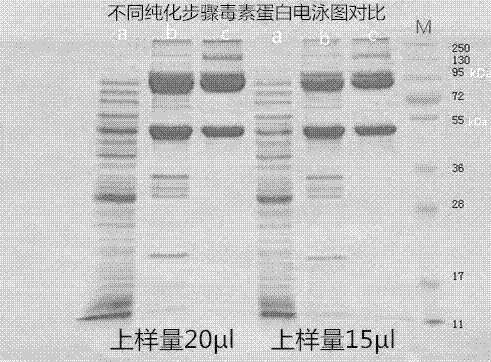
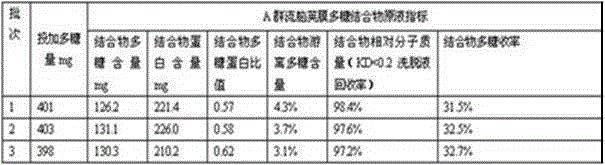
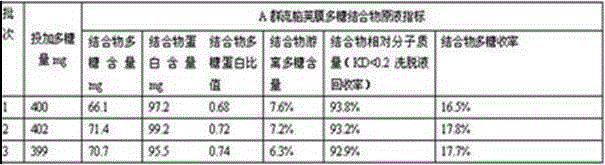
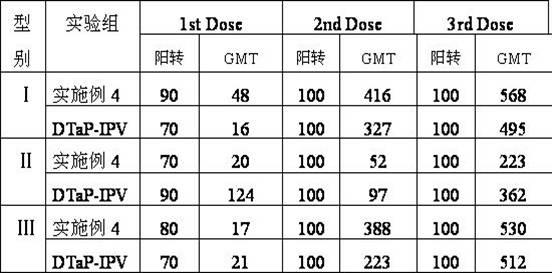
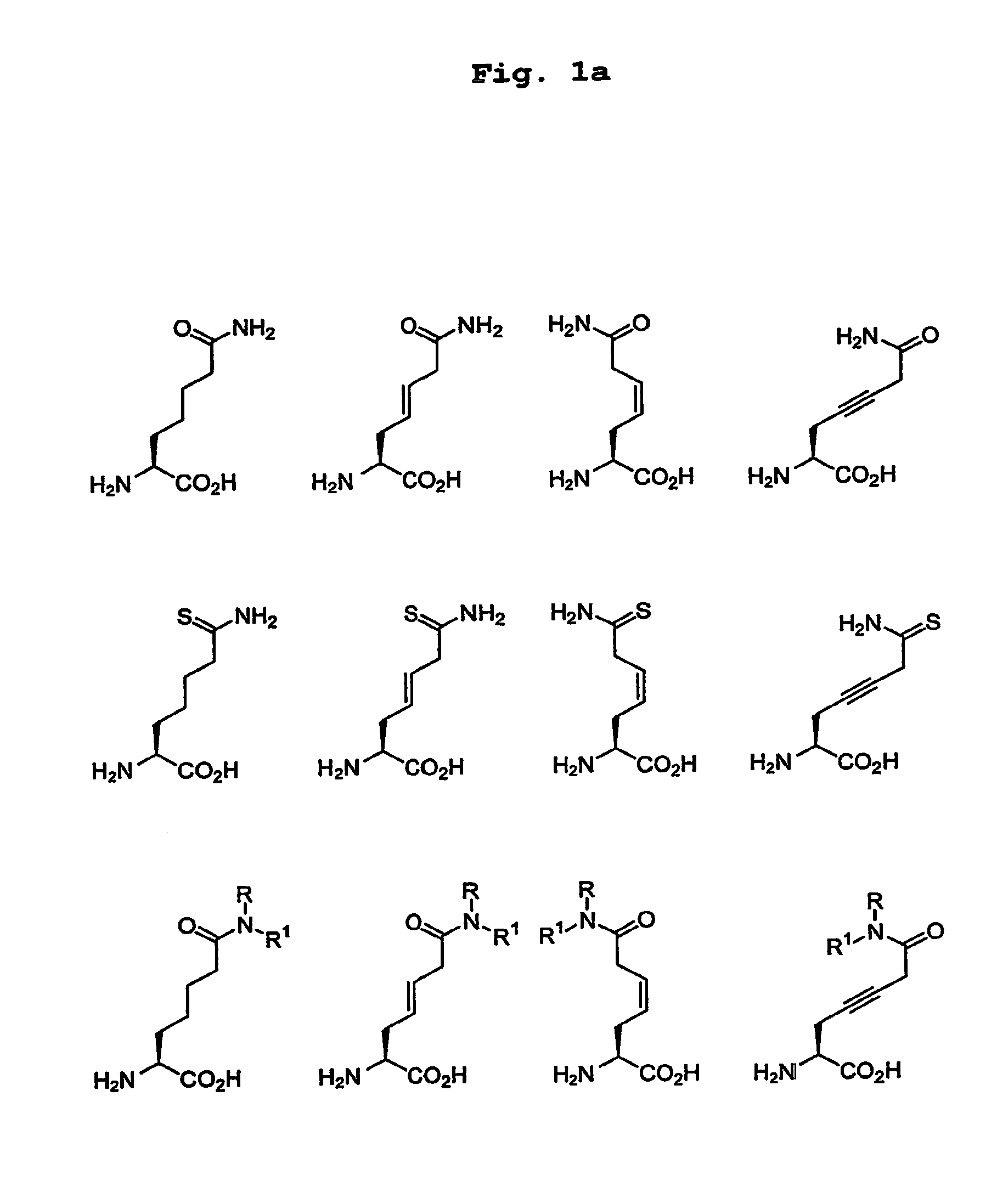
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
123 results about "Tetanus toxoids" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tetanus Toxoid is used to prevent tetanus (also known as lockjaw). Tetanus is a serious illness that causes convulsions (seizures) and severe muscle spasms that can be strong enough to cause bone fractures of the spine.
Acellular pertussis vaccines and methods of preparation thereof
InactiveUS6696065B1Increase contentEnhance immune responseBiocideSsRNA viruses positive-sensePoliomyelitisTetanus toxoids
A multi-component vaccine composition is described comprising acellular pertussis vaccine components, diphtheria toxoid, tetanus toxoid and inactivated poliovirus. The composition also may contain a conjugate of a capsular polysaccharide on Haemophilus influenzae type b and tetanus toxoid or diphtheria toxoid, which may be reconstituted from a lyophilized state by the other component. The administration of the multiple component vaccine resulted in no diminution of the immunogenicity of any component as a result of interference by other components of the vaccine.
Owner:SANOFI PASTEUR LTD
High level expression of recombinant toxin proteins
ActiveUS20110287443A1High yieldQuality improvementAntibody mimetics/scaffoldsBacteria peptidesBacteroidesHigh level expression
The present invention relates to the field of recombinant toxin protein production in bacterial hosts. In particular, the present invention relates to production processes for obtaining high levels of a recombinant CRM197, Diphtheria Toxin, Pertussis Toxin, Tetanus Toxoid Fragment C, Cholera Toxin B, Cholera holotoxin, and Pseudomonas Exotoxin A, from a bacterial host.
Owner:PFENEX
High level expression of recombinant toxin proteins
The present invention relates to the field of recombinant toxin protein production in bacterial hosts. In particular, the present invention relates to production processes for obtaining high levels of a recombinant CRM197, Diphtheria Toxin, Pertussis Toxin, Tetanus Toxoid Fragment C, Cholera Toxin B, Cholera holotoxin, and Pseudomonas Exotoxin A, from a bacterial host.
