Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
190 results about "Toxin protein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Protein toxins are usually produced by bacteria or plants. Many bacterial toxins are comprised of two parts: the cell binding portion interacts with cell surface while the enzymatic portion enters the cytosol and generates toxicity. Common protein toxins include pseudomonas exotoxin (PE), diphtheria toxin...
Novel bacillus thuringiensis isolate
A novel bacterial strain of Bacillus thuringiensis, VBTS 2528, is described. This strain comprises genes encoding Cry1Ac, Cry 1Ca, and Cry2Aa endotoxin proteins. The invention further relates to an insecticidal composition comprising a mixture of VBTS 2528 and to methods for controlling insect pests utilizing VBTS 2528.
Owner:VALENT BIOSCIENCES CORP
Mixing and matching TC proteins for pest control
InactiveUS7491698B2High activityEffective control of widerBiocidePeptide/protein ingredientsHeterologousToxin protein
The subject invention relates to the surprising discovery that toxin complex (TC) proteins, obtainable from Xenorhabdus, Photorhabdus, and Paenibacillus, can be used interchangeably with each other. In particularly preferred embodiments of the subject invention, the toxicity of a “stand-alone” TC protein (from Photorhabdus, Xenorhabdus, or Paenibacillus, for example) is enhanced by one or more TC protein “potentiators” derived from a source organism of a different genus from which the toxin was derived. As one skilled in the art will recognize with the benefit of this disclosure, this has broad implications and expands the range of utility that individual types of TC proteins will now be recognized to have. Among the most important advantages is that one skilled in the art will now be able to use a single set of potentiators to enhance the activity of a stand-alone Xenorhabdus protein toxin as well as a stand-alone Photorhabdus protein toxin. (As one skilled in the art knows, Xenorhabdus toxin proteins tend to be more desirable for controlling lepidopterans while Photorhabdus toxin proteins tend to be more desirable for controlling coleopterans.) This reduces the number of genes, and transformation events, needed to be expressed by a transgenic plant to achieve effective control of a wider spectrum of target pests. Certain preferred combinations of heterologous TC proteins are also disclosed herein. Other objects, advantages, and features of the subject invention will be apparent to one skilled in the art having the benefit of the subject disclosure.
Owner:DOW AGROSCIENCES LLC
Hemipteran-and coleopteran active toxin proteins from Bacillus thuringiensis
A novel Bacillus thuringiensis crystal protein exhibiting insect inhibitory activity is disclosed. Growth of Lygus insects is significantly inhibited by providing the novel crystal protein in Lygus insect diet. Polynucleotides encoding the crystal protein, transgenic plants and microorganisms that contain the polynucleotides, isolated peptides derived from the crystal protein, and antibodies directed against the crystal protein are also provided. Methods of using the crystal protein and polynucleotides encoding the crystal protein to control Hemipteran insects are also disclosed.
Owner:MONSANTO TECH LLC
Soluble recombinant botulinum toxin proteins
The present invention includes recombinant proteins derived from Clostridium botulinum toxins. In particular, soluble recombinant Clostridium botulinum type A, type B and type E toxin proteins are provided. Methods which allow for the isolation of recombinant proteins free of significant endotoxin contamination are provided. The soluble, endotoxin-free recombinant proteins are used as immunogens for the production of vaccines and antitoxins. These vaccines and antitoxins are useful in the treatment of humans and other animals at risk of intoxication with clostridial toxin.
Owner:ALLERGAN INC
Novel Hemipteran and Coleopteran Active Toxin Proteins From Bacillus Thuringiensis
A novel Bacillus thuringiensis crystal protein exhibiting insect inhibitory activity is disclosed. Growth of Lygus insects is significantly inhibited by providing the novel crystal protein in Lygus insect diet. Polynucleotides encoding the crystal protein, transgenic plants and microorganisms that contain the polynucleotides, isolated peptides derived from the crystal protein, and antibodies directed against the crystal protein are also provided. Methods of using the crystal protein and polynucleotides encoding the crystal protein to control Hemipteran insects are also disclosed.
