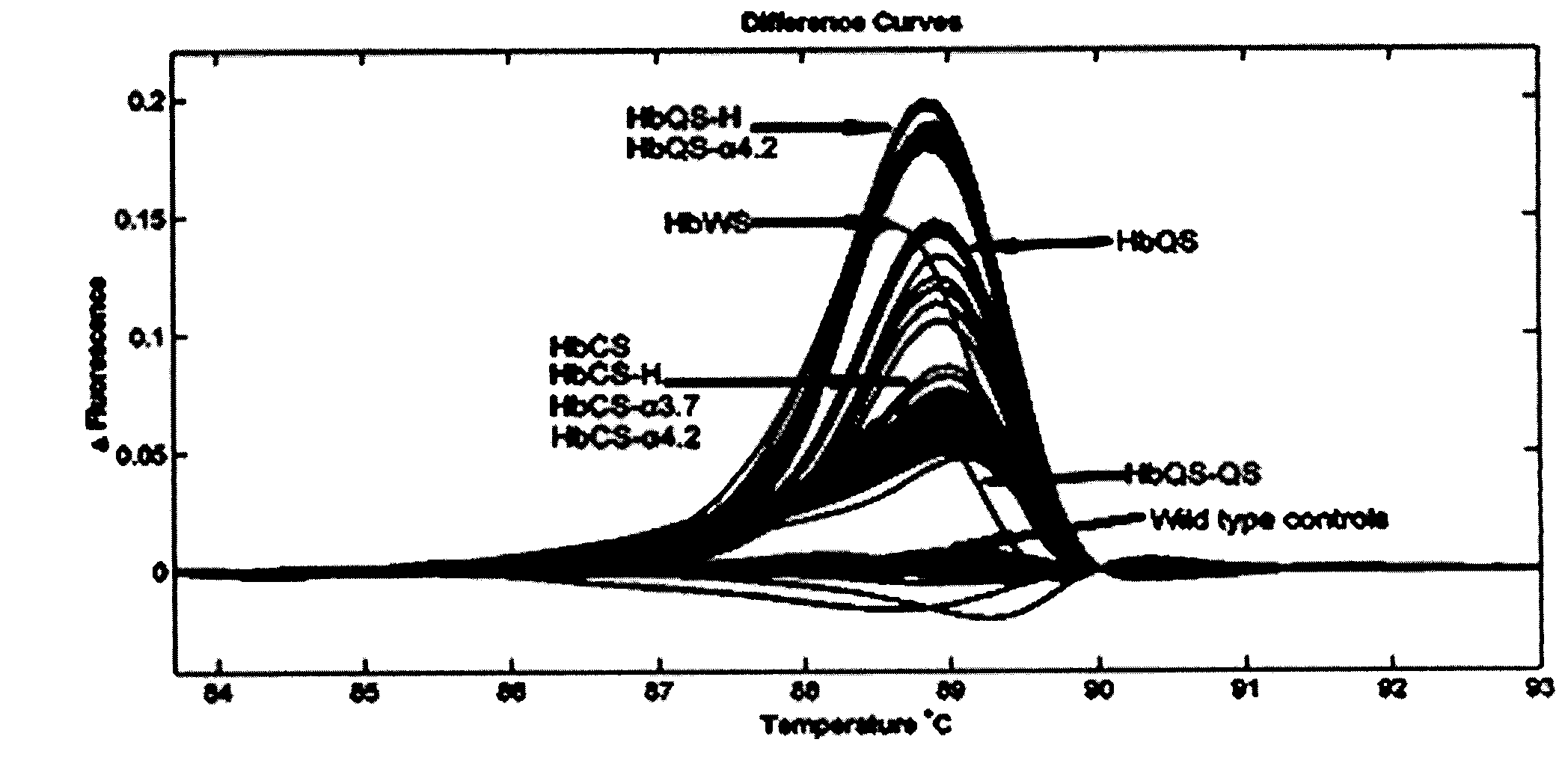
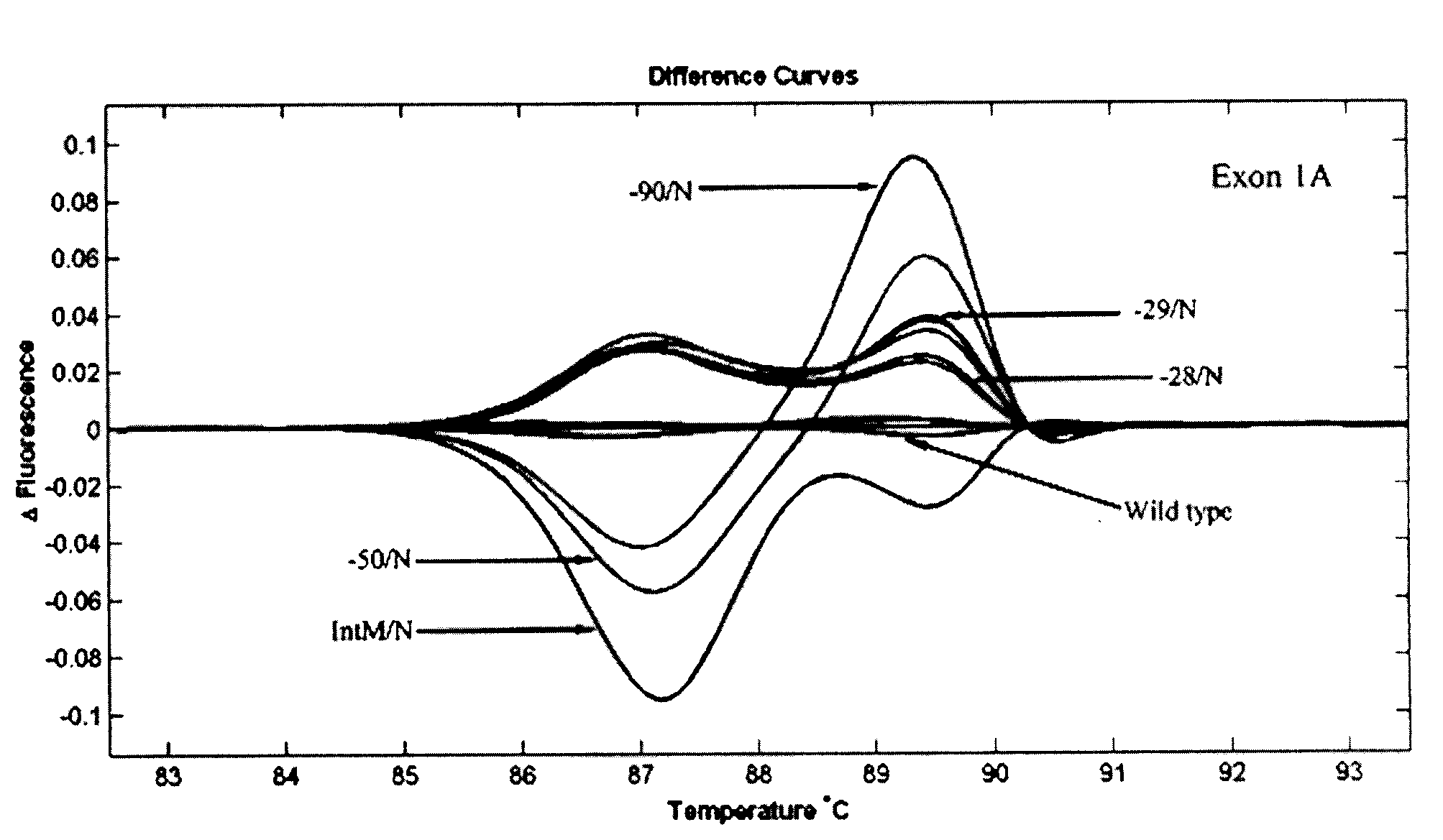
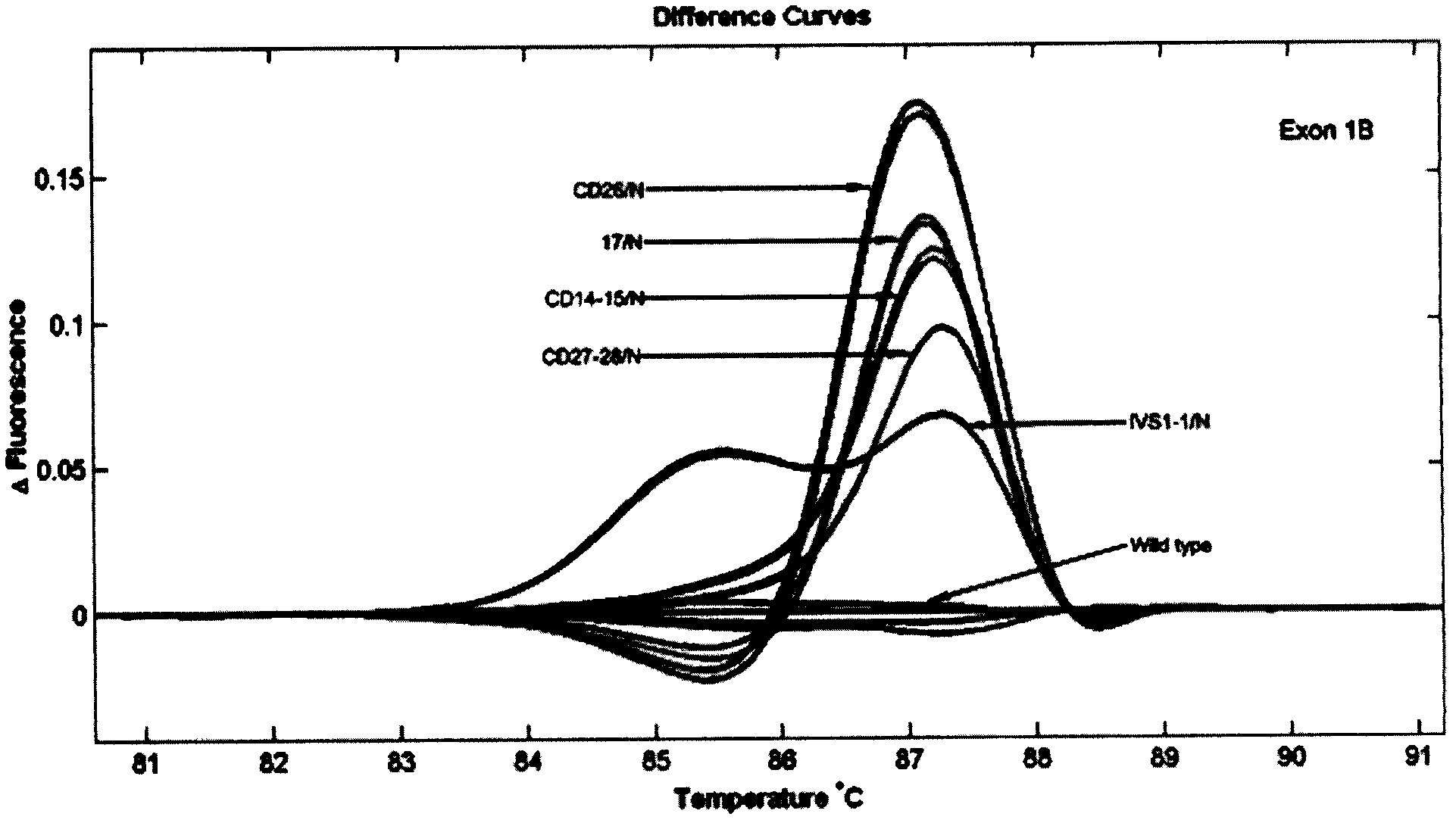
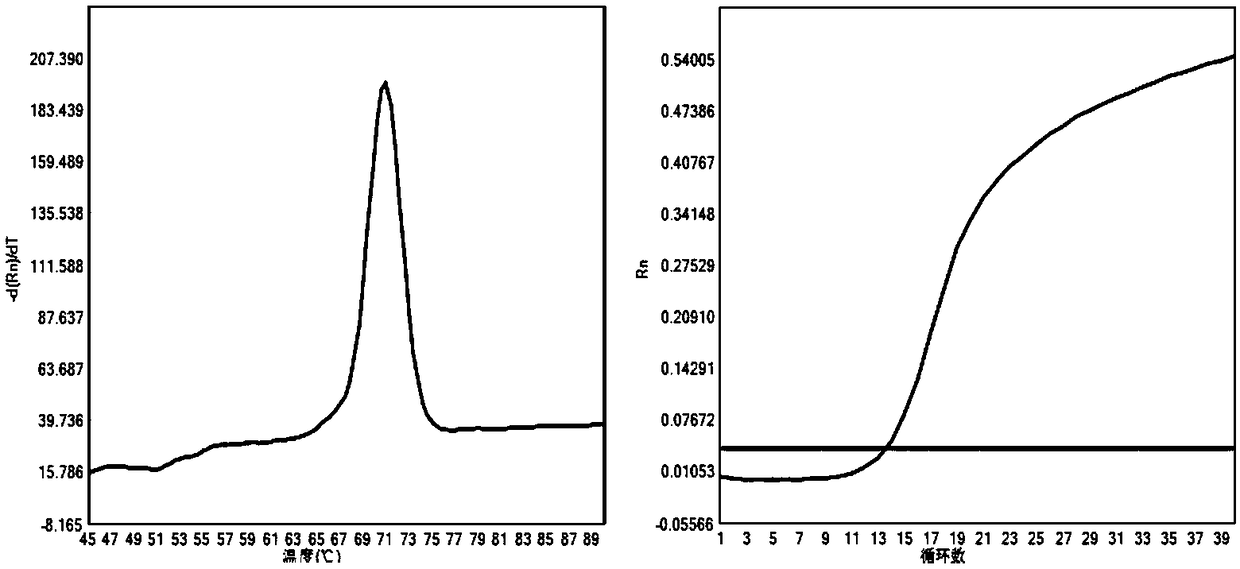
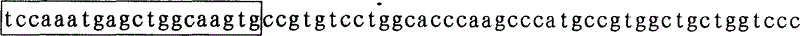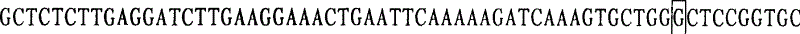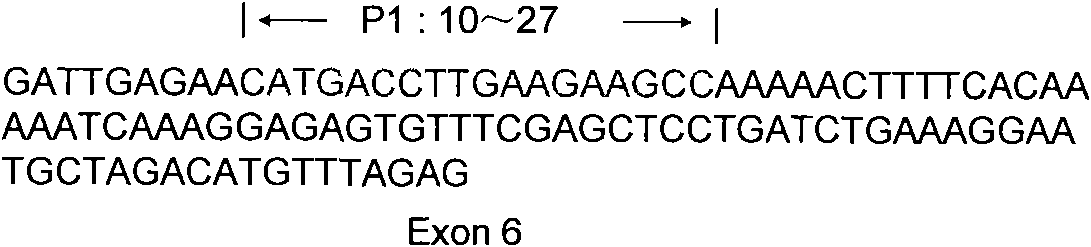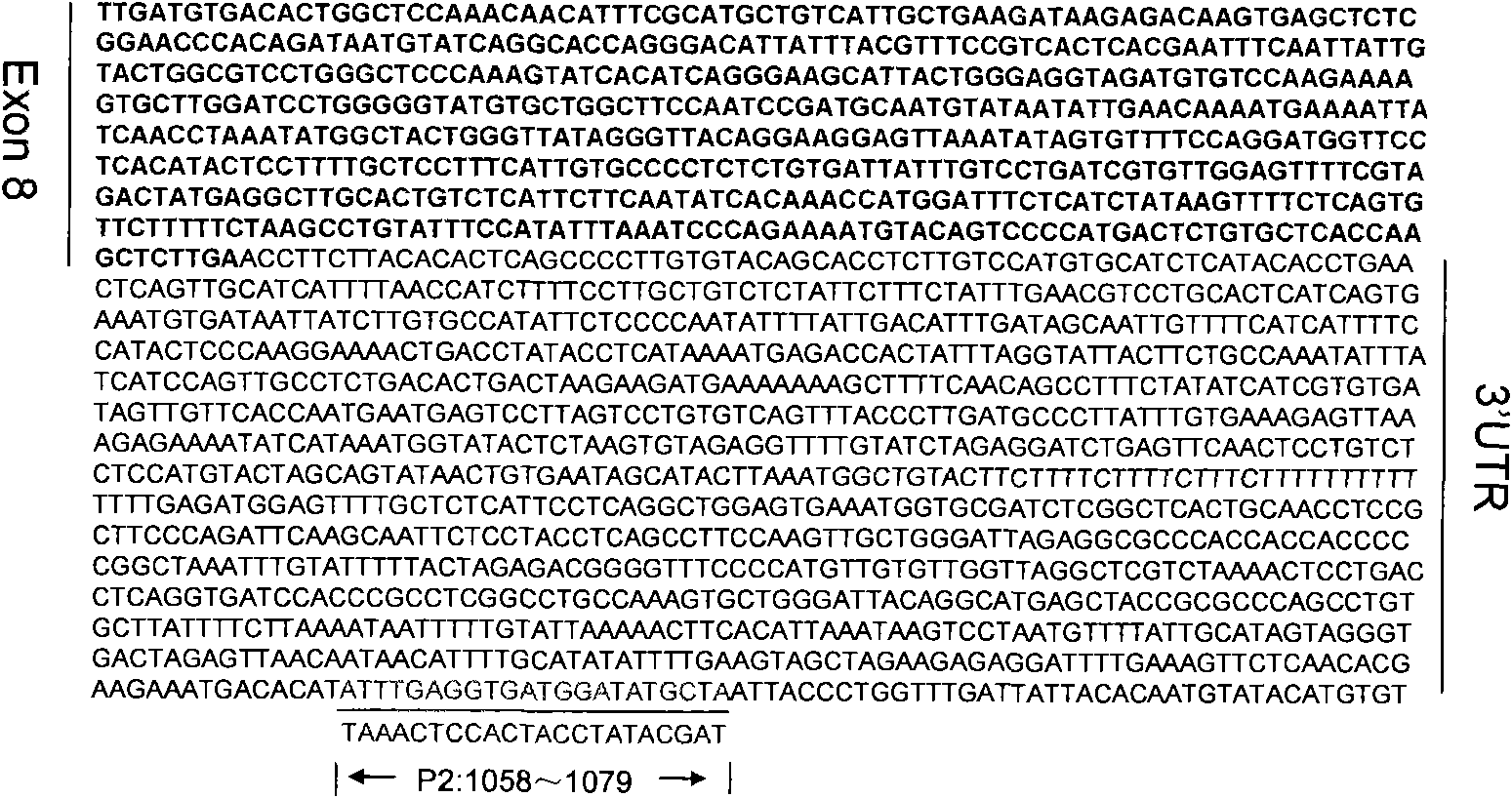Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
110 results about "Heterozygous mutation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Heterozygous (different) mutations mean that you have one copy of the mutation and one copy of the normal MTHFR gene. One of your parents passed down the mutation to you. Heterozygous mutations aren’t as serious as the other types of mutations but they still cause disruption in the bodies processes.
