Method and kit for detecting polymorphism of mitochondrial ND1 gene mononucleotide, and application of kit
A single nucleotide polymorphism and mitochondrial technology, applied in biochemical equipment and methods, recombinant DNA technology, microbial measurement/inspection, etc., can solve the problem of increasing PCR product contamination, cannot achieve closed-tube operation, and is difficult to separate Type and other problems, to achieve the effect of low detection cost, convenient and simple detection, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1: Blood Sample Collection and Genomic DNA Extraction
[0062] 1. Sample selection
[0063] Household survey of long-lived elderly in Bama, Guangxi, aged ≥ 90 years old. Clear consciousness, independent life, able to cooperate with examination, no cardiovascular and cerebrovascular diseases, Alzheimer's disease, Parkinson's disease, cancer and other aging-related degenerative diseases, a total of 358 cases. The basic information of all subjects was recorded and informed consent was signed by themselves or their relatives. This study was also approved by the institutional ethics committee.
[0064] 2. Preparation of Genomic DNA
[0065] In the presence of the anticoagulant EDTA, 5 ml of peripheral blood collected from the subject was centrifuged at 2500 rpm for 30 minutes to remove serum. Then add 5ml of cell lysate (containing 10mmol / L Tris-HCl pH8.0, 10mmol / L EDTA pH8.0, 15mmol / L Nacl, 0.4% SDS, 0.1mg / ml proteinase K) and incubate overnight at 37°C. Then u...
Embodiment 2
[0066] Embodiment 2: Identification and determination of SNP
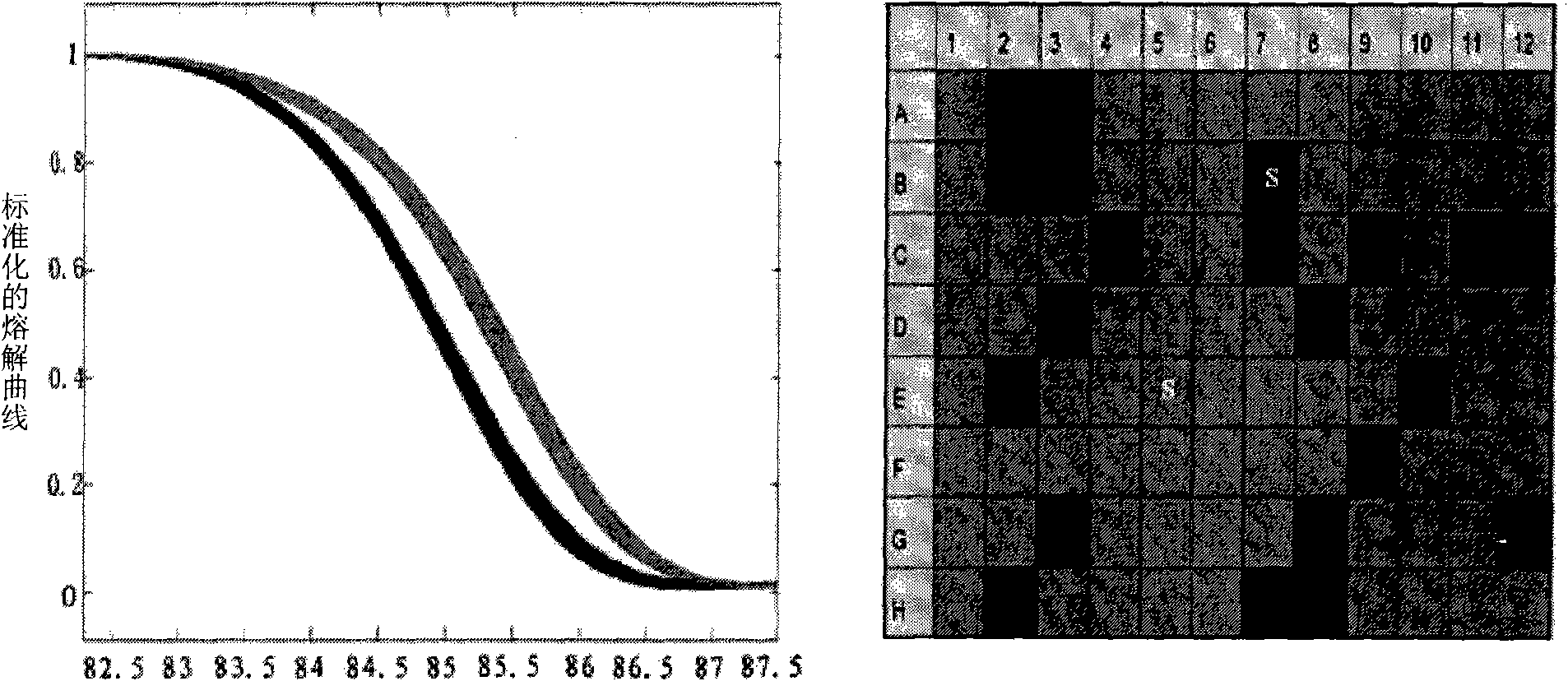
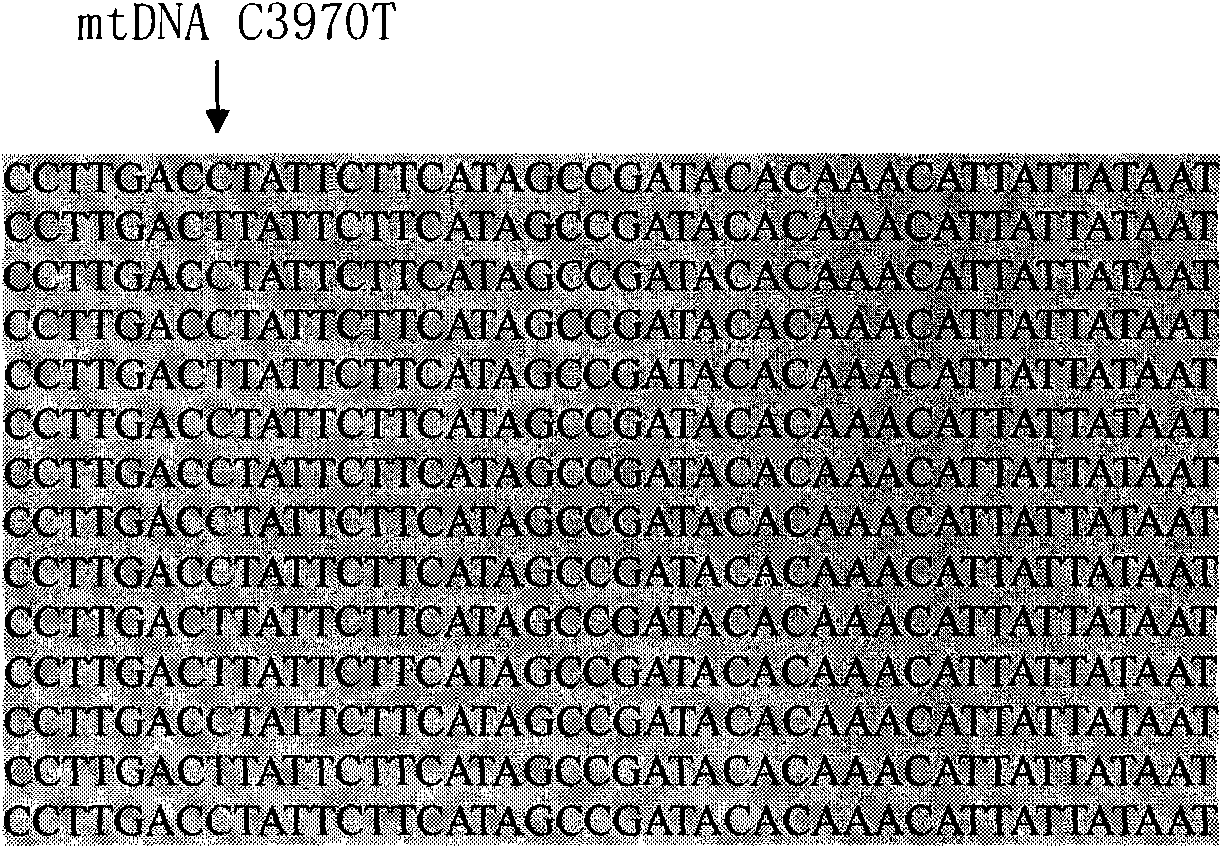
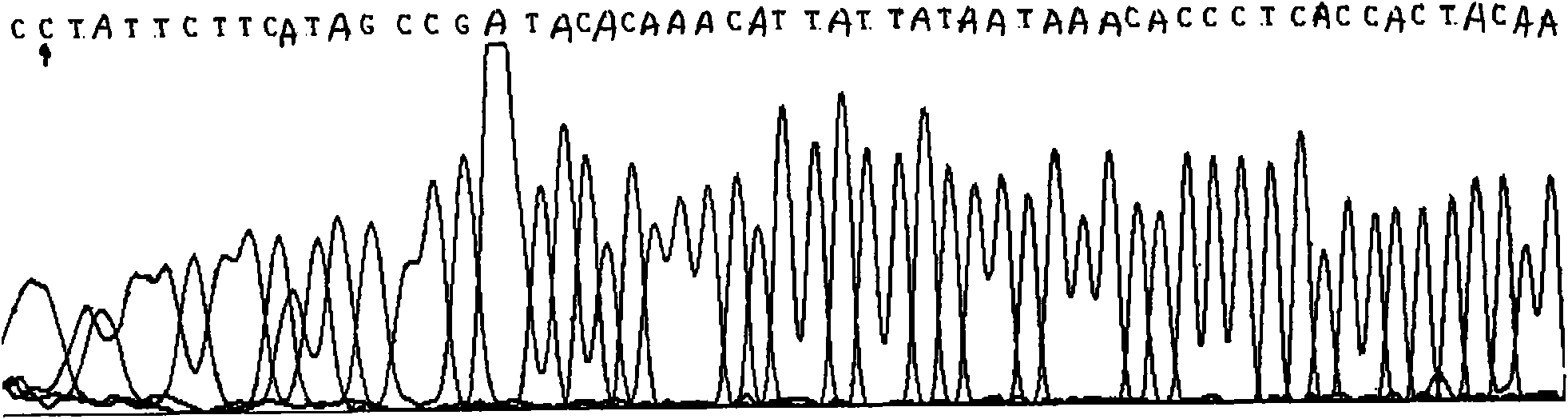
[0067] The invention adopts HRMA and PCR sequencing technology to simultaneously detect the C3970T site (the allelic site pair is A / G).
[0068] 1. Specific primers are as follows:
[0069] F1: 5'-CTAGTCTCAGGCTTCAACATC-3' (SEQ ID NO.1)
[0070] R1: 5'-TGTTTGTGTATTCGGCTAT-3' (SEQ ID NO.2)
[0071] 2. Amplify some fragments near C3970T by PCR; prepare mixed solution: add 2 ul of genomic DNA solution, 2 ul of 10X PCR buffer, 0.4 ul of 10mM dNTP, 2 ul of Taq DNA polymerase, respectively 0.2 ul of the above step 1) in Example 1 For each primer described, 2ul LCGreen PLUS+ saturated fluorescent dye, 0.1ul C1 and C2 oligonucleotide internal references respectively. Next, pure water was added to make the total volume 20ul. The reaction was performed at 95°C for 5 minutes, 95°C for 1 minute, 56°C for 30 seconds, 72°C for 3 s, and 2°C for 7 minutes for 35 cycles. After the reaction was completed, the PCR product was sub...
Embodiment 3
[0075] Example 3: Association of mtDNA 3970 single nucleotide polymorphisms with aging-related degenerative diseases
[0076] 1. Statistical method: use STATA8.0 and SPSS11.0 software Yates chi-square test to calculate the carrier frequency of mtDNA 3970 single nucleotide polymorphism, carry out continuous correction and one-sided asymptotic probability analysis, statistical significance Levels were set at P<0.05. Single factor Logistic regression analysis was used to calculate the risk OR value of longevity and its 95% confidence interval (CI).
[0077] 2. Results
[0078] 1. Distribution of mtDNA 3970 single nucleotide polymorphisms in healthy people
[0079] According to the method of Examples 1 and 2, the gene polymorphisms of 362 healthy people (without cardiovascular and cerebrovascular diseases, cancer, etc., age≤60 years old) were determined. 285 people had C polymorphism at base 3970 (78.1%), and 77 people had T polymorphism (21.3%).
[0080] 2. Distribution of mt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com