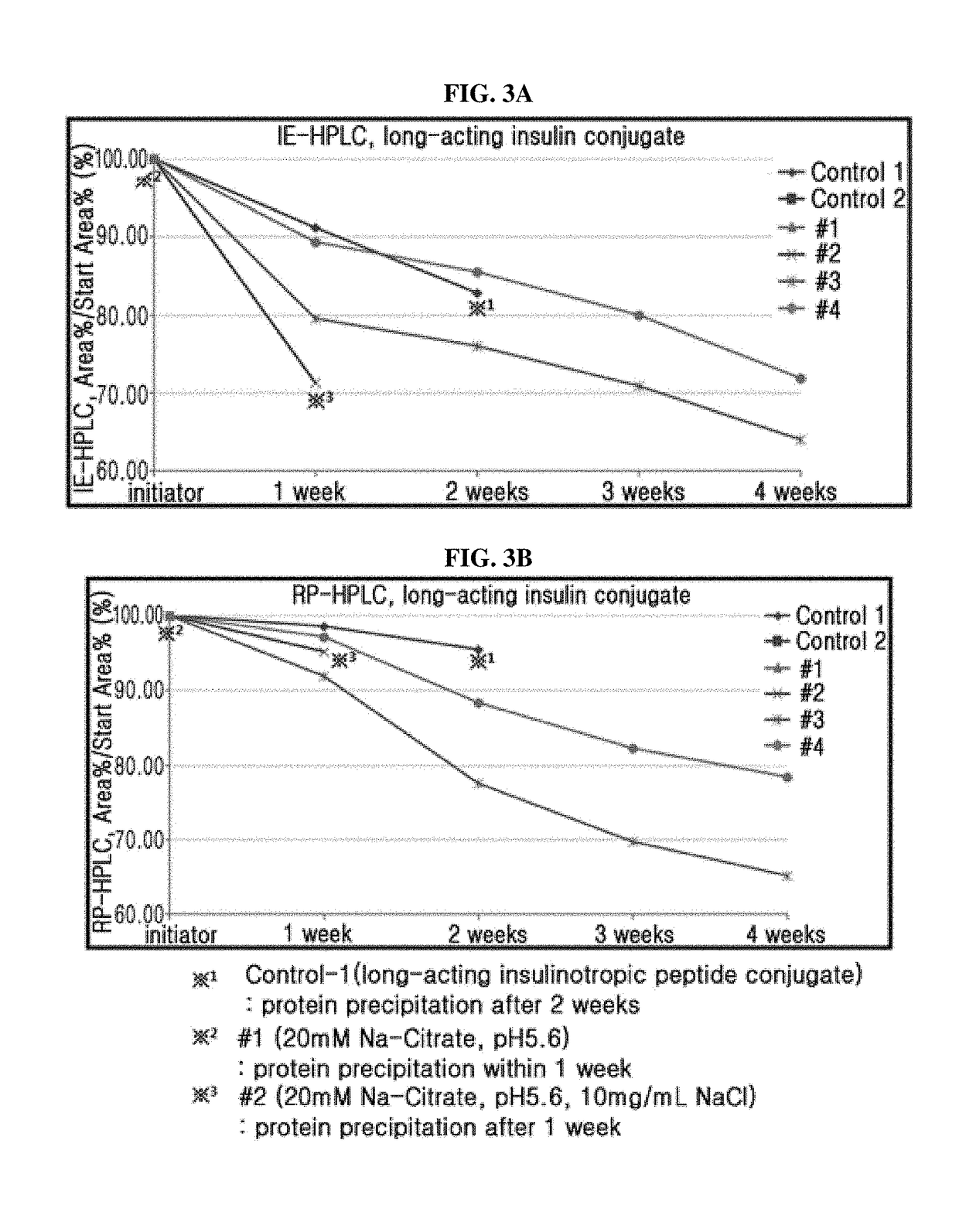
The present invention relates to a liquid formulation of a combination of long-acting insulin and insulinotropic peptide, comprising insulin which is a physiologically active peptide, insulinotropic peptide, and albumin-free stabilizer, wherein the stabilizer comprises a buffer, a sugar alcohol, a non-ionic surfactant, and an isotonic agent; and a method for preparing the liquid formulation. The liquid formulation of the present invention does not contain a human serum albumin and potentially toxic factors to the body, and thus it has excellent storage stability for insulin conjugate and insulinotropic peptide conjugate at high concentration, without a risk of viral contamination.
Owner:HANMI PHARMA
New method for partition and inactivation of viral and prion contaminants
InactiveUS20050250935A1Peptide preparation methodsDepsipeptidesBuffer solutionProteinaceous infectious particle
Herein is described a new method for partition and inactivating of viral and prion contaminants contained in a solution. The method is based on ultracentrifugation of the solution in a centrifuge tube at the bottom of which a high molarity urea solution is layered. The system can include a cushion solution interposed as an intermediate liquid phase between the starting solution and the urea solution.
Owner:IBSA INSTITUT BIOCHIM
Cell cryopreservation fluid and application thereof
InactiveCN109221092AAvoid pollutionImprove survival rateDead animal preservationAntigenHydroxyethyl starch
The invention relates to the technical field of biomedicine, in particular to cell cryopreservation fluid and application thereof. The cell cryopreservation fluid disclosed by the invention is prepared from the following components: dimethyl sulfoxide, human albumin, low-molecular dextran, a human mesenchymal stem cell exosome, hydroxyethyl starch and a basal culture medium. Cells cryopreserved and revived by using the cell cryopreservation fluid are high in viability, the morphologic change is not large, differentiation cannot be caused during culture, and surface antigen detection accords with the standard; in addition, by optimizing a cell cryopreservation scheme, death of a large amount of cells caused by ice crystal formation during a cryopreservation process of the cells can be avoided as much as possible, a cryopreservation system is optimized, revived cells can be directly fed back to a human body, and animal derived serum is not added, animal derived virus infection is avoided; the cell cryopreservation fluid is wide in application, and the technical problem that the cell viability after the reviving of the cells which are cryopreserved by an existing cell cryopreservationsystem is not high is solved.
