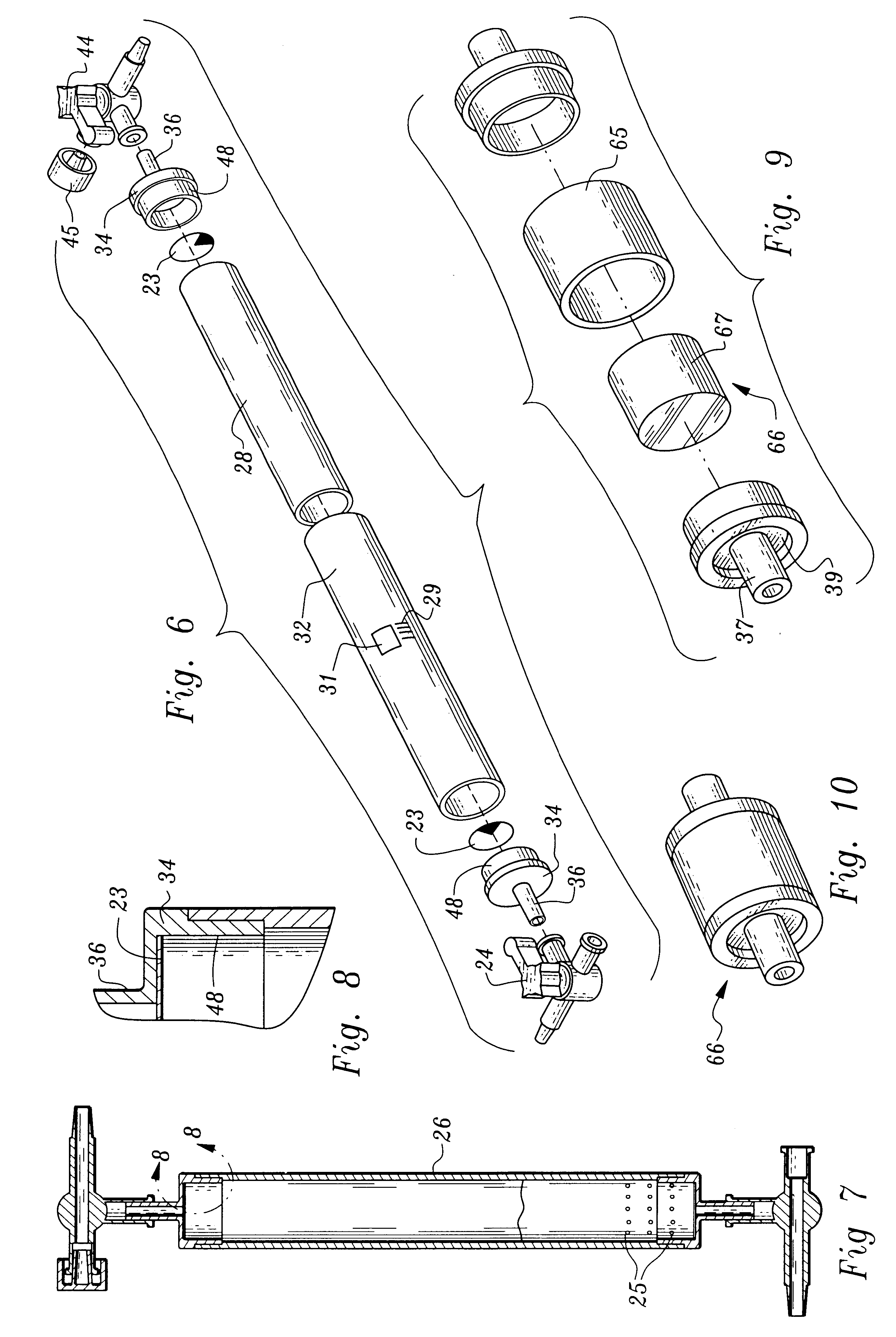
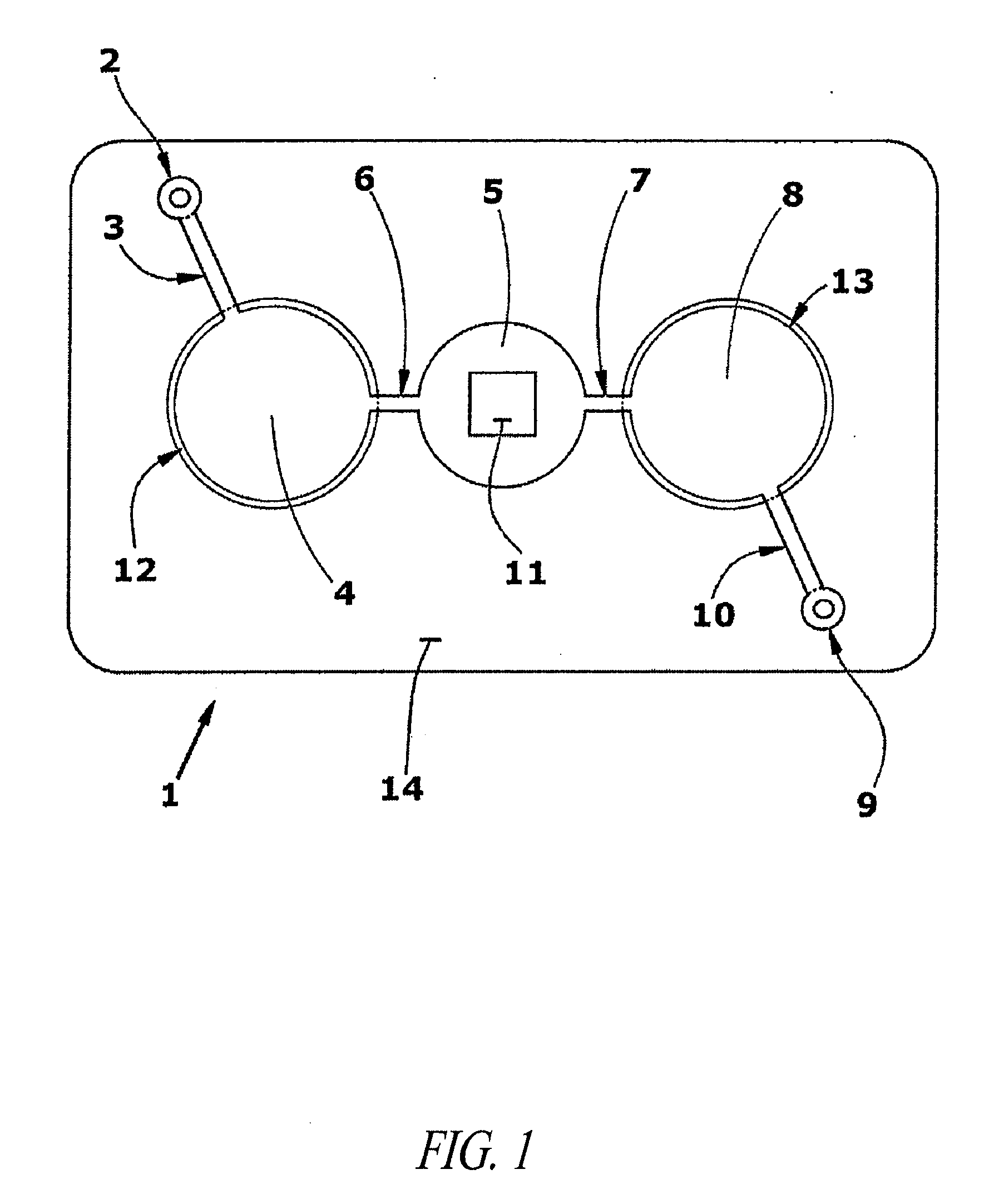
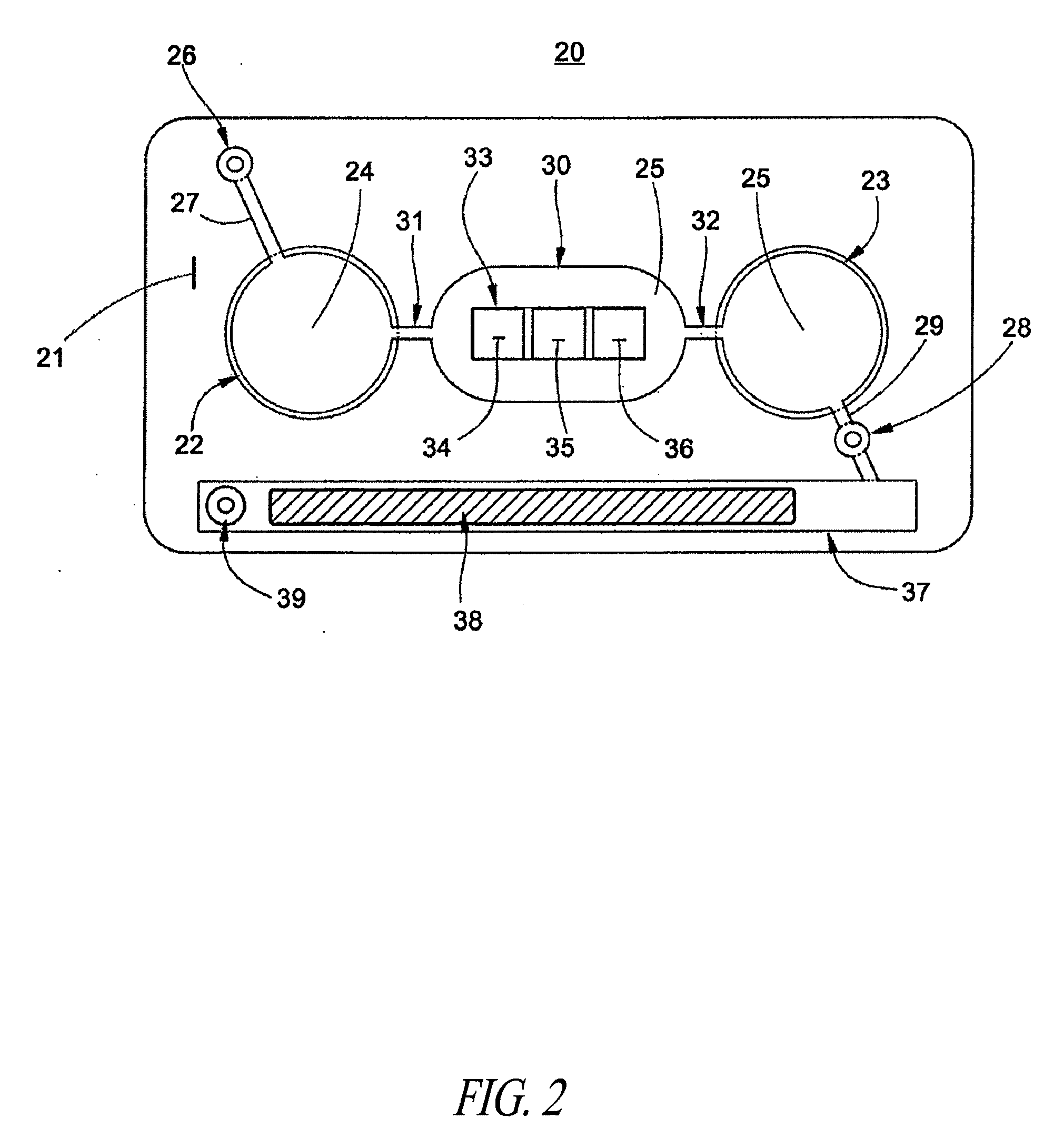
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
6772results about "Apparatus sterilization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
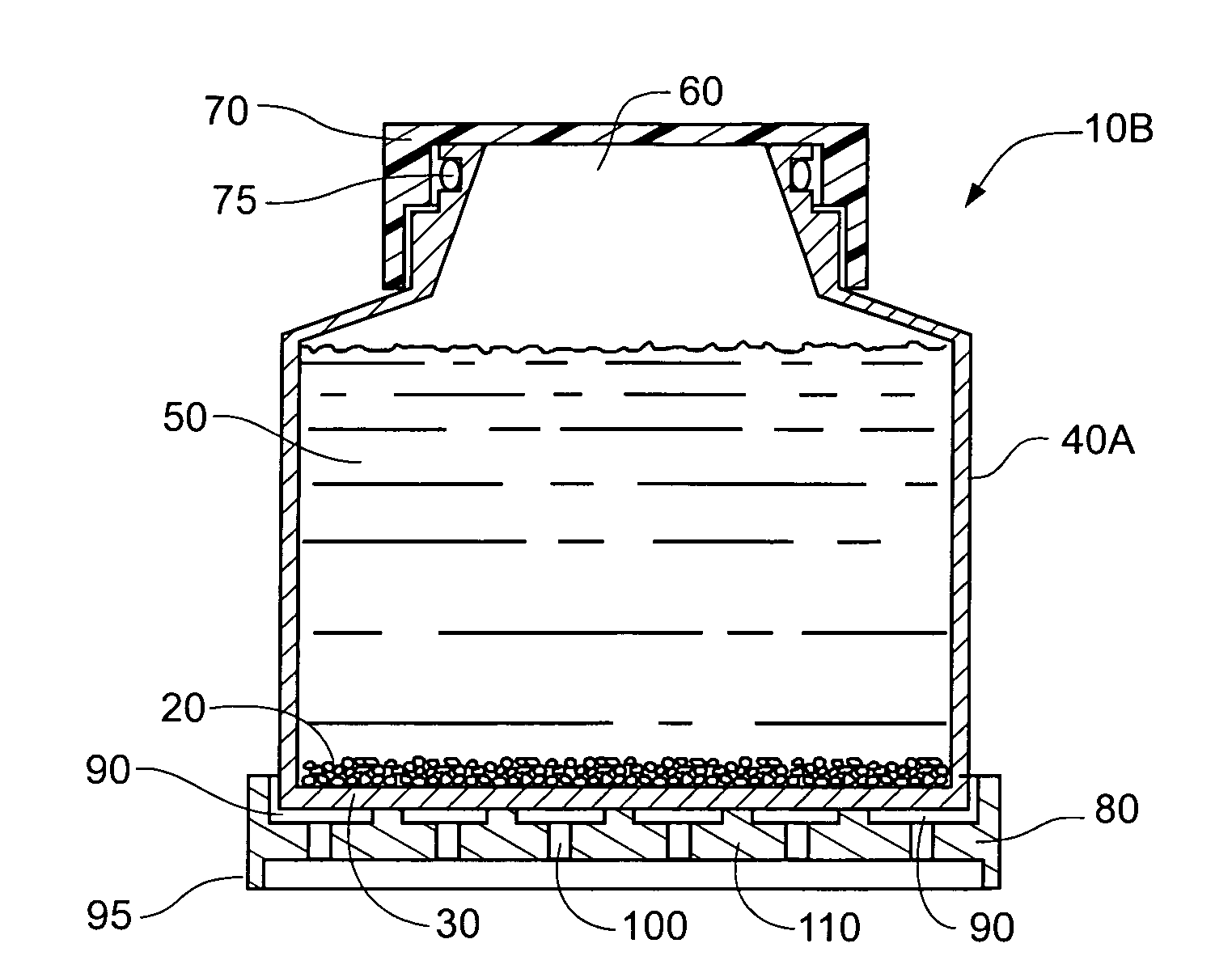
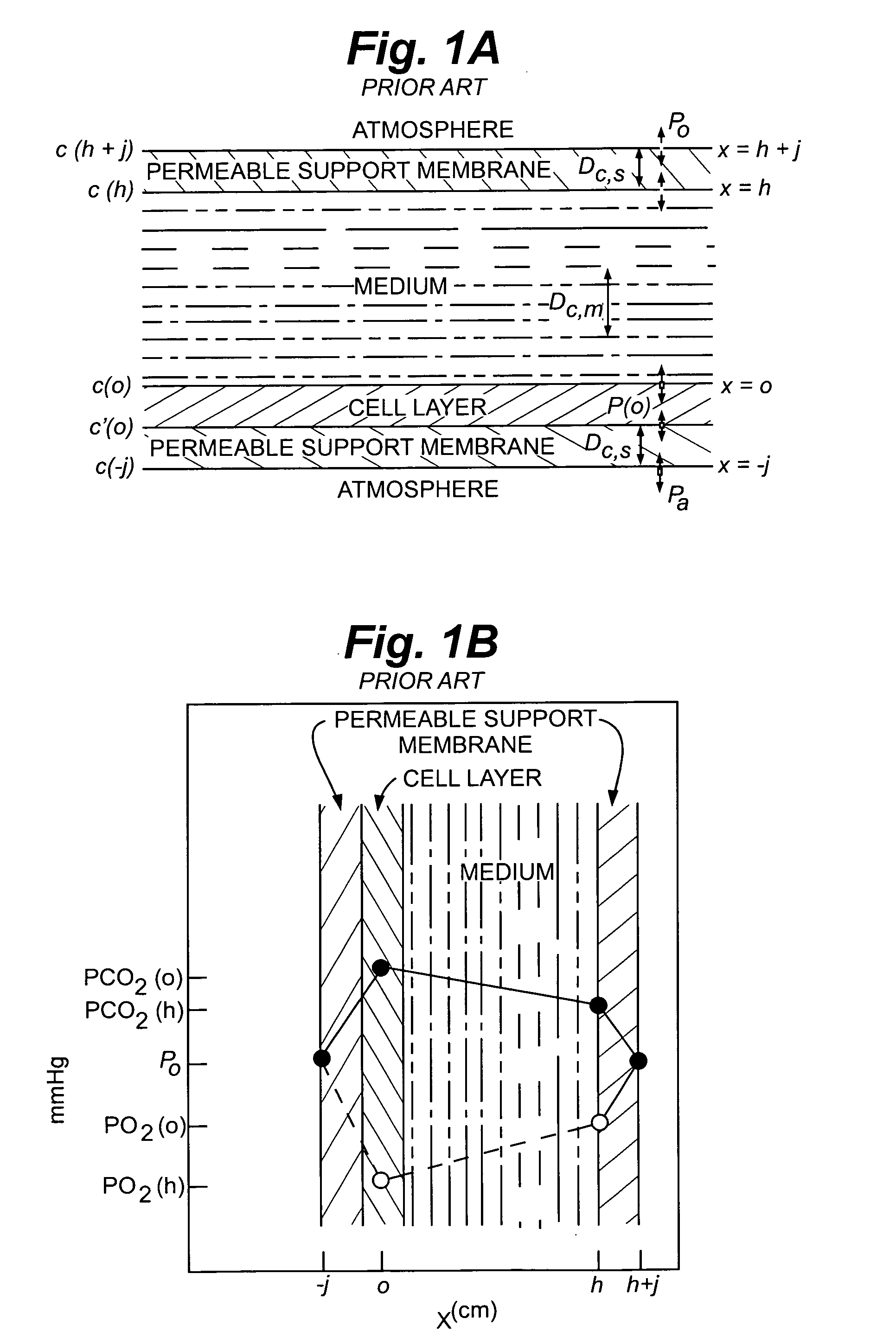
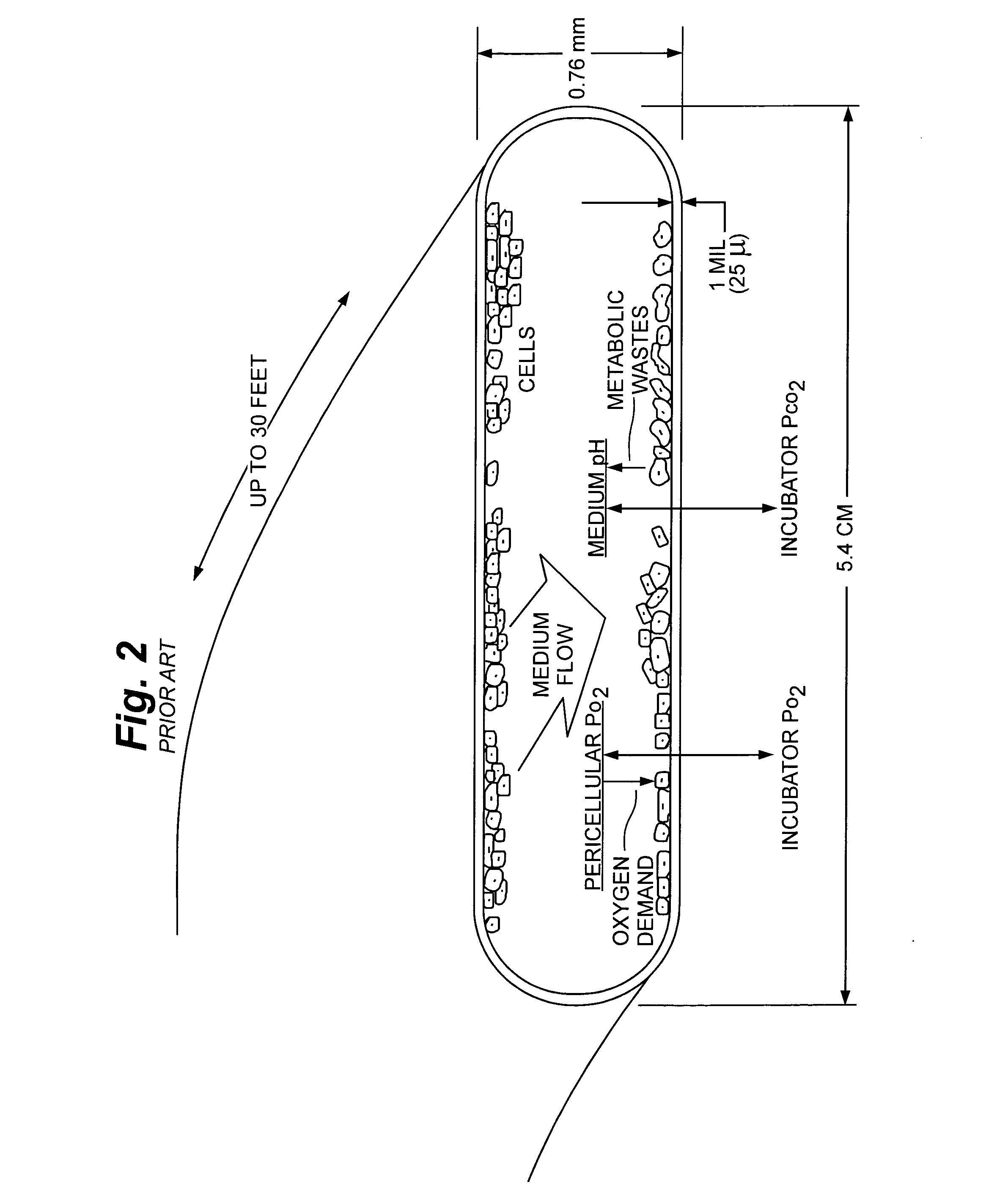
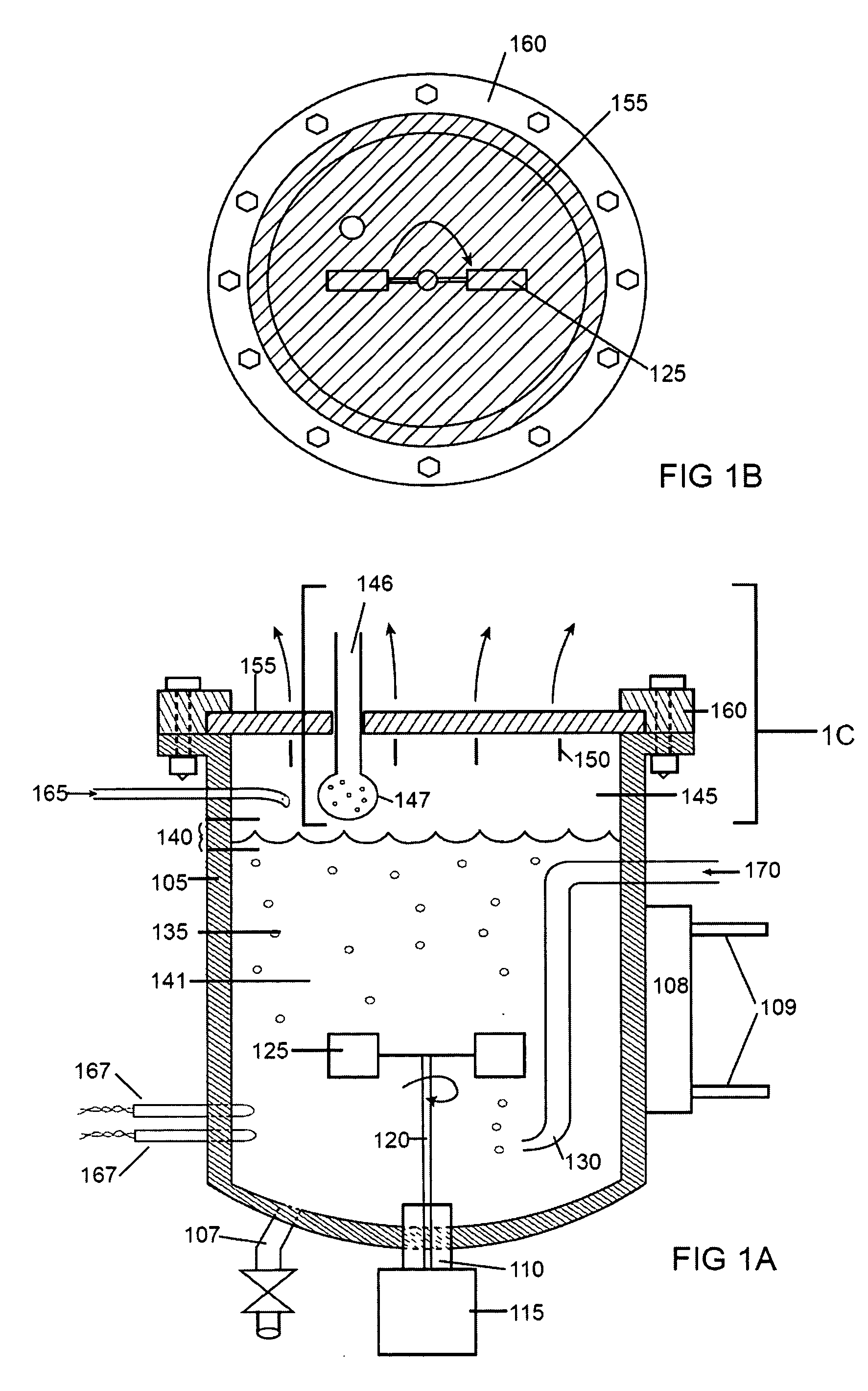
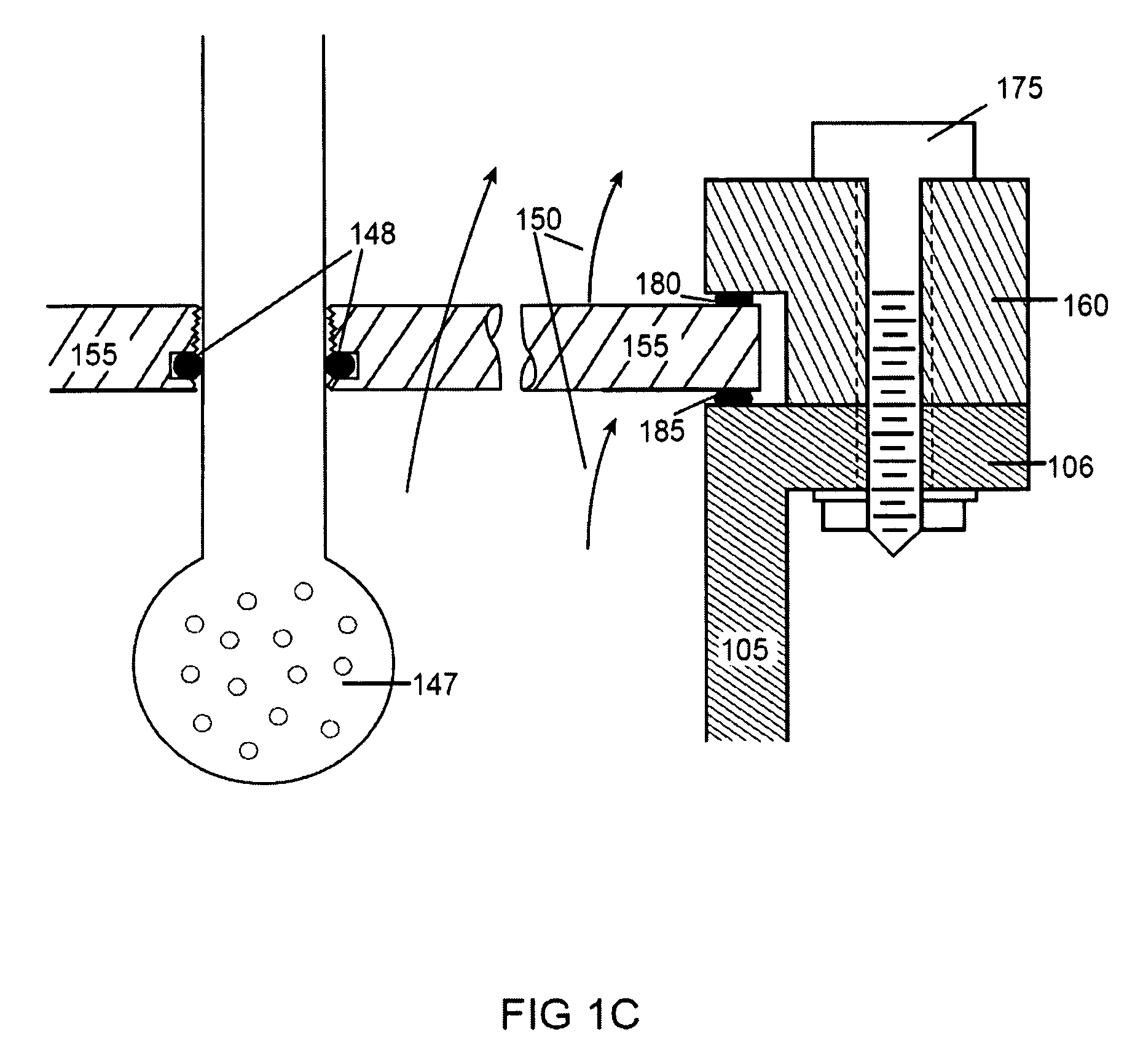
Cell culture methods and devices utilizing gas permeable materials
ActiveUS20050106717A1Increase exchange surfaceIncrease surface areaBioreactor/fermenter combinationsBiological substance pretreatmentsProduct gasBiology
Gas permeable devices and methods are disclosed for cell culture, including cell culture devices and methods that contain medium at heights, and certain gas permeable surface area to medium volume ratios. These devices and methods allow improvements in cell culture efficiency and scale up efficiency.
