GLP-I analogue liraglutide sustained-release microspheres and preparation method thereof
A sustained-release microsphere preparation and technology of sustained-release microspheres, which are used in drug combinations, pharmaceutical formulations, non-active components of polymer compounds, etc., can solve the problems of reduced dosing frequency and poor patient compliance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
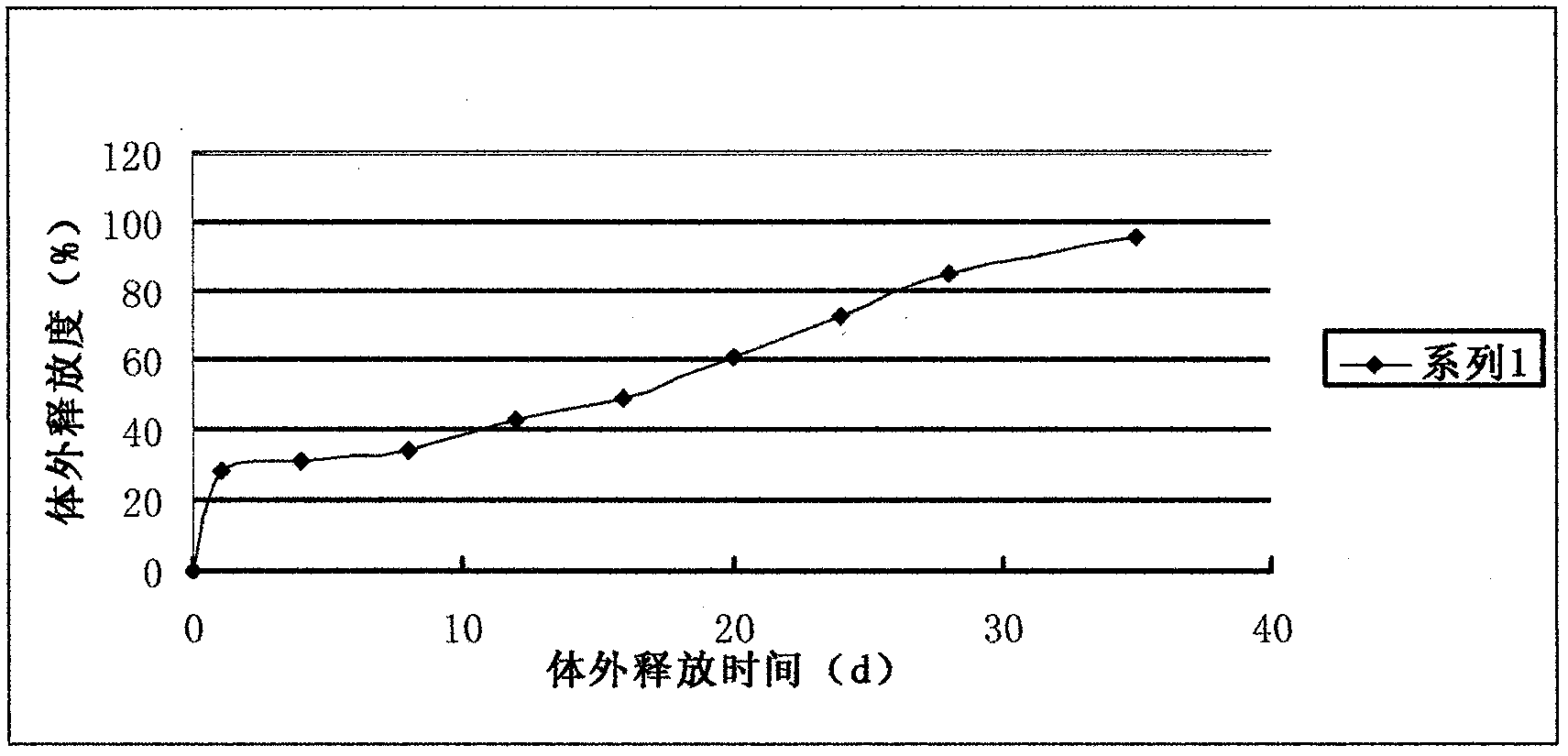
[0022] Weigh 20 mg of liraglutide and dissolve it in distilled water to obtain the inner water phase; weigh 1000 mg of PLGA (polymerization ratio = 1:3) and dissolve it in dichloromethane to obtain the oil phase. Preparation concentration is 50ml of the PVA solution of 5% and the PVA solution of 0.5% is 500ml. First transfer the liraglutide solution into the dichloromethane solution dissolved with PLGA, put it on an emulsification disperser at high speed (30000rpm) for 30 seconds at room temperature, and then transfer the obtained W / O emulsion to 20ml with a concentration of 5% Put it in the PVA solution of 5000rpm on the emulsification disperser, and mix it evenly for 1 minute to get the W / O / W type double emulsion. Stir at low speed for 2 hours, centrifuge, collect the obtained microspheres, wash with distilled water several times, then collect by centrifugation, freeze-dry, and pack into liraglutide sustained-release microsphere preparations with an actual drug loading of 4....