Owner:GUANGDONG CHINAHEALTH LIFE SCI CO LTD
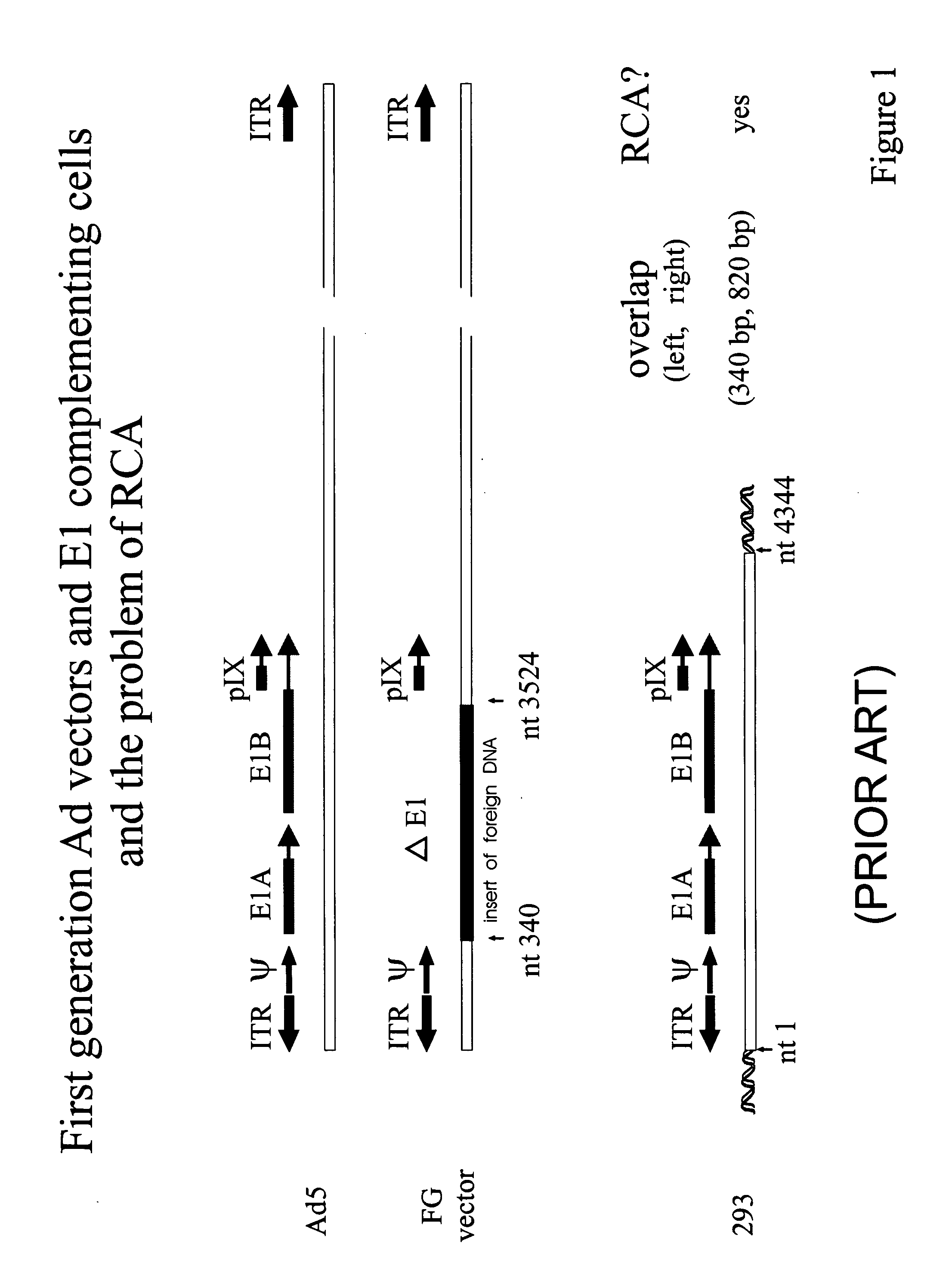
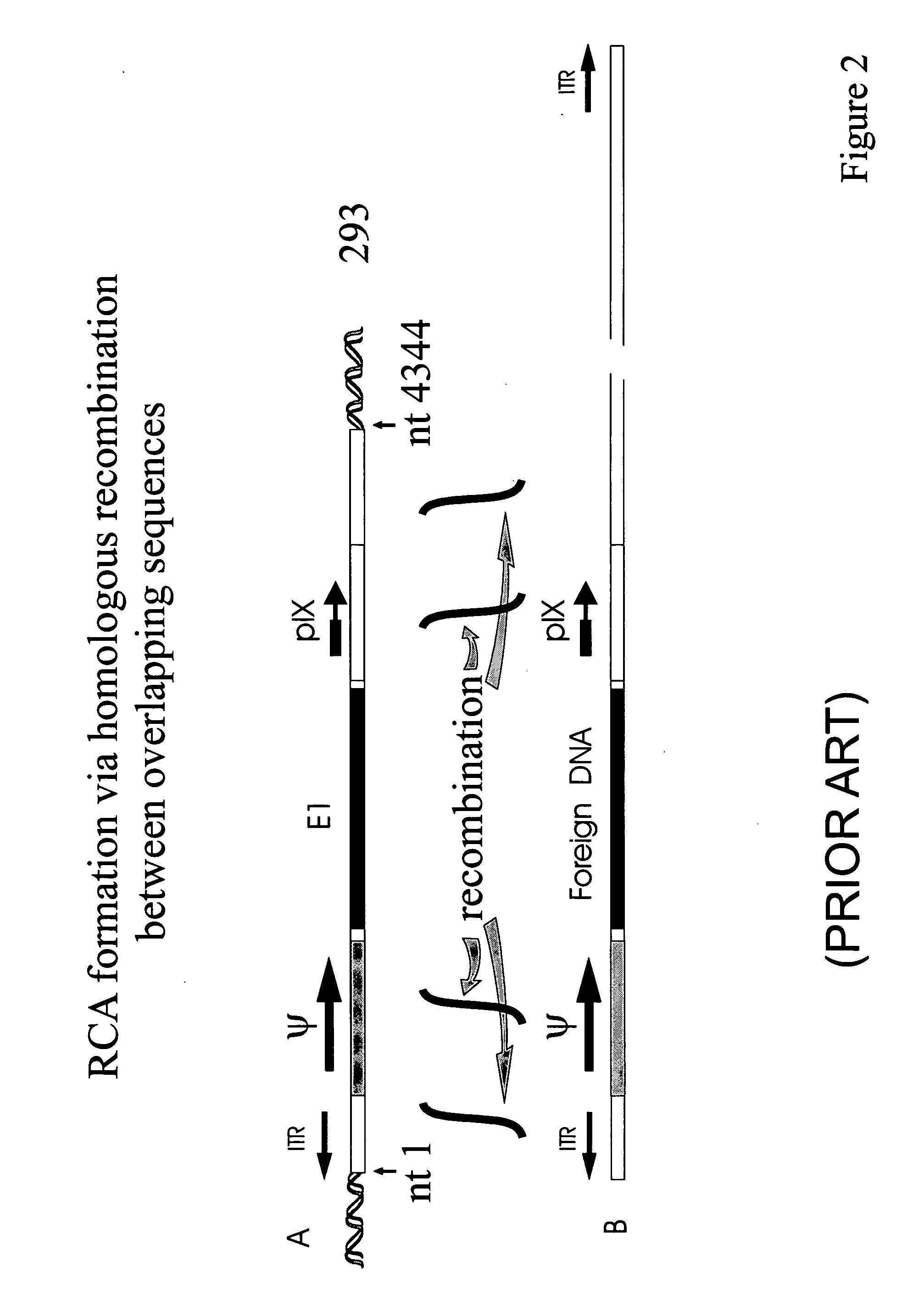
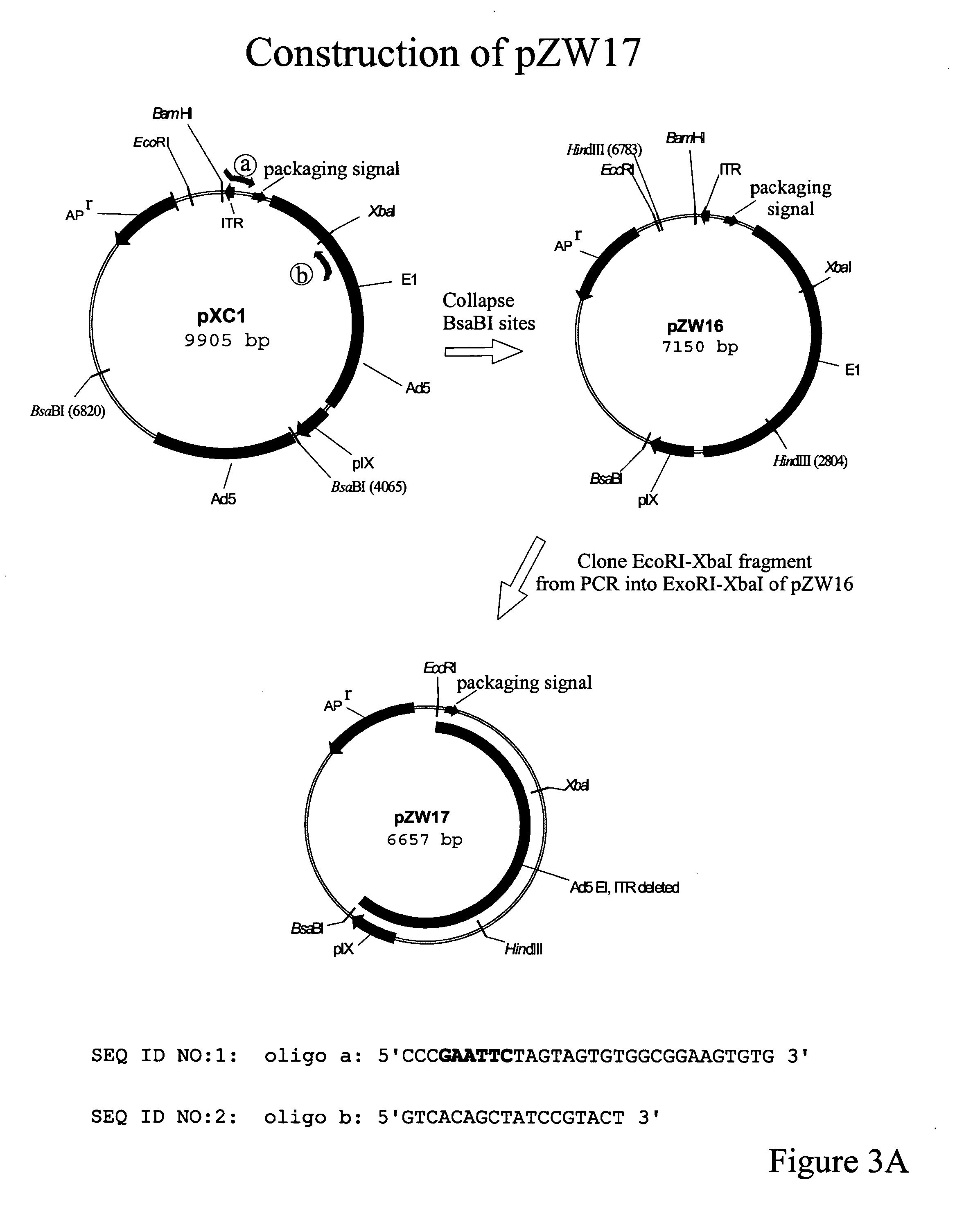
Production of adenovirus vectors with reduced levels of replication competent adenovirus contamination
Methods, cells and recombinant adenoviral vectors are disclosed that permit the production of recombinant adenoviral vector stocks with reduced levels of contamination by replication competent adenoviruses (RCA). In certain embodiments are disclosed early region 1 (E1) deficient recombinant adenoviral vectors and complementing E1 positive host cells whose sequences are designed to avoid formation of RCA by homologous recombination between sequences in the vector and E1 sequences in the cells. One aspect of the invention involves the inversion of the packaging signal in a recombinant adenoviral vector relative to an adjacent or nearby inverted terminal repeat (ITR). Methods include use of site-specific intregrase family recombinases such as Cre or FLP and recombinase recognition sites such as lox sites or frt sites.
Owner:ADVEC
Methods and compositions for inactivating viruses
The present invention relates to methods and processes of inactivating viral contaminants in a biological source material (e.g. a host cell, cell supernatant, cell lysate, blood plasma, tissue homogenate, or other biological materials) with a solution containing one or more alkylamine compounds. In a particular embodiment, the active ingredients are amphipathic, charged amines or amine oxides coupled to saturated hydrocarbon chains of varying lengths.
Owner:NV ORGANON
Viral reduction method for plasma using a leukocyte-reduction filter and two virus-reduction filters of decreasing pore diameters
ActiveUS7592134B2Efficient methodEfficient removalOther blood circulation devicesHaemofiltrationWhite blood cellLeukocyte Reduction Filtration
The present invention relates to a plasma product or a serum product with an extremely low risk of viral contamination and a method for producing the same. Before treating plasma or serum to be used as a raw material for producing a plasma product or a serum product using a virus removal membrane, leucocytes contaminating the blood are removed. Thus, a plasma product or a serum product with an extremely low risk of viral contamination can be efficiently produced while preventing clogging. Since clogging scarcely arises, it is possible to carry out efficient filtration without applying an elevated pressure as the filtration proceeds.
Owner:ASAHI KASEI MEDICAL CO LTD
Method for preparing serum-free soybean protein peptide animal cell medium
InactiveCN101914485ASave the cultivation cycleSave distractionsVertebrate cellsArtificial cell constructsCell culture mediaL-Glutamin
