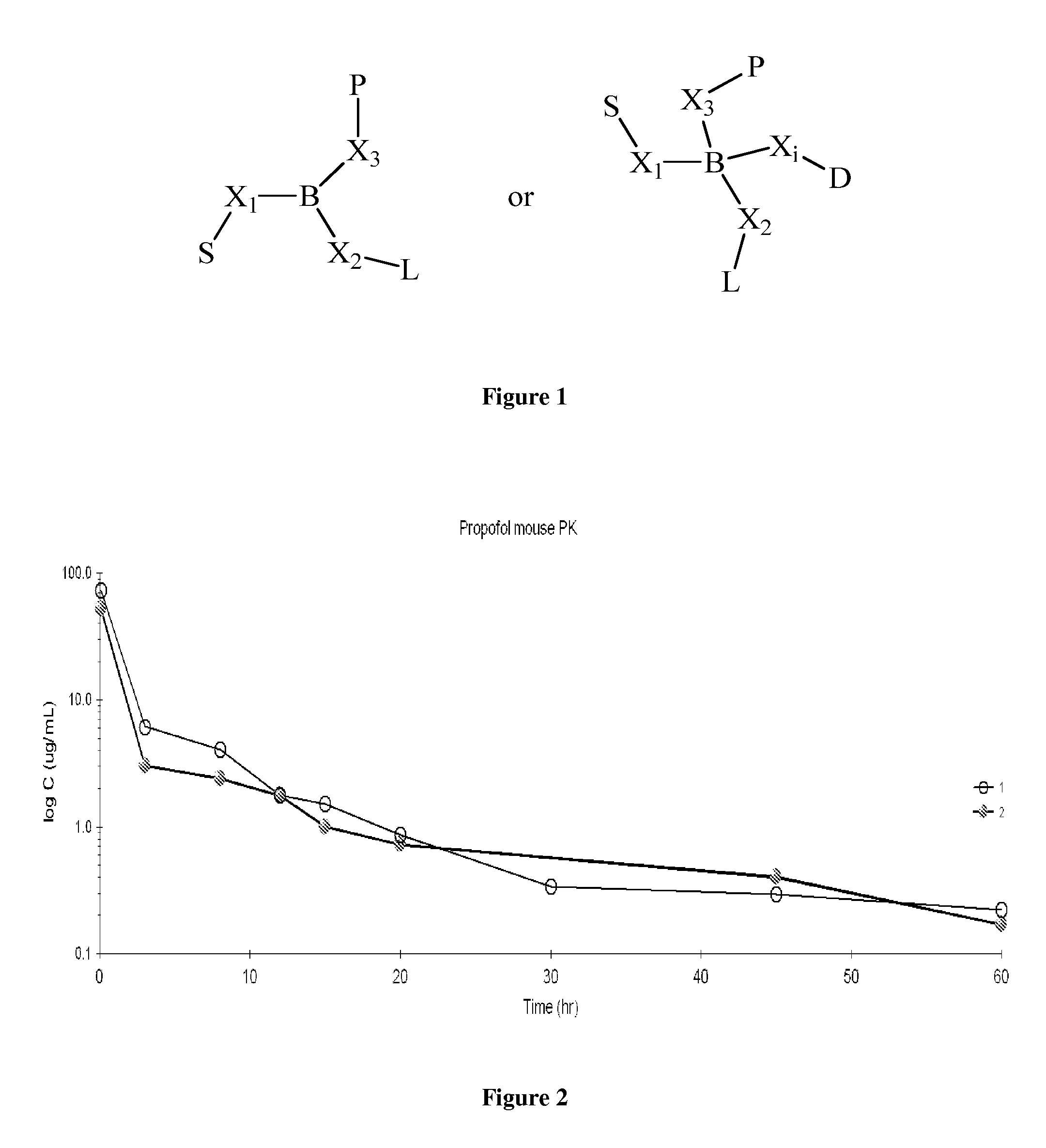
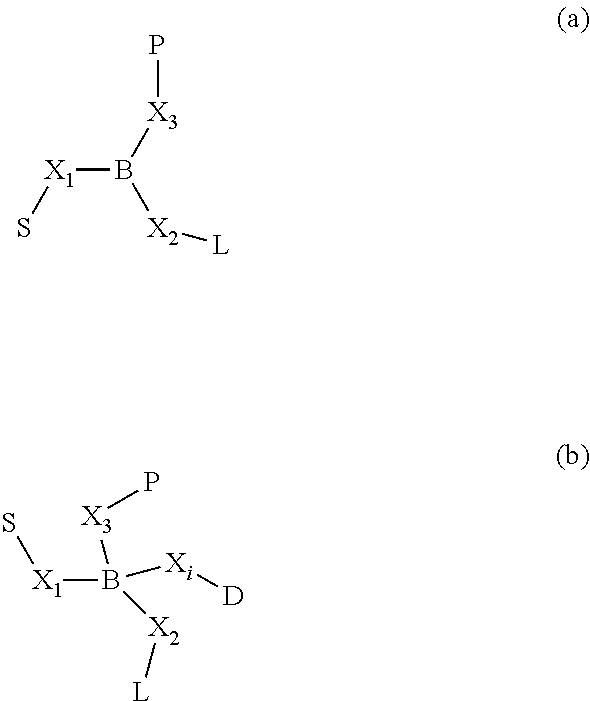
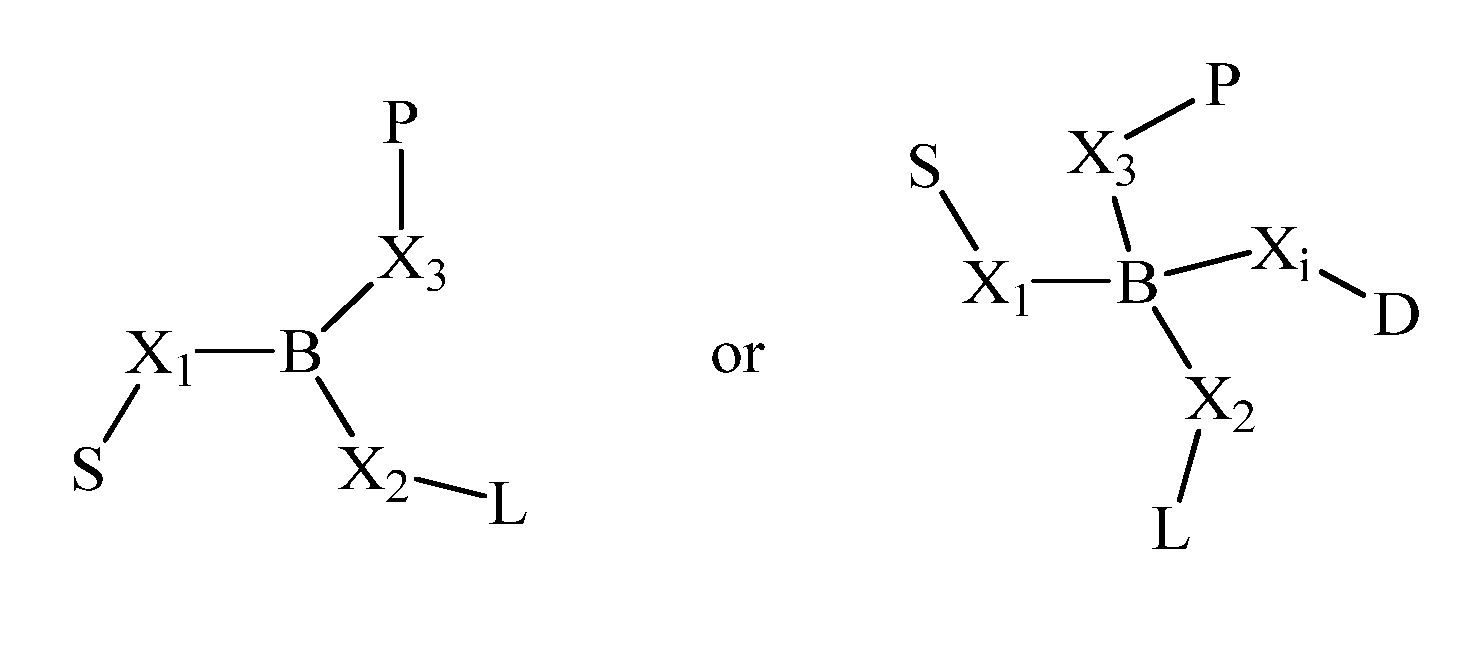Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
81 results about "Safety profile" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Safety profile. The chemistry, pharmacology, therapeutic effects, and adverse effects of an administered drug or other substance.
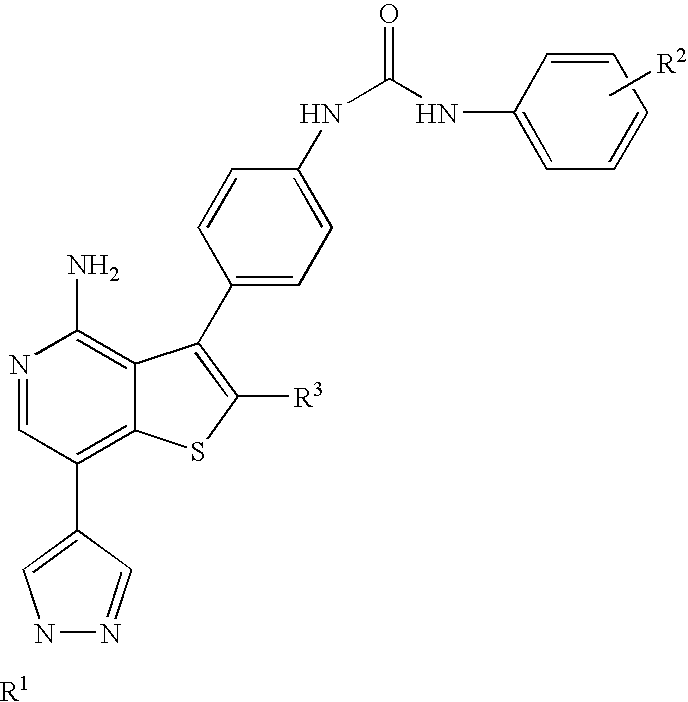
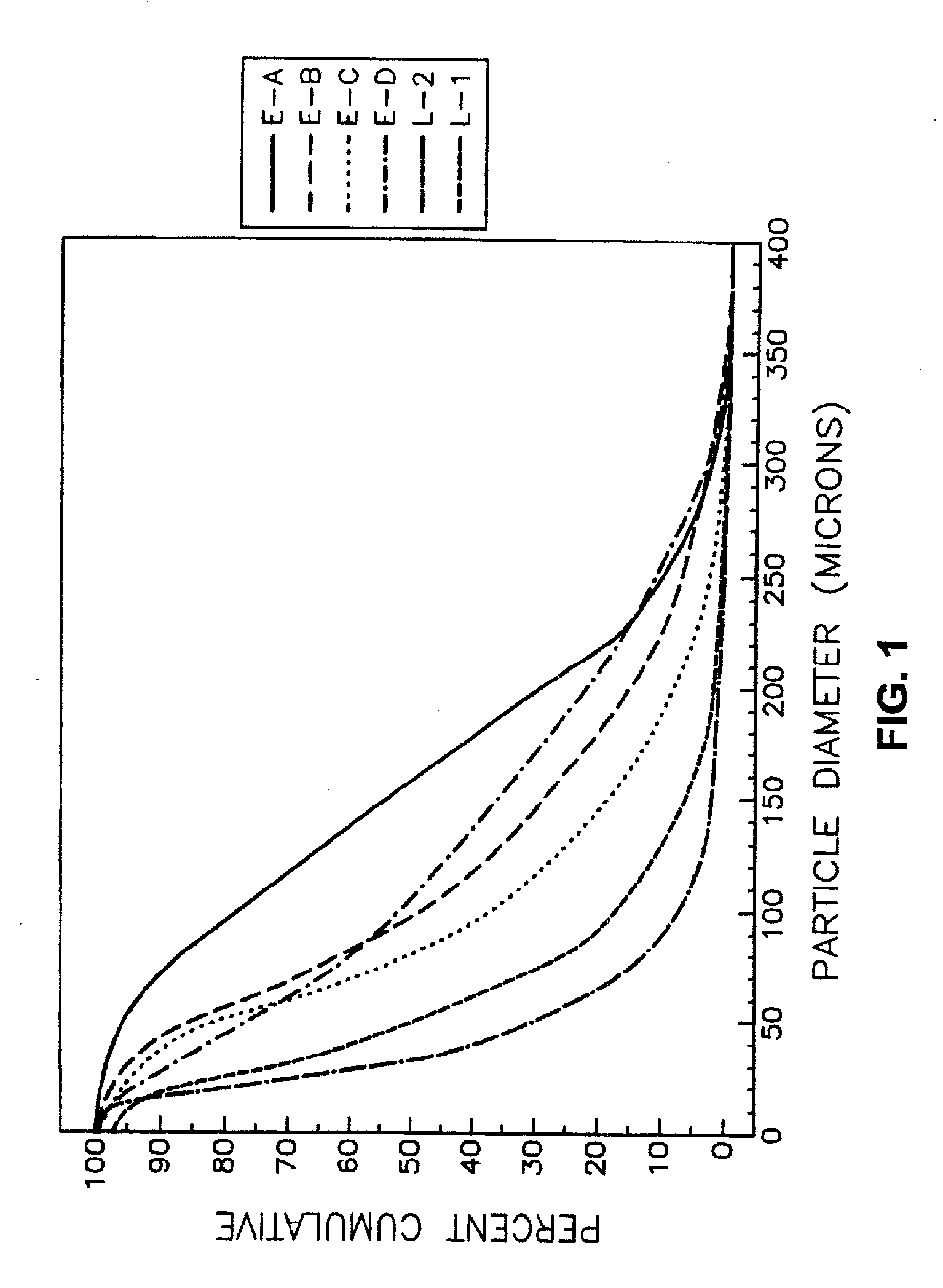
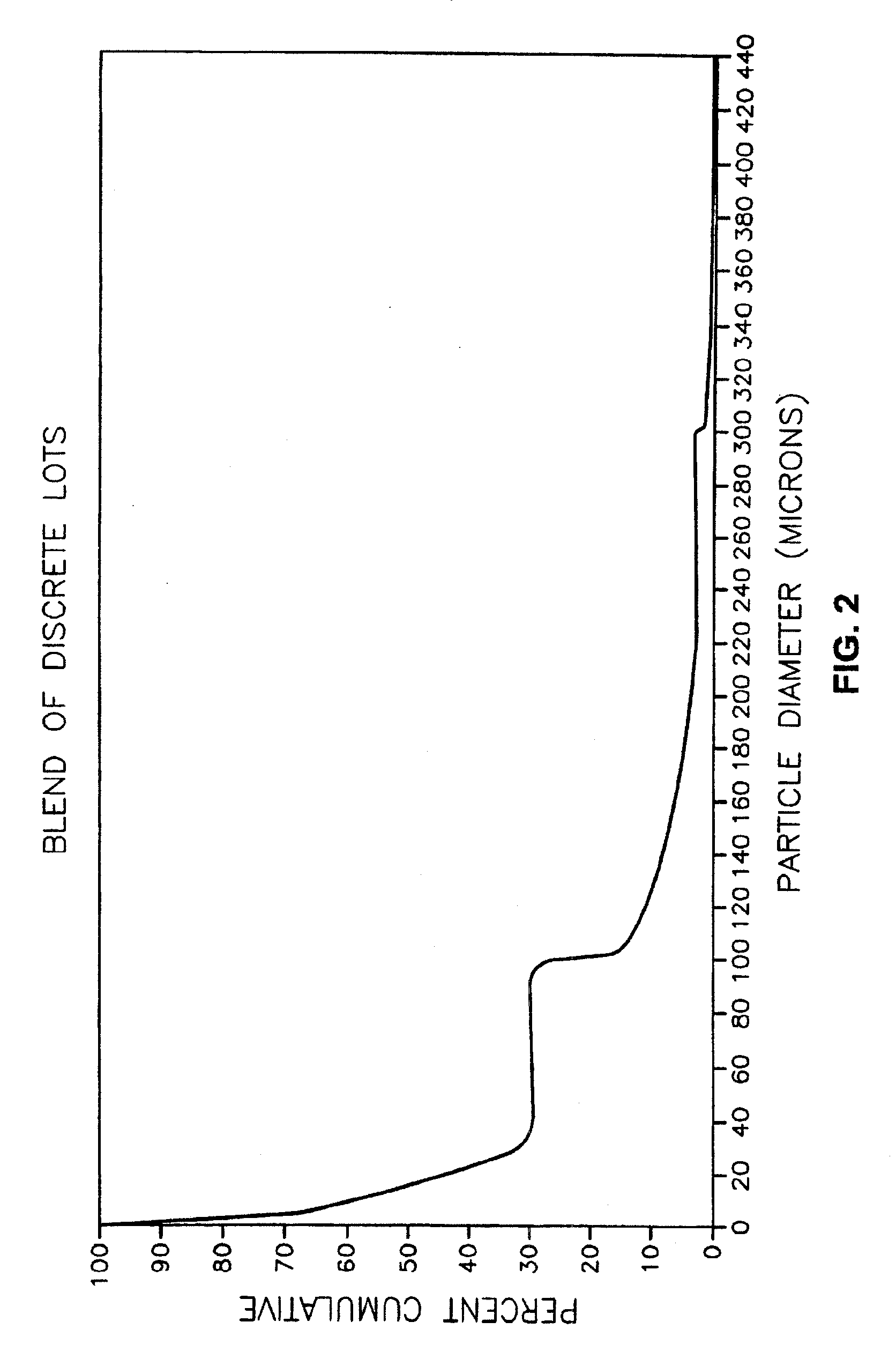
Acetamide derivative having defined particle size
InactiveUSRE37516E1Reduce and eliminate symptomIncrease alertnessPowder deliveryBiocideMedicineSafety profile
Pharmaceutical compositions comprising modafinil in the form of particles of defined size. The particle size of modafinil can have a significant effect on the potency and safety profile of the drug.
