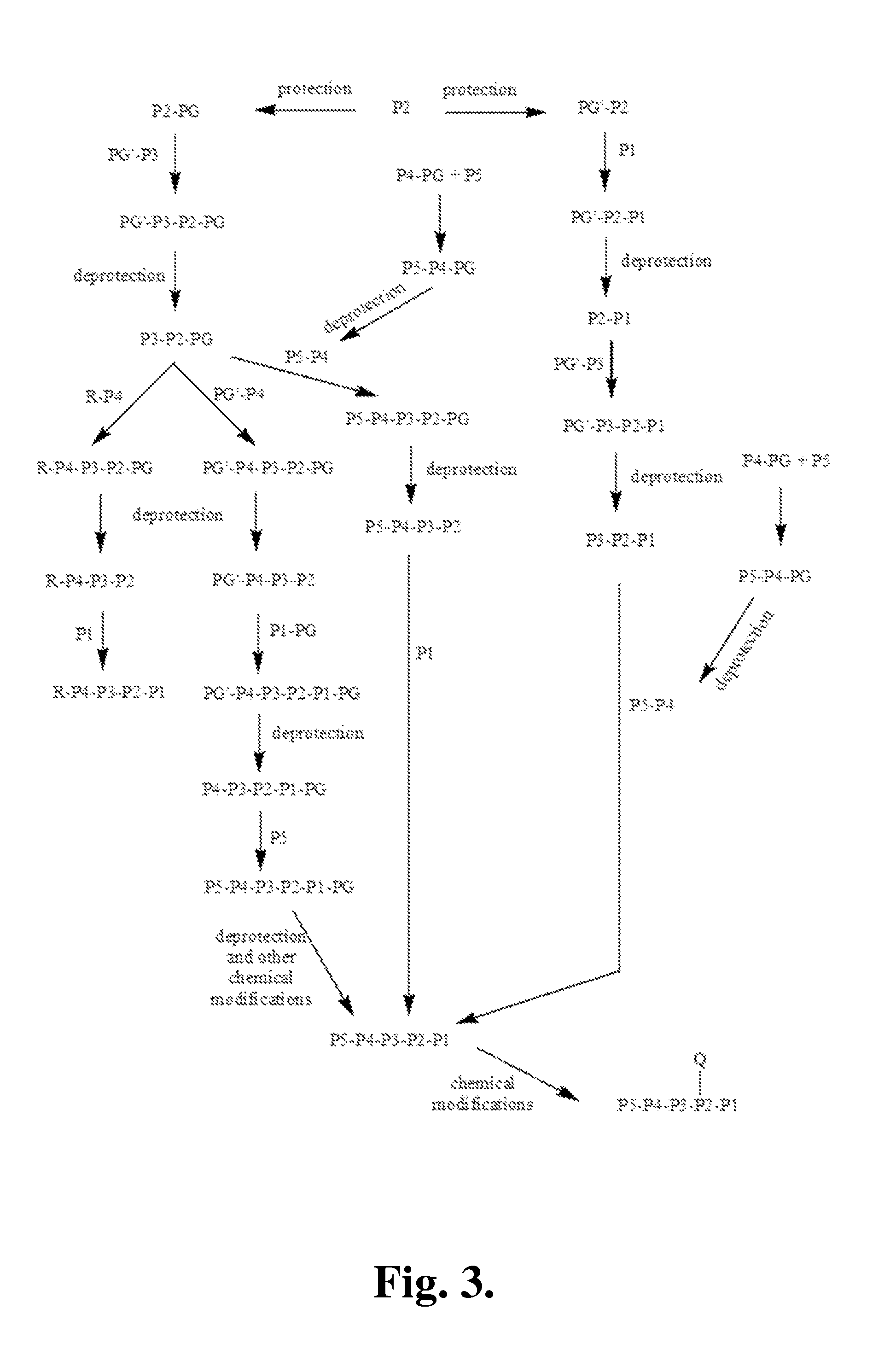
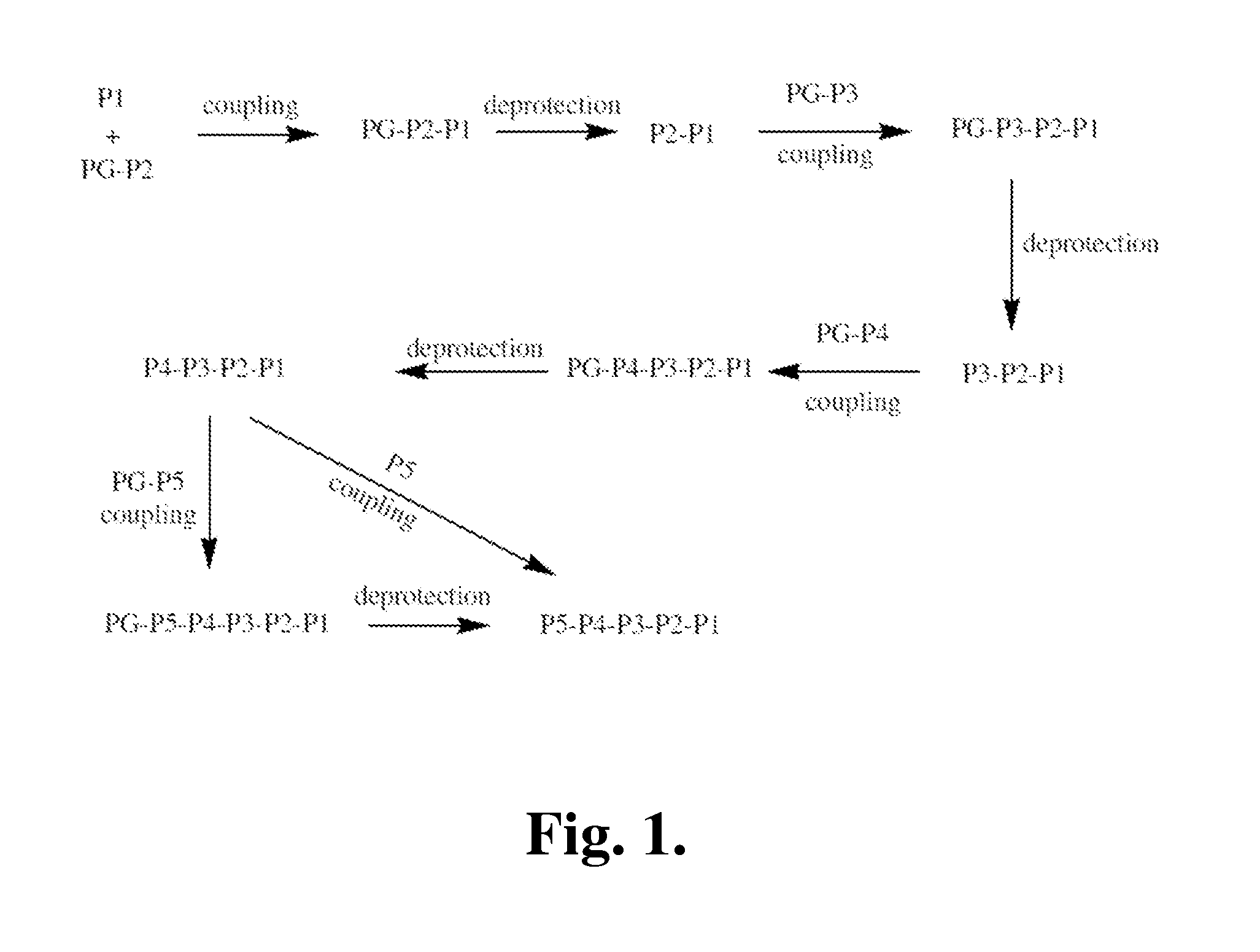
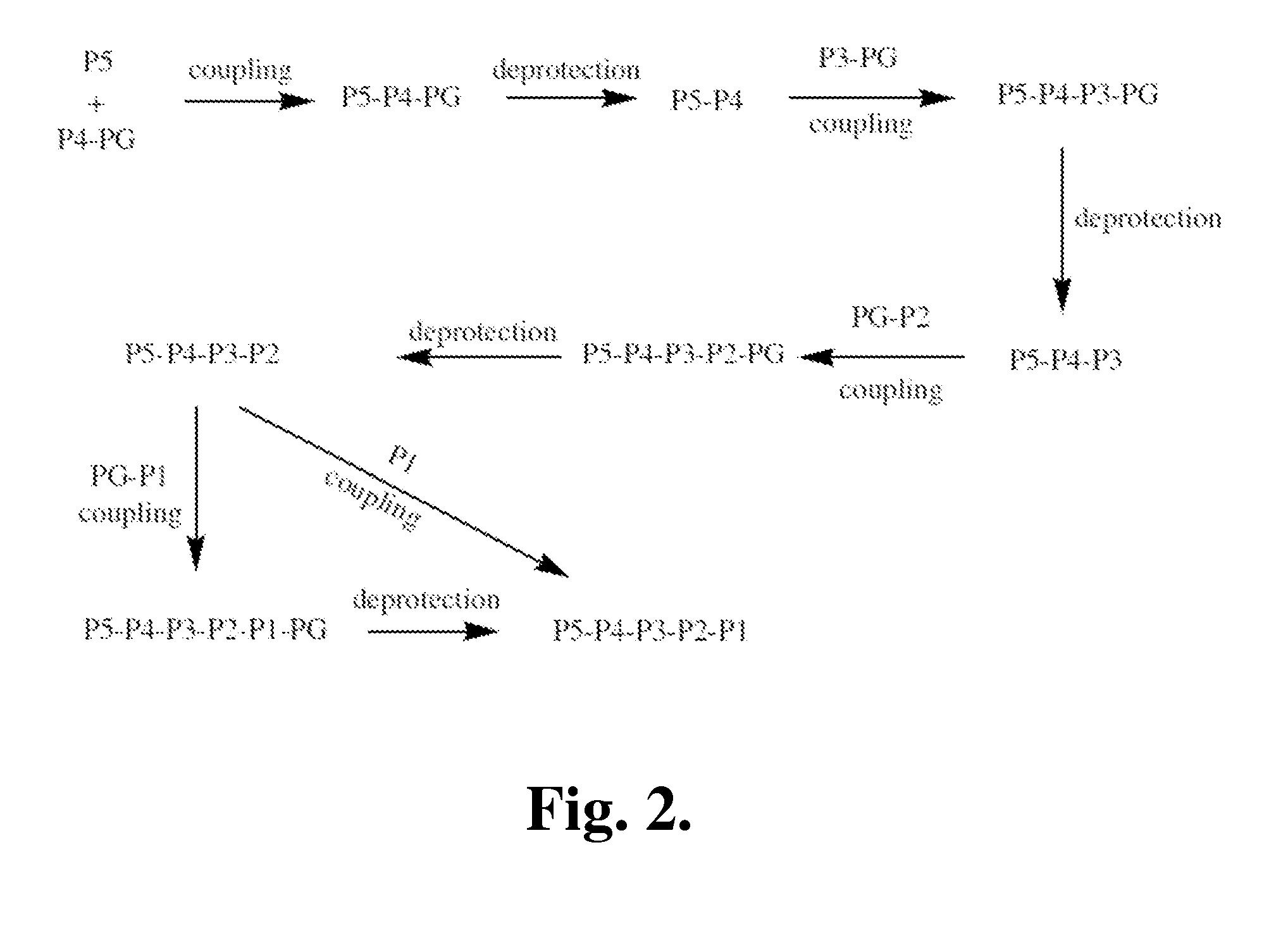
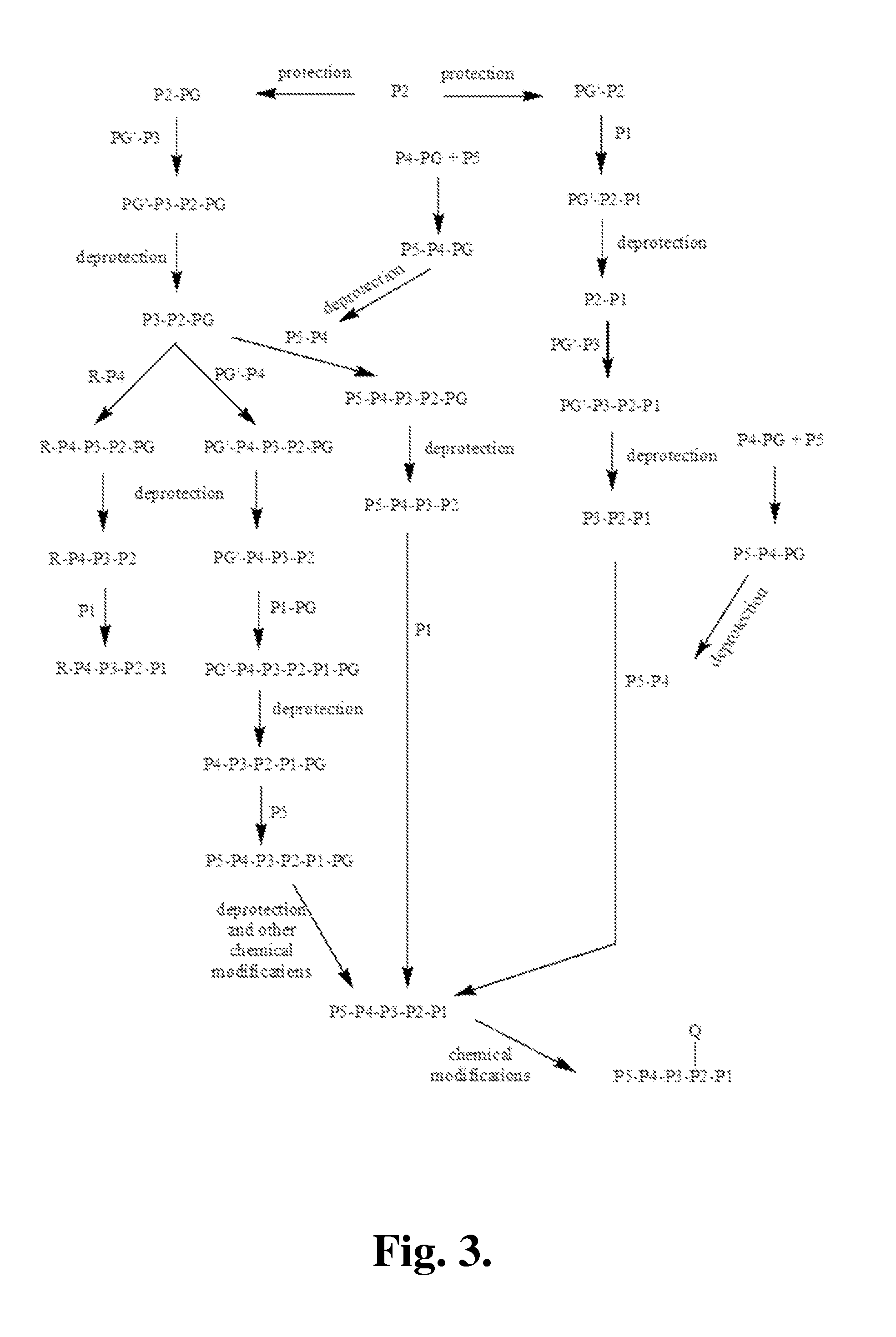
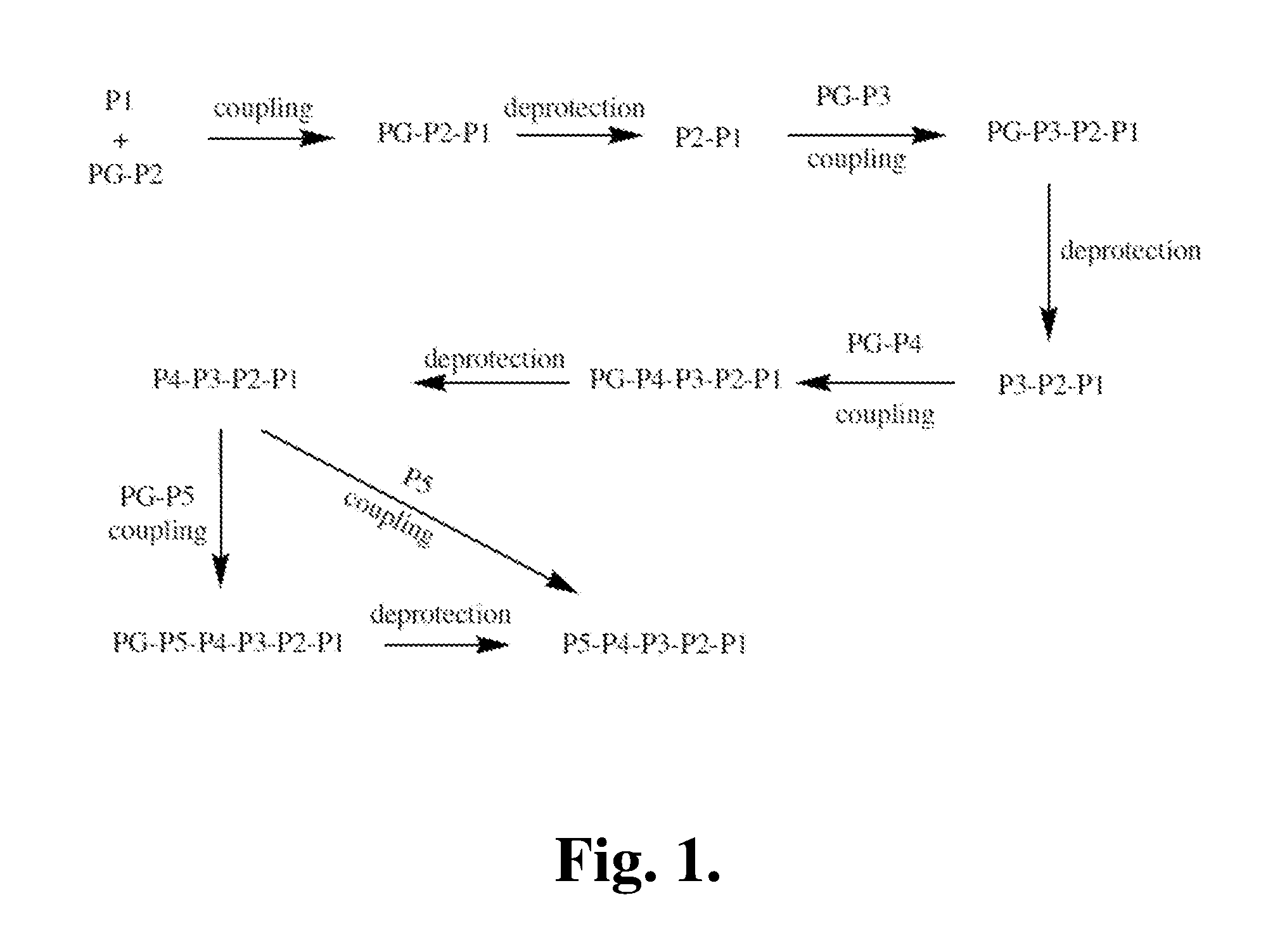
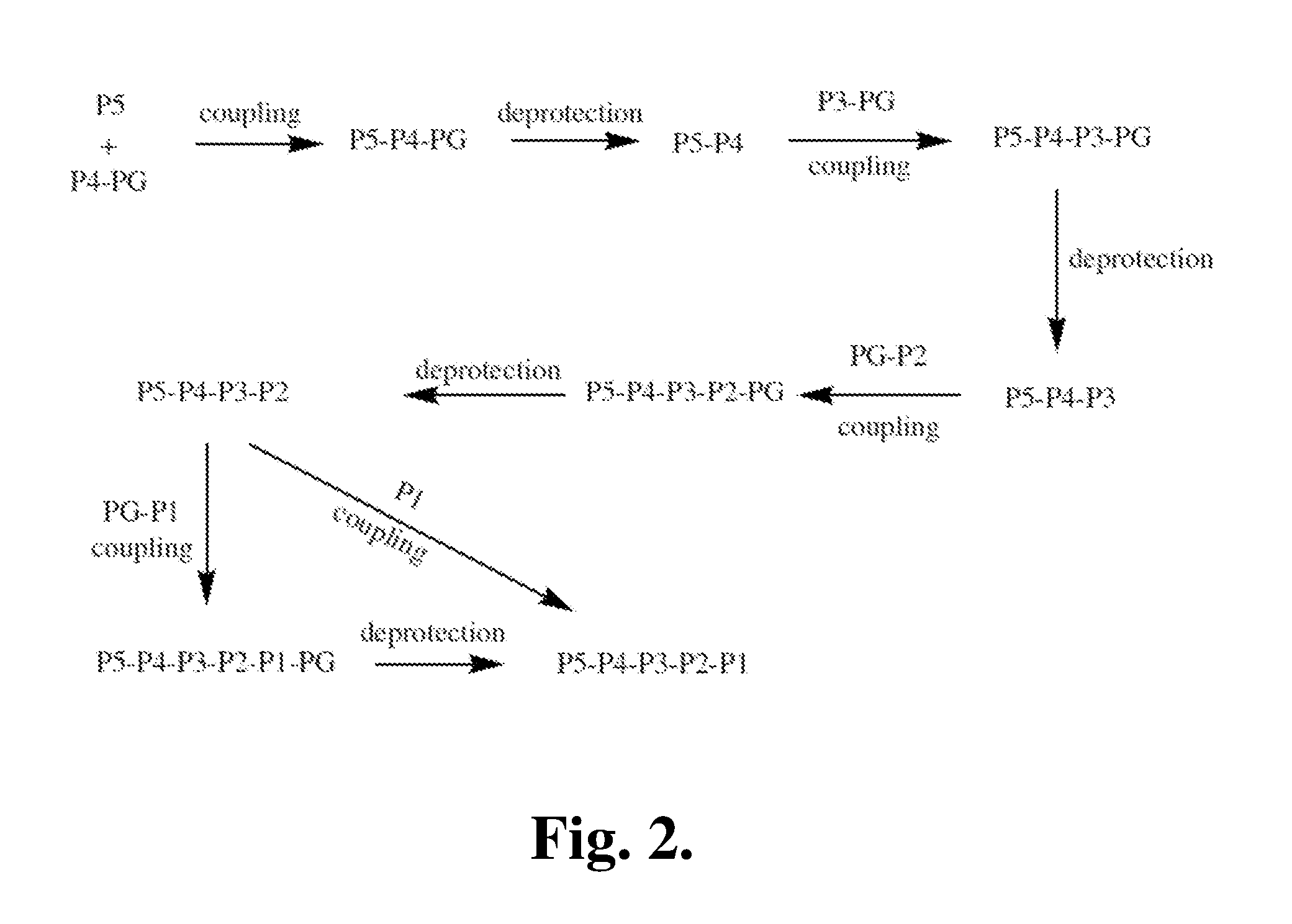
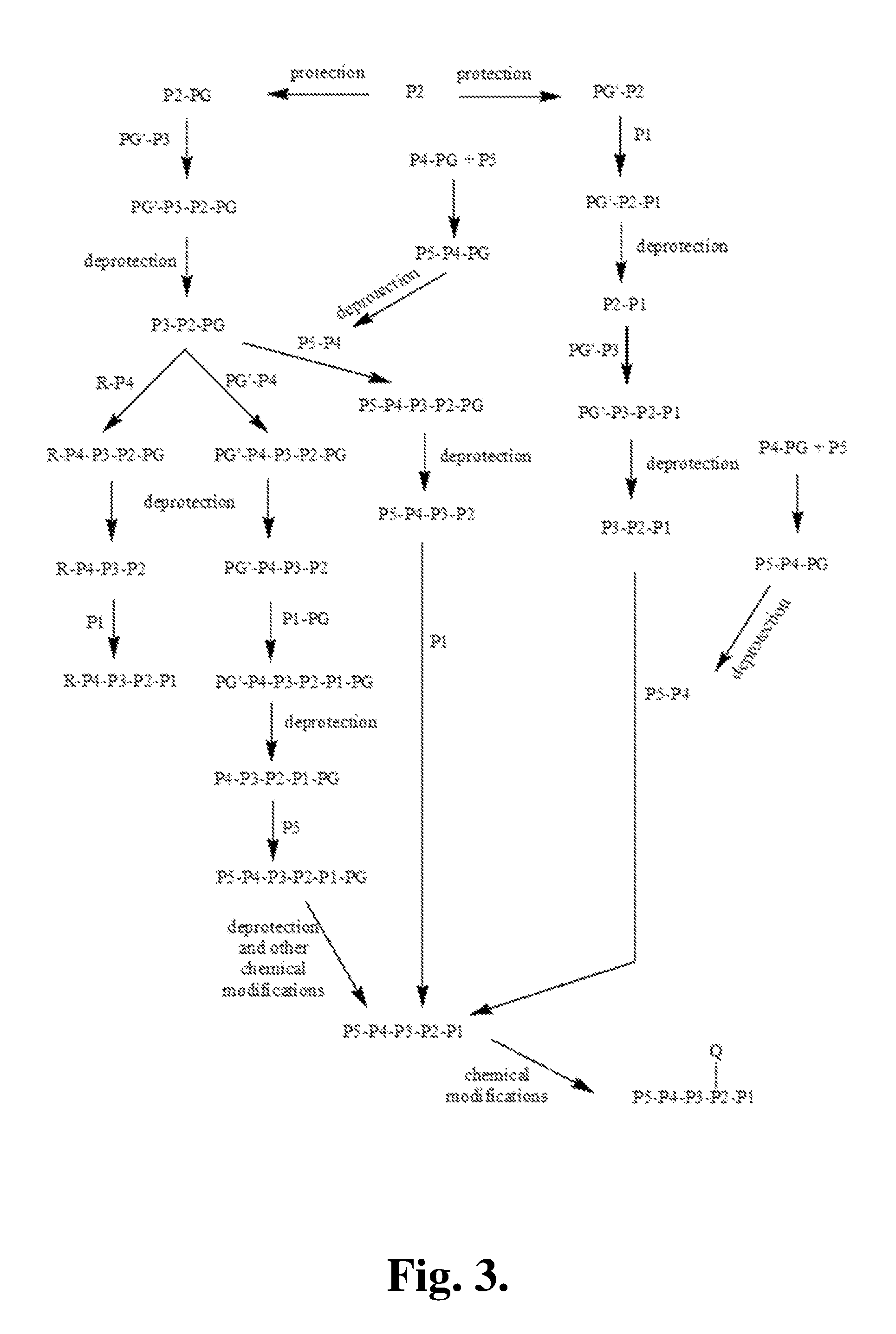
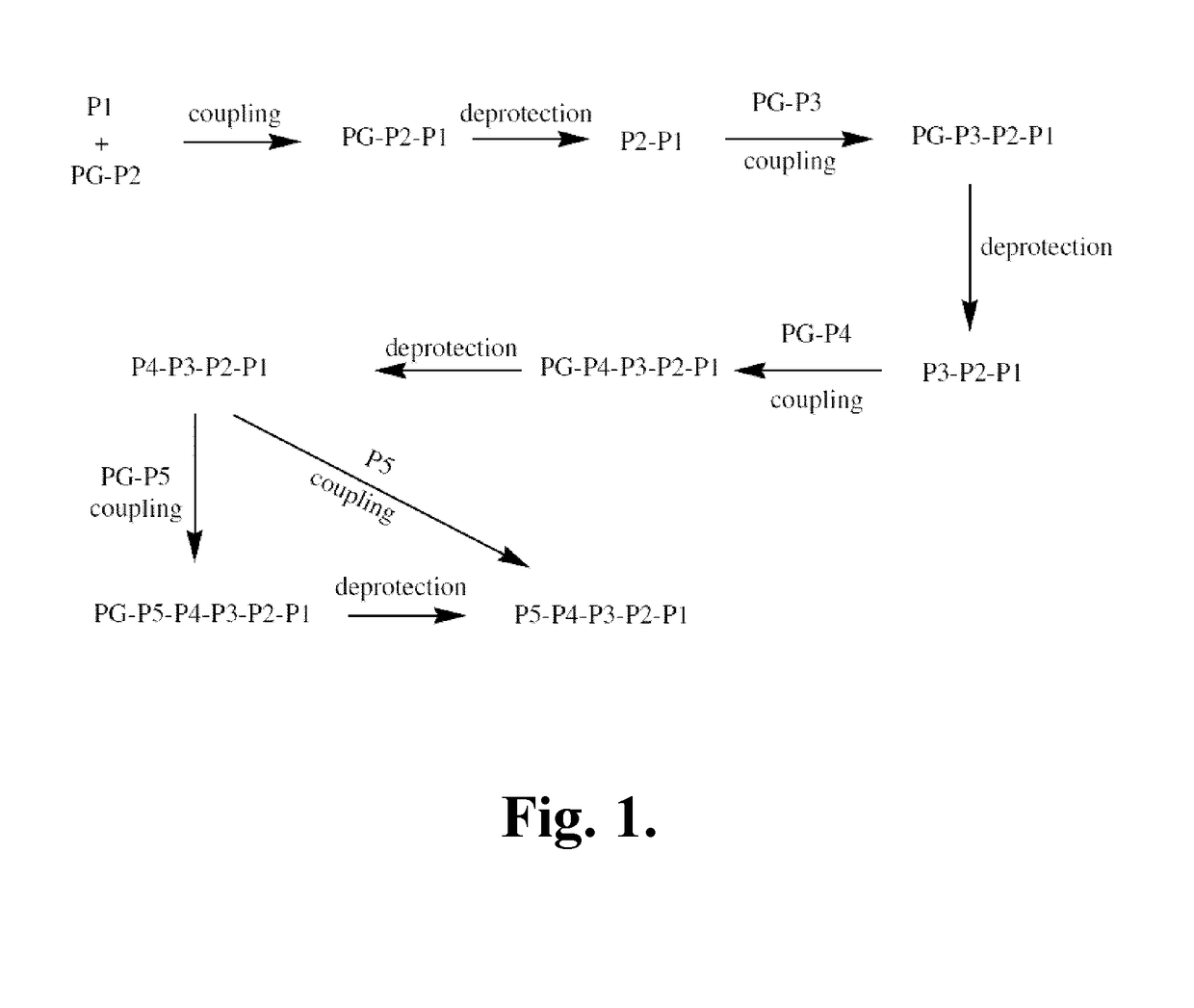
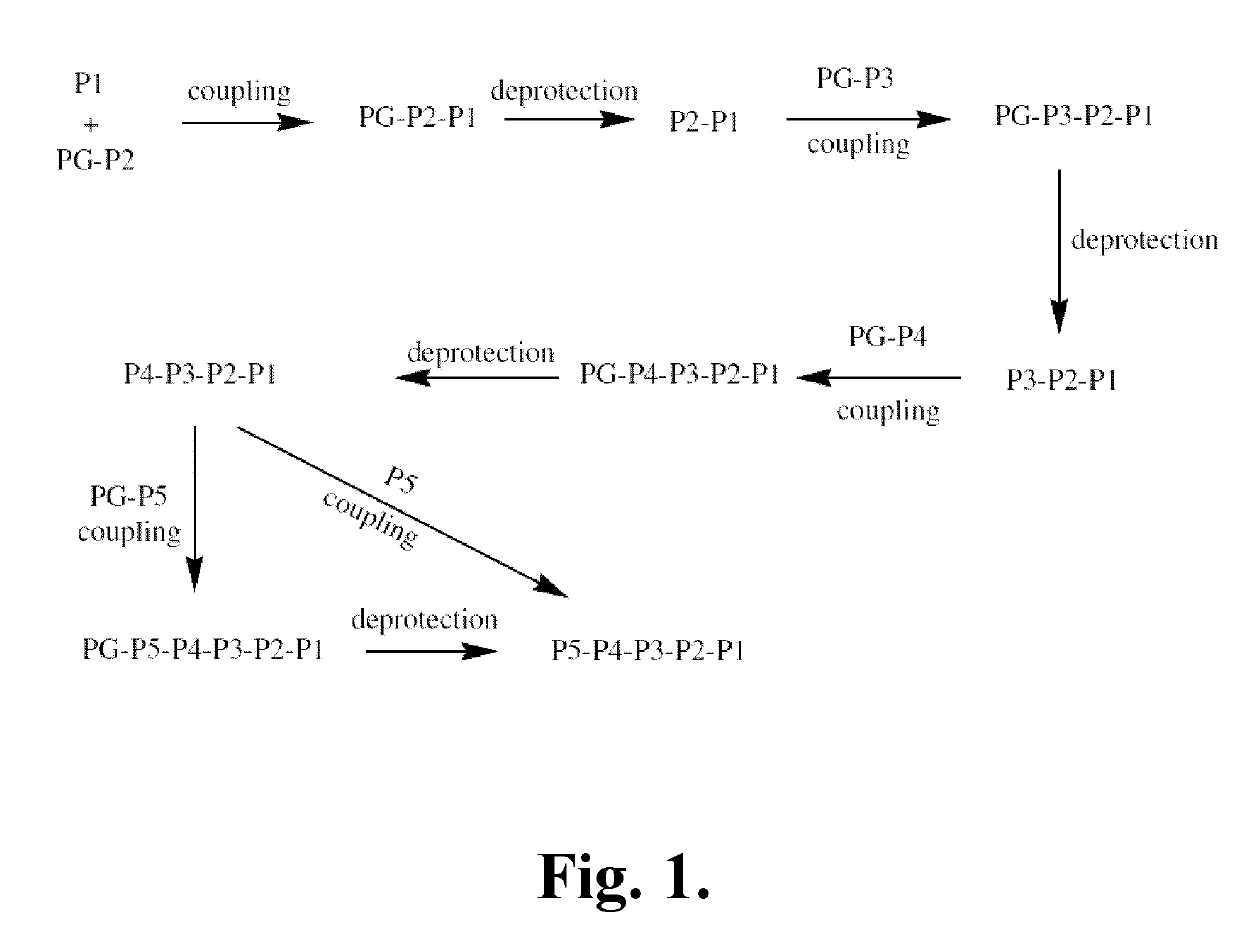
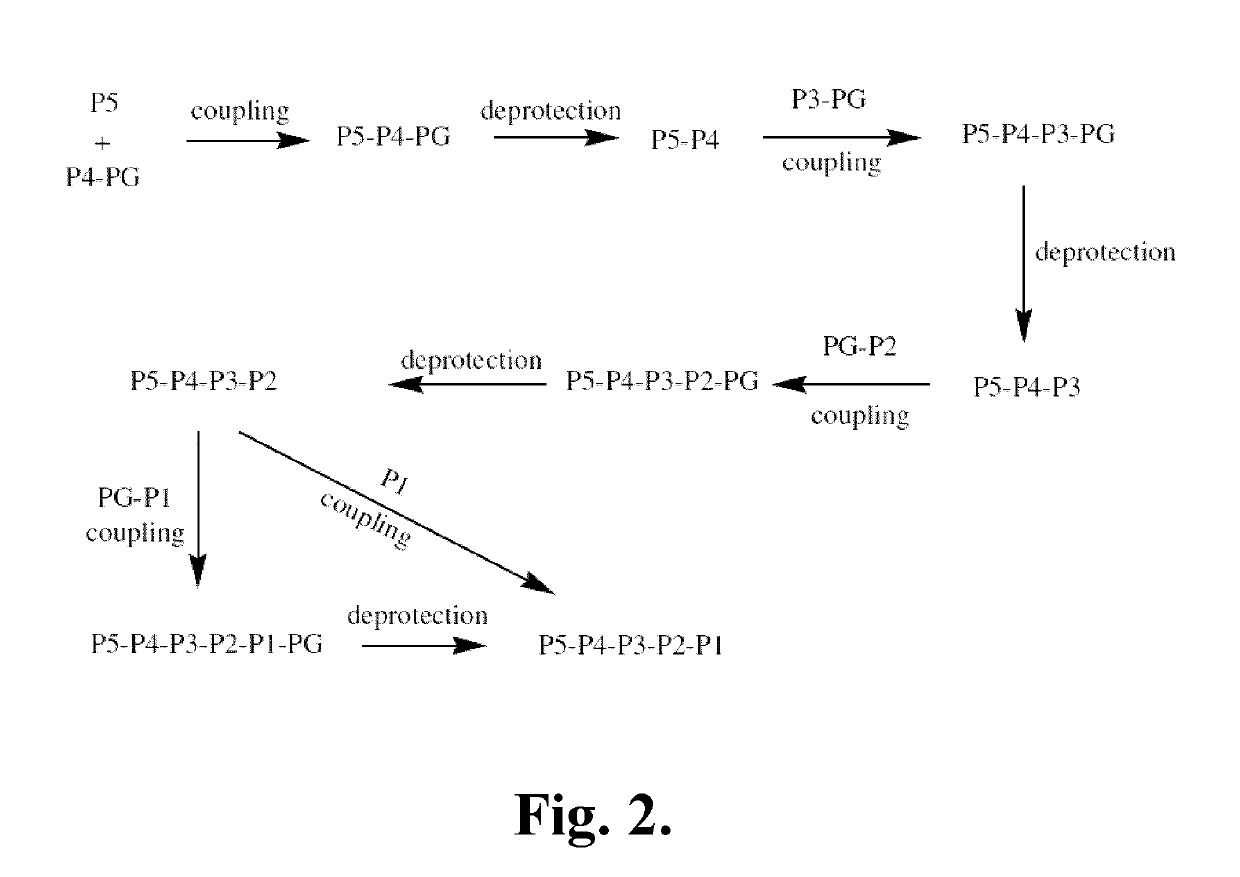
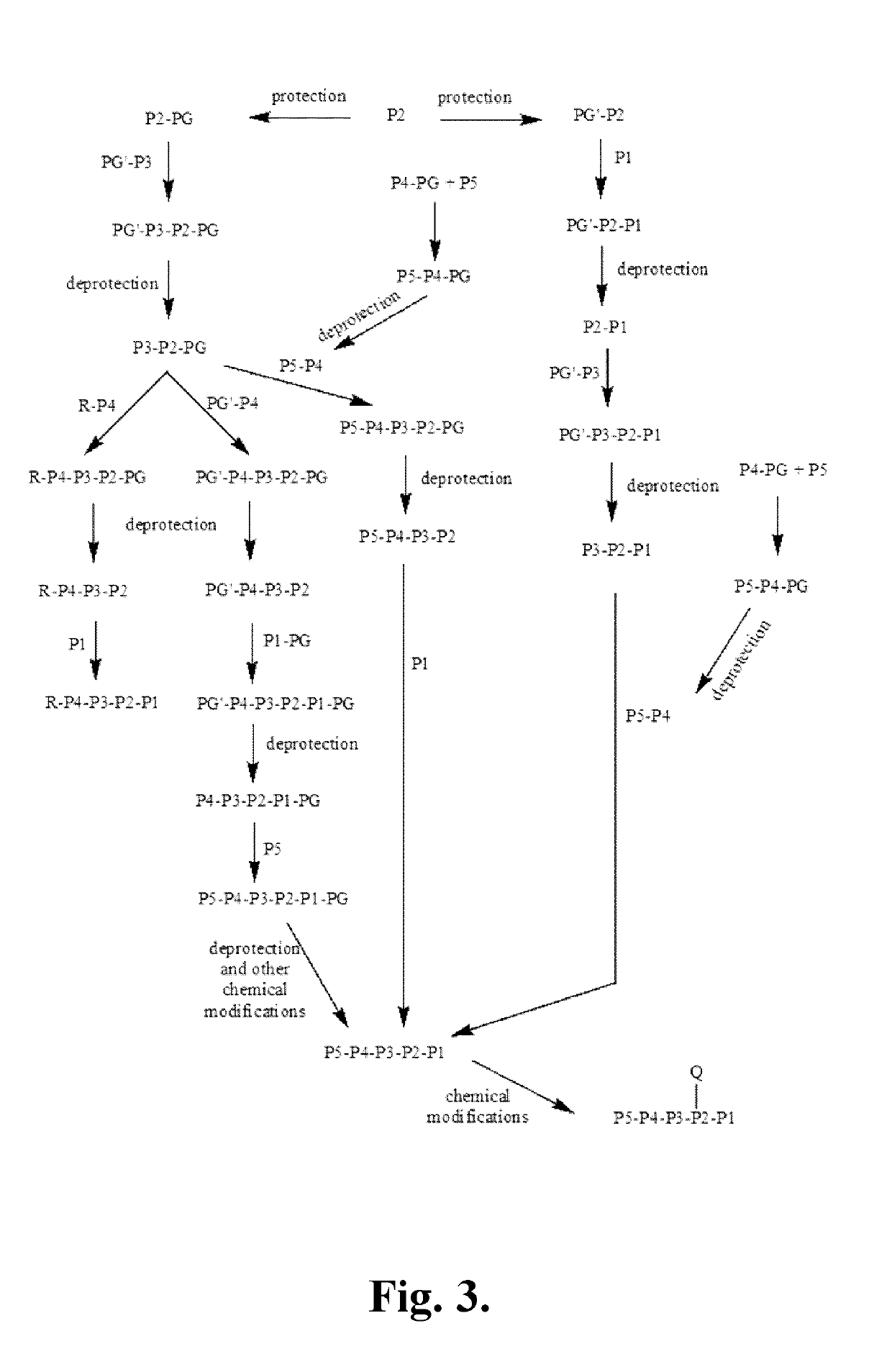
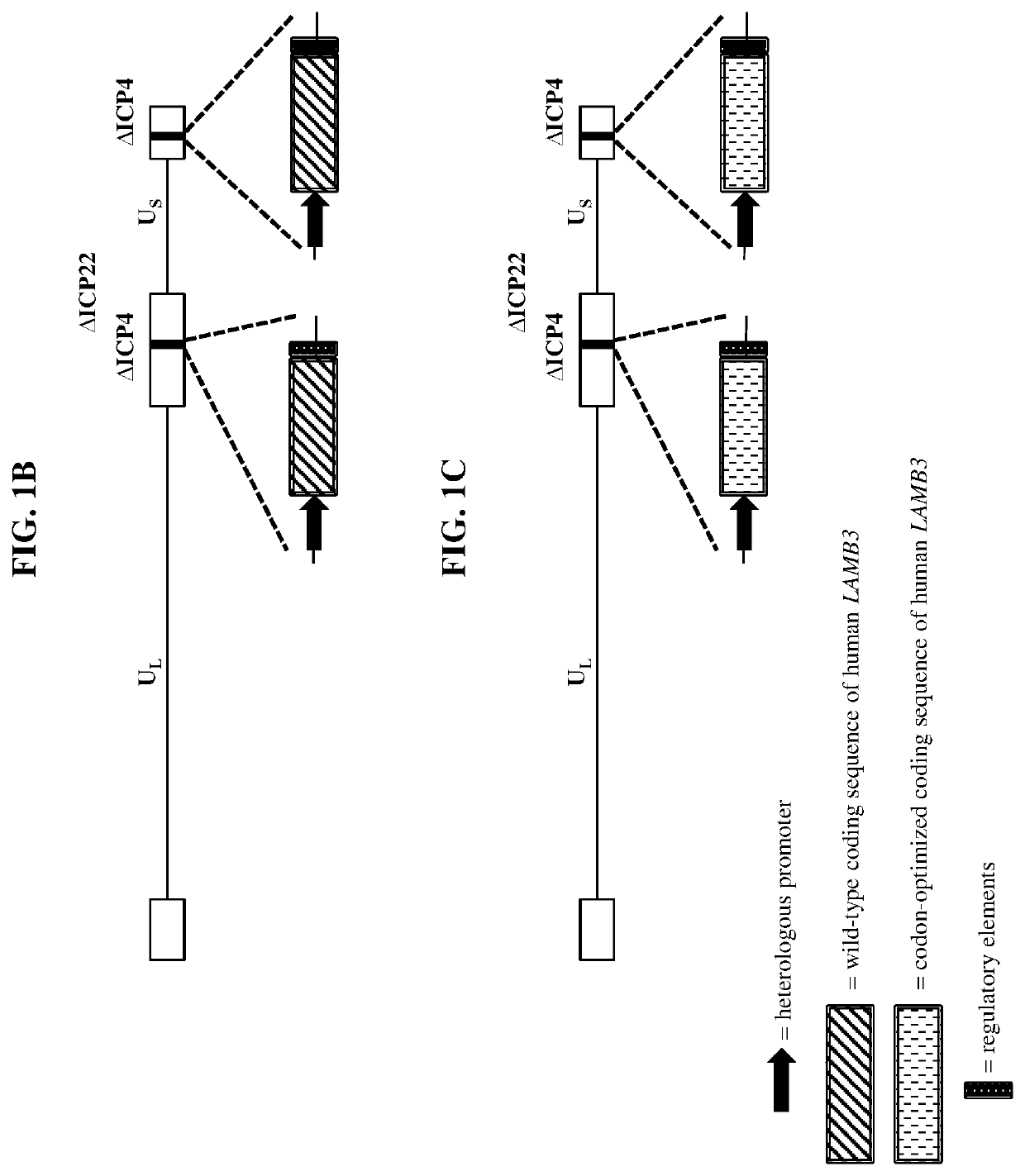
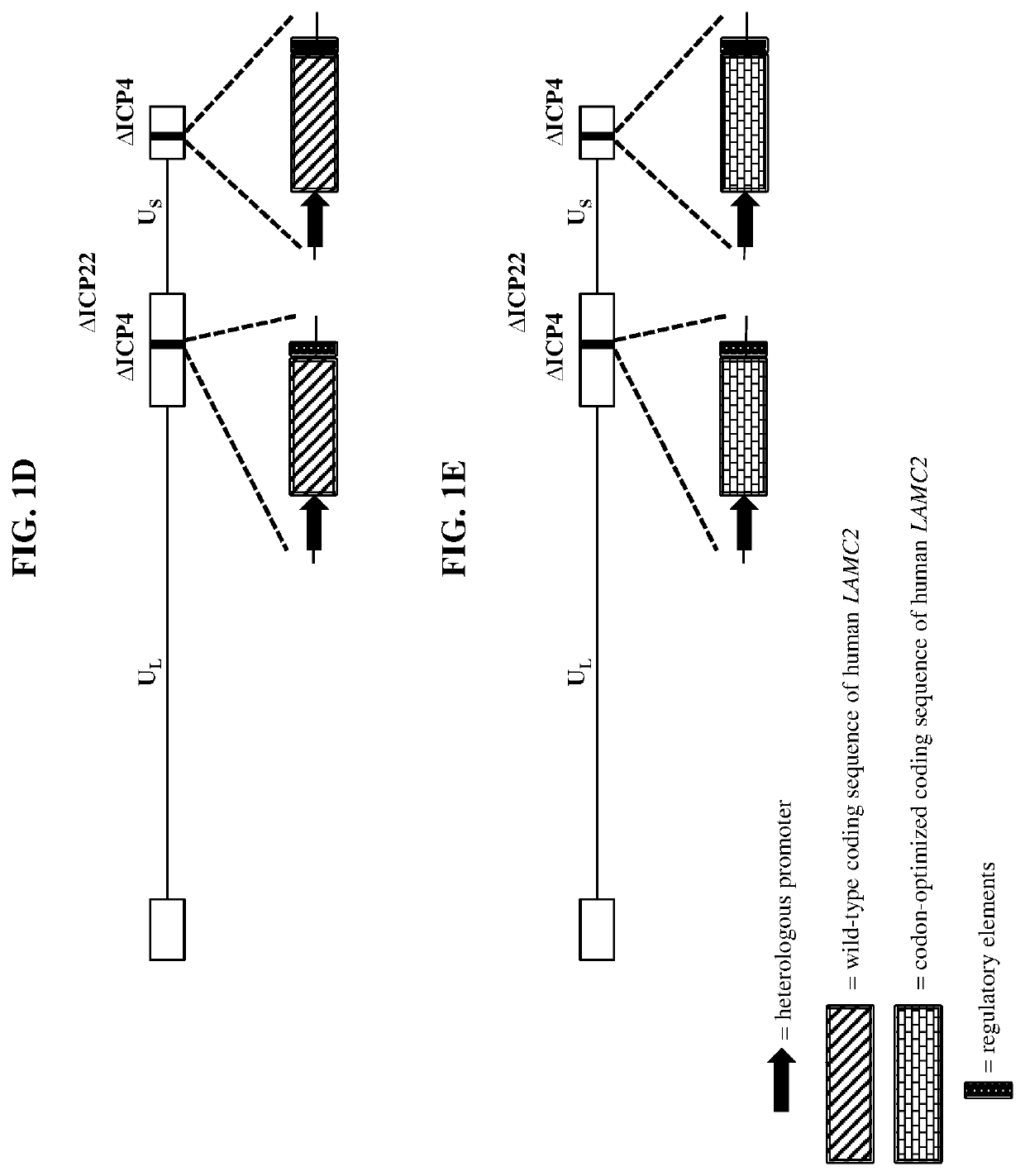
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
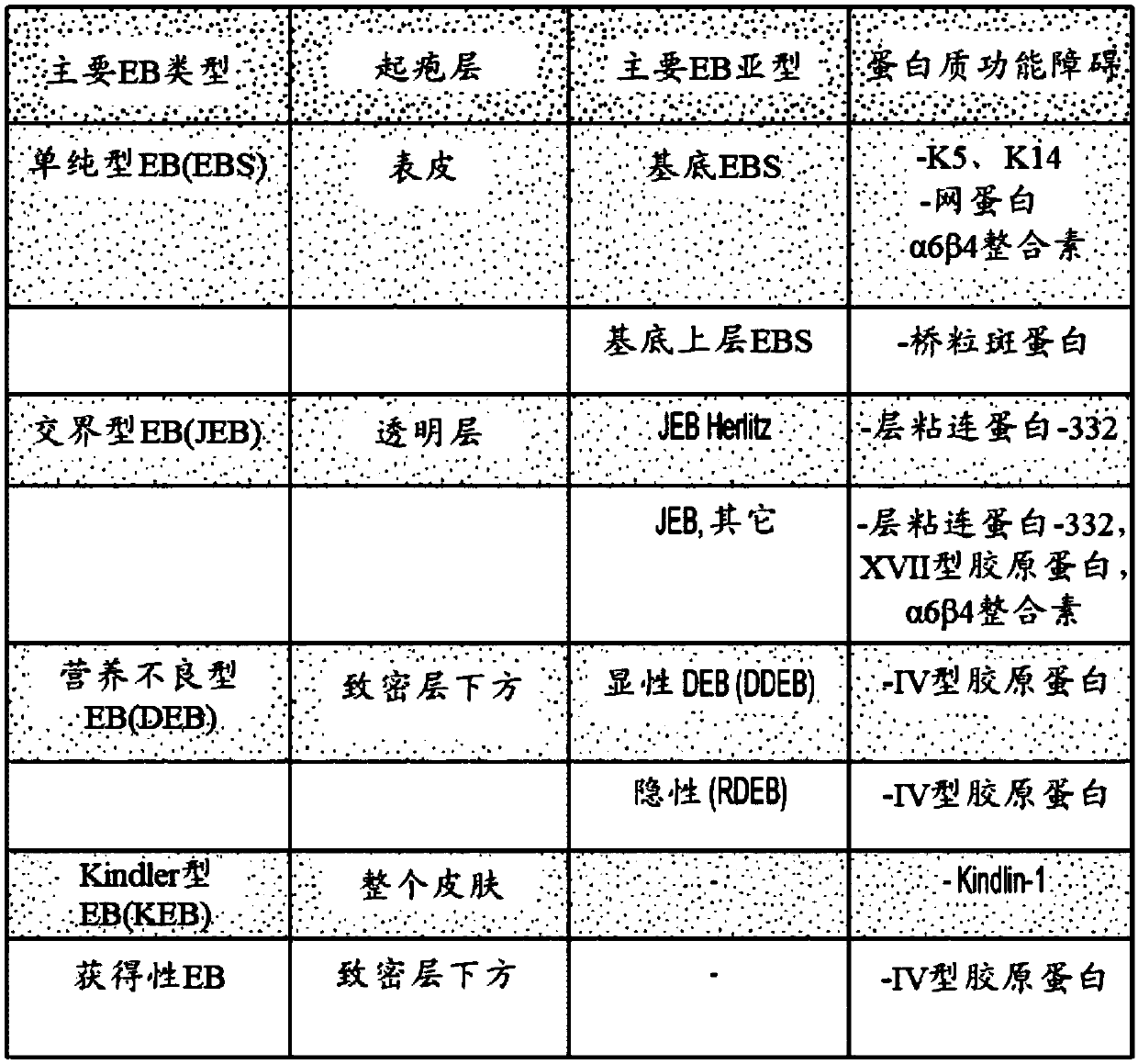
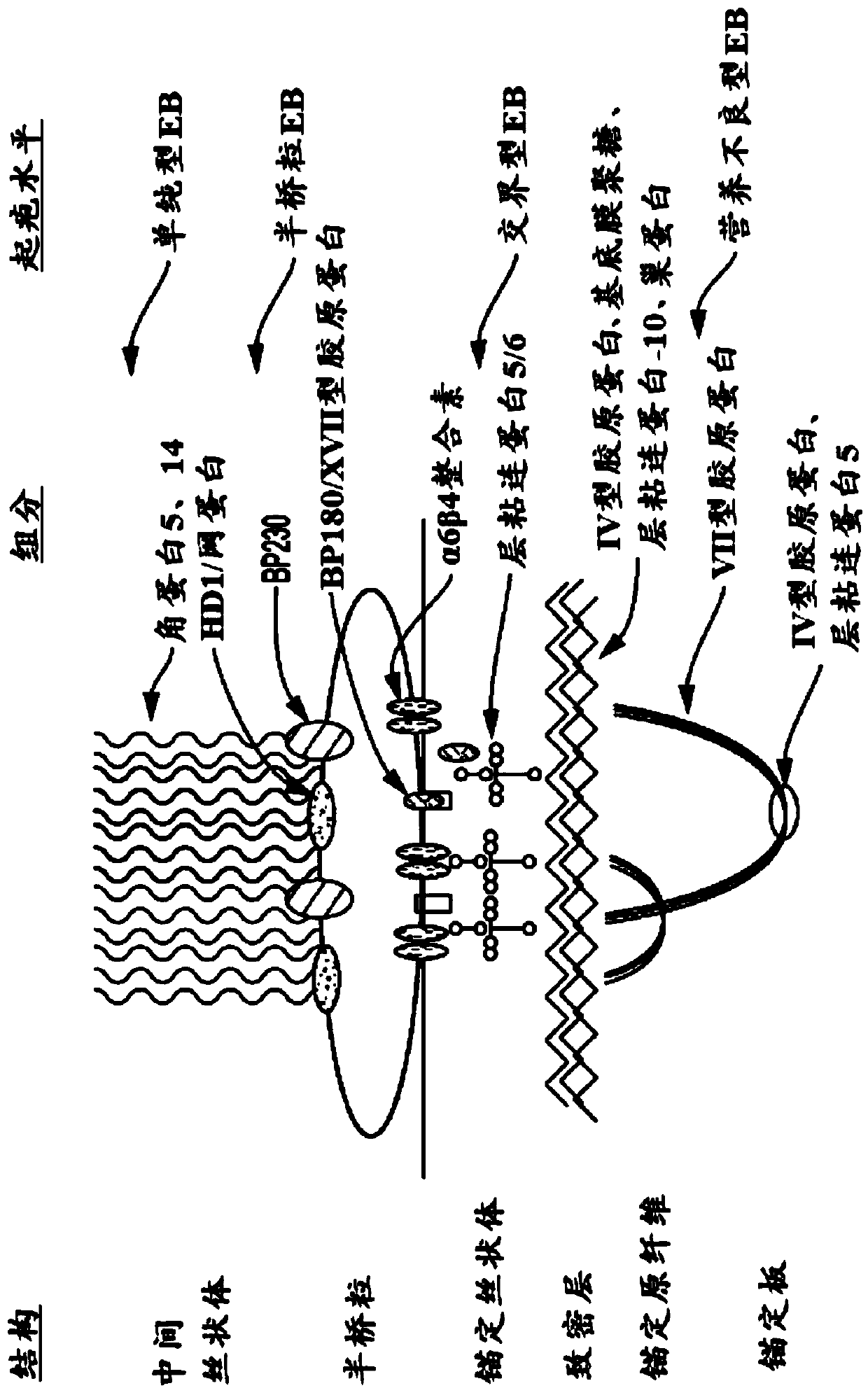
