Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "Hepatitis B Antigens" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hepatitis B virus e antigen testing corpuscle, preparation and application thereof
ActiveCN101251540AWide detection rangeReduce sensitivityAnalysis by material excitationAntigenAnti hbe
The invention relates to a diagnosing reagent for hepatitis B, disclosing detection grains for e antigens of the hepatitis B virus, which are of the luminous grains coated by anti-HBE antibodies. The invention also discloses preparation and application for the detection grains for e antigens of the hepatitis B virus; moreover, the invention further discloses an outside-body diagnosis reagent box for detecting e antigens of the hepatitis B in a blood serum sample of human beings as well as a method for utilizing the light excitation chemiluminescence principle to quantitatively and qualitatively detect e antigens of the hepatitis B virus. The reagent box of the invention can be jointly used to diagnose the individual acute or chronic hepatitis B together with other blood serums and clinic information, and screen the hepatitis B for women in the perinatal period so as to judge the risk of newborn babies contaminating the hepatitis B.
Owner:BEYOND DIAGNOSTICS (SHANGHAI) CO LTD
Application of forsythiaside A in preparing hepatitis B therapeutic drug
The invention discloses an application of forsythiaside A in preparing a hepatitis B therapeutic drug. An anti-hepatitis B virus test in vitro of the forsythiaside A shows that the forsythiaside A can effectively inhibit hepatitis B antigen and hepatitis B viruses, which is characterized by the following aspects: using a HepG2.2.2.15 cell model, TC50 cytotoxicity of the forsythiaside A in vitro is equal to 739mu g / mL; IC50 of inhabitation on S antigen is equal to 149.4mu g / mL; IC50 of inhabitation on e antigen is equal to 139.6mu g / mL; and IC50 of inhabitation on a cell HBV-DNA is equal to 56.3mu g / mL.
Owner:SHANDONG NEWTIME PHARMA
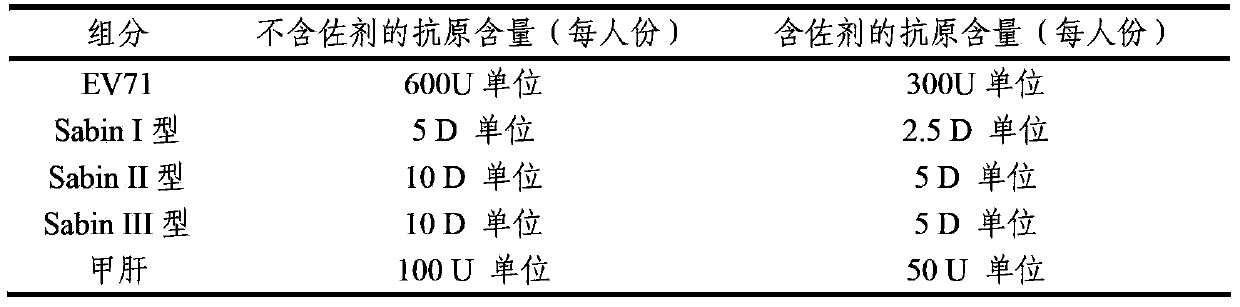
Multivalent immunogenic composition containing enterovirus antigens
ActiveCN103386126AImproving immunogenicityImprove securityBacterial antigen ingredientsViral antigen ingredientsHepatitis A AntigensTetanus toxoids
The invention provides a multivalent immunogenic composition containing enterovirus antigens. The composition comprises inactivated EV71 antigens and / or inactivated CA16 antigens, and inactivated polio antigens. The composition can further comprise antigens selected from hepatitis A antigens, hepatitis B antigens, acellular pertussis antigens, tetanus toxoid, diphtheria toxoid, Haemophilus influenzae type b capsular polysaccharide, and meningococcal polysaccharide antigens, as well as physiologically acceptable carriers combined with bacterial polysaccharide antigens. The invention also provides a preparation method of the composition. The composition can prevent invasion of a plurality of pathogens simultaneously without interference among the antigens, and the immunogenicity is no less than that of individually activated antigens. With the composition, vaccination processes are significantly simplified, and the vaccination efficiency is improved with reduced costs.
Owner:SINOVAC BIOTECH
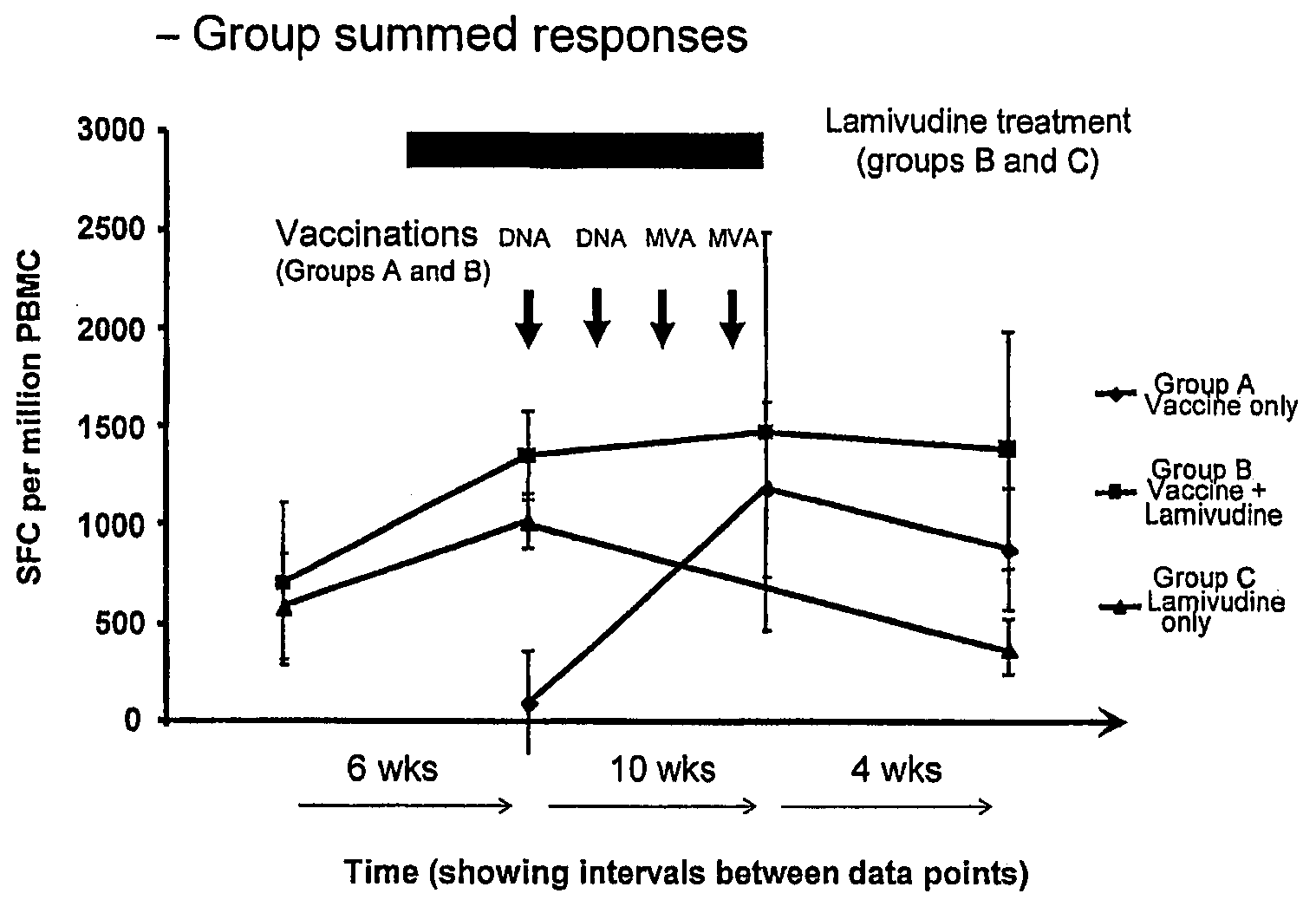
Compositions for inducing an immune response against hepatitis B
The present invention is directed to a method of inducing an immune response against a hepatitis B antigen (e.g., an antigen from a hepatitis B virus) in a mammal, which comprises administering to the mammal a priming composition (e.g., a DNA plasmid), comprising a source of one or more epitopes of the hepatitis B target antigen; and a boosting composition, comprising a source of one or more eptiopes of the hepatitis B target antigen (e.g., a non-replication or replication-impaired poxvirus such as MVA), wherein at least one epitope of the boosting composition is identical to an epitope of the priming composition. The present invention also is directed to a method of inducing an immune response against a hepatitis B antigen (e.g., an antigen from a hepatitis B virus) in a mammal, which comprises administering to the mammal a priming composition (e.g., a DNA plasmid), comprising a source of one or more epitopes of the hepatitis B target antigen. In addition, the present invention is directed to compositions for use in the methods of the present invention.
Owner:OXXON THERAPEUTICS LTD
Novel anti- hepatitis B virus medicine compounds-lamivudine and protocatechuic acid
InactiveCN101224209AGood curative effectReduce the risk of drug resistanceOrganic active ingredientsDigestive systemHepatitis B Virus AntigenSide effect
The invention provides an anti-HBV medicine combination which has more remarkable treatment effect and safety and no toxic and side effect and can evidently increase the negative conversion ratio of HBSAg. The invention takes HepG2.2.15 as a target cell and adds lamivudinevir, protocatechuic acid and the compatibility preparation of lamivudinevir and protocatechuic acid with a proper concentration to cell culturing medium. The cell is cultured by a regular method and HBsAg and HBeAg secreted by the cell is detected by ELISA method, HBV DNA content is measured by fluorescent quantitation PCR, HBV resistance effect of the drug is judged by depression rate calculation method to determine the medical treatment effect on HBV and the preparation proportion of medicine combination. The proportion of lamivudinevir to protocatechuic acid is between 1 to 50 and 1 to 100 (mass ratio) and generally the dosage of lamivudinevir is 0.05-5Mug and the protocatechuic acid is 5-500 Mug. The medicine combination can evidently improve the depression effect of the lamivudinevir on Hepatitis B antigen and reduce the toxic and side effect of the lamivudinevir when being used separately.
