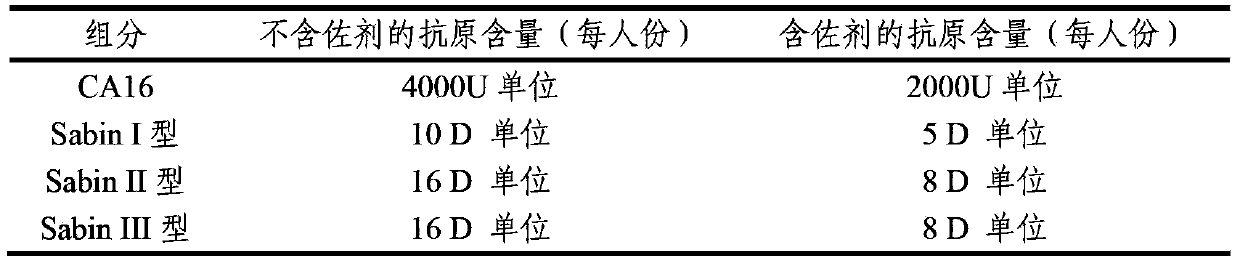
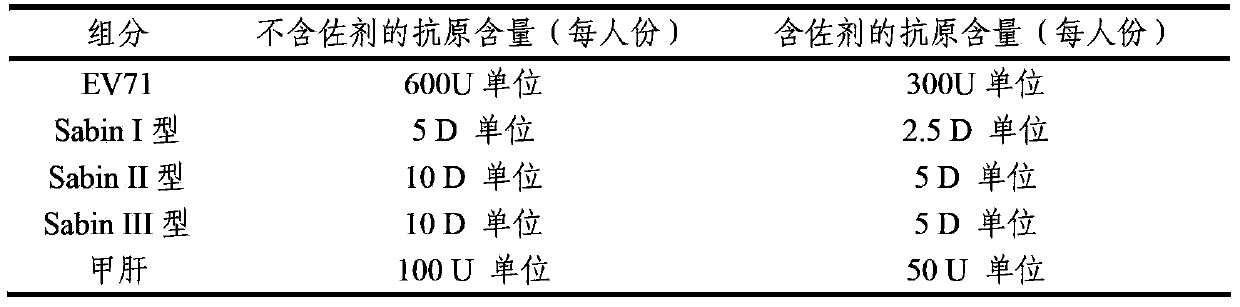
Multivalent immunogenic composition containing enterovirus antigens
A technology of immunogenicity and viral antigen, applied in the direction of viral antigen components, antiviral agents, drug combinations, etc., can solve time-consuming and labor-intensive problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] The cultivation of embodiment 1EV71 virus
[0069] The EV71 virus strain (preservation number is CGMCC No.3544) was adapted to grow in 10-layer cell factory Vero cells and continuously passaged, using MEM medium plus 10% (v / v) calf serum, and cultured at 37±1°C After culturing in the box for 5-7 days, change the MEM medium and add 2% (v / v) calf serum, inoculate EV71 virus according to the MOI of 0.001, culture at 33±1°C for 15 days, observe the cytopathic changes every day, when the cytopathic changes reach 50 % or more, the virus liquid was harvested, and after clarification and filtration, the virus titer value was detected by the CCID50 method. The EV71 virus harvest liquid can be in the range of 6-8LgCCID50 / ml.
Embodiment 2
[0070] Inactivation and purification of embodiment 2EV71 virus
[0071] Add formaldehyde solution (1:1000-1:10000) (v / v) to the EV71 virus harvest solution described in Example 1, so that the final concentration of free formaldehyde is 100-200 μg / ml, and inactivate at 36.5°C±1°C On day 13, a virus inactivation solution was prepared. Centrifuge the inactivation solution at 30,000rpm in a sucrose density gradient for 6-12 hours, collect the zone where the virus is located, and desugar through membrane bag ultrafiltration. After molecular sieve chromatography, equilibrate with 0.01M PBS (pH7.0) buffer, then continue to elute with 0.01M PBS (pH7.0), collect virus peaks, and finally obtain EV71 virus antigen stock solution after sterile filtration.
Embodiment 3
[0072] The cultivation of embodiment 3CA16 virus
[0073] The CA16 virus strain (preservation number is CGMCC No.5371) was adapted to grow in 10-layer cell factory Vero cells and continuously passaged, using MEM medium plus 10% calf serum, and cultured in a 37±1°C incubator for 5- After 7 days, change the MEM medium and add 2% calf serum, inoculate CA16 virus according to the MOI of 0.001, and continue to culture in the incubator at 37±1°C, observe the cytopathic changes every day, and harvest the virus liquid when the cytopathic changes reach more than 50% , After clarification and filtration, the virus titer value was detected by the CCID50 method. CA16 virus harvest liquid can be in the range of 6-8LgCCID50 / ml.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com