Owner:MONSANTO TECH LLC
High level expression of recombinant toxin proteins
ActiveUS20110287443A1High yieldQuality improvementAntibody mimetics/scaffoldsBacteria peptidesBacteroidesHigh level expression
The present invention relates to the field of recombinant toxin protein production in bacterial hosts. In particular, the present invention relates to production processes for obtaining high levels of a recombinant CRM197, Diphtheria Toxin, Pertussis Toxin, Tetanus Toxoid Fragment C, Cholera Toxin B, Cholera holotoxin, and Pseudomonas Exotoxin A, from a bacterial host.
Owner:PFENEX
Thermostable Vaccine Compositions and Methods of Preparing Same
InactiveUS20130309273A1Reduce concentrationSmall particle sizePowder deliveryLyophilised deliveryAdjuvantRicin
The disclosure provides compositions relating to thermostable vaccines and methods of preparing same. Specifically, the disclosure provides for methods of preparing thermostable vaccines based on a recombinant ricin neurotoxin protein and uses of co-adjuvants to develop a composition capable of eliciting an immune response in a subject.
Owner:SOLIGENIX INC +1
Agricultural chemical composition containing fipronil and bacillus thuringiensis
InactiveCN101248800AOvercome the disadvantage of easy resistanceImprove efficacyBiocideAnimal repellantsFipronilAdditive ingredient
The invention belongs to the pesticide field and particularly relates to a synergic pesticidal composition with active ingredients to be the combinations of fipronil and a series of subspecies of Bacillus thuringiensis (Bt for short), wherein the Bacillus thuringiensis subspecies include six subspecies: Bacillus thuringiensis var.kurstaki, Bacillus thuringiensis var.aizawa, Bacillus thuringiensis var.tianmensis, B.thuringiensis var.israelensis, Bacillus thuringiensis subsp.aizawai and Bacillus thuringiensis subsp. kenyae. The main agent thereof includes the following active ingredients by the weight percentages as follows: fipronil 0.1%-80%, Bacillus thuringiensis 100-100000 IU (international unit) / mg, or 10-1000 billion spore / g, or 0.01-20% toxin protein, and the rest is an accessory ingredient. The pesticidal composition can be efficiently used for controlling agricultural pests such as rice leaf rollers, chilo suppressalis walkers, diamondback moths, prodenia lituras, corn borers, and so on, and has particular characteristics of high efficacy, long lasting period and safe usage, so that the pesticidal composition belongs to environment-friendly pesticides and can prolong the generation of resistance, reduce production cost and increase economic benefit.
Owner:JIANGMEN PLANT PROTECTION
High level expression of recombinant toxin proteins
The present invention relates to the field of recombinant toxin protein production in bacterial hosts. In particular, the present invention relates to production processes for obtaining high levels of a recombinant CRM197, Diphtheria Toxin, Pertussis Toxin, Tetanus Toxoid Fragment C, Cholera Toxin B, Cholera holotoxin, and Pseudomonas Exotoxin A, from a bacterial host.
Owner:PELICAN TECH HLDG INC
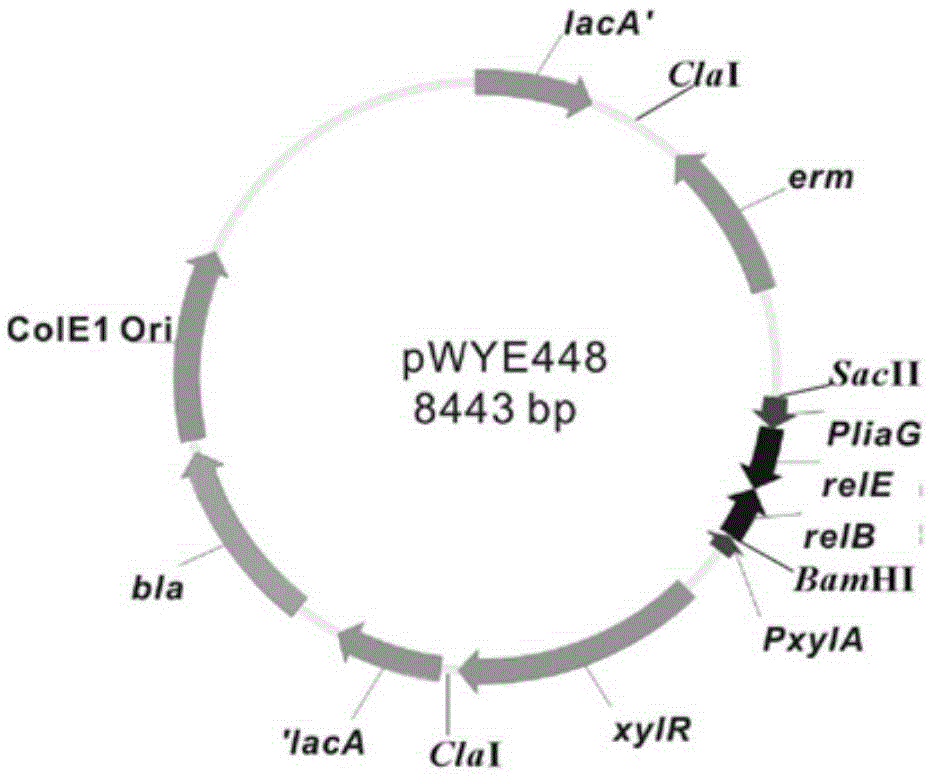
Bacterial traceless genetic manipulation vector and construction method and application thereof
ActiveCN106676119AAvoid toxicityImprove function and effectBacteriaVector-based foreign material introductionAntibiotic YToxin protein