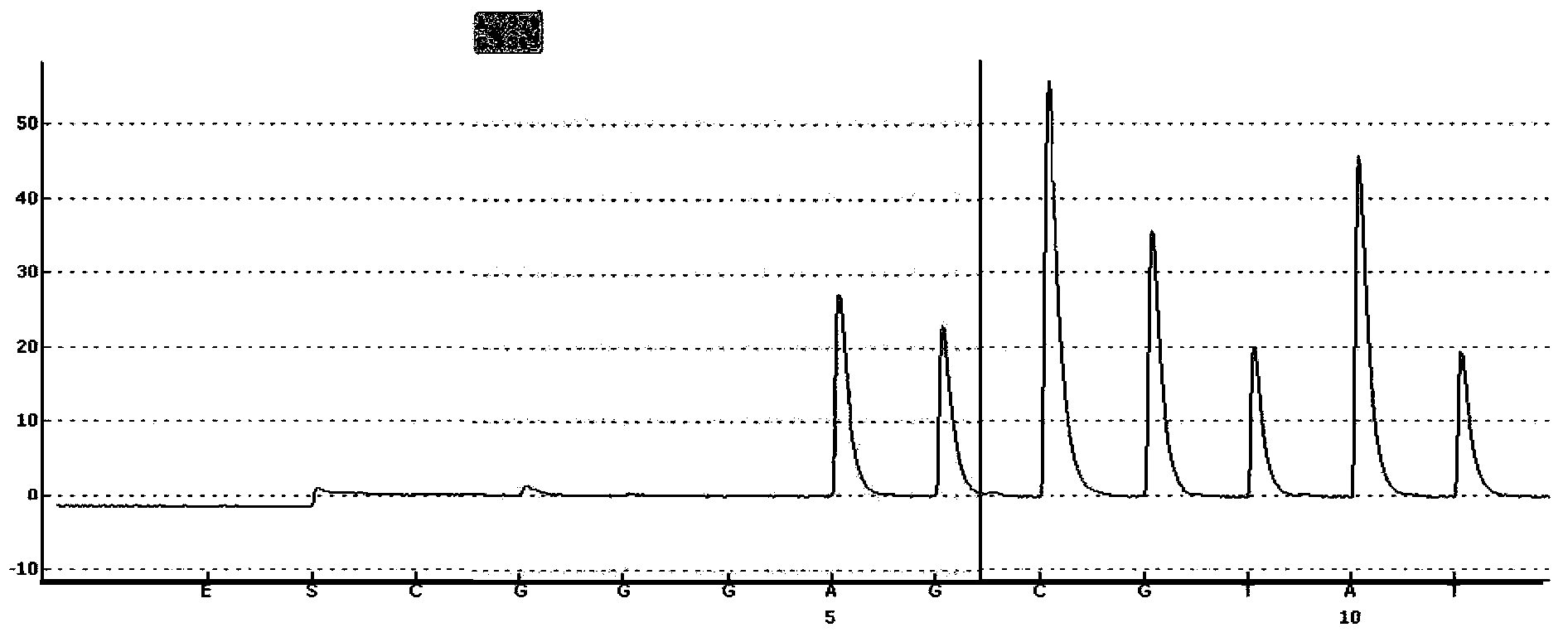
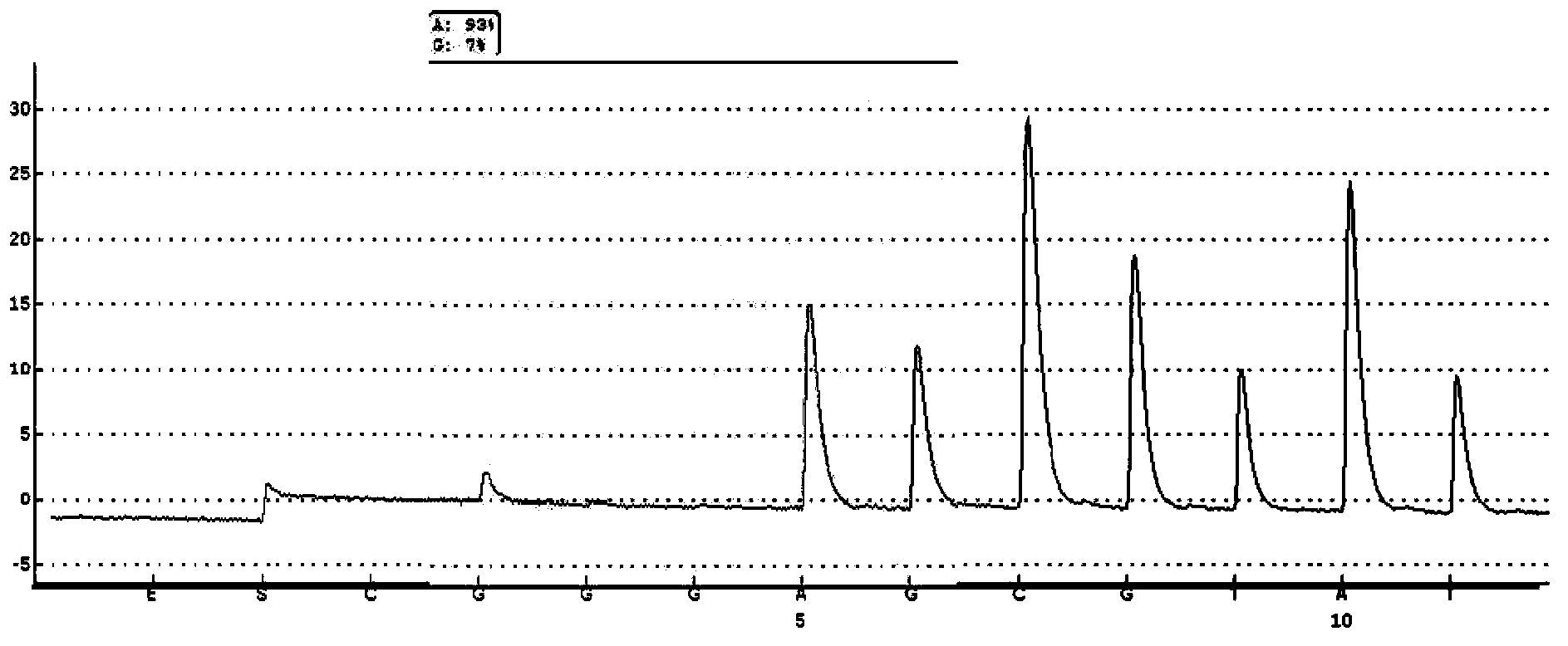
Probe-based analysis of heterozygous mutations using two-color labelling
InactiveUS6013449ASugar derivativesMicrobiological testing/measurementVariant alleleHeterozygous mutation
The invention provides methods of analyzing a nucleic acid in a target sample for variant alleles. In such methods, a first-labelled control sample and a second-labelled target sample are hybridized to at least one set of probes. The control sample comprises a homozygous reference allele. The target sample comprises the homozygous reference allele, or variant alleles differing from the reference allele at a locus, or one variant allele differing from the reference allele at the locus and one reference allele. The probes in the probe set span the locus and are complementary to the reference allele. After hybridization the intensity of first and second label bound to each probe in the set is measured. This information is then used to indicate the presence of one variant allele and one reference allele, or the presence of two variant alleles in the target sample.
Owner:HEALTH & HUMAN SERVICES UNITED STATES OF AMERICA REPRESENTED BY THE +1
Probe-based analysis of heterozygous mutations using two-color labelling
InactiveUS6342355B1Bioreactor/fermenter combinationsBiological substance pretreatmentsVariant alleleGenomic clone
The invention provides methods of analyzing a nucleic acid in a target sample for variant alleles. In such methods, a first-labelled control sample and a second-labelled target sample are hybridized to at least one set of probes. The control sample comprises a homozygous reference allele. The target sample comprises the homozygous reference allele, or variant alleles differing from the reference allele at a locus, or one variant allele differing from the reference allele at the locus and one reference allele. The probes in the probe set span the locus and are complementary to the reference allele. After hybridization the intensity of first and second label bound to each probe in the set is measured. This information is then used to indicate the presence of one variant allele and one reference allele, or the presence of two variant alleles in the target sample.
Owner:UNITED STATES OF AMERICA +1
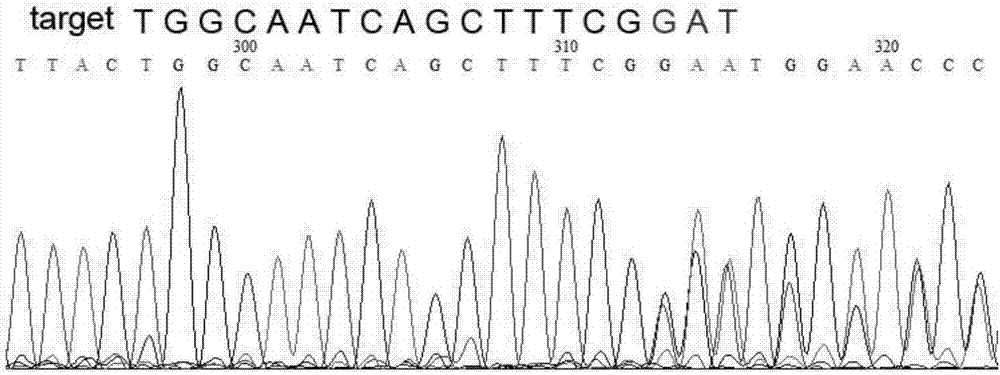
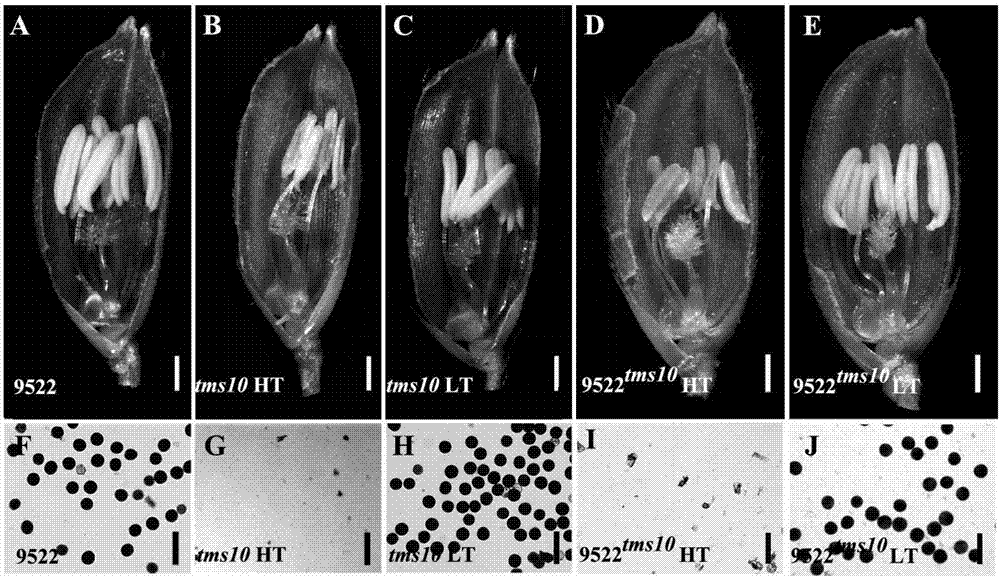
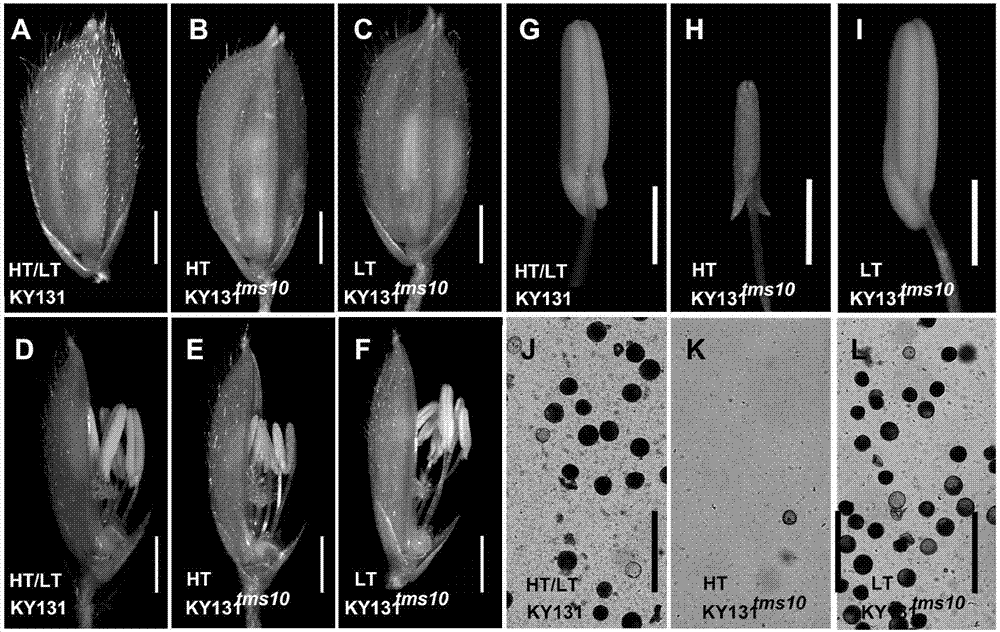
Site-directed knockout system for rice TMS10 gene, and applications thereof
InactiveCN107326042AExhibit male sterilityEfficient breedingTransferasesNucleic acid vectorBiotechnologyGermplasm
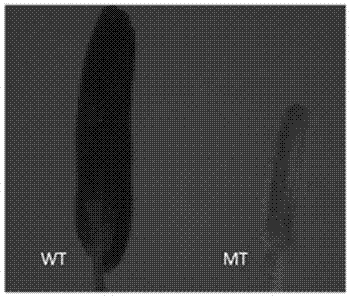
The present invention relates to a site-directed knockout system for a rice TMS10 gene, and applications thereof. The site-directed knockdown system comprises a CRISPR / Cas9 system and a sgRNA target, wherein the sgRNA target is the sequence containing PAM or NGG in a rice male sterile gene TMS10, the CRISPR / Cas9 system is CC-TMS10-1, and the CC-TMS10-1 target sequence is a SEQ ID NO.1 sequence from site 2281 to site 2299. The applications comprise carrying out targeted knockout on the TMS10 genes of different japonica rice varieties and different Indica rice varieties by separately using CC-TMS10-1, wherein the experiment results prove that the heterozygous mutation transformation plant produced through the site-directed knockout induction in different rice varieties shows the temperature sensitive male sterility characteristic. According to the present invention, the site-directed knockout system provides the efficient breeding method for the creation of the temperature sensitive male sterile line germplasm resources based on the rice temperature-sensitive male sterile gene TMS10 and the rice hybridization breeding.
Owner:SHANGHAI JIAO TONG UNIV
Gene for controlling male reproduction and development of rice and application of gene
InactiveCN107446932AIncrease productionExpand advantage utilizationPlant peptidesFermentationBiotechnologySequence analysis
The invention discloses a gene for controlling male reproduction and development of rice and application of the gene. The gene has a nucleotide sequence represented by SEQ ID NO.1, and a protein encoded by the gene has an amino acid sequence represented by SEQ ID NO.2. By targeting a LOC_Os08g20730 gene design target by virtue of a CRISPR / Cas9 gene editing system, a receptor is a middle flower 11, and a targeted T0 generation phenotype are shown in the figures 1, 2, 3 and 4 in the description; and sequencing analysis shows that anther development abnormality occurs in a homozygously mutated plant or a biallelically mutated plant and pollen does not exist. The analysis of a homozygously mutated T1 generation shows that the separation proportion of fertile plants to sterile plants is 3 to 1. The integration shows that the gene is capable of regulating and controlling the reproduction and development of rice, once the gene in the rice is damaged, the anther development abnormality occurs, and non-pollen sterility occurs. According to the obtained cell nucleus gene for controlling the reproduction and development of the rice, an intelligent sterile line can be created by virtue of a molecular measure, and excellent parents in the production are improved into excellent sterile lines, so that the heterosis utilization is expanded.
Owner:JIANGXI ACAD OF AGRI SCI
Epidermal growth factor receptor (EGFR)gene sequencing detection method
InactiveCN1737162ASignificant effectMicrobiological testing/measurementHeterozygous mutationEpidermal Growth Factor Receptor Genes
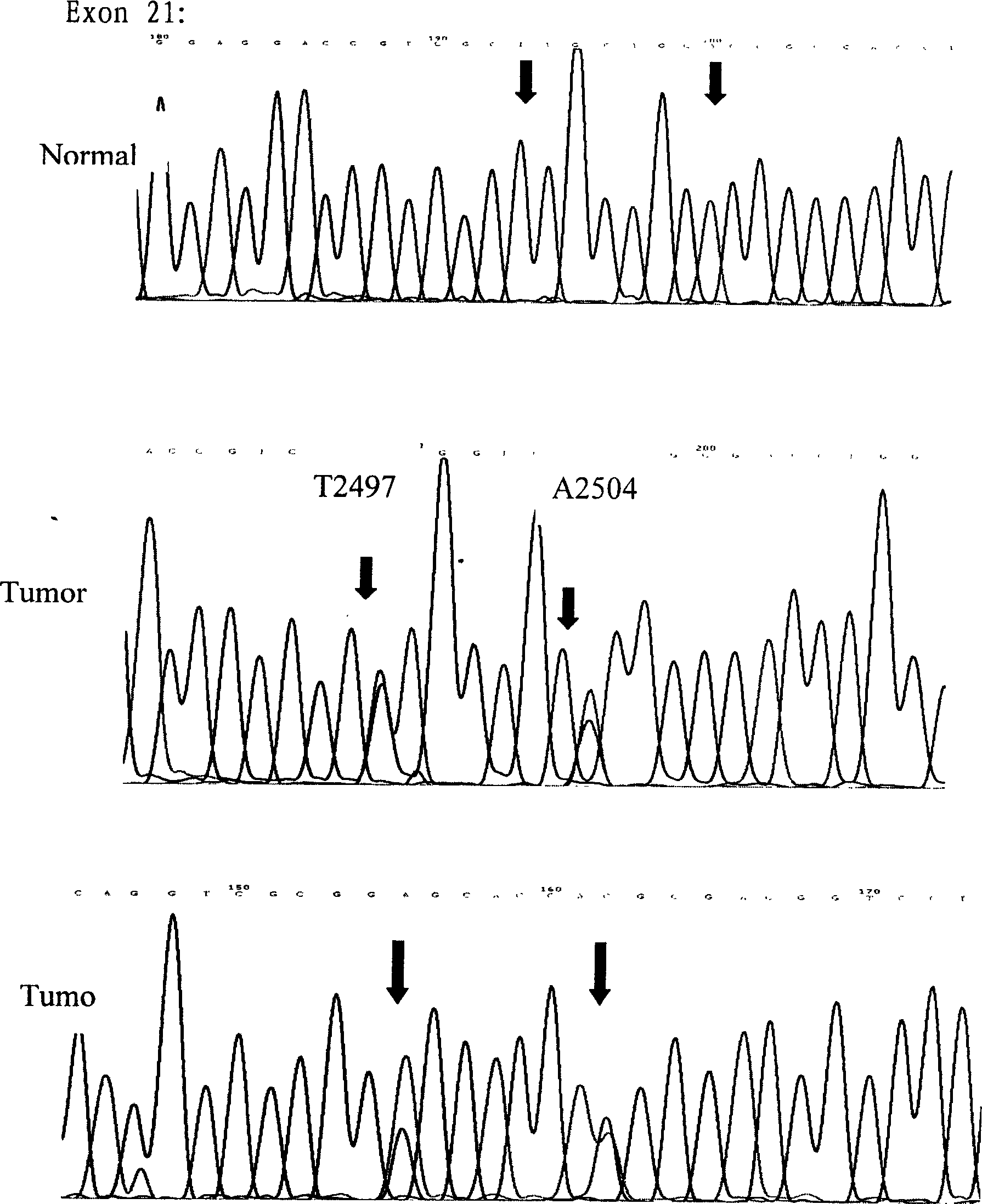
The invention relates to an epidermal growth factor receptor gene detection and analysis method, wherein the No. 18, 19 and 21 extron gene fragment of the EGFR can observe and analyze the gene sequence and mutation characteristic unique for the Chinese, on the level of bases and amino acids one by one. The No. 21 extron T>G mutation and A>T and No. 852 codon inserted G mutation discovered by this inventor are all novel heterozygous mutations not reported domestically and abroad, thus pertinency is achieved when used for identification of targeting medicinal treatment for the Chinese. The invention provides a process for detecting EGFR gene mutation, the result of which is used as the basis to decide whether to take medicines.