Owner:PELICAN TECH HLDG INC
Separated and purified acellular pertussis-diphtheria-tetanus, b-type haemophilus influenzae and A-group and C-group meningococcus combined vaccine and preparation method thereof
InactiveCN104689309ARelieve painReduce the burden onAntibacterial agentsBacterial antigen ingredientsHemagglutininAluminium hydroxide
The invention discloses a combined vaccine and a preparation method thereof. The combined vaccine is formed by a component A and a component B, wherein the component A is a liquid preparation and composed of pertussis toxin, filamentous hemagglutinin, pertussis adhesion, diphtheria toxoid, tetanus toxoid, aluminium hydroxide and sodium chloride; the component B is a freeze-drying preparation and composed of A-group meningitis polysaccharide conjugate, C-group meningitis polysaccharide conjugate, b-type haemophilus influenzae polysaccharide conjugate and lactose. The preparation method of the vaccine includes the steps of preparing of acellular pertussis-diphtheria-tetanus vaccine semi-finished products, A-group and C-group meningococcocci and b-type haemophilus influenzae combined vaccine semi-finished products, split charging and packaging. The vaccine has the characteristics of being safe, effective, controllable and capable of preventing diseases through an injection, the preparation method is easy to operate, preparation is facilitated, cost is low, and the combined vaccine is suitable for industrialized mass production.
Owner:CHENGDU OLYMVAX BIOPHARM
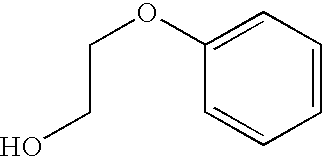
Combination vaccines with 1-hydroxy-2-phenoxyethane preservative
Processes for preparing combination vaccines that include diphtheria and tetanus toxoids, where these two toxoids are used in the processes as a single component containing both toxoids, and also containing 1-hydroxy-2-phenoxyethane.
Owner:NOVARTIS AG
Meningococcal conjugate vaccination
ActiveUS20100104593A1Improving immunogenicityReduce chain lengthAntibacterial agentsBacterial antigen ingredientsCoccidiaMedicine
Conjugated meningococcal capsular saccharides will be introduced into immunisation schedules in the near future, but the phenomenon of “carrier suppression” must first be addressed, particularly where multiple conjugates are to be used. In the invention, tetanus toxoid is used as the carrier protein, even where multiple meningococcal conjugates are administered at the same time and where a patient has previously been exposed to the carrier protein, either in the form of a previous immunogen (e.g. in a DTP vaccine) or as a previous carrier protein (e.g. in a Hib or pneumococcal conjugate vaccine). The invention provides a method for immunising a patient, comprising administering multiple conjugates of meningococcal capsular saccharides, wherein each conjugate comprises a tetanus toxoid carrier protein, and the capsular saccharide, and wherein the patient has been pre-immunised with a tetanus toxoid.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Fine purification method of polyvalent anti-snake venom freeze-dry blood serum
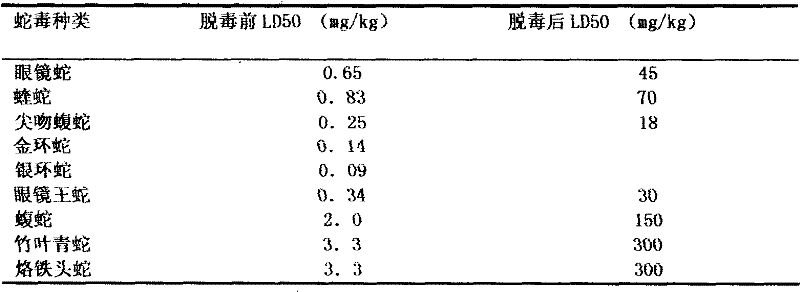
InactiveCN101385855AImprove immunityQuality improvementAntibody ingredientsDermatological disorderVirulent characteristicsFreeze-drying
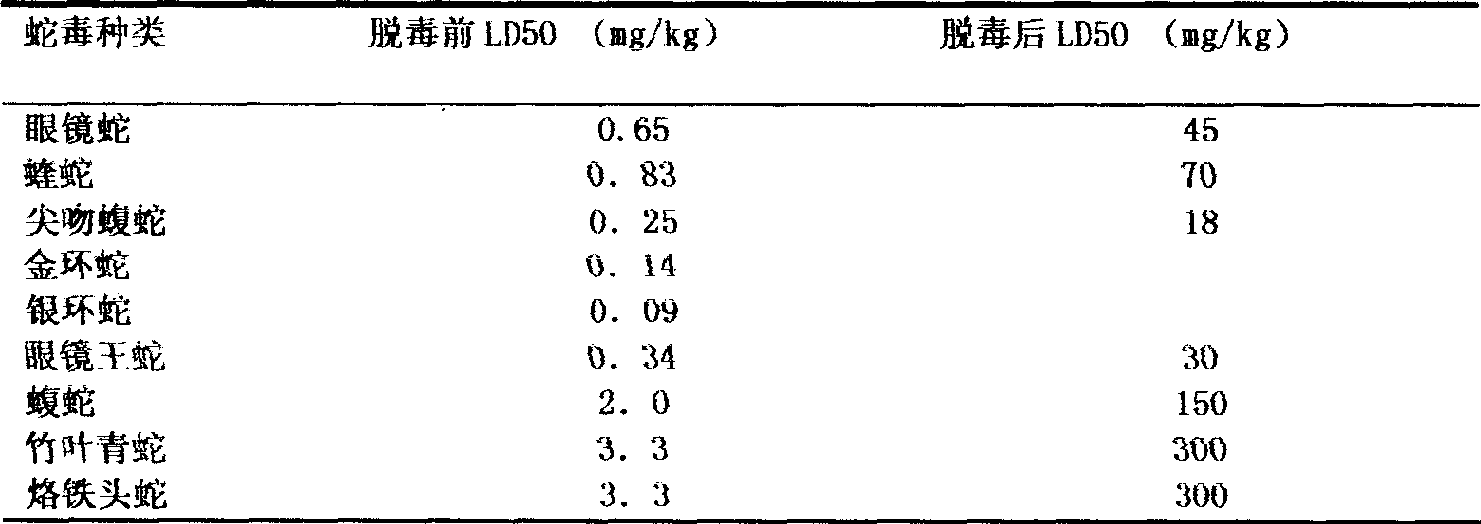
The invention discloses a method for refining a polyvalent ASV (anti-snake venom) lyophilized serum, pertaining to an immune serum containing antibody which aims at least two kinds of antigens. The serum takes the venom of Ancistrodon acutus, Agkistrodon halys, Pit-vipers, bamboo snakes, adders and the like as the immune antigen which is inoculated into the bodies of animals after degerming and dialysis, and then titer immune serum after purification is used for collecting the antibody which is then desalted and lyophilized; mice are used for evaluating the LD50 of the virulence of the venom and the titer of the ASV serum; the antibody inoculated into the bodies of the animals is one of the venom, toxoid or snake venom or the composition thereof, and glutaraldehyde or formaldehyde is used for toxin detoxication; before immunity, anti-tetanic toxoid condensed antibody for human beings is used for carrying out minim gradual immunization on the animals, or an Emuade adjuvant is used for immunization purpose. The polyvalent ASV lyophilized serum complies with the biological product standards upon the tests and inspections of purity and specific activity, safety, pyrogen, hypersusceptibility, dissolution and the like; moreover, the serum can cure sufferers who are bitten by vipers with the advantages of high purity, low foreign protein, easy preservation and quick dissolving capacity.
