Cystatin C detection kit and preparation method therefor
A detection kit and cystatin technology are applied in the field of medical immunology in vitro diagnosis to achieve the effects of good stability, improved sensitivity and good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation of reagent R1: add corresponding weight of PEG6000 and sodium chloride to Tris buffer and mix well, then add NaN 3 , adjust the pH value; the components of reagent R1 are:
[0029]
[0030] Preparation of reagent R2: add CDI to activate polystyrene latex for 12 minutes, centrifuge at 18000g / min for 25min, discard supernatant, add PB buffer to dissolve, add goat anti-human cystatin C polyclonal antibody and shake at room temperature for 1 hour, 18000g / min Centrifuge for 25 minutes, discard the supernatant, and add 0.01mol / L Tris buffer to get it; the components of reagent R2 are:
[0031]
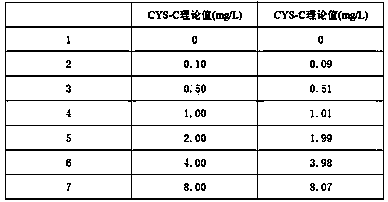
[0032] Preparation of calibrator: Remove corresponding amounts of cystatin C and add them to 0.01mol / L Tris buffer to obtain six gradient calibrator with final concentrations of 0.1, 0.5, 1.0, 2.0, 4.0 and 8.0mg / L .
[0033] Pack the above reagents separately to obtain the cystatin C detection kit
Embodiment 2-5
[0035] Except each reagent component, other is all with embodiment 1, and concrete component is as follows:
[0036] Reagent R1 consists of:
[0037]
[0038] Reagent R2 consists of:
[0039]
Embodiment 6
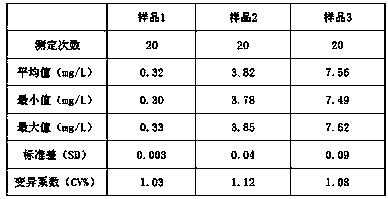
[0041] The minimum detection limit test of the detection kit prepared by embodiment 1-5
[0042] Adopt Hitachi 7080 automatic biochemical analyzer and the kit of the present invention to measure the human serum sample and blank solution (physiological saline or purified water) of known concentration, the specific method is:
[0043]
[0044] After mixing and incubating at 37°C for 30 seconds, read the absorbance A1, and after continuing to incubate for 5 minutes, read the absorbance A2, and calculate ΔA=A2-A1.
[0045] The analysis method is: two-point endpoint method, the main wavelength is 546nm, and the secondary wavelength is 700nm.
[0046] The sensitivity detection result of the kit prepared in Example 1 is as follows:
[0047]
[0048] The minimum detection limit of the kit prepared by the present invention is: [Microsoft user 1]
[0049]The minimum detection limit of the kit prepared by embodiment 2-5 is shown in the following table:
[0050]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com