Owner:南京中医药大学附属医院(江苏省中医院) +2
Primer in use for in vitro diagnosing GJB2 mutation of deaf gene of autosomal recessive inheritance in non-syndrome
InactiveCN1873027AAbundant sources of supplyLow costMicrobiological testing/measurementForward primerAutosomal recessive inheritance
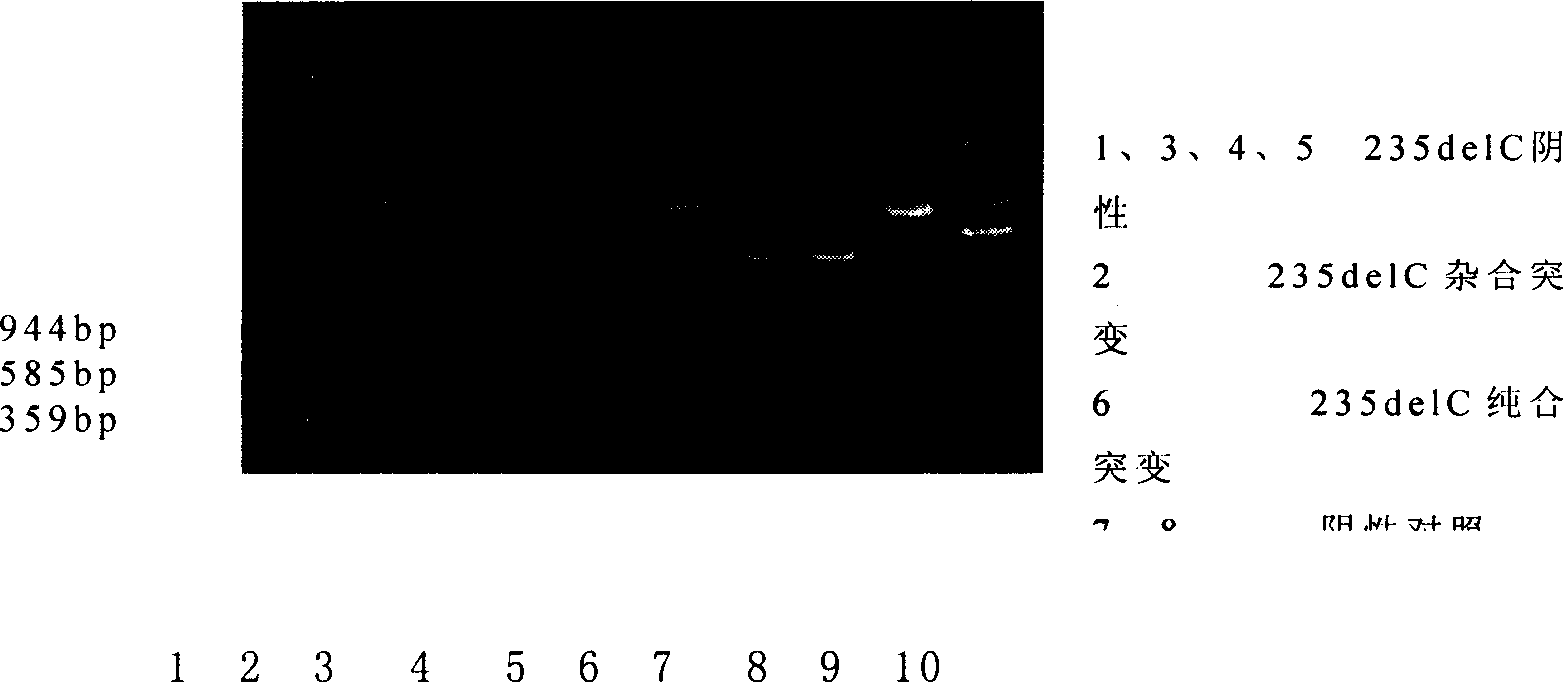
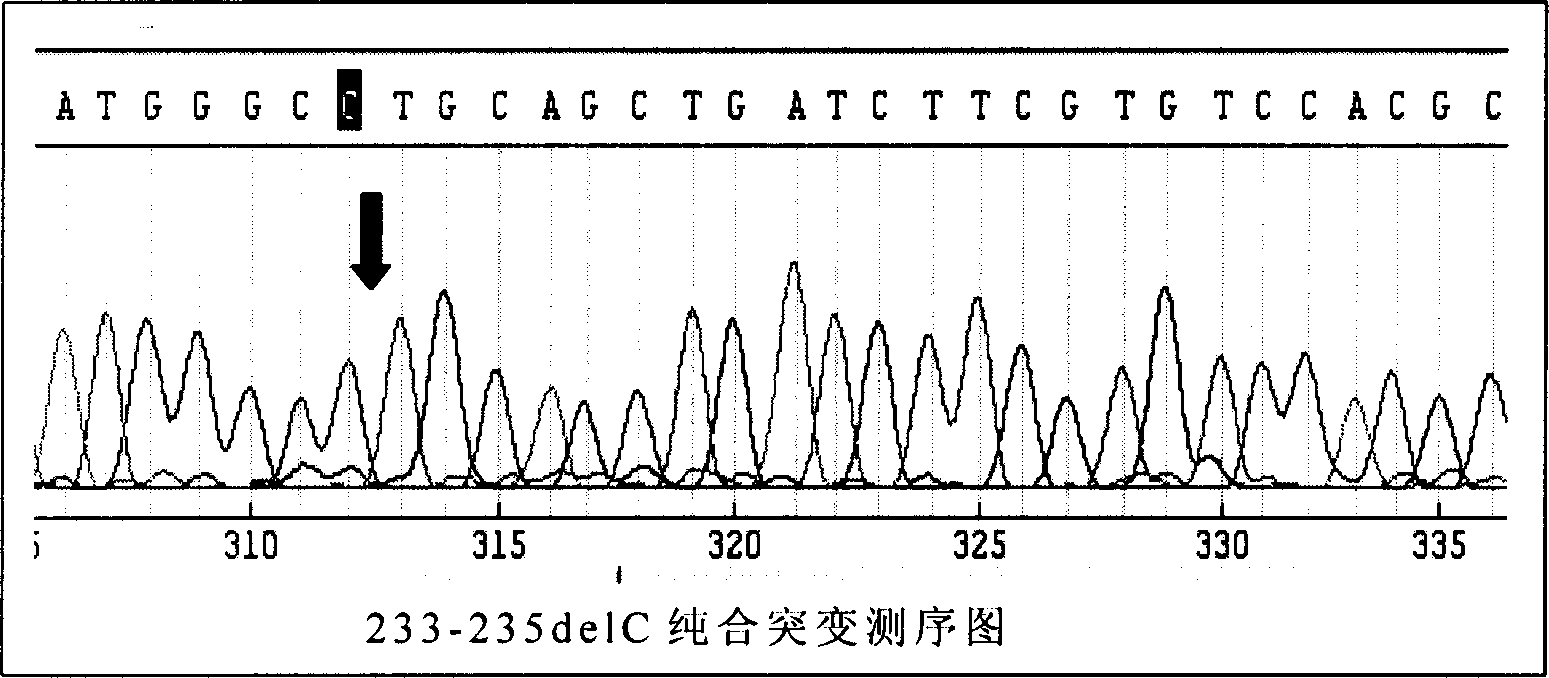
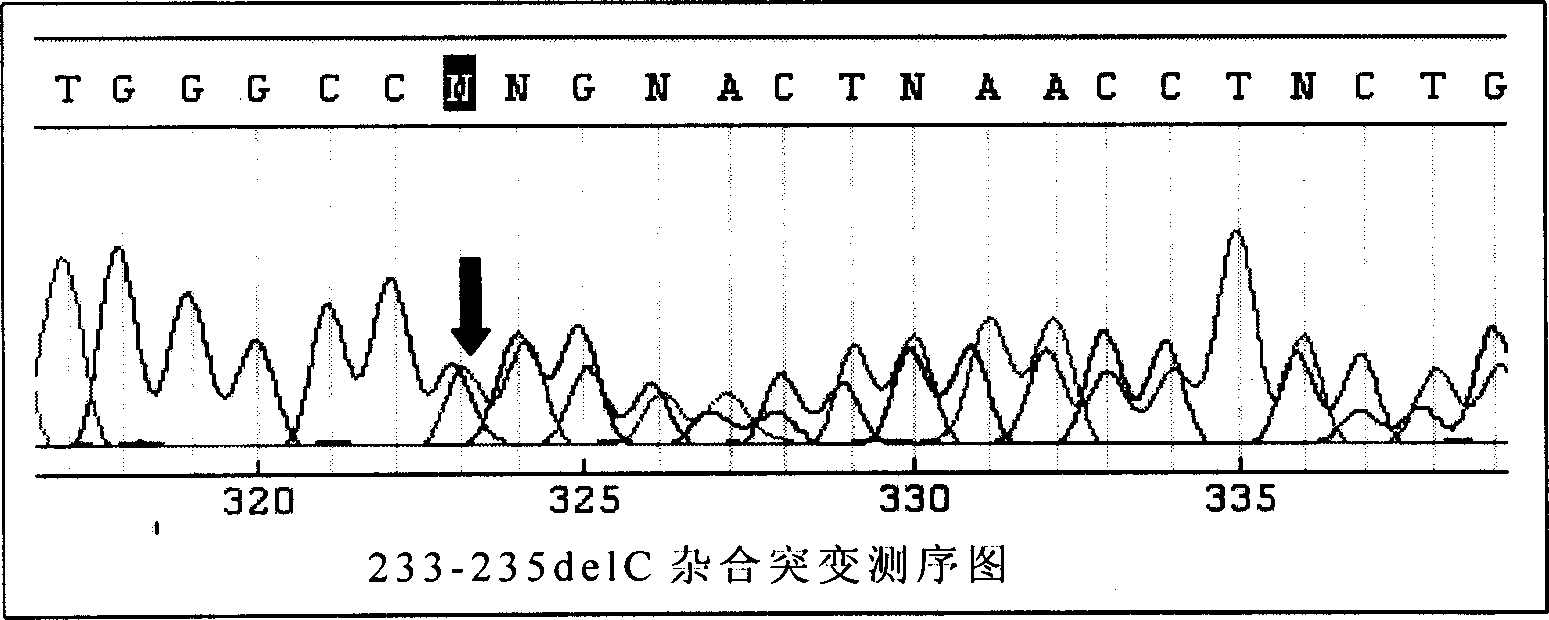
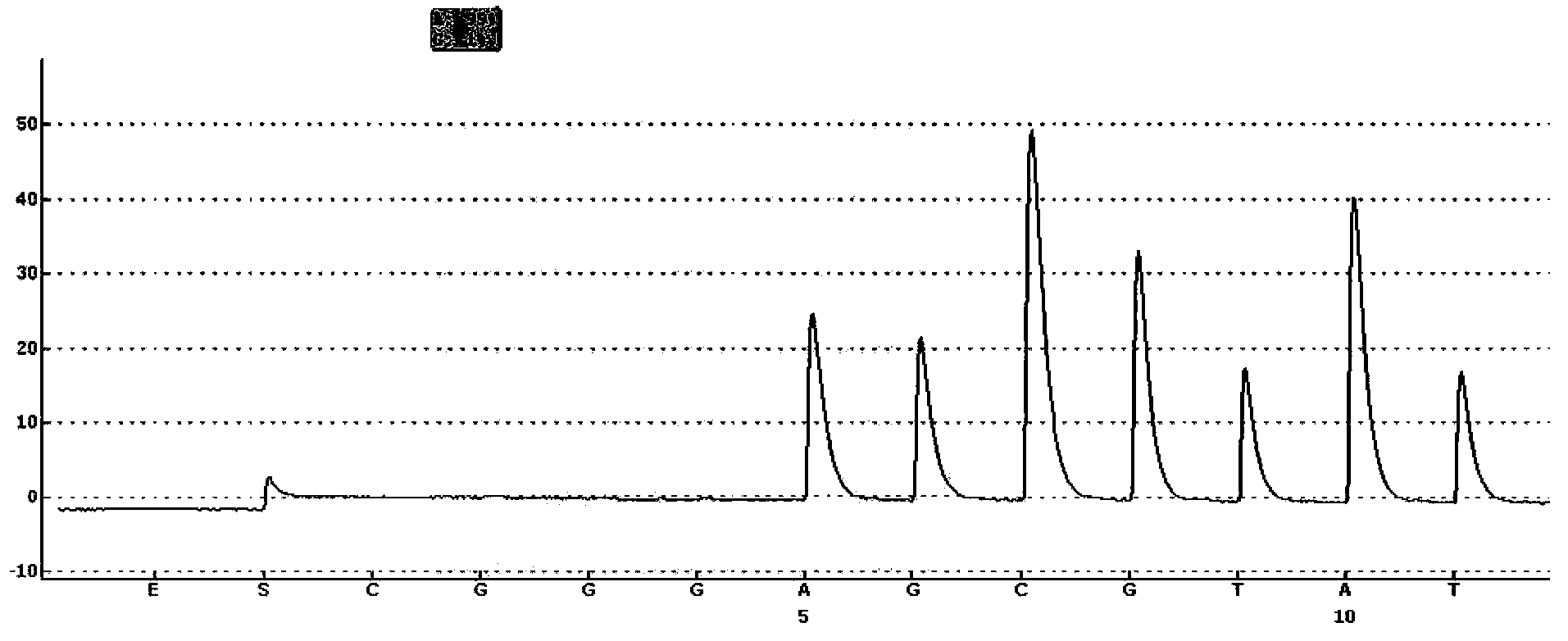
This invention provides primers for in vitro diagnosis of mutation of autosomal recessive nonsyndromic hearing loss gene GJB2. The primers can be used for completely amplifying the coding region and upstream or downstream important splicing sequences of GJB2 gene. This invention also provides a test kit for containing the primers and ApaI restriction endonuclease and their application for in vitro detection of 233-235delC mutation of the complete GJB2 coding region. The test kit can also be used for detecting the mutation sites of the GJB2 coding region through sequencing by the forward primer, and detecting deletion or insertion heterozygous mutation through sequencing by the reverse primer. The test kit is suitable for large-scale screening of autosomal recessive nonsyndromic hearing loss gene GJB2 mutation and genetic counseling.
Owner:山东三月三基因技术有限公司
Kit for detecting folic acid utilization ability gene and detection method thereof
InactiveCN102676645AImprove throughputImprove performanceMicrobiological testing/measurementBiologyDNA
The invention discloses a kit for detecting folic acid utilization ability gene and a detection method of the kit, relating to the technical field of gene detection, wherein the kit for detecting folic acid utilization ability gene comprises (1) a PCR (polymerase chain reaction) reaction reagent including dNTP, 5* and 10*PCR buffer liquid, Mg<2+> ion, de-ionized water, FastTaq enzyme, SNaPshot Mix mixed liquid; (2), a mixture obtained by mixing 16 pairs of PCR primers according to a certain proportion; (3), a mixture obtained by mixing 16 extended primers according to a certain proportion; (4), SAP enzyme for purification, Exon I enzyme and buffer liquid matched with the enzymes; (5), positive and negative control DNA with single locus subjected to homozygous or heterozygous mutation; (6), an instruction manual. The method provided by the invention is simple, high in flux, high in effect and low in cost, and is suitable for detecting folic acid utilization ability gene for Chinese population.
Owner:SUZHOU MUNICIPAL HOSPITAL
Standard substance and kit for detecting mitochondrial A3243G heterozygous mutation rate and detection method
ActiveCN103451268AHigh sensitivityQuantitatively accurateMicrobiological testing/measurementVector-based foreign material introductionQuantitative determinationWild type
The invention discloses a standard substance and a kit for detecting mitochondrial A3243G heterozygous mutation rate and a detection method. The standard substance for detecting mitochondrial A3243G heterozygous mutation rate provided by the invention comprises: (1) a vector containing a mitochondrial DNA fragment with 3243 locus being a wild type A; and (2) a vector containing a mitochondrial DNA fragment with 3243 locus being a mutant G. The plasmid molecule standard substance, the detection method and a regression equation used for detecting data correction provided by the invention can conveniently and accurately detect A3243G heterozygous mutation rate in the mitochondrial DNA, and have advantages of high efficiency and sensitivity. In addition, the plasmid molecule standard substance, the detection method and the regression equation can accurately and quantificationally detect heterozygous mutation with mutation rate lower than 5%, which is a unique advantage unachievable by other heterozygous mutation detection means. The invention also has good reference meaning for quantitative determination of other heterozygous mutation in scientific research and production practice.
Owner:SHANGHAI CHILDRENS HOSPITAL +1
Hereditary hearing loss gene mutation detection kit
PendingCN106566880AA large number of detection pointsImprove accuracyMicrobiological testing/measurementMultiplex ligation-dependent probe amplificationPositive control
The invention a mutation detection kit used for performing qualitative detection on 20 gene loci related to hearing loss in a genome DNA. The kit comprises a DNA diluent, a hybridization reaction liquid, a hearing loss probe mixed liquid A, a hearing loss probe mixed liquid B, a connecting reaction liquid A, ligase, a connecting reaction liquid B, polymerase, a PCR amplification primer mixed liquid, positive control, and negative control. The kit provided by the invention performs gene mutation detection mainly by combination of a multiplex ligation-dependent probe amplification (MLPA) technology and capillary electrophoresis, so that multiple mutation loci can be detected at the same time; wild type, homozygous mutation type and heterozygosis mutation type of 20 loci can be accurately determined by two tubes concurrently; and meanwhile, a primer probe is also designed, so that quite high accuracy and specificity are achieved.
Owner:哈尔滨星云生物信息技术开发有限公司
Pathogenic mutation of osteogenesis imperfecta disease and detection reagent of pathogenic mutation
ActiveCN109897894ASignificant genetic heterogeneityBirth preventionMicrobiological testing/measurementGenetic engineeringCol1a1 geneAmino acid change
The invention discloses a pathogenic mutation of osteogenesis imperfecta and a detection reagent of the pathogenic mutation. According to a novel mutant COL1A1 gene, the mutated COL1A1 gene is a single point mutation c.1822G>A (chr17:48270211), a heterozygous mutation is pathogenic and in a mode of dominant heredity, the amino acid change is p.Gly608Ser, dyssynthesis of I-type collagen in connective tissue is caused by locus mutation of the p.Gly608Ser, and a lesion is formed. A kit for detection of osteogenesis imperfecta includes a reagent for detection of the 1822bpth locus of a COL1A1 geneCDS or a reagent for detection of the 608th amino acid locus of a COL1A1 protein. The pathogenic mutation (c.1822G>A on the COL1A1 gene) of the osteogenesis imperfecta disease is obtained, and the osteogenesis imperfecta disease can be diagnosed by detecting the mutation.
Owner:黄欢
Congenital adrenal hyperplasia gene screening kit, screening method and application thereof
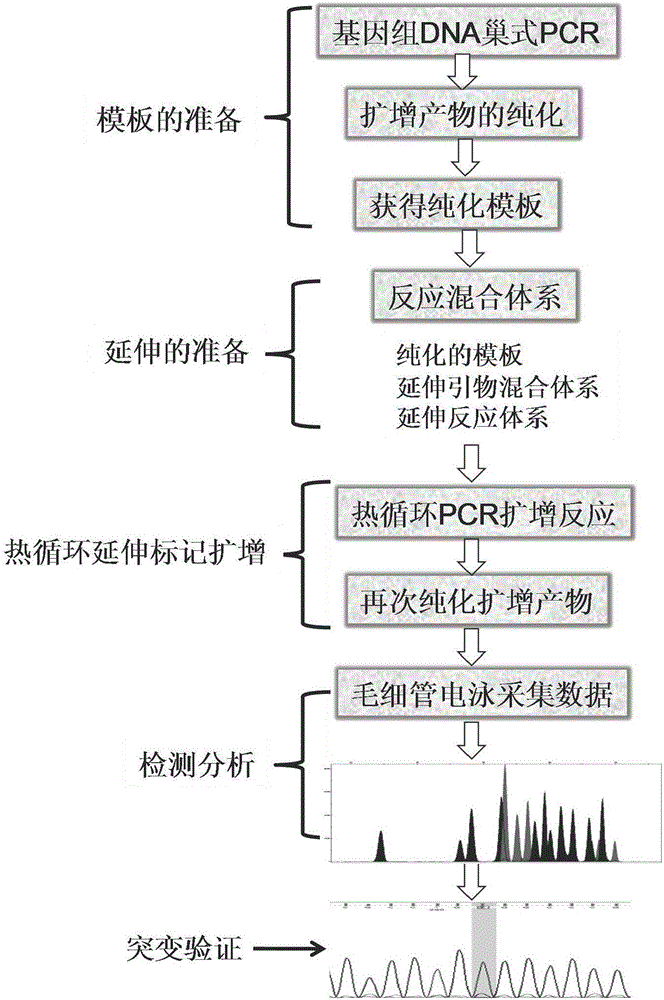
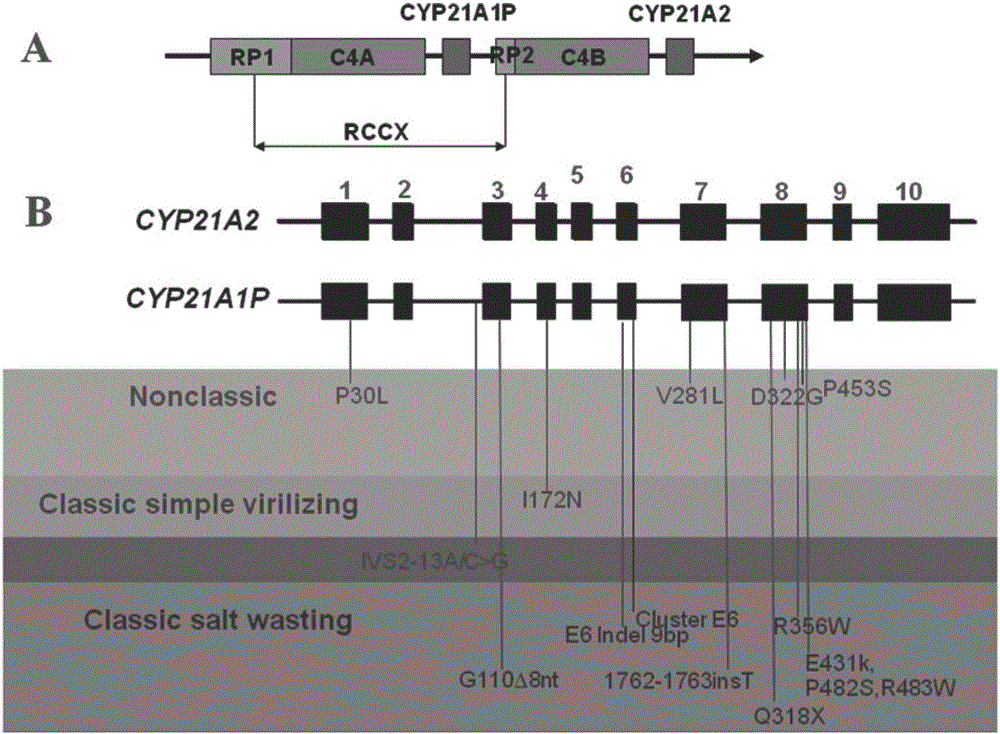
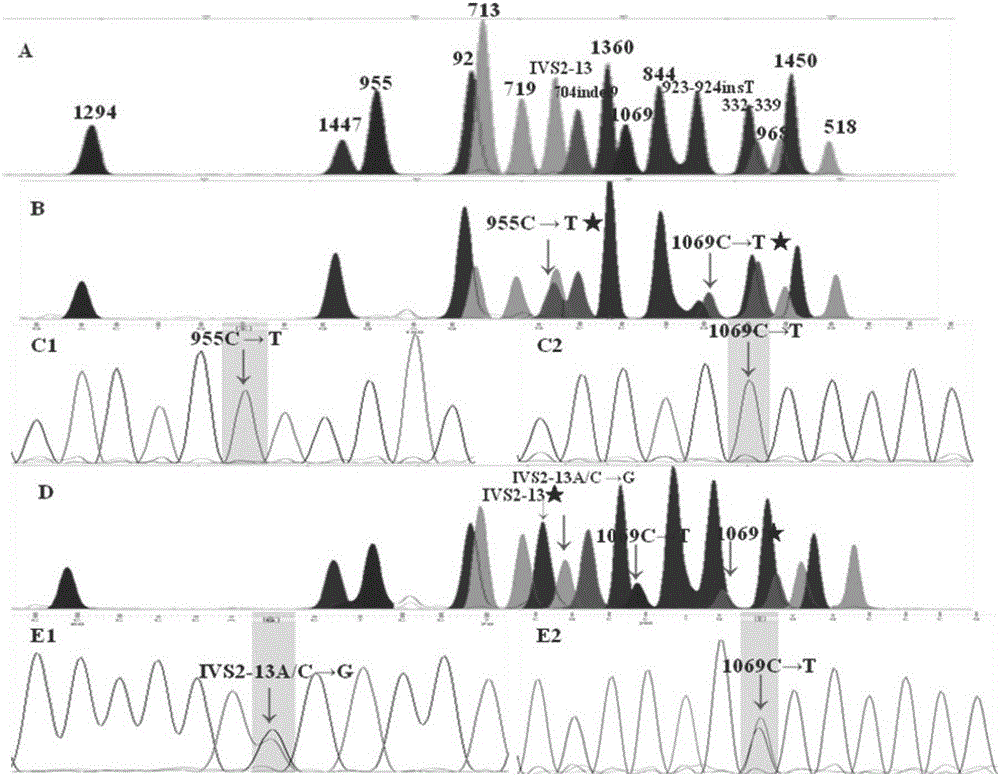
InactiveCN106434859AImprove accuracySimple and fast operationMicrobiological testing/measurementScreening methodMutation screening
The present invention discloses a congenital adrenal hyperplasia gene screening kit. The congenital adrenal hyperplasia gene screening kit comprises a PCR amplification primer mixed liquor used for detecting 17 mutation sites including CYP 21A2 gene; an extended primer mixed liquor for the above 17 mutation sites; dNTP; 10*RCR buffer; 25 mM Mg2+ ions; Fast StartTaq enzyme; purification reagent composed of SAP enzyme, Exon I enzyme and a matched buffer; SNaPshot Multiplex mixed liquor; single-site homozygous and heterozygous mutation positive and negative control DNA; and deionized water. According to the technical scheme of the invention, a large number of researches and practices show that, the mutation sites of the congenital adrenal hyperplasia gene in the Chinese population are screened and combined. By utilizing the kit, 17 mutation sites of the specific congenital adrenal hyperplasia gene are simultaneously subjected to multiplex nested PCR amplification, labeling and extending, so that the defects of the existing mutation screening method are overcome. The kit is simple in operation and low in cost. The detection throughput and the accuracy of the detection result are greatly improved compared with the prior art.