Owner:WILSON WOLF MFG
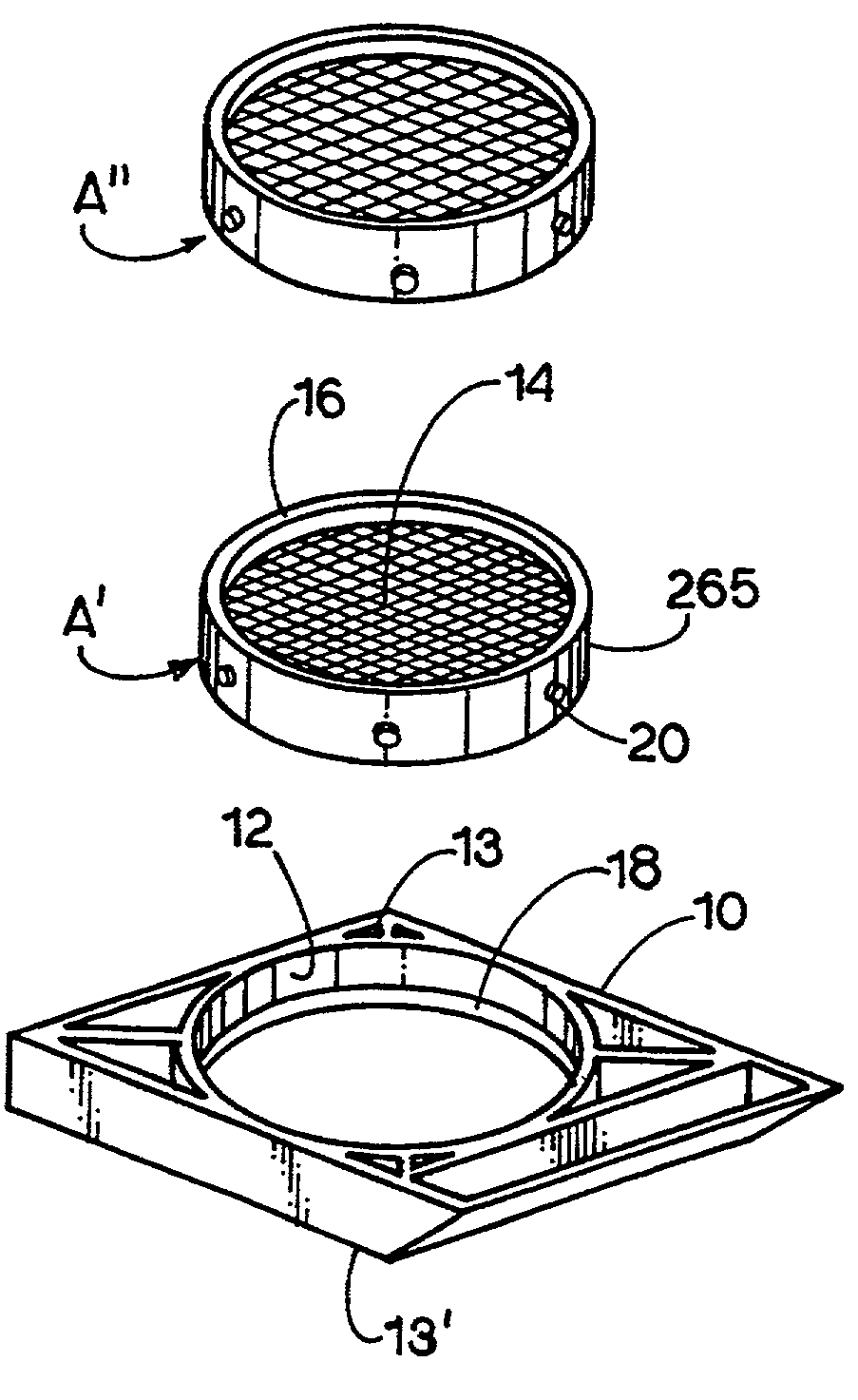
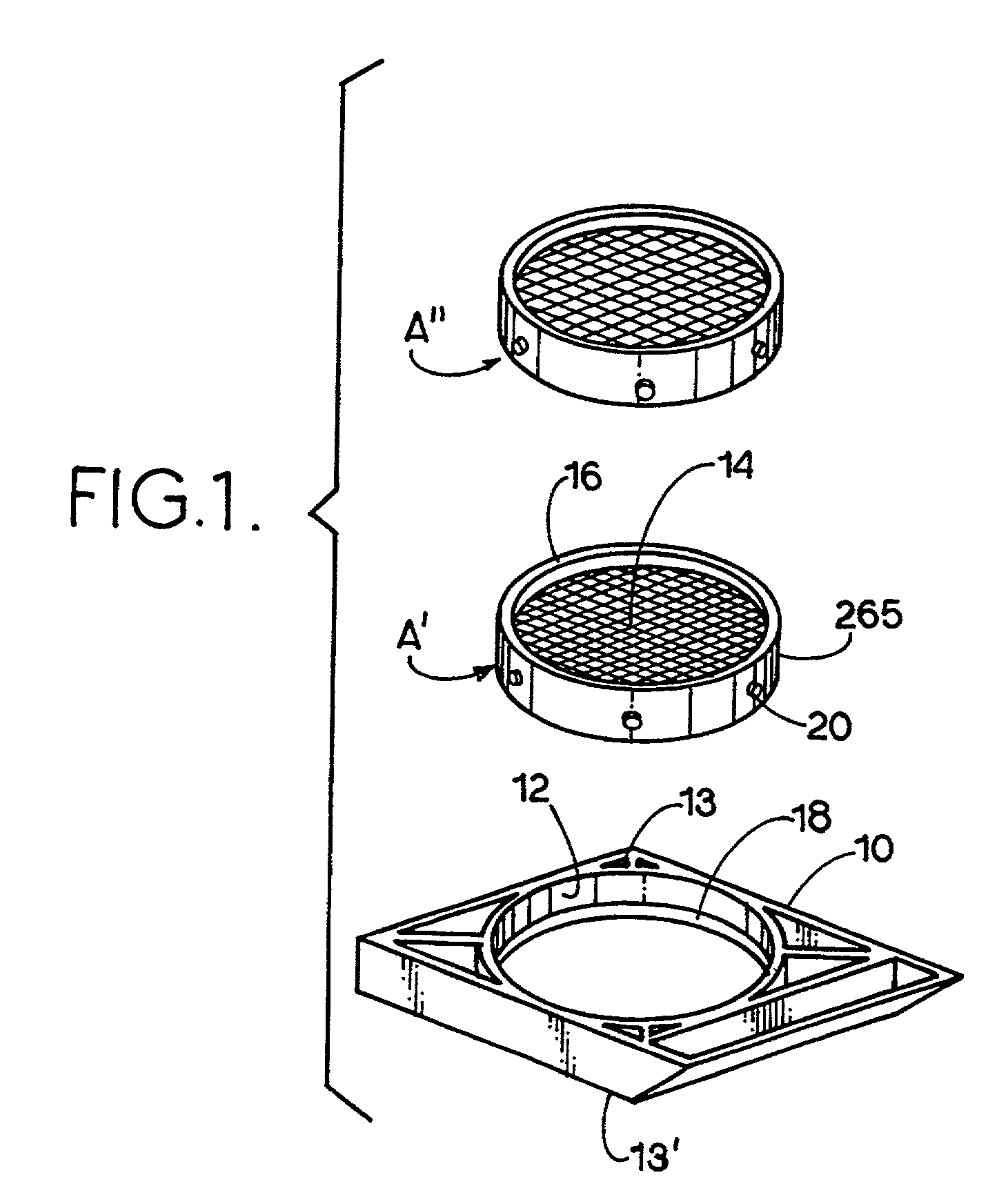
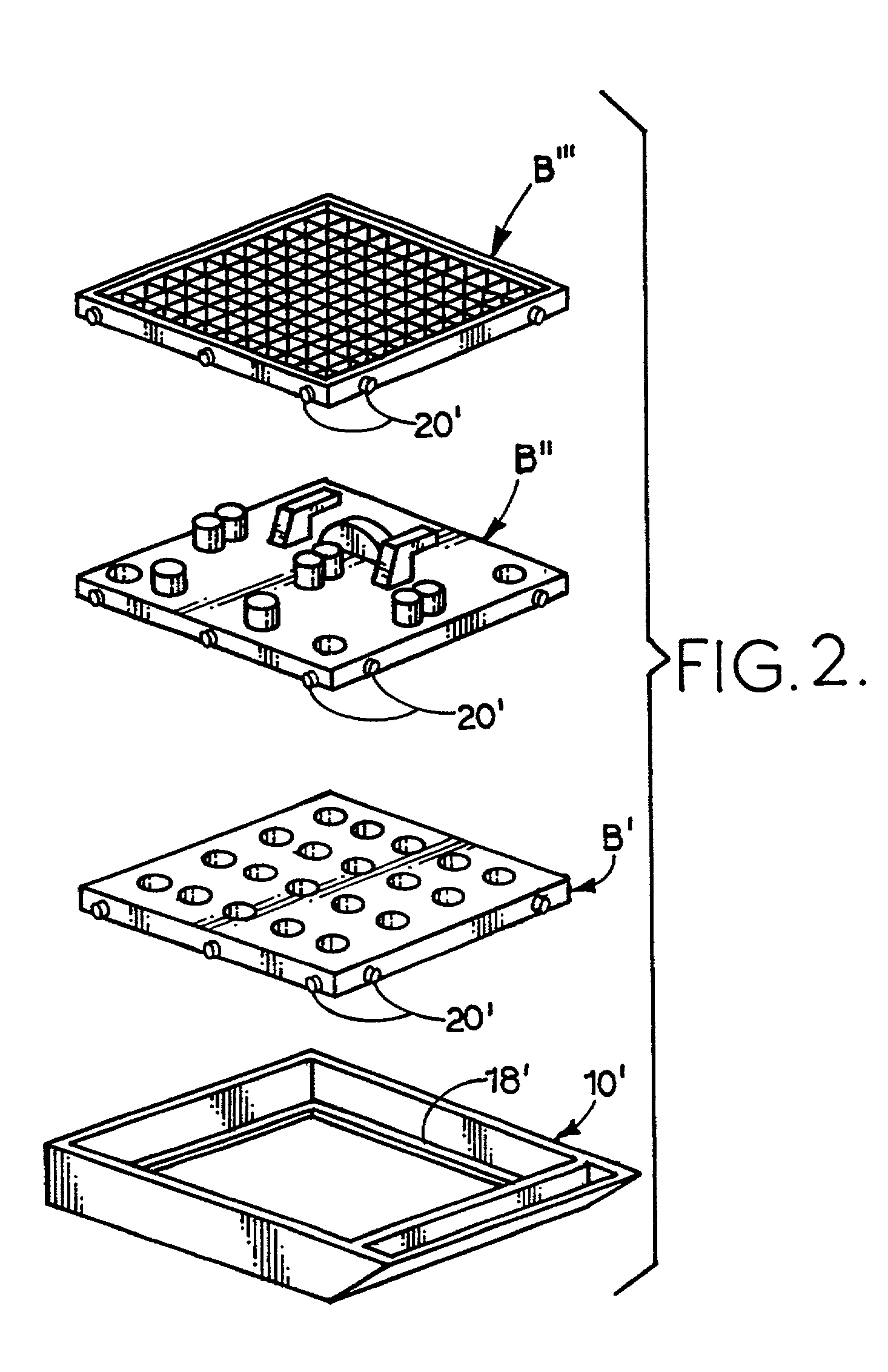
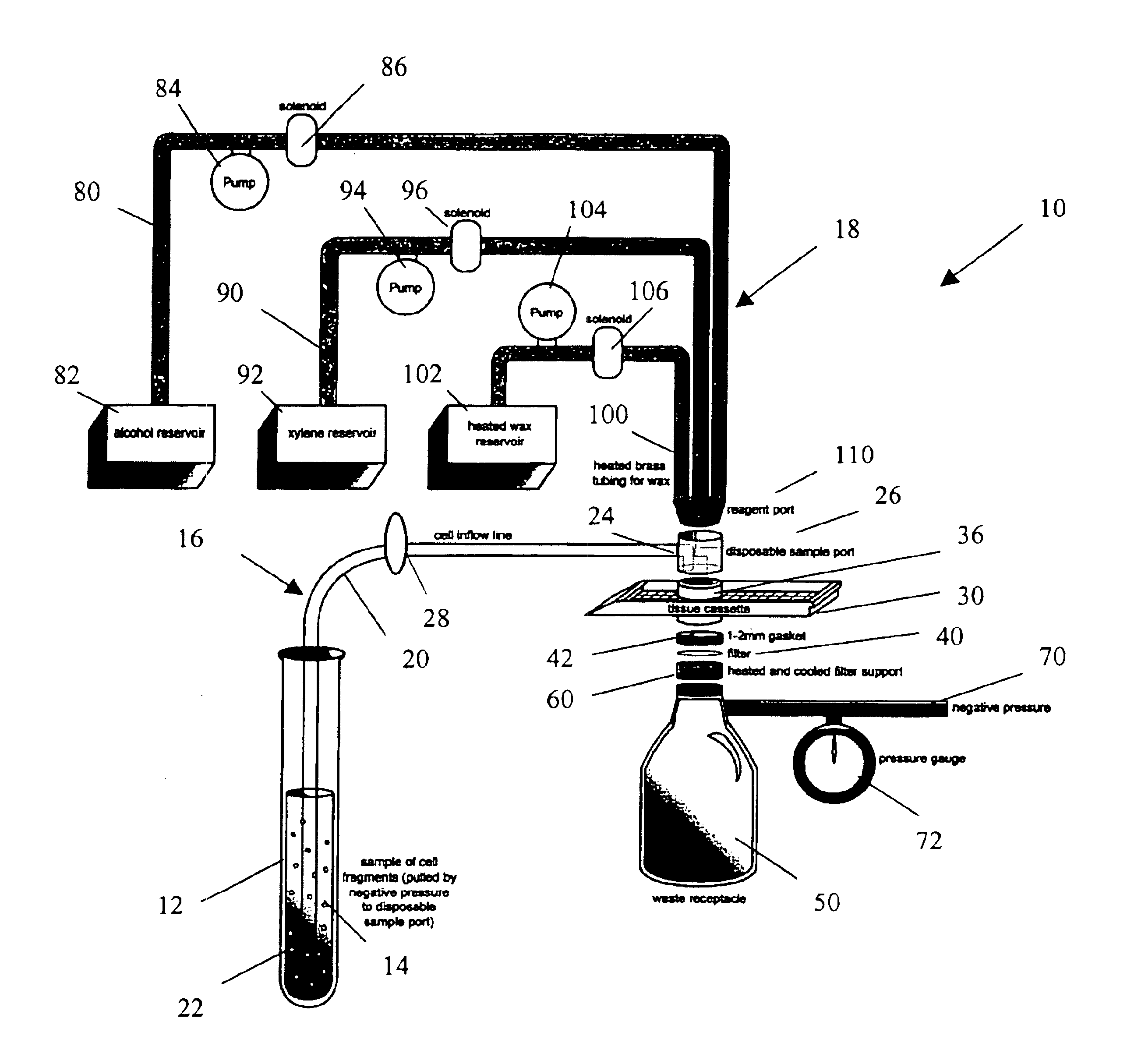
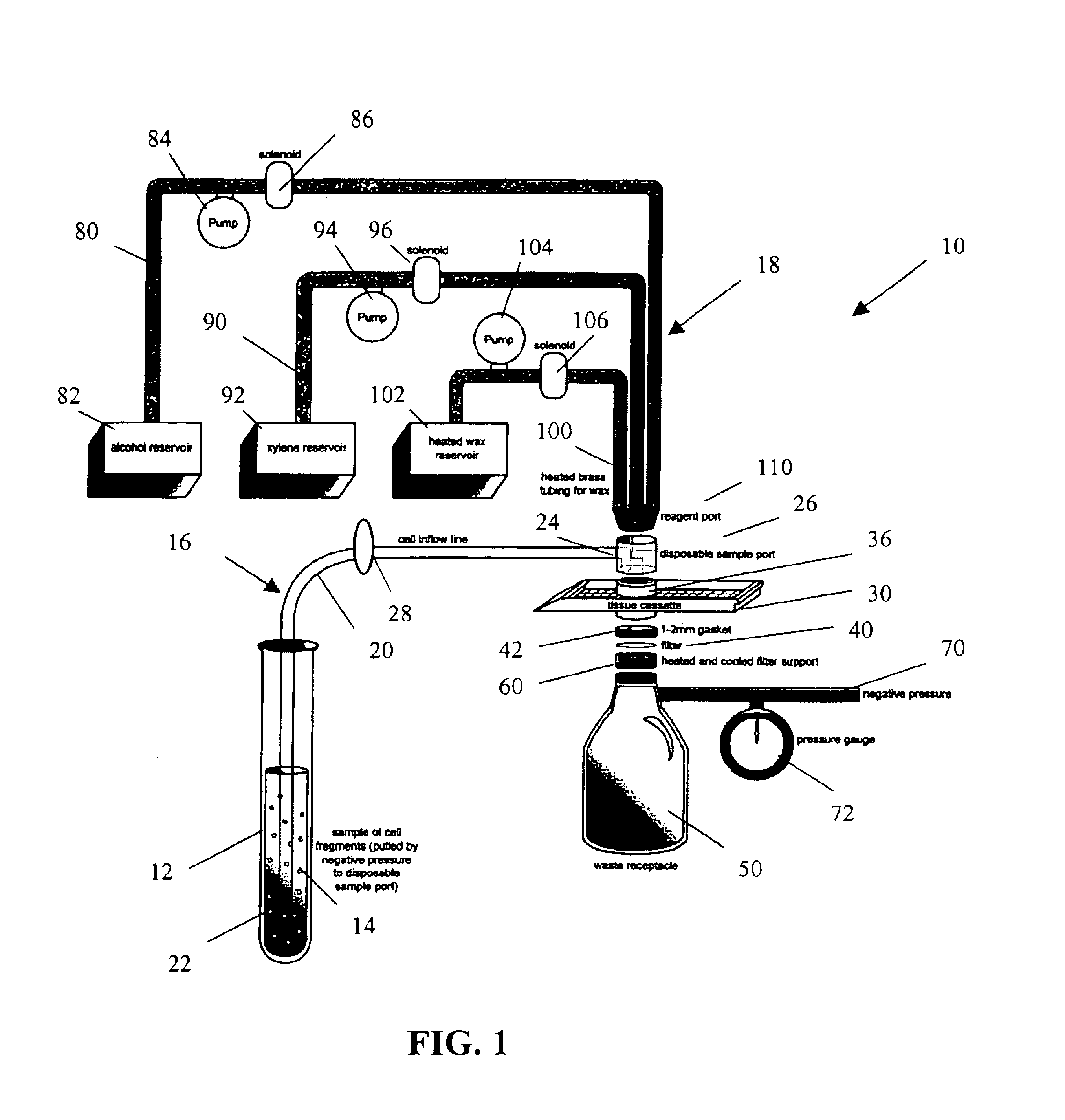
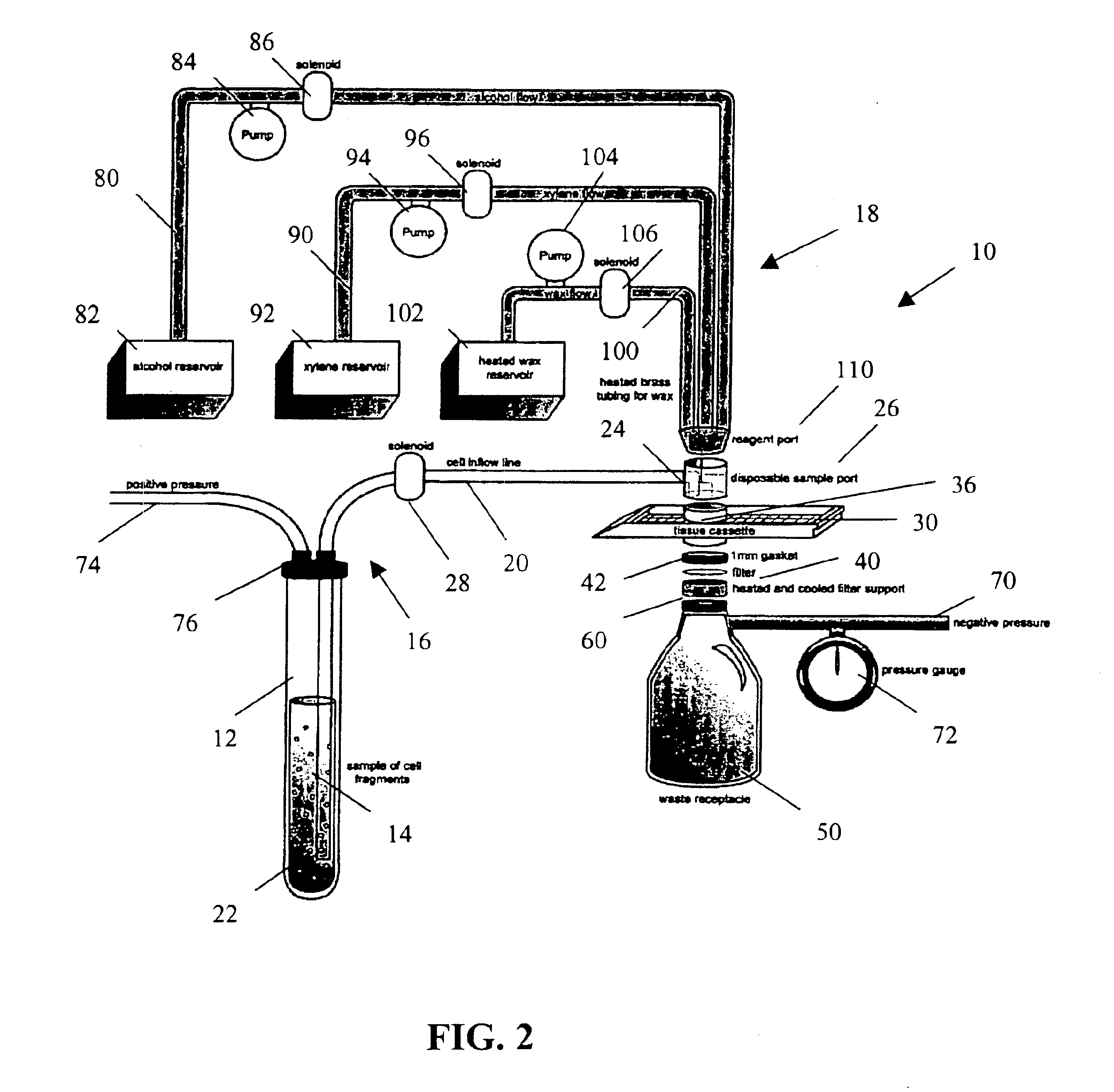
Apparatus and method for harvesting and handling tissue samples for biopsy analysis
InactiveUS7156814B1Exemption stepsReduce the numberBioreactor/fermenter combinationsBiological substance pretreatmentsTissue biopsyTissue fluid
A sectional cassette (10) for use in a process for harvesting and handling tissue samples for biopsy analysis is disclosed. In the procedure, a tissue biopsy sample is placed on a tissue trapping supporting material (A′) that can withstand tissue preparation procedures, and which can be cut with a microtome. The tissue is immobilized on the material, and the material and the tissue are held in the cassette (10) subjected to a process for replacing tissue fluids with wax. The tissue and supporting material are sliced for mounting on slides using a microtome. Harvesting devices and containers (200) using the filter material (202) are disclosed. An automated process is also disclosed.
Owner:BIOPATH AUTOMATION
Method for preparing thrombin for use in a biological glue
InactiveUS6472162B1Derive fast acting, stable autologous thrombinSimple preparatory procedureBioreactor/fermenter combinationsBiological substance pretreatmentsTissue sealantDonors plasma
A sterile method for preparing stable thrombin component from a single donor's plasma in which the thrombin component and the clotting and adhesive proteins component are harvested simultaneously from the same donor plasma in less than one hour. The combined components provide an improved biological hemostatic agent and tissue sealant by virtue of its freedom from the risk of contaminating viruses or bacteria from allogenic human or bovine blood sources. The thrombin provides polymerization of the clotting and adhesive proteins in less than five seconds, and is sufficiently stable to provide that fast clotting over a six hour period. Further, the clotting times can be predictably lengthened by diluting the thrombin with saline.
Owner:ASAHI KASEI MEDICAL CO LTD
Methods and devices for microfluidic point-of-care immunoassays
ActiveUS20090181411A1Endpoint detectionReduce incubation timeBioreactor/fermenter combinationsBiological substance pretreatmentsPoint of careSystems design
Microfluidic methods and devices for heterogeneous binding and agglutination assays are disclosed, with improvements relating to mixing and to reagent and sample manipulation in systems designed for safe handling of clinical test samples.
Owner:PERKINELMER HEALTH SCIENCES INC
Device for lysing cells, spores, or microorganisms
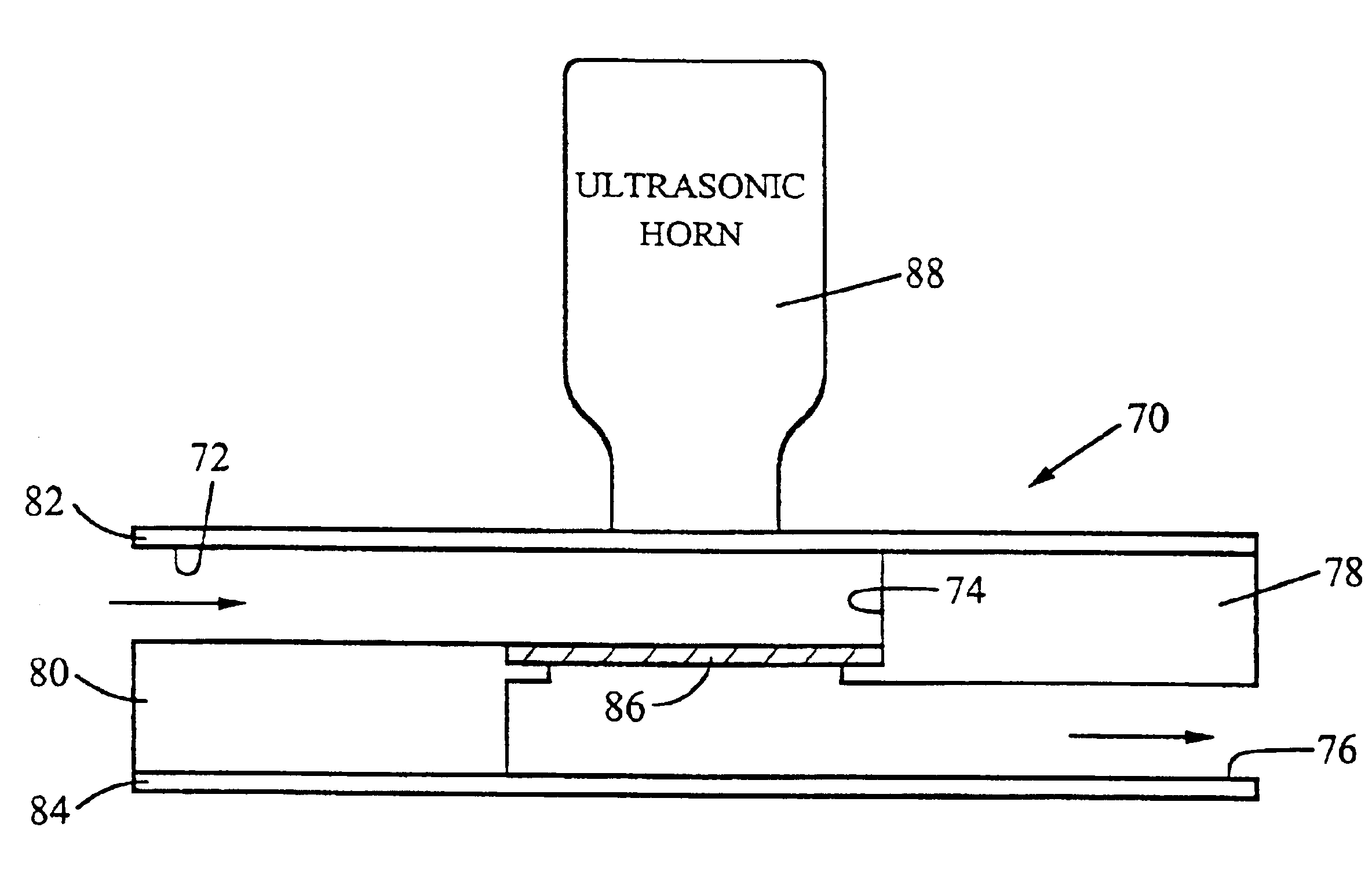
InactiveUS6878540B2Improve convenienceImprove efficiencyBioreactor/fermenter combinationsBiological substance pretreatmentsMicroorganismSpore
A device for use with an ultrasonic transducer to lyse components of a fluid sample comprises a cartridge having a lysing chamber, an inlet port in fluid communication with the lysing chamber, and an outlet port for exit of the sample from the lysing chamber. The inlet and outlet ports are positioned to permit flow of the sample through the lysing chamber, and the chamber contains at least one solid phase for capturing the sample components to be lysed as the sample flows through the chamber. The lysing chamber is defined by at least one wall having an external surface for contacting the transducer to effect the transfer of ultrasonic energy to the chamber.
Owner:CEPHEID INC
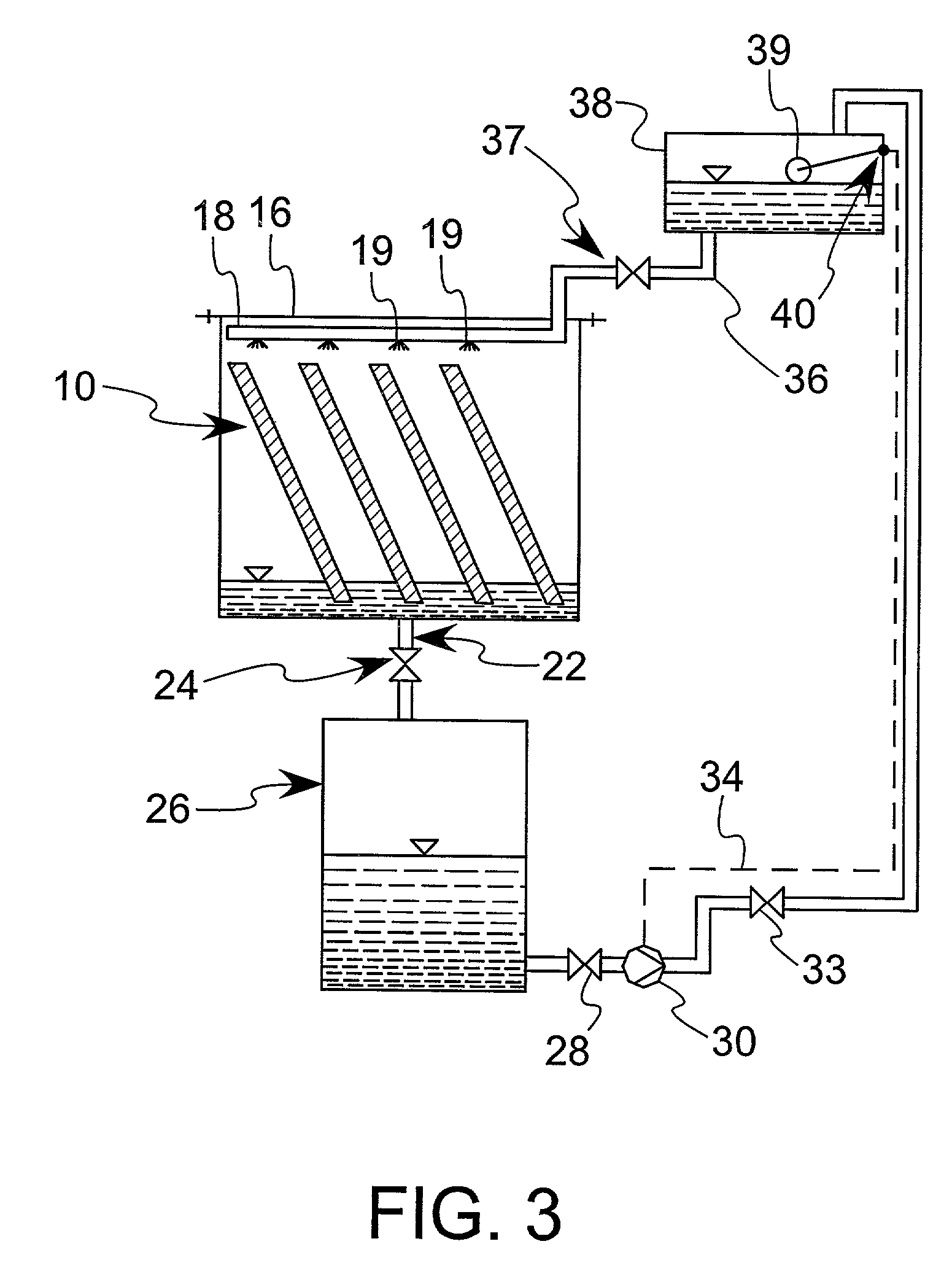
Bioreactors with substance injection capacity
InactiveUS20060154361A1Minimized pressure dropBioreactor/fermenter combinationsBiological substance pretreatmentsLiquid mediumControl substances
A bioreactor with substance injection capability. In one embodiment, the bioreactor includes a first substrate having a first surface, an opposite second surface and edges. The bioreactor further includes a second substrate having a first surface and an opposite second surface, defining a cavity with a bottom surface, where the bottom surface is located therebetween the first surface and the second surface. The first surface of the first substrate is received by the second surface of the second substrate to cover the cavity so as to form a chamber for receiving cells and a liquid medium. A port is formed in the second substrate between the bottom surface and the first surface of the second substrate. As formed, the port is in fluid communication with the chamber to allow a stream of substance to be introduced into the chamber. The stream of substance is controlled so as to provide a gradient, or a concentration gradient of the substance, to the chamber. The stream of substance includes a substance affecting the growth of cells such as chemokine.
Owner:VANDERBILT UNIV
Stirred-tank reactor system
ActiveUS7384783B2Less regulatory controlMinimal or no preparation priorBioreactor/fermenter combinationsBiological substance pretreatmentsReactor systemNuclear engineering
The present invention relates to a stirred-tank reactor system and methods of preparing such systems. The present invention further encompasses the use of the stirred-tank reactor system as a disposable bioreactor and in kits with disposable elements.
Owner:TAKEDA PHARMA CO LTD +1
Stirred-tank reactor system
ActiveUS20050239199A1Less regulatory controlConsiderable cost advantageBioreactor/fermenter combinationsBiological substance pretreatmentsReactor systemNuclear engineering
The present invention relates to a stirred-tank reactor system and methods of preparing such systems. The present invention further encompasses the use of the stirred-tank reactor system as a disposable bioreactor and in kits with disposable elements.
Owner:TAKEDA PHARMA CO LTD +1
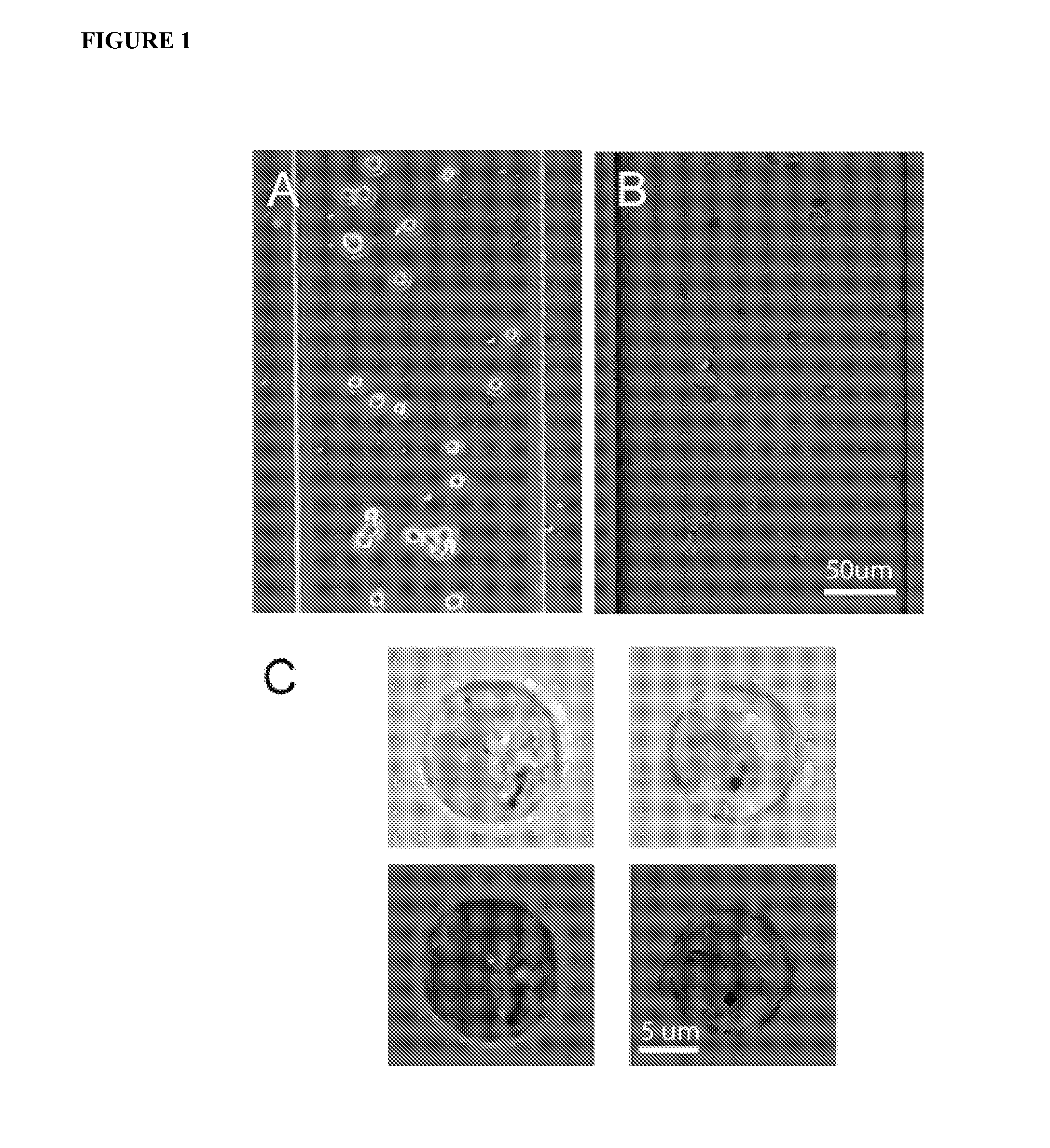
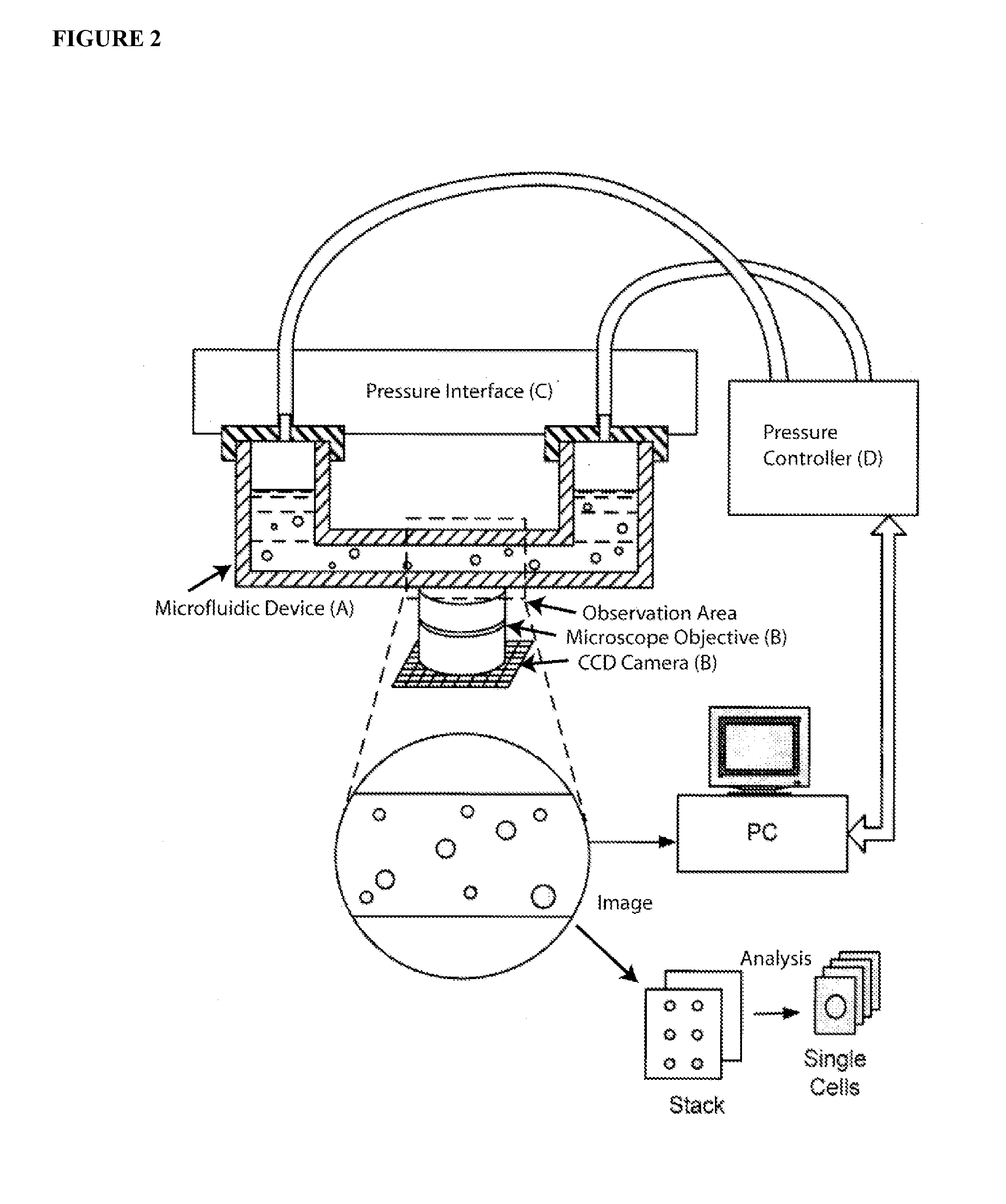
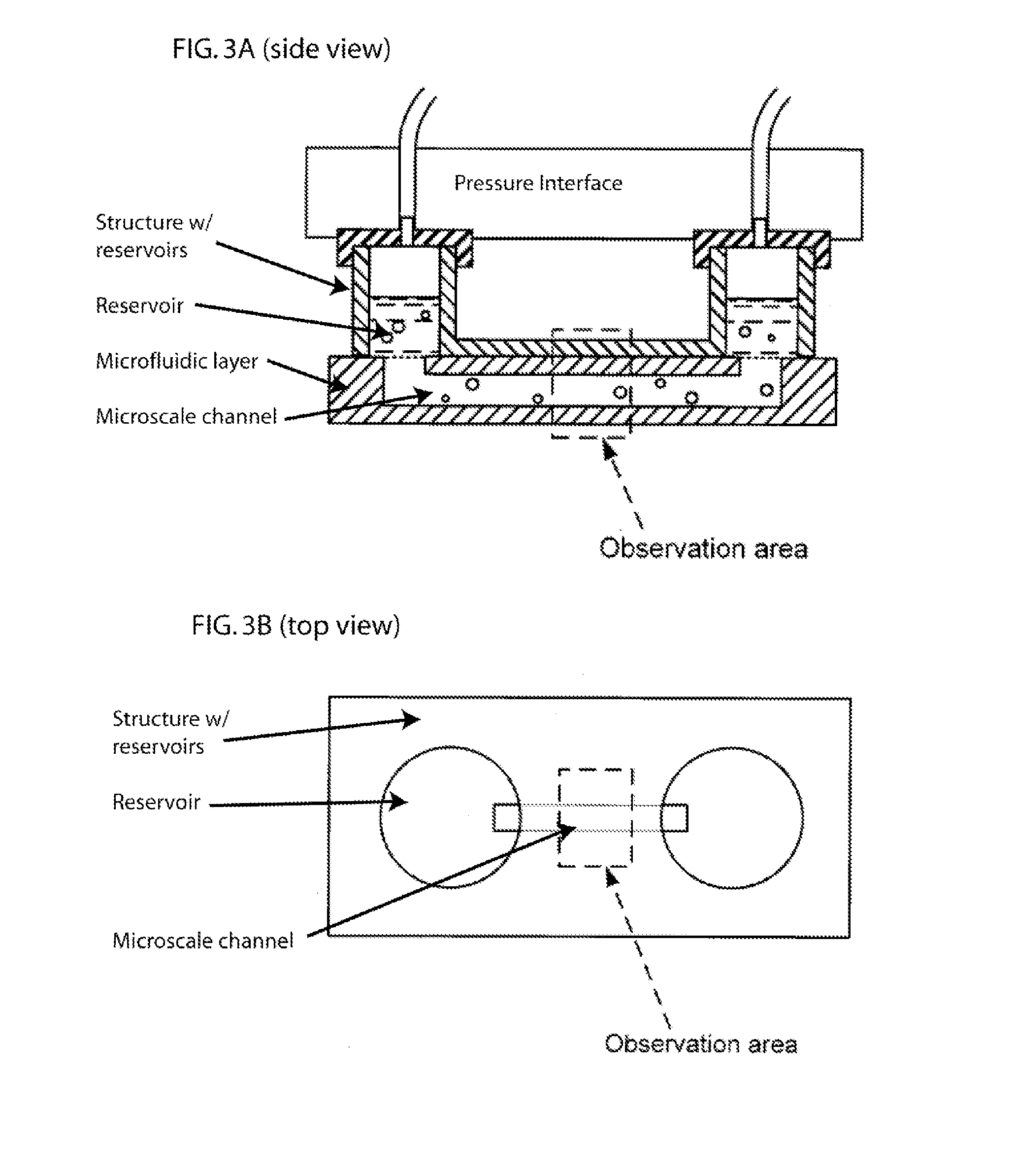
Methods and Apparatus for the Manipulation of Particle Suspensions and Testing Thereof
InactiveUS20070243523A1Bioreactor/fermenter combinationsBiological substance pretreatmentsElectrophysiology studyElectroporation
Apparatus and methods are provided for analysis of individual particles in a microfluidic device. The methods involve the immobilization of an array of particles in suspension and the application of experimental compounds. Such methods can also include electrophysiology studies including patch clamp recording, electroporation, or both in the same microfluidic device. The apparatus provided includes a microfluidic device coupled to a multi-well structure and an interface for controlling the flow of media within the microchannel device.