Owner:KUNMING INST OF MILITARY MEDICINE CHENGDU MILITARY REGIONS CENT FOR DISEASE CONTROL & PREVENTION
Method for preparing tetanus toxoid vaccine
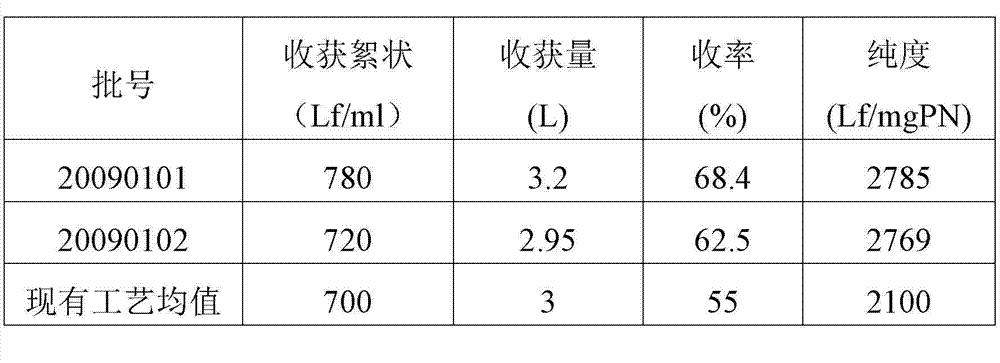
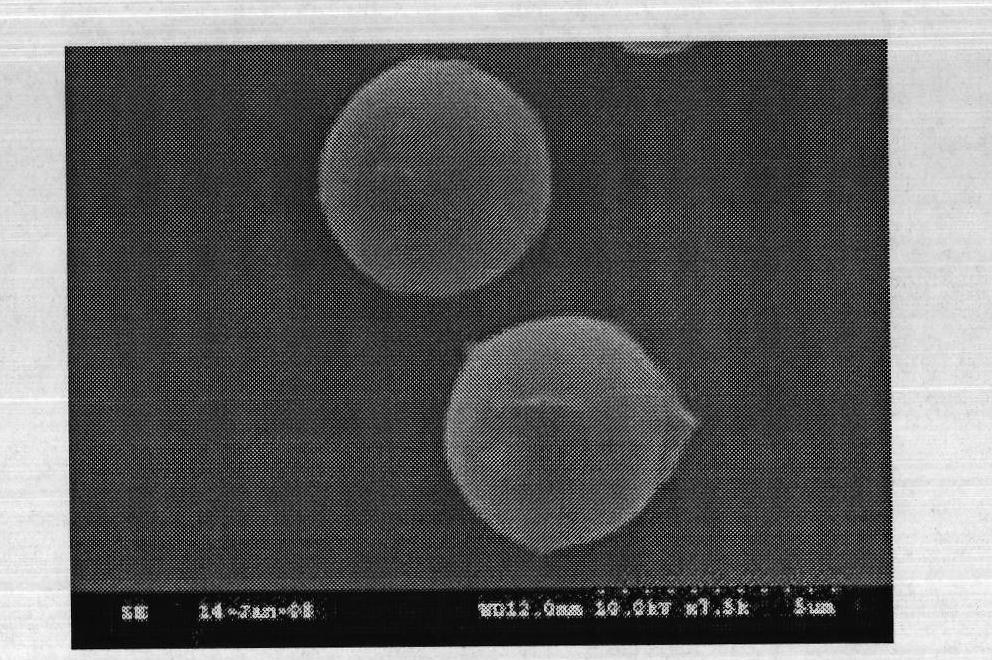
ActiveCN102961741AReduce cloggingAvoid damageAntibacterial agentsBacterial antigen ingredientsFiltrationUltrafiltration
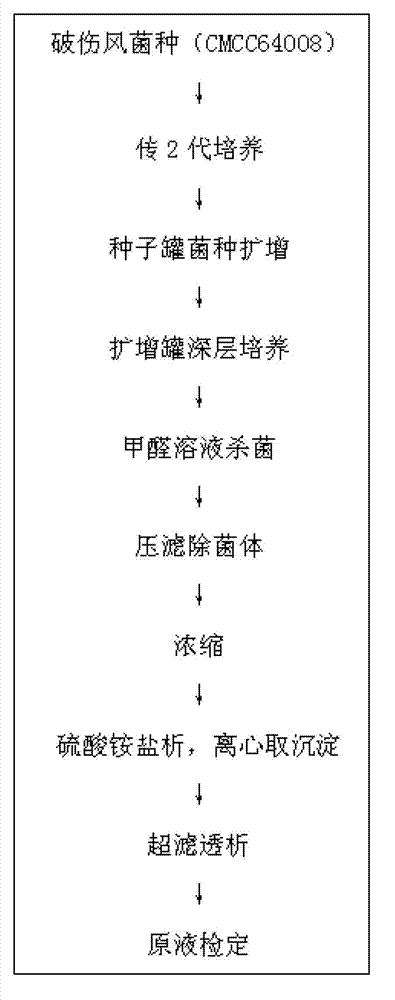
The invention discloses a method for preparing a tetanus toxoid vaccine. According to the process, with clostridium tetani strains as raw materials, the tetanus toxoid vaccine is prepared through the following steps of: culturing of tetanus toxoid, bacterium liquid separation, ultrafiltration and concentration, salting out, ultrafiltration desalting and the like. According to the method, firstly culture liquid is subjected to virus-free treatment and then refined, so that the porous channel plugging caused by accumulation of thalli and other impurity segments at a plate and frame membrane package during plate and frame filtering to remove thalli is reduced, and the smoothness during filtration is increased; toxoid protein and other allergens in the culture liquid are removed by changing the salting-out method; and as the desalting methods of the culture liquid after concentration and salting out adopt the tangential flow ultrafiltration method, the destruction of antigen caused by shearing of toxoid protein is reduced, and the protein precipitation is avoided. By utilizing the method, the time for preparing the tetanus toxoid vaccine is shortened, and the production efficiency is improved.
Owner:WUHAN INST OF BIOLOGICAL PROD CO LTD
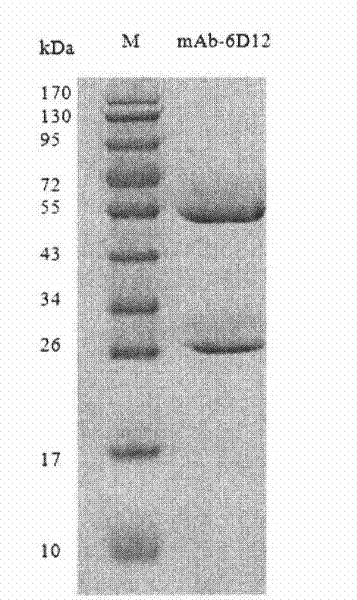
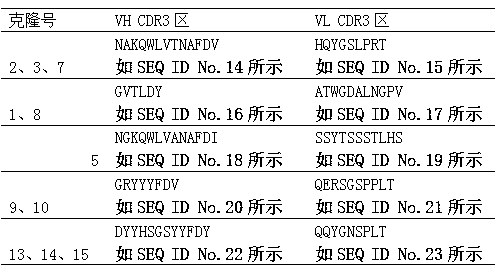
Tetanus toxoid monoclonal antibody and preparation method and application thereof
The invention relates to a tetanus toxoid monoclonal antibody and a preparation method and application thereof, in particular to a tetanus toxoid monoclonal antibody. A complementary determining region (CDR) of a heavy chain variable region of the monoclonal antibody comprises CDR1 shown as SEQ ID NO:6, CDR2 shown as SEQ ID NO:8 and / or CDR3 shown as SEQ ID NO:10; and a CDR of a light chain variable region of the monoclonal antibody comprises CDR1 shown as SEQ ID NO:12, CDR2 shown as SEQ ID NO:14 and CDR3 shown as SEQ ID NO:16. The invention also provides a deoxyribonucleic acid (DNA) moleculefor coding the sequence, an expression vector and a host cell.
Owner:SHANGHAI INST OF BIOLOGICAL PROD CO LTD
Rabies vaccine for human beings
InactiveCN102526722AGood immune effectImprove securityAntiviralsDepsipeptidesVirus ProteinTetanus toxoids
The invention provides a rabies vaccine for human beings, which contains an outer membrane fragment of a split rabies virus particle and tetanus toxoid; the outer membrane fragment comprises furcella and matrix protein, wherein each dosage of rabies vaccine for human beings contains 10-150 micrograms of virus protein; and the dosage of the tetanus toxoid is 3-10 Lf / dosage. Compared with the existing virus purification stock solution, the rabies vaccine for human beings contains less other proteins, has higher immunogenicity and effect, and is more secure for use.