The invention discloses a method for preparing a serum-free soybean protein peptide animal cell medium, and relates to a serum-free or low-serum collective cell medium. The serum-free soybean protein peptide animal cell medium is prepared from calcium chloride, potassium chloride, anhydrous magnesium sulfate, sodium chloride, anhydrous sodium dihydrogen phosphate, L-glutamine, D-glucose, phenol red, D-calcium pantothenate, choline chloride, folic acid, i-inositol, niacinamide, pyridoxal hydrochloride, lactoflavin, thiamine hydrochloride, soybean protein peptide and distilled water. The method of the invention solves the problem that the conventional serum-free medium cannot be directly used for cultivating cells in the poor growth condition, and a large number of the cells can adapt to the conventional serum-free medium only when the serum concentrations thereof are gradually reduced, and the cells are easily affected by external factors, so fatal damages are easily caused. The method of the invention has the advantages that the cells can directly be cultivated in the medium of the invention without gradual domestication and cultivation, so the culture period of the cell is shortened and the interference of other factors is avoided; meanwhile, the virus pollution brought by serums is reduced, and the survival rate of the cells is over 94 percent.
Owner:乐能生物工程股份有限公司
Non-blood serum plant protein peptide universal cell culture medium
InactiveCN101245335AReduce distractionsImprove survival rateVertebrate cellsArtificial cell constructsCell culture mediaCulture mediums
A serum-free vegetable protein peptide general cell culture medium relates to a serum-free or low-serum general cell culture medium. The serum-free vegetable protein peptide general cell culture medium solves the problems that the existing serum-free culture medium can not directly culture the cells with adverse growth status, most of cells can be adaptable to the existing serum-free culture medium after the gradual reduction of the serum concentration, and the existing serum-free culture medium is vulnerable to the external factors and easy to cause fatal damage. The serum-free vegetable protein peptide general cell culture medium is prepared by calcium chloride, potassium chloride, magnesium sulfate anhydrous, sodium chloride, anhydrous sodium dihydrogen phosphate, L-glutamine, D-glucose, phenol red, D-calcium pantothenate, choline chloride, folic acid, i-inositol, nicotinamide, pyridoxal hydrochloride, riboflavin, thiamine hydrochloride, soybean protein peptide and distilled water. Cells can be directly cultured on the culture medium of the invention without gradual habituation and culture, thus shortening culture period and avoiding the interference by other factors, reducing the virus pollution which is caused by serum; the cell survival rate reaches more than 94 percent.
