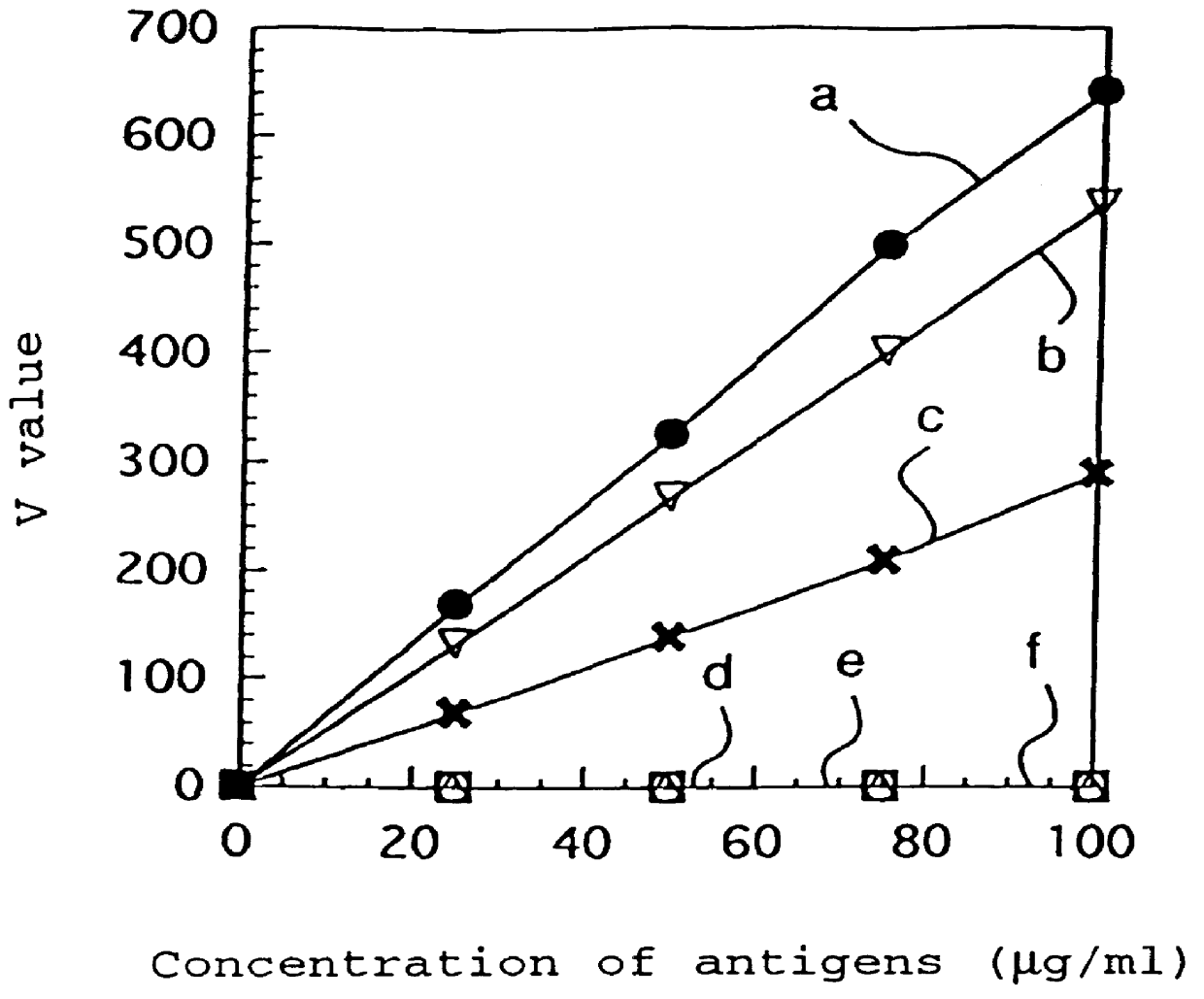
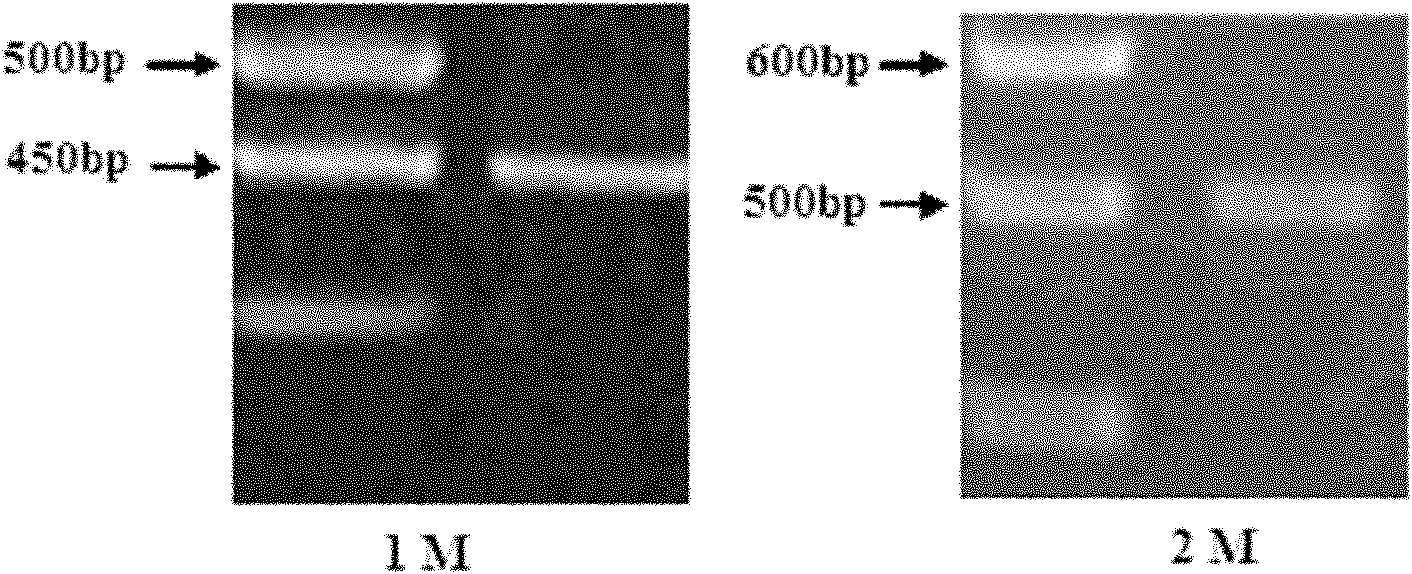
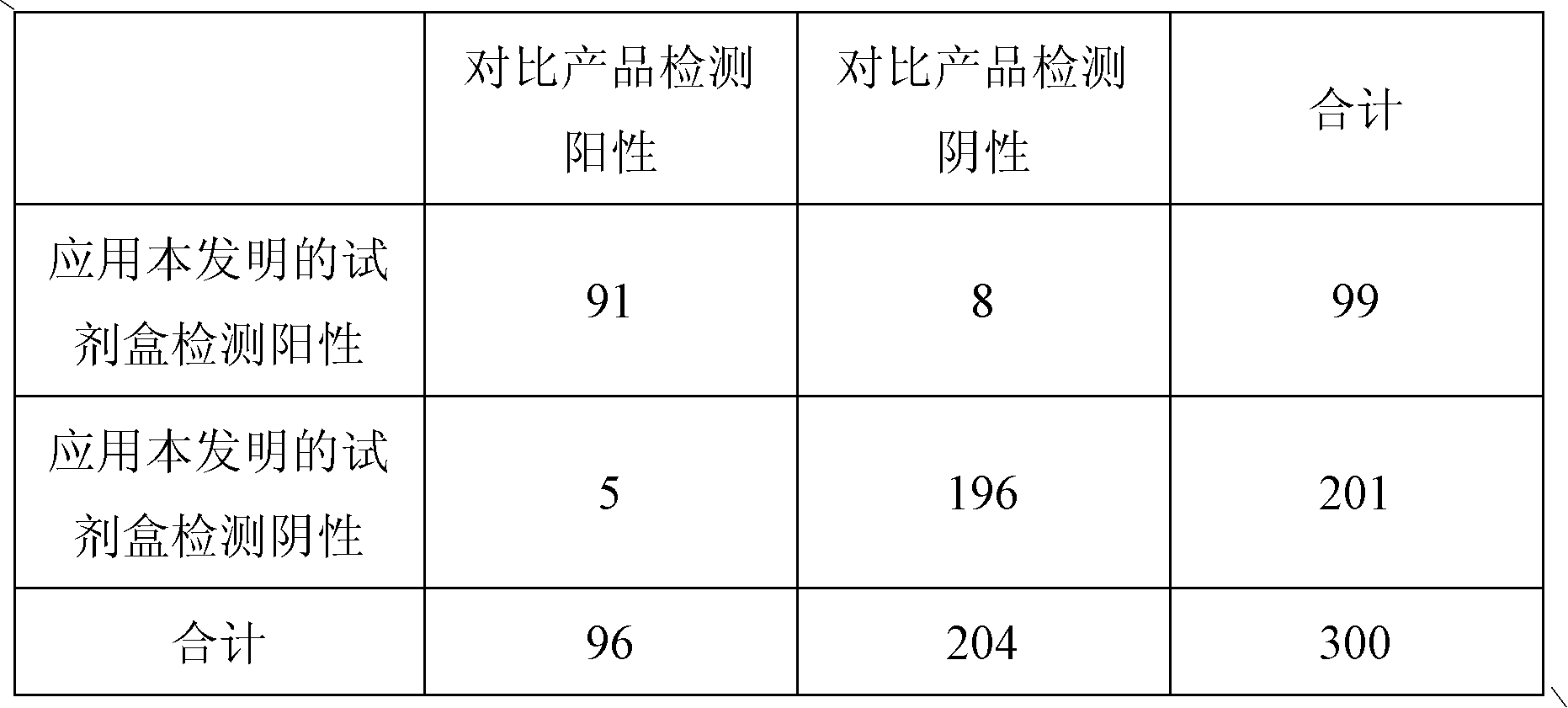
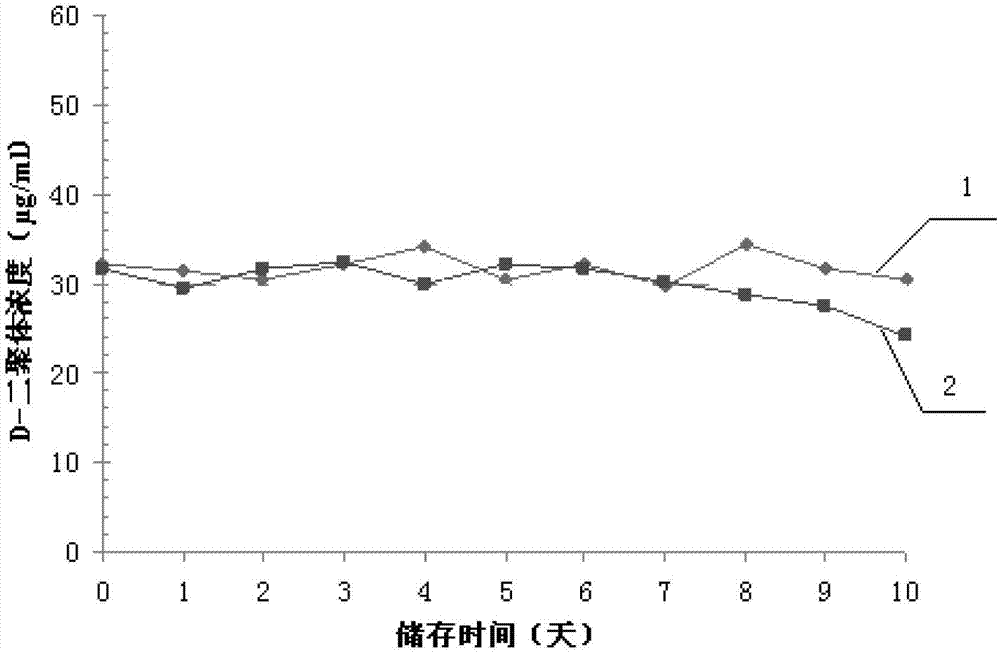
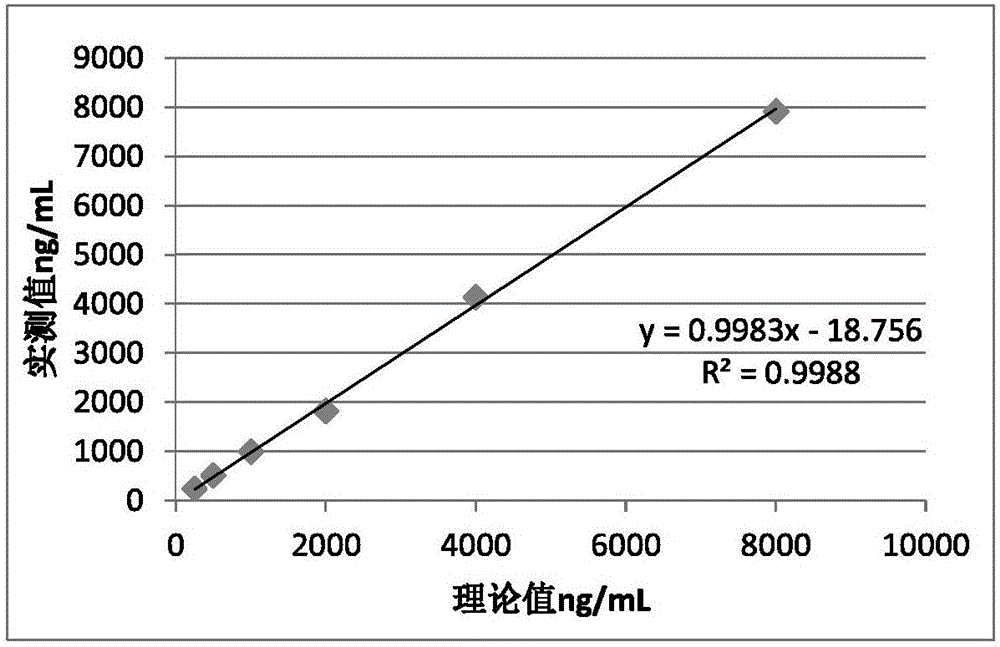
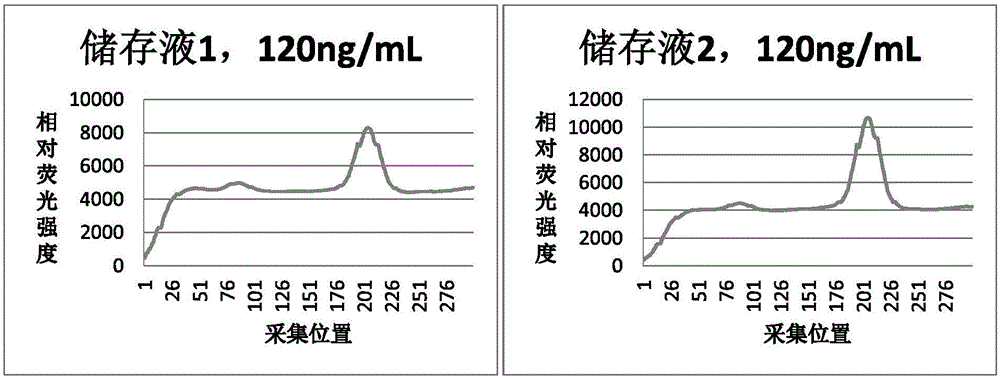
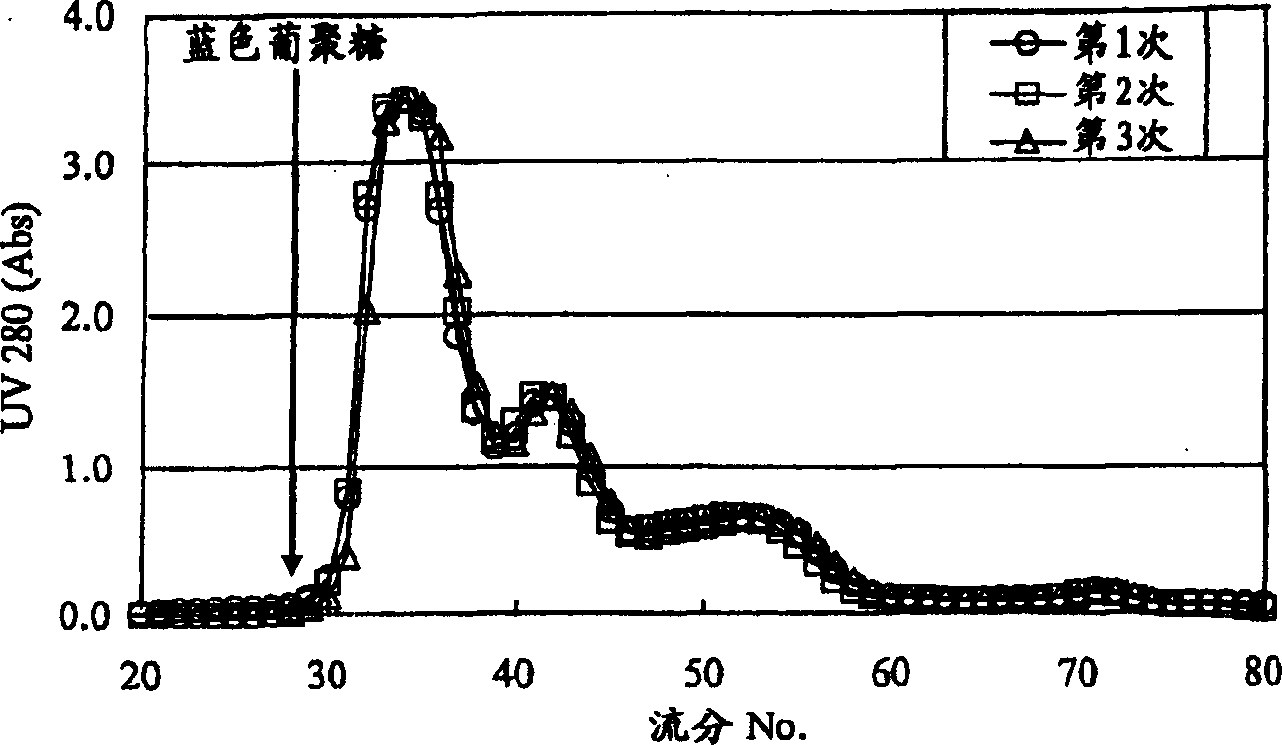
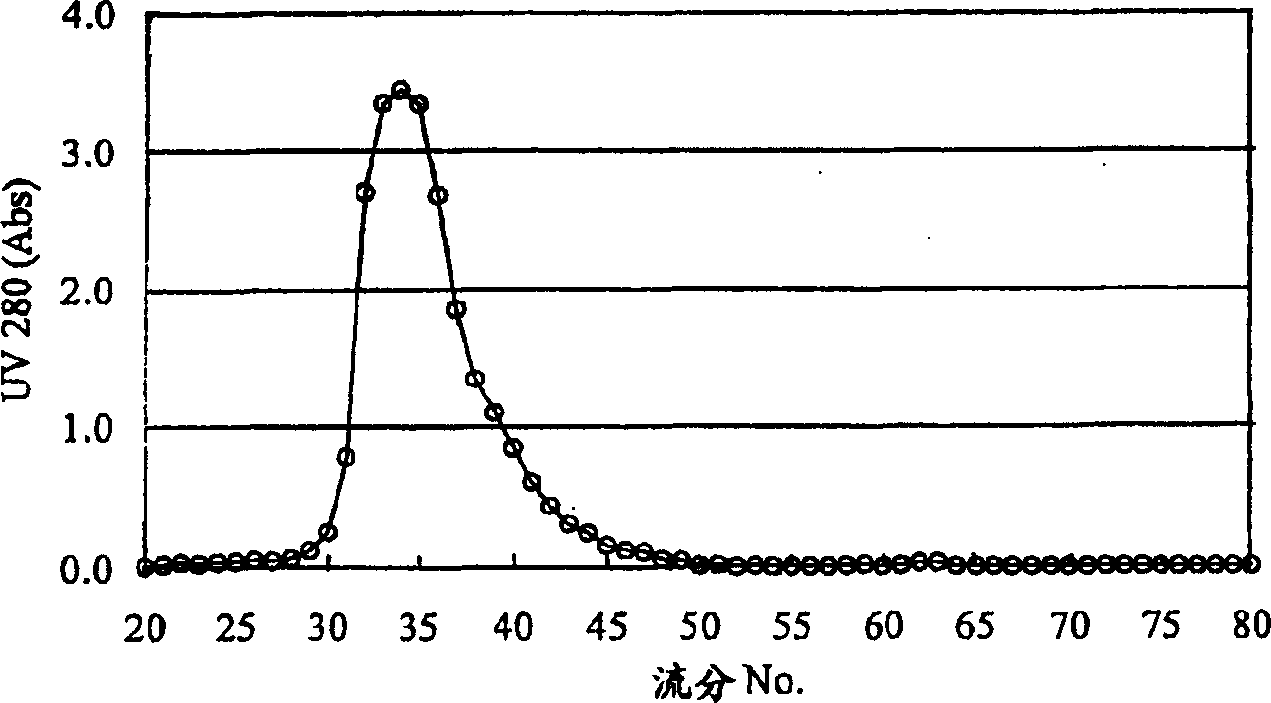
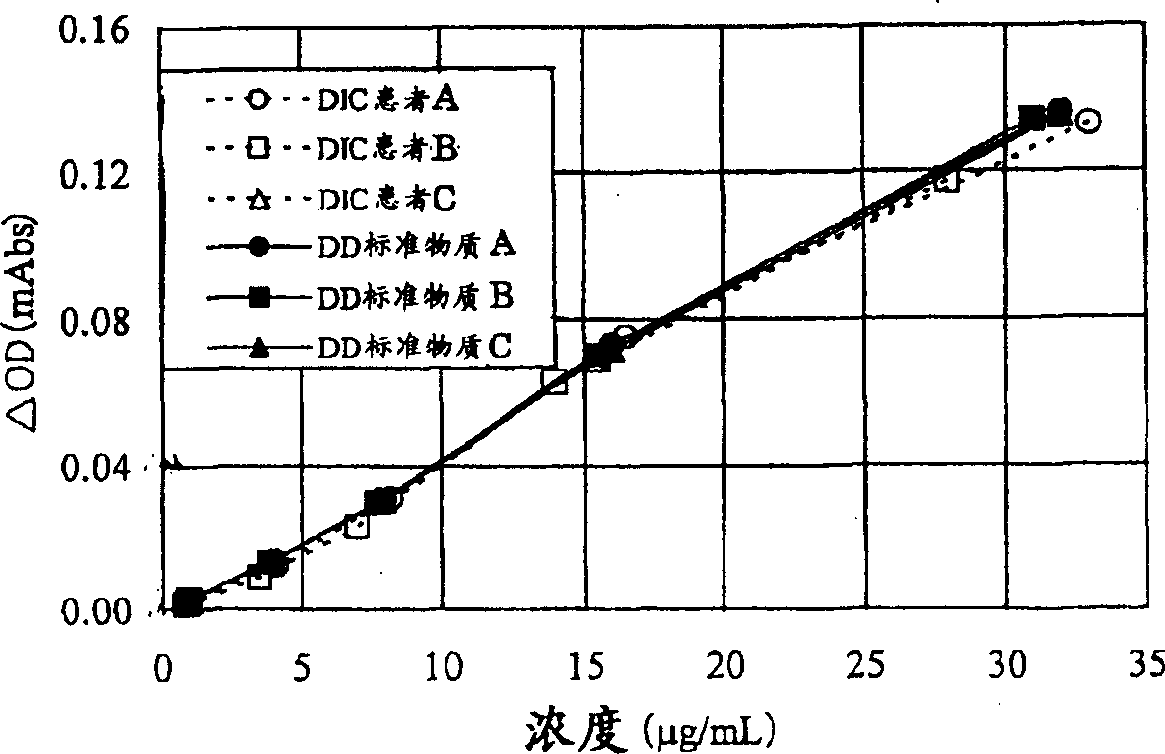
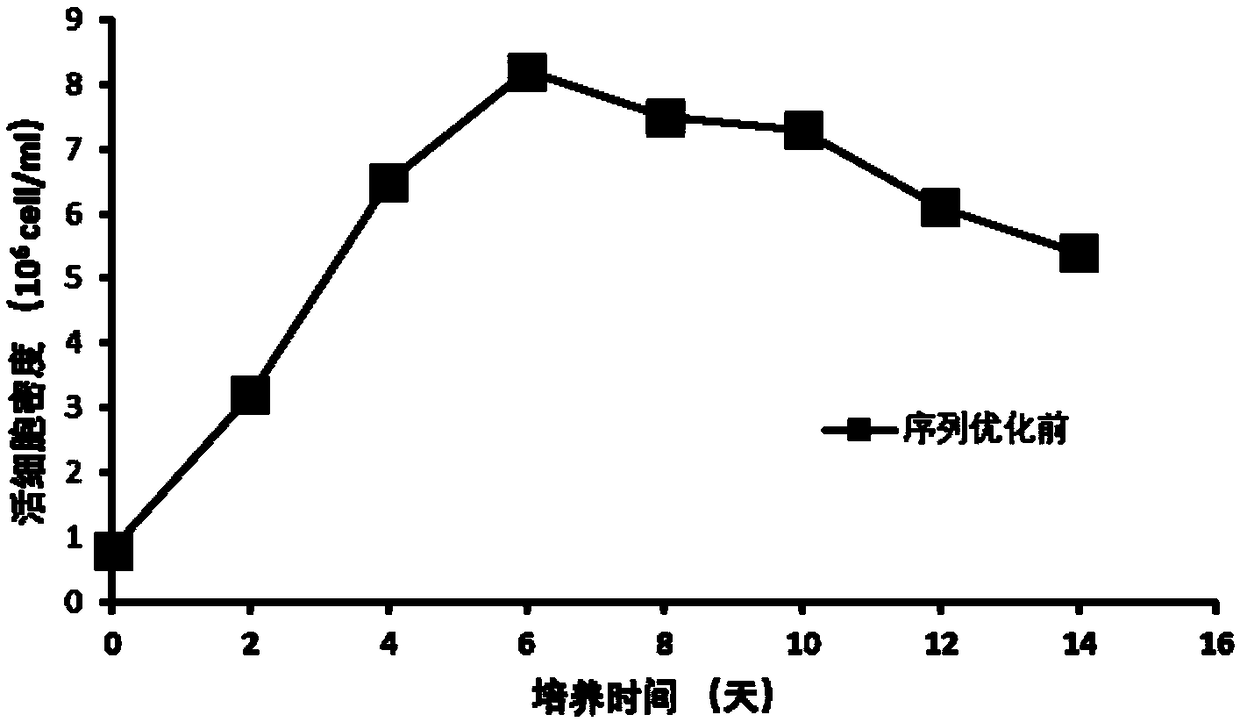
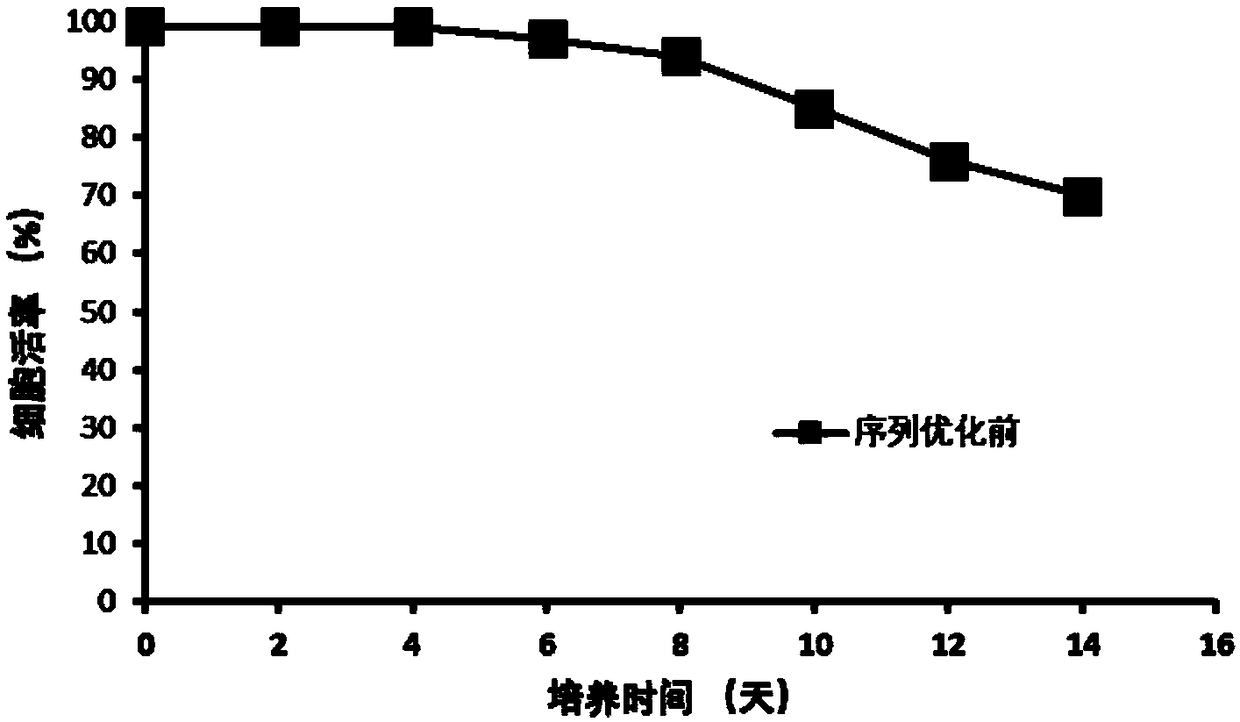
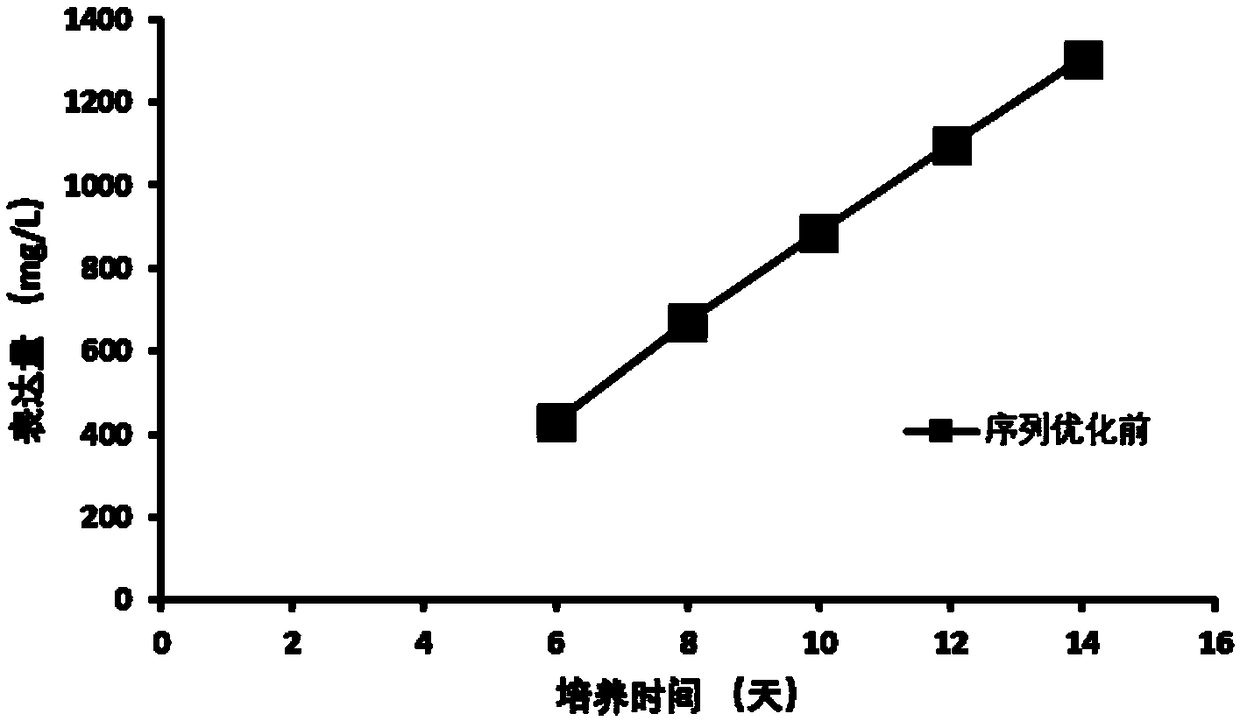
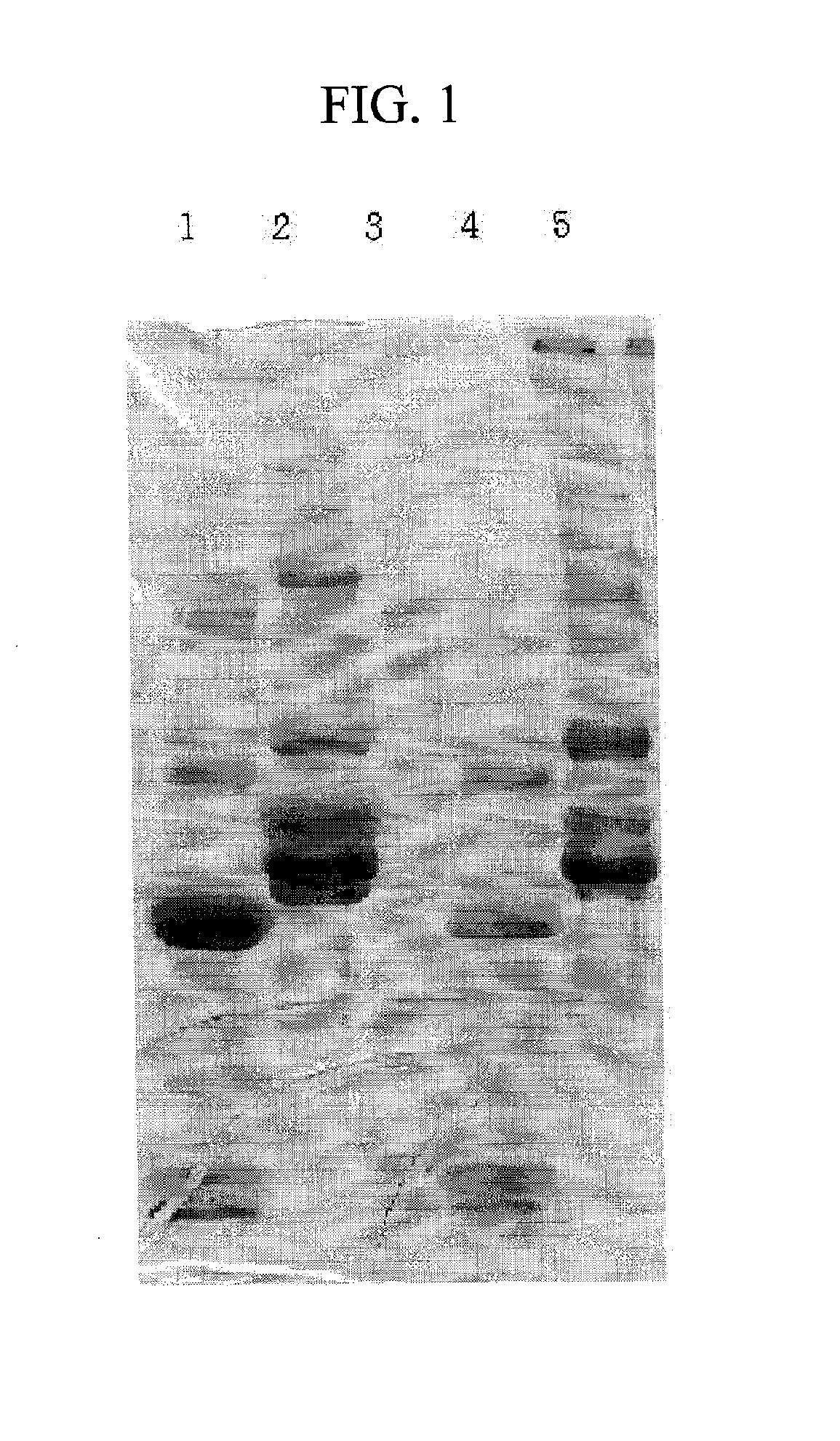
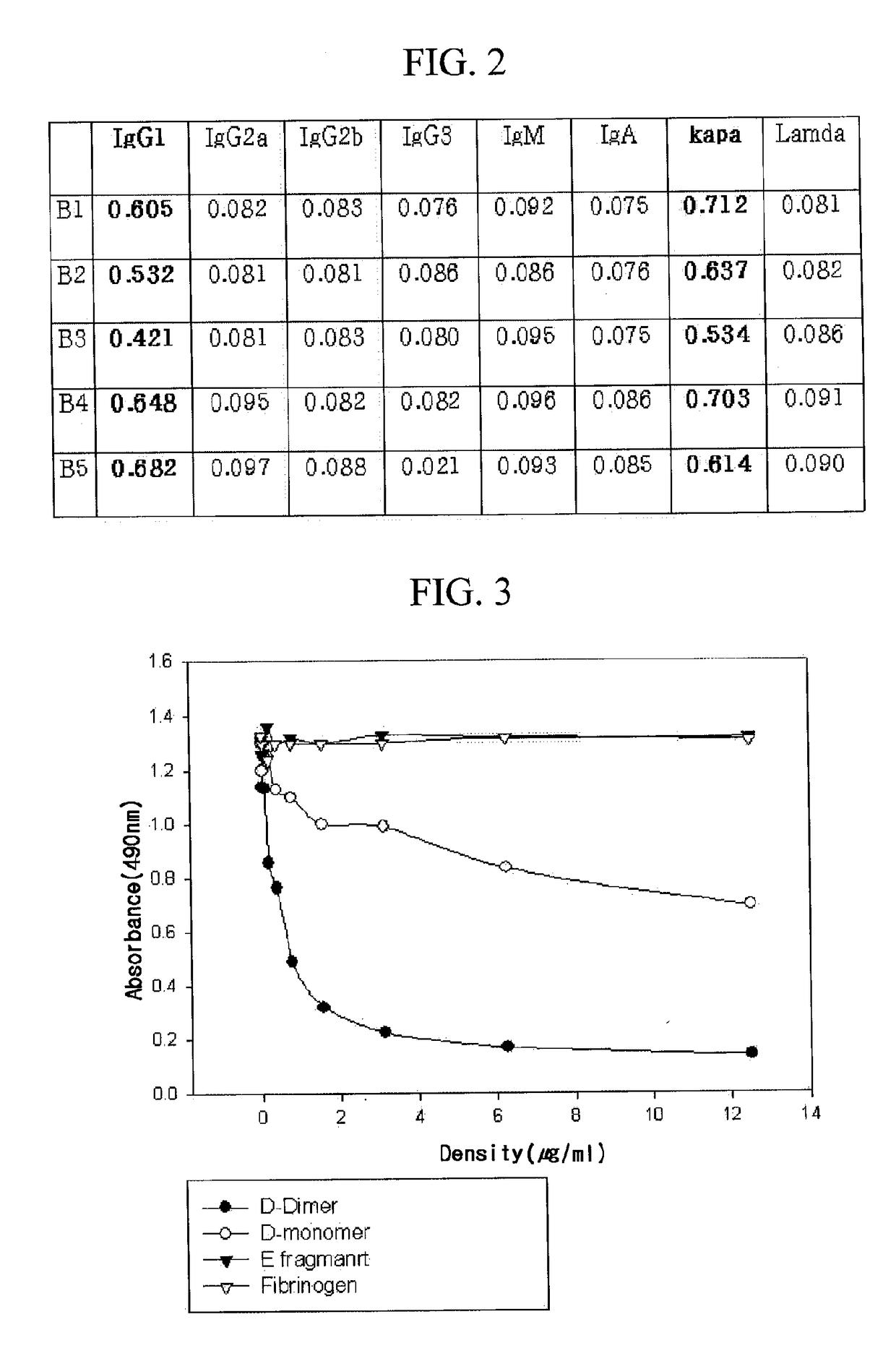
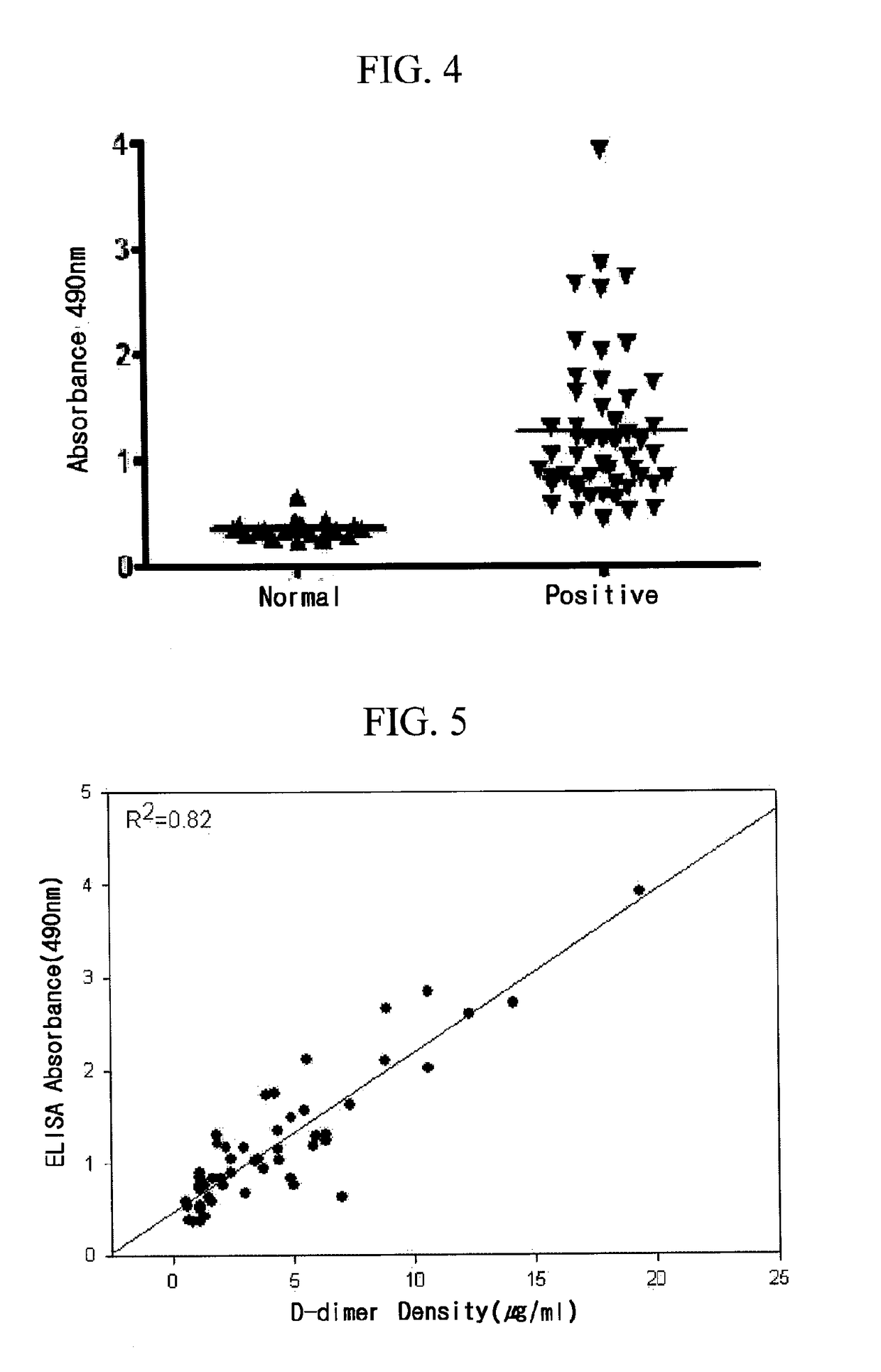
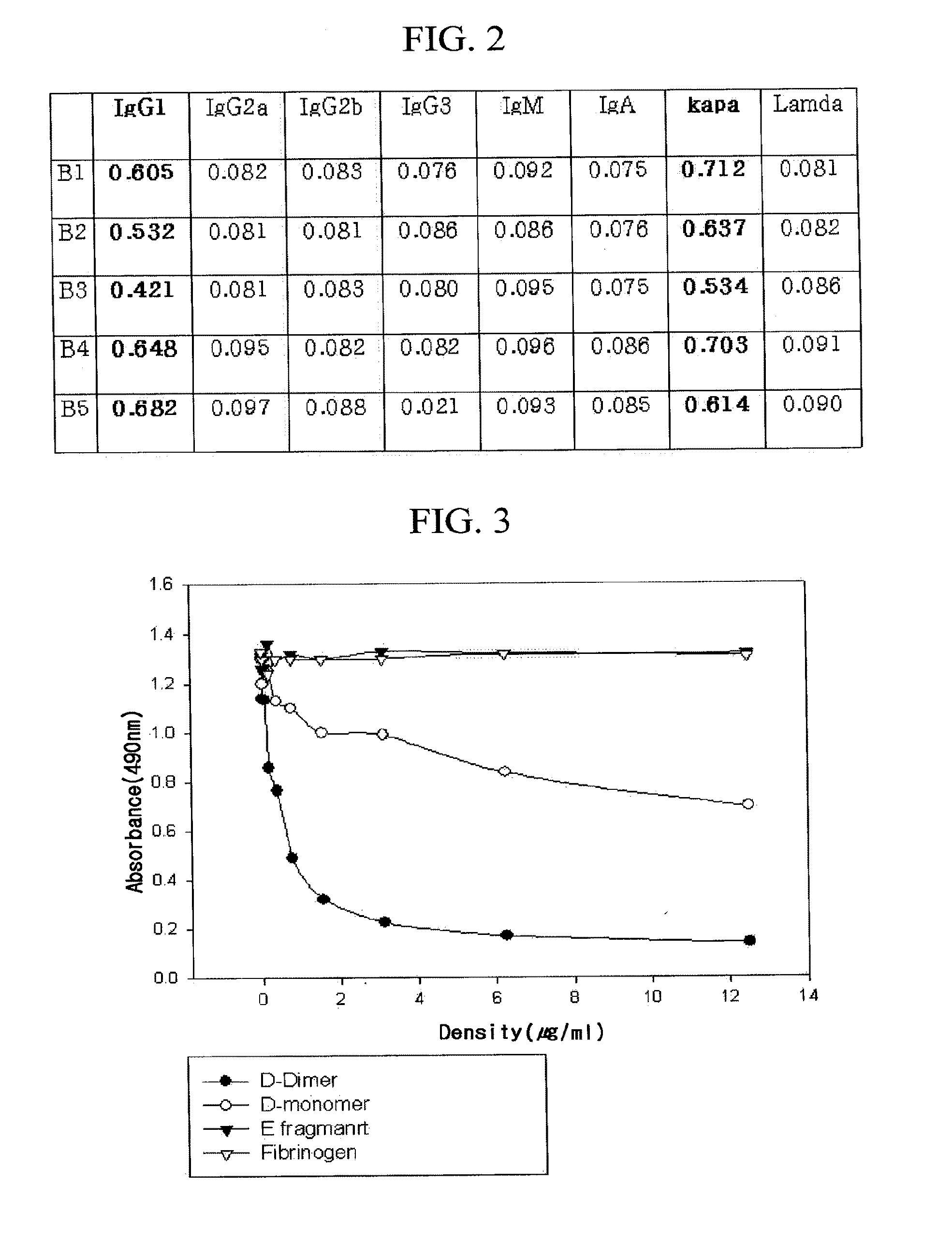