Owner:SHANGHAI ZERUN BIOTECHNOLOGY CO LTD
Optimization method of Hib polysaccharide and protein binding process
InactiveCN102633898AIncrease binding rateHigh yieldAntibacterial agentsCarrier-bound antigen/hapten ingredientsTetanus toxoidsCarrier protein
The invention discloses an optimization method of Haemophilus Influenzae Serotype B polysaccharide and protein binding process, and the method comprises the following steps of: derivating polysaccharide, and preparing polysaccharide-TT (tetanus toxoid) conjugate. The invention provides a novel bridging substance combined by polysaccharide and vector protein, and the novel bridging substance can be used for more effectively performing binding reaction by changing a bridge molecule based on a conventional method, so that the binding rate of polysaccharide and vector protein is improved, the binding reaction time is shortened, the yield of qualified conjugate of the binding reaction is improved to a certain extent, the binding reaction has high repeatability, large-scale production is facilitated, and the production cost is reduced.
Owner:CHENGDU OLYMVAX BIOPHARM
Multivalent immunogenic composition
ActiveCN103394082AImprove securitySave the number of seedsBacterial antigen ingredientsViral antigen ingredientsHemagglutininTetanus toxoids
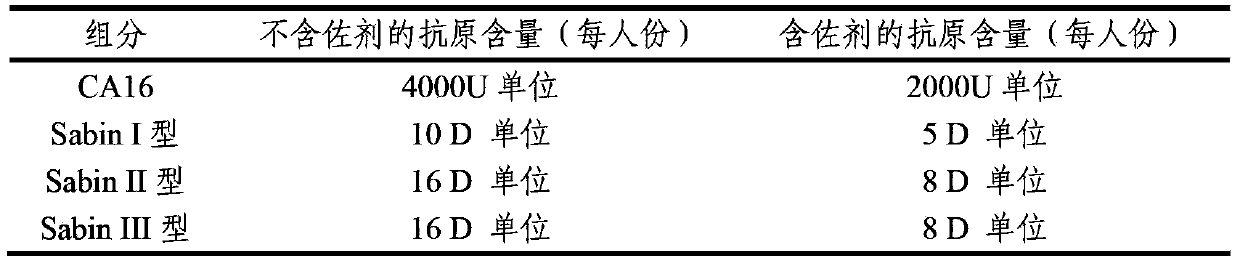
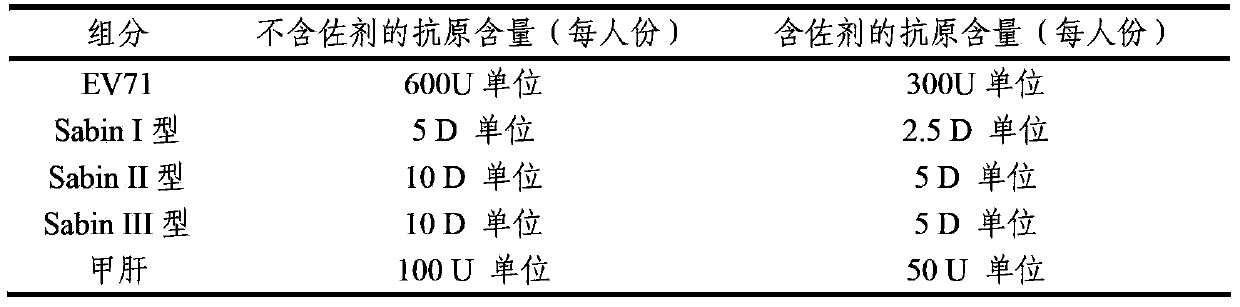
The invention provides a multivalent immunogenic composition, which includes an inactivated hepatitis A antigen and an inactivated poliovirus. The composition also can further include over one or two of a purified pertussis antigen, diphtheria toxoid, tetanus toxoid, filamentous hemagglutinin, Haemophilus influenzae type b polysaccharide, Neisseria meningitidis capsular polysaccharide, a hepatitis B virus antigen, enterovirus 71 and a coxsackievirus A16 antigen, and a physiologically acceptable carrier. The composition involved in the invention is employed to immunize the inoculated population in the form of a bivalent vaccine or more combined vaccines. Without reducing the immune effects of each immunizing antigen, the inoculation number of times can be reduced at the same time, and the time and human resources can also be saved.
Owner:SINOVAC BIOTECH
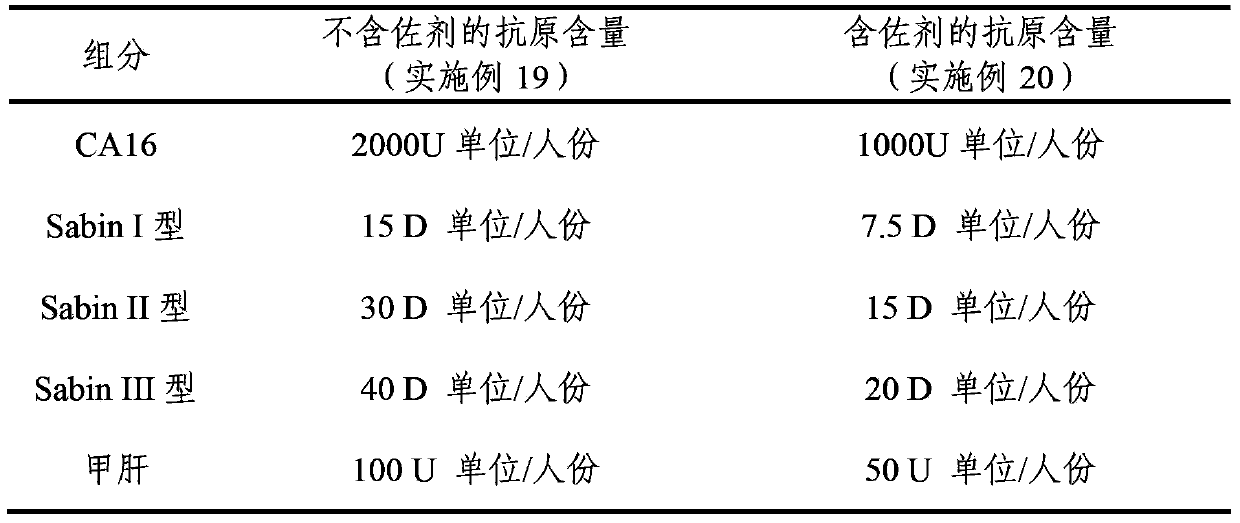
Multivalent immunogenic composition containing enterovirus antigens
ActiveCN103386126AImproving immunogenicityImprove securityBacterial antigen ingredientsViral antigen ingredientsHepatitis A AntigensTetanus toxoids
The invention provides a multivalent immunogenic composition containing enterovirus antigens. The composition comprises inactivated EV71 antigens and / or inactivated CA16 antigens, and inactivated polio antigens. The composition can further comprise antigens selected from hepatitis A antigens, hepatitis B antigens, acellular pertussis antigens, tetanus toxoid, diphtheria toxoid, Haemophilus influenzae type b capsular polysaccharide, and meningococcal polysaccharide antigens, as well as physiologically acceptable carriers combined with bacterial polysaccharide antigens. The invention also provides a preparation method of the composition. The composition can prevent invasion of a plurality of pathogens simultaneously without interference among the antigens, and the immunogenicity is no less than that of individually activated antigens. With the composition, vaccination processes are significantly simplified, and the vaccination efficiency is improved with reduced costs.
Owner:SINOVAC BIOTECH
Human vaccine for preventing hydrophobia and tetanus
ActiveCN102671194AIncreased potencyImprove immunityAntibacterial agentsBacterial antigen ingredientsRabiesTetanus toxoids
The invention discloses a human vaccine for preventing hydrophobia and tetanus, which belongs to the field of vaccines. The vaccine consists of a hydrophobia vaccine and a tetanus toxin, wherein the hydrophobia vaccine is obtained by inoculating a CDKHBP-1 strain onto a human diploid cell WI-38 and purifying. By using the hydrophobia vaccine and the tetanus toxin prepared with the preparation method disclosed by the invention together, immunity to hydrophobia and tetanus can be realized; and moreover, the tetanus toxin can be used for remarkably enhancing valence effect of the hydrophobia vaccine, plays a role in enhancing immunity, and is convenient for administration.