Owner:CEPHALON INC
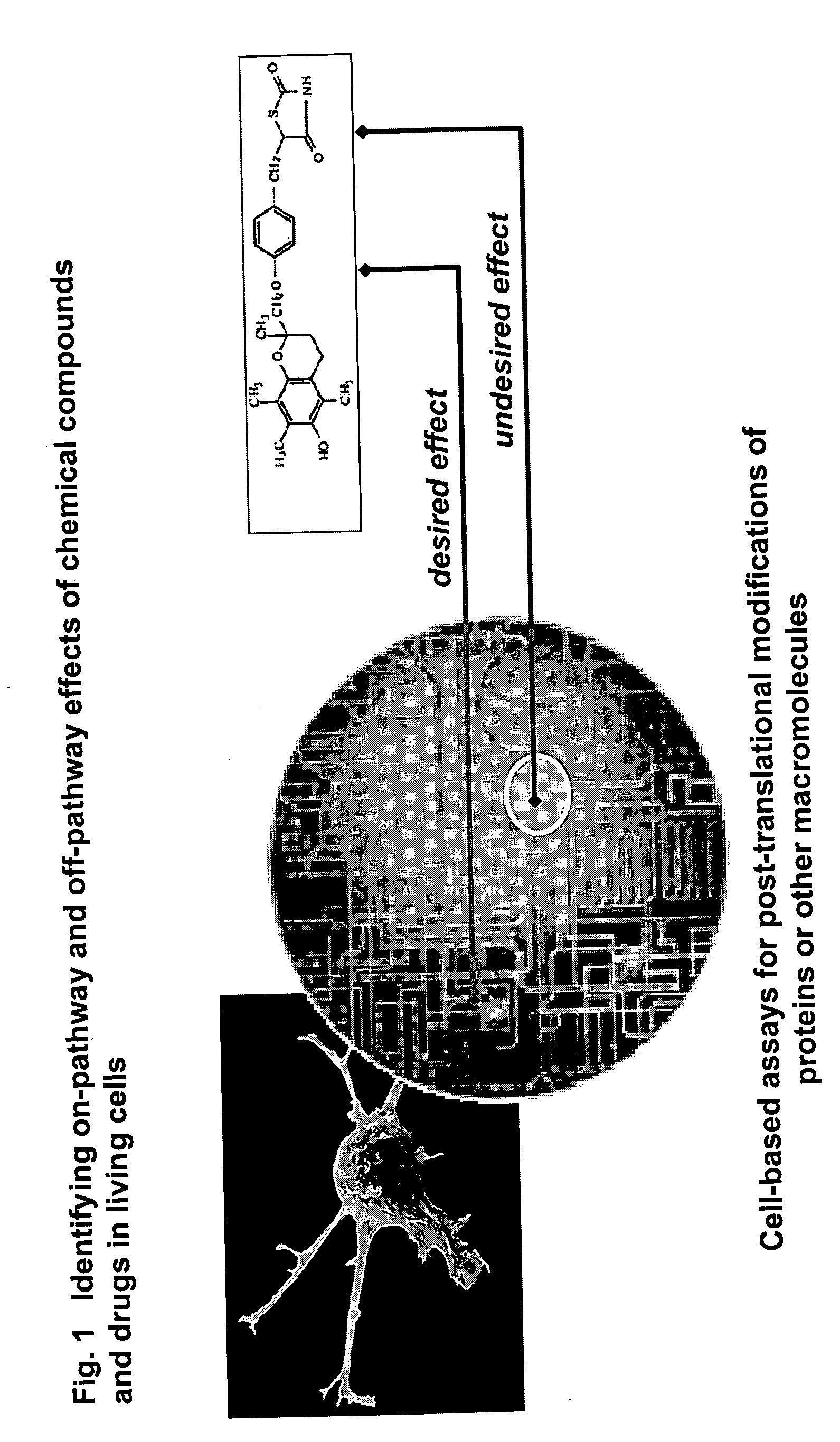
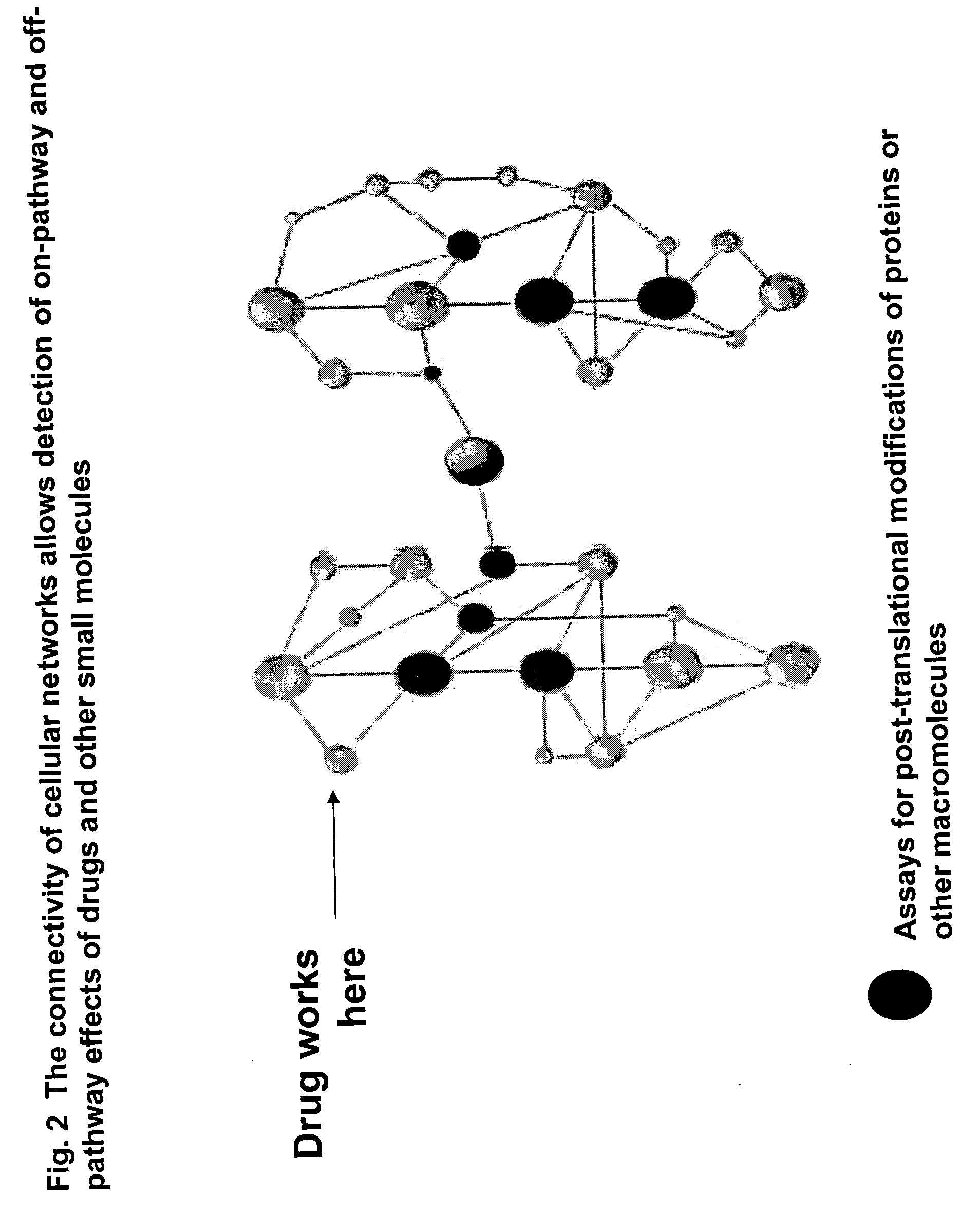
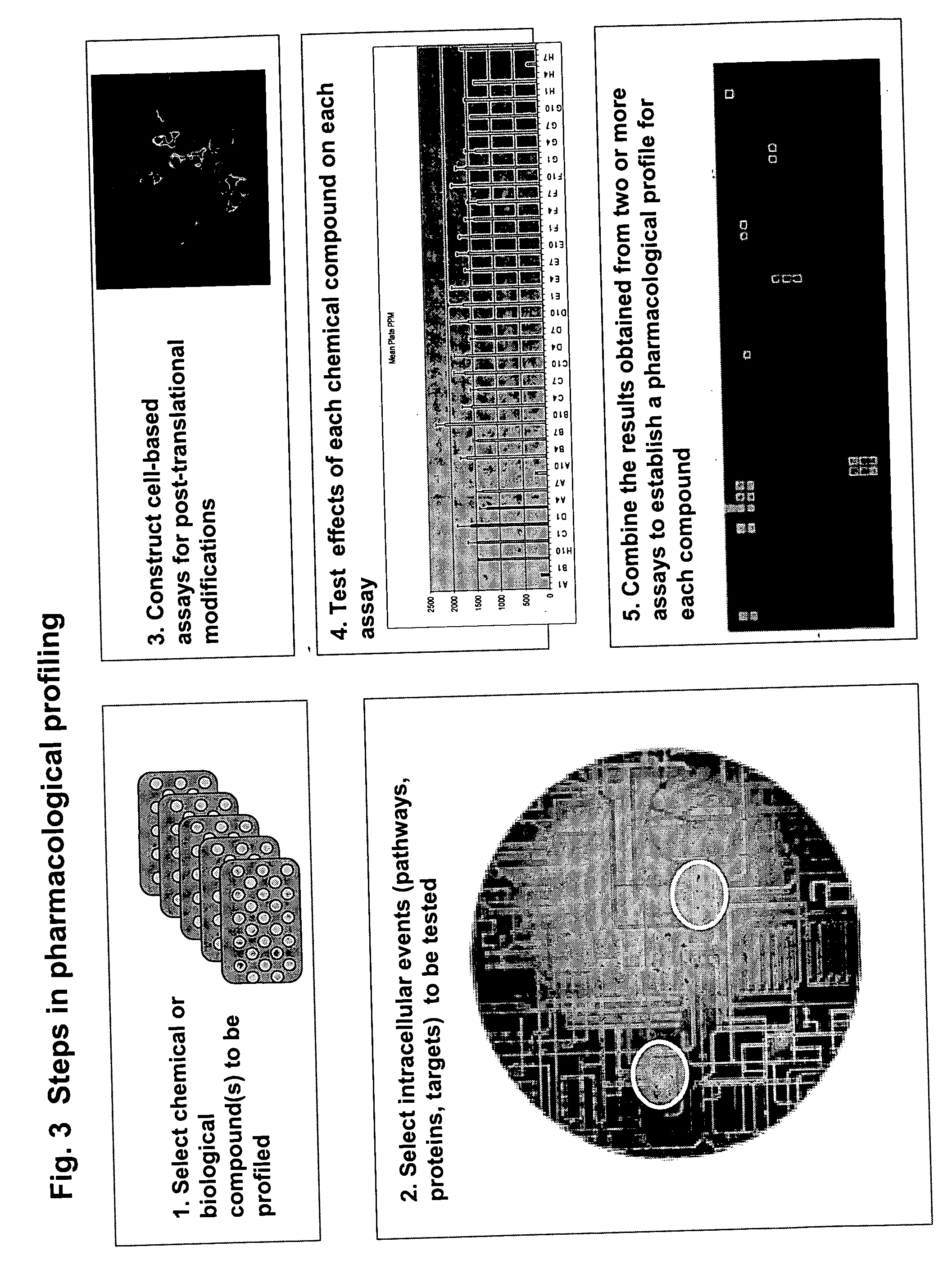
Pharmacological profiling of drugs with cell-based assays
InactiveUS20060040338A1Enable optimizationBioreactor/fermenter combinationsCompound screeningPost translationalAssay
The instant invention provides a method for establishing safety profiles for chemical compounds, as well as pharmacological profiling said method comprising (A) testing the effects of said chemical compounds on the amount and / or post-translational modifications of two or more macromolecules in intact cells; (B) constructing a pharmacological profile based on the results of said tests; and (C) comparing said profile to the profile(s) of drugs with established safety characteristics. Additionally, the invention is also directed to a composition comprising an assay panel, said panel comprising at least one high-content assay for the amount and / or post-translational modification of a protein and at least one high-content assay for the amount and / or subcellular location of a protein-protein interaction.
Owner:ODYSSEY THERA INC
Kinase inhibitors with improved cyp safety profile
Compounds that inhibit protein kinases such as Aurora-kinases and the VEGFR and PDGFR families of kinases, with an improved safety profile due to low CYP3A4 inhibition, compositions containing the compounds and methods of treating diseases using the compounds are disclosed.
Owner:ABBVIE INC
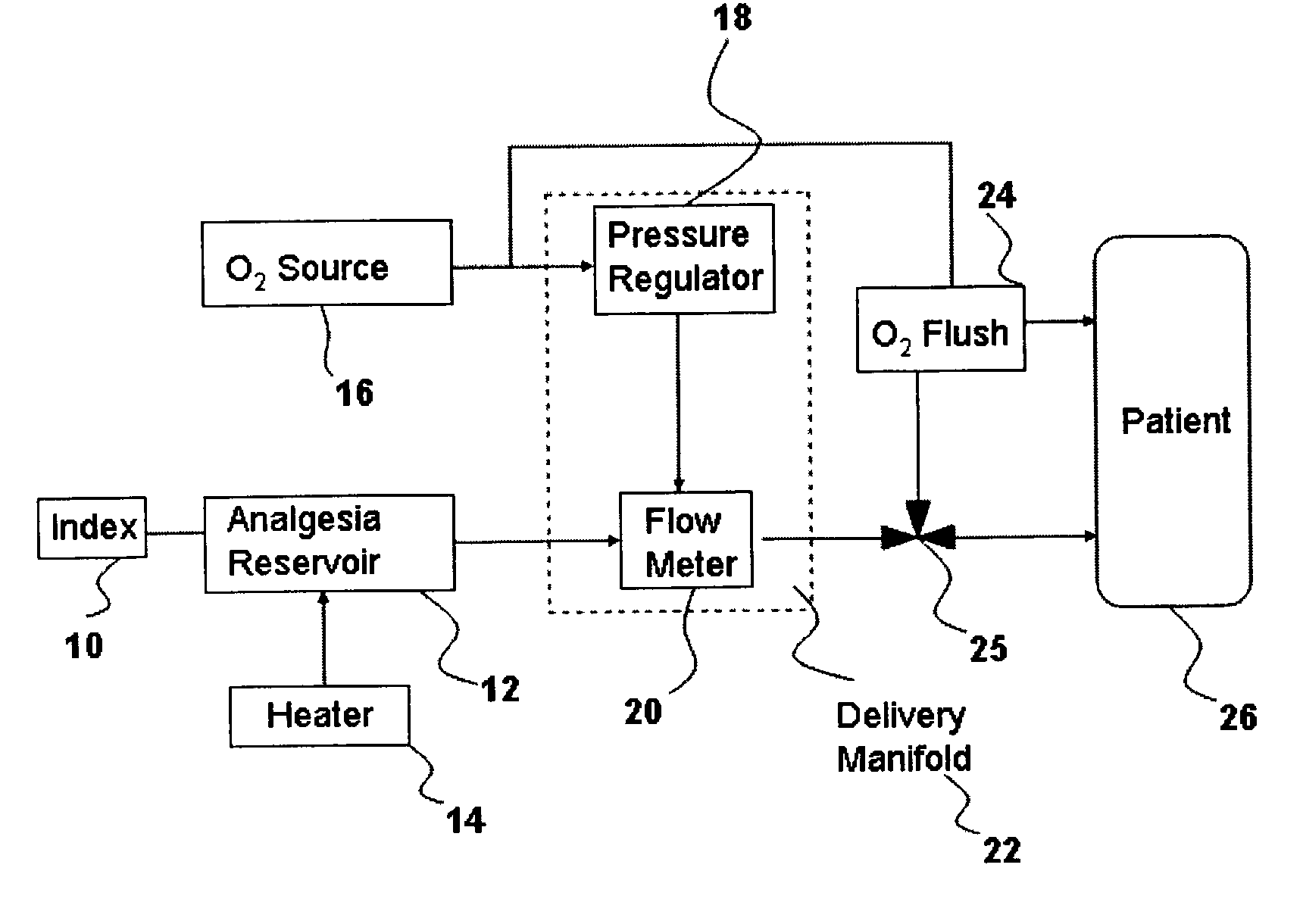
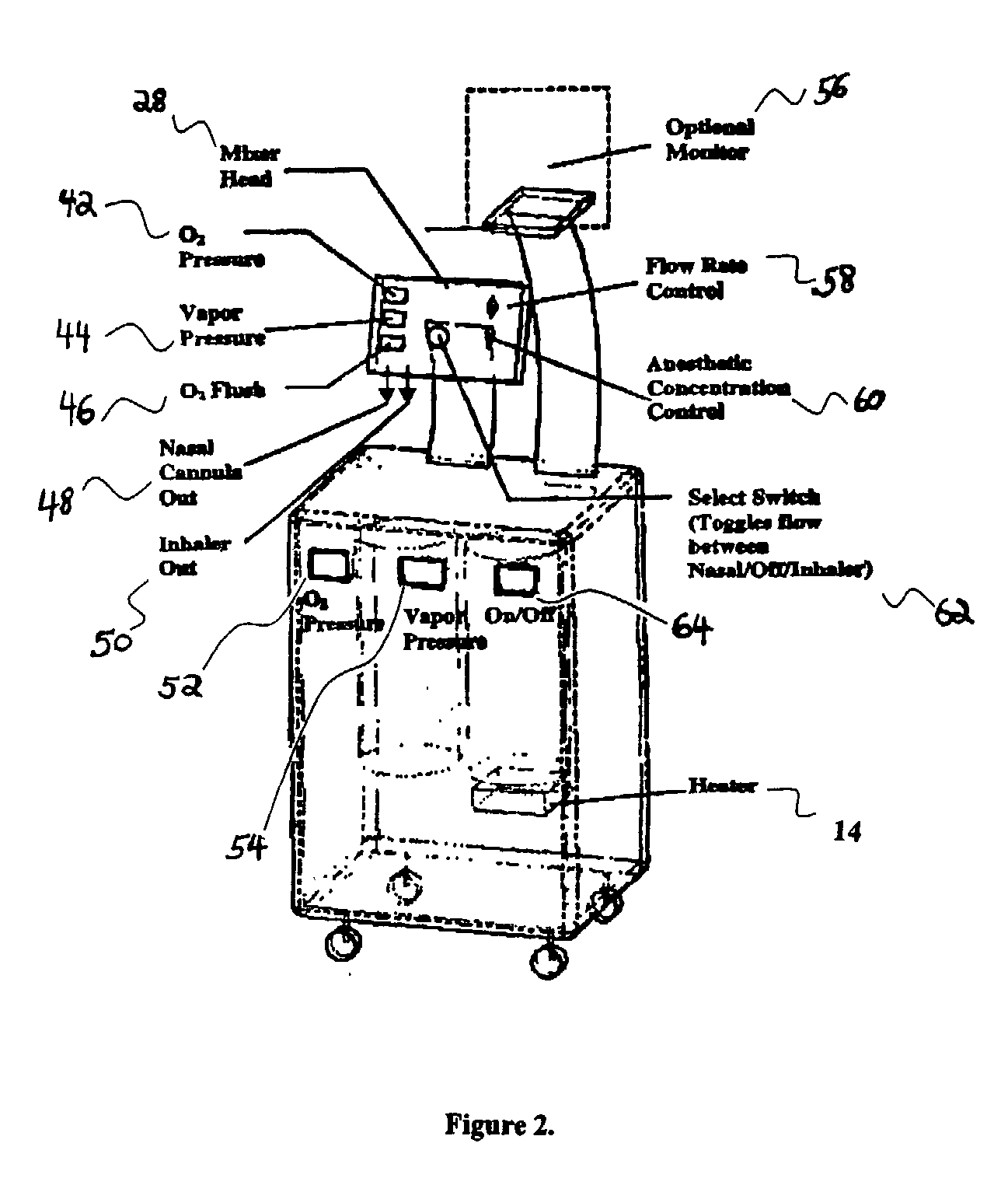
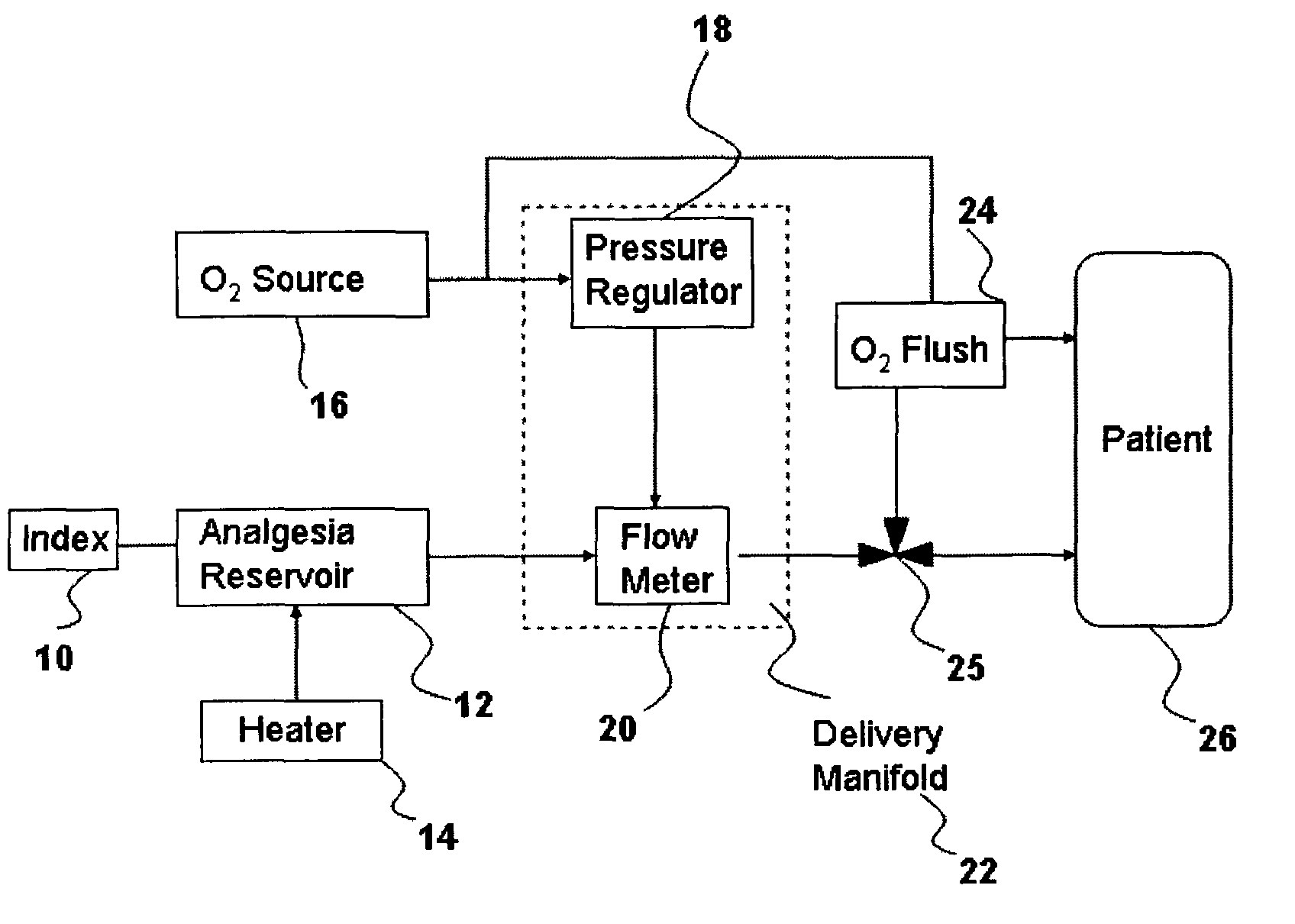
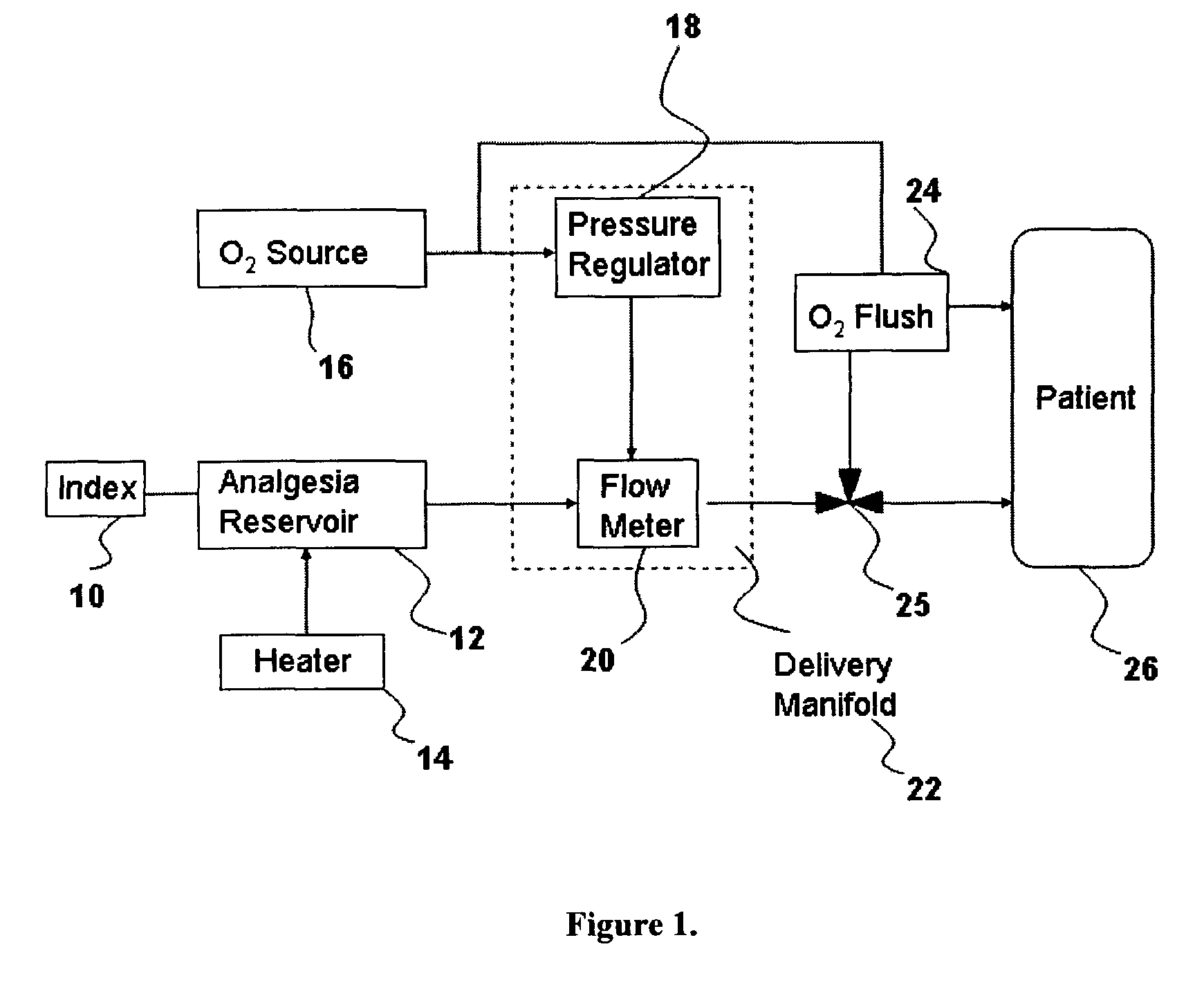
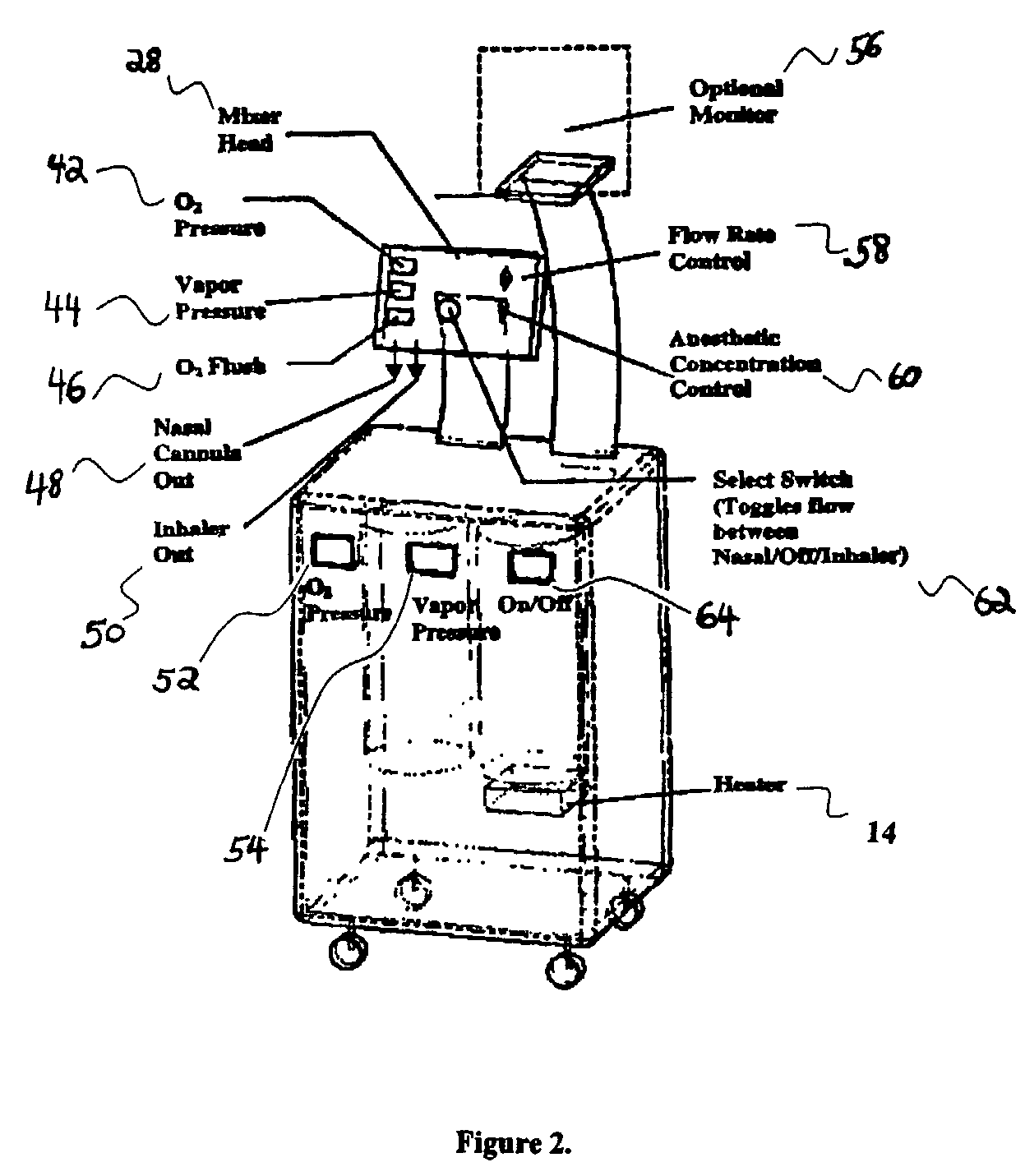
Drug delivery system for conscious sedation
InactiveUS20030233086A1Increase oxygenationRelief the painHalogenated hydrocarbon active ingredientsNervous disorderAmnesiaSedation
Inhalant anesthetics are developed with a number of properties including rapid onset and recovery, controllability, and, ideally, a broad safety profile. The efficacy of these agents is measured by their ability to create anesthesia within the framework of the other desirable properties. The instant invention focuses on the dosage level where analgesia occurs but amnesia or lack of consciousness does not. In addition to identifying the dosage level where pain is sharply reduced or eliminated but awareness remains, a delivery system for safe and effective delivery of the agent is described.
