Insulin analogue having quick response and stability under acidic condition and preparation thereof
A technology of insulin analogs and long-acting insulin, applied in the direction of insulin, medical preparations containing active ingredients, specific peptides, etc., can solve problems such as product instability, incomplete product safety data, hypoglycemia, etc., to delay the complications of diabetes symptoms, reduce the risk of hypoglycemia, and reduce the incidence of hypoglycemia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
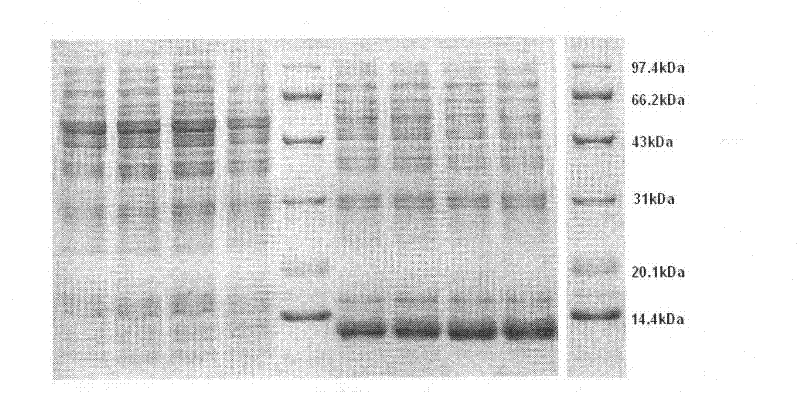
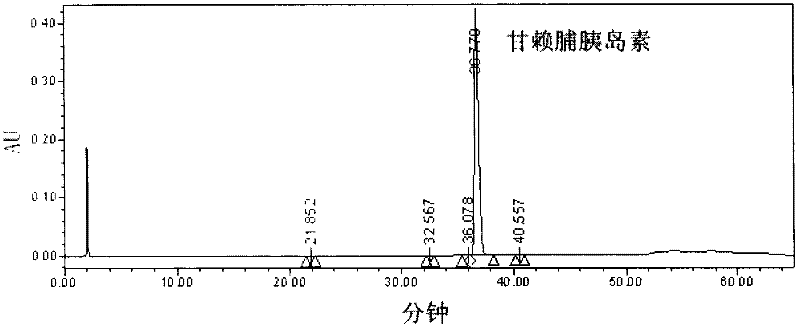
[0029] Embodiment 1 Preparation and Confirmation of Insulin Analogs of the Present Invention
[0030] Material:
[0031] Strains:
[0032] The cloned strain Escherichia coli (DH5a) is a common tool strain for genetic engineering.
[0033] The expression strain Escherichia coli (3110) was purchased from the American Type Culture Collection (ATCC). Enzymes and Reagents:
[0034] Molecular cloning tools, enzymes and reagents were purchased from Jingke Hongda Company, Beijing.
[0035] Plasmid extraction kits and PCR purification kits were purchased from Beijing Tiangen Company (TianGen).
[0036] Site-directed mutagenesis kit (Fast Mutagenesis System) was purchased from TransGene.
[0037] Medium:
[0038] LB medium, ampicillin-resistant LB medium, M9 medium, and enrichment medium are commonly used in the field of genetic engineering. For the formula of each medium, please refer to Appendix 2 of the third edition of Molecular Cloning.
[0039] method:
[0040] Plasmid...
Embodiment 2
[0077] Embodiment 2 The stability of insulin analog of the present invention under acidic conditions
[0078] The insulin analogs of the present invention prepared by the method of Example 1 were respectively placed in solutions with a pH value of 4.0±0.2, and the obtained solutions were in a clear state. After the above solution was stored at 2-8°C for 9 months, the total increase of related proteins in various insulin analog solutions was no more than 1.26%; the increase of high molecular protein was no more than 0.55%; More than 1%, all in the 95%-105% range.
[0079] The above results indicate that the insulin analogs of the present invention exhibit good stability under acidic conditions because the asparagine at position A21 that is easily deaminated under acidic conditions is replaced by glycine.
Embodiment 3
[0080] The preparation method and component analysis of embodiment 3 pharmaceutical preparations of the present invention
[0081] 1. the preparation method of pharmaceutical preparation of the present invention
[0082] :
[0083] Insulin lispro: prepared by the method of Example 1.
[0084] Insulin glargine: provided by Gan & Lee Pharmaceutical Co., Ltd., batch number GLGB09001.
[0085] Hydrochloric acid (batch number 20070802) and sodium hydroxide (batch number 20090804) were purchased from Hunan Erkang Pharmaceutical Co., Ltd.; zinc chloride (batch number 20090522) and m-cresol (07496PK) were purchased from Shanghai Jinghua Co., Ltd.; glycerin was purchased from Zhejiang Suichanghui Kang Pharmaceutical Co., Ltd., batch number 20090921.
[0086] :
[0087] Preparation of insulin mother solution: Weigh 500,000 units each of insulin lispro and insulin glargine accurately, add them to 3 liters of distilled water, stir and suspend evenly, add dropwise 3M hydrochloric acid ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com