Owner:SUZHOU MUNICIPAL HOSPITAL
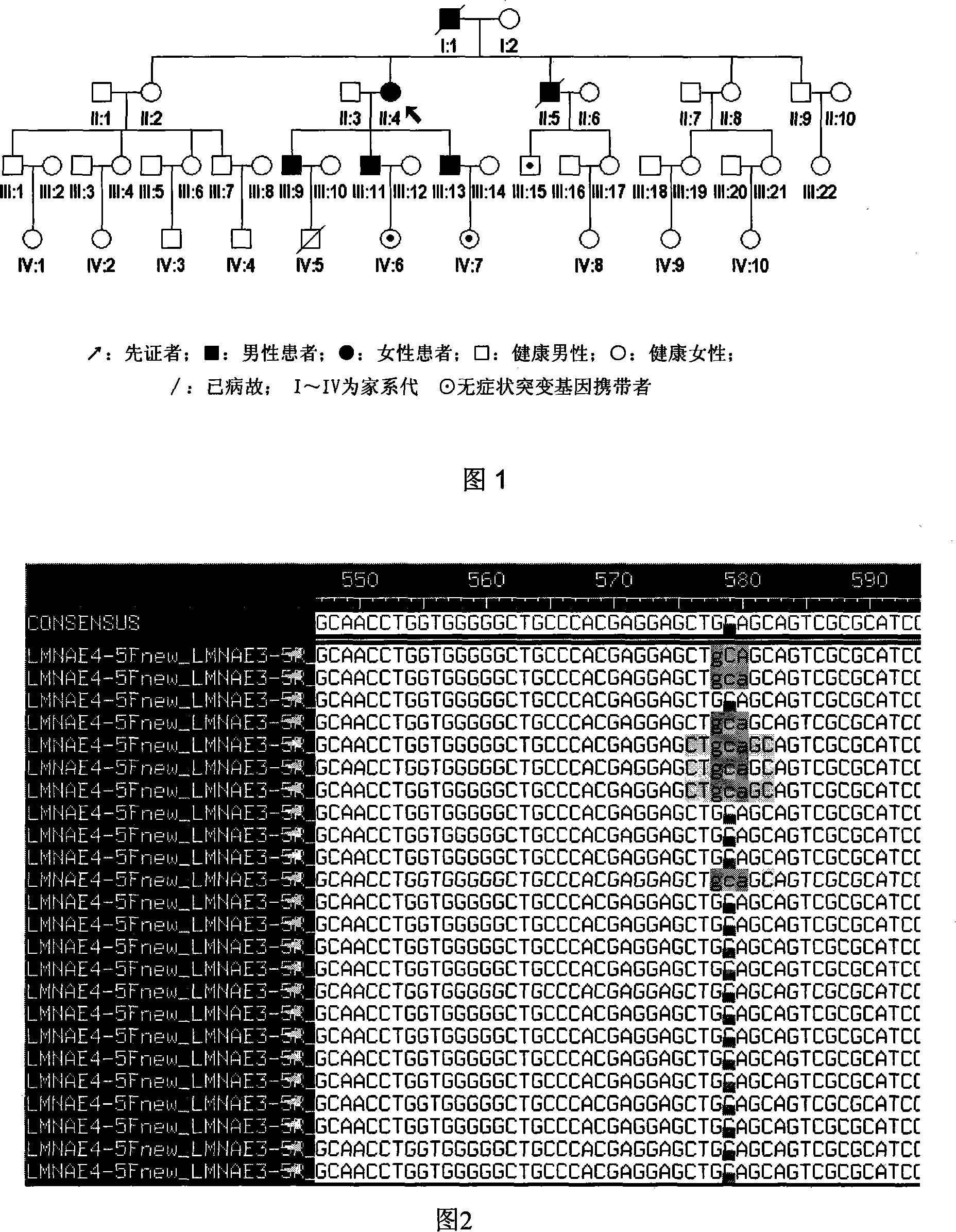
Expanding cardiomyopathy LMNA gene mutation and detecting method thereof
InactiveCN101134960AMicrobiological testing/measurementGenetic engineeringMolecular geneticsConducting system
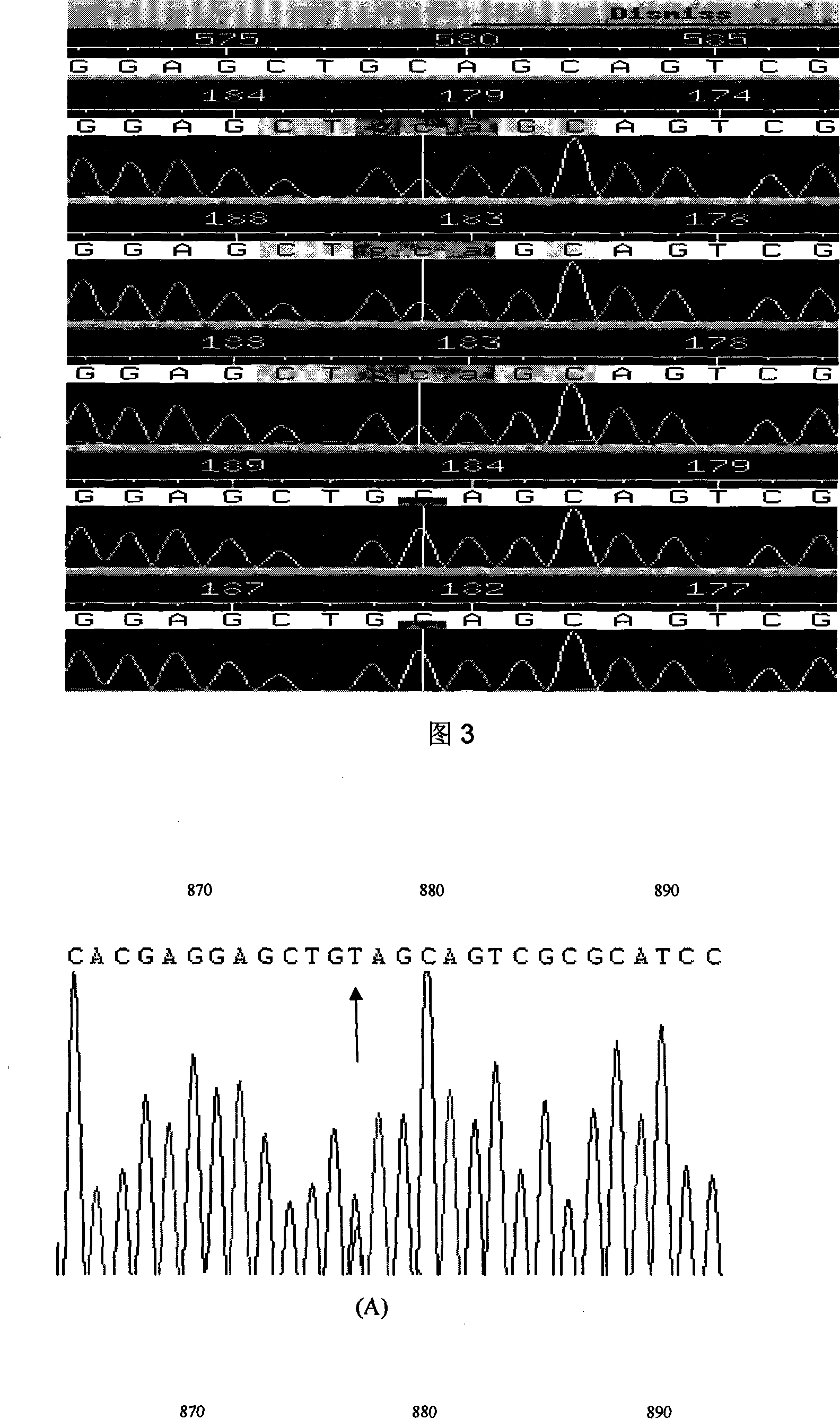
The present invention relates to LMNA gene mutation of dilated cardiomyopathy and its detection method. The LMNA gene mutation is the C-->T heterozygous mutation of the No. 877 place base in the 5th exon of LMNA gene, and causes the coded amino acid in No. 293 place to change from glutamine (Q) to termination codon (X). Its detection method includes the following steps: 1. extracting peripheral blood DNA; 2. in vitro PCR amplification of LMNA gene exon; and 3. DNA sequencing analysis. The discovery of the LMNA gene mutation can define the molecular genetic mechanism of dilated cardiomyopathy combined with conducting system abnormality further.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY +1
Mutation site of XI-type osteogenesis imperfecta pathogenic gene FKBP10 and application of mutation site
ActiveCN106755395AEasy to identifyGuaranteed FeaturesMicrobiological testing/measurementDNA preparationDiagnosis methodsGenome resequencing
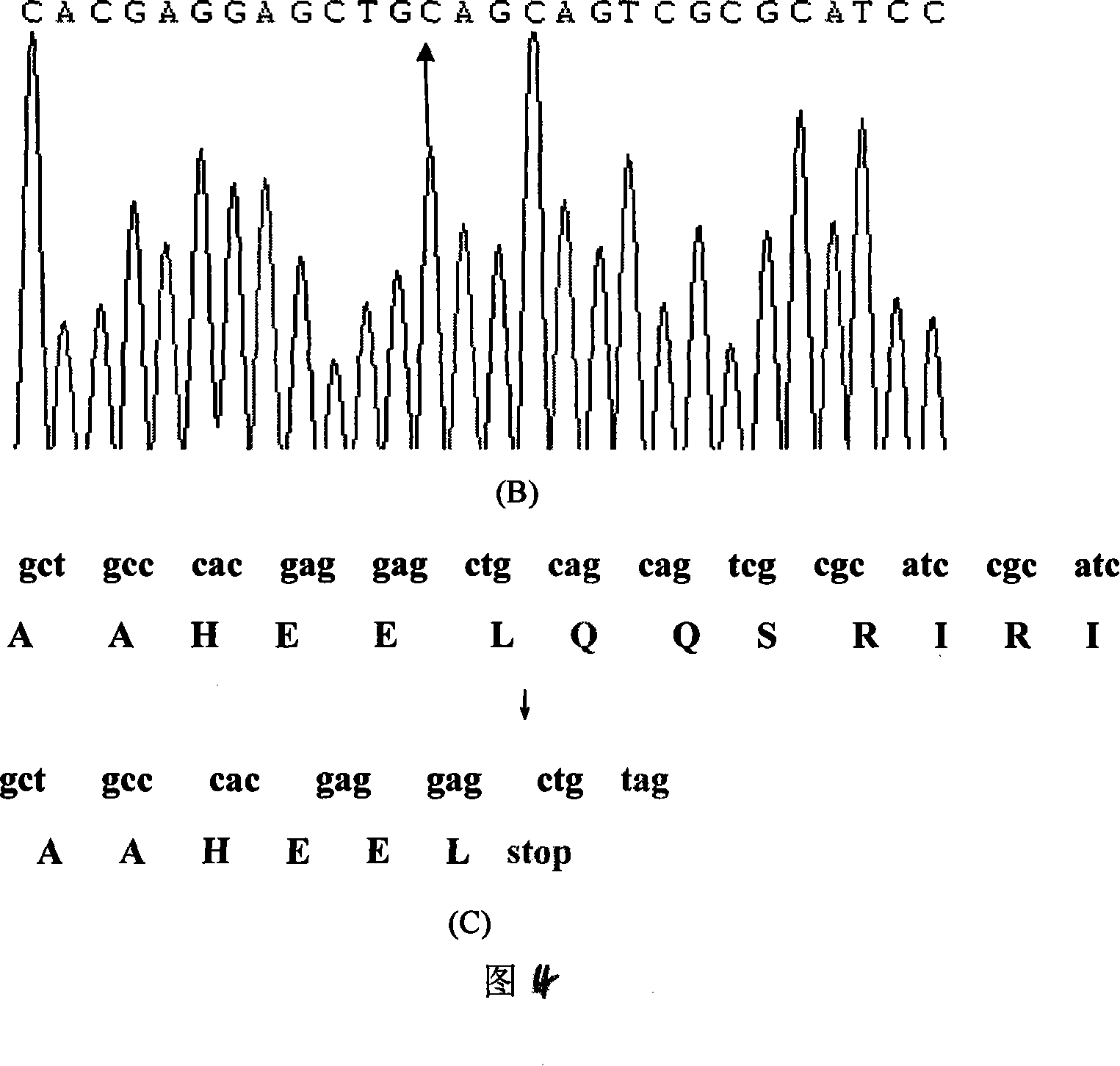
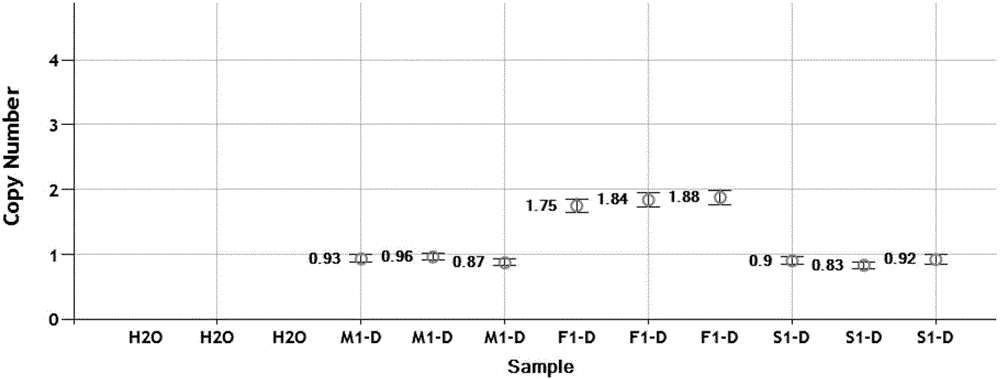
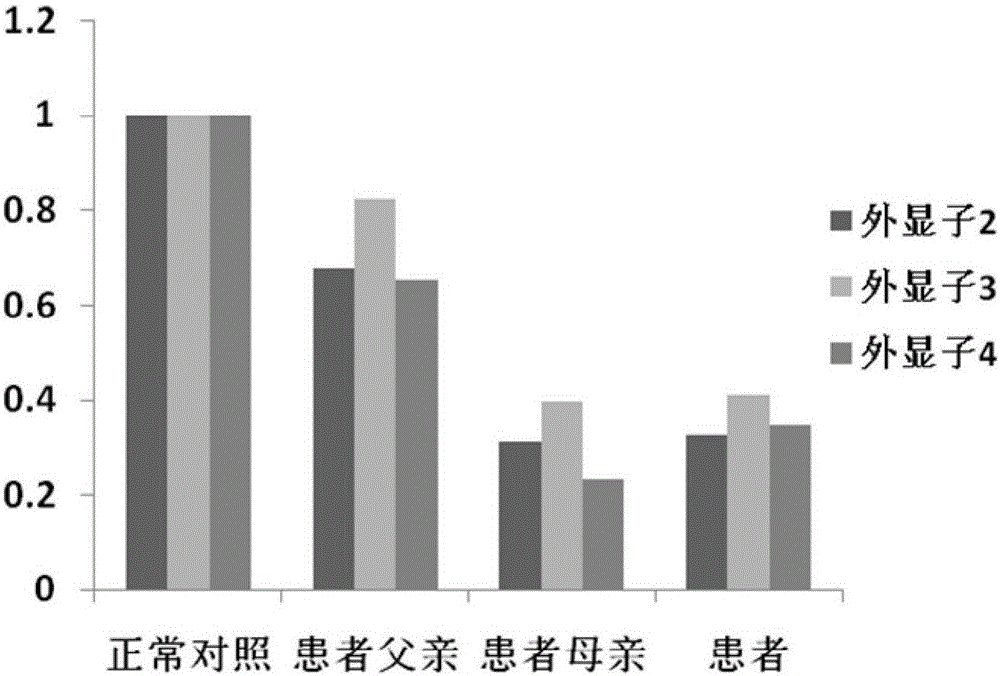
The invention relates to a mutation site of an XI-type osteogenesis imperfecta pathogenic gene FKBP10 and application of the mutation site. A whole genome resequencing technology is combined with comprehensive technologies of digital PCR, Sanger sequencing and the like; whole genome resequencing is carried out for clinic suspected osteogenesis imperfect patients; the condition that copy number variation exists between an exon 2 to an exon 4 of the FKBP10 gene and meanwhile, a heterozygous shear site mutation that c.918-3C is greater than G is discovered by combining with bioinformatics. Copy number variation and heterozygous shear mutation between the exon 2 to the exon 4 of the FKBP10 gene are further validated through digital PCR and a traditional PCB-based Sanger sequencing technology separately. Through discovery of the copy number variation and the heterozygous shear mutation, basis and reference are provided for discussing pathogenesis of XI-type osteogenesis imperfect and enriching and developing a diagnosis method for clinical diagnosis and treatment, and a basic basis is provided for early pathogenic gene screening and treatment.
Owner:SHANDONG FIRST MEDICAL UNIV & SHANDONG ACADEMY OF MEDICAL SCI
Analysis system based on genetic disease virulence genes and application thereof
PendingCN111785323AAvoid damage factorsEffective Marriage GuidanceMedical automated diagnosisProteomicsMutation CarrierGenes mutation
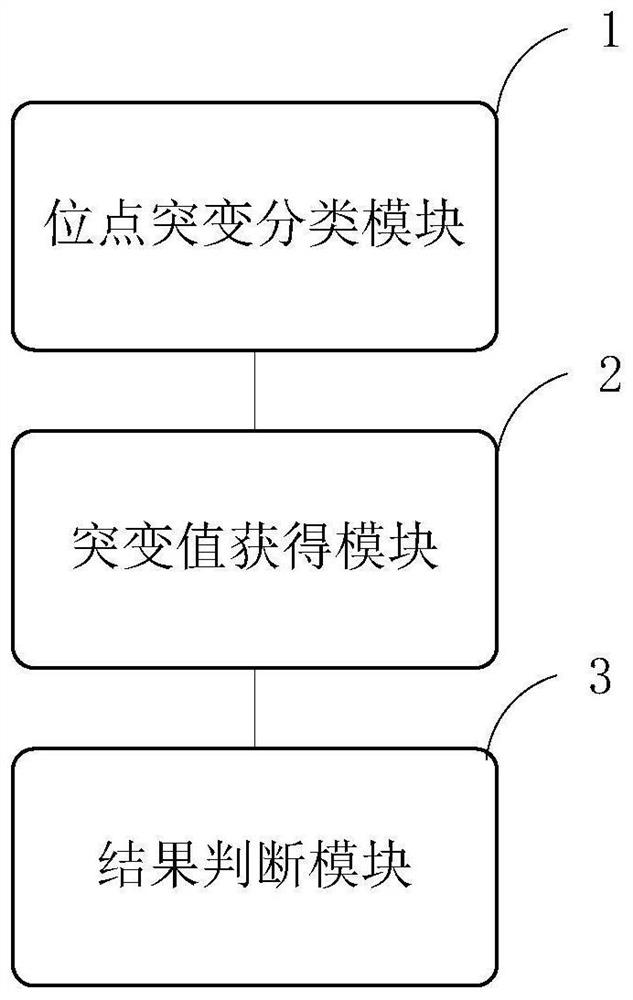
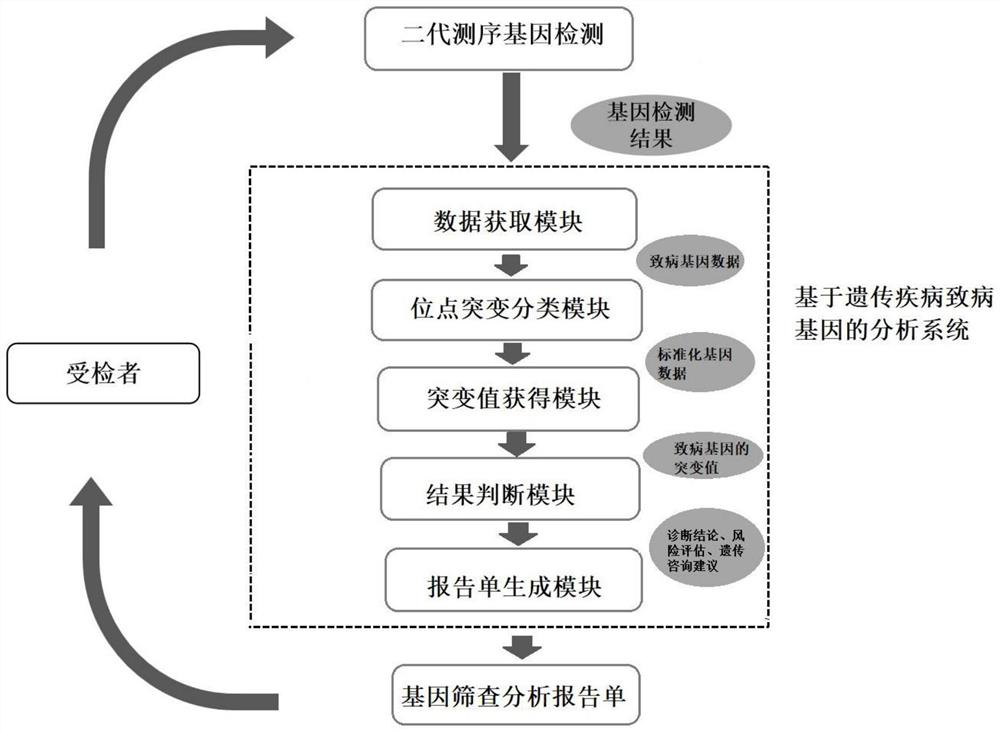
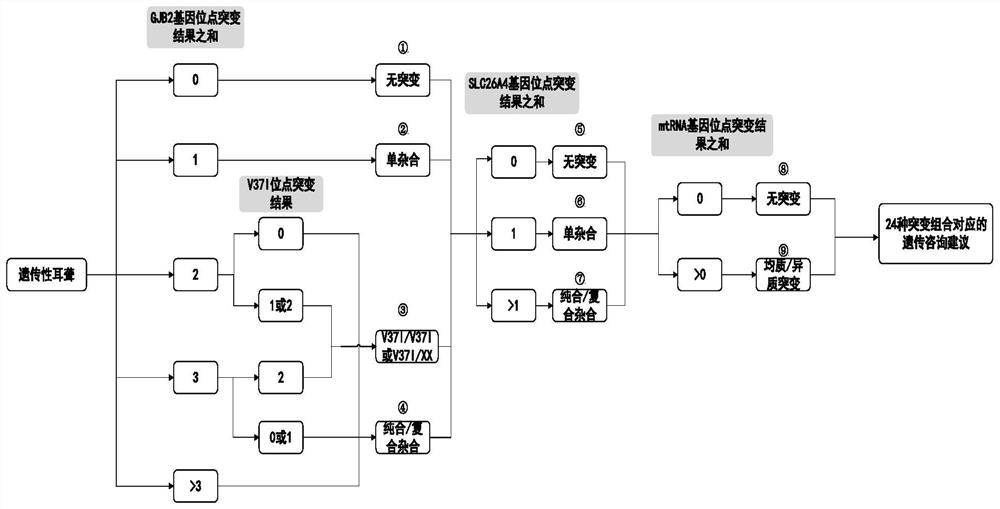
The invention provides an analysis system based on genetic disease virulence genes. The system at least comprises the following modules: a site mutation classification module, which is used for classifying mutation results of endosites of pathogenic genes of genetic diseases, wherein the site without mutation is 0, the site of heterozygous mutation or heterozygous mutation is 1, and the site of homozygous mutation or homogeneous mutation is 2; a mutation value obtaining module used for obtaining a mutation value of the pathogenic gene; a result judgment module used for judging the mutation state and / or pathogenicity of the pathogenic gene according to the mutation value of the pathogenic gene; and a report generation module used for obtaining a matched diagnosis conclusion, disease risk assessment and genetic counseling suggestion according to the mutation state and / or pathogenicity of the pathogenic gene, and generating a gene screening analysis report of the subject. According to themethod, risk assessment can be carried out on a mutation carrier with a normal phenotype, acquired injury factors are avoided, he pathogenic risk of the offspring of the mutation carrier is assessed,and wedding guidance and genetic counseling suggestions are provided.