42 results about "Epidermolysis bullosa" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A group of inherited connective tissue diseases that cause blisters in the skin and mucosal membranes.
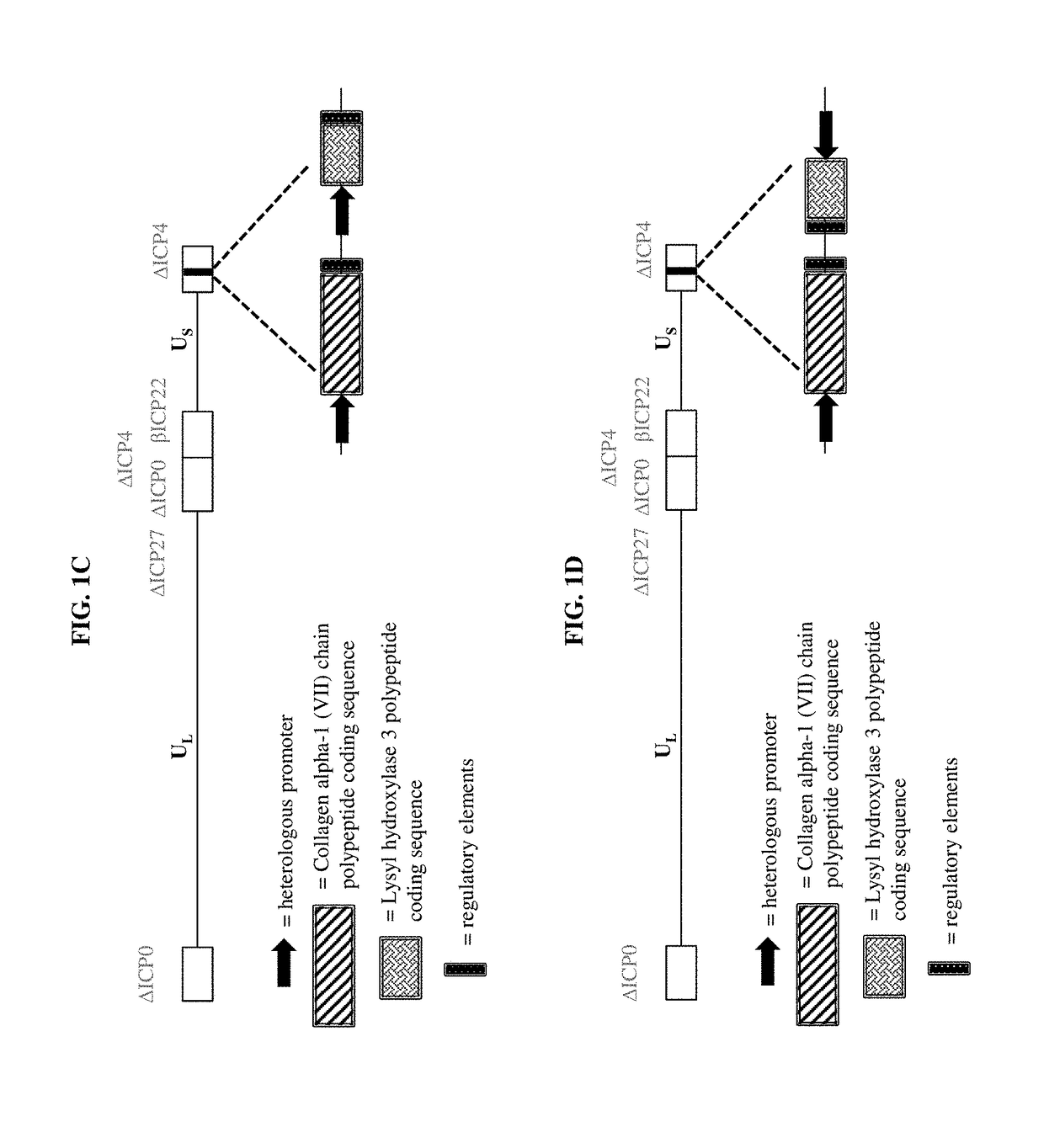
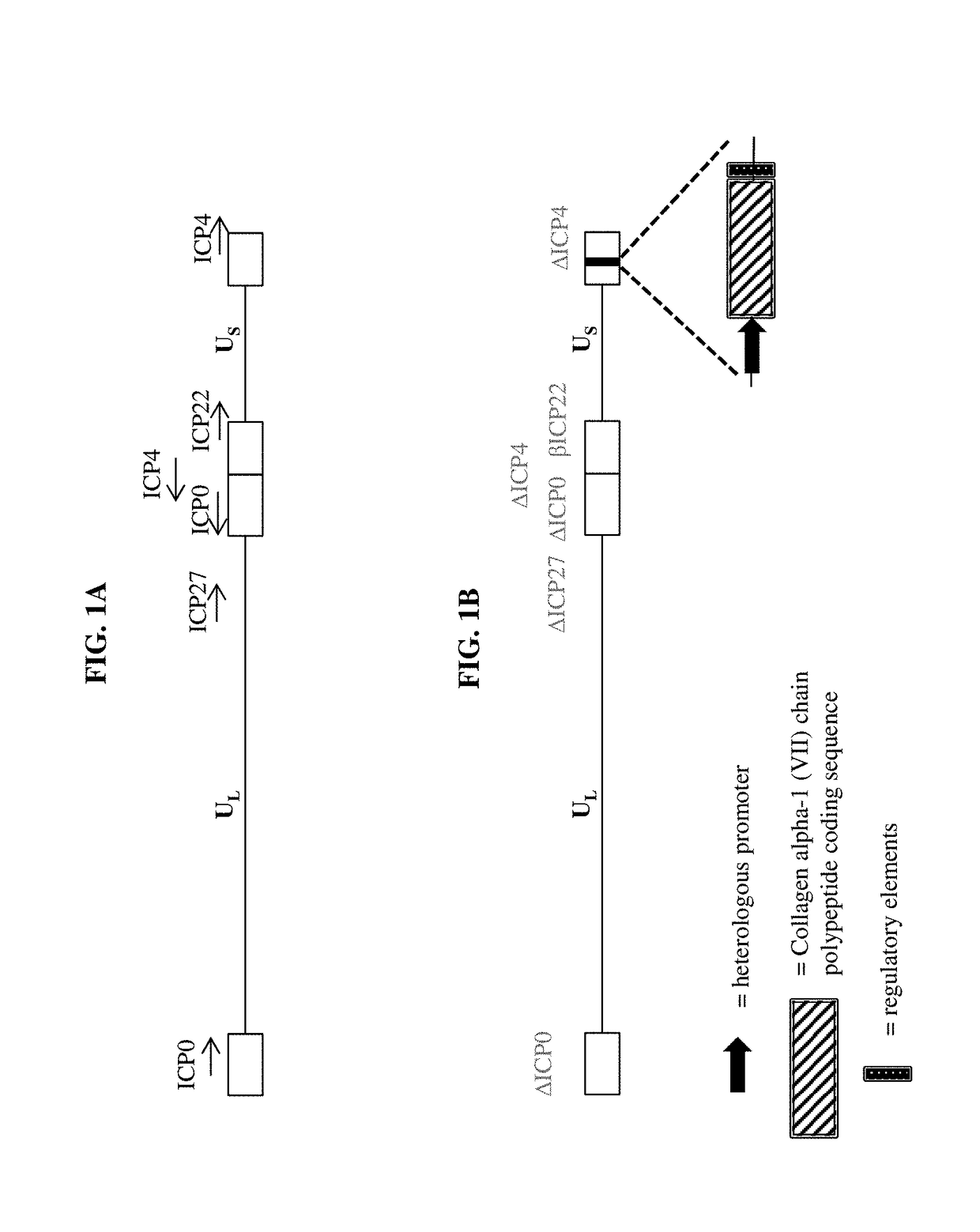
Recombinant C7 and Methods of Use
InactiveUS20140031295A1Progression of moreMore symptomPeptide/protein ingredientsPharmaceutical non-active ingredientsBlistersEpidermolysis bullosa
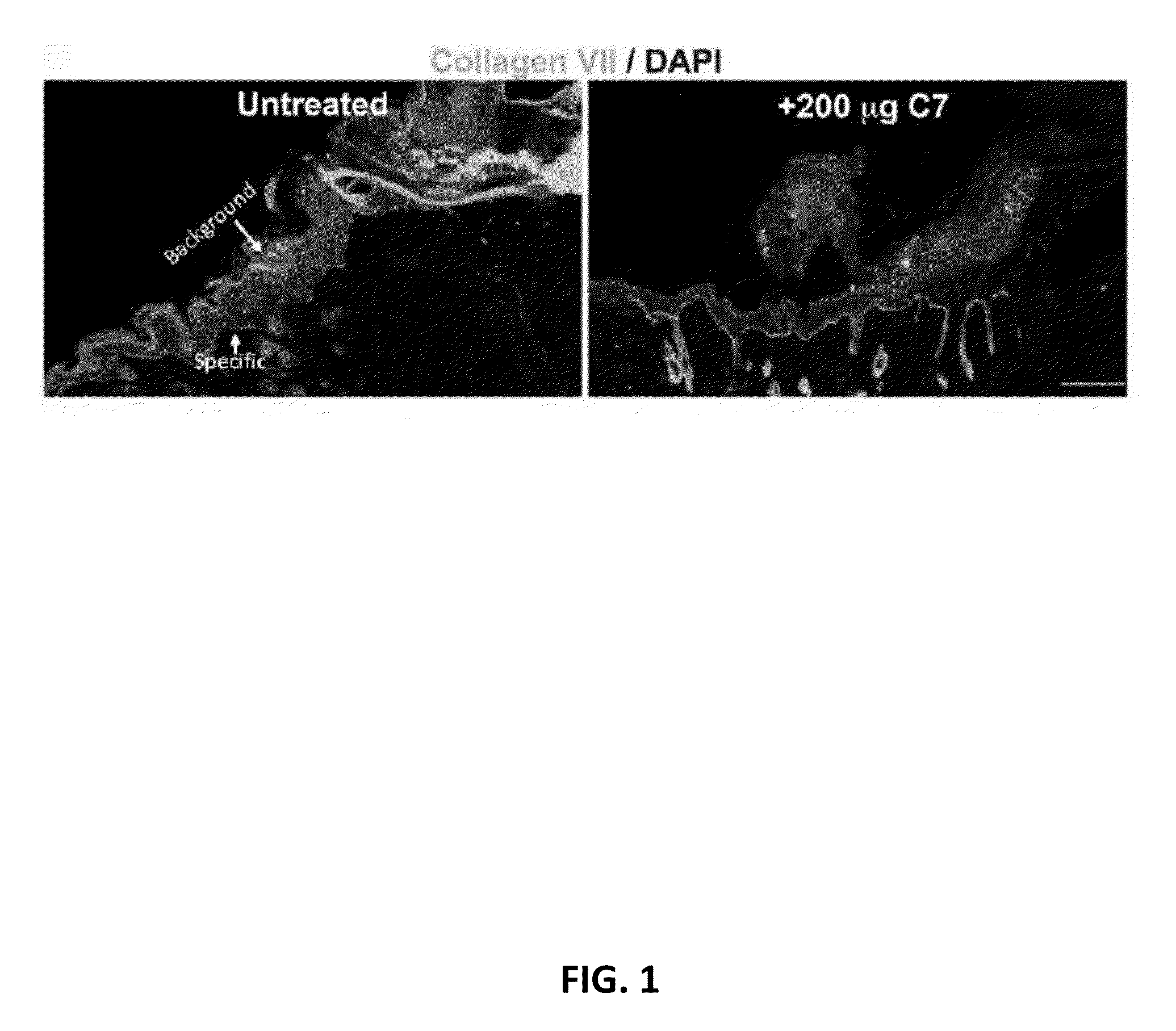
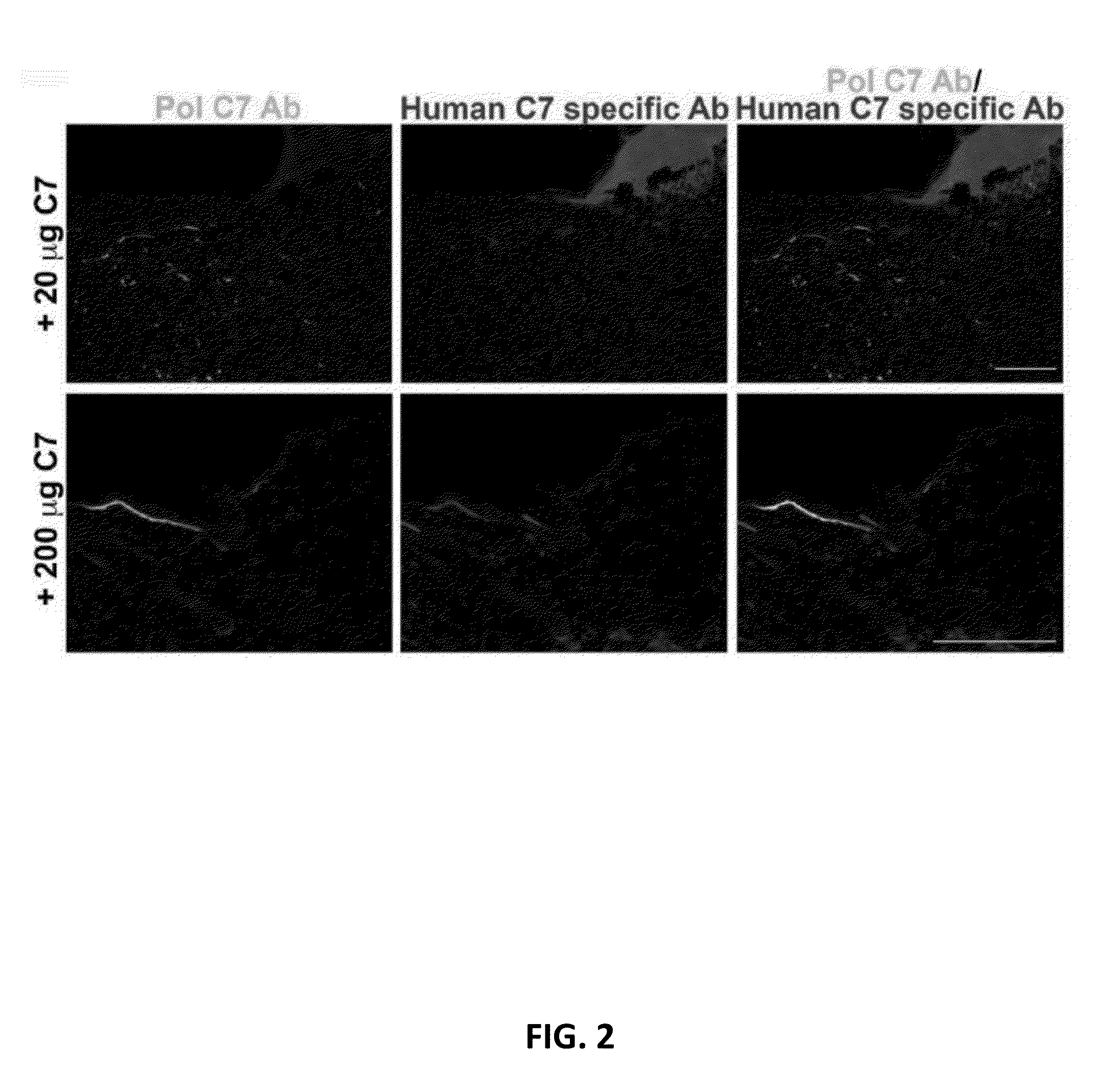
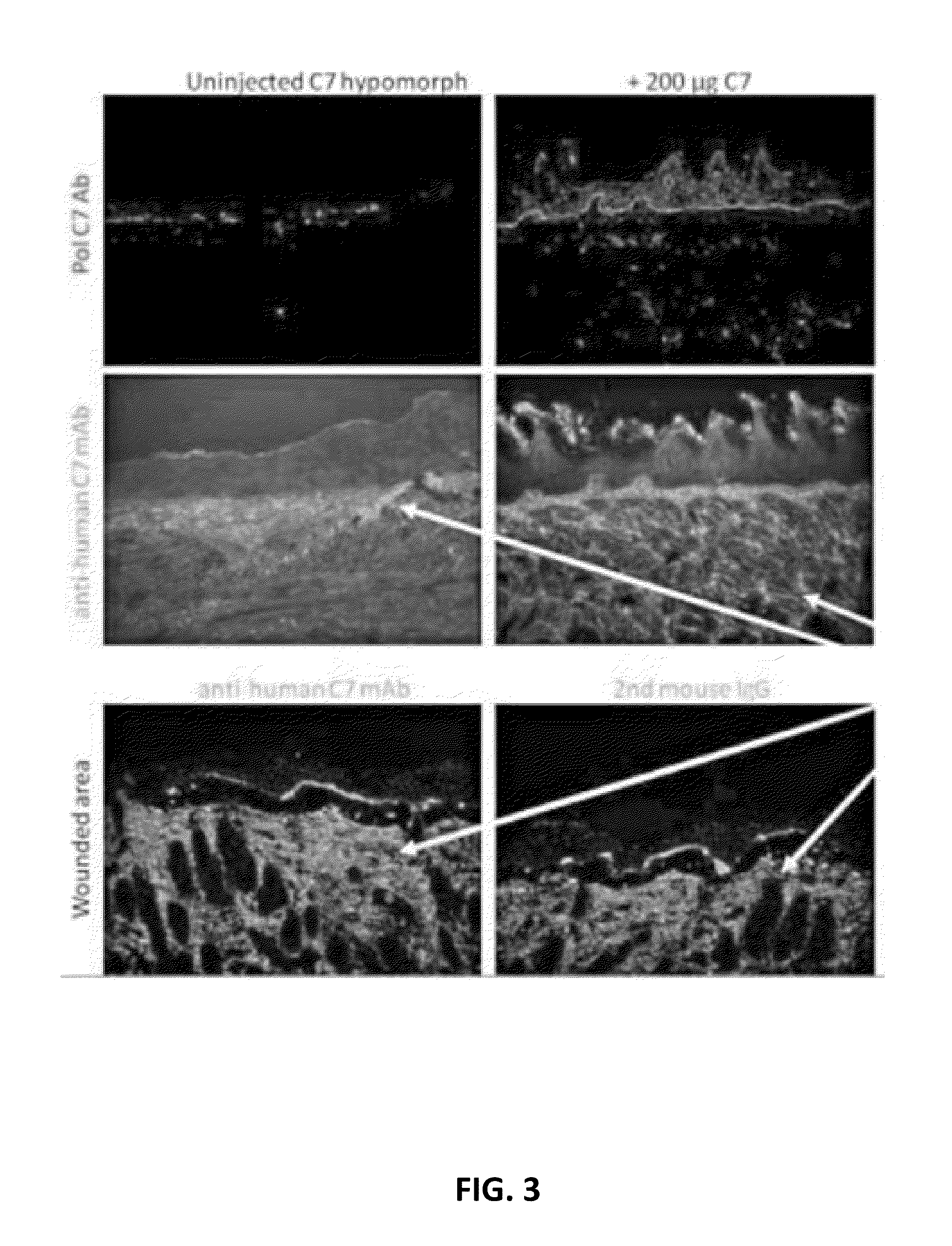
The present disclosure provides methods of treating epidermolysis bullosa, and / or preventing, preventing