Owner:HUBEI UNIV
Drug for treating hepatitis B
InactiveCN101574494APromote repairImprove microcirculationDigestive systemMammal material medical ingredientsSalvia miltiorrhizaSemen
A drug for treating hepatitis B is characterized by being made from the mixture of bupleurum, gardenia, radix scutellariae, isatis root, salvia miltiorrhiza, herba artemisiae capillaris, rhubarb, rhizoma coptidis, cyperus rotundus, desmodium, rhizoma sparganii, endothelium corneum gigeriae galli, Chinese angelica, dried rhizome of rehmannia, radix aucklandiae, dried tangerine peel, sealwort, malt, hawthorn, medicated leaven, semen plantaginis, poria, turtle shell, date and gentian. The drug has simple process, the effects of adjusting immunologic function, inducing the occurrence of interferon, inhibiting and eliminating hepatitis B virus, promoting hepatocyte repair, improving liver microcirculation and promoting the reliable treating effect of hepatitis B antigen negative conversion, short treating period, fast effect, no relapse after cure and low cost. The drug is handed down from the ancestor over 200 years before and cures tens of thousands of patients with the effective rate up to 100 percent.
Owner:张淑芬
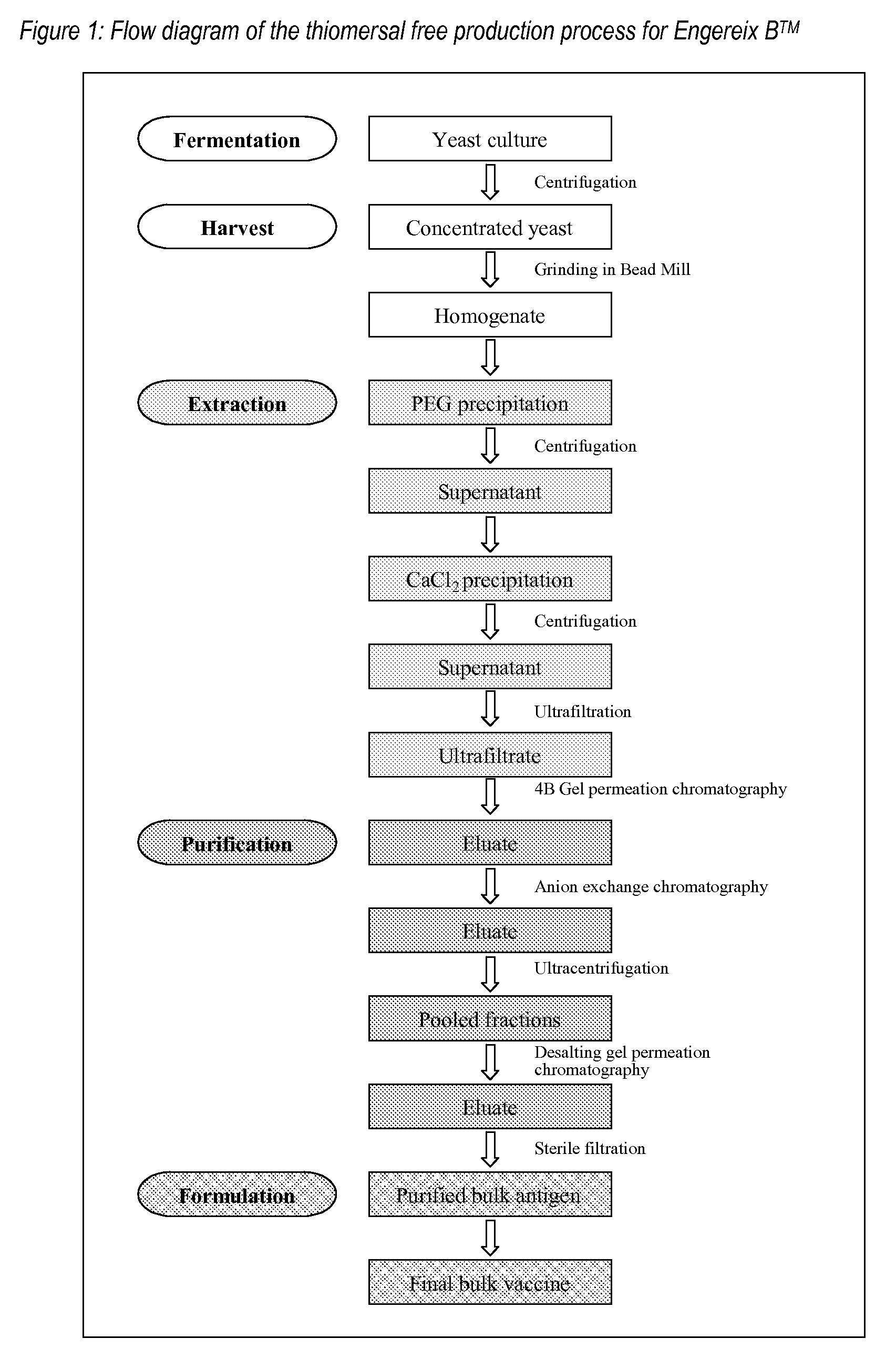
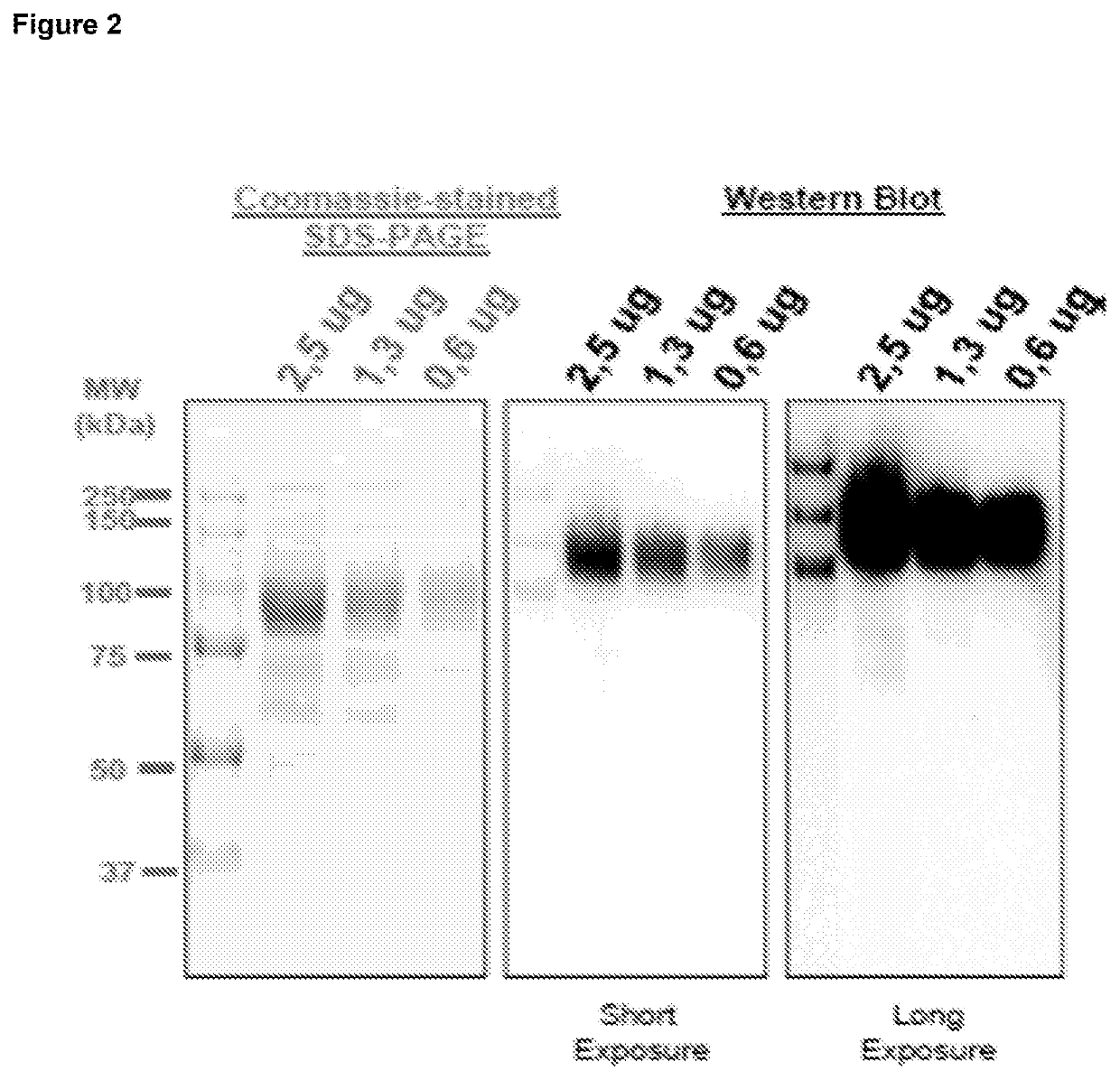
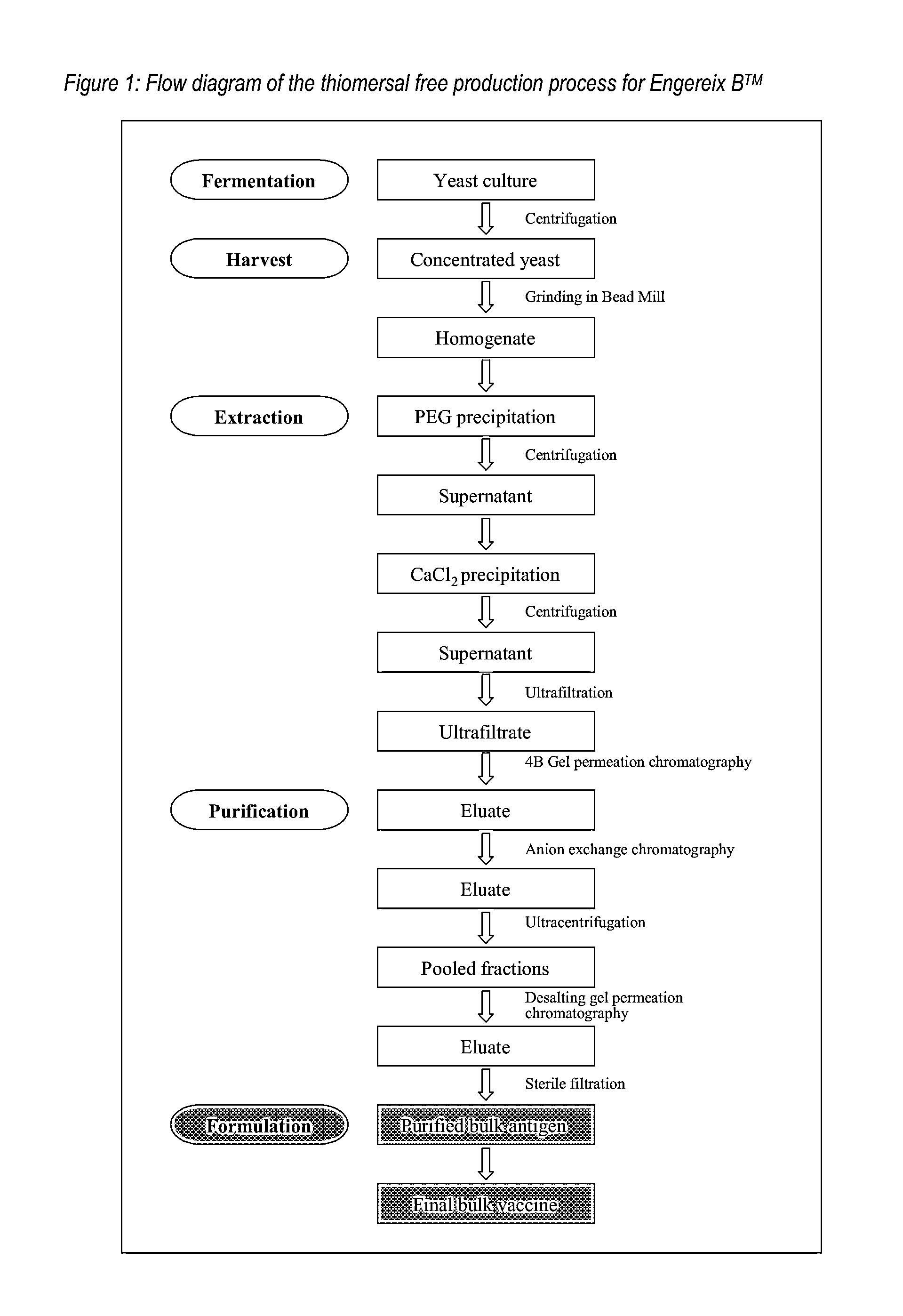
Purification of HBV antigens for use in vaccines
InactiveUS20060159705A1Suitable for useAntibacterial agentsSsRNA viruses positive-senseAntigenCysteine thiolate
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Recombinant Plasmid and Method for Expressing Hepatitis B Viral Antigens and Virions in Vivo
Disclosed is a recombinant plasmid for expressing hepatitis B viral antigens in vivo, comprising an adeno-associated virus (AVV) vector and a replication-competent hepatitis B virus genome fragment. Mice hydrodynamically injected with the recombinant plasmid of the present invention show persistent expression of hepatitis B viral antigens for more than 6 months in the hepatocytes, thus a immuno-competent mouse model for persistent expression of hepatitis B antigens and also for human chronic hepatitis B virus infection is established, which can be applied in evaluation and elucidation of mechanism of chronic hepatitis and anti-viral drug discovery research.