Embodiment 2
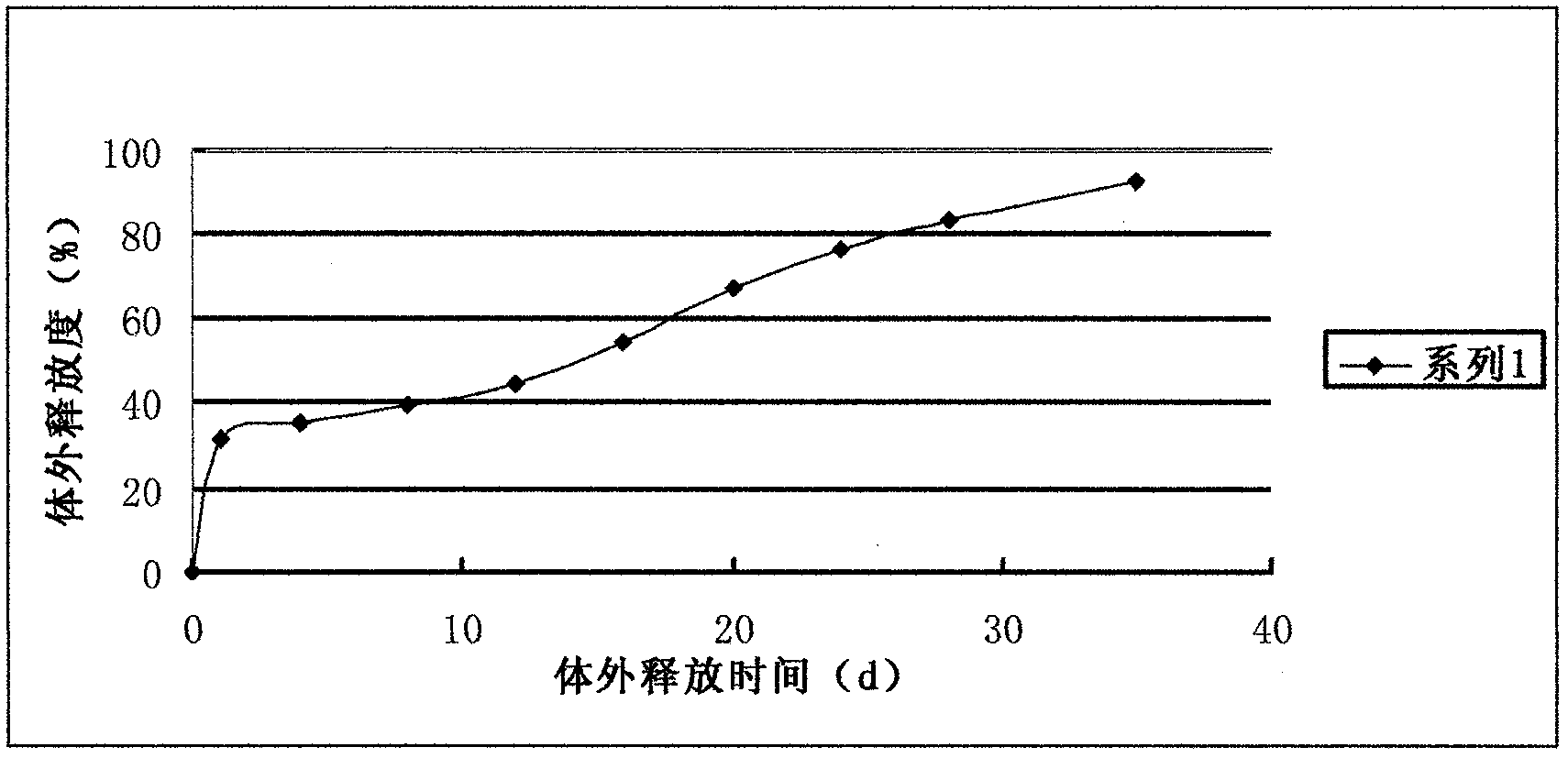
[0025]Weigh 15 mg of liraglutide and dissolve it in distilled water to obtain the inner water phase; weigh 1000 mg of PLGA (polymerization ratio = 1:1) and dissolve it in dichloromethane to obtain the oil phase. Preparation concentration is 50ml of the PVA solution of 5% and the PVA solution of 0.5% is 500ml. First transfer the liraglutide solution into the dichloromethane solution dissolved with PLGA, put it on an emulsification disperser at high speed (30000rpm) for 30 seconds at room temperature, and then transfer the obtained W / O emulsion to 20ml with a concentration of 5% In the PVA solution, place it on an emulsifying disperser at a speed of 5000rpm, and mix it evenly for 1 minute to obtain a W / O / W type double emulsion, move it into 500ml of 0.5% PVA solution, place it on a mechanical stirrer, Stir at low speed for 2 hours, centrifuge, collect the obtained microspheres, wash with distilled water several times, then collect by centrifugation, freeze-dry, and pack into lir...
Embodiment 3
[0028] Weigh 25 mg of liraglutide and dissolve it in distilled water to obtain the inner water phase; weigh 500 mg of PLA and 1500 mg of PLGA (polymerization ratio = 3:1) and dissolve it in dichloromethane to obtain the oil phase. Preparation concentration is 100ml of 5% PVA solution and 1000ml of 0.5% PVA solution. First transfer the liraglutide solution into the dichloromethane solution dissolved with PLA and PLGA, place it on an emulsification disperser at high speed (30000rpm) for 30 seconds at room temperature, and then transfer the obtained W / O emulsion to 20ml with a concentration of 5% PVA solution, placed on an emulsifying disperser at a speed of 5000rpm, and homogenized for 1 minute to obtain a W / O / W type double emulsion, moved into 500ml of 0.5% PVA solution, placed on a mechanical stirrer, and Stir at a low speed of 500rpm for 2 hours, centrifuge, collect the obtained microspheres, wash with distilled water several times, then collect by centrifugation, freeze-dry,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com