Owner:乐能生物工程股份有限公司
Coronavirus pseudovirus packaging system and packaging method, and application of coronavirus pseudovirus to evaluating disinfection efficacy
ActiveCN112760297AFast packLow background valueSsRNA viruses negative-senseBiocideDisinfectantFluorescent protein
The invention relates to a coronavirus pseudovirus packaging system. The coronavirus pseudovirus packaging system comprises a vesicular stomatitis virus VSV vector and an assembly cell, wherein the vesicular stomatitis virus VSV vector is formed by replacing GP genes with Fluc and EGFP double reporter genes, and the assembly cell is used for expressing coronavirus spike protein S. The double reporter genes are selected from luciferase and fluorescent protein, and the luciferase reporter gene is preferably the Fluc gene. According to the packaging system, a one-step packaging method is adopted, so that pseudoviruses which are infected in a single cycle, low in background value and high in titer and have the characteristic of rapid detection compared with a lentivirus-mediated pseudovirus system can be rapidly packaged, and the packaging system can be used for researching coronaviruses such as COVID-19 (SARS-CoV-2), SARS (SARS-CoV) and MERS; and the pseudoviruses can be used for evaluating the efficacy of a disinfectant through the steps of a virus pollution distribution model, scene building and sampling detection, a safe, convenient and effective tool method is provided for evaluating the disinfectant, and the pseudoviruses have wide application value.
Owner:FANTASIA BIOPHARMA ZHEJIANG CO LTD
Method for proliferating avian influenza viruses in bioreactor with cell carrier
InactiveCN102127524AUniform product qualityStable virulenceMicroorganism based processesViruses/bacteriophagesAutomatic controlVaccine Production
The invention discloses a method for replicating and proliferating avian influenza viruses in the passage cells which are absorbed and grow in the microcarrier of a bioreactor. The invention is based on the method which uses cells as carrier to proliferate viruses in the bioreactor. The obtained avian influenza viruses and the avian influenza virus vaccine product have uniform quality and stable toxicity. The automatic control of vaccine production can be realized, the problem of large-scale production and application can be solved, the production matrix of the influenza vaccine can be completely changed, the technology that chick embryo is adopted to culture viruses can be changed, the problems caused by chick embryo such as adventitious agent pollution and viral pollution and the interferences caused by heterologous proteins such as chick embryo can be reduced, the purity, safety and immune effect of vaccine can be increased and the method can play an important role in the prevention of avian influenza.
Owner:深圳市南海潮生物技术有限公司
Human source anti- tetanus exotoxin antibody and preparation method and use thereof
InactiveCN1594361AAvoid allergic reactionsAddressing issues that predispose to allergic reactionsAntibacterial agentsImmunoglobulins against virusesEscherichia coliHypersensitive response