The invention provides a bacterial traceless genetic manipulation vector. The vector contains a toxin inverse-screening element controlled by an antitoxin switch and an antibiotics resistance gene. The invention also provides a construction method of the vector and a method for application of the vector in bacterial traceless gene knock-out, knock-in, replacement, point mutation and insertion and deletion of large DNA fragments (larger than 194kb). By the utilization of a constitutive promoter for continuous expression of toxin protein and by the utilization of an inducible promoter for inducible expression of antitoxin protein, the toxin inverse-screening element (TCCRAS) controlled by the antitoxin switch is established, and the problem that specificity of existing genetic manipulation systems of gram-positive bacterium is not high, the systems are instable and screening is difficult is solved.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Hemipteran and coleopteran active toxin proteins from Bacillus thuringiensis
A novel Bacillus thuringiensis crystal protein exhibiting insect inhibitory activity is disclosed. Growth of Lygus insects is significantly inhibited by providing the novel crystal protein in Lygus insect diet. Polynucleotides encoding the crystal protein, transgenic plants and microorganisms that contain the polynucleotides, isolated peptides derived from the crystal protein, and antibodies directed against the crystal protein are also provided. Methods of using the crystal protein and polynucleotides encoding the crystal protein to control Hemipteran insects are also disclosed.
Owner:MONSANTO TECH LLC
Pesticidal Toxin Proteins Active Against Coleopteran Insects
ActiveUS20150274786A1Low costImprove breeding efficiencyBiocidePeptide/protein ingredientsNucleotideInsect pest
The invention generally relates to the field of insect inhibitory toxin proteins. A novel class of proteins exhibiting insect inhibitory activity against agriculturally relevant pests of crop plants and seeds are disclosed. Insecticidal activity is particularly effective against the Coleopteran order of insect pests. Plants, plant parts, and seed are provided containing a polynucleotide construct encoding one or more of the toxin proteins disclosed herein. The proteins are referred to herein variously as the TIC2463-related toxin protein class or family, the TIC2463-related toxin proteins, the TIC2463-related protein genus, toxin proteins related to the TIC2463 toxin protein, proteins related to TIC2463, TIC2463-related toxin polypeptides, TIC2463-related pesticidal proteins, and the like.
Owner:MONSANTO TECH LLC
Compositions and Methods of Topical Application and Transdermal Delivery of Botulinum Toxins without Reduced Non-Toxin Proteins
InactiveUS20070116724A1Low antigenicityBlood stabilityCosmetic preparationsNervous disorderHypodermoclysisEpithelium
This invention relates to novel compositions of botulinum toxin that can be applied topically for various therapeutic, aesthetic and / or cosmetic purposes. The compositions may include botulinum toxin complexes, wherein the amounts of hemagglutinin, non-toxin non-hemagglutinin and / or exogenous albumin are selectively and independently reduced compared to conventional commercially available botulinum toxin. The compositions may further contain molecules that are not native to botulinum toxin and that bind non-covalently to the botulinum toxin complexes, thereby acting as skin-tropic “adhesion molecules” to improve the ability of the toxin complexes to adhere to and to penetrate the skin epithelium. The compositions have an improved safety profile compared to existing botulinum-containing compositions that are injected subcutaneously. Methods for the use of such compositions are also contemplated by this invention.
