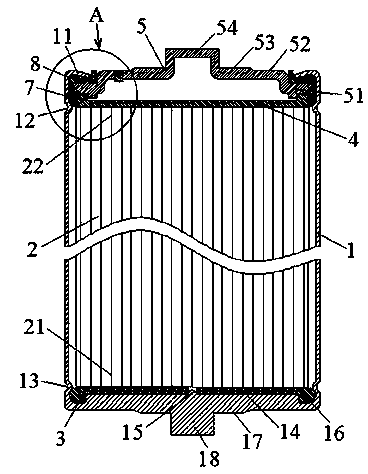
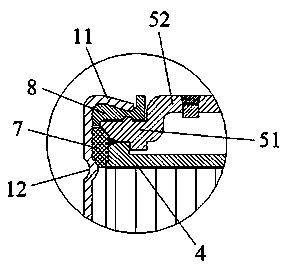
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
138results about How to "Reduce the number of injections" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
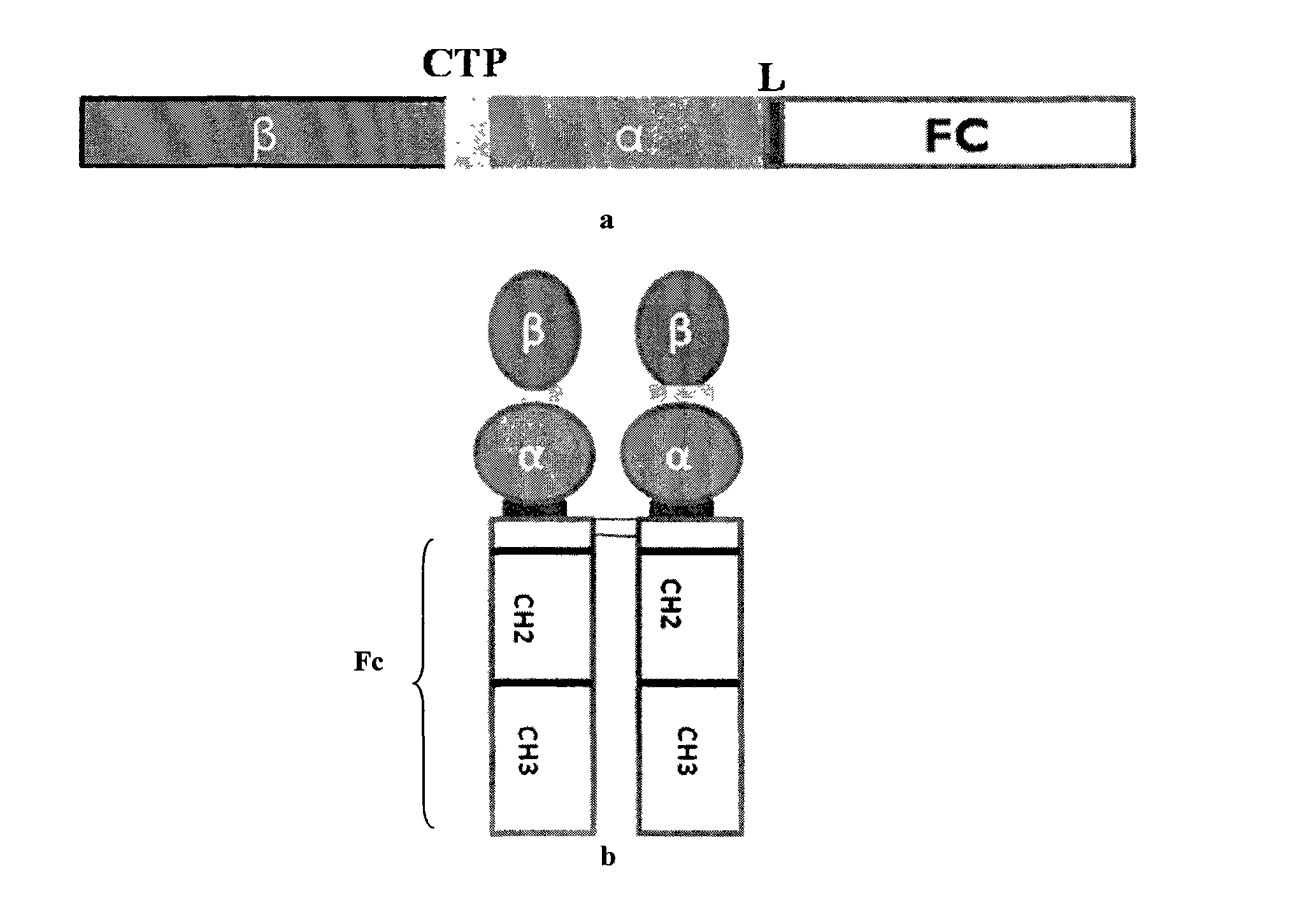
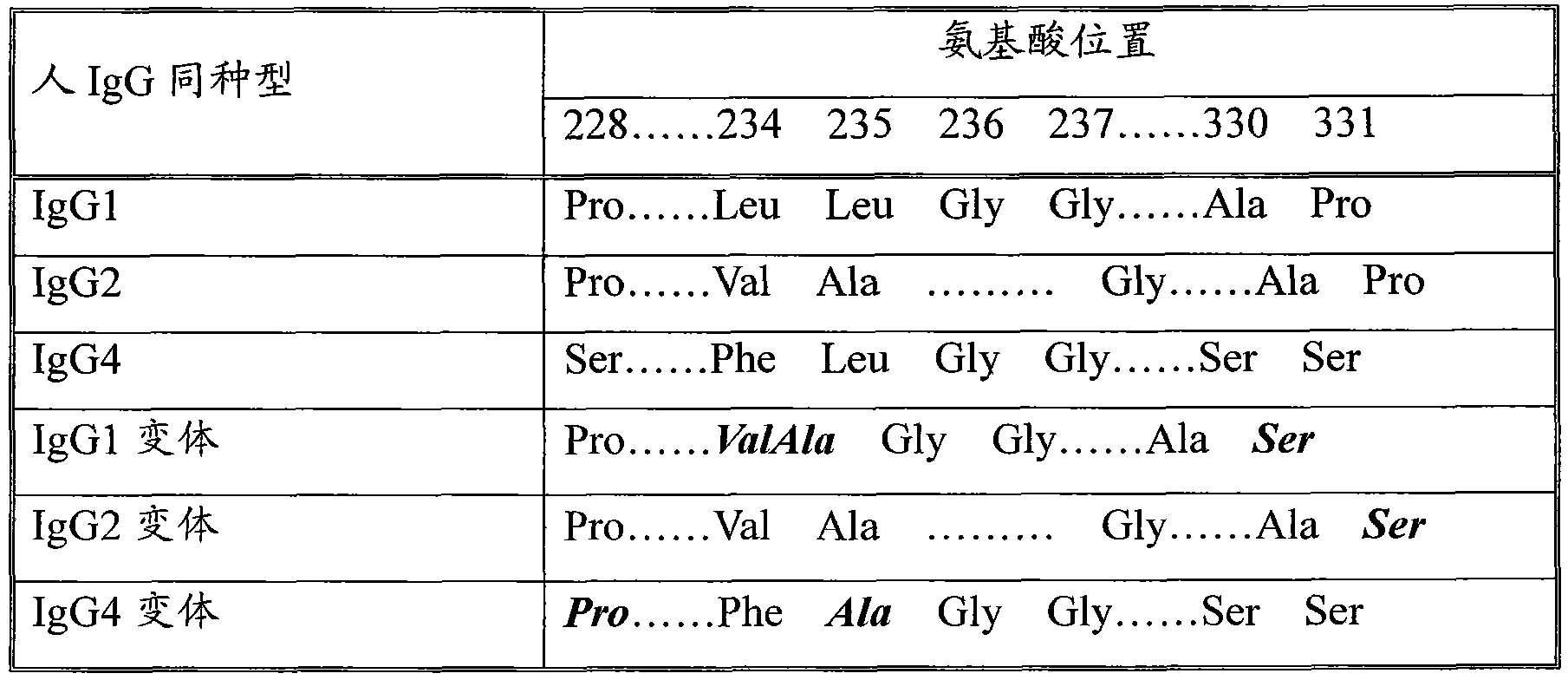
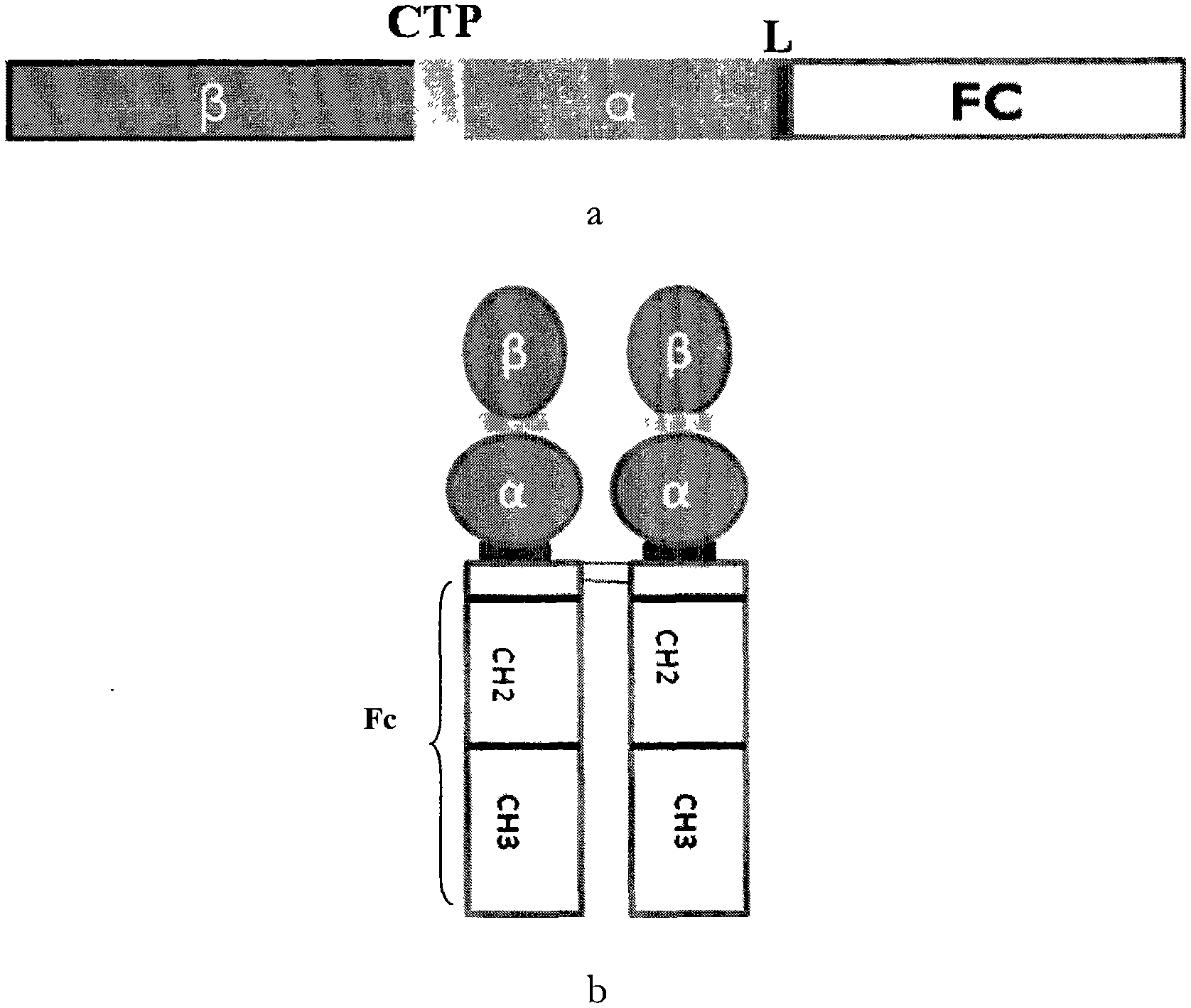
Long-acting recombinant human follicle-stimulating hormone-Fc fusion protein (hFSH-Fc)
ActiveCN103539860AImprove biological activitySmall toxicityPeptide/protein ingredientsAntibody mimetics/scaffoldsRecombinant human follicle stimulating hormoneSide effect
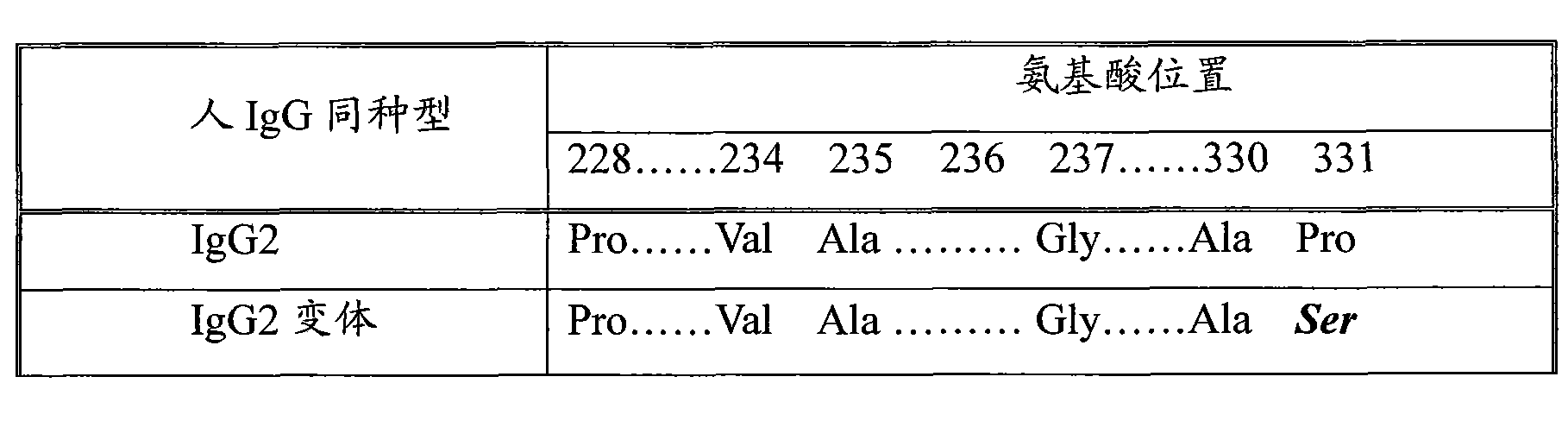
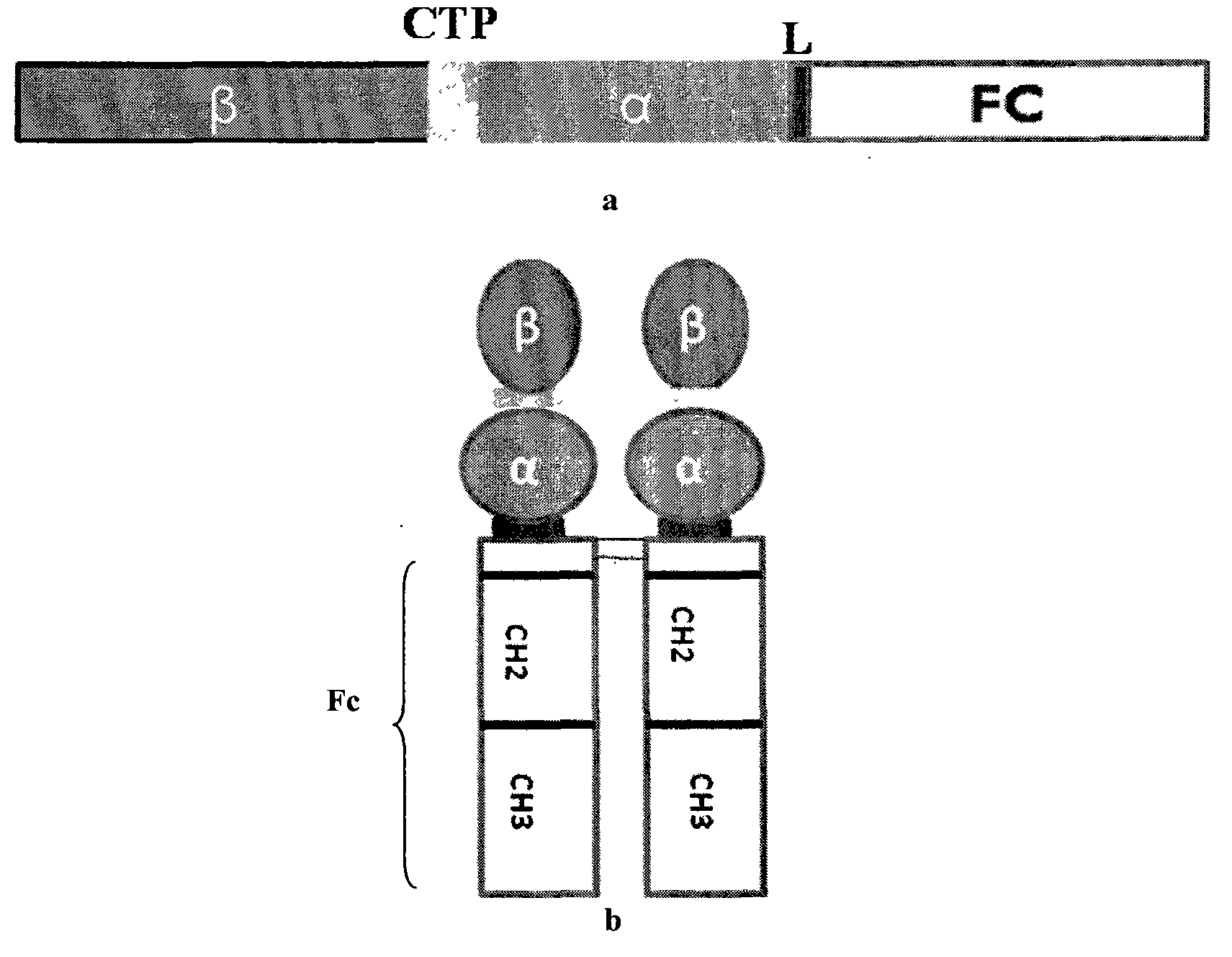
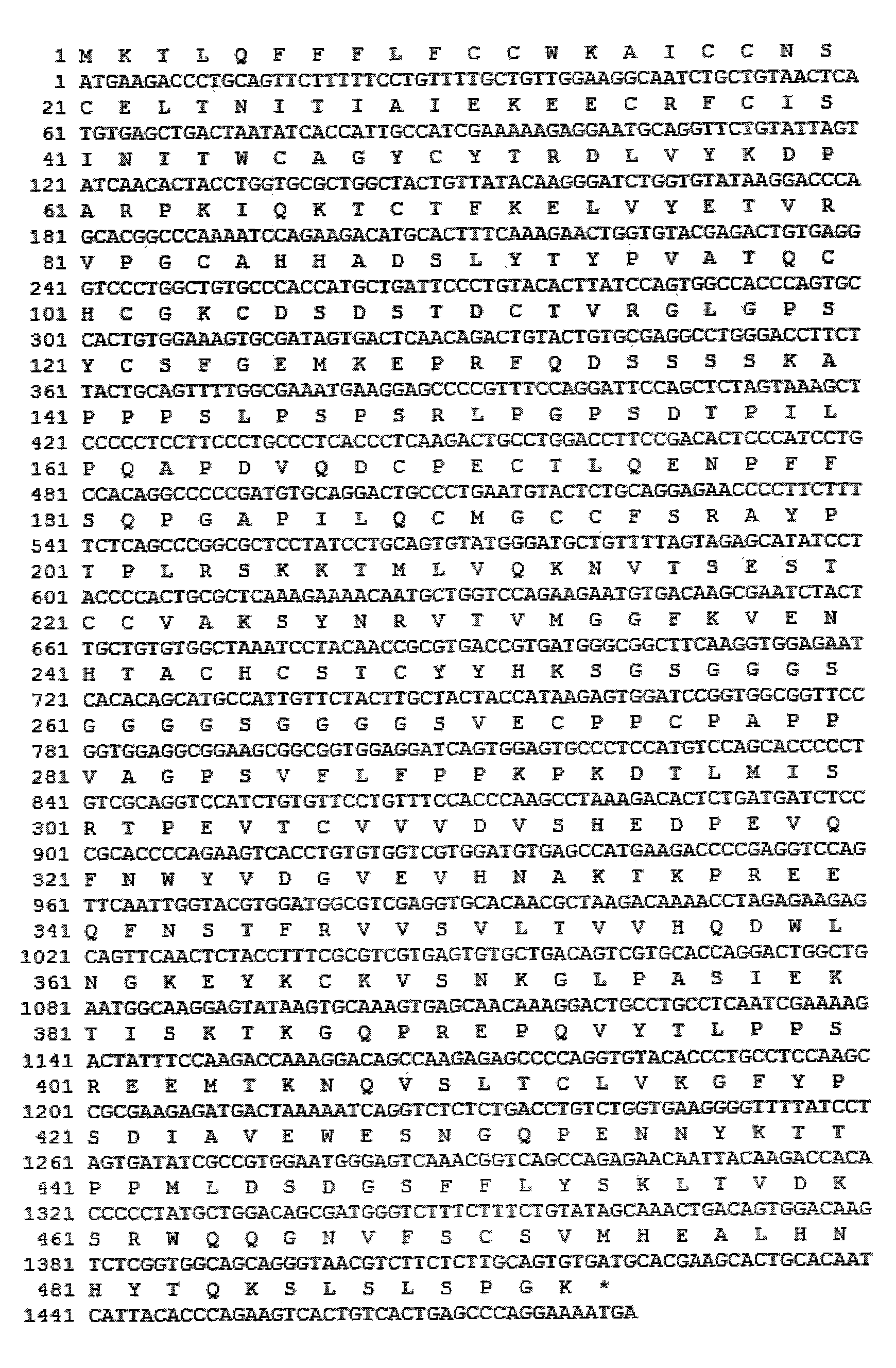