the progression of, or delaying the onset of one or more symptom associated with scarring, e.g., of blisters, in subjects with epidermolysis bullosa through chronic systemic administration of collagen 7.
Owner:PHOENIX TISSUE REPAIR INC +1
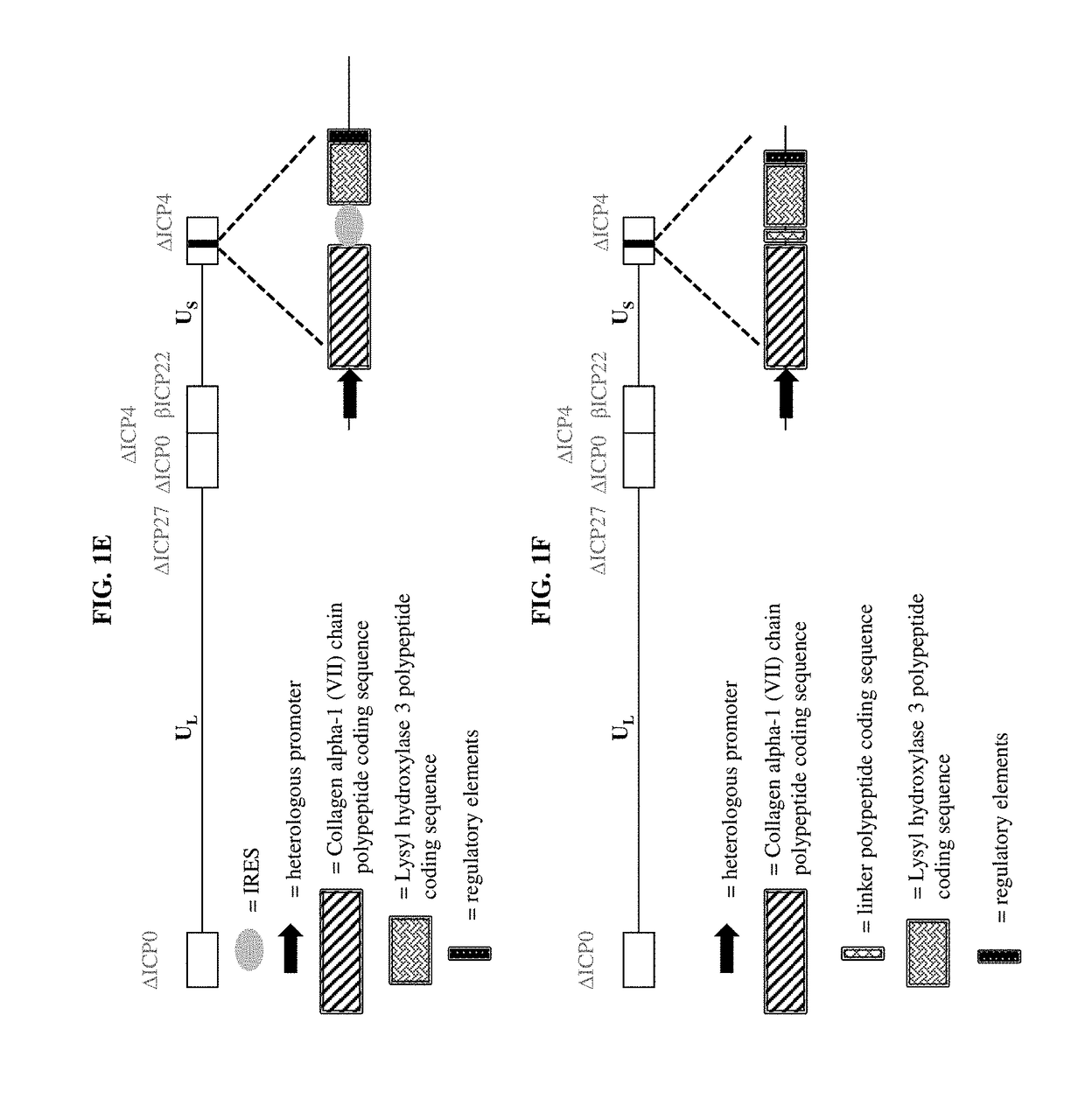
Compositions and methods for the treatment of wounds, disorders, and diseases of the skin
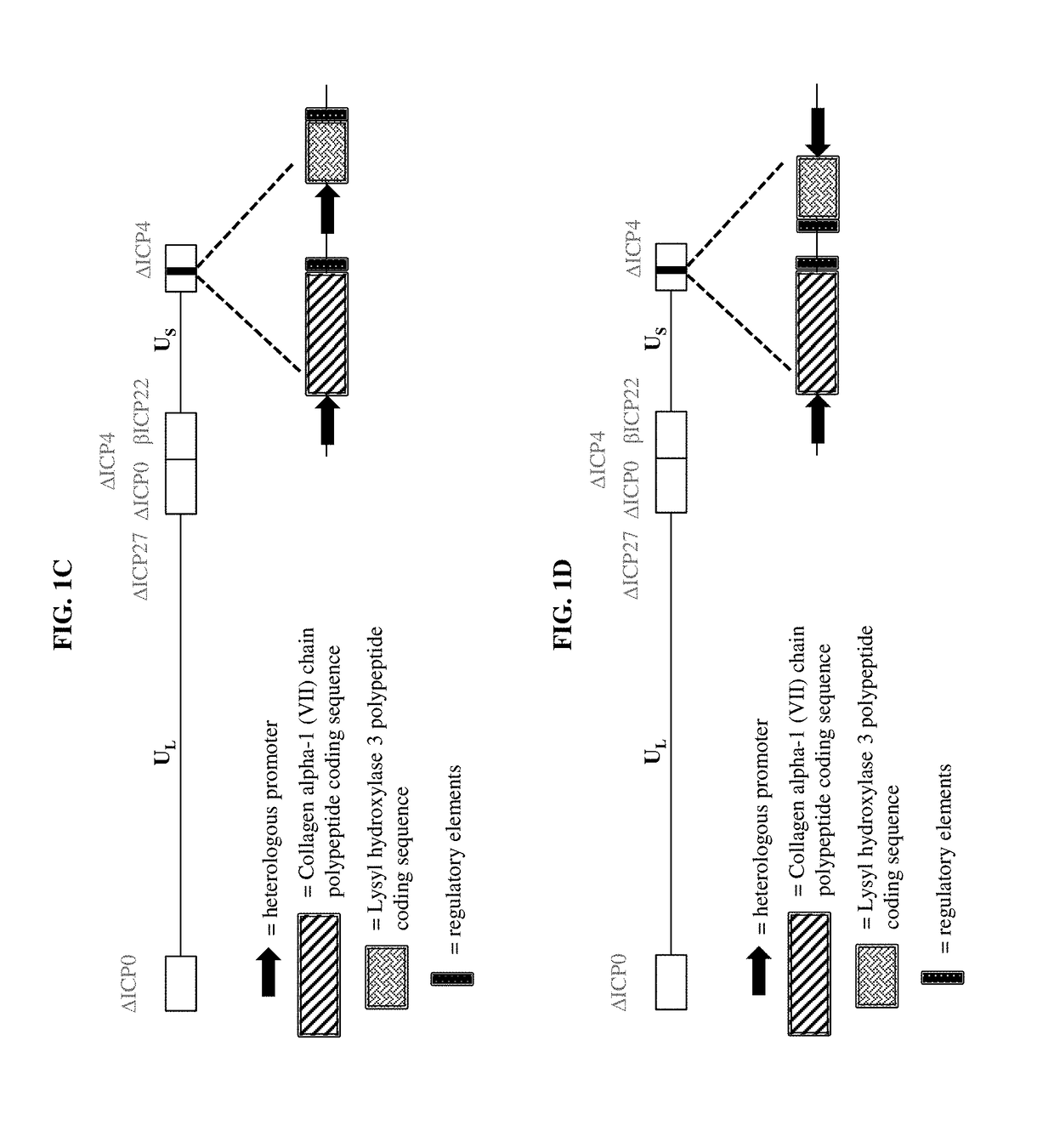
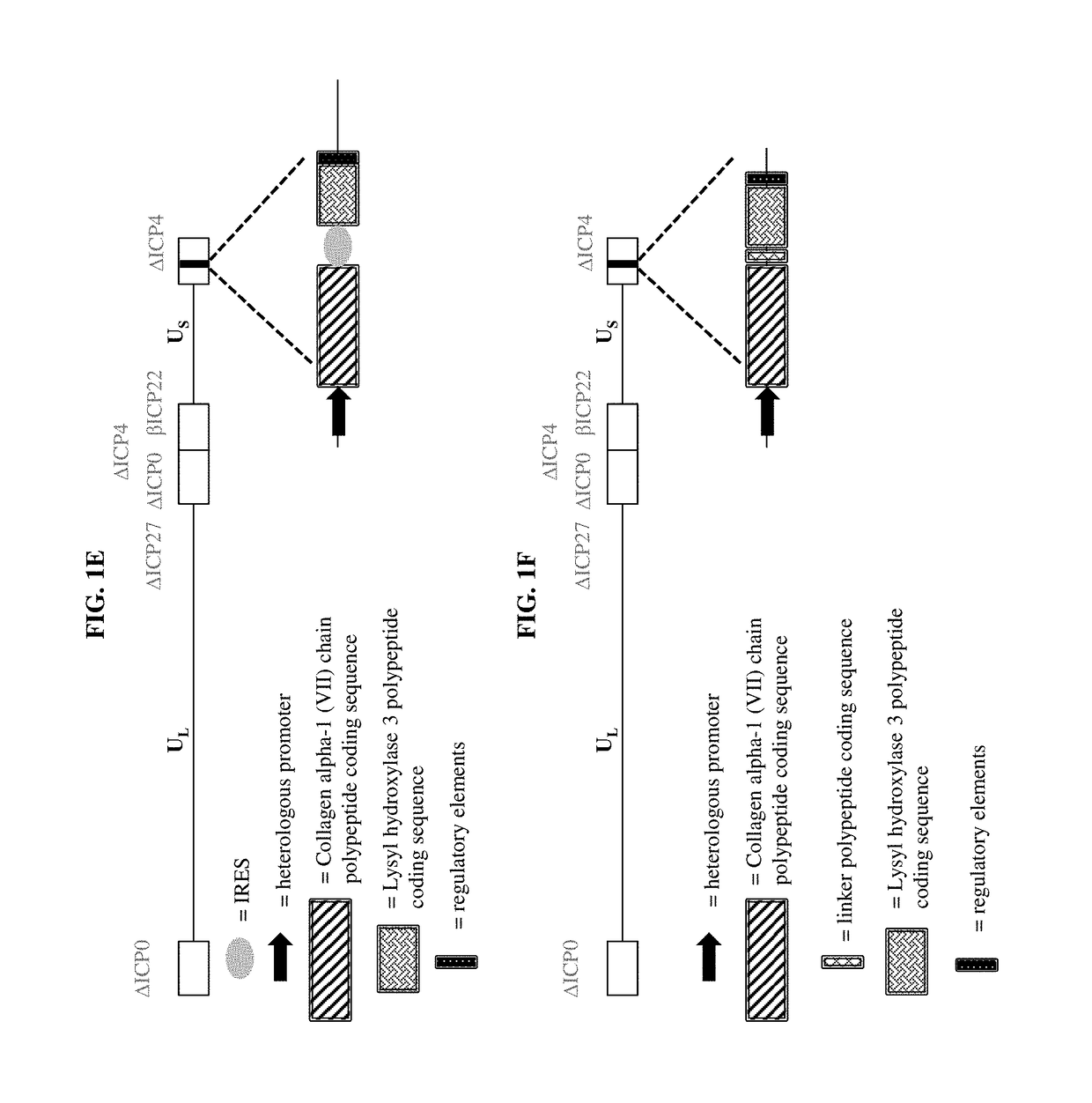
ActiveUS9877990B2Enhances and increase and augments and supplements formationEnhancing and increasing and augmenting and levelPeptide/protein ingredientsOintment deliveryLysyl hydroxylaseCvd risk
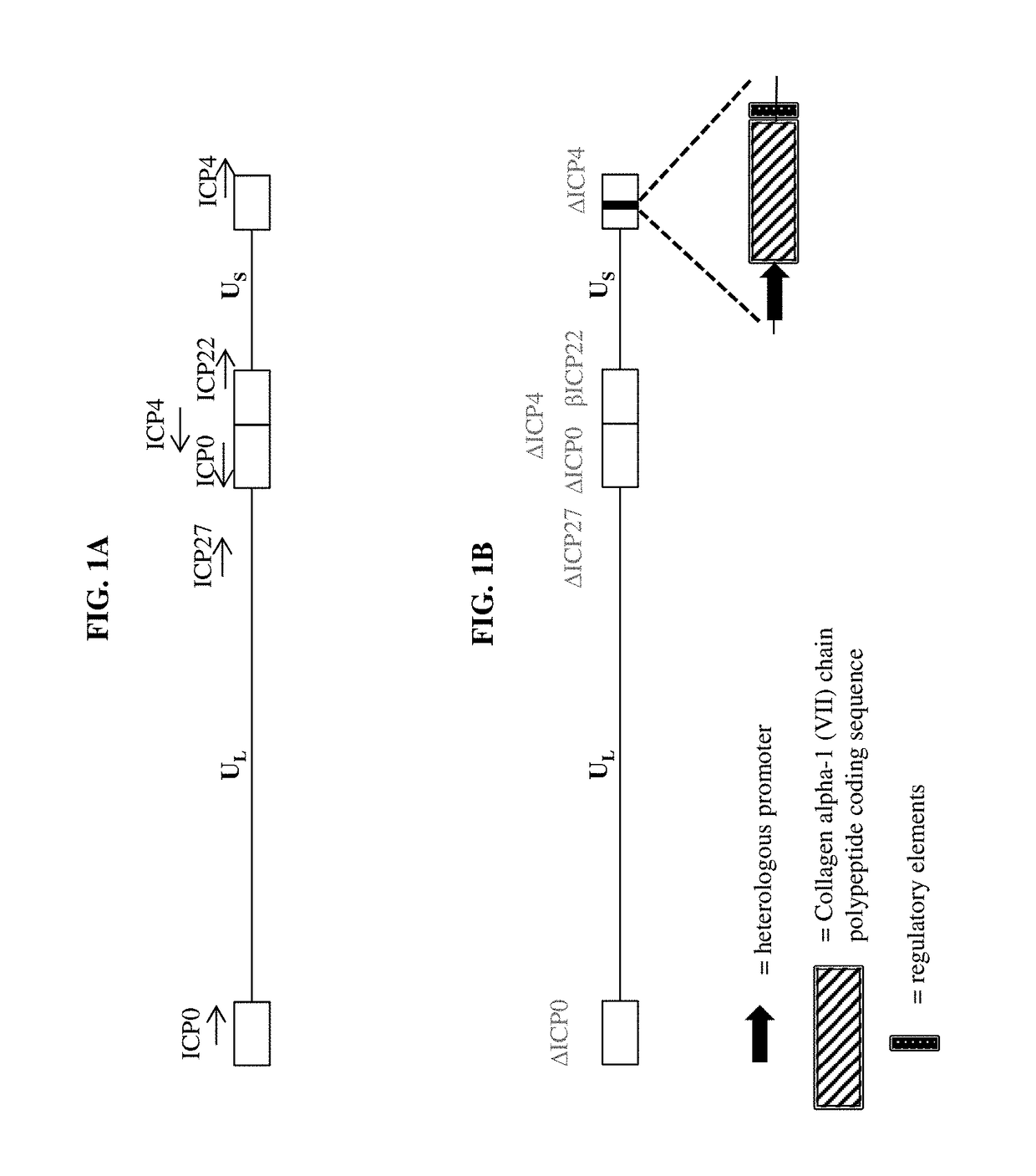
The present disclosure relates, in part, to pharmaceutical compositions comprising one or more polynucleotides suitable for enhancing, increasing, augmenting, and / or supplementing the levels of Collagen alpha-1 (VII) chain polypeptide and / or Lysyl hydroxylase 3 polypeptide and / or Keratin type I cytoskeletal 17 polypeptide in a subject. The present disclosure also relates, in part, to pharmaceutical compositions and methods of use for providing prophylactic, palliative, or therapeutic relief of a wound, disorder, or disease of the skin in a subject, including a subject having, or at risk of developing, one or more symptoms of epidermolysis bullosa.
