Citrin deficiency pathogenic gene detection primer and kit
A technology for detection kits and disease-causing genes, which is applied in the determination/inspection of microorganisms, biochemical equipment and methods, DNA/RNA fragments, etc., and can solve problems such as cumbersome operations, high detection costs, and insufficient sensitivity and specificity. Achieve the effect of simple operation, high sensitivity and high specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] An embodiment of the Citrin deficiency disease-causing gene detection kit of the present invention comprises an upstream primer shown in SEQ ID No.1, a downstream primer shown in SEQ ID No.2 and a probe shown in SEQ ID No.3. needle; the 5' end of the probe is connected with a fluorescent reporter group FAM, and the 3' end is connected with a fluorescent quencher group BHQ1.
[0039] SEQ ID No.1: 5'-GGAGGAGGGCAGCAATCAG-3';
[0040] SEQ ID No.2: 5'-GGCCTCAGCCAAGTTAAAGG-3';
[0041] SEQ ID No.3: 5'FAM-TTTGTTTTTCCCCTACAGACGTATGACCTTAGC
[0042] AGACATTGAAC-BHQ1 3'.
[0043] The primers were synthesized by a biological company and configured according to the instructions.
Embodiment 2
[0045] In this example, the kit described in Example 1 is used to detect the causative gene of Citrin deficiency.
[0046] 1. Reaction system configuration
[0047] Configure the reaction system according to the components and component final concentrations shown in Table 1:
[0048]Table 1 reaction system
[0049]
[0050] The DNA template is DNA extracted from peripheral blood cells of the patient.