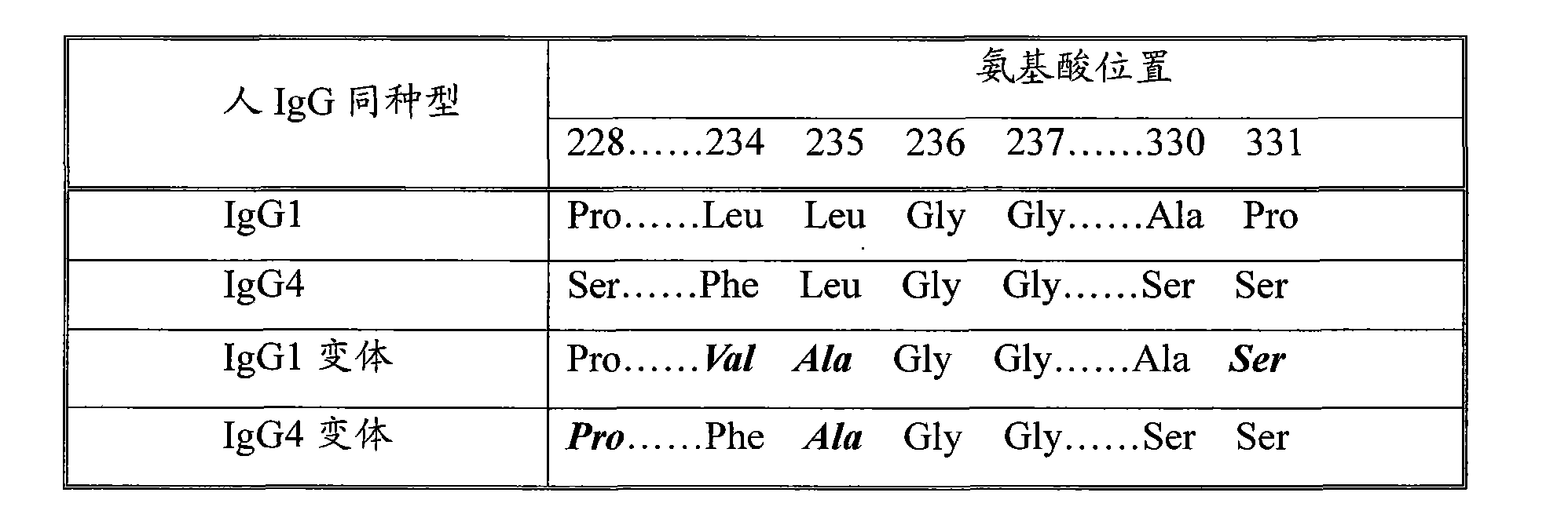
The invention discloses a long-acting recombinant human follicle-stimulating hormone-Fc fusion protein (hFSH-Fc) and a preparation method thereof. The recombinant hFSH-Fc is a dimerization fusion protein. An amino acid sequence of the hFSH-Fc comprises an hFSHbeta subunit, CTP (carboxy-terminal peptide), an hFSHalpha subunit, a flexible peptide linker and a human IgG (immunoglobulin G)2 Fc variant from the N terminal to the C terminal in sequence. The hFSH-Fc has longer half-life in vivo and smaller side effects than existing hFSH. The invention also relates to an application of a recombinant hFSH-Fc composition to preparation of drugs for treating and / or preventing infertility.