The invention discloses a human source anti-tetanus exotoxin antibody (HTAT-Fab) and preparation method, comprising: constructing human immunity phage antibody bank, screening phage positive clone, further getting HTAT-Fab gene with a specific neutralization activity and high affinity. The gene can be expressed in the procaryotic cell such as E.coli, eukaryotic cell such as microzyme, or mammalian cell such as CHO, purifying to get highly purified HTAT-Fab with a strong tissue penetrability, a high affinity. The HTAT-Fab product can not only eliminate the allergic reaction generated by horse serum anti-tatanus antitoxin (TAT) (foreign protein), but also avoid the blood source for producing human tetanus immunoglobulin (HTIG) and the latent virus pollution.
Owner:北京明新高科技发展有限公司 +2
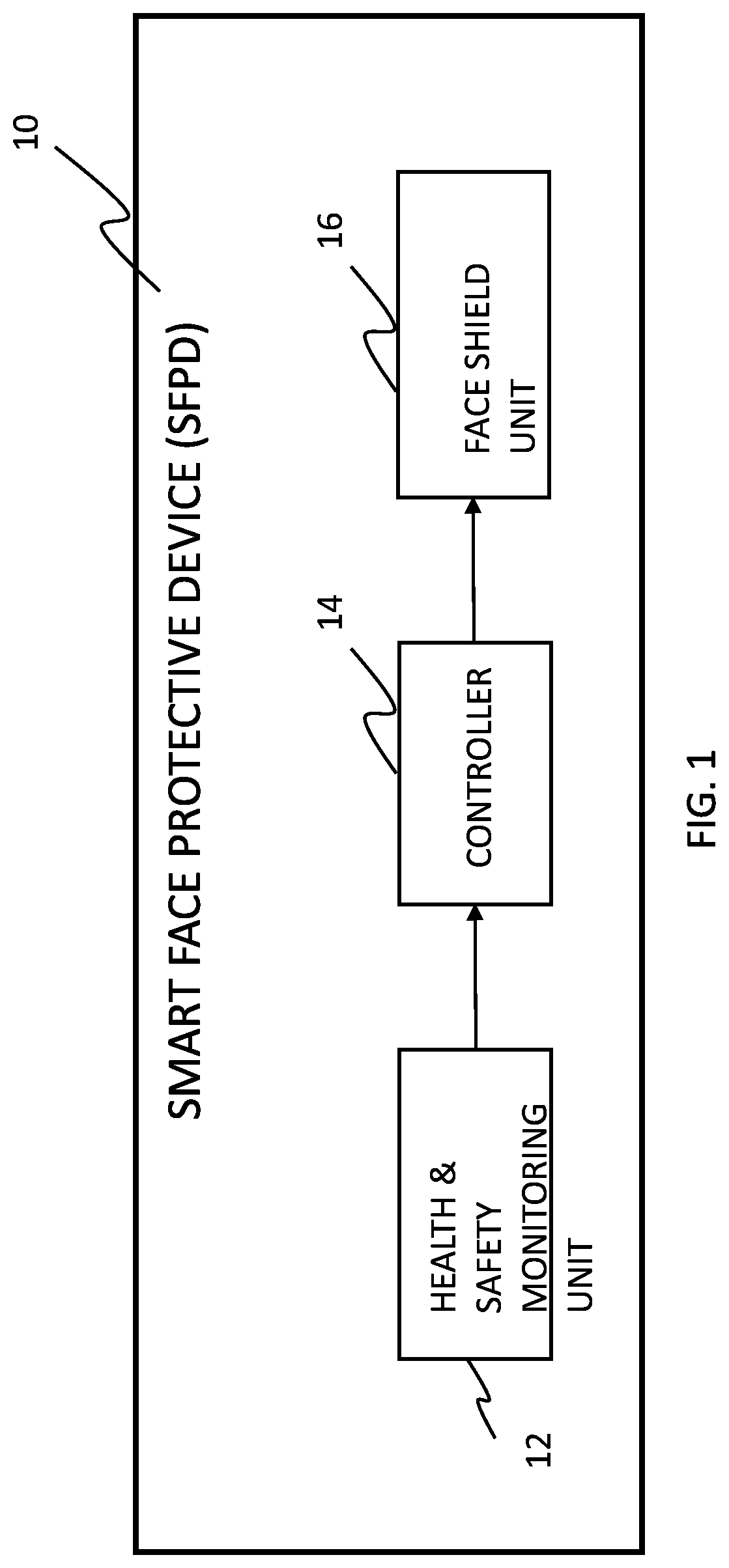
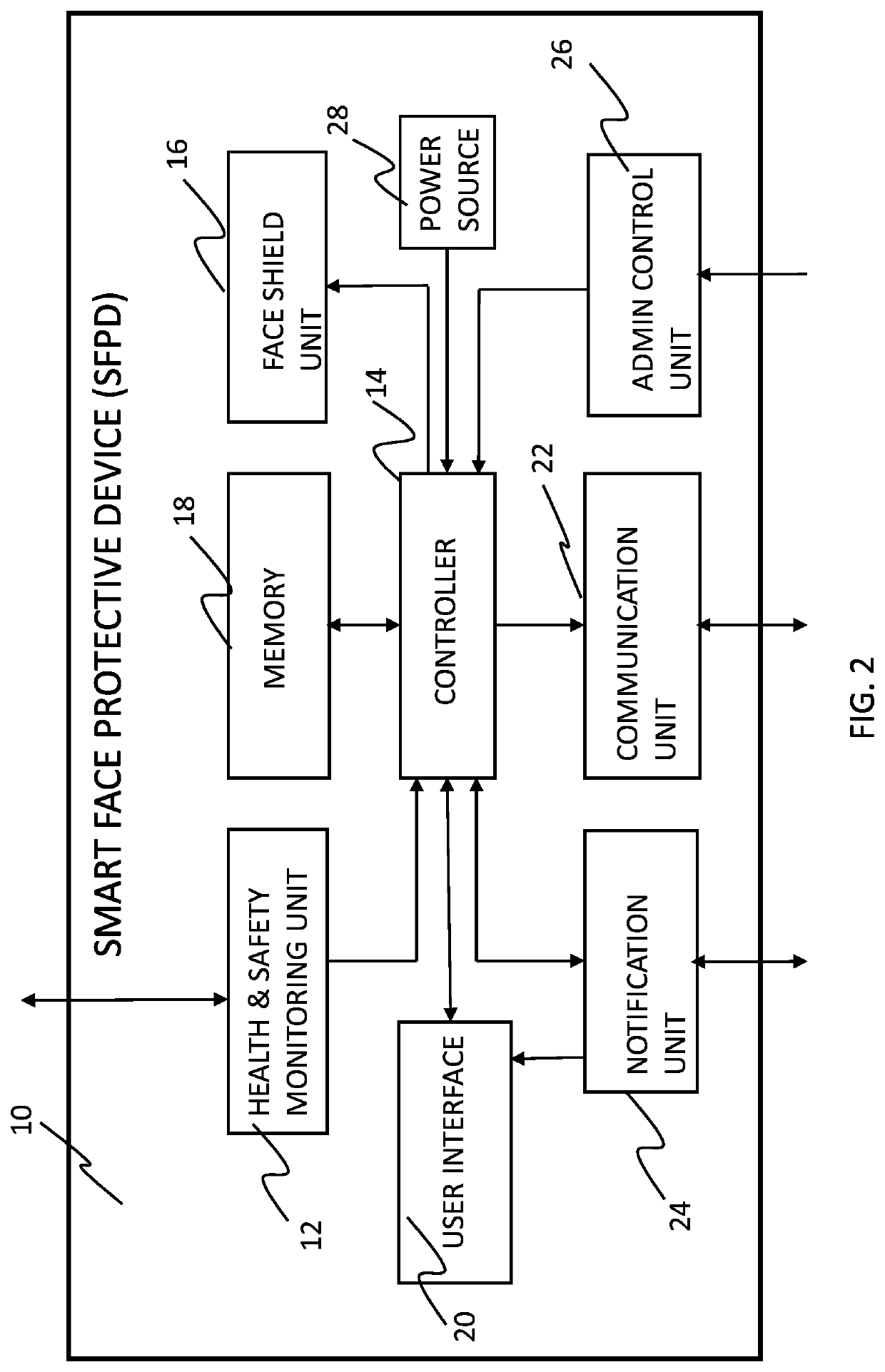
Smart face protective device and system for infection control
There is provided a smart face protective device, preferably as part of a helmet or another head held device, to be used by a user for preventing or reducing spread of infectious droplets through a portal portion of the user's face, where the face protective device is configured to be automatically adjusted while being in use between a secured position and an unsecured position based on an electric signal associated to a monitored health and safety condition of the user. There is also provided a system, a network and a method for allocating smart face protective devices to members of a community and managing these devices and data collected thereby for preventing or reducing risks of virus contamination and spread, namely COVID-19, between the members of the community.
Owner:SALEH AHMAD
Plant enzyme and preparation method and use thereof
ActiveCN104957610AComprehensive supplementImprove the immunityFood ingredient functionsFood preparationBiotechnologyLactobacillus fermentum
The present invention belongs to the field of food and food processing, in particular to a plant enzyme and a preparation method and a use thereof. The plant enzyme is mainly composed of moringa oleifera leaves, maca, roselle, passiflora coerulea, blueberries and kiwi fruits. The preparation method includes: pulping the plant enzyme raw materials, fermenting in a fermentation tank containing mixed bacteria of bifidobacterium adolescentis, streptococcus thermophilus and lactobacillus and drying. The provided plant enzyme uses food-grade mixed bacteria strains as the fermentation bacteria, fully decomposes nutrients in plant raw materials, and can fully replenish nutrients needed by human body, enhance cellular metabolic function, promote digestion, and enhance human body immunity. At the same time, the food-grade bacteria strains will not appear other bacterial and viral contamination problems in the fermentation process, which realize the security of the plant enzymes. Besides, animal experiments and clinical trials both prove that the plant enzymes have good blood pressure lowering effects.