Owner:FIRST NIAGARA BANK
Fastener driving apparatus
ActiveUS8733610B2Improve efficiencyEasy to removeStapling toolsConstructionsLinear motionSafety profile
A fastener driving apparatus includes a vacuum piston and a drive piston, which vacuum piston, when moved (by way of a motor and linear motion converter), draws a vacuum against the drive piston, which drive piston may be held in place by retention means. An anvil is coupled to the drive piston. The retention means is released electrically or mechanically at or near the point of maximum vacuum volume. This drive piston and anvil assembly is then driven by atmospheric pressure and may strike as fastener to drive it into a substrate. At least one position sensor may be used. Once the fastener is driven, the apparatus may reset to an initial position. At least one valve may be included to dump the energy stored in the vacuum in the case of a jam condition, thus providing good safety profile.
Owner:TRICORD SOLUTIONS INC
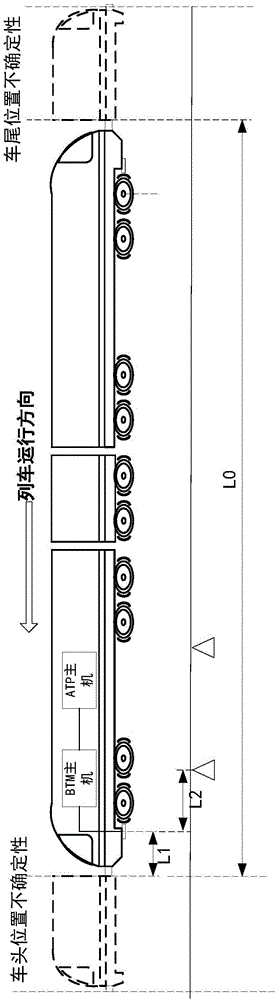
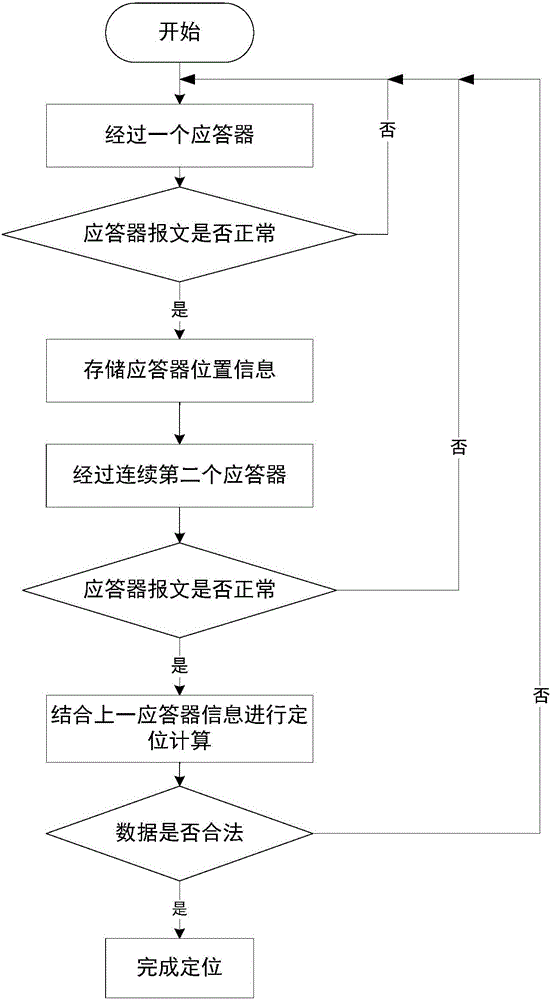
Method and system for detecting train positioning on basis of dynamic adjustment
ActiveCN106672025AHigh positioning accuracyIncrease the uncertaintyRailway traffic control systemsSafety profileSimulation
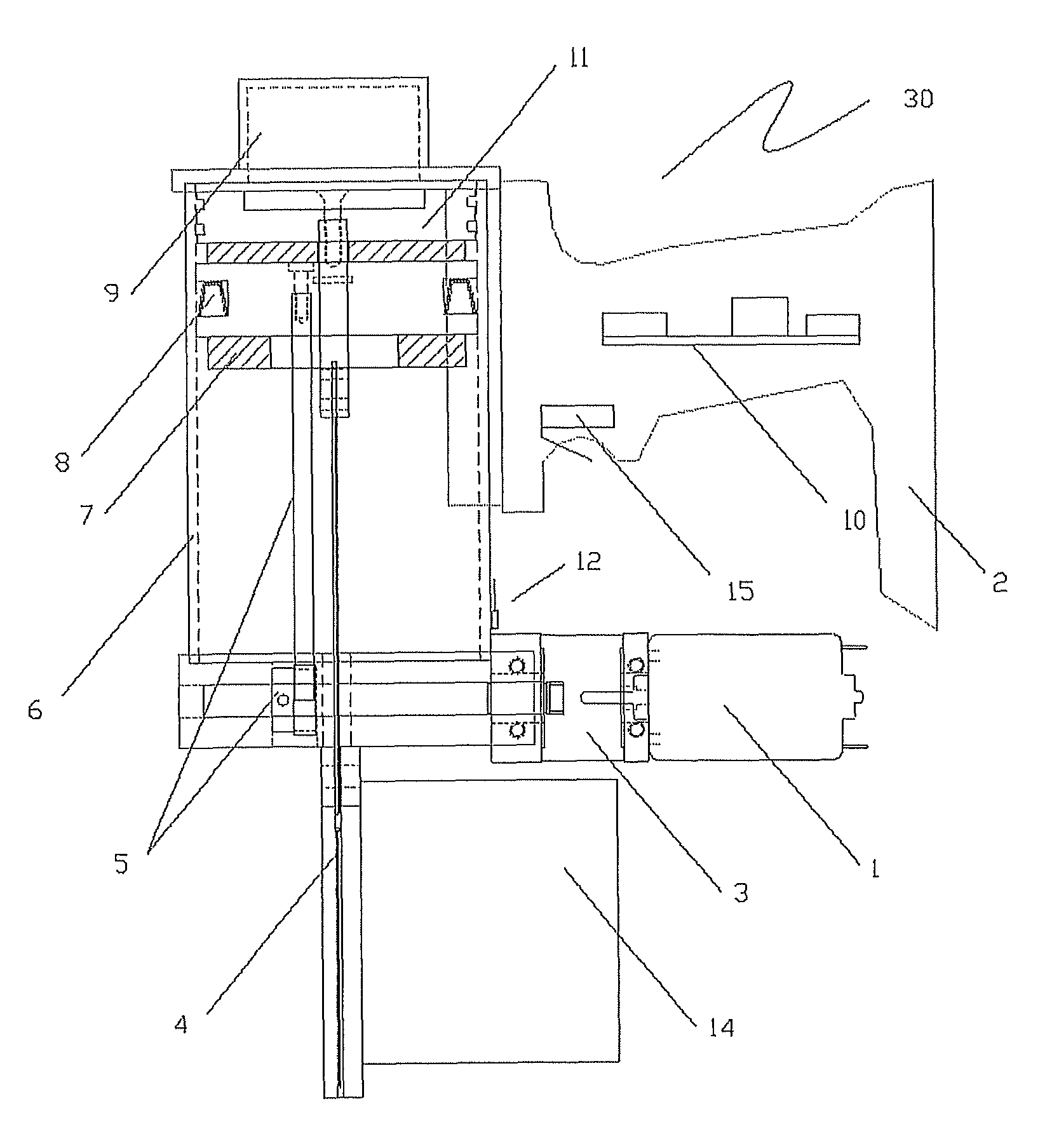
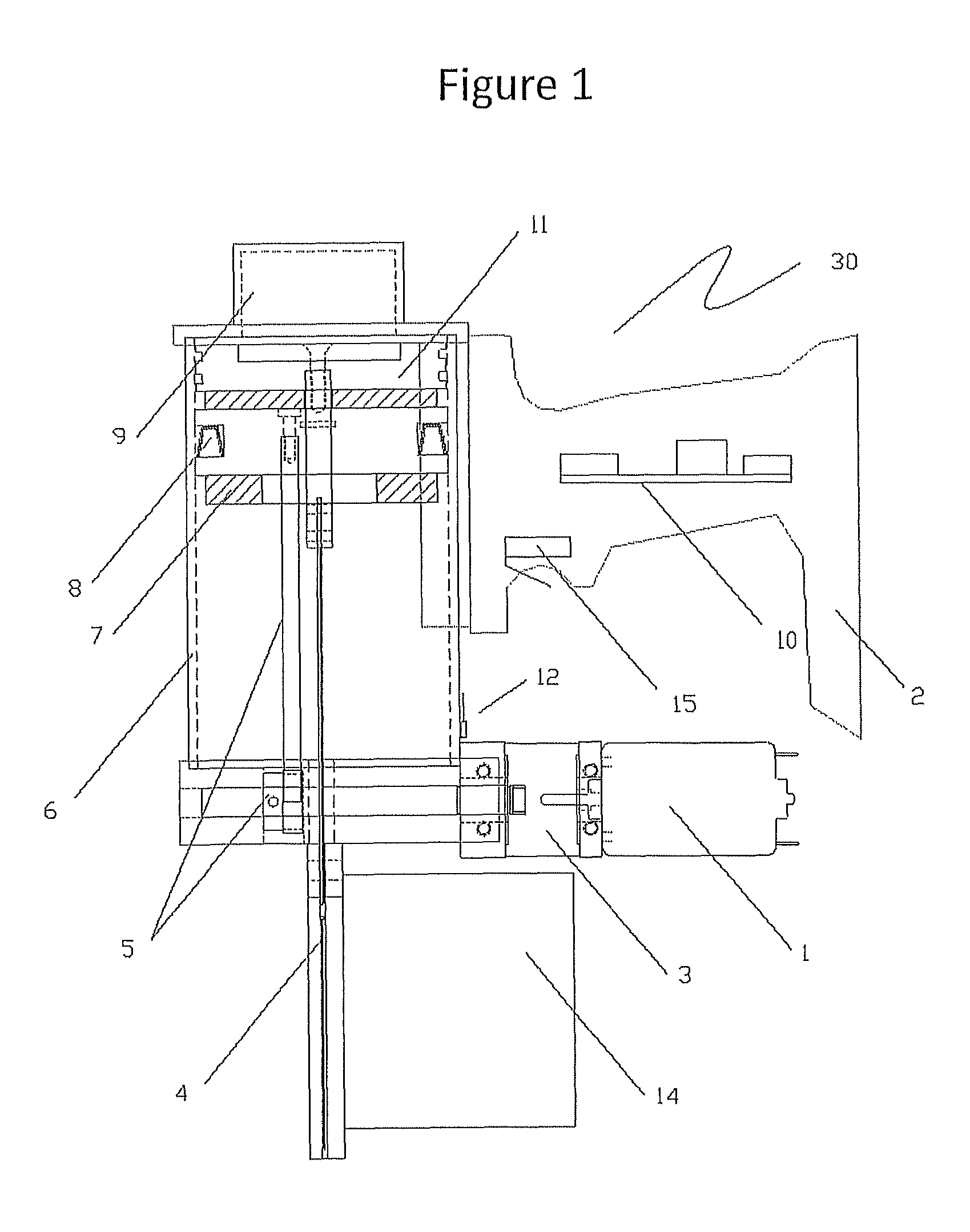
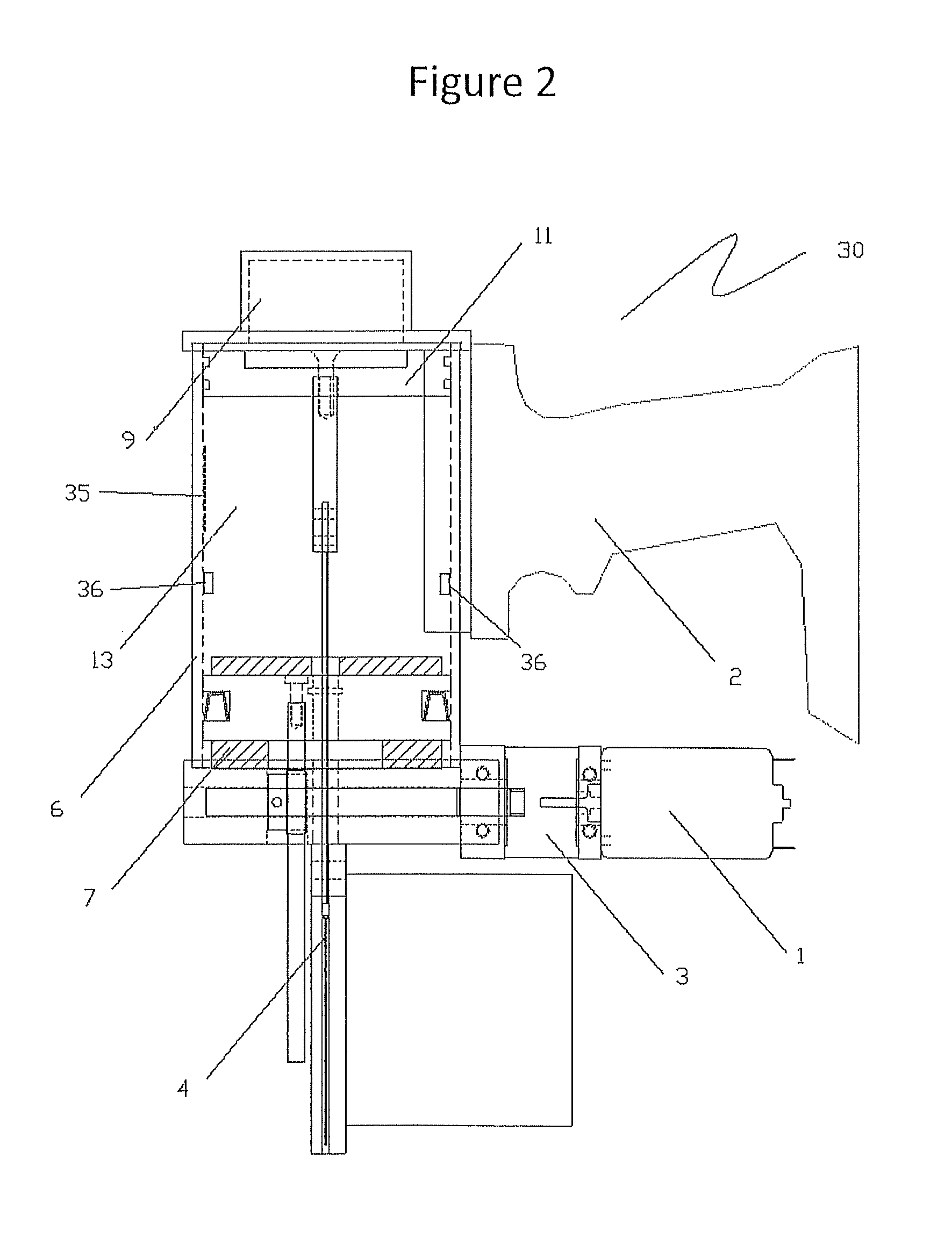
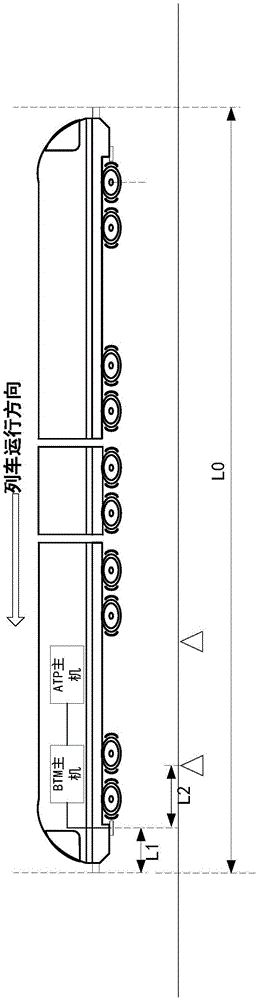
The invention discloses a method and a system for detecting train positioning on the basis of dynamic adjustment. The technical scheme includes that the method comprises initially positioning trains from non-positioning phases to positioning phases; computing the dynamic distance measurement accuracy whenever the trains are completely initially positioned and then run through every two continuous transponders and effective messages of the transponders are received; computing uncertain values of the locations of the trains on the basis of dynamic distance measurement accuracy computation results and determining safety profiles of the locations of the trains according to the uncertain values of the locations of the trains. The method and the system have the advantages that the train positioning accuracy can be improved to the greatest extent, the arrangement quantity of the trackside transponders can be reduced, and accordingly the engineering cost can be lowered.