Owner:UNICOHEALTH CO LTD
Long-acting recombinant human follicle-stimulating hormone-Fc fusion protein (hFSH-Fc)
ActiveCN103539861AImprove biological activityHigh activityPeptide/protein ingredientsPeptide preparation methodsSide effectRecombinant human follicle stimulating hormone
The invention discloses a long-acting recombinant human follicle-stimulating hormone-Fc fusion protein (hFSH-Fc) and a preparation method thereof. The hFSH-Fc is a dimerization fusion protein. An amino acid sequence of the hFSH-Fc comprises an hFSHbeta subunit, CTP (carboxy-terminal peptide), an hFSHalpha subunit, a flexible peptide linker and a human IgG (immunoglobulin G) Fc variant from the N terminal to the C terminal in sequence. The hFSH-Fc has longer half-life in vivo and smaller side effects than existing hFSH. The invention also relates to an application of a recombinant hFSH-Fc composition to preparation of drugs for treating and / or preventing infertility.
Owner:UNICOHEALTH CO LTD
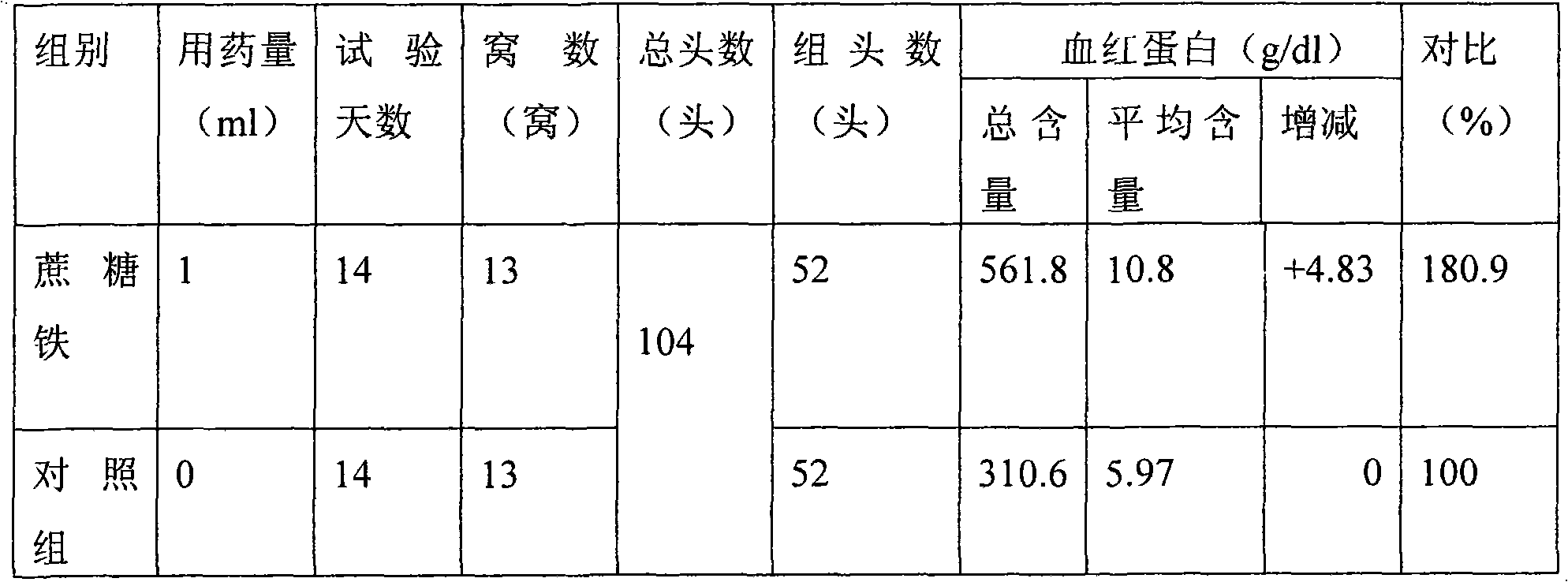
Preparation method for iron sucrose bulk drug and injection thereof
InactiveCN101671373AImprove stabilityGood water solubilityOrganic active ingredientsSugar derivativesAcetic acidIron salts
The invention relates to a preparation method for an iron sucrose bulk drug. The method comprises the following steps: (1) crystallization: the pH value of sucrose solution is adjusted to 1-4 with acetic acid, iron salt solution is added to the sucrose solution to form mixed liquor, the pH value of the mixed liquor is adjusted to 1-3 with Na2CO3 solution, then the mixed liquor is stirred and is continued to be added with the Na2CO3 solution until turbidity appears in the mixed liquor, the pH value of the mixed liquor is adjusted to 4-7, and then the mixed liquor is filtered and added with water to obtain iron cake solution; (2) complexing: the sucrose solution is heated and added with alkaline liquor for alkalization, and then the alkalized sucrose solution is added with the iron cake solution and stands for the night to obtain complexing solution; and (3) refining: the complexing solution after standing for the night is filtered, and the filtrate precipitates with 2-3 times of 95 percent of ethanol and is filtered to obtain the iron sucrose bulk drug. The invention has the advantages of simple process steps, easy control of the reaction processes, good product quality, high yieldand lower production cost and is a high-novelty preparation method for the iron sucrose bulk drug and the injection thereof.
Owner:TIANJIN ZHONGAO BIOTECH
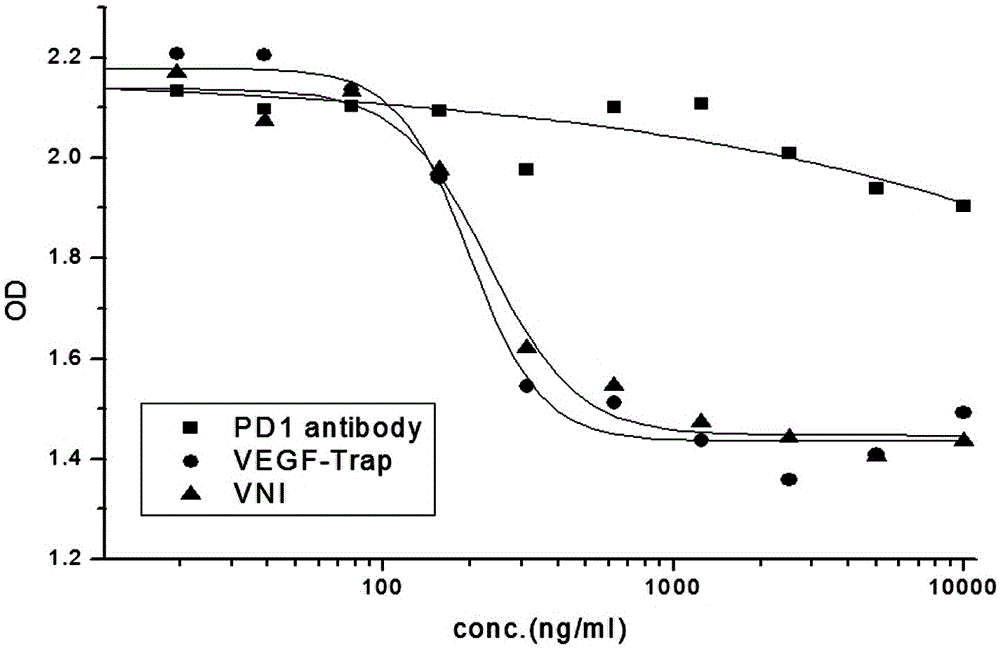
Novel fusion protein, pharmaceutical composition and preparation method therefor and use thereof
ActiveCN105111314AGrowth inhibitionSynergistic effectAntibody ingredientsPharmaceutical non-active ingredientsAntiendomysial antibodiesAntibody fragments
The invention discloses a novel fusion protein, a pharmaceutical composition formed by the novel fusion protein, a preparation method for the novel fusion protein and use of the novel fusion protein. According to novel fusion protein, the structural general formula is X-connecting peptide-Y-connecting peptide-IgG Fc fragment or Y-connecting peptide-X-connecting peptide-IgG Fc fragment, wherein X is VEGF-Trap and derivatives thereof, and Y is a PD-1 antibody fragment and derivatives thereof or a PD-1 antagonist fragment and derivatives thereof. The fusion protein plays roles in suppressing regenerated blood vessels and activating T cellular immunity and plays a role in coordinating and promoting two functions, so that the function of inhibiting tumor growth is more remarkable.
Owner:DONGGUAN YUNJING BIOTECHNOLOGY CO LTD
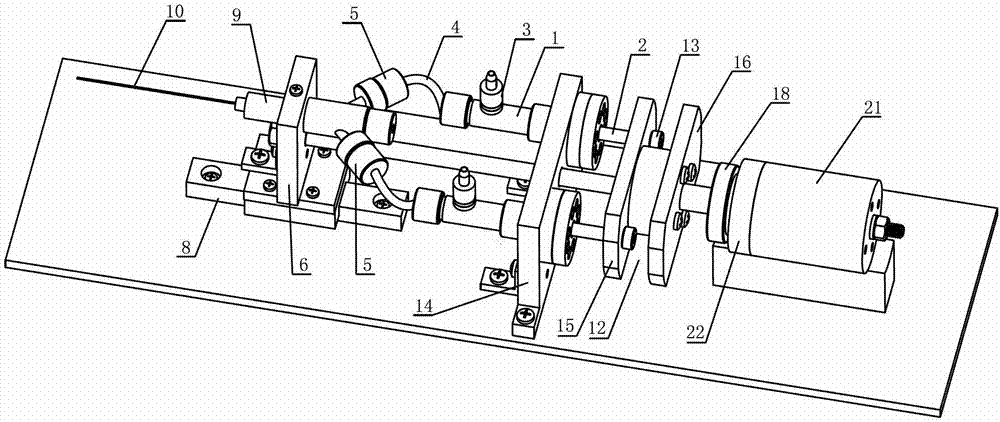
Automatic continuous injection unit for simultaneously injecting poultry with two drugs
InactiveCN102846407AReduce the number of injectionsLess traumatic injectionsVeterinary instrumentsDrugSyringe
The invention discloses an automatic continuous injection unit for simultaneously injecting poultry with two drugs and belongs to the technical field of poultry syringes. The automatic continuous injection unit comprises two injection systems driven by one push system. The automatic continuous injection unit can be used for injecting two drugs simultaneously, and injection times and injection wounds are reduced for young fowls.