Owner:REVANCE THERAPEUTICS INC
Clostridium perfringens alpha toxin double-antibody sandwich ELIS quantitative determination method
ActiveCN103941004AStrong specificityStrong responsivenessMaterial analysisAlpha-toxinClostridium perfringens toxoid
The invention relates to a clostridium perfringens alpha toxin double-antibody sandwich ELIS quantitative determination method. According to the clostridium perfringens alpha toxin double-antibody sandwich ELIS quantitative determination method, alpha toxin protein obtained via prokaryotic expression is taken as an immunogen; monoclonal antibodies obtained via hybridoma technique are taken as detection antibodies and capture antibodies; reaction conditions are optimized via experiments; alpha toxin protein samples with a series of concentration are used for construction of a standard curve; the double-antibody sandwich ELIS method is established; and indexes of the double-antibody sandwich ELIS are verified. The clostridium perfringens alpha toxin double-antibody sandwich ELIS quantitative determination method specifically comprises following steps: (1) prokaryotic expression of alpha toxin; (2) preparation of anti-alpha toxin monoclonal antibodies; (3) establishment of the double-antibody sandwich ELIS method; (4) establishment of the standard curve; and (5) performance evaluation on the double-antibody sandwich ELIS method. The clostridium perfringens alpha toxin double-antibody sandwich ELIS quantitative determination method is excellent in specificity, and high in sensitivity and stability, is fast and convenient, and can be used for effective quantitative determination of alpha toxin in A-E type clostridium perfringens cultural supernatants; and the high-efficient detection method is provided for alpha toxin determination.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Vaccine
InactiveUS6040427AReduce the binding forceLow toxicityBacterial antigen ingredientsBacteriaToxin proteinSaxitoxin
The Bordetella pertussis toxin is genetically modified to express a toxin protein which is deficient in target-cell receptor binding and is used in a vaccine for protection against whooping cough.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Method of preparing an immunologically-active adjuvant-bound dried vaccine composition
ActiveUS8444991B2Reduce concentrationAntibacterial agentsPowder deliveryAdjuvantClostridial Neurotoxin
The disclosure provides a method of preparing an immunologically-active adjuvant-bound freeze dried vaccine composition. A specific embodiment provides a stable vaccine composition comprising an aluminum-salt adjuvant, a recombinant Clostridium botulinum neurotoxin protein and a glass-forming agent. These vaccine compositions are useful in the treatment of humans and other animals at risk of infection from Clostridium botulinum neurotoxin.
Owner:UNIV OF COLORADO THE REGENTS OF
Recombinant toxin A and toxin B protein carrier for polysaccharide conjugate vaccines
InactiveUS20050202042A1Provide protectionConstant stableBiocideOrganic active ingredientsConjugate vaccineMicroorganism
The present invention provides for immunogenic compositions and their methods of use as vaccines and their method of preparation. These immunogenic compositions comprise a recombinant protein of toxin A or toxin B of Clostridium difficile conjugated to a polysaccharide of a microbial pathogen. The immunogenic compositions may include only a truncated portion of toxin A or toxin B, particularly the repeating units (rARU or rBRU), that is associated with a microbial pathogen polysaccharide. Such compositions are effective in eliciting T-cell dependent and antibody responses. These compositions are therefore effective as vaccines for humans, particularly children, and animals in affording protection against one or more microbial pathogens.