Owner:FLUXION BIOSCI
Apparatus and method of preparation of stable, long term thrombin from plasma and thrombin formed thereby
InactiveUS6274090B1Derive fast acting, stable autologous thrombinSimple preparatory procedureImmobilised enzymesBioreactor/fermenter combinationsTissue sealantDonors plasma
A sterile method for preparing stable thrombin component from a single donor's plasma in which the thrombin component is harvested simultaneously from the clotting and adhesive proteins component from the same donor plasma in less than one hour. The combined components provide an improved biological hemostatic agent and tissue sealant by virtue of its freedom from the risk of contaminating viruses or bacteria from allogenic human or bovine blood sources. The thrombin provides polymerization of the clotting and adhesive proteins in less than five seconds, and is sufficiently stable to provide that fast clotting over a six hour period. Further, the clotting times can be predictably lengthened by diluting the thrombin with saline.
Owner:ASAHI KASEI MEDICAL CO LTD
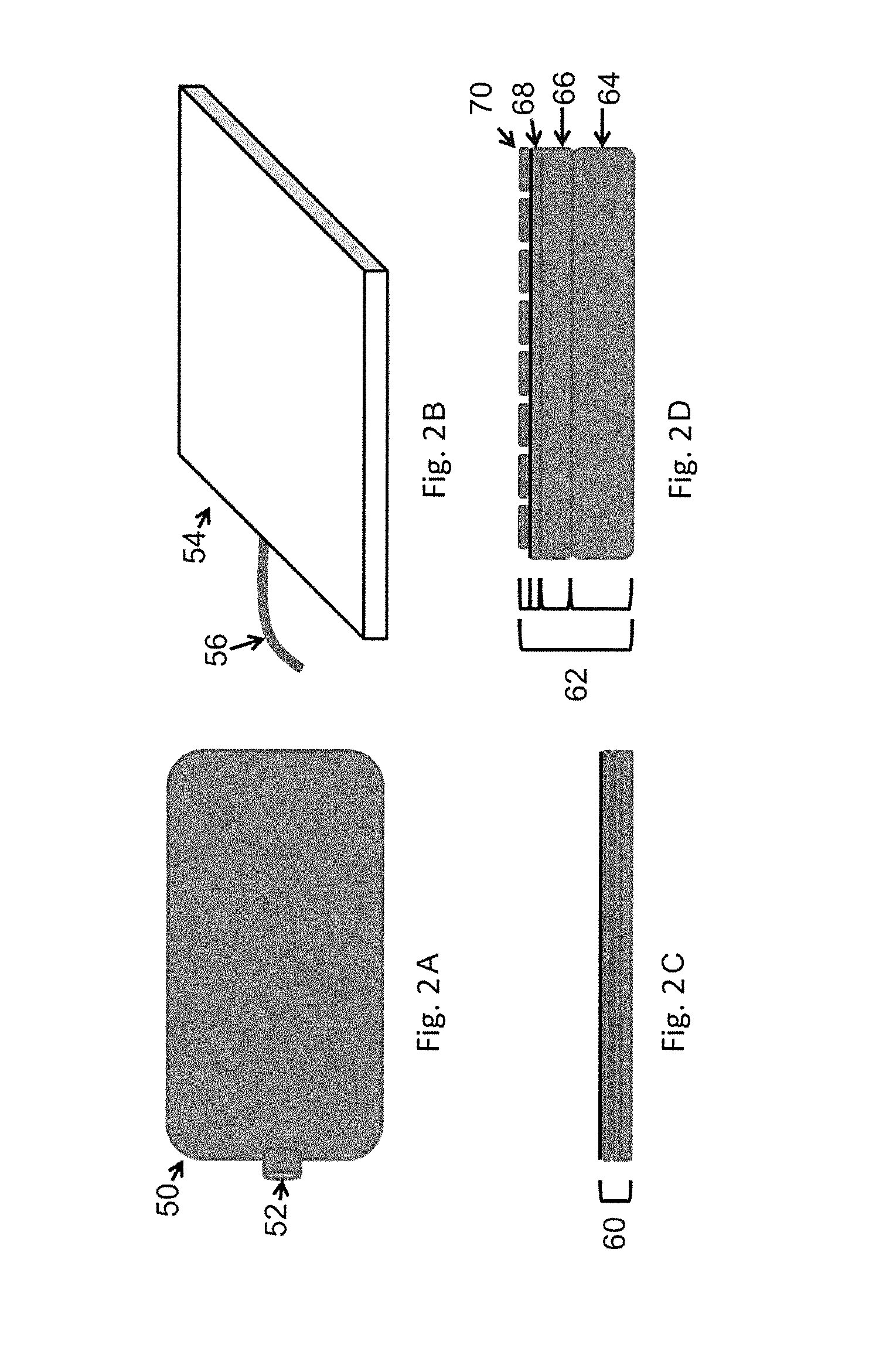
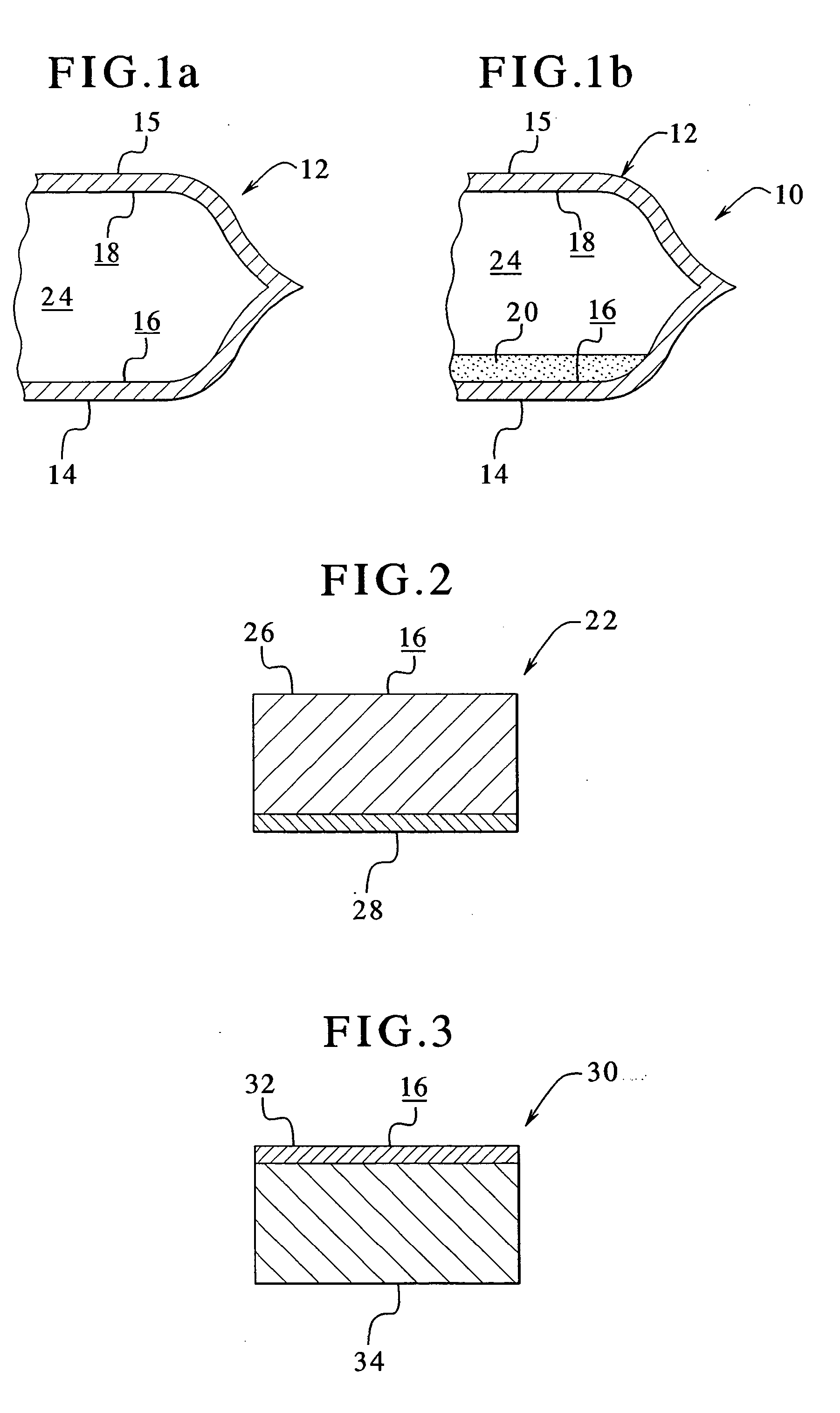
Multilayer gas-permeable container for the culture of adherent and non-adherent cells
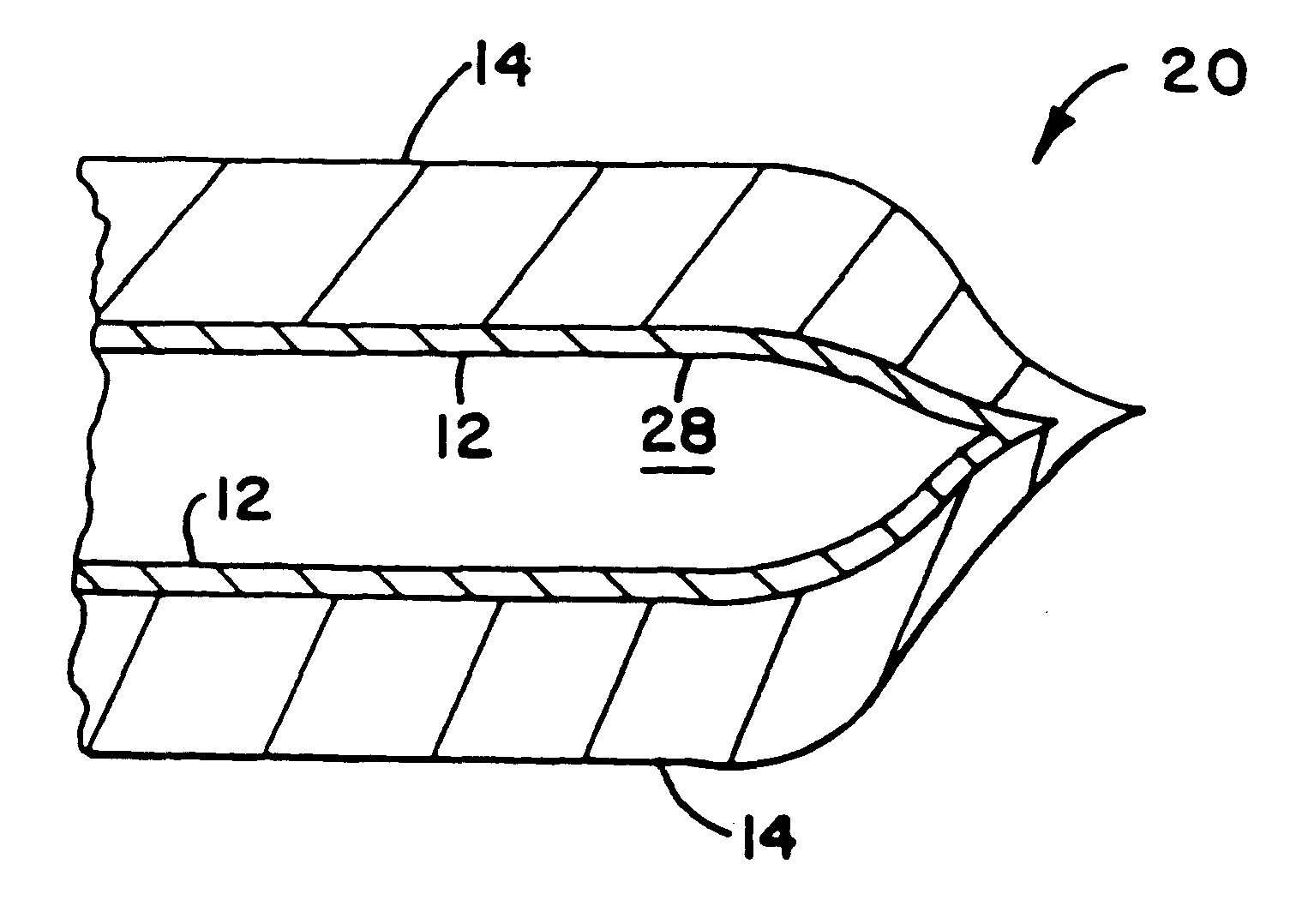
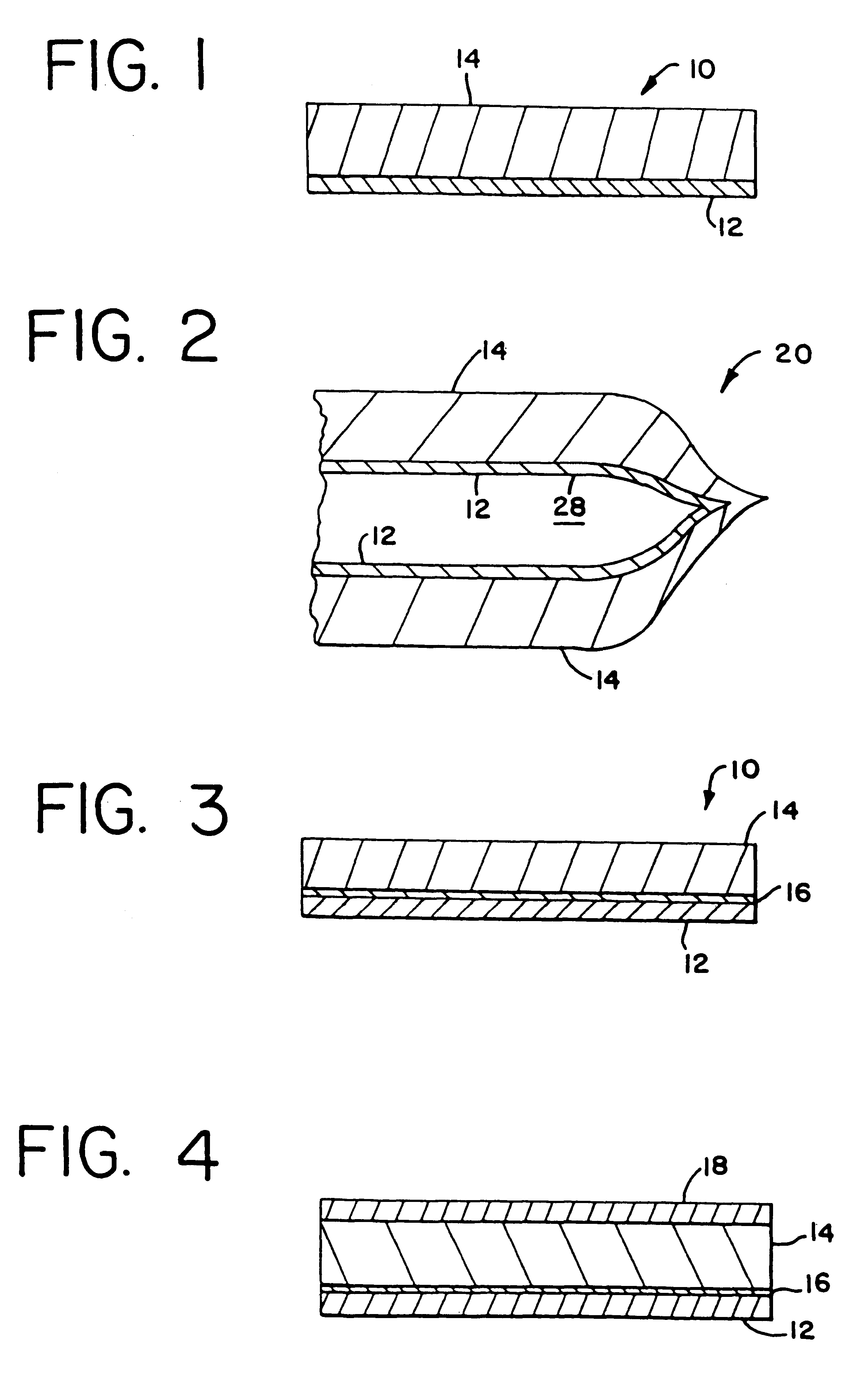
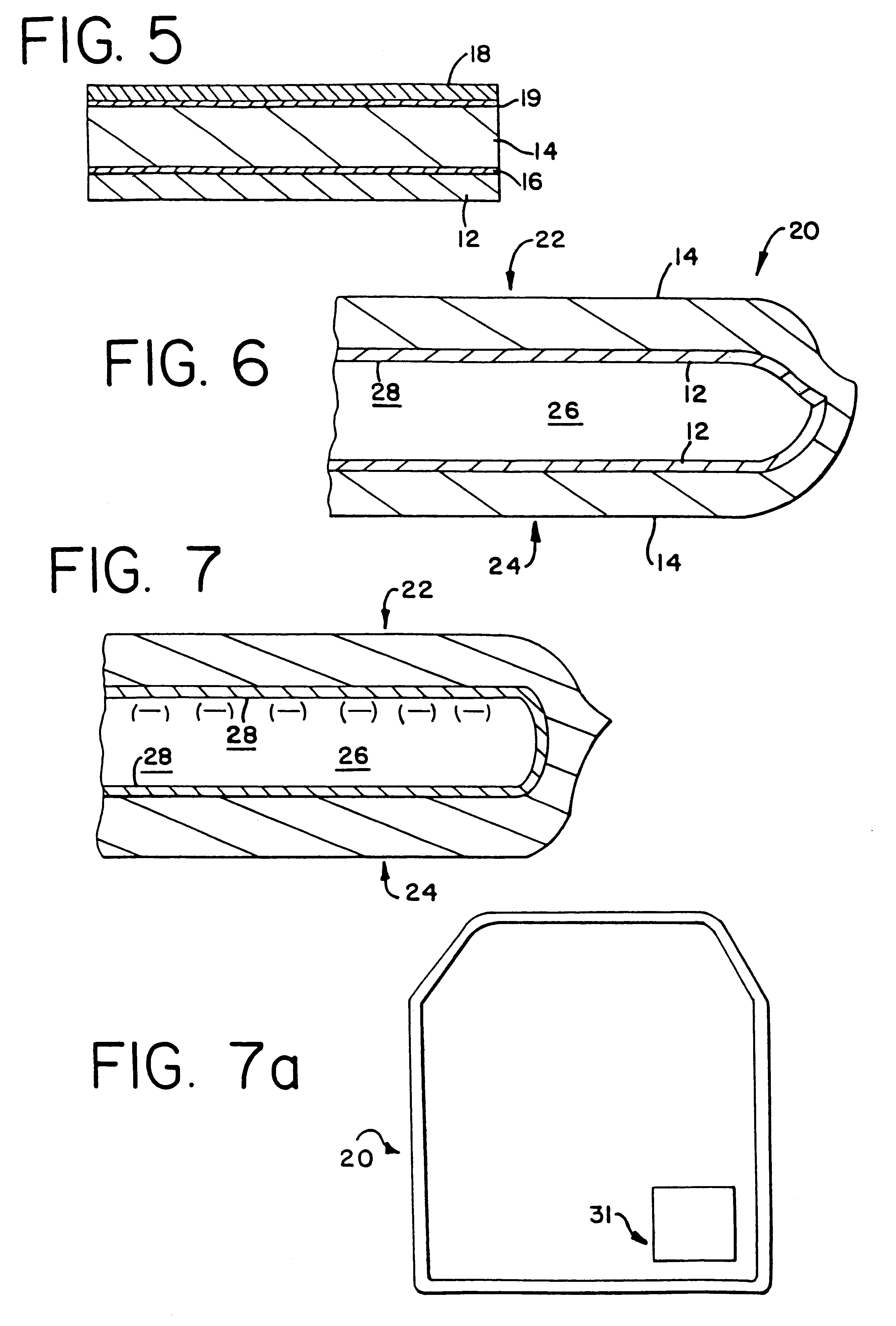
InactiveUS6297046B1Promote cell growthStrength and resistanceBioreactor/fermenter combinationsBiological substance pretreatmentsPolyolefinPolystyrene
A multi-layer, flexible, gas-permeable film (10) suitable for forming a cell culture container (20), the film (10) comprising a first layer (12) composed of a polystyrene having a thickness within the range of 0.0001 inches to about 0.0010 inches and, a second layer (14) adhered to the first layer (12) composed of a polyolefin having a thickness within the range of 0.004 inches to about 0.015 inches.
Owner:BAXTER INT INC
Bio-electrochemically assisted microbial reactor that generates hydrogen gas and methods of generating hydrogen gas
ActiveUS20060011491A1Provide supportGrowth inhibitionBioreactor/fermenter combinationsBiological substance pretreatmentsElectricityHydrogen
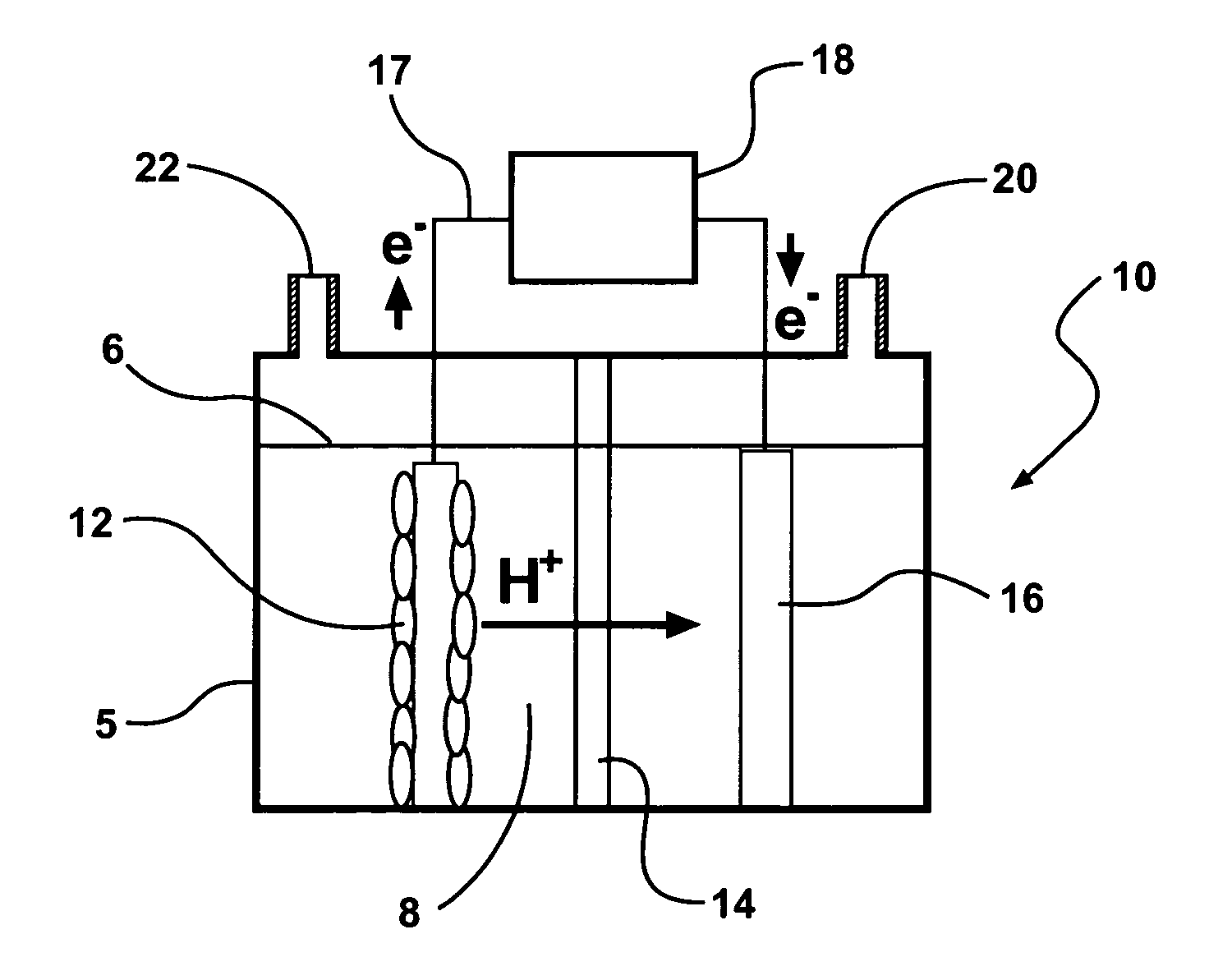
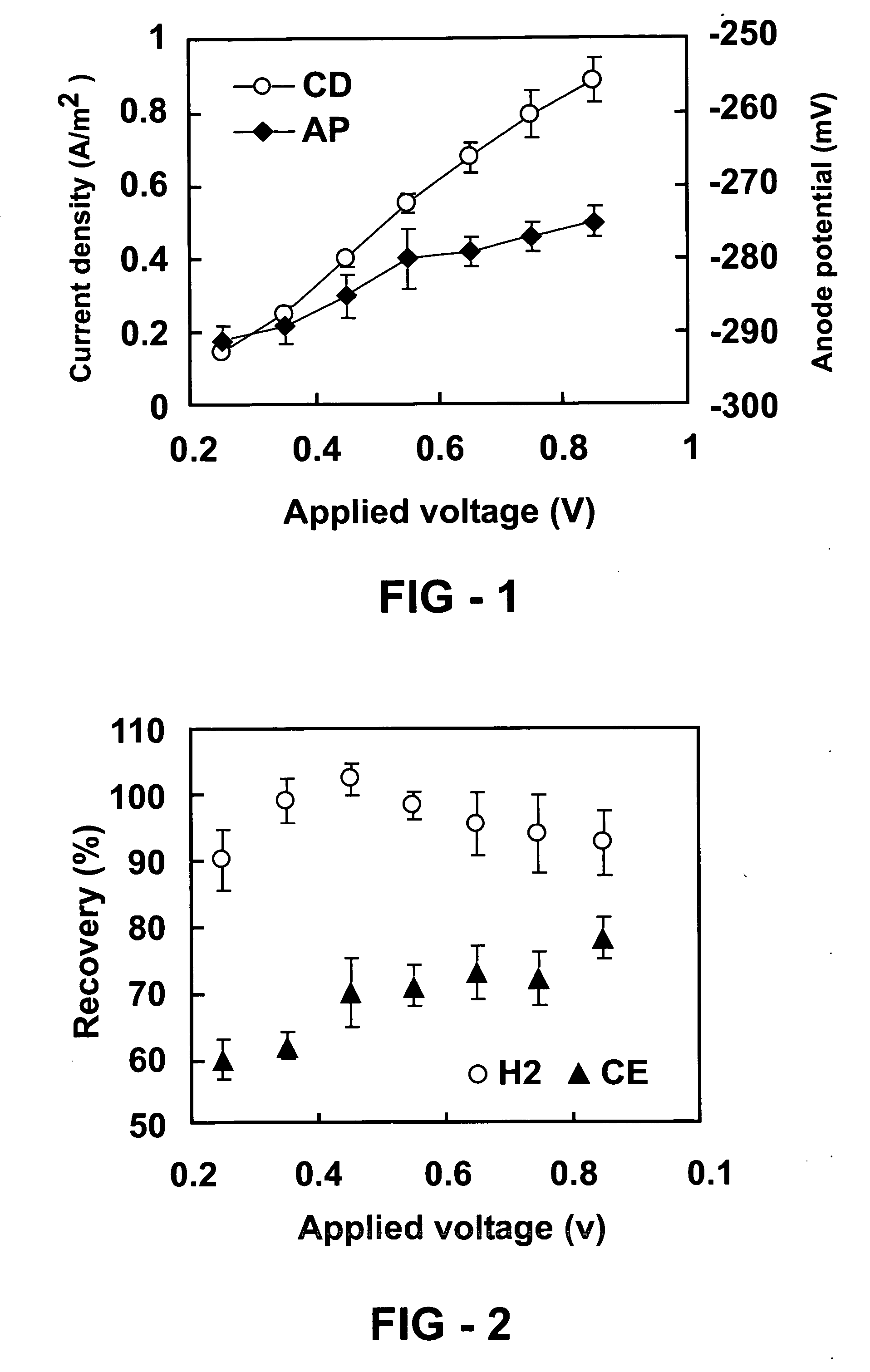
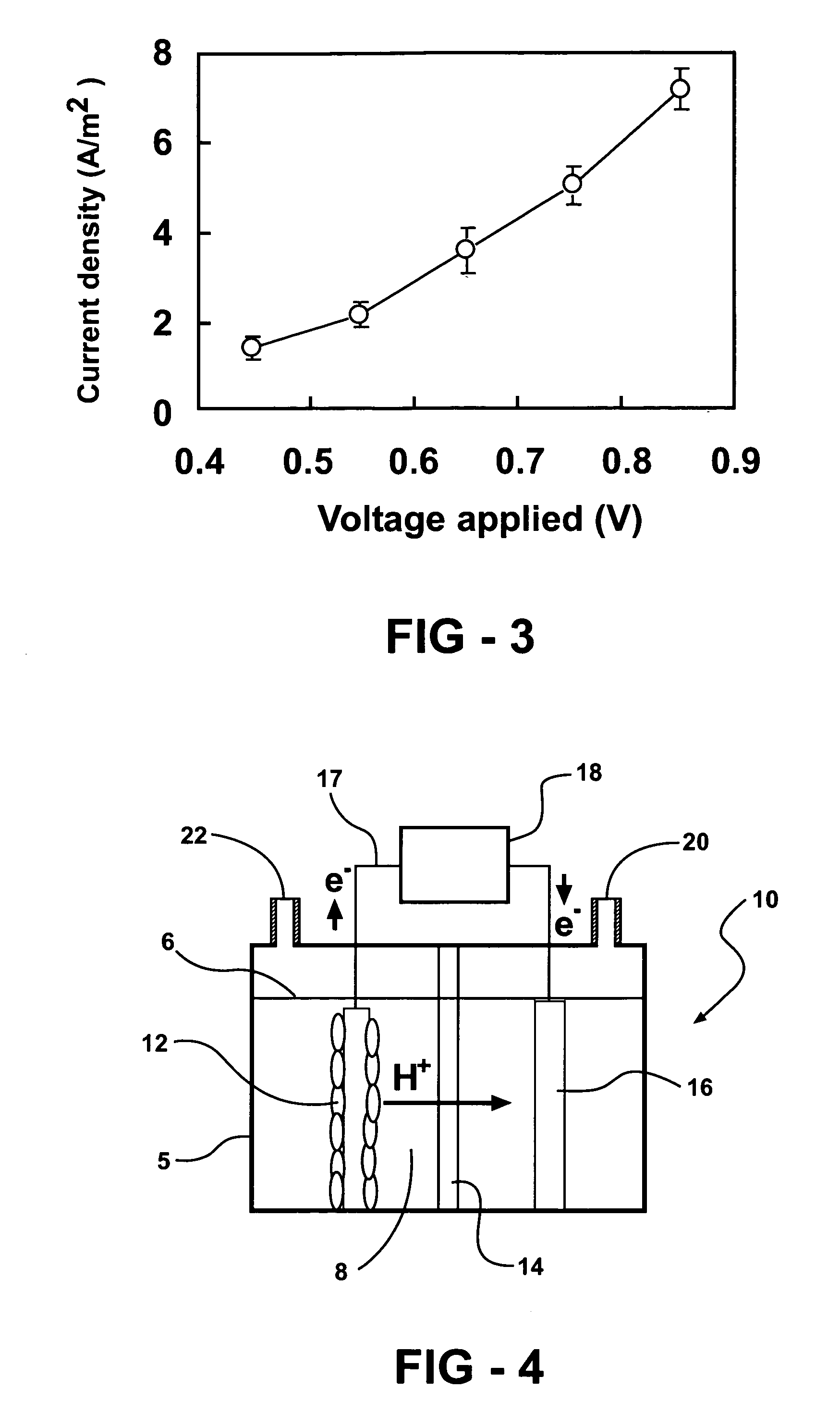
Systems and processes for producing hydrogen using bacteria are described. One detailed process for producing hydrogen uses a system for producing hydrogen as described herein, the system including a reactor. Anodophilic bacteria are disposed within the interior of the reactor and an organic material oxidizable by an oxidizing activity of the anodophilic bacteriais introduced and incubated under oxidizing reactions conditions such that electrons are produced and transferred to the anode. A power source is activated to increase a potential between the anode and the cathode, such that electrons and protons combine to produce hydrogen gas. One system for producing hydrogen includes a reaction chamber having a wall defining an interior of the reactor and an exterior of the reaction chamber. An anode is provided which is at least partially contained within the interior of the reaction chamber and a cathode is also provided which is at least partially contained within the interior of the reaction chamber. The cathode is spaced apart at a distance in the range between 0.1-100 centimeters, inclusive, from the anode. A conductive conduit for electrons is provided which is in electrical communication with the anode and the cathode and a power source for enhancing an electrical potential between the anode and cathode is included which is in electrical communication at least with the cathode. A first channel defining a passage from the exterior of the reaction chamber to the interior of the reaction chamber is also included.