Owner:HUZHOU ZHONGAN AGRI INTELLIGENT TECH
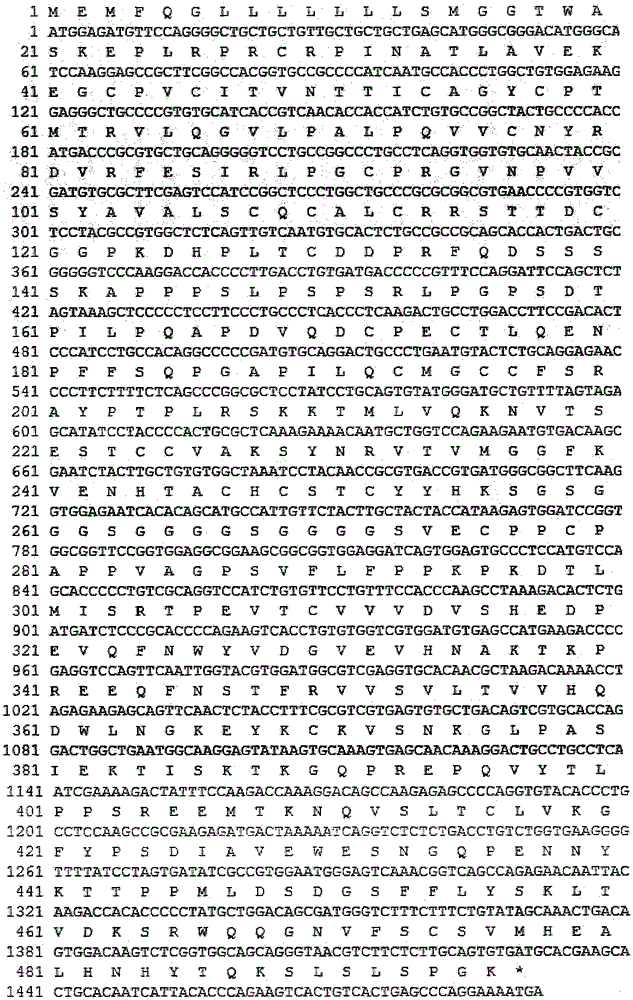
Preparation and purification method of recombinant proserum/growth hormone fusion protein for treating children dwarfism
ActiveCN109851674AHigh expression yieldCorrectly foldedFungiPeptide/protein ingredientsPurification methodsEvery Two Weeks
The invention discloses recombinant proserum / growth hormone fusion protein, a preparation and purification method of the recombinant fusion protein, and the use of the recombinant fusion protein to preparation of medicines for treating children dwarfism. The amino acid sequence of the recombinant proserum / growth hormone fusion protein is SEQID NO.1, and the nucleotide sequence of the recombinant proserum / growth hormone fusion protein is SEQID NO.2. According to a preparation technology of the recombinant proserum / growth hormone fusion protein disclosed by the invention, yeast engineering bacteria are constructed and expressed, so that high-density expression recombinant fusion protein is obtained; and through a purification technology, the recombinant proserum / growth hormone fusion proteinwhich can be used clinically is obtained. The recombinant proserum / growth hormone fusion protein obtained by the preparation and purification method adopts a creative medicine structure for treatingthe children dwarfism, has long residual action that administration can be performed once every two weeks, is more suitable for children medication demands, and has more excellent treatment effects, less administration frequency and lower production cost.
Owner:TIANJIN LINDA SINOBIOTECH CO LTD +1
Film transistor and manufacturing method thereof
ActiveCN104576399AReduce the number of injectionsImprove featuresTransistorSemiconductor/solid-state device manufacturingInsulation layerConcentration gradient
Owner:KUNSHAN GO VISIONOX OPTO ELECTRONICS CO LTD
Insulin analogue having quick response and stability under acidic condition and preparation thereof
ActiveCN102199206AQuick effectProtect capillariesPeptide/protein ingredientsMetabolism disorderPharmaceutical formulationInsulin Analogue
The invention relates to an isulin analogue having quick response and stability under acidic condition, and a medicinal composition and a medicinal preparation thereof. Asparagine (Asn) at the A 21 position of the chain A of the isulin analogue is mutated into glycine (Gly), the isulin analogue can be mixed with a long-acting isulin analogue such as insulin glargine to form a premixed preparationwith two functions of quick-acting sugar reduction and stable long-acting sugar reduction, and the problems that the conventional natural insulin (of pigs, cows and human) and quick-acting isulin analogues are unstable in the acidic environment, and the conventional isulin premixed preparation is not clarified and needed to be introduced with a foreign protein (such as protamine) serving as a slow release preparation so as to easily cause immune reaction, and is needed to be injected twice each day, namely in the morning and evening are solved, so that the safety of the preparation is higher.