Owner:TECHLAB THERAPEUTICS
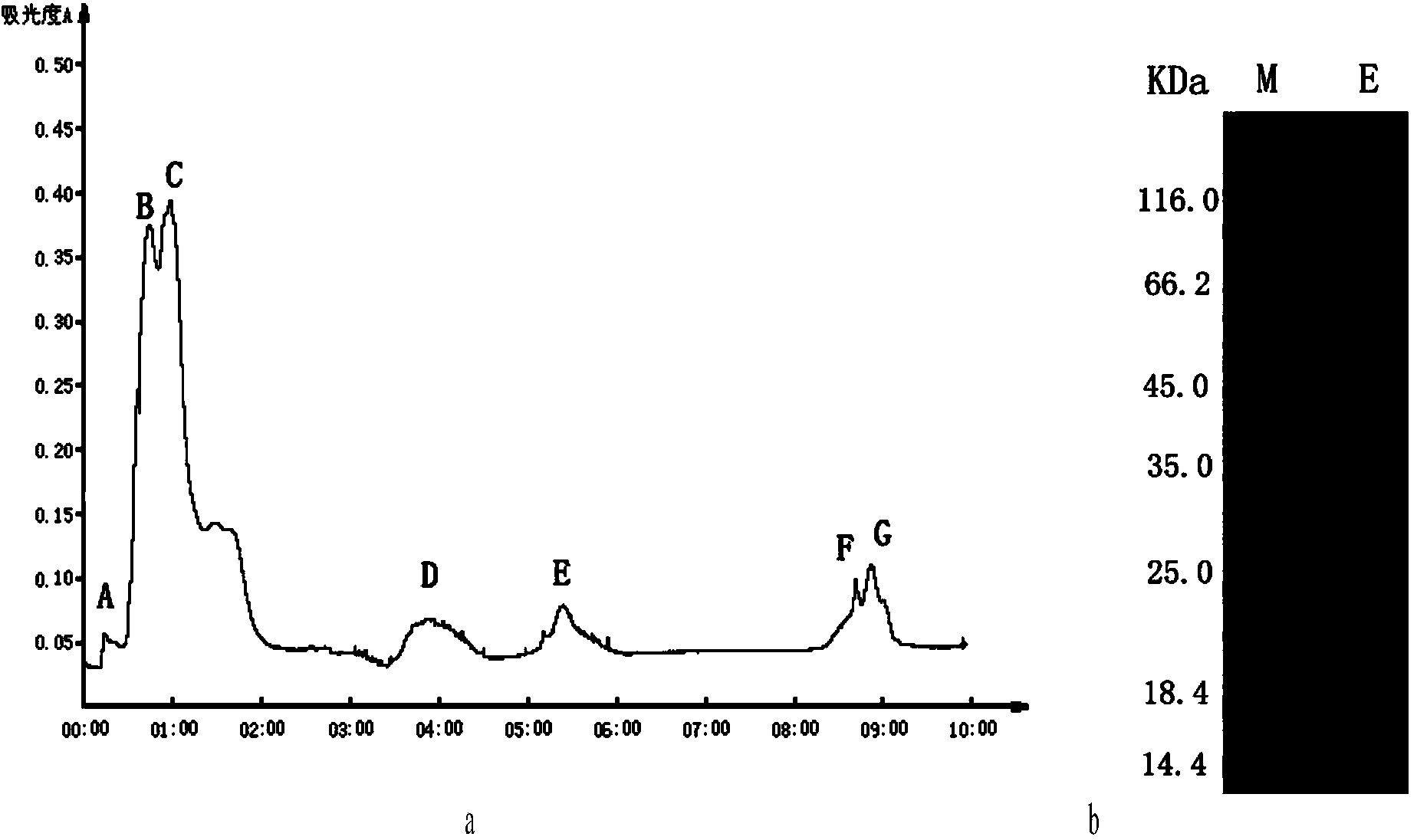
Method for extracting toxin protein from white cyanea tentacles
ActiveCN103739694AEasy extractionFree from destructionPeptide preparation methodsAnimals/human peptidesToxin proteinBuffer solution
The invention relates to the technical field of biologics, in particular to a method for extracting a toxin protein which causes vascular permeability increase from white cyanea tentacles. The method comprises the following steps: unfreezing fresh white cyanea tentacles which are frozen at a super-low temperature at a low temperature, washing by a small quantity of buffer solution, collecting the buffer solution, centrifuging to obtain a supernatant and using the supernatant as cyanea crude toxin, and sequentially separating by an ion chromatographic column and a gel chromatographic column to obtain the toxin protein CnP45 which causes vascular permeability increase, wherein the molecular weight of the toxin protein is 45kD. The extracting process of the jellyfish toxin is simplified, the interference of impurity proteins is reduced, and an important method instruction is provided for the further separation and purification of jellyfish toxin ingredients.