Owner:NAT TAIWAN UNIV
Recombinant plasmid and method for expressing hepatitis B viral antigens and virions in vivo
Disclosed is a recombinant plasmid for expressing hepatitis B viral antigens in vivo, comprising an adeno-associated virus (AVV) vector and a replication-competent hepatitis B virus genome fragment. Mice hydrodynamically injected with the recombinant plasmid of the present invention show persistent expression of hepatitis B viral antigens for more than 6 months in the hepatocytes, thus a immuno-competent mouse model for persistent expression of hepatitis B antigens and also for human chronic hepatitis B virus infection is established, which can be applied in evaluation and elucidation of mechanism of chronic hepatitis and anti-viral drug discovery research.
Owner:NAT TAIWAN UNIV
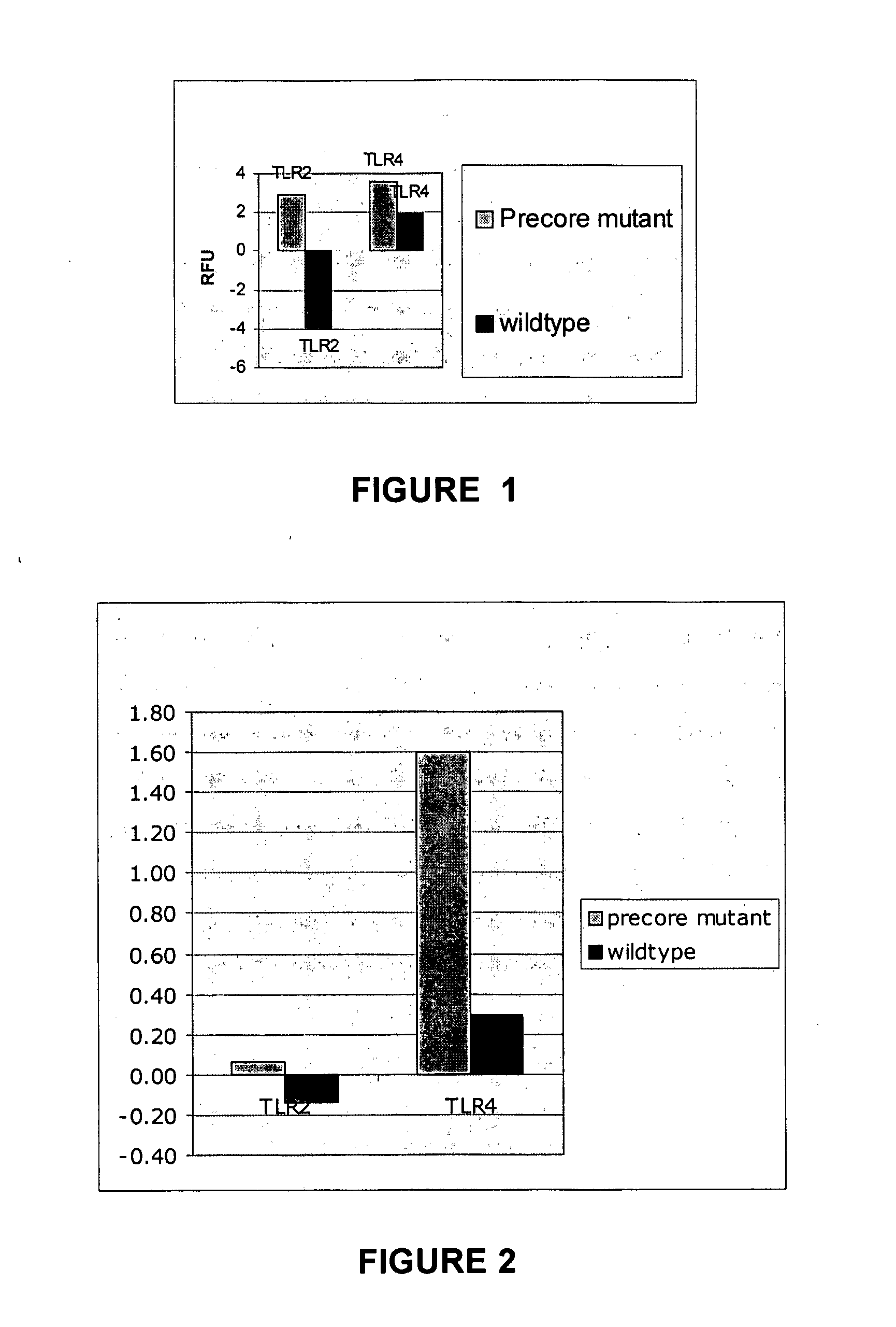
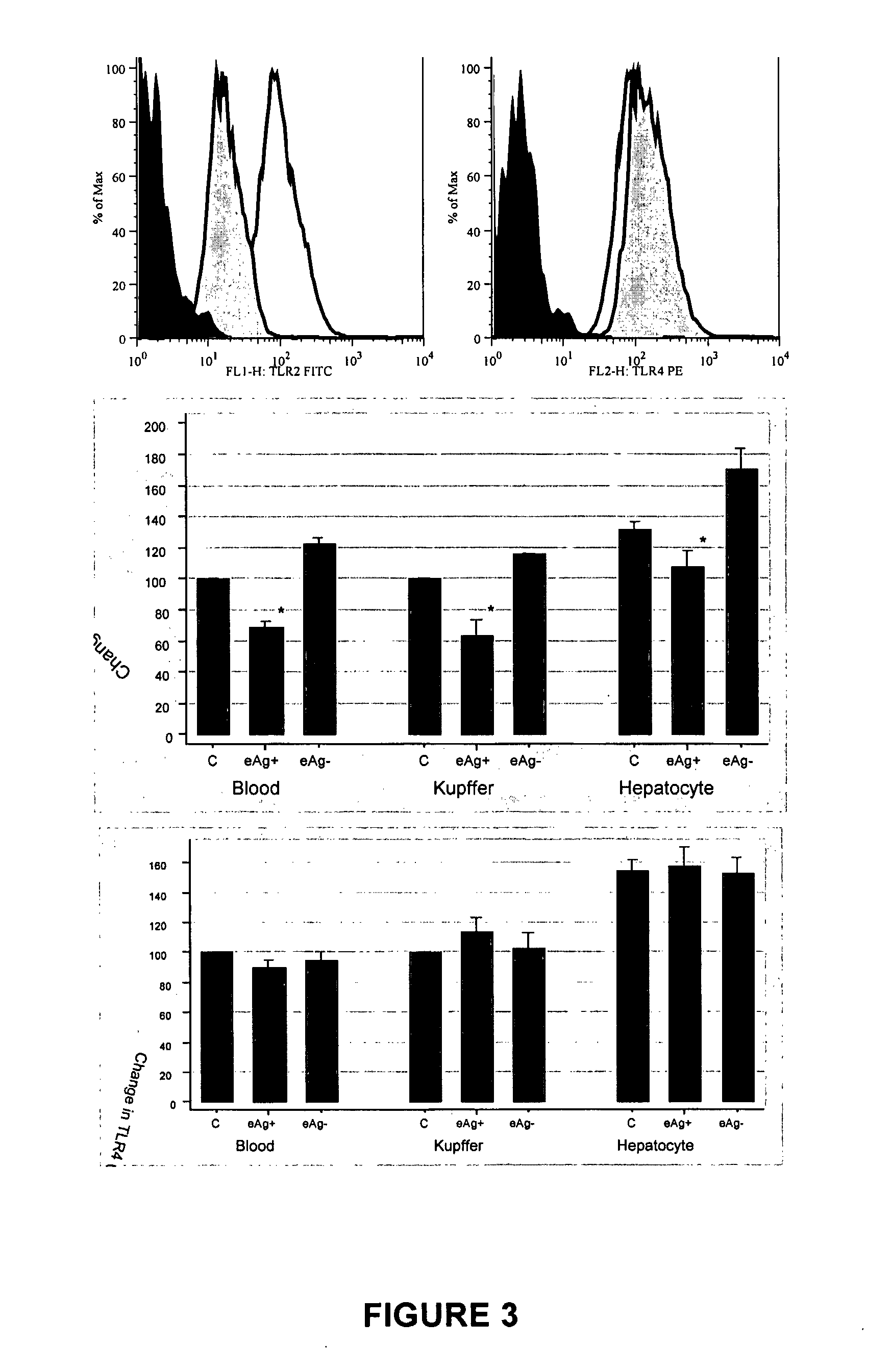
Therapeutic, Prophylactic and Diagnostic Agents for Hepatitis B
InactiveUS20070264284A1Promote infectionPeptide/protein ingredientsMicrobiological testing/measurementHepatitis b e antigenDiagnostic agent
The present invention provides regulation of expression of toll-like receptors by the hepatitis B (HBV) pre-core protein, or its extracellular expression product the hepatitis B E antigen (HbeAg). Compounds regulating such expression have use in the treatment and prophylaxis of HBV infection in animal. The invention also provides methods for diagnosing HBV and agents useful in diagnostic protocols. The present invention further contemplates methods for monitoring disease states in humans and other animal species, including animal models, and providing an indication of the subject for infection by HBV, or development of other diseased states.