Owner:KRYSTAL BIOTECH INC
Pre-MRNA trans-splicing molecule (RTM) molecules and their uses
The present invention relates to specific and markedly improved pre-mRNA trans-splicing molecule (RTM) molecules which are designed to correct specific genes expressed within cells to be targeted, and which are associated with epidermolysis bullosa, cystic fibrosis, pachyonychia congenital, and psoriasis or neurodermitis, as well as cancers of the skin. In particular, the RTMs of the present invention are genetically engineered to interact with a specific target pre-mRNA expressed in cells to be targeted so as to result in correction of genetic defects or reprogramming of gene expression responsible for a variety of different skin disorders.
Owner:BAUER JOHANN +1
Methods for treatment of inflammatory diseases
InactiveUS20020055531A1Function is not alteredEasy to relaxCosmetic preparationsBiocideAdditive ingredientPolyethylene glycol
An improved method of treating skin diseases comprises applying to the skin of a patient suffering such a skin disease an allantoin-containing composition in a therapeutically effective quantity. The allantoin-containing composition is an oil-in-water emulsion that includes allantoin and an emulsifier system that includes at least one emulsifier that is either an anionic emulsifier or a nonionic emulsifier. If the emulsifier is an anionic emulsifier, the emulsifier system can include an acidic wax such as beeswax. The nonionic emulsifiers used can include at least one nonionic emulsifier that is an ethoxylated ether or an ethoxylated ester whose carbon chain length ranges from 8 to 22 carbon atoms. Alternatively, the emulsifier system can include an acidic anionic polymer such as carboxypolymethylene and an anionic emulsifier. In another alternative, the emulsifier system can include the acidic anionic polymer and a nonionic emulsifier, or the acidic anionic polymer alone. In still another alternative, the emulsifier system can include cetyl alcohol and stearic acid. In yet another alternative, the emulsifier system can include sodium stearoyl lactylate and sodium isostearoyl lactylate. In another alternative, the emulsifier system can include at least one polyethyleneglycol ether of cetearyl alcohol. In still another alternative, the emulsifier system can include a polyethylene glycol ester of stearic acid and glyceryl stearate. The composition can include other ingredients. The pH of the composition used in a method according to the present invention is from about 3.0 to about 6.0; preferably, a narrower pH range is used, varying with each embodiment of the composition. Among the diseases that can be treated is epidermolysis bullosa.
Owner:ALWYN
Compositions and methods for the treatment of wounds, disorders, and diseases of the skin
ActiveUS20170290866A1Enhances and increase and augments and supplements formationEnhancing and increasing and augmenting and levelPeptide/protein ingredientsOintment deliveryDiseaseNucleotide
The present disclosure relates, in part, to pharmaceutical compositions comprising one or more polynucleotides suitable for enhancing, increasing, augmenting, and / or supplementing the levels of Collagen alpha-1 (VII) chain polypeptide and / or Lysyl hydroxylase 3 polypeptide and / or Keratin type I cytoskeletal 17 polypeptide in a subject. The present disclosure also relates, in part, to pharmaceutical compositions and methods of use for providing prophylactic, palliative, or therapeutic relief of a wound, disorder, or disease of the skin in a subject, including a subject having, or at risk of developing, one or more symptoms of epidermolysis bullosa.
Owner:KRYSTAL BIOTECH INC
Methods for treatment of inflammatory diseases
InactiveUS20060134149A1Simple methodOrganic active ingredientsBiocideAdditive ingredientPolyethylene glycol
An improved method of treating skin diseases comprises applying to the skin of a patient suffering such a skin disease an allantoin-containing composition in a therapeutically effective quantity. The allantoin-containing composition is an oil-in-water emulsion that includes allantoin and an emulsifier system that includes at least one emulsifier that is either an anionic emulsifier or a nonionic emulsifier. If the emulsifier is an anionic emulsifier, the emulsifier system can include an acidic wax such as beeswax. The nonionic emulsifiers used can include at least one nonionic emulsifier that is an ethoxylated ether or an ethoxylated ester whose carbon chain length ranges from 8 to 22 carbon atoms. Alternatively, the emulsifier system can include an acidic anionic polymer such as carboxypolymethylene and an anionic emulsifier. In another alternative, the emulsifier system can include the acidic anionic polymer and a nonionic emulsifier, or the acidic anionic polymer alone. In still another alternative, the emulsifier system can include cetyl alcohol and stearic acid. In yet another alternative, the emulsifier system can include sodium stearoyl lactylate and sodium isostearoyl lactylate. In another alternative, the emulsifier system can include at least one polyethyleneglycol ether of cetearyl alcohol. In still another alternative, the emulsifier system can include a polyethylene glycol ester of stearic acid and glyceryl stearate. The composition can include other ingredients. The pH of the composition used in a method according to the present invention is from about 3.0 to about 6.0; preferably, a narrower pH range is used, varying with each embodiment of the composition. Among the diseases that can be treated is epidermolysis bullosa.
Owner:ALWYN
Primer combination and kit for simultaneous detection of 93 bovine genetic defect genes and lethal haplotypes
ActiveCN107099607AAccurate amplificationImprove throughputMicrobiological testing/measurementDNA/RNA fragmentationAgricultural scienceAssortative mating
The invention discloses a primer combination and a kit for simultaneous detection of 93 bovine genetic defect genes and lethal haplotypes. Through use of the primer combination and the kit, casual mutation sites (SNP and insertion or deletion of short fragments) of the 93 genetic defect genes (such as PIRM syndrome, crooked tail syndrome, epidermolysis bullosa and the like) and the lethal haplotypes (HH1, HH3, HH4, HH5 and JH1) can be detected in one time, cattle with monogenic inheritance defect gene carriers and lethal haplotype carriers can be screened, and accurate guidance is provided for cattle breeding and genetic evaluation, herd selection and assortative mating, population genetic improvement, cultivation of new varieties and genetic resource protection. The primer combination and the kit have the characteristics of high throughput, low cost, high accuracy, and the like, is suitable for the varieties of beef cattle and dairy cattle, and can be widely used in the breeding and reproduction field of the cattle.
Owner:DAIRY CATTLE RES CENT SHANDONG ACADEMY OF AGRI SCI
Covalent granzyme B inhibitors
ActiveUS9458192B1Treat, reduce, and/or inhibit the appearance of ageing in the skinTetrapeptide ingredientsTripeptide ingredientsMedicineAlopecia areata
Covalent Granzyme B inhibitors, compositions that include the compounds, and methods for using the compounds. A method for treating cutaneous scleroderma, epidermolysis bullosa, radiation dermatitis, alopecia areata, and discoid lupus erythematosus are provided.
Owner:VIDA THERAPEUTICS
Proline compounds as Granzyme B inhibitors
ActiveUS9458193B1Reduce, and/or inhibit the appearance of ageing in the skinPharmaceutical delivery mechanismTripeptide ingredientsMedicineAlopecia areata
Proline compounds as Granzyme B inhibitors, compositions that include the compounds, and methods for using the compounds. Methods for treating cutaneous scleroderma, epidermolysis bullosa, radiation dermatitis, alopecia areata, and discoid lupus erythematosus are provided.
Owner:VIDA THERAPEUTICS
Pyrrole compounds as granzyme B inhibitors
ActiveUS9458138B1Reduce, and/or inhibit the appearance of ageing in the skinOrganic chemistryDipeptide ingredientsAlopecia areataPyrrole
Pyrrole compounds as Granzyme B inhibitors, compositions that include the compounds, and methods for using the compounds. Method for treating cutaneous scleroderma, epidermolysis bullosa, radiation dermatitis, alopecia areata, and discoid lupus erythematosus are provided.
Owner:VIDA THERAPEUTICS
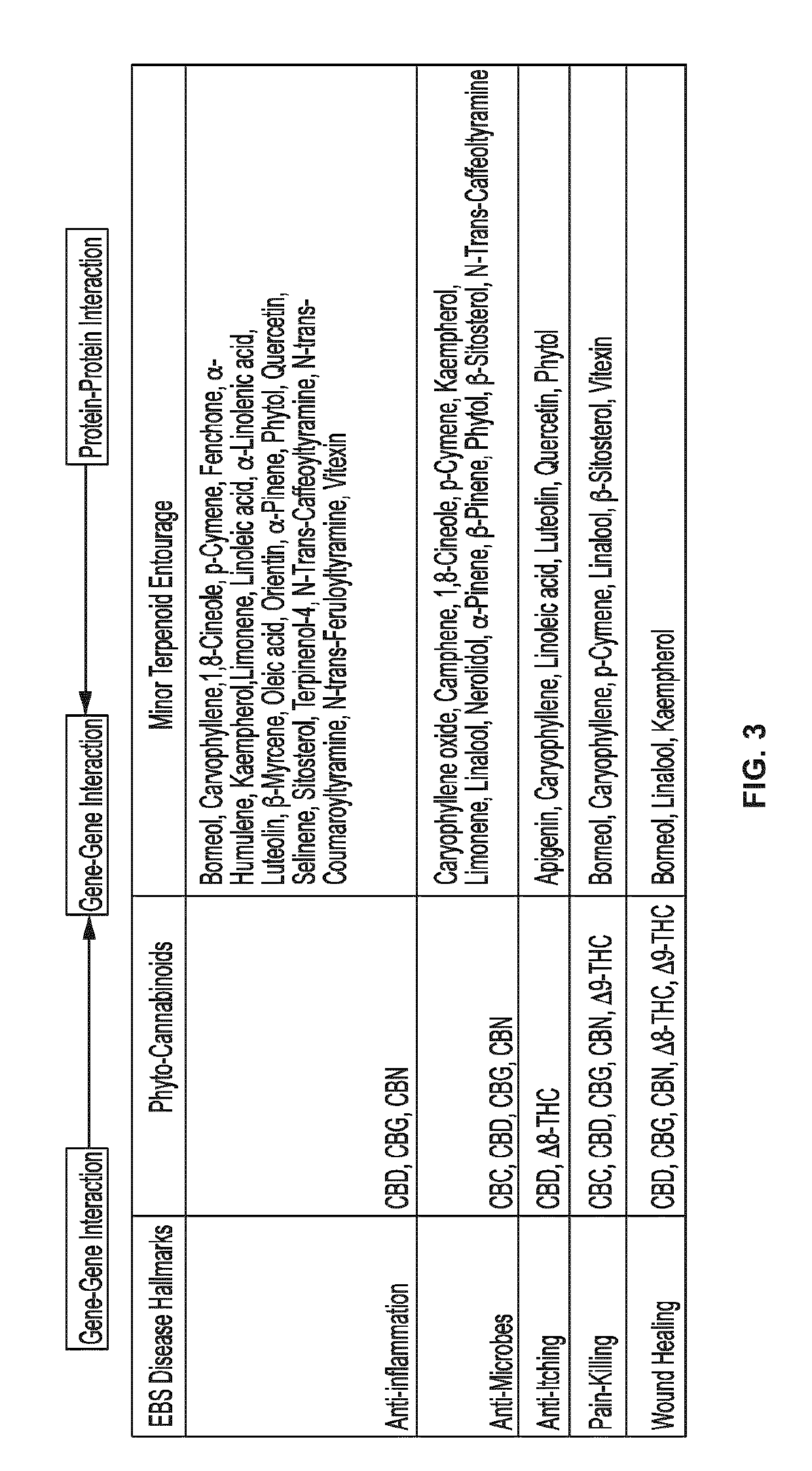
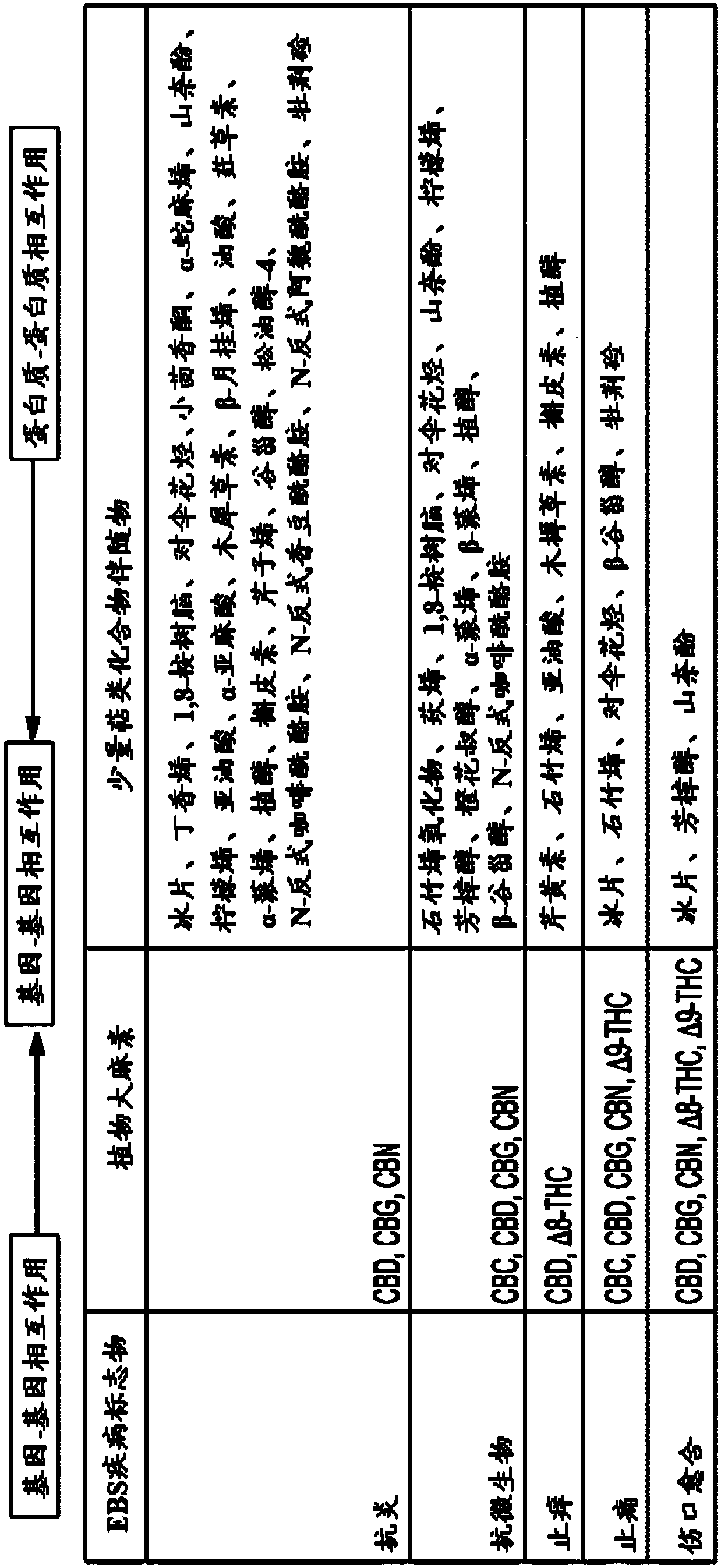
Use of topical formulations of cannabinoids in the treatment of epidermolysis bullosa and related connective tissue disorders
PendingUS20190142788A1Reduce inflammationPromote regenerationHydroxy compound active ingredientsPharmaceutical delivery mechanismConnective tissueIntermediate filament
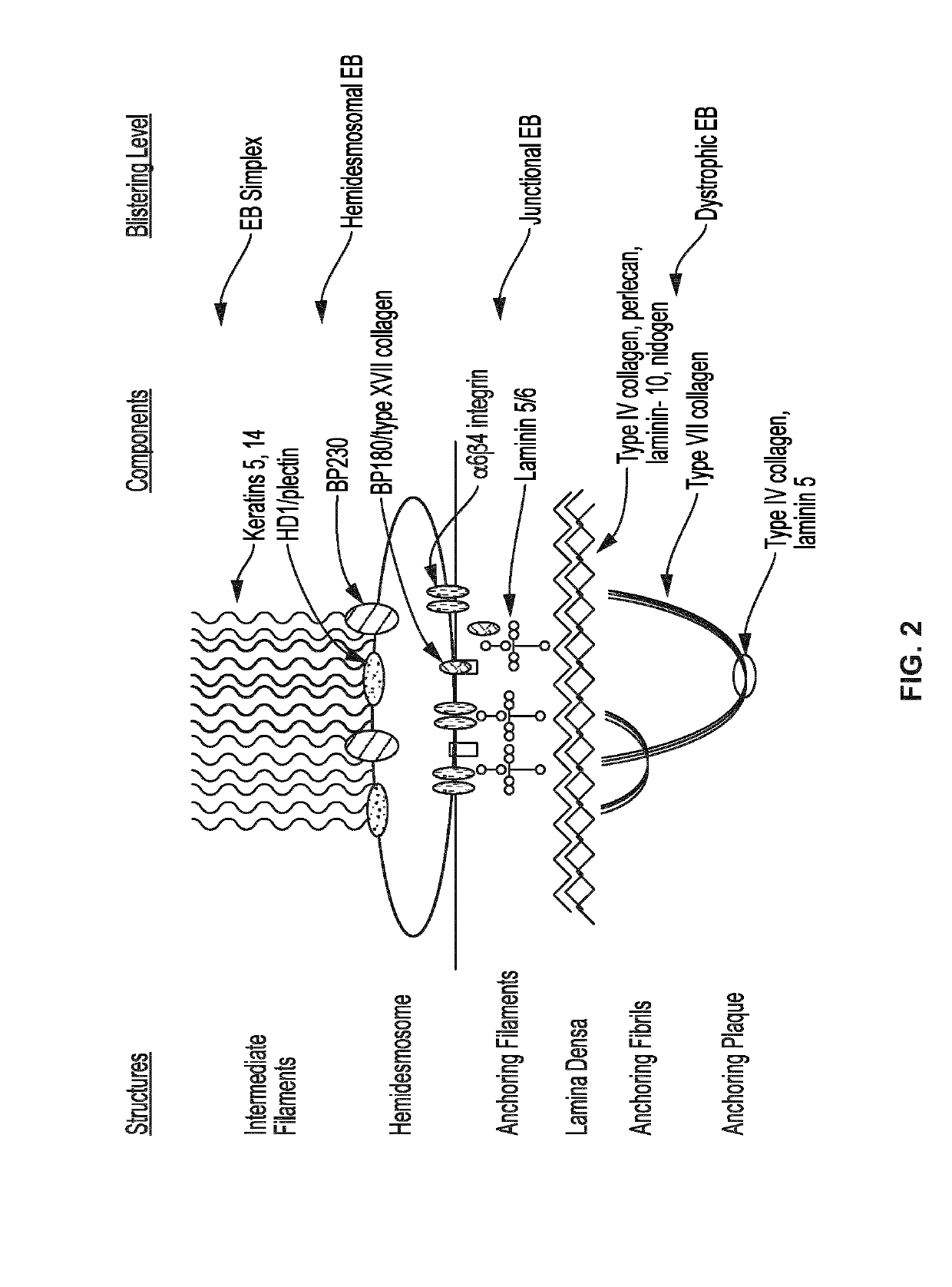
Mutations in keratin genes or the genes that regulate keratin expression can result in epithelial cells lacking sufficient structural integrity. The resulting disruption of connective tissue gives rise to inherited disorders such epidermolysis bullosa. It has been found that various cannabinoids (including mixtures of cannabidiols and cannabinol) upregulate expression of various keratins such that loss of function in other keratin genes may be compensated for. By way of this upregulation, these cannabinoids can be used to treat epidermolysis bullosa and other connective tissue disorders arising from intermediate filament dysfunction.
Owner:INMED PHARMA INC
Topical medicament for skin and mucosal injuries associated with Epidermolisis bullosa
ActiveUS10016466B2Heal fastHydroxy compound active ingredientsInanimate material medical ingredientsVitaminExcipient
A composition and method for treating Epidermolisis bullosa (EB), comprising: applying to skin or mucosal surfaces of a patient in need of treatment for EB a dressing gauze or bandage without prior cleaning of said skin or mucosal surfaces, wherein distributed on said dressing is an effective amount of a composition containing beeswax; an oleaginous base, vitamins and a pharmaceutically acceptable excipient; then removing said dressing at least twice per day without damaging the patient's skin or mucosal surfaces due to removal of the dressing.
Owner:REV PHARMA
Pyrrole compounds as granzyme b inhibitors
ActiveUS20170015705A1Reduce, and/or inhibit the appearance of ageing in the skinDipeptide ingredientsPharmaceutical delivery mechanismDISCOID LUPUS ERYTHEMATOSISPyrrole
Owner:VIDA THERAPEUTICS
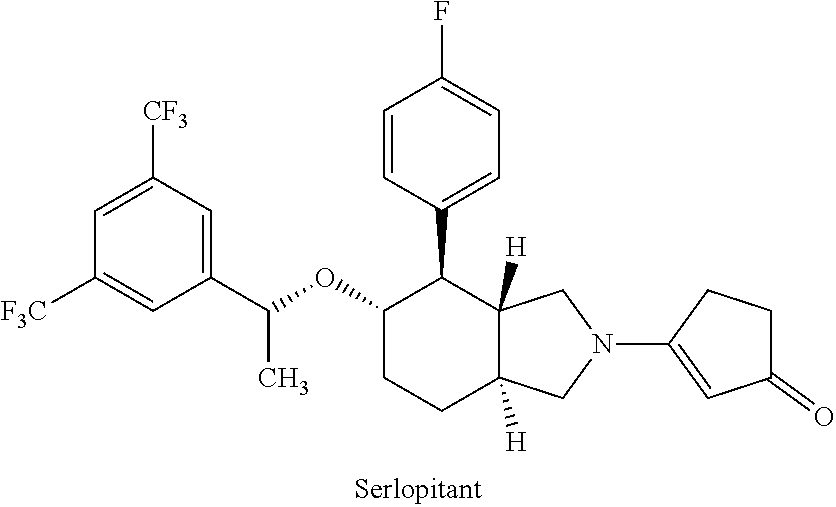
Use of neurokinin-1 antagonists to treat a variety of pruritic conditions
InactiveUS20190216779A1Shorten the progressReduce severityDigestive systemBoron compound active ingredientsDiseasePrurigo
The disclosure relates to the use of neurokinin-1 (NK-1) antagonists, such as serlopitant, in treating acute or chronic pruritus associated with a variety of medical conditions, including dermatitis / eczema, psoriasis, prurigo, urticaria, cutaneous T-cell lymphoma, epidermolysis bullosa, bums and hepato-biliary diseases, or / and treating the medical conditions themselves. One or more additional antipruritic or therapeutic agents can optionally be used in combination with an NK-1 antagonist to treat acute or chronic pruritus associated with a medical condition or / and the medical condition itself.