Owner:HUNAN CRRC TIMES SIGNAL & COMM CO LTD
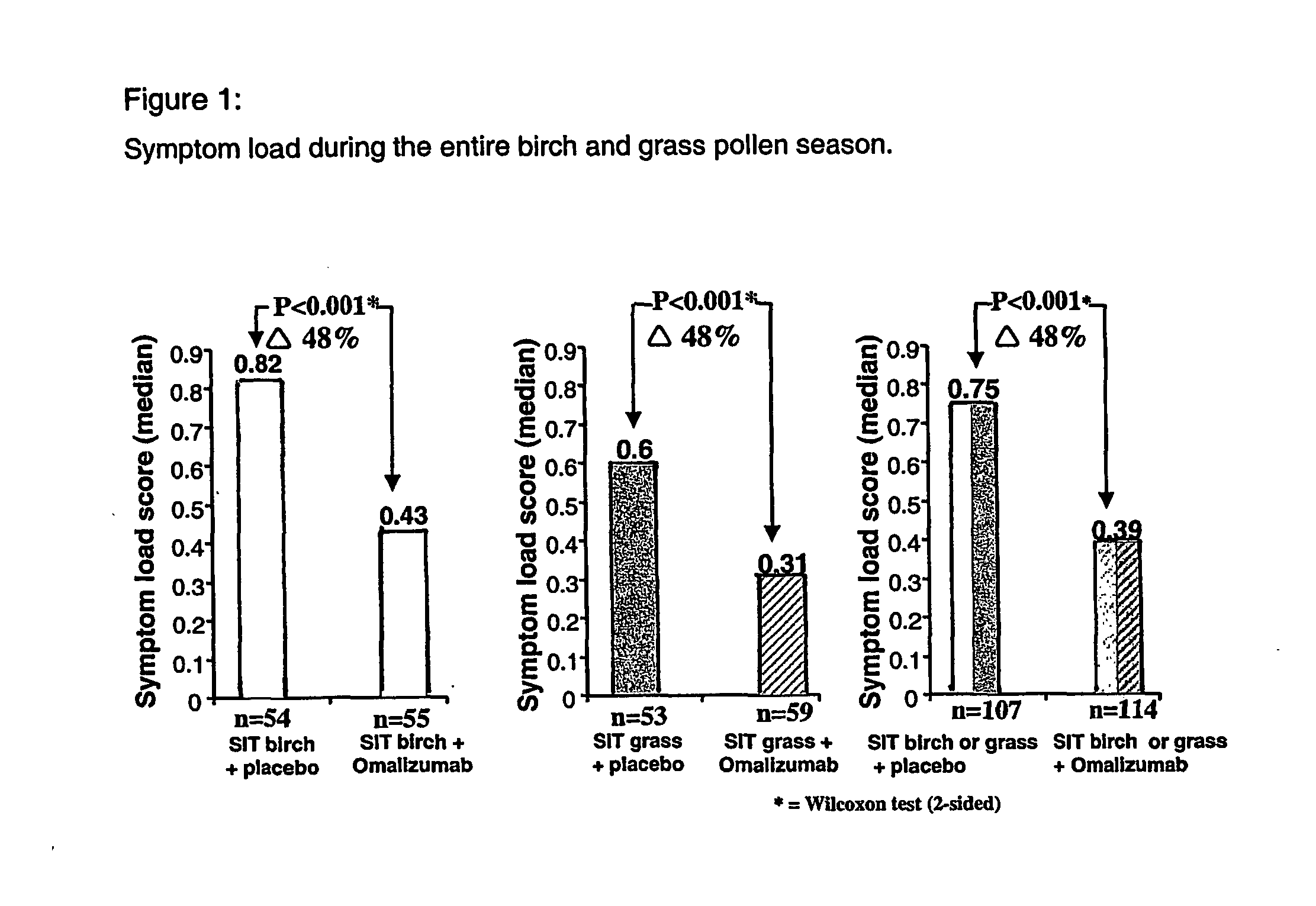
Compositions for use in treating ige-associated disorders
InactiveUS20050031609A1Inhibitory activityReduced activityAllergen ingredientsUnknown materialsAntigenDisease
The present invention provides methods of treating IgE-associated disorders and products for use therein. The methods comprise administering to a subject an amount of a first composition comprising an immunogenie antigen and an amount of a second composition that inhibits the activity of IgE. The methods are particularly useful in treatment of allergies such as allergic rhinitis. These combination methods offer significant advantages, such as improving the efficacy of therapy while showing a good safety profile.
Owner:HULTSCH THOMAS +1
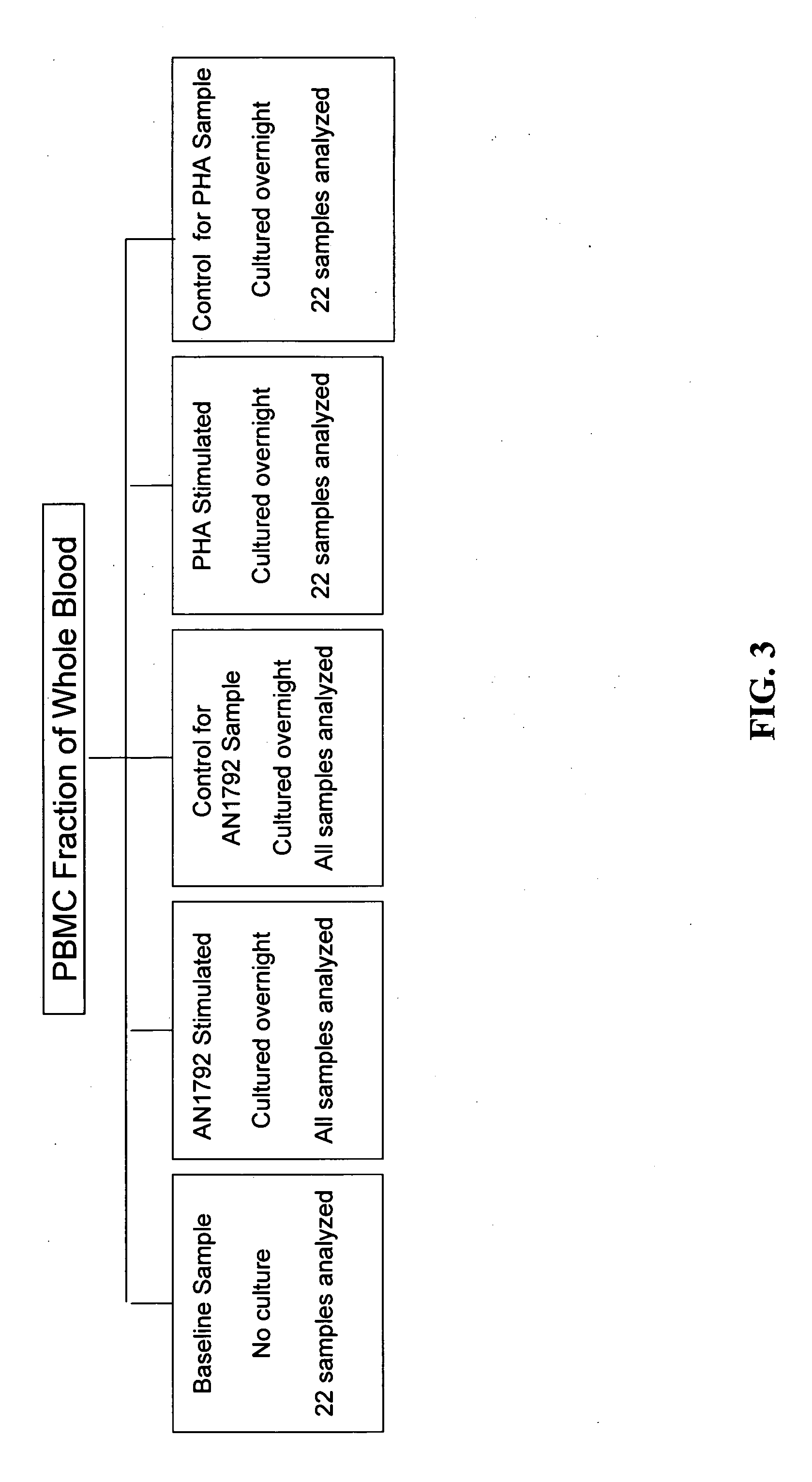
Methods of identifying patients at risk of developing encephalitis following immunotherapy for Alzheimer's disease
InactiveUS20060073496A1Improve accuracyImprove responseData processing applicationsHealth-index calculationSafety profileΒ amyloid
The present invention generally relates to a method for an improved treatment for Alzheimer's disease (AD) using immunotherapy, e.g., immunotherapy targeting β amyloid (Aβ), e.g., immunotherapy based on AN1792. In one embodiment, the method allows for predicting an adverse clinical response, and therefore allows for an improved safety profile of AN1792. In another embodiment, the method allows for predicting a favorable clinical response, and therefore allows for an improved efficacy profile of AN1792. The methods of the present invention may be combined to predict a favorable clinical response and the lack of an adverse clinical response.
Owner:WYETH LLC +1
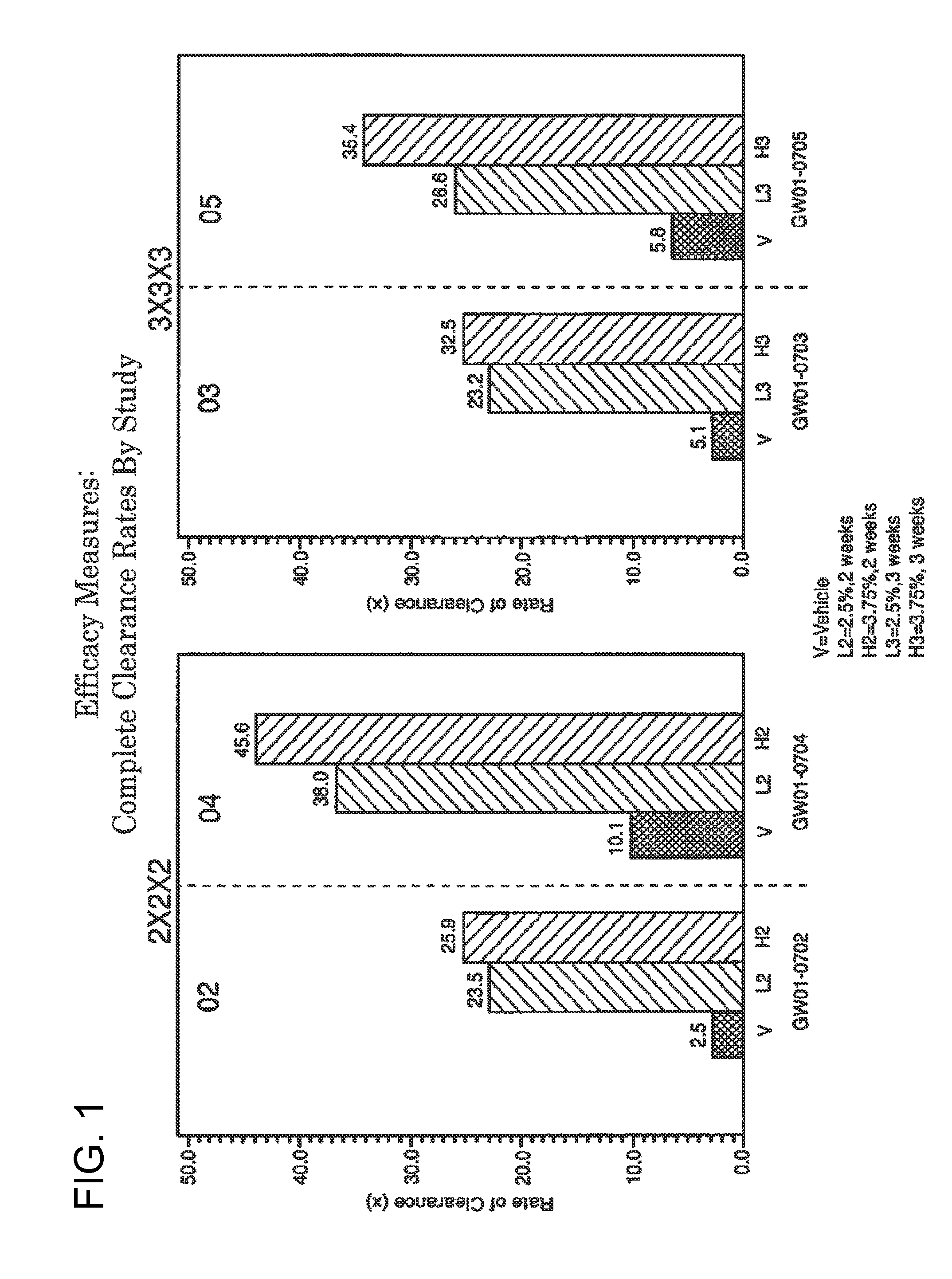
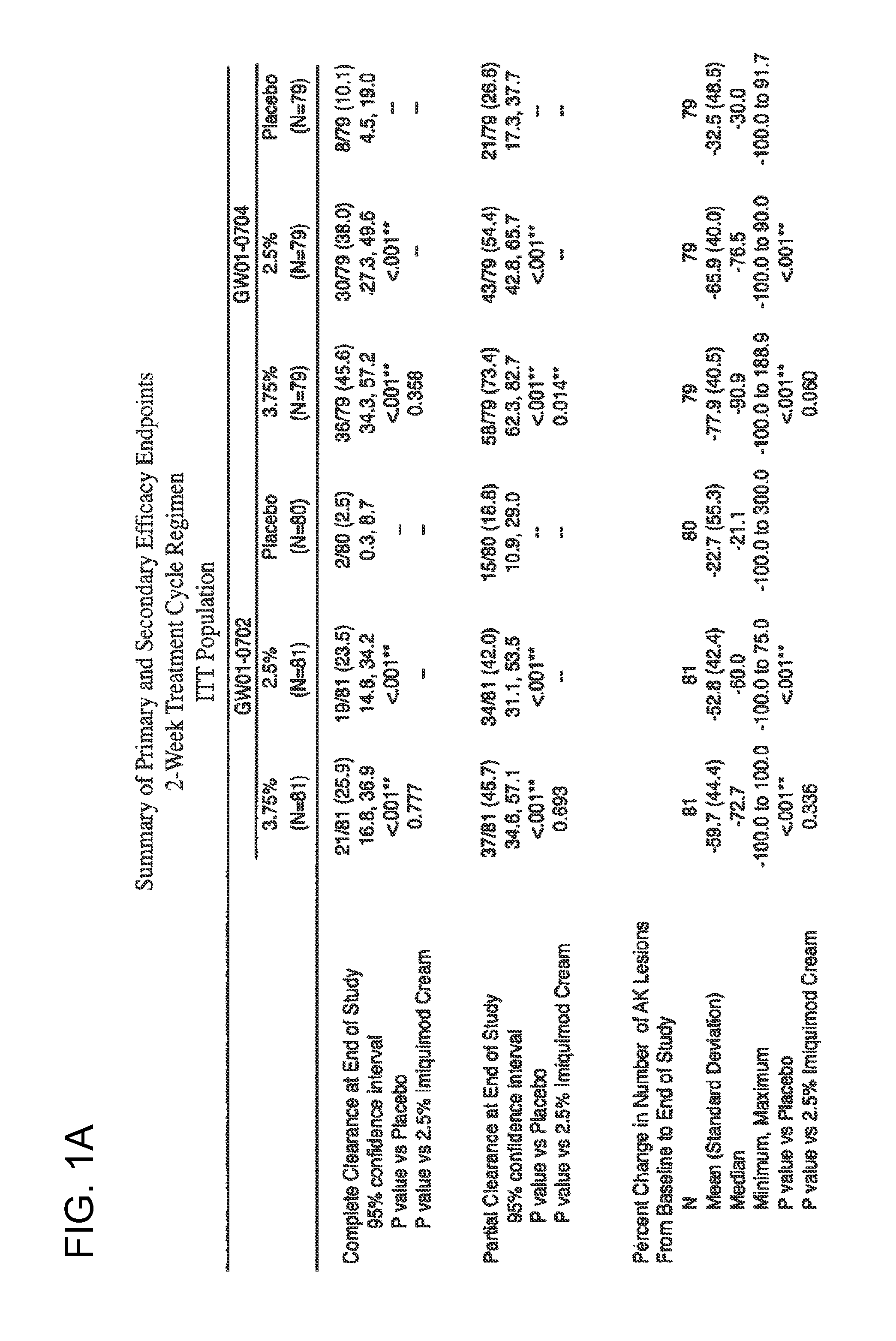
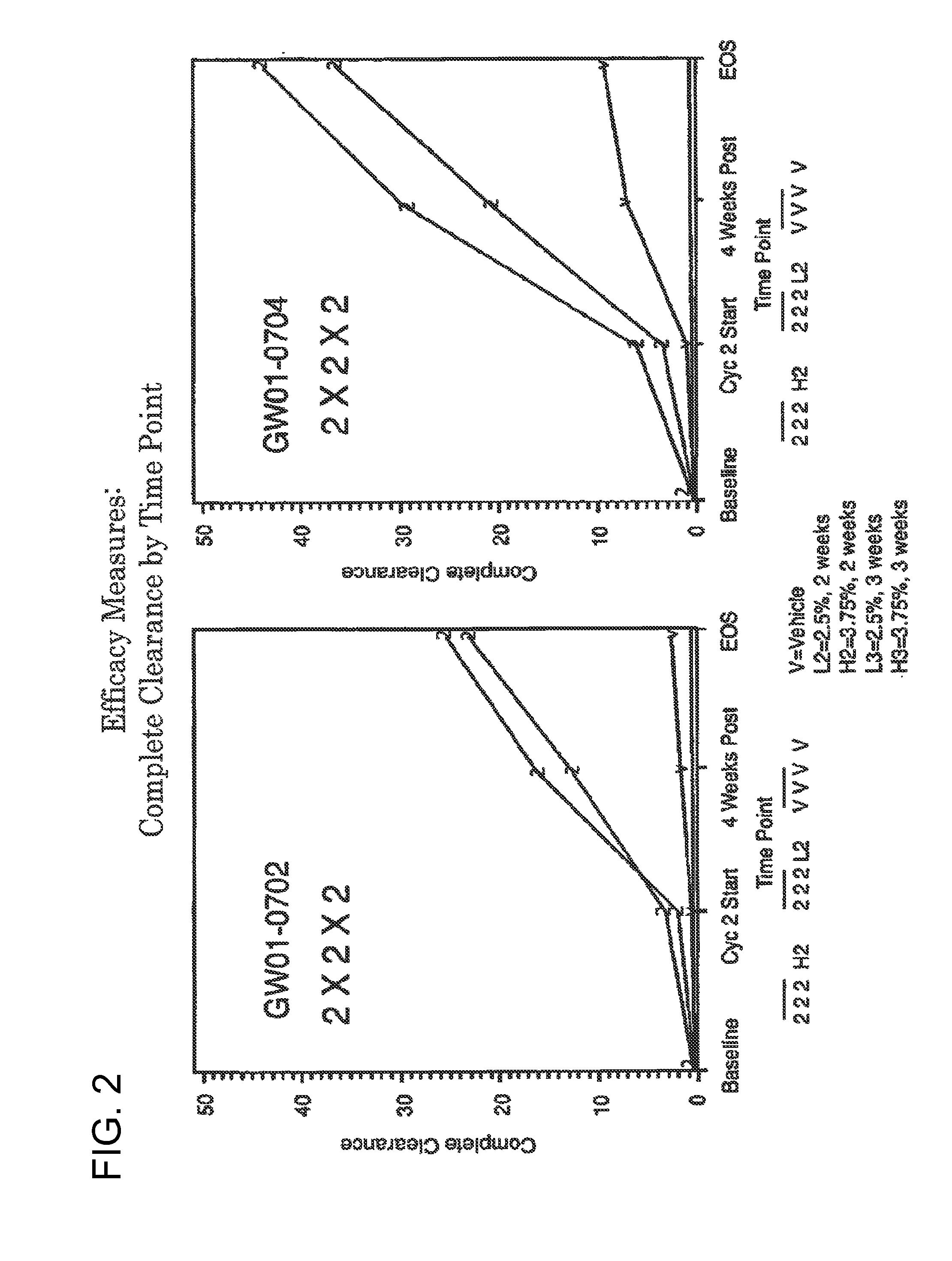
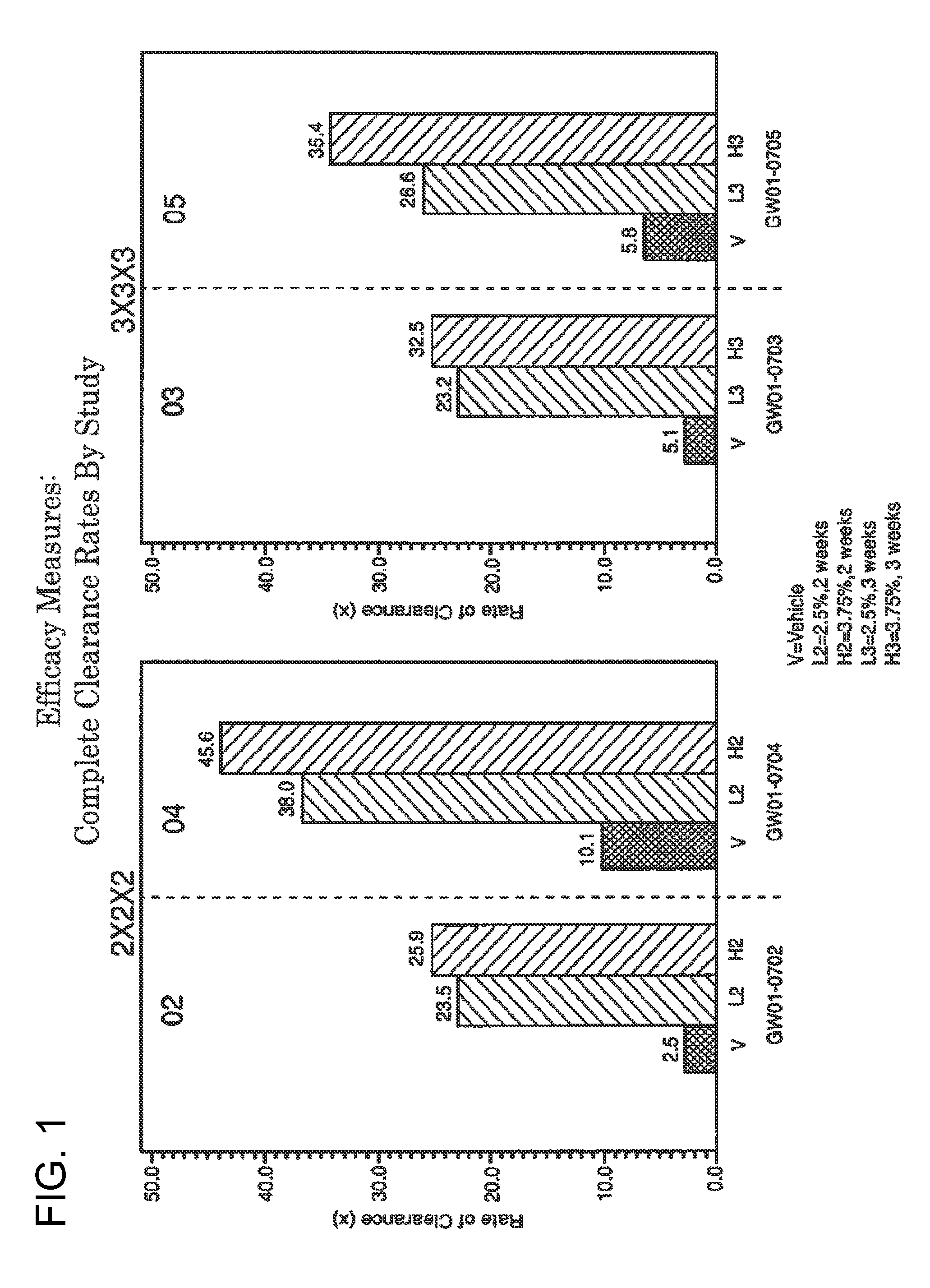
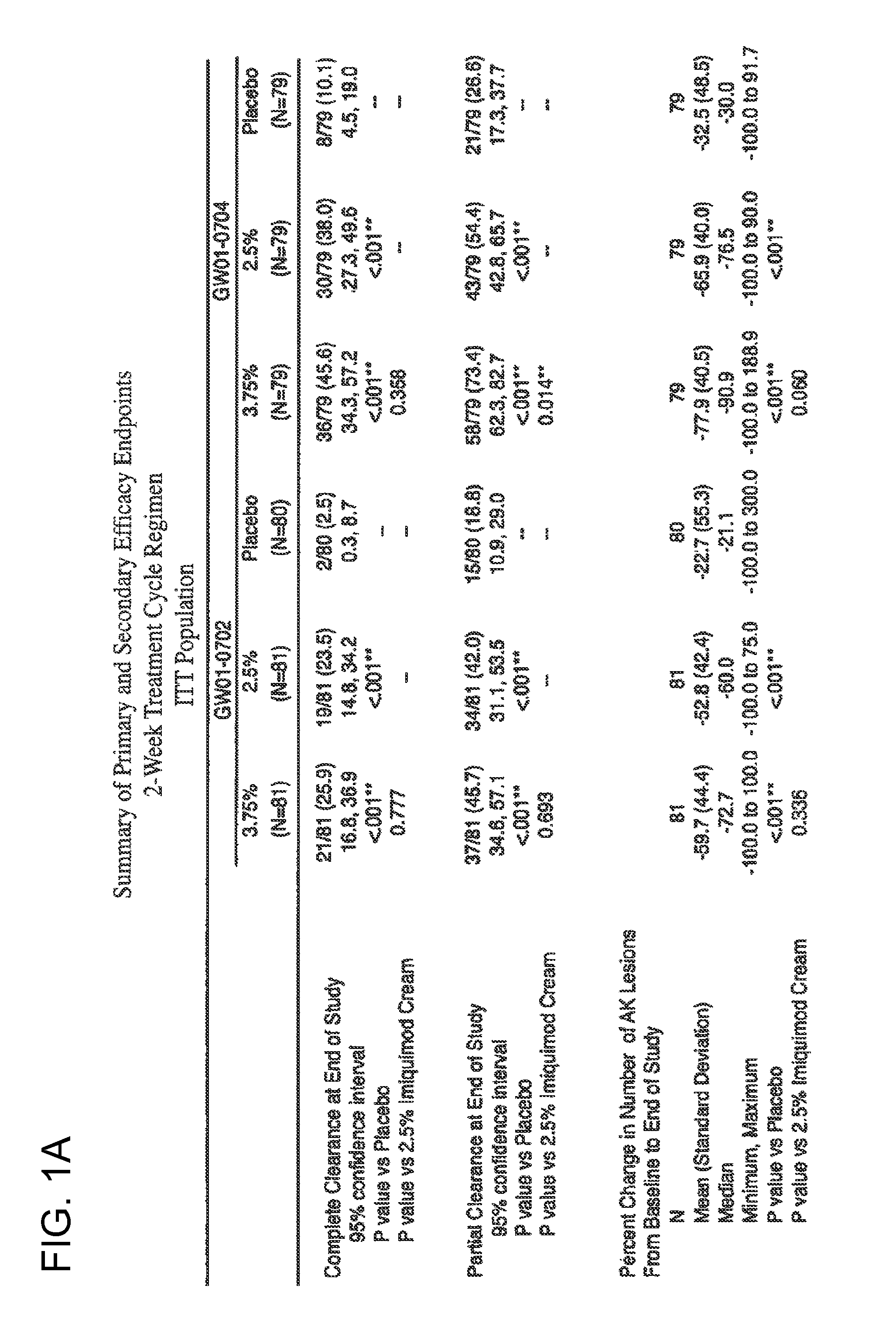
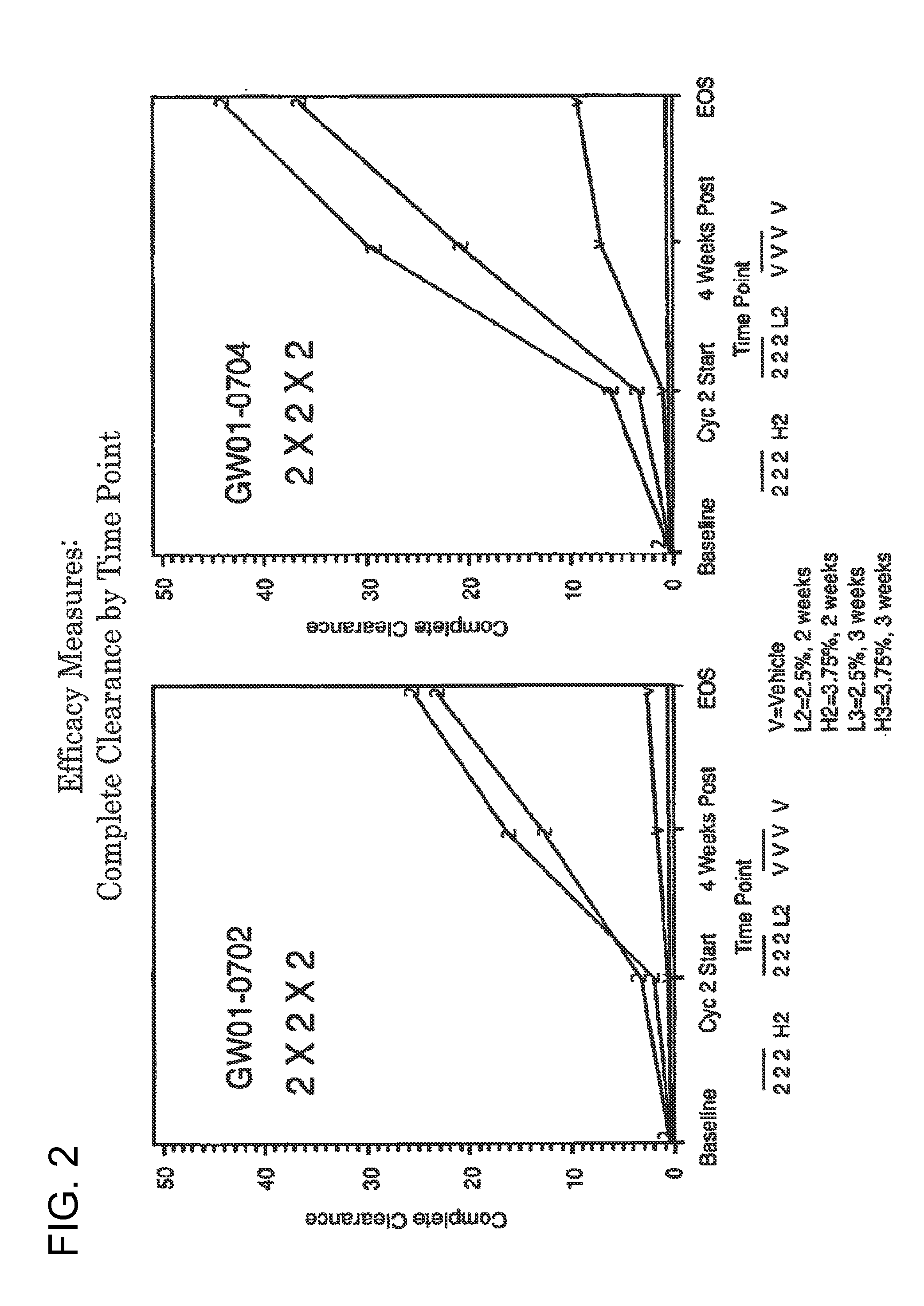
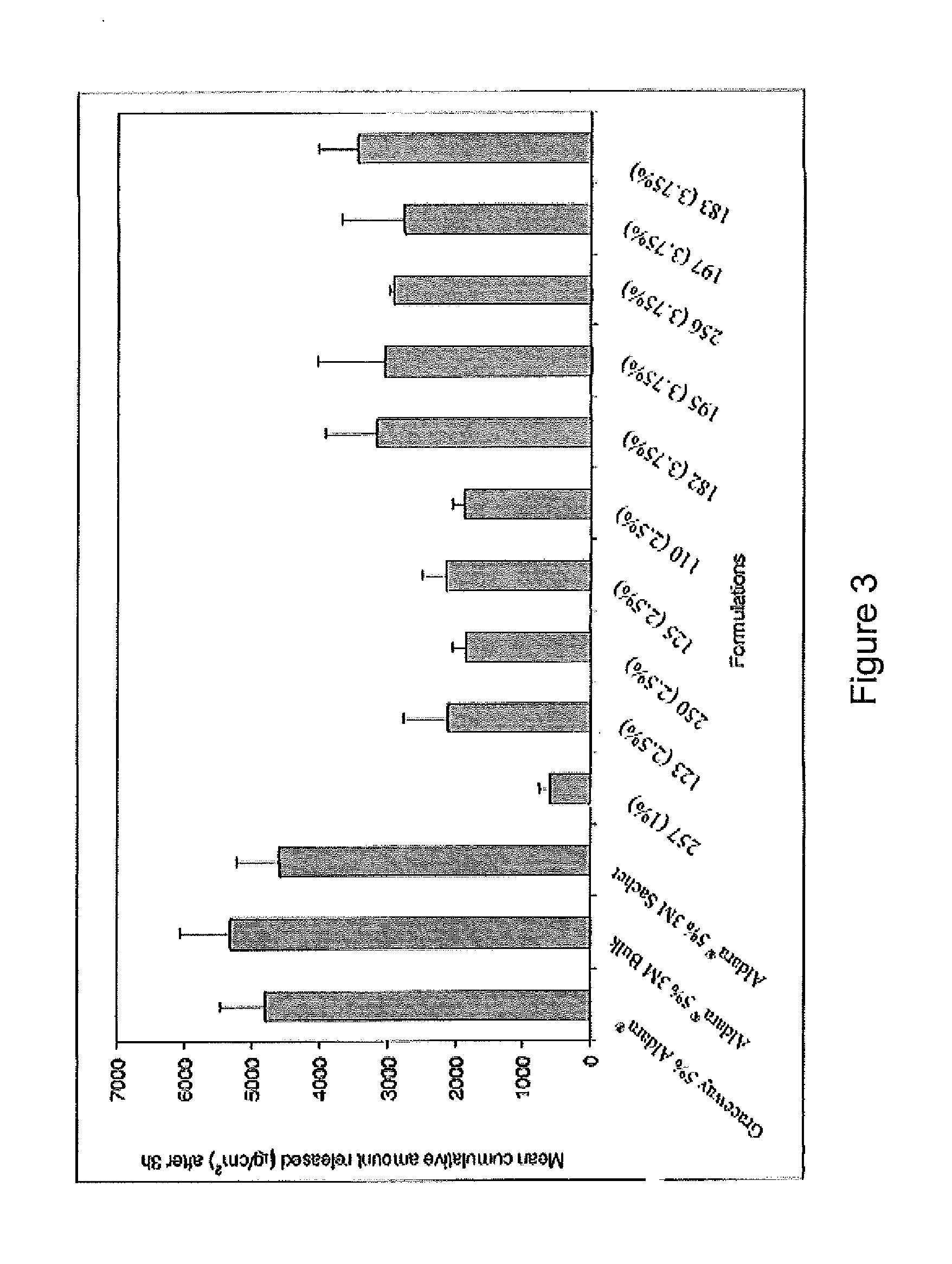
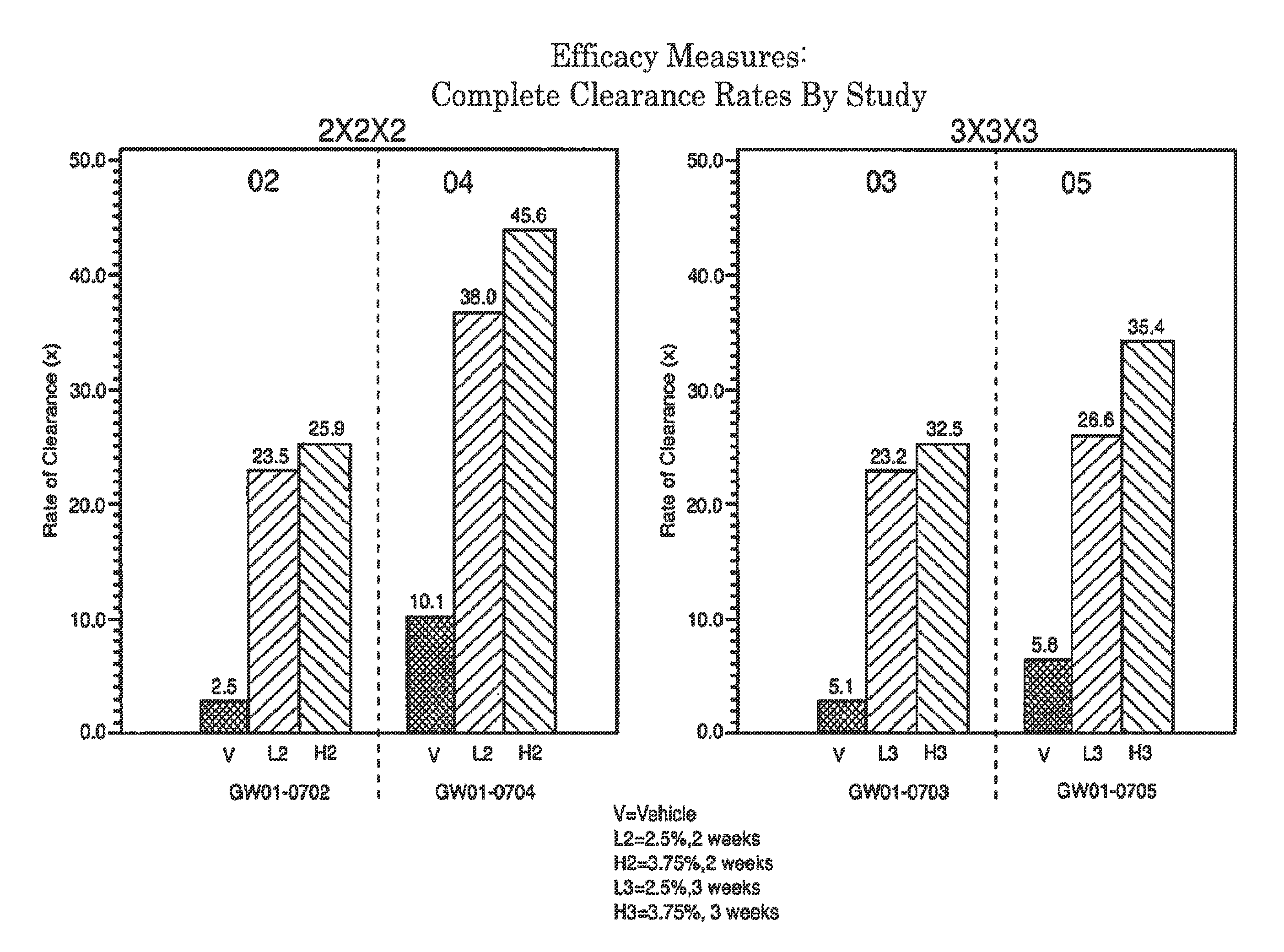
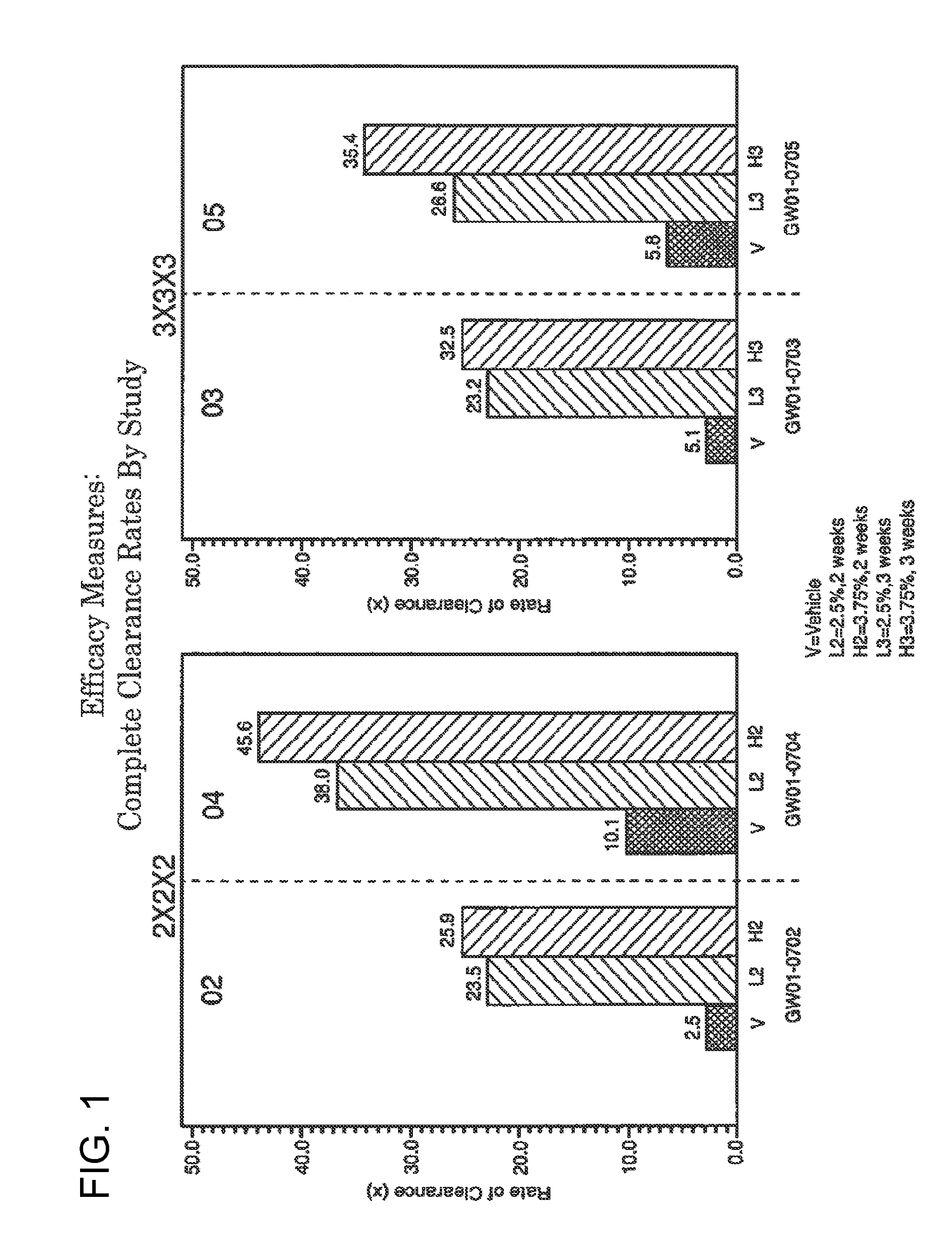
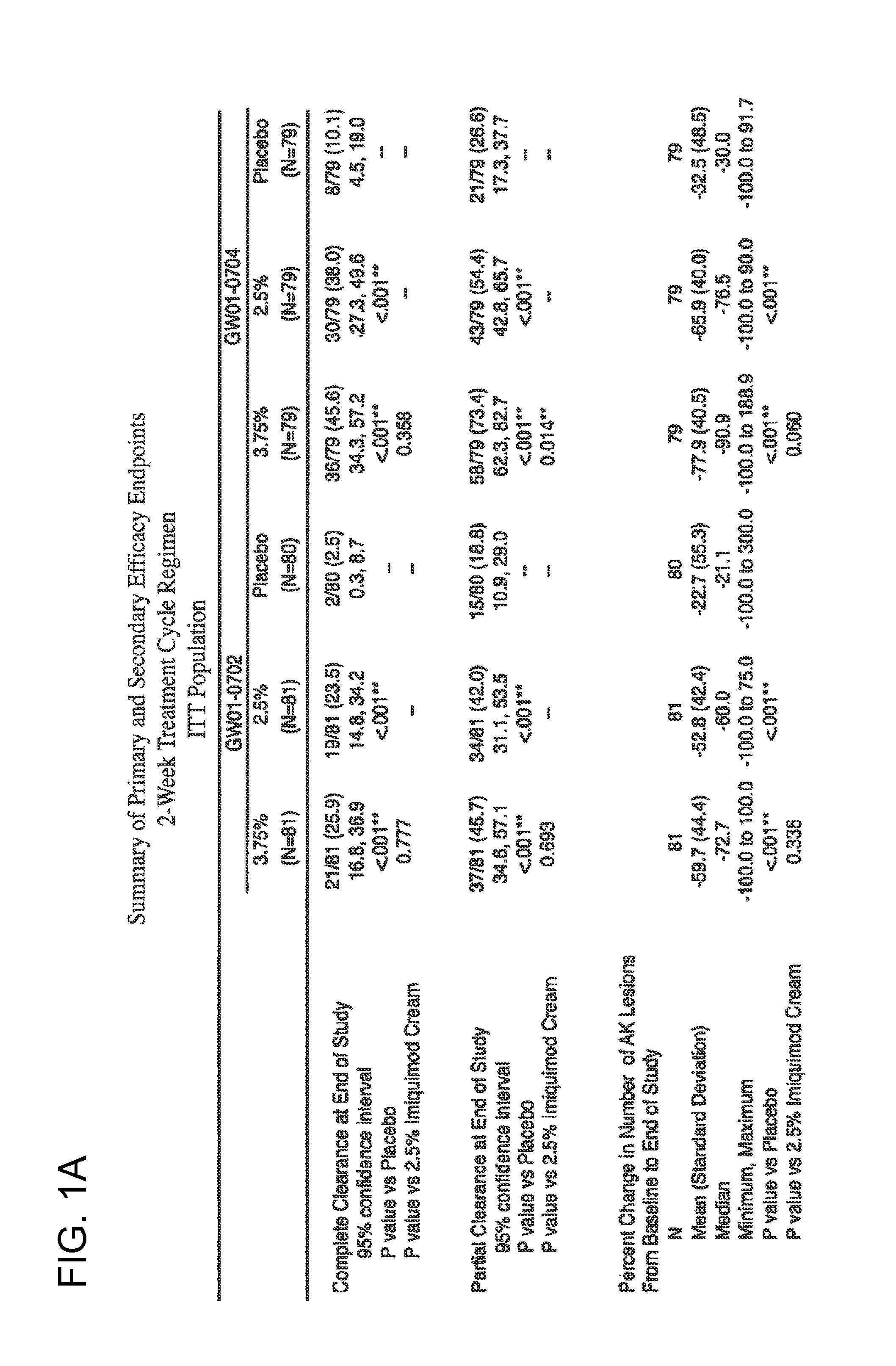
2 x 2 x 2 WEEK TREATMENT REGIMEN FOR TREATING ACTINIC KERATOSIS WITH PHARMACEUTICAL COMPOSITIONS FORMULATED WITH 2.5% IMIQUIMOD
ActiveUS20110257216A1Improved imiquimodReduced strengthBiocideOintment deliveryActinic keratosesDosing regimen
Pharmaceutical formulations and methods for the topical or transdermal delivery of 1-isobutyl-1H-imidazo[4,5-c]-quinolin-4-amine or 1-(2-methylpropyl)-1H-imidazo[4,5-c]quinolin-4-amine, i.e., imiquimod, to treat actinic keratosis with short durations of therapy, than currently prescribed for the commercially available Aldara® 5% imiquimod cream, as now approved by the U.S. Food & Drug Administration (“FDA”), are disclosed and described. More specifically, lower dosage strength imiquimod formulations to deliver an efficacious dose of imiquimod for treating actinic keratosis with an acceptable safety profile and dosing regimens that are short and more convenient for patient use than the dosing regimen currently approved by the U.S. Food & Drug Administration (“FDA”) for Aldara® 5% imiquimod cream to treat actinic keratosis are also disclosed and described.
Owner:MEDICIS PHARMA CORP
2 x 2 x 2 WEEK DOSING REGIMEN FOR TREATING ACTINIC KERATOSIS WITH PHARMACEUTICAL COMPOSITIONS FORMULATED WITH 3.75 % IMIQUIMOD
ActiveUS20110257218A1Improved imiquimodReduced strengthBiocideOintment deliveryDosing regimenRegimen