Owner:MELBOURNE HEALTH +1
Hepatitis B virus e antigen quantitative detection kit, preparation method and detection method thereof
InactiveCN105572358AHigh detection sensitivityStrong specificityMaterial analysisAntigenHepatitis B immunization
The present invention discloses a hepatitis B virus e antigen detection kit, which comprises a hepatitis B e antigen calibration substance, an anti-HBe monoclonal antibody coated plate, europium labeled anti-HBe monoclonal antibody, an assay buffer liquid, a fluorescence enhanced liquid and a concentrated washing liquid. The present invention further discloses a preparation method of the kit, and a method for detecting hepatitis B virus e antigen by using the kit. According to the present invention, the kit has advantages of high detection sensitivity, good specificity, simple operation, no pollution, and low cost, can be used for quantitative detection of the HBeAg content in human serum / plasma, and provides precise reference for the clinical hepatitis B individualized management treatment program.
Owner:SUZHOU SYM BIO LIFESCI CO LTD
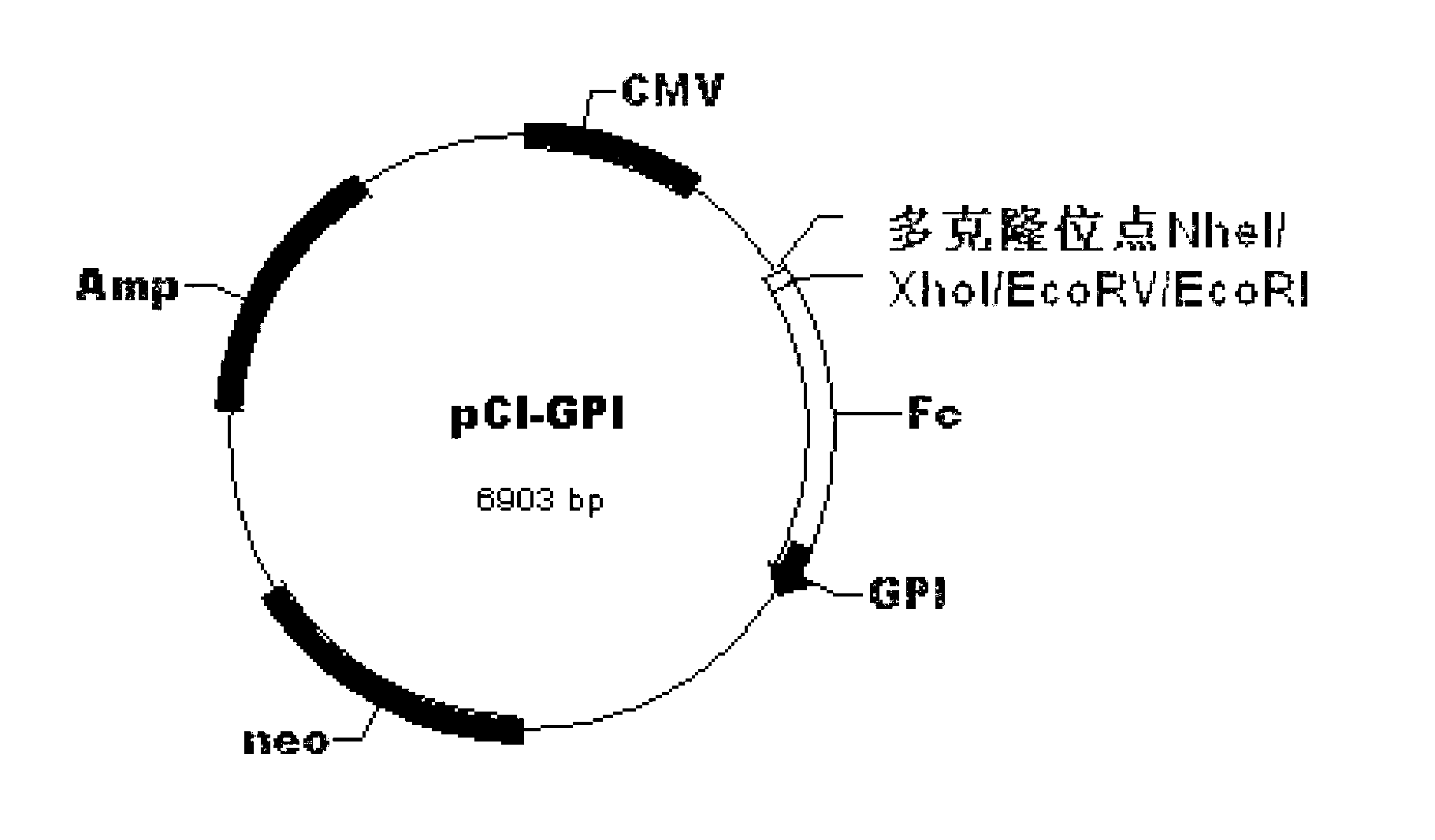
Replicon DNA (Deoxyribonucleic Acid) vaccine for treating chronic hepatitis B
InactiveCN103239717AEnhance antigen presentationHigh activityAntiviralsAntibody medical ingredientsNucleotideChronic hepatitis
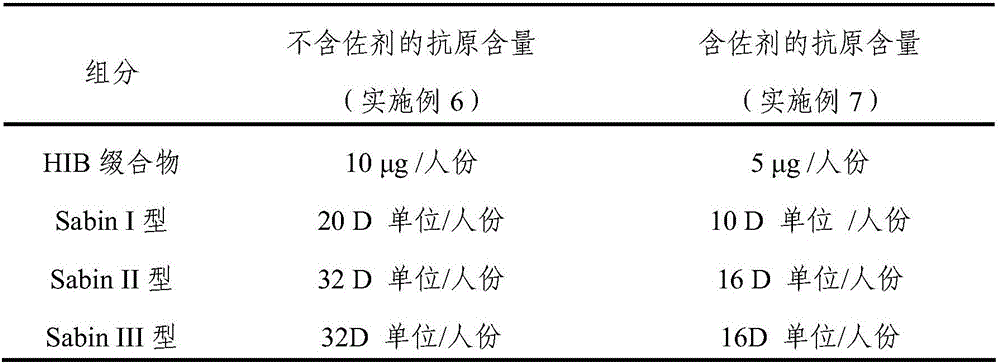
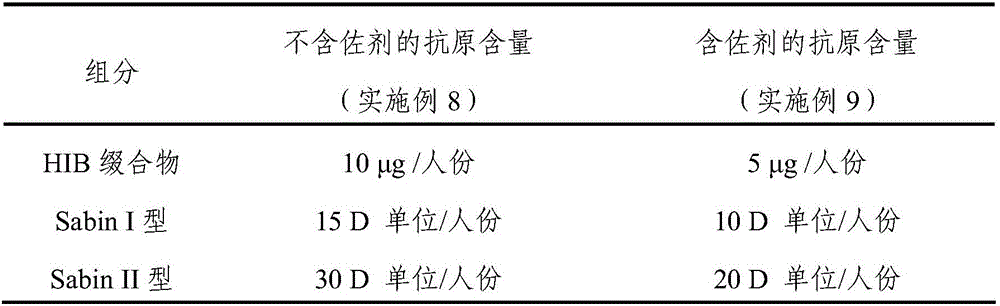
The invention discloses a replicon DNA (Deoxyribonucleic Acid) vaccine for treating chronic hepatitis B. The replicon DNA vaccine is a recombinant replicon DNA vaccine vector plasmid carrying a composite hepatitis B antigen gene. The composite hepatitis B antigen gene is named as HBV-CS, the nucleotide sequence of the composite hepatitis B antigen gene is shown as the sequence 1 in a sequence table; a vector of the replicon DNA vaccine is named as pSVK. The recombinant replicon DNA vaccine vector plasmid which sequentially carries a fusion gene FL-CS, a human IgGFc-segment gene, a GPI gene and a GM-CSF gene and a B7.1 gene from upstream to downstream and is constructed by using the pSVK as a starting gene is named as pSVK-HBVA, and the nucleotide sequence of the recombinant replicon DNA vaccine vector plasmid is shown as the sequence 7 in the sequence table.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
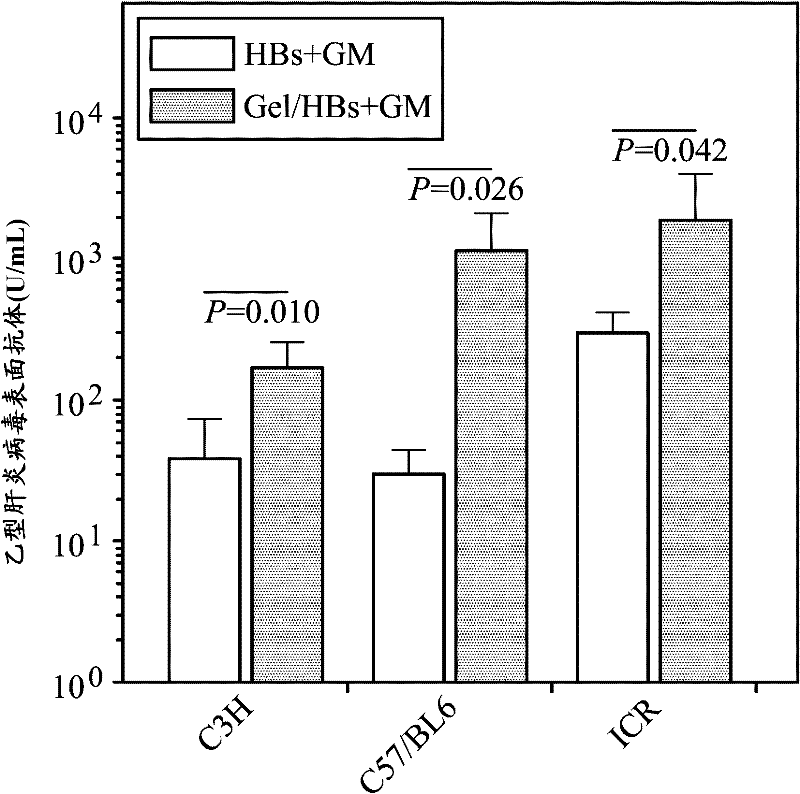
Thermosensitive hepatitis B vaccine
A thermosensitive hepatitis B vaccine is provided. The thermosensitive hepatitis B vaccine includes an aqueous phase solution comprising a biodegradable thermosensitive hydrogel copolymer; at least one antigen of hepatitis B virus (HBsAg); and at least one bioactive substance. The thermosensitive hepatitis B vaccine of the disclosure is particularly suitable for being applied in the patients, which are low responsive or non-responsive to conventional hepatitis B vaccine, for enhancing the induction of cell-mediated immune responses and overcoming the HBsAg non-responsiveness.