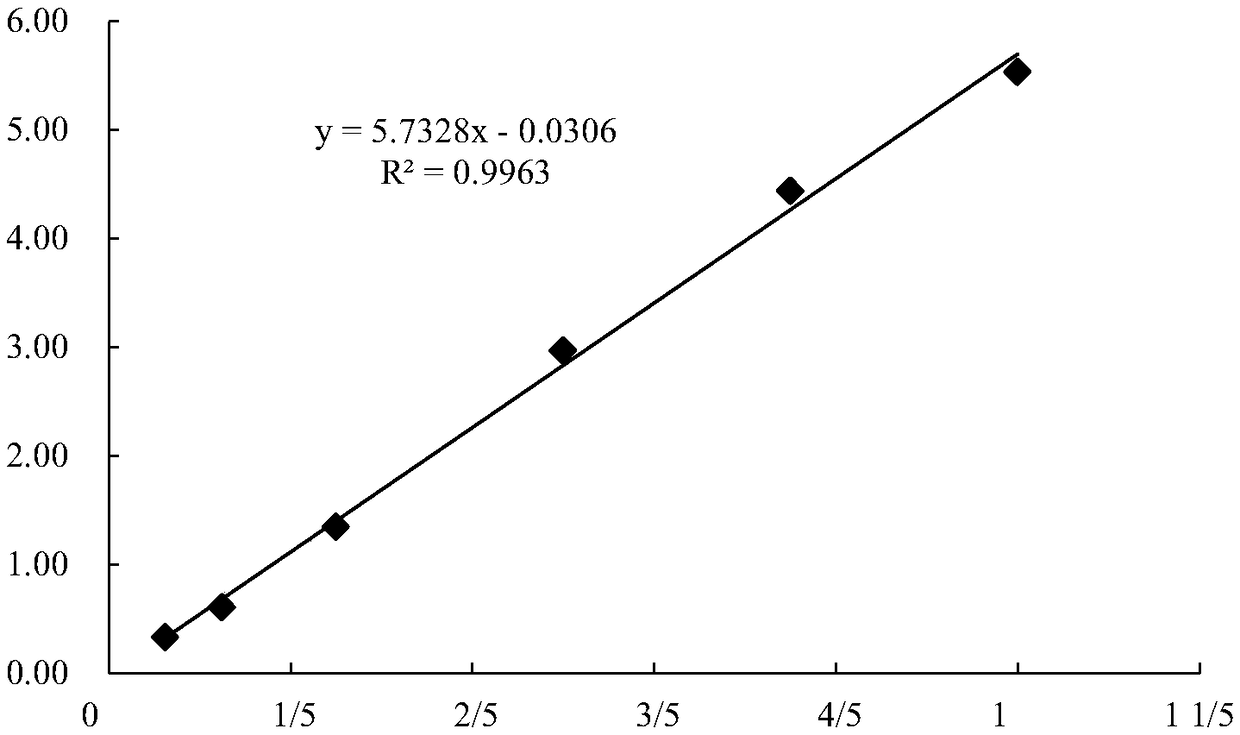
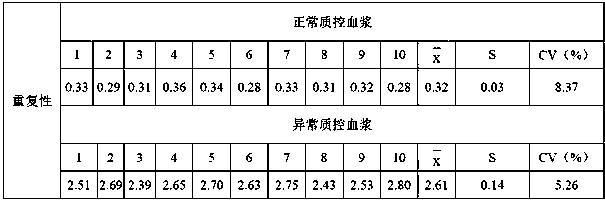
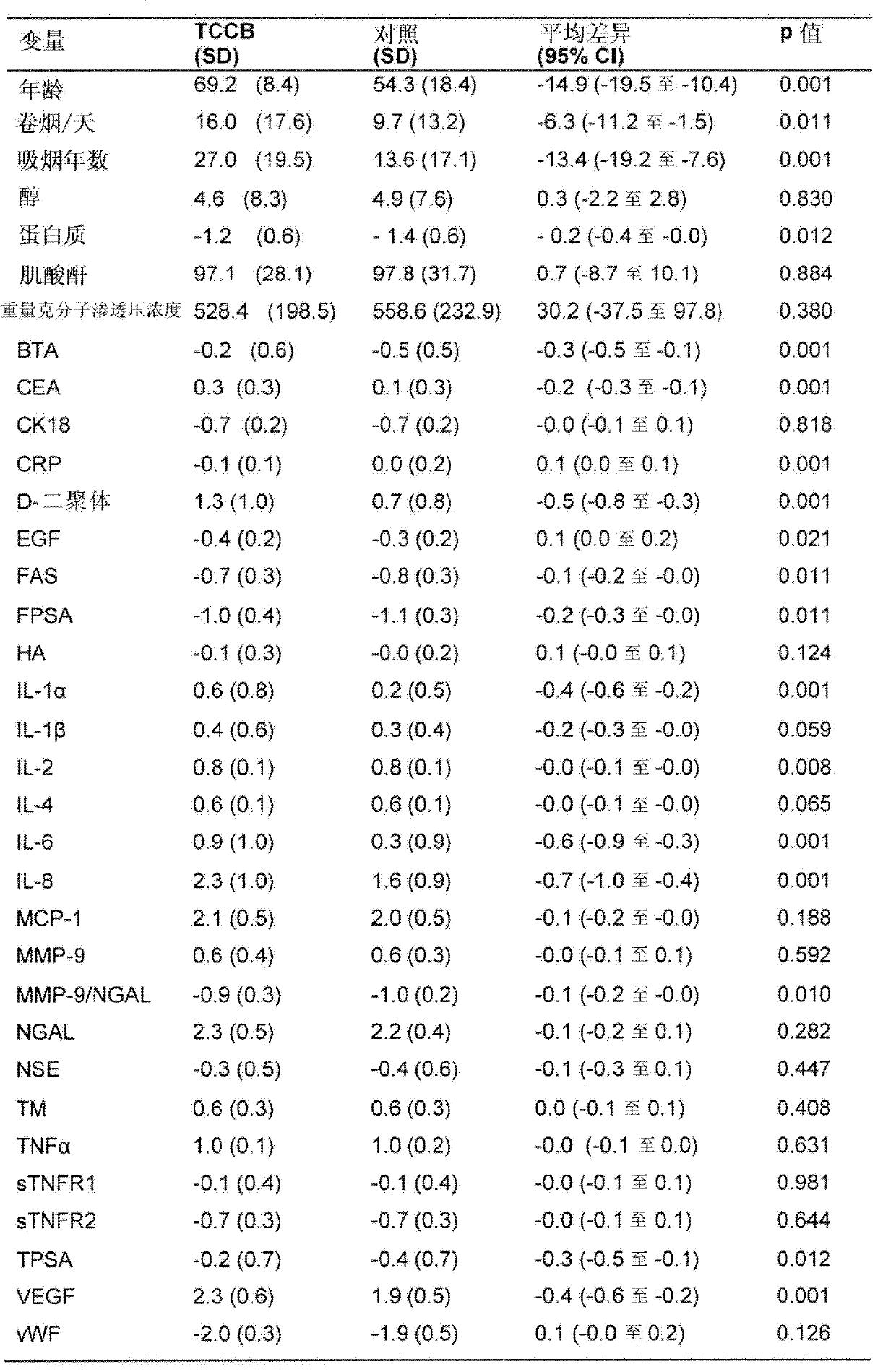
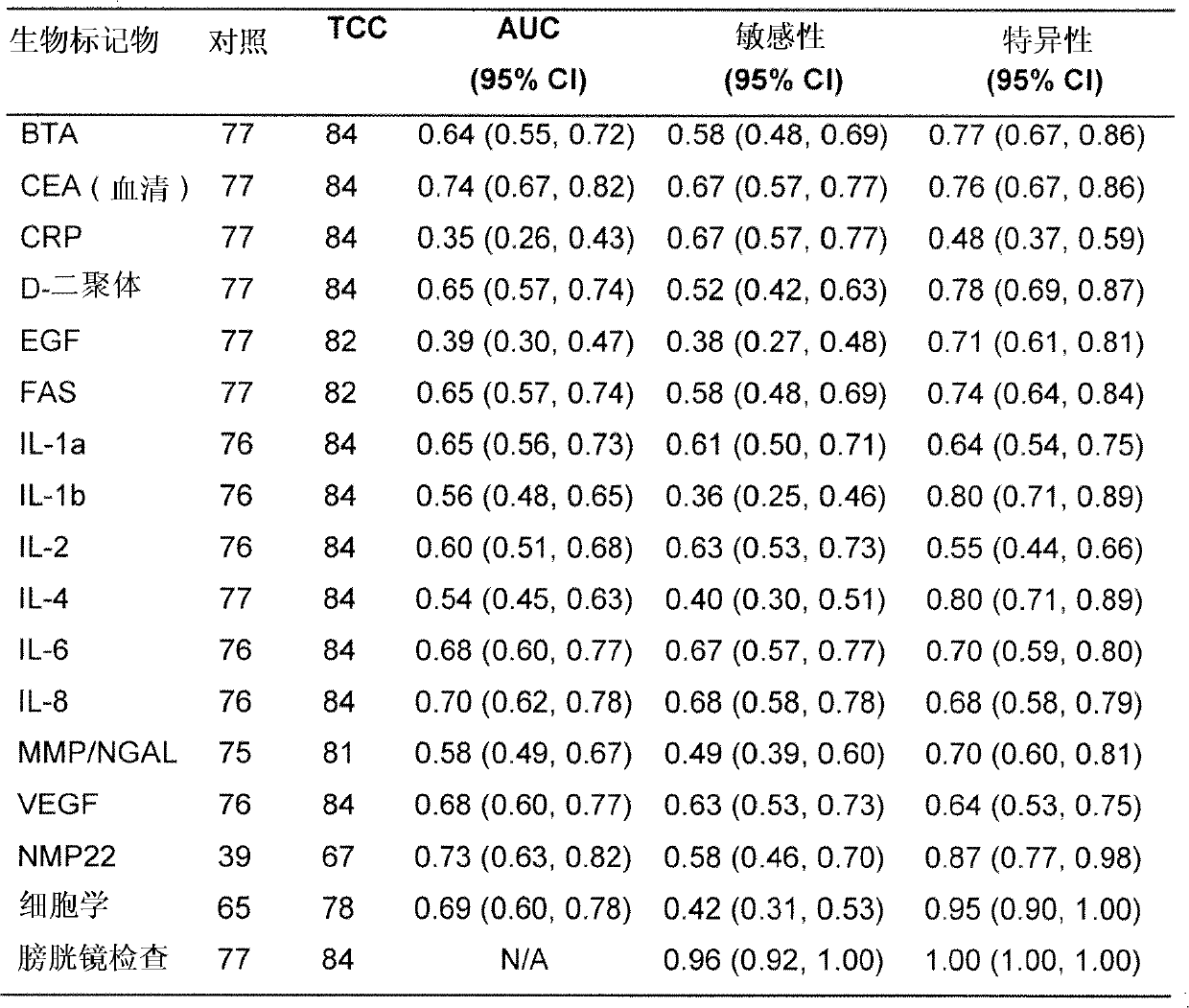
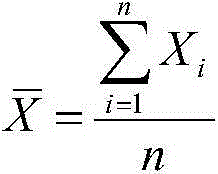Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
88 results about "D-dimer" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
D-dimer (or D dimer) is a fibrin degradation product (or FDP), a small protein fragment present in the blood after a blood clot is degraded by fibrinolysis. It is so named because it contains two D fragments of the fibrin protein joined by a cross-link.
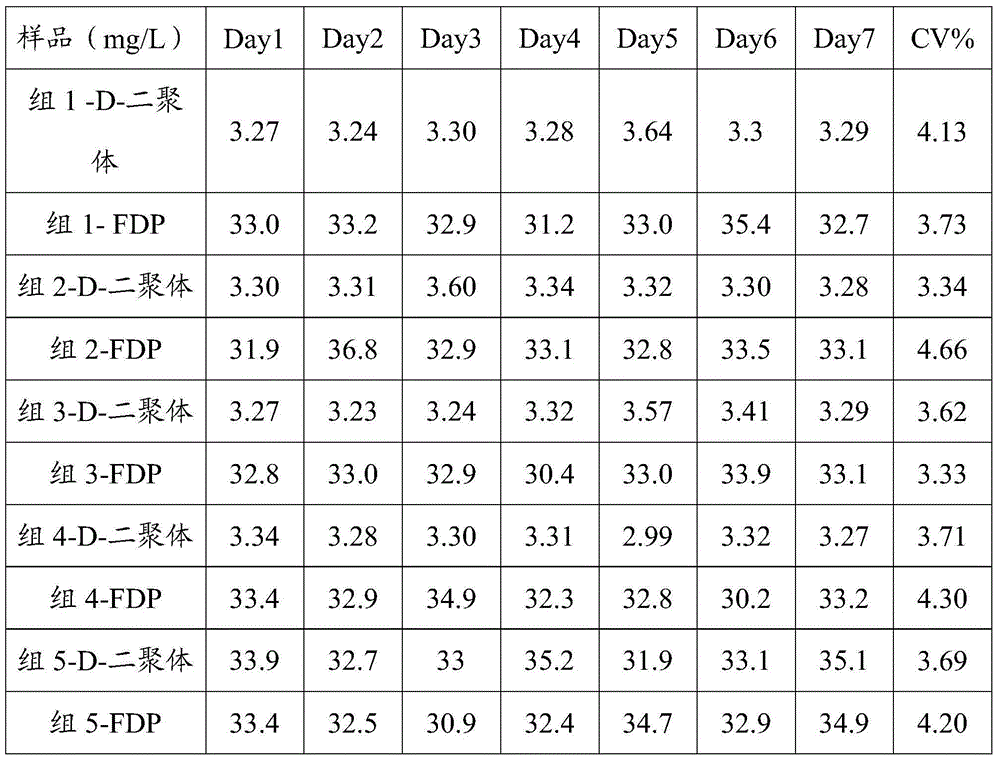