Owner:MEDICIS PHARMA CORP
Compositions and Methods of Topical Application and Transdermal Delivery of Botulinum Toxins without Reduced Non-Toxin Proteins
InactiveUS20070116724A1Low antigenicityBlood stabilityCosmetic preparationsNervous disorderHypodermoclysisEpithelium
This invention relates to novel compositions of botulinum toxin that can be applied topically for various therapeutic, aesthetic and / or cosmetic purposes. The compositions may include botulinum toxin complexes, wherein the amounts of hemagglutinin, non-toxin non-hemagglutinin and / or exogenous albumin are selectively and independently reduced compared to conventional commercially available botulinum toxin. The compositions may further contain molecules that are not native to botulinum toxin and that bind non-covalently to the botulinum toxin complexes, thereby acting as skin-tropic “adhesion molecules” to improve the ability of the toxin complexes to adhere to and to penetrate the skin epithelium. The compositions have an improved safety profile compared to existing botulinum-containing compositions that are injected subcutaneously. Methods for the use of such compositions are also contemplated by this invention.
Owner:REVANCE THERAPEUTICS INC
3 x 3 x 3 WEEK TREATMENT REGIMEN FOR TREATING ACTINIC KERATOSIS WITH PHARMACEUTICAL COMPOSITIONS FORMULATED WITH 2.5% IMIQUIMOD
InactiveUS20110257217A1Improved imiquimodReduced strengthBiocideOintment deliveryDosing regimenRegimen
Pharmaceutical formulations and methods for the topical or transdermal delivery of 1-isobutyl-1H-imidazo[4,5-c]-quinolin-4-amine or 1-(2-methylpropyl)-1H-imidazo[4,5-c]quinolin-4-amine, i.e., imiquimod, to treat actinic keratosis with short durations of therapy, than currently prescribed for the commercially available Aldara® 5% imiquimod cream, as now approved by the U.S. Food & Drug Administration (“FDA”), are disclosed and described. More specifically, lower dosage strength imiquimod formulations to deliver an efficacious dose of imiquimod for treating actinic keratosis with an acceptable safety profile and dosing regimens that are short and more convenient for patient use than the dosing regimen currently approved by the U.S. Food & Drug Administration (“FDA”) for Aldara° 5% imiquimod cream to treat actinic keratosis are also disclosed and described.
Owner:MEDICIS PHARMA CORP
Fluticasone lotion having improved vasoconstrictor activity
InactiveUS7300669B2Cosmetic preparationsOrganic active ingredientsFluticasone propionateSafety profile
A fluticasone lotion having improved vasoconstrictor and anti-inflammatory activity and higher than expected potency. The fluticasone lotion contains 0.05 weight percent fluticasone propionate and an oil-in-water vehicle that includes excipients. The fluticasone lotion is unexpectedly efficacious while exhibiting an improved safety profile.
Owner:FOUGERA PHARM INC
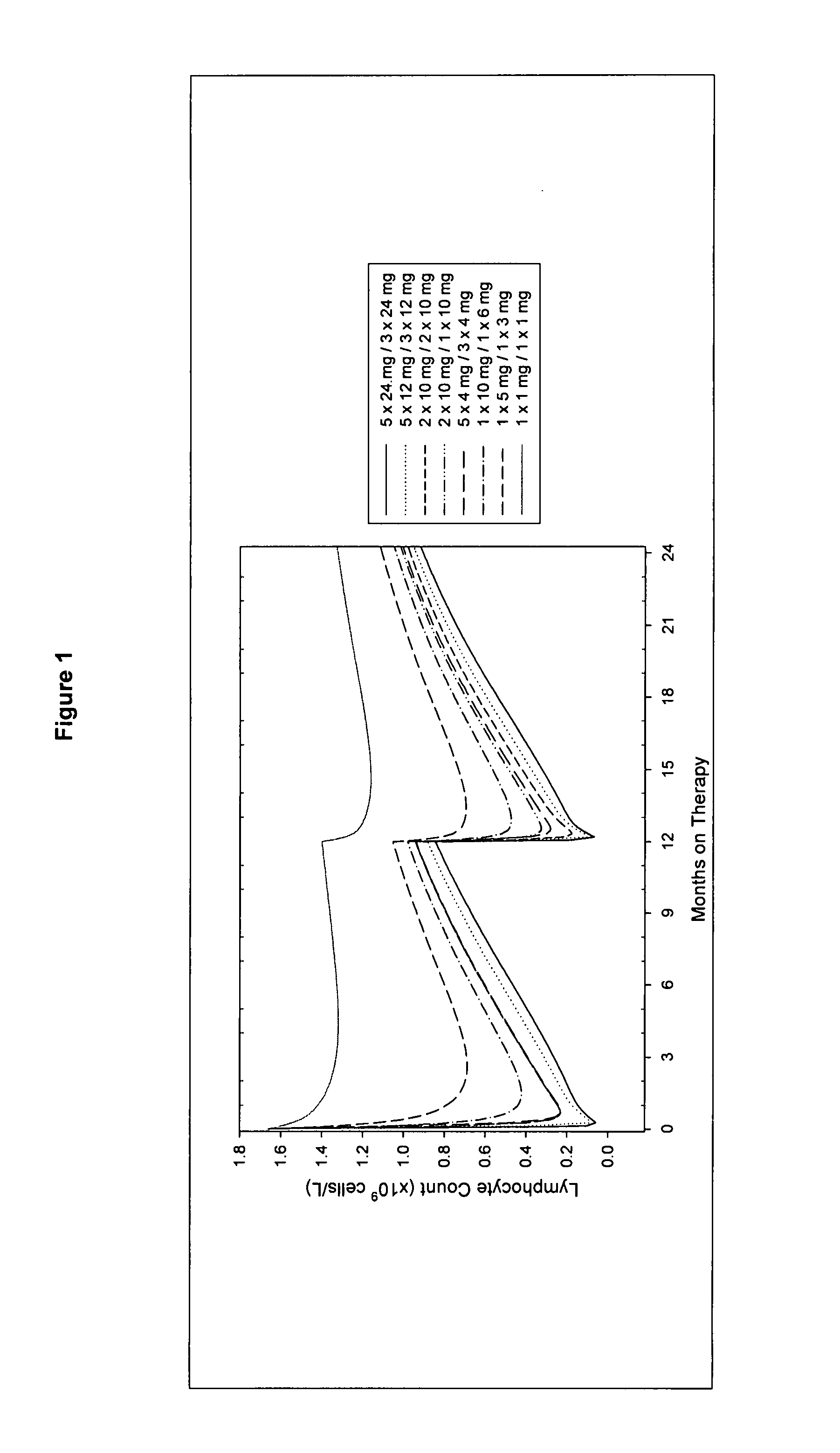
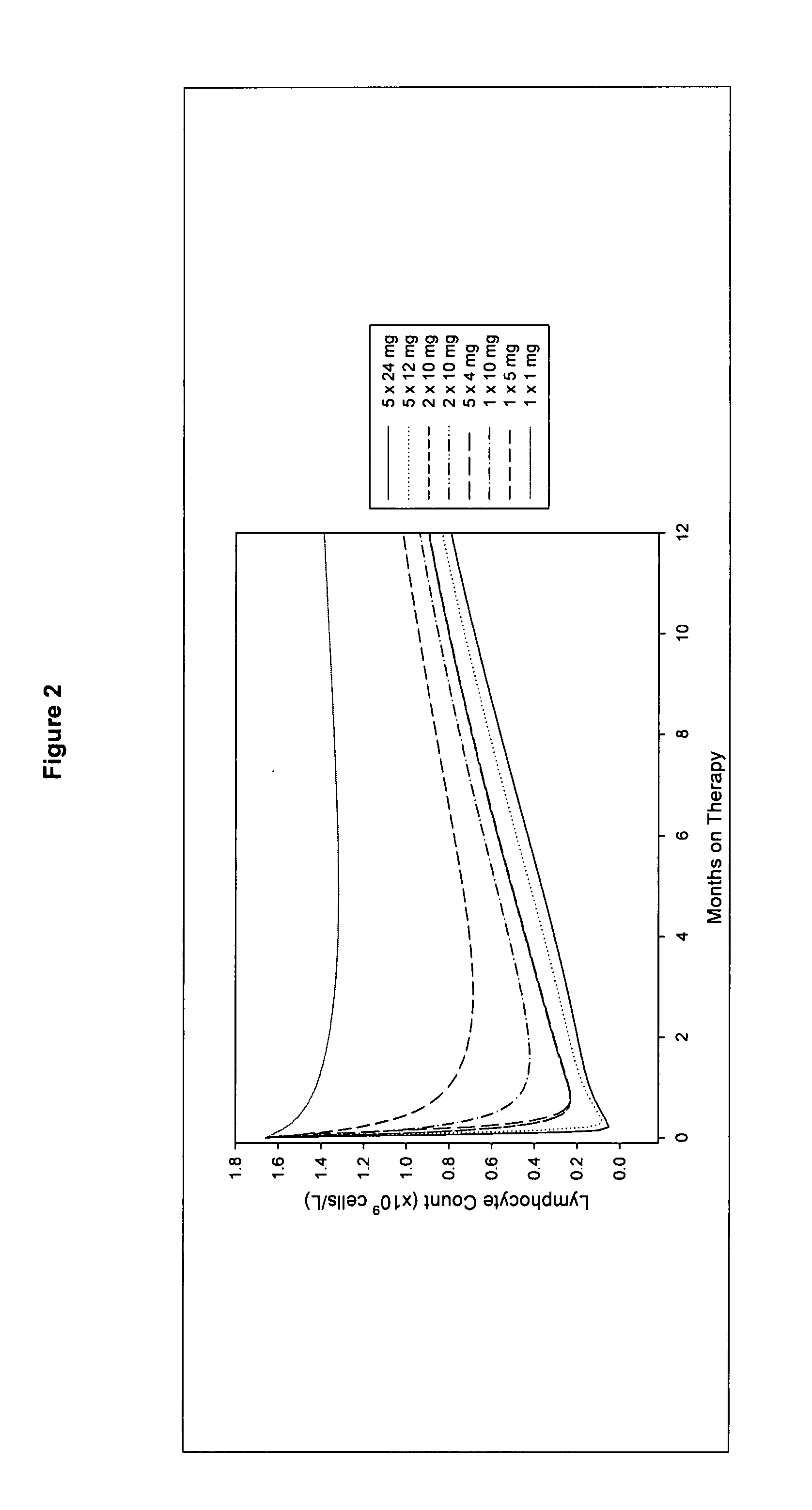
Treatment of multiple sclerosis (MS)
ActiveUS20080267954A1Maximum efficacyImprove securityAntibody ingredientsImmunoglobulinsGynecologySafety profile
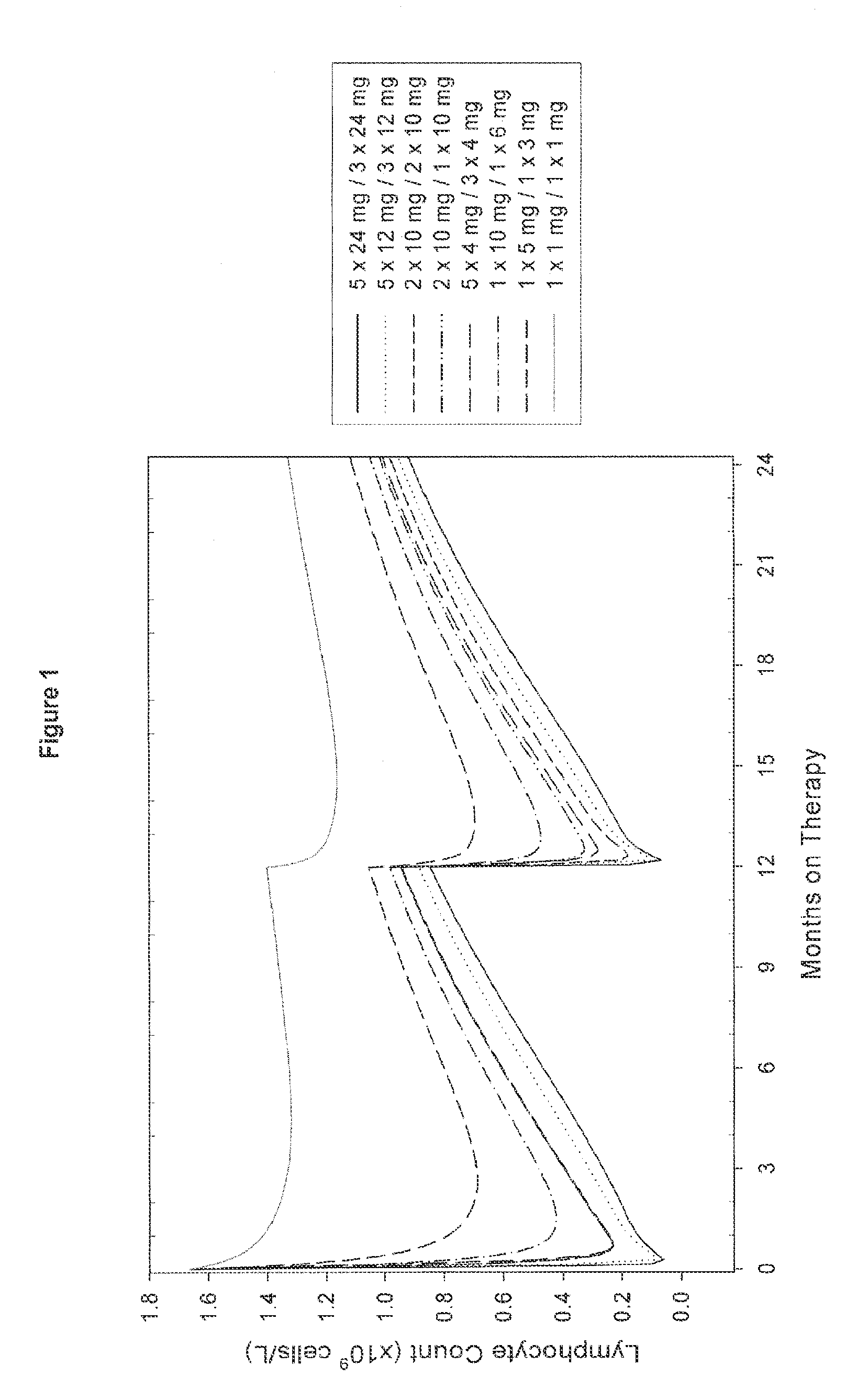
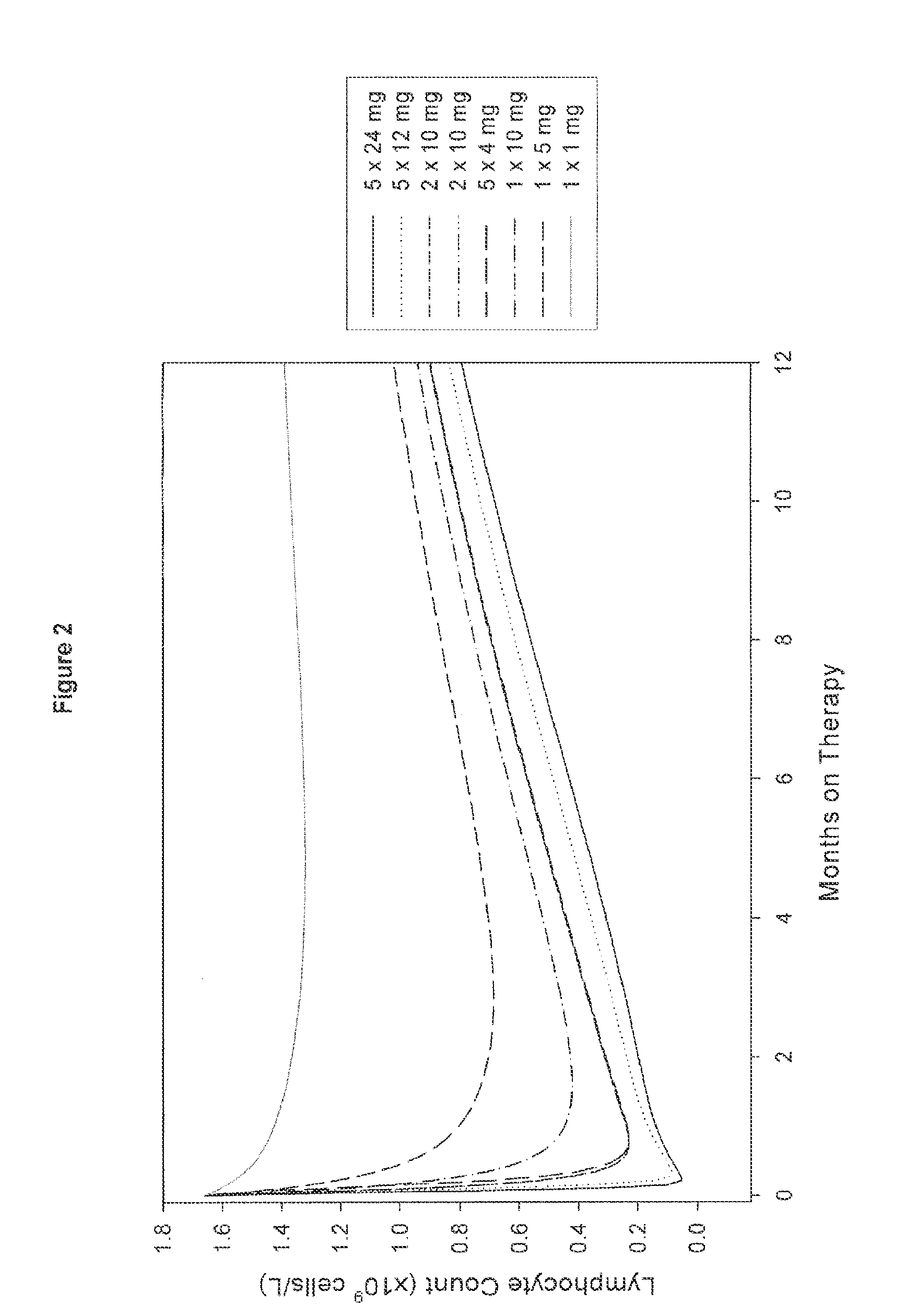
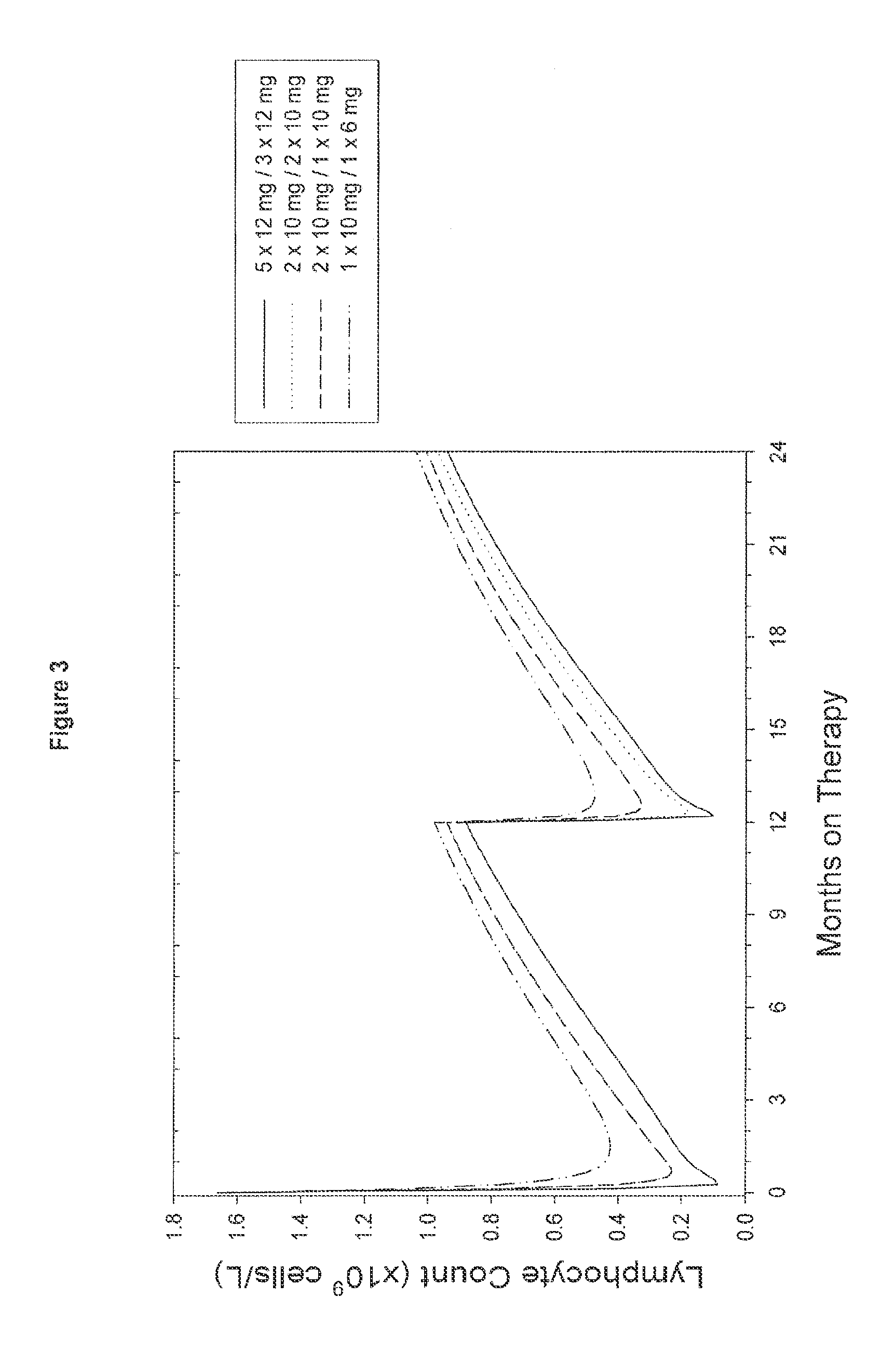