Owner:IND TECH RES INST
Adsorbing material of hepatitis B antigen protein and preparation method of material
ActiveCN103111270BLow chance of rejectionReduce the risk of immune rejectionOther chemical processesSolid sorbent liquid separationSynthesis methodsSepharose
The invention relates to a specific adsorbing material for removing hepatitis B virus protein in blood plasma, and a preparation method of the adsorbing material, and discloses a high polymer material which is prepared by coupling sepharose gel with gene-modified sodion taurocholic acid cotransporting polypeptide. The preparation method of the material comprises the steps of reacting the sepharose gel, which is taken as a carrier matrix, with a bi-glycidyl ether coupling reagent to obtain an active carrier, and subsequently, conducting coupling reaction on the active carrier with the sodion taurocholic acid cotransporting polypeptide which is fused with polypeptide tags. The synthesis method of the material is simple and convenient, the process route is short and the preparation is safe; and the material has the characteristics of strong specificity, high adsorption rate to the hepatitis B virus protein, good regenerability, harmlessness and no heat source reaction, and can be applied to clinical adsorption treatment to eliminate the hepatitis B virus protein in blood plasma in a specificity mode.
Owner:WUHAN RUIFA MEDICAL DEVICES CO LTD
Immunogenic composition and preparation method thereof
InactiveCN106075428AAvoid the risk of virulence reversionImprove securityAntibacterial agentsSsRNA viruses positive-senseAntigenVaccination
The invention provides an immunogenic composition and belongs to the technical field of preparation of biological products. The immunogenic composition contains an inactivated poliomyelitis antigen and a type b haemophilus influenzae proteoglycan protein conjugate. The immunogenic composition can further contain a hepatitis b antigen and a physiologically acceptable carrier. The invention further provides a preparation method of the immunogenic composition. The immunogenic composition provided by the invention can be used for preventing infection of a plurality of types of pathogens at the same time; the antigens do not have a mutual interference phenomenon; the corresponding immunogenicity is not reduced when being compared with the immunogenicity excited by a single antigen; when the immunogenic composition provided by the invention is used, a vaccine inoculation program can be remarkably simplified, the inoculation efficiency is improved and the cost is reduced.
Owner:SINOVAC RES & DEV
Drug for treating hepatitis B
A drug for treating hepatitis B is characterized by being made from the mixture of bupleurum, gardenia, radix scutellariae, isatis root, salvia miltiorrhiza, herba artemisiae capillaris, rhubarb, rhizoma coptidis, cyperus rotundus, desmodium, rhizoma sparganii, endothelium corneum gigeriae galli, Chinese angelica, dried rhizome of rehmannia, radix aucklandiae, dried tangerine peel, sealwort, malt,hawthorn, medicated leaven, semen plantaginis, poria, turtle shell, date and gentian. The drug has simple process, the effects of adjusting immunologic function, inducing the occurrence of interferon, inhibiting and eliminating hepatitis B virus, promoting hepatocyte repair, improving liver microcirculation and promoting the reliable treating effect of hepatitis B antigen negative conversion, short treating period, fast effect, no relapse after cure and low cost. The drug is handed down from the ancestor over 200 years before and cures tens of thousands of patients with the effective rate upto 100 percent.
Owner:张淑芬
A Therapeutic Vaccine for Hepatitis B Virus (HBV) using the HBV PreS1 and/or PreS2, and/or S-HBsAg regions of the HBV envelope protein
ActiveUS20200345838A1Viral antigen ingredientsAntibody mimetics/scaffoldsHepatitis B immunizationProtein composition
Compositions including a CD180 binding ligand and a linked Hepatitis B antigen and their use are disclosed. The Hepatitis B antigen includes Hepatitis B virus pre-S1 and / or pre-S2 region of the HBV envelope protein (HBVpreS1 / S2Ag), L-HBsAg, MHBsAg, S-HBsAg, or antigenic fragments or mutants thereof.
Owner:UNIV OF WASHINGTON +1
Method for building hepatitis B e-antigen negative mouse model
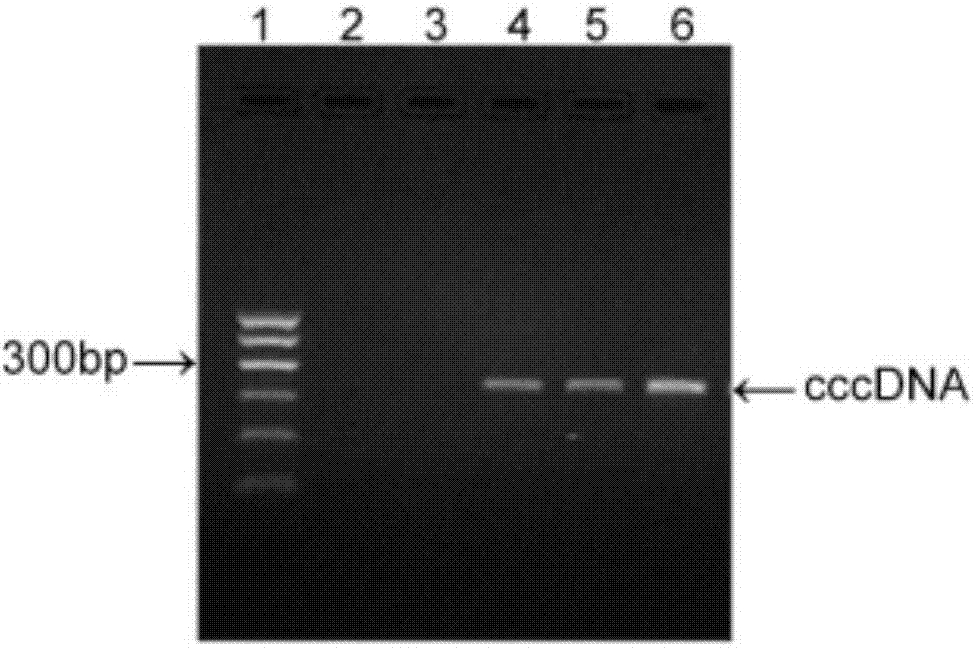
InactiveCN106868045AAddressing the absence of e-antigen-negative animal modelsVector-based foreign material introductionAnimal husbandryAntigenEnzyme digestion
The invention discloses a method for building a hepatitis B e-antigen negative mouse model. The method comprises the following steps: 1) extracting serum DNA of a patient diagnosed with HBeAg negative HBV infection, and carrying out PCR amplification by using a primer pair HBV-F / HBV-R; 2) sequencing a PCR product, and redesigning a specific PCR amplification primer pair to the sequence for PCR amplification; 3) cloning the PCR product to a pEASY-Blunt Zero carrier; 4) extracting plasmids, carrying out enzyme digestion, recovering and purifying fragments, and carrying out cyclization and purification to obtain HBVe negative cccDNA; and 5) injecting the cccDNA into a mouse, thereby building the hepatitis B e-antigen negative mouse model. According to the invention, the hepatitis B e-antigen negative mouse model is successfully built for the first time, which is highly significant for study on the mechanism of the HBeAg negative hepatitis infection and study on medication effects.