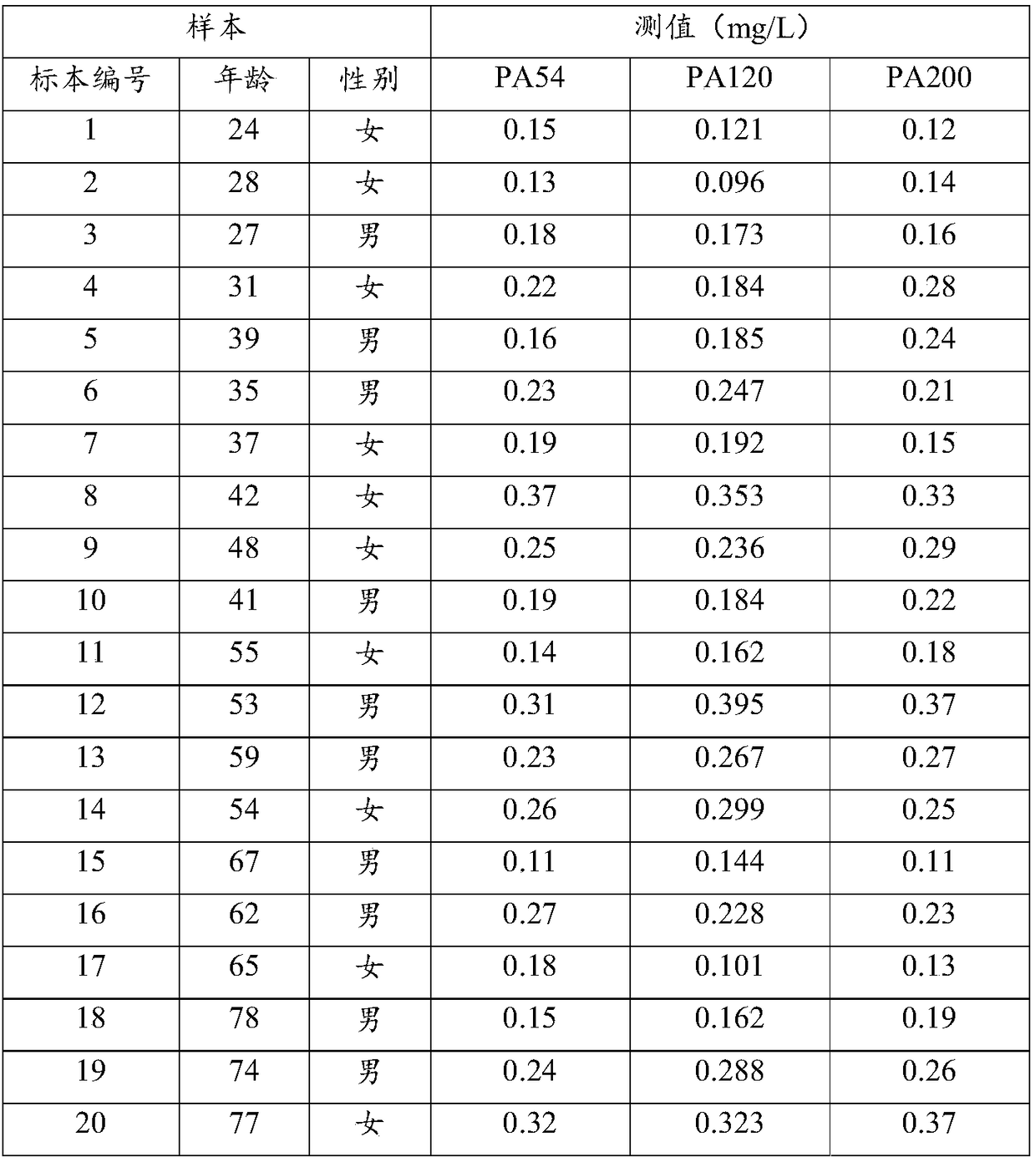
D-Dimer measuring kit (latex immunonephelometry method)
ActiveCN101819202AHigh refractive indexHigh chemical inertnessMaterial analysisFreeze-dryingPreservative
The invention relates to a kit for measuring the D-Dimer content by adopting a latex immunonephelometry method. The kit comprises a D-Dimer reagent R1, a D-Dimer reagent R2, a D-Dimer diluent and a D-Dimer calibration product, wherein the D-Dimer reagent R1 comprises a buffering solution, a stabilizing agent (1), coagulant and preservative; the D-Dimer reagent R2 comprises mouse anti-human D-Dimer monoclonal antibody latex enhanced particles, a buffering solution, a stabilizing agent (2) and preservative; the D-Dimer diluent comprises a buffering solution and preservative; and the D-Dimer calibration product is prepared by subpacking and freeze-drying a solution comprising a fibrin degradation product D-Dimer, a buffering solution, a stabilizing agent (3), excipient and preservative. The kit has the advantages of simple and rapid operation, accurate quantification, high sensitivity, strong specificity, low detection cost and strong instrument compatibility and is suitable for being popularized and used in various big-scale and small-scale hospitals.