Owner:VYNE THERAPEUTICS INC
Use of topical formulations of cannabinoids in the treatment of epidermolysis bullosa and related connective tissue disorders
InactiveCN109689045AHydroxy compound active ingredientsPharmaceutical delivery mechanismConnective tissue fiberMedicine
Mutations in keratin genes or the genes that regulate keratin expression can result in epithelial cells lacking sufficient structural integrity. The resulting disruption of connective tissue gives rise to inherited disorders such epidermolysis bullosa. It has been found that various cannabinoids (including mixtures of cannabidiols and cannabinol) upregulate expression of various keratins such thatloss of function in other keratin genes may be compensated for. By way of this upregulation, these cannabinoids can be used to treat epidermolysis bullosa and other connective tissue disorders arising from intermediate filament dysfunction.
Owner:因美制药公司
Proline compounds as granzyme b inhibitors
ActiveUS20170015706A1Reduce, and/or inhibit the appearance of ageing in the skinPharmaceutical delivery mechanismTripeptide ingredientsMedicineDepressant
Proline compounds as Granzyme B inhibitors, compositions that include the compounds, and methods for using the compounds. Methods for treating cutaneous scleroderma, epidermolysis bullosa, radiation dermatitis, alopecia areata, and discoid lupus erythematosus are provided.
Owner:VIDA THERAPEUTICS
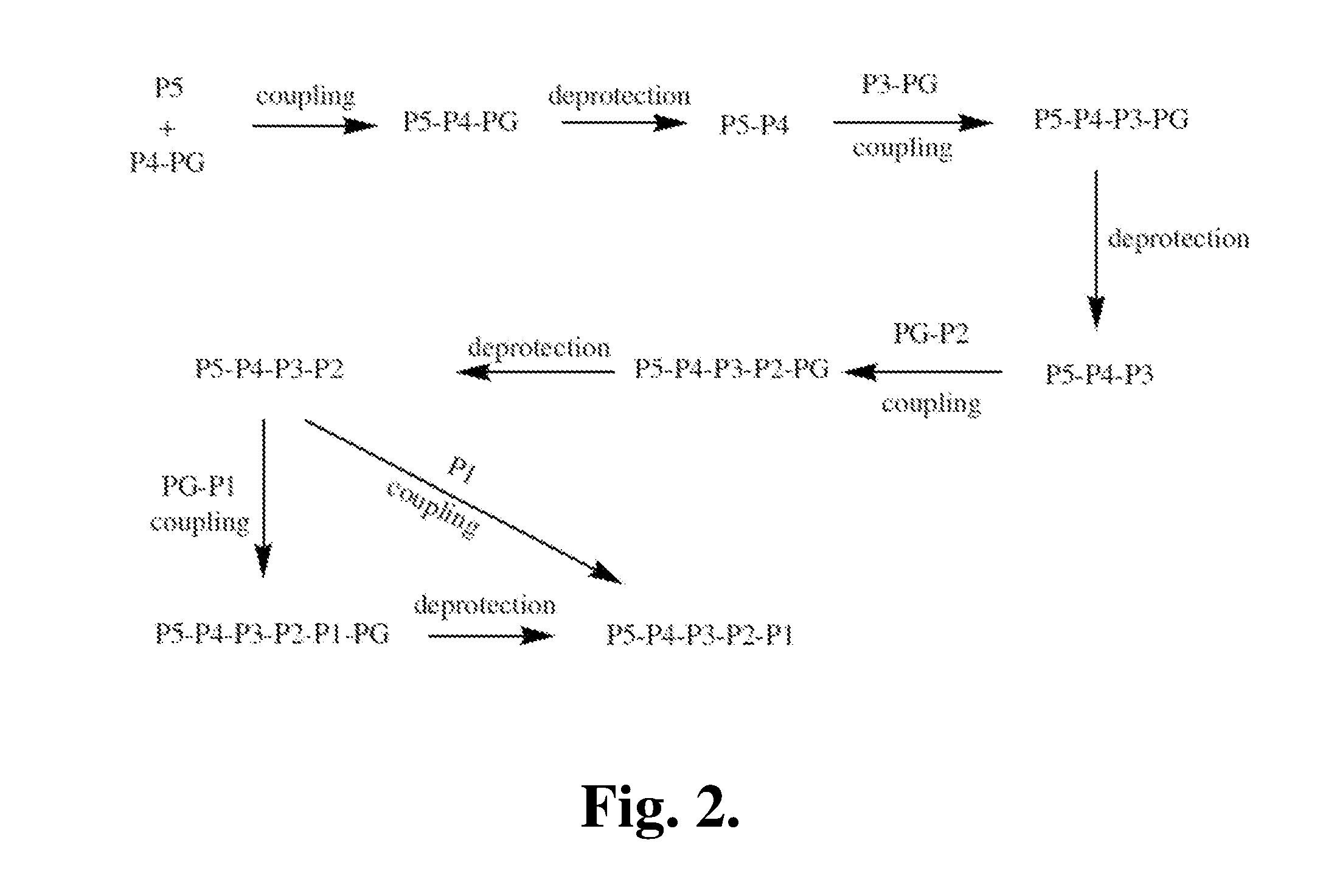
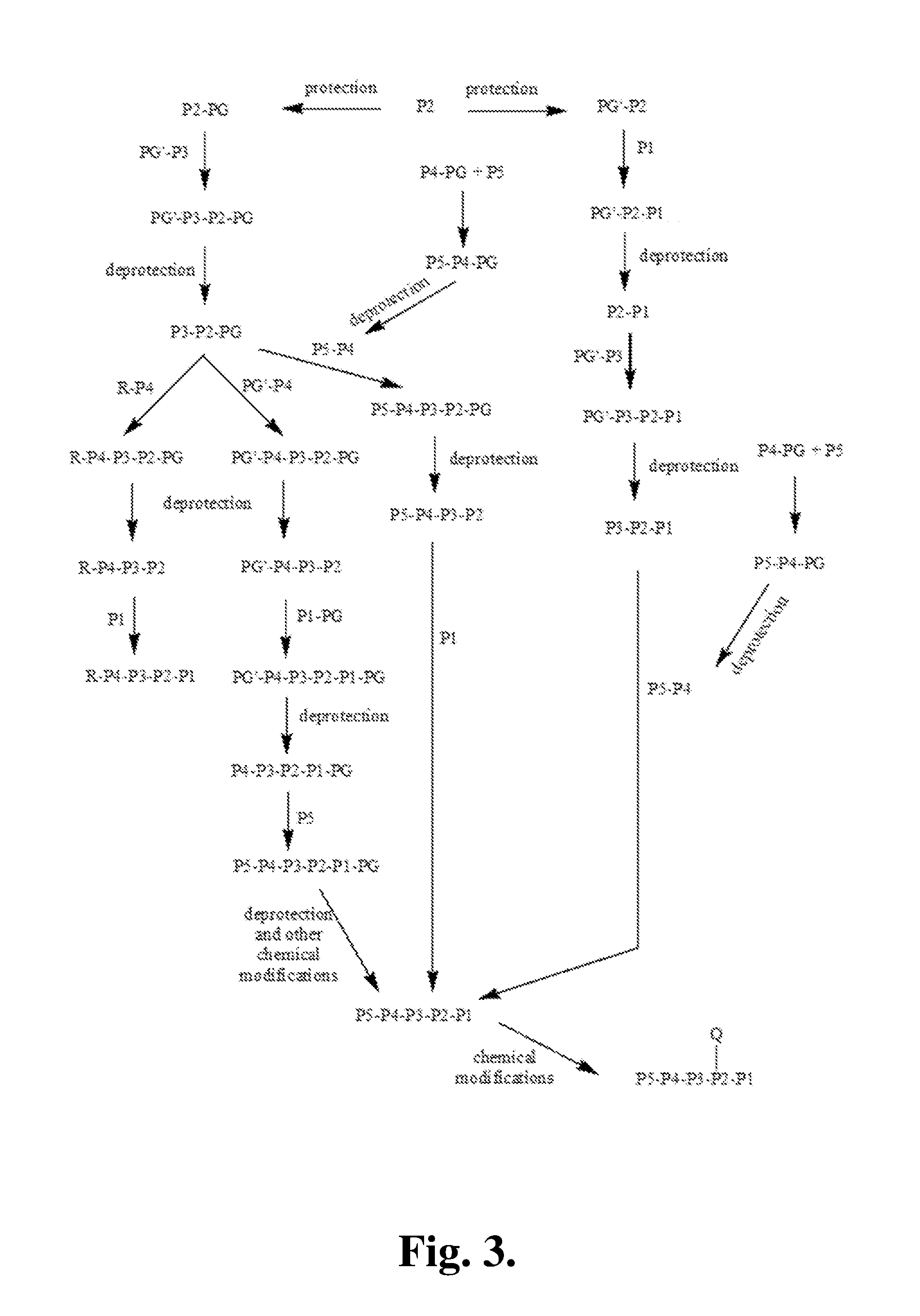
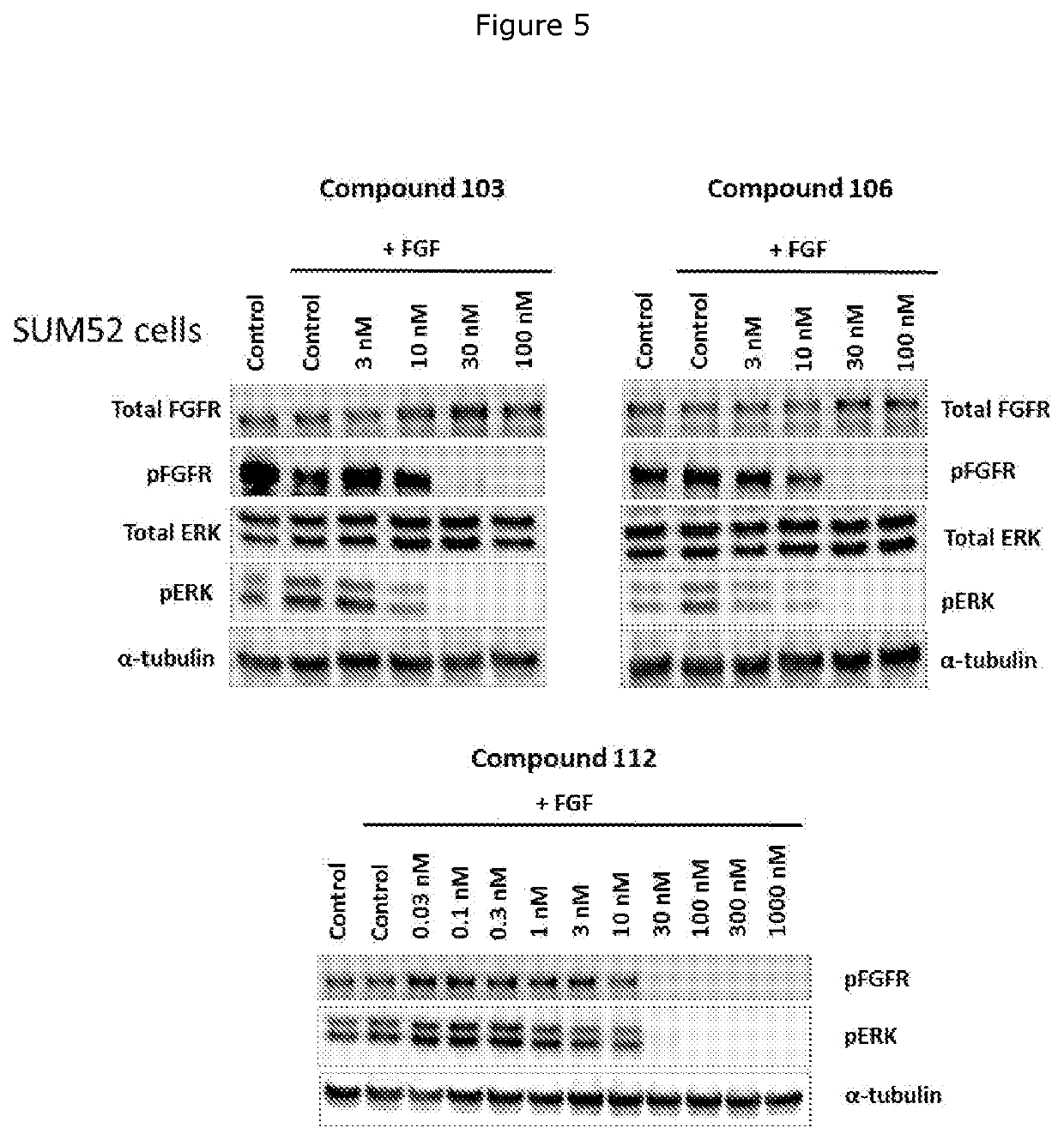
FGFR kinase inhibitors and pharmaceutical uses
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI +1
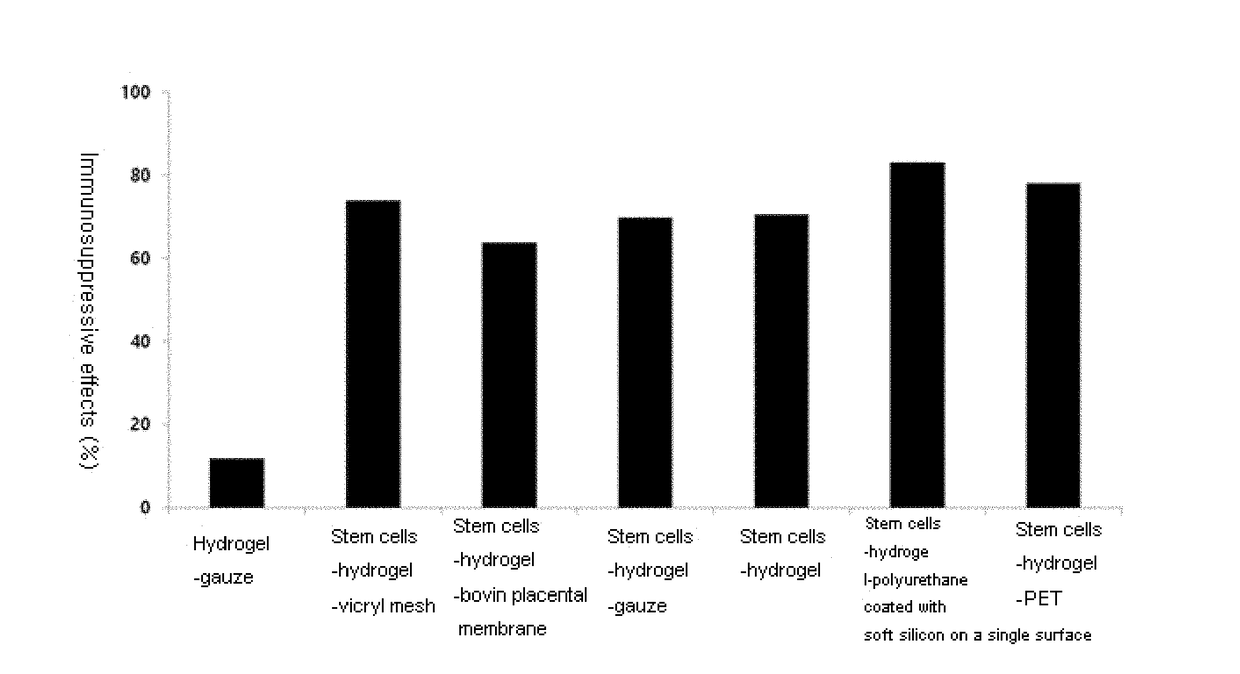
Mesenchymal stem cells-hydrogel-biodegradable or mesenchymal stem cells-hydrogel-nondegradable support composition for alleviating or improving epidermolysis bullosa
InactiveUS20180117217A1High expressionGood effectPeptide/protein ingredientsEpidermal cells/skin cellsCell-Extracellular MatrixECM Protein
Provided are a composition and a sheet, including a mesenchymal stem cells-hydrogel-biodegradable support or a mesenchymal stem cells-hydrogel-nondegradable support and a preparing method thereof. More specifically, in the sheet including a mesenchymal stem cells-hydrogel-biodegradable support or a mesenchymal stem cells-hydrogel-nondegradable support according to the present invention, the high-active mesenchymal stem cells may be applied to a wounded part of a patient with epidermolysis bullosa as it is without isolation using proteases, and in the culturing, an extracellular matrix such as collagen, laminin, fibronectin, and elastin secreted from the mesenchymal stem cells is wholly present on the hydrogel to have an advantageous effect that skin reproduction and re-epithelization abilities are significantly excellent as compared with conventional dressing agents used for epidermolysis bullosa.
Owner:ANTEROGEN
Covalent granzyme b inhibitors
ActiveUS20170015707A1Reduce, and/or inhibit the appearance of ageing in the skinTetrapeptide ingredientsTripeptide ingredientsEnzyme Inhibitor AgentDISCOID LUPUS ERYTHEMATOSIS
Covalent Granzyme B inhibitors, compositions that include the compounds, and methods for using the compounds. A method for treating cutaneous scleroderma, epidermolysis bullosa, radiation dermatitis, alopecia areata, and discoid lupus erythematosus are provided.
Owner:VIDA THERAPEUTICS
Azaindoline compounds as granzyme b inhibitors
ActiveUS20170218014A1Reduce, and/or inhibit the appearance of ageing in the skinCosmetic preparationsHair cosmeticsMedicineAlopecia areata
Azaindoline compounds as granzyme B inhibitors, compositions that include the compounds, and methods for using the compounds. Methods for treating cutaneous scleroderma, epidermolysis bullosa, radiation dermatitis, alopecia areata, and discoid lupus erythematosus are provided.