Owner:CHONGQING MEDICAL UNIVERSITY +1
Vaccines for protection from and treatment of alzheimer's disease
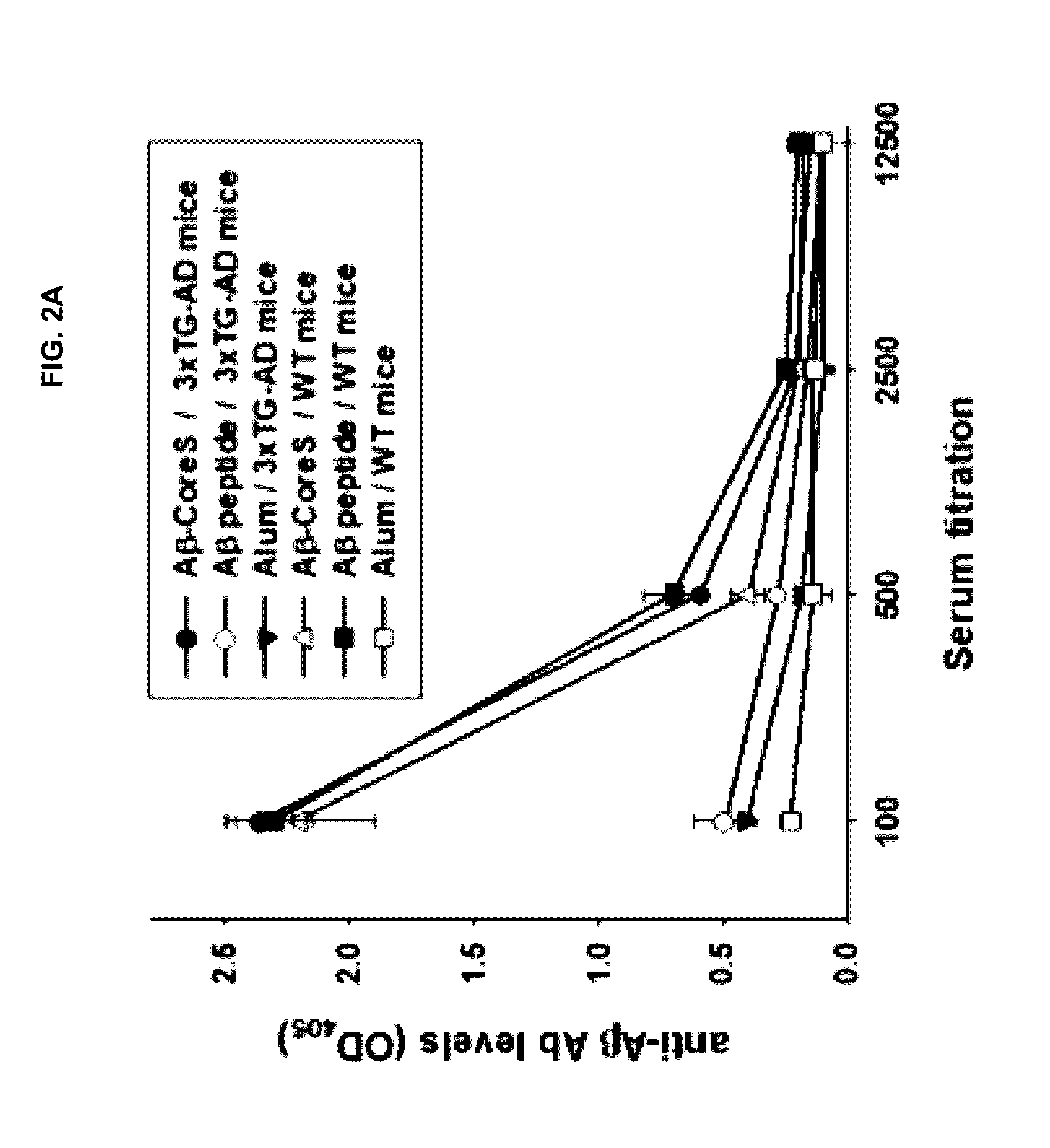
ActiveUS20170042990A1Alleviate Alzheimer's DiseaseImprove survivalNervous system antigen ingredientsWhole-cell/virus/DNA/RNA ingredientsAntigenDisease
Disclosed herein are DNA-based vaccines against amyloid β peptide for use in treating and alleviating Alzheimer's Disease and related conditions. A DNA construct comprising DNA encoding one or more amyloid β peptides, such as amino acids 1-11 of Aβ, and DNA encoding a hepatitis B antigens, is administered with an adjuvant or by electroporation. The vaccine can also be formulated using a fusion protein expressed by the disclosed DNA, in combination with an adjuvant.
Owner:CYVAX
Reagent and method for in-vitro diagnosis of hepatitis B virus antibody
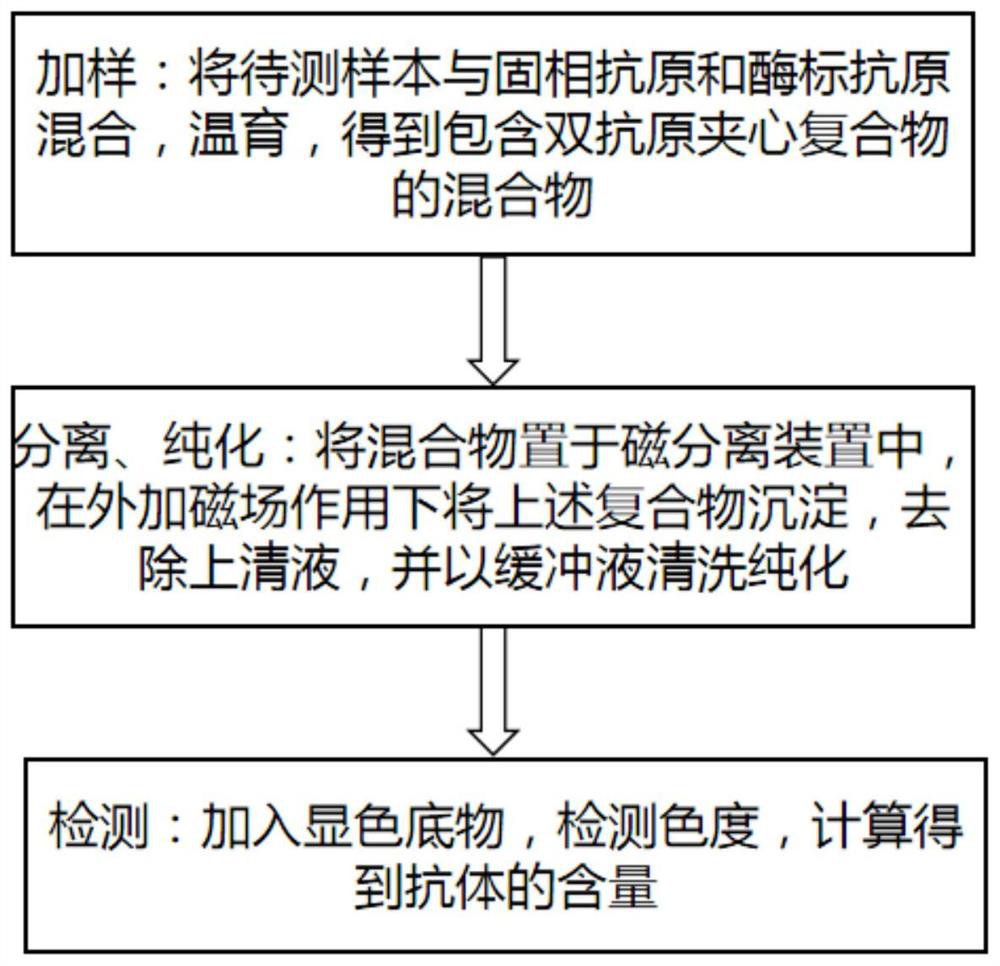
ActiveCN113358878AHigh antigen purityStrong specificityChemiluminescene/bioluminescenceBiological testingAntigenHorse radish peroxidase
The invention discloses a reagent for hepatitis B virus antibody in-vitro diagnosis, the reagent comprises a solid phase antigen, an enzyme-labeled antigen and a chromogenic substrate, the solid phase antigen comprises a solid phase carrier and an antigen coated on the surface of the solid phase carrier, the enzyme-labeled antigen comprises an enzyme for labeling the antigen and an enzyme-labeled antigen, the antigen is hepatitis B antigen recombinant protein, the antigens in the solid-phase antigen and the enzyme-labeled antigen are the same hepatitis B antigen recombinant protein, the solid-phase carrier comprises magnetic microspheres, the enzyme comprises horse radish peroxidase, and the chromogenic substrate comprises TMB. The invention also discloses a method for preparing the reagent and a method for identifying the hepatitis B virus antibody by using the reagent. The reagent is used for detecting the hepatitis B virus antibody, has the advantages of good specificity, high sensitivity, accurate result, good repeatability and strong clinical applicability, and can accurately reflect the content index of the hepatitis B virus antibody in a human body.
Owner:深圳市辰纳生物科技有限公司
Therapeutic vaccine for Hepatitis b virus (HBV) using the HBV PreS1 and/or PreS2, and/or s-HBsAg regions of the HBV envelope protein
ActiveUS11389532B2Antibody mimetics/scaffoldsViral antigen ingredientsHepatitis B immunizationProtein composition
Owner:UNIV OF WASHINGTON +1
Preparation method of hepatitis B antibody fragment, hepatitis B antibody fragment, kit and application
The invention belongs to the technical field of medical instruments, and particularly discloses a preparation method of a hepatitis B antibody fragment, the hepatitis B antibody fragment, a kit and an application, wherein the preparation method comprises the following steps: adding pepsin, dithioerythritol and 2-mercaptoethanol into a hepatitis B antibody solution, and incubating for the first time; and adding glycine, and purifying after secondary incubation. The preparation method at least has one of the following beneficial effects: when the hepatitis B antibody fragment prepared by the preparation method of the hepatitis B antibody fragment provided by the invention is applied to detection of hepatitis B antigen, the generation of false positive can be remarkably reduced, so that the accuracy of a detection result is improved.