Owner:INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Detection kit for helicobacter pylori virulence protein antibody and detection method by using the same
InactiveCN102721815AStrong specificityDifferentiate between strong and weak virulenceBiological testingAntigenPositive control
The invention relates to a detection kit for helicobacter pylori virulence protein antibody and a detection method by using the same. The kit comprises a microwell plate, an enzyme-labeled secondary antibody, a negative control serum, a positive control serum, a substrate, a stop buffer and a washing liquor, wherein the microwell plate is coated with purified recombinant helicobacter pylori virulence proteins, the substrate is a solution containing 1% m / m 3, 3', 5, 5'-tetramethyl benzidine and 30% v / v hydrogen peroxide, and the recombinant helicobacter pylori virulence proteins are cytotoxin-associated protein CagA, vacuolating toxin protein VacA, flagellum protein subunit A, flagellum protein subunit B, urease subunit A, and urease subunit B. According to the invention, six purified helicobacter pylori virulence proteins are used as antigens which itself have high conservatism and specificity and are related to the symptom of a patient, so the kit has characteristics of high specificity, identification capacity for helicobacter pylori clinical strain virulence strength, and semi-quantitative detection capacity.
Owner:TIANJIN TIANFOLUO BIOLOGICAL TECH
Triple and four-prevention subunit vaccine for sheep and preparation method thereof
ActiveCN107308445ASufficient space to expandImprove biological activityAntibacterial agentsBacterial antigen ingredientsEuropean rabbitAdjuvant
The invention discloses triple and four-prevention subunit vaccine for sheep and a preparation method thereof and belongs to the field of prevention and control of animal epidemic diseases. The triple and four-prevention subunit vaccine is prepared from clostridium perfringens beta-epsilon fusion protein, clostridium septicum alpha toxin recombinant protein and an adjuvant. After beta-epsilon (beta and epsilon fusion proteins) of a clostridium perfringens C58-1 strain and alpha toxin protein of a clostridium septicum C55-2 strain, which are cloned and expressed, are purified and then are mixed with the adjuvant to prepare vaccine for immunizing white mice and domestic rabbits; the triple and four-prevention subunit vaccine for the sheep is checked to be qualified by utilizing inspection standards related to the triple and four-prevention subunit vaccine for the sheep in the third volume of 2015 version of China Veterinary Pharmacopoeia; an attacking toxin protection test result shows that animals immunized with the proteins have 100 percent protection efficiency on type B, type C and type D of clostridium perfringen and clostridium septicum. Therefore, the triple and four-prevention subunit vaccine can be used for replacing traditional triple and four-prevention vaccine.
Owner:SHANDONG BINZHOU ANIMAL SCI & VETERINARY MEDICINE ACADEMY
Expression and preparation methods for polyvalent multi-specific antibody and immune hybrid protein
ActiveCN106397598AComplete structureImprove stabilityHybrid immunoglobulinsAntibody mimetics/scaffoldsImmunoproteinToxin protein
The invention discloses expression and preparation methods for a polyvalent multi-specific antibody and an immune hybrid protein and belongs to the technical field of biology. According to the invention, by using characteristics of protein Intein, a novel method for preparing polyvalent specific antibodies, or antibody and cell factor merged hybrid immunoproteins, or antibody and toxin protein merged immunotoxins or antibody and other active protein merged immune hybrid proteins is designed and developed. According to the methods, products, i.e., the polyvalent specific antibody and the immune hybrid protein are prepared through expressing all parts of the hybrid protein separately in appropriate procaryotic or eucaryotic cell systems, carrying out high-performance affinity-chromatography purification and separation, and then, carrying out splicing in vitro under the condition of intein splicing. The methods have high production efficiency, are wide in applicable application range and facilitate the separation and purification of the products. The invention provides a novel method for preparing biological drugs for directional attack on cancers or other diseases.