Owner:SHANGHAI NINTH PEOPLES HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE +1
SNP G10320A molecular marker of mitochondrion ND3 gene of senescence-associated degenerative disease, detecting method and kit thereof
InactiveCN101525665AEasy to detectThe test result is accurateMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceWild type
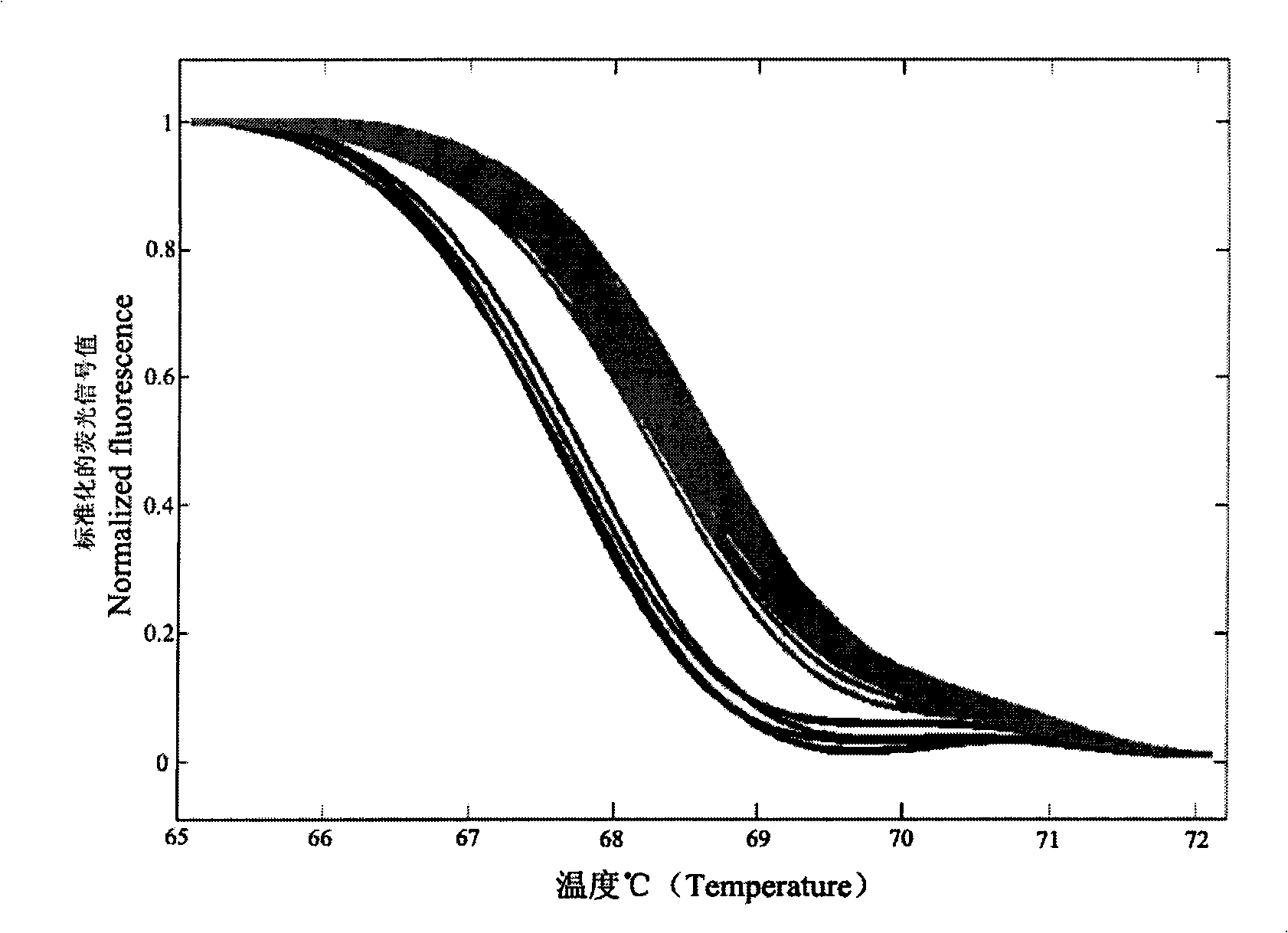
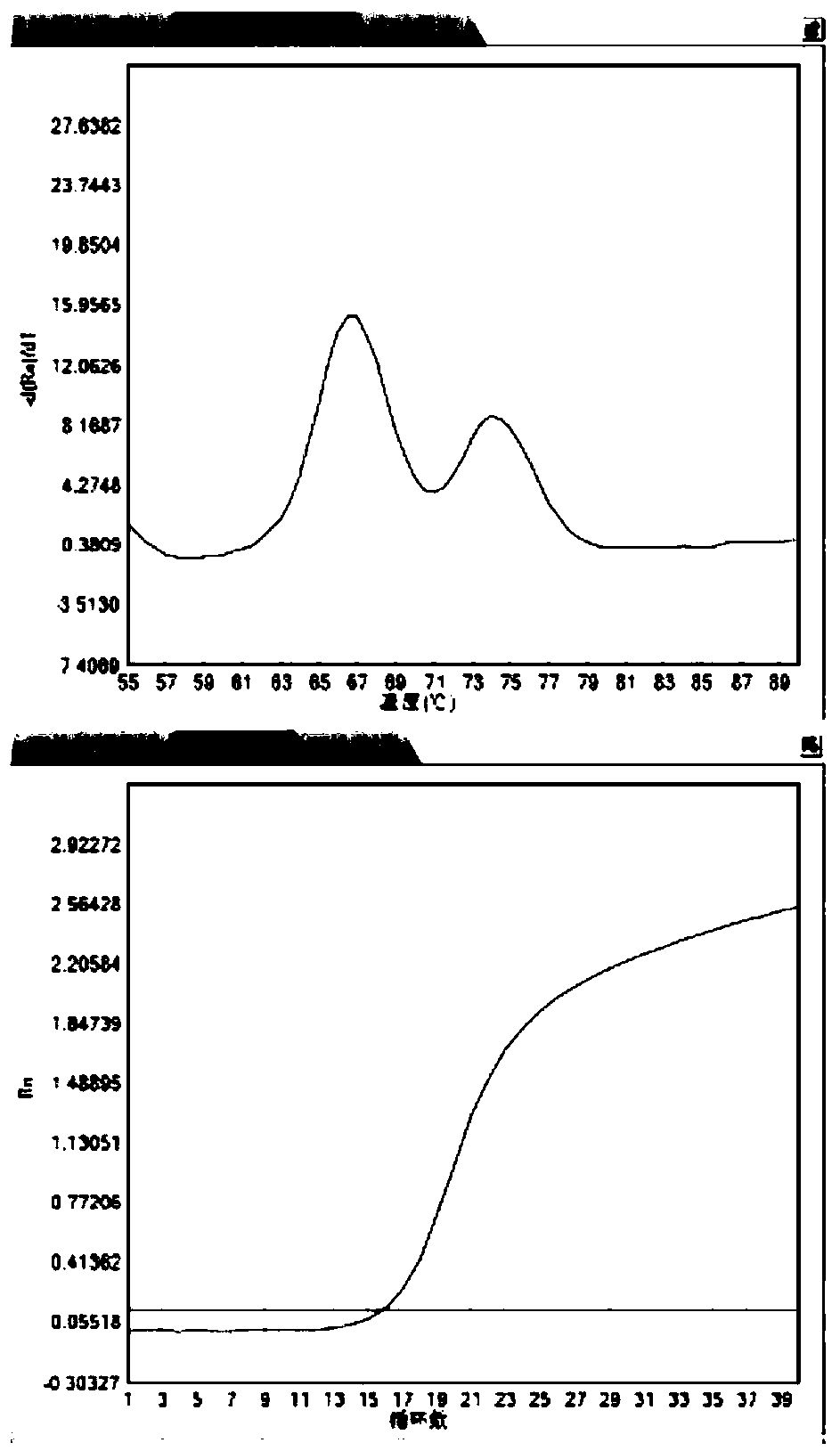
The present invention discloses a molecular marker of senescence-associated degenerative disease, a method for detecting the molecular marker SNP and a detecting kit thereof. The method of the invention has no special restriction to the sample. The body fluid and histiocyte can be equally taken as the detecting sample of the invention to cause that the detection is convenient and concise. The specific primer designed by the invention has no strict requirement for the length of basic group in the amplification area. The PCR product of the invention can be obtained through the specific primer for analyzing the high resolution fluxing curve. The saturated fluorescent dye is added before PCR reaction. A light-scanner detects the variation of fluorescent signal through optical detection and draws a temperature fluxing curve. The wild type, heterozygous mutation and homozygous mutation can be accurately differentiated according to the curve. The invention has the advantages of easy operation, low detection cost, accurate detection result, excellent application value and market value.
Owner:BEIJING HOSPITAL
Method and kit for detecting polymorphism of mitochondrial ND1 gene mononucleotide, and application of kit
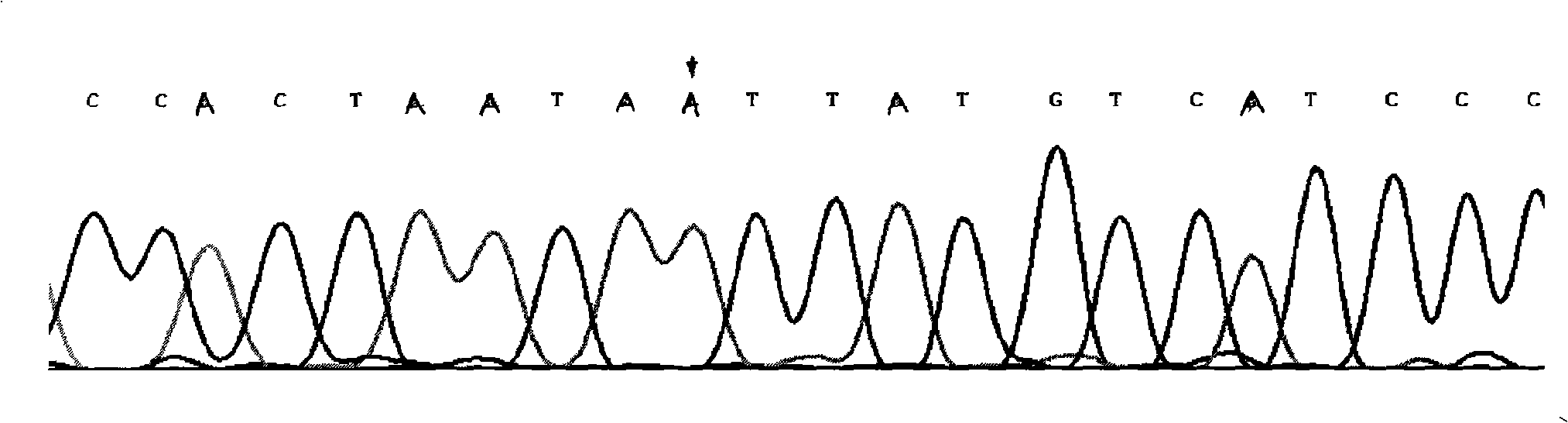
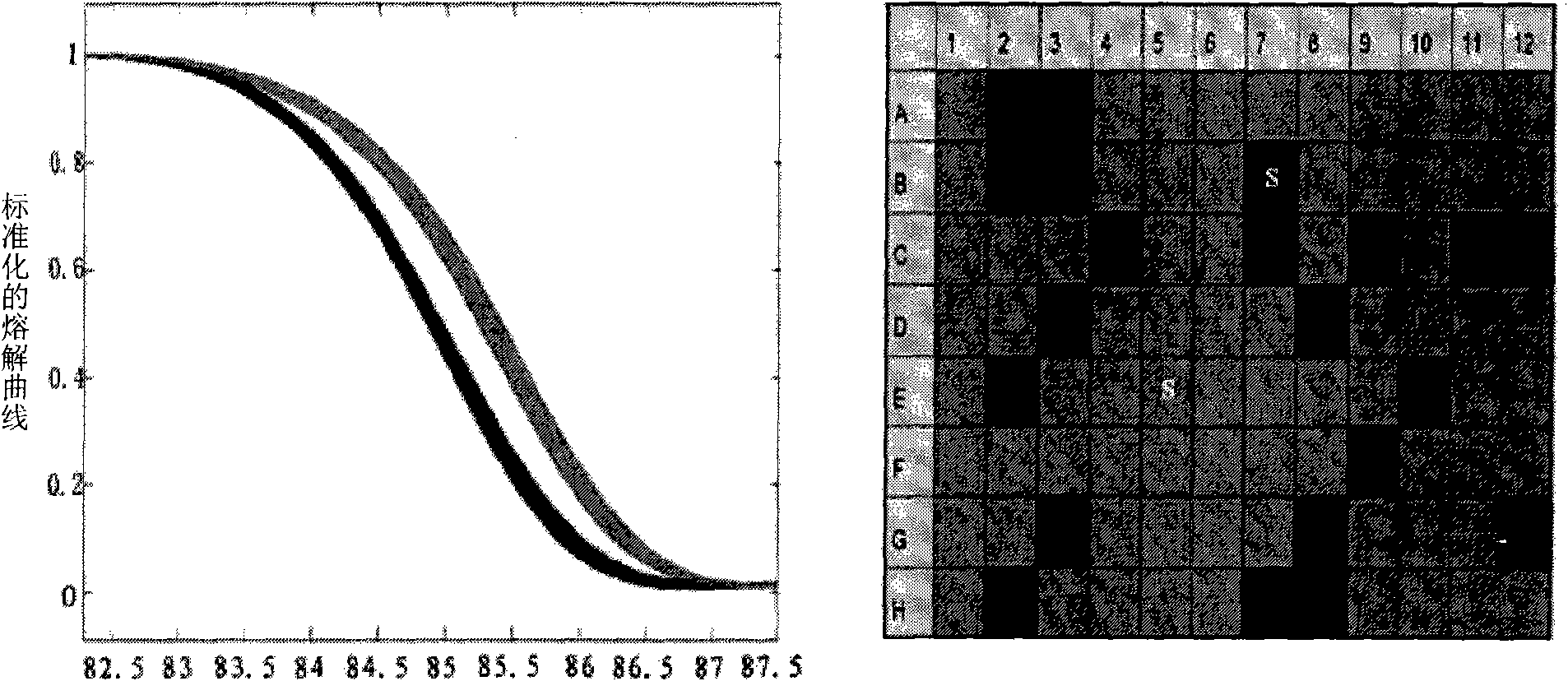
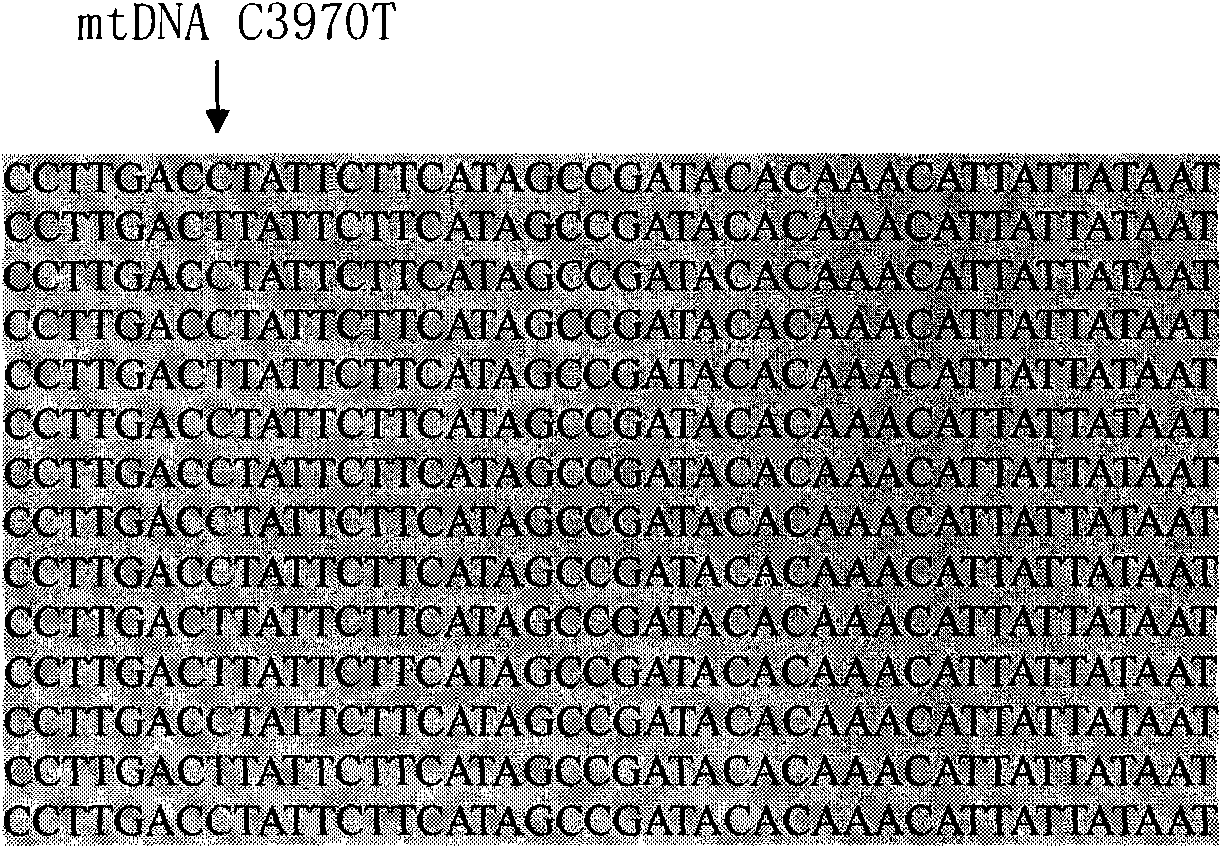
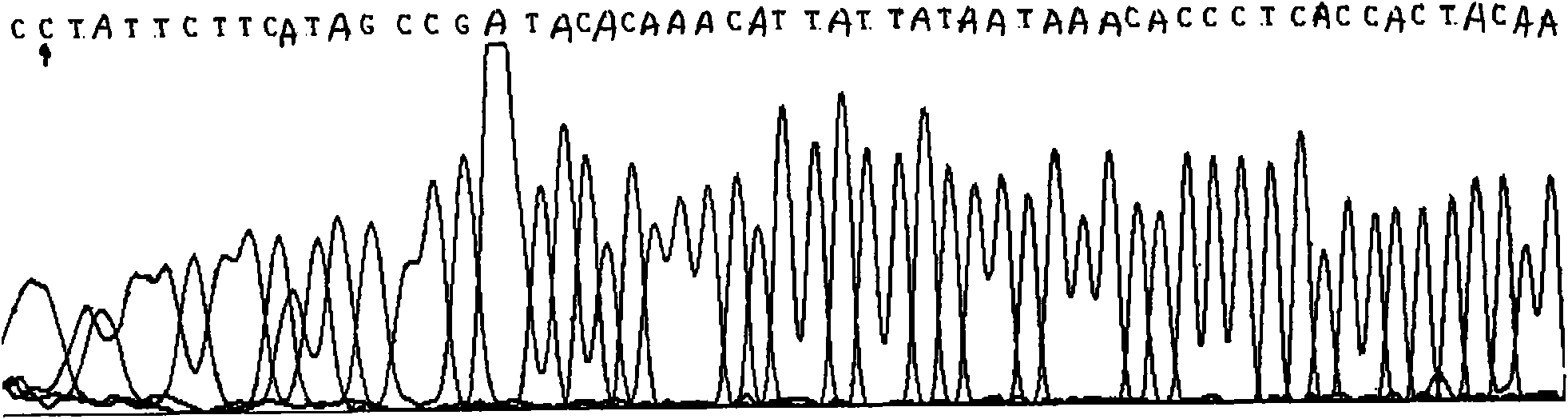
InactiveCN101775435ASignificant progressEasy to detectMicrobiological testing/measurementDNA/RNA fragmentationHeterozygous mutationMutation
The invention discloses a method for detecting SNP of mitochondrial ND1 gene mt DNA 3970 locus, a kit for detecting the SNP of the locus and application of the kit. The method does not have special restriction on samples, namely, either body fluid or histiocyte can be used as a detection sample, so the detection is convenient and rapid; auele specific primers designed by the method do not have strict requirement on the length of basic group of a region to be amplified, and PCR products can be obtained and high-resolution melting curve can be analyzed by using the auele specific primers; and before PCR reaction, saturated fluorescent dye is added, a light scanner detects fluorescent signals by optics and draws a temperature melting curve, so that wild type, heterozygous mutation and homozygous mutation can be accurately differentiated according to the curve. The invention has the advantages of simple and convenient operation, low detection cost, accurate detection result, and high application and market values.