Owner:SHANGHAI SUNBIO TECH
Methods and compositions for the diagnosis of diseases of the aorta
InactiveUS20070224643A1Facilitate patient treatmentConvenient treatmentDiagnosticsSurgeryAortic dissectionSmooth Muscle Myosins
The present invention relates to methods and compositions for symptom-based differential diagnosis, prognosis, and determination of treatment regimens in subjects. In particular, the invention relates to the use of biomarkers, either individually or in combinations with one another to rule in or out diseases of the aorta and its branches, most particularly aortic aneurysm and / or aortic dissection, and for risk stratification in such conditions. Preferred markers include one or more of creatine kinase-BB (CK-BB), creatine kinase-MB (CK-MB), acidic calponin, basic calponin, B-type natriuretic peptide (BNP), NT-proBNP, proBNP, BNP79-108, BNP3-108, caldesmon, caspase-3, D-dimer, soluble elastin fragments, endothelial cell-selective adhesion molecule (ESAM), fibrillin-1, heart-type fatty acid binding protein, MMP-9, myeloperoxidase, myoglobin, smooth muscle myosin, smooth muscle myosin heavy chain, TIMP-1, free cardiac troponin I, complexed cardiac troponin I, free and complexed cardiac troponin I, free cardiac troponin T, complexed cardiac troponin T, and free and complexed cardiac troponin T, and preferred assays are configured to detect these markers.
Owner:BIOSITE INC
Turbidimetric rapid detection kit for myocardial infarction nano-immunoenhancement and use method thereof
InactiveCN103185798ANo special training requiredNo need to waitMaterial analysis by observing effect on chemical indicatorBiological testingDisease courseBiomarker (petroleum)
The invention provides a turbidimetric rapid detection kit for myocardial infarction nano-immunoenhancement. The turbidimetric rapid detection kit comprises a reaction-detection integrated device, and a detection kit arranged in the reaction device, wherein a reagent R1, a reagent R2 and a calibrator are contained in the detection kit; simultaneously, quantitative detection for five important myocardial infarction-related biomarkers in one sample can be realized, and the five important biomarkers include content of troponin-I, content of D-dimer, content of myoglobin, content of hypersensitive C-reactive protein (hs-CRP) and content of heart-type fatty acid binding protein (FABP); and the turbidimetric rapid detection kit has an important clinical significance in the aspects of early diagnosis and disease course monitoring for myocardial infarction, treatment monitoring for medicines, and the like. The turbidimetric rapid detection kit provided by the invention realizes the integration of the reagents and the reaction device, and is simple, rapid and accurate to operate, high in sensitivity, strong in specificity, and low in detection cost; and the turbidimetric rapid detection kit is a detection / reagent measurement integrated kit suitable for outpatient and emergency treatment / clinical detection, and wide in instrument application range.
Owner:SUZHOU DIAGVITA BIOTECH
Polymerase chain reaction based chiral gold dimer controllable assembly method
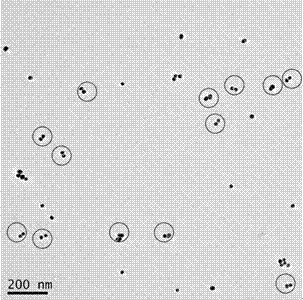
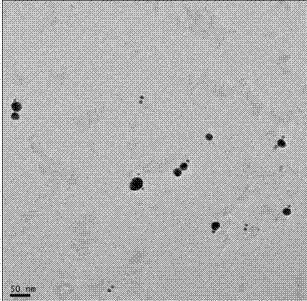
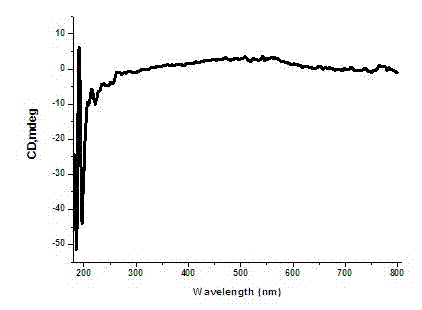
A polymerase chain reaction based chiral gold dimer controllable assembly method belongs to the technical field of material chemistry. The invention has the following main implementation steps of: (1) preparing gold nanoparticles with different particle sizes; (2) coupling gold nanoparticles with upstream and downstream primers; (3) modifying gold nanoparticle-primer conjugates; (4) performing PCR by the use of the obtained gold nanoparticle-primer conjugates, representing an assembly structure by TEM; (5) carrying out CD signal determination on the PCR product. By the controllable modification of the primer coupling quantity on gold nanoparticle surface, in order to achieve the stability of gold nanoparticles in PCR system and carry out modification on the nanoparticle-primer conjugates,gold dimer is assembled and comparison of TEM character and CD signal is performed in a suitable PCR condition. Except for the assembly of gold nanoparticles with same particle size, the invention also studies the assembly of gold nanoparticles with different particle sizes and their properties.
Owner:王利兵 +2
Preparation method and application of D-dimer immuno latex microspheres
ActiveCN106290822AGuaranteed Coupling EfficiencyAvoid damageMaterial analysisMicrospherePolystyrene microsphere
The invention discloses a preparation method and application of D-dimer immuno latex microspheres. In the preparation method of D-dimer immuno latex microspheres, a chemical crosslinking method is optimized, the carboxyl of polystyrene microsphere is activated in a low-pH condition and then coupled with amino of an antibody dissolved in a high-pH condition, and the mixture of the two is neutral and is coupled for 12-24h at a low temperature, so that not only can the coupling efficiency of the antibody be guaranteed, but also the damage of the antibody activity caused by an activator is reduced to a great extent. A latex enhanced turbidimetric immunoassay D-dimer test kit prepared from the immuno latex microspheres prepared by the method has the characteristics of high sensitivity, accurate quantification, good repeatability and stable property, and the lowest detection limit can reach 0.01mg / L; the latex enhanced turbidimetric immunoassay D-dimer test kit can be applied to a full-automatic biochemical analyzer or coagulation analyzer, is quick and easy to operate, only needs 5-10 minutes from detection to result collection and has relatively good clinical application prospect.
Owner:WUHAN KING DIAGNOSTIC TECH CO LTD
Monoclonal antibody and method of immunological analysis of e-D-dimer and e-DD/E complex
InactiveUS6132719AImmunoglobulins against animals/humansBiological material analysisFragment XMonoclonal antibody
PCT No. PCT / JP97 / 01639 Sec. 371 Date Jan. 15, 1998 Sec. 102(e) Date Jan. 15, 1998 PCT Filed May 15, 1997 PCT Pub. No. WO97 / 43315 PCT Pub. Date Nov. 20, 1997A monoclonal antibody which specifically reacts with D-monomer produced by digesting human fibrinogen with granulocyte elastase and D-domain containing digestion products produced by digesting human stabilized fibrin with granulocyte elastase, but does not react with fibrinogen, or fragment X, Y or E produced by digesting fibrinogen with granulocyte elastase is disclosed. The D-dimer or DD / E complex produced by digestion with granulocyte elastase in a sample from a living body can be analyzed without being interferred with fibrinogen, digestion products of fibrinogen with plasmin, or digestion products of stabilized fibrin with plasmin, using the monoclonal antibody.
Owner:MITSUBISHI CHEM MEDIENCE
Anti-D-dimer monoclonal antibody and application thereof
ActiveCN102010472AQuick checkDetection is simple and fastImmunoglobulins against blood coagulation factorsMicroorganism based processesHeavy chainDiagnostic concordance
The invention discloses an anti-D-dimer monoclonal antibody. The amino acid residue sequence of the light chain of the antibody is shown in SEQ ID No.1, and the amino acid residue sequence of the heavy chain of the antibody is shown in SEQ ID No.2; and the antibody is secreted by a hybridoma cell strain 6B8D11H12 the preservation number of which is CCTCC NO.C201060. The anti-D-dimer monoclonal antibody can be used in preparing various D-dimer aspartame assay kits. By utilizing the anti-D-dimer monoclonal antibody disclosed by the invention and a D-dimer aspartame assay kit prepared by gold immunochromatographic assay(GICA), the detection sensitivity is up to 96.08%, the detection specificity is up to 94.79%, the detection accuracy is up to 95.67%, the Kappa detection value is up to 0.901 which is greater than 0.75, and the diagnosis is more consistent with clinical diagnosis, thus the anti-D-dimer monoclonal antibody can be used for aided diagnosis on thrombotic diseases in clinical applications.
Owner:上海贝西生物科技有限公司
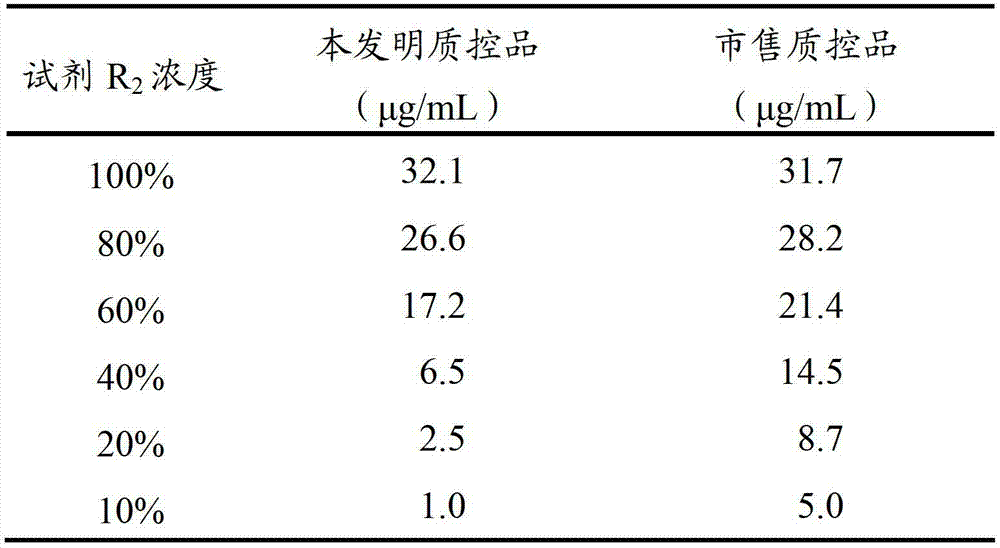
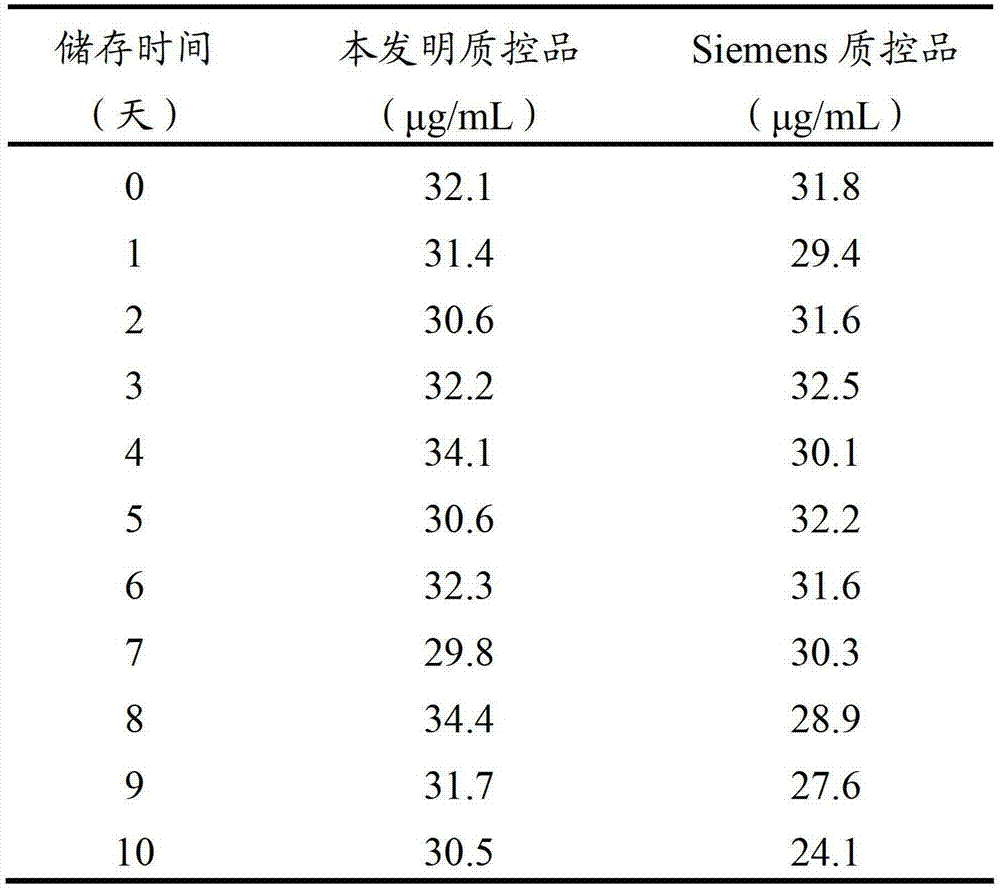
D-dimer quality control product and preparation method thereof
The invention relates to the field of quality control of clinical blood coagulation detection projects, and in particular relates to a method for preparing a D-dimer quality control product. The method comprises the following steps: carrying out recalcification on bovine plasma and solidifying; adding an enzyme capable of degrading fibrous protein, thereby dissolving a fibrous protein clot; stopping the reaction by using a protease inhibitor; diluting a generated D-dimer mother liquor by using a freeze-dried protecting solution; and freeze-drying, thereby obtaining the D-dimer quality control product. The prepared D-dimer quality control product is very sensitive to the change of a detection reagent and good in stability, thereby satisfying the quality control requirement of clinical D-dimer detection.
Owner:SHANGHAI SUNBIO TECH
Method for the prediction of vascular events and the diagnosis of acute coronary syndrome
The present invention relates to a method for the prognosis of a vascular event in a patient suspected of being at risk for a vascular event, said patient presenting: - no elevation of the ST segment as seen on an electrocardiogram, and / or - a normal level of at least one myocardial necrosis marker, wherein the presence and / or levels of at least two different biochemical markers are measured in a biological sample of said patient, whereby the probability that the patient will experience a vascular event is deduced from the measured presence and / or levels of the biochemical markers such as MPO, sCD40L, IL-6, PaPP-A, CRP, D-dimer, troponin, and proBNP.
Owner:CENT NAT DE LA RECHERCHE SCI
Cardiac muscle control material, preparation method therefor, detection kit and detection device for cardiac muscle
The invention relates to a cardiac muscle control material, a preparation method therefor, a detection kit and a detection device for cardiac muscle. The cardiac muscle control material comprises quality control component and protection component, the quality control component comprises at least one of B-type natriuretic peptide, D-dimer, NT-proBNP, cardiac troponin I, myoglobin, creatine kinase isozyme and cardioid fatty acid binding protein and heart fatty acid binding protein. The protective component comprises 5 mM to 100 mM of a buffer agent, 0.5 % to 5% by weight of an excipient, 0.05 mMto 5 mM of an antioxidant, 0.05 mM to 15 mM of a protease inhibitor, 0.5% to 10% by weight of a stabilizer and 0.01% to 2% by weight of a surfactant. The cardiac muscle control material disclosed bythe invention is good in stability.
Owner:深圳天深医疗器械有限公司
High-sensitivity high-linearity D-D dimer detection kit and application method thereof
InactiveCN103235130AHigh detection sensitivityExpand the scope of detectionMaterial analysisDimerHigh concentration
The invention discloses a high-sensitivity high-linearity D-D dimer detection kit and an application method thereof. A reagent one is a 30mmol / L trihydroxymethyl aminomethane buffer solution. A reagent two comprises a mixed liquid of large-diameter mouse-anti-human D-D dimer monoclonal antibody latex particles with a concentration of 0.01-0.04% (w / v) and small-diameter mouse-anti-human D-D dimer monoclonal antibody latex particles with a concentration of 0.05-0.2% (w / v). Specific antibody content in the small-diameter mouse-anti-human D-D dimer monoclonal antibody latex particles is 0.05-10% of that in the large-diameter mouse-anti-human D-D dimer monoclonal antibody latex particles. Through optimizing the average diameter of large and small particles and concentrations thereof in the reaction system, D-D dimer high-sensitivity detection under low-concentration situation can be realized, and detection range under high-concentration situation can be expanded.