Owner:VIDA THERAPEUTICS
Methods for treating pain caused by inflammation induced mechanical and/or thermal hypersensitivity
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
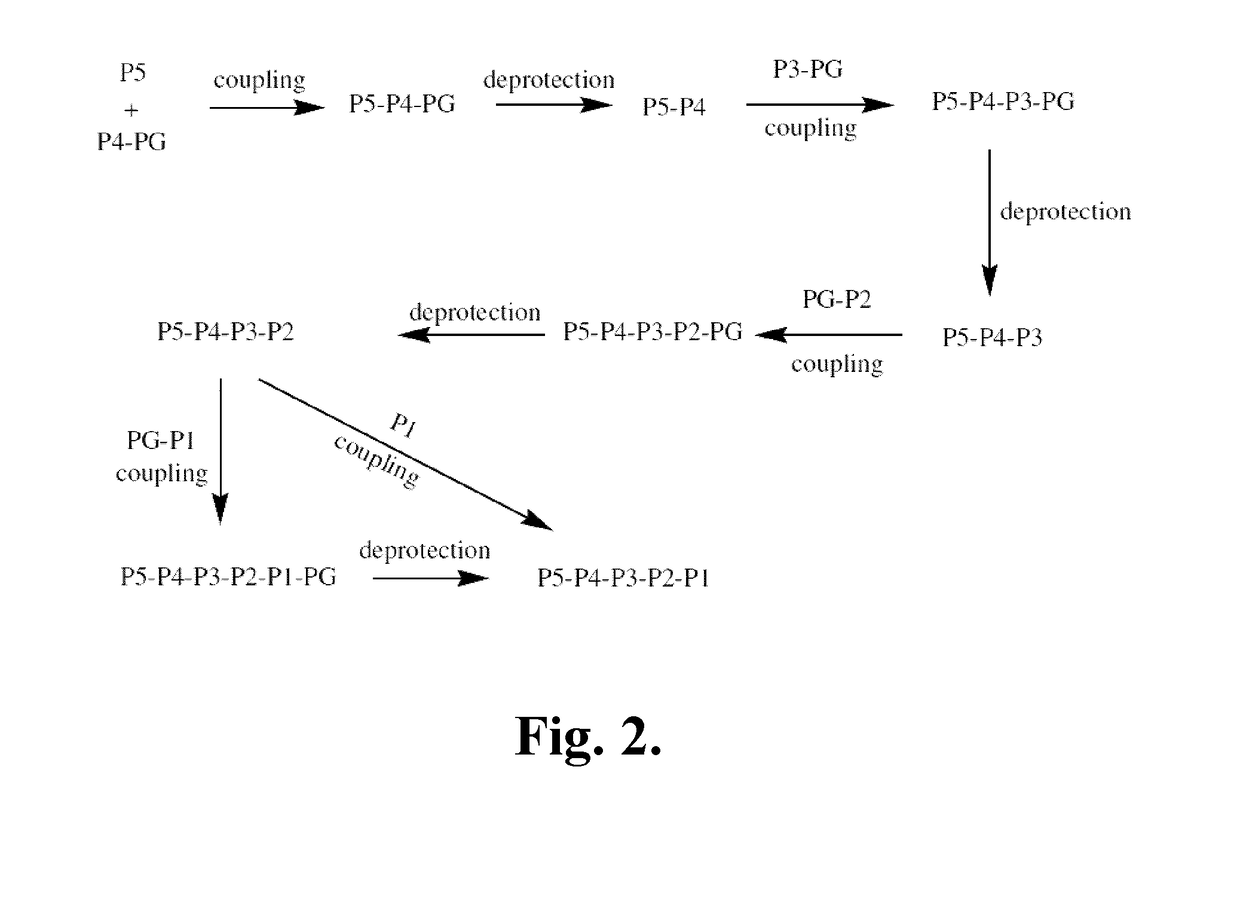
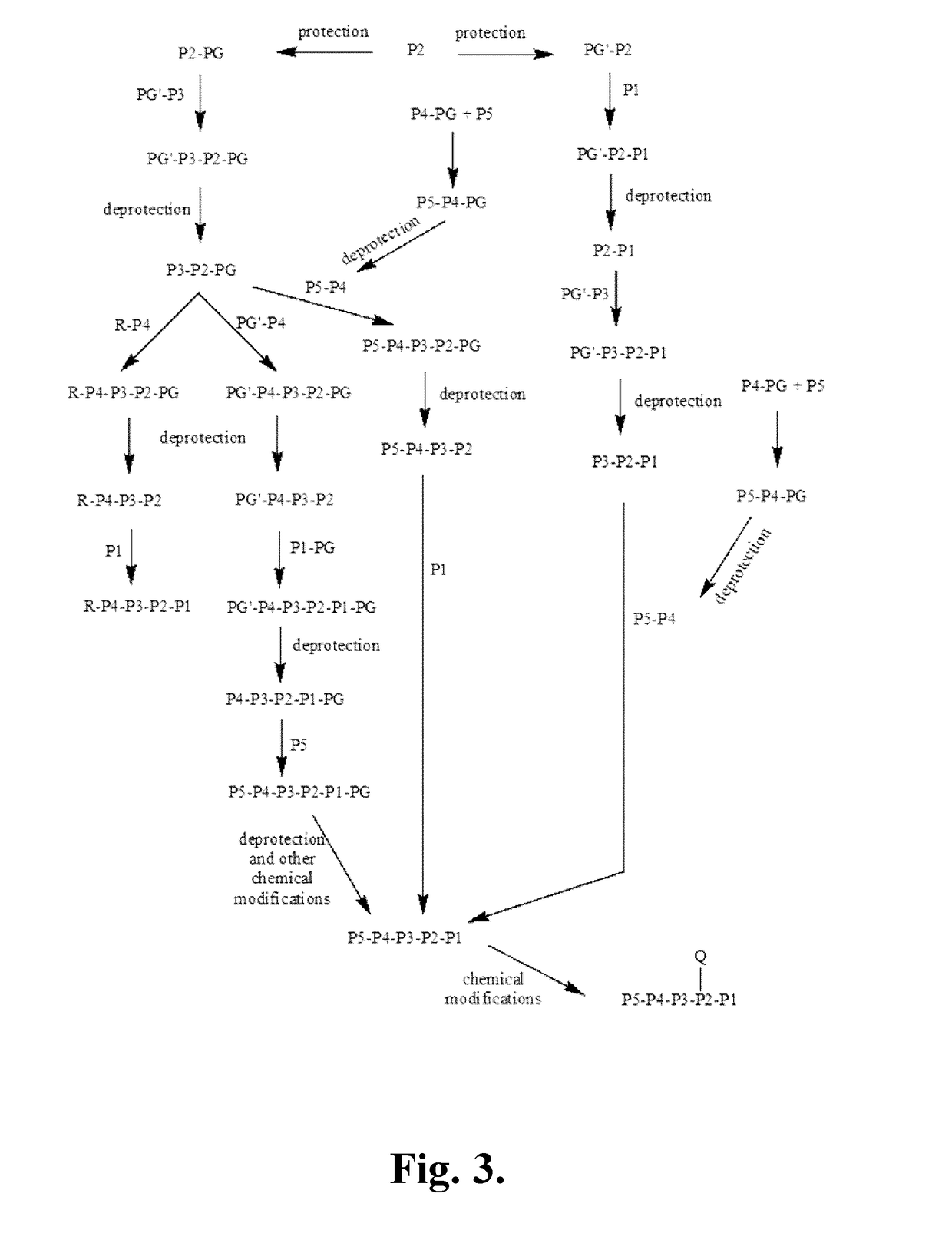
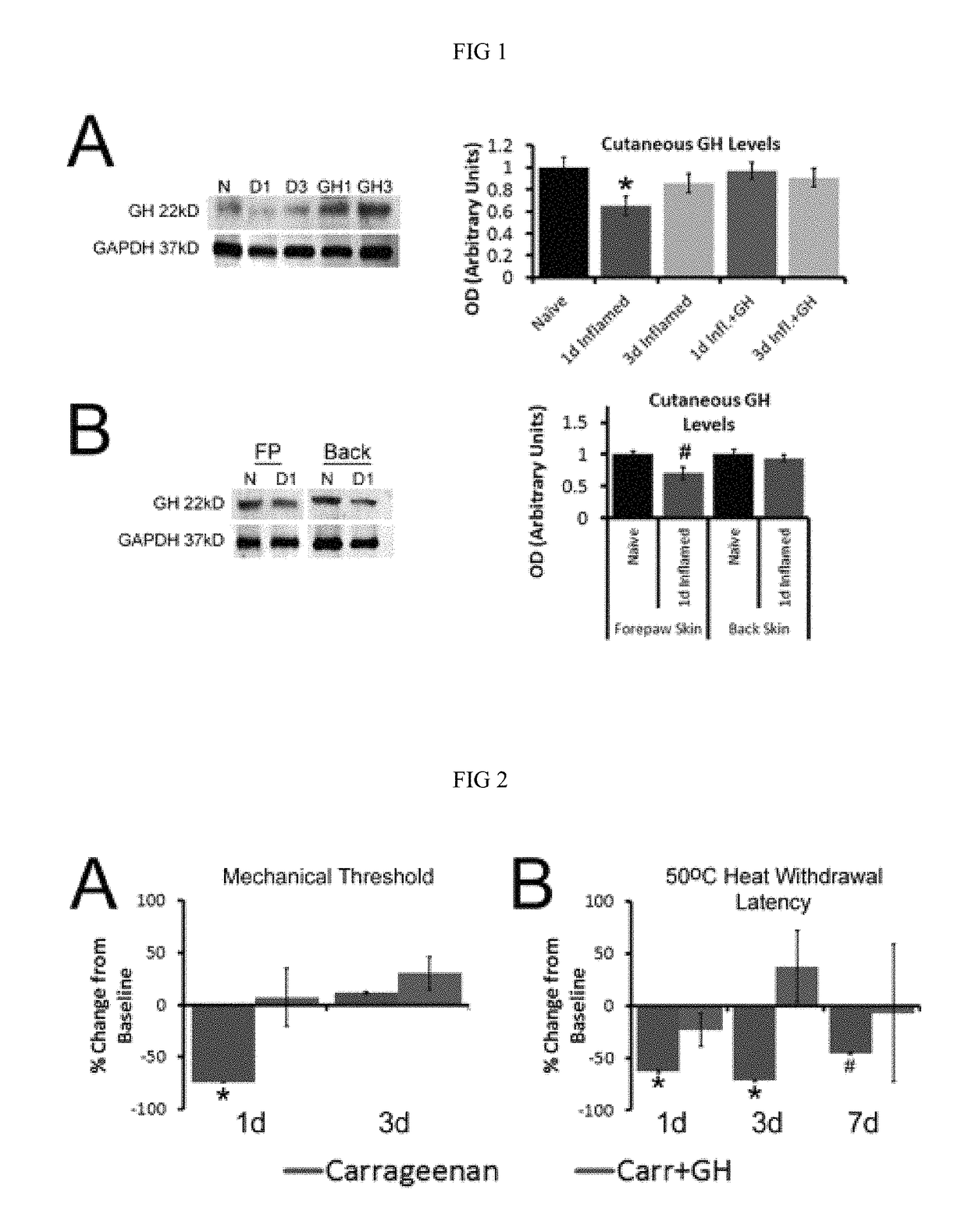
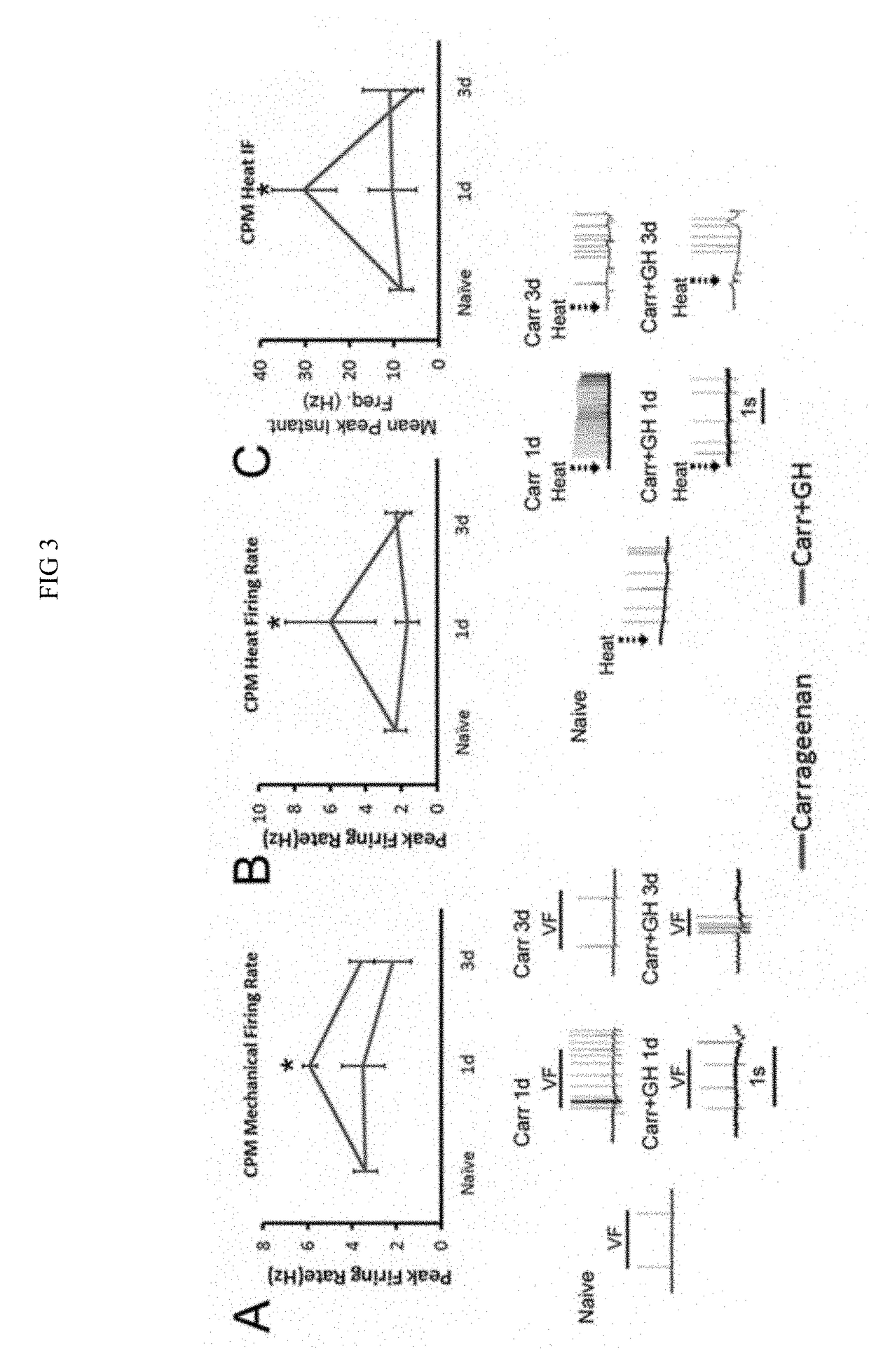
Compositions and methods for treatment of pediatric pain
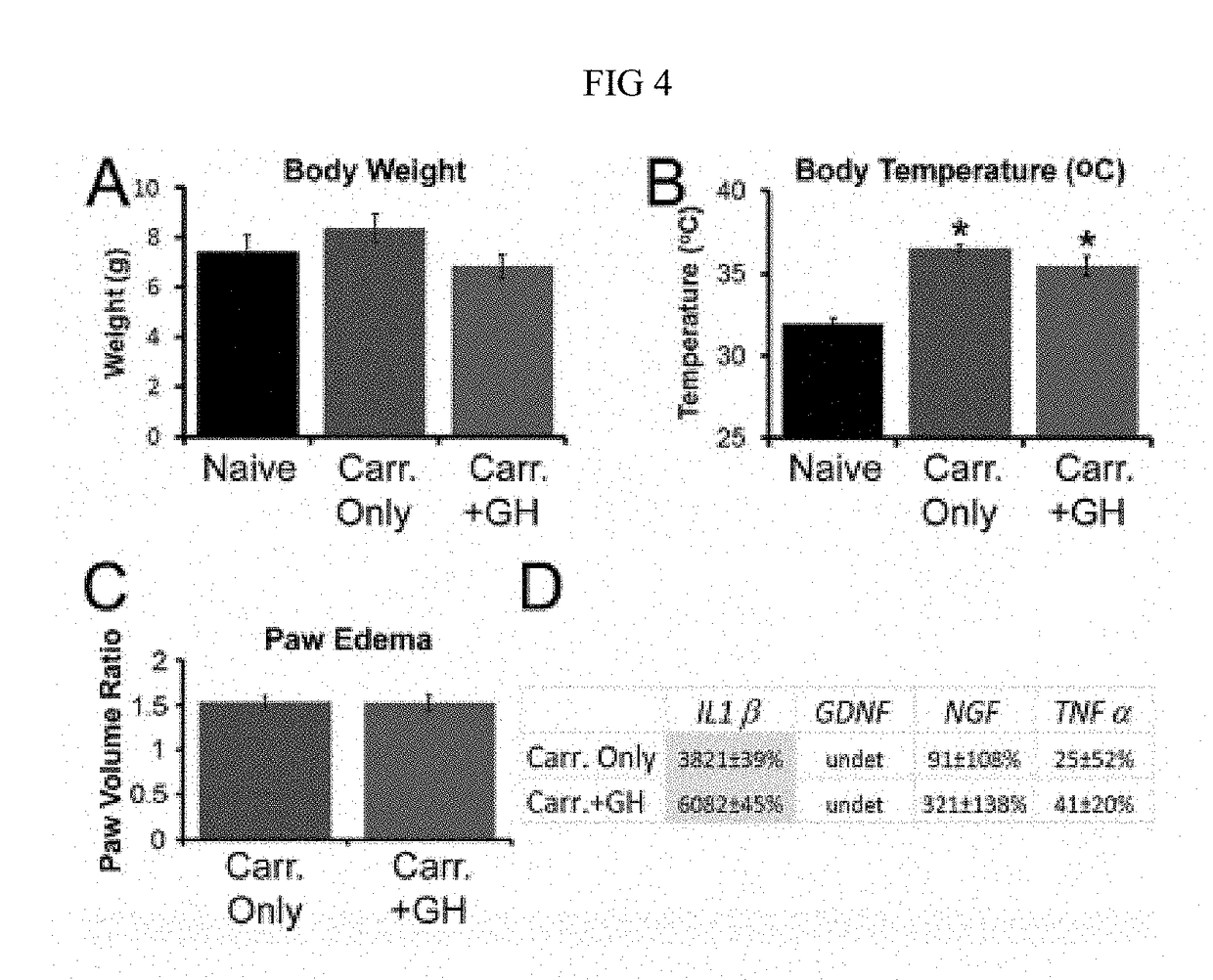
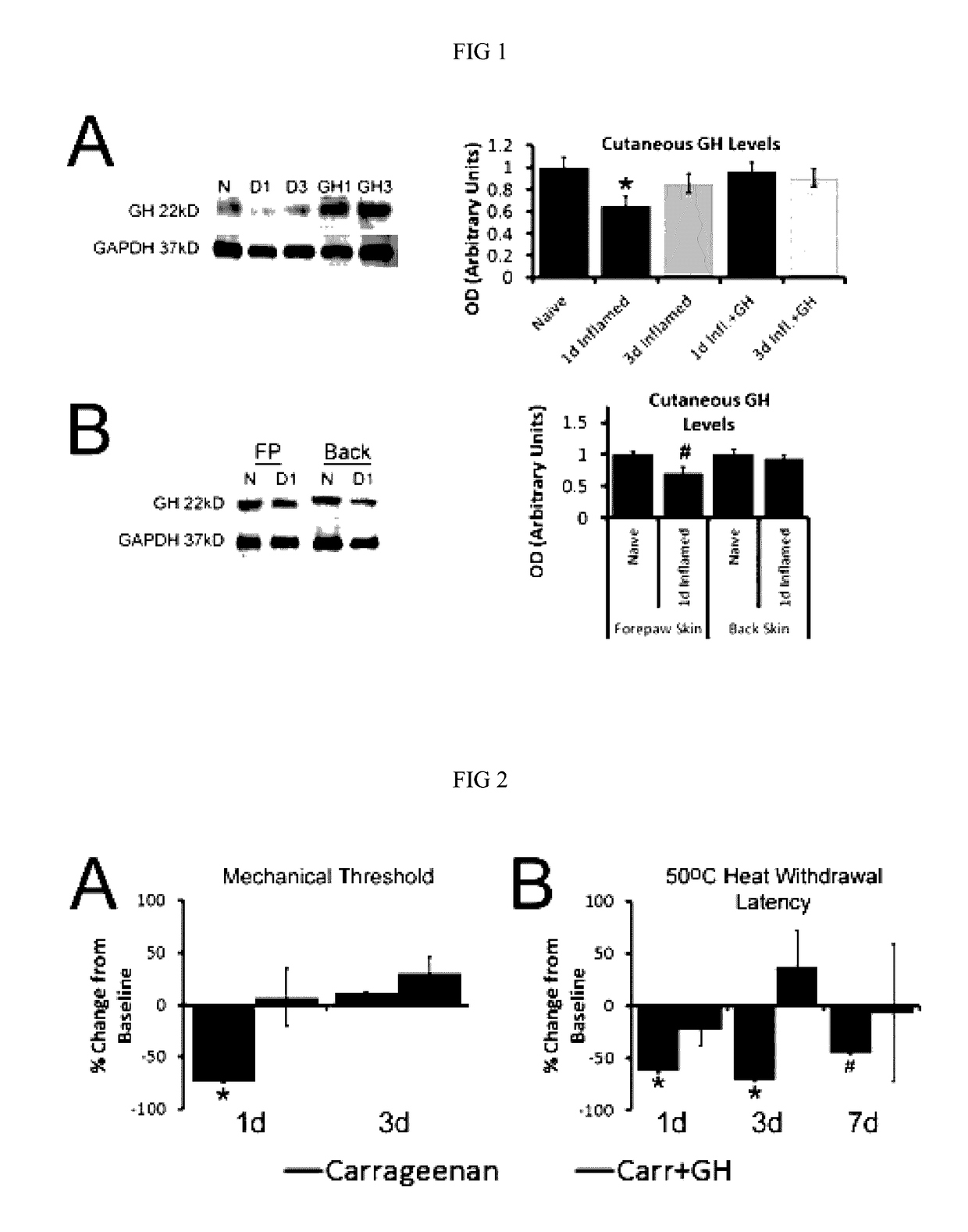
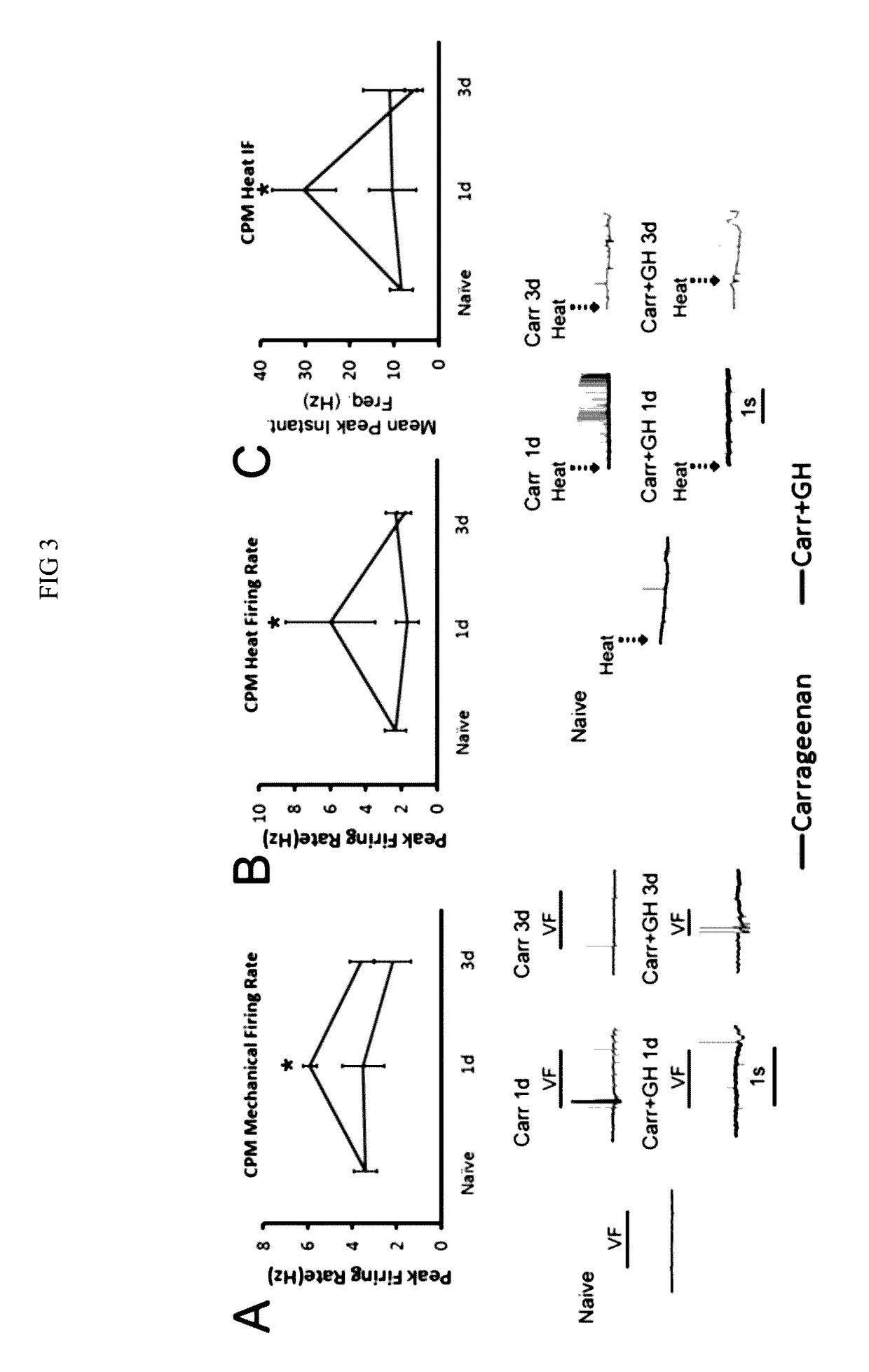
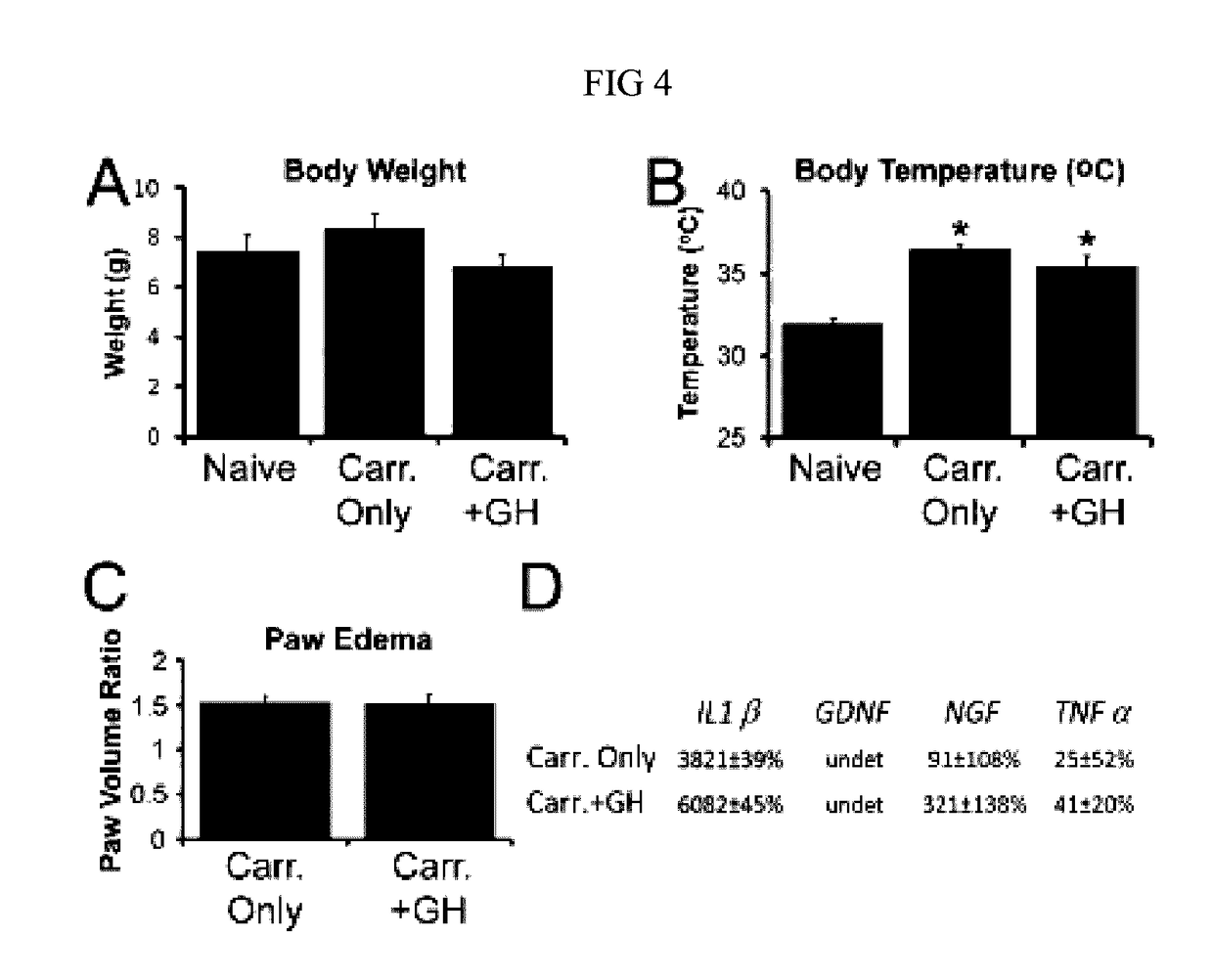
Disclosed are methods of treating pain in a mammal, which may include the step of administering human growth hormone to a mammal in need thereof. The pain treated by the disclosed methods may be of a type caused by inflammation induced mechanical and / or thermal hypersensitivity, and may include, for example, a pain type resulting from one or more conditions selected from peripheral injury pain, post-operative pain, cutaneous inflammation, cutaneous incision, muscle incision, or chronic pain. Disease states in which the disclosed methods may be used include fibromyalgia, sickle cell anemia, epidermolysis bullosa, erythromelalgia, complex regional pain syndrome, or generalized muscle pain.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Cyclic urea compounds as granzyme b inhibitors
ActiveUS20190151484A2Treat, reduce, and/or inhibit the appearance of ageing in the skinPeptide/protein ingredientsPeptidesAlopecia areataDiscoid lupus erythematosus
Cyclic urea compounds as Granzyme B inhibitors, compositions that include the compounds, and methods for using the compounds. Methods for treating cutaneous scleroderma, epidermolysis bullosa, radiation dermatitis, alopecia areata, and discoid lupus erythematosus are provided.