Owner:卫生部北京医院
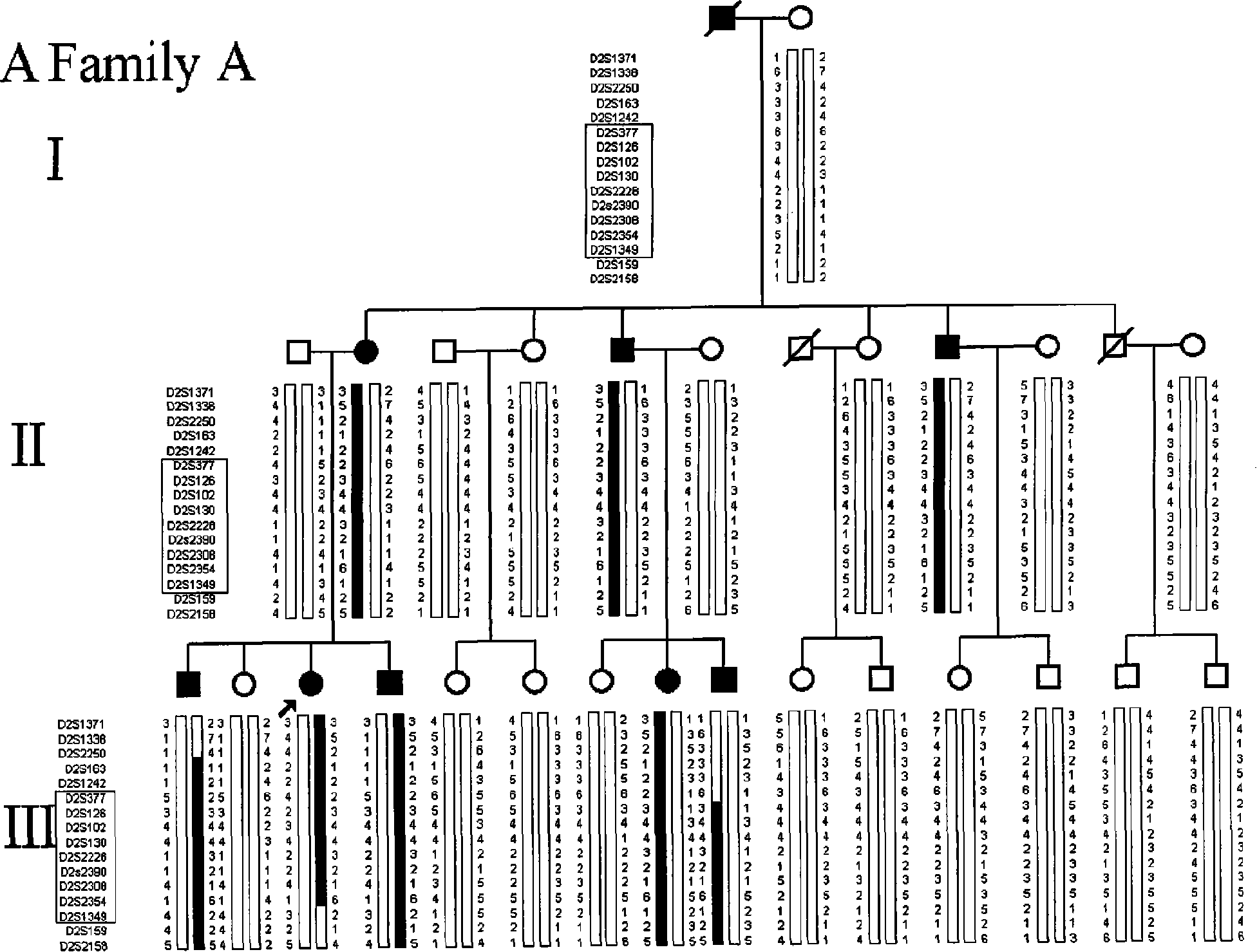
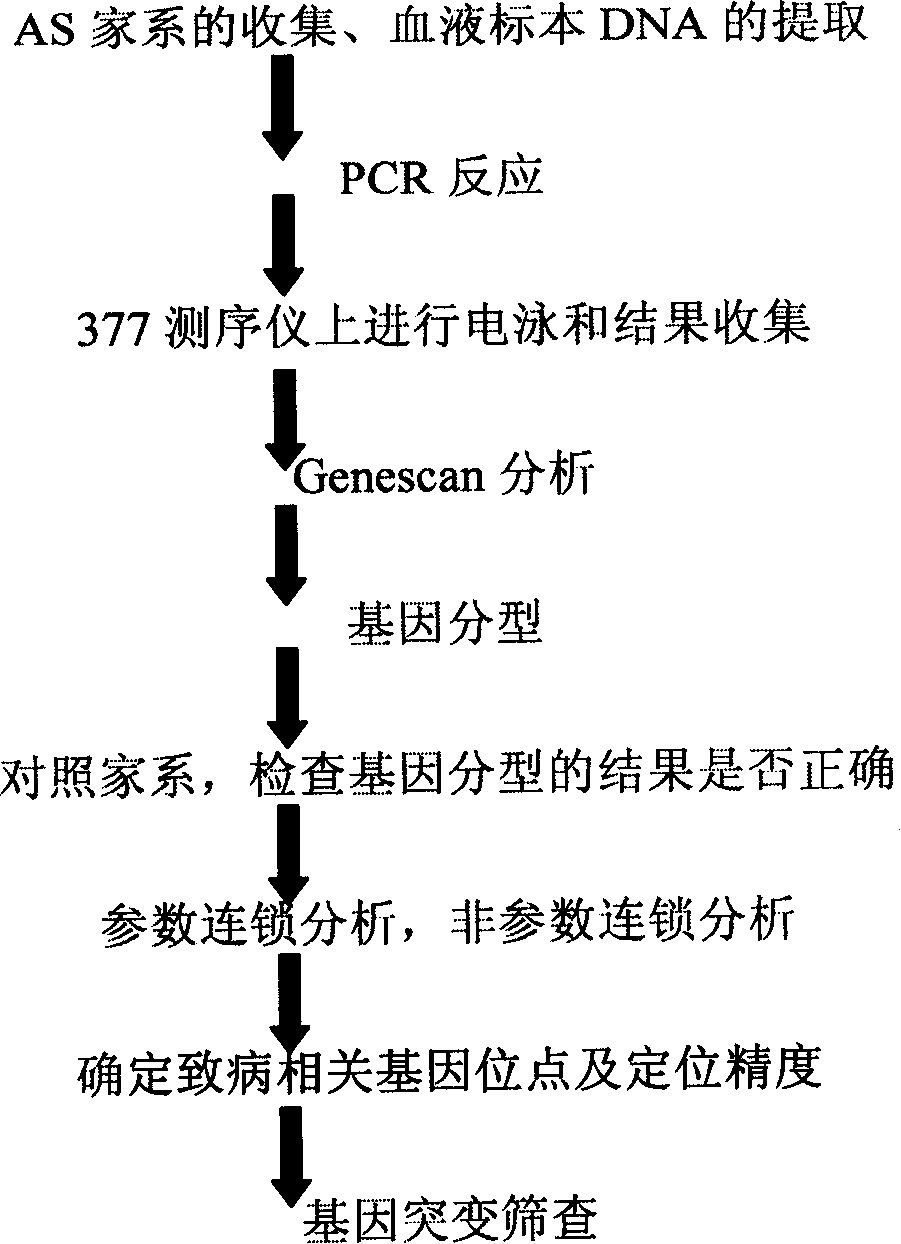
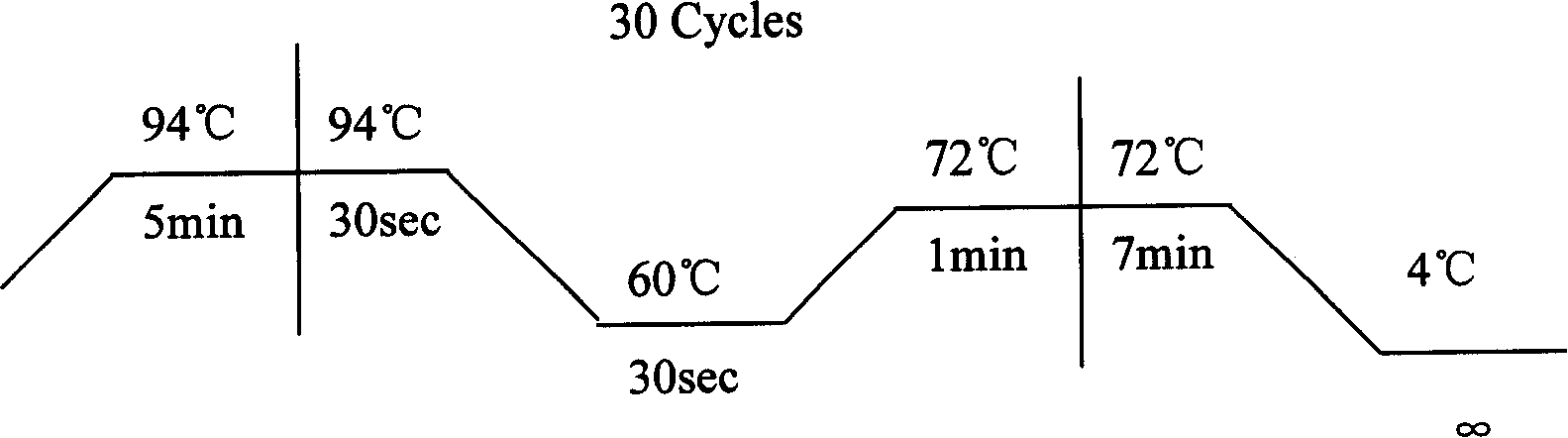
Ankylosing spondylitis pathopoiesia correlation gene IRS-1 mutant gene, detecting method and reagent kit thereof
The invention discloses an IRS-1 mutant to relative gene of ankylosing spondylitis pathogenesis, which comprises IRS-1 gene series and 2759C to G heterozygous mutation. The invention also discloses a method of detecting the IRS-1 mutant, which comprises the comparison between the nucleotide sequence from samples under testing and the sequence of IRS-1 normal gene and checking that mutating is arranged at 2750 situs of the IRS-1 gene and mutated into G from basic group C; the invention also discloses a kit detecting the IRS-1 mutant, which comprises a PCR primer designed at neighboring coding area aiming at IRS-1 gene or No. 2759 basic group of the IRS-1 gene and other components. The invention has the advantages of benefitting for further studying pathogenesis theory of ankylosing spondylitis, developing work of IRS-1 mutation screening for ankylosing spondylitis patients, providing relative information for reference for prenatal diagnosis of patients with family history, predicting, screening and assuring disease of family patients and sporadic patients and providing service for diagnosis and therapy for ankylosing spondylitis.
Owner:古洁若 +1
PCR method for detecting TRIMCyp genotypes of animals of macaca
InactiveCN101818204AReduce stimulationThe test results are credibleMicrobiological testing/measurementPcr methodTRIM5 Gene
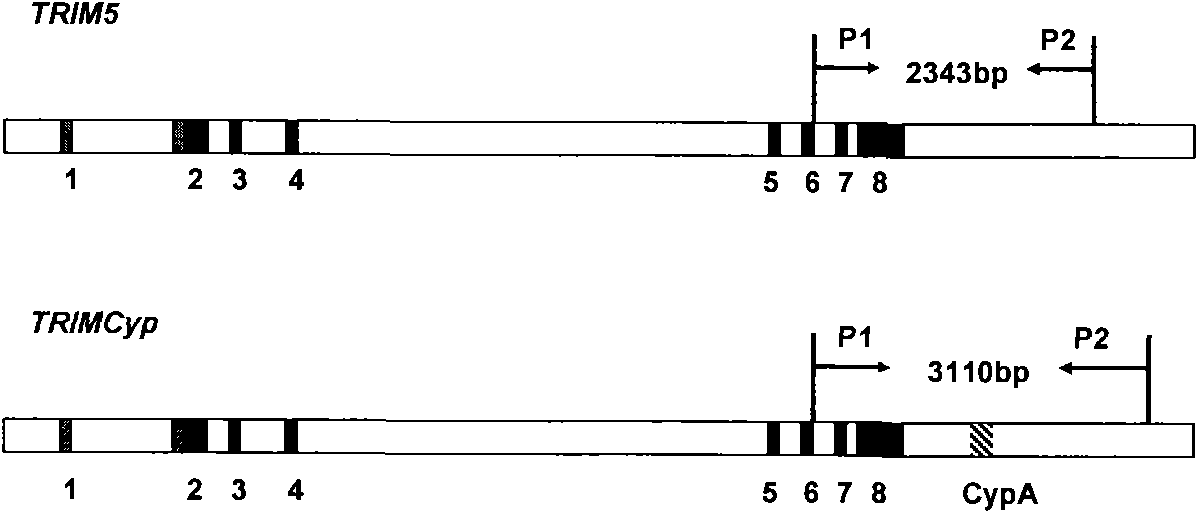
The invention discloses a PCR method for detecting the mutation of TRIM4 loca of animals of macaca, which comprises the following steps of: (1) extracting DNA from the hair of the animals of the macaca; (2) performing PCR amplification by taking SEQ ID NO:1 and SEQ ID NO:2 as primers; and (3) determining the TRIM5 loca of the animals to be detected are not mutated if an amplification product is one 2,300 to 2,400 bp segment, determining the TRIM5 loca are subjected to heterozygous mutation if the amplification products are the 2,300 to 2,400 bp segment and a 3,000 to 3,150 bp segment respectively, and determining the TRIM5 loca are subjected to homozygous mutation if the amplification product is only the 3,000 to 3,150 bp segment. The method of the invention has the advantage of accurately detecting the TRIMCyp genotypes of the animals of the macaca, and can be used for surveying the distribution of TRIMCyp in macaques in China and screening positive individuals.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI +1
Hypertrophic type cardiomyopathy PRKAG2 gene mutation and detecting method thereof
InactiveCN101134961AMicrobiological testing/measurementGenetic engineeringHypertrophic cardiomyopathyMolecular genetics
The present invention relates to PRKAG2 gene mutation of hypertrophic cardiomyopathy and its detection method. The PRKAG2 gene mutation is the G-->A heterozygous mutation of the No. 298 place base in the 3rd exon of PRKAG2 gene, and causes the coded amino acid in No. 100 place to change from glycine (G) to serine (S). Its detection method includes the following steps: 1. extracting peripheral blood DNA; 2. in vitro PCR amplification of PRKAG2 gene exon; and 3. DNA sequencing analysis. The discovery of the PRKAG2 gene mutation can define the molecular genetic mechanism of Chinese familial hypertrophic cardiomyopathy combined with conducting system abnormality and ventricle pre-exciting disease further.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY +1
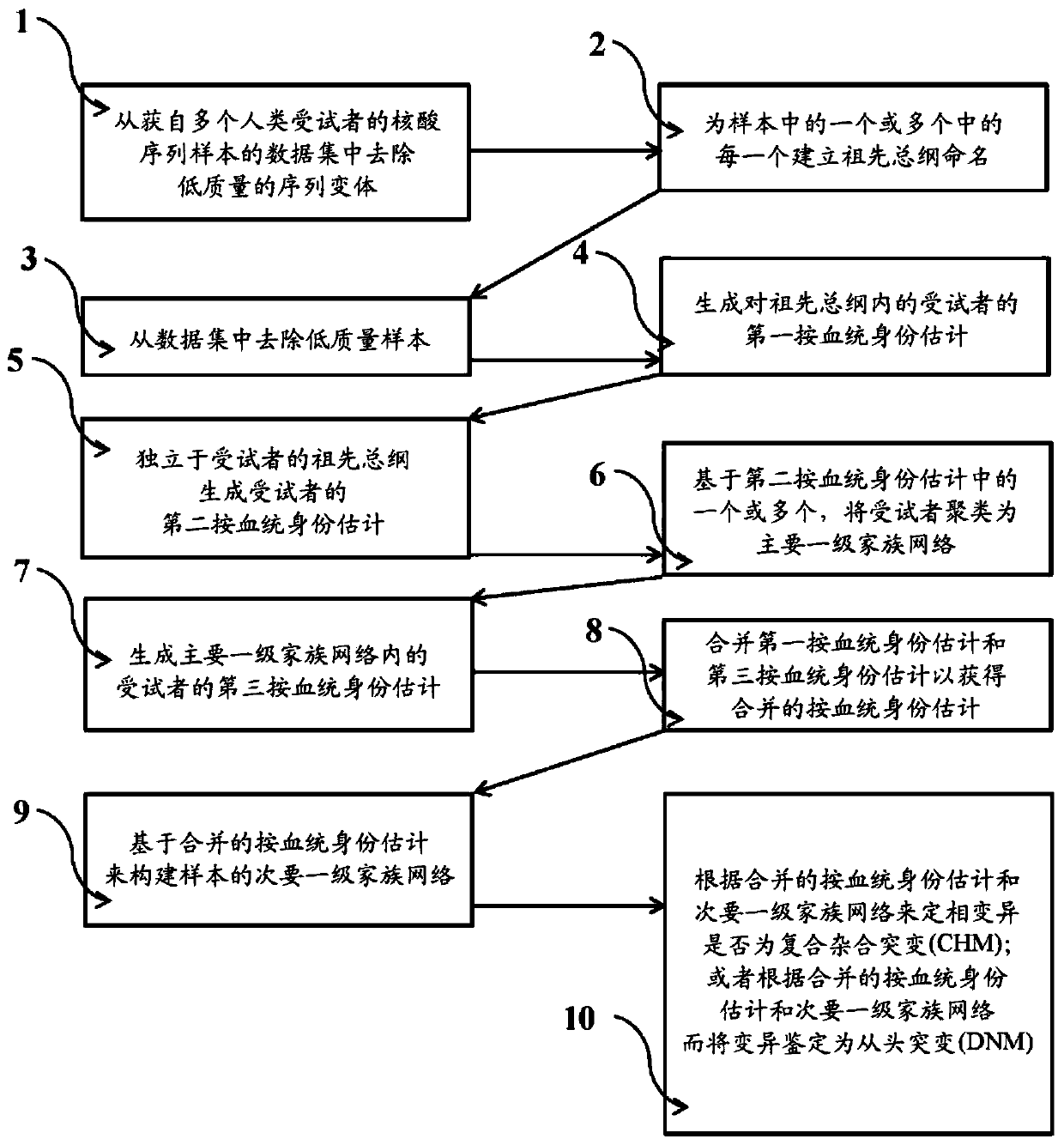
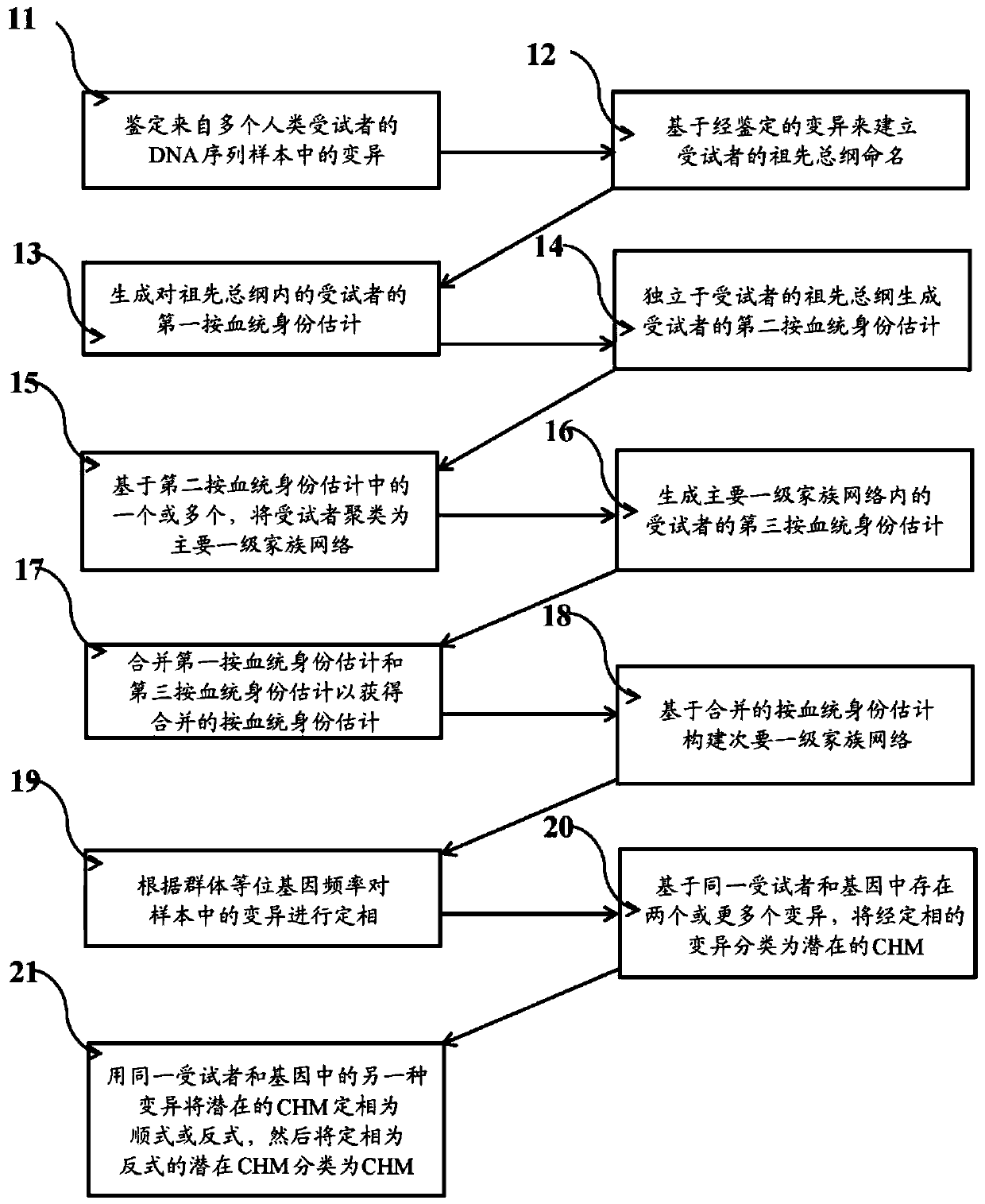
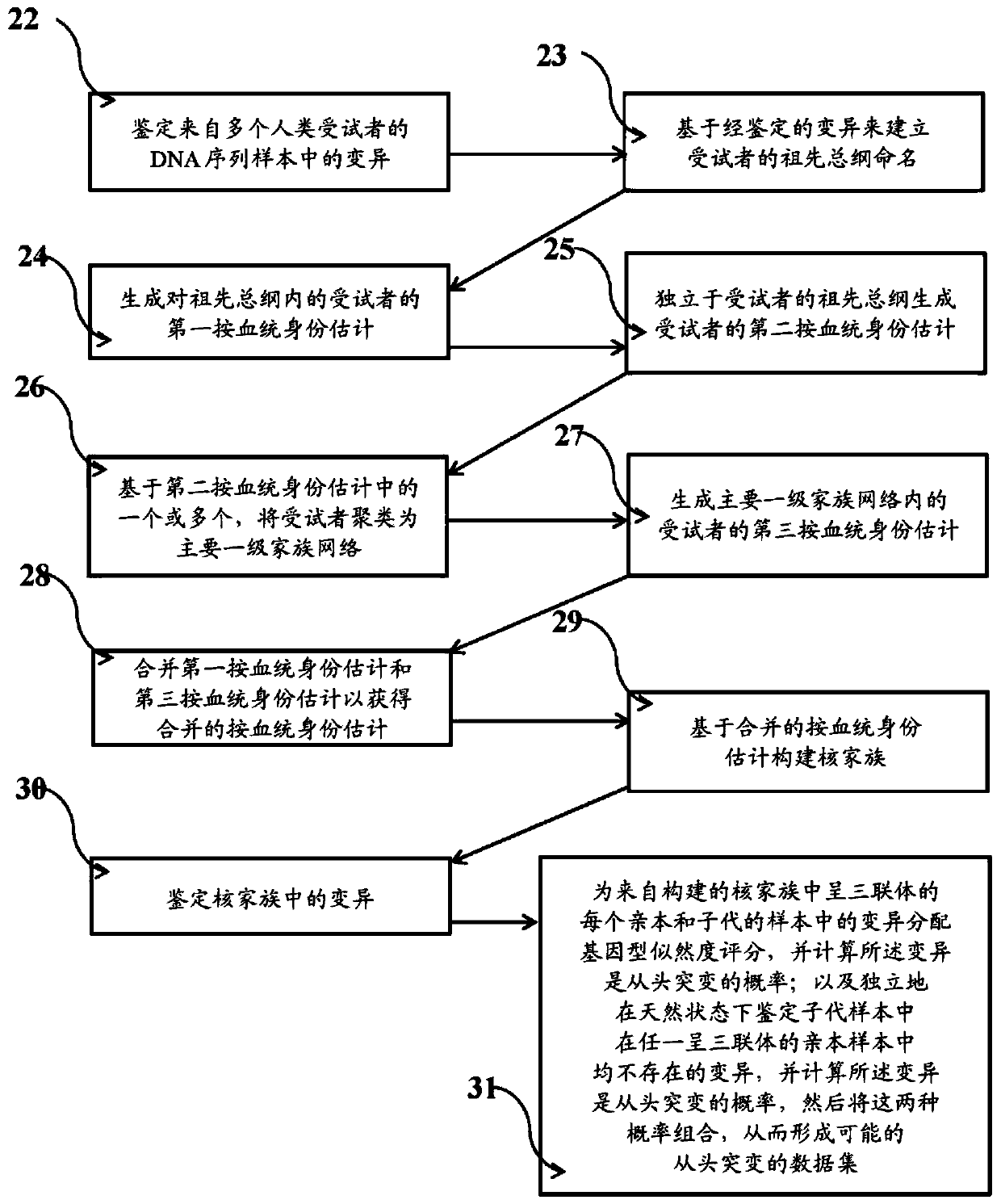
Systems and methods for leveraging relatedness in genomic data analysis
Methods, non-transitory computer-implemented methods and systems for identifying compound heterozygous mutations (CHMs) and de novo mutations (DNMs) in populations are provided. Also provided are methods for phasing genetic variants in a population by leveraging the populations relatedness. Further provided is a prediction model of relatedness in a human population.
