Cyclic urea compounds as granzyme b inhibitors
a technology of granzyme b and compound, applied in the field of granzyme b inhibitors, can solve the problems of limited use of biological inhibitors such as serpins, insufficient potency or specificity to be effective treatments for granzyme-b-related diseases, disorders, and conditions, and achieve the effects of reducing the appearance of ageing in the skin, reducing the number of side effects, and/or inhibiting the appearance of ageing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Synthetic Intermediates
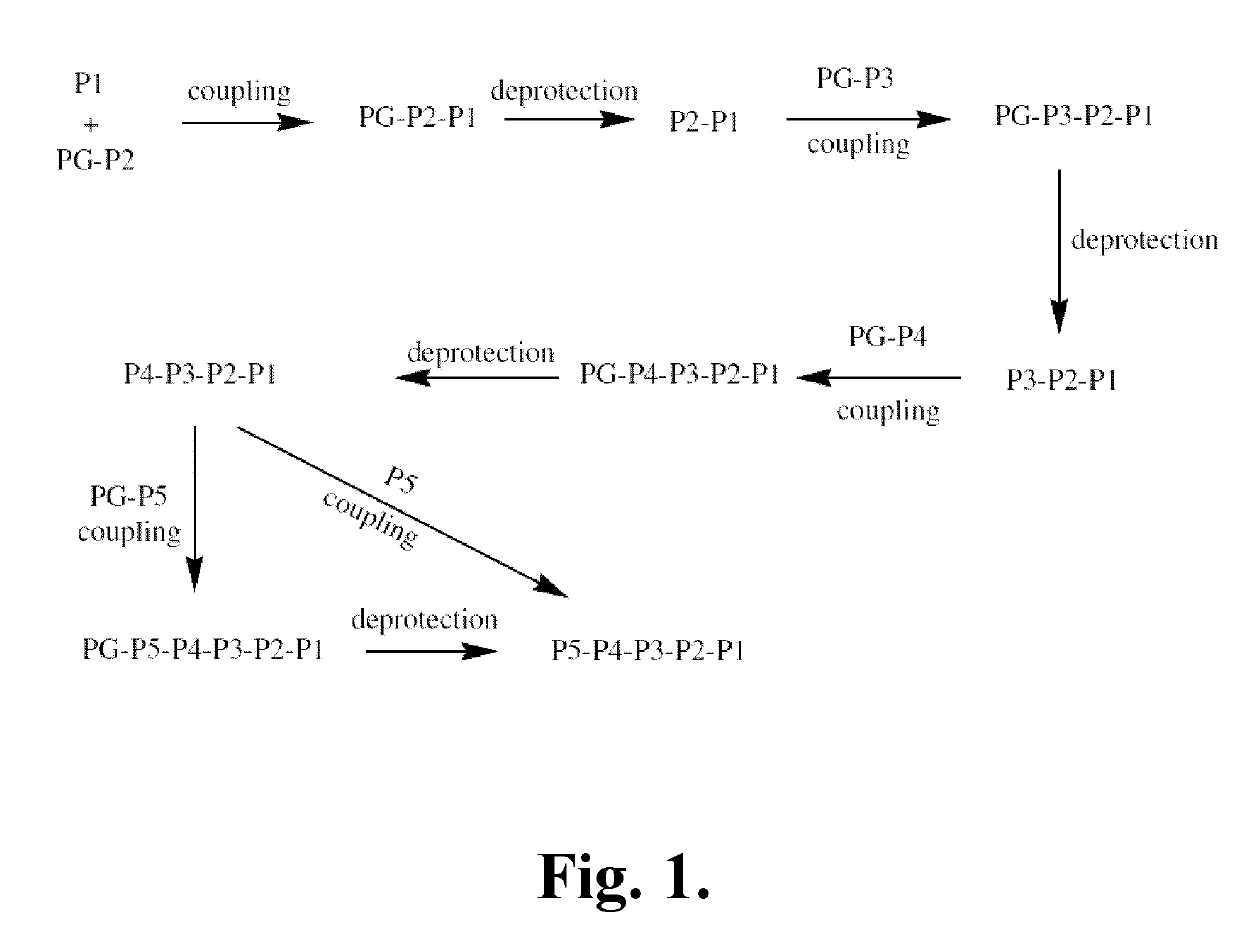
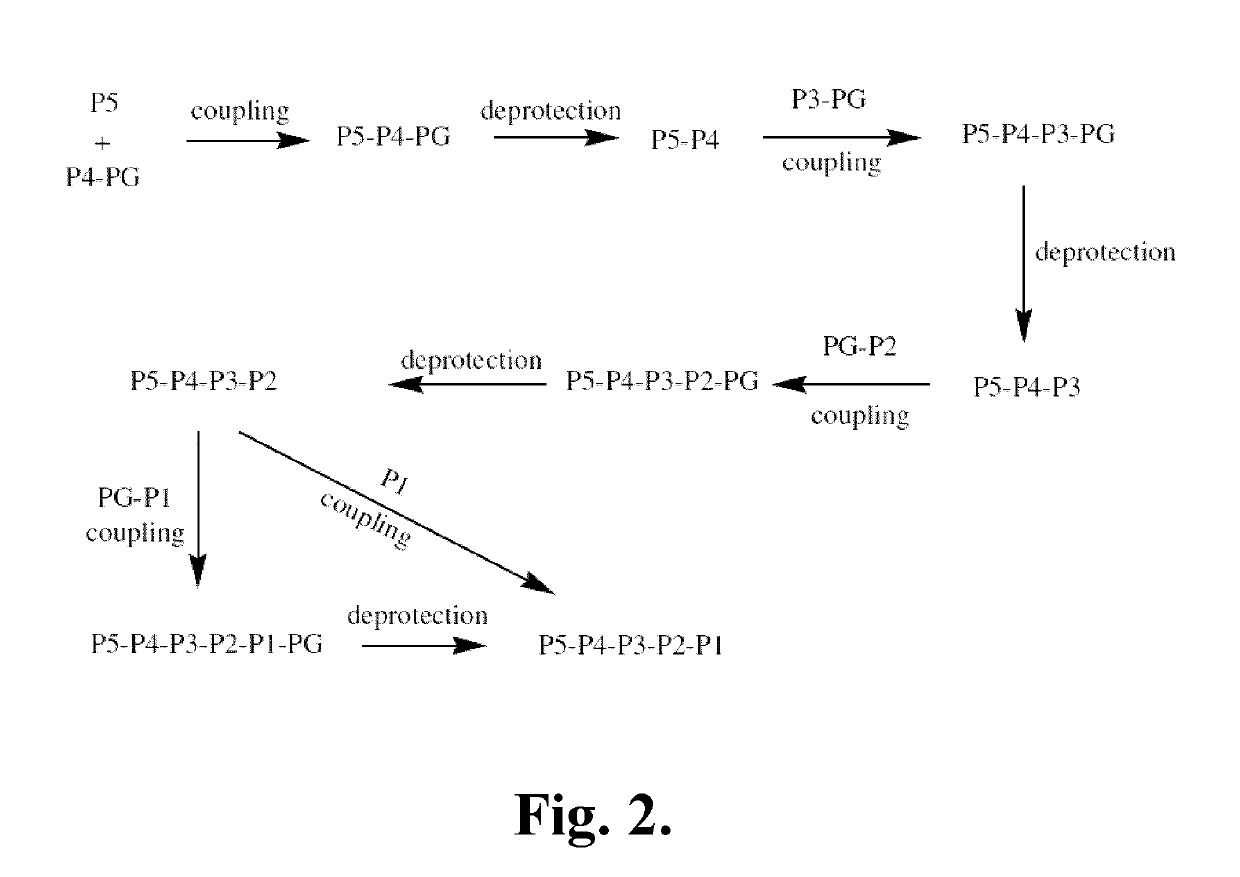
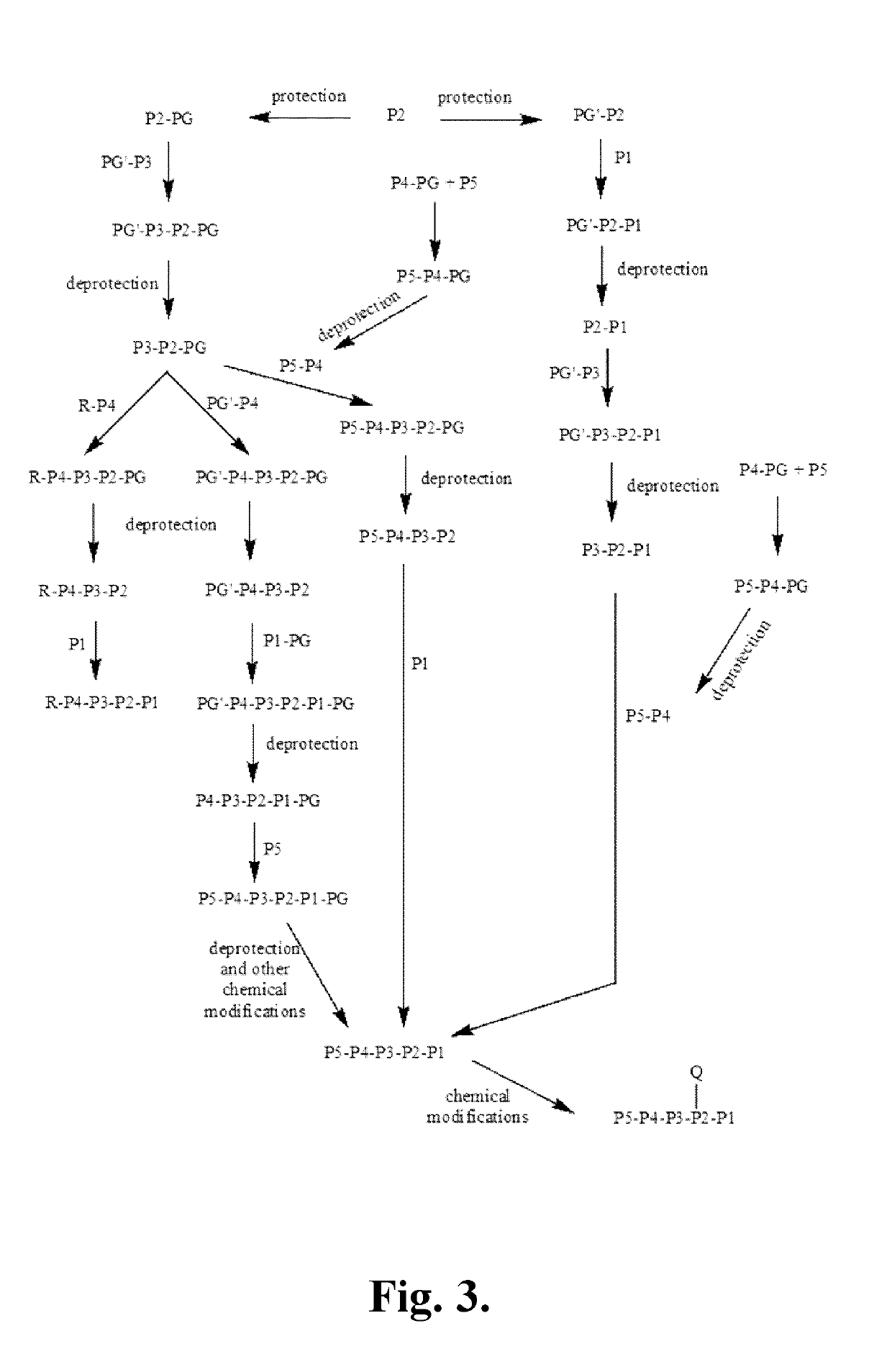
[0342]The following is a description of synthetic intermediates (I-1 to I-4) useful for making representative compounds of the invention.