Owner:CHENGDU KANGHUA BIOLOGICAL PROD
Multivalent DTP-POLIO vaccines
InactiveCN101310769AEffective preventionSsRNA viruses negative-senseAntibacterial agentsTetanus toxoidsHaemophilus influenzae type
A multi-component vaccine composition is described comprising acellular pertussis vaccine components, diphtheria toxoid, tetanus toxoid and inactivated poliovirus. The composition also may contain a conjugate of a capsular polysaccharide of Haemophilus influenzae type b and tetanus toxoid or diphteria toxoid, which may be reconstituted from a lyophilized state by the other components of the vaccine. The administration of the multiple component vaccine results in no diminution in the immunogenicity of any component as a result of interference by other components of the vaccine.
Owner:CONNAUGHT LAB
Preparation of rabies vaccine for human being
InactiveCN103182081AGood immune effectImprove securityBacterial antigen ingredientsAntiviralsRabiesAdjuvant
The invention provides rabies vaccine for human being. The vaccine comprises an outer membrane segment of cracked rabies complete viral particles and tetanus toxoid, and the outer membrane segment comprises spike and matrix protein. Each dose of the rabies vaccine comprises 10 to 100 micrograms of viral protein; the dosage of the tetanus toxoid is 2 Lf / dose to 10 Lf / dose; and the dosage of aluminum phosphate adjuvant is 0.5mg / dose. Compared with the existing virus purified stock solution, the rabies vaccine for human being has the advantages that the content of impurity protein is greatly reduced, the immunogenicity and the potency are high, and the rabies vaccine is safer to use.
Owner:SHANGHAI ZERUN BIOTECHNOLOGY CO LTD
IPV-DPT vaccine
InactiveUS8753646B2Efficient productionAntibacterial agentsBacterial antigen ingredientsProtective antigenTetanus toxoids
The invention provides a process for producing a combined vaccine containing an inactivated Sabin strain of poliovirus, a Bordetella pertussis protective antigen, a diphtheria toxoid and a tetanus toxoid, the process including a step of producing a high-titer Sabin strain poliovirus. The inventive process for producing a combined vaccine, including a step of culturing, in the presence of from about 4 g / L to about 6 g / L of a microcarrier, Vero cells to be inoculated with a Sabin strain of poliovirus, is useful as a process for efficiently producing a combined vaccine containing an inactivated Sabin strain of poliovirus.
Owner:TAKEDA PHARMA CO LTD +1
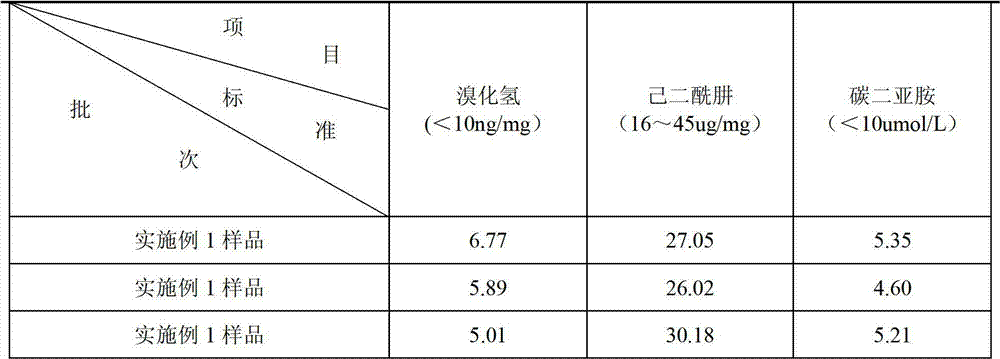
Method for producing tetanus toxoid raw liquid through chromatography purification method
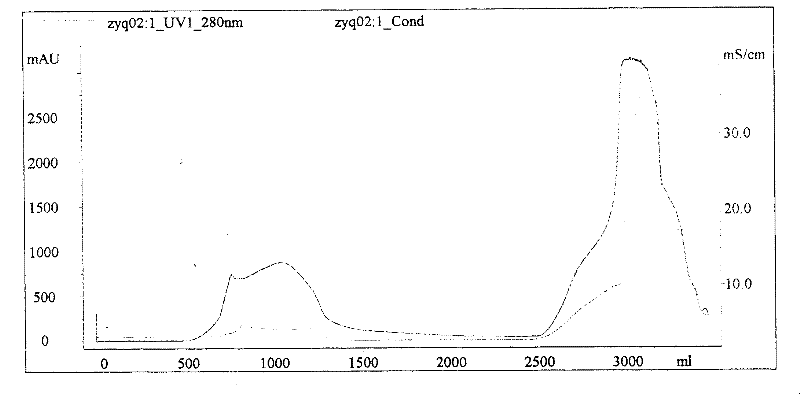
ActiveCN104327171AEasy to separateHigh purityDepsipeptidesPeptide preparation methodsBiotechnologyPurification methods
The present invention discloses a method for producing a tetanus toxoid raw liquid through a chromatography purification method, and belongs to the technical field of biology. The production method comprises: filtering a bacillus tetani culture solution, carrying out ultrafiltration concentration on the filtrate through a 30 KD ultrafiltration membrane to achieve 1 / 20-1 / 10 of the original volume, washing the concentrated solution, collecting, and carrying out ion exchange chromatography, hydrophobic chromatography, primary membrane filtration, detoxification, ultrafiltration and secondary membrane filtration on the concentrated solution to obtain the tetanus toxoid raw liquid. The method of the present invention has advantages of simple process, easy operation, high production efficiency and low cost. The tetanus toxoid raw liquid produced by the method of the present invention has advantages of single tetanus toxoid component and high purity so as to obtain the good immunogenicity and reducing the occurrence of adverse events following immunization.
Owner:CHENGDU OLYMVAX BIOPHARM
Single dose immunization against tetanus toxin cation dextran microspheres and preparation method thereof
InactiveCN101869704AReduce the number of injectionsImprove vaccination coverageAntibacterial agentsBacterial antigen ingredientsControlled releaseTetanus
The invention relates to the filed of pharmaceutical preparation, in particular to single dose immunization against tetanus toxin cation dextran microspheres and a preparation method thereof. The single dose immunization against tetanus toxin cation dextran microspheres are prepared by carrying tetanus toxin after electrostatic interaction on cation hydroxyethyl acrylate dextran microspheres. The tetanus toxin controlled release microspheres can reduce the injection frequency of tetanus vaccine, improve the vaccination coverage and reduce the drop-out rate, thereby effectively preventing tetanus and providing a single dose tetanus toxin controlled release vaccine preparation with long-term effect and realizing the whole course immunity by one injection.
Owner:CHINA PHARM UNIV
Fine purification method of polyvalent anti-snake venom freeze-dry blood serum
InactiveCN101385855BEasy to storeImprove securityAntibody ingredientsDermatological disorderFreeze-dryingTetanus toxoids
The invention discloses a method for refining a polyvalent ASV (anti-snake venom) lyophilized serum, pertaining to an immune serum containing antibody which aims at least two kinds of antigens. The serum takes the venom of Ancistrodon acutus, Agkistrodon halys, Pit-vipers, bamboo snakes, adders and the like as the immune antigen which is inoculated into the bodies of animals after degerming and dialysis, and then titer immune serum after purification is used for collecting the antibody which is then desalted and lyophilized; mice are used for evaluating the LD50 of the virulence of the venom and the titer of the ASV serum; the antibody inoculated into the bodies of the animals is one of the venom, toxoid or snake venom or the composition thereof, and glutaraldehyde or formaldehyde is used for toxin detoxication; before immunity, anti-tetanic toxoid condensed antibody for human beings is used for carrying out minim gradual immunization on the animals, or an Emuade adjuvant is used for immunization purpose. The polyvalent ASV lyophilized serum complies with the biological product standards upon the tests and inspections of purity and specific activity, safety, pyrogen, hypersusceptibility, dissolution and the like; moreover, the serum can cure sufferers who are bitten by vipers with the advantages of high purity, low foreign protein, easy preservation and quick dissolving capacity.