Owner:NINGBO RUI BIO TECH
D dimer latex-enhanced immunoturbidimetric assay kit utilizing surface functional group
The invention relates to an in vitro diagnostic kit, and in particular relates to a D dimer latex-enhanced immunoturbidimetric assay kit utilizing a surface functional group. The D dimer latex-enhanced immunoturbidimetric assay kit utilizing the surface functional group consists of two components, namely a reagent R1 and a reagent R2, wherein the reagent R1 mainly consists of a buffer solution 1, a stabilizer 1, a preservative 1, a gelling agent and a protective agent 1; the reagent R2 mainly consists of a buffer solution 2, two polystyrene latex microspheres cross-linked to different D dimer monoclonal antibodies, a stabilizer 2, a protective agent 2 and a preservative 2; the polystyrene latex microspheres are connected to the D dimer antibodies in a manner of covalent cross-linking or physical adsorption; and the surface functional group of the polystyrene latex microsphere in the reagent R2 is an amino group, a carboxyl group, hydrazide, an aldehyde group or an epoxy group. Compared with the prior art, by virtue of the latex-enhanced immunoturbidimetric assay, the kit can be used for detecting by wavelength of 400-800nm, so that the kit is more convenient to detect and is easy in clinical application.
Owner:ZYBIO INC
D-dimer immunofluorescent quantitative test strip and preparation method thereof
The invention discloses a D-dimer immunofluorescent quantitative test strip and a preparation method thereof. Compared with a test strip prepared from a common stock solution, the D-dimer immunofluorescent quantitative test strip prepared in the invention has better storage stability and higher precision, and in peak appearance of a detected sample, a higher signal, a flat baseline and high accuracy are realized.
Owner:ZHONGSHAN CHUANGYI BIOCHEM ENG
Reference material for assaying d-dimer
ActiveCN1825119AHigh sensitivityImprove featuresImmunoglobulins against animals/humansBiological testingFiltrationPeak area
The present invention relates to a reference material for assaying D-dimer, comprising a fibrin degradation product, wherein no less than 70 % of a total peak area has the molecular weight of no less than 500,000 in a molecular weight distribution of the fibrin degradation product, measured by gel filtration chromatography.
Owner:SYSMEX CORP
Anti-D-dimer monoclonal antibody and preparation method thereof
ActiveCN109053893AIncrease productionReduced stabilityImmunoglobulins against blood coagulation factorsBiological material analysisHybridoma cellAscites
The invention discloses an anti-D-dimer monoclonal antibody and a preparation method thereof, and belongs to the technical field of biology. The anti-D-dimer monoclonal antibody is first prepared by means of hybridoma, the sequence of the obtained antibody is then optimized, the output of the anti-D-dimer monoclonal antibody is increased by 54 percent under the premise of keeping affinity unchanged, and the production cost is greatly reduced. Compared with the conventional hybridoma culture and mouse ascites extraction methods, the method disclosed by the invention not only greatly increases expression and reduces the production cost, but also can effectively control the stability of product batches.
Owner:智享生物(苏州)有限公司
Kit for quantitatively detecting D-dimer and preparation method thereof
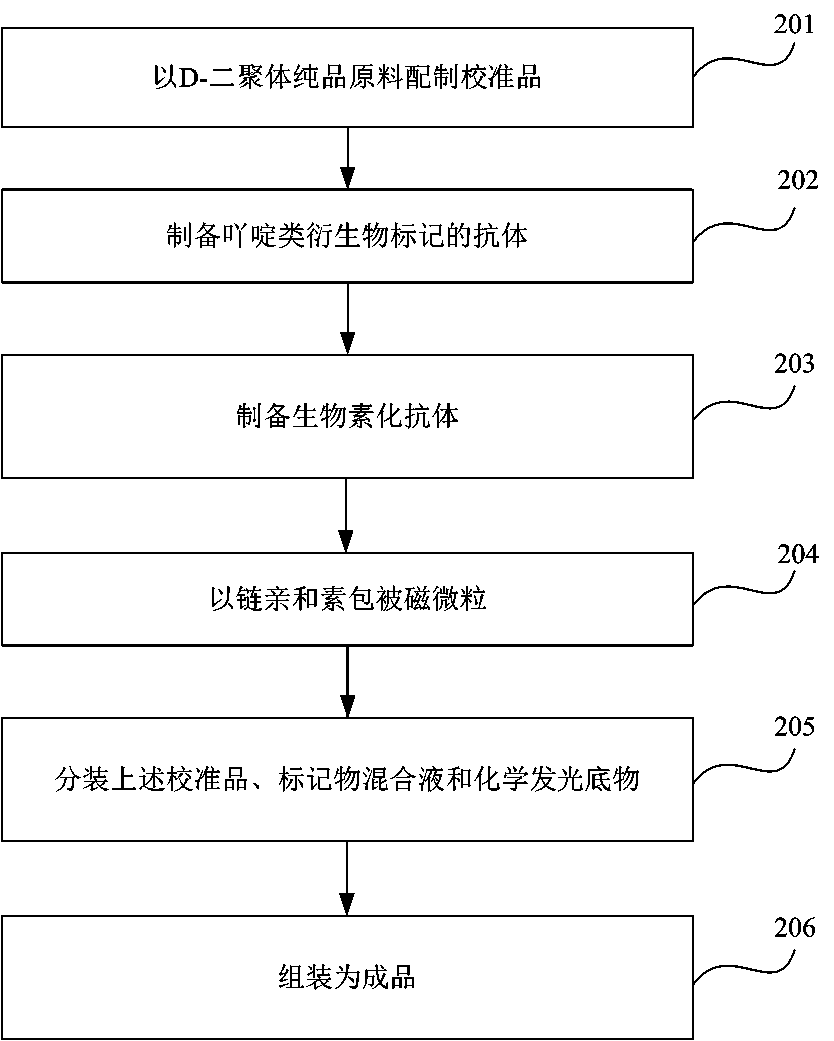
InactiveCN108445214AImplement diagnosticsEnable clinical monitoringChemiluminescene/bioluminescenceDisease diagnosisBiotin-streptavidin complexQuantitative determination
The invention provides a kit for quantitatively detecting D-dimer. The kit includes: a D-dimer calibrator, magnetic-particles coated with streptavidin, a D-dimer monoclonal antibody marked with an acridine derivative, a D-dimer monoclonal antibody marked with a biotin, a chemical illumination substrate, and a quality control substance. The preparation method of the kit includes the following steps: preparing the calibrator from a D-dimer pure product raw material; preparing the antibody marked with the acridine derivative, preparing the biotinylated antibody; coating magnetic-particles with the streptavidin; dispensing the calibrator, the marker mixture liquid and the chemical illumination substrate; and combining the finish product. The kit has high sensitivity and good specificity, is high in accuracy of a quantitative determination result, is low in use cost and is easier to promote and apply.
Owner:浙江艾明德生物科技有限公司
Method of Distinguishing Among Type a and Type B Acute Aortic Dissection and Acute Myocardial Infraction and Kit For Distinguishment
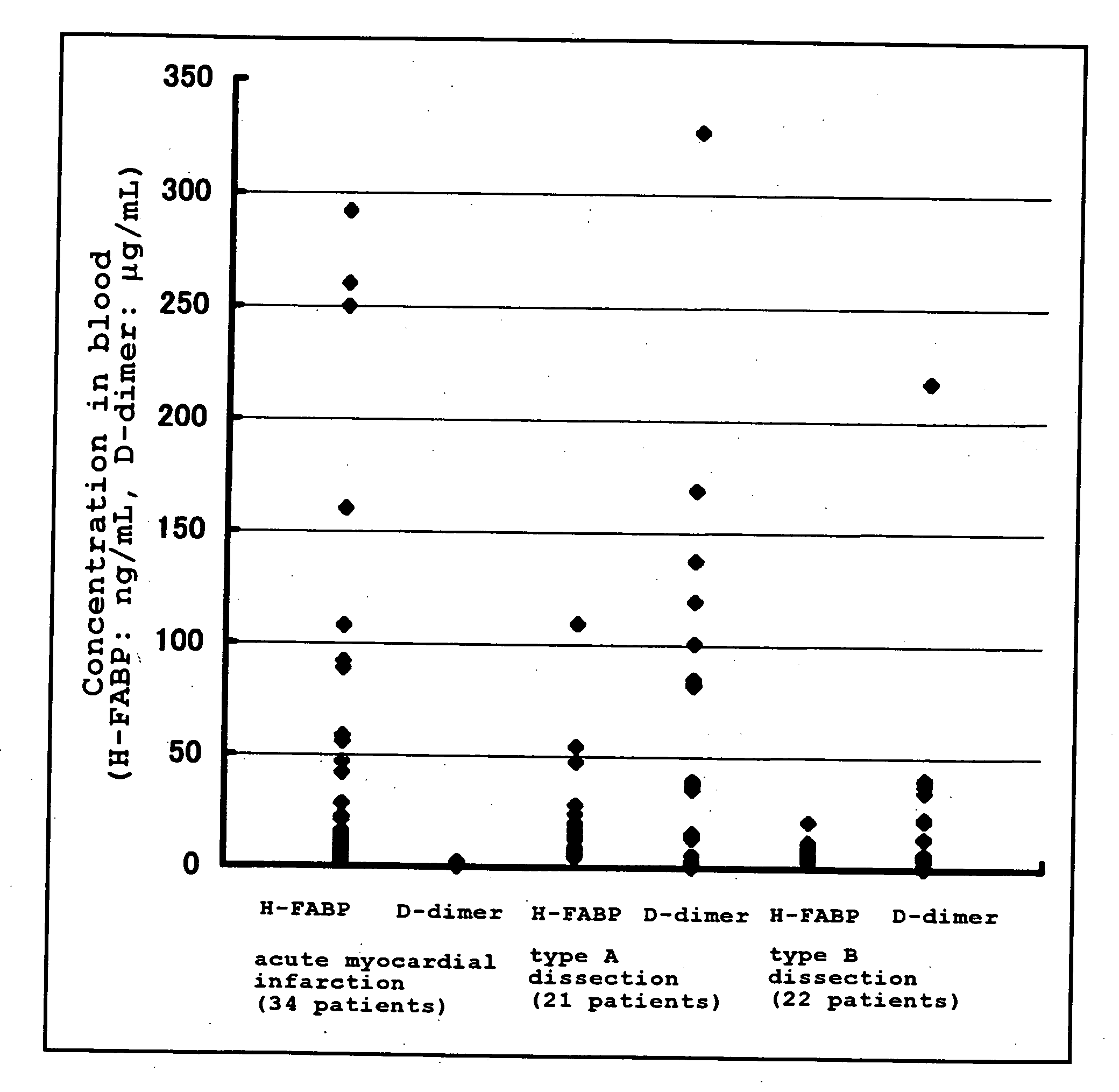
InactiveUS20080183062A1Highly accurately distinguishedDisease diagnosisDiagnostic recording/measuringAnatomyMyocardial infarction syndrome
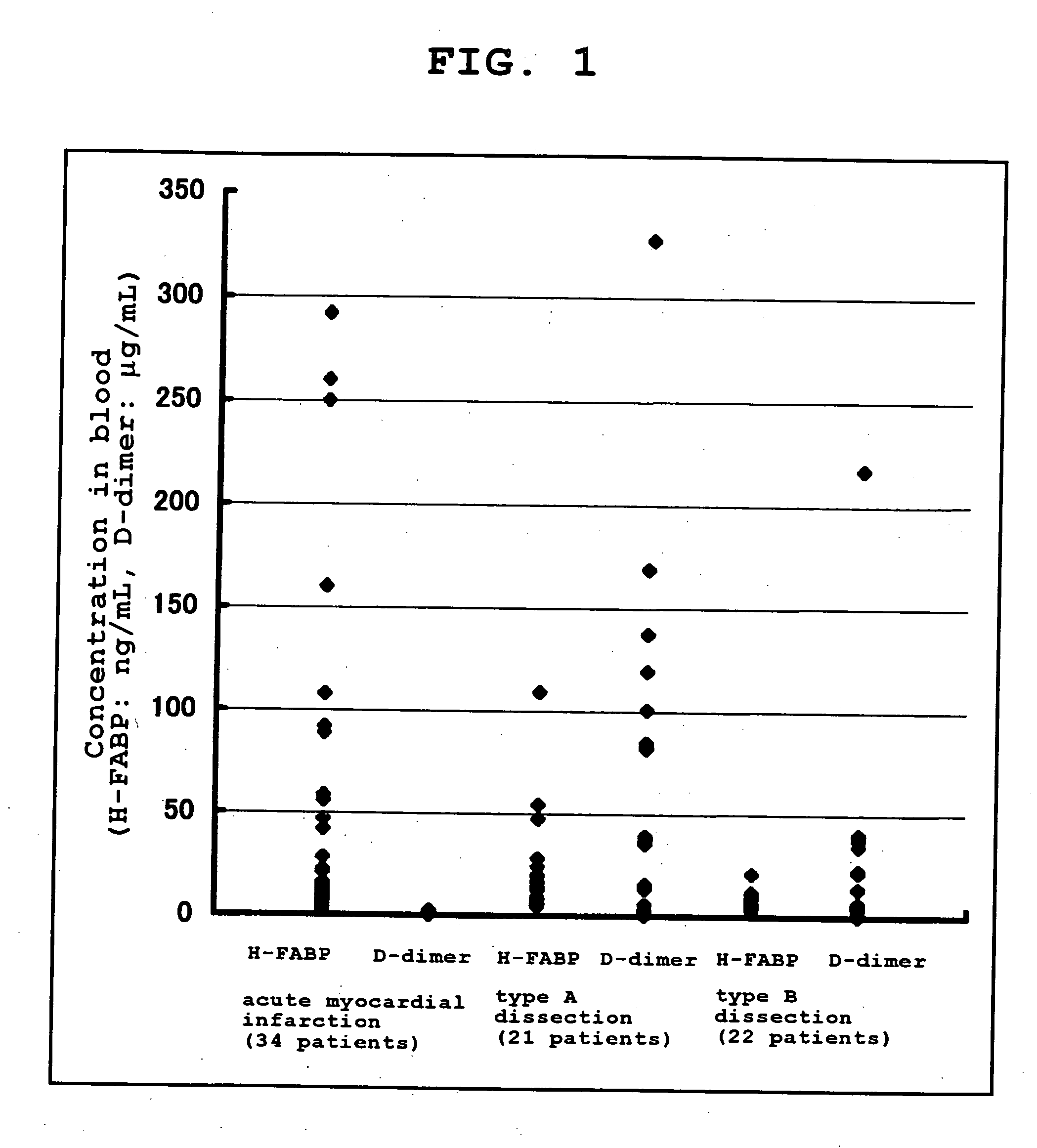
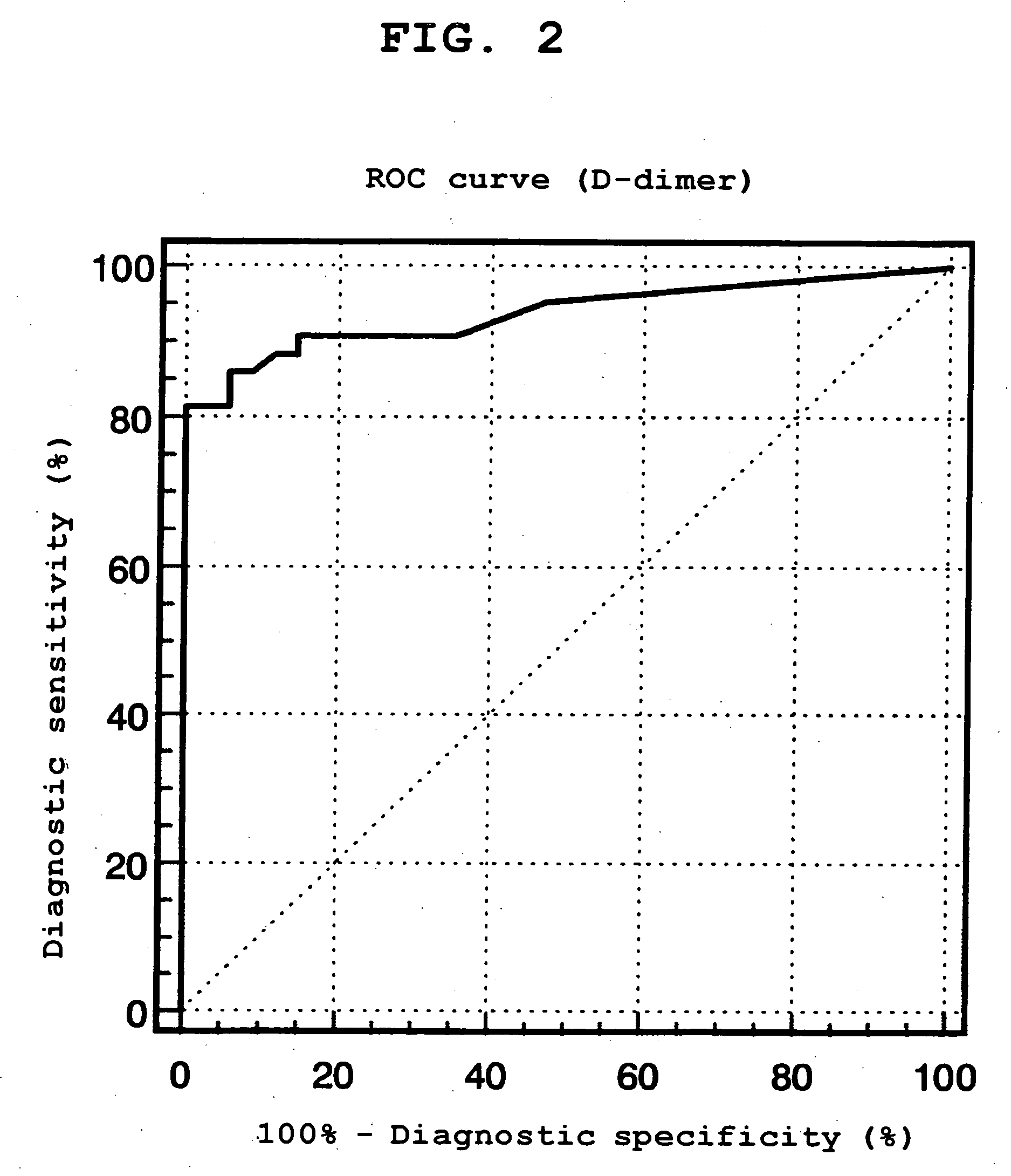
Provided is a method of distinguishing among Stanford type A acute aortic dissection, Stanford type B acute aortic dissection, and acute myocardial infarction, which are mutually similar in terms of clinical symptoms, and a kit for the distinguishment. Specifically, provided is a method of distinguishing among Stanford type A acute aortic dissection, Stanford type B acute aortic dissection, and acute myocardial infarction, which comprises detecting both D-dimer and H-FABP in blood separated from a person suspected of having acute aortic dissection and suspected of having acute myocardial infarction, and establishing the diagnosis on the basis of the concentrations detected, and a kit for the distinguishment.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
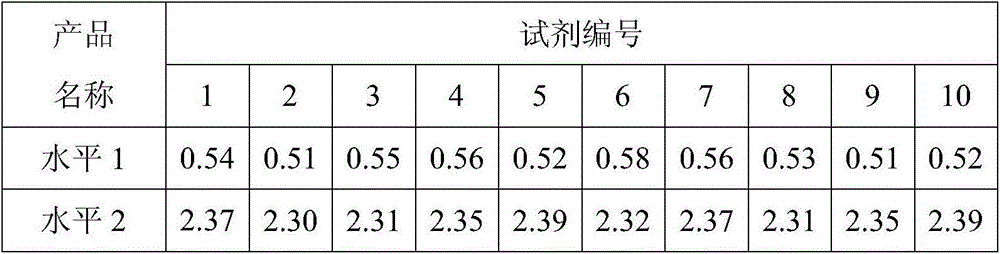
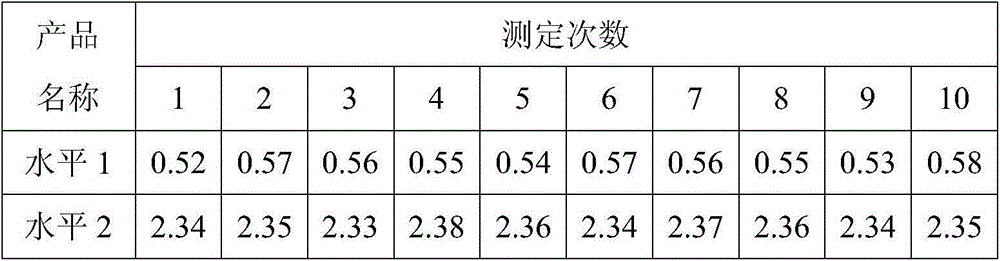
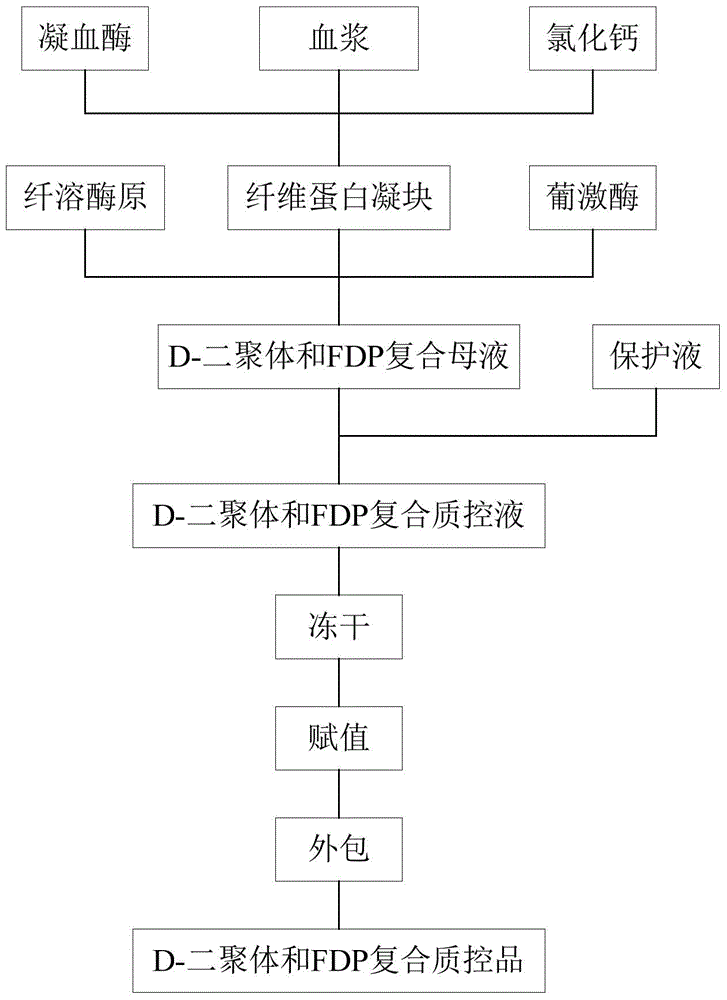
D-dimer and preparation method of FDP composite quality control product
ActiveCN107677839AAvoid wastingAvoid problems such as deactivationMaterial analysisProteinase activityFibrinogen
The invention discloses a D-dimer and a preparation method of an FDP composite quality control product. The preparation method comprises the following steps that 1, fresh blood is poured into a glasscontainer pre-containing glass beads and then is horizontally shaken; 2, white fibrinogen floating on the blood and obtained in the step 1 is re-dissolved; 3, the fibrinogen obtained in the step 2 issubjected to enzymolysis with protease to obtain the D-dimer and an FDP composite mother solution; 4, the composite mother solution obtained in the step 3 is diluted with protection liquid, so that the D-dimer and FDP in the composite mother solution reach target concentration, and the D-dimer and the FDP composite quality control product are obtained. The preparation method is simple in operationand accurate in concentration, large-scale production is facilitated, and the accurate and reliable quality control product is provided for clinical medical detection of D-dimer and the FDP.
Owner:北京众驰伟业科技发展有限公司
Monoclonal antibody against d-dimer and diagnosis agent for detecting d-dimer, crosslinked fibrin and its derivatives containing d-dimer by using the antibody
ActiveUS20190011465A1Immunoglobulins against blood coagulation factorsDisease diagnosisCross-linkMonoclonal antibody