[0343]Intermediate I-1
(S)-Dibenzyl 2-oxoimidazolidine-1,5-dicarboxylate (I-1)
[0344](S)-(-)-1-Z-2-Oxo-5-imidazolidinecarboxylic acid (2.5 g, 9.461 mmol, 1 eq.), para-toluenesulfonic acid (360 mg, 1.892 mmol, 0.2 eq.) and benzyl alcohol (2.39 mL, 23.12 mmol, 2.4 eq.) were dissolved in toluene (25 mL) in a round bottom flask equipped with a Dean-stark apparatus and a condenser. The reaction was heated to reflux for 24 hrs and then allowed to come to RT. It was then washed with a saturated solution of NaHCO3 solution (1×25 mL) and the aqueous layer was re-extracted with ethyl acetate (1×25 mL). The combined organic layers were dried over Na2SO4 and concentrated. The product was then purified by column chromatography using 15% to 70% ethyl acetate in hexanes as the eluent to give (S)-dibenzyl 2-oxoimidazolidine-1,5-dicarboxylate (...
example c1
4-(((2S,3S)-1-((2-((S)-5-(((2H-Tetrazol-5-yl)methyl)carbamoyl)-2-oxoimidazolidin-1-yl)-2-oxoethyl)amino)-3-methyl-1-oxopentan-2-yl)amino)-4-oxobutanoic acid
[0353]A solution of I-2 (100 mg, 0.282 mmol, 1 eq.) in anhydrous THF (2 mL) was cooled to −50° C. under N2. Potassium tert-butoxide (31.6 mg, 0.282 mmol, 1 eq.) was then added, followed by Boc-glycine N-hydroxysuccinimide ester (76.7 mg, 0.282 mmol, 1 eq.) and the reaction mixture was slowly allowed to warm up to −10° C. and it was stirred at that temperature for 1 h. Analysis of the reaction mixture by TLC showed the presence of starting material left. The reaction mixture was cooled to −50° C. and potassium tert-butoxide (31.6 mg, 0.282 mmol, 1 eq.), followed by Boc-glycine N-hydroxysuccinimide ester (76.7 mg, 0.282 mmol, 1 eq.) were added and the reaction mixture was slowly allowed to warm up to −10° C. and it was stirred at that temperature for 1 h. TLC showed disappearance of the starting material. The reaction mixture was a...
example c2
4-(((2S,3S)-1-((2-((S)-5-(((2H-TETRAZOL-5-YL)METHYL)CARBAMOYL)-3-CYCLOHEXYL-2-OXOIMIDAZOLIDIN-1-YL)-2-OXOETHYL)AMINO)-3-METHYL-1-OXOPENTAN-2-YL)AMINO)-4-OXOBUTANOIC ACID
[0358]A solution of I-3 (220 mg, 0.733 mmol, 1 eq.) in anhydrous THF (15 mL) was cooled to −50° C. under N2. Potassium tert-butoxide (98 mg, 0.880 mmol, 1.2 eq.) was then added, followed by Boc-glycine N-hydroxysuccinimide ester (240 mg, 0.880 mmol, 1.2 eq.) and the reaction mixture was slowly allowed to warm up to −10° C. and it was stirred at that temperature for 1 h. Analysis of the reaction mixture by TLC showed the presence of starting material left. The reaction mixture was cooled to −50° C. and potassium tert-butoxide (25 mg, 0.219 mmol, 0.25 eq.), followed by Boc-glycine N-hydroxysuccinimide ester (60 mg, 0.219 mmol, 0.25 eq.) were added and the reaction mixture was slowly allowed to warm up to −10° C. and it was stirred at that temperature for 1 h. TLC showed disappearance of the starting material. The react...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water solubility | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com