Owner:GAN&LEE PHARMA
GLP-I analogue liraglutide sustained-release microspheres and preparation method thereof
InactiveCN103142488AImprove adaptabilityEasy to acceptPeptide/protein ingredientsMetabolism disorderSide effectMedicine
The invention discloses a GLP-I analogue liraglutide sustained-release microspheres and a preparation method thereof. The GLP-I analogue liraglutide sustained-release microspheres comprise, by weight, 0.5 to 30% (w / w) of GLP-I analogue liraglutide, 70 to 99.5% of a polymer which has the molecular weight of 5000 to 300000 dalton, can be biodegraded and has biocompatibility, and 0 to 10% of pharmaceutically acceptable auxiliary materials. The GLP-I analogue liraglutide sustained-release microspheres have the average grain size of 5 to 40 micrometers and an encapsulation ratio more than 90%. The GLP-I analogue liraglutide sustained-release microspheres have a sustained-release period of several days or several months, obviously reduce use frequency, improve GLP-I analogue liraglutide bioavailability, reduce toxic and side effects and are conducive to clinical treatment.
Owner:SHENZHEN JYMED TECH
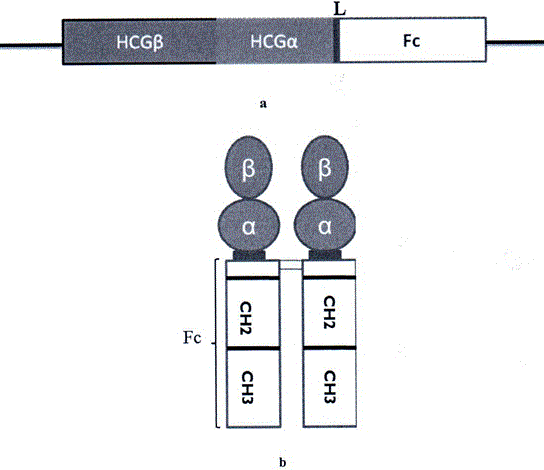
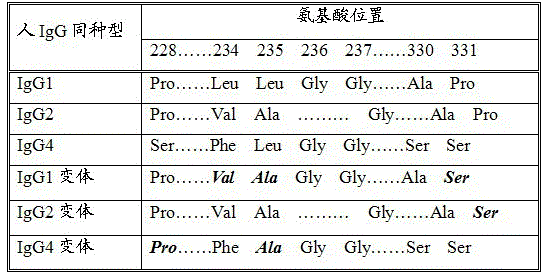
Long-acting recombinant human chorionic gonadotrophin-Fc fusion protein
ActiveCN103897064AImprove biological activityProlong half-life in vivoPeptide/protein ingredientsPeptide preparation methodsProtein compositionHalf-life
The invention discloses a recombinant human chorionic gonadotrophin-Fc fusion protein (HCG-Fc for short) and a preparation method thereof. The HCG-Fc protein is a dimerized fusion protein, and the amino acid sequence of the fusion protein sequentially comprises an HCG beta subunit, an HCG alpha subunit, a flexible peptide joint and a human IgG Fc mutant from an N end to a C end. Compared with the existing chorionic gonadotrophin, the fusion protein has longer in-vivo half life, higher bioavailability and lower side effect. The invention also relates to an application of a recombinant HCG-Fc fusion protein composition in preparation of medicaments for treating and / or preventing infertility.
Owner:UNICOHEALTH CO LTD
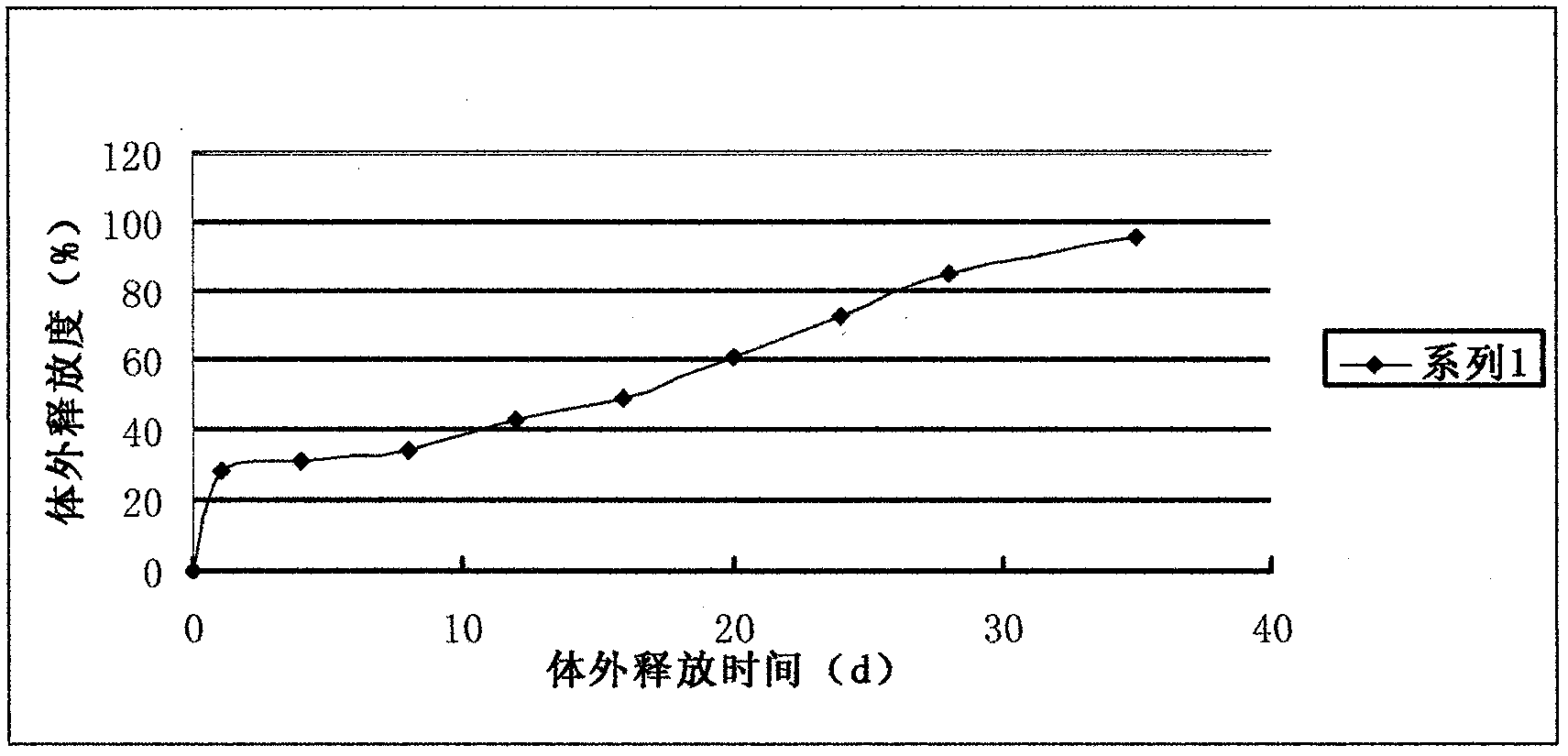
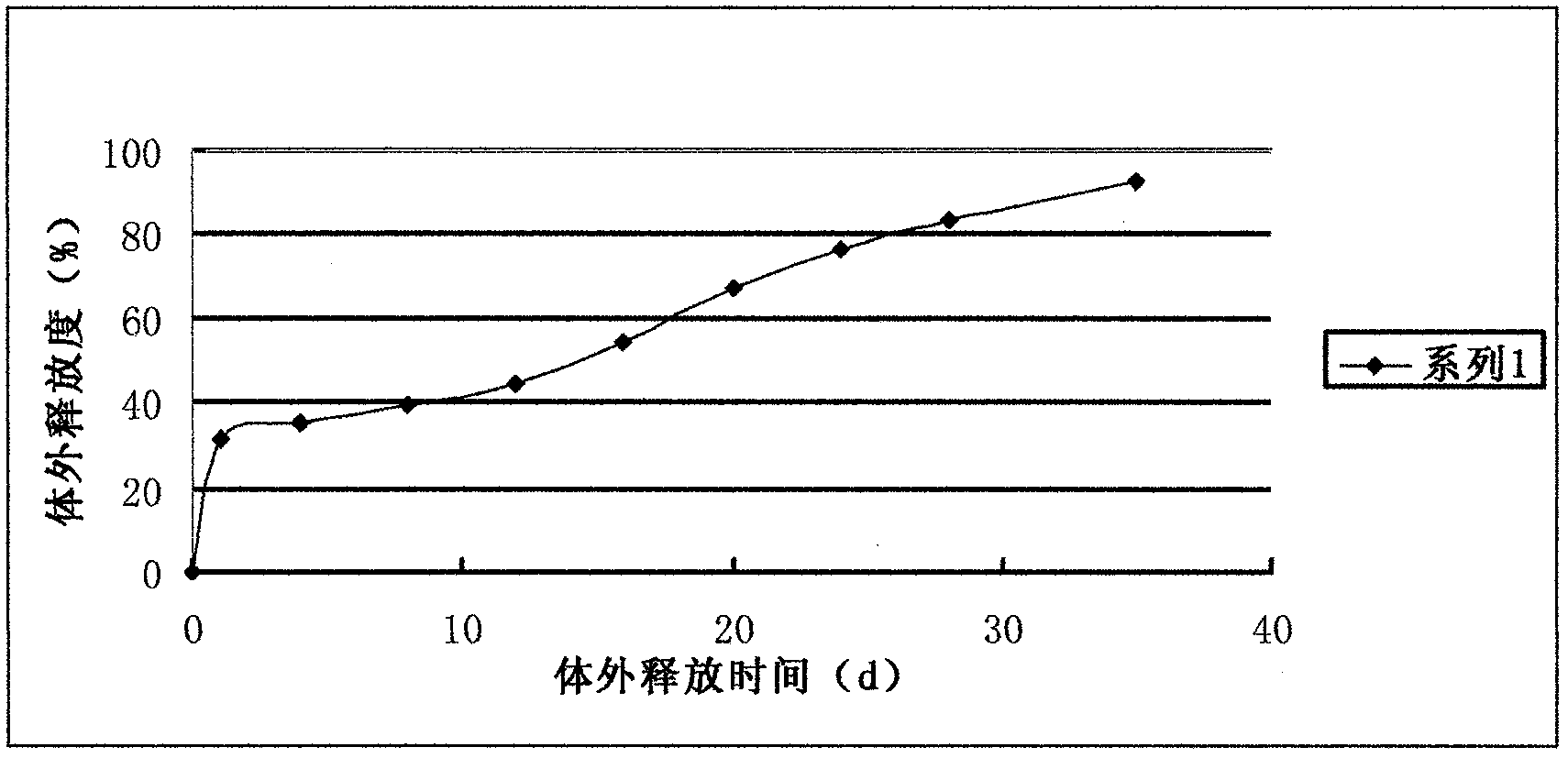
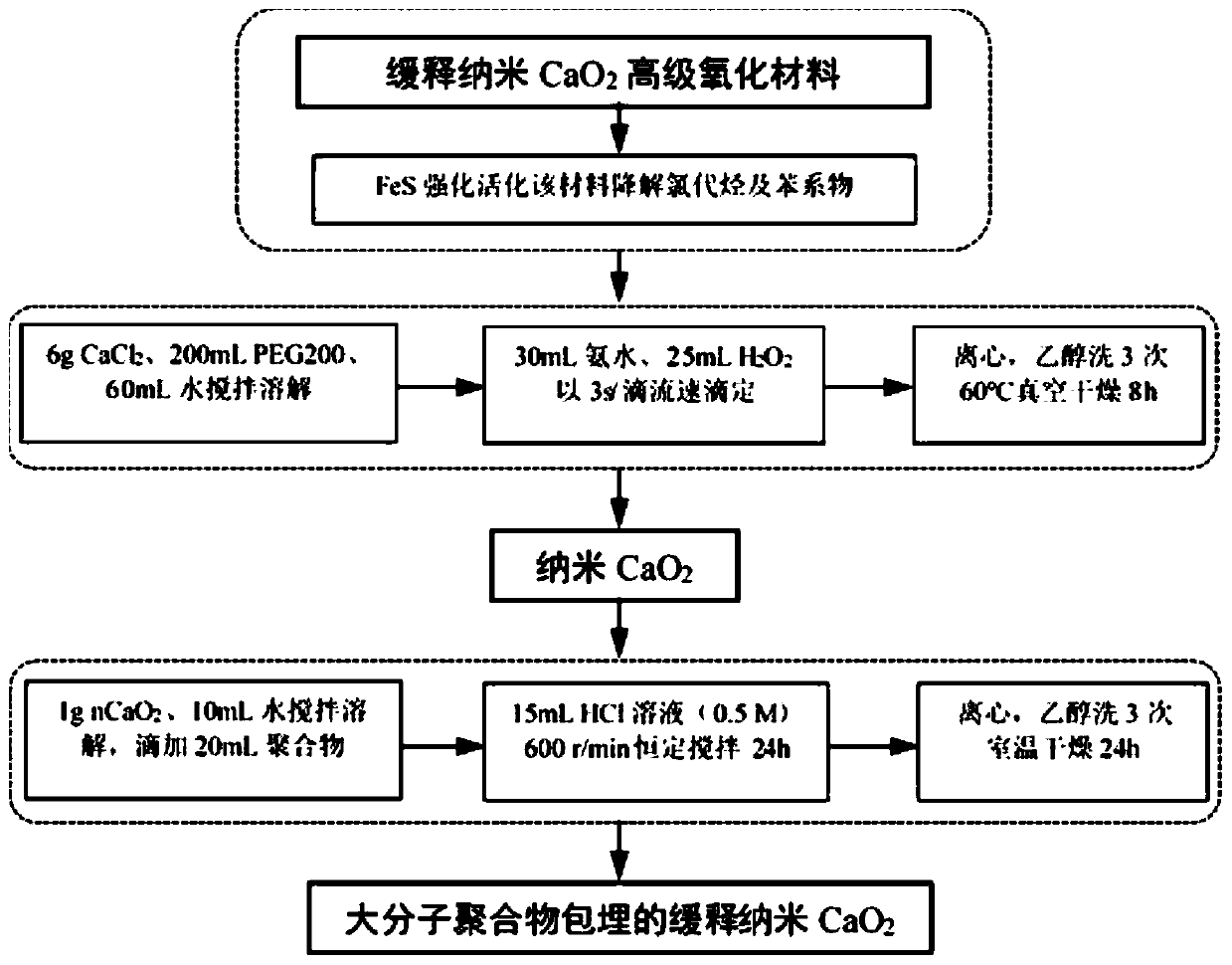
Sustained-release nano-calcium peroxide materials, preparation thereof, and method for removing chlorohydrocarbon and/or benzene series from underground water through sustained-release nano-calcium peroxide materials
PendingCN110759319AReduce manufacturing costEasy to operateMaterial nanotechnologyWater treatment compoundsPolyvinyl alcoholPolyethylene glycol