Disclosed are an immunochemical assay device and a method of using the immunochemical assay device for detecting one or more targets or markers such as Cardiac Troponin I, NT-pro-BNP, D-dimer and / or cross-linked fibrin in a fluid sample.
Owner:PRINCETON BIOMEDITECH
Monoclonal Antibody Against D-Dimer and Diagnosis Agent for Detecting D-Dimer, Crosslinked Fibrin and its Derivatives Containing D-Dimer by Using the Antibody
ActiveUS20090298100A1High activityEasy to useImmunoglobulins against blood coagulation factorsDisease diagnosisEpitopeMonoclonal antibody
Disclosed are a monoclonal antibody against human D-dimer produced in a mouse and high molecular weight crosslinked fibrin including a corresponding epitope, a cell line secreting the monoclonal antibody, and a method for manufacturing the same. The anti-D-dimer monoclonal antibody of the present invention may be effectively used as a diagnosis agent for screening and detecting in vivo D-dimer, and high molecular weight crosslinked fibrin and its derivatives containing the D-dimer since the monoclonal antibody specifically reacts with D-dimer, and crosslinked fibrin and its derivatives containing the D-dimer, which do not bind to human fibrinogen or fibrin.
Owner:DOH HYUN JU +2
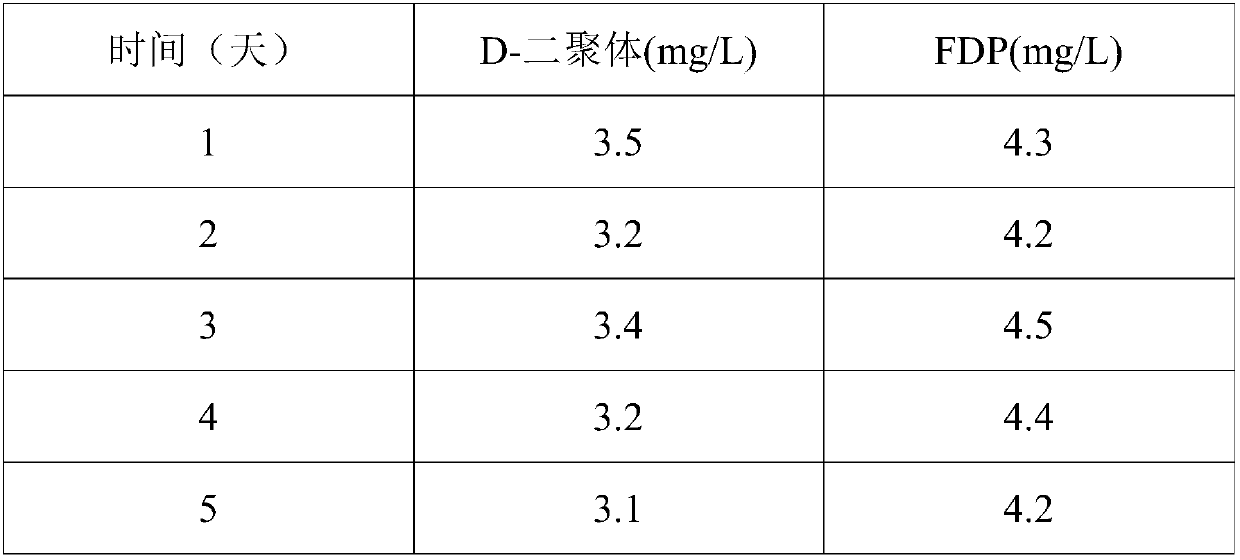
Preparation method of D-dimer quality controls
InactiveCN104459103ASolve problems that need to be added in stepsSolve the problem of step-by-step additionPreparing sample for investigationBiological testingQuality controlEnzyme complex
The invention relates to a preparation method of a detection agent and specifically relates to a preparation method of D-dimer quality controls. The preparation method comprises the following steps: by taking the human plasma as a raw material, mixing the human plasma with an enzyme complex solution, incubating to form fibrin clots, washing the fibrin clots by use of a buffer solution, resuspending the fibrin clots by use of the buffer solution, dissolving the fibrin clots to form a D-dimer mother solution after incubation, adjusting the concentration of the D-dimer mother solution by use of the buffer solution, and adding the human normal mixed plasma to obtain the D-dimer quality controls different in concentration level. The preparation method of the D-dimer quality controls is simple and convenient in process, and low in cost. The human plasma is taken as the major raw material to directly prepare the fibrin; the fibrin dissolving process is as close to the natural fibrin dissolving process inside a human body as possible, and therefore, a product as similar to the human body inside product in properties and structure as possible can be obtained; the product is consistent with the D-dimer in the human body blood in state and properties under normal conditions or sick conditions.
Owner:BLOOD TRASFUSION INST CHINESE ACAD OF MEDICAL SCI +1
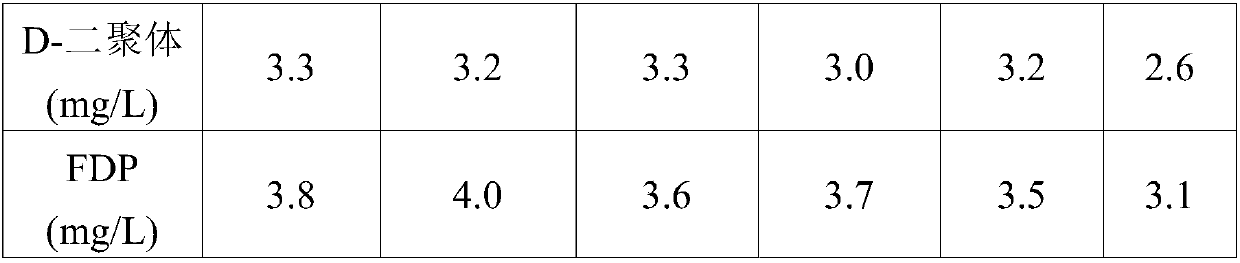
D-dimer and FDP (Fibrin/Fibringen Degradation Product) composite quality control material and preparation method thereof
ActiveCN104458367AGood reconstitution uniformityEasy to preparePreparing sample for investigationBiological testingEnzymatic hydrolysisFreeze-drying
The invention discloses a D-dimer and FDP (Fibrin / Fibringen Degradation Product) composite quality control material and a preparation method thereof. The method for preparing the D-dimer and FDP composite quality control material comprises the following steps: adding thrombin into plasma in the presence of calcium ions, so that the plasma completely coagulates; mashing the coagulated plasma, adding an enzyme solution to carry out an enzymatic hydrolysis reaction so as to degrade fibrous proteins; destructing the enzymatic activity so as to stop the enzymatic hydrolysis reaction after the reaction is complete, thereby obtaining a D-dimer and FDP composite mother solution; and diluting the D-dimer and FDP composite mother solution by using a protective solution till the concentration of D-dimer and FDP in the composite mother solution reaches the target concentration, thereby obtaining the composite quality control material. The composite quality control material which can be simultaneously used for testing two items including the D-dimer and FDP is prepared at a time, the preparation method is simple, and the production cost is low. The freeze-dried composite quality control material is high in re-dissolving uniformity, is uniformly mixed within 15 minutes, can replace an imported product and is simultaneously used for quality control of the D-dimer and FDP.
Owner:SHANGHAI LONG ISLAND BIOTEC CO LTD
Fluorescence immunochromatography kit for simultaneously detecting CRP, lipoprotein phospholipase A2 and D-dimers and preparation method thereof
InactiveCN108872581ASimple structureRealize joint detectionMaterial analysisPhospholipase A2Monoclonal antibody
The invention discloses a fluorescence immunochromatography kit for simultaneously detecting CRP, lipoprotein phospholipase A2 and D-dimers and a preparation method thereof. The kit consists of an immunochromatography test paper strip and a plastic card case, wherein the immunochromatography test paper strip comprises a sample pad, a marker pad, a immunochromatography film, a water suction pad anda bottom plate; a fluorescent marker hs-CRP monoclonal antibody-1, a fluorescent marker Lp-PLA2 monoclonal antibody-1 and a fluorescent marker D-Dimer monoclonal antibody-1 are coated on the marker pad; hs-CRP monoclonal antibody-2, Lp-PLA2 monoclonal antibody-2 and D-Dimer monoclonal antibody-2 are respectively coated on a first detection line, a second detection line and a third detection lineon the immunochromatography film. According to the preparation method, the kit is obtained through fluorescent material processing, antibody marking, marker pad preparation, and immunochromatography film preparation and assembly. The kit realizes the combined detection of hs-CRP, Lp-PLA2 and D-Dimer; the detection and diagnosis efficiency is greatly improved; the structure of the kit is simple; the materials are popularized materials on the market; the industrial production cost can be greatly reduced.
Owner:江苏恒易生物科技有限公司
Novel venous thromboembolism risk and prognosis prediction model
InactiveCN109512388AAid in early diagnosisHelp to judge the prognosisRadiation diagnostic image/data processingComputerised tomographsCase fatality rateDisease course
A novel venous thromboembolism risk and prognosis prediction model is produced through the following steps: (1) establishing a new special personal risk scoring model for assessing the VTE of diabetology inpatients according to the age, the gender, the duration of diabetes, the blood sugar level, the body mass index, the blood pressure, uric acid and other diabetic patient VTE related risk factors; and (2) establishing a diabetolog VTE prognosis model according to the pulmonary embolism severity index score, the dual-energy CT lung perfusion imaging relative enhancement value, the pulmonary artery occlusion index, the C-response protein, the D-dimer and other indicators. The venous thromboembolism risk and prognosis prediction model for diabetology inpatients is clinically verified to be helpful for early diagnosis and judgment prognosis of diabetic patients with venous thromboembolism, so the disability rate and the case fatality rate are reduced.