Owner:SHANGHAI JIAO TONG UNIV +2
Bacterial toxin vaccine
ActiveUS20110231960A1Efficient productionAntibacterial agentsPeptide/protein ingredientsDNA constructToxin protein
A bacterial toxin protein such as a Shiga toxin protein is efficiently produced using plant cells. The plant cells are transformed using a DNA construct containing DNA encoding a hybrid protein in which the bacterial toxin proteins such as the Shiga toxin proteins are tandemly linked through a peptide having the following characteristics (A) and (B) to produce the bacterial toxin protein in the plant cells: (A) a number of amino acids is 12 to 30; and (B) a content of proline is 20 to 35%.
Owner:IDEMITSU KOSAN CO LTD +1
Indirect ELISA (enzyme-linked immuno sorbent assay) kit for detecting haemophilus parasuis antibody
ActiveCN103941020AHigh homologyImproved conservatismBiological material analysisBiological testingToxin proteinCytolethal distending toxin
The invention discloses an indirect ELISA (enzyme-linked immuno sorbent assay) kit for detecting a haemophilus parasuis antibody. The kit consists of an ELISA coating plate with haemophilus parasuis cytolethal distending toxin)-C protein serving as a coating antigen, a to-be-detected sample dilution plate, a positive contrast serum, a negative contrast serum, 20-time concentrated washing liquid, a serum sample dilution solution, an enzyme-labeled antibody working solution, a developing solution and a terminating solution. A judgment standard is that if an S / P value is less than 0.200, a sample to be detected is negative; if the S / P value is greater than or equal to 0.200, the to-be-detected sample is positive; the S / P value is obtained according to a formula: S / P value=(the mean value of the to-be-detected sample OD450nm-the mean value of a negative contrast OD450nm) / (the mean value of a positive sample OD450nm-the mean value of the negative contrast OD450nm). Due to the specificity test, the sensitivity test, the repetitiveness test, the coincidence rate test, the test for comparing the kit disclosed by the invention with a kit on sale, the clinical application test and the like, the kit disclosed by the invention has the characteristics of high specificity, high sensitivity, high repetitiveness and the like and is high in coincidence rate to the same type of products on sale home and abroad; the indirect ELISA kit can be used for clinical large-scale detection and epidemiological investigation for the haemophilus parasuis antibodies.
Owner:SOUTHWEST UNIVERSITY FOR NATIONALITIES
Bovine type-A clostridium perfringens subunit vaccine and preparation method and application thereof
ActiveCN107596361AStrong immune responseOvercoming the problem of incomplete inactivationAntibacterial agentsBacterial antigen ingredientsAntigenAlpha-toxin
The invention provides a bovine type-A clostridium perfringens subunit vaccine and a preparation method and an application thereof. The subunit vaccine comprises a bovine type-A clostridium perfringens alpha-toxin protein truncation body and a pharmacologically acceptable adjuvant. The bovine type-A clostridium perfringens alpha-toxin protein truncation body is an antigen determinant amino acid sequence from 255th to 372nd amino acids of alpha-toxin protein. The subunit vaccine has the following advantages that 1) safety problems due to uncompleted inactivation are not existed; 2) the qualityis controllable, and the difference between the batches is not existed; 3) the production equipment and space requirement is low, the expression level is high, and the cost is low; and 4) toxin dispersion risk is not existed, and the safety of operators can be guaranteed.
Owner:NOVO BIOTECH CORP
Method for preparing recombinant scorpion neurotoxin LqhIT2 protein
ActiveCN106701774AToxicLow toxicityMicroorganism based processesPeptide preparation methodsEscherichia coliPichia pastoris
The invention discloses a method for efficiently preparing recombinant active scorpion insect neurotoxin LqhIT2. The method mainly comprises a step (1) of optimizing and synthesizing LqhIT2 genes with C- ends provided with 6*His labels according to amino acid sequences of mature LqhIT2 toxins and characteristics of a pichia pastoris expression system and constructing pichia pastoris secretory expression vectors; a step (2) of converting recombinant vectors into a pichia pastoris host, and through screening, obtaining yeast transformants in high-level secretory expression; a step (3) of expressing recombinant scorpion insect neurotoxin LqhIT2 proteins in the pichia pastoris host, and quickly obtaining the recombinant scorpion insect neurotoxin LqhIT2 proteins with the purity over 98% through a Ni2+ affinity chromatography method, wherein the purified recombinant proteins have very strong poisonous activity on insect cells. The method is modified based on conventional expression of insect neurotoxin LqhIT2 through prokaryotic host escherichia coli, the method of using eukaryotic host pichia pastoris to express the insect neurotoxin LqhIT2 is explored, and the yeast transformants in high-level secretory expression are screened.
