Haemophilus influenzae type b (Hib) polysaccharide and refined tetanus toxoid coupling process
A technology of tetanus toxoid and polysaccharide, which is applied in the directions of inactive medical preparations, carrier-binding antigen/hapten components, antibacterial drugs, etc., can solve the problems of wasting time and solution, easily polluted, etc. Dosage, reduce the chance of pollution, improve work efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] A Hib polysaccharide and refined tetanus toxoid coupling process, which includes two steps of Hib polysaccharide-AH derivative generation and Hib polysaccharide-TT conjugate preparation,
[0022] The generation of the Hib polysaccharide-AH derivative comprises the following substeps:
[0023] A1, dissolving the polysaccharide, weighing the Hib polysaccharide, adding sterilized water for injection, so that the final concentration is 1mg / ml;
[0024] A2, polysaccharide activation, according to the amount of 0.3 times Hib polysaccharide, add CNBr to activate, and keep the pH at 9.8 and react for 25 minutes;
[0025] A3, generate, add ADH 3 times the amount of Hib polysaccharide for connection, and keep the pH at 8.0 for 20-30 minutes;
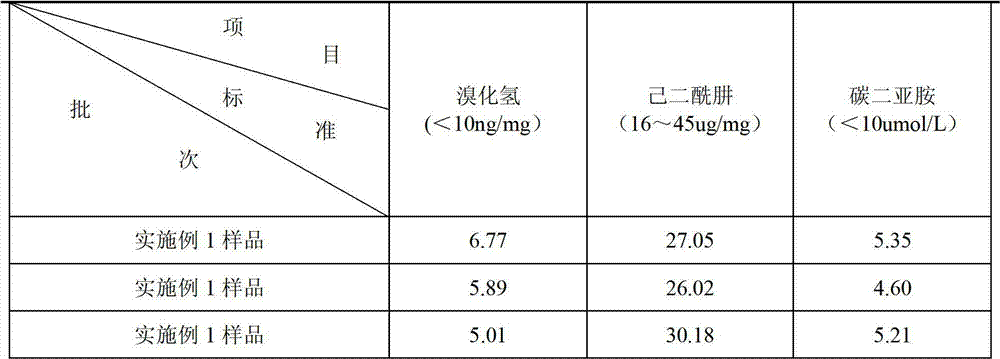
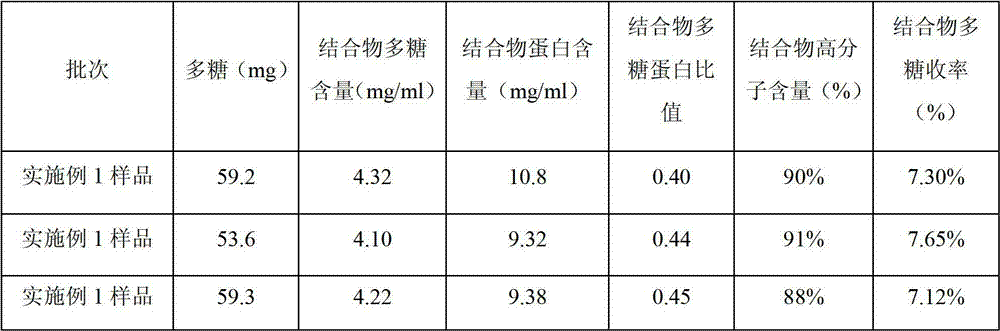
[0026] A4. For purification, use a 30KD membrane bag, first dilute with 0.05mol / L NaCl at a volume ratio of 1:1, then ultrafilter to 1 / 2 volume, repeat dilution and ultrafiltration 13 times. After completion, filter with a 0.45 μm filter ...
Embodiment 2
[0035] A Hib polysaccharide and refined tetanus toxoid coupling process, which includes two steps of Hib polysaccharide-AH derivative generation and Hib polysaccharide-TT conjugate preparation,
[0036] The generation of the Hib polysaccharide-AH derivative comprises the following substeps:
[0037] A1, dissolving the polysaccharide, weighing the Hib polysaccharide, adding sterilized water for injection, so that the final concentration is 2 mg / ml;
[0038] A2, polysaccharide activation, add CNBr according to the amount of 0.2 times Hib polysaccharide to activate, and keep the pH at 9.5 and react for 30 minutes;
[0039] A3, generate, add ADH 5 times the amount of Hib polysaccharide for connection, and keep the pH at 8.5 for 20 minutes;
[0040] A4. For purification, use a 50KD membrane bag, first dilute with 0.02mol / L NaCl at a volume ratio of 1:1, then ultrafilter to 1 / 2 volume, repeat dilution and ultrafiltration 15 times. After completion, filter with a 0.45 μm filter mem...
Embodiment 3
[0049] A Hib polysaccharide and refined tetanus toxoid coupling process, which includes two steps of Hib polysaccharide-AH derivative generation and Hib polysaccharide-TT conjugate preparation,
[0050] The generation of the Hib polysaccharide-AH derivative comprises the following substeps:
[0051] A1, dissolving the polysaccharide, weighing the Hib polysaccharide, adding sterilized water for injection, so that the final concentration is 1mg / ml;
[0052] A2, polysaccharide activation, add CNBr to activate according to the amount of 0.6 times Hib polysaccharide, and keep the pH at 10.5 for 20 minutes;
[0053] A3, generate, add ADH 2 times the amount of Hib polysaccharide for connection, and keep the pH at 7.5 for 30 minutes;
[0054] A4. For purification, use a 40KD membrane bag, first dilute with 0.03mol / L NaCl at a volume ratio of 1:1, then ultrafilter to 1 / 2 volume, repeat dilution and ultrafiltration 10 times. After completion, filter with a 0.45 μm filter membrane to o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com