Owner:PENN STATE RES FOUND +1
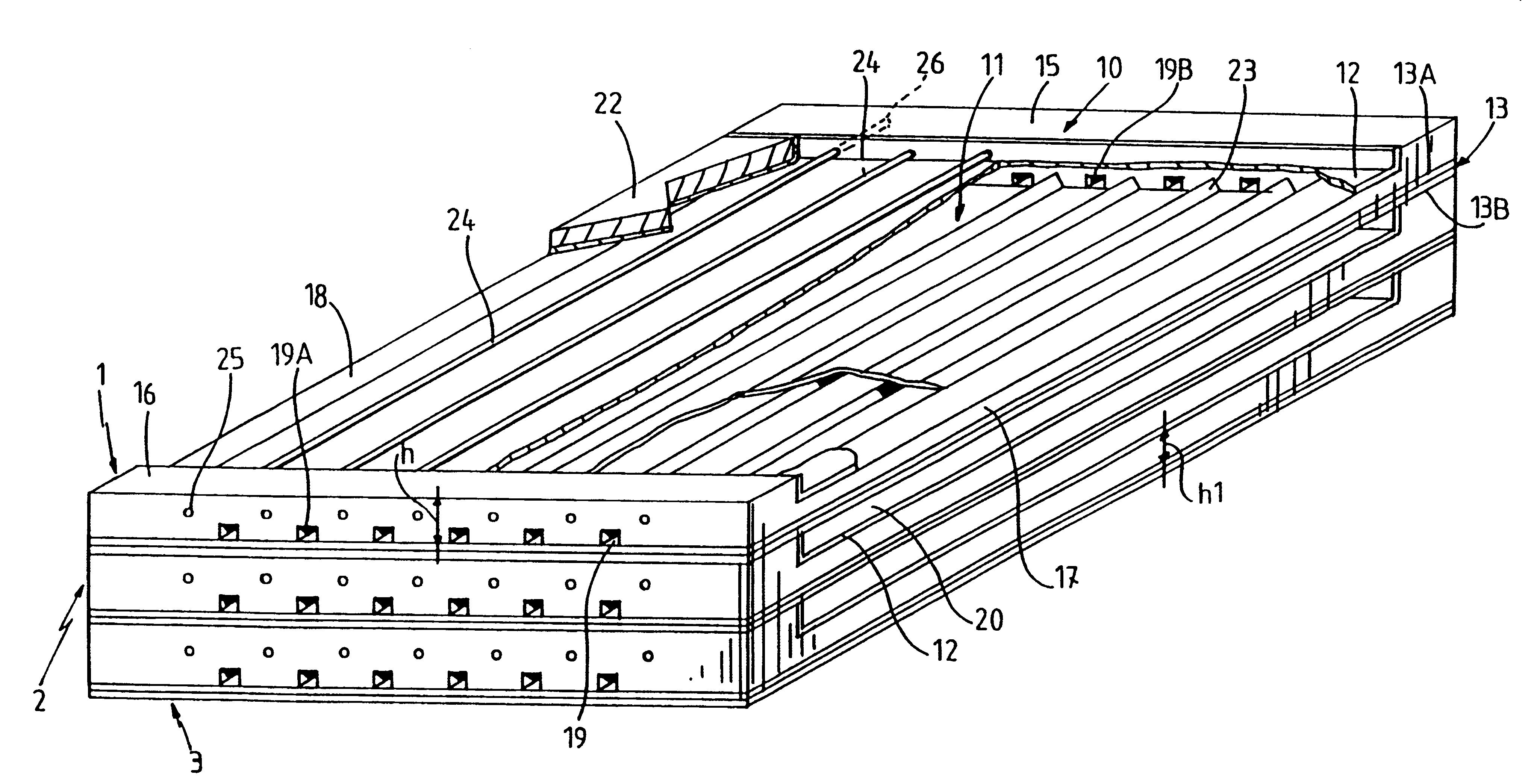
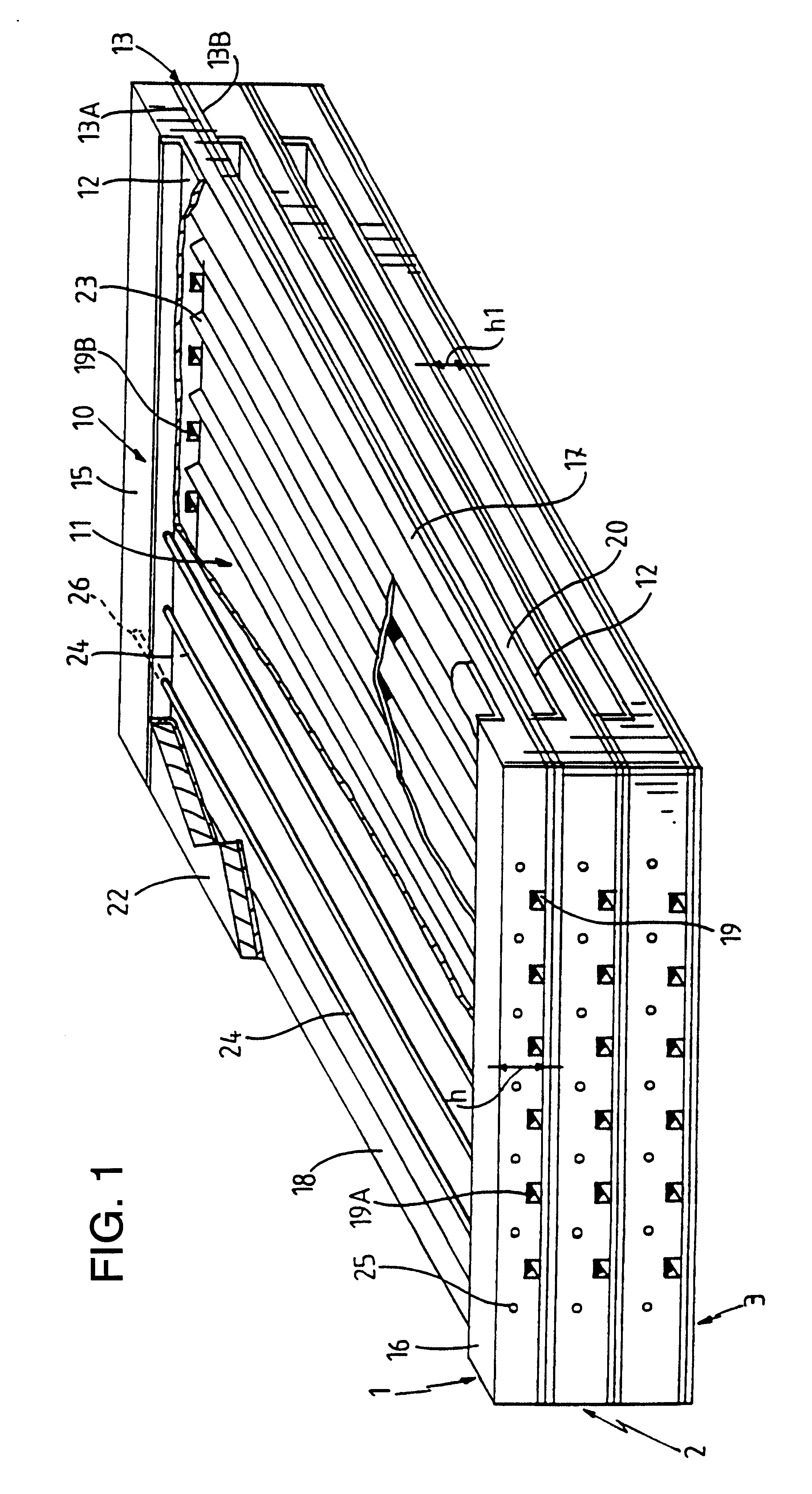
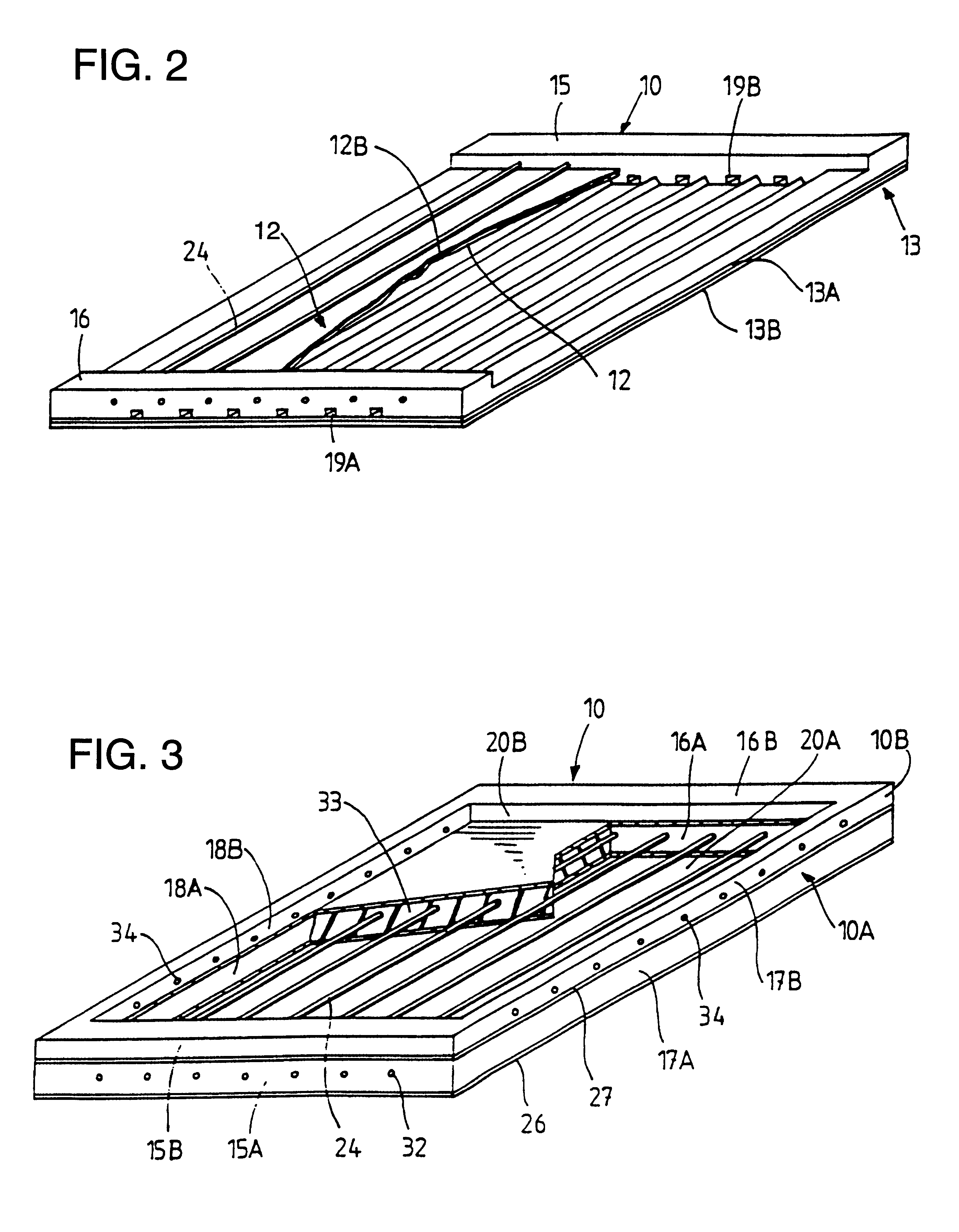
Multilayered cell culture apparatus
ActiveUS20070026516A1Effective trainingProvide consistencyBioreactor/fermenter combinationsBiological substance pretreatmentsCulture cellCell growth
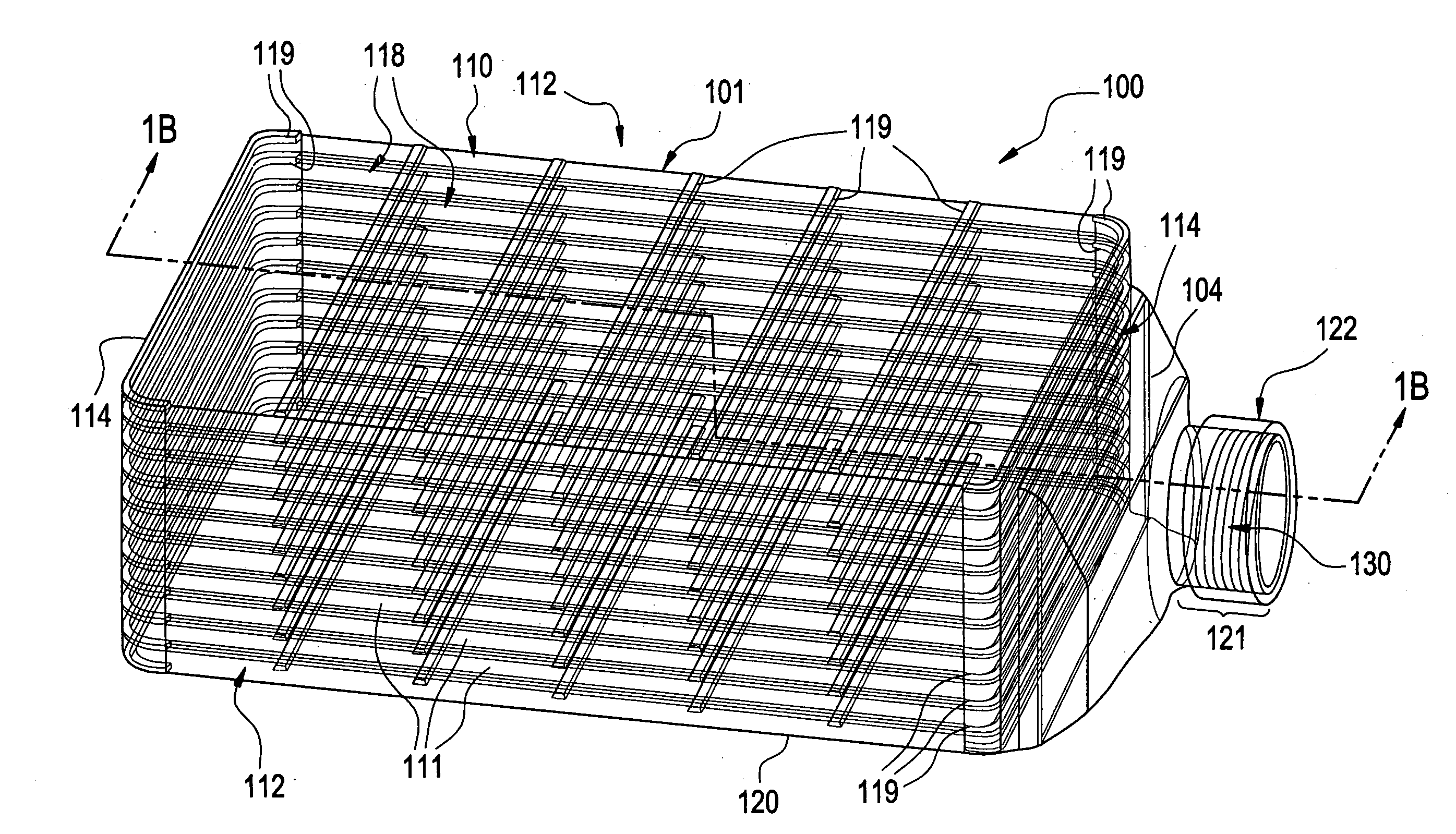
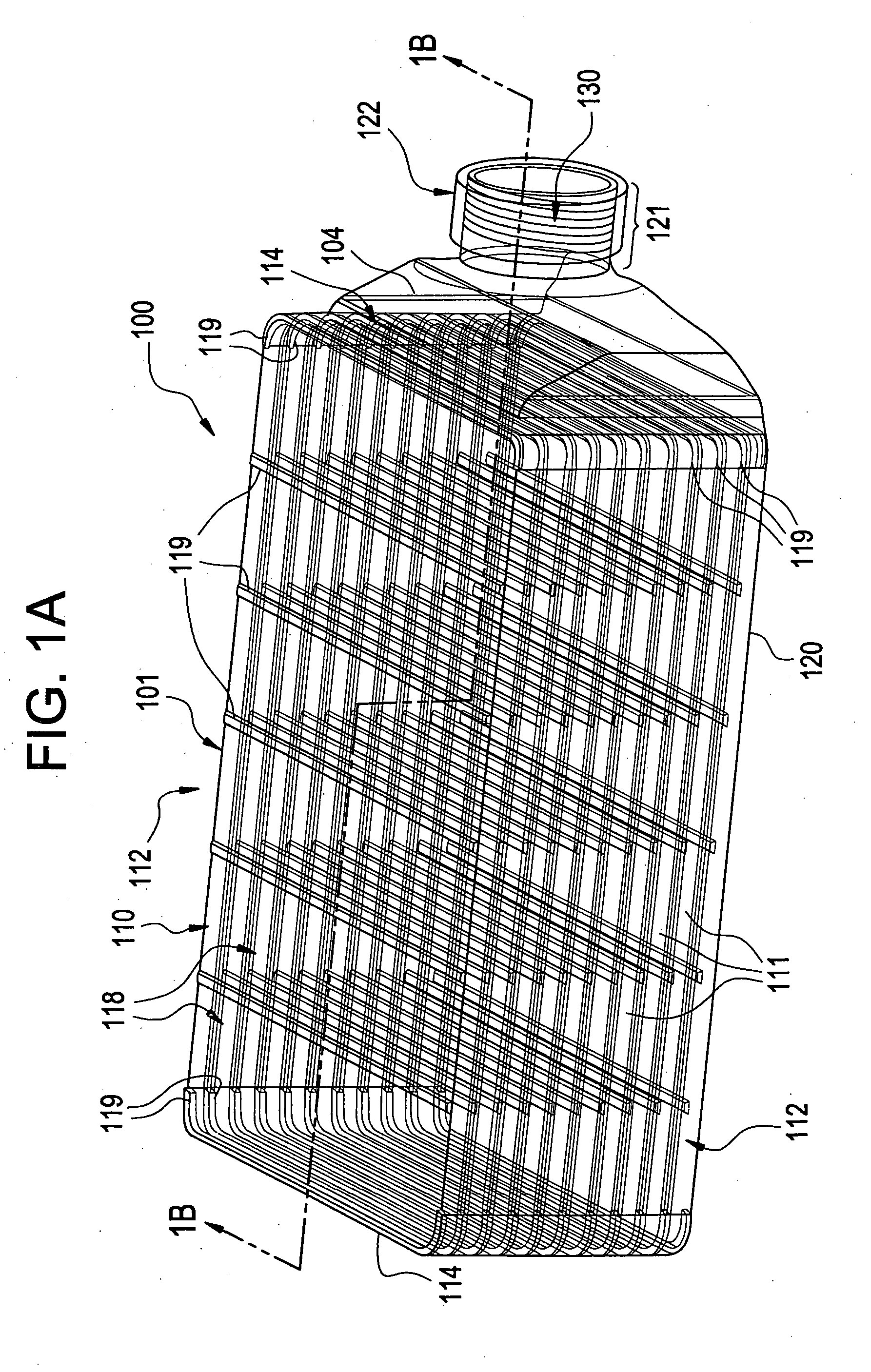
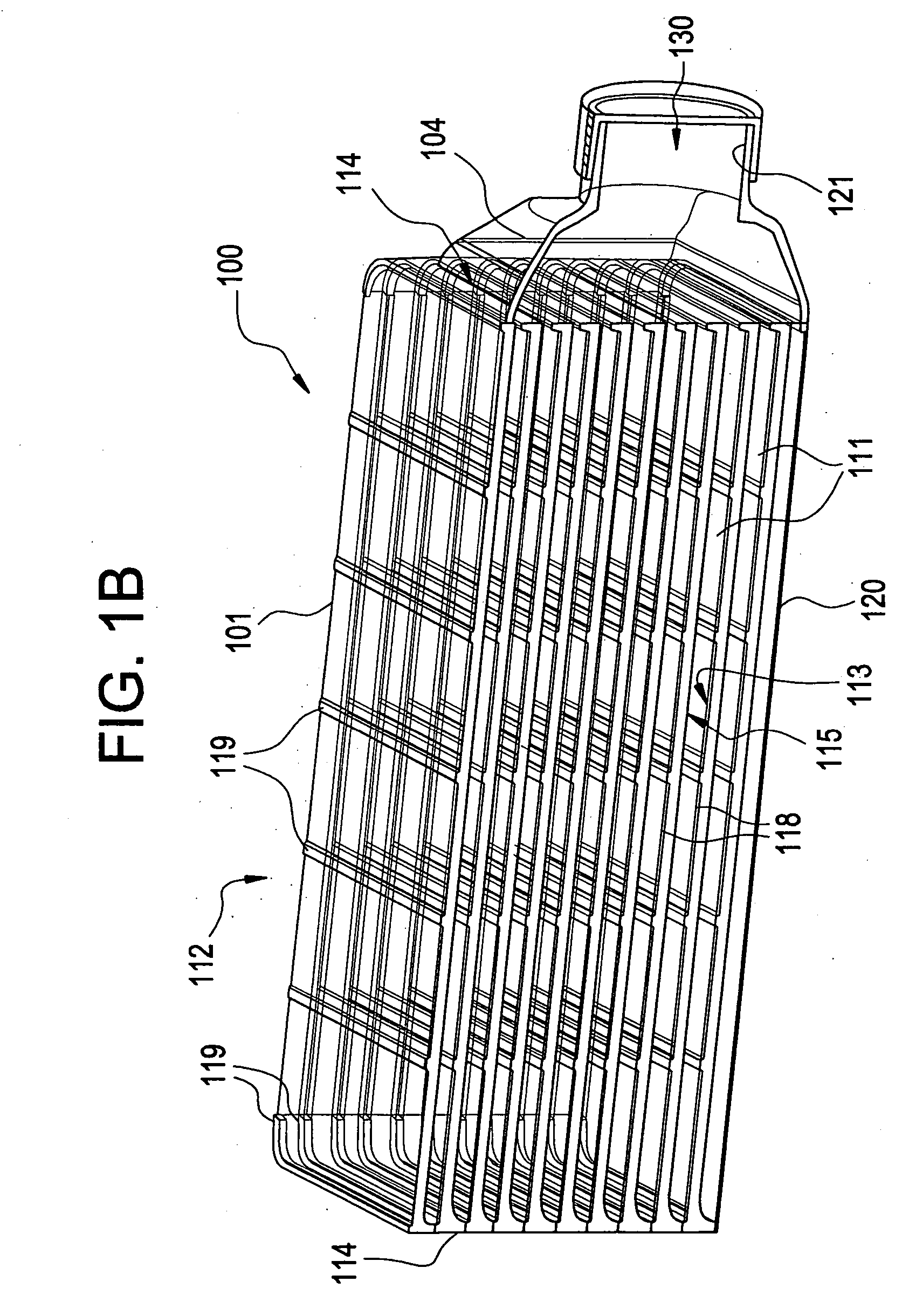
A multilayered cell culture apparatus for the culturing of cells is disclosed. The cell culture apparatus is defined as an integral structure having a plurality of cell culture chambers in combination with tracheal space(s). The body of the apparatus has imparted therein gas permeable membranes in combination with tracheal spaces that will allow the free flow of gases between the cell culture chambers and the external environment. The flask body. also includes an aperture that will allow access to the cell growth chambers by means of a needle or cannula. The size of the apparatus, and location of an optional neck and cap section, allows for its manipulation by standard automated assay equipment, further making the apparatus ideal for high throughput applications.