The invention belongs to the technical field of water treatment, and particularly relates to sustained-release nano-calcium peroxide materials, preparation thereof, and a method for removing chlorohydrocarbon and benzene series from underground water by strengthening and activating the sustained-release nano-calcium peroxide materials through ferrous sulfide. By taking calcium chloride, hydrogen peroxide with the mass fraction being 30% and ammonium hydroxide with the mass fraction being 30% as raw materials and by adding different dispersing agents, an irreversible coagulation phenomenon is avoided in the synthesis process, then by adding different macromolecular polymers such as polyethylene glycol 400 (PEG400), polyvinyl alcohol (PVA) and diethylene glycol monoethyl ether (DEGMME), a film is formed on the surface of nCaO2, and through processes such as washing with water and ethyl alcohol and vacuum drying, the sustained-release nano-calcium peroxide advanced oxidation materials embedded with the various macromolecular polymers are obtained. By applying the oxidation materials, the purposes of efficient and lasting treatment of pollutants and lowering of the repair cost of the underground water in contaminated sites are achieved.
Owner:EAST CHINA UNIV OF SCI & TECH
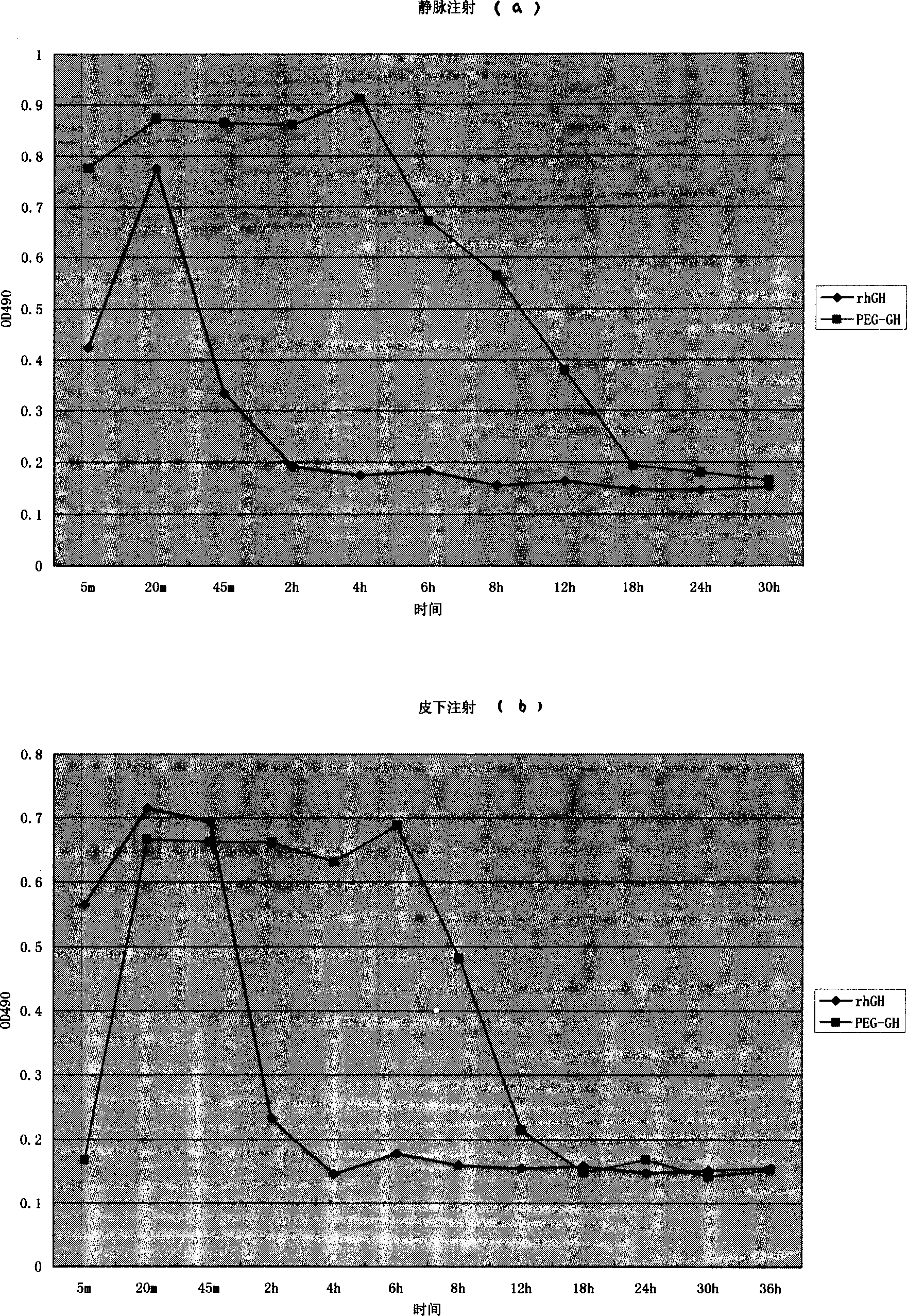
Carbowaxing recombiant human growth hormone medicine and its preparation process
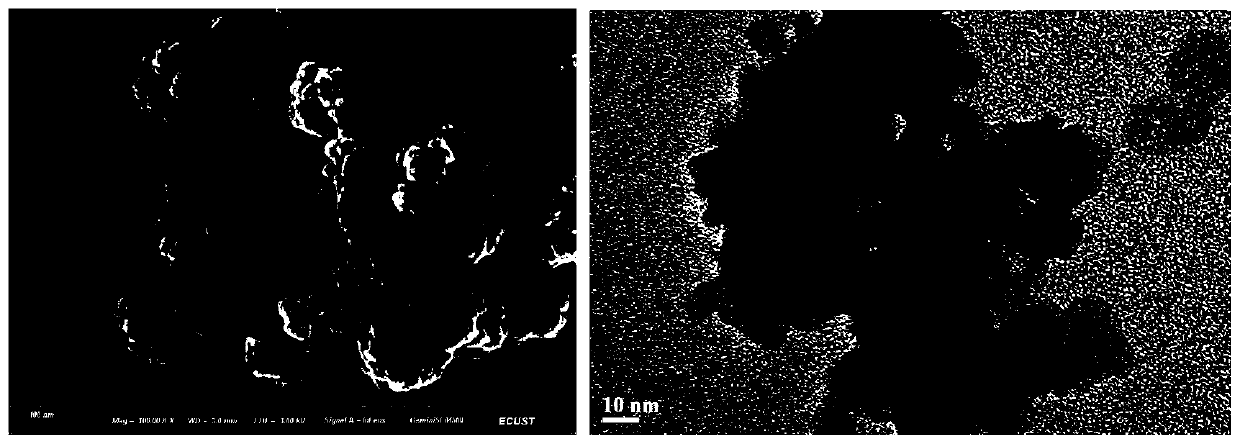
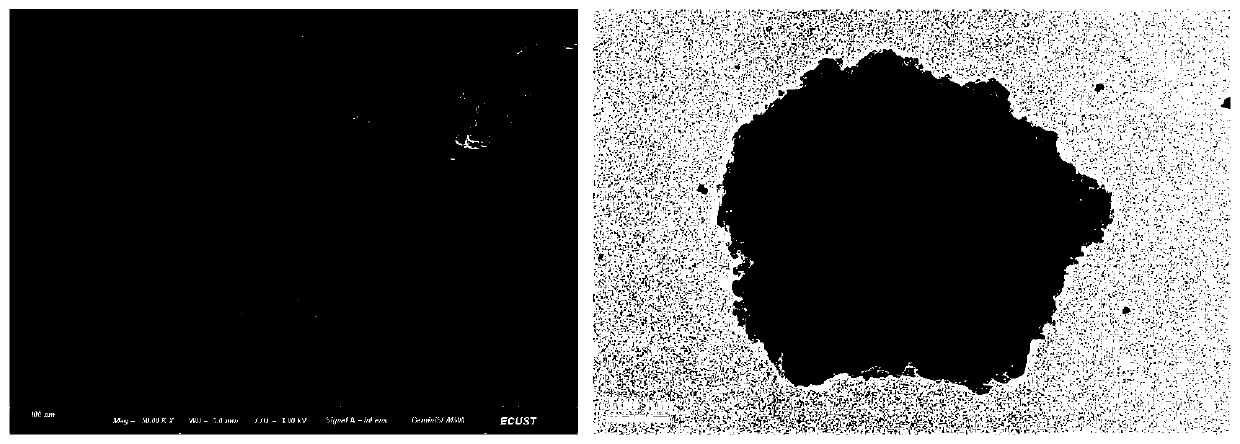
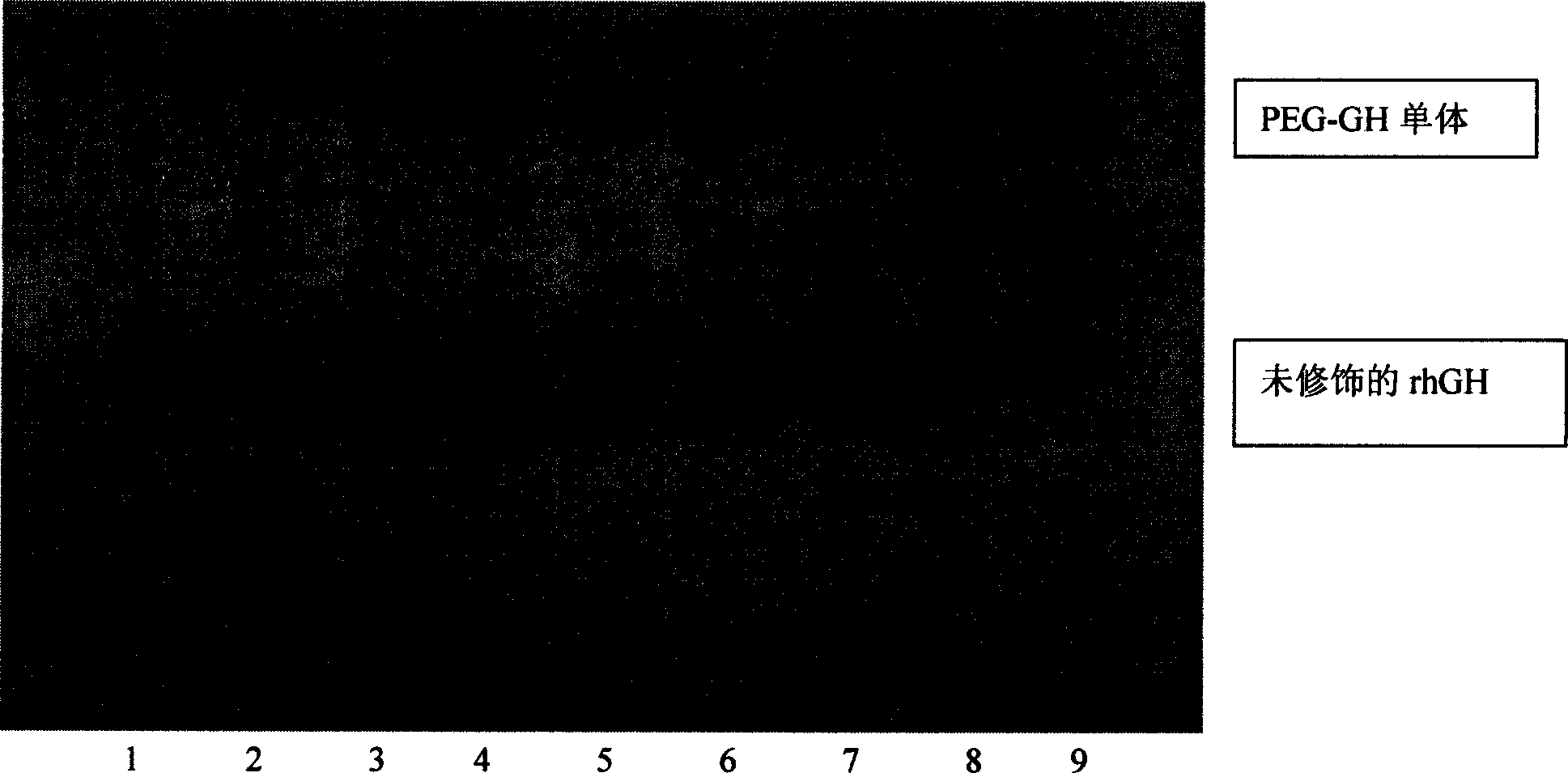
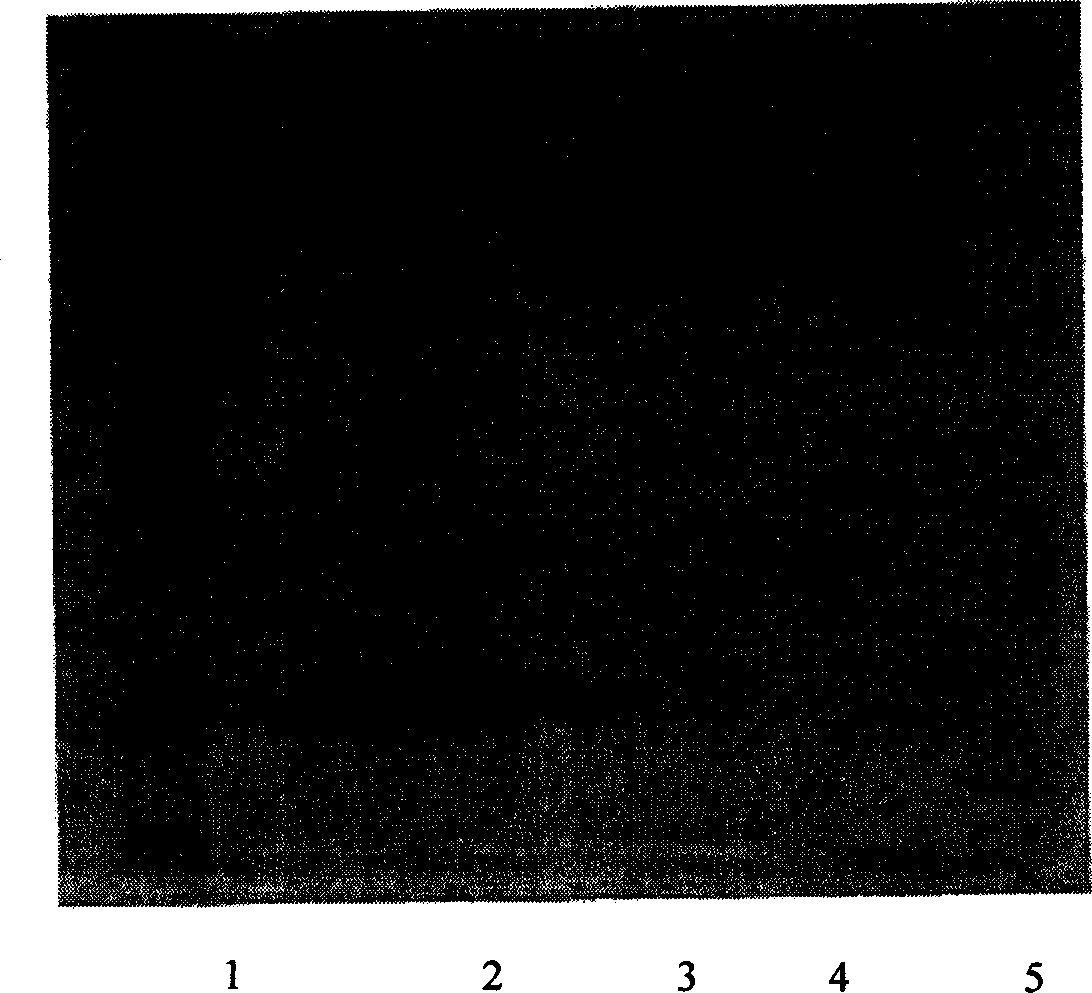
InactiveCN1565624AHighlight substantiveSignificant technological progressPeptide/protein ingredientsGrowth hormonesHuman growth hormoneMedicine
The invention relates to a clinical therapeutic medicine for recombined human growth hormone (rhGH) deficiency disease. Carbowax rhGH medicine is made up of carbowax and rhGH by conjuncting The manufacturing process mainly includes providing a buffer system of PH4.0-9.0, enabling rhGH and carbowax react 2-48 hours in the buffer system, obtaining purification through separating and purifying process, the temperature should be controlled in 4-37 DEG C. The invention has advantages of easy purification, long effect time and be convenient for industrial production.
Owner:ANHUI ANKE BIOTECHNOLOGY (GRP) CO LTD
Hyaluronic acid slow-release filler and preparation method thereof
ActiveCN111184909AReduce the number of injectionsShorten the timePharmaceutical delivery mechanismProsthesisSodium hyaluronateComposite material
The invention discloses hyaluronic acid slow-release filler and a preparation method thereof. The invention aims to solve the problem that absorbable injection filler has a short maintenance time andno effects of long-term filling in the field of medical aesthetics. Absorbable high-molecular polymer porous microspheres are added to an original injection system so as to form a gradient degradationformula, the microporous structure is filled with cross-linked sodium hyaluronate through a unique technology, the tissue is stimulated to produce collagen fiber by the microporous structure during gradual degradation of the cross-linked sodium hyaluronate in micropores so that a network structure is formed, and finally a filling effect can still be achieved after the polymer molecules are degraded.