Owner:REGENERON PHARM INC
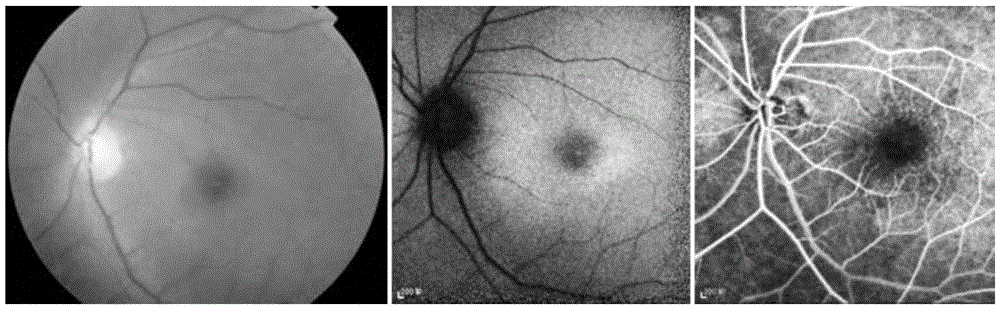
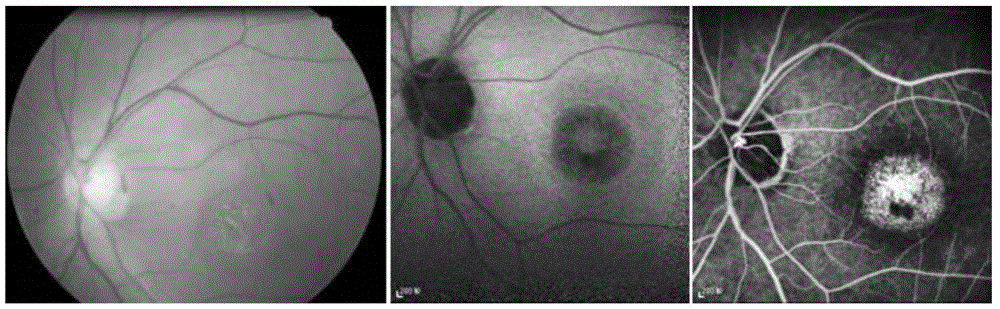
Pathogenic mutant gene of hereditary central areolar retinopathy and detection reagent thereof
InactiveCN105624167ABenefit the real needsClear relationshipMicrobiological testing/measurementDisease diagnosisHeterozygous mutationMolecular biology
The invention discloses a pathogenic mutant gene of hereditary central areolar retinopathy and a detection reagent thereof. The pathogenic mutant gene is a GUCA1A gene used for detecting hereditary central areolar retinopathy, and the mutant GUCA1A gene is heterozygously mutant GUCA1A p.Arg120Leu. Further disclosed is a kit for detecting hereditary central areolar retinopathy. The kit comprises a reagent for detecting nucleotide sites with physical positions of 42146547 and 42146548 of the GUCA1A gene, or a reagent for detecting 120th amino acid site of GCAP 1 protein (a) and a specification (b). Hereditary central areolar retinopathy can be diagnosed by detecting the mutant GUCA1A gene.
Owner:JIANGSU PROVINCE HOSPITAL
Primer set and kit for detecting four mutations of SLC25A13 gene and application thereof
InactiveCN109628587AEasy to operateHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationWild typeMultiplex pcrs
The invention discloses a primer set for detecting four mutations of an SLC25A13 gene and application thereof. The invention also discloses a detection kit for Citrin deficiency pathogenic gene mutations. The detection kit comprises the primer set, a PCR buffer, Mg2+, dNTPs and DNA polymerase. The PCR buffer contains Tris-HCl and KCl. The kit detects the mutations of the SLC25A13 gene by using a multi-color probe melting curve technology, relates to a fluorescent PCR technology, an asymmetric PCR technology, a multiplex PCR technology and a melting curve analysis technology, and achieves simultaneous detection of the wild type mutation, the heterozygous mutation and the homozygous mutation of 851_854del (type I), 1638_1660dup (type III), IVS6+5G) A (type X) and IVS16ins3kb (type XIX) through one PCR reaction. The method has the advantages of simple operation, high sensitivity, high specificity and low cost.
Owner:江门市妇幼保健院
Alpha and beta thalassemia point mutation screening method
ActiveCN102994615AImprove accuracyReduce missed diagnosesMicrobiological testing/measurementFluorescence/phosphorescenceBeta thalassemiaFluorescence
The invention discloses an alpha and beta thalassemia point mutation screening method, and aims to provide an alpha and beta thalassemia point mutation screening method having the advantages of simplicity, rapidness, low cost and high accuracy. The method is characterized in that the method comprises the following steps: 1, extracting the genome DNA of a specimen; 2, mixing PCR mix with template DNA, and adding an LC Green Plus saturated dye; 3, carrying out PCR amplification; and 4, directly putting a product obtained after the PCR ending into an HRM analyzer, denaturing the PCR amplification product at 60-96DEG C, acquiring dense fluorescent signal changes through the optical detection system of the analyzer and drawing a temperature melting curve in the denaturing period, and accurately and automatically sorting wild and heterozygous mutation and homozygous mutation according to the curve by software.
Owner:GUANGZHOU WOMEN AND CHILDRENS MEDICAL CENTER
Citrin deficiency pathogenic gene detection primer and kit
ActiveCN109182492AEasy to operateHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationDiseaseCitrin deficiency
The invention discloses a Citrin deficiency gene detection primer, comprising an upstream prim as shown in SEQ ID No. 1, a downstream primer as shown in SEQ ID No. 2 and a probe as shown in SEQ ID No.3. The invention also discloses the use of the detection primer in preparing a Citrin defect detection reagent and a Citrin defect detection reagent kit. The detection primer provided by the invention can realize the simultaneous detection of the wild type of the c.851_854del (rs80338720) of the disease-causing gene SLC25A13 of the Citrin deficiency in the same tube PCR reaction, Heterozygous mutation and homozygous mutation have the advantages of simple operation, high sensitivity, high specificity and low cost, which are very suitable for the detection and diagnosis of clinical Citrin deficiency.
Owner:江门市妇幼保健院
CYP2D6 gene mutation detection primer and kit
InactiveCN109182474AEasy to operateHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationMutation detectionWild type
The invention discloses a CYP2D6 gene mutation detection primer, comprising an upstream primer as shown in SEQ ID No. 1, a downstream primer as shown in SEQ ID No. 2 and a probe as shown in SEQ ID No.3. The invention also discloses the use of the detection primer in preparing a CYP2D6 gene mutation detection reagent and a CYP2D6 gene mutation detection reagent kit. The detection primer provided by the invention can realize the simultaneous detection of wild type of CYP2D6*10 (rs1065852 C100T) site in the same tube PCR reaction, heterozygous mutation and homozygous mutation, and has the advantages of simple operation, high sensitivity, high specificity and low cost.
Owner:江门市妇幼保健院
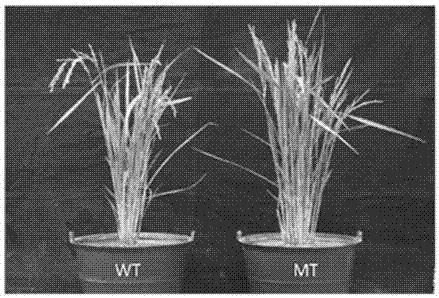
Method for increasing growth speed of zebra fish through gene knockout technology
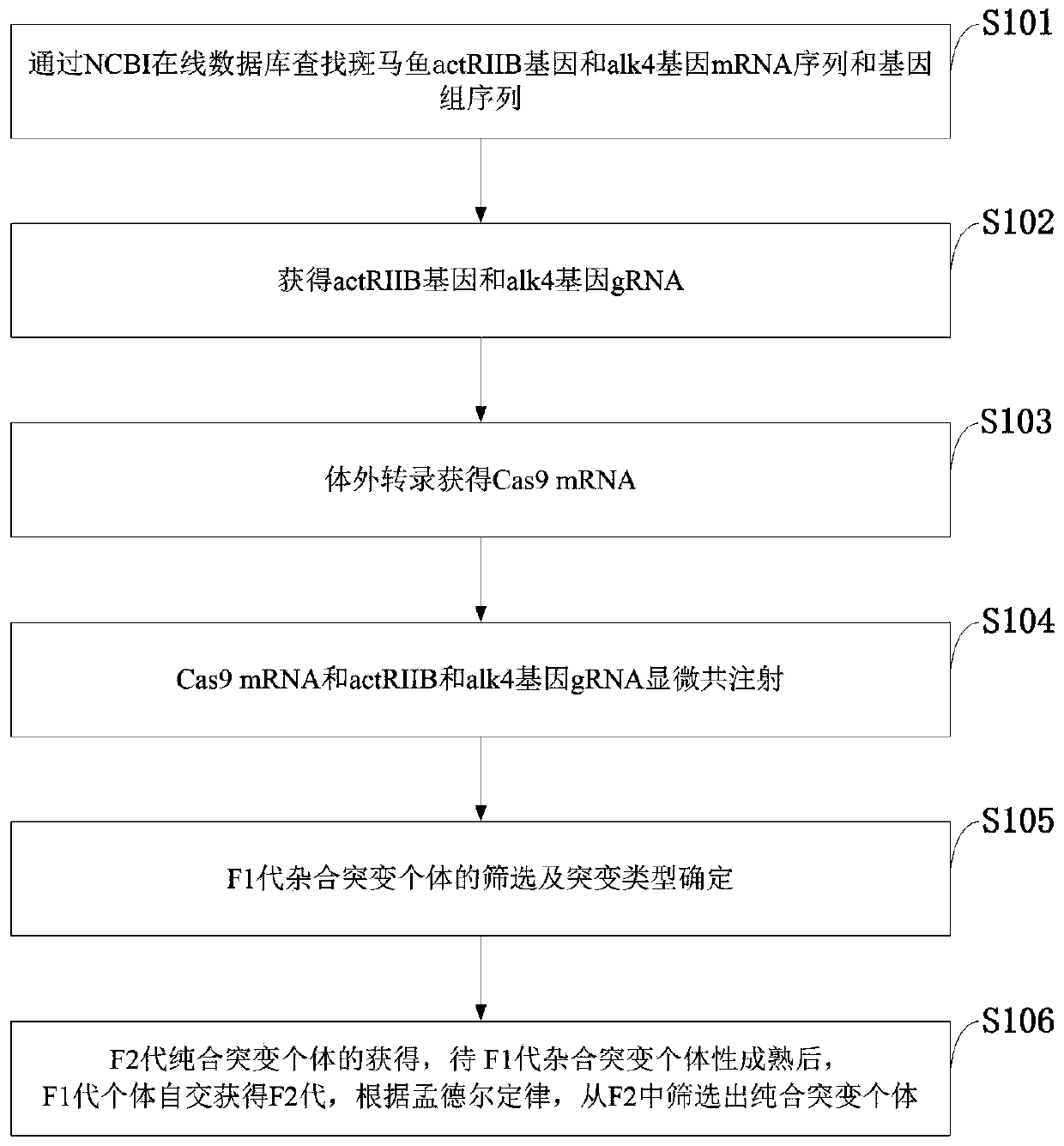
ActiveCN111269943AIncrease the number ofGrow fastClimate change adaptationStable introduction of DNAAnimal scienceGenetic engineering
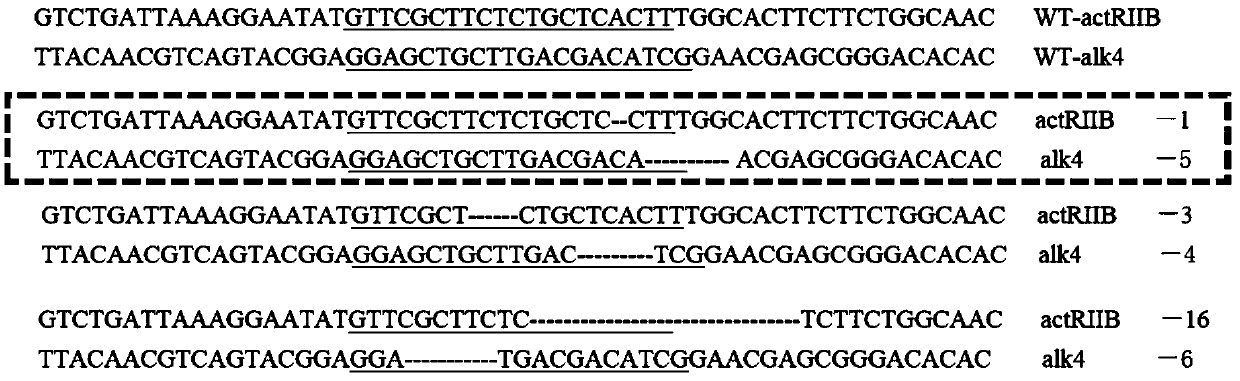
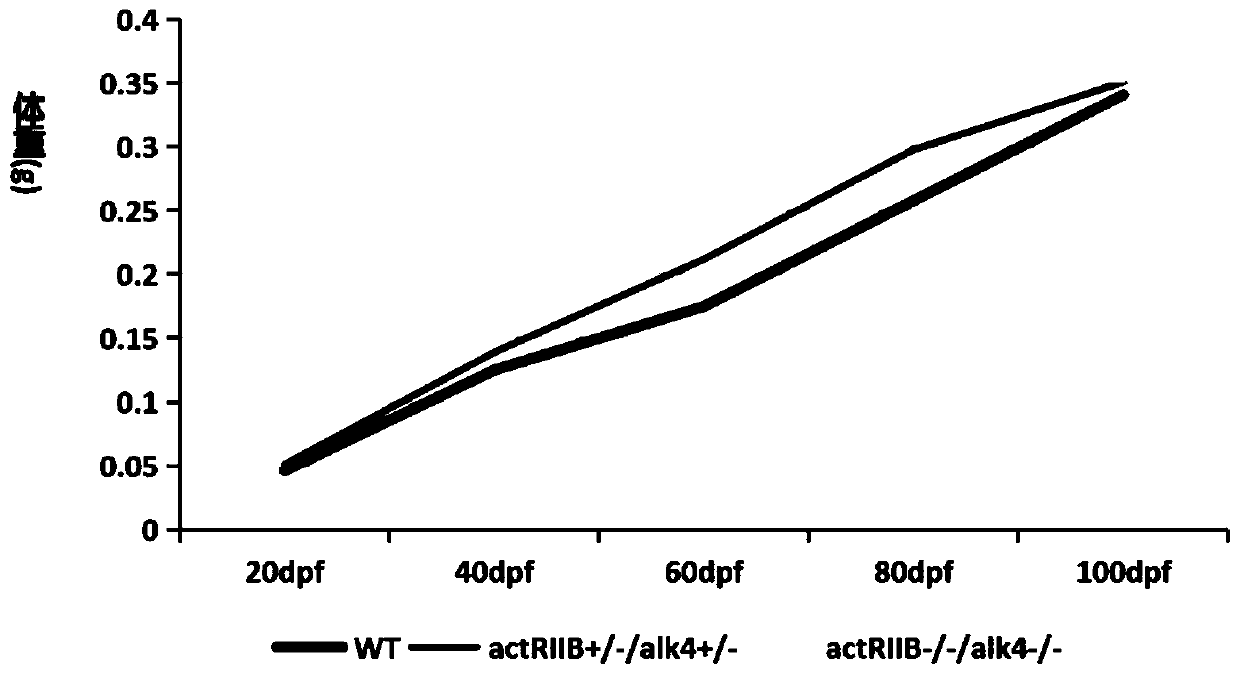
The invention belongs to the technical field of gene engineering, and discloses a method for increasing the growth speed of zebra fish through a gene knockout technology. The method for increasing thegrowth speed of zebra fish through the gene knockout technology comprises the following steps: searching mRNA sequences and genome sequences of actRIIB genes and alk4 genes of zebra fish through an NCBI online database; designing the knockout target sites of the actRIIB gene and the alk4 gene; carrying out in vitro transcription to obtain Cas9 mRNA; carrying out micro co-injection on the Cas9 mRNA, the actRIIB and the alk4 gene gRNA; screening F1-generation heterozygous mutation individuals and determining mutation types; obtaining F2-generation homozygous mutant individuals, and after the F1-generation heterozygous mutant individuals are mature, selfing the F1-generation individuals to obtain F2-generation individuals; and screening homozygous mutant individuals from F2 according to theMendel's law. According to the invention, co-knockout of actRIIB and alk4 of zebra fish can cause rapid growth of zebra fish.