Owner:CORNING INC
Prevalidated, modular good manufacturing practice-compliant facility
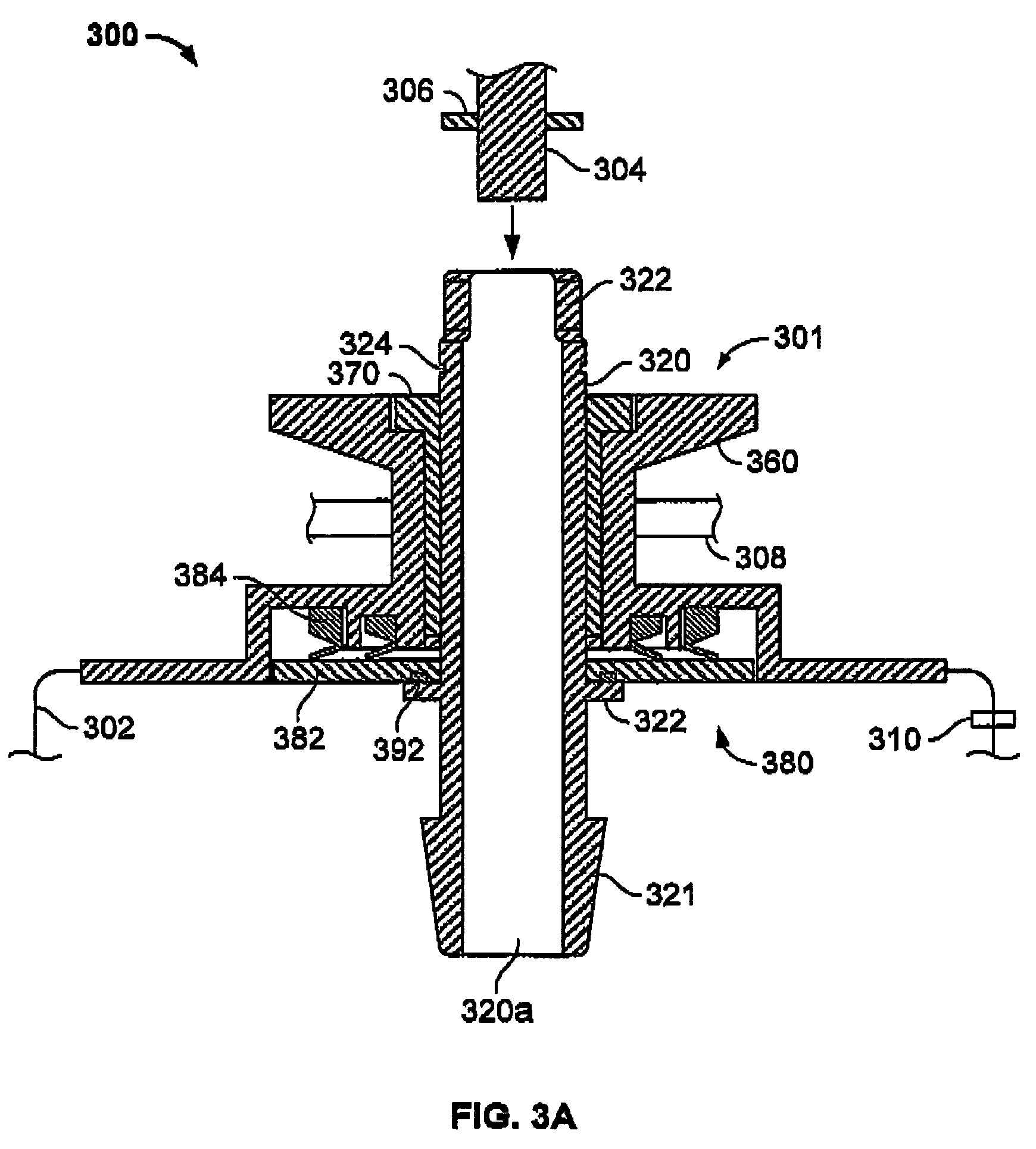
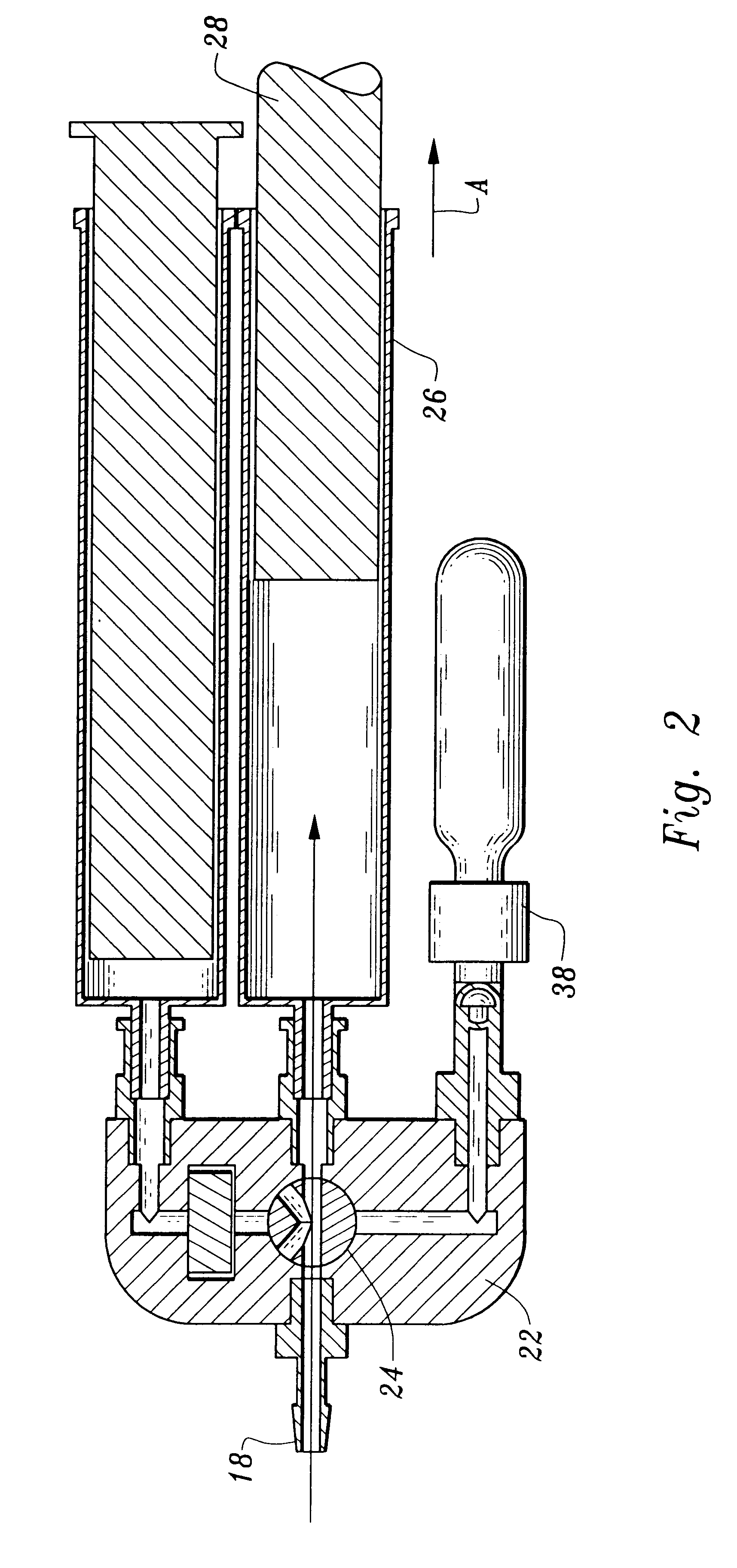
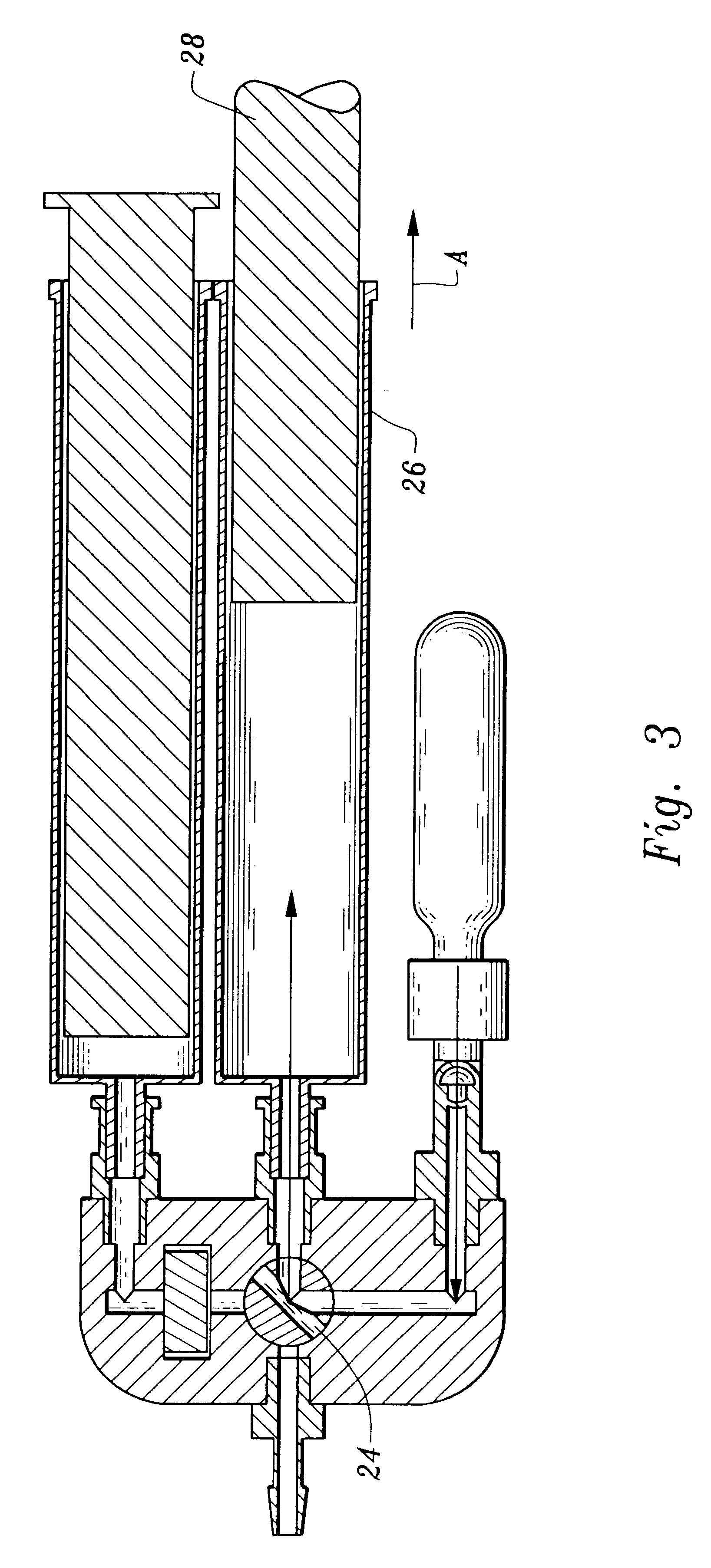
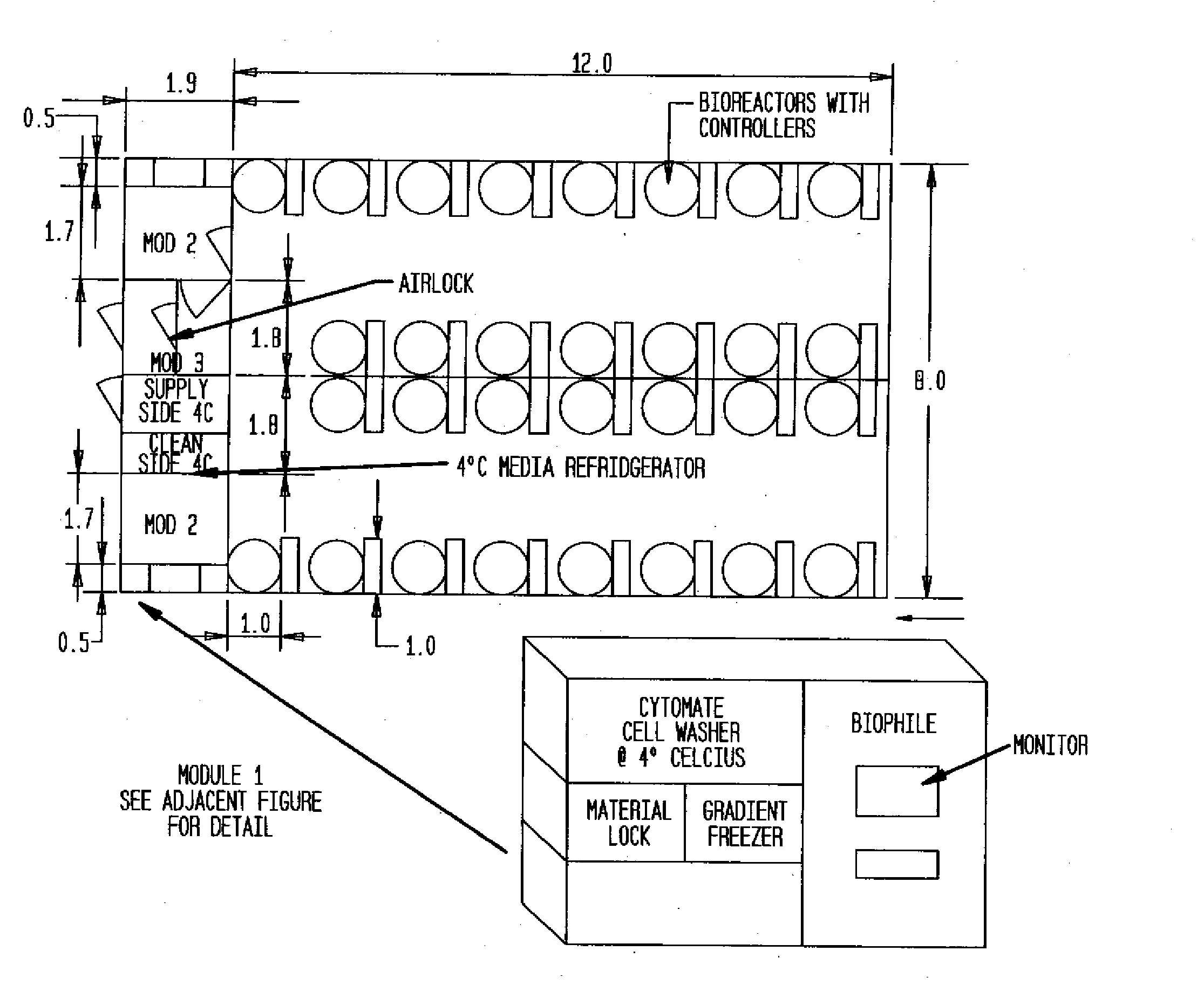
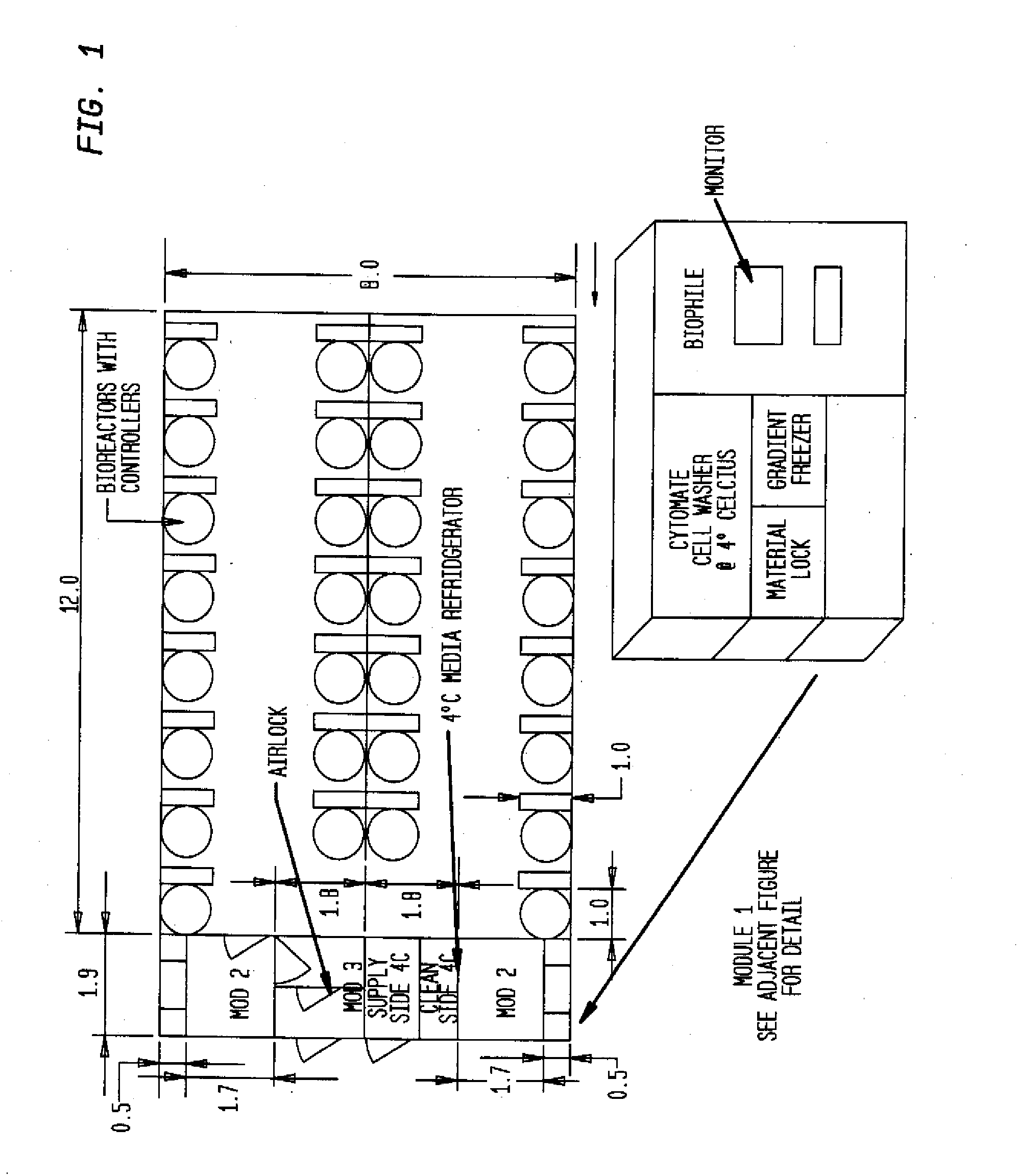
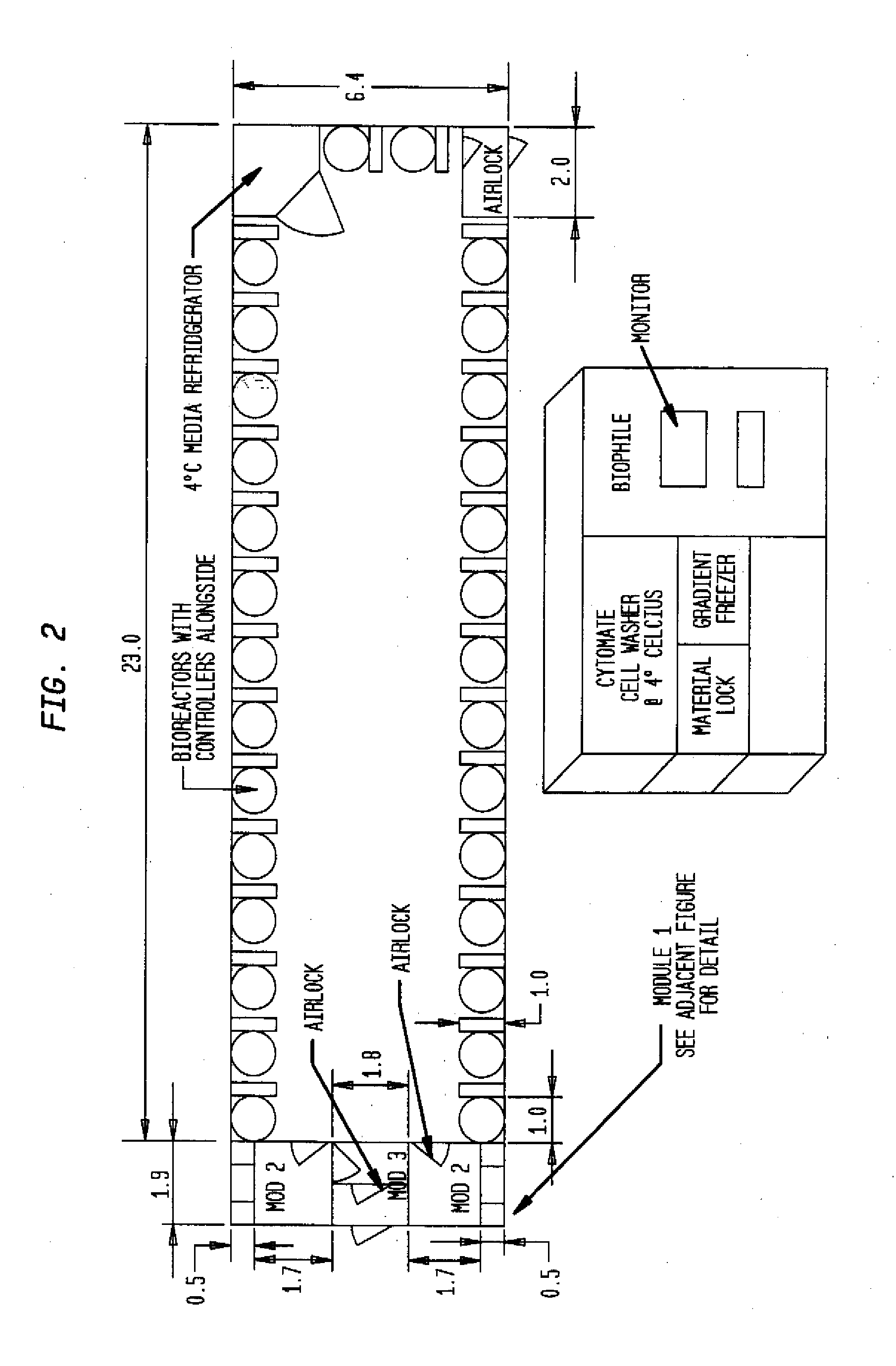
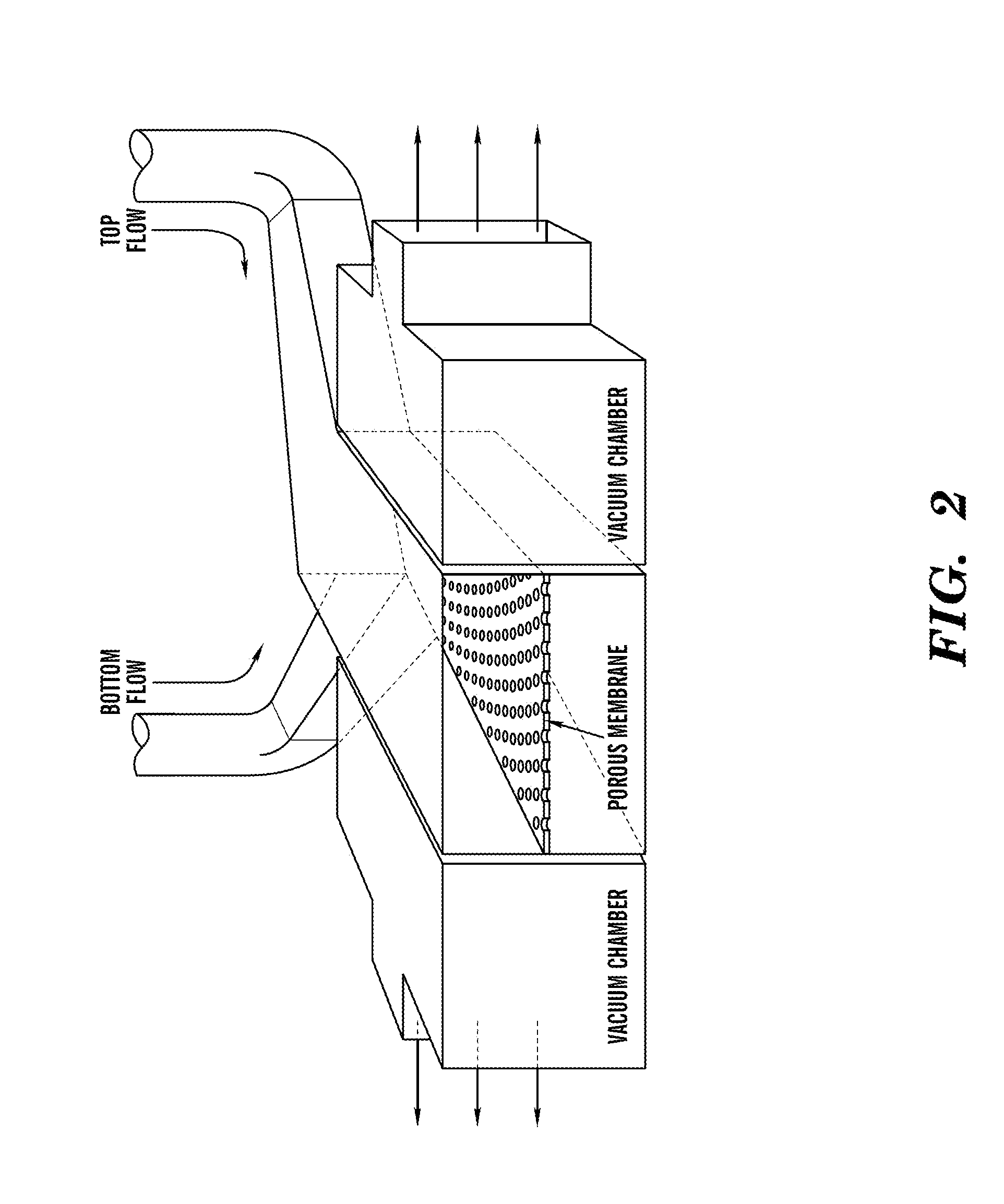
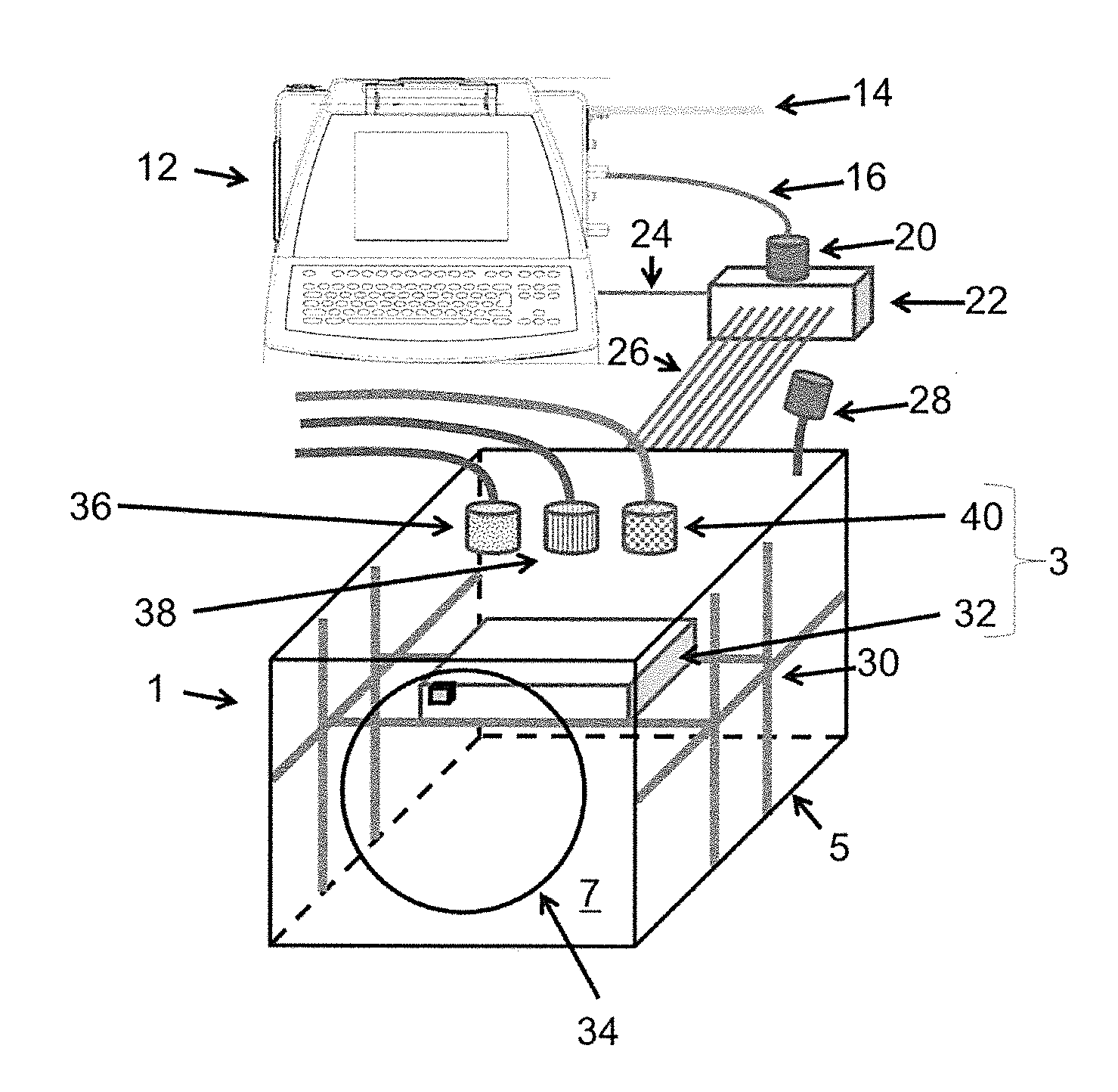
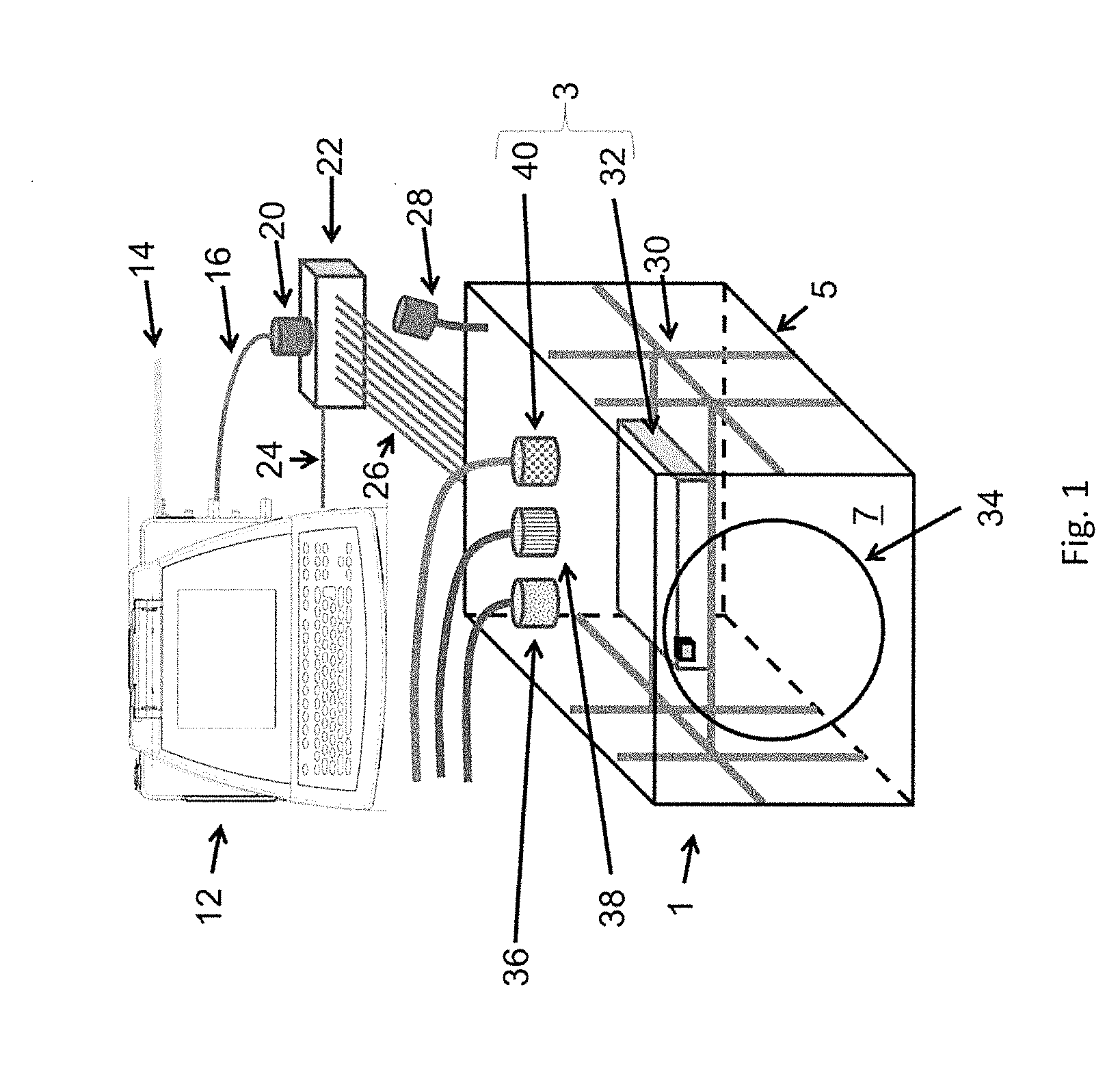
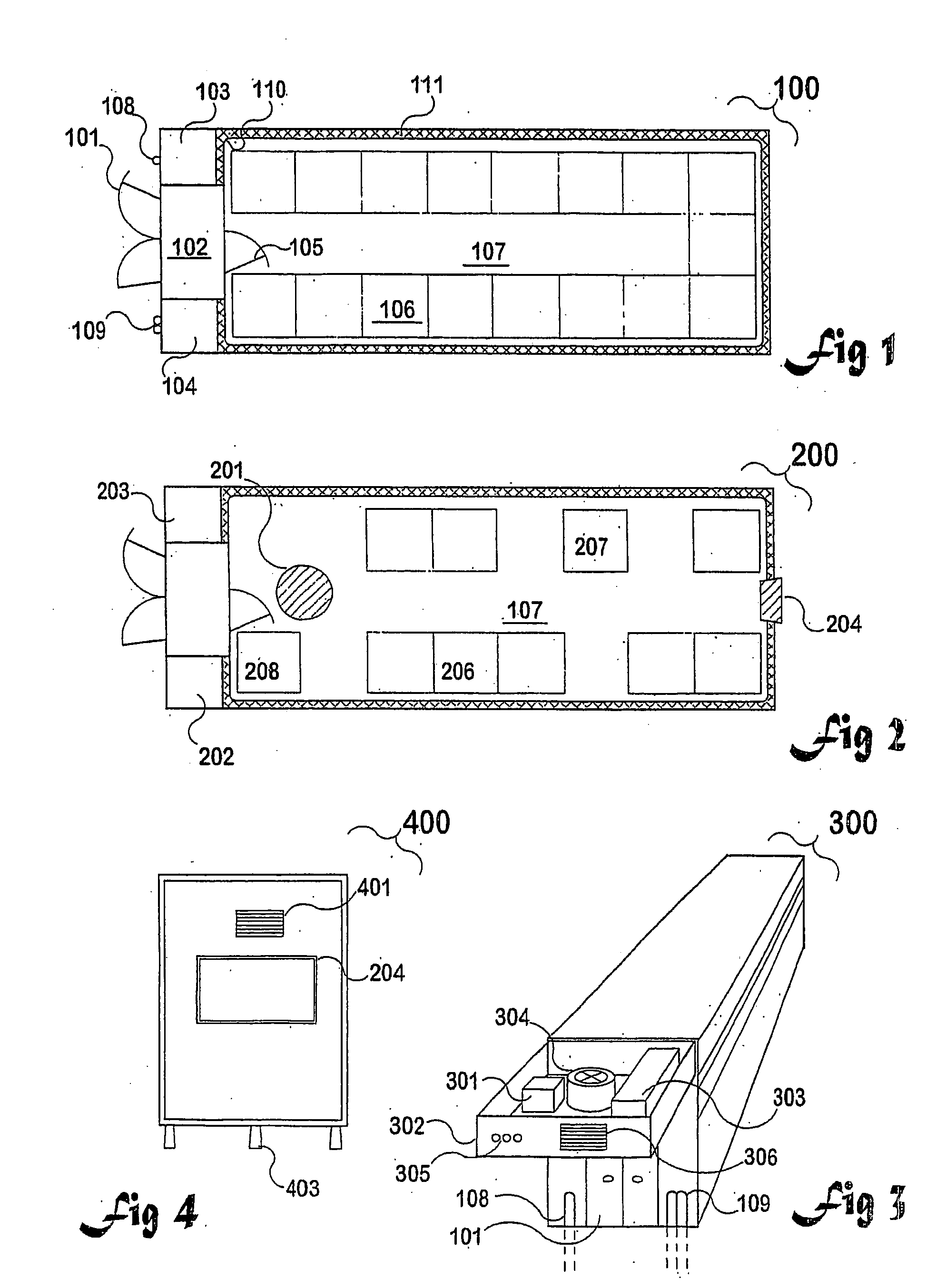
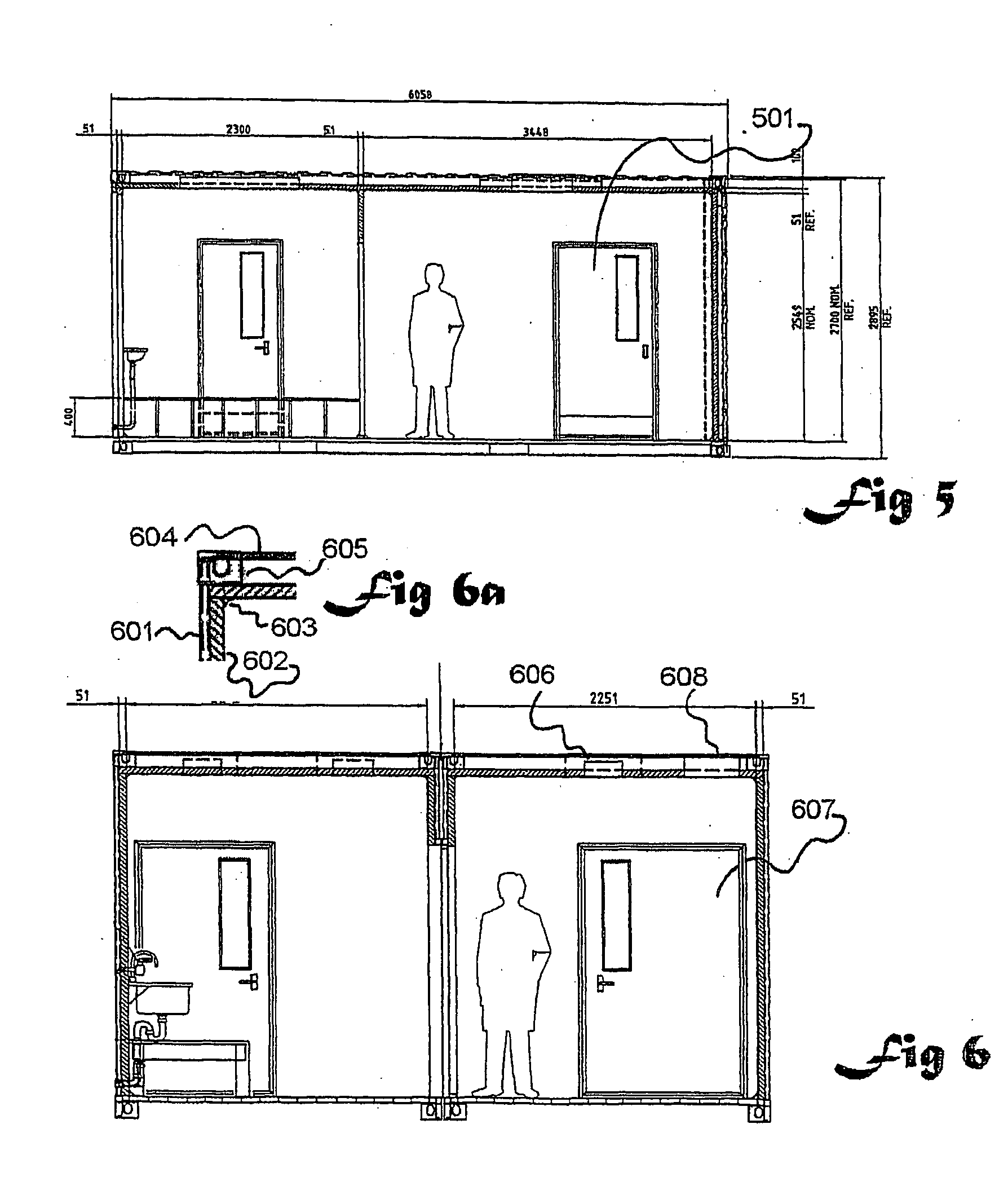
InactiveUS20090305626A1Precious timeShorten the timeApparatus sterilizationDust-free enclosuresInterior spaceGood laboratory practice
The invention is directed to a ready-to-use modular cleanroom and facility, in particular for the production of drugs and biological substances, which is equipped with pre-approved manufacturing equipment cores. The modular cleanroom is implemented in the interior space of a container, such as a standard shipping container, and includes at least one bioreactor station. The modular facility can be installed on-site from pre-approved cleanroom modules without further regulatory approval. The cleanroom and facility comply with FDA-approved good manufacturing practices (GMP) and good laboratory practices (GLP).