Owner:湖北翎美生物科技有限公司
Circulating iodine adsorber performance test method and device
InactiveCN104634926AReduce the temperatureHumidity variation range is smallAerodynamic testingMaterial analysisNuclear technologyIodine
Belonging to the field of nuclear technologies, the invention specifically relates to an iodine adsorber performance test method and device. The method includes: forming circulating air between the inlet and outlet of a test iodine adsorber, adopting a frequency adjustment fan to accurately adjust the flow of circulating air, controlling the humidity of the circulating air by a heat pump system to make the air humidity on the inlet side and outlet side of the iodine adsorber consistent, injecting a test source into the inlet side of the iodine adsorber, conducting sampling at the inlet and outlet of the iodine adsorber respectively to test the purification performance of the iodine adsorber. The method and the device provided by the invention combine air circulation, frequency adjustment technology, heat pump technology and other energy saving technologies, and reach the comprehensive benefits of environmental protection, energy saving, safety and high efficiency.
Owner:CHINA INST FOR RADIATION PROTECTION
Recombinant porcine follicle-stimulating hormone-Fc fusion protein (pFSH-Fc)
ActiveCN103554269AProlong half-life in vivoImprove mechanical propertiesPeptide/protein ingredientsPeptide preparation methodsAmino acidOvarian follicle
The invention discloses a recombinant porcine follicle-stimulating hormone-Fc fusion protein (pFSH-Fc) and a preparation method thereof. The fusion protein is a dimerized fusion protein, wherein the amino acid sequence of the fusion protein sequentially comprises pFSH beta subunits, carboxy-terminal peptide (CTP), pFSH alpha subunits, peptide connectors and human IgG Fc variants from a terminal N to a terminal C. Compared with existing pFSH, the pFSH-Fc has a longer in-vivo half-life period and better efficacies. The invention further relates to application of the pFSH-Fc to preparation of medicaments in the animal breeding fields.
Owner:GUANGZHOU VBIO PHARM CO LTD
Method for preparing triple inactivated vaccine for preventing chicken Newcastle disease, infectious bronchitis and egg drop syndrome
InactiveCN101099863AImprove securityReduce the number of injectionsViral antigen ingredientsRespiratory disorderDiseaseInfectious bronchitis
The invention is concerned with a kind of preparation method of inactivated vaccine to prevent new castle disease, infectious bronchitis and egg drop syndrome vaccine. The selected genus is Newcastle Disease virus La Sota individual plant, infectious bronchitis virus M41 individual plant, HN99 kidney type of aberrance individual plant and EDS76 Jing 911 individual plant. Dilute three kinds of virus and inoculate in the allantoic cavity of SPF chick embryo and affectable duck embryo. Collect liquid of embryo to alive and dead embryos as seed. Dilute and inoculate in allantoic cavity of affectable chick embryo or duck embryo to get liquid of virus embryo. Concentrate with equipment and add with formaldehyde solution to prepare bacterin through emulsion process. This invention can prevent Newcastle Disease, chick kidney type and breathing type infectious bronchitis, and egg drop syndrome vaccine. It can reduce the times of injection and the cost of epidemic prevention for easy using and practicality.
Owner:HENAN AGRICULTURAL UNIVERSITY
Preparation method of long-acting sustained-release microspheres containing bevacizumab
InactiveCN102988301AGood volatilization effectReduce evaporation rateSenses disorderAntibody ingredientsMicrosphereWater soluble drug
The invention discloses a preparation method of long-acting sustained-release microspheres containing bevacizumab, which is a preparation method of the sustained-release microspheres formed by encapsulating water-soluble drug protein bevacizumab in a degradable biomedical polymer material. The microspheres are prepared by a W / O / W (water-in-oil-in-water) solvent evaporation method, which comprises the steps of: dispersing bevacizumab and a solution thereof and alginate as inner water phases into a solution which uses the degradable biomedical polymer material as an oil phase to form colostrum; dispersing the colostrum into an outer water phase which is water liquid containing emulsifier to form multiple emulsion; and stirring, distilling in reduced pressure, centrifuging, washing and drying to obtain the bevacizumab sustained-release microspheres. According to the long-acting sustained-release microspheres containing bevacizumab prepared by the method, the encapsulation efficiency of the water-soluble protein drug can be effectively improved, the drug protein activity is not influenced, the releasing time of water-soluble protein can be effectively prolonged, and the sustained release period can be 2-3 months, even longer, so that the number of injection times can be reduced. The preparation method is convenient for clinical application.
Owner:WENZHOU MEDICAL UNIV
Single dose immunization against tetanus toxin cation dextran microspheres and preparation method thereof
InactiveCN101869704AReduce the number of injectionsImprove vaccination coverageAntibacterial agentsBacterial antigen ingredientsControlled releaseTetanus
The invention relates to the filed of pharmaceutical preparation, in particular to single dose immunization against tetanus toxin cation dextran microspheres and a preparation method thereof. The single dose immunization against tetanus toxin cation dextran microspheres are prepared by carrying tetanus toxin after electrostatic interaction on cation hydroxyethyl acrylate dextran microspheres. The tetanus toxin controlled release microspheres can reduce the injection frequency of tetanus vaccine, improve the vaccination coverage and reduce the drop-out rate, thereby effectively preventing tetanus and providing a single dose tetanus toxin controlled release vaccine preparation with long-term effect and realizing the whole course immunity by one injection.
Owner:CHINA PHARM UNIV
A kind of micro/nano fiber sustained-release preparation for treating scar and preparation method thereof
InactiveCN102266282AAvoid churnAvoid effects such as denaturationOrganic active ingredientsPharmaceutical delivery mechanismFiberPolymer science
The invention relates to a micro / nanometer fiber slow release preparation for treating cicatrices and a preparation method thereof. The micro / nanometer fiber slow release preparation is a complex consisting of panaxoside Rg3 and degradable high molecular polymer fibers, wherein the panaxoside Rg3 is dispersed in the degradable high molecular polymer fibers in a non-crystal form, and accounts for 0.1 to 50 percent of the mass of the complex; and the diameters of the degradable high molecular polymer fibers are between 5 nanometers and 10 micrometers. The method for preparing the micro / nanometer fiber slow release preparation comprises the following steps of: dissolving the panaxoside Rg3 and degradable high molecular polymer in an organic solvent to form mixed solution, spinning the mixed solution in a method of electrostatic spinning, and drying under vacuum to prepare the micro / nanometer fiber slow release preparation. By the micro / nanometer fiber slow release preparation, the release concentration and action time of active medicaments of the panaxoside Rg3 at local parts of the cicatrices are improved, the availability of the medicaments is improved, the long-term release of the medicaments is realized, and the requirement of the panaxoside Rg3 in clinical treatment is met, so the micro / nanometer fiber slow release preparation has the characteristics of high adaptability, simple process, low cost, high repeatability and the like.
Owner:SHANGHAI JIAO TONG UNIV
Groove type CoolMOS and manufacturing method thereof
InactiveCN105870194AImprove pressure resistanceReduce switching lossesSemiconductor/solid-state device manufacturingSemiconductor devicesInsulation layerEngineering
The invention belongs to the technical field of semiconductor devices, a trench type CoolMOS, which sequentially includes a back metal layer, an N+ substrate, an N epitaxial layer, a P well region, an n+ source region, an insulating layer and a front metal layer from bottom to top; A number of super junction trenches penetrating the N+ substrate are provided on the N+ substrate, one end of the super junction trench extends to the N-epitaxial layer, and the super junction trench is filled with P-type silicon; the P-well region is provided with a penetrating P-well A number of gate trenches in the N+ region and the n+ source region, one end of the gate trench extends to the N-epitaxial layer, the gate trench is filled with polysilicon, and the polysilicon and the gate trench are set by a gate oxide interval; the n+ source region A plurality of contact holes are provided through the n+ source region and the insulating layer, one end of the contact hole extends to the P-well region, the contact holes are filled with conductive metal, and the conductive metal is in contact with the front metal layer. The front adopts a gate trench structure, and the back is dug and filled with P-type silicon to achieve the effect of multiple implantation and diffusion of P-type ions.
Owner:SUZHOU TONGGUAN MICROELECTRONICS
Insulin-protamine zinc injection and method for preparing the same
InactiveCN101219209AImprove stabilityReduce the number of injectionsPeptide/protein ingredientsMetabolism disorderInsulin injectionProtamine sulfate
The invention pertains to a pharmaceutical preparation field, and in particular relates to a zinc protamine insulin ampoule injection and a preparation method thereof. The zinc protamine insulin ampoule injection comprises insulin, isotonizing agent, antiseptic agent, pH regulator, protamine sulfate and bi-distilled water for injection, wherein, the bi-distilled water for injection contains divalent zinc salt and the concentration of zinc ion is 0.01-0.04g per100000IU of insulin. The injection of the invention has the advantages that minim zinc ion is added into the insulin to form a stable polymer, which stabilizes the insulin and has a prolonged curative effect; the invention has simple, scientific and reasonable preparation and easy manipulation. The insulin can exist in two patterns: firstly, in liquor with quick effect; secondly, combining with protamine sulfate and in precipitation with action characters of both two insulin preparations, which reduces injection time and pain caused by rejection. Animal insulin is used for the injection, thus reducing the cost.
Owner:JIANGSU WANBANG BIOPHARMLS
Semiconductor device and process for production thereof
InactiveCN102576723AGuaranteed contact areaIncrease contact areaSemiconductor/solid-state device detailsSolid-state devicesHigh concentrationBody region
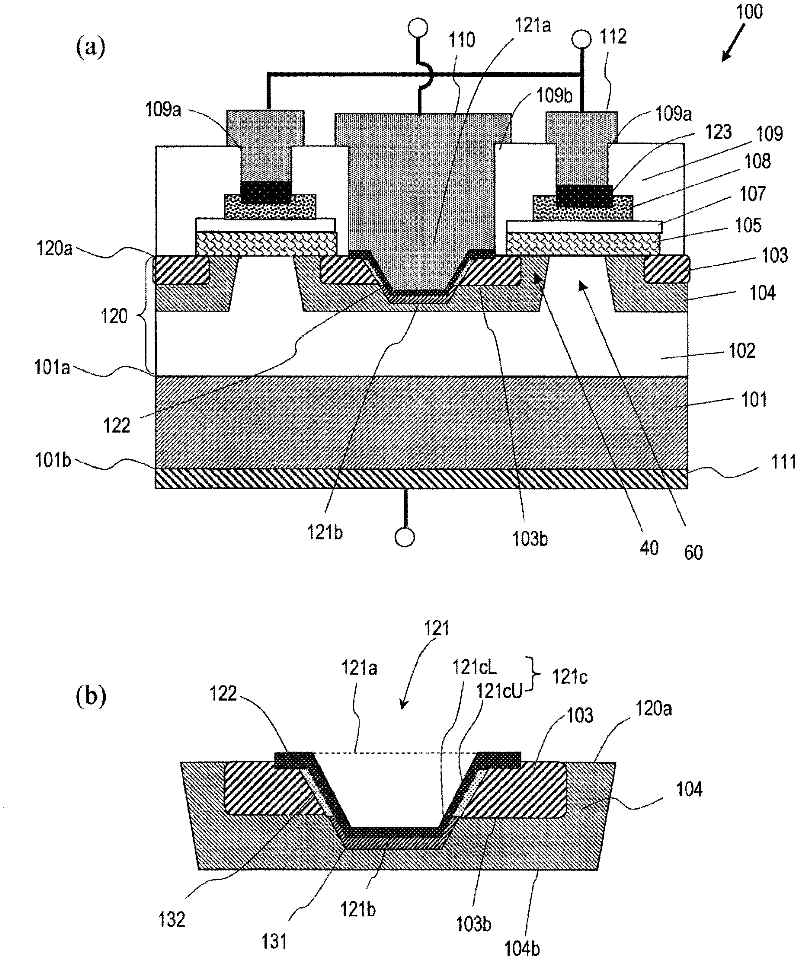
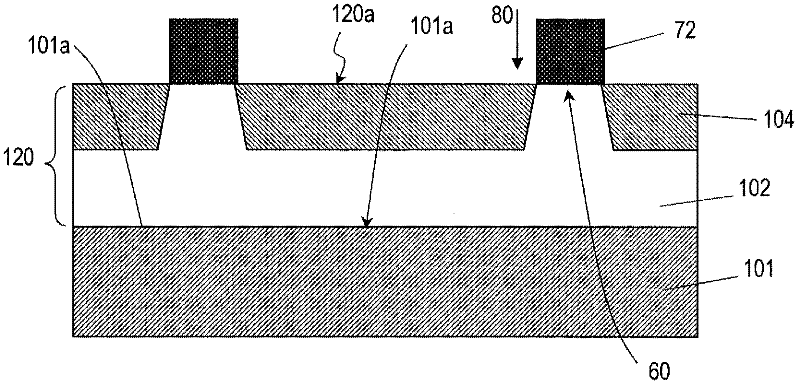
Disclosed is a semiconductor device (100) comprising: a semiconductor substrate (101); a first silicon carbide layer (120) which is arranged on the main surface of the semiconductor substrate (101); a first first-conductivity-type impurity region (103), a second-conductivity-type body region (104), and a second-conductivity-type contact region (131) formed in a deeper place than the first impurity region (103) in the body region and containing a second-conductivity-type impurity at a higher concentration than that in the body region, all of which are arranged in the first silicon carbide layer; a first-conductivity-type drift region (102); and a first ohmic electrode (122) which is in ohmically contact with the first impurity region (103) and the contact region (131). In the first silicon carbide layer (120), a contact trench (121) that penetrates the first impurity region (103) is provided. The first ohmic electrode (122) is arranged in the contact trench (121) so as to contact with the contact region (131) at at least a part of a lower part (121cL) of the side wall or the bottom surface (121b) of the contact trench.