Owner:HUAIHUA UNIV
Preparation method of diphtheria toxoid vaccine
ActiveCN102961740AAvoid damageEfficient removalAntibacterial agentsBacterial antigen ingredientsFiberUltrafiltration
The invention discloses a preparation method of a diphtheria toxoid vaccine. The diphtheria toxoid vaccine is prepared by taking a diphtheria bacillus strain as a raw material and then carrying out the steps of diphtheria toxoid culture, bacteria-liquid separation, ultrafiltration and concentration, two-time salting out, ultrafiltration and desalination and the like. According to the preparation method, the thalli of the culture solution is removed by using a hollow fiber filter membrane and then refining is carried, so that the pore blockage caused by the accumulation of the thalli and other impurity fragments in the subsequent filter process is prevented; through an improved salting out method, the toxin proteins and other allergens in the culture solution are effectively removed; and a tangential flow ultrafiltration method is used for both the concentration of the culture solution and the desalting method after the salting out, so that the antigen damage caused by the shearing to the toxin proteins is decreased and the precipitation of the proteins is avoided. According to the preparation method, the preparation time of the diphtheria toxoid vaccine is shortened and the production efficiency is improved.
Owner:WUHAN INST OF BIOLOGICAL PROD CO LTD
Soluble recombinant botulinum toxin proteins
The present invention includes recombinant proteins derived from Clostridium botulinum toxins. In particular, soluble recombinant Clostridium botulinum type A, type B and type E toxin proteins are provided. Methods which allow for the isolation of recombinant proteins free of significant endotoxin contamination are provided. The soluble, endotoxin-free recombinant proteins are used as immunogens for the production of vaccines and antitoxins. These vaccines and antitoxins are useful in the treatment of humans and other animals at risk of intoxication with clostridial toxin.
Owner:ALLERGAN SALES ALLERGAN BOTOX
Method for producing recombinant scorpion toxin protein by adopting silkworm as parasitifer
InactiveCN101629186ALow costIncrease productionMicroorganism based processesGenetic engineeringForward primerToxin protein
The invention discloses a method for producing recombinant scorpion toxin protein by adopting a silkworm as a parasitifer, which mainly comprises the following steps: adopting a scorpion cDNA as a template, adopting 5'-ACGTAGATCTATGGATGGATATATAAG-3' containing BglII enzyme cutting site as a forward primer and adopting 5'-ACGTAGATCT TTACTTTTTTCCAC-3' containing the BglII enzyme cutting site as a reverse primer to carry out PCR gene amplification reaction in 32 cycles to obtain a scorpion toxin gene; digesting pCR2.1-scorpion toxin by restriction endonuclease BglII to obtain a scorpion toxin gene fragment; cloning the scorpion toxin gene fragment in a baculovirus transfer vector pAcGP67b to obtain a recombinant pAcGP67b-scorpion toxin gene, carrying out homologous recombination with baculovirus BmNPV DNA of a silkworm in an insect cell by cotransfection to produce a recombinant virus; expressing in a silkworm body and purifying the recombinant scorpion toxin. Most of the recombinant scorpion toxin protein expressed by a silkworm larva is retained in a fat body of the silkworm; the scorpion toxin is purified from the fat body by an affinity chromatography; and the recombinant scorpion toxin has obvious biological toxicity, is suitable for the requirement of a biopharmaceutical industrialization level and has wide application prospect in the field of future medical and clinic treatment.
Owner:ZHEJIANG UNIV
Mixing and matching tc proteins for pest control
ActiveUS20090221501A1High activityEffective control of widerBiocidePeptide/protein ingredientsHeterologousPhotorhabdus species
Owner:DOW AGROSCIENCES LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com