Owner:GUANGDONG ZHENGDANGNIAN BIO TECH CO LTD
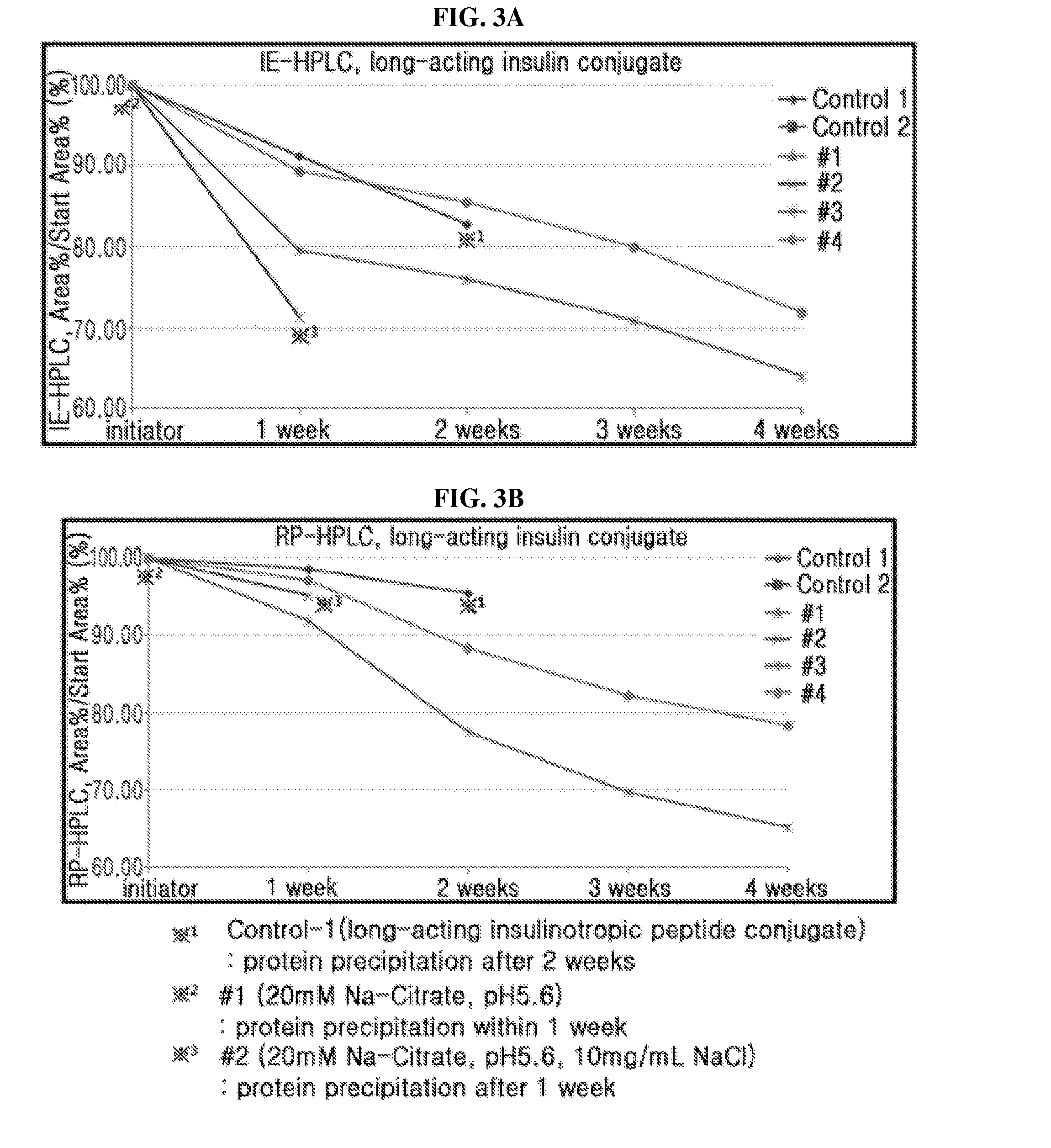
Liquid formulation of long acting insulinotropic peptide conjugate
ActiveUS9801950B2No risk of viral contaminationGood storage stabilityPeptide/protein ingredientsMetabolism disorderHigh concentrationAlcohol sugars
The present invention relates to a liquid formulation of long-acting insulinotropic peptide conjugate, comprising a pharmaceutically effective amount of long-acting insulinotropic peptide conjugate consisting of a physiologically active peptide, insulinotropic peptide, and an immunoglobulin Fc region; and an albumin-free stabilizer, wherein the stabilizer comprises a buffer, a sugar alcohol, a non-ionic surfactant, and an isotonic agent, and a method for preparing the formulation. For the purpose of preventing microbial contamination, a preservative may be added. The liquid formulation of the present invention is free of human serum albumin and other potentially hazardous factors to body, having no risk of viral contamination, and thus can provide excellent storage stability for insulinotropic peptide conjugates at high concentration.
Owner:HANMI PHARMA
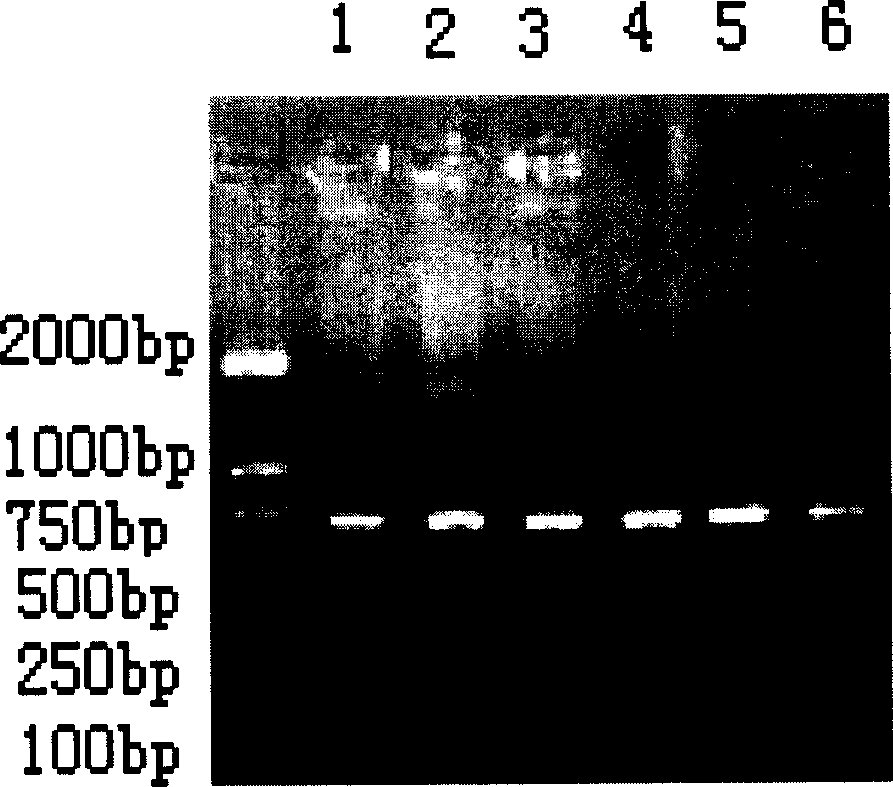
Multi-RT-PCR method for monitoring pollution of six viruses in pig farm environment through one system and application
InactiveCN105002297AMicrobiological testing/measurementMicroorganism based processesBiotechnologyPig farms
The invention provides a multi-RT-PCR method for monitoring pollution of six main viruses in a pig farm environment through one system and application. According to the method, primer design is conducted for CSFV, JEV, PRRSV, PCV-2, PRV and PPV, a multi-RT-PCR reaction system for the six viruses is established, air samples are collected through liquid collision, the viruses are subjected to separation concentration through ultrafiltration centrifugation, and the method for detecting the pollution of the main viruses in the pig farm environment is established. The method can be used for monitoring the pollution of the six viruses in air, fodder, drinking water, appliances, excrement and pig groups in the pig house and pig farm environment, and technical support is provided for establishing a main virus pollution early-warning mechanism and a main virus disease preventing, controlling and purifying system in the pig farm environment.