Owner:普十生物科技(北京)有限公司
Application of leonurine in preparation of anti-hepatitis B virus drugs
The invention belongs to the technical field of medicines, and relates to a compound aiming at hepatitis B virus surface antigen (HBsAg), in particular to a leonurine hydrochloride (Leonurine) (CAS: 24697-74-3) aiming at hepatitis B virus surface antigen (HBsAg). The compound is a monomer extracted and chemically synthesized from traditional Chinese medicine motherwort, and tests show that the compound can effectively reduce the HBsAg in vitro supernatant and has an effect on reducing the hepatitis B e antigen (HBeAg) in vitro supernatant. The compound leonurine can be used for targeted reduction of extracellular HBsAg and HBeAg, and provides support for research and development of therapeutic drugs and methods for effective reduction of HBsAg and HBeAg in HBV persistent infection.
Owner:FUDAN UNIV
Vaccines for protection from and treatment of alzheimer's disease
ActiveUS9433665B2Alleviate Alzheimer's DiseaseImprove survivalGenetic material ingredientsNervous system antigen ingredientsAntigenAdjuvant
Owner:CYVAX
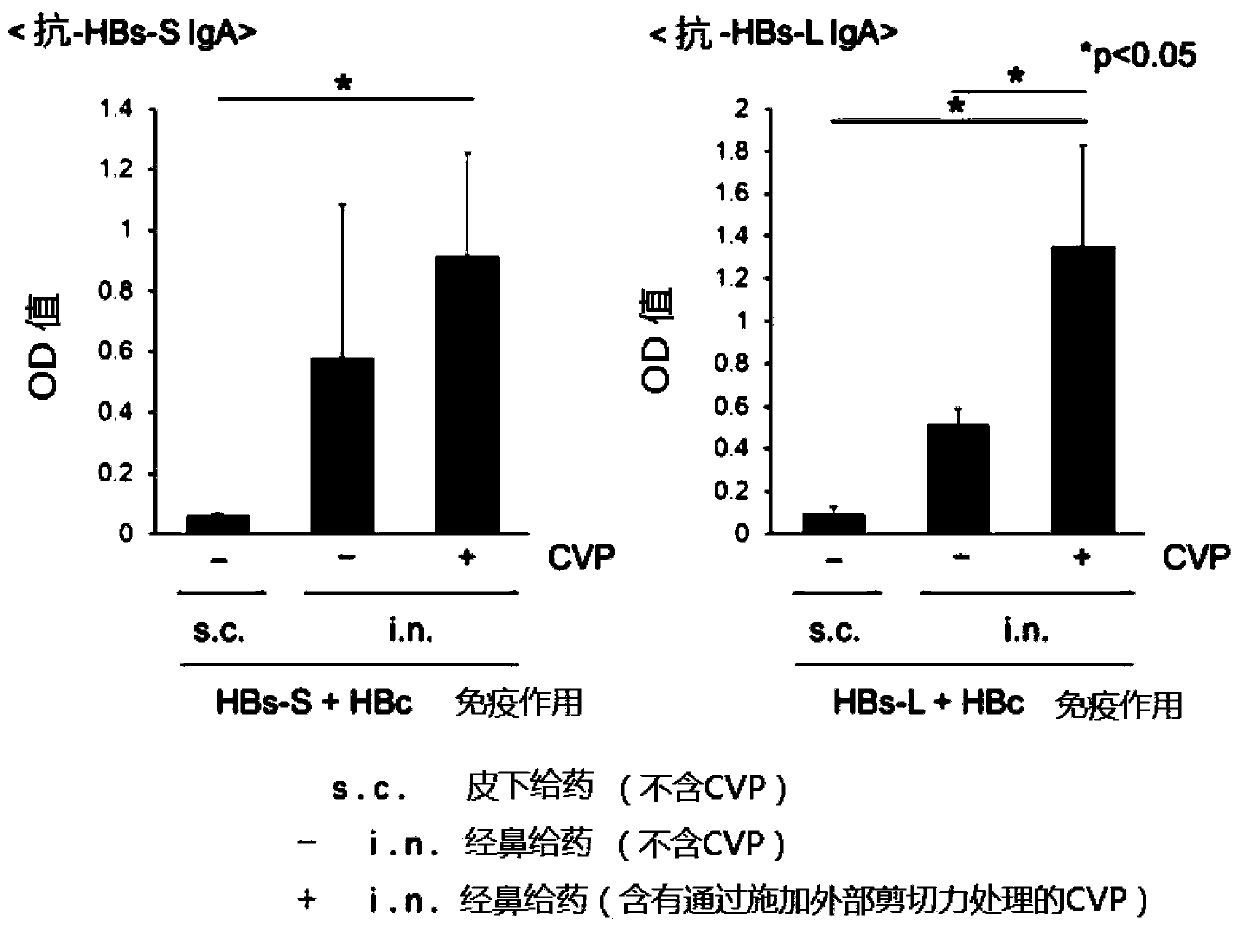
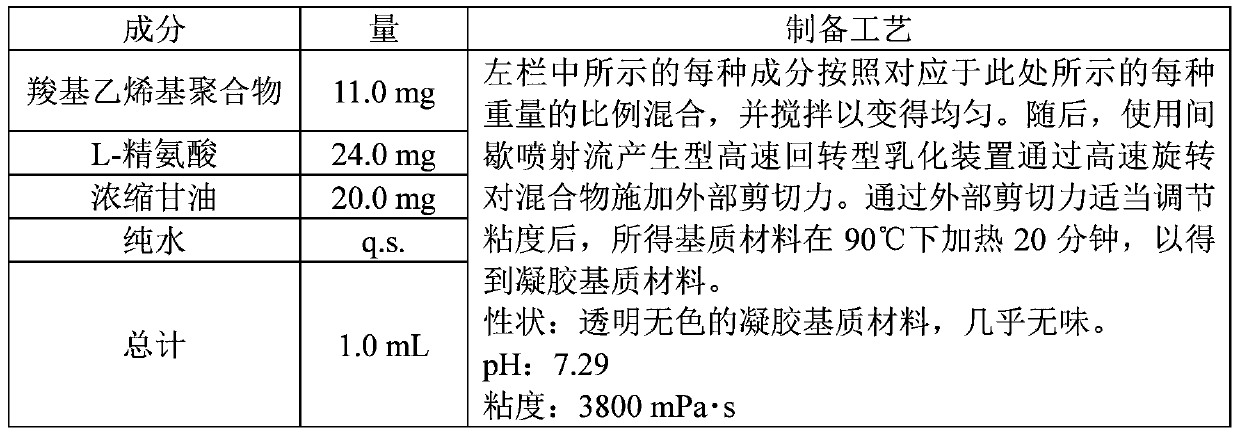
Nasal hepatitis b vaccine composition and method for producing same
PendingCN111344005AComplete treatmentLittle side effectsDispersion deliveryViral antigen ingredientsAntigenHepatitis B immunization
The present invention relates to a hepatitis B vaccine composition for nasal mucosa spraying administration, characterized by comprising a hepatitis B antigen and a gel base containing a carboxyl vinyl polymer, and by being applicable to hepatitis B prevention and treatment.
Owner:TOKO YAKUHIN IND CO LTD +4
Use of liposomes for treatment of chronic viral hepatitis b
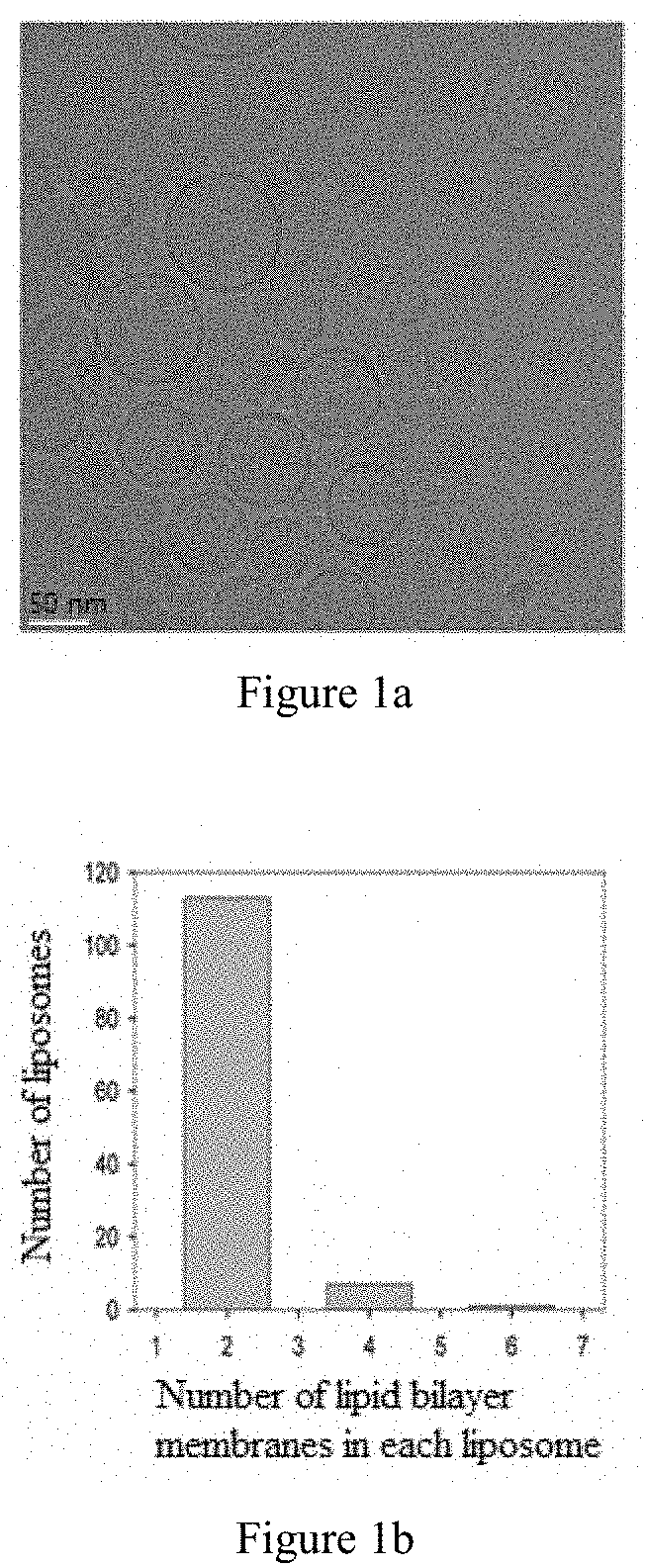
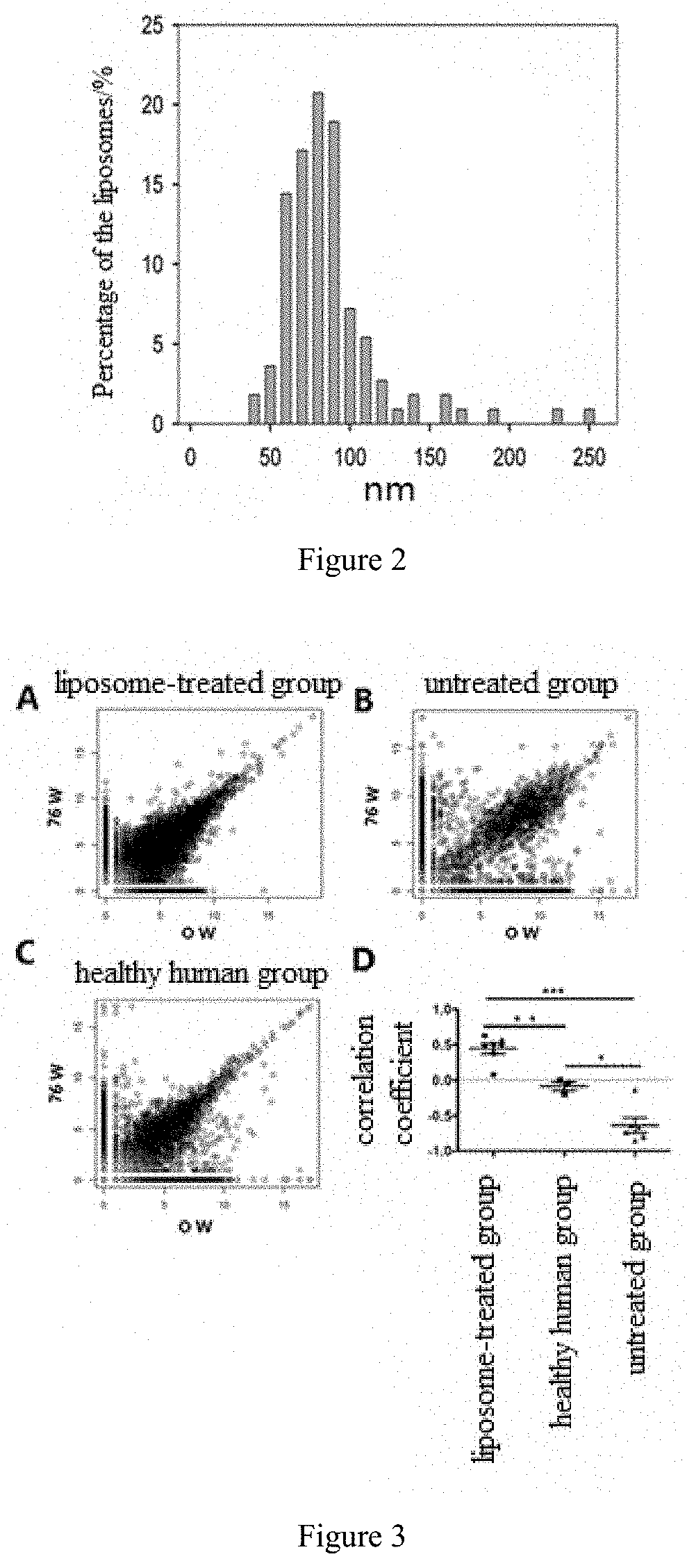
InactiveUS20200101019A1Simple and convenient and easy to operate processImprove stabilityPowder deliveryOrganic active ingredientsAntigenAntiendomysial antibodies
The invention relates to medicinal use of liposomes for the treatment of chronic viral hepatitis B, wherein said liposomes are prepared from phospholipid and cholesterol. This invention further relates to uses of said liposomes for the manufacture of medicaments for promoting the seroconversion of Hepatitis B e antibody to positive and the seroconversion of Hepatitis B e antigen to negative in patients with chronic viral hepatitis B, for reducing the hepatitis B virus titer in the peripheral blood serum of patients with chronic viral hepatitis B, for reducing the concentration of glutamic-pyruvic transaminase in the peripheral blood serum of patients with chronic viral hepatitis B, and for maintaining the stability of the T cell receptor repertoire in patients with chronic viral hepatitis B.