[0051] 2. Reaction procedure
[0052] The PCR reaction program is shown in Table 2:
[0053] Table 2 Reaction program
[0054]
[0055]
[0056] 3. Judgment criteria for results
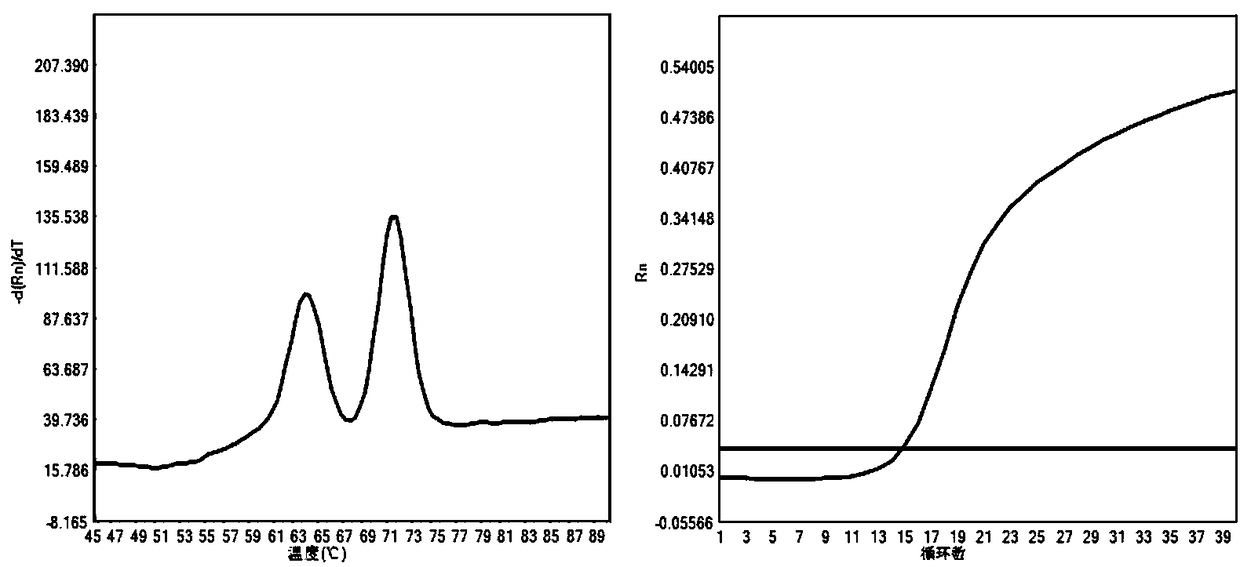
[0057] The kit of the invention can be used to detect the wild type, heterozygous mutation and homozygous mutation of SLC25A13 gene c.851_854del (rs80338720), so as to diagnose Citrin deficiency. The criteria for judging the test results are as follows:
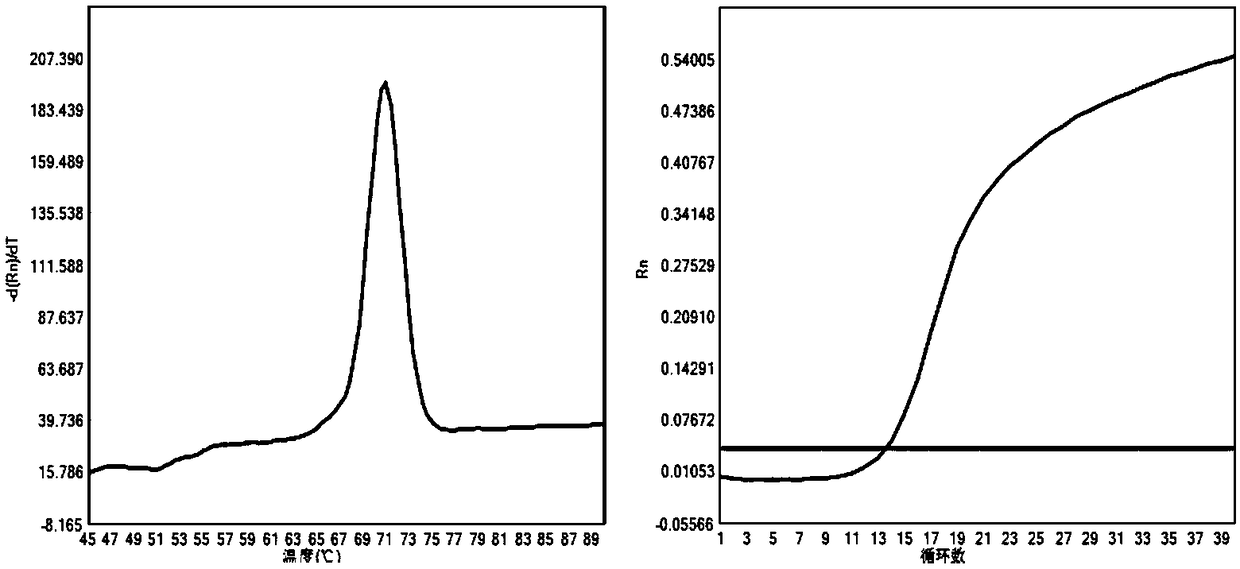
[0058] (1) Wild type of the causative gene of Citrin deficiency: Ct value is less than 35; Tm value: 70.94±0.55°C, single peak;
[0...
Embodiment 3
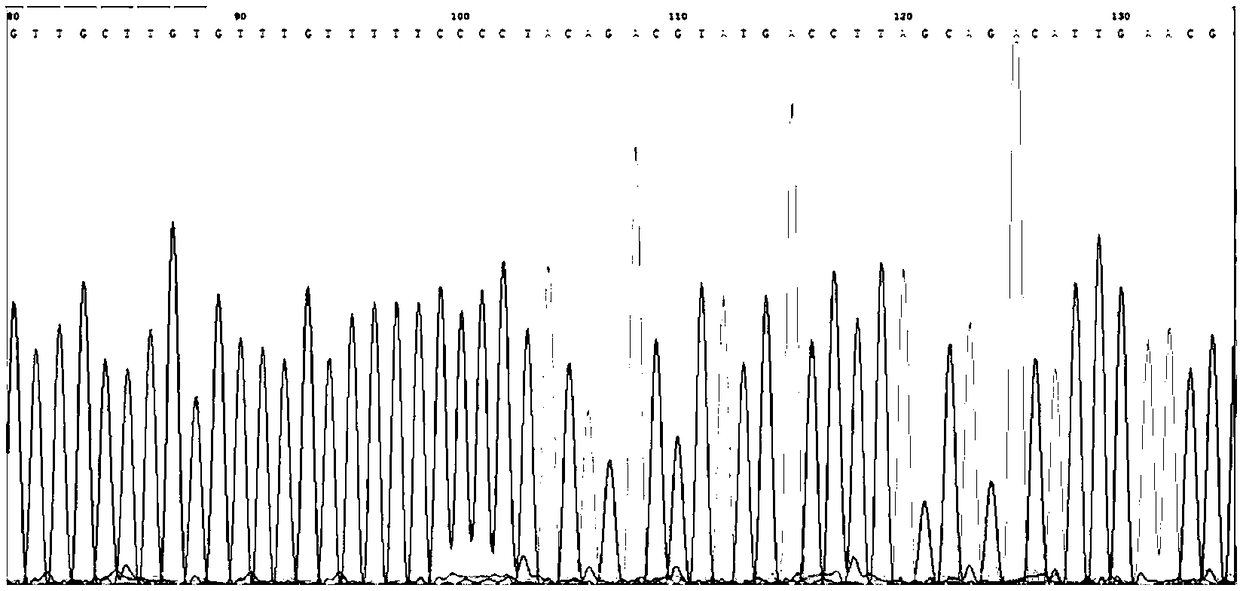
[0067] In this example, the pathogenic gene of Citrin deficiency was detected in 15 cases of clinical samples. Take the peripheral blood sample of the subject to be tested, extract the DNA, use the kit described in Example 1, and perform detection according to the detection method described in Example 2, and at the same time perform the first-generation sequencing on the sample. The test results are shown in Table 3:
[0068] Table 3 Test results
[0069]
[0070]
[0071] The above results show that the kit of the present invention has good accuracy when used in the detection of Citrin deficiency disease-causing genes, and the detection results are completely consistent with the sequencing results.
[0072] Table 4Mg 2+ Final concentration optimization reaction system
[0073]
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com