Owner:KUNMING INST OF MILITARY MEDICINE CHENGDU MILITARY REGIONS CENT FOR DISEASE CONTROL & PREVENTION
Fully humanized anti-tetanus bispecific antibody as well as construction method and application thereof
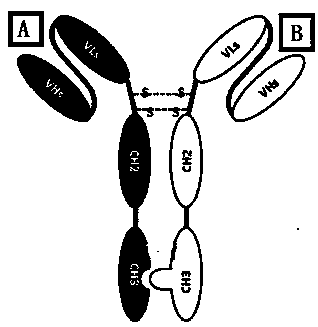
ActiveCN111116742AReduce allergiesInhibit bindingAntibacterial agentsAntibody mimetics/scaffoldsPassive ImmunizationsTetanus toxoids
The invention provides a fully humanized anti-tetanus bispecific antibody. The bispecific antibody includes a single-stranded unit A and a single-stranded unit B, both the single-stranded units A andB contain a single-stranded variable fragment (scFv), a human IgG1 hinge region and an Fc fragment, wherein the scFv of the single-stranded unit A has specific binding ability to a region centered onTrp 1289 of tetanus toxoid, and the scFv of the single-stranded unit B has specific binding ability to a region centered on Arg1216 of the tetanus toxoid. The invention also provides a construction method and application of the bispecific antibody. After neutralization experiments in mice, results show that the antibody has a neutralizing protection effect, and the bispecific antibody can be usedas a diagnostic reagent to detect the quality of a tetanus toxoid antigen, and can replace the tetanus antitoxin, equine tetanus immunoglobulin or human tetanus immunoglobulin to be used for passive immunization.
Owner:SHANGHAI SERUM BIOTECH
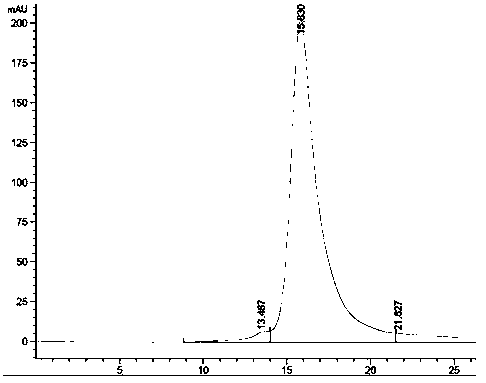
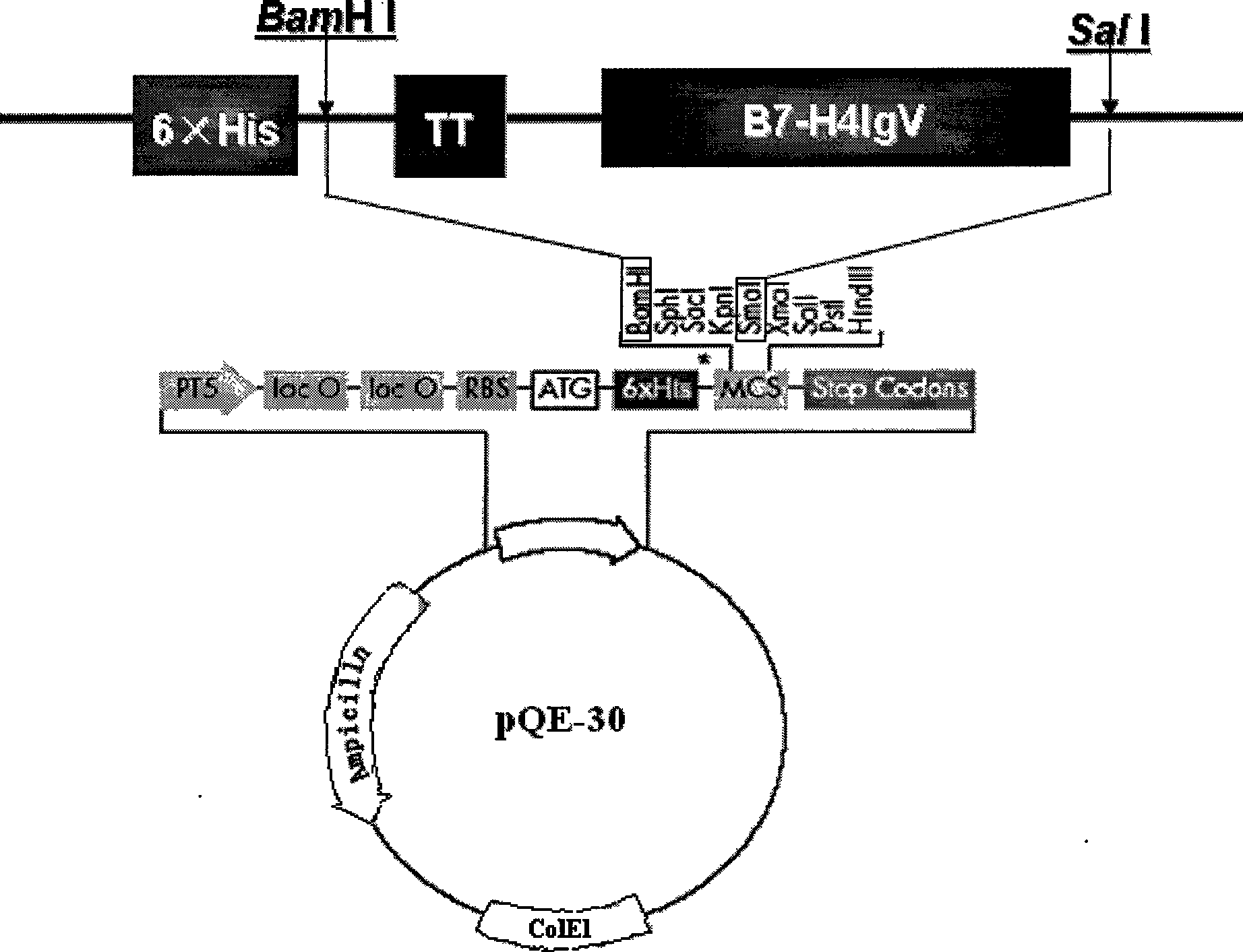
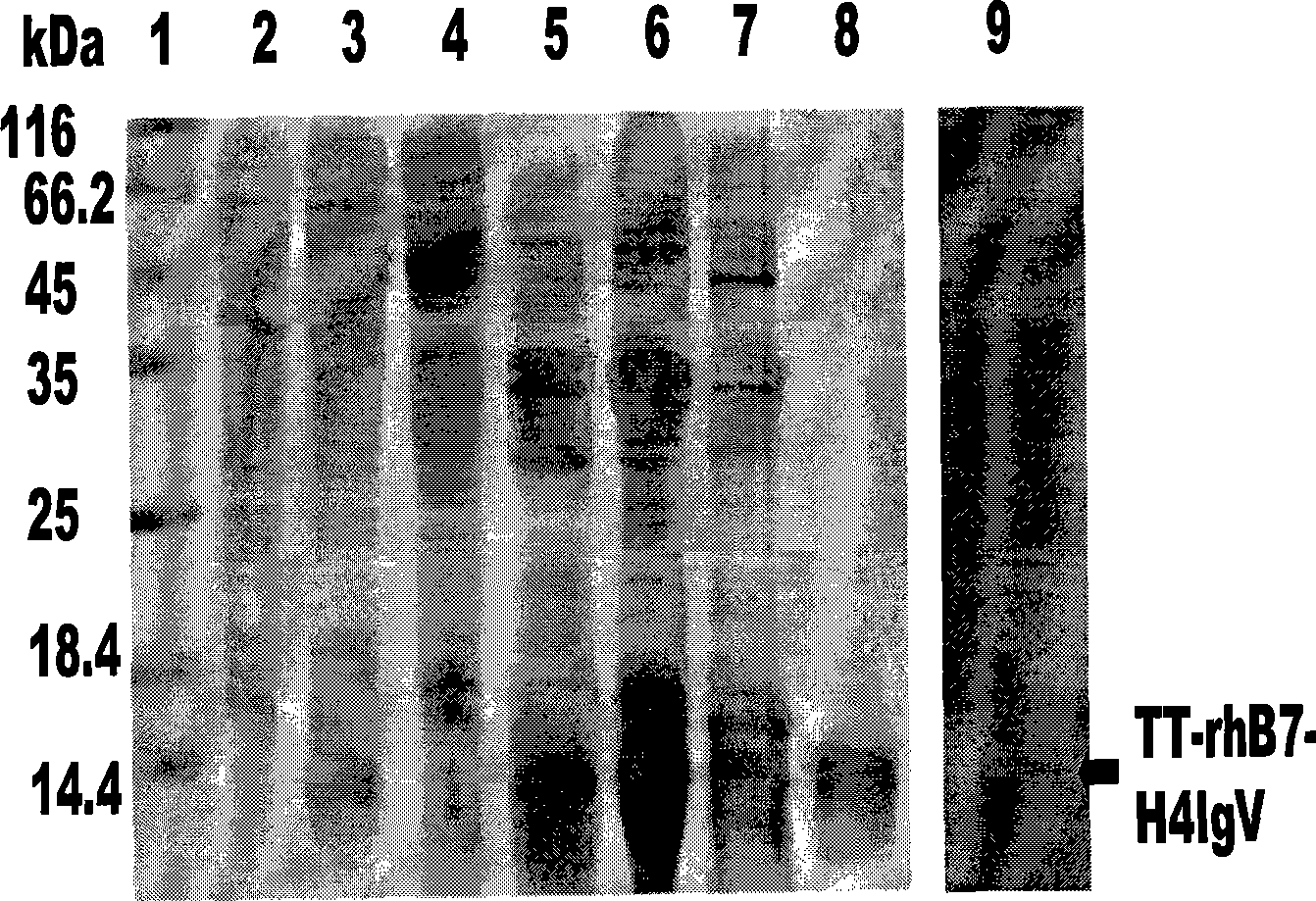
Fusion protein TT-B7-H4IgV as well as preparation method and application thereof
InactiveCN101544697AHigh binding activityPeptide/protein ingredientsPeptide preparation methodsWestern blotTetanus toxoids
The invention relates to a fusion protein TT-B7-H4IgV as well as a preparation method and an application thereof. The B7-H4IgV is a variable region IgV of a B7-H4 molecule extracellular region, TT is a T auxiliary cell epitope peptide or a tetanus toxoid epitope, the N end of the fusion protein TT-B7-H4IgV is connected with 6 His residues, a connection mode is as follows: 