Owner:HOPE ERNEST G
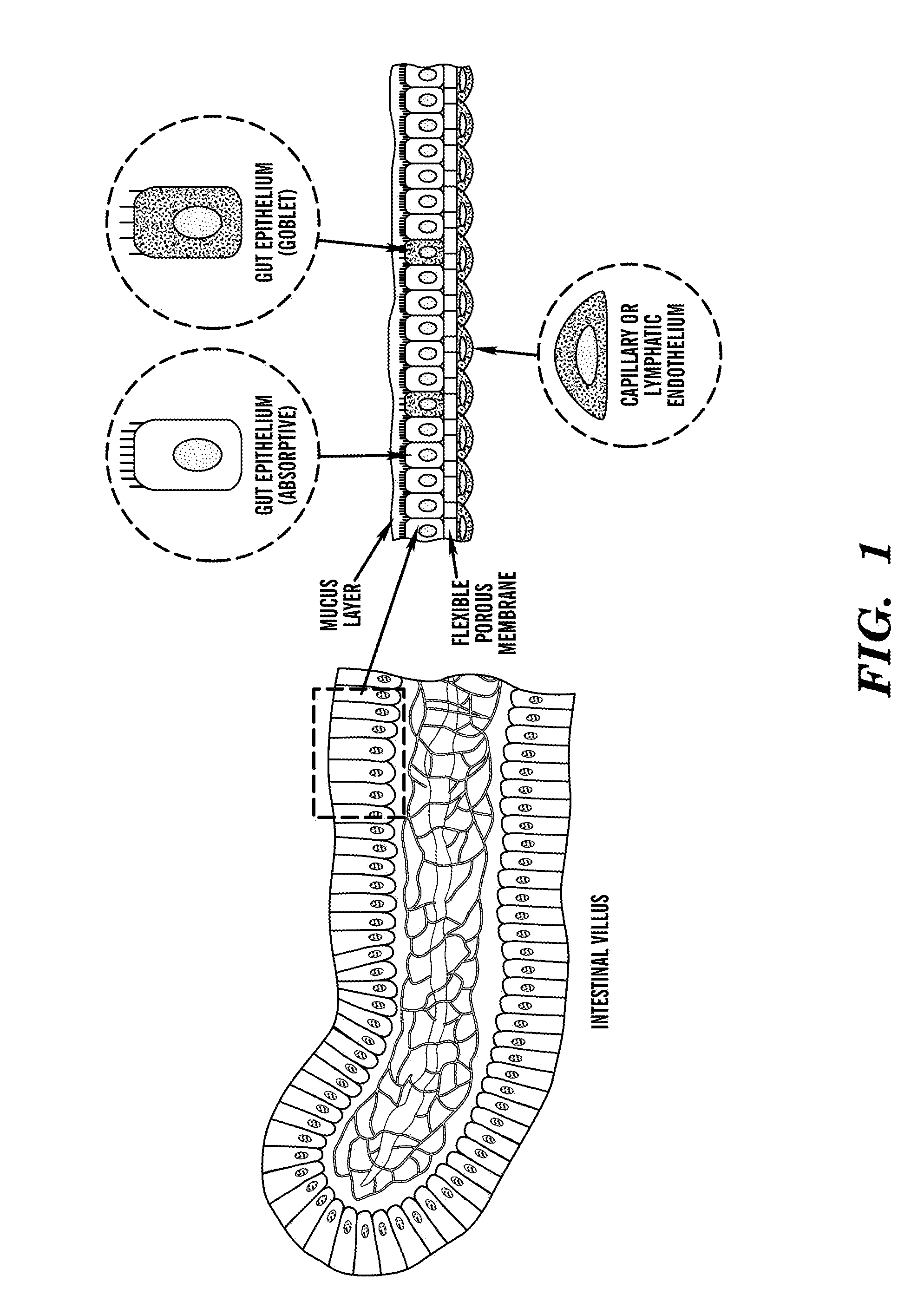
Cell culture system
The embodiments of the invention described herein relate to systems and methods for culturing and / or maintaining intestinal cells, tissues and / or organoids in vitro. The cells, tissues and / or organoids cultured according to the methods and systems described herein can mimic or reproduce natural intestinal epithelial structures and behavior as well as support co-culture of intestinal microflora.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
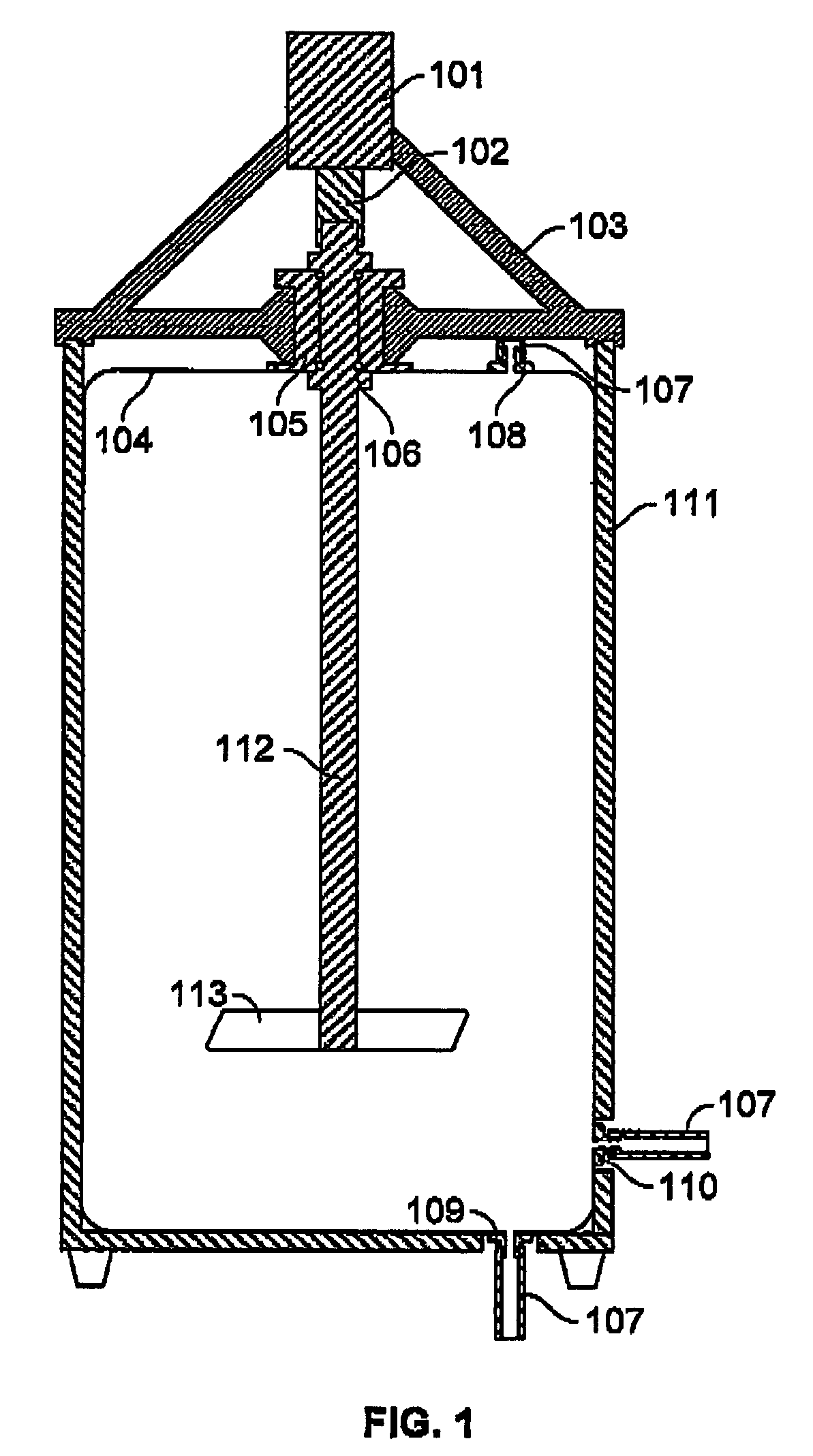
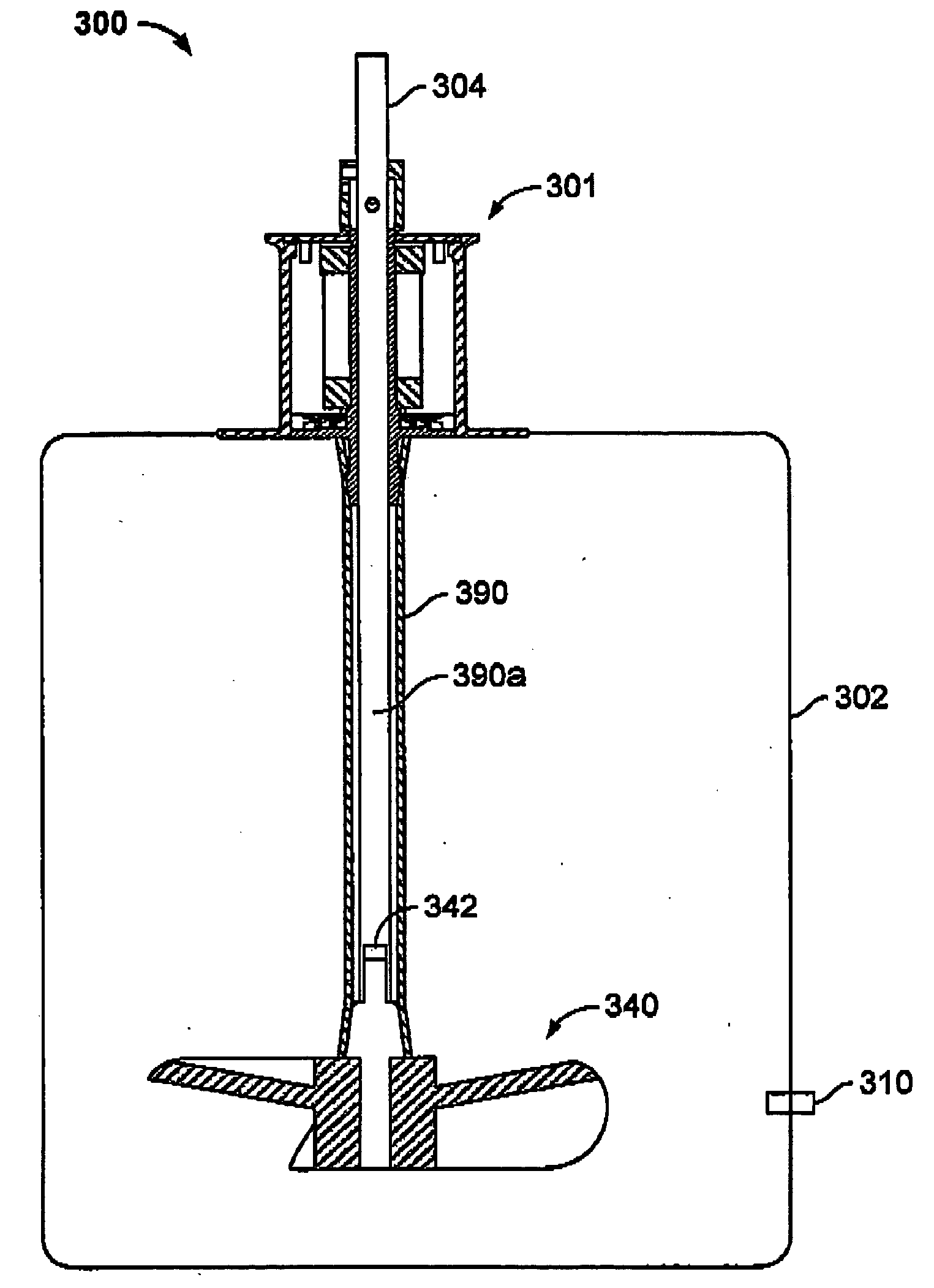
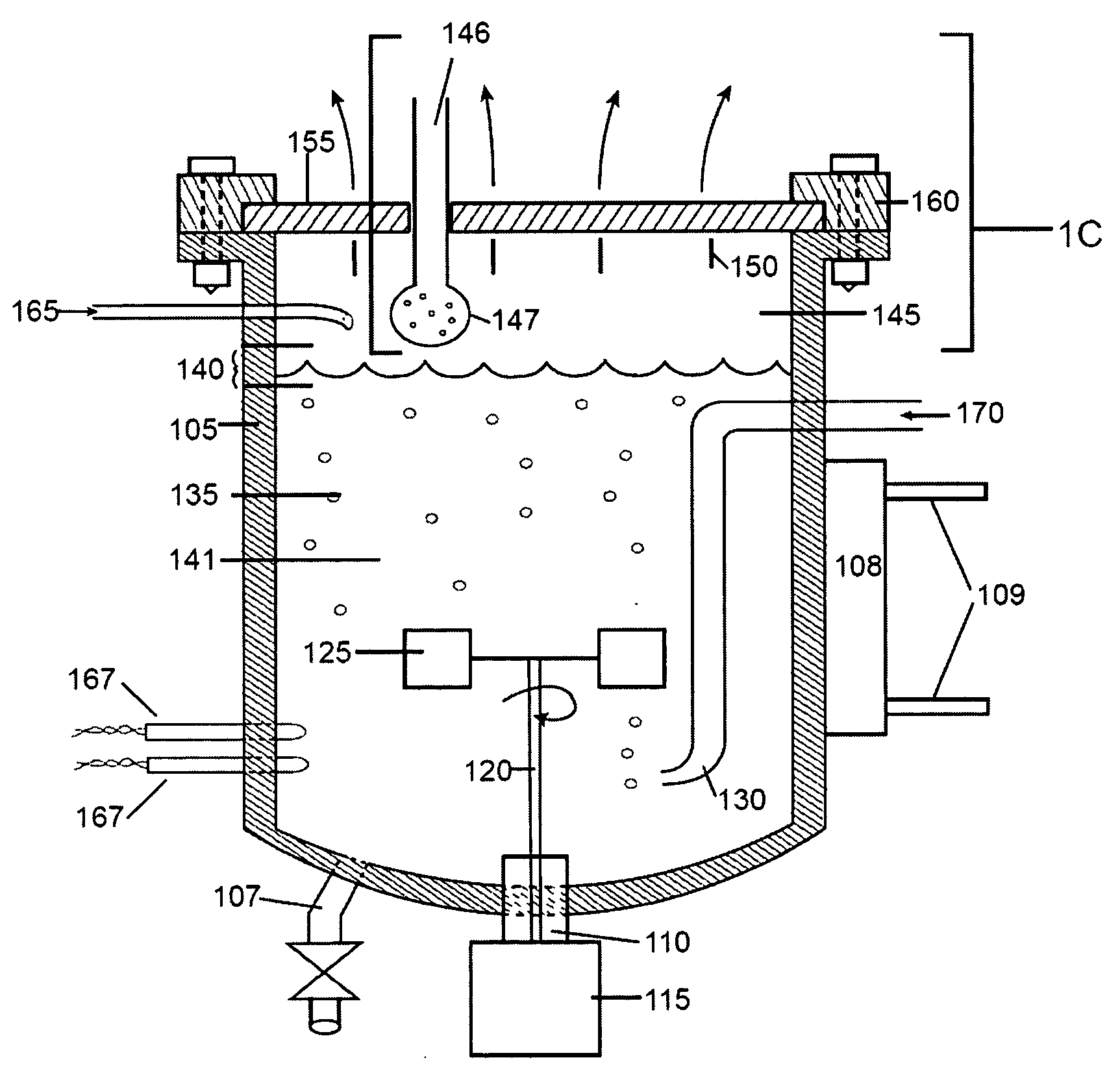
Bioreactor
InactiveUS6228607B1Convenience to workBioreactor/fermenter combinationsBiological substance pretreatmentsOxygenBioreactor
The invention relates to a bioreactor for a cell treatment of a medium. Said bioreactor comprises an element defining a chamber in which cells for treating the medium are located, a liquid permeable membrane separating the said chamber from a first channel in which flows the medium to be treated, and a gas permeable membrane separating the said chamber from a second channel in which flows a gas containing oxygen.
Owner:ORGANOGENESIS
Bioreactor design and process for engineering tissue from cells
InactiveUS6979308B1Easy to adjustEnhance effective transferBioreactor/fermenter combinationsBiological substance pretreatmentsLipid formationFiber
A scaled-up multi-coaxial fiber bioreactor, and variations of this bioreactor. The device is characterized by a hollow housing and an array of from about 20 to about 400 modules of hollow fibers, where each module includes at least three coaxial semipermeable hollow fibers. The innermost fiber provides a boundary for an innermost compartment which is connected to inlet and outlet ports. Arranged coaxially around the central hollow fiber are several other hollow fibers with their respective compartments, each compartment defined by a respective annular space between adjacent fibers and each including inlet and outlet ports. An outermost compartment for permitting integral aeration is the space between the outer side of the outermost fibers and the inner side of the housing, and has inlet and outlet ports. The hollow housing has inlet and outlet manifolds and flow distributors for each of the compartments. In a preferred embodiment the bioreactor is used as an extracorporeal liver. Liver cells, are introduced into one or more annular compartments and media and aeration are provided in others. Plasma from an ailing patient is introduced into another compartment for biotransformation of blood-borne toxins and biosynthesis of proteins, lipids, and other metabolic products.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Methods and devices for microfluidic point-of-care immunoassays
ActiveUS8110392B2Reduce incubation timeMean flow velocityBioreactor/fermenter combinationsShaking/oscillating/vibrating mixersPoint of careSystems design
Microfluidic methods and devices for heterogeneous binding and agglutination assays are disclosed, with improvements relating to mixing and to reagent and sample manipulation in systems designed for safe handling of clinical test samples.
Owner:PERKINELMER HEALTH SCIENCES INC
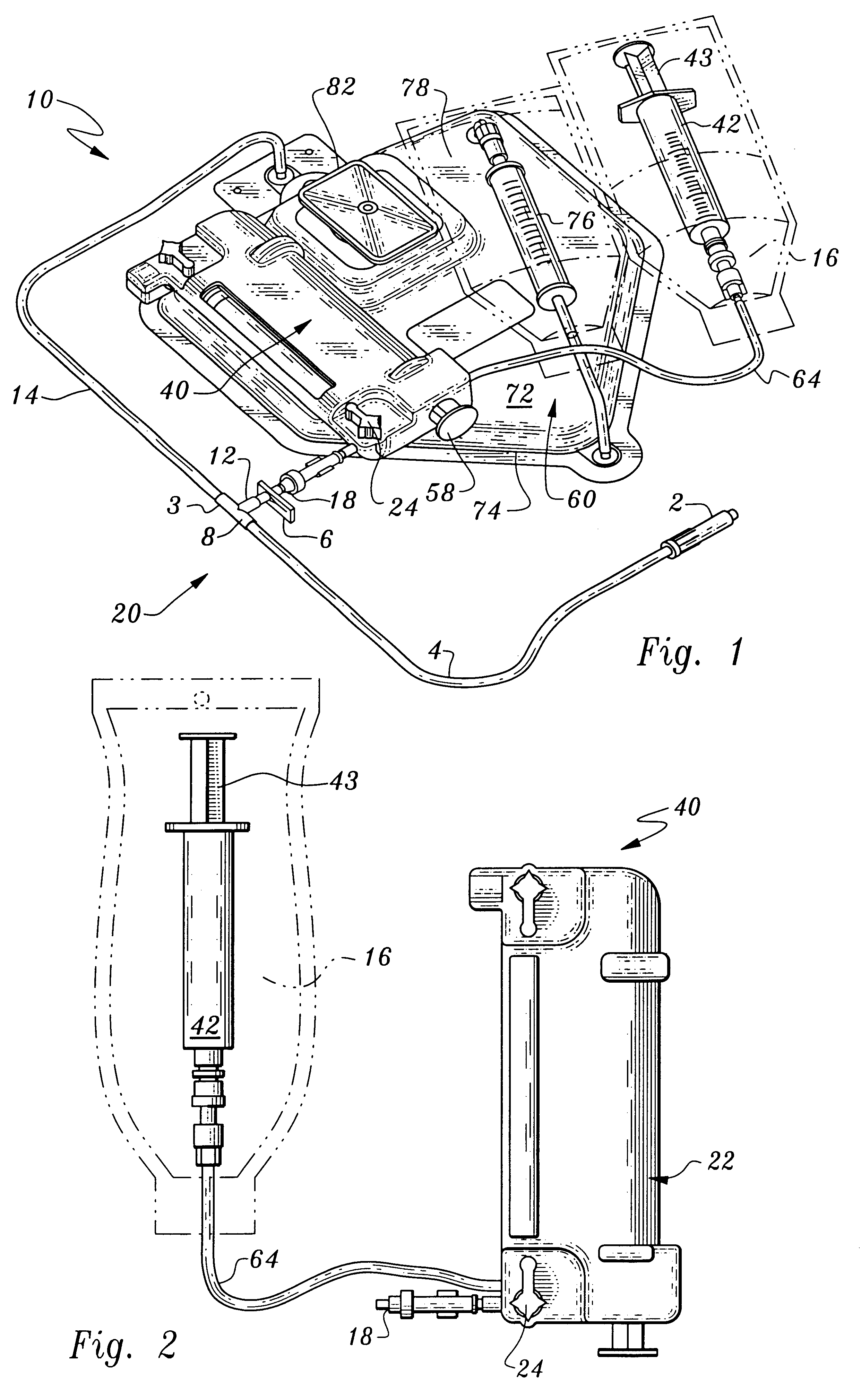
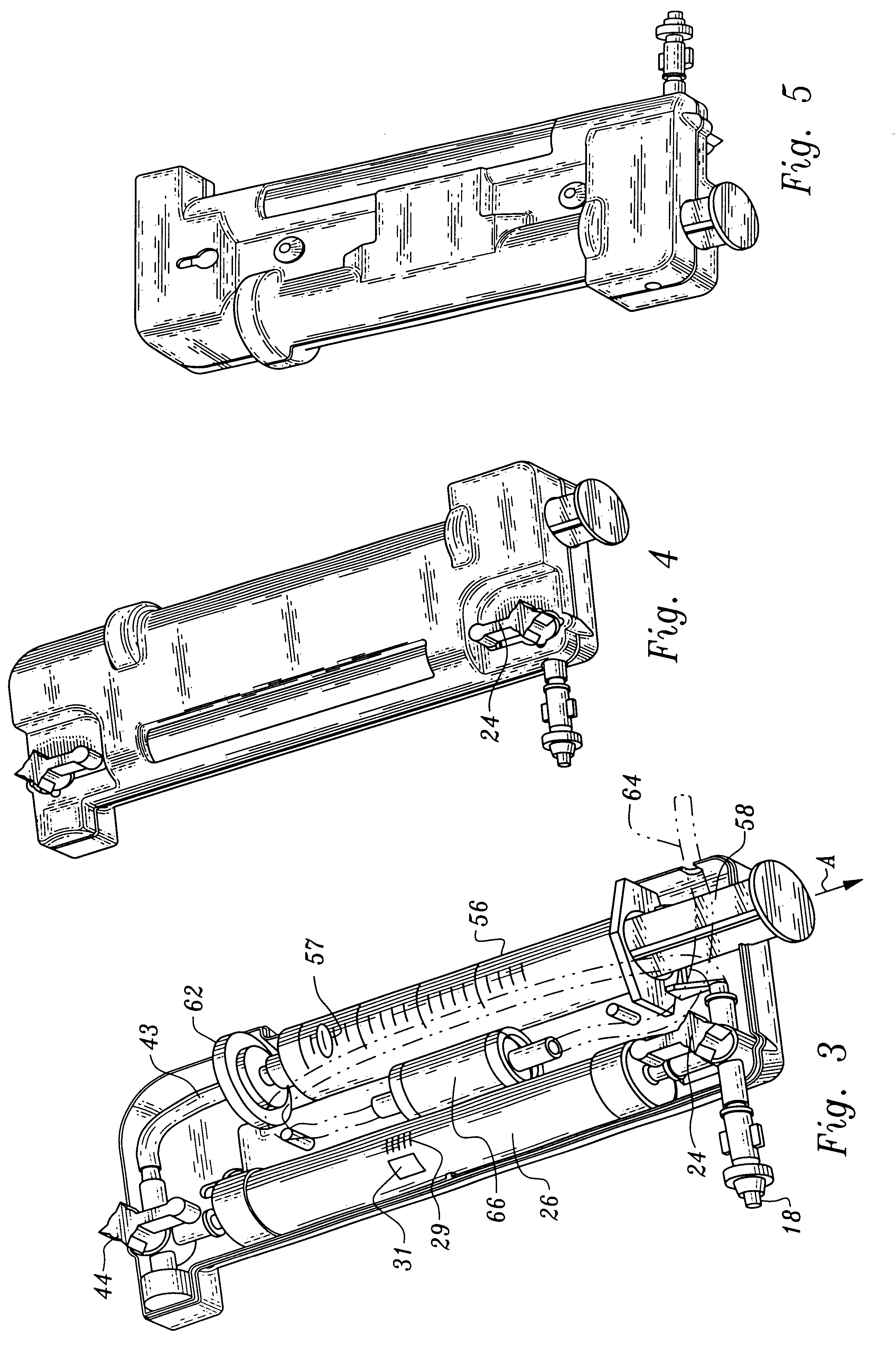
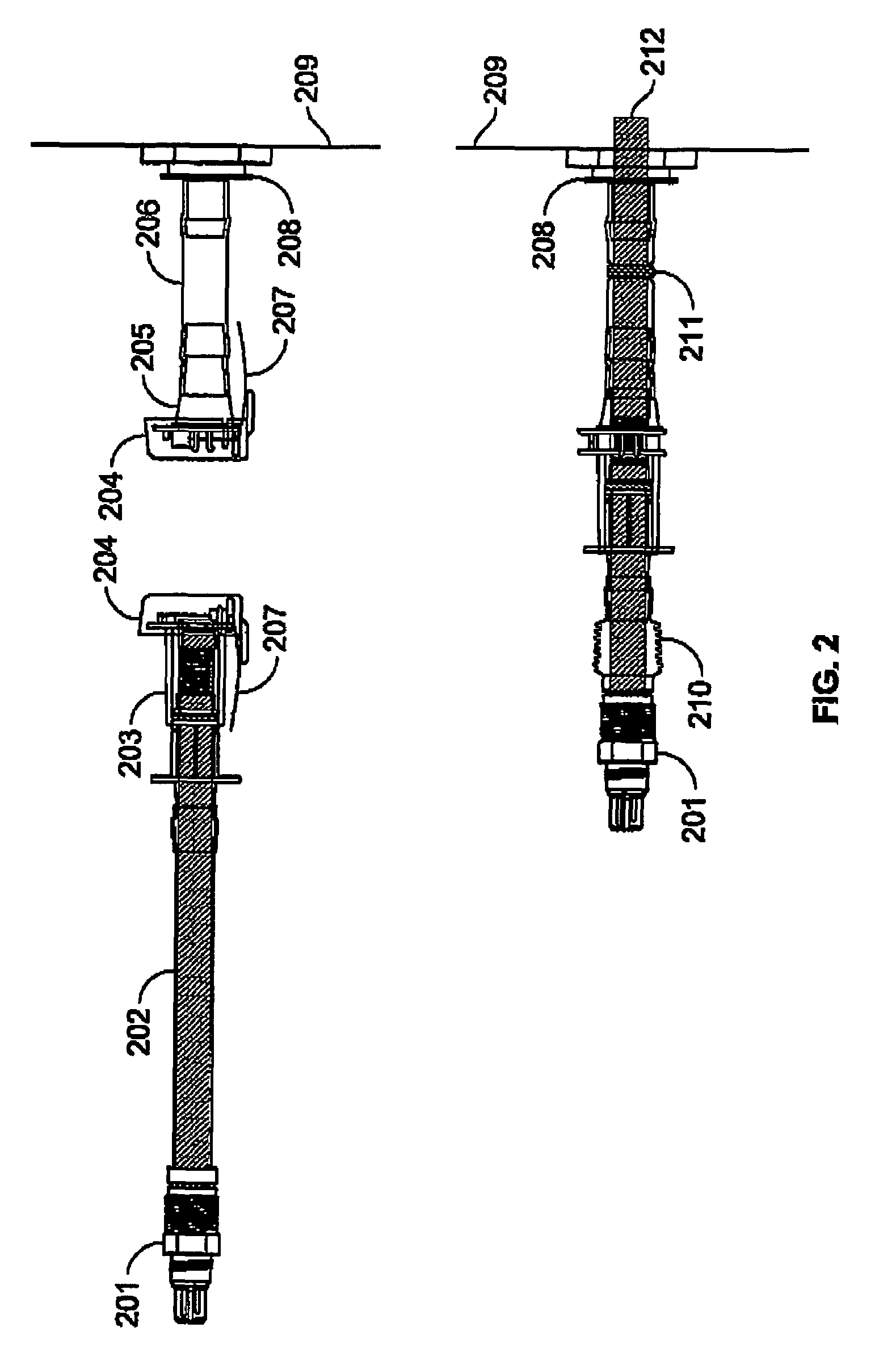
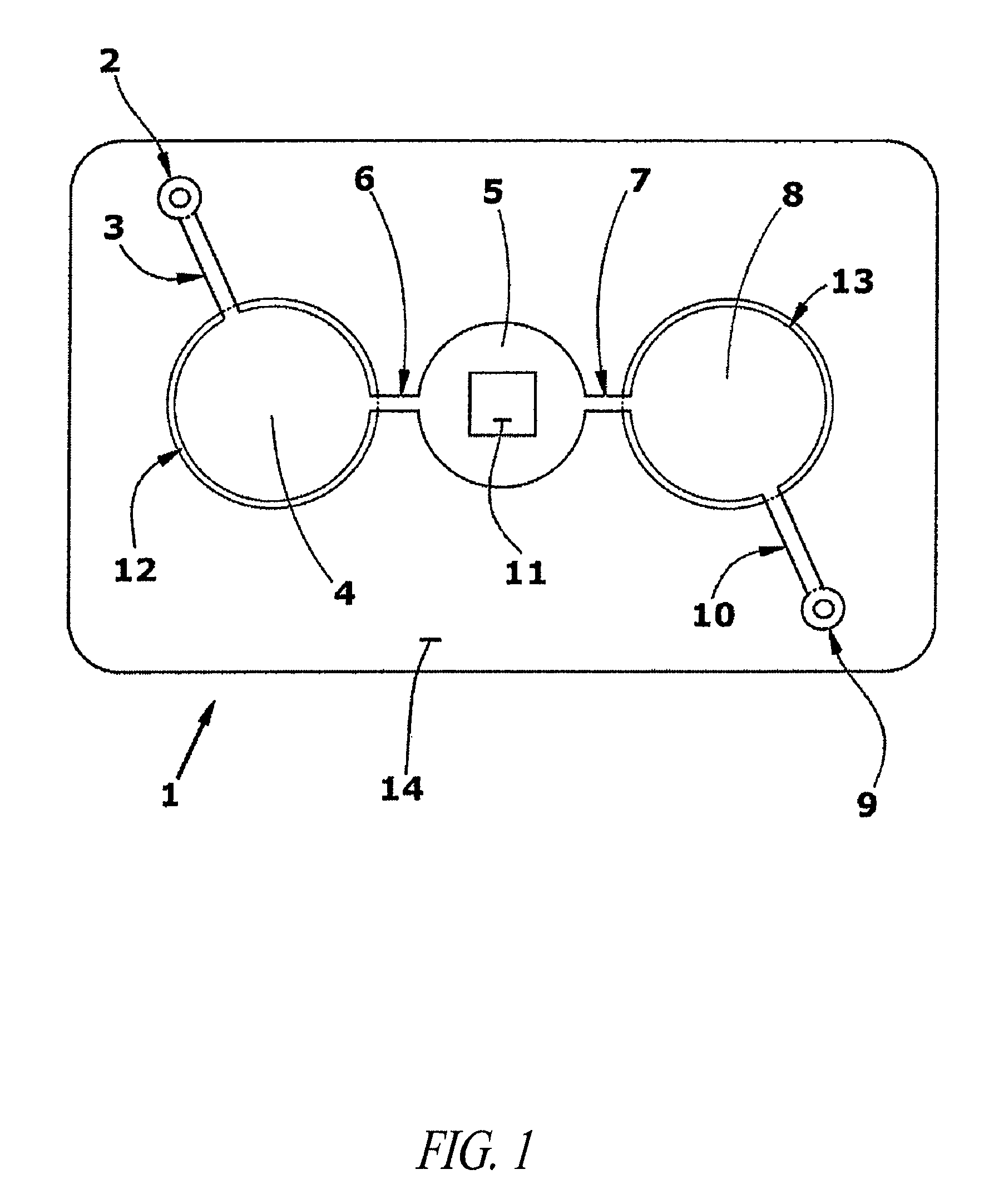
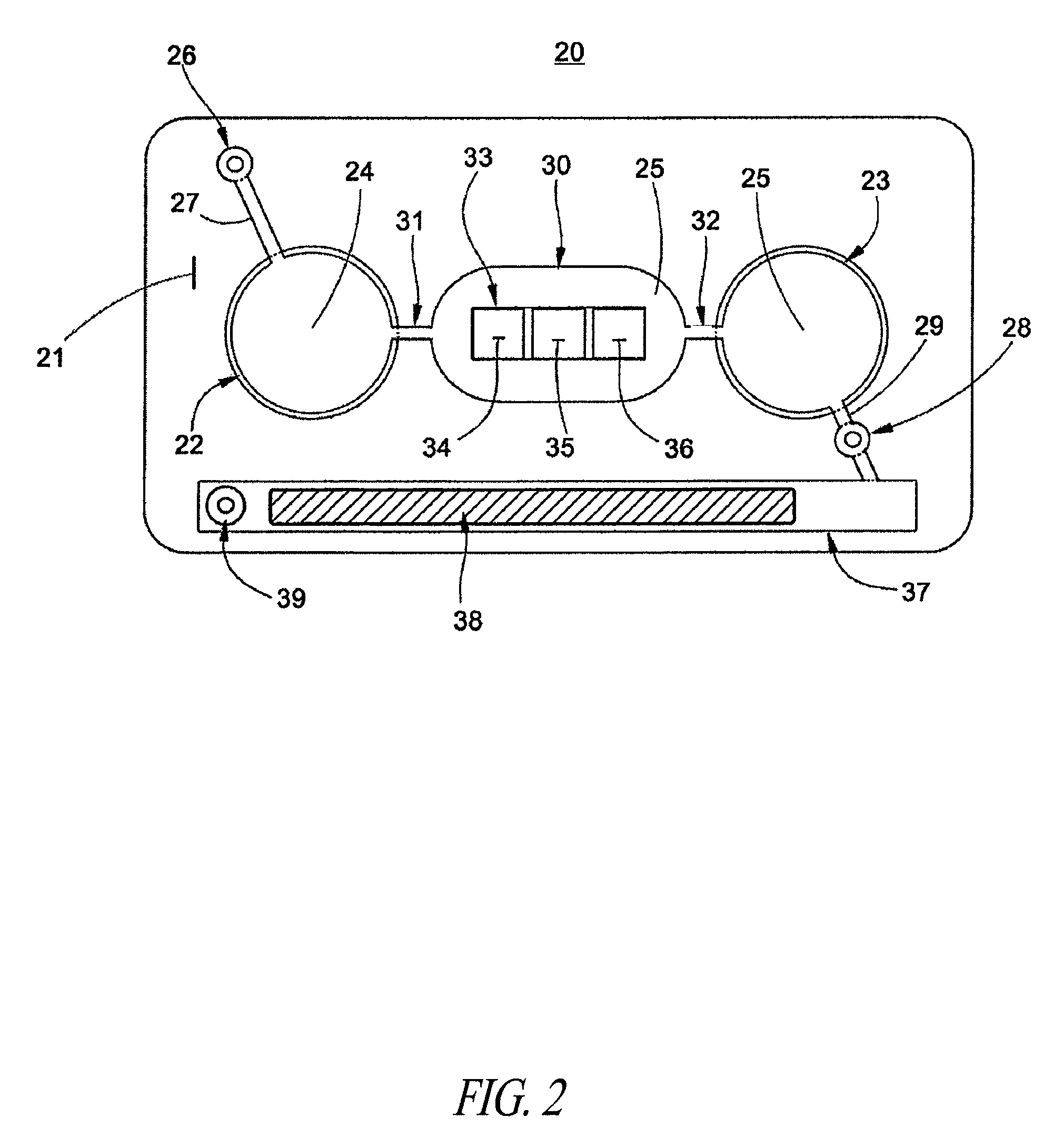
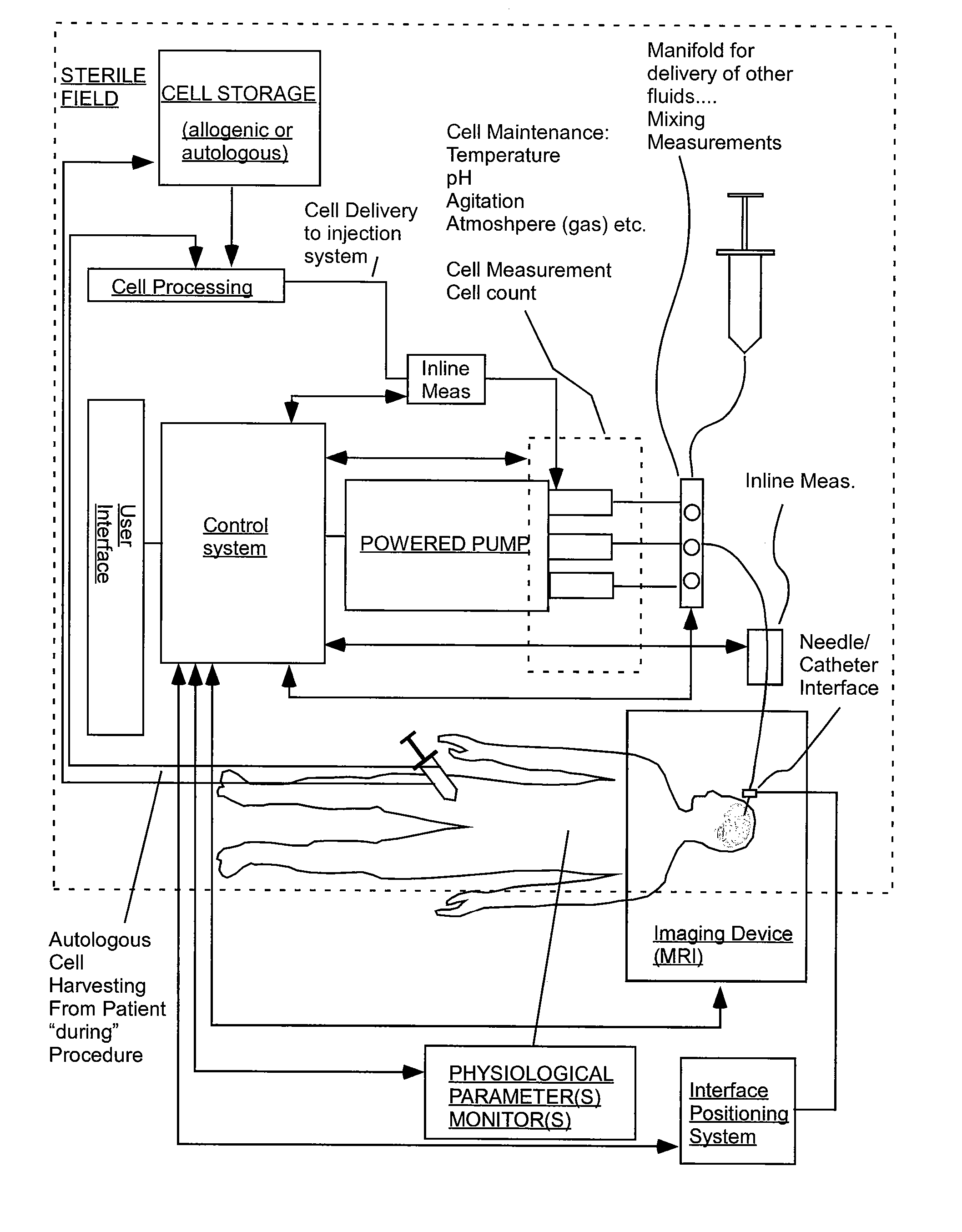
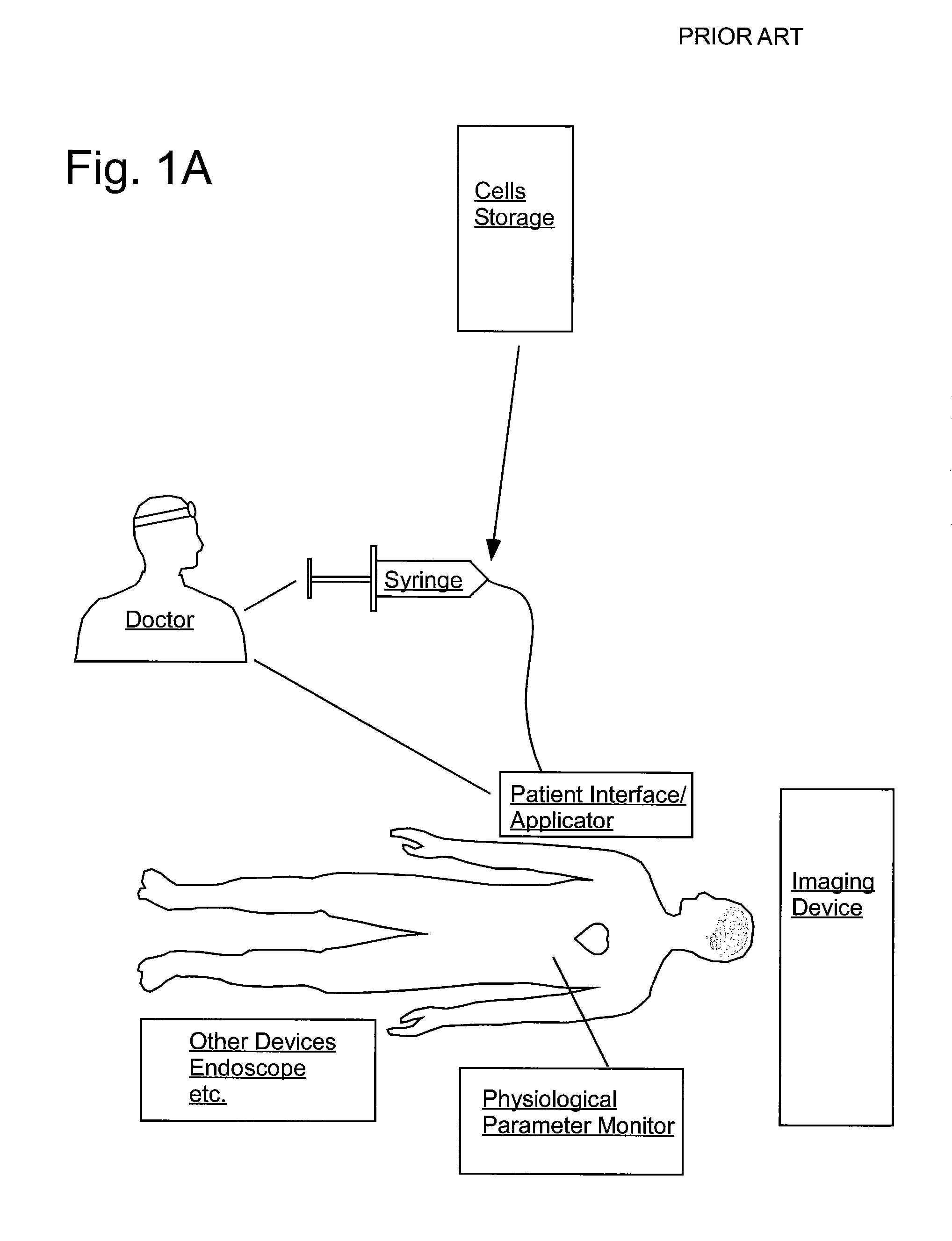
Delivery of agents to tissue
InactiveUS20070106208A1Limit localizationKeep sterileBioreactor/fermenter combinationsBiological substance pretreatmentsStereotactic localizationBiomedical engineering
A system for injecting an injectate into patient includes a first pressurizable container for holding the injectate; a patient interface in fluid connection with the first pressurizable container, the patient interface being adapted to pass the injectate into tissue of the patient; a powered injector in operative connection with the first pressurizable container to pressurize the injectate; a controller system in operative connection with powered injector; and a stereotactic localization frame adapted to be placed in operative connection with the patient interface to assist in controlling localization of the patient interface. A system for processing cells (and / or other injectate components) includes a container and a plunger adapted to be slidably positioned within the container. The system includes at least one inlet port through which a fluid can enter the system and at least one effluent port through which an effluent can exit the system. The plunger section forms a sealing engagement with the inner wall of the container such that rearward motion of the plunger is adapted to draw fluid into the system via the inlet and forward motion of the plunger is adapted to force effluent out of the system via the effluent port.