Owner:HENAN INST OF SCI & TECH
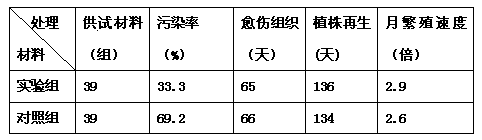
Prevention technology for multiple blueberry viruses at early stage
InactiveCN103404434AMany types of pollutionThe type of pollution is lowHorticulture methodsPlant tissue cultureBiotechnologyMicrobiology
The invention provides a method for cultivating blueberries, which is used for preventing or reducing viral pollution during the process of cultivating blueberries. The method comprises the following steps: performing pretreatment on the blueberries so as to obtain disinfectant root tips; culturing the root tips into regeneration plant sprouts on a culture medium; culturing the sprouts into plantlets on the culture medium; enabling the plantlets to grow into test-tube plantlets on the culture medium; grouping and sampling randomly, and detecting whether samples are infected viruses, especially specific viruses or not. In addition, the invention further provides a method for detecting blueberry viruses synchronously and quickly through multiple RT-PCR, and a mixed prime pair is adopted.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
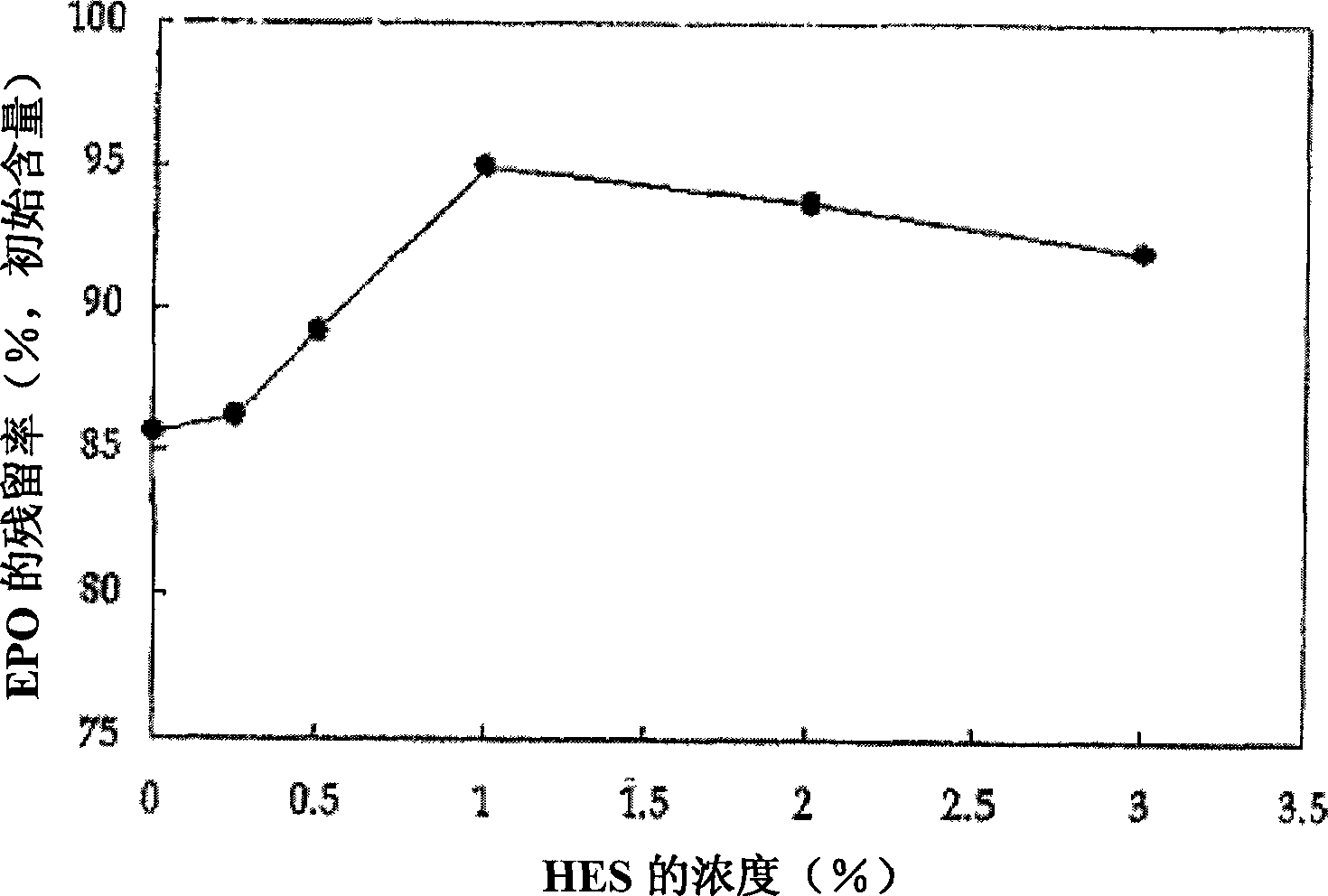
Formulation of albumin-free erythropoietin
InactiveCN1832754ANo risk of contaminationPeptide/protein ingredientsAntipyreticDrugs solutionTonicity
Disclosed is a stable pharmaceutical solution preparation of erythropoietin (EPO), which includes a stabilizing agent not containing a blood-derived protein, thereby maintaining EPO activity for a prolonged period of time without the risk of viral contamination. The stable solution preparation further includes a non-ionic surfactant and a tonicity agent, thereby preventing EPO loss during storage and facilitating administration to the body.
Owner:CJ CHEILJEDANG CORP
Virus-free plasma protein compositions treated with porous membrane and process for producing the same
InactiveUS6867285B2Efficient removalSafer plasma protein preparationAntibacterial agentsPeptide/protein ingredientsFiberProtein composition
Contaminant viruses can be efficiently removed almost without losing the activity of protein by subjecting a plasma protein composition having a high risk of viral contamination to a treatment with a porous membrane having a pore size greater than a single-particle size of the virus, particularly by subjecting a plasma protein composition to a fractionation treatment by precipitation, before the porous membrane treatment. Particularly, a fibrinogen composition substantially free of non-enveloped viruses, Parvovirus among others, can be provided. By the application of the present invention, a safe plasma protein preparation free of viruses can be conveniently provided.
Owner:JAPAN BLOOD PROD ORG
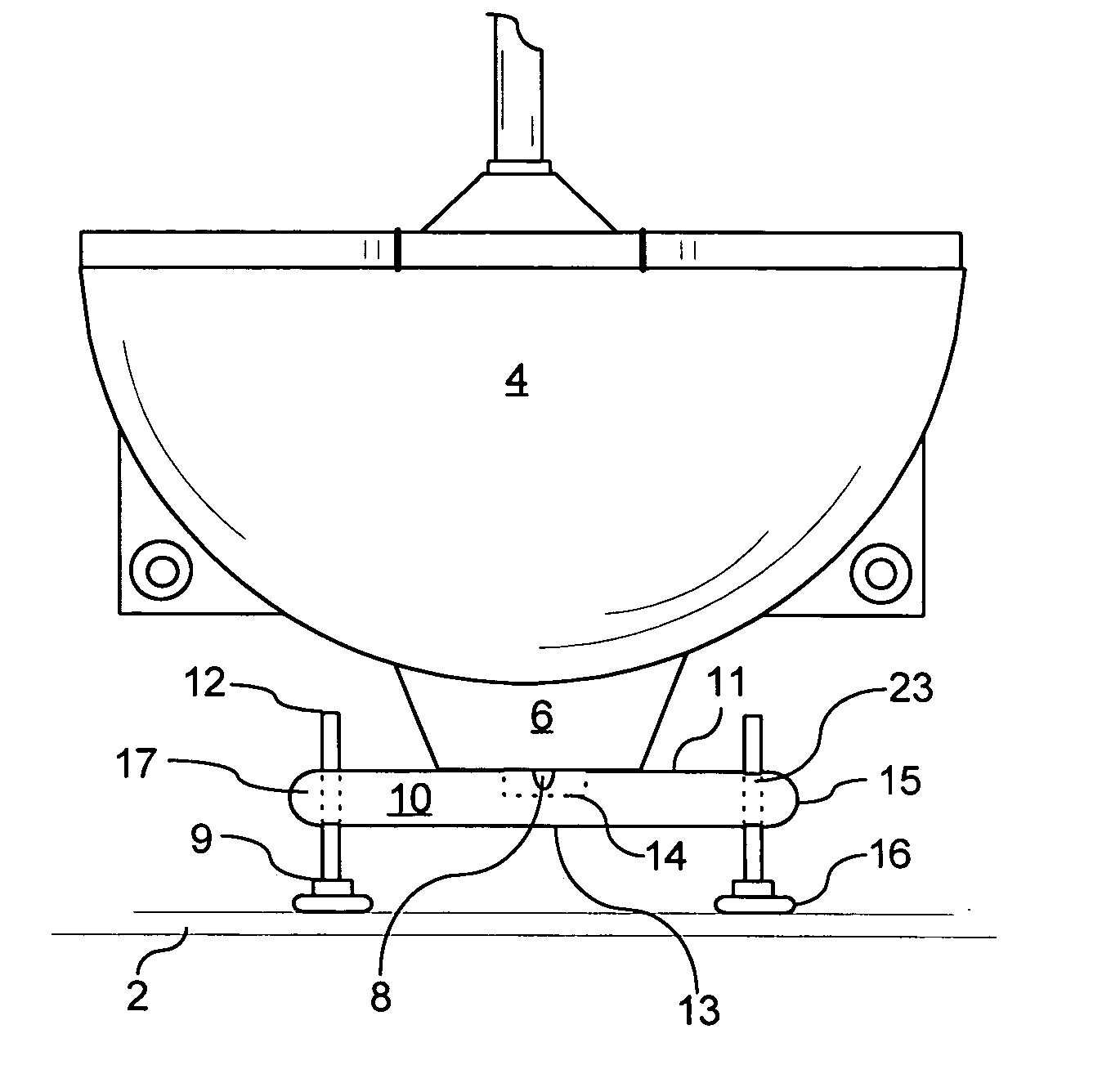
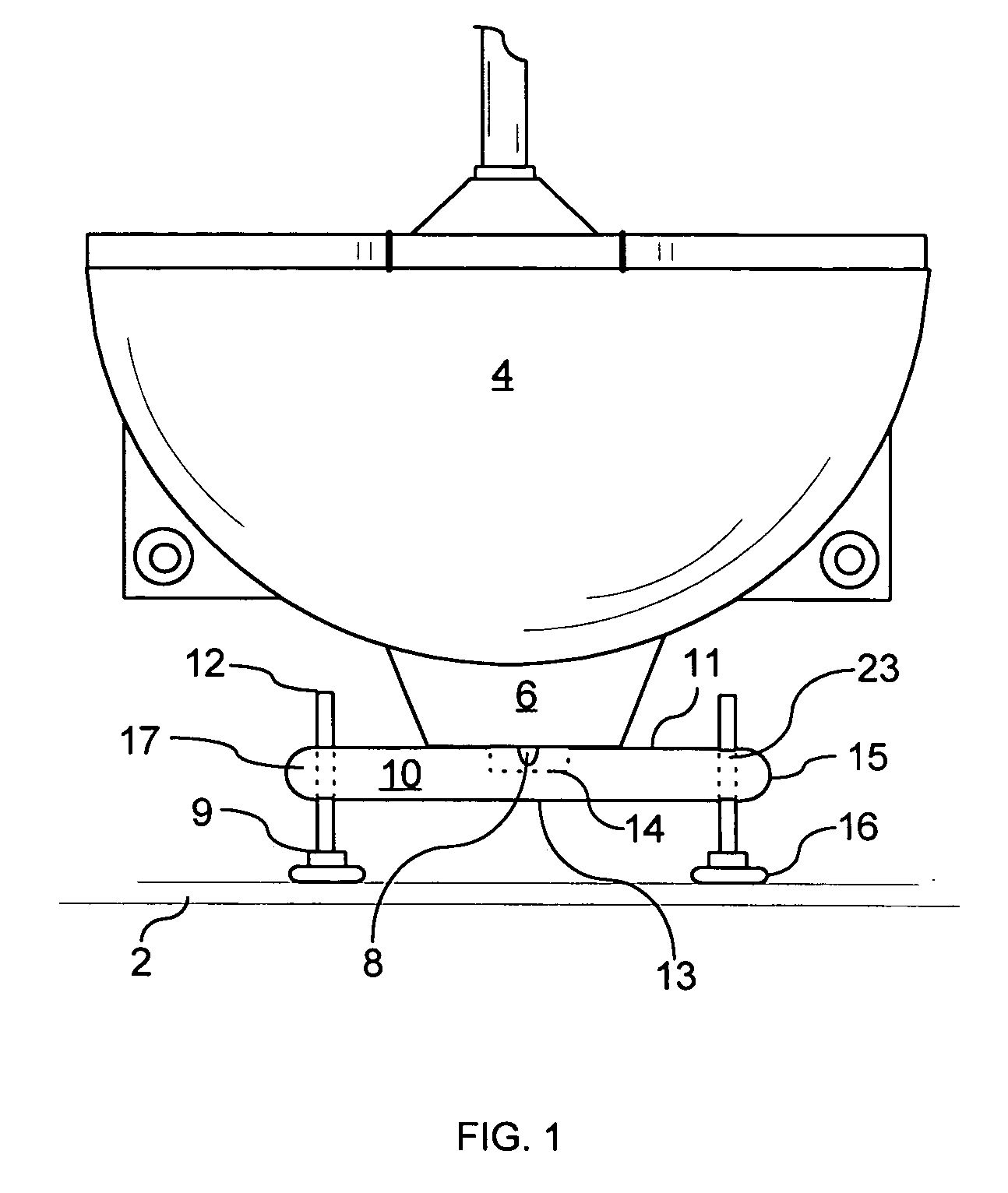
Support for wall mounted toilets