Owner:PANASONIC CORP
PEG-IFN omega conjugate and preparation technique thereof
InactiveCN101491682AGood long-term effectReduce the number of injectionsPeptide/protein ingredientsDigestive systemHalf-lifeSide effect
The invention discloses a pegylation recombinant interferon omega conjugate and a preparation process thereof. Compared with an unmodified interferon omega, the pegylation recombinant interferon omega conjugate has longer half-life in a human body, can obviously reduce the number of medication, reduce toxic and side effect, is particularly suitable for treating chronic viral infection sensitive to the interferon omega, and has wide application prospect clinically.
Owner:BEIJING KAIZHENG BIOTECH DEV
Low-temperature polysilicon thin film transistor display panel and manufacturing method thereof
PendingCN110349859AReduce the number of injectionsGuaranteed thicknessTransistorSolid-state devicesEngineeringIon implantation
The invention provides a low-temperature polysilicon thin film transistor display panel and a manufacturing method thereof. The manufacturing method comprises the steps: providing a substrate; forminga polysilicon layer, wherein the polysilicon layer is formed on the substrate; forming a first insulating layer, wherein the first insulating layer covers the polysilicon layer; forming a second insulating layer, wherein the second insulating layer covers the first insulating layer; forming a metal layer, wherein the metal layer is formed on the second insulating layer; patterning the metal layerand the second insulating layer so as to form a first patterned metal layer and a patterned second insulating layer; patterning the first patterned metal layer so as to form a second patterned metallayer; and injecting ions into the polysilicon layer so as to simultaneously form a heavily doped region and a lightly doped region.
Owner:AU OPTRONICS KUNSHAN CO LTD
Veterinary ciprofloxacin lactate injection and preparation method thereof
InactiveCN102697725AEasy to useReduce stress responseAntibacterial agentsOrganic active ingredientsAnimal scienceCiprofloxacin lactate
The invention relates to the technical field of veterinary administration, in particular to a veterinary ciprofloxacin lactate injection. The veterinary ciprofloxacin lactate injection consists of the following components in percentage by weight: 4-10 percent of ciprofloxacin lactate counted by ciprofloxacin, 10-15 percent of polyoxyethylene 40 hydrogenated castor oil, 0.5-1.0 percent of carbomer, 0.1 percent of sodium bisulfite, 0.01 percent of ethylene diamine tetraacetic acid, and the balance of water for injection. The injection is convenient to use; compared with the conventional injection, the veterinary ciprofloxacin lactate injection has the advantages: the injection times are reduced from twice a day to once a day, treatment cost is saved, stress caused by repeated injection for animals is reduced, and medicament tolerance of bacteria is avoided; the veterinary ciprofloxacin lactate injection has low toxicity and a reliable curative effect, acts for a long time, and can be used for effectively treating white scour of piglets and white diarrhea; and the swine muscle injection half-life period is 22.96 hours, which is equivalent to five times that of an ordinary injection (4.6 hours).
Owner:山东省健牧生物药业有限公司
Energy storage device and assembling method thereof
ActiveCN103700511AReduce contact resistanceImprove power characteristicsHybrid capacitor electrodesHybrid cases/housings/encapsulationsElectrical resistance and conductanceEngineering
The invention discloses an energy storage device. The energy storage device comprises a shell, a battery core, an anode inner terminal, a cathode inner terminal, a cathode outer terminal and electrolyte, wherein the anode inner terminal and the cathode inner terminal are respectively welded on the end surfaces of an anode and a cathode of the battery core, and the periphery of the anode inner terminal is inserted in an annular groove on the bottom wall of the shell; a welding seam is formed in a way that the cathode inner terminal and the cathode outer terminal are welded together, an O-shaped sealing ring can cover the welding seam, and a roll rim at the opening part of the shell is pressed on the cathode outer terminal through an annular sealing washer; an upper annular side bulge and a lower annular side bulge, which face the inner part of the shell and have pressing functions, are respectively arranged in the positions, which correspond to the cathode inner terminal and the anode inner terminal, on the side wall of the shell. The invention also discloses an assembling method of the energy storage device. The energy storage device disclosed by the invention has the advantages that the stability is good, the resistance is low, and the reliability is good; the structure is simple, the machining and forming cost is low, and the assembling is easy.
Owner:KBR POWER QUALITY MANAGEMENT SHANGHAI
Variable doped junction terminal preparation method
ActiveCN105914133AReduce the number of injectionsAvoid injection dielectric film thickness variationSemiconductor/solid-state device manufacturingStep heightDielectric layer
The present invention discloses a variable doped junction terminal preparation method. An etching barrier layer is processed on the surface of a dielectric layer to form an echelonment medium morphology and then form the junction terminal of a grading structure through ion implantation. Through strictly controlling the etching speed ratio of two layers of medium, the variable doped junction terminal preparation method realizes step height accurate control and avoids the injection medium film thickness changing caused by etching ratio drifting. The injection dosage of each area of the junction terminal may be accurately controlled, and the number of times of ion injection in the device processing is reduced.
Owner:NO 55 INST CHINA ELECTRONIC SCI & TECHNOLOGYGROUP CO LTD
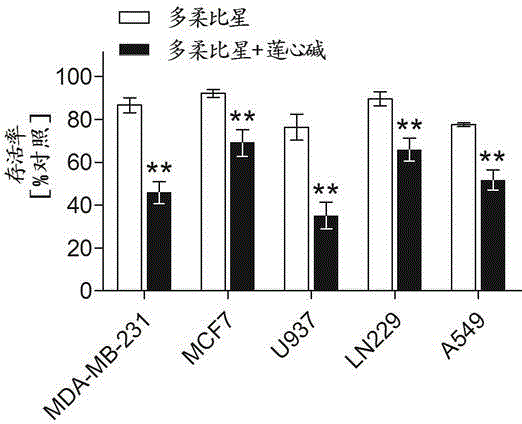
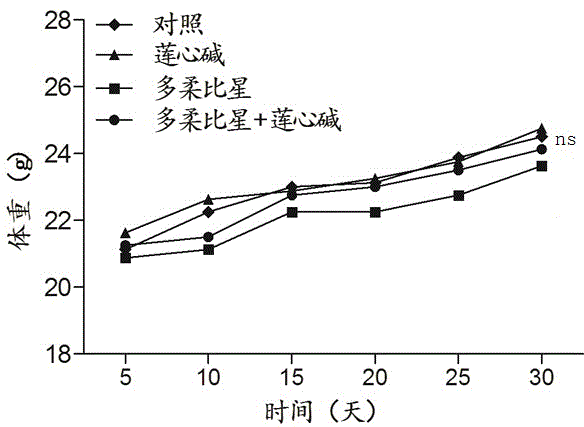
Pharmaceutical composition using liensinine to work in coordination with doxorubicin for chemotherapy and application method of pharmaceutical composition
InactiveCN104586870AGood effectInhibition of killingOrganic active ingredientsAntineoplastic agentsDrug withdrawalTherapeutic effect
The invention relates to a pharmaceutical composition using liensinine to work in coordination with doxorubicin for chemotherapy and an application method of the pharmaceutical composition. The pharmaceutical composition comprises a liensinine injection solution and a chemotherapy drug doxorubicin injection solution. The application method comprises the following steps: (1) performing intravenous injection of liensinine once daily according to the dose that actual dosage in the liensinine injection solution to the body weight of a patient is equal to 4.88mg / kg; (2) performing intravenous injection of doxorubicin once according to the dose that the actual dosage in the doxorubicin injection solution to the body weight of the patient is equal to 0.16mg / kg on the day of injecting liensinine according to the step (1); (3) performing intravenous injection of the doxorubicin injection solution once again according to the dose in the step (2) on the sixth day; and (4) determining whether to circulate according to the method of the steps (1)-(3) or not, the number of circulation times and the intermediate drug withdrawal time from the eleventh day according to the bearing capacity and the treatment effect of a patient body. Compared with the prior art, in the aspect of inhibiting and killing various malignant tumor cells, the pharmaceutical composition provided by the invention has the advantages of reducing the dosage, shortening the treatment period and reducing the occurrence of toxic and side reactions.
Owner:ARMY MEDICAL UNIV
Long-acting recombinant human chorionic gonadotropin fusion protein
ActiveCN103897064BProlong half-life in vivoImprove bioavailabilityPeptide/protein ingredientsPeptide preparation methodsProtein compositionHalf-life
The invention discloses a recombinant human chorionic gonadotrophin-Fc fusion protein (HCG-Fc for short) and a preparation method thereof. The HCG-Fc protein is a dimerized fusion protein, and the amino acid sequence of the fusion protein sequentially comprises an HCG beta subunit, an HCG alpha subunit, a flexible peptide joint and a human IgG Fc mutant from an N end to a C end. Compared with the existing chorionic gonadotrophin, the fusion protein has longer in-vivo half life, higher bioavailability and lower side effect. The invention also relates to an application of a recombinant HCG-Fc fusion protein composition in preparation of medicaments for treating and / or preventing infertility.
Owner:UNICOHEALTH CO LTD
Subcutaneous injection administration system based on Brij97 liquid crystal and preparation method of subcutaneous injection administration system based on Brij97 liquid crystal
InactiveCN104173278AReduce the number of injectionsGuaranteed curative effectPeptide/protein ingredientsPharmaceutical delivery mechanismHypodermoclysisSide effect
The invention discloses a subcutaneous injection administration system based on Brij97 liquid crystal and a preparation method of the subcutaneous injection administration system based on Brij97 liquid crystal. The preparation of the administration system comprises the following components in percentage by weight: 0.05-5 percent of medicines, 60-85 percent of Brij97, 0-25 percent of an adding material and 10-40 percent of water, wherein the content ratio of Brij97 to water is more than 7:3. Compared with a common oleophylic liquid-crystal vector material, the administration system has the advantages of excellent long-acting sustained release performance, good bio-compatibility, small toxic and side effect and small stimulation due to addition of the hydrophilic liquid crystal material Brij97, and is applicable to subcutaneous injection administration of protein polypeptide medicines.
Owner:CHINA PHARM UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com