Owner:JIANGSU MENDEL GENETIC TECH INC +1
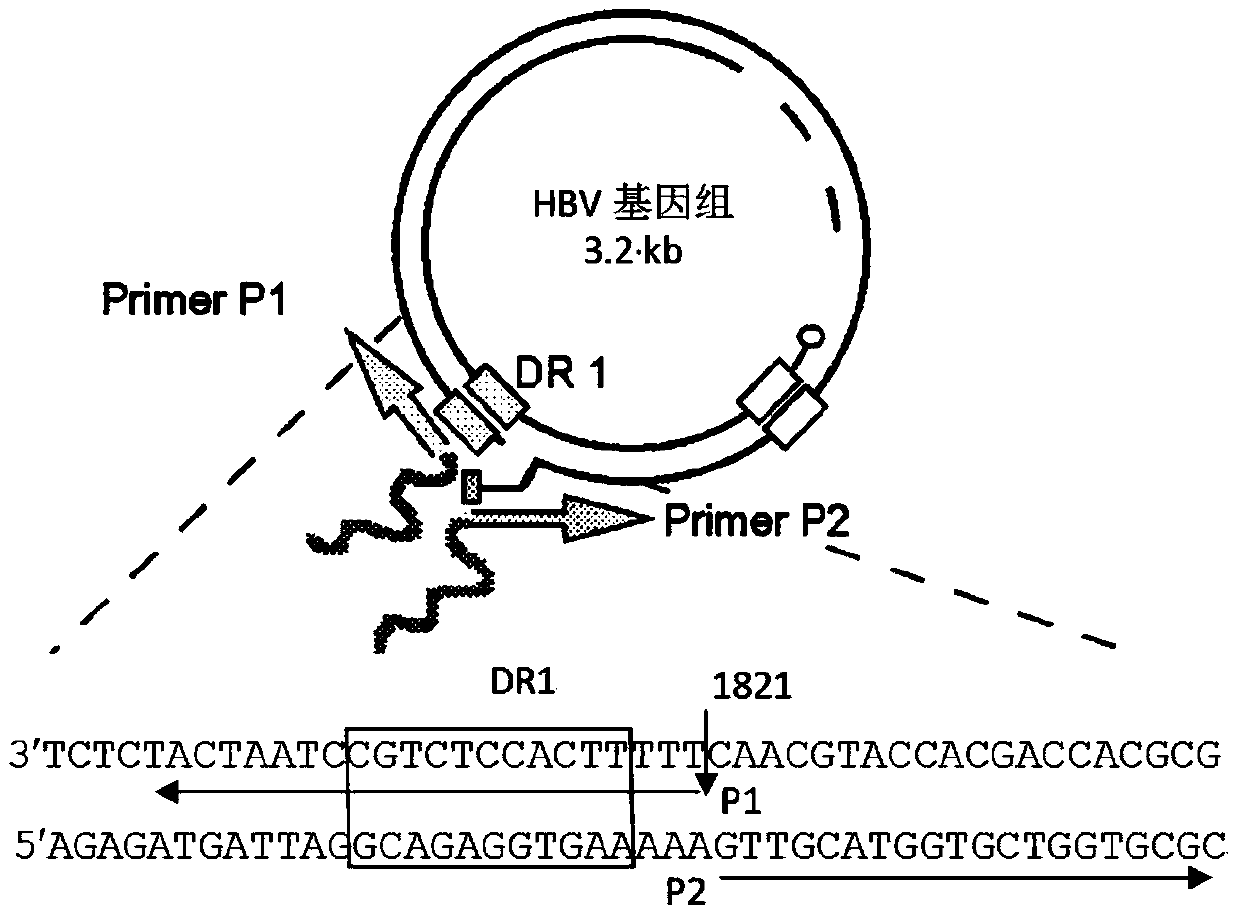
Construction method and application of a mouse model of HBV infection
ActiveCN105943560BCompounds screening/testingBacteria material medical ingredientsHepatitis B immunizationHepatitis B Antigens
The invention relates to the technical field of medicine and provides a hepatitis B virus (HBV)-infected mouse model construction method. According to the method, a polymerase chain reaction (PCR) is adopted to amplify an HBV single-copy linear genome, a PCR product transfects Huh7 cells, HepG2 cells and 293T cells, and hepatitis b surface antigen (HBsAg) and hepatitis B e antigen (HBeAg) in supernatant of culture cells are detected through an enzyme-linked immunosorbent assay (ELISA); the PCR product is injected to a C57BL / 6J mouse through a tail vein high-pressure hydrodynamic method, so that hepatitis b core antigen (HBcAG) in mouse liver tissue is detected in an immunohistochemistry mode, and the HBsAg and the HBeAG in serum of the mouse are detected in an ELISA mode. By means of the method, a novel HBV-infected mouse model is constructed, the model is simple in preparation method, and the HBC genome similar to the HBV cccDNA exists in the liver tissue. A novel tool is provided for research on an HBV cccDNA degradation mechanism, medicine removal, HBV biological characteristics in a serum sample of a hepatitis B patient and the like.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Thermosensitive hepatitis B vaccine
A thermosensitive hepatitis B vaccine is provided. The thermosensitive hepatitis B vaccine includes an aqueous phase solution comprising a biodegradable thermosensitive hydrogel copolymer; a surface antigen of hepatitis B virus (HBsAg); and a bioactive substance. The thermosensitive hepatitis B vaccine of the disclosure is particularly suitable for being applied in the patients, which are low responsive or non-responsive to conventional hepatitis B vaccine, for enhancing the induction of cell-mediated immune responses and overcoming the HBsAg non-responsiveness.
Owner:IND TECH RES INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com