Owner:HUNAN UNIV OF ARTS & SCI
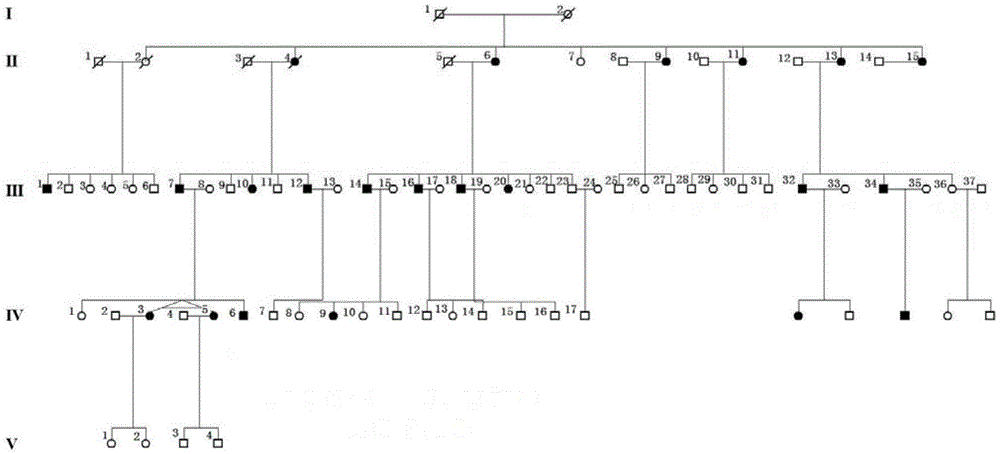
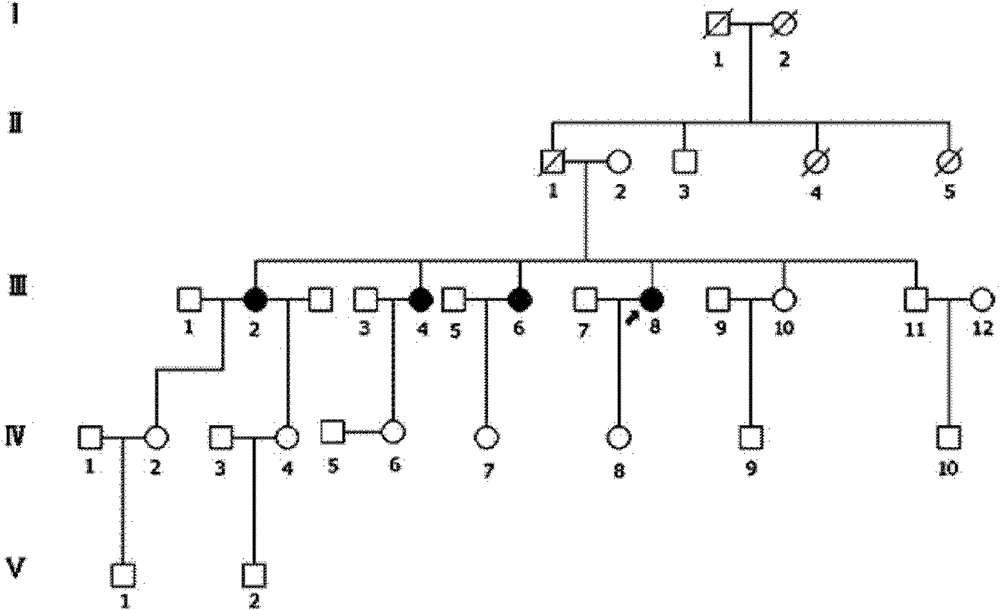
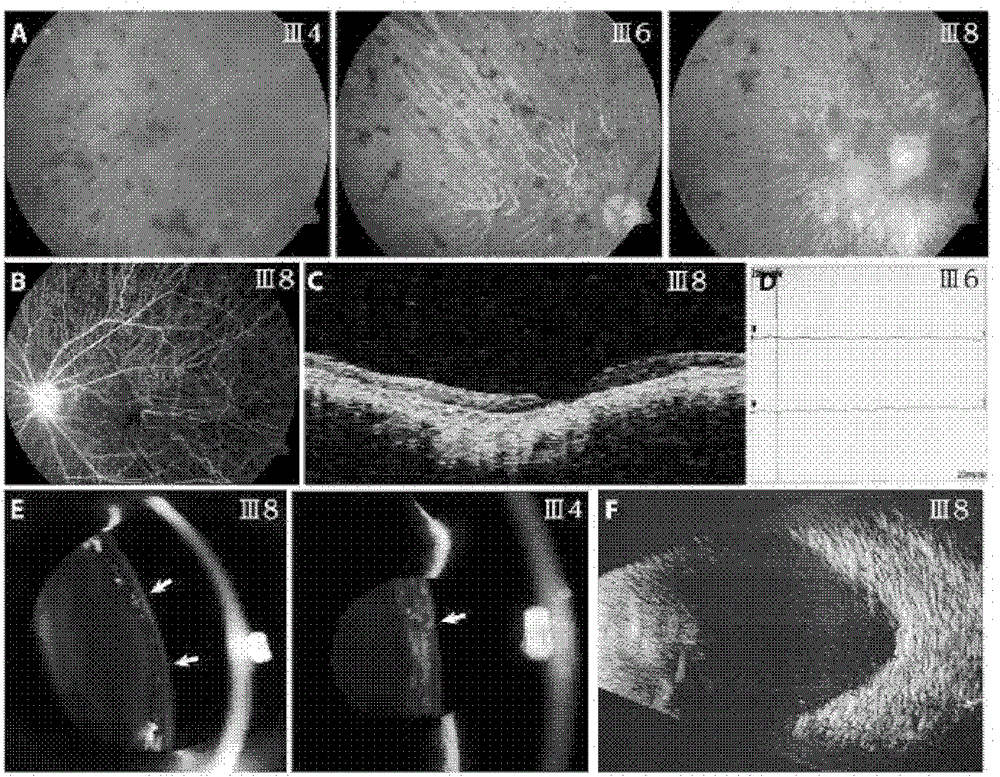
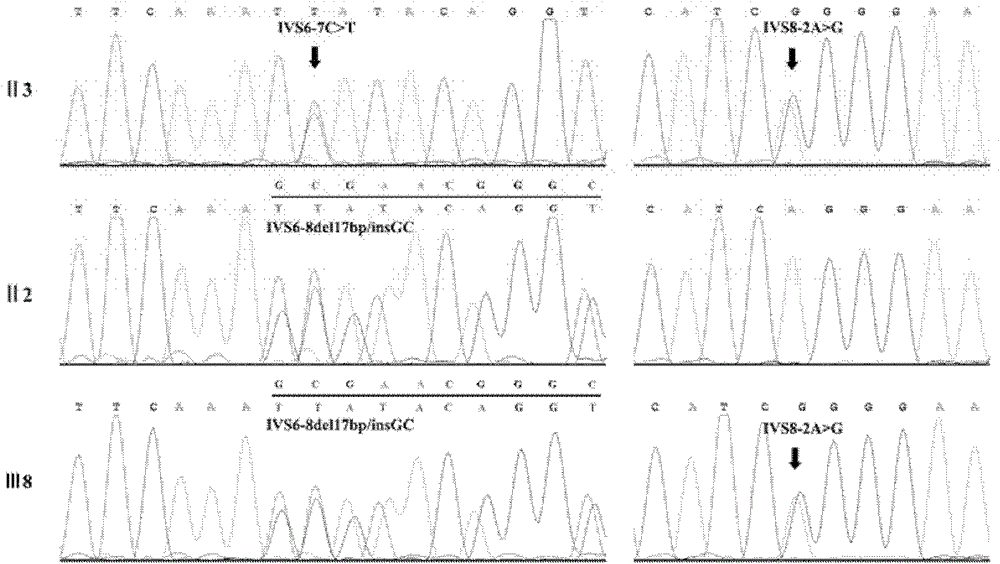
Retinitis pigmentosa related gene identification, and product, method and use thereof
The invention relates to compound heterozygous mutation identification, especially relates to retinitis pigmentosa related compound heterozygous mutation identification, and concretely provides a method for identifying the retinitis pigmentosa related compound heterozygous mutation, a use of identified gene and / or mutation in the retinitis pigmentosa identification, and a product for diagnosing the retinitis pigmentosa and a use thereof.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV +1
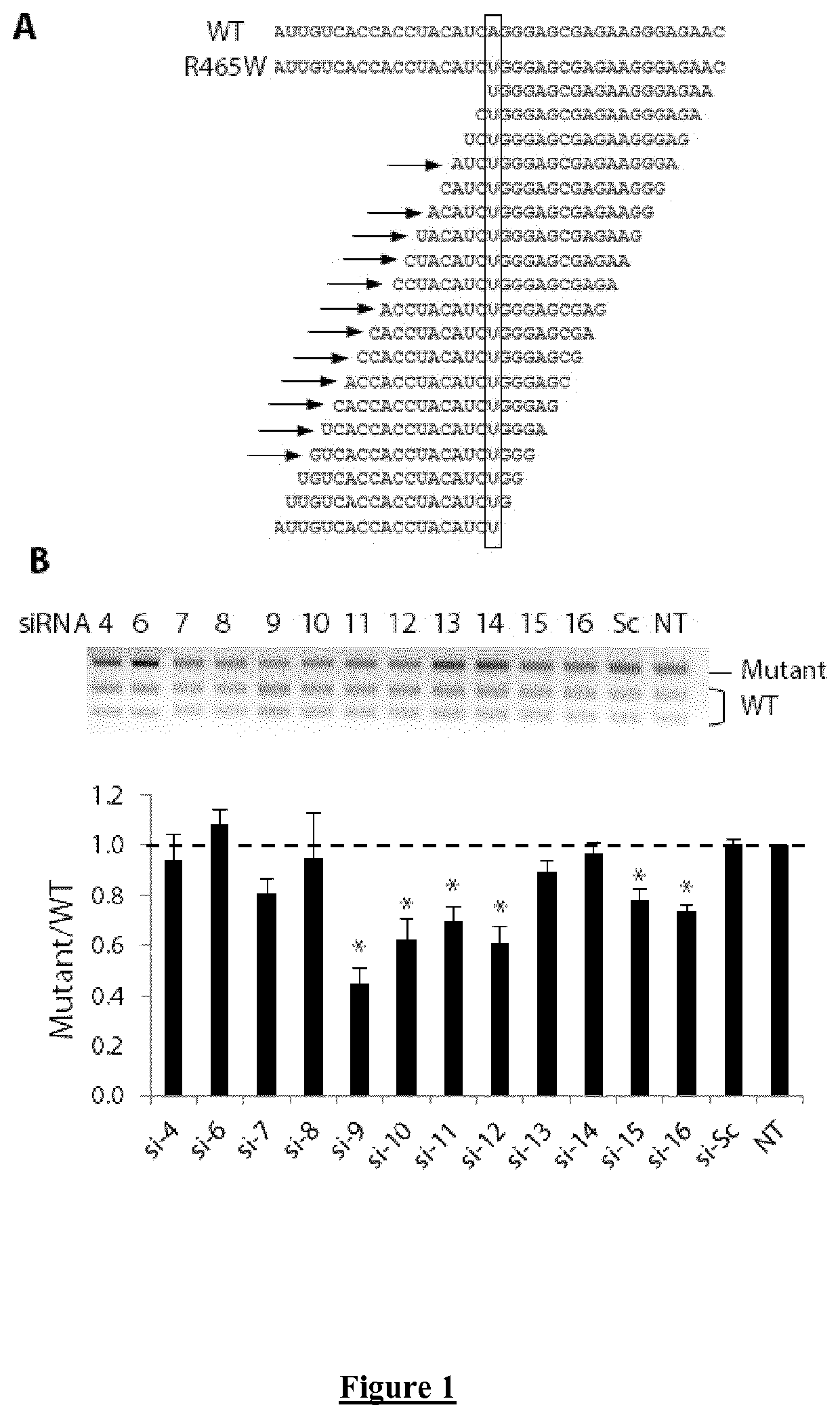
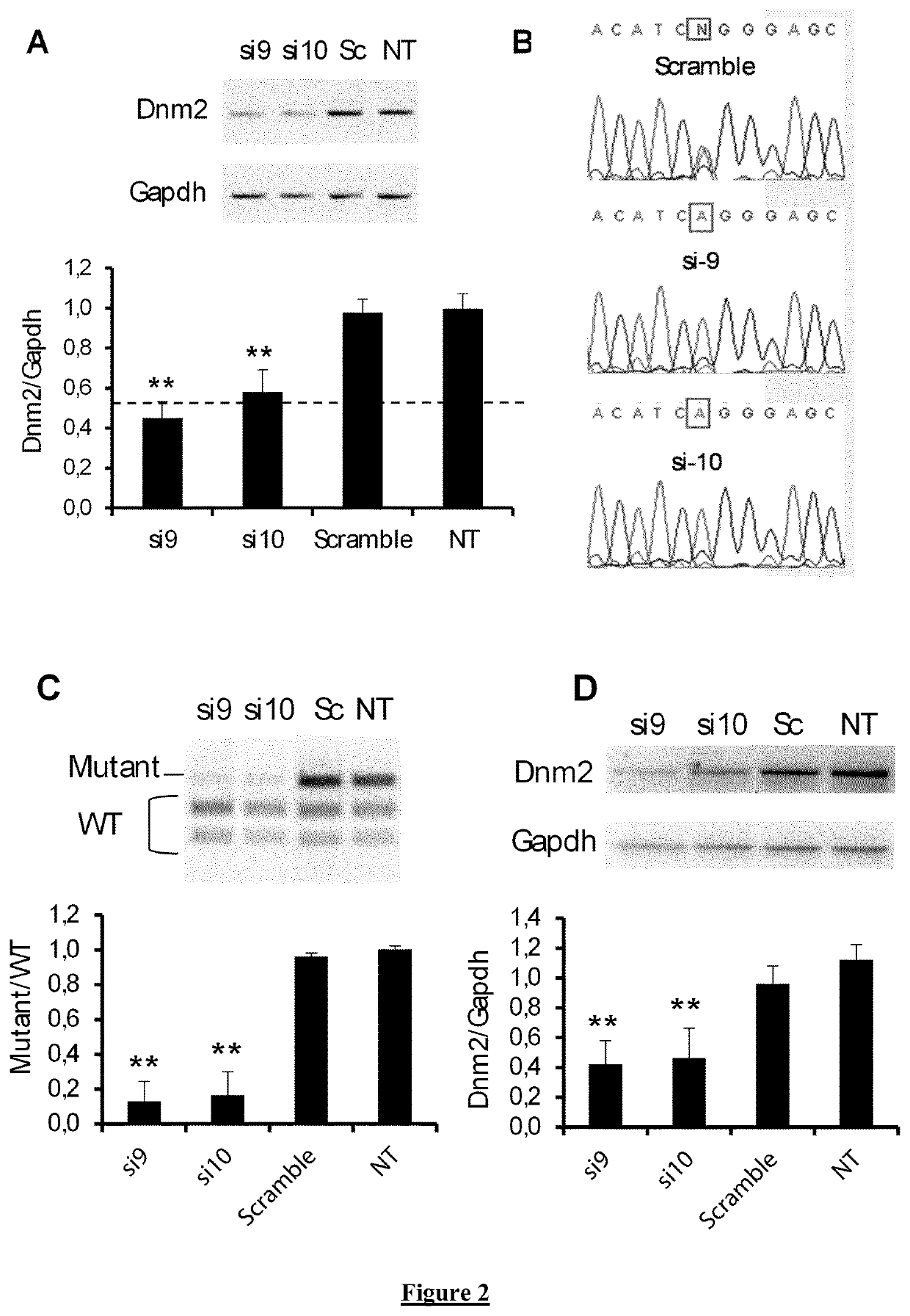
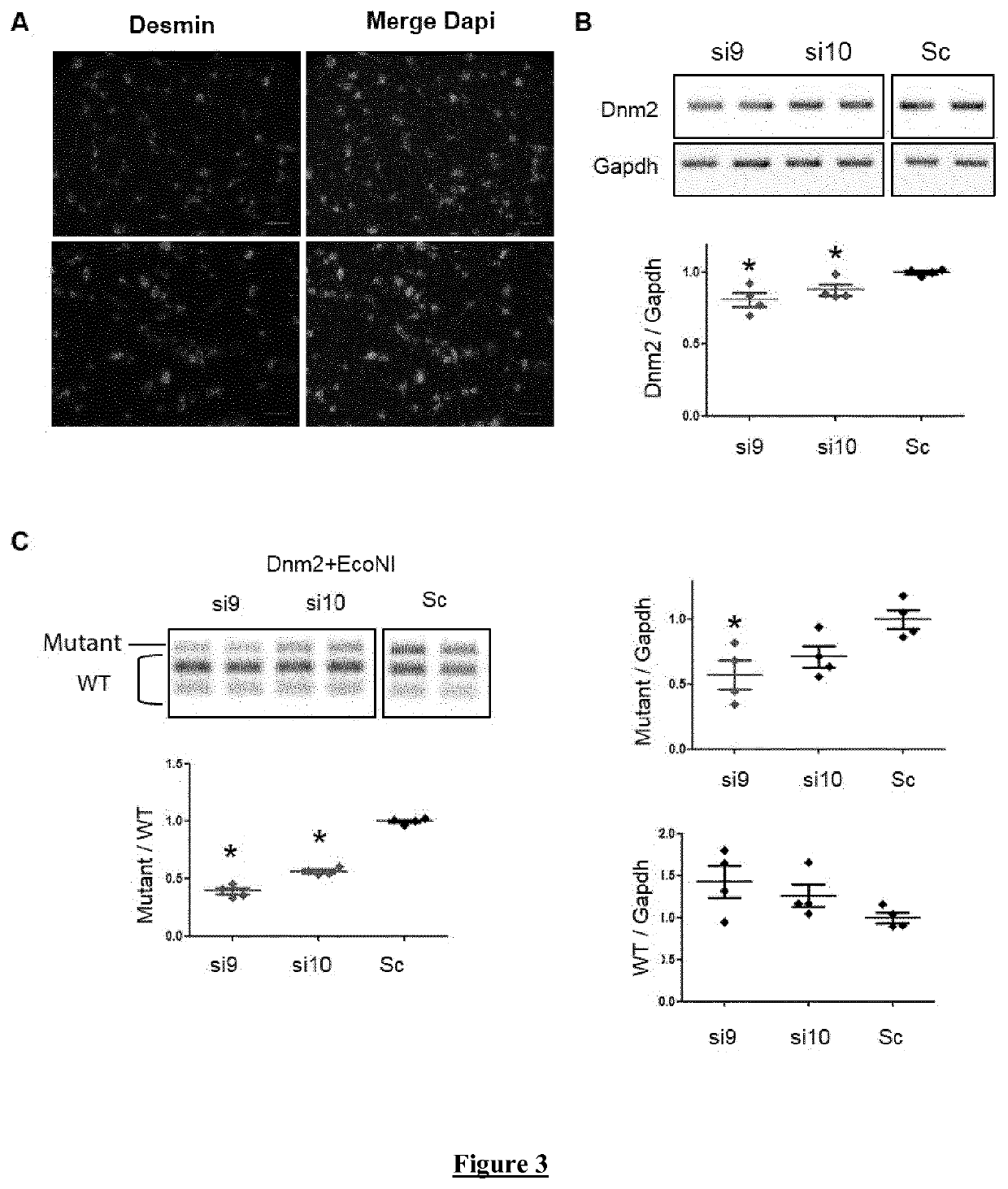
Allele-specific silencing therapy for Dynamin 2-related diseases
Owner:ASSOC INST DE MYOLOGIE +3
Primers and kit for detecting rare type beta-thalassemia mutation
InactiveCN106939341AStrong specificityImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationBeta thalassemiaNucleic acid sequencing
The invention discloses primers and kit for detecting rare type beta-thalassemia mutation; the primers can amplify a nucleic acid sequence containing a beta-globin transcription promoter upstream-90 position (C>T) beta+(HBB:c.-140C>T). The primers and the detection kit can effectively detect rare type beta-globin transcription promoter-90 position (C>T) beta+ heterozygous mutation (HBB:c.-140C>T), provide a corresponding basis for prenatal detection, are used for accurate detection of one gene sequence for detecting the rare type beta-thalassemia, promote function analysis and research of the gene for detecting the rare type beta-thalassemia.
Owner:GUANGZHOU KINGMED DIAGNOSTICS CENT
Heterozygous-mutation dried-blood-spot positive quality control product applicable to detection of mutant genes of deafness
ActiveCN107955833AGuaranteed biostabilityEasy to collectMicrobiological testing/measurementOrganismBiology
The invention discloses a heterozygous-mutation dried-blood-spot positive quality control product applicable to detection of mutant genes of deafness. The heterozygous-mutation dried-blood-spot positive quality control product is characterized in that DNA fragments containing mutation sites or plasmids containing the DNA fragments are mixed with whole blood to prepare dried blood spots; the mutation sites comprise 11 mutation sites of four genes such as GJB2, GJB3, 12SrRNA and SLC26A4. The heterozygous-mutation dried-blood-spot positive quality control product applicable to detection of mutantgenes of deafness is close to a clinical sample, and has the advantages that the needed blood volume is less, the sample collection is convenient, the biological stability of the sample can be maintained, the biological risk can be reduced, and the transportation and storage are convenient. The heterozygous-mutation dried-blood-spot positive quality control product can be still used normally after being stored for 2 years at the temperature of 4 DEG C, and the detection result can not be influenced.
Owner:GUANGZHOU DARUI BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com