A method for treatment of multiple sclerosis (MS) with Campath-1H with significant efficacy and a favourable safety profile is described, which offers an acceptable benefit / risk ratio. Especially described is the use of Campath-1H (alemtuzumab) for the production of a medicament for the treatment of multiple sclerosis (MS), comprising a first treatment cycle followed by at least one further treatment cycle of Campath-1H (alemtuzumab), in which each treatment cycle comprises 1-5 daily doses which are applied on consecutive days, wherein the daily dose is >0 and ≦12 mg, and wherein each treatment cycle is separated from the next cycle by at least 1-24 months. Also described are treatment regimens comprising the administration of less than 12 mg / day of Campath-1H for a period of 1-5 consecutive days.
Owner:ALCAFLEU MANAGEMENT +1
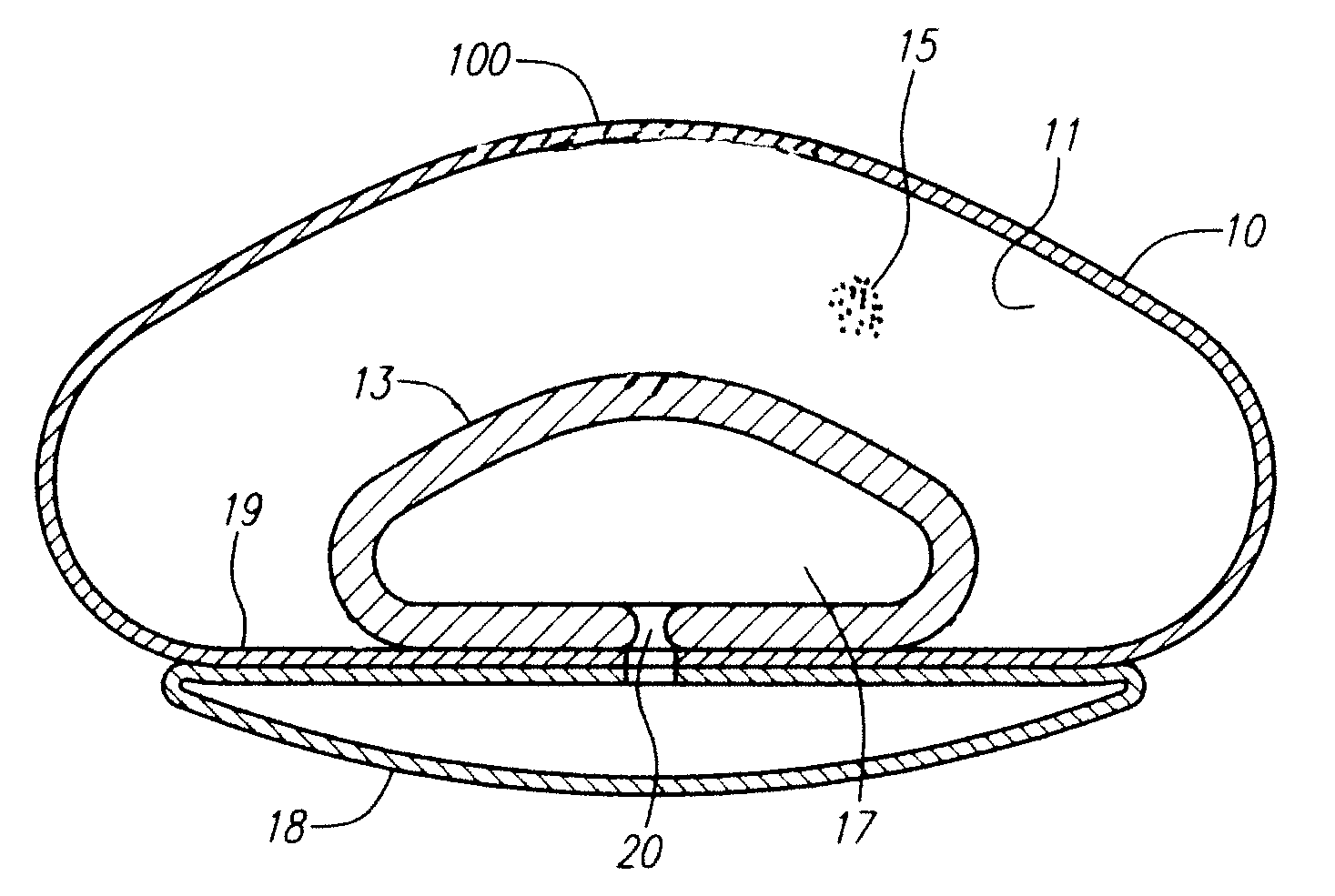
Modulating buttress saline mammary prosthesis
InactiveUS20090216323A1High viscosityImprove performanceMammary implantsFluid compartmentsSafety profile
A saline-filled breast prosthesis has enhanced performance by virtue of having tactile characteristics approximating those of gel-filled implants. The invention retains all the benefits of the recognized safety profile, straight-forward construction, and broad clinical indications for saline implants. An enhanced implantable mammary prosthesis comprises a shell, a slurry filler compartment interior to the shell containing slurry filler, a fluid compartment, the fluid compartment being deformable from a neutral profile under pressure from the slurry filler and which recoils to the neutral profile when not under pressure, a reservoir, the reservoir being disposed external to the shell, the reservoir and fluid compartment being fluidically coupled by a port, and a limiting membrane region disposed between the fluid compartment and the reservoir.
Owner:LEDERGERBER WALTER J
Lower dosage strength imiquimod formulations and short dosing regimens for treating genital and perianal warts
InactiveUS20110207766A1Low dosage strengthSimplified dosing regimenBiocideOintment deliveryDosing regimenRegimen
Pharmaceutical formulations and methods for the topical or transdermal delivery of 1isobutyl-1H-imidazo[4,5-c]-quinolin-4-amine or 1-(2-methylpropyl)-1H-imidazo[4,5 c]quinolin-4-amine, i.e., imiquimod, to treat genital / perianal warts with shorter durations of therapy than currently prescribed for the commercially available Aldara® 5% imiquimod cream, as now approved by the U.S. Food & Drug Administration (“FDA”), are disclosed and described. More specifically, lower dosage strength imiquimod formulations to deliver an efficacious dose of imiquimod for treating genital / perianal warts with an acceptable safety profile and dosing regimens that are shorter and more convenient for patient use than the dosing regimen currently approved by the U.S. Food & Drug Administration (“FDA”) for Aldara® 5% imiquimod cream to treat genital / perianal warts are also disclosed and described.
Owner:MEDICIS PHARMA CORP
Drug delivery system for conscious sedation
InactiveUS8393321B2Increase oxygenationRelief the painHalogenated hydrocarbon active ingredientsNervous disorderAmnesiaSafety profile
Inhalant anesthetics are developed with a number of properties including rapid onset and recovery, controllability, and, ideally, a broad safety profile. The efficacy of these agents is measured by their ability to create anesthesia within the framework of the other desirable properties. The instant invention focuses on the dosage level where analgesia occurs but amnesia or lack of consciousness does not. In addition to identifying the dosage level where pain is sharply reduced or eliminated but awareness remains, a delivery system for safe and effective delivery of the agent is described.