6*His-TT-B7-H4IgV, and the fusion protein TT-B7-H4IgV is applied to prepare anti-tumor vaccines which take the B7-H4 as a target spot. The fusion protein TT-B7-H4IgV is established by selecting the B7-H4 as a target spot and taking the TT as antigen presentation and is in accordance with prospects by being identified by a Western blot and checking orders by the Western blot; a purified fusion protein TT-hB7-H4IgV is used for immunizing small Kunming mice, and the biological activity of antiserums is measured by ELISA and flow cytometry so as to prove that a B7-H4 antiserum and the B7-H4 have good combination activity, thus the purified fusion protein TT-hB7-H4IgV can induce organisms to generate anti-B7-H4 polyclonal antibodies.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
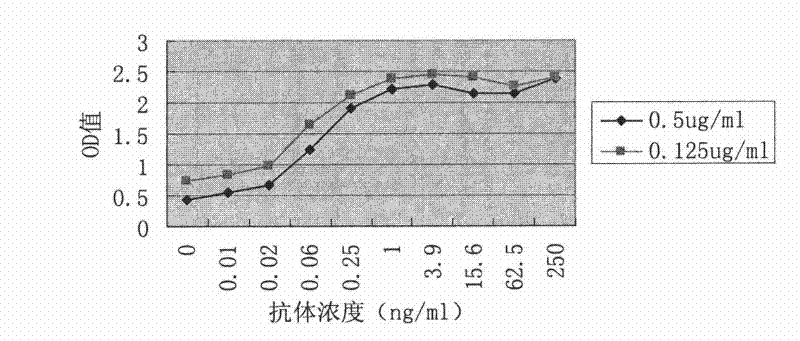
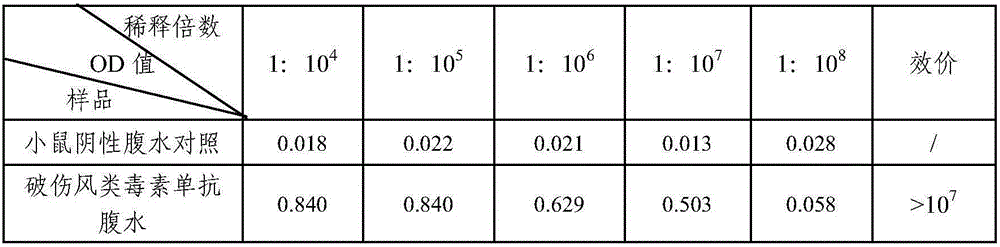
Tetanus toxoid monoclonal antibody and application thereof
ActiveCN105039262AImproving immunogenicityMeet the integration needsAntibacterial agentsMicroorganism based processesTetanus toxoidsImmunologic function
The invention relates to a tetanus toxoid monoclonal antibody and application thereof, and belongs to the field of immunology. Mouse spleen cells and mouse myeloma cells are fused with one another after mice are subjected to tetanus toxoid immunization, tetanus-toxoid-resistant specific monoclonal antibody hybridoma cell strains can be generated by means of screening, and a preservation number of the hybridoma cell strains is CGMCC No.10590. The tetanus toxoid monoclonal antibody and the application have the advantages that the potency of the monoclonal antibody secreted by the hybridoma cell strains can reach 10<7>, an affinity constant of the monoclonal antibody is 1.24*10<9>M<-1>, and the tetanus toxoid monoclonal antibody can be used for preparing tetanus toxoid antigen detection reagent kits, also can be applied to detecting tetanus vaccine production procedures by the aid of enzyme-linked immunosorbent assay and detecting the content of toxoid in finished vaccine and has a broad application prospect and high market value.