Owner:BAYER HEALTHCARE LLC +1
Highly efficient gas permeable devices and methods for culturing cells
ActiveUS20080227176A1Minimize potentialEasy to useBioreactor/fermenter combinationsBiological substance pretreatmentsCulture cellPolystyrene
Owner:WILSON WOLF MFG
Manufacturing within a single-use container
ActiveUS20160068793A1Function increaseProgramme-controlled manipulatorBioreactor/fermenter combinationsNumerical controlThree degrees of freedom
A manufacturing assembly has at least a sterilizable chamber containing at least one of a three-dimensional printing device (additive manufacturing), a Computer Numerical Controlled (CNC) finishing head (subtractive manufacturing), a vacuum-forming unit, an injection-molding unit, a laser-cutting unit, a ultrasonic-welding unit, a robotic arman analysis device, a sampling device or a combination thereof. A plurality of individual sterilizable chambers may be aseptically connected into a network of sterilizable chambers that provides additional functionality for the manufacturing assembly. A sterilizable printer assembly may include at least one printing head, a printing platform, and a driving mechanism adapted to perform a movement of the at least one printing head relative to the printing platform along three degrees of freedom; a printer housing enclosing the printer assembly in a sterile manner, at least one aseptic connector fluidly connected to a corresponding one of the at least one printing head.
Owner:SARTORIUS STEDIM BIOTECH GMBH
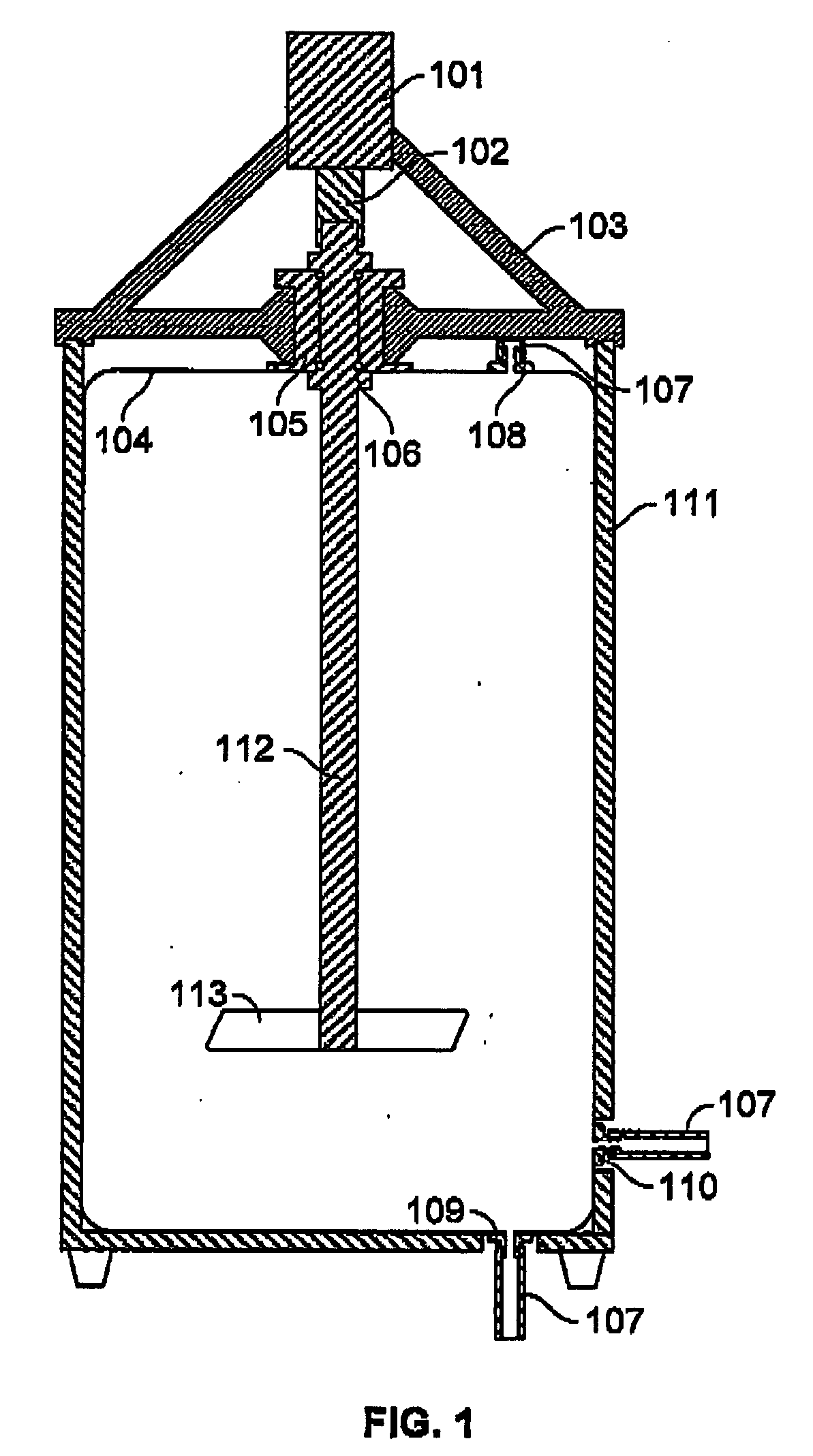
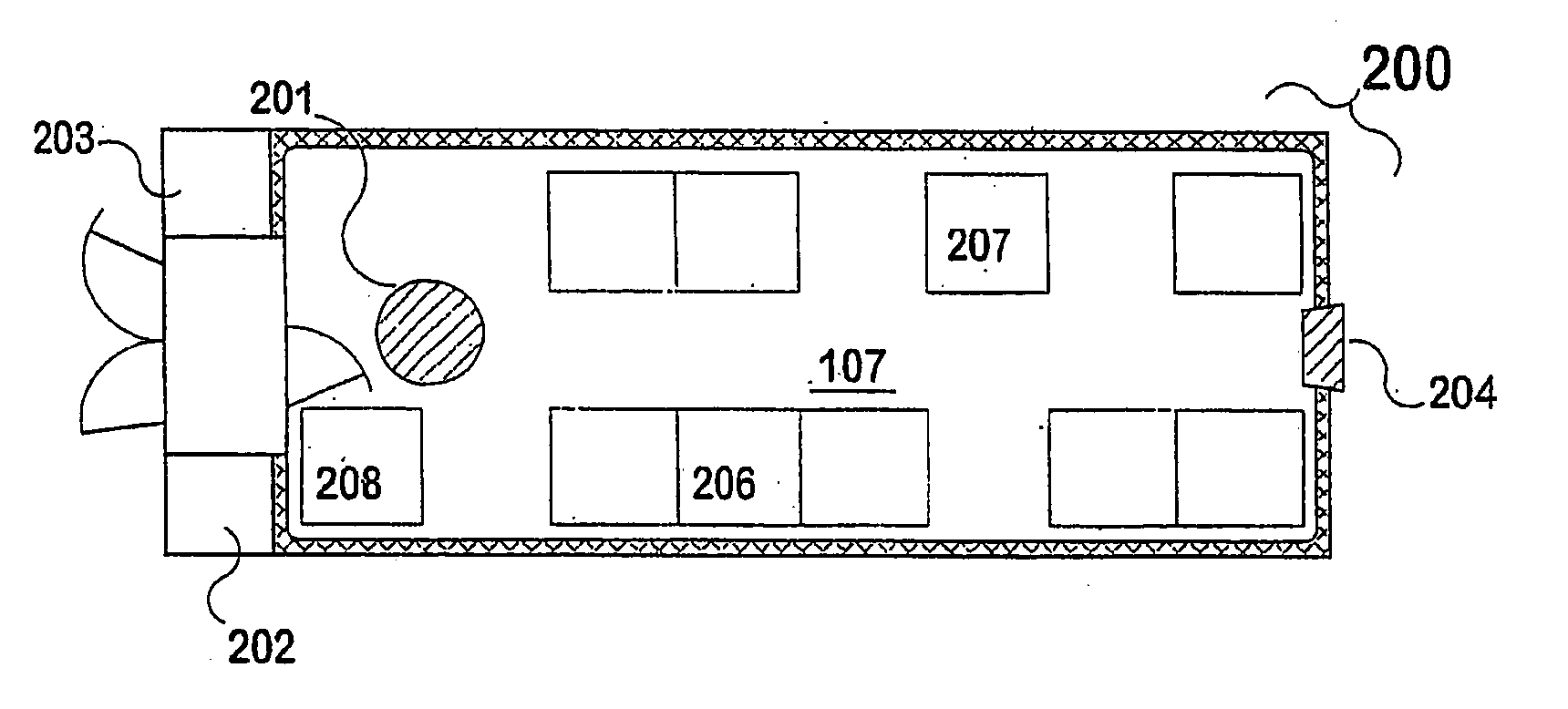
Modular containment unit
InactiveUS20050193643A1Inhibit exchangeInhibit transferMechanical apparatusApparatus sterilizationModularityWorking environment
A prefabricated, self-contained, standardised working environment that combines transport-useful physical standards for overall dimensions (a shipping container / cargo container) together with functional standards (such as specified clean air or biological containment standards) related to specific types of work to be carried out, and takes advantage of volume production at an assembly site with appropriate materials and expertise. Optionally, users are provided with “no-touch” control of items like doors or taps, and with management of supplies, event logging, instrument control, communications and the like through a network of computer peripherals and a control unit.
Owner:PETTUS DARYL OWEN
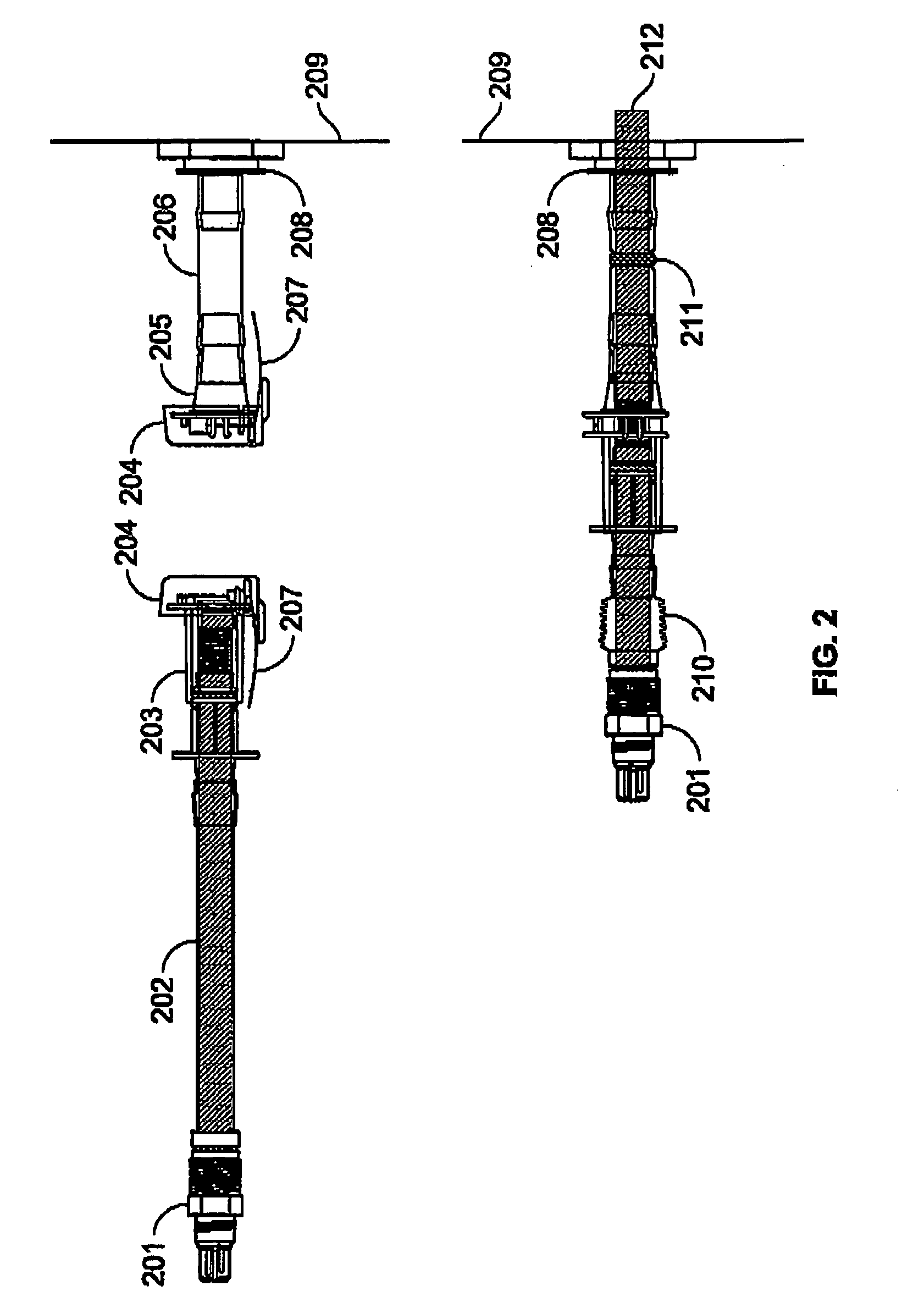
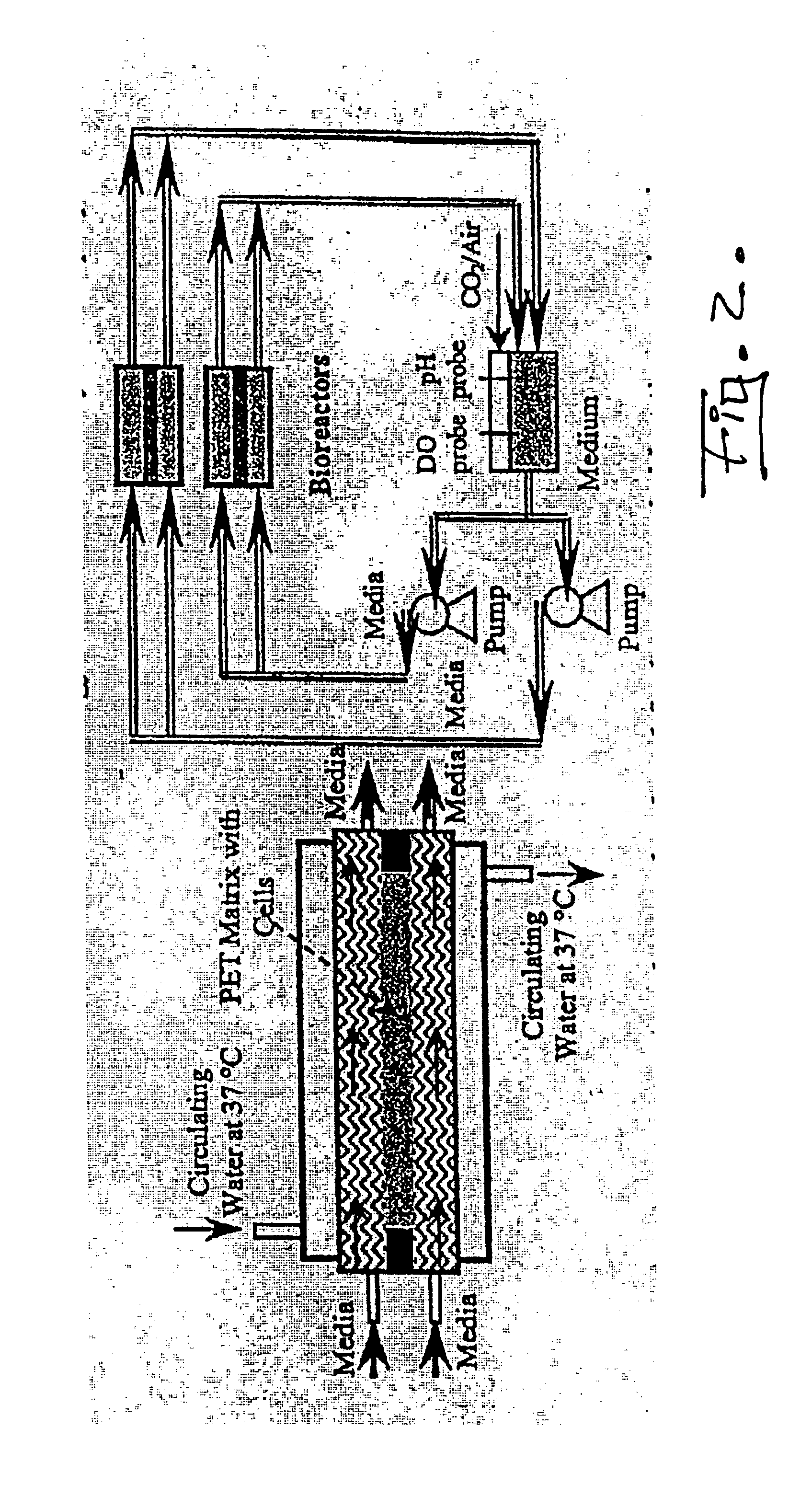
Modular cell culture bioreactor and associated methods
InactiveUS6875605B1Bioreactor/fermenter combinationsBiological substance pretreatmentsPolyethylene terephthalate3D cell culture
An apparatus and method for a modular cell culture bioreactor comprises a plurality of chambers for cell culture; at least one reservoir containing a cell support medium; a plurality of conduits fluidly connecting the at least one reservoir with the plurality of chambers; and at least one pump fluidly connected through the plurality of conduits with the at least one reservoir and with the plurality of chambers to pump cell support medium therethrough; wherein each individual chamber of the plurality of chambers includes at least one three-dimensional matrix comprising polyethylene terephthalate, a plurality of channels carrying the cell support medium and having the matrix positioned in fluid communication therebetween, and at least two openings into each the channel, wherein a first the opening is in fluid connection with the pump and a second the opening is in fluid connection with the reservoir.
Owner:FLORIDA STATE UNIV RES FOUND INC
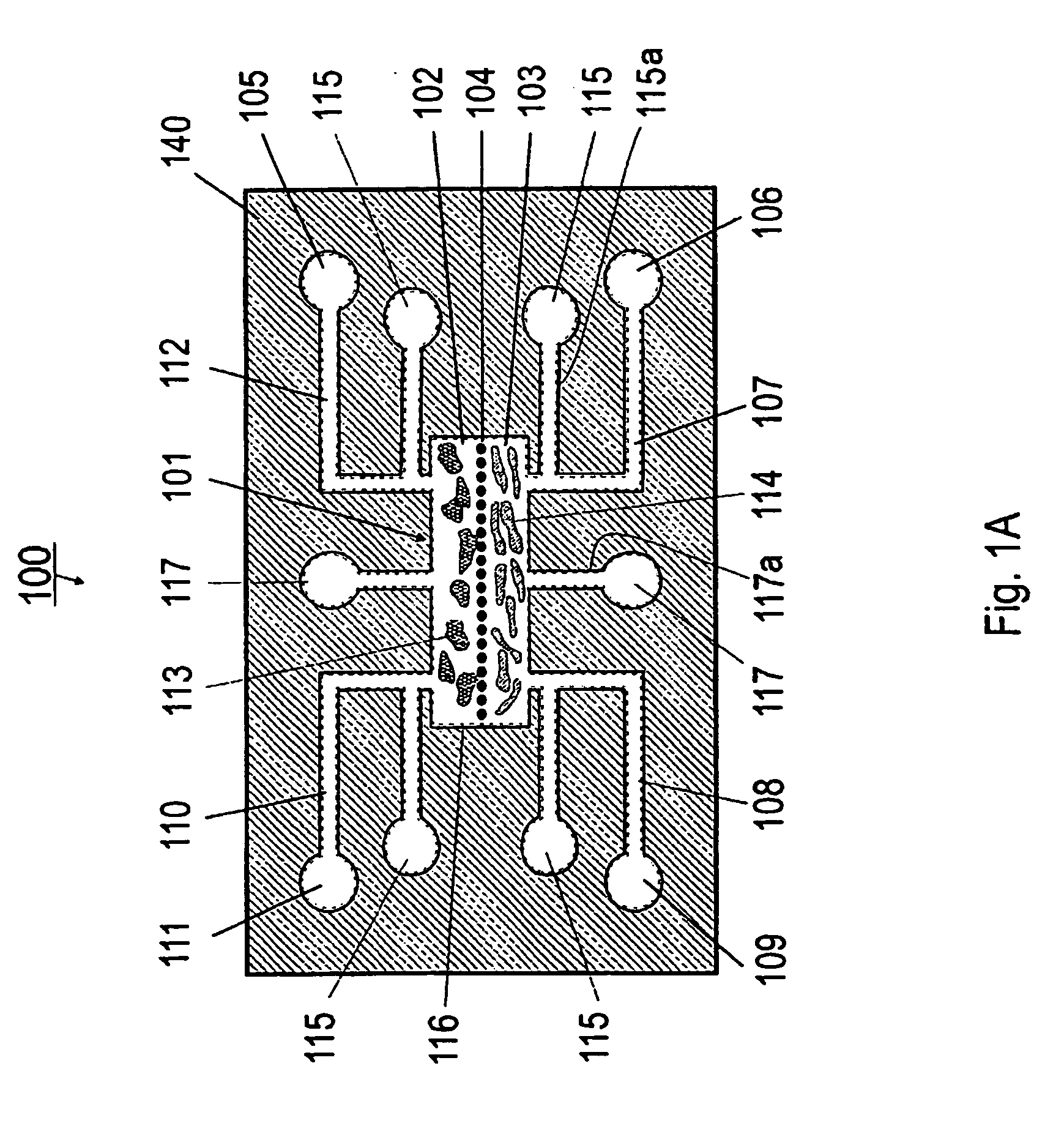
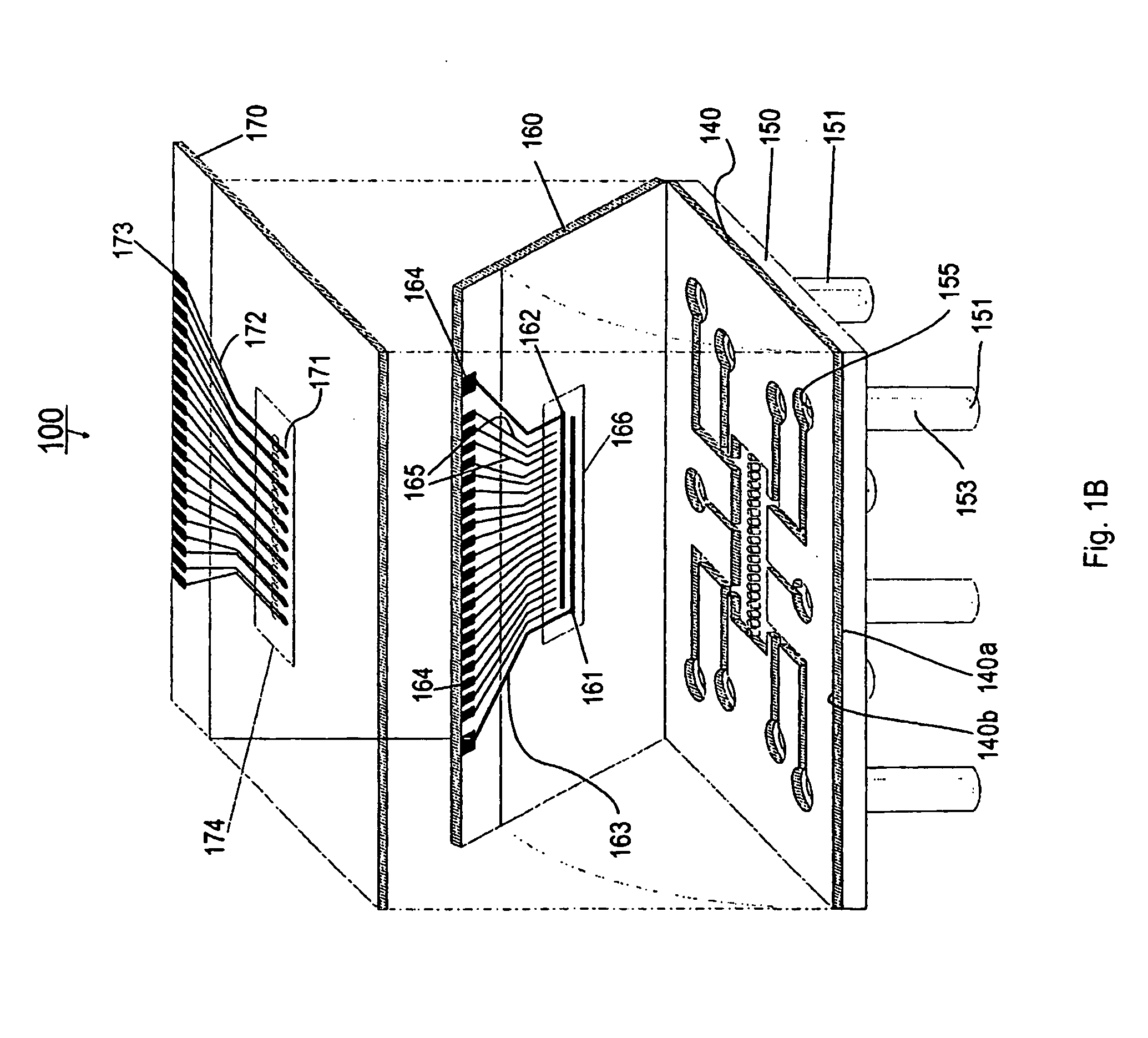
In vitro cell culture employing a fibrin network in a flexible gas permeable container
InactiveUS20050032205A1Convenient to accommodateBioreactor/fermenter combinationsBiological substance pretreatmentsCellular respirationFibrin matrix
This invention relates to in vitro cell culture employing a fibrin network in a flexible gas permeable container. Specifically, the invention is directed to a cell culture container comprising a flexible, gas permeable material with fibrin matrix which is conducive to the culture of anchorage dependent cells, and the container is suitable for use in closed system in vitro cell culture. The gas permeability of the container is sufficient to permit cellular respiration.
Owner:BAXTER INT INC
Rapid cell block embedding method and apparatus
ActiveUS6913921B2Maximize efficiencyReduce amountBioreactor/fermenter combinationsBiological substance pretreatmentsEngineeringReagent
A method and apparatus for embedding cells that utilizes a flow-through embedding technique maximizes the efficiency of extractions and decreases time for embedding the cell fragments, minimizes cell loss, and automatically positions cell samples at the position in which a microtome blade will section them. The apparatus includes a cell flow pathway defined by an inflow tube for delivering cell fragments from a cell sample to a sample port. The sample port is in fluid communication with a tissue cassette having attached thereto a filter. The cell flow pathway is in communication with a reagent flow pathway for delivering the reagents through the sample port to the cassette. The apparatus is configured such that the application of pressure directs the cell fragments from the cell sample through the cell flow pathway, and effects delivery of the reagents through the reagent flow pathway. The apparatus produces an embedded cell block having concentrated cells near the plane of the block to be sectioned in a quick and efficient manner.
Owner:MASSACHUSETTS UNIV OF
Novel bioreactor
InactiveUS20090130704A1Avoid pollutionBioreactor/fermenter combinationsBiological substance pretreatmentsBiological bodyHydrogen
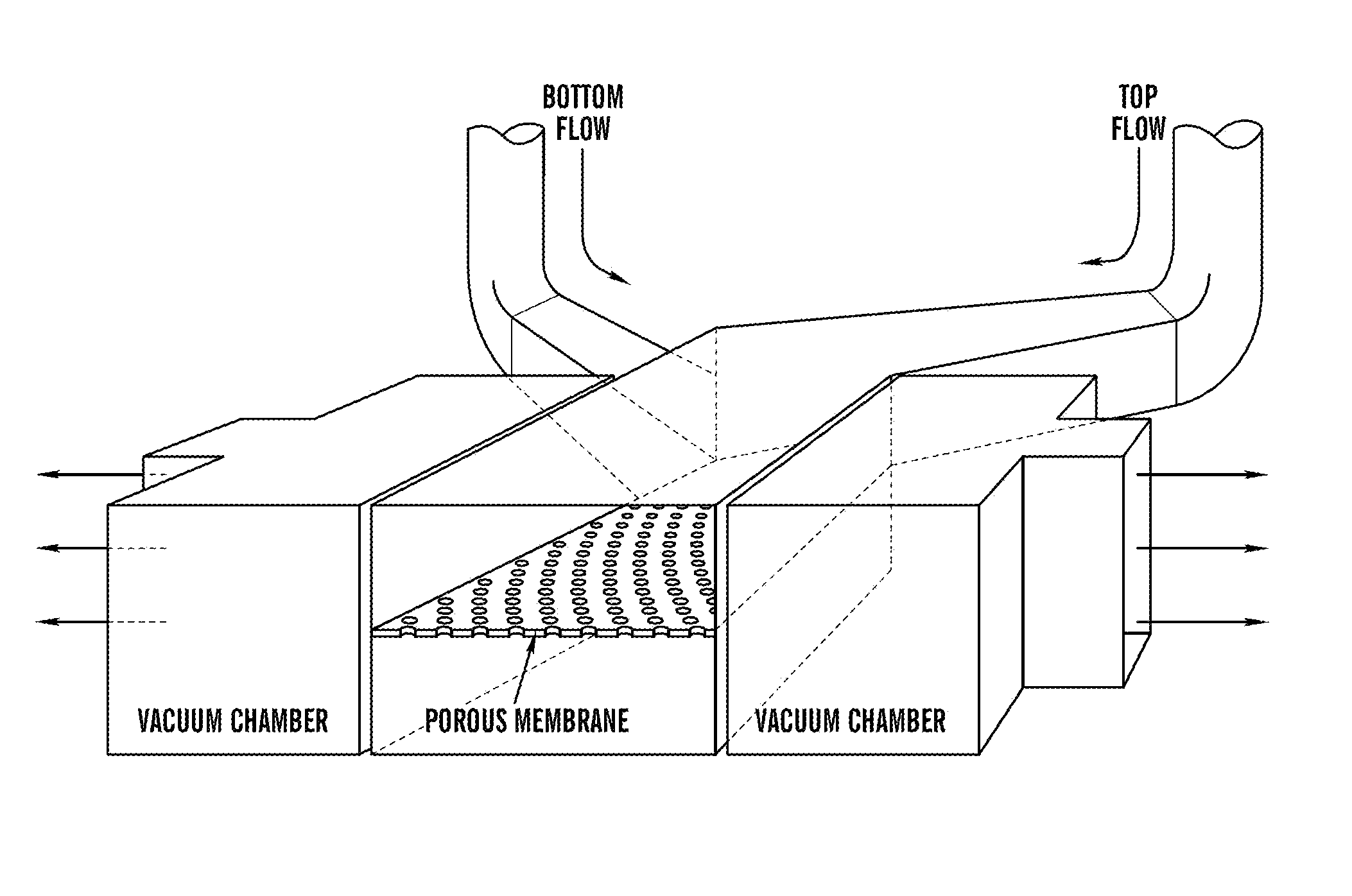
This invention provides bioreactors having a selectively permeable porous material with an open pore structure, useful for producing products including hydrogen gas, biomass, chemicals, and pharmaceuticals. The porous materials are utilized, for example, as one or more portions of or entire walls, covers, floors, filters, windows, or tubes of the bioreactors. This invention provides bioreactors comprising porous materials that are aerogels, xerogels, or sol-gel glasses, including silica aerogels. The selectively permeable porous materials are gas-permeable, and in addition optionally photopermeable, transparent, hydrophobic, and / or capable of functioning as sterile barriers. This invention provides methods for culturing cells and organisms employing the bioreactors of the invention. This invention further provides methods for producing gaseous products, including hydrogen, biomass, chemicals, and pharmaceuticals employing the bioreactors of the invention.