Owner:FIRST NIAGARA BANK
Pharmaceutical compositions for parenteral administration
ActiveUS20130004592A1Improve solubilityEnhance dispersivityPowder deliveryHeavy metal active ingredientsLipid formationSolubility
Owner:LIPOSEUTICALS
Modulating buttress saline mammary prosthesis
InactiveUS8202316B2Enhanced implantable mammary prosthesisSolve the lack of rigidityMammary implantsButtressFluid compartments
A saline-filled breast prosthesis has enhanced performance by virtue of having tactile characteristics approximating those of gel-filled implants. The invention retains all the benefits of the recognized safety profile, straight-forward construction, and broad clinical indications for saline implants. An enhanced implantable mammary prosthesis comprises a shell, a slurry filler compartment interior to the shell containing slurry filler, a fluid compartment, the fluid compartment being deformable from a neutral profile under pressure from the slurry filler and which recoils to the neutral profile when not under pressure, a reservoir, the reservoir being disposed external to the shell, the reservoir and fluid compartment being fluidically coupled by a port, and a limiting membrane region disposed between the fluid compartment and the reservoir.
Owner:LEDERGERBER WALTER J
Compounds for treatment of cardiac arrhythmia, synthesis, and methods of use
The subject invention pertains to novel compounds (and salts thereof), and compositions comprising the compounds, for the treatment of cardiac arrhythmias. The subject invention further concerns methods of making the novel compounds. The novel compounds are rapidly metabolized analogs of amiodarone, having the distinct and advantageous characteristic of being metabolized to a less lipophilic compound. This results in an improved safety profile. The new compounds have particular utility for treating life-threatening ventricular tachyarrhythmias, especially in patients with congestive heart failure (CHF). The compounds also provide effective management for ventricular arrhythmias and supraventricular arrhythmias, including atrial fibrillation and re-entrant tachyarrhythmias involving accessory pathways.
Owner:ARYX THERAPEUTICS
Topical formulations of 5-alpha-reductase inhibitors and uses thereof
ActiveUS20200147071A1Increase in hair countExpand coverageCosmetic preparationsOrganic active ingredientsSide effectBULK ACTIVE INGREDIENT
Disclosed herein are topical compositions of 5-α reductase inhibitors, such as dutasteride or finasteride, or a pharmaceutically acceptable salt, ester, or derivative thereof and the use of the compositions for the treatment of hair loss secondary to endocrine therapy in patients with breast cancer (Endocrine Therapy-Induced Alopecia or ETIA), androgenetic alopecia (AGA), alopecia areata, and hirsutism. The topical composition is advantageous over the existing oral compositions of 5-α reductase inhibitors because the topical composition is safer and more effective. The topical formulation may allow for a slow release of the active ingredient dutasteride, better penetration at the therapeutically effective amount of dutasteride with an improved safety profile because it does not need to travel through the bloodstream to be efficacious, thereby minimizing the risk of systemic side effects.