Owner:顿晓熠
D-dimer detection kit and preparation method thereof
InactiveCN106405076AHigh detection sensitivityImprove bindingMaterial analysisImmune complex depositionLatex particle
The invention relates to the technical field of biology, and discloses a D-dimer detection kit and a preparation method thereof. The kit comprises a buffer solution, D-dimer, a coagulation accelerator, a stabilizing agent, and an antiseptic; wherein the coagulation accelerator is polyvinylpyrrolidone (PVP). By adding polyvinylpyrrolidone (PVP) taken as a coagulation accelerator into the D-dimer detection kit, the combination between an antigen and an antibody is promoted to form a compound rapidly, at the same time, the dissociation of immune complex is inhibited, the precipitate appears rapidly, the detection speed is accelerated; moreover, the non-specific range is not enlarged, and the wide linear range of the D-dimer detection kit is maintained. Furthermore, PVP can fix D-dimer monoclonal antibodies on latex particles, the change of turbidity is specifically enhanced, and thus the detection sensitivity of the kit is improved.
Owner:SHANGHAI KEHUA BIO ENG
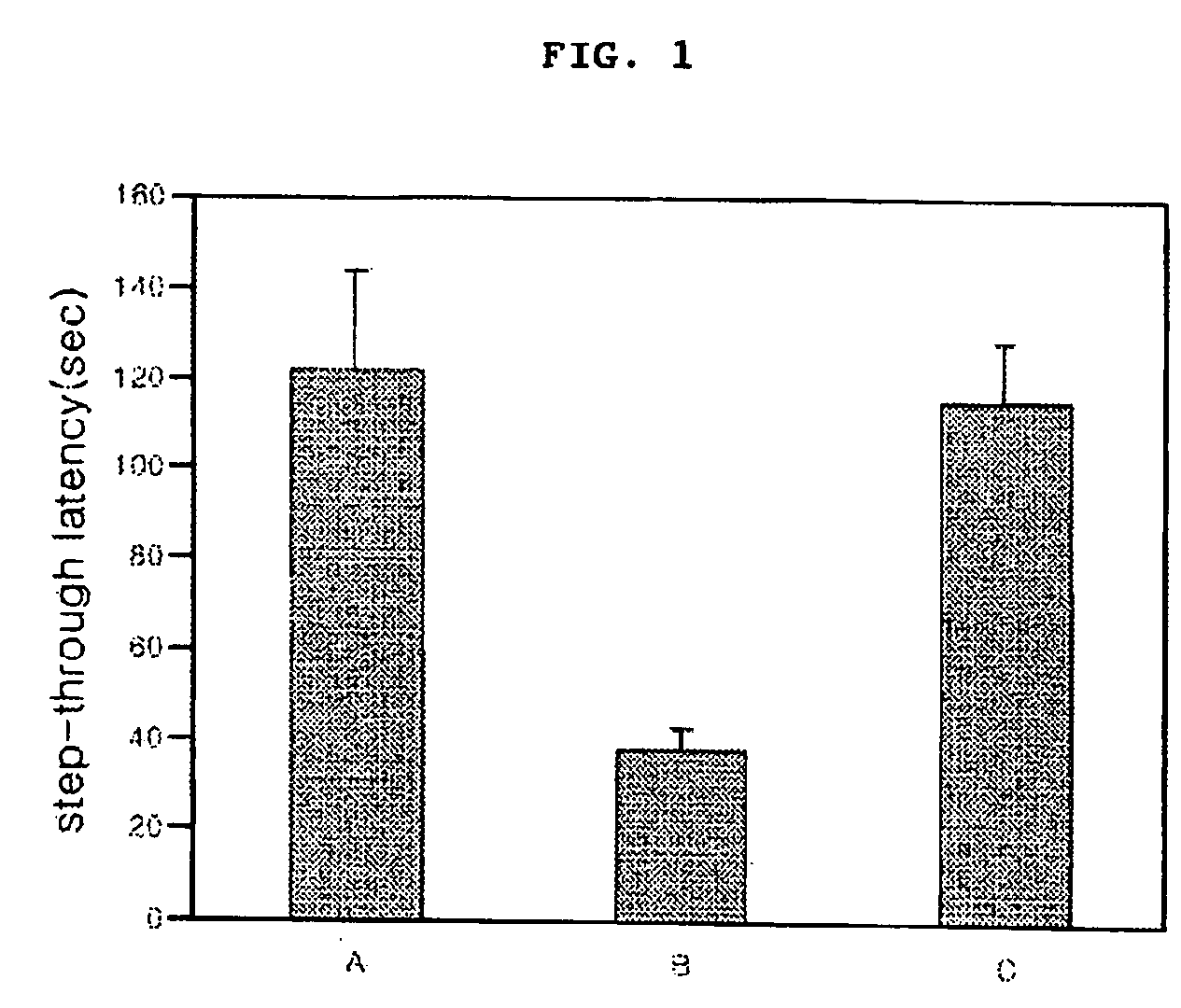
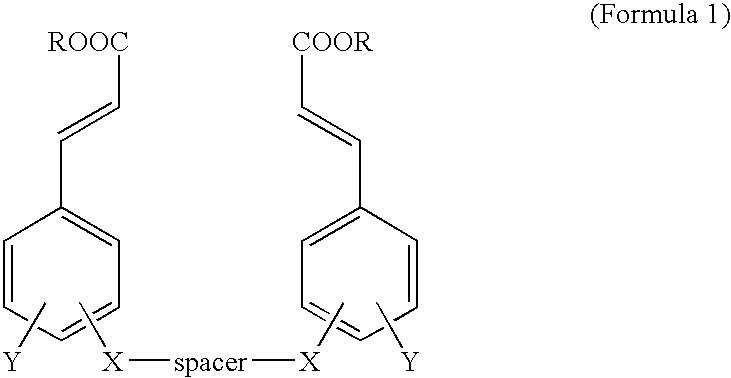
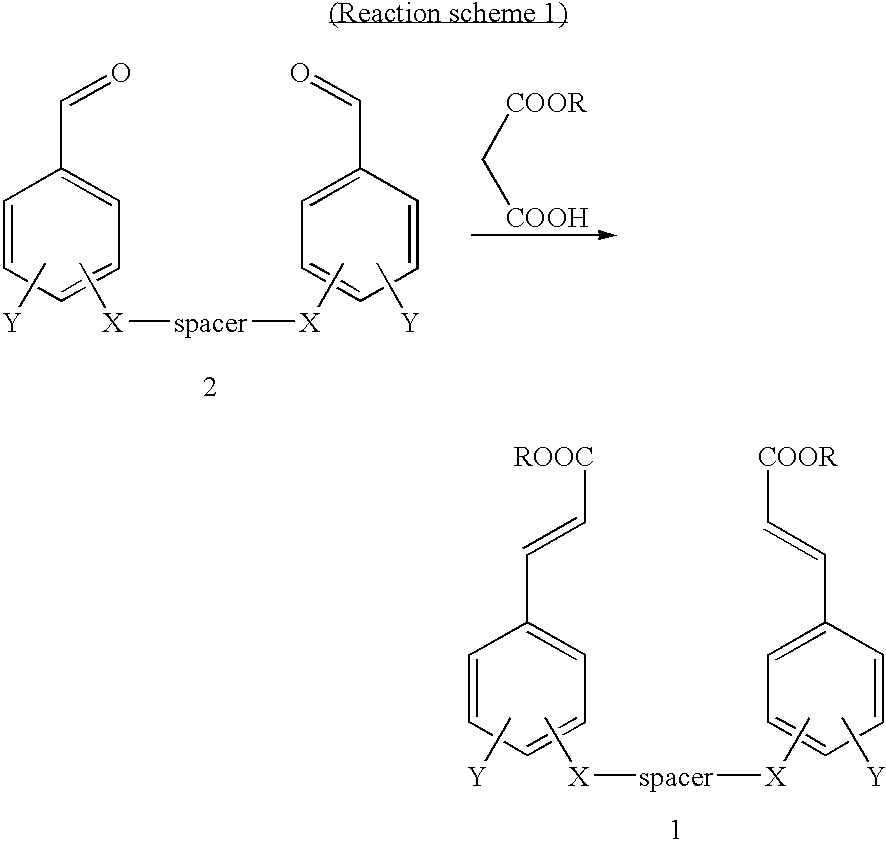
Cinnamic acid dimers, their preparation and the use thereof for treating neurodegenerative disease
InactiveUS20050203180A1Good treatment effectMinimizing side-effectsBiocideNervous disorderMemory retentionSide effect
The present invention relates to cinnamic acid dimers, their preparation and the use thereof for treating neurodegenerative disease, which have excellent effect on enhancing the learning and memory-retention ability in vivo and have fewer side-effects by showing no hormone properties, even when administered for a long period of time, and thus which can be used for neurodegenerative disease including dementia.
Owner:KOREA INST OF SCI & TECH +1
Kit and detection method for detecting D-dimer
InactiveCN109187955AEasy to operateThe test result is accurateMaterial analysisPhosphateMonoclonal antibody
The invention relates to the technical field of chemical analysis and detection and discloses a kit and detection method for detecting D-dimer. The kit is composed of antiserum, a buffer solution, a quality control product, a magnetic card and a stirrer, wherein the antiserum is composed of a phosphate buffer solution and mouse anti-human D-dimer monoclonal antibody coupled latex particles; the buffer solution is composed of the phosphate buffer solution and sodium chloride; the quality control product is a solution containing D-dimer; the mass ratio of the phosphate buffer solution to the mouse anti-human D-dimer monoclonal antibody coupled latex particles is (10-30):1; the mass ratio of the phosphate buffer solution to sodium chloride is 1:(2-3); the magnetic card is used for storing standard curve information of the kit; and the stirrer is used for stirring a to-be-detected sample. Preparing a required reagent on site and drawing a standard curve alone are not needed again when thekit is adopted to detect the D-dimer, so that not only is the detecting time saved, but also the labor force of detecting persons is reduced, in addition, the operation process of testing the D-dimeris simplified.
Owner:GENRUI BIOTECH INC
Kit for measuring content of D-dimer through latex immunoturbidimetry
The invention provides a kit for measuring the content of D-dimer through latex immunoturbidimetry. The kit comprises anti-human D-dimer antibody latex. The latex is formed by polystyrene latex microspheres which are mixed according to the volume ratio of (4-8):1, wherein the average grain diameters of the polystyrene latex microspheres are 300 nm and 60 nm. The anti-human D-dimer antibody latex is formed by polystyrene latex and an anti-human D-dimer antibody which are subjected to chemical crosslinking. According to the kit provided by the invention, the detection limit of the kit is lower than that of an existing similar kit, the detected linearity range, relativity and sensitivity are all higher than those of the existing kit, the detection sensitivity of the D-dimer is improved remarkably, the measurement linearity range of the D-dimer is enlarged, and the problems that the existing kit for measuring the D-dimer is low in detection sensitivity and precision degree and narrow in linearity range are solved.
Owner:山东艾科达生物科技有限公司
Method for detection of, or the risk of, bladder cancer
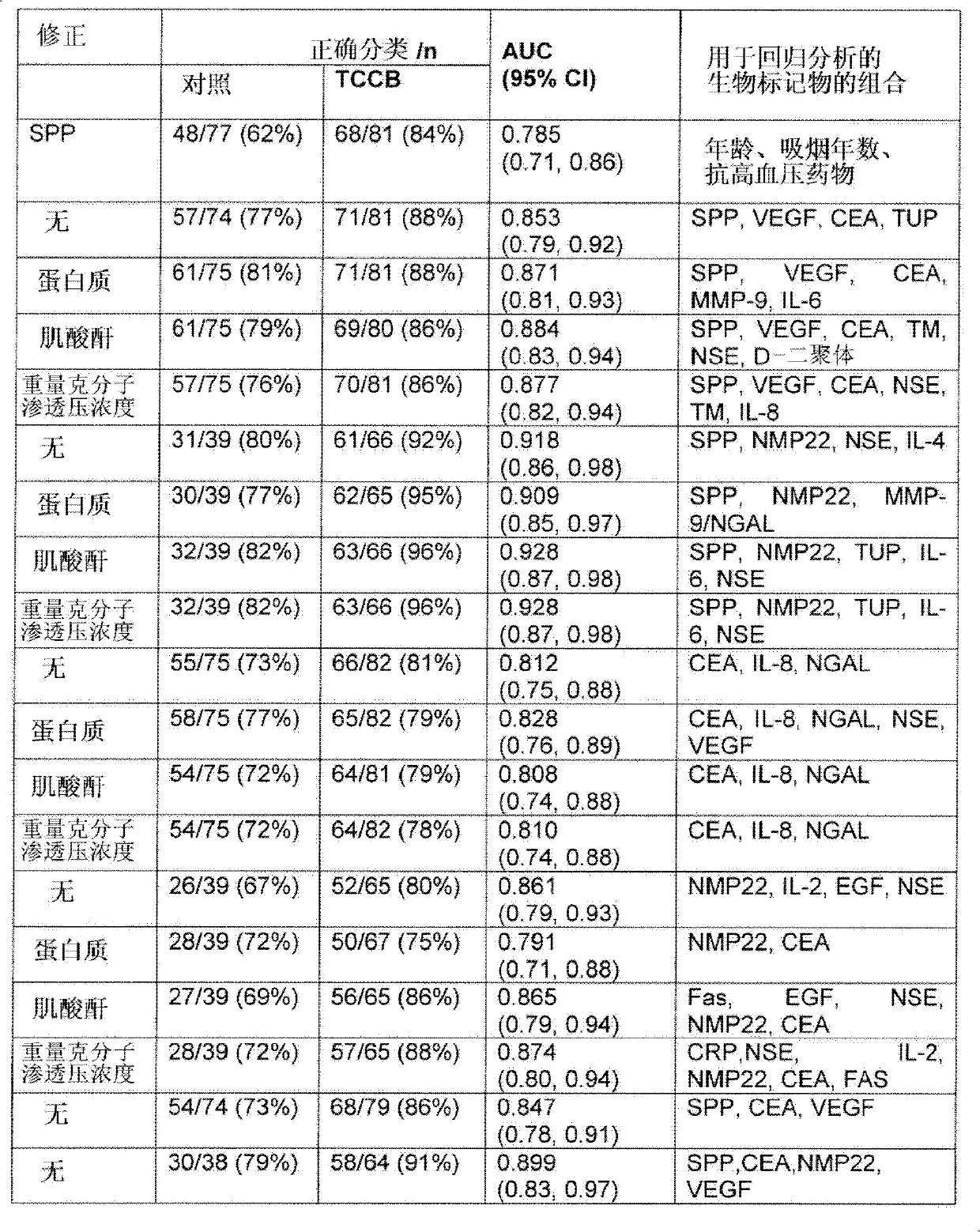
The present invention provides a method for the detection of, or the risk of, bladder cancer in a patient, comprising the step of detecting the presence of a combination of at least two biomarkers selected from CEA, VEGF, IL-8, NGAL, NSE, IL-2, EGF, TM, d-Dimer, MMP-9, IL-6, IL-4, MMP-9 / NGAL, FAS, CRP, TUP and NMP22 in one or more samples isolated from a patient wherein the presence of a combination of at least two biomarkers in the one or more samples indicates the presence or risk of bladder cancer.
Owner:RANDOX LAB LTD
D-dimer detection reagent kit and preparation method thereof
InactiveCN102788878AImprove stabilityImprove accuracyMaterial analysisLatex particlePolyethylene glycol
A D-dimer detection reagent kit, the kit body of the reagent kit comprises two reagents inside: reagent R1: buffer solution, polyethylene glycol, sodium azide, sodium ethylene diamine tetracetate, and bovine serum albumin; reagent R2: buffer solution, latex particles bound with D-dimer monoclonal antibody, Tween-20, sodium azide, sodium ethylene diamine tetracetate, and bovine serum albumin. Mixing a sample with the reagents in a specific volumetric ratio to conduct a series of reactions, placing the reactant under a semi / full automatic biochemical analyzer, and detecting the change speed of light absorbance at the position of 570nm dominant wavelength, thus the concentration of D-dimer can be figured out. The reagent kit has the advantages of accuracy, stability and convenience.
Owner:乐普(北京)诊断技术股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com