Owner:GYURE DALE C
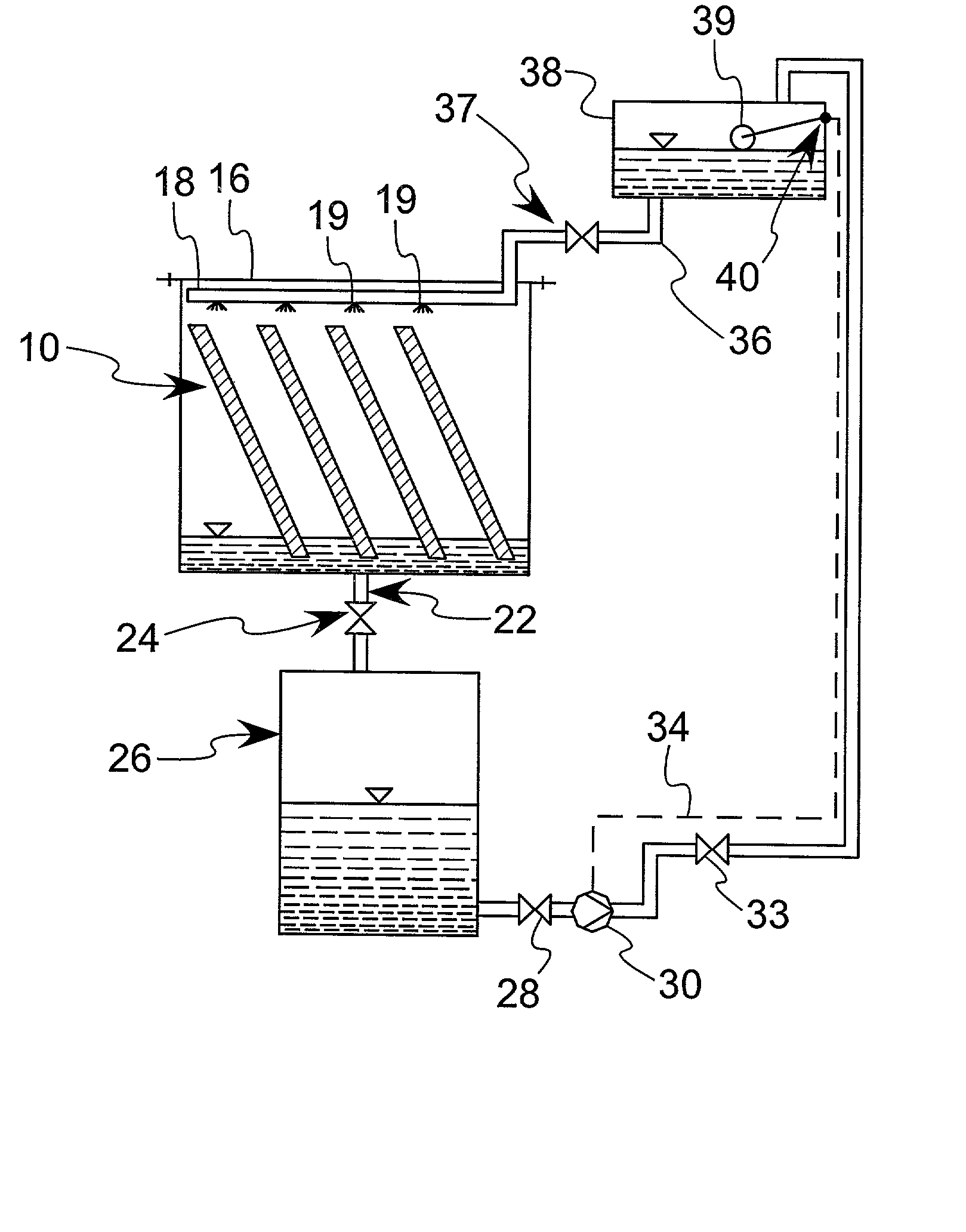
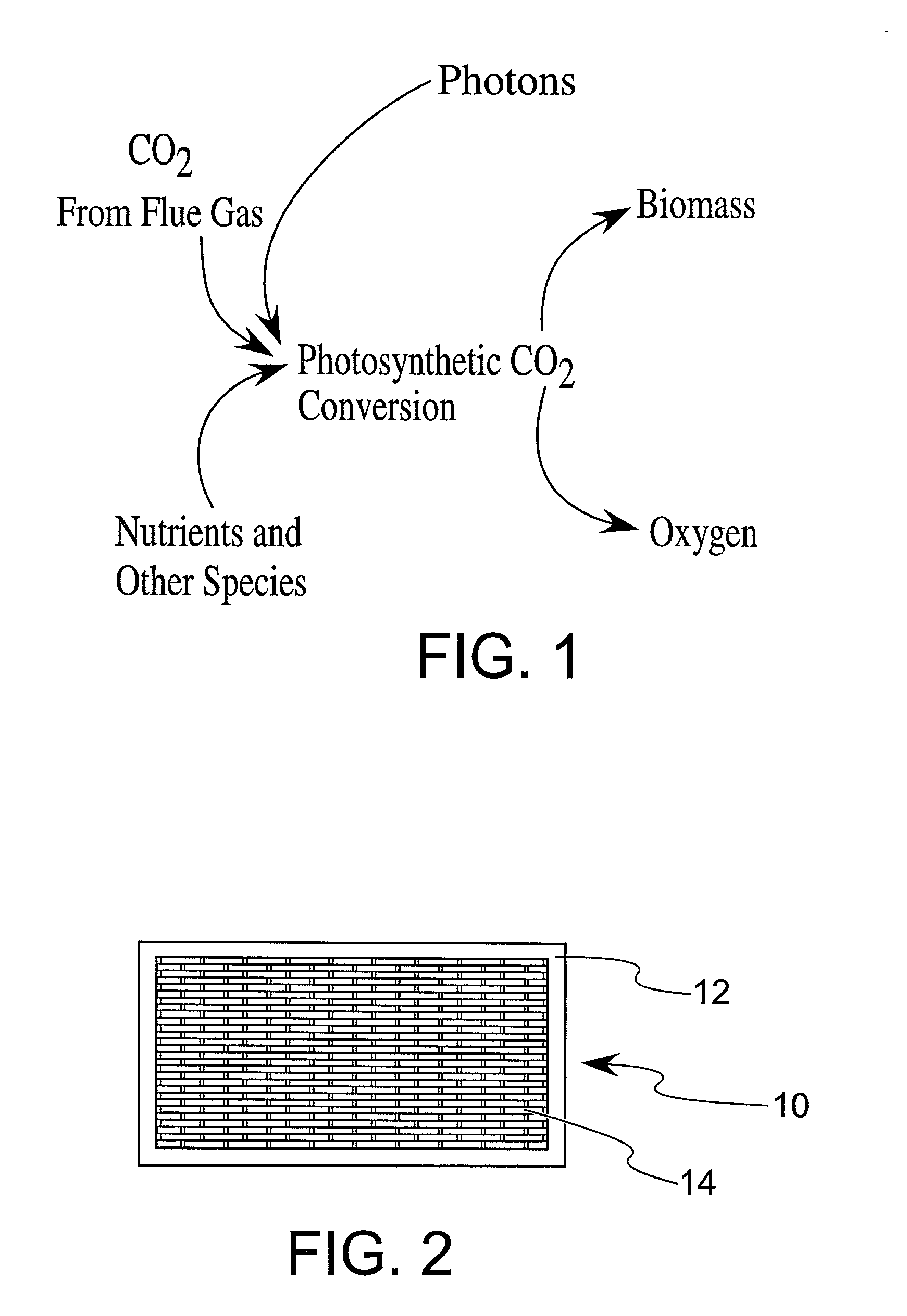
Enhanced practical photosynthetic CO2 mitigation
InactiveUS20020072109A1Increase the amount of waterBioreactor/fermenter combinationsBiological substance pretreatmentsMicroorganismCyanobacteria
This process is unique in photosynthetic carbon sequestration. An on-site biological sequestration system directly decreases the concentration of carbon-containing compounds in the emissions of fossil generation units. In this process, photosynthetic microbes are attached to a growth surface arranged in a containment chamber that is lit by solar photons. A harvesting system ensures maximum organism growth and rate of CO2 uptake. Soluble carbon and nitrogen concentrations delivered to the cyanobacteria are enhanced, further increasing growth rate and carbon utilization.
Owner:OHIO UNIV
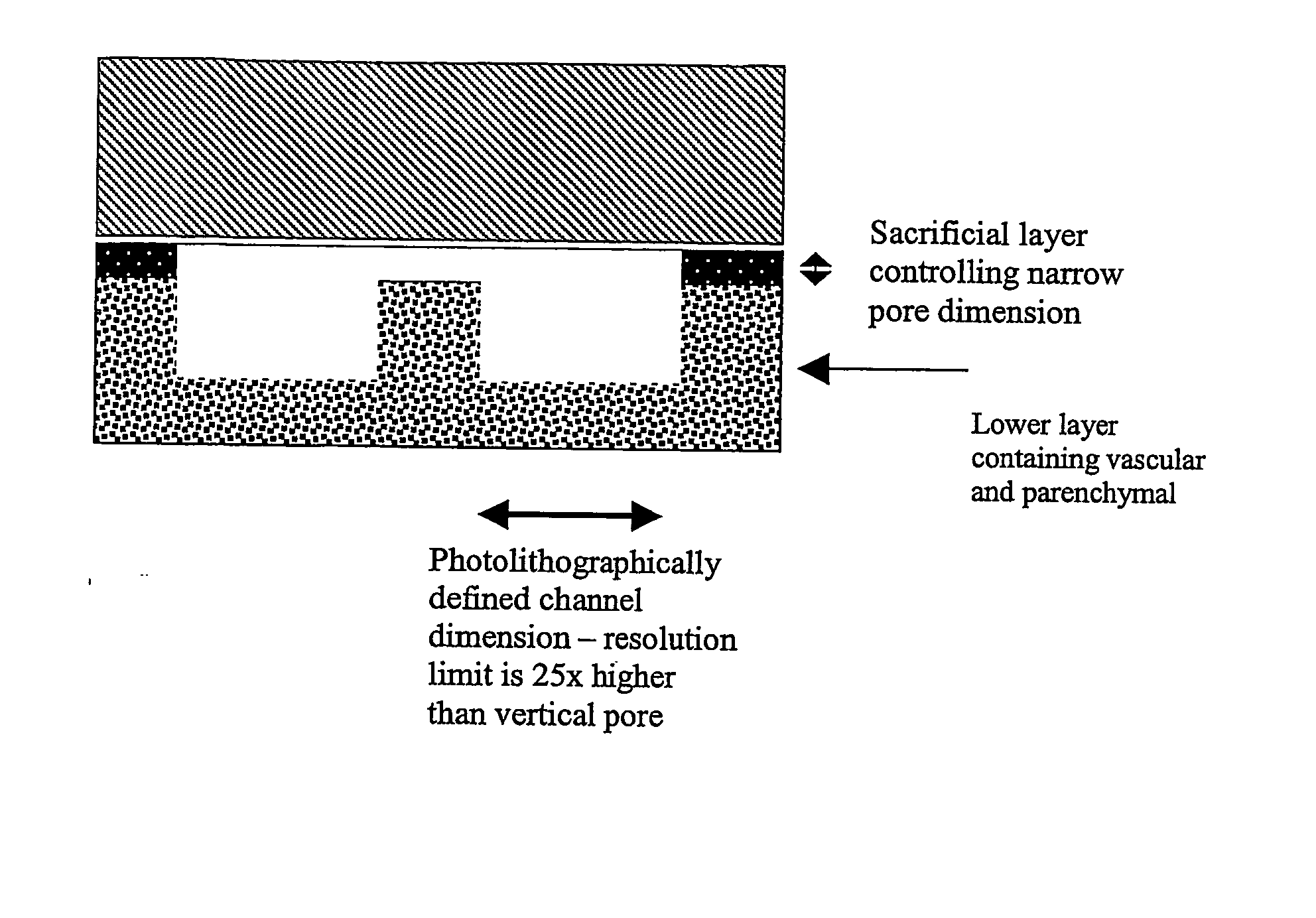
Microfabricated Compositions and Processes for Engineering Tissues Containing Multiple Cell Types
ActiveUS20070281353A1Reduce complexityLower the volumeBioreactor/fermenter combinationsBiological substance pretreatmentsEngineering tissueCell type
The present invention relates to a three-dimensional system, and compositions obtained therefrom, wherein individual layers of the system comprise channels divided longitudinally into two compartments by a centrally positioned membrane, and wherein each compartment can comprise a different cell type.
Owner:CHARLES STARK DRAPER LABORATORY +1
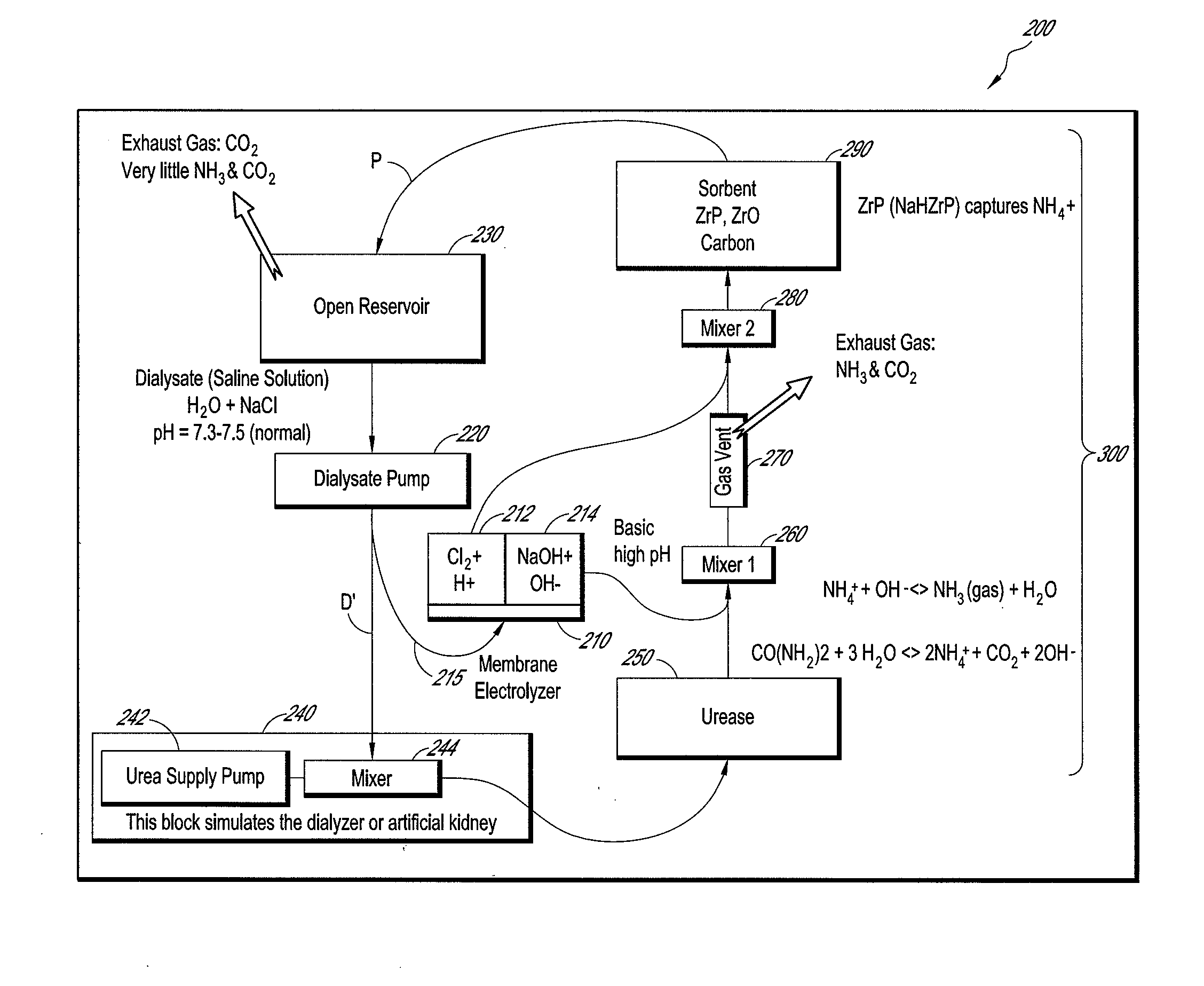
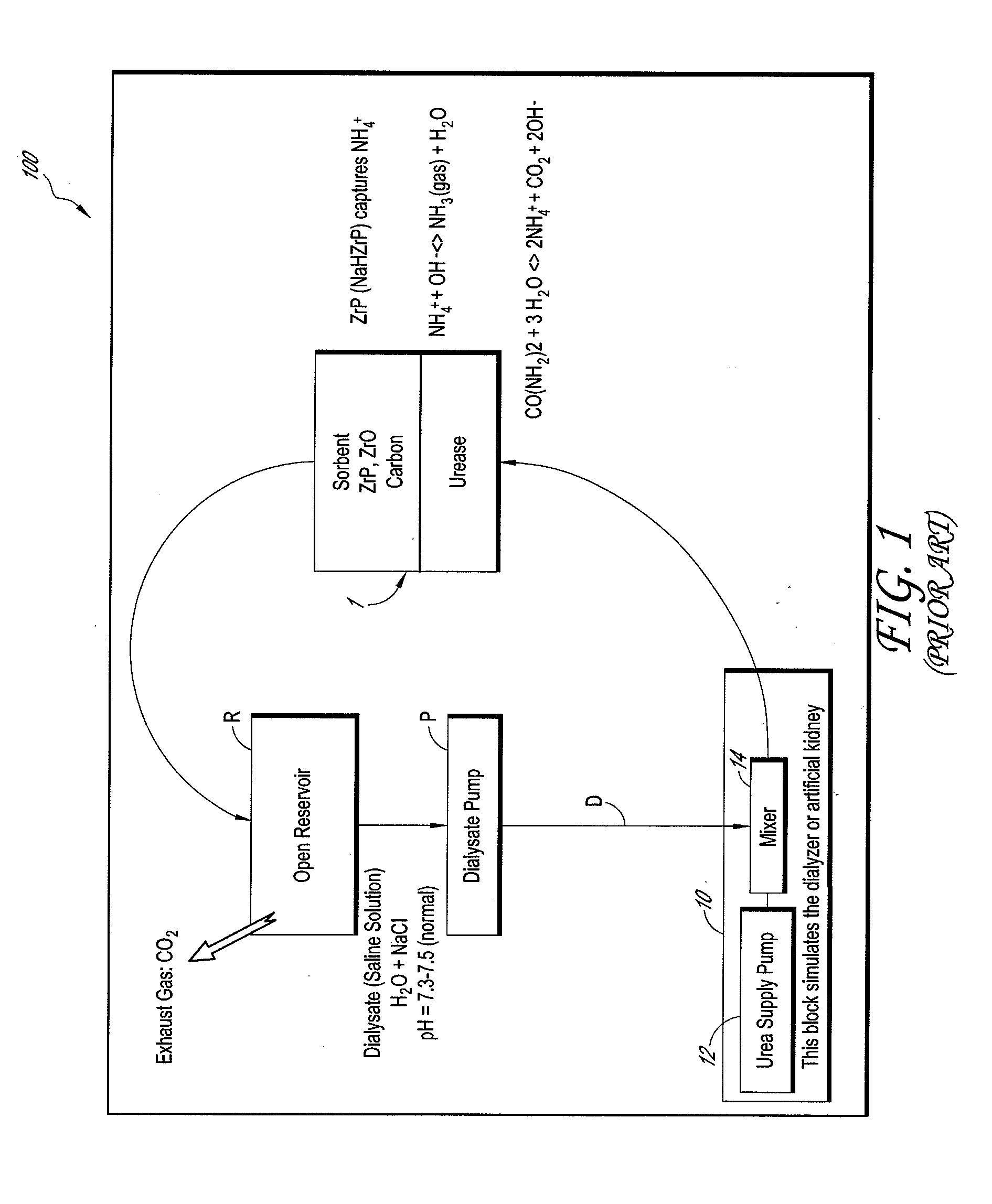
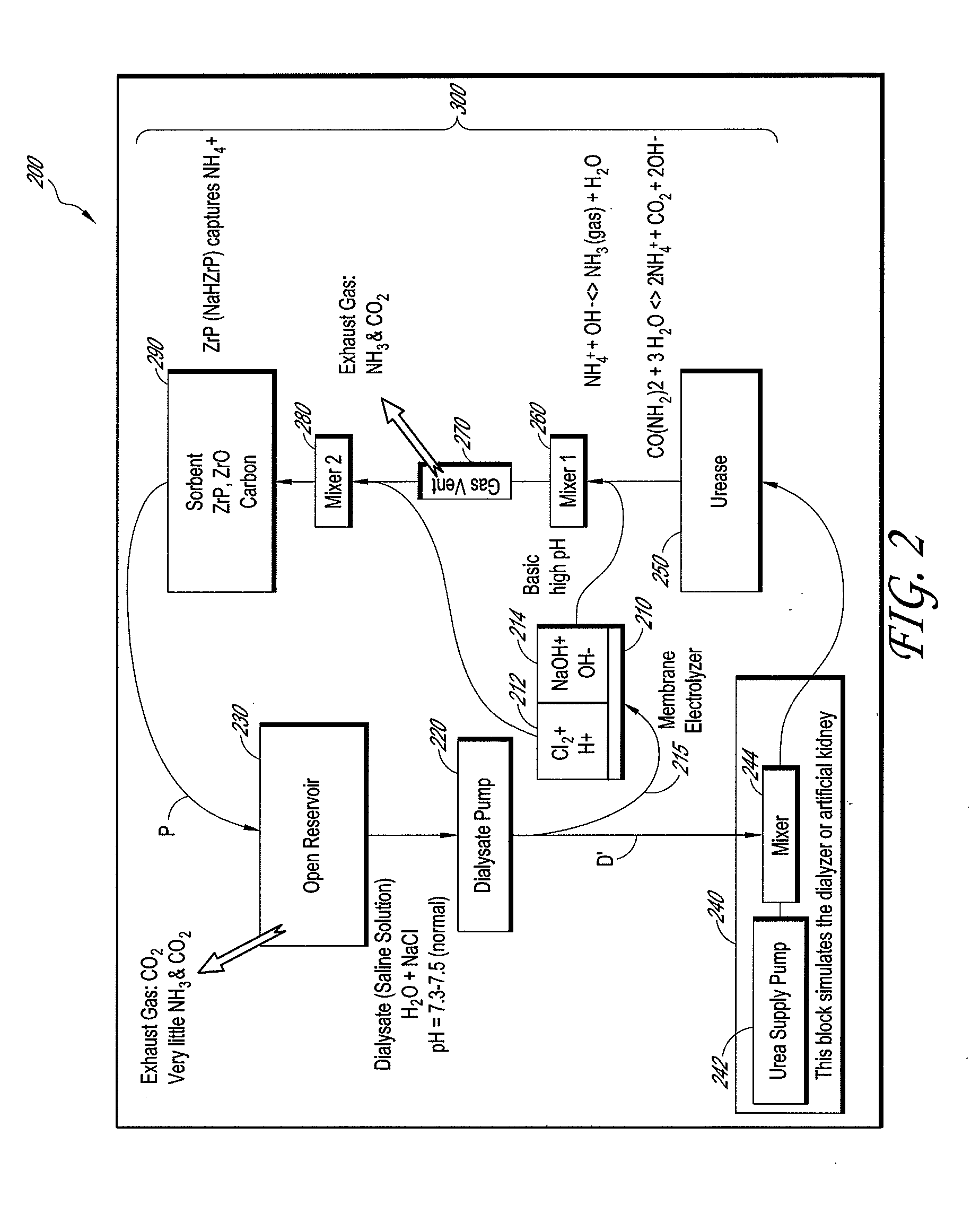
Membrane electrolyzer and hemodialysis system using the same
InactiveUS20110272352A1Raise the pHElectrolysis componentsApparatus sterilizationSorbentHaemodialysis machine
A sorbent hemodialysis system includes a dialyzer configured to receive a flow of clean dialysate from a reservoir and to output an unclean dialysate flow. The system also includes a sorbent component having a urease section and a sorbent section through which the unclean dialysate flow from the dialyzer passes, wherein the sorbent component removes urea from the dialysate. The system further comprises a membrane electrolyzer that receives at least a portion of said clean dialysate flow and separates the dialysate flow into an acidic component flow and a base component flow. A mixing conduit combines the base component flow from the membrane electrolyzer and an output dialysate solution from the urease section of the sorbent component to separate the dialysate solution into an ammonia gas amount and ammonia liquid amount. A gas vent is used to vent the ammonia gas amount, and the sorbent section with a suitable amount of zirconium phosphate (ZrP) removes the ammonia liquid amount from the unclean dialysate flow before flowing the clean dialysate to the reservoir. The system can further include a second mixing conduit upstream of the sorbent section of the sorbent component, the second mixing conduit combining the acidic component flow and the ammonia liquid amount in the dialysate solution to increase the pH of the dialysate solution to about 7.5 prior to returning to the reservoir.
Owner:C TECH BIOMEDICAL
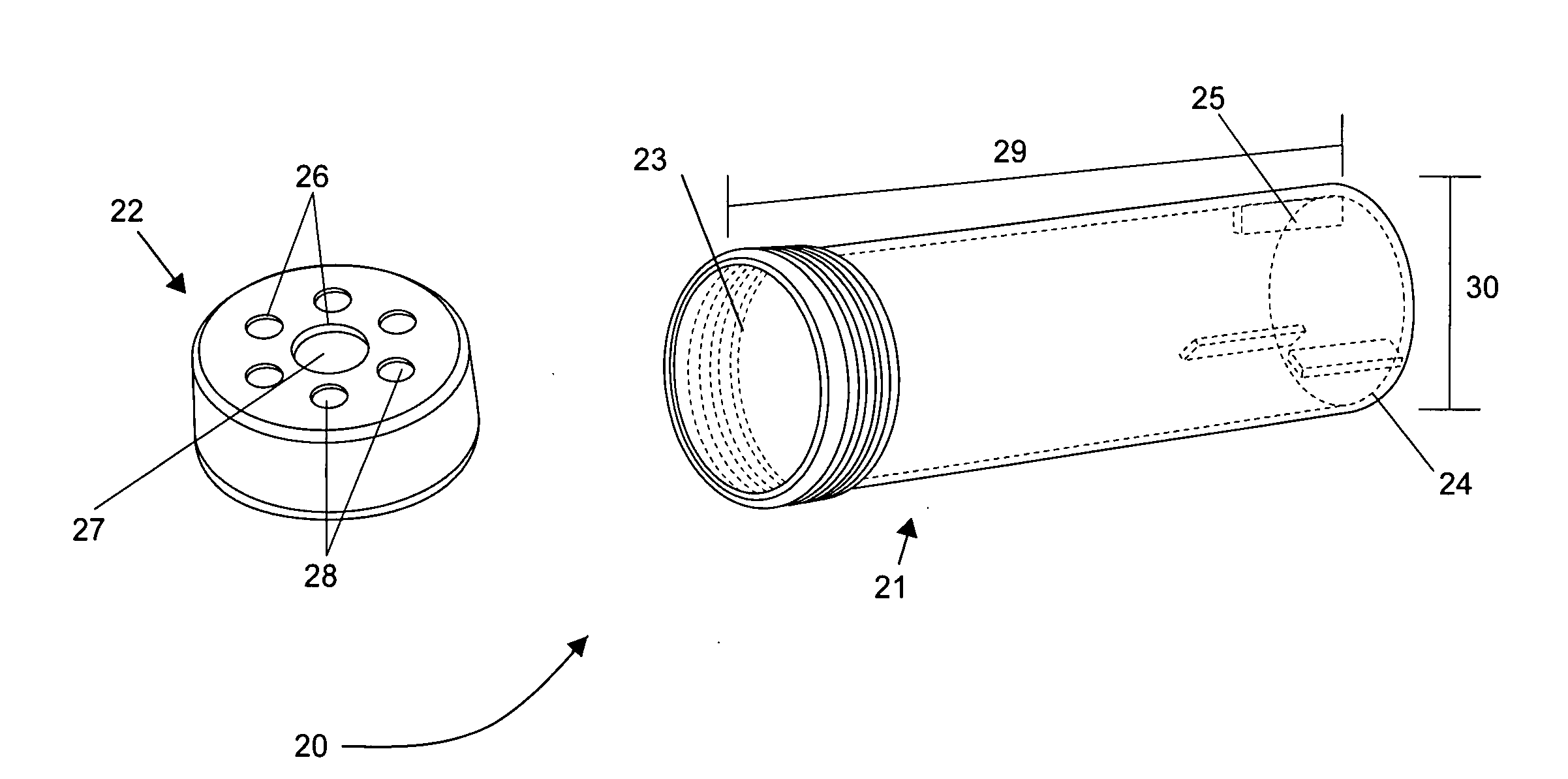
Disposable mini-bioreactor device and method
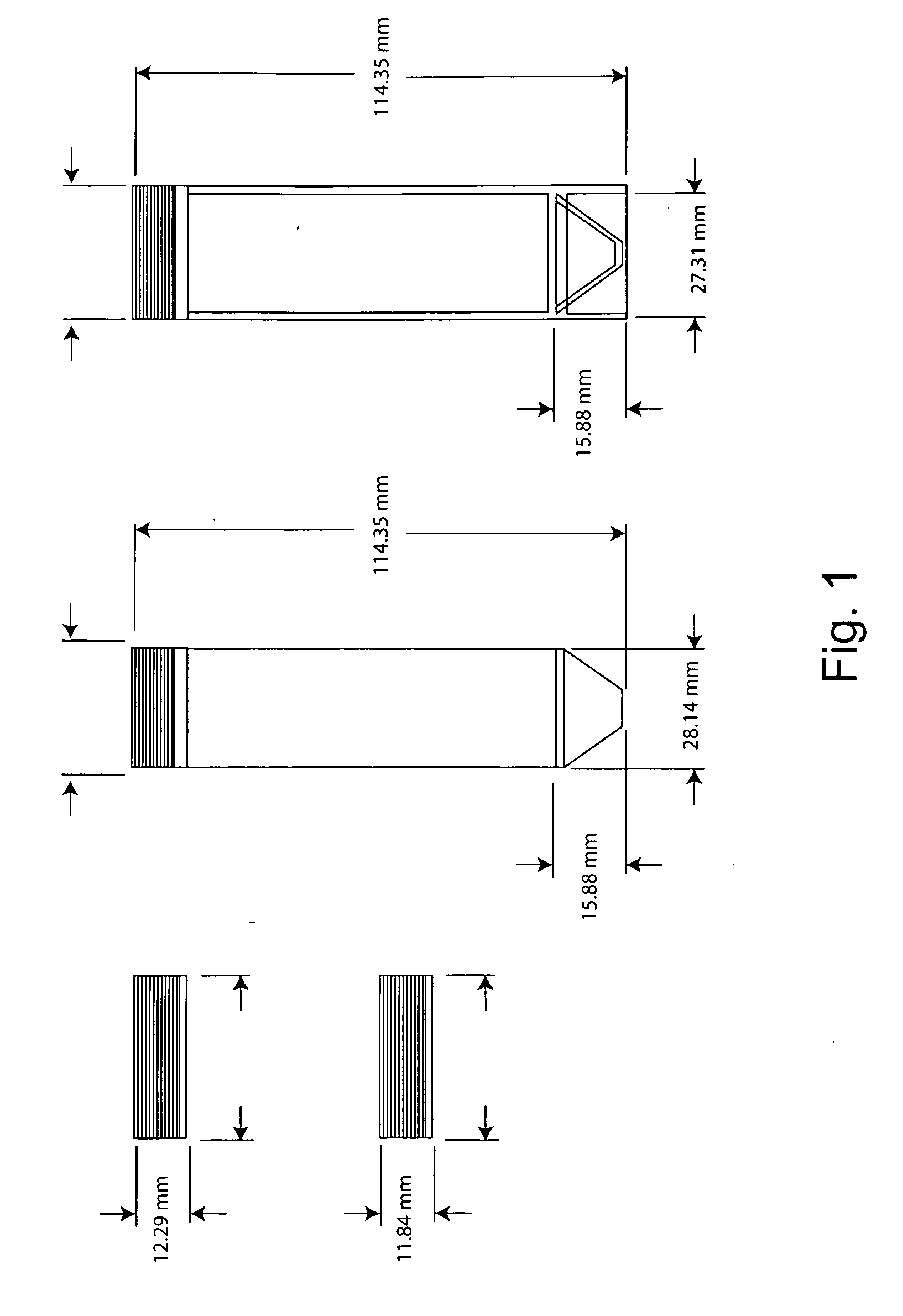
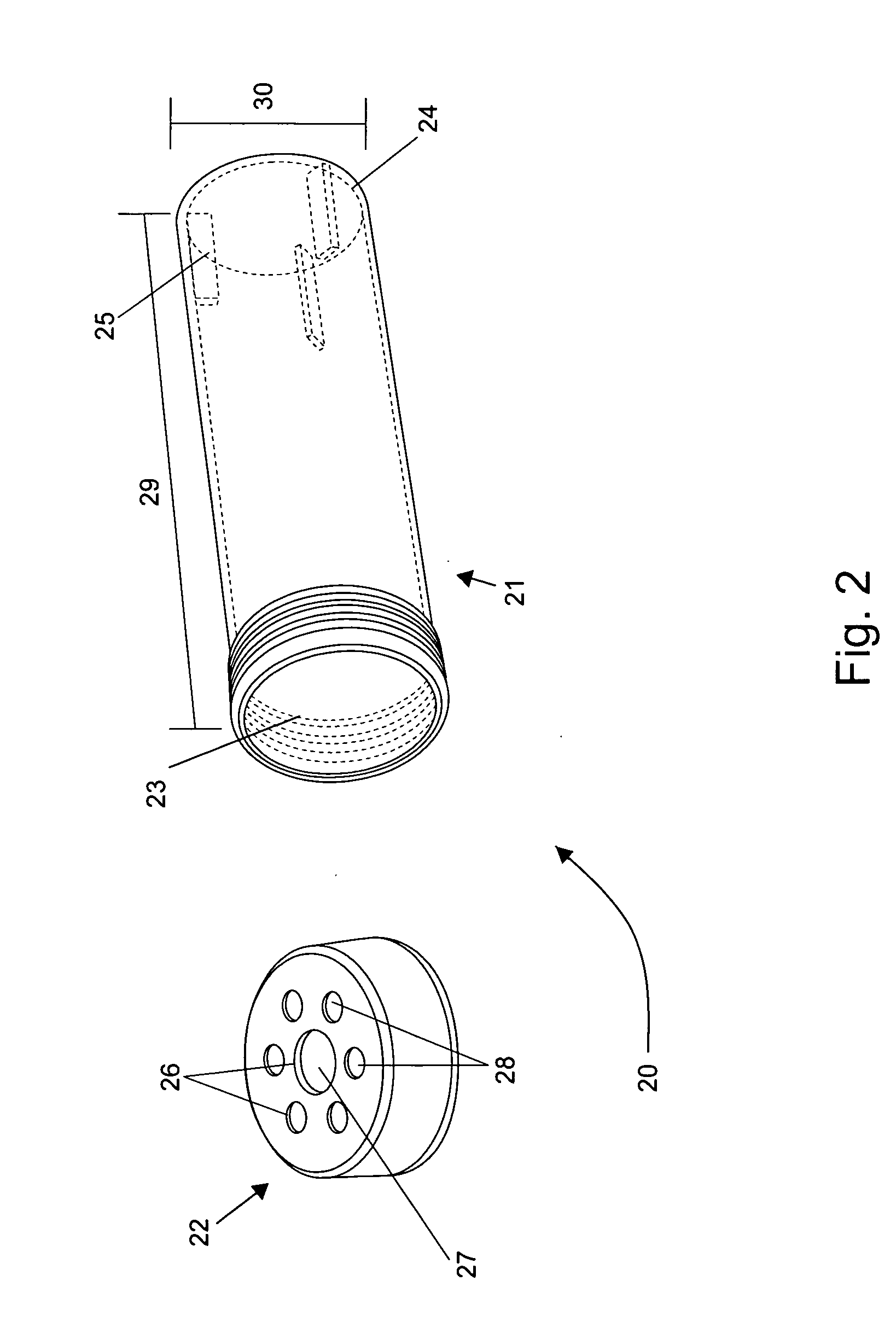
InactiveUS20090148941A1Quick testInhibition releaseBioreactor/fermenter combinationsCapsCulture cellGas exchange
This invention provides cylindrical cell culture tubes with a cap having both a septum and gas exchange membranes. The culture tubes can be used to inoculate media, culture cells, harvest cells and store cells in the same container with reduced risk of contamination, while facilitating automated handling.
Owner:OPTIMUM PROCESSING +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com