Owner:VARSONA THERAPEUTICS INC
Chafing Dish
The present invention relates to a more efficient chafing dish basin or Bain Marie for the hotel / catering and hospitality industry, that has a first exterior basin with a heating element, a second basin comprising an insulating base, one or more heat conductive layers and a third food basin. The first exterior basin has a heating element that extends through the insulation base, and heat conductive layer(s) to contact the bottom of the food basin. The heating element can be heated by naked flames or electrically. Sufficient heat is transferred to the food basin to obviate the need for water or allows for significantly less water to be used, increasing the safety profile of these chafing dishes.
Owner:CATALYTIC BURNERS
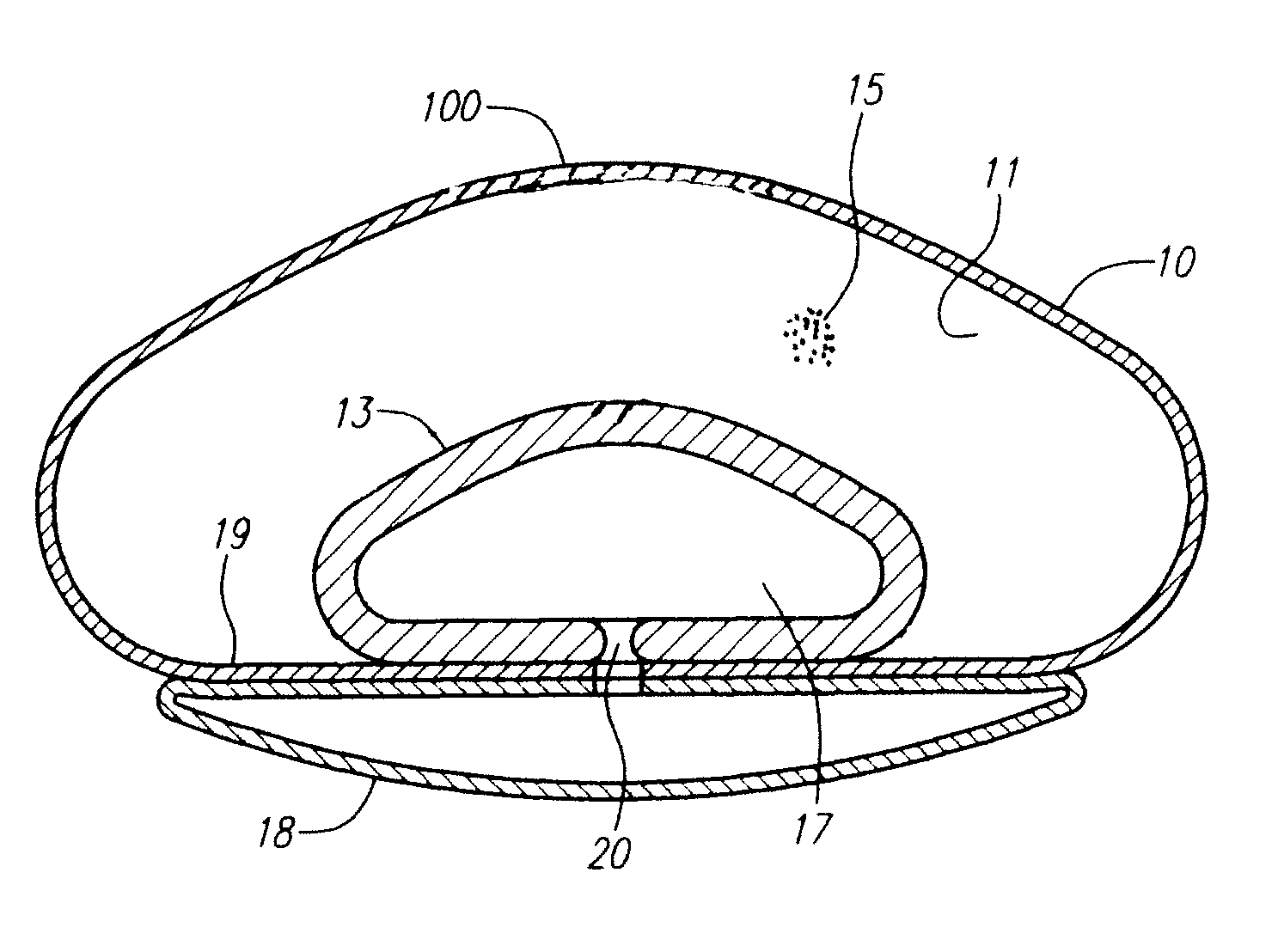
Rear crash safety profile
A rear impact system for an electric vehicle includes a left crash beam coupled with a rear portion of a rear wheel arch of the electric vehicle and extending to a rear bumper of the electric vehicle and a right crash beam coupled with the rear portion of the rear wheel arch and extending to the rear bumper. Each of the left crash beam and the right crash beam defines a generally octagonal shape. Each of the left crash beam and the right crash beam defines an interior comprising a plurality of ribs extending in a longitudinal direction.
Owner:THUNDER POWER ELECTRIC VEHICLE LTD
Identification of invasive and slow-growing tumorigenic cell subsets in tumors
InactiveUS20130157285A1Convenient treatmentLow costSugar derivativesMicrobiological testing/measurementDiseaseSafety profile
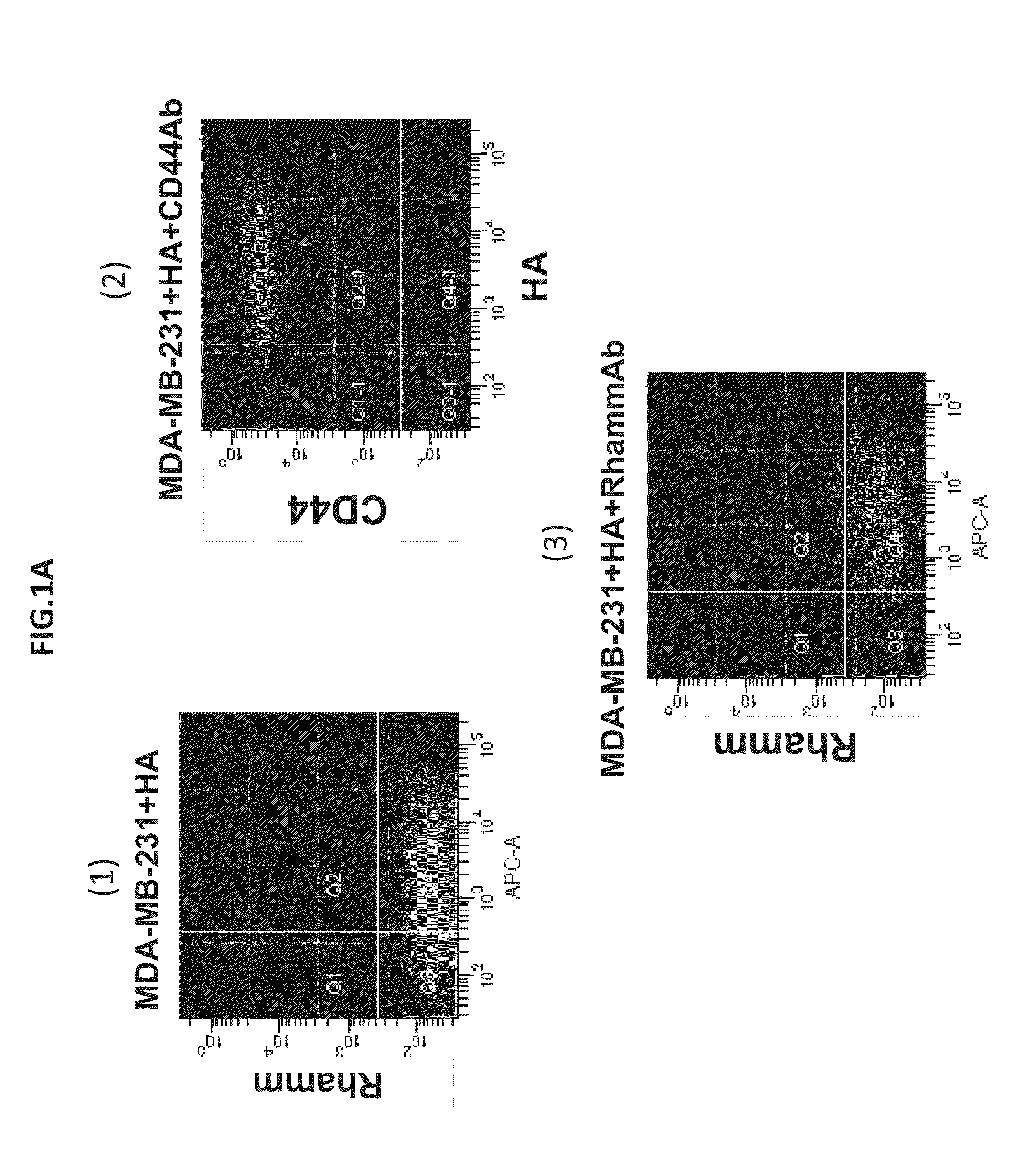
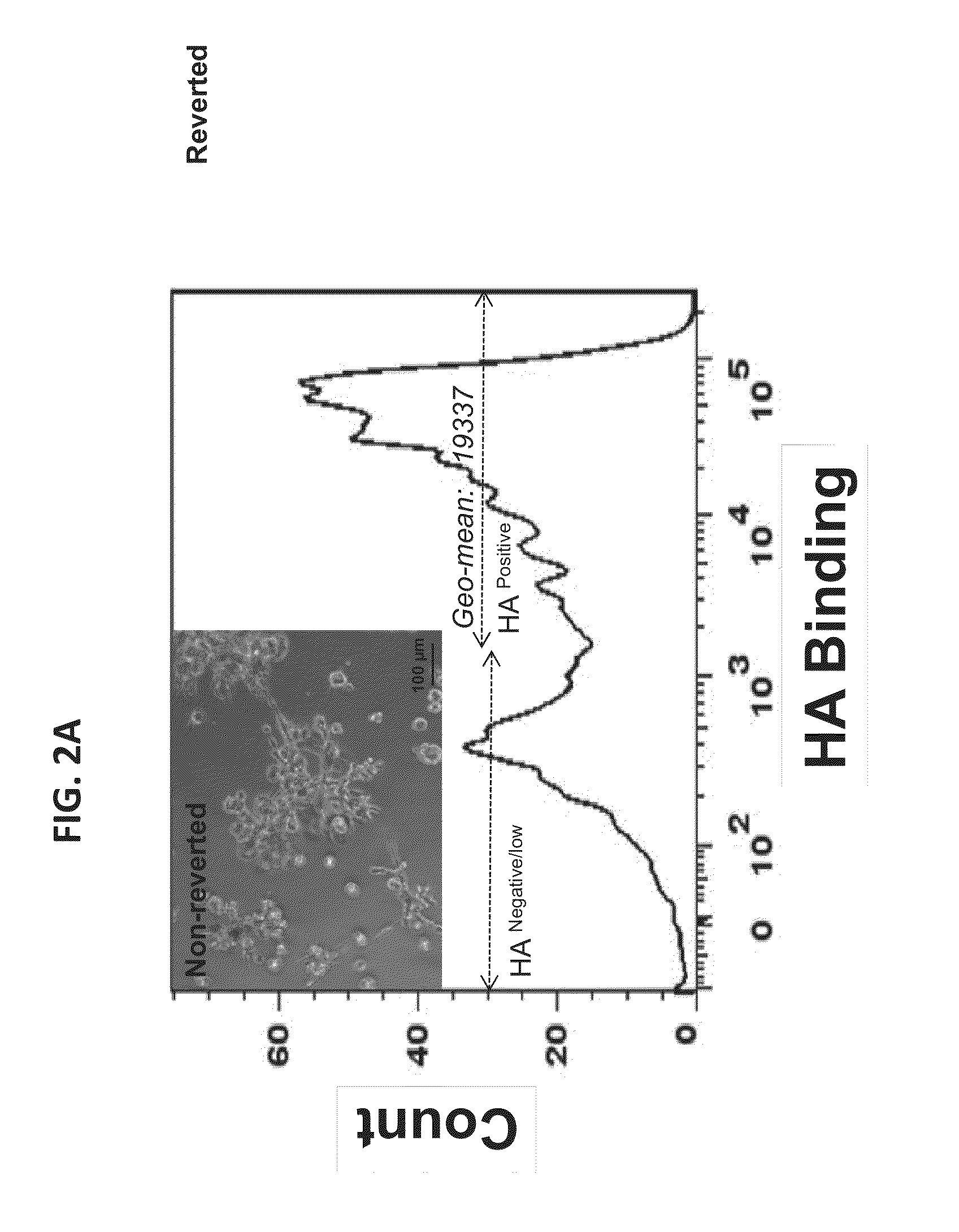
HA-based functional probes and a multiplexed targeting strategy for detection and isolation of invasive subpopulations in breast cancer cell lines. Methods for using HA metabolism for profiling and sorting breast cancer heterogeneity. As such, HA-based functional probes have appropriate targeting capacity and safety profiles for development as imaging and therapeutic agents for following repair and neoplastic disease processes such as breast cancer.
Owner:RGT UNIV OF CALIFORNIA
Modafinil Pharmaceutical Compositions
Pharmaceutical compositions comprising modafinil in the form of particles of defined size and methods for preparing same. The particle size of modafinil can have a significant effect on the potency and safety profile of the drug.
Owner:CEPHALON INC
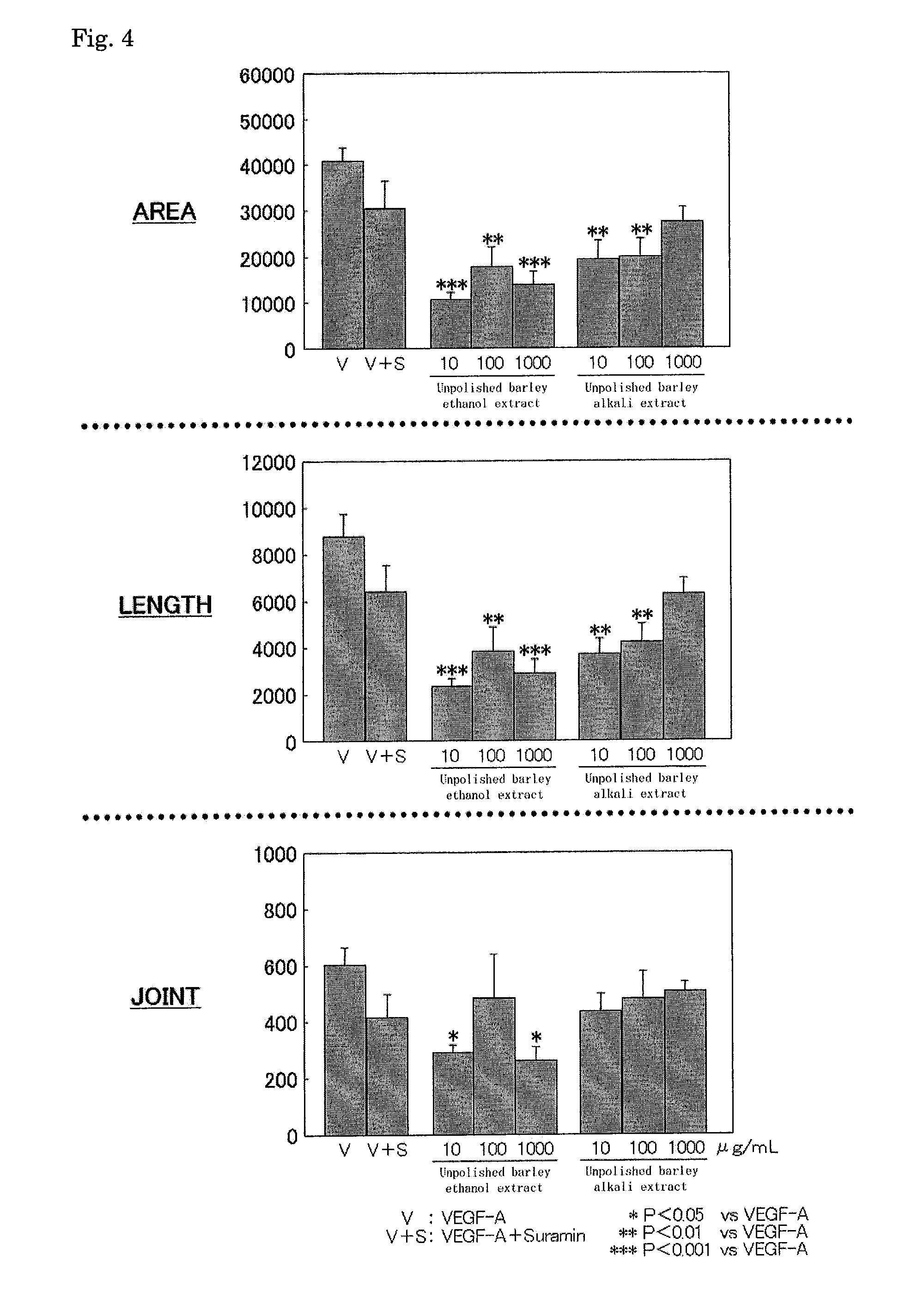
Anti-angiogenic composition comprising grain-derived component as active ingredient
It is an object of the invention to provide a composition with an excellent action of inhibiting vascularization, as prepared by a simple method suitable for actual production from an inexpensive, relatively readily available material without any problematic safety profile at an industrial scale without any complicated purification step. The composition is a composition for inhibiting vascularization, which contains a barley-derived ingredient selected from the group consisting of an unpolished barley ethyl alcohol extract fraction, an unpolished barley alkali extract fraction and fermented barley (preferably, a residual solution from the distillation of barley distilled spirits) as the active component with an action of inhibiting vascularization. The composition for inhibiting vascularization is a composition for therapeutically treating or preventing diseases which vascularization should be inhibited, specifically diseases with an etiology of abnormal vascularization in tumor or cancer, chronic inflammation or retinopathy. The composition is in a form selected from the group consisting of food additives, food materials, foods and drinks, pharmaceutical products under regulations by the Ministry of Health and Labor in Japan and quasi-pharmaceutical products under regulations by the Ministry of Health and Labor in Japan, and feeds.
Owner:SANWA SHURUI
Lower dosage strength pharmaceutical compositions forumlated with 3.75% imiquimod
InactiveUS20110257219A1Improved imiquimodReduced strengthBiocideOintment deliveryDosing regimenRegimen
Pharmaceutical formulations and methods for the topical or transdermal delivery of 1-isobutyl-1H-imidazo[4,5-c]-quinolin-4-amine or 1-(2-methylpropyl)-1H-imidazo[4,5-c]quinolin-4-amine, i.e., imiquimod, to treat actinic keratosis with short durations of therapy, than currently prescribed for the commercially available Aldara® 5% imiquimod cream, as now approved by the U.S. Food & Drug Administration (“FDA”), are disclosed and described. More specifically, lower dosage strength imiquimod formulations to deliver an efficacious dose of imiquimod for treating actinic keratosis with an acceptable safety profile and dosing regimens that are short and more convenient for patient use than the dosing regimen currently approved by the U.S. Food & Drug Administration (“FDA”) for Aldara® 5% imiquimod cream to treat actinic keratosis are also disclosed and described.
Owner:MEDICIS PHARMA CORP
Treatment of multiple sclerosis (MS)
InactiveUS20130108625A1Maximum efficacyImprove securityAntibody ingredientsImmunoglobulinsSafety profileAlemtuzumab
Owner:ALCAFLEU MANAGEMENT +1
Compounds for treatment of cardiac arrhythmia, synthesis, and methods of use
InactiveUS7037933B2Fast metabolismReduce compoundingImage enhancementBiocideVentricular TachyarrhythmiasSafety profile
The subject invention pertains to novel compounds (and salts thereof), and compositions comprising the compounds, for the treatment of cardiac arrhythmias. The subject invention further concerns methods of making the novel compounds. The novel compounds are rapidly metabolized analogs of amiodarone, having the distinct and advantageous characteristic of being metabolized to a less lipophilic compound. This results in an improved safety profile. The new compounds have particular utility for treating life-threatening ventricular tachyarrhythmias, especially in patients with congestive heart failure (CHF). The compounds also provide effective management for ventricular arrhythmias and supraventricular arrhythmias, including atrial fibrillation and re-entrant tachyarrhythmias involving accessory pathways.
Owner:HESP LLC
Novel compounds for treatment of cardiac arrhythmia, synthesis, and methods of use
InactiveUS20030158194A1Short onset timeDecreased and more manageable long-term toxicityBiocideOrganic chemistryRe entrantCardiac arrhythmia
The subject invention pertains to novel compounds (and salts thereof), and compositions comprising the compounds, for the treatment of cardiac arrhythmias. The subject invention further concerns methods of making the novel compounds. The novel compounds are rapidly metabolized analogs of amiodarone, having the distinct and advantageous characteristic of being metabolized to a less lipophilic compound. This results in an improved safety profile. The new compounds have particular utility for treating life-threatening ventricular tachyarrhythmias, especially in patients with congestive heart failure (CHF). The compounds also provide effective management for ventricular arrhythmias and supraventricular arrhythmias, including atrial fibrillation and re-entrant tachyarrhythmias involving accessory pathways.
Owner:ARYX THERAPEUTICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com