Owner:VIDA THERAPEUTICS
Compositions and methods for the treatment of skin diseases
PendingUS20210395775A1Low cytotoxicityEffectively transducing mammalian cellConnective tissue peptidesPeptide/protein ingredientsDiseaseLaminin
The present disclosure provides recombinant nucleic acids comprising one or more polynucleotides encoding a laminin polypeptide (e.g., a human laminin polypeptide) and / or a filaggrin polypeptide (e g , a human filaggrin polypeptide); viruses comprising the recombinant nucleic acids; compositions and formulations comprising the recombinant nucleic acids and / or viruses; methods of their use (e.g., for the treatment of Junctional Epidermolysis Bullosa); and articles of manufacture or kits thereof.
Owner:KRYSTAL BIOTECH INC
klhl24 gene mutant and its application
ActiveCN107974436BEfficient screeningMicrobiological testing/measurementLigasesGene MutantBiological organism
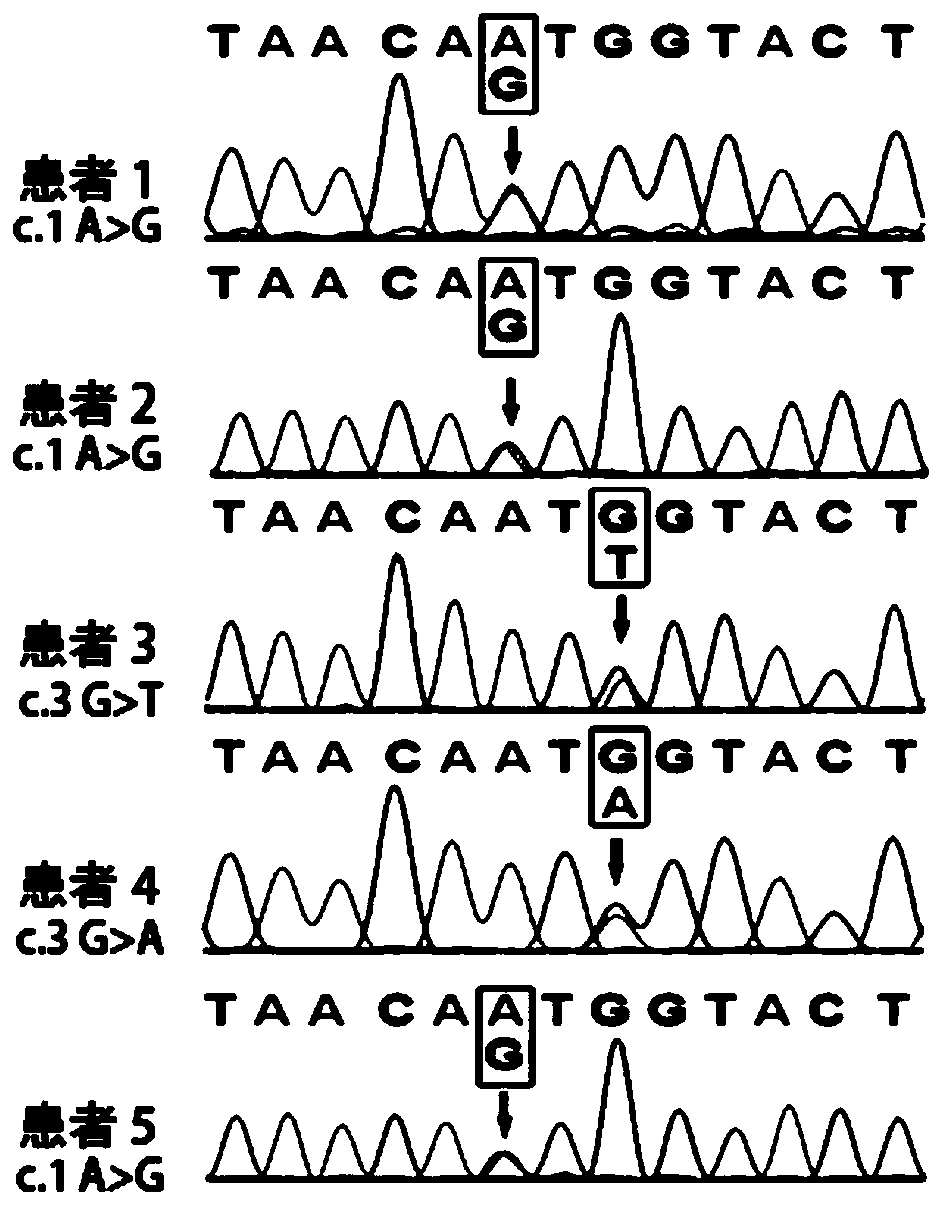
The invention discloses a KLHL24 gene mutant and application thereof and specifically relates to a separated nucleic acid coding the KLHL24 mutant, a separated polypeptide, a system used for screeningbiological samples susceptible to epidermolysis bullosa, a kit used for screening the biological samples susceptible to epidermolysis bullosa, a construct, and a recombinant cell. Compared with SEQ ID No. 1, the separated nucleic acid coding the KLHL24 mutant has at least one mutation selected from a group consisting of c.1A>G mutation, c.3G>T mutation and c.3G>A mutation. Whether a biological sample is prone to epidermolysis bullosa can be effectively detected by detecting existence of the gene mutant in the biological sample.
Owner:SHENZHEN HUADA GENE INST +1
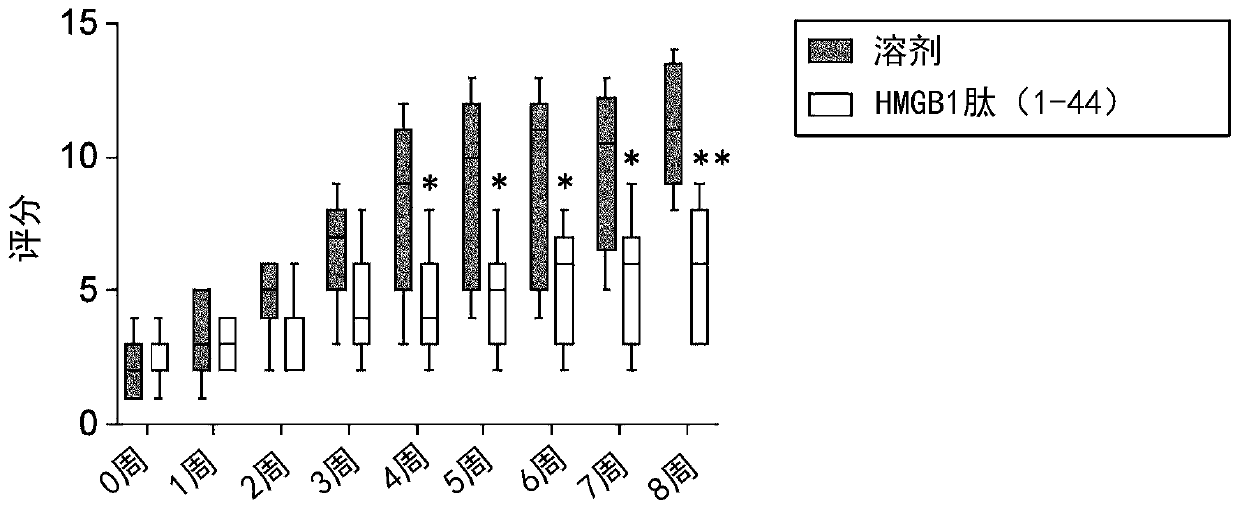
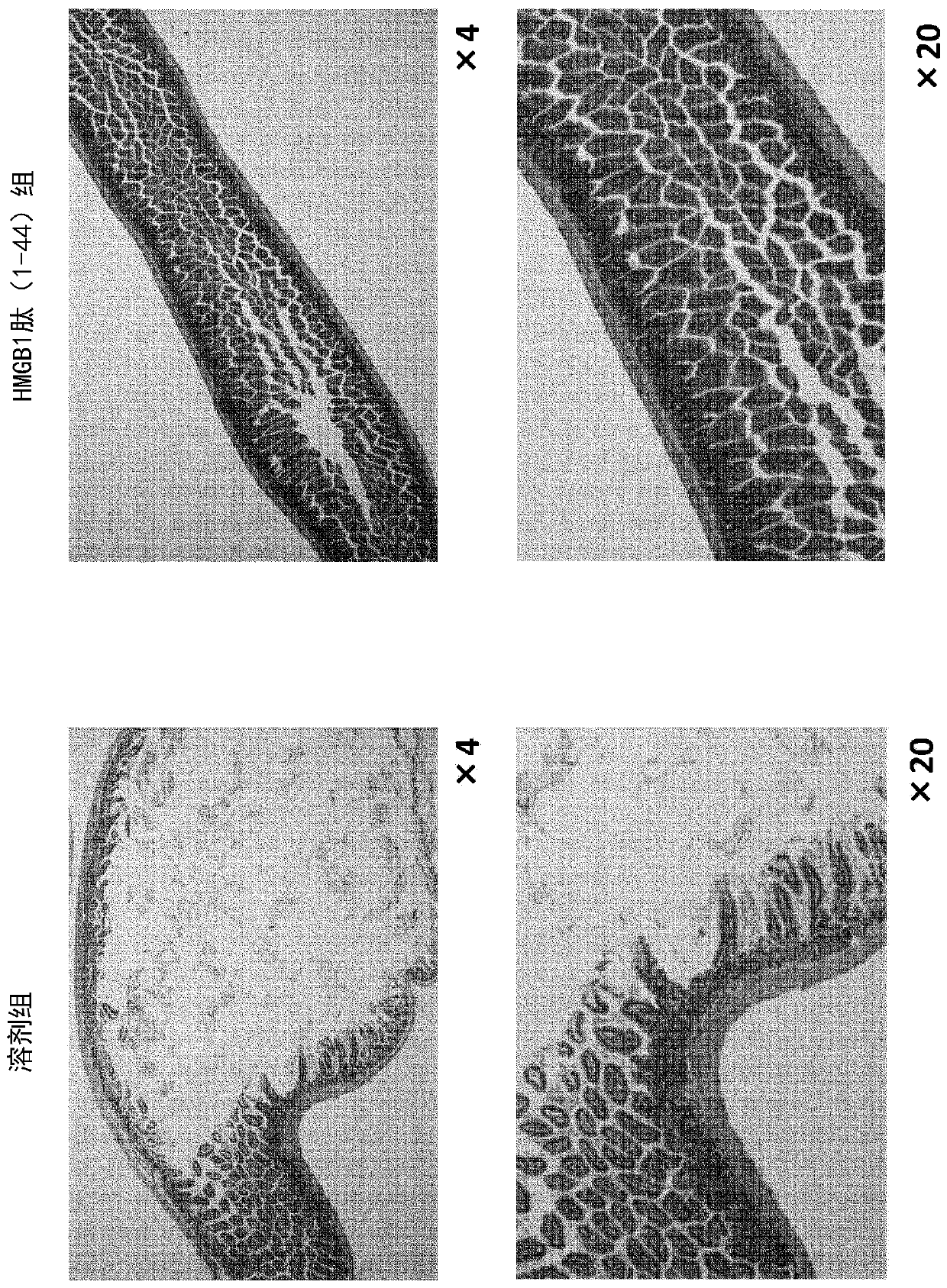
Therapeutic medicine for fibrous disease
The inventors of the present invention found that an HMGB1 peptide fragment having a specific amino acid sequence is effective at suppressing finger fusion and scarring of the digestive tract, and atprolonging survival in malnutrition-type epidermolysis bullosa model mice. The inventors also found that in a cutaneous ulcer model, administration of the specific HMGB1 peptide fragment suppresses the fibrosing of skin that occurs in the healing process of the ulcer. The present invention, on the basis of these findings, provides a pharmaceutical composition for fibrous disease treatment, which contains the specific HMGB1 peptide fragment.
Owner:斯特姆里姆有限公司 +1
Therapeutic uses of oxidising hypotonic acid solutions
PendingUS20220241324A1Antibacterial agentsPharmaceutical delivery mechanismInfection inducedPharmacology
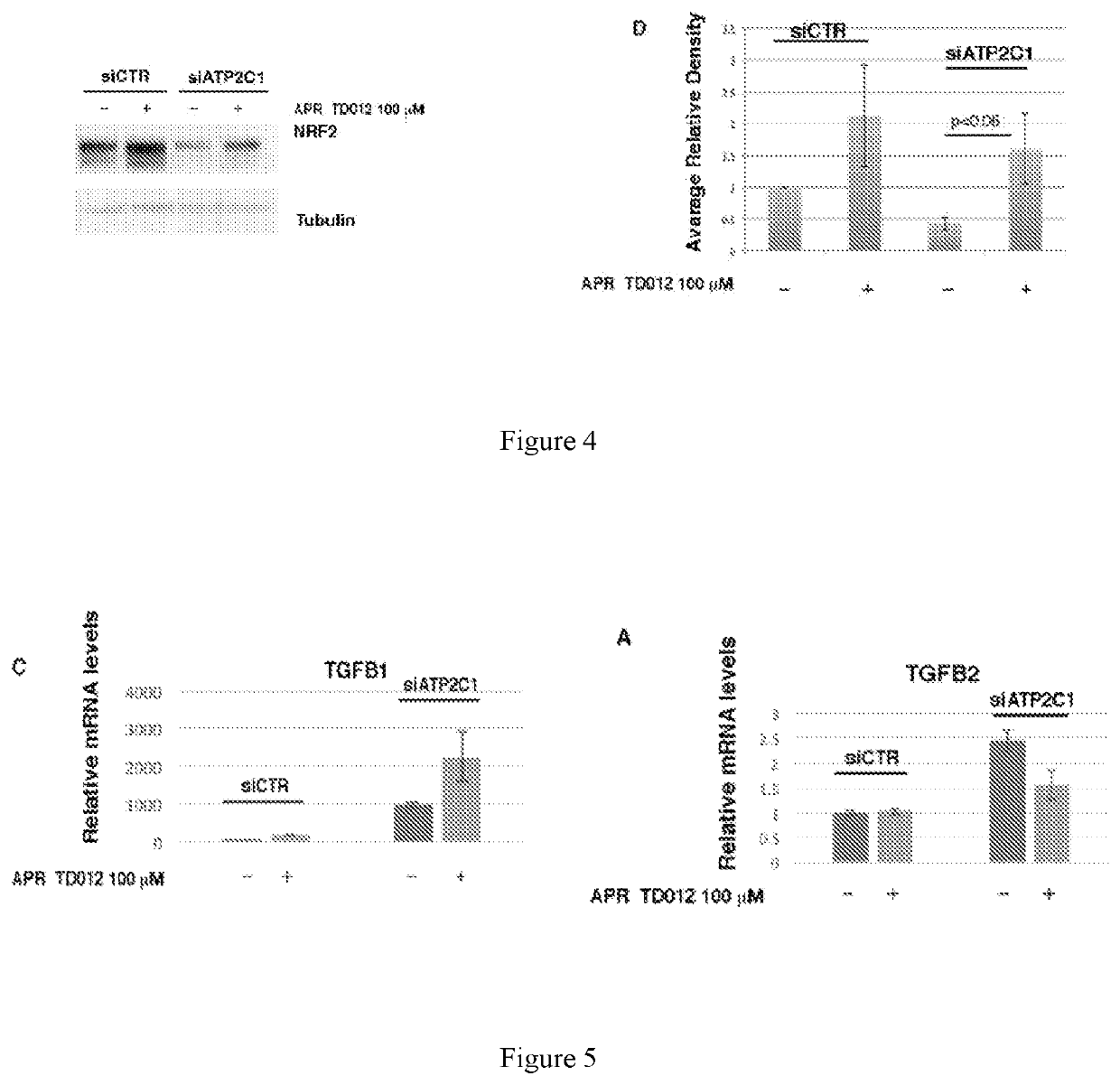
Methods for treating or preventing ulcers caused by Epidermolysis Bullosa (“EB”), EGFR inhibitor-induced skin toxicities, lesions caused by Hailey-Hailey Disease (“HHD”), Buruli Ulcers, and SARS-CoV-2 infections, by topically applying a hypotonic, acid oxidizing solution containing hypochlorous acid (HClO) to the affected area.
Owner:APR APPLIED PHARMA RES
Topical Medicament for skin and mucosal injuries associated with Epidermolisis bullosa
ActiveUS20170333489A1So as not to damageHeal fastHydroxy compound active ingredientsInanimate material medical ingredientsVitaminBandage
A composition and method for treating Epidermolisis bullosa (EB), comprising: applying to skin or mucosal surfaces of a patient in need of treatment for EB a dressing gauze or bandage without prior cleaning of said skin or mucosal surfaces, wherein distributed on said dressing is an effective amount of a composition containing beeswax; an oleaginous base, vitamins and a pharmaceutically acceptable excipient; then removing said dressing at least twice per day without damaging the patient's skin or mucosal surfaces due to removal of the dressing.
Owner:REV PHARMA
FGFR kinase inhibitors and pharmaceutical uses
Fibroblast Growth Factor Receptor kinase inhibitors and prodrugs thereof of Formula (I) and their use for the treatment of hyper-proliferative diseases such as retinopathy, psoriasis, rheumatoid arthritis, osteoarthritis, septic arthritis, tumour metastasis, periodontal disease, corral ulceration, proteinuria, coronary thrombosis from atherosclerotic plaque, aneurismal aorta, dystrophobic epidermolysis bullosa, degenerative cartilage loss following traumatic joint injury, osteopenias mediated by MMP activity, tempero mandibular joint disease, and demyelating disease of the nervous system.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI +1
A set of primer combinations and kits for simultaneous detection of 93 bovine genetic defect genes and lethal haplotypes
ActiveCN107099607BImprove throughputLow costMicrobiological testing/measurementDNA/RNA fragmentationBiotechnologyAssortative mating
The invention discloses a primer combination and a kit for simultaneous detection of 93 bovine genetic defect genes and lethal haplotypes. Through use of the primer combination and the kit, casual mutation sites (SNP and insertion or deletion of short fragments) of the 93 genetic defect genes (such as PIRM syndrome, crooked tail syndrome, epidermolysis bullosa and the like) and the lethal haplotypes (HH1, HH3, HH4, HH5 and JH1) can be detected in one time, cattle with monogenic inheritance defect gene carriers and lethal haplotype carriers can be screened, and accurate guidance is provided for cattle breeding and genetic evaluation, herd selection and assortative mating, population genetic improvement, cultivation of new varieties and genetic resource protection. The primer combination and the kit have the characteristics of high throughput, low cost, high accuracy, and the like, is suitable for the varieties of beef cattle and dairy cattle, and can be widely used in the breeding and reproduction field of the cattle.
Owner:DAIRY CATTLE RES CENT SHANDONG ACADEMY OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com