ActiveUS20060277673A1Improve weight capacityEasy positioningWater closetsStands/trestlesEngineeringBody weight
A support platform for wall mounted toilets is described. The platform attaches easily under the toilet and contains bolts and feet for adjustment. The platform provides support to wall mounted toilets so that persons of weight greater than the rated load of the wall mounted toilet can use the toilet in comfort and safety. It is removable for use in different bathrooms, easily transported and can be sterilized where bacterial or viral contamination is a concern.
Owner:RUSH UNIV MEDICAL CENT
Liquid formulation of long-acting insulin and insulinotropic peptide
ActiveUS9833516B2Good storage stabilityMaintain activityPeptide/protein ingredientsMetabolism disorderHigh concentrationAlcohol sugars
The present invention relates to a liquid formulation of a combination of long-acting insulin and insulinotropic peptide, comprising insulin which is a physiologically active peptide, insulinotropic peptide, and albumin-free stabilizer, wherein the stabilizer comprises a buffer, a sugar alcohol, a non-ionic surfactant, and an isotonic agent; and a method for preparing the liquid formulation. The liquid formulation of the present invention does not contain a human serum albumin and potentially toxic factors to the body, and thus it has excellent storage stability for insulin conjugate and insulinotropic peptide conjugate at high concentration, without a risk of viral contamination.
Owner:HANMI PHARMA
Destruction of prions using vibrolysin or variants thereof
InactiveUS20050255095A1Reduced activityDestroy their infective activityNervous disorderPeptide/protein ingredientsAdvanced stageProteinaceous infectious particle
The present invention provides a method of reducing the activity of prions using vibriolysin or variants thereof. Vibriolysin-containing solutions are used to sanitize prion-contaminated facilities and instruments and decontaminate food products and biological tissues. The present invention provides a method of treating prion-related disease in animals and humans, comprising the administration of a formulation of vibriolysin or a variant thereof together with a pharmaceutically acceptable carrier. Such novel formulations are engineered to track the natural path of the prion from cells where the prions accumulate in the preclinical stage into neuronal cells and the brain at the advanced stage of the disease. The present invention provides methods and formulations that encompasses natural and recombinant vibriolysins and variants thereof with enhanced ability to access prion target cells, and with enzyme activity capable of being regulated by specific conditions, such as pH range or enzymatic cleavage.
Owner:BIOMARIN PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com