Owner:SINOVAC RES & DEV
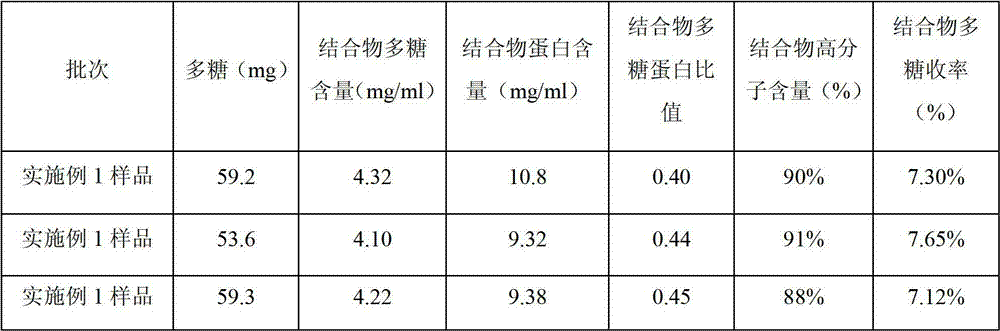
Haemophilus influenzae type b (Hib) polysaccharide and refined tetanus toxoid coupling process
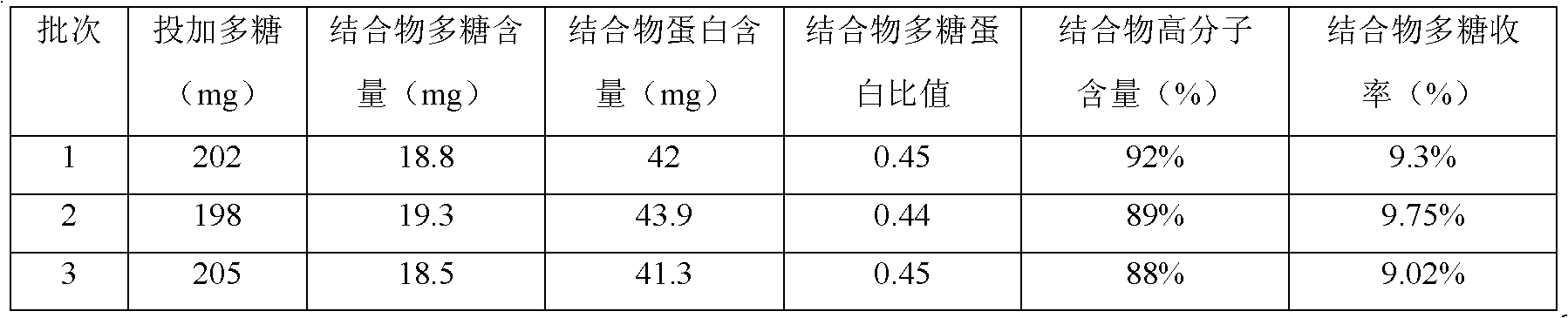
ActiveCN102861330AReduce dosageReduced Chances of ContaminationAntibacterial agentsCarrier-bound antigen/hapten ingredientsTetanus toxoidsHaemophilus influenzae type
The invention discloses a haemophilus influenzae type b (Hib) polysaccharide and refined tetanus toxoid coupling process which includes steps of Hib polysaccharide-AH derivant generation and Hib polysaccharide-tetanus toxoid (TT) combination preparation. The Hib polysaccharide-AH derivant generation includes steps of A1, polysaccharide dissolving, A2, polysaccharide activation, A3, antidiuretic hormone addition for connection and A4, CNBr and antidiuretic hormone (ADH) removing. The Hib polysaccharide-TT combination preparation includes the following steps: B1, adding TT solution, B2, adding carbodiimide (EDAC), B3, removing the EDAC to obtain polysaccharide-TT carbodiimide; and B4, separating and pourifying the polysaccharide-TT carbodiimide, eluting, degerming and filtering to obtain the polysaccharide-TT combination. The Hib polysaccharide and refined tetanus toxoid coupling process has the advantages of improving working efficiency, saving time and labor and simultaneously saving using amount of NaCl solution and reduces the contaminated rate of samples compared with dialysis adopted by the traditional process.
Owner:CHENGDU OLYMVAX BIOPHARM
Method for preparing group A meningococcal capsular polysaccharide conjugate vaccine
InactiveCN105056228AAvoid steric hindranceMolecular chain lengthAntibacterial agentsCarrier-bound antigen/hapten ingredientsTetanus toxoidsCarrier protein
The invention relates to a method for preparing group A meningococcal capsular polysaccharide conjugate vaccine. The method includes the following steps that group A meningococcal capsular polysaccharide is diluted, activated and coupled into a group A meningococcal polysaccharide-sebacic dihydrazide derivative together with sebacic dihydrazide; then the group A meningococcal polysaccharide-sebacic dihydrazide derivative and a tetanus toxoid solution are subjected to a catalytic reaction through carbodiimide, so that group A meningococcal capsular polysaccharide and carrier protein conjugate is obtained. According to the method, the sebacic dihydrazide is used as a coupling agent in the preparation process of the group A meningococcal conjugate vaccine; compared with adipic dihydrazide, the sebacic dihydrazide has two more methylenes on carbon chains, molecular chains are longer, the steric hindrance of biomacromolecules can be better resisted, and the conjugation yield is increased.
Owner:CHENGDU OLYMVAX BIOPHARM
Preparation method for absorbed acellular diphtheria, tetanus and pertussis combined vaccine
InactiveCN102526717AAntibacterial agentsBacterial antigen ingredientsTetanus toxoidsDiptheria toxoid
The invention relates to a method using stain cultivation toxoid to prepare diphtheria, tetanus and pertussis vaccine, which uses modes of ammonium sulfate salting out, density gradient centrifugation and glutaraldehyde detoxification to prepare acellular pertussis raw liquid containing effective antigen of PT and FHA. acellular diphtheria, tetanus and pertussis combined vaccine for preventing diphtheria, tetanus and pertussis can be finally prepared by preparing the pertussis raw liquid and refining and purifying to obtain mixture of diphtheria toxoid and tetanus toxoid.
Owner:CHENGDU KANGHUA BIOLOGICAL PROD
Acellular pertussis combined vaccine and preparation method thereof
InactiveCN104707134AClear ingredientsLittle side effectsAntibacterial agentsBacterial antigen ingredientsHemagglutininSide effect
The invention discloses an acellular pertussis combined vaccine and a preparation method thereof and belongs to the technical field of production and preparation of vaccines. The acellular pertussis combined vaccine is prepared from the following raw material components: 5-40Mu g of pertussis toxin, 5-40Mu g of filamentous hemagglutinin, 2-10Mu g of pertussis adhesin, 10-25lf of diphtheria toxoid, 4-10lf of tetanus toxoid, 1.0-2.0mg / ml of aluminium hydroxide and 7.5-9.5g / L of sodium chloride. The preparation method of the acellular pertussis combined vaccine comprises the following steps: preparing monovalent vaccine original fluids, mixing and diluting. The acellular pertussis combined vaccine is clear and definite in ingredients, quality control can be easily realized, the side effect is small, and the safety is high; and the preparation method of the acellular pertussis combined vaccine is simple to operate, convenient in preparation and low in cost, so that the acellular pertussis combined vaccine is applicable to industrial mass production.
Owner:CHENGDU OLYMVAX BIOPHARM
METHOD OF PRODUCING MENINGOCOCCAL MENINGITIS VACCINE FOR NEISSERIA MENINGITIDIS SEROTYPES A, C, Y, and W-135
InactiveUS20080318285A1Yield maximizationYield minimizationAntibacterial agentsBacteriaDiseaseSynechococcus
Owner:REDDY JEERI R
Combined vaccine for adsorbing Diphtheria, tetanus and acellular pertussis-Sabin inactivated poliovirus and preparation thereof
InactiveCN102178949ASimplified and expanded immunization programsReduce the number of vaccinationsAntibacterial agentsAntiviralsDiseaseTetanus toxoids
The invention provides combined vaccine for adsorbing Diphtheria, tetanus and acellular pertussis-Sabin (DTaP-sIPV) inactivated poliovirus and preparation thereof. The DTaP-sIPV is characterized in that each 100ml of the combined vaccine comprises the following components: 400-1800ug (PN) of acellular pertussis (AP) stock solution, 300-700Lf of tetanus toxoid (TT), 1000-2500Lf of diphtheria toxoid (DT), 126-154mg of Al(OH)<3>, 3000-6000DU of sIPV I, 5700-7100DU of sIPV II, 4500-9000DU of sIPV III, 765-935mg of NaCl, 0-600mg of 2-phenoxyethanol and the balance of H<2>O. Compared with the existing products, the DTaP-sIPV has the advantages of higher biological safety, better side reaction and the like; and the DTaP-sIPV has the beneficial effects of preventing a plurality of target diseases, reducing inoculating needles, simplifying immunization programs, improving inoculation rate, reducing opportunity of cross infection, being popular with a majority of parents and children, saving various expenses and facilitating smooth promotion of immunization plan.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Method of detecting auto-antibodies from patients suffering from rheumatoid arthritis, a peptide and an assay kit
A liposomal composition, preferably a vaccine, comprising liposomes formed of liposome forming compounds, containing coentrapped polysaccharide antigen and T-cell dependent protein carrier, such as tetanus toxoid or diphtheria toxin modified to render it non-toxic. The invention is of use in the production of vaccines against Haemophilus influenzae, Streptococcus pneumoniae or Neisseria meningitidis.
Owner:STICHTING VOOR